Aggregation-induced emission of azobenzene towards a sensitive indication on the self-assembly of a cellulose material†
Jingjing
Gu‡§
a,
Guoqiang
Zhang‡
a,
Jiahao
Chang
b,
Lei
Zhang
 a,
Zhongtao
Wu
a,
Zhongtao
Wu
 *a,
Xiliang
Luo
*a,
Xiliang
Luo
 *a and
Hao
Wang
*c
*a and
Hao
Wang
*c
aKey Laboratory of Optic-electric Sensing and Analytical Chemistry for Life Science, MOE; Shandong Key Laboratory of Biochemical Analysis, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao, 266042, China. E-mail: wuzhongtao@qust.edu.cn; xiliangluo@qust.edu.cn; kingwhao@sohu.com
bSchool of Clinical Medicine, Shandong Second Medical University, Weifang, 261053, China
cDongguan Eighth People's Hospital, Dongguan 523000, China
First published on 11th July 2024
Abstract
Azobenzene is one of the most commonly used photochromic molecules, but is rarely used as a fluorescence probe in materials chemistry, due to its efficient photoisomerization providing competition for consumption of light energy. In this study, an azobenzene-containing ammonium surfactant was designed for fabricating an ionic cellulose material through an electrostatic complexation with carboxymethyl cellulose. Based on the AIE effect of the azobenzene motif, the cellulose material exhibited fluorescence. Furthermore, in aqueous conditions, the self-assembly of this cellulose material could be well regulated by effecting azobenzene isomerization under UV/Vis irradiation, which resulted in a remarkable change in the fluorescence intensity. As compared to the commonly used UV-Vis absorption, the fluorescence change of azobenzene was found to provide a more sensitive indication for tracking the dissolution and precipitation of the ionic cellulose-surfactant assemblies in aqueous conditions. This work has provided a useful strategy for fabricating photoresponsive fluorescent biomaterials based on azobenzene, opening a new opportunity for detecting drug-loading materials.
Introduction
Fluorescent biomaterials are attractive in the fields of biochemistry and biomedicine and could be used for imaging tissues, cells, and animals, and for monitoring biological processes.1 As one of the most commonly used photochromic molecules, azobenzene is of great importance in the development of photoresponsive biomaterials, owing to its distinct color change and conformational change via trans–cis isomerization. For example, azobenzene has been widely used for regulating the structure, physical properties and biological activity of DNA.2–7 Sugar materials functionalized by azobenzene could be used as reworkable adhesives.8,9 However, thus far, azobenzene-based fluorescent biomaterials are rather limited, despite azobenzene showing good absorbance for π–π* and n–π* transitions at UV and Vis light regions. The efficient isomerization of azobenzene consumes the absorbed light energy in a non-radiative way, which makes the radiative emission non-competitive. Therefore, azobenzene has been deemed as a non-fluorescent chromophore and is rarely used for the development of fluorescent biomaterials.Molecular structure design efforts have realized fluorescence for some azobenzene derivatives, but normally with very weak intensity or requiring intensified light stimulation.10–13 In contrast, forming aggregates would be an effective strategy for facilitating the radiative emission of azobenzene-containing materials, via an aggregation-induced emission (AIE) effect.14–18 In principle, formation of the aggregated packing state could suppress the photoisomerization of azobenzene, and hence be the main cause for the observation of enhanced fluorescence. But interestingly, studies found that once the aggregates form and light-driven AIE is saturated, the trans–cis isomerization of azobenzene would not impact the emission intensity.13 This observation means that the concentration of the aggregates is the determining factor for impacting the AIE intensity of azobenzene-containing materials, and an efficient regulation of the concentration of an azobenzene-containing assembly would result in a change in the fluorescence emission intensity. In other words, measurement of fluorescence here could provide an indication of the concentration of such materials. This strategy affords a guide for fabricating fluorescent biomaterials based on azobenzene.
In solvent-free conditions, a class of photoresponsive ionic biomaterials based on the photoisomerization of azobenzene has been developed in the past decade.19–24 These biomaterials are complexed by anionic biomolecules and cationic azobenzene-containing ammonium surfactants, forming ionic self-assemblies in ordered structures at room temperature. Currently, such biomaterials are mainly studied in solvent-free conditions, exhibiting interesting physicochemical property changes under triggers of external stimuli. This phenomenon provides a great opportunity to use such biomaterials for biomedical purposes. For example, by taking advantage of the ability of these biomaterials to undergo photoinduced phase changes, they could be used as loading materials for controllable drug release. However, the biomedical use of these biomaterials in the solution state requires real-time determinations of their concentration, dissociation and degradation to ensure their biosafety. However, tracking the concentration of such biomaterials in aqueous conditions is difficult. Despite various studies investigating the physicochemical property changes in solvent-free conditions, no sensitive method has been developed yet for indicating the real-time concentrations of ionic complexes between biomacromolecules and surfactants in aqueous conditions, which limits their use in the biomedical area.
In the ionic biomaterials fabricated with azobenzene-containing surfactants, the ordered packing state of the surfactant sublayer would provide a structural basis for radiative emission of the azobenzene motif under light excitation by forming an aggregated state. This idea has prompted us to investigate whether the photoluminescence (PL) behavior of azobenzene could reflect the changes in concentration of such ionic biomaterials in aqueous conditions. In this study, an ionic cellulose material (CMC-AZO) was fabricated by effecting an electrostatic complexation between carboxymethyl cellulose (CMC) and synthesized surfactant AZO (Fig. 1). CMC-AZO could exhibit fluorescence owing to the AIE effect of azobenzene. And in aqueous systems, under the assistance of photoisomerization of AZO, CMC-AZO could form self-assemblies of different sizes, efficiently regulating the concentration of CMC-AZO aggregates and further tuning its fluorescence. This work has provided a useful strategy for fabricating photoresponsive fluorescent biomaterials in a manner other than covalently modifying biomolecules with fluorophores or fluorescent proteins, and provides a facile way for determining the concentrations of such biomaterials in aqueous conditions.
 | ||
| Fig. 1 Depictions of the molecular design of AZO, complexation of CMC-AZO and photoresponsive change in PL intensity of CMC-AZO. | ||
Results and discussion
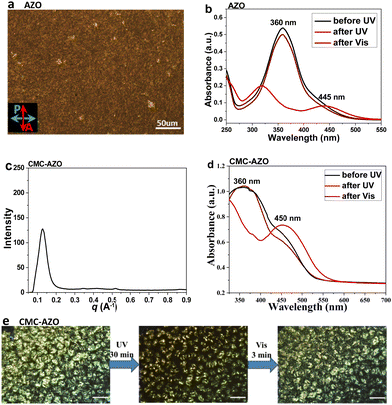
Structurally, AZO contains two flexible chains, which could reduce the molecular packing density. As compared to the single-chain ammonium surfactant, AZO was found to exhibit better photoisomerization ability at room temperature, facilitated by the sufficient free volume from the double-chain design.25 Additionally, the presence of the polyethylene glycol (PEG) chain can increase the water-solubility of AZO, facilitating fabrication of the materials in aqueous conditions. Surfactant AZO was synthesized by following a procedure we previously reported, affording a light orange solid in ∼36% yield over five steps.26 Generally, AZO was furnished by carrying out successive etherification, hydrolysis, diazotization, alkylation and quaternization treatments of the 4-acetoamidophenol starting material (Scheme S1, ESI†). The structure of AZO was confirmed from the results of an NMR analysis. Solid-state AZO showed a birefringent texture at room temperature under polarized optical microscopy (POM), indicating an ordered structure (Fig. 2a). A lamellar ordered structure of AZO could be deduced from its small-angle X-ray scattering (SAXS) profile, showing a first-order peak at 0.17 Å−1 and following harmonic peaks at 0.34 Å−1 and 0.51 Å−1, which indicated a d-spacing distance of 3.60 nm (Fig. S1, ESI†). At room temperature, AZO was indicated to undergo rapid trans–cis isomerization in aqueous solution (10 μM) according to its giving weaker absorption at 365 nm (π–π* transition) and stronger absorption at 445 nm (n–π* transition) under UV light and reversed changes in absorption under Vis light (Fig. 2b).Subsequently, AZO was electrostatically complexed with CMC, affording the ionic material CMC-AZO. Subjecting this material to centrifugation, water-separation and lyophilization purifications yielded solvent-free CMC-AZO as a solid material. Thermogravimetric analysis (TGA) of CMC-AZO showed a water content of less than 3% and thermal integrity up to 200 °C (Fig. S2, ESI†). SAXS analysis revealed CMC-AZO to be in an ordered structure (Fig. 2c), and the ordered structure was further confirmed from the birefringence in a POM analysis (Fig. 2e). After complexing with CMC, AZO could still undergo UV-induced trans–cis and Vis-induced cis–trans isomerizations, by showing typical π–π* transition and n–π* transition changes (Fig. 2d). The photoisomerization of AZO successfully caused a reversible change in the physical state of CMC-AZO. POM analysis (images in Fig. 2e) showed a partial disappearance of the birefringence of CMC-AZO under UV illumination, indicating formation of an isotropic liquid state, and a restoration of the ordered structure under Vis light. This result meant that the reversible trans–cis isomerization could be an effective driving force for promoting a change in the physical state of CMC-AZO at room temperature, albeit in an incomplete way.
Aggregation of AZO is the basis for its strengthened fluorescence. In aqueous conditions, the PL of the AZO aggregate would be expected to be impacted by the concentration and molecular conformation of AZO. A high concentration of AZO would favor the formation of aggregates, and a low concentration a separation of AZO molecules in water. Regarding the impact of conformation, the planar trans-AZO would be expected to prefer to form aggregates due to the π–π interaction between azobenzene motifs. In contrast, the formation of non-planar cis-AZO would disturb the aggregated state. Therefore, in principle, UV and Vis light irradiations could, respectively, reduce and enhance the PL intensity of AZO in aqueous conditions.
To verify these assumptions, studies of CMC-AZO in aqueous conditions involving the dependence of its PL on its concentration and on light were carried out. We prepared a series of AZO suspensions/solutions with concentrations from 14.35 to 0.06 mM, and acquired their PL spectra after they were subjected to Vis and UV irradiation. At very high concentrations of 14.35–3.58 mM, our analysis showed trans-AZO exhibiting decreasing PL intensity with decreasing concentration (Fig. S3, ESI†). Interestingly, our measurements showed a higher PL intensity for cis-AZO than for trans-AZO at each tested concentration, with this phenomenon attributed to a UV-light-promoted dissolution of cis-AZO in water. At high concentrations, some of the trans-AZO apparently formed aqueous suspensions (precipitates) by stacking together after becoming saturated in water. UV light transformed trans-AZO into cis-AZO, causing dissociation of precipitates of stacked AZO molecules, and in turn leading to more cis-AZO dissolving into water. At high concentrations, cis-AZO could still form aggregates, and the concentration increase would intensify the PL signal, as discussed in the Introduction section. With a decrease in the concentration to 1.79 mM, a stronger PL intensity of the trans-AZO aggregate than that of the 3.58 mM suspension was observed, probably generated from the transition of the suspension to the solution state, which could improve the light penetration efficiency. Between concentrations of 1.79–0.45 mM, our spectroscopic measurements showed trans-AZO exhibiting decreasing PL intensity with decreasing concentration, and meanwhile PL intensities much higher than those of cis-AZO (Fig. 3a). For these aqueous solutions, the formation of cis-AZO caused the aggregate to dissociate, thus leading to the disappearance of the PL signal. At a concentration lower than 0.45 mM, trans-AZO gave very weak or no PL signals (Fig. 3b), due to the difficulty in forming aggregates at low concentrations.
In solvent-free conditions, the PL behavior of AZO and CMC-AZO could be monitored with a broad emission band centered at a wavelength of 492 nm under the excitation of 365 nm, owing to the aggregated state (Fig. 3c). Interestingly, small PL intensity decreases of these two samples were also observed upon UV light illumination, attributed to the photoisomerization of AZO. The UV-Vis absorption changes of solid-state AZO and CMC-AZO indicated an obvious trans–cis isomerization upon UV illumination (Fig. S4 and S2d, ESI†). A trans–cis isomerization of AZO would be expected to cause its molecular packing state to change, impacting the UV light penetration efficiency in solid-state samples.27 Time-resolved PL data for AZO and CMC-AZO were collected for analyzing the states of AZO before and after its binding with CMC. The PL lifetime of CMC-AZO was measured to be 0.37 ns, much shorter than the 13.7 ns lifetime of AZO (Fig. 3b), indicating the higher molecular packing density of AZO than that of AZO in CMC-AZO. In CMC-AZO, AZO molecules would be expected to be arranged in a relatively loose way via electrostatic complexation to the CMC skeleton, providing more free volume for the photoisomerization of AZO, and hence leading to energy loss in a non-radiative manner.
For the aggregates/self-assemblies of azobenzene-containing materials in solutions, the PL intensity has turned out to be determined by the concentration of the aggregate, which would not be changed under different photoirradiations.13 Therefore, the PL intensity was expected to be an effective indicator for monitoring the concentration change of the self-assembly of azobenzene-containing materials. To test this expectation, a saturated aqueous solution of the CMC-AZO aggregates was prepared by putting solvent-free CMC-AZO into water and subsequently irradiating the mixture with UV light for over 30 minutes (Fig. 4a). The planar conformation of trans-azobenzene would be expected to favor the ordered arrangement of AZO at high packing density, promoting the formation of CMC-AZO precipitates in aqueous systems. Meanwhile, the nonplanar cis-azobenzene would be expected to disturb the original arrangement of AZO, disfavoring the formation of large self-assemblies, and hence promoting the dispersion of the CMC-AZO aggregates in water. As a result, the dispersion of the CMC-AZO aggregates in water could be promoted by UV light and suppressed by Vis light, leading to the observed change in the PL intensity of CMC-AZO (Fig. S5, ESI†).
 | ||
| Fig. 4 . (a) Schematic representation of the photoregulated change in the concentration of the CMC-AZO aggregates in aqueous conditions. (b) PL and (c) UV-Vis absorption spectra of the CMC-AZO aggregates corresponding to the states in Fig. 4a. TEM images of saturated aqueous solution of the CMC-AZO aggregates after (d) UV and (e) Vis irradiations. | ||
Following this rule, we further switched the UV and Vis illuminations on the saturated solution of the CMC-AZO aggregates and carried out precipitate separation and re-dissolution (Fig. 4a), for checking the change in PL intensity upon changing the aggregate concentration of CMC-AZO. As illustrated in Fig. 4b, after I-UV illumination, the initial PL intensity at 500 nm of saturated CMC-AZO aggregates was strong, and this intensity decreased markedly upon I-Vis illumination, indicating the formation of CMC-AZO precipitate. By separating the precipitate out, the remaining solution contained less CMC-AZO aggregate mass, leading to much lowered PL intensity even after II-UV treatment. When re-dissolving the precipitate into the remaining solution again under the assistance of III-UV light, the PL intensity well recovered to a value similar to that of the initial state, implying that CMC-AZO precipitate re-dispersed as aggregates. The different assembly states of CMC-AZO after UV and Vis irradiations were further analyzed using a transmission electron microscope (TEM) (Fig. S6 and S7, ESI†). UV irradiation can promote the formation of small assemblies in aqueous conditions, resulting in a good dispersion of the CMC-AZO aggregates in water (Fig. 4d). Upon Vis irradiation, much larger assemblies formed (Fig. 4e), which led to the formation of precipitate.
During the test described in Fig. 4a, the photoisomerization of AZO was confirmed from the UV-Vis absorption changes of CMC-AZO in aqueous conditions (Fig. 4c). Interestingly, we found PL intensity change to be a much more sensitive indication than UV-Vis absorption change for tracking the aggregate concentration. For example, after the I-UV and II-UV treatments, the ratio of the corresponding PL intensities was about 1.5 times that of the ratio of UV-Vis absorption levels (Fig. 4b and c). Additionally, the measured range of values of UV-Vis absorption intensity at 360 nm was relatively narrow, only from 0.27 to 0.18, which might introduce measurement errors more easily as compared to those from the large change in value of PL intensity at 500 nm, from 2093 to 1018. This kind of fluorescence indicator for effectively monitoring the concentration change of dispersed azobenzene-containing assemblies in aqueous conditions provides a convenient methodology for tracking the concentration of ionic biomaterials in biological and biomedical studies. Additionally, based on the photoregulated dissolution and precipitation of the CMC-AZO aggregates in water, detectable functional biomaterials such as photocontrollable drug-releasing materials might be developed.
Conclusions
In this work, a fluorescent azobenzene-containing cellulose material, CMC-AZO, was developed based on the AIE effect of azobenzene. In aqueous conditions, the PL intensity of CMC-AZO relies on the concentration of aggregates, which provides a more sensitive indication than UV-Vis absorption for tracking the dissolution and precipitation of CMC-AZO assemblies. We also proved that the dissolution and precipitation of CMC-AZO in water could be photoregulated via the trans–cis isomerization of AZO. This study has provided a new method for developing detectable functional biomaterials based on cellulose and azobenzene and has also opened an opportunity for developing new photocontrollable drug-releasing biomaterials.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Natural Science Foundation of Shandong Province (no. ZR2023MB104) and Dongguan Science and Technology of Social Development Program (20221800905352).Notes and references
- F. Xia, J. Wu, X. Wu, Q. Hu, J. Dai and X. Lou, Modular Design of Peptide- or DNA-Modified AIEgen Probes for Biosensing Applications, Acc. Chem. Res., 2019, 52, 3064–3074 CrossRef CAS PubMed.
- H. Asanuma, X. Liang, T. Yoshida, A. Yamazawa and M. Komiyama, Photocontrol of Triple-Helix Formation by Using Azobenzene-Bearing Oligo(thymidine), Angew. Chem., Int. Ed., 2000, 39, 1316–1318 CrossRef CAS.
- C. Dohno, S.-n Uno and K. Nakatani, Photoswitchable Molecular Glue for DNA, J. Am. Chem. Soc., 2007, 129, 11898–11899 CrossRef CAS PubMed.
- Y. Kim, J. A. Phillips, H. Liu, H. Kang and W. Tan, Using photons to manipulate enzyme inhibition by an azobenzene-modified nucleic acid probe, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 6489 CrossRef CAS PubMed.
- Y. Nakasone, H. Ooi, Y. Kamiya, H. Asanuma and M. Terazima, Dynamics of Inter-DNA Chain Interaction of Photoresponsive DNA, J. Am. Chem. Soc., 2016, 138, 9001–9004 CrossRef CAS PubMed.
- A. Bergen, S. Rudiuk, M. Morel, T. Le Saux, H. Ihmels and D. Baigl, Photodependent Melting of Unmodified DNA Using a Photosensitive Intercalator: A New and Generic Tool for Photoreversible Assembly of DNA Nanostructures at Constant Temperature, Nano Lett., 2016, 16, 773–780 CrossRef CAS PubMed.
- Z. Wu and L. Zhang, Photoregulation between small DNAs and reversible photochromic molecules, Biomater. Sci., 2019, 7, 4944–4962 RSC.
- H. Akiyama and M. Yoshida, Photochemically Reversible Liquefaction and Solidification of Single Compounds Based on a Sugar Alcohol Scaffold with Multi Azo-Arms, Adv. Mater., 2012, 24, 2353–2356 CrossRef CAS PubMed.
- H. Akiyama, S. Kanazawa, Y. Okuyama, M. Yoshida, H. Kihara, H. Nagai, Y. Norikane and R. Azumi, Photochemically Reversible Liquefaction and Solidification of Multiazobenzene Sugar-Alcohol Derivatives and Application to Reworkable Adhesives, ACS Appl. Mater. Interfaces, 2014, 6, 7933–7941 CrossRef CAS PubMed.
- M. Nepraš, S. Luňák, R. Hrdina and J. Fabian, Electronic excited states of azo compounds: Strong π–π* fluorescence of bis-4,4′-diethylaminoazobenzene, Chem. Phys. Lett., 1989, 159, 366–370 CrossRef.
- M. Ghedini, D. Pucci, G. Calogero and F. Barigelletti, Luminescence of azobenzene derivatives induced by cyclopalladation, Chem. Phys. Lett., 1997, 267, 341–344 CrossRef CAS.
- J. Yoshino, N. Kano and T. Kawashima, Synthesis of the most intensely fluorescent azobenzene by utilizing the B–N interaction, Chem. Commun., 2007, 559–561 RSC.
- B.-K. Tsai, C.-H. Chen, C.-H. Hung, V. K. S. Hsiao and C.-C. Chu, Photoswitchable fluorescence on/off behavior between cis- and trans-rich azobenzenes, J. Mater. Chem., 2012, 22, 20874–20877 RSC.
- M. Shimomura and T. Kunitake, Fluorescence and photoisomerization of azobenzene-containing bilayer membranes, J. Am. Chem. Soc., 1987, 109, 5175–5183 CrossRef CAS.
- K. Tsuda Dol, T. Gensch, J. Hofkens, L. Latterini, W. Meijer and S. De, Fluorescence from Azobenzene Functionalized Poly(propylene imine) Dendrimers in Self-Assembled Supramolecular Structures, J. Am. Chem. Soc., 2000, 122, 3445–3452 CrossRef.
- M. Han and M. Hara, Intense Fluorescence from Light-Driven Self-Assembled Aggregates of Nonionic Azobenzene Derivative, J. Am. Chem. Soc., 2005, 127, 10951–10955 CrossRef CAS PubMed.
- M. Han, S. J. Cho, Y. Norikane, M. Shimizu, A. Kimura, T. Tamagawa and T. Seki, Multistimuli-responsive azobenzene nanofibers with aggregation-induced emission enhancement characteristics, Chem. Commun., 2014, 50, 15815–15818 RSC.
- M. Yamauchi, K. Yokoyama, N. Aratani, H. Yamada and S. Masuo, Crystallization-Induced Emission of Azobenzene Derivatives, Angew. Chem., Int. Ed., 2019, 58, 14173–14178 CrossRef CAS PubMed.
- L. Zhang, Y. Qu, J. Gu, Y. Liu, Z. Tang, C. Zhang, H. Liu, J. Liu, Z. Wu and X. Luo, Photoswitchable Solvent-free DNA Thermotropic Liquid Crystals toward Self-erasable Shape Information Recording Biomaterials, Mater. Today Bio, 2021, 100140 CrossRef CAS PubMed.
- L. Zhang, Y. Qu, J. Gu, Z. Tang, Z. Wu and X. Luo, Photoliquefiable DNA-surfactant ionic crystals: Anhydrous self-healing biomaterials at room temperature, Acta Biomater., 2021, 128, 143–149 CrossRef CAS PubMed.
- L. Zhang, J. Gu, X. Luo, Z. Tang, Y. Qu, C. Zhang, H. Liu, J. Liu, C. Xie and Z. Wu, Photoregulative phase change biomaterials showing thermodynamic and mchanical stabilities, Nanoscale, 2022, 14, 976–983 RSC.
- J. Sun, C. Ma, S. Maity, F. Wang, Y. Zhou, G. Portale, R. Göstl, W. H. Roos, H. Zhang, K. Liu and A. Herrmann, Reversibly Photo-Modulating Mechanical Stiffness and Toughness of Bioengineered Protein Fibers, Angew. Chem., Int. Ed., 2021, 60, 3222–3228 CrossRef CAS PubMed.
- L. Zhang, S. Maity, K. Liu, Q. Liu, R. Göstl, G. Portale, W. H. Roos and A. Herrmann, Nematic DNA Thermotropic Liquid Crystals with Photoresponsive Mechanical Properties, Small, 2017, 13, 1701207 CrossRef PubMed.
- L. Zhang, C. Ma, J. Sun, B. Shao, G. Portale, D. Chen, K. Liu and A. Herrmann, Genetically Engineered Supercharged Polypeptide Fluids: Fast and Persistent Self-Ordering Induced by Touch, Angew. Chem., Int. Ed., 2018, 57, 6878–6882 CrossRef CAS PubMed.
- Q. Du, J. Zhao, L. Jiang, Y. Liu, X. Zhang, X. Zhou, Z. Wu, L. Zhang and X. Luo, Molecular design on dual stimuli-responsive azobenzene-containing ionic complexes toward self-healing materials under photoirradiation or humid condition at room temperature, Appl. Mater. Today, 2023, 35, 101945 CrossRef.
- L. Zhang, Z. Tang, L. Hou, Y. Qu, Y. Deng, C. Zhang, C. Xie and Z. Wu, Selective mercury(II) detection in aqueous solutions upon the absorption changes corresponding to the transition moments polarized along the short axis of an azobenzene chemosensor, Analyst, 2020, 145, 1641–1645 RSC.
- P. Weis, W. Tian and S. Wu, Photoinduced Liquefaction of Azobenzene-Containing Polymers, Chem. – Eur. J., 2018, 24, 6494–6505 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Syntheses and characterizations of AZO and CMC-AZO. See DOI: https://doi.org/10.1039/d4qm00542b |
| ‡ These authors contributed equally. |
| § Present address: Xianghe Middle School, Yantai, 264002, China. |
| This journal is © the Partner Organisations 2024 |