Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
LungDepo: modelling the regional particle deposition in the human lung via the Enalos Cloud platform†
Dimitris G. Mintis ab,
Dimitra-Danai Varsou
ab,
Dimitra-Danai Varsou bc,
Panagiotis D. Kolokathisbc,
Andreas Tsoumanis
bc,
Panagiotis D. Kolokathisbc,
Andreas Tsoumanis ab,
Georgia Melagraki
ab,
Georgia Melagraki d,
Johannes P. Seif
d,
Johannes P. Seif e,
Alejandro J. del Real
e,
Alejandro J. del Real ef,
Iseult Lynch
ef,
Iseult Lynch *bg and
Antreas Afantitis
*bg and
Antreas Afantitis *abc
*abc
aNovaMechanics Ltd., Nicosia 1070, Cyprus. E-mail: afantitis@novamechanics.com; Tel: +357 99048039
bEntelos Institute, Larnaca 6059, Cyprus
cNovaMechanics MIKE, Piraeus 18545, Greece
dDivision of Physical Sciences and Applications, Hellenic Military Academy, Vari 16672, Greece
eIDENER, 41300 Sevilla, Spain
fDepartment of Systems and Automation, University of Seville, 41092 Seville, Spain
gSchool of Geography, Earth and Environmental Sciences, University of Birmingham, Birmingham B15 2TT, UK
First published on 7th July 2025
Abstract
This work presents the development of LungDepo, a freely accessible online web application hosted on the Enalos Cloud platform (https://enaloscloud.novamechanics.com/proplanet/lungdeposition or https://enaloscloud.novamechanics.com/insight/lungdeposition/), which enables users to simulate particle deposition in the human lung across the head airway, the tracheobronchial and the alveolar (pulmonary) regions based on the aerodynamic diameter of inhaled particles. The LungDepo web-based tool offers two modelling approaches: the International Commission on Radiological Protection (ICRP) model and the multiple path particle dosimetry (MPPD) model, to simulate regional particle deposition and assess inhalation toxicity risk. In addition to modeling the fractional regional particle deposition based on the two implemented models, LungDepo offers the computation of the regional deposited mass (mg) and the computation of the regional lung surface area covered by deposited particles (m2) providing statistical analyses of the relative contributions of coarse (2.5–10.0 micron), fine (<2.5 micron) and ultrafine (<0.1 micron) particles. The web-based tool includes predefined modelling scenarios for various particle-bound substances or co-pollutants such as polycylic aromatic hydrocarbons (PAHs) or per- and polyfluoroalkyl substances (PFAS) and micro-sized engineered materials, along with provision for the inclusion of user-defined particle size distributions. The integration of an application programming interface (API) and the development of an intuitive graphical user interface (GUI) facilitate data exchange and integration with external environmental and regulatory toxicity models by simplifying complex tasks and broadening the tool's applicability for diverse users in environmental research, monitoring, and regulatory activities.
Environmental significanceEvery human breath may expose individuals to a toxic cocktail of ultrafine particles and engineered nanomaterials that are silently deposited in the human lungs. In this study, LungDepo is introduced as a free-to-use web application that uses dosimetry models to quantify the deposition of airborne particles within the human respiratory tract. The tool provides statistical analyses of the contributions of coarse (2.5–10.0 μm), fine (<2.5 μm), and ultrafine (<0.1 μm) particles. Designed for non-programmers, LungDepo is accessible to a wide audience and can potentially support broader regulatory frameworks for assessing human inhalation toxicity and safeguarding public health. |
1 Introduction
Air pollution results from the release of harmful substances into the atmosphere, originating from a variety of sources, including industrial emissions, vehicle exhaust, and natural events.1–4 Air pollution is responsible for approximately 9–12 million deaths annually on a global scale.5,6 The World Health Organization (WHO) identifies six principal air pollutants: particle pollution (particulate matter), ground-level ozone, carbon monoxide, sulfur oxides, nitrogen oxides, and lead.5,7 These pollutants can be broadly categorized based on their physical state into gases (e.g., ozone, carbon monoxide, nitrogen oxides, sulfur oxides), volatile liquids (e.g., certain organic compounds contributing to ozone formation), and particulate matter (solid or liquid particles suspended in the air, such as fine dust, soot, and aerosols) (Fig. 1B). Among these, particulate matter (PM) has emerged as the most critical air/environmental pollutant, as it is associated with the highest mortality and morbidity rates due to a range of health issues, including cardiovascular and pulmonary diseases, arrhythmias, coughing, chest discomfort and dyspnea.2,8–16 PM consists of aerosol particles suspended in the air that may be composed of either liquid or solid particulate matter.17 These particles may be formed in the atmosphere as a result of chemical reactions among various pollutants,5 or result from a range of both anthropogenic (road dust, waste-water treatment plants, landfills, construction sites, mining, office equipment, cooking etc.)18–21 and natural sources (soil, plants, volcanoes, forest fires, sea spray etc.)2,22 (Fig. 1A). Based on aerodynamic diameter, PM is categorized into three size fractions as coarse (PM10, 2.5–10.0 μm),5,23 fine (PM2.5, <2.5 μm)23 and ultrafine (PM0.1, <0.1 μm)24 (Fig. 1C). Fine and ultrafine particles are likely to enter the lower airways upon inhalation, causing detrimental respiratory health effects. Particles larger than 10 μm are mostly filtered by the nasal and upper respiratory tract25,26 (Fig. 1D).Understanding the deposition of inhaled particulate matter in the different regions of the respiratory tract is crucial for evaluating, quantifying and predicting inhalation toxicity. The chemical composition of PM is primarily carbonaceous with contributions from inorganic ions (including sulphates, nitrates and metal oxides) and volatile and semi-volatile organic compounds such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs), and per- and polyfluoroalkyl substances (PFAS),27–29 which can alter biological activity and contribute to carcinogenic, neurotoxic, and endocrine-disrupting effects.30–34 Fine and ultrafine particles remain airborne longer, penetrate deeper into the lungs, and often carry highly reactive transition metals and oxidized organics, enhancing their toxicity.35 Furthermore, microparticles or micro-sized engineered materials and their agglomerates with aerodynamic diameters between 0.1 and 100 μm such as zinc oxide (ZnO),36,37 iron oxide (Fe2O3),38 titanium dioxide (TiO2),39 aluminum oxide (Al2O3),40 copper oxide (Cu2O),41 cobalt oxide (Co3O4)41 and nickel hydroxide (Ni(OH)2)42 may pose additional inhalation hazards due to their ability to penetrate deep into the respiratory tract. Engineered micro-sized materials are applied in various industrial and consumer products.39 Consequently, it is crucial to evaluate the effects of inhalation exposure, particularly in terms of pulmonary toxicity, that may arise as a result of their very small dimensions.39
From a morphological perspective, the human respiratory system consists of two distinct structural regions. The first consists of the extrathoracic airways, termed as the head airways (HA) consisting of the nose, mouth, pharynx and larynx. The second consists of the tracheobronchial (TB) airway trees and the alveolar (AL) regions43 (Fig. 1D). Deposition in the HA and TB regions act as barriers to safeguard the AL region, which constitutes the air–blood barrier, from potential irritation or harmful particles.44 In the AL region, where the airway walls lacks a mucus layer, deposited insoluble particles are effectively cleared by the alveolar macrophages.43 However, soluble particles deposited in the AL region may dissolve and pass through the alveolar membranes, entering the systemic circulation.43,45 Numerous factors affect the regional deposition of particles within the human lungs, primarily dependent on an individuals' pulmonary physiology such as breathing patterns and lung geometry.46,47 Additionally, the physicochemical characteristics of inhaled particles, such as their size, size distribution, density, shape, charge, surface properties, hygroscopicity (ability to absorb moisture from the surrounding air) and the interactions between particles and pulmonary surfactant play a critical role.35,48–50 Of these factors, the size of inhaled particles is considered as the key predominant factor affecting the fate and regional deposition of particles in the human lungs.51 Coarser size particles (PM10) tend to lodge in the throat and/or the bronchi, whereas fine and ultrafine particles can travel deeper into the airways of the respiratory tract, reaching the alveoli, binding with proteins that support (opsonising) or hamper (disopsonising) recognition by macrophages and influence crossing of anatomical barriers, and penetrating into the bloodstream.2,8,52
The experimental determination of particle deposition within the human respiratory tract poses significant challenges in both in vivo and in vitro studies. In vivo studies are ethically problematic, while in vitro models have significant limitations in accurately replicating realistic physiological conditions.53 To overcome these limitations, particle dosimetry models have been extensively developed over the past decades to calculate the regional deposition patterns of inhaled aerosols.54–56 It is still challenging however, to accurately measure and quantify analytically the deposition of particles in the respiratory tract due to the intricate interplay between the structural morphology of lungs (that differs between individuals) and the hydrodynamic flow field within the airways, which constantly undergoes dynamic changes.44 According to Hofmann,57 existing particle dosimetry models are categorized based on lung morphometry and mathematical modelling techniques into semi-empirical models,58–60 one-dimensional cross-section or “trumpet” models,61–64 mechanistic models,65–70 and computational fluid and particle dynamics (CFPD)-based models.71–75 Among the most widely used particle dosimetry models are two semi-empirical models that combine (first-principle) mechanistic frameworks with experimental (empirical) data:57,58,76 the ICRP66 model developed by the International Committee of Radiological Protection (ICRP) in 1994 (ref. 58) (which has since been updated to ICRP130),59 and the NCRP model developed by the National Council on Radiation Protection and Measurements (NCRP) in the USA, in 1997.60 Additionally, mechanistic models such as the deterministic asymmetric branching model, also known as the multiple pathway particle dosimetry (MPPD) model, introduced in 2001,77,78 and the stochastic asymmetric branching model, referred to as IDEAL (inhalation, deposition and exhalation of aerosols in the lung), developed between 1990 and 1992,69,70,79 which derive deposition fractions directly from classical flow equations. The clearance mechanisms of particles are not discussed here, as they are beyond the scope of the present work.
The objective of this work is to introduce a publicly accessible web application, LungDepo, designed to facilitate users including policy-makers, public health professionals and risk assessors in employing the ICRP and MPPD models for the quantification of particle deposition within the three anatomical compartments of the human lungs: the head airways (HA), the tracheobronchial region (TB), and the alveolar region (AL). Designing and developing a web application in the context of inhalation toxicity is essential for providing accessible and efficient tools that can be utilized by a wide range of users without the need for advanced mathematical or programming skills. Well-designed graphical user interfaces (GUI) are crucial for enabling users with limited technical expertise to navigate and apply complex models for assessing inhalation risks. This accessibility promotes broader use of the models, enabling researchers, healthcare professionals, and policymakers to efficiently conduct evaluations and make informed decisions. Additionally, incorporating application programming interfaces (API) capabilities enhances the flexibility of the web application, allowing it to integrate with external datasets, automate processes, and support the implementation of customized workflows. This facilitates collaboration across different research domains and accelerates innovation in the design of safer materials and substances and evaluation of the potential impacts of mitigation measures to reduce exposures to particles. LungDepo is hosted on the Enalos Cloud platform facilitating its integration with other freely accessible web applications within the framework of nano-informatics, including several human health related models such as the integrated approach to testing and assessment (IATA) for lung exposure and toxicity.80–82 The Enalos Cloud platform also offers access to several other models including NanoBioAccumulate,83 which simulates nanomaterial uptake and elimination in aquatic and soil invertebrates; MicroPlasticFate,84 which provides the dynamic and steady-state modeling of the environmental fate of micro- and nanoplastics across key environmental scales and compartments; and UANanoDock,85 which predicts protein adsorption onto nanoparticles. Beyond nano-informatics, the Enalos Cloud platform also hosts web applications in cheminformatics,86,87 and machine learning,88,89 providing a versatile infrastructure for developing, deploying, and accessing predictive models in materials and life sciences.
The LungDepo web application presented in this study is specifically designed to offer a user-friendly GUI that supports the application of complex models for computing the mass deposition (of particles themselves or based on concentrations of adsorbed co-pollutants), the lung surface area covered by deposited particles and the relative contributions of inhaled particulates classified by their size (coarse, fine and ultrafine) in the various regions of the human lungs. As regulators and industry increasingly shift towards the implementation of safe and sustainable by design (SSbD) principles for the design of chemicals and materials, the LungDepo web application provides valuable insights into how new materials can be engineered to reduce harmful effects on human health as well as visualizing the impacts of altering the particle size distributions through specific emissions mitigation measures. Additionally, LungDepo features predefined scenario models to simulate the deposition of several airborne substances and micro-sized engineered materials in the human lungs depending on the breathing frequency and concentration of airborne particles.
2 Particle (aerosol) deposition models
2.1 ICRP model
The ICRP model, integrated within the LungDepo web application employs the simplified deposition equations proposed by Hinds in 1999.44 These equations are applicable for the calculation of fractional deposition of particles in the size range from 0.001 to 100 μm. Several studies have employed the Hinds equations for the ICRP model to study the respiratory deposition of aerosols.90,91This model provides calculations for the deposition efficiency of inhaled particles across three distinct regions of the respiratory tract: the head airways (HA), the tracheobronchial region (TB), and the alveolar region (AR). The deposition efficiency of particles across these three distinct regions is calculated as follows:
 | (1) |
 | (2) |
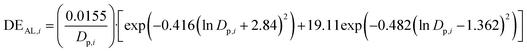 | (3) |
 | (4) |
Fig. 2, presents the analytical calculation of the deposition efficiency (fraction) in the three regions of the human lung as a function of the aerodynamic particle size, ranging from 0.001 μm to 100 μm, using the simplified equations proposed by Hinds44 for the ICRP model. The x-axis, which represents the aerodynamic diameter of particles, is displayed on a logarithmic scale. As shown in Fig. 2, the ICRP model suggests that particles with aerodynamic diameter of less than 10 μm are inhaled at nearly 100%. In contrast, particles larger than 10 μm in diameter exhibit a significantly reduced inhalable fraction, becoming lodged within the head airways of the human lung. Ultrafine particles with aerodynamic diameter of less than 0.1 μm (PM0.1) exhibit significant deposition efficiencies, reaching up to 50% in the alveolar region and approximately 30% in the tracheobronchial regions.
 | ||
| Fig. 2 Deposition efficiency (fraction) computed based on the simplified equations proposed by Hinds44 for the ICRP model, plotted against the aerodynamic particle diameter in the range 0.001–100 μm. | ||
2.2 MPPD model
The MPPD model, integrated within the LungDepo web application, is specifically tailored to align with human symmetric lung geometry as proposed by Yeh and Schum.65 This model is the recommended default human geometry by the U.S. Environmental Protection Agency (EPA).92 The functional residual capacity (FRC), the volume of air present in the lungs at the end of passive expiration in a 70 kg average-sized male, is set at 3300 mL, while the upper respiratory tract (URT) volume is designated at 50 mL. The inhalation pathway is specified as nasal, with inhaled particles considered to be spherical in shape, spanning a size range from 0.001 to 100 μm. The breathing parameters are defined as 12 breathes per minute, a tidal volume (the volume of gas inhaled and exhaled with each breath) of 625 mL, and an inspiratory fraction (inspiratory capacity/total lung capacity) of 0.5.Fig. 3, presents the analytical calculation of the deposition efficiency (fraction) for particles deposited in the three regions of the human lungs using the MPPD model version 3.04, developed by Applied Research Associates Inc.78,93 based on the conditions described in the previous paragraph. The analysis reveals three notable discrepancies between the outcomes of the ICRP model and the MPPD model. Firstly, the ICRP model estimates the AL deposition efficiency for ultrafine particles to be approximately 50%, whereas the MPPD model yields a lower estimate of approximately 30%. Secondly, regarding the deposition efficiency for ultrafine particles in the TB region, the MPPD model estimates the efficiency to be approximately 50%, contrasting with the ICRP model's lower estimate of around 30%. According to the ICRP model, the inhalation efficiency for particles with an aerodynamic diameter up to 100 μm declines to approximately 60%. In contrast, the MPPD model suggests that all particles within this diameter range (100%) continue to be inhaled.
 | ||
| Fig. 3 Deposition efficiency (fraction) computed using the MPPD model version 3.04, developed by Applied Research Associates Inc.,78,93 plotted against the aerodynamic particle diameter in the range 0.001–100 μm. | ||
2.3 Deposition mass flow rate
The total mass deposition rate, mdeposition (mg h−1), is estimated by:
 | (5) |
2.4 Lung surface area on which particles are deposited
The LungDepo web application integrates the computation of the total surface area of the lung covered by deposited particles (measured in m2), to assess the extent of particle coverage across the HA, TB and AL regions.The total deposited surface area of particles, Adeposition, is calculated as:
 | (6) |
The number Ni of particles deposited for a given particle aerodynamic diameter, Dp,i is given by:
 | (7) |
 | (8) |
The surface area, Ai, assuming the particles are spherical, is given by:
| Ai = π·D2p,i | (9) |
3 The LungDepo web application
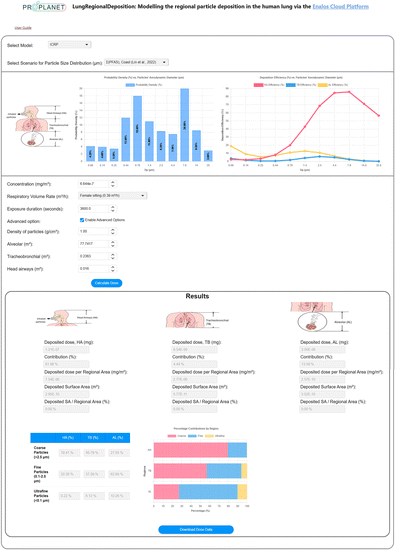
The LungDepo web application is designed with a user-centric approach and is developed utilizing the ZK framework – an open-source Ajax web application framework implemented in Java.95 This web application is hosted on the Enalos Cloud platform (https://enaloscloud.novamechanics.com/proplanet/lungdeposition/ or https://enaloscloud.novamechanics.com/insight/lungdeposition/)96 and is freely accessible to all users without cost or login requirements, as it is provided as freeware (free-to-use software with closed-source code).Fig. 4 presents the graphical user interface (GUI) of the LungDepo web application. In this example, the chosen model is the ICRP, accompanied by a predefined scenario for the particle size distribution of PFAS in particulate matter collected from a coastal area, as reported in the study by Lin et al.91 Once the model and scenario related to particle size distribution are selected, two intuitive graphical plots are generated. The first plot represents the normalized probability density of the aerodynamic diameter size distribution of the particulate matter. The second plot shows the deposition efficiency, expressed as a fraction, across the relevant range of particle sizes. In the example shown, the concentration of exposure to particulate matter is set at 6.644 × 10−7 mg m−3, with a respiratory volume rate reflective of a female in a sitting position, set at 0.39 m3 h−1. The exposure duration is defined as 3600 seconds. By selecting the ‘enable advanced options’ check box, the user can specify the density of particles, which is set to 1 g cm−3 in this example. Also, the user can define the total surface area of the HA, TB and AL regions, measured in m2. Upon selecting the ‘calculate dose’ button, users can obtain the lung regional deposited doses of particles, measured in milligrams (mg) and as a percentage (%). Here, the percentage represents the fraction of the total deposited mass that occupies the surface area of a specific lung region relative to the total mass deposited across the entire lung. Additionally, users can retrieve the deposited dose of particles per regional lung surface area (mg m−2), the surface area (of the lung region) covered by deposited particles (m2) and the ratio of the surface area covered by deposited particles to the total regional lung area, expressed as percentage (%). The output also includes the relative contributions of particles classified by size in the three different regions of the human lung, measured in percentage. This classification includes coarse particles larger than 2.5 μm, fine particles less than 2.5 μm, and ultrafine particles smaller than 0.1 μm. Additionally, there is an option to download the results via the ‘Download Dose Data’ button.
The user may choose from a dropdown menu that includes the ICRP and MPPD models. Additionally, for the scenario pertaining to particle size distribution, users can select from a range of predefined examples representing various substances that may be constituents of particulate matter. These substances include91 perfluorobutanoic acid (PFBA),91 perfluorobutane sulfonate (PFBS),91 corn starch,97 chitosan,98 glycerol,99 zinc oxide (ZnO),100 acetic acid,101 silane-based102 and siloxane-based103 substances, 2-octenylsuccinic anhydride,104 sodium alginate,105 alkyl ketene dimers (AKDs)106 and polycyclic aromatic hydrocarbons (PAHs)107 substances as reported in previous studies. Users have the option to upload a custom scenario as well pertaining to the size distribution of the substance of interest within particulate matter. Additionally, for the respiratory volume rate, users may choose from predefined scenarios established by the ICRP model, which takes into account gender as well as varying activity levels, such as sitting, light exercise, or heavy exercise.43 To support broader applicability, LungDepo includes a step-by-step user guide, which is directly available within the web application interface, explaining how to upload a custom particle size distribution in .txt format and how to manually define all relevant input parameters, including particle concentration, respiratory volume rate, exposure duration, particle density, and lung region surface areas to ensure that users can apply easily LungDepo to a wide variety of compounds and exposure scenarios.
The LungDepo web application incorporates a representational state transfer (REST) application programming interface (API) that enhances both its functionality and usability. This is shown in Fig. 5. The functionality of the ‘POST’ and ‘GET’ requests for the endpoints https://enaloscloud.novamechanics.com/proplanet/apis/lungdeposition, https://enaloscloud.novamechanics.com/proplanet/apis/lungdeposition/scenario and https://enaloscloud.novamechanics.com/proplanet/apis/lungdeposition/respiratoryVolumeRate, respectively, were tested using Postman (see Fig. S1, S3 and S4†). The same tests were performed using Swagger. The API documentation is publicly accessible via the Swagger user interface (see Fig. 5) at https://enaloscloud.novamechanics.com/proplanet/swagger-ui/index.html#/ and the underlying specification at https://enaloscloud.novamechanics.com/proplanet/apis/swagger.json. Fig. S2† demonstrates the use of Swagger to perform a ‘/lungdeposition’ POST request, using the same input data as shown in Fig. S1† (which used Postman), confirming consistency of the output across tools. This testing ensured that the expected responses were returned for each request type. In section S2 of the ESI,† robustness tests for the ‘/lungdeposition’ POST query are presented by submitting extreme input scenarios. Specifically, two high-value and two low-value test cases were constructed using exaggerated concentrations, nanoparticle densities and tidal surface areas across the alveolar, tracheobronchial, and head airway regions, using both the ICRP and MPPD models implemented in LungDepo. All tests were performed using Postman and, in every case, the API successfully returned results, confirming the robustness and reliability of its implementation under a wide range of input conditions. A REST API allows the LungDepo web application to interact with external systems and services in a standardized way, facilitating the exchange of data between the application and other tools, databases, or platforms. This means that users can access the application's features programmatically, enabling integration with broader research ecosystems and allowing for automation of tasks, such as data input, analysis, and reporting. Additionally, the REST API provides scalability and flexibility, allowing different users – whether researchers, developers, or public health professionals – to customize their workflows by integrating the web application into their existing systems. For instance, researchers can automate the analysis of large-scale datasets to simulate particle deposition under various conditions or integrate LungDepo with other modeling tools to enhance the breadth of their inhalation toxicity studies.
 | ||
| Fig. 5 LungDepo is available through a REST API to enable programmatic access and integration with other models, tools and into an IATA for hazard and risk assessment. | ||
Furthermore, a REST API facilitates collaboration by enabling multiple users to access the same application from different locations or systems, allowing for shared research efforts and cross-platform compatibility. This makes the application more adaptable to different research needs, supporting a wide range of use cases – from routine regulatory assessments to advanced scientific research in inhalation toxicology. By providing a flexible and interoperable interface, a REST API significantly expands the reach and impact of the LungDepo web application, helping users make more informed decisions in evaluating inhalation toxicity, evaluating the impact of exposure mitigation measures such as air filtration, and designing materials by application of the SSbD principles. Integrating the LungDepo web application into the Enalos Cloud Platform further enhances its capabilities by allowing users to access other web applications hosted on the same platform, including the integrated approach to testing and assessment (IATA) for lung exposure and toxicity.80–83 This integration fosters a collaborative environment where different tools can be used together to provide comprehensive insights and support multifaceted research efforts.
4 Case studies
4.1 Particle-bound PFAS
The ICRP and MPPD dosimetry models incorporated into the LungDepo web application are used to estimate the deposited doses and surface area on which inhaled particulate-bound per- and polyfluoroalkyl substances (PFAS) are deposited across the three distinct regions of the human lung. The relative contributions of inhaled PFAS associated with particles of different sizes are also computed. The predictions generated by LungDepo (using the input data from Lin et al.91), are compared with the findings reported by Lin et al.91 to verify the accuracy and robustness of the ICRP and MPPD models as integrated within the web application.Lin et al.91 conducted extensive work quantifying the (normalized) particle size distribution of particle-bound PFAS and the sum concentrations of gaseous and particulate PFAS emitted from various reference sites, including wastewater treatment plants (WWTPs), landfills, coastal areas and natural reserve sites. They investigated collectively 49 PFAS and reported the particulate concentrations of each, measured in picograms of PFAS per cubic meter in the air (pg m−3). The total concentrations were designated as Σ PFAS. The normalized distribution of total PFAS (Σ PFAS) on the different size fractions in the particulate matter, along with the total particulate concentrations of PFAS from different sampling locations as reported by Lin et al.,91 have been included as predefined scenarios in the LungDepo web application. Among the predefined scenarios the normalized distributions of PFBA and PFBS are included, as obtained from the study of Lin et al.91
Fig. 6A, presents the predicted mass deposition rate (pg h−1) of inhaled PFAS at or near a landfill site using the ICRP and MPPD models in LungDepo as compared to those calculated by Lin et al.91 also using the ICRP model, while Fig. 6B presents the corresponding predicted mass deposition percentage (%) within the three distinct regions of the human lung calculated from the LungDepo model (this was not calculated by Lin et al.,91 and is one of the new features of LungDepo). The corresponding figures for predicted exposures based on PFAS emissions at coastal and natural reserve sites and at WWTPs are provided in the supplemental information file (Fig. S9–S18†). These calculations were performed using the ICRP and MPPD dosimetry models integrated within the LungDepo web application, alongside the findings reported in the study by Lin et al.91 to validate and verify the accuracy of the calculation outputs generated by the LungDepo web application. Lin et al.91 used the simplified equations proposed by Hinds,44 similar to our approach in this study, to calculate the mass deposition rate of inhaled PFAS based on the ICRP model. As can be observed in Fig. 6A, the mass deposition rate calculated by the LungDepo web application is consistent with that reported by Lin et al.91 thereby verifying the calculations performed in relation to the ICRP dosimetry model. The total mass of inhaled PFAS deposited, as estimated by both the ICRP and MPPD models, is approximately 110 pg h−1. Specifically, the ICRP model predicts that the contributions to the HA, TB and AL region are roughly 77%, 5% and 18%, respectively. The MPPD model predicts that the contributions to the HA, TB and AL region are around 66%, 13% and 21%, respectively. These discrepancies are attributed to the different deposition efficiencies predicted by the two models, as shown in Fig. 2 and 3, respectively. The particle size distribution of the PFAS, as reported in the study by Lin et al.,91 spanned from 0.08 to 25 μm. The distribution exhibited a peak at an aerodynamic diameter of 0.78 μm that corresponded to an inhalation probability of around 17%. It is evident from Fig. 2 and 3, that for particles with an aerodynamic diameter of approximately 0.78 μm, the ICRP model predicts slightly higher deposition efficiency in the HA, estimated at around 8%, compared to the 5% predicted by the MPPD model. Conversely, the deposition efficiency in the TB region, according to the ICRP model, is lower at approximately 1.7%, whereas the MPPD model predicts a value of around 5%. The differences predicted between the MPPD and ICRP models are minor and reflect the improved lung geometry in the MPPD model.
 | ||
| Fig. 6 Predictions of PFAS mass deposition at a landfill site in Hong Kong (based on input data from Lin et al.), computed using the ICRP and MPPD models integrated within LungDepo. (A) Deposition in pg h−1 compared with Lin et al.; (B) deposition in % of Σ PFAS, comparing ICRP and MPPD results.91 | ||
The total mass deposition and the contributions of different particle sizes of the particles to which the PFAS are bound, as calculated here using input data (on PFAS concentration and particle size distribution) retrieved from the study of Lin et al.,91 are found to be consistent and comparable to the findings reported in the study of Guo et al.90 Guo et al.,90 reported that the mass deposition of eight PFAS within the human lungs, in the urban atmosphere of Shanghai, China is approximately 361 pg h−1, indicating that the coarse PFAS particles contribute the most to the particle and PFAS load in the HA region. They also reported that PFAS particles with an aerodynamic diameter of less than 2.1 μm account for approximately 72.5% of the particle deposition in the AL region. This observation is comparable with the results presented here that demonstrated that fine and ultrafine particles contribute approximately 75–80% to the total deposition in the AL region.
The LungDepo web application offers further advanced analytical features for calculating the regional percentage contributions of the three distinct particle size categories (coarse, fine and ultrafine particles) as well as the computation of the deposited dose per regional area (mg m−2) and evaluation of the percentage of the regional surface area on which particles have deposited relative to the total regional area (as a percentage) which can also be thought of as lung surface area in which effective lung function (e.g., oxygen exchange) will likely be reduced. As can be seen in Fig. 7, both ICRP and MPPD models predict that coarse particles predominate in the HA region. In more detail, the MPPD model predicts this percentage to be slightly higher at around 89% of the total particle load, whereas ICRP predicts that coarse particles constitute around 74%. In the TB region, the MPPD model indicates that fine particles predominate corresponding to around 55% of the total particle load, whereas ICRP model indicates equal contribution between fine and coarse particles. In the AL region, both models predict that fine particles predominate this region with percentages around 60–65% and ultrafine particles contributing to around 15% of the total particle load here.
 | ||
| Fig. 7 Contributions (expressed as percentages of the Σ PFAS) of inhaled Σ PFAS associated with particles of different sizes (collected from a landfill site in Hong Kong by Lin et al.91) as calculated using the ICRP and MPPD models integrated within the LungDepo web application. | ||
The predicted deposition dose per lung regional area of people in close proximity to the landfill, using the ICRP model, is found to be 5.03 × 10−5 mg m−2 in the HA region, 2.19 × 10−7 mg m−2 in the TB region and 2.34 × 10−9 mg m−2 in the AL region. The corresponding computations using the MPPD model yielded values of 4.24 × 10−5 mg m−2 in the HA region, 5.84 × 10−7 mg m−2 in the TB region and 2.80 × 10−9 mg m−2 in the AL region. Although the predicted particles' coverage in the AL region is relatively low, in the order of 10−9 mg m−2, as observed by both models, the computed mass of PFAS deposited in the AL region, which is found to be 20–25 pg h−1, should be of concern regarding potential toxicological effects and health implications. While there are as yet limited health-based guidance values or limit values for individual or total PFAS concentrations in air, some suggestions are that annual exposure to PFOA (for example) should be limited to 0.0053 μg m−3.108 The European Food Safety Authority has advised a tolerable weekly intake (TWI) for total PFAS via food of 0.63 ng kg−1 from food and water for example.109 In parallel, the U.S. Environmental Protection Agency (EPA) has set maximum contaminant levels (MCLs) in drinking water at or near zero for several PFAS compounds, including PFOA and PFOS, reflecting increasing concern over their health impacts and a shift toward more protective regulatory thresholds.110
4.2 Particle-bound total PAH
Polycyclic aromatic hydrocarbons (PAHs) represent another class of particle-bound organic compounds that may have significant adverse health effects upon human inhalation. The atmospheric presence of PAHs in both gas and particulate phases raises significant concerns, particularly due to the fine sizes of particle-bound PAHs that range from 0.1 to 10 μm (ref. 107 and 111) and which can penetrate deeply into the human respiratory system upon inhalation, potentially leading to significant health impacts. Furthermore, the concentrations of PAHs in the atmosphere are notably higher than those of PFAS, typically ranging from a few to hundreds of nanograms per cubic meter112 Atmospheric particle-bound PAHs are primarily formed from the incomplete combustion of carbon-based materials113–115 which are characterized by high biochemical persistence due to the existence of dense π electrons on the aromatic rings.116,117Using the probability density size distribution as reported by Lv et al.107 and the concentrations from the sum of 12 PAHs measured by Voliotis et al.29 during cold (January–March 2013) and warm (May–July 2013) seasons at an urban traffic site in Thessaloniki (northern Greece), the mass deposition rate (ng h−1) of particle-bound PAHs is calculated. The respiratory volume rate is set to 1.7 m3 s−1 to be consistent with the study of Voliotis et al.29 This volumetric rate corresponds to light activity levels in adults. The mass deposition rate is computed using the ICRP and MPPD models integrated within the LungDepo web application. The results were compared to those reported by Voliotis et al.,29 who utilized the MPPD model with the stochastic lung option, applying a functional residual capacity (FRC) of 3389 mL. In contrast, the MPPD model integrated within the LungDepo web application is fitted based on a Yeh/Schum symmetric lung geometry model with an FRC of 3300 mL. Nevertheless, as presented in Fig. 8A and B respectively, the predicted outcomes from the MPPD model using LungDepo and the outcomes predicted by Voliotis are in close agreement during both cold and warm seasons. This comparison further assures the validity of the implementation of the MPPD model integrated within the LungDepo web application. The predicted outcome based on the ICRP model overestimates the mass deposition of particle-bound PAHs. This discrepancy as discussed previously is attributed to the different computations of deposition efficiency as evidenced in Fig. 2 and 3.
 | ||
| Fig. 8 Predictions of the mass deposition in human male adult lung measured in ng h−1 of PAHs during the cold season (A) and warm season (B) at an urban traffic site as computed using the ICRP and MPPD models integrated within LungDepo and compared against the previous study29 (whose data was used as the input to the LungDepo model).29 | ||
As can be seen in Fig. 8A and B, the mass deposition of particle-bound PAHs during the cold season is significantly higher, predicted to be approximately 18–20 ng h−1, compared to the warm season, where deposition levels are predicted to be around 6–8 ng h−1. This observation aligns with previous studies that reported higher concentrations of PAHs during cold seasons due to the increase from seasonal emission sources such as domestic heating etc.118–122 and lower concentrations of PAHs during warm seasons due to the rise of temperature and concentrations of atmospheric oxidants such as ozone and radicals.123–126
4.3 Engineered microparticles (MPs)
In addition to the toxic organic compounds that can bound onto particulate matter, micro-sized engineered materials, with an aerodynamic diameter between 0.1 and 100 μm, have also been addressed as potential risks for environmental and occupational lung diseases following inhalation.127 Engineered microparticles (MPs), which are classified as ultrafine particles (<0.1 μm), can be found in both indoor and outdoor aerosols.128 Due to their small sizes, these particles can penetrate deep into the respiratory tract, potentially inducing pulmonary inflammation and other adverse health effects due to their ability to cross the air-lung barrier.129,130A case study presented by Tsiros et al.131 is used to examine the robustness and accuracy of the LungDepo web application to predict the mass of TiO2 deposited in the three different human lung regions, following the exposure of female and male workers to 22 nm ultrafine TiO2 particles under light exercise during an 8 hour work shift. Tsiros et al.,131 employed the ICRP model using the Hinds approach,44 and the MPPD model for assessing the risk exposure of female and male workers exposed to a concentration of 5.85 mg m−3 of TiO2 ultrafine particles, considering a particle density (assuming spherical particles) of approximately 4.26 g cm−3 and a count median diameter of the particles (CMD) of 0.02 μm. Fig. 9A and B, present the mass deposition measured in mg calculated using the ICRP and MPPD models integrated within the LungDepo web application, following the exposure of a male or female, respectively to 22 nm ultrafine TiO2 particles during an 8 hour work shift under light exercise. The ICRP model predicts that the largest mass of deposited TiO2 microparticles is in the AL region, estimated at approximately 33 mg for males and 28 mg for females. This finding underscores the significant risk of pulmonary inflammation that may result from the 8 hour (workday) exposure to TiO2 ultrafine particles, to both males and females. This observation is in perfect agreement with the calculations conducted by Tsiros et al.,131 using the ICRP model. Using the MPPD model integrated within the LungDepo web application, it is observed that the predicted mass of TiO2 deposited in the alveolar (AL) region is also considerable, approximately 20 mg for males and 16 mg for females however, the largest accumulation of TiO2 is predicted to be in the TB region. This discrepancy between the two models can be described through the calculation of deposition efficiency for particles measuring 0.022 μm, as illustrated in Fig. 2 and 3. It is noteworthy to state that although Tsiros et al.,131 used the same lung geometry for MPPD model as used in this study, they considered variable exposure (30 minutes of active emissions per hour) in contrast to the MPPD model used in this study which was fitted based on a constant exposure scenario. This distinction largely explains the differences observed in the predictions yielded by the MPPD model integrated within the LungDepo web application and the predictions yielded by the MPPD model utilized by Tsiros et al.131
Using the advanced functionalities of the LungDepo web application, it has been observed that both the ICRP and MPPD models predict the surface area occupied by deposited particles per the regional area to be around 2–3% in the AL region, around 250% in the TB region and around 2500% in the HA region. These findings clearly indicate that inhalation of 22 nm ultrafine TiO2 particles for 8 hours work shift, could potentially lead to significant pulmonary inflammation and other adverse health implications for both males and females.
4.4 Domain of applicability and user interaction
Following the presentation of the case studies demonstrating the applicability of the LungDepo web application for predicting the mass deposition of particle-bound toxic organic compounds and engineered micro-sized materials, it is important to note also that the web application integrates predefined (normalized) size distribution data for a variety of additional substances. This feature enables users to explore diverse scenarios involving various combinations of materials and chemicals, including perfluorobutyl sulfonate (PFBS), perfluorobutanoic acid (PFBA), corn starch, chitosan, glycerol, zinc oxide (ZnO), acetic acid, silane- and siloxane-based materials, 2-octenylsuccinic anhydride, sodium alginate, and alkyl ketene dimer (AKD). Furthermore, the web application accommodates the integration of user-defined particle size distributions, thereby enhancing the analytical flexibility of the tool. This signifies that the LungDepo web application can serve as a valuable tool for accurate and robust evaluation of the potential impacts of inhaled materials on the human respiratory tract by environmental regulatory agencies. Also, the web application can be utilized to investigate and assess the carcinogenic risks associated with inhalation exposure to these substances by calculating the inhalation cancer risk (ICR) metric.Future extensions of LungDepo could include parameterisation of different lung sizes/breathing rates and functional residual capacities to cover also children and vulnerable populations (e.g., pregnant women, the elderly, people with asthma, emphysema or other lung-related conditions, and obesity) which will influence both exposure and susceptibility to additional health effects arising from air pollution.132,133
5 Conclusions
This paper presents the LungDepo web application for the prediction of the mass deposition of inhaled particles and particle-associated organic pollutants within the respiratory tract. LungDepo is a freely accessible web application hosted on the Enalos Cloud platform (https://enaloscloud.novamechanics.com/proplanet/lungdeposition or https://enaloscloud.novamechanics.com/insight/lungdeposition/), which enables researchers to assess potential hazards and risks associated with the inhalation of particulate matter, toxic organic compounds bound to particles, micro-sized engineered materials or their combinations. The robustness and validity of LungDepo, utilizing either the ICRP or MPPD models, has been confirmed through several case studies comparing its predictions with the model calculations performed in previous studies,29,90,91,107,131 including the mass deposition of particle-bound PFAS,90,91 PAH aerosol29,107 and micro-sized engineered material such as of TiO2.131 Its broad applicability is further demonstrated by integrating predefined case studies with (normalized) size distribution data for various substances, including perfluorobutyl sulfonate (PFBS), perfluorobutanoic acid (PFBA), corn starch, chitosan, glycerol, zinc oxide (ZnO), acetic acid, silane- and siloxane-based materials, 2-octenylsuccinic anhydride, sodium alginate, and alkyl ketene dimer (AKD). Further enhancements are planned to extend the range of pollutants and combinations available, and to enable tuning of the lung volume and residual capacity to allow modelling of the exposures and consequent risks of especially vulnerable individuals.The enhanced user interaction and flexibility of the LungDepo web application is affirmed by its user-friendly GUI, which facilitates users with no programming knowledge to perform calculations using the ICRP and MPPD models for prediction of the mass deposition of inhaled particles and aerosols in the human lungs. The tool also features REST API integration that facilitates interaction with other web applications and software such as for integrated exposure, hazard and risk assessment, thereby promoting efficient data exchange and enhancing interoperability. This integration opens new opportunities for collaborative research and a thorough evaluation of inhalation toxicity, ultimately contributing to the design and development of inherently safer materials through application of the SSbD principles.
In this study, clearance mechanisms of deposited particles were not considered. To more accurately assess the risk from inhaled particles to human lungs, future work will integrate comprehensive physiologically-based pharmacokinetic (PBPK) models within LungDepo, such as the one presented in the work of Tsiros et al.131 The inclusion of additional variants of MPPD models fitted to alternative lung geometric models, such as the Yeh/Schum 5-Lobe, the stochastic lung, the Weibel etc. will also be considered. Finally, the variable exposure conditions featured in the MPPD model version 3.04, developed by Applied Research Associates Inc.78,93 will also be integrated within LungDepo in future upgrades.
Data availability
All data utilised in the manuscript is extracted from literature, and the curated datasets are in the process of being uploaded to the free-to-access NanoPharos database https://db.nanopharos.eu/Queries/Datasets.zul.Author contributions
Conceptualization, D. M., D.-D. V.; methodology, D. M.; software, D. M., A. T.; supervision, G. M., J. S., I. L, A. A.; writing – original draft preparation, D. M.; writing – review and editing, D. M., D.-D. V., P. K., A. T., G. M., J. S., A. R., I. L., A. A.; funding acquisition, A. A.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the European Union's Horizon 2020 research and innovation program via the PROPLANET project under grant agreement number 101091842 and the INSIGHT project under grant agreement number 101137742. The authors would like to thank Periklis Tsiros for the fruitful discussions, and his suggestions and advice for integrating additional statistical measures into the LungDepo web application to enable in-depth analysis of the regional deposition of particles in the human lungs.References
- A. S. Shah, J. P. Langrish, H. Nair, D. A. McAllister, A. L. Hunter, K. Donaldson, D. E. Newby and N. L. Mills, Global association of air pollution and heart failure: a systematic review and meta-analysis, Lancet, 2013, 382, 1039–1048 CrossRef CAS PubMed.
- K.-H. Kim, E. Kabir and S. Kabir, A review on the human health impact of airborne particulate matter, Environ. Int., 2015, 74, 136–143 CrossRef CAS.
- L. B. Lave and E. P. Seskin, Air pollution and human health, RFF Press, 2013 Search PubMed.
- B. Brunekreef and S. T. Holgate, Air pollution and health, Lancet, 2002, 360, 1233–1242 CrossRef CAS.
- I. Manisalidis, E. Stavropoulou, A. Stavropoulos and E. Bezirtzoglou, Environmental and health impacts of air pollution: a review, Frontiers in public health, 2020, 8, 14 CrossRef.
- R. J. Henning, Particulate matter air pollution is a significant risk factor for cardiovascular disease, Curr. Probl. Cardiol., 2024, 49, 102094 CrossRef.
- WHO, https://www.who.int/health-topics/air-pollution#tab=tab_1, (accessed 25, 2024).
- R. W. Atkinson, G. W. Fuller, H. R. Anderson, R. M. Harrison and B. Armstrong, Urban ambient particle metrics and health: a time-series analysis, Epidemiology, 2010, 21, 501–511 CrossRef PubMed.
- G. Cadelis, R. Tourres and J. Molinie, Short-term effects of the particulate pollutants contained in Saharan dust on the visits of children to the emergency department due to asthmatic conditions in Guadeloupe (French Archipelago of the Caribbean), PLoS One, 2014, 9, e91136 CrossRef.
- A. W. Correia, C. A. Pope III, D. W. Dockery, Y. Wang, M. Ezzati and F. Dominici, Effect of air pollution control on life expectancy in the United States: an analysis of 545 US counties for the period from 2000 to 2007, Epidemiology, 2013, 24, 23–31 CrossRef PubMed.
- Y. Fang, V. Naik, L. Horowitz and D. L. Mauzerall, Air pollution and associated human mortality: the role of air pollutant emissions, climate change and methane concentration increases from the preindustrial period to present, Atmos. Chem. Phys., 2013, 13, 1377–1394 CrossRef.
- K. Meister, C. Johansson and B. Forsberg, Estimated short-term effects of coarse particles on daily mortality in Stockholm, Sweden, Environ. Health Perspect., 2012, 120, 431–436 CrossRef CAS.
- R. Guaita, M. Pichiule, T. Maté, C. Linares and J. Díaz, Short-term impact of particulate matter (PM2. 5) on respiratory mortality in Madrid, Int. J. Environ. Health Res., 2011, 21, 260–274 CrossRef.
- J. I. Halonen, T. Lanki, T. Yli-Tuomi, P. Tiittanen, M. Kulmala and J. Pekkanen, Particulate air pollution and acute cardiorespiratory hospital admissions and mortality among the elderly, Epidemiology, 2009, 20, 143–153 CrossRef.
- L. Perez, A. Tobías, X. Querol, J. Pey, A. Alastuey, J. Díaz and J. Sunyer, Saharan dust, particulate matter and cause-specific mortality: A case–crossover study in Barcelona (Spain), Environ. Int., 2012, 48, 150–155 CrossRef PubMed.
- E. Samoli, R. Peng, T. Ramsay, M. Pipikou, G. Touloumi, F. Dominici, R. Burnett, A. Cohen, D. Krewski and J. Samet, Acute effects of ambient particulate matter on mortality in Europe and North America: results from the APHENA study, Environ. Health Perspect., 2008, 116, 1480–1486 CrossRef PubMed.
- A. A. Rostami, Computational modeling of aerosol deposition in respiratory tract: a review, Inhalation Toxicol., 2009, 21, 262–290 CrossRef CAS.
- N. Tlotleng, T. Kootbodien, K. Wilson, F. Made, A. Mathee, V. Ntlebi, S. Kgalamono, M. Mokone, K. Du Preez and N. Naicker, Prevalence of respiratory health symptoms among landfill waste recyclers in the city of Johannesburg, South Africa, Int. J. Environ. Res. Public Health, 2019, 16, 4277 CrossRef PubMed.
- A. Stobnicka-Kupiec, M. Gołofit-Szymczak, M. Cyprowski and R. L. Górny, Detection and identification of potentially infectious gastrointestinal and respiratory viruses at workplaces of wastewater treatment plants with viability qPCR/RT-qPCR, Sci. Rep., 2022, 12, 4517 CrossRef CAS.
- T. M. De Kok, H. A. Driece, J. G. Hogervorst and J. J. Briedé, Toxicological assessment of ambient and traffic-related particulate matter: a review of recent studies, Mutat. Res. - Rev. Mutat. Res., 2006, 613, 103–122 CrossRef CAS.
- J. Madureira, I. Paciência and E. D. O. Fernandes, Levels and indoor–outdoor relationships of size-specific particulate matter in naturally ventilated Portuguese schools, J. Toxicol. Environ. Health, Part A, 2012, 75, 1423–1436 CrossRef CAS.
- C. Misra, M. D. Geller, P. Shah, C. Sioutas and P. A. Solomon, Development and Evaluation of a Continuous Coarse (PM10–PM25) Particle Monitor, J. Air Waste Manage. Assoc., 2001, 51, 1309–1317 CrossRef CAS.
- T. Feng, Y. Sun, Y. Shi, J. Ma, C. Feng and Z. Chen, Air pollution control policies and impacts: A review, Renewable Sustainable Energy Rev., 2024, 191, 114071 CrossRef CAS.
- R. Esworthy, Air Quality: EPA's 2013 Changes to the Particulate Matter (PM) Standard, Library of Congress, Congressional Research Service, Washington, DC, USA, 2013 Search PubMed.
- D. A. Glencross, T.-R. Ho, N. Camina, C. M. Hawrylowicz and P. E. Pfeffer, Air pollution and its effects on the immune system, Free Radical Biol. Med., 2020, 151, 56–68 CrossRef CAS.
- J. O. Anderson, J. G. Thundiyil and A. Stolbach, Clearing the air: a review of the effects of particulate matter air pollution on human health, J. Med. Toxicol., 2012, 8, 166–175 CrossRef CAS PubMed.
- S. Garg, P. Kumar, V. Mishra, R. Guijt, P. Singh, L. F. Dumée and R. S. Sharma, A review on the sources, occurrence and health risks of per−/poly-fluoroalkyl substances (PFAS) arising from the manufacture and disposal of electric and electronic products, J. Water Process Eng., 2020, 38, 101683 CrossRef.
- T. Stoiber, S. Evans and O. V. Naidenko, Disposal of products and materials containing per-and polyfluoroalkyl substances (PFAS): A cyclical problem, Chemosphere, 2020, 260, 127659 CrossRef CAS.
- A. Voliotis, S. Bezantakos, A. Besis, Y. Shao and C. Samara, Mass dose rates of particle-bound organic pollutants in the human respiratory tract: Implications for inhalation exposure and risk estimations, Int. J. Hyg. Environ. Health, 2021, 234, 113710 CrossRef CAS.
- K.-H. Kim, S. A. Jahan, E. Kabir and R. J. Brown, A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects, Environ. Int., 2013, 60, 71–80 CrossRef CAS PubMed.
- D. Belpomme, P. Irigaray, L. Hardell, R. Clapp, L. Montagnier, S. Epstein and A. J. Sasco, The multitude and diversity of environmental carcinogens, Environ. Res., 2007, 105, 414–429 CrossRef CAS PubMed.
- A. Besis, D. Voutsa and C. Samara, Atmospheric occurrence and gas-particle partitioning of PBDEs at industrial, urban and suburban sites of Thessaloniki, northern Greece: implications for human health, Environ. Pollut., 2016, 215, 113–124 CrossRef CAS PubMed.
- Z. Cao, F. Xu, A. Covaci, M. Wu, H. Wang, G. Yu, B. Wang, S. Deng, J. Huang and X. Wang, Distribution patterns of brominated, chlorinated, and phosphorus flame retardants with particle size in indoor and outdoor dust and implications for human exposure, Environ. Sci. Technol., 2014, 48, 8839–8846 CrossRef CAS PubMed.
- W. Wang, M.-J. Huang, F.-Y. Wu, Y. Kang, H.-S. Wang, K. C. Cheung and M. H. Wong, Risk assessment of bioaccessible organochlorine pesticides exposure via indoor and outdoor dust, Atmos. Environ., 2013, 77, 525–533 CrossRef CAS.
- A. L. Moreno-Ríos, L. P. Tejeda-Benítez and C. F. Bustillo-Lecompte, Sources, characteristics, toxicity, and control of ultrafine particles: An overview, Geosci. Front., 2022, 13, 101147 CrossRef.
- M. Ho, K.-Y. Wu, H.-M. Chein, L.-C. Chen and T.-J. Cheng, Pulmonary toxicity of inhaled nanoscale and fine zinc oxide particles: mass and surface area as an exposure metric, Inhalation Toxicol., 2011, 23, 947–956 CrossRef CAS PubMed.
- W.-S. Cho, R. Duffin, S. E. Howie, C. J. Scotton, W. A. Wallace, W. MacNee, M. Bradley, I. L. Megson and K. Donaldson, Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn 2+ dissolution inside lysosomes, Part. Fibre Toxicol., 2011, 8, 1–16 CrossRef CAS.
- A. Srinivas, P. J. Rao, G. Selvam, A. Goparaju, B. P. Murthy and N. P. Reddy, Oxidative stress and inflammatory responses of rat following acute inhalation exposure to iron oxide nanoparticles, Hum. Exp. Toxicol., 2012, 31, 1113–1131 CrossRef CAS PubMed.
- J. Wang and Y. Fan, Lung injury induced by TiO2 nanoparticles depends on their structural features: size, shape, crystal phases, and surface coating, Int. J. Mol. Sci., 2014, 15, 22258–22278 CrossRef CAS PubMed.
- P. Rajsekhar, G. Selvam, A. Goparaju, P. B. Murthy and P. N. Reddy, Pulmonary responses of manufactured ultrafine aluminum oxide particles upon repeated exposure by inhalation in rats, Indian J. Forensic Med. Toxicol., 2014, 8, 97–102 CrossRef.
- W.-S. Cho, R. Duffin, F. Thielbeer, M. Bradley, I. L. Megson, W. MacNee, C. A. Poland, C. L. Tran and K. Donaldson, Zeta potential and solubility to toxic ions as mechanisms of lung inflammation caused by metal/metal oxide nanoparticles, Toxicol. Sci., 2012, 126, 469–477 CrossRef CAS.
- G. S. Kang, P. A. Gillespie, A. Gunnison, H. Rengifo, J. Koberstein and L.-C. Chen, Comparative pulmonary toxicity of inhaled nickel nanoparticles; role of deposited dose and solubility, Inhalation Toxicol., 2011, 23, 95–103 CrossRef CAS PubMed.
- C.-S. Wang, Inhaled particles, Elsevier, 2005 Search PubMed.
- W. C. Hinds, Aerosol technology, properties, behavior, and measurements of airborne particles, Wiley Interscience, 1999 Search PubMed.
- H. G. Folkesson, M. A. Matthay, B. Westrom, K. J. Kim, B. W. Karlsson and R. H. Hastings, Alveolar epithelial clearance of protein, J. Appl. Physiol., 1996, 80, 1431–1445 CrossRef CAS.
- X. M. Zeng, G. P. Martin and C. Marriott, Particulate interactions in dry powder formulation for inhalation, CRC Press, 2000 Search PubMed.
- A. J. Hickey, Pharmaceutical inhalation aerosol technology, CRC Press, 2003 Search PubMed.
- G. Pilcer and K. Amighi, Formulation strategy and use of excipients in pulmonary drug delivery, Int. J. Pharm., 2010, 392, 1–19 CrossRef CAS.
- T. C. Carvalho, J. I. Peters and R. O. Williams III, Influence of particle size on regional lung deposition–what evidence is there?, Int. J. Pharm., 2011, 406, 1–10 CrossRef CAS.
- F. Wang, J. Liu and H. Zeng, Interactions of particulate matter and pulmonary surfactant: Implications for human health, Adv. Colloid Interface Sci., 2020, 284, 102244 CrossRef CAS PubMed.
- L. Jin, X. Luo, P. Fu and X. Li, Airborne particulate matter pollution in urban China: a chemical mixture perspective from sources to impacts, Natl. Sci. Rev., 2017, 4, 593–610 CrossRef CAS.
- A. Valavanidis, K. Fiotakis and T. Vlachogianni, Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2008, 26, 339–362 CrossRef CAS.
- M. Hussain, P. Madl and A. Khan, Lung deposition predictions of airborne particles and the emergence of contemporary diseases, Part-I, Health, 2011, 2, 51–59 Search PubMed.
- E. R. Weibel, A. F. Cournand and D. W. Richards, Morphometry of the human lung, Springer, 1963 Search PubMed.
- T. Soong, P. Nicolaides, C. Yu and S. Soong, A statistical description of the human tracheobronchial tree geometry, Respir. Physiol., 1979, 37, 161–172 CrossRef CAS PubMed.
- J. Heyder and G. Rudolf, Mathematical models of particle deposition in the human respiratory tract, J. Aerosol Sci., 1984, 15, 697–707 CrossRef.
- W. Hofmann, Modelling inhaled particle deposition in the human lung—A review, J. Aerosol Sci., 2011, 42, 693–724 CrossRef CAS.
- H. Smith, Human respiratory tract model for radiological protection, ICRP Publication 66, Ann. ICRP, 1994, 24 Search PubMed.
- F. Paquet, G. Etherington, M. R. Bailey, R. W. Leggett, J. Lipsztein, W. Bolch, K. F. Eckerman and J. D. Harrison, ICRP Publication 130: Occupational Intakes of Radionuclides: Part 1, Ann. ICRP, 2015, 44, 5–188 CrossRef CAS.
- N. C. o. R. Protection and Measurements, 1997.
- C. Yu and C. Diu, Total and regional deposition of inhaled aerosols in humans, J. Aerosol Sci., 1983, 14, 599–609 CrossRef.
- W. Nixon and M. Egan, Modelling study of regional deposition of inhaled aerosols with special reference to effects of ventilation asymmetry, J. Aerosol Sci., 1987, 18, 563–579 CrossRef.
- C. Darquenne and M. Paiva, One-dimensional simulation of aerosol transport and deposition in the human lung, J. Appl. Physiol., 1994, 77, 2889–2898 CrossRef CAS PubMed.
- C. Mitsakou, C. Helmis and C. Housiadas, Eulerian modelling of lung deposition with sectional representation of aerosol dynamics, J. Aerosol Sci., 2005, 36, 75–94 CrossRef CAS.
- H. C. Yeh and G. M. Schum, Models of human lung airways and their application to inhaled particle deposition, Bull. Math. Biol., 1980, 42, 461–480 CrossRef CAS PubMed.
- T. Martonen, Analytical model of hygroscopic particle behavior in human airways, Bull. Math. Biol., 1982, 44, 425–442 CrossRef CAS.
- B. Asgharian, W. Hofmann and R. Bergmann, Particle deposition in a multiple-path model of the human lung, Aerosol Sci. Technol., 2001, 34, 332–339 CrossRef CAS.
- W. Hofmann, B. Asgharian and R. Winkler-Heil, Modeling intersubject variability of particle deposition in human lungs, J. Aerosol Sci., 2002, 33, 219–235 CrossRef CAS.
- L. Koblinger and W. Hofmann, Monte Carlo modeling of aerosol deposition in human lungs. Part I: Simulation of particle transport in a stochastic lung structure, J. Aerosol Sci., 1990, 21, 661–674 CrossRef.
- W. Hofmann and L. Koblinger, Monte Carlo modeling of aerosol deposition in human lungs. Part II: Deposition fractions and their sensitivity to parameter variations, J. Aerosol Sci., 1990, 21, 675–688 CrossRef.
- Z. Zhang, C. Kleinstreuer and C. Kim, Cyclic micron-size particle inhalation and deposition in a triple bifurcation lung airway model, J. Aerosol Sci., 2002, 33, 257–281 CrossRef CAS.
- Z. Zhang, C. Kleinstreuer and C. Kim, Flow structure and particle transport in a triple bifurcation airway model, J. Fluids Eng., 2001, 123, 320–330 CrossRef CAS.
- J. Comer, C. Kleinstreuer and Z. Zhang, Flow structures and particle deposition patterns in double-bifurcation airway models. Part 1. Air flow fields, J. Fluid Mech., 2001, 435, 25–54 CrossRef.
- Z. Zhang, C. Kleinstreuer and C. S. Kim, Airflow and nanoparticle deposition in a 16-generation tracheobronchial airway model, Ann. Biomed. Eng., 2008, 36, 2095–2110 CrossRef.
- B. Ma, V. Ruwet, P. Corieri, R. Theunissen, M. Riethmuller and C. Darquenne, CFD simulation and experimental validation of fluid flow and particle transport in a model of alveolated airways, J. Aerosol Sci., 2009, 40, 403–414 CrossRef CAS.
- B. Asgharian, R. Wood and R. Schlesinger, Empirical modeling of particle deposition in the alveolar region of the lungs: a basis for interspecies extrapolation, Fundam. Appl. Toxicol., 1995, 27, 232–238 CrossRef CAS PubMed.
- S. Anjilvel and B. Asgharian, A multiple-path model of particle deposition in the rat lung, Fundam. Appl. Toxicol., 1995, 28, 41–50 CrossRef CAS PubMed.
- F. J. Miller, B. Asgharian, J. D. Schroeter and O. Price, Improvements and additions to the multiple path particle dosimetry model, J. Aerosol Sci., 2016, 99, 14–26 CrossRef CAS.
- W. Hofmann and L. Koblinger, Monte Carlo modeling of aerosol deposition in human lungs. Part III: Comparison with experimental data, J. Aerosol Sci., 1992, 23, 51–63 CrossRef CAS.
- A. Afantitis, G. Melagraki, P. Isigonis, A. Tsoumanis, D. D. Varsou, E. Valsami-Jones, A. Papadiamantis, L.-J. A. Ellis, H. Sarimveis and P. Doganis, NanoSolveIT Project: Driving nanoinformatics research to develop innovative and integrated tools for in silico nanosafety assessment, Comput. Struct. Biotechnol. J., 2020, 18, 583–602 CrossRef CAS.
- N. Cheimarios, S. Harrison, A. C. Ø. Jensen, P. Karatzas, A. Tsoumanis, P. Doganis, P. Tsiros, D. A. Winkler, S. Lofts and K. A. Jensen, in Handbook of Functionalized Nanomaterials, Elsevier, 2021, pp. 81–120 Search PubMed.
- N. Cheimarios, B. Pem, A. Tsoumanis, K. Ilić, I. V. Vrček, G. Melagraki, D. Bitounis, P. Isigonis, M. Dusinska and I. Lynch, An in vitro dosimetry tool for the numerical transport modeling of engineered nanomaterials powered by the Enalos RiskGONE Cloud Platform, Nanomaterials, 2022, 12, 3935 CrossRef CAS PubMed.
- D. G. Mintis, N. Cheimarios, A. Tsoumanis, A. G. Papadiamantis, N. W. van den Brink, H. J. van Lingen, G. Melagraki, I. Lynch and A. Afantitis, NanoBioAccumulate: Modelling the uptake and bioaccumulation of nanomaterials in soil and aquatic invertebrates via the Enalos DIAGONAL Cloud Platform, Comput. Struct. Biotechnol. J., 2024, 25, 243–255 CrossRef CAS PubMed.
- C. Papavasiliou, D. G. Mintis, A. Tsoumanis, A. Karaoli, I. Lynch, S. Krause, D. D. Varsou, G. Melagraki, M. Kavousanakis and A. Afantitis, MicroPlasticFate web application: Multimedia environmental fate modelling of microplastic particles via the enalos cloud platform, Bioresour. Technol. Rep., 2025, 102157 CrossRef.
- J. Subbotina, P. D. Kolokathis, A. Tsoumanis, N. K. Sidiropoulos, I. Rouse, I. Lynch, V. Lobaskin and A. Afantitis, UANanoDock: A Web-Based UnitedAtom Multiscale Nanodocking Tool for Predicting Protein Adsorption onto Nanoparticles, J. Chem. Inf. Model., 2025, 65, 3142–3153 CrossRef PubMed.
- P. D. Kolokathis, E. Voyiatzis, N. K. Sidiropoulos, A. Tsoumanis, G. Melagraki, K. Tämm, I. Lynch and A. Afantitis, ASCOT: a web tool for the digital construction of energy minimized Ag, CuO, TiO2 spherical nanoparticles and calculation of their atomistic descriptors, Comput. Struct. Biotechnol. J., 2024, 25, 34–46 CrossRef CAS.
- P. D. Kolokathis, N. K. Sidiropoulos, D. Zouraris, D.-D. Varsou, D. G. Mintis, A. Tsoumanis, F. Dondero, T. E. Exner, H. Sarimveis and E. Chaideftou, Easy-MODA: Simplifying standardised registration of scientific simulation workflows through MODA template guidelines powered by the Enalos Cloud Platform, Comput. Struct. Biotechnol. J., 2024, 25, 256–268 CrossRef CAS PubMed.
- D.-D. Varsou, P. D. Kolokathis, M. Antoniou, N. K. Sidiropoulos, A. Tsoumanis, A. G. Papadiamantis, G. Melagraki, I. Lynch and A. Afantitis, In silico assessment of nanoparticle toxicity powered by the Enalos Cloud Platform: Integrating automated machine learning and synthetic data for enhanced nanosafety evaluation, Comput. Struct. Biotechnol. J., 2024, 25, 47–60 CrossRef CAS PubMed.
- D.-D. Varsou, L.-J. A. Ellis, A. Afantitis, G. Melagraki and I. Lynch, Ecotoxicological read-across models for predicting acute toxicity of freshly dispersed versus medium-aged NMs to Daphnia magna, Chemosphere, 2021, 285, 131452 CrossRef CAS.
- M. Guo, Y. Lyu, T. Xu, B. Yao, W. Song, M. Li, X. Yang, T. Cheng and X. Li, Particle size distribution and respiratory deposition estimates of airborne perfluoroalkyl acids during the haze period in the megacity of Shanghai, Environ. Pollut., 2018, 234, 9–19 CrossRef CAS PubMed.
- H. Lin, J.-Y. Lao, Q. Wang, Y. Ruan, Y. He, P. K. Lee, K. M. Leung and P. K. Lam, Per-and polyfluoroalkyl substances in the atmosphere of waste management infrastructures: Uncovering secondary fluorotelomer alcohols, particle size distribution, and human inhalation exposure, Environ. Int., 2022, 167, 107434 CrossRef CAS PubMed.
- EPA, Multiple-path particle dosimetry (MPPD) model: U.S. EPA technical support documentation and user's guide, MPPD EPA, China, 2021 Search PubMed.
- O. Price, B. Asgharian, F. Miller, F. Cassee and R. de Winter-Sorkina, Multiple Path Particle Dosimetry model (MPPD v1.0): A model for human and rat airway particle dosimetry, RIVM rapport 650010030, 2002 Search PubMed.
- K. Zhang, B.-Z. Zhang, S.-M. Li, C. S. Wong and E. Y. Zeng, Calculated respiratory exposure to indoor size-fractioned polycyclic aromatic hydrocarbons in an urban environment, Sci. Total Environ., 2012, 431, 245–251 CrossRef CAS PubMed.
- M. Stäuble and H.-J. Schumacher, ZK Developer's Guide, Packt Publishing Ltd., 2008 Search PubMed.
- D.-D. Varsou, A. Tsoumanis, A. Afantitis and G. Melagraki, Enalos cloud platform: Nanoinformatics and cheminformatics tools, Ecotoxicological QSARs, 2020, pp. 789–800 Search PubMed.
- C. Fuentes, I. Kang, J. Lee, D. Song, M. Sjöö, J. Choi, S. Lee and L. Nilsson, Fractionation and characterization of starch granules using field-flow fractionation (FFF) and differential scanning calorimetry (DSC), Anal. Bioanal. Chem., 2019, 411, 3665–3674 CrossRef CAS.
- S. B. Patil and K. K. Sawant, Chitosan microspheres as a delivery system for nasal insufflation, Colloids Surf., B, 2011, 84, 384–389 CrossRef CAS.
- E. Zora-Guzman and J. R. Guzman-Sepulveda, Optical characterization of native aerosols from e-cigarettes in localized volumes, Biomed. Opt. Express, 2024, 15, 1697–1708 CrossRef CAS.
- C. Monsé, M. Raulf, B. Jettkant, V. van Kampen, B. Kendzia, L. Schürmeyer, C. E. Seifert, E.-M. Marek, G. Westphal and N. Rosenkranz, Health effects after inhalation of micro-and nano-sized zinc oxide particles in human volunteers, Arch. Toxicol., 2021, 95, 53–65 CrossRef PubMed.
- S. Zhang, C. Campagne and F. Salaün, Preparation of electrosprayed poly (caprolactone) microparticles based on green solvents and related investigations on the effects of solution properties as well as operating parameters, Coatings, 2019, 9, 84 CrossRef.
- C. Motzkus, C. Chivas-Joly, E. Guillaume, S. Ducourtieux, L. Saragoza, D. Lesenechal and T. Mace, Characterization of aerosol emitted by the combustion of nanocomposites, J. Phys.: Conf. Ser., 2011, 304, 012020 CrossRef.
- A. McDonagh and M. Byrne, A study of the size distribution of aerosol particles resuspended from clothing surfaces, J. Aerosol Sci., 2014, 75, 94–103 CrossRef CAS.
- P.-P. Wang, Z.-G. Luo and T. M. Tamer, Effects of octenyl succinic anhydride groups distribution on the storage and shear stability of Pickering emulsions formulated by modified rice starch, Carbohydr. Polym., 2020, 228, 115389 CrossRef PubMed.
- M. Santa-Maria, H. Scher and T. Jeoh, Microencapsulation of bioactives in cross-linked alginate matrices by spray drying, J. Microencapsulation, 2012, 29, 286–295 CrossRef CAS PubMed.
- O. Werner and C. Turner, Investigation of different particle sizes on superhydrophobic surfaces made by rapid expansion of supercritical solution with in situ laser diffraction (RESS-LD), J. Supercrit. Fluids, 2012, 67, 53–59 CrossRef CAS.
- Y. Lv, X. Li, T. T. Xu, T. T. Cheng, X. Yang, J. M. Chen, Y. Iinuma and H. Herrmann, Size distributions of polycyclic aromatic hydrocarbons in urban atmosphere: sorption mechanism and source contributions to respiratory deposition, Atmos. Chem. Phys., 2016, 16, 2971–2983 CrossRef CAS.
- U.S. New York State Department of Environmental Conservation, DAR-1: Guidelines for the Evaluation and Control of Ambient Air Contaminants under 6 NYCRR Part 212, 2020 Search PubMed.
- EFSA, Risk to human health related to the presence of perfluoroalkyl substances in food, EFSA J., 2020, 18, e06223 Search PubMed.
- U. S. EPA, Per- and Polyfluoroalkyl Substances (PFAS) Final PFAS National Primary Drinking Water Regulation, https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas, (accessed June 26, 2025) Search PubMed.
- K. Zhang, B.-Z. Zhang, S.-M. Li, L.-M. Zhang, R. Staebler and E. Y. Zeng, Diurnal and seasonal variability in size-dependent atmospheric deposition fluxes of polycyclic aromatic hydrocarbons in an urban center, Atmos. Environ., 2012, 57, 41–48 CrossRef CAS.
- Y. Kawanaka, Y. Tsuchiya, S.-J. Yun and K. Sakamoto, Size distributions of polycyclic aromatic hydrocarbons in the atmosphere and estimation of the contribution of ultrafine particles to their lung deposition, Environ. Sci. Technol., 2009, 43, 6851–6856 CrossRef CAS PubMed.
- P. Fernández, J. O. Grimalt and R. M. Vilanova, Atmospheric gas-particle partitioning of polycyclic aromatic hydrocarbons in high mountain regions of Europe, Environ. Sci. Technol., 2002, 36, 1162–1168 CrossRef.
- K. Hytönen, P. Yli-Pirilä, J. Tissari, A. Gröhn, I. Riipinen, K. Lehtinen and J. Jokiniemi, Gas–particle distribution of PAHs in wood combustion emission determined with annular denuders, filter, and polyurethane foam adsorbent, Aerosol Sci. Technol., 2009, 43, 442–454 CrossRef.
- G. Shen, W. Wang, Y. Yang, J. Ding, M. Xue, Y. Min, C. Zhu, H. Shen, W. Li and B. Wang, Emissions of PAHs from indoor crop residue burning in a typical rural stove: emission factors, size distributions, and gas− particle partitioning, Environ. Sci. Technol., 2011, 45, 1206–1212 CrossRef CAS PubMed.
- A. B. Patel, S. Shaikh, K. R. Jain, C. Desai and D. Madamwar, Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches, Front. Microbiol., 2020, 11, 562813 CrossRef.
- A. Haritash and C. Kaushik, Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review, J. Hazard. Mater., 2009, 169, 1–15 CrossRef CAS PubMed.
- E. Manoli, A. Kouras and C. Samara, Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece, Chemosphere, 2004, 56, 867–878 CrossRef CAS.
- E. Manoli, A. Kouras, O. Karagkiozidou, G. Argyropoulos, D. Voutsa and C. Samara, Polycyclic aromatic hydrocarbons (PAHs) at traffic and urban background sites of northern Greece: source apportionment of ambient PAH levels and PAH-induced lung cancer risk, Environ. Sci. Pollut. Res., 2016, 23, 3556–3568 CrossRef CAS PubMed.
- M. Rehwagen, A. Müller, L. Massolo, O. Herbarth and A. Ronco, Polycyclic aromatic hydrocarbons associated with particles in ambient air from urban and industrial areas, Sci. Total Environ., 2005, 348, 199–210 CrossRef CAS PubMed.
- J. Zhou, T. Wang, Y. Huang, T. Mao and N. Zhong, Size distribution of polycyclic aromatic hydrocarbons in urban and suburban sites of Beijing, China, Chemosphere, 2005, 61, 792–799 CrossRef CAS PubMed.
- J. Duan, X. Bi, J. Tan, G. Sheng and J. Fu, Seasonal variation on size distribution and concentration of PAHs in Guangzhou city, China, Chemosphere, 2007, 67, 614–622 CrossRef CAS.
- A. Besis, D. Gallou, A. Avgenikou, E. Serafeim and C. Samara, Size-dependent in vitro inhalation bioaccessibility of PAHs and O/N PAHs-Implications to inhalation risk assessment, Environ. Pollut., 2022, 301, 119045 CrossRef CAS.
- H. Guo, S. Lee, K. Ho, X. Wang and S. Zou, Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong, Atmos. Environ., 2003, 37, 5307–5317 CrossRef CAS.
- K. Hayakawa, N. Tang, K. Akutsu, T. Murahashi, H. Kakimoto, R. Kizu and A. Toriba, Comparison of polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in airborne particulates collected in downtown and suburban Kanazawa, Japan, Atmos. Environ., 2002, 36, 5535–5541 CrossRef CAS.
- K. Hayakawa, N. Tang, E. G. Nagato, A. Toriba, S. Sakai, F. Kano, S. Goto, O. Endo, K.-I. Arashidani and H. Kakimoto, Long term trends in atmospheric concentrations of polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons: A study of Japanese cities from 1997 to 2014, Environ. Pollut., 2018, 233, 474–482 CrossRef CAS PubMed.
- J. C. Bonner, Nanoparticles as a potential cause of pleural and interstitial lung disease, Proc. Am. Thorac. Soc., 2010, 7, 138–141 CrossRef CAS.
- H. Qiao, W. Liu, H. Gu, D. Wang and Y. Wang, The transport and deposition of nanoparticles in respiratory system by inhalation, J. Nanomater., 2015, 2015, 394507 CrossRef.
- N. R. Yacobi, F. Fazllolahi, Y. H. Kim, A. Sipos, Z. Borok, K.-J. Kim and E. D. Crandall, Nanomaterial interactions with and trafficking across the lung alveolar epithelial barrier: implications for health effects of air-pollution particles, Air Qual., Atmos. Health, 2011, 4, 65–78 CrossRef PubMed.
- M. Riediker, D. Zink, W. Kreyling, G. Oberdörster, A. Elder, U. Graham, I. Lynch, A. Duschl, G. Ichihara and S. Ichihara, Particle toxicology and health-where are we?, Part. Fibre Toxicol., 2019, 16, 1–33 CrossRef.
- P. Tsiros, N. Cheimarios, A. Tsoumanis, A. Ø. Jensen, G. Melagraki, I. Lynch, H. Sarimveis and A. Afantitis, Towards an in silico integrated approach for testing and assessment of nanomaterials: from predicted indoor air concentrations to lung dose and biodistribution, Environ. Sci.: Nano, 2022, 9, 1282–1297 RSC.
- E. Garcia, M. B. Rice and D. R. Gold, Air pollution and lung function in children, J. Allergy Clin. Immunol., 2021, 148, 1–14 CrossRef CAS PubMed.
- C. M. Salome, G. G. King and N. Berend, Physiology of obesity and effects on lung function, J. Appl. Physiol., 2010, 108, 206–211 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: ESI file is included, providing a comprehensive analysis of the outcomes generated using the LungDepo web-tool. See DOI: https://doi.org/10.1039/d5en00299k |
| This journal is © The Royal Society of Chemistry 2025 |