Self-assembled phthalocyanine-based nano-photosensitizers in photodynamic therapy for hypoxic tumors
Lin
He
 a and
Ding
Ma
*b
a and
Ding
Ma
*b
aSchool of Health and Nursing, Wuxi Taihu University, Wuxi 214064, P. R. China. E-mail: hel@wxu.edu.cn
bState Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, P. R. China. E-mail: martin@mail.nankai.edu.cn
First published on 5th November 2024
Abstract
Photodynamic therapy (PDT) is a well-established minimally invasive cancer treatment, yet its effectiveness in treating hypoxic tumors is limited due to oxygen scarcity, hindering the production of reactive oxygen species (ROS). Phthalocyanines, notable for their remarkable optoelectronic attributes and structural flexibility, have emerged as a class of photosensitizers with potential to enhance PDT. This review highlights innovations in the development of self-assembled phthalocyanine-based nano-photosensitizers, underscoring their potential to mitigate the obstacles posed by hypoxia in PDT. It details advancements in self-assembly methodologies and their applications to augment the therapeutic impact of PDT in hypoxic tumors, encompassing oxygen supply augmentation, metabolic pathway modulation, development of phthalocyanine-based nano-photosensitizers for photothermal therapy (PTT), type I PDT photosensitizers and combination therapy. It concludes with an overview of the current challenges and future prospects of phthalocyanine-based nano-photosensitizers in PDT. By reviewing recent progress, this paper aspires to offer pioneering insights into the conception of novel nano-photosensitizers, engineered to counteract hypoxia and circumvent the intrinsic limitations of PDT.
1. Introduction
PDT is a photochemistry-based approach that involves three components (Fig. 1), namely a photosensitizer (PS), molecular oxygen, and light with appropriate wavelength, to produce ROS that cause cytotoxicity.1 PSs are typically injected or administered topically in clinical settings. Following a time interval, light of the appropriate wavelength is applied to the lesion site, leading to the selective activation of the PS and subsequent formation of extremely reactive ROS, hence inducing cell apoptosis, necrosis, and immune response.2,3 PDT shows various advantages over conventional cancer therapies, such as radiotherapy, chemotherapy, and surgery, including minimum toxicity, repetition without cumulative effects, fewer side effects, and negligible drug resistance.4 | ||
| Fig. 1 Schematic diagrams illustrating (a) the fundamental mechanism of PDT and (b) the typical protocol for PDT in a clinical setting. Copyright 2010, ACS.3 | ||
A simplified Jablonski diagram explains the process of typical photodynamic reactions, as shown in Fig. 2. Upon excitation by light with a specific wavelength, the PS reaches its first excited singlet state. The PS, in its excited state, is highly unstable and can decay by emitting fluorescence, allowing tracking of the PS. Alternatively, the PS can reach a more stable excited triplet state through an intersystem crossing process. The triplet-state PS can decay back to the ground state (by emitting phosphorescence), but this is a ‘forbidden process’ according to the quantum selection rules. The triplet state is much more stable than the singlet state and the excited triplet state has a lifetime of microseconds compared with only nanoseconds for the excited singlet state.4 The PS in the triplet-state will transfer its energy to the surrounding oxygen to form singlet oxygen (1O2) (type II reaction), which is considered the most important ROS in PDT treatment. Alternatively, the PS in the triplet-state can directly interact with various biomolecules through electron transfer to generate free radicals, which can further react with water or oxygen to produce ROS, such as superoxide anion radicals (O2˙−), hydroxyl radicals (OH˙), and hydrogen peroxides (H2O2) (type I reaction).5 The balance between type I and type II reactions, which can happen simultaneously, is determined by the specific PS used, as well as by the concentrations of available substrates and oxygen.6 Type I PDT is particularly advantageous under hypoxic conditions, as it can reduce the oxygen requirement through efficient recycling of O2 during the cascade of reactions.7 This makes type I PDT more hypoxic-tolerant compared to type II PDT. Moreover, the generation of ROS via type I mechanisms is often more efficient.8,9 O2˙− can convert to H2O2 either through dismutation or via the catalytic action of superoxide dismutase (SOD). Subsequently, H2O2 can react with O2˙− to produce the highly reactive OH˙ through the Haber–Weiss mechanism or Fenton chemistry. The ˙OH generated by type-I PDT is a stronger oxidant than 1O2 (generated by Type-II PDT), ensuring the efficient utilization of the scarce oxygen present within hypoxic tumors. Type I PDT, which is less oxygen-dependent and generates more highly cytotoxic free radicals, is regarded as an effective strategy for overcoming hypoxic tumors.
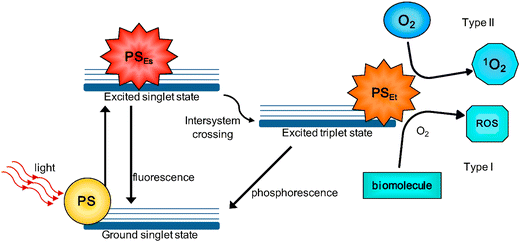 | ||
| Fig. 2 Schematic depiction of typical photodynamic reactions. Copyright 2016, MDPI.4 | ||
Phthalocyanines are versatile organic compounds renowned for their diverse applications. They are characterized by an aromatic heterocyclic structure comprising four isoindole rings interconnected by nitrogen atoms. Noteworthy for their outstanding attributes such as high thermal stability, vivid coloration, chemical inertness, and limited solubility, phthalocyanines are highly suitable for pigment applications across various industries.10–12 Over the last few decades, their distinct optoelectronic, catalytic, and self-assembly characteristics have expanded the utility of phthalocyanines beyond industrial dyes to a wide array of cutting-edge technological applications, including molecular electronics, photonics, photovoltaics, electrophotography, electrochromic displays, electrocatalysis, and sensor technology.13–19 Furthermore, the potential of phthalocyanines in diverse biomedical fields is evident, exhibiting promise as fluorescent probes for bioanalysis and bioimaging, photosensitizers for PDT,20,21 and even anti-amyloid agents22,23 to hinder disease-associated protein aggregation in the treatment of neurological disorders.
Phthalocyanines, possessing a variety of favorable attributes, show great potential as second-generation photosensitizers in PDT. Characterized by absorption wavelengths exceeding 670 nm and high extinction coefficients, these compounds exhibit minimal absorption in the 400–600 nm range, reducing the risk of phototoxic reactions under solar exposure. Furthermore, the chemical structure of these compounds allows facile modifications through the incorporation of central metal ions or substituents at the periphery, resulting in changes to their photophysical properties.24 Clinical investigations have demonstrated the efficacy of phthalocyanine-based photosensitizers. For instance, photosens, an aluminum phthalocyanine, has received clinical approval in Russia for treating cancers of the skin, breast, gastrointestinal tract, and lungs. Additionally, photocyanine and Pc 4 are in various stages of clinical trials, demonstrating the potential of these compounds to target a diverse range of tumors (Fig. 3).
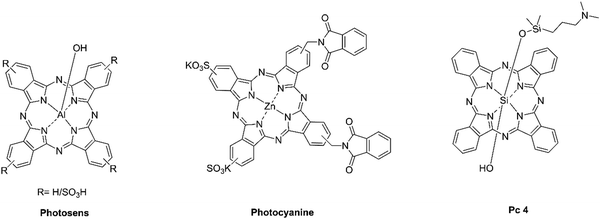 | ||
| Fig. 3 Molecular structures of the three phthalocyanine-derived photosensitizers currently employed or under investigation in clinical settings. | ||
The effectiveness of phthalocyanines in PDT heavily depends on the oxygen levels in the environment. A tumor microenvironment often exhibits increased hypoxia compared to normal tissues due to rapid tumor growth and insufficient blood supply, which can hinder the continuous supply of oxygen needed for PDT.25 To address this challenge, various nanomaterials such as liposomes, polymeric micelles, dendrimers, and mesoporous silica nanoparticles, have been employed as carriers to develop nano-photosensitizers capable of sustaining oxygen levels.26–29 Fenton or Fenton-like reactions have been extensively utilized to enhance the efficacy of PDT.30 Furthermore, researchers are exploring nano-photosensitizers that can reduce oxygen consumption during cellular respiration, thereby improving the oxygenation of tumors for more effective PDT.31,32 Nonetheless, many existing nanocarriers involve complex synthetic procedures, present challenges in loading efficiency, display significant heterogeneity, and exhibit low biocompatibility. Safety concerns arise from potential toxicity towards healthy tissues, uncertain metabolic pathways, and the use of harmful organic solvents in their production processes. Consequently, there is a critical need to develop biocompatible and versatile nanomaterials specifically tailored for the delivery of phthalocyanine-derived photosensitizers, in order to combat hypoxic tumors.
Self-assembly of molecular photosensitizers has recently attracted considerable attention for PDT because it provides a facile and straightforward approach for incorporating photosensitizers and additional therapeutic agents within a nanostructure.33,34 Self-assembly is a natural process where individual molecules spontaneously self-organize into stable, well-defined aggregates under equilibrium conditions, held together by non-covalent bonds such as van der Waals forces, π–π stacking, hydrogen bonding, and metal–ligand coordination.35,36 This phenomenon is particularly advantageous in PDT as it facilitates the efficient encapsulation of photosensitizers, improving their stability and protection against degradation.37 Moreover, self-assembled nano-photosensitizers often exhibit enhanced cellular uptake, increasing the bioavailability of the photosensitizer at the tumor site.34 This property, along with the ability to incorporate a variety of therapeutic agents, such as chemotherapeutics and gene therapy materials, enables the design of combination therapies which can improve treatment outcomes for hypoxic tumors.38 Moreover, certain self-assembling systems possess stimuli-responsive properties, allowing for the controlled release of the photosensitizer under specific conditions like pH changes or light exposure.34,39 This feature not only optimizes therapeutic efficacy but also minimizes adverse effects on normal tissues, highlighting the potential of self-assembly in advancing PDT strategies.
The refinement of nano-photosensitizers with phthalocyanines holds the key to enhancing the efficacy of PDT, especially in hypoxic tumors. This review emphasizes the strategic application of self-assembly techniques in the design of phthalocyanine-based nano-photosensitizers, aiming to surmount the hypoxic limitations encountered in PDT. The strategies include: in situ O2 generation, reduction of intracellular oxygen consumption, development of phthalocyanine-based nano-photosensitizers for photothermal therapy (PTT), and development of type I nano-photosensitizers. The concurrent use of the aforementioned strategies in specific studies also reflects an innovative trend in the field. Moreover, the integration of PDT with other therapeutic modalities, such as chemotherapy, gas therapy, immunotherapy, and starvation therapy, constitutes a holistic approach designed to address the multifaceted issues of hypoxic tumor microenvironments (TMEs). This comprehensive strategy aims to counteract tumor resistance, proliferation, and metastasis, which are significant challenges posed by single-agent therapies.
2. Self-assembly strategies for phthalocyanine-based photosensitizers
2.1. Single component assembly
Single-component assembly provides the advantage of streamlined design and production, which could simplify performance by reducing complex interactions. However, it may not offer the flexibility and synergistic effects found in multi-component systems. Phthalocyanines, which possess a hydrophobic core, are prone to precipitation in aqueous environments due to π–π interactions. This precipitation can quench fluorescence and reduce 1O2 production, diminishing the effectiveness of PDT.40 To counter this, incorporating hydrophilic substituents at the periphery or axial positions of phthalocyanines can increase their water solubility.41 Various hydrophilic moieties, such as polyethylene glycol (PEG), peptides, phospholipids, tetrasulfonic acid, amines, β-galactose and proteins can be attached to phthalocyanine-based photosensitizers to fine-tune their amphiphilic properties.42–49 By carefully manipulating the balance between hydrophilic and hydrophobic characteristics of the photosensitizers, researchers can regulate the formation of nano-photosensitizers.50,51 This regulation is essential for optimizing their performance in PDT, ensuring that the nano-photosensitizers maintain their therapeutic potential at tumor sites while remaining stable and bioavailable within biological systems.2.2. Multicomponent assembly
Multi-component assembly, while providing the benefits of synergistic interactions and the ability to address multiple targets or pathways simultaneously, can introduce complexity in optimization, and increase potential challenges in system stability and control. The self-assembly process of phthalocyanine-based photosensitizers can be significantly influenced by incorporating multicomponent molecules. This approach allows for the fine-tuning of interactions between the photosensitizers and other therapeutic agents or other building blocks, thereby controlling the self-assembly dynamics. By combining distinct molecular building blocks through various interactions such as between phthalocyanine-based photosensitizer-metal,52,53 phthalocyanine-based photosensitizer-photosensitizer,54,55 and phthalocyanine-based photosensitizer-anticancer drug,56,57 the synergistic effects of these multi-component systems are harnessed. The advancements in structural design and functional attributes hold great promise for developing sophisticated materials that are specifically tailored for hypoxic tumors. Moreover, a summary of the representative phthalocyanine-based assemblies for enhancement of PDT are listed in Table 1.| Names | Building units | PDT properties | Practical applications | Ref. |
|---|---|---|---|---|
| MPEG: monomethoxy polyethylene glycol; Fmoc-Cys: Fmoc-protected cysteine; ACF: acriflavine; Ac-CD:DOX: pH-sensitive acetalated β-CD (Ac-CD) nanoparticle-loaded chemotherapy drugdoxycycline (DOX); FI: fluorescence imaging. | ||||
| MPEG-monosubstituted ZnPcs | Direct coupling; one MPEG-modified ZnPc | Selectively accumulated in tumor tissues for better PDT | Obvious cell mortality in HepG2; Growth inhibition of HT-29 tumors | 42 |
| ZnPc nanoparticles | Direct coupling; ZnPc with four peripheral hydrophilic long chain hydroxy substituents | PDT combined PTT effect | Significant growth inhibition in HeLa tumors | 43 |
| ZnPc-GGK(B)-COOH/DOX NP | Direct coupling; ZnPc-conjugated peptide; Non covalent interaction | Targeted chemotherapy and PDT | Synergistic PDT and chemotherapy towards HT-29 tumors | 44 |
| ZnPc-SPC | Direct coupling; ZnPc-soybean phosphatidylcholine | Targeted FI and enhanced PDT | Photodynamic cytotoxicity against HeLa and MCF-7 cells | 45 |
| NanoPcA | Direct coupling; 2,4,6-tris-(N,N-dimethylaminomethyl)phenoxy substituted ZnPc | Type I PDT for bacterial eradication | PDT effects towards antibiotic-resistant bacteria | 47 |
| Gal-(ZnPc*)2-NP | Direct coupling; β-galactose-conjugated dimeric ZnPc | Selective detection and elimination of senescent cells | Selective PDT towards senescent HeLa cells | 48 |
| Fmoc-Cys/Fe@Pc/ACF | Fe3+-driven self-assembly; Fe3+, Fmoc-Cys, ZnPc, ACF | Self-oxygen-supplying ability for enhanced PDT | Significant inhibition of HeLa tumor growth | 52 |
| PcDA | Electrostatic adsorption interaction; ZnPc tetra-substituted with quaternary ammonium salt group and sulphonate groups | Simultaneous effect of PDT and PTT against tumors | Significant inhibition of H22 tumor progression | 54 |
3. Application of self-assembled phthalocyanine-based nano-photosensitizers in PDT for hypoxic tumors
The hypoxic nature of tumor tissues, combined with the oxygen-dependent nature of PDT, presents formidable challenges to effective tumor treatment. To counter these challenges, researchers leverage self-assembled nano-photosensitizers as delivery vehicles for encapsulating photosensitizers and therapeutic agents, which are specifically designed to overcome hypoxic conditions. These strategies include in situ oxygen generation to elevate local oxygen concentrations and reducing intracellular oxygen consumption to preserve available oxygen. Moreover, the structural modification of phthalocyanines with functional groups is being explored to enhance their photophysical properties. Through careful design, the properties of traditional phthalocyanine-based photosensitizers can be modified, allowing a shift from oxygen-dependent type II photoreactions to type I reactions that are less reliant on oxygen, or even towards oxygen-independent PTT induced by vibrational relaxation. The integration of PDT with other therapeutic modalities, such as chemotherapy, gas therapy, immunotherapy, and starvation therapy, represents a multifaceted approach to address the complex challenges of the hypoxic tumor microenvironment. This synergistic combination of therapies aims to enhance treatment effectiveness, overcoming the limitations of single-modality therapies and offering a more comprehensive strategy for hypoxic tumor management.3.1. In situ O2 generation
In the ongoing quest to enhance the efficacy of PDT in hypoxic tumors, substances capable of catalyzing the decomposition of endogenous H2O2 to generate O2 have garnered significant interest. Catalysts such as MnO2, Pt, Fe3+, Cu2+, and catalase can be selectively delivered to tumor sites via self-assembly strategies.58–62 Li et al.52 pioneered a groundbreaking approach to crafting supramolecular photosensitizing nanozymes capable of self-supplying oxygen, addressing a pivotal need in the effectiveness of PDT for hypoxic tumors. The synthesis of these nanozymes was achieved through a self-assembly process driven by Fe3+ and involving fluorenylmethyloxycarbonyl (Fmoc)-protected amino acids. The nanovesicles successfully encapsulated a zinc(II) phthalocyanine-based photosensitizer (ZnPc) and an inhibitor for hypoxia-inducible factor 1 (HIF-1) and acriflavine (ACF) (Fig. 4a and b). A key feature of these particles was their inherent ability to convert the abundant H2O2 in cancer cells into oxygen, a process facilitated by Fe3+ ions after cellular internalization, thus overcoming the oxygen deficiency typically encountered in PDT for hypoxic tumors. The Fmoc-Cys/Fe@Pc and Fmoc-Cys/Fe@Pc/ACF nanovesicles exhibited remarkable stability under physiological conditions and possessed a notable GSH-responsive disassembly property. They successfully down-regulated the HIF-1α level in HT29 cells, indicating their ability to mitigate hypoxia (Fig. 4c). Moreover, these nanosystems exhibited high PDT potency against HT29 cells under both normoxic and hypoxic conditions. In vivo biodistribution in tumor-bearing nude mice confirmed the selective accumulation of these nanosystems at the tumor site, likely due to the enhanced permeability and retention (EPR) effect (Fig. 4d). Upon light irradiation, significant suppression of tumor growth and attenuation of tumor hypoxia were observed (Fig. 4e). The study concluded that Fe3+-driven self-assembled nanosystems were potent and versatile photosensitizing agents, effectively tackling the hypoxia hurdle in PDT. | ||
| Fig. 4 (a) Design and (b) mechanistic actions of the multifunctional self-assembled nanozymes. (c) Western blot analysis of HIF-1α expression of HT29 cell lysate following different treatment regimens. (d) Fluorescence images of HT29 tumor-bearing nude mice prior to and post intravenous injection of Fmoc-Cys/Fe@Pc/ACF. (e) Corresponding tumor weight and tumor image at day 15 after indicated treatment. Copyright 2020, Wiley.52 | ||
He et al.63 devised a cupric-ion-promoted self-assembled nanotherapeutic system, termed ZnPc*/Cu/SN38@NP, integrating a glutathione (GSH)-responsive carboxy zinc(II) phthalocyanine (ZnPc*) photosensitizer with the anticancer drug SN38. The system harnessed Cu2+ ions to facilitate the self-assembly through metal complexation and uniquely employed these ions to catalyze the conversion of hydrogen peroxide to oxygen in a catalase-like reaction, providing the nanosystems with an inherent oxygen-replenishing capability (Fig. 5a). Demonstrating remarkable stability in aqueous environment, the nanotherapeutic system was designed to be sensitive to the acidic and GSH-enriched tumor microenvironment. Under such conditions, the nanoparticles gradually disassembled, releasing the encapsulated therapeutic agents. This disassembly, coupled with the intracellular GSH activation, alleviated the intrinsic quenching of the nanophotosensitizers, restoring their photoactivities. In vitro studies revealed that ZnPc*/Cu/SN38@NP could efficiently internalize into HT29 cells and attenuate intracellular hypoxia levels (Fig. 5b). The combination of the photodynamic effect of ZnPc* with the anticancer effect of SN38, synergistically induced significant apoptotic cell death. In vivo studies in HT29 tumor-bearing nude mice further confirmed the system's potential, as it demonstrated selective accumulation at the tumor site (Fig. 5c). Upon light irradiation, the nanoparticle significantly suppressed tumor progression and alleviated tumor hypoxia, without inducing notable adverse effects in the mice (Fig. 5d–f). The utilization of Cu2+ ions to achieve both self-assembly and catalytic conversion of H2O2 to oxygen represented a novel tactic not previously documented. By incorporating an oxygen-replenishing strategy and a chemotherapy agent into a single nanosystem, this study created a theranostic platform with synergistic effects against tumor hypoxia.
 | ||
| Fig. 5 (a) Schematic diagram of self-assembled ZnPc*/Cu/SN38@NP for dual oxygen-replenished PDT and chemotherapy. (b) Cellular uptake for HT29 cells after incubation with ZnPc*/Cu/SN38@NP or SN38 for 12 h under different conditions. (c) Biodistribution profile of ZnPc*/Cu/SN38@NP, visualized through fluorescence imaging. (d) Tumor volume changes in mice over 14 days following various treatment modalities. (e) Immunofluorescence staining of HIF-1α (green) in tumor sections post-treatment. (f) Fluorescent hypoxia probe staining post-treatment, indicating the hypoxic status of the tumor microenvironment. Copyright 2023, Elsevier.63 | ||
Liang et al.64 introduced a novel therapeutic approach using copper-coordinated nanoassemblies (CCNAs) for dual apoptosis and cuproptosis in cancer treatment, integrated with immunotherapy. Cuproptosis, a copper-dependent cell death mechanism, has emerged as a potent adjunct to apoptosis in cancer therapy. The CCNAs were ingeniously engineered by integrating a photosensitizer (ZnPc) conjugated with a chemotherapeutic drug (Doxorubicin, DOX) via a thioketal (TK) spacer, along with an indoleamine 2,3-dioxygenase (IDO) inhibitor, 1-methyl tryptophan (1-MT). These components self-assembled via copper (Cu2+) coordination (Fig. 6a). By employing Cu2+ to catalyze the conversion of endogenous hydrogen peroxide into molecular oxygen through a Fenton-like process, CCNAs provided necessary oxygen for effective PDT while also stimulating cuproptosis. This process involved copper accumulation, aggregation of lipoylated mitochondrial proteins, and disruption of iron–sulfur clusters, ultimately leading to cell death. Experimental results substantiated the CCNAs’ efficacy in oxygen generation, evidenced by a significant decrease in intracellular H2O2 levels and an increase in oxygen concentration upon treatment (Fig. 6b). Notably, CCNAs maintained high photo-cytotoxicity against PC-3 cancer cells under both normoxic and hypoxic conditions, outperforming the free ZnPc-TK-DOX formulation, particularly in hypoxic environments (Fig. 6c). This enhanced performance under low oxygen conditions was attributed to the Cu2+-mediated oxygen provision, ensuring PDT efficacy. The study further delineated the cell death pathways induced by CCNAs, revealing their capability to trigger both apoptosis and cuproptosis. The cuproptosis was confirmed by the inhibition of cytotoxic effects in the presence of a Cu2+ chelating agent and a specific cuproptosis inhibitor (Fig. 6d). The assembly's ability to induce immunogenic cell death (ICD) and inhibit IDO-1 activity, thereby reversing immune tolerance, complemented their direct cytotoxic effects and established a foundation for sustained antitumor immunity (Fig. 6e). The CCNAs offered a multifaceted therapeutic platform, addressing the hypoxia challenge in PDT, while concurrently leveraging apoptosis, cuproptosis, and immunomodulatory effects for comprehensive cancer treatment. This innovative strategy represented a promising advancement in metal-based nanomedicine, particularly in exploiting the synergies between different cell death modalities and immunotherapy to enhance therapeutic outcomes.
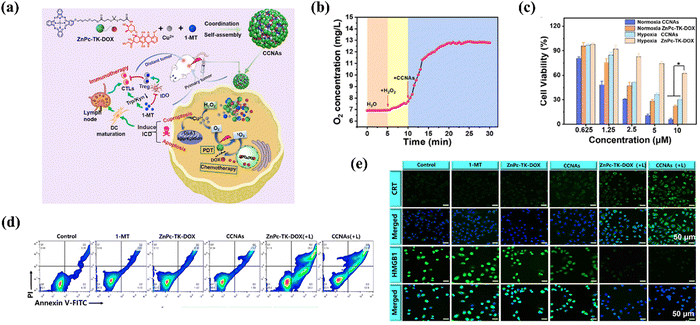 | ||
| Fig. 6 (a) Schematic diagram of the fabrication of CCNAs and their application for self-enhanced apoptosis-cuproptosis and immunotherapy. (b) Kinetics of oxygen generation catalyzed by CCNAs over time. (c) Cell viabilities of PC-3 cells after different treatments. (d) Cell death pathway of PC-3 cells after different treatments. (e) Fluorescence staining of calreticulin (CRT) and high-mobility group protein 1 (HMGB1) on PC-3 cells post-treatment, indicative of immunogenic cell death markers. Copyright 2024, Elsevier.64 | ||
Moreover, the self-assembled phthalocyanine-based nano-photosensitizers are poised to enhance the potency of sonodynamic therapy (SDT) for hypoxic tumors. Li et al.65 introduced an innovative bifunctional nanoassembly, integrating an iridium(III) phthalocyanine complex (IrPc) with bovine serum albumin (BSA) to create IrPc-NPs (Fig. 7). The IrPc-NPs demonstrated dual functionality, acting as catalysts to decompose H2O2 into O2 and as sonosensitizers to generate 1O2 under ultrasound (US) irradiation. This dual functionality not only ameliorated hypoxia, thereby improving SDT effectiveness, but also showed promise for integrated imaging and therapeutic strategies.
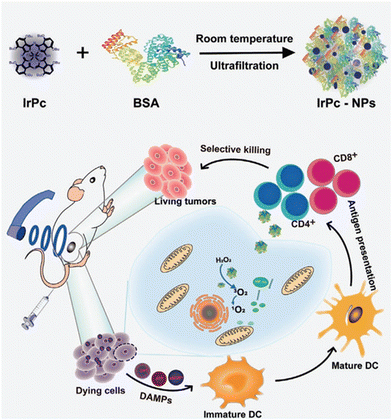 | ||
| Fig. 7 Schematic diagram of the synthesis of IrPc-NPs and their utilization in sonodynamic immunotherapy. Copyright 2023, Wiley.65 | ||
3.2. Reduction of intracellular oxygen consumption
Inhibiting mitochondrial respiration to lower tumor O2 consumption is a recognized strategy to alleviate tumor hypoxia. Liu et al.66 developed a groundbreaking nanostructured photosensitizer, designated as ATO/ZnPc-CA@DA, which functioned as a carrier-free drug delivery system with controlled release properties. The core innovation of this study was the integration of atovaquone (ATO), an FDA-approved drug that inhibits mitochondrial respiration and reduces oxygen consumption in tumor cells, with a nanostructure composed of cationic and anionic zinc phthalocyanines (Fig. 8a). The nanoassembly, coated with polydopamine for enhanced stability and tumor-targeting, released ATO in response to the acidic pH of tumor microenvironment, activating fluorescence for tumor imaging and therapy monitoring. Cellular investigations unveiled that ATO/ZnPc-CA@DA induced apoptosis, disrupted mitochondrial membrane potential, and enhanced the elimination of tumor cells. Animal experiments confirmed the nanoassembly's tumor-targeting efficacy, with significant accumulation at the tumor site and minimal distribution in normal organs (Fig. 8b). Daily LED light illumination at the tumor site yielded a marked decrease in tumor volume, highlighting the therapeutic potential of ATO/ZnPc-CA@DA, which could be attributed to its hypoxia-alleviating properties (Fig. 8c and d). This work advanced the development of more efficacious PDT approaches and shows promise for improving clinical outcomes for cancer patients with hypoxic tumors. | ||
| Fig. 8 (a) The preparation process of ATO/ZnPc-CA@DA and its application for enhanced photodynamic antitumor activity by respiratory depression. (b) Biodistribution profile of ATO/ZnPc-CA@DA, as visualized by fluorescence imaging. (c) The changes in tumor size monitored during the different treatments. (d) Representative tumor image at day 7 after different treatments. Copyright 2024, Elsevier.66 | ||
A recent study67 unveiled an innovative approach to cancer treatment involving the use of manganese(III) phthalocyanine complex nanoparticles loaded with glucose oxidase (MnClPc-HSA@GOx). This nanoformulation strategically targeted tumor cells by disrupting their energy metabolism and modulating the immune microenvironment through repolarization of tumor-associated macrophages (TAMs) from a pro-tumor M2 to an anti-tumor M1 phenotype. Glucose oxidase facilitated the enzymatic conversion of glucose into H2O2 and gluconic acid. In the presence of MnClPc, H2O2 was further converted into ROS, including both type I (˙O2−) and type II (1O2) forms. This system effectively curtailed glucose supply to tumors, suppressing their metabolic activity and ATP production. Simultaneously, the elevated ROS levels triggered the repolarization of macrophages, stimulating an inflammatory response against the tumor.
3.3. Development of phthalocyanine-based nano-photosensitizers for PTT
Phthalocyanines predominantly undergo a type II mechanism to generate 1O2, a process highly dependent on the O2 level, resulting in a restricted therapeutic outcome in treating hypoxic tumors. Supramolecular self-assembly has recently emerged as a feasible strategy for facilitating the organization of phthalocyanine molecules into functional nanostructures. This aggregation triggers intermolecular collisions among the phthalocyanine units, consequently leading to the dissipation of absorbed light through nonradiative relaxation (heat) due to quenched fluorescence and obstructed ROS pathways. Specific self-assembled phthalocyanine-based nano-photosensitizers have demonstrated unique spherical nanostructures with exceptional colloidal stability and supramolecular photothermal properties. These attributes effectively tackle the challenge of PDT, opening up new possibilities for enhancing the therapeutic outcomes of hypoxic tumors.Li et al.68 introduced an innovative strategy in tumor theranostics with the development of monocomponent nanoparticles based on phthalocyanine-peptide (PF) conjugates. The PF nanoparticles self-assembled into well-defined spherical structures, showcasing remarkable colloidal stability and supramolecular photothermal effects. The unique feature of these nanoparticles was their spatiotemporally coupled photoactivity, enabling a dynamic transition between PTT and photoacoustic imaging (PAI) to PDT and corresponding fluorescence imaging (FLI), regulated by interactions with the cell membrane (Fig. 9a). Moreover, the study highlighted the efficient photothermal conversion of PF nanoparticles, with a concentration dependent temperature rise observed under laser irradiation. The photothermal conversion efficiency (PCE) was measured to be approximately 43.6%, comparable to or surpassing other reported photothermal agents. Importantly, the PF nanoparticles exhibited both photothermal and photostability during continuous irradiation-cooling cycles. In vitro experiments verified the capability of PF nanoparticles to disassemble within cells, switching between PTT and PDT modalities and resulting in enhanced tumor cell cytotoxicity. In vivo studies using a mouse model with MCF-7 xenograft tumors further validated the switchable photoactivity of PF nanoparticles (Fig. 9b–d). The findings suggested that PF nanoparticles offered a comprehensive theranostic platform integrating PAI, FLI, PTT, and PDT functionalities into a single system. The intelligent integration of theranostic modalities, facilitated by interactions with cell membranes, along with the capacity to guide treatment guidance via imaging, positioned these nanoparticles as an exceptionally promising option for targeted and hypoxia-tolerant phototherapy.
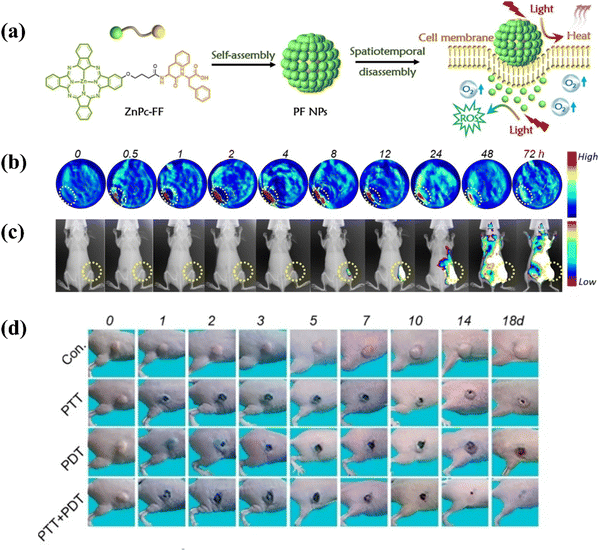 | ||
| Fig. 9 (a) Schematic diagram of the fabrication process of PF self-assemblies and its application in adaptive tumor theranostics. (b) Photoacoustic images and (c) fluorescence images of mice bearing MCF-7 tumors before and after intravenous injection of PF NPs (1.5 mg mL−1, 20 mL). (d) Representative images of mice bearing MCF-7 tumors at different time intervals after treatment. Copyright 2019, Wiley.68 | ||
Li et al.69 synthesized two water-soluble phthalocyanine derivatives, PcS4 and PcN4, which self-assembled into nanostructures through molecular recognition. The resultant nanostructure, PcS4–PcN4, displayed quenched fluorescence and reduced singlet oxygen generation, properties advantageous for enhancing PA imaging and PTT (Fig. 10a). The nanoassembly PcS4–PcN4 displayed superior properties compared to individual phthalocyanines. In vivo experiments illustrated the high-contrast tumor visualization capability of PcS4–PcN4 through whole-body PA imaging, indicating its potential for cancer detection (Fig. 10b). Furthermore, upon exposure to 660 nm laser irradiation, PcS4–PcN4 induced a substantial temperature rise of approximately 25 °C at the tumor site, evidencing its efficacy in PTT (Fig. 10c and d). Notably, the treatment did not induce body weight loss in mice, pointing towards its favorable biocompatibility. In summary, the study showcased a feasible and improved nanostructured contrast agent for dual-modality PA imaging and PTT, highlighting PcS4–PcN4 as a promising and biocompatible tool for cancer theranostics.
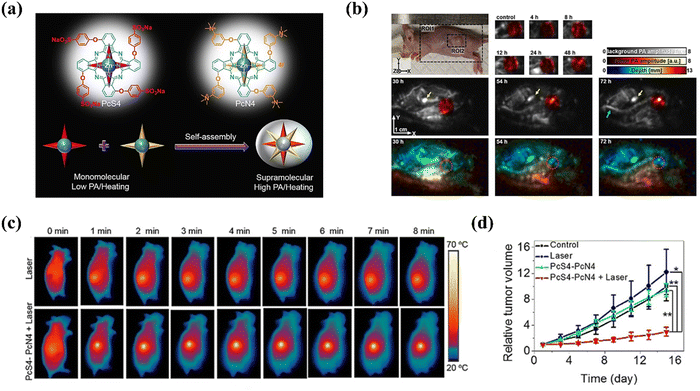 | ||
| Fig. 10 (a) Schematic diagram of the fabrication of a nanostructure (PcS4–PcN4) and its application in enhanced PA and PTT in hypoxic tumors. (b) PA imaging of tumor-bearing mice after the injection of PcS4–PcN4. (c) Thermal infrared imaging of tumor-bearing mice after the indicated treatment. (d) Relative tumor volume of mice after different treatments over 16 days. Copyright 2020, Wiley.69 | ||
Zhao and colleagues54 innovated a supramolecular nanostructured phototherapeutic agent known as PcDA, employing a Förster resonance energy transfer (FRET)-based assembly strategy. This agent was created through anion and cation supramolecular interactions between two water-soluble phthalocyanine derivatives, PcD and PcA. The PcDA agent demonstrated augmented energy absorption, leading to increased generation of ROS (PDT) and heat (PTT), consequently augmenting therapeutic efficacy (Fig. 11). Notably, PcDA showed a high PA signal-to-liver ratio of 11.9 after intravenous injection, indicating its potential for precise tumor visualization. The study ingeniously addressed the challenge of tumor heterogeneity, specifically low oxygen levels, by integrating the oxygen-dependent mechanism of PDT with the oxygen-independent PTT. Notably, by utilizing water-soluble components and obviating the necessity for intricate nanohybrid systems, the supramolecular assembly strategy also overcame issues associated with conventional phthalocyanine-based therapies, such as poor reproducibility, insufficient loading, and unpredictable toxicity. In conclusion, the research pioneered a versatile method for constructing a nanostructured supramolecular agent that integrated PDT and PTT with PA imaging guidance, offering a potent and biocompatible approach for cancer treatment.
 | ||
| Fig. 11 Schematic diagram of the fabrication a nanostructure (PcDA) and its enhanced PA and PTT properties for hypoxic tumors. Copyright 2024, Wiley.54 | ||
Zhao and colleagues recently70 published a paper detailing the development and assessment of a novel self-degradable nanostructured phthalocyanine assembly, NanoPcDA, designed to enhance biosecurity and minimize adverse effects in PTT. The synthesis of NanoPcDA involved utilization of a silicon(IV) phthalocyanine molecule with dopamine substitution, enabling its spontaneous self-assembly in aqueous solutions. NanoPcDA demonstrated a high photothermal conversion efficiency, effectively converting absorbed light into heat for tumor ablation (Fig. 12a). In animal models, NanoPcDA accumulated significantly in tumors, as evidenced by a tumor/liver signal intensity ratio approximately 4.8 times greater than that of the commonly used contrast agent indocyanine green (ICG) (Fig. 12b). Upon exposure to an 808-nm laser at a power density of 0.5 W cm−2, tumors treated with NanoPcDA rapidly increased in temperature from 37 °C to approximately 50 °C within 5 minutes, resulting in a temperature change (ΔT) of around 13 °C (Fig. 12c). This led to a substantial suppression in tumor growth, with 86.5% inhibition observed after the designated treatment period (Fig. 12d). NanoPcDA featured a critical characteristic of self-degradation post-photothermal therapy, facilitated by the 1O2 produced, resulting in the loss of its photoactivity. Importantly, the degradation by-products of NanoPcDA demonstrated negligible cytotoxicity, with less than 5% hemolysis observed, well below the acceptable threshold of 50%. This self-degradation mechanism was crucial for mitigating potential phototoxicity issues associated with residual photothermal agents (PTAs) left in the body after treatment. Overall, the study highlighted NanoPcDA as a groundbreaking, single-component, and easily fabricated PTA with high biosecurity and promising clinical implications for improved cancer therapy.
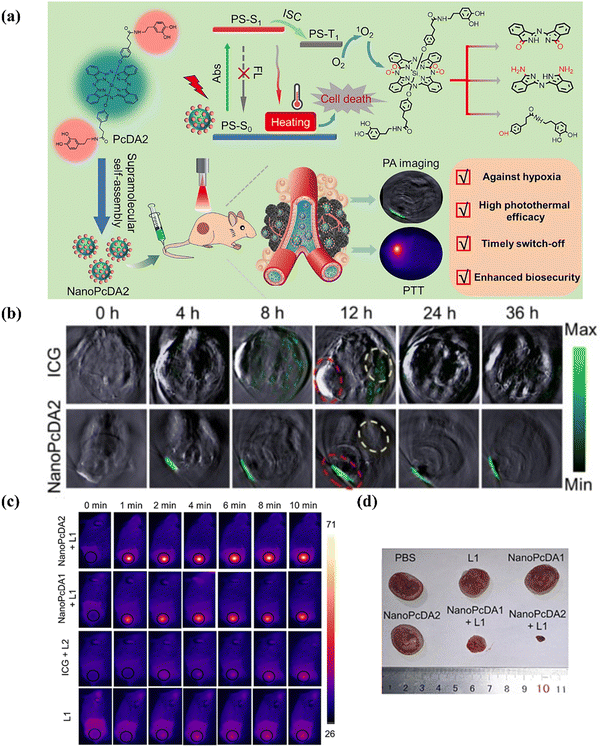 | ||
| Fig. 12 (a) Molecular structure of PcDA, fabrication of NanoPcDA and its application for PTT guided by PA imaging. (b) PA images of tumor-bearing mice before and after intravenous injection with the indicated formulations. (c) Thermal infrared imaging of tumor-bearing mice after the indicated treatment. (d) Representative images of the tumor after the indicated treatment. Copyright 2023, Elsevier.70 | ||
Table 2 offers a comprehensive summary of various self-assembled nano-photosensitizers derived from phthalocyanines that have been explored for PTT applications. The table systematically categorizes these nano-photosensitizers based on their composition, specific names, and the distinct properties that render them suitable for PTT. It also highlights key findings from in vitro and vivo studies, including the efficiency of photothermal conversion and the consequent therapeutic outcomes. This compilation serves as a valuable resource for researchers and clinicians engaged in the development and application of phthalocyanine-based nano-photosensitizers for PTT, facilitating a deeper understanding of their potential and limitations.
| Phthalocyanine | Names | Advantages | In vivo application | Ref. |
|---|---|---|---|---|
| PCE: photothermal conversion efficiency; MA: mitoxantrone; PTA: photothermal agent; FLI: fluorescence imaging; CuPc: copper phthalocyanine; MnPc: manganese phthalocyanine; MRI: magnetic resonance imaging; FePc: iron phthalocyanine. | ||||
| ZnPc | PcS-MA | Suppressed fluorescence emission indicating PcS-MA as a potential PTA; notable tumor suppression | FLI, PDT, PTT and CHT | 71 |
| ZnPc, CuPc | ZnPc(PEG)5:CuPc-N | CuPc for enhanced PTT with a high PCE; PDT and PTT stimulated by a single laser beam; the combination treatment group exhibiting the slowest tumor growth rate | FLI, PDT and PTT | 55 |
| ZnPc | Bio-ZnPc-Pdots | Redshift absorption and excellent PTT within the in vivo transparent window (800–900 nm); PCE (η = 38.17%); solid tumor reduction and scab formation within 2 days post-treatment | PA, PTT | 72 |
| ZnPc | ZnPc NPs | An obvious PTT effect due to Pcs molecule propensity for π–π stacking in nanostructure formation; high PCE (η = 31.3%); combined remarkable PDT; an obvious decrease in the tumor size after treatment | FLI, PTT, PDT | 43 |
| ZnPc | Zn4–H2Pc/DP NPs | Characteristic absorption in the NIR-II region at 1064 nm; high PCE of 58.3%; elimination of tumor after treatment | NIR-II PA, PTT | 73 |
| ZnPc | NanoPc3 | Aggregation-induced non-radiation (PTT) under laser irradiation; PCE of 26.7%; excellent therapeutic efficacy toward the tumor | PA, PTT | 74 |
| MnPc | MnPc-NDs | MnPc with paramagnetic ions for effective PTT; high PCE (η = 59.8%); excellent magnetic resonance contrast performances; a significant solid tumor reduction | MRI, PDT, PTT | 75 |
| FePc | HSA-FePc NPs | FePc with open shell paramagnetic ions used for PTT; high PCE (η = 44.4%) and high contrast and spatial resolution for PA imaging; complete inhibition of tumor after treatment | PA, PTT | 76 |
3.4. Development of phthalocyanine-based type I nano-photosensitizers
Type I PDT demonstrates reduced oxygen dependency compared to type II PDT, emphasizing the significance of developing type I photosensitizers. Recently, Zhao et al.77 engineered nanostructured phthalocyanine assemblies (NanoPcAF), capable of transforming the photophysical and photochemical properties of traditional phthalocyanine-based photosensitizers, from a type II photoreaction to an efficient type I photoreaction and photothermal conversion (Fig. 13a). Under hypoxic conditions, NanoPcAF showcased the capacity to produce 3.4 times the amount of O2˙− compared to the known photosensitizer methylene blue (MB) (Fig. 13b). Furthermore, NanoPcAF showed a photothermal conversion efficiency 2.4 times higher than that of ICG (Fig. 13c). NanoPcAF also exhibited pronounced concentration-dependent cytotoxicity under both normoxic and hypoxic conditions, with IC90 values of 0.45 ± 0.08 μM and 0.42 ± 0.10 μM, respectively (Fig. 13d). Tumor growth inhibition reached 94% when NanoPcAF was administered at a dose of 0.8 nmol g−1 in combination with a light dose of 300 J cm−2 (Fig. 13e). The high tumor accumulation, efficient generation of type I ROS, and robust photothermal performance of NanoPcAF positioned it as a clinically promising agent for the treatment of hypoxic tumor. | ||
| Fig. 13 (a) Chemical structure of PcAF, fabrication of NanoPcAF and application of NanoPcAF in type I PDT and PTT. (b) O2˙− generation of the indicated formulation in water. (c) Time-dependent temperature elevation of NanoPcAF and ICG after laser treatment. (d) Dark and photocytotoxicity after different treatments. (e) Relative tumor volume of mice after different treatments over 14 days. Copyright 2021, ACS.77 | ||
Wang et al.78 presented a study on a nanostructured phthalocyanine/albumin supramolecular assembly, termed NanoPcM, for fluorescence turn-on imaging and photodynamic immunotherapy (Fig. 14). NanoPcM was synthesized through the self-assembly process involving morpholine-substituted silicon phthalocyanine (PcM) and albumin. The assembly featured a pH-responsive fluorescence turn-on mechanism, pivotal for precise tumor-targeted imaging. NanoPcM demonstrated an efficient type I photoreaction. The generation of 1O2 (a type II ROS) was minimal, while the production of O2˙− (a type I ROS) was substantial, suggesting the potential for effective PDT even under hypoxic conditions (Fig. 14b and c). Notably, the tumor volume and weight were considerably reduced in the NanoPcM + laser group compared to the control group (Fig. 14d and e). The tumor growth inhibition (TGI) rate reached 50.28% for the NanoPcM + laser group, which further increased to 64.85% with the addition of αPD-1, demonstrating a synergistic effect in inhibiting tumor growth.
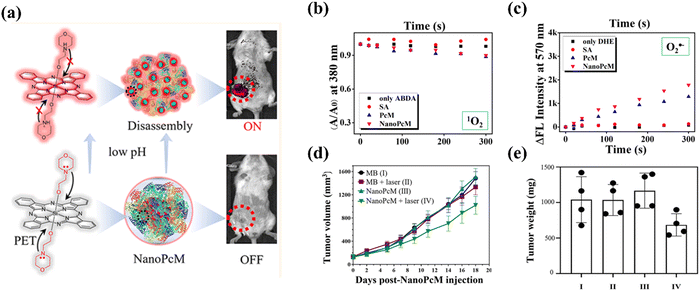 | ||
| Fig. 14 (a) Structure of PcM, fabrication of NanoPcM and its application in acid responsive tumor targeted fluorescence turn-on imaging. Time-dependent 1O2 (b) or O2˙− (c) generation of the indicated formulation after laser irradiation. (d) Curves of the tumor volume of mice after different treatments over 18 days. (e) Measurement of tumor weights at day 18 after treatment. Copyright 2022, ACS.78 | ||
Contrary to the majority of type I nano-photosensitizers that predominantly generate O2˙− upon light irradiation, Li and colleagues79 recently reported on a silicon phthalocyanine-based nano-photosensitizer. This innovative agent displayed the unique capability to efficiently produce ˙OH, a different type of type I ROS, without requiring oxygen participation. This characteristic made it particularly attractive for use in hypoxic environments.
Beyond their applications in cancer therapy, several self-assembled phthalocyanine-based type I nanoplatforms were developed, showcasing their potential for bacterial eradication.47,80–82 These self-assembled phthalocyanines demonstrated the capability to efficiently generate O2˙− under both normal and hypoxic conditions. Given their ability to produce ROS even under hypoxia, these nano-photosensitizers show promise for PDT of hypoxic tumors. Nonetheless, the lack of a specific guideline for the design of type I nano-photosensitizers highlights the need for substantial research efforts to advance the development of these therapeutic agents. Further exploration is required to optimize their design, efficacy, and safety, ensuring their full potential can be realized in clinical settings.
3.5. Combination of PDT with other therapies
The effectiveness of PDT as a standalone treatment for cancer may be limited, particularly under hypoxic conditions. Furthermore, monotherapy is often insufficient to completely eradicate malignant tumors due to the aggressive nature of cancer metastasis and the emergence of drug resistance.83,84 A multi-modal approach, involving the combination of two or more therapies, can effectively overcome these limitations by providing a more comprehensive strategy to combat cancer.85–87 This strategy not only improves treatment efficacy but also helps to prevent the development of resistance, offering a more potent and versatile treatment option. By integrating PDT with complementary modalities, such as hypoxia-activated chemotherapy,88 gas therapy,89 PTT,90,91 starvation therapy,67 and immunotherapy,92,93 a synergistic effect can be realized. The combination optimizes therapeutic outcomes while mitigating adverse effects, thereby enhancing the overall treatment efficacy of PDT and minimizing collateral damage to healthy tissues.Yoon et al.94 unveiled a groundbreaking strategy that enhanced the efficacy of PDT for cancer treatment by employing in vivo-assembled phthalocyanine/albumin supramolecular complexes along with a hypoxia-activated prodrug, AQ4N (as shown in Fig. 15). This method effectively addressed key limitations of traditional PDT, including inefficient photosensitizer delivery, tumor hypoxia, and inadequate immune stimulation. The combination of PcN4 and AQ4N led to a significant reduction in the growth of primary tumor. The treatment significantly activated CD8+ T cells, with the majority being both PD-1- and TIM3-positive, indicating the potential benefit of combination therapy with a PD-L1/PD-1 pathway blockade. Incorporating an anti-PD-L1 blocking antibody into the treatment regimen amplified the abscopal effect, suppressing the growth of both distant and metastatic tumors. In summary, the combination of PcN4 and AQ4N, along with anti-PD-L1 therapy, was highly effective in activating cancer-specific cytotoxic T cells in primary and distant tumors. This combination therapy also demonstrated an enhanced abscopal effect on treatment-resistant murine triple-negative breast cancers, suggesting its potential in boosting antitumor immunity and managing metastatic disease.
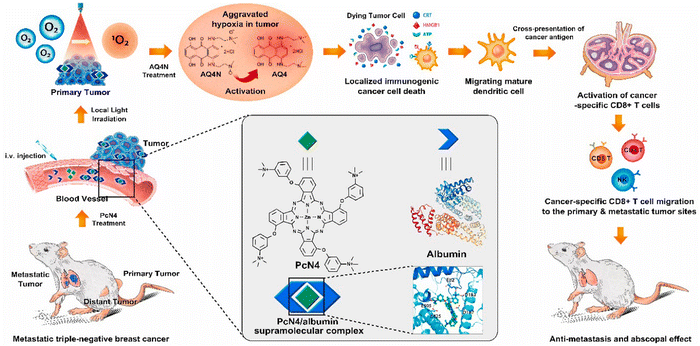 | ||
| Fig. 15 Schematic illustration detailing the fabrication process of PcN4/albumin supramolecular complexes integrated with the hypoxia-activated prodrug AQ4N, designed for immunogenic PDT in hypoxic tumor microenvironments. Copyright 2021, Elsevier.94 | ||
In a trailblazing study, Sen Yang and colleagues95 crafted a smart DNA hydrogel designed to synergize immunotherapy and PDT for the treatment of melanoma. The DNA hydrogel, assembled from ultra-long DNA chains through a process known as rolling circle amplification, integrated a triad of functional units: immune-activating adjuvants, photodynamic modules (ZnPc), and tumor-killing exosomes (Fig. 16a). The innovative design allowed for the hydrogel to disassemble into its constituent units within the tumor microenvironment, facilitated by the presence of HhaI restriction endonuclease, thereby releasing the loaded therapeutic agents (Fig. 16b). The immune-activating CpG oligonucleotides within the hydrogel played a pivotal role in stimulating antigen-presenting cells, enhancing the body's immune response to the tumor. ZnPc, upon exposure to a 660-nm laser, initiated a cascade of reactions that resulted in the generation of ROS, effectively targeting and killing tumor cells. Notably, the cellular debris produced by PDT acted as an immune antigen, further amplifying the immunotherapeutic effect (Fig. 16c). In an orthotopic mouse melanoma model, the smart DNA hydrogel achieved a striking tumor suppression rate of 91.2%. This high efficacy was attributed to the hydrogel's ability to overcome the challenges of a hypoxic tumor microenvironment. Moreover, the study confirmed the hydrogel's biocompatibility, with no significant side effects noted in the histopathological analysis of major organs and serum biochemical indicators.
 | ||
| Fig. 16 Illustrative schematic showcasing a smart DNA hydrogel designed for the synergistic and efficacious treatment of melanoma by incorporating multivalent therapeutic components. The schematic included: (a) construction of a DNA hydrogel based on the assembly, (b) responsive mechanisms of a DNA hydrogel, and (c) synergistic effect of a DNA hydrogel for in vivo therapy. Copyright 2024, Wiley.95 | ||
For a comprehensive overview, Table 3 elaborates on additional instances where phthalocyanine-based PDT has been successfully integrated with complementary treatments, achieved through the self-assembly process. This table serves as a detailed resource, offering insights into various self-assembled phthalocyanine-based nano-photosensitizers and their synergistic combinations with other therapeutic modalities. Included in Table 3 are specifics on the phthalocyanine derivatives utilized, the functional substances incorporated, the types of complementary treatments integrated, and the observed outcomes regarding enhanced therapeutic effects and potential applications in cancer treatment. This compilation of data highlights the versatility and efficacy of combining phthalocyanine-based PDT with other modalities to achieve superior outcomes in cancer therapy.
| Phthalocyanine | Functional substances | Therapeutic types | Inhibition on tumor or cells | Ref. |
|---|---|---|---|---|
| ICG: indocyanine; RBC: red blood cell membranes; CD: cyclodextrin; NO: nitric oxide; CDT: chemodynamic therapy; Chemo: chemotherapy. | ||||
| ZnPc | ZnPc/ICG/RBC | PDT/PTT | Eradication of tumor completely | 96 |
| ZnPc | ZnPc/Poly-β-CD/Adamantyl-nitroaniline | PDT/Gas Therapy (NO) | Obvious cell mortality | 97 |
| ZnPc | Cu2+/ZnPc/Dox | PDT/PTT/CDT | An outstanding tumor-suppressive effect | 53 |
| ZnPc | ZnPc/Curcumin | PDT/Chemo | A significant tumor growth inhibition | 57 |
| ZnPc | ZnPc/C14-IP2000 (iridium-based photosensitizer) | PDT/PTT | An 83.2% decrease in tumor volume | 90 |
| ZnPc | ZnPc/PD-L1 antibody | PDT/immunotherapy | A significant tumor growth inhibition and prolonged survival time of treated mice | 98 |
| ZnPc | ZnPc/Dox | PDT/PTT/Chemo | Tumor growth inhibition rate of more than 90% | 99 |
| MnPc | Bi/MnPcE4 | PDT/PTT | Complete tumor elimination | 100 |
| FePc | Fe(II)Pc | PDT/PTT/CDT | Complete tumor elimination | 101 |
In this part, we outline some current strategies to augment the efficacy of PDT in hypoxic tumors based on self-assembled phthalocyanine-based nano-photosensitizers. The advantages and disadvantages of different strategies are summarized in Table 4. These approaches offer a promising perspective for overcoming the challenges posed by hypoxia in PDT.
| Strategy | Advantages | Disadvantages |
|---|---|---|
| CAT: catalase; OXPHOS: oxidative phosphorylation. | ||
| In situ O2 generation | The method exhibits tumor specificity, attributed to the selective reaction of CAT or metal nanozyme with H2O2 within the tumor microenvironment | (1) Endogenous H2O2 within the tumor may be inadequate to attain optimal therapeutic efficacy, and the targeted delivery of exogenous H2O2 to tumor remains challenging |
| (2) Relieving hypoxia within a deep-seated tumor presents a challenge, due to abnormal tumor vasculature | ||
| Reduction of intracellular oxygen consumption | The methodology is capable of reducing intracellular ATP levels by inhibiting the process of OXPHOS, thereby inhibiting the proliferative of tumor cells | (1) The majority of tumor cells exhibit a reliance on glycolysis for energy production, with the inhibition of respiratory pathways being inherently constrained |
| (2) The development of drug resistance can significantly undermine the therapeutic efficacy of cancer treatments | ||
| Development of phthalocyanine-based nano-photosensitizers for PTT | PTT is independent of oxygen and can drive therapy in an O2-deficient environment | (1) PTT needs high intensities of light to excite the PS, which can easily cause damage to the healthy skin and tissue |
| (2) Thermal resistance increases the expression of heat shock protein | ||
| (3) PTT can cause inflammatory responses | ||
| Development of phthalocyanine-based type I nano-photosensitizers | (1) Type-I PDT is less affected by O2 concentrations | The design of type I PDT lacks a specific guideline. |
| (2) The generated ˙OH of Type-I PDT is a stronger oxidant than 1O2 (generated by Type-II PDT) | ||
| Combination of PDT with other therapies | (1) Combination therapy provides a more comprehensive strategy to combat cancer | (1) The fabrication of nanoparticles becomes complicated due to more components being involved |
| (2) Combination therapy may prevent the development of resistance | (2) The metabolic process involving multiple components continues to be intricate | |
4. Summary and outlook
This review delved deeply into the advancements in the development and application of self-assembled phthalocyanine-based nano-photosensitizers of PDT for hypoxic tumors. The self-assembly approach was underscored as an innovative strategy to enhance the solubility, stability, and bioavailability of these photosensitizers, while facilitating the integration of additional therapeutic agents and the creation of stimuli-responsive systems. Initially, the fundamental principles of phthalocyanines and PDT were thoroughly illustrated. The oxygen content within tumor tissue was emphasized as a critical factor impacting the efficacy of PDT.Following this, this review explored the self-assembly strategies for phthalocyanine-based photosensitizers, including single component assembly and multicomponent assembly. Finally, this review meticulously summarized and enumerated the methodologies and applications of self-assembled phthalocyanine-based nano-photosensitizers in the treatment of hypoxic tumors. The methodologies included: (1) in situ O2 generation; (2) reduction of intracellular oxygen consumption; (3) development of phthalocyanine-based nano-photosensitizers for PTT; (4) development of type I nano-photosensitizers; (5) combination of PDT with other therapies.
Despite the extensive research and significant advancements in the field of PDT for hypoxic tumors, the development of self-assembled phthalocyanine-based nano-photosensitizers continues to confront several future challenges:
(1) Evolution of Type-I and Type-III PSs. The advent of Type-I PDT paradigms, characterized by a relative independence from oxygen, offers a compelling alternative to conventional Type-II PDT methods. However, there is a lack of standardized protocols to guide the synthesis and application of Type-I PDT. Despite the reduced oxygen requirement for Type-I PSs through facilitated oxygen circulation, a minimal amount of oxygen is still essential for the photosensitization process. The development of Type-III PSs for cancer PDT has introduced a novel therapeutic strategy. Specifically, in the triplet excited state, the PSs predominantly transfers energy or electrons to RNA molecules, not oxygen.102 This mechanism leads to tumor-killing effects through apoptosis under hypoxic conditions and even in anoxic environments by damaging RNA. The design of Type-III PSs, alongside the engineering of multifunctional Type-I PSs capable of synergistic therapeutic effects represents the future research directions of developing more effective PDT treatments for hypoxic tumors.
(2) Limited tissue penetration depth of light. The depth of light penetration into living tissues increases with longer wavelengths. To surpass the energy threshold requisite for ROS generation, the maximum permissible excitation wavelength is established at 850 nm. This constraint implies a maximum light penetration depth of approximately 3 mm. To counteract the limitation of restricted excitation light penetration, the implementation of two-photon excitation, X-ray, or internal illumination facilitated by chemo/bioluminescence-activated PSs stands out as a prospective strategy for enhancing deep-tissue PDT. Meanwhile, sonodynamic therapy (SDT), which exhibits no tissue penetration limit, is an intriguing approach.103
(3) Combination with immunotherapy. The synergistic integration of PDT with other therapies, such as chemotherapy or PTT, has demonstrated enhanced anticancer effects. Furthermore, the combination of PDT with immunotherapy, such as PD-L1, STING agonist, or pyroptosis inducer, has exhibited superior therapeutic efficacy and demonstrated efficacy in preventing tumor recurrence. Nonetheless, the exploration of ZnPc-based immunotherapy is still in its nascent stages, highlighting the need for further investigation.
(4) Simplifying synthesis routes. The current synthesis process of phthalocyanine-based photosensitizers is complex,104–106 necessitating optimization of synthesis and purification steps. Without such optimization, reproducible and scalable nanoparticle production remains elusive, hindering the potential for clinical application.
(5) Pharmacokinetic/pharmacodynamic analysis. Presently, the majority of research endeavors in this field lacks comprehensive pharmacokinetic/pharmacodynamic evaluation, which is indispensable for optimizing therapy conditions, demonstrating the long-term safety of photosensitizers, minimizing side effects, understanding the in vivo behavior of nano-photosensitizer, and facilitating clinical translation of PDT for hypoxic tumors.
(6) Optimization of self-assembly processes. The production of self-assembled nanodrugs in laboratories predominantly relies on the nanoprecipitation.107,108 This method, however, is subject to various influencing factors that complicate the achievement of optimal preparation conditions, thereby posing significant challenges to the transition toward industrial mass production. Self-assembled phthalocyanine-based nano-photosensitizers primarily utilize the EPR effect to accumulate at tumor sites. The dimensions and morphology of these nano-photosensitizers governed by the self-assembly process significantly affect their biodistribution, cellular uptake, and clearance rates. Furthermore, the self-assembly process is vital for creating stable colloidal suspensions, which are necessary to ensure consistent efficacy of these agents in biological environments. This self-assembly process prevents premature release of the photosensitizers in the bloodstream, dilution by bodily fluids, interaction with intricate physiological components, and enzymatic degradation, requiring careful consideration and precise control for the progression of nano-photosensitizers in PDT. Thus, the optimization of the self-assembly process stands as a critical and arduous task for the progression of nano-photosensitizers in PDT, enabling meticulous regulation of the characteristics governing their performance in biological systems, thereby resulting in more efficient, targeted, and safe treatments for cancer.
Upon addressing these critical research gaps, we anticipate the emergence of a diverse spectrum of self-assembled phthalocyanine-based nano-photosensitizers tailored for biomedical applications. This advancement will facilitate the transformation of experimental successes into viable clinical therapies, surmounting the challenges posed by hypoxic tumors for PDT and potentially redefining the landscape of cancer treatment.
Data availability
This article is a review and does not involve the collection or use of new data. All information presented is based on previously published studies and publicly available sources.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by Jiangsu Province's Innovation and Entrepreneurship Doctoral Program (JSSCBS20222376).References
- J. J. Hu, Q. Lei and X. Z. Zhang, Recent advances in photonanomedicines for enhanced cancer photodynamic therapy, Prog. Mater. Sci., 2020, 114, 100685 CrossRef CAS.
- A. P. Castano, P. Mroz and M. R. Hamblin, Photodynamic therapy and anti-tumour immunity, Nat. Rev. Cancer, 2006, 6, 535–545 CrossRef CAS.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Imaging and photodynamic therapy: mechanisms, monitoring, and optimization, Chem. Rev., 2010, 110, 2795–2838 CrossRef CAS.
- G. M. F. Calixto, J. Bernegossi, L. M. D. Freitas, C. R. Fontana and M. Chorilli, Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: a review, Molecules, 2016, 21, 342 CrossRef.
- D. Chen, Q. Xu, W. Wang, J. Shao, W. Huang and X. Dong, Type I photosensitizers revitalizing photodynamic oncotherapy, Small, 2021, 17, 2006742 CrossRef CAS.
- C. A. Robertson, D. H. Evans and H. Abrahamse, Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT, J. Photochem. Photobiol., B, 2009, 96, 1–8 CrossRef CAS PubMed.
- T. Luo, K. Ni, A. Culbert, G. Lan, Z. Li, X. Jiang, M. Kaufmann and W. Lin, Nanoscale metal–organic frameworks stabilize bacteriochlorins for type I and type II photodynamic therapy, J. Am. Chem. Soc., 2020, 142, 7334–7339 CrossRef CAS PubMed.
- J. Jia, Z. Ma, J. Zhuang, L. Huo, C. Zhou, N. Li and N. Zhao, Lipid droplet-targeted NIR AIE photosensitizer evoking concurrent ferroptosis and apoptosis, Aggregate, 2024, 5, e516 CrossRef CAS.
- X. He, Y. Luo, Y. Li, Y. Pan, R. T. K. Kwok, L. He, X. Duan, P. Zhang, A. Wu, B. Z. Tang and J. Li, D-type neuropeptide decorated AIEgen/RENP hybrid nanoprobes with light-driven ROS generation ability for NIR-II fluorescence imaging-guided through-skull photodynamic therapy of gliomas, Aggregate, 2024, 5, e396 CrossRef CAS.
- O. A. Hamad, R. O. Kareem and P. K. Omer, Properties, characterization, and application of phthalocyanine and metal phthalocyanine, J. Chem. Rev., 2024, 6, 39–75 CAS.
- A. Kumar, V. K. Vashistha and D. K. Das, Recent development on metal phthalocyanines based materials for energy conversion and storage applications, Coordin. Chem. Rev., 2021, 431, 213678 CrossRef CAS.
- D. Dini and M. Hanack, Phthalocyanines as materials for advanced technologies: some examples, J. Porphyrins Phthalocyanines, 2004, 8, 915–933 CrossRef CAS.
- S. Yang, Y. Yu, X. Gao, Z. Zhang and F. Wang, Recent advances in electrocatalysis with phthalocyanines, Chem. Soc. Rev., 2021, 50, 12985–13011 RSC.
- D. Mamand, T. K. Anwer, H. Qadr and C. H. Mussa, Investigation of spectroscopic and optoelectronic properties of phthalocyanine molecules, Russ. J. Gen. Chem., 2022, 92, 1827–1838 CrossRef CAS.
- M. C. Vebber, N. A. Rice, J. L. Brusso and B. H. Lessard, Thermodynamic property–performance relationships in silicon phthalocyanine-based organic photovoltaics, ACS Appl. Energy Mater., 2022, 5, 3426–3435 CrossRef CAS.
- D. S. Weiss, in Organic photoconductors: photogeneration, transport, and applications in printing, ed. S. O. Kasap, Wiley, Hoboken, 7th edn, 2022, ch. 7, pp. 275–338 Search PubMed.
- B. Köksoy, E. B. Orman, H. Kuruca, M. Bulut, M. Durmuş and A. R. Ozkaya, Mono and double-decker lutetium phthalocyanines bearing iodine groups: electrochemical and electrochromic properties, J. Electrochem. Soc., 2016, 163, H927 CrossRef.
- A. Kumar, G. Zhang, W. Liu and X. M. Sun, Electrocatalysis and activity descriptors with metal phthalocyanines for energy conversion reactions, J. Electroanal. Chem., 2022, 922, 116799 CrossRef CAS.
- D. Gounden, N. Nombona and W. V. Zyl, Recent advances in phthalocyanines for chemical sensor, non-linear optics (NLO) and energy storage applications, Coordin. Chem. Rev., 2020, 420, 213359 CrossRef CAS.
- D. Li, S. Cai, P. Wang, H. Cheng, B. Cheng, Y. Zhang and G. Liu, Innovative design strategies advance biomedical applications of phthalocyanines, Adv. Healthcare Mater., 2023, 12, 2300263 CrossRef CAS.
- D. Chen, M. Song, J. Huang, N. Chen, J. Xue and M. Huang, Photocyanine: A novel and effective phthalocyanine-based photosensitizer for cancer treatment, J. Innovative Opt. Health Sci., 2020, 13, 2030009 CrossRef CAS.
- S. Tabassum, A. M. Sheikh, S. Yano, T. Ikeue, S. Mitaki, M. Michikawa and A. Nagai, A cationic gallium phthalocyanine inhibits amyloid β peptide fibril formation, Curr. Alzheimer Res., 2020, 17, 589–600 CrossRef CAS.
- O. Zhytniakivska, A. Kurutos, U. Tarabara, K. Vus, V. Trusova, G. Gorbenko, N. Gadjev and T. Deligeorgiev, Probing the amyloid protein aggregates with unsymmetrical monocationic trimethine cyanine dyes, J. Mol. Liq., 2020, 311, 113287 CrossRef CAS.
- M. Wang and K. Ishii, Photochemical properties of phthalocyanines with transition metal ions, Coord. Chem. Rev., 2022, 468, 214626 CrossRef CAS.
- Z. Chen, F. Han, Y. Du, H. Shi and W. Zhou, Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions, Signal Transduction Targeted Ther., 2023, 8, 70 CrossRef.
- Q. Yu, T. Huang, C. Liu, M. Zhao, M. Xie, G. Li, S. Liu, W. Huang and Q. Zhao, Oxygen self-sufficient NIR-activatable liposomes for tumor hypoxia regulation and photodynamic therapy, Chem. Sci., 2019, 10, 9091–9098 RSC.
- Y. Qin, M. Huang, C. Huang, H. L. Perry, L. Zhang and D. Zhu, O2-generating multifunctional polymeric micelles for highly efficient and selective photodynamic-photothermal therapy in melanoma, Chin. Chem. Lett., 2024, 35, 109171 CrossRef CAS.
- F. Zhu, L. Xu, X. Li, Z. Li, J. Wang, H. Chen, X. Li and Y. Gao, Co-delivery of gefitinib and hematoporphyrin by aptamer-modified fluorinated dendrimer for hypoxia alleviation and enhanced synergistic chemo-photodynamic therapy of NSCLC, Eur. J. Pharm. Sci., 2021, 167, 106004 CrossRef CAS.
- K. Liang, F. Zhao, F. Nan, J. Wang, Y. Zhang, J. Li, X. Xue, T. Chen, L. Kong and J. Ge, Carbon dots/platinum nanoparticles-loaded mesoporous silica for synergistic photodynamic/catalytic therapy of hypoxic tumors, Mater. Chem. Front., 2023, 7, 2706–2720 RSC.
- W. Jin, Z. Chen, Y. Wang, J. Li, J. Li, Y. Pei and Z. Pei, Nano metal-photosensitizer based on Aza-BODIPY-Cu complex for CDT-enhanced dual phototherapy, Chin. Chem. Lett., 2024, 35, 109328 CrossRef CAS.
- H. Zhang, X. Yan, Y. Zhang, C. Bao and C. Li, An oxygen-economical nano-photosensitizer with a high photodynamic therapeutic outcome via simultaneous reduction of the cellular respiration and oxygen depletion of PDT, J. Mater. Chem. B, 2022, 10, 4623–4631 RSC.
- W. Feng, S. Zhang, Y. Wan, Z. Chen, Y. Qu, J. Li, T. James, Z. Pei and Y. Pei, Nanococktail based on supramolecular glyco-assembly for eradicating tumors in vivo, ACS Appl. Mater. Interfaces, 2022, 14, 20749–20761 CrossRef CAS.
- X. Xiong, J. Liu, L. Wu, S. Xiong, W. Jiang and P. Wang, Self-assembly strategies of organic small-molecule photosensitizers for photodynamic therapy, Coord. Chem. Rev., 2024, 510, 215863 CrossRef CAS.
- J. Zhao, X. Xu, Y. Yang and J. Li, Assembled photosensitizers applied for enhanced photodynamic therapy, CCS Chem., 2023, 5, 1043–1060 CrossRef CAS.
- S. Yadav, A. K. Sharma and P. Kumar, Nanoscale self-assembly for therapeutic delivery, Front. Bioeng. Biotechnol., 2020, 8, 127 CrossRef PubMed.
- X. Niu, M. Yuan, R. Zhao, L. Wang, Y. Liu, H. Zhao, H. Li, X. Yang and K. Wang, Fabrication strategies for chiral self-assembly surface, Microchim. Acta, 2024, 191, 202 CrossRef CAS.
- B. D. Zheng, J. Ye, X. Q. Zhang, N. Zhang and M. Xiao, Recent advances in supramolecular activatable phthalocyanine-based photosensitizers for anti-cancer therapy, Coord. Chem. Rev., 2021, 447, 214155 CrossRef CAS.
- C. Lan and S. Zhao, Self-assembled nanomaterials for synergistic antitumour therapy, J. Mater. Chem. B, 2018, 6, 6685–6704 RSC.
- Z. Yan, Y. Liu, L. Zhao, J. Hu, Y. Du, X. Peng and Z. Liu, In situ stimulus-responsive self-assembled nanomaterials for drug delivery and disease treatment, Mater. Horiz., 2023, 10, 3197–3217 RSC.
- X. Zhao, J. Liu, J. Fan, H. Chao and X. J. Peng, Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application, Chem. Soc. Rev., 2021, 50, 4185–4219 RSC.
- Y. Zhang and J. F. Lovell, Recent applications of phthalocyanines and naphthalocyanines for imaging and therapy, Wires Nanomed. Nanobiotechnol., 2017, 9, e1420 CrossRef.
- W. Wang, J. Wang, G. Hong, L. Mao, N. Zhu and T. Liu, Methoxypolyethylene glycol-substituted zinc phthalocyanines for multiple tumor-selective fluorescence imaging and photodynamic therapy, Biomacromolecules, 2021, 22, 4284–4294 CrossRef CAS PubMed.
- Z. Wang, S. Gai, C. Wang, G. Yang, C. Zhong, Y. Dai, F. He, D. Yang and P. Yang, Self-assembled zinc phthalocyanine nanoparticles as excellent photothermal/photodynamic synergistic agent for antitumor treatment, Chem. Eng. J., 2019, 361, 117–128 CrossRef CAS.
- Y. Li, R. C. Wong, X. Yan, D. K. Ng and P. C. Lo, Self-assembled nanophotosensitizing systems with zinc(II) phthalocyanine-peptide conjugates as building blocks for targeted chemo-photodynamic therapy, ACS Appl. Nano Mater., 2020, 3, 5463–5473 CrossRef CAS PubMed.
- J. Ma, Y. Li, G. Liu, A. Li, Y. Chen, X. Zhou, D. Chen, Z. Hou and X. Zhu, Novel theranostic zinc phthalocyanine–phospholipid complex self-assembled nanoparticles for imaging-guided targeted photodynamic treatment with controllable ROS production and shape-assisted enhanced cellular uptake, Colloids Surf., B, 2018, 162, 76–89 CrossRef CAS PubMed.
- K. Hou, L. Huang, Y. Qi, C. Huang, H. Pan and M. Du, A bisphenol A sensor based on novel self-assembly of zinc phthalocyanine tetrasulfonic acid-functionalized graphene nanocomposites, Mater. Sci. Eng., C, 2015, 49, 640–647 CrossRef CAS PubMed.
- X. Li, D. Lee, J. D. Huang and J. Yoon, Phthalocyanine-assembled nanodots as photosensitizers for highly efficient type I photoreactions in photodynamic therapy, Angew. Chem., 2018, 130, 10033–10038 CrossRef.
- J. C. Chu, J. Xiong, C. T. Wong, S. Wang, D. Y. Tam, A. García-Fernández, R. Martínez-Máñez and D. Ng, Detection and elimination of senescent cells with a self-assembled senescence-associated β-galactosidase-activatable nanophotosensitizer, J. Med. Chem., 2023, 67, 234–244 CrossRef.
- W. Yu, Y. Wang, J. Zhu, L. Jin, B. Liu, K. Xia, J. Wang, J. Gao, C. Liang and H. Tao, Autophagy inhibitor enhance ZnPc/BSA nanoparticle induced photodynamic therapy by suppressing PD-L1 expression in osteosarcoma immunotherapy, Biomaterials, 2019, 192, 128–139 CrossRef CAS PubMed.
- C. Wang, Z. Wang and X. Zhang, Amphiphilic building blocks for self-assembly: from amphiphiles to supra-amphiphiles, Acc. Chem. Res., 2012, 45, 608–618 CrossRef CAS.
- S. Ghosh, A. Ray and N. Pramanik, Self-assembly of surfactants: An overview on general aspects of amphiphiles, Biophys. Chem., 2020, 265, 106429 CrossRef CAS PubMed.
- Y. Li, P. Sun, L. Zhao, X. Yan, D. K. Ng and P. C. Lo, Ferric ion driven assembly of catalase-like supramolecular photosensitizing nanozymes for combating hypoxic tumors, Angew. Chem., Int. Ed., 2020, 59, 23228–23238 CrossRef CAS.
- J. Yin, C. Liu, J. Guo, M. Li, B. Chen, X. Zhang, B. Wang, X. Zhu and D. Chen, A copper-loaded self-assembled nanoparticle for disturbing the tumor redox balance and triple anti-tumor therapy, J. Mater. Chem. B, 2024, 12, 3509–3520 RSC.
- Y. Zhao, X. Zhang, Z. Chen, Y. Xu, H. Kim, H. Jeong, Y. R. Lee, J. Lee, X. Li and J. Yoon, Supramolecular phthalocyanine assemblies-enhanced synergistic photodynamic and photothermal therapy guided by photoacoustic imaging, Aggregate, 2024, e514 CrossRef CAS.
- K. Zheng, X. Liu, M. Li, S. Zhou and C. Ding, Phthalocyanine-based nanoassembly with switchable fluorescence and photoactivities for tumor imaging and phototherapy, Anal. Chem., 2022, 94, 15067–15075 CrossRef CAS.
- K. Zheng, X. Liu, H. Liu, D. Dong, L. Li, L. Jiang, M. Huang and C. Ding, Novel pH-triggered doxorubicin-releasing nanoparticles self-assembled by functionalized β-cyclodextrin and amphiphilic phthalocyanine for anticancer therapy, ACS Appl. Mater. Interfaces, 2021, 13, 10674–10688 CrossRef CAS.
- Z. Zhang, R. Wang, X. Huang, R. Luo, J. Xue, J. Gao, W. Liu, F. Liu, F. Feng and W. Qu, Self-delivered and self-monitored chemo-photodynamic nanoparticles with light-triggered synergistic antitumor therapies by downregulation of HIF-1α and depletion of GSH, ACS Appl. Mater. Interfaces, 2020, 12, 5680–5694 CrossRef CAS PubMed.
- J. Wu, Y. Liu, M. Cao, N. Zheng, H. Ma, X. Ye, N. Yang, Z. Liu, W. Liao and L. Sun, Cancer-responsive multifunctional nanoplatform based on peptide self-assembly for highly efficient combined cancer therapy by alleviating hypoxia and improving the immunosuppressive microenvironment, ACS Appl. Mater. Interfaces, 2023, 15, 5667–5678 CrossRef CAS PubMed.
- D. Wang, W. Ma, Y. Huang, W. Wang, S. Li, H. Liu, Y. Zhao, D. Peng, C. Y. Yu and H. Wei, Supramolecular nanoassemblies-mediated GSH depletion boosts synergistic chemo-and photodynamic therapy for immunogenicity enhancement, Chem. Eng. J., 2023, 468, 143731 CrossRef CAS.
- L. Shi, F. Hu, Y. Duan, W. Wu, J. Dong, X. Meng, X. Zhu and B. Liu, Hybrid nanospheres to overcome hypoxia and intrinsic oxidative resistance for enhanced photodynamic therapy, ACS Nano, 2020, 14, 2183–2190 CrossRef CAS PubMed.
- T. Chen, W. Zeng, Y. Liu, M. Yu, C. Huang, Z. Shi, C. Lin, J. Tang, L. Mei and M. Wu, Cu-doped polypyrrole with multi-catalytic activities for sono-enhanced nanocatalytic tumor therapy, Small, 2022, 18, 2202964 CrossRef CAS.
- J. Zhang, Z. Li, L. Liu, L. Li, L. Zhang, Y. Wang and J. Zhao, Self-assembly catalase nanocomplex conveyed by bacterial vesicles for oxygenated photodynamic therapy and tumor immunotherapy, Int. J. Nanomed., 2022, 17, 1971–1985 CrossRef PubMed.
- L. He, F. Xu, Y. Li, H. Jin and P. C. Lo, Cupric-ion-promoted fabrication of oxygen-replenishing nanotherapeutics for synergistic chemo and photodynamic therapy against tumor hypoxia, Acta Biomater., 2023, 162, 57–71 CrossRef CAS.
- W. Liang, C. Han, D. Zhang, C. Liu, M. Zhu, F. Xu, C. Fang, S. Zhang, C. Liu and Y. Li, Copper-coordinated nanoassemblies based on photosensitizer-chemo prodrugs and checkpoint inhibitors for enhanced apoptosis-cuproptosis and immunotherapy, Acta Biomater., 2024, 175, 341–352 CrossRef CAS PubMed.
- C. Li, Y. Gao, Y. Wang, J. Wang, J. Lin, J. Du, Z. Zhou, X. Liu, S. Yang and H. Yang, Bifunctional nano-assembly of iridium (III) phthalocyanine complex encapsulated with BSA: hypoxia-relieving/sonosensitizing effects and their immunogenic sonodynamic therapy, Adv. Funct. Mater., 2023, 33, 2210348 CrossRef CAS.
- X. Liu, L. Chen and Z. Chen, Acid-triggered controlled release and fluorescence-switchable phthalocyanine nanoassemblies combined with O2-economizer for tumor imaging and collaborative photodynamic antitumor therapy, Bioorg. Chem., 2024, 143, 106986 CrossRef CAS.
- Z. Liu, C. Li, Y. Cao, X. Xu, Z. Zhou, J. Du, S. Yang and H. Yang, Manganese (III) phthalocyanine complex nanoparticle-loaded glucose oxidase to enhance tumor inhibition through energy metabolism and macrophage polarization, ACS Appl. Bio Mater., 2024, 7, 1862–1877 CrossRef CAS PubMed.
- S. Li, L. Zhao, R. Chang, R. Xing and X. Yan, Spatiotemporally coupled photoactivity of phthalocyanine-peptide conjugate self-assemblies for adaptive tumor theranostics, Chem. – Eur. J., 2019, 25, 13429–13435 CrossRef CAS.
- X. Li, E. Y. Park, Y. Kang, N. Kwon, M. Yang, S. Lee, W. J. Kim, C. Kim and J. Yoon, Supramolecular phthalocyanine assemblies for improved photoacoustic imaging and photothermal therapy, Angew. Chem., 2020, 132, 8708–8712 CrossRef.
- Y. Y. Zhao, Z. Chen, L. Zhang, X. W. Qin, H. Liu, B. Y. Zheng, M. R. Ke, J. D. Huang and X. Li, A self-degradable nanostructured phthalocyanine assembly with high photothermal efficacy to enhanced biosecurity in photothermal therapy, Chem. Eng. J., 2023, 474, 145921 CrossRef CAS.
- X. Li, S. Yu, D. Lee, G. Kim, B. Lee, Y. Cho, B. Y. Zheng, M. R. Ke, J. D. Huang, K. T. Nam, X. Y. Chen and J. Y. Yoon, Facile supramolecular approach to nucleic-acid-driven activatable nanotheranostics that overcome drawbacks of photodynamic therapy, ACS Nano, 2018, 12, 681–688 CrossRef CAS PubMed.
- H. Jin, W. Zhong, S. Yin, X. Zhang, Y. Zhao, Y. Wang, L. Yuan and X. Zhang, Lesson from nature: biomimetic self-assembling phthalocyanines for high-efficient photothermal therapy within the biological transparent window, ACS Appl. Mater. Interfaces, 2019, 11, 3800–3808 CrossRef CAS.
- H. Pan, S. Li, J. Kan, L. Gong, C. Lin, W. Liu, D. Qi, K. Wang, X. Yan and J. Jiang, A cruciform phthalocyanine pentad-based NIR-II photothermal agent for highly efficient tumor ablation, Chem. Sci., 2019, 10, 8246–8252 RSC.
- B. D. Zheng, Z. L. Huang, L. L. Lv, W. L. Lan, J. Q. Hu, X. Li, B. Y. Zheng, M. R. Ke and J. D. Huang, A pH-sensitive nanoagent self-assembled from a highly negatively-charged phthalocyanine with excellent biosafety for photothermal therapy, J. Mater. Chem. B, 2021, 9, 2845–2853 RSC.
- F. Wu, J. Chen, L. Yue, H. Li, H. Wang and X. Zhu, A simple strategy to fabricate phthalocyanine-encapsulated nanodots for magnetic resonance imaging and antitumor phototherapy, ACS Appl. Bio Mater., 2020, 3, 3681–3689 CrossRef CAS PubMed.
- Q. Jia, J. Ge, W. Liu, X. Zheng, M. Wang, H. Zhang and P. Wang, Biocompatible iron phthalocyanine–albumin assemblies as photoacoustic and thermal theranostics in living mice, ACS Appl. Mater. Interfaces, 2017, 9, 21124–21132 CrossRef CAS PubMed.
- Y. Y. Zhao, L. Zhang, Z. Chen, B. Zheng, M. R. Ke, X. Li and J. D. Huang, Nanostructured phthalocyanine assemblies with efficient synergistic effect of type I photoreaction and photothermal action to overcome tumor hypoxia in photodynamic therapy, J. Am. Chem. Soc., 2021, 143, 13980–13989 CrossRef CAS PubMed.
- R. Wang, K. H. Kim, J. Yoo, X. Li, N. Kwon, Y. H. Jeon, S. K. Shin, S. S. Han, D. S. Lee and J. Yoon, A nanostructured phthalocyanine/albumin supramolecular assembly for fluorescence turn-on imaging and photodynamic immunotherapy, ACS Nano, 2022, 16, 3045–3058 CrossRef CAS.
- L. Li, Y. Liao, S. Fu, Z. Chen, T. Zhao, L. Fang and X. Li, Efficient hydroxyl radical generation of an activatable phthalocyanine photosensitizer: oligomer higher than monomer and nanoaggregate, Chem. Sci., 2024, 15, 10980–10988 RSC.
- W. Su, X. Luo, P. Li, Y. Zhang, C. Lin, K. Wang and J. Jiang, Phthalocyanine self-assembled nanoparticles for type I photodynamic antibacterial therapy, Chin. Chem. Lett., 2024, 109522 CrossRef CAS.
- R. Wang, D. Kim, M. Yang, X. Li and J. Yoon, Phthalocyanine-assembled “one-for-two” nanoparticles for combined photodynamic–photothermal therapy of multidrug-resistant bacteria, ACS Appl. Mater. Interfaces, 2022, 14, 7609–7616 CrossRef CAS.
- W. Su, X. Jiang, Y. Zhang, C. Lin and P. Li, Photothermal-driven disassembly of naphthalocyanine nano-photosensitizers for photothermal and photodynamic therapy, J. Colloid Interface Sci., 2023, 647, 201–210 CrossRef CAS PubMed.
- N. Vasan, J. Baselga and D. Hyman, A view on drug resistance in cancer, Nature, 2019, 575, 299–309 CrossRef CAS PubMed.
- M. Sharma, A. K. Bakshi, N. Mittapelly, S. Gautam, D. Marwaha, N. Rai, N. Singh, P. Tiwari, N. Agarwal and A. Kumar, Recent updates on innovative approaches to overcome drug resistance for better outcomes in cancer, J. Controlled Release, 2022, 346, 43–70 CrossRef CAS PubMed.
- R. Kaur, A. Bhardwaj and S. Gupta, Cancer treatment therapies: traditional to modern approaches to combat cancers, Mol. Biol. Rep., 2023, 50, 9663–9676 CrossRef CAS PubMed.
- J. Gong, T. Shi, J. Liu, Z. Pei, J. Liu, X. Ren, F. Li and F. Qiu, Dual-drug codelivery nanosystems: An emerging approach for overcoming cancer multidrug resistance, Biomed. Pharmacother., 2023, 161, 114505 CrossRef CAS.
- S. Gavas, S. Quazi and T. Karpiński, Nanoparticles for cancer therapy: current progress and challenges, Nanoscale Res. Lett., 2021, 16, 173 CrossRef CAS PubMed.
- M. Wang, M. He, M. Zhang, S. Xue, T. Xu, Y. Zhao, D. Li, F. Zhi and D. Ding, Controllable hypoxia-activated chemotherapy as a dual enhancer for synergistic cancer photodynamic immunotherapy, Biomaterials, 2023, 301, 122257 CrossRef CAS PubMed.
- T. Zhang, Y. Pan, M. Suo, M. Lyu, J. W. Y. Lam, Z. Jin, S. Ning and B. Tang, Photothermal-triggered sulfur oxide gas therapy augments type I photodynamic therapy for potentiating cancer stem cell ablation and inhibiting radioresistant tumor recurrence, Adv. Sci., 2023, 10, 2304042 CrossRef CAS.
- Y. Deng, X. Wang, Y. Liu, Y. Xu, J. Zhang, F. Huang, B. Li, Y. Miao, Y. Sun and Y. Li, Dual-light triggered metabolizable nano-micelles for selective tumor-targeted photodynamic/hyperthermia therapy, Acta Biomater., 2021, 119, 323–336 CrossRef CAS.
- W. Feng, Y. Lv, Z. Chen, F. Wang, Y. Wang, Y. Pei, W. Jin, C. Shi, Y. Wang, Y. Qu, W. Ji, L. Pu, X. W. Liu and Z. Pei, A carrier-free multifunctional nano photosensitizer based on self-assembly of lactose-conjugated BODIPY for enhanced anti-tumor efficacy of dual phototherapy, Chem. Eng. J., 2021, 417, 129178 CrossRef CAS.
- M. Warszyńska, P. Repetowski and J. Dąbrowski, Photodynamic therapy combined with immunotherapy: Recent advances and future research directions, Coord. Chem. Rev., 2023, 495, 215350 CrossRef.
- X. Zhang, C. Yi, L. Zhang, X. Zhu, Y. He, H. Lu, Y. Li, Y. Tang, W. Zhao and G. Chen, Size-optimized nuclear-targeting phototherapy enhances the type I interferon response for “cold” tumor immunotherapy, Acta Biomater., 2023, 159, 338–352 CrossRef CAS.
- X. Li, Y. H. Jeon, N. Kwon, J. G. Park, T. Guo, H. R. Kim, J. D. Huang, D. S. Lee and J. Yoon, In Vivo-assembled phthalocyanine/albumin supramolecular complexes combined with a hypoxia-activated prodrug for enhanced photodynamic immunotherapy of cancer, Biomaterials, 2021, 266, 120430 CrossRef CAS.
- S. Yang, J. Wu, Z. Wang, Y. Cheng, R. Zhang, C. Yao and D. Yang, A smart DNA hydrogel enables synergistic immunotherapy and photodynamic therapy of melanoma, Angew. Chem., 2024, 136, e202319073 CrossRef.
- Y. Chen, Y. Li, J. Liu, Q. Zhu, J. Ma and X. Zhu, Erythrocyte membrane bioengineered nanoprobes via indocyanine green-directed assembly for single NIR laser-induced efficient photodynamic/photothermal theranostics, J. Controlled Release, 2021, 335, 345–358 CrossRef CAS.
- N. Kandoth, V. Kirejev, S. Monti, R. Gref, M. B. Ericson and S. Sortino, Two-photon fluorescence imaging and bimodal phototherapy of epidermal cancer cells with biocompatible self-assembled polymer nanoparticles, Biomacromolecules, 2014, 15, 1768–1776 CrossRef CAS.
- Z. Su, Z. Xiao, J. Huang, Y. Wang, Y. An, H. Xiao, Y. Peng, P. Pang, S. Han and K. Zhu, Dual-sensitive PEG-sheddable nanodrug hierarchically incorporating PD-L1 antibody and zinc phthalocyanine for improved immuno-photodynamic therapy, ACS Appl. Mater. Interfaces, 2021, 13, 12845–12856 CrossRef CAS.
- C. Liu, Q. Liu, L. Chen, M. Li, J. Yin, X. Zhu and D. Chen, A pH-sensitive self-assembled and carrier-free nanoparticle based on charge reversal for enhanced synergetic chemo-phototherapy, Adv. Healthcare Mater., 2020, 9, 2000899 CrossRef CAS.
- Z. Wang, T. Jia, Q. Sun, Y. Kuang, B. Liu, M. Xu, H. Zhu, F. He, S. Gai and P. Yang, Construction of Bi/phthalocyanine manganese nanocomposite for trimodal imaging directed photodynamic and photothermal therapy mediated by 808 nm light, Biomaterials, 2020, 228, 119569 CrossRef CAS.
- F. Nan, Q. Jia, X. Xue, S. Wang, W. Liu, J. Wang, J. Ge and P. Wang, Iron phthalocyanine-derived nanozyme as dual reactive oxygen species generation accelerator for photothermally enhanced tumor catalytic therapy, Biomaterials, 2022, 284, 121495 CrossRef CAS PubMed.
- Q. Yao, J. Fan, S. Long, X. Zhao, H. Li, J. Du, K. Shao and X. Peng, The concept and examples of type-III photosensitizers for cancer photodynamic therapy, Chem, 2022, 8, 197–209 CAS.
- F. Zhou, Y. Luo, W. Zhuang and T. Xu, A fully integrated conformal wearable ultrasound patch for continuous sonodynamic therapy., Adv. Mater., 2024, 2409528 CrossRef.
- F. Xu, M. Wang, E. Dotse, K. T. Chow and P. C. Lo, Inducing immunogenic cancer cell death through oxygen-economized photodynamic therapy with nitric oxide-releasing photosensitizers, Angew. Chem., Int. Ed., 2024, e202404561 CAS.
- J. Xiong, E. Y. Xue and D. K. Ng, Synthesis, cellular uptake, and photodynamic activity of oligogalactosyl zinc (II) phthalocyanines, ChemPlusChem, 2023, 88, e202200285 CrossRef CAS.
- S. Liu, J. Ma, E. Y. Xue, S. Wang, Y. Zheng, D. K. Ng, A. Wang and N. Zheng, Polymeric phthalocyanine-based nanosensitizers for enhanced photodynamic and sonodynamic therapies, Adv. Healthcare Mater., 2023, 12, 2300481 CrossRef CAS.
- H. Ji, W. Wang, O. Qiao and X. Hao, Review of carrier-free self-assembly of anticancer nanodrugs, ACS Appl. Nano Mater., 2024, 7, 4564–4587 CrossRef CAS.
- Q. Li, L. Yuan, S. Gao, Z. Wang, Y. Tang, C. Chen, C. Q. Zhao and X. Fu, Self-assembled nanodrug delivery systems for anti-cancer drugs from traditional Chinese medicine, Biomater. Sci., 2024, 12, 1662–1692 RSC.
| This journal is © the Partner Organisations 2024 |


