Advancements in separator materials for aqueous zinc batteries
Qingshun Nian†
a,
Xinru Yang†a,
Hu Honga,
Peng Chena,
Yuwei Zhaoa,
Haiming Lva and
Chunyi Zhi *abcd
*abcd
aDepartment of Materials Science and Engineering, City University of Hong Kong, Kowloon, Hong Kong SAR 999077, China. E-mail: cy.zhi@cityu.edu.hk
bHong Kong Institute for Advanced Study, City University of Hong Kong, Kowloon, Hong Kong SAR 999077, China
cHong Kong Institute for Clean Energy, City University of Hong Kong, Kowloon, Hong Kong SAR 999077, China
dCentre for Advanced Nuclear Safety and Sustainable Development, City University of Hong Kong, Kowloon, Hong Kong SAR 999077, China
First published on 25th June 2025
Abstract
Aqueous zinc (Zn) batteries (AZBs) are becoming promising candidates for grid-scale energy storage because of their inherent safety, cost-effectiveness, and high theoretical capacity. However, their widespread application is hindered by critical challenges, including Zn dendrite formation, hydrogen evolution reaction (HER), corrosion, and cathode material dissolution. The separator plays a crucial role in regulating ion transport, suppressing side reactions, and promoting uniform Zn deposition. While recent advancements in separator design have introduced various modification strategies to enhance electrochemical performance, a systematic classification based on the modification location remains lacking. This review provides a comprehensive analysis of recent advancements in AZB separators, categorized by modification position—anode side, cathode side, and full-separator modifications. Key modification strategies, including ion-selective layers, interfacial engineering, and composite functional membranes, are discussed in detail, with an emphasis on their effects on Zn2+ flux regulation, dendrite suppression, and long-term cycling stability. Additionally, emerging separator materials such as covalent organic frameworks (COFs), metal–organic frameworks (MOFs), and inorganic–organic hybrid separators are highlighted for their potential in optimizing battery performance. By elucidating the underlying mechanisms governing separator modifications, this review provides theoretical insights and design principles for the development of next-generation AZB separators. Finally, we discuss future research directions, focusing on separator thinness, enhanced ion selectivity, interface stability, corrosion resistance, and scalable manufacturing to accelerate the commercialization of high-performance AZBs.
1. Introduction
With the growing demand for sustainable energy storage, the development of safe, cost-effective, and high-performance battery technologies has become extremely important.1–5 Although lithium-ion batteries (LIBs) have achieved widespread commercialization, their reliance on flammable organic electrolytes raises safety concerns.6–17 Sodium-ion batteries (SIBs), which share similar electrochemical configurations with LIBs, also rely on organic electrolytes and thus suffer from comparable issues in terms of safety.18–20 From the perspectives of safety, environmental sustainability, and resource abundance, aqueous batteries offer inherent advantages owing to their water-based electrolytes and the earth-abundant nature of water.Compared with other aqueous battery systems—such as aqueous lithium-, sodium-, or potassium-ion batteries—AZBs benefit from the low redox potential and high theoretical capacity of the Zn metal anode, resulting in higher achievable energy density. Furthermore, in contrast to aqueous multivalent systems such as aluminum-ion batteries, AZBs exhibit better electrochemical reversibility.21–31 AZBs have emerged as promising candidates for grid-scale energy storage due to their high safety, low cost, environmental friendliness, and high theoretical capacity (820 mAh g−1; 5855 mAh cm−3).32–40 Despite these advantages, the practical use of AZBs is still limited by key challenges, including Zn dendrite growth, side reactions, and electrode corrosion.41–46 Key strategies include electrode modification, electrolyte regulation, and separator optimization. Electrode modification focuses on constructing functional interface layers on the Zn anode surface or designing innovative electrode structures to regulate Zn deposition behavior, suppress side reactions, and improve electrode stability.47–53 Coating a protective layer on the Zn anode surface can effectively suppress dendrite growth and side reactions. Common coating materials include carbon materials (such as reduced graphene oxide (rGO)54 and carbon nanotubes (CNTs),55 which have good conductivity and chemical stability, can distribute the electric field uniformly, and suppress dendrite growth),56–58 metal oxides (such as ZnO,59,60 CuO,61 and In2O3,62 which regulate Zn2+ flux and guide uniform Zn deposition), and polymers (such as PVDF-HFP63 and PPPA,64 which form flexible protective layers to suppress dendrite growth and corrosion). Alloys, such as Zn alloyed with other metals like Cu,65 In,66 Bi,67 Sb,68 and Sn,69 can alter Zn's deposition potential and suppress dendrite growth and HER. In addition, the addition of small amounts of additives to the electrolyte can effectively suppress dendrite growth and side reactions. Common additives include metal ion additives (such as Ca2+,70 Ga3+,71 Y3+,72 Ce3+,73 which adsorb on the Zn surface to alter deposition behavior and suppress dendrite growth) and organic additives (such as PPGA74 and DMSO,75 which adsorb onto the Zn surface to regulate Zn deposition, mitigate corrosion, and lower water molecule activity to suppress the HER). Using high-concentration electrolytes can reduce the reactivity of water, suppress HER and side reactions, while enhancing the electrochemical window of the electrolyte.76 In parallel, many reviews have focused on electrode modification and electrolyte optimization to improve Zn anode performance. However, the importance of separators has often been overlooked.
As an important but often overlooked component, the separator plays a key role in maintaining battery stability by physically separating the electrodes, optimizing ion transport, and reducing side reactions.77 Recent advances in separator engineering have demonstrated significant potential in enhancing AZB cycling stability.78–83 However, current separator modification strategies exhibit diversity and complexity, particularly with significant differences in the selection of modification locations—such as modifications on the anode side, cathode side, and full-separator modifications. Notably, existing reviews on AZB separators have not systematically summarized the research from the perspective of modification locations.
To address this gap, this review offers a detailed and organized analysis of advancements in AZB separators, categorized by modification position—anode side, cathode side, and full-separator modifications. By explaining the mechanisms of these modifications in controlling Zn2+ movement, suppressing dendrite growth, and reducing side reactions, this review aims to provide theoretical guidance and practical insights for designing high-performance AZB separators. Finally, it discusses future prospects for separator development, focusing on improving ion selectivity, structural stability, and scalable manufacturing to support the commercialization of AZBs.
2. Performance requirements and design guidelines for separators
Ideal separator materials for AZBs must meet several key performance criteria to ensure efficient and stable operation:Cost (<$1 per m2): the cost should align with the low-cost positioning of AZBs and meet the economic requirements for grid-scale energy storage applications. Currently, the cost of commercial glass fiber (GF) separators is about $0.5–0.8 per m2, while the cost of new polymer separators still needs to be reduced further.
Electrical insulation: excellent electrical insulating properties are essential to prevent internal electron conduction in the battery, thus reducing the self-discharge rate (typically required to be <5% per month). Studies indicate that the volume resistivity of a high-quality separator should be greater than 109 Ω cm.
Porosity (40–70%) and pore size distribution: an ideal separator should have a uniform microporous structure (pore size 0.1–1 μm) to equalize ion flux distribution and suppress dendrite penetration. Excessively high porosity (>80%) may reduce mechanical strength, while excessively low porosity (<30%) can significantly increase ion transport resistance.
Electrolyte wettability: excellent wettability (contact angle <30°) ensures high ionic conductivity. For example, surface-modified GF separators can achieve an ionic conductivity of 173 mS cm−1, significantly outperforming traditional Celgard separators (around 0.3 mS cm−1).
Mechanical strength: the wet-state tensile strength should exceed 5 MPa, and the puncture resistance should exceed 100 N to effectively resist penetration by Zn dendrites. Research shows that adding a nanofiber-reinforced layer can increase the mechanical strength of the separator by approximately 40%.
Thickness (<20 μm) and uniformity: ultra-thin and uniform separators can reduce the volume of inactive materials, thereby enhancing the energy density of the battery. Currently, advanced separators have a thickness controlled in the range of 10–15 μm, with thickness deviation less than ±2 μm.
Chemical stability: excellent chemical stability allows the separator to withstand the redox environment of the electrolyte (pH 3–14) without degradation. Experimental data shows that a high-quality separator should have a mass loss of less than 1% after being soaked in 1 M ZnSO4 electrolyte for 30 days.
Thermal stability: a wide temperature tolerance range (−50 °C to 80 °C), with a low thermal shrinkage rate (<5%).
Environmental friendliness: the separator should be recyclable or biodegradable.
Processability: good processability and dimensional stability. These performance indicators are closely interconnected, and in practical applications, balancing them requires trade-offs tailored to specific usage scenarios. For example, increasing porosity can enhance ion conductivity but may reduce mechanical strength; increasing thickness can improve mechanical performance but will lower energy density. Therefore, separator design needs to seek the optimal balance between various performance parameters.
3. Structure and functional properties of common separators in AZBs
3.1 Structure and functional properties of filter paper separator
Filter paper is a fibrous material primarily composed of cellulose, a macromolecular polysaccharide formed by the β-1,4-glycosidic bonding of glucose units. From a molecular structure perspective, filter paper consists of numerous interconnected six-membered glucose ring units, forming a complex pore network. This porous structure allows it to serve as a channel for ion transport while preventing direct contact between the electrodes, thus providing physical isolation (Fig. 1a).84,85In AZBs, filter paper separators have been widely used in early research due to their low cost and ease of processing. However, they exhibit several critical drawbacks in practical applications:
Poor chemical stability: the primary component of filter paper, cellulose, is prone to degradation in strongly alkaline electrolytes (such as KOH), which compromises the separator's structure and impacts the long-term cycling stability of the battery.
Uneven pore size distribution: the pores of filter paper are relatively large and unevenly distributed, limiting its ability to physically block Zn dendrites, thus failing to effectively suppress dendrite growth. Once Zn dendrites penetrate the separator, they can cause internal short circuits, leading to battery failure.
Insufficient mechanical strength: after immersion in electrolyte, filter paper loses mechanical strength, becoming prone to rupture or deformation, which hinders its use in long-term cycling.
In summary, despite the low cost of filter paper separators, its poor chemical stability in strongly alkaline environments makes it unsuitable for high-performance AZBs.
3.2 Structure and functional properties of glass fiber (GF) separator
GF is an inorganic material primarily composed of SiO2 (silicon dioxide), Al2O3 (aluminum oxide), CaO/MgO (calcium oxide/magnesium oxide), and B2O3 (boron oxide) (Fig. 1b).86,87 These components impart excellent chemical and mechanical properties to GF:Strong electrochemical corrosion resistance: during charge and discharge cycles, GF remains electrochemically inert, neither participating in redox reactions nor reacting with the electrolyte, thereby maintaining its structural integrity over time.
High porosity and excellent ion transport capability: GF separators typically have a porosity of 80–85%, providing an effective channel for Zn2+, which reduces ion diffusion resistance and enhances the battery's rate capability.
Although GF separators exhibit several advantages, they also have certain limitations:
Ineffective Zn dendrite suppression: the pore size of GF separators is typically in the micron range, while the initial nucleation size of Zn dendrites can be as small as nanometers (<100 nm). Therefore, physical confinement alone is insufficient to prevent dendrite growth, which can still lead to short circuit issues.
Surface chemical inertia: the chemical surface of GF is relatively inert, limiting its ability to regulate the uniform deposition of Zn through adsorption or interface electric field control, which may exacerbate dendrite growth unevenness.
An ideal separator material should possess high mechanical strength, excellent chemical stability, a uniform pore structure, and good electrolyte wettability. While GF outperforms filter paper in chemical stability and electrochemical resistance, improvements are still needed in dendrite suppression.
3.3 Development timeline of separators for AZBs
In the early stages of AZBs research, filter paper was commonly used as the separator (Fig. 2) due to its low cost, simple structure, and easy accessibility in laboratory settings.88–92 However, as research progressed and attention increasingly focused on the Zn anode, the limitations of filter paper became apparent—particularly its low mechanical strength and limited thickness, which make it ineffective at blocking Zn dendrites. This can lead to short circuits and reduced battery safety. In contrast, GF separators offer several distinct advantages. The increased thickness confers improved resistance to dendrite penetration. Additionally, GF materials typically exhibit high porosity and excellent electrolyte wettability, which enhance ionic conductivity and electrolyte retention, thereby improving cycling stability and rate performance. As a result, GF has gradually replaced filter paper as a more widely used base material for separators in AZBs.Nevertheless, GF alone still cannot fully address the challenges posed by both the anode and cathode, such as continuous dendrite growth, side reactions at the electrode interfaces, and poor ion selectivity of the separator. To overcome these issues, researchers have developed a variety of modification strategies based on GF, including surface coatings, functional treatments, and the incorporation of heterogeneous structures. At the same time, significant efforts have been made to design and develop novel high-performance separators aimed at fundamentally resolving the limitations of existing materials. Given the limitations of unmodified GF, extensive research efforts have been devoted to enhancing its performance and developing novel separators. In the following sections, I provide a comprehensive overview of recent progress in both GF modifications and the design of advanced separator materials.
4. GF separator modification strategies
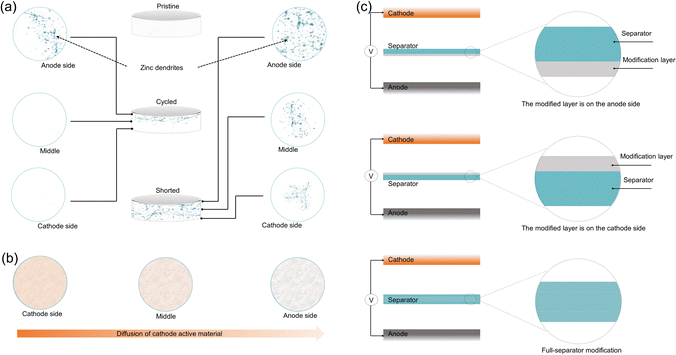
The continuous growth of Zn dendrites is a major safety concern in AZBs. These needle-like structures can eventually pierce the separator, causing a short circuit that seriously affects the safety and lifespan of the battery. As shown in Fig. 3a, Zn dendrites grow inside the separator and can break through its structure, leading to short circuit failure. GF is a commonly used separator in AZBs because of its ability to hold electrolyte. However, it still has several problems during real use. For example, GF has a strong attraction to Zn2+, which can guide dendrite growth toward the separator and increase the chance of short circuits.93 In addition, as shown in Fig. 3b, GF cannot effectively stop the spreading and loss of cathode materials. This causes active material loss, faster self-discharge, and reduced battery capacity, which all speed up battery failure.To address these issues, GF separators are typically modified to optimize their physical and chemical properties. This enables them to better suppress Zn dendrite growth, improve ion selectivity, and reduce side reactions, thus ensuring stable battery operation.
In AZBs, GF separator modification strategies are diverse. Different modification layer positions can meet various electrochemical needs, thereby optimizing battery performance. As an essential component of the battery, the separator is in direct contact with both the anode and cathode, and it can simultaneously regulate the chemical reactions at both electrode interfaces. Therefore, theoretically, separator modification holds the potential to address issues on both the cathode and Zn anode sides in a synergistic manner. Fig. 3c illustrates three common approaches to separator modification: modifying the layer near the anode side, the cathode side, or the full-separator.
The rationale behind our spatial classification is to highlight the functional asymmetry that naturally arises in AZB systems, where the anode and cathode experience significantly different electrochemical environments. Modifications on the anode side typically aim to suppress Zn dendrites and parasitic reactions (e.g., hydrogen evolution), while those on the cathode side often target cathode dissolution, shuttle effects, or sluggish kinetics. Modifications applied to the entire separator are generally intended to address challenges on both sides simultaneously. Compared to previous reviews that mostly focus on material types or general functional effects, our spatially resolved framework offers a structure–function perspective that helps clarify where specific challenges arise and how targeted separator modifications can address them. By implementing these modification strategies, the performance of GF separators can be further enhanced, making them better suited for the requirements of AZBs.
4.1 Modification layer located on the anode side
In this design, the modification layer of the separator is oriented towards the Zn anode. This strategy is primarily employed to suppress Zn dendrite growth and prevent their penetration through the separator, which could otherwise cause battery internal short circuits. Typically, the materials used for the modification layer possess good Zn2+ affinity. These materials can induce uniform deposition of Zn, reducing the tip effect of dendrites. Additionally, the modification layer on the anode side can optimize the diffusion dynamics of Zn2+ through electric field regulation, improving the Coulombic efficiency and cycling stability of the battery.This section reviews various strategies for separator modification on the anode side, including homogenizing the pore size distribution to regulate Zn2+ flux, employing Janus separators to optimize the interfacial electric field, and incorporating inorganic nanomaterials to enhance separator wettability while improving Zn2+ transport kinetics and deposition behavior. Through different material designs and engineering optimizations, these strategies have made significant progress in improving the stability of Zn anodes, suppressing dendrite growth, optimizing electrolyte wetting, and enhancing ion migration efficiency.
Furthermore, Liu et al.94 designed a Halloysite nanotube (HNT)-modified glass fiber separator (HNT-GF, Fig. 4a), which further improves the performance of AZBs. As a natural inorganic mineral, HNT not only enhances the mechanical strength and puncture resistance of the separator but also homogenizes the pore size distribution (Fig. 4b), thereby optimizing the Zn2+ flux (Fig. 4c). More importantly, the unique electro-negativity differences between the inner and outer surfaces of HNT give the modified separator ion screening capability: the negatively charged outer surface stabilizes the Zn2+ flow, promoting multi-site progressive nucleation for uniform deposition, while the positively charged inner surface effectively traps and restricts the migration of SO42−, serving as an “anion brake”. As a result, the Zn2+ transference number of the HNT-GF separator increases to 0.71, effectively suppressing the passivation reaction on the anode surface and achieving dendrite-free, highly reversible Zn deposition. This strategy inspires the possibility of achieving specific ion screening and directional migration through surface chemical modifications or hetero structural designs, such as optimizing ion channels using positive/negative charge distribution, functional group regulation, or layered material structures.
 | ||
Fig. 4 (a) Schematic representation illustrating the fabrication process of the HNT-GF separator and the ion-sieving mechanism of HNT. (b) Cross-sectional SEM image showcasing the HNT-GF separator. (c) Diagrammatic depiction of Zn deposition behavior when using the HNT-GF separator. Schematic visualizations of the Janus separator concept designed for stabilizing the Zn anode. Reproduced with permission.94 Copyright 2024, American Chemical Society. (d) Image of an unmodified GF separator and (e) a Janus separator featuring a vertically grown VG layer on one side, which aids in reducing local current density and ensuring uniform ion distribution. (f) Illustration of current distribution in the 3D scaffold. Electric field distribution of (g) the 3D scaffold structure (Janus separator) and (h) the 2D planar structure (pristine separator). DFT simulations of Zn adsorption on O/N-functionalized graphene: (i) structural configuration of O- and N-doped graphene, where gray, red, blue, and white represent carbon, oxygen, nitrogen, and hydrogen atoms, respectively. (j) Zn binding energy on O/N-functionalized graphene. (k) Yellow contours displaying the partial charge density around the Fermi level with an isosurface value of 0.002e bohr−3, shown in top-view (upper) and side-view (lower) for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–G and C-Npr-G configurations (left to right). (l) Schematic representation of the fabrication process and functional mechanism of the MXene-GF separator. Reproduced with permission.96 Copyright 2022 Wiley-VCH GmbH. O–G and C-Npr-G configurations (left to right). (l) Schematic representation of the fabrication process and functional mechanism of the MXene-GF separator. Reproduced with permission.96 Copyright 2022 Wiley-VCH GmbH. | ||
The separator not only acts as a physical isolation layer but can also regulate the electric field distribution by introducing conductive networks or polar functional materials, optimizing the morphology of Zn2+ deposition and improving anode stability. Li et al.95 developed a Janus separator by directly growing vertical graphene (VG) carpets on the anode side of commercial GF separators (Fig. 4e). Further, by using air plasma treatment to introduce oxygen and nitrogen heteroatoms onto the graphene surface, they formed a high surface area and porous three-dimensional VG framework. This design optimizes the electric field distribution at the anode/electrolyte interface, reducing the local current density and making the Zn2+ flux more uniform (Fig. 4f–k). This strategy not only has excellent scalability and cost-effectiveness but also serves as a general approach to optimize the stability of metal anodes in rechargeable batteries.
Although separator modification has proven to be an effective dendrite suppression strategy, current research pathways still face challenges in large-scale production, and there is a lack of focus on the fundamental mechanisms behind separator regulation. To address this, Su et al.96 proposed a scalable MXene-modified Janus separator, which deposits Ti3C2Tx MXene nanosheets on one side of commercial glass fiber (MXene-GF) using a spray printing process (Fig. 4l). This method is not only simple and suitable for mass production but also imparts good electrolyte wettability, high ionic conductivity, and rich surface polar groups to the separator, thus optimizing Zn2+ transport dynamics and deposition behavior.
It is noteworthy that the MXene-GF separator has an adjustable dielectric constant (ε), with an optimized value of 53.5. This dielectric constant gradient can induce polarization in the GF through the Maxwell–Wagner effect, establishing a directional built-in electric field at the interface with 50% enhanced strength, accelerating Zn2+ migration and deposition dynamics. Therefore, this strategy can effectively stabilize the Zn anode, providing a uniform interface ion field, and offering a new direction and technical support for the development of high-performance AZBs.
4.2 Modification layer located on the cathode side
When the modification layer is positioned on the cathode side, its primary role is to optimize cathode reaction kinetics, suppress the dissolution and shuttling of active materials, and enhance the battery's cycling stability. This strategy is particularly critical in aqueous Zn–iodine (Zn–I2) batteries. Zn–I2 batteries have garnered widespread attention due to their high theoretical capacity (∼211 mAh g−1), cost-effectiveness, and the low toxicity of iodine.97–99 However, in practical applications, this system faces challenges such as Zn dendrite growth, polyiodide (I3−/I5−) diffusion (Fig. 5a), and slow iodine redox kinetics. A common strategy to address polyiodide shuttling is interfacial engineering (Fig. 5a).100 However, recent studies have shown that modifying the separator can also be highly effective. Bi et al.101 proposed an innovative cathode-side interface engineering strategy by introducing cobalt-modified hollow nitrogen-doped MXene spheres (Co-HNMXS) on the cathode side of the GF separator (Fig. 5b). This material exhibits excellent interface hydrophilicity, ensuring fast ion transport. Additionally, the highly active Co–N–C sites effectively suppress polyiodide shuttling while accelerating the electrochemical conversion of iodine. This design not only enhances the redox kinetics of iodine but also reduces side reactions, effectively extending the battery lifespan. Yang et al.102 reported a capture–adsorption–catalysis synergistic strategy, using Zn–Mn atomic pairs modified GF separators (ZnMn-NC/GF) to suppress self-discharge phenomena. The single Mn atomic sites were primarily responsible for adsorbing polyiodides, while the Zn–Mn atomic pairs promoted the conversion of iodine redox reaction intermediates (Fig. 5c and d). This strategy significantly improves the utilization of cathode active materials and demonstrating exceptional stability and long cycle life. Kang et al.103 developed a Janus membrane with functional layers on both the anode and cathode sides to address multiple challenges associated with iodine cathodes. On the cathode side of a commercial glass fiber (GF) separator, they introduced a coating of Fe-decorated single-walled carbon nanotubes (Fe-SCNTs). This functional layer not only physically adsorbs polyiodide species and facilitates electron transport via the high surface area SCNTs, but also chemically adsorbs and catalyzes polyiodide through embedded Fe nanoparticles, thereby enhancing the redox reaction kinetics of iodine species (Fig. 5e). Through these synergistic effects, the Fe-SCNT cathode layer effectively anchors polyiodides and suppresses the shuttle effect (Fig. 5f), significantly improving the overall battery performance. | ||
| Fig. 5 (a) Schematic representation of the Zn metal anode corrosion mechanism induced by the I3− shuttle in AC-based AZBs, along with the inhibition mechanism of the I3− shuttle in Bpd-COC-based AZBs. Reproduced with permission.100 Copyright 2024, Wiley-VCH. (b) Diagram illustrating the functional mechanism of the Co-HNMXS@GFA separator. Reproduced with permission.101 Copyright 2025, Wiley-VCH. (c) Schematic visualization of the operational principles of a Zn-halogen battery incorporating a ZnMn-NC/GF separator. (d) Differential charge density mapping between I−/I3− and ZnMn-N6 sites. Reproduced with permission.102 Copyright 2025, Wiley-VCH. (e) Schematic and basic characterization of the Fe-SCNT/GF separator. (f) Optical images showing the shuttle effect using H-type cells with I3−. Reproduced with permission.103 Copyright 2023, Wiley-VCH. | ||
The successful implementation of cathode-side modification strategies provides new insights for enhancing the performance of AZBs. However, single modification strategies often address specific issues, and future research should focus on achieving multifunctional integration (such as simultaneously suppressing dendrite growth and polyiodide shuttling). Through continued optimization of cathode-side modification strategies, further improvements in the performance of AZBs can be achieved, promoting their development for practical applications.
In addition to directly optimizing cathode reaction kinetics, the cathode-side modification layer can also indirectly influence the behavior of the Zn anode through unique interfacial chemical interactions. Wu et al.93 proposed a novel strategy to regulate Zn deposition by in situ modifying the cathode side of a commercial GF separator with polyaniline (PANI), forming a GF/PANI separator. In cells using the unmodified GF separator, the electric field at the Zn anode surface is unevenly distributed, which promotes the formation of Zn protrusions in the early stages of deposition. Moreover, the strong binding affinity between GF and Zn drives Zn2+ toward the separator, increasing the risk of dendrite penetration. In contrast, the PANI layer, rich in functional groups and with moderate Zn affinity, guides Zn2+ to deposit uniformly along the edge of Zn nuclei, resulting in a smooth Zn layer (Fig. 6a). The GF/PANI-600 separator provides balanced Zn affinity and a uniform electric field distribution, effectively directing homogeneous Zn deposition and suppressing dendrite growth through the PANI modification layer. Bu et al.104 have moved beyond the traditional approach of passively inhibiting Zn dendrite growth and instead proposed an active dendrite digestion strategy. They modified separator with mesoporous Ti3C2 MXene (Meso Ti3C2), enabling it to actively participate in the oxidation and dissolution of dendrites during the battery's cycling process (Fig. 6b). This innovative design not only overcomes the limitations of traditional passive protective separators but also provides a new solution for stabilizing the Zn anode.
 | ||
| Fig. 6 (a) The schematic demonstration of the evolution of the plating process on Zn anodes with GF/PANI-600 and pristine GF separators. Reproduced with permission.93 Copyright 2023, Royal Society of Chemistry. (b) Diagram illustrating the role of the meso Ti3C2-coated PP separator in suppressing Zn dendrite formation and the associated working mechanisms. (c) Spontaneous redox reaction occurring between Zn0 and the Ti–Oe functional groups on meso Ti3C2. Reproduced with permission.104 Copyright 2023, American Chemical Society. | ||
Studies have shown that meso Ti3C2 has high oxidative properties, capable of spontaneously converting the dead Zn dendrites that have already formed back into Zn2+ ions through a redox process, which are then returned to the electrolyte to participate in electrochemical reactions (Fig. 6c). Compared to planar Zn dendrites, the edge-rich Ti–O sites of meso Ti3C2 can more effectively promote the oxidation of Zn dendrites and accelerate the electron transfer process. Therefore, under a practical working current density of 5 mA cm−2 and low overpotential (<50 mV), asymmetric cells can still cycle stably for 2200 hours, significantly extending the battery's lifespan. This study is the first to reveal the unique edge effect of mesoporous MXene materials and proposes an innovative active dendrite digestion method that ensures long-term operation of AZBs even when dendrites inevitably form.
The cathode-side modification layer strategy not only significantly enhances the performance of Zn–I2 batteries but also provides new design directions for other aqueous metal batteries (such as Zn–VOx, Zn–air, etc.).79,80,83 For example, in Zn–VOx batteries, similar modification layer designs can effectively suppress the dissolution of V; in Zn–air batteries, the modification layer can simultaneously optimize the kinetics of the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER). These applications demonstrate the broad applicability and scalability of the cathode-side modification layer strategy.
4.3 Full-separator modification
In this strategy, the full-separator substrate undergoes functional modification, with the modifier distributed throughout the separator. This approach focuses more on optimizing the overall battery performance rather than the modification of a single electrode. Full-separator modification can effectively regulate ion transport pathways, enhance electrolyte wettability, reduce internal resistance, and inhibit dendrite growth. Additionally, this method can balance local current density, thereby improving the battery's rate performance and long-term cycling stability. Full-separator modification mainly includes two forms: uniform modification of the full-separator and gradient modification of the full-separator. Due to the slow ion transport rate on the surface of the Zn anode, large concentration gradients are easily formed, leading to uneven Zn2+ flux distribution, especially under high current density conditions, which can easily trigger dendrite growth. To address this, Tan et al.105 proposed a simple and efficient separator engineering strategy—introducing an ion acceleration layer to regulate Zn2+ flux and achieve dendrite-free deposition (Fig. 7a and b). In this strategy, ZnHCF (Zn hexacyanoferrate complex) is used as a modifier. With its excellent Zn affinity and efficient ion diffusion channels, it can quickly capture Zn2+ and guide its uniform deposition. The ion acceleration layer can improve the conductivity of Zn2+, reduce the activation energy of the deposition process, and effectively regulate the Zn2+ concentration gradient, thereby inducing uniform distribution of Zn2+ (Fig. 7c and d). Under this optimized strategy, Zn‖Zn symmetric cells can stably cycle for 2700 hours at a current density of 2 mA cm−2, and 1770 hours at a high current density of 10 mA cm−2. Furthermore, the modified separator significantly improves the overall battery's cycle life and rate performance, fully verifying its advantages in practical applications. Future separator designs can draw from this concept to explore new materials with higher Zn affinity and fast ion transport capabilities (such as MOFs, Prussian blue materials, 2D nanomaterials, etc.) to further optimize ion migration. | ||
| Fig. 7 Design of the ZnHCF–GF separator. Schematic depiction of the Zn2+ deposition process using different separators: (a) GF and (b) ZnHCF–GF. COMSOL simulations illustrating (c) Zn2+ flux and (d) current density distribution on the Zn electrode with GF and ZnHCF–GF separators. Reproduced with permission.105 Copyright 2024, Wiley-VCH. (e) Structural representation of UiO-66 and its fundamental role in regulating Zn2+ ion coordination and migration. (f) Schematic diagram of AZBs assembled with commercial GF and UiO-66-GF separators. Reproduced with permission.106 Copyright 2022, Elsevier. Computational models evaluating the interaction between Zn2+ ions and (g) UiO-66/UiO-66-(COOH)2. (h) Binding energy comparison of Zn2+ ions with UiO-66 and UiO-66-(COOH)2. (i) Schematic representation of AZBs incorporating commercial GF and UC/GF separators. Reproduced with permission.107 Copyright 2024, Wiley-VCH. (j) Comparison between the Zn2+ ion size and the pore size of UiO-66-NH2. (k) and (l) Graphical depiction of hydrogen bonding interactions between H2O molecules and –NH2 groups in UiO-66-NH2 from different perspectives. (m) H2O adsorption model and (n) H2O–Zn2+ adsorption model for UiO-66-NH2. Reproduced with permission.108 Copyright 2023, Wiley-VCH. | ||
Song et al.106 proposed a Zr-based MOF functionalized separator (UiO-66-GF) (Fig. 7e). In this design, the UiO-66 MOF, through its confinement effect, uniformly distributes Zn2+ flux, enhances Zn2+ transport at the separator–anode interface, and effectively regulates the electric field distribution on the Zn anode surface (Fig. 7f). Additionally, UiO-66-GF induces Zn2+ deposition to preferentially grow along the (002) crystallographic plane, thereby inhibiting dendrite formation and side reactions. The (002) plane has a weak adsorption effect on H+, further reducing hydrogen evolution and corrosion side reactions, thus improving the battery's corrosion resistance. Ultimately, this strategy constructs an ultra-stable Zn anode and high-performance AZB, with symmetric cells exhibiting highly reversible electroplating/stripping behavior, a cycle life exceeding 1650 hours, and the full-cell cycling 1000 times at a current density of 1.0 A g−1 with a capacity retention rate of 85%, demonstrating excellent long-term cycling stability.
MOFs, as a type of porous and stable functional material, have significant potential in constructing ion-selective separators, particularly due to their tunable crystal structures, which make it easy to introduce various functional groups (such as –COOH, –NH2, etc.), enhancing their interaction with electrolyte components and improving separator ion conductivity and selectivity. For example, Yang et al.107 used carboxyl-functionalized Zr-based MOFs (UiO-66-(COOH)2, abbreviated as UC) to modify a separator and constructed a multifunctional UC/GF separator (Fig. 7g–i). This separator achieves dual regulation of both the anode and cathode in AZBs: on the one hand, the Zn- and water-affinity properties of the carboxyl group can effectively adsorb Zn2+, promote Zn2+ migration, and accelerate the desolvation process of hydrated Zn2+, improving the uniformity of Zn2+ flow and reducing corrosion and HER; on the other hand, the electrostatic repulsion between carboxyl groups and polyiodides can effectively inhibit the shuttle effect, reducing the loss of cathode active material and severe Zn corrosion (Fig. 7g–i).
Ma et al.108 further investigated the modification effect of amino (–NH2)-functionalized Zr-based MOFs (UiO-66-NH2) on the separator and incorporated it into a cellulose-based separator. The study found that the amino group not only exhibits good Zn affinity but also forms strong interactions with H2O molecules through hydrogen bonds, allowing the subnano-sized channels inside UiO-66-NH2 to act as a desolvation sieve, increasing the Zn2+ migration rate and optimizing its distribution (Fig. 7j–n). Even with an ultra-thin separator of only 20 μm, the modified separator still significantly enhances the reversibility of Zn electrochemistry and effectively suppresses HER induced by water.
The uniform full-separator modification strategy can effectively enhance the cycle life and rate performance of AZBs by regulating Zn2+ transport, optimizing deposition morphology, and reducing side reactions. In the future, functionalized separators based on MOFs, Prussian blue materials, and other efficient ion-transport materials still have vast research potential and could provide new ideas and solutions for the development of AZBs.
Tian et al.109 proposed an ion gradient separator for stabilizing the dual-electrode structure of AZBs (Fig. 8a). Compared to traditional GF separators, this gradient separator forms a low Zn2+ concentration region near the cathode and a high Zn2+ concentration region near the anode, thus optimizing overall battery performance. In the low Zn2+ concentration region, the NH4V4O10 (NVO) model cathode maintains good electrolyte wettability, ensuring smooth ion transport. Meanwhile, N-methyl pyrrolidone (NMP), diffusing from the high Zn2+ concentration region to the cathode, can regulate the solvent structure of the electrolyte, promoting the formation of a stable cathode–electrolyte interface (CEI) rich in N/ZnS/ZnF2, which effectively inhibits the dissolution of vanadium(V) (Fig. 8b and c). Additionally, the presence of the high Zn2+ concentration region synergistically interacts with the solid electrolyte interface (SEI) formed at the anode, significantly suppressing Zn dendrite growth and side reactions. Importantly, this high Zn2+ concentration region also effectively prevents vanadium ion diffusion toward the anode, thus improving the reversibility of Zn plating/stripping (Fig. 8d and e).
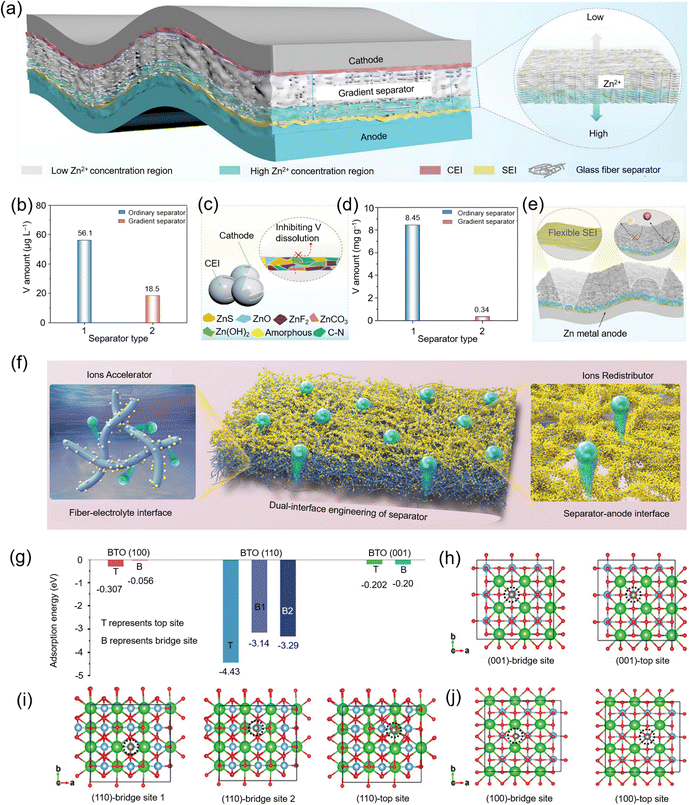 | ||
| Fig. 8 (a) Schematic representation of a Zn metal battery incorporating a gradient separator. (b) Analysis of vanadium(V) content in the electrolyte after 100 cycles. (c) Diagram illustrating the role of the gradient separator on the cathode. (d) V concentration on the surface of the anode. (e) Mechanistic illustration of the interface chemistry between the gradient separator and the Zn metal anode. Reproduced with permission.109 Copyright 2024, Wiley-VCH. (f) Schematic diagram showcasing dual-interface engineering in separators. (g) Binding energy between a Zn atom and various adsorption sites of BTO. (h) Top view of the geometric configuration for Zn atom adsorption on the BTO(001) plane, (i) BTO(110) plane, and (j) BTO(100) plane. Reproduced with permission.110 Copyright 2022, Wiley-VCH. | ||
On the other hand, Liang et al.110 introduced BaTiO3 (BTO) nanoparticles into a GF separator, utilizing their excellent Zn affinity and spontaneous polarization effect to regulate Zn2+ transport behavior (Fig. 8f). The BTO nanoparticles exhibit a gradient distribution within the GF separator—anchoring to the fiber surface within the separator to enhance Zn2+ transport efficiency at the electrolyte-fiber interface; and near the Zn anode, the BTO nanoparticles not only anchor to the fibers but also fill the fiber gaps, regulating the uniform distribution of Zn2+ at the separator–Zn anode interface, reducing local current density nonuniformity. Moreover, studies show that the BTO (110) crystallographic plane has the strongest adsorption ability for Zn atoms (Fig. 8g and j), superior to many known Zn-affinity materials, further verifying the excellent performance of BTO particles in capturing Zn2+.
Different separator modification strategies are suitable for different types of AZBs. By rationally designing the types and distribution positions of modification layers, it is possible to effectively optimize the electrolyte environment, balance ions flux, and stabilize electrode interfaces, thereby significantly enhancing the battery's cycle life and rate performance. These research findings provide important theoretical support and technical guidance for the development of high-performance AZBs and are expected to drive the commercialization of next-generation energy storage devices.
The reported effects of modified GF separators are summarized in Table 1. These separators have demonstrated excellent capacity retention and enhanced cycling stability across a range of AZBs. The incorporation of functional layers onto GF separators effectively regulates Zn2+ flux, suppresses dendrite growth, and promotes uniform Zn deposition. In many cases, the modified GF structure also contributes to mitigating parasitic reactions, such as hydrogen evolution, thereby improving Coulombic efficiency and extending battery lifespan. While current studies primarily explore a limited set of modification strategies and functional materials, the promising performance outcomes highlight the substantial potential of this approach. These strategies typically involve physical coating, chemical grafting, or in situ formation of inorganic or polymeric layers, each contributing specific benefits such as enhanced ionic conductivity, mechanical robustness, or interfacial stability.
| Separator | Working mechanisms | Electrolyte Zn‖Zn performance | Full cells performance |
|---|---|---|---|
| Ref. | |||
| HNT@GF94 | • Enhancement of mechanical strength | 2 M ZnSO4 (+0.1 M MnSO4) | Zn‖MnO2 |
| • Homogenization of pore size distribution | 3000 h (1 mA cm−2; 1 mAh cm−2) | 1000 cycles | |
| • Optimization of Zn2+ flux | 2000 h (5 mA cm−2; 1 mAh cm−2) | 2 A g−1; 93.4% | |
| VG@GF95 | • Optimization of electric field distribution | 2 M ZnSO4 | Zn‖AC |
| • Reduction of local current density | 300 h (0.5 mA cm−2; 0.5 mAh cm−2) | 5000 cycles | |
| • Uniform Zn2+ flux distribution | 600 h (10 mA cm−2; 1 mAh cm−2) | 5 A g−1; 93% | |
| Ti3C2Tx MXene @GF96 | • Built-in electric field | 2 M ZnSO4 | Zn‖KVOH |
| • Good electrolyte wettability | 1180 h (1 mA cm−2; 1 mAh cm−2) | 1000 cycles | |
| • Homogenization of local current | 250 h (5 mA cm−2; 5 mAh cm−2) | 5 A g−1; 77.9% | |
| Gra-CeF3@GF111 | • Tailored Zn2+ flux | 2 M ZnSO4 | Zn‖V2O5 |
| • Suppressed SO42− transport | 2500 h (1 mA cm−2; 1 mAh cm−2) | 2000 cycles | |
| • Suppression of side reactions | 1000 h (5 mA cm−2; 5 mAh cm−2) | 2 A g−1; — | |
| Co-HNMXS@GF101 | • Inhibition of polyiodide shuttling | Catholyte (0.1 M I2 + 1 M LiI) anolyte (2 M ZnSO4) | Zn‖I2 |
| • Promotion of iodine conversion | 2900 h (1 mA cm−2; 1 mAh cm−2) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 2 A g−1; 80% 000 cycles 2 A g−1; 80% |
|
| ZnMn-NC@GF102 | • Efficient polyiodide adsorption | 2 M ZnSO4 | Zn‖I2 |
| • Accelerated intermediate conversion | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
||
| 5 A g−1; 95% | |||
| SCNT@GF112 | • Effective polyiodide anchoring | 1 M ZnSO4 | Zn‖I2 |
| • Catalysis of iodine redox kinetics | 2500 h (1 mA cm−2; 1 mAh cm−2) | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
|
| 5 A g−1; 76% | |||
| GO@GF113 | • Inhibit dendrite growth | 3 M Zn (CF3SO3)2 | Zn‖PAVO |
| 480 h (1 mA cm−2; 0. 5 mAh cm−2) | 4800 cycles | ||
| 5 A g−1; 74% | |||
| SR-P@GF114 | • Enhanced Zn ion transport | 2 M ZnSO4 (+0.2 M MnSO4) | Zn‖MnO2 |
| • Promotion of uniform Zn deposition | 500 h (1 mA cm−2; 1 mAh cm−2) | 1000 cycles | |
| • Assistance in desolvation of Zn2+ | 1 A g−1; 73.9% | ||
| PANI@GF93 | • Weakens zincophilicity | 2 M ZnSO4 | Zn‖MnO2 |
| • Homogenization of surface electric field | 3000 h (1 mA cm−2; 1 mAh cm−2) | 300 cycles | |
| • Promotion of uniform Zn Ion stripping | 0.5 A g−1; 89.5% | ||
| ZnHCF@GF105 | • Fast Zn2+ trapping | 2 M ZnSO4 | Zn||AC |
| • Quick Zn2+ delivery | 2700 h (2 mA cm−2; 1 mAh cm−2) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 1 A g−1; 95.8% 000, 1 A g−1; 95.8% |
|
| 1750 h (10 mA cm−2; 2 mAh cm−2) | |||
| UiO-66@GF106 | • Enhanced charge carrier transport | 2 M ZnSO4 (+0.1 M MnSO4) | Zn‖MnO2 |
| • Improved corrosion resistance | 1650 h (2 mA cm−2; 1 mAh cm−2) | 1000 cycles | |
| • Promotion of dendrite-free | 1 A g−1; 85% | ||
| UiO-66-(COOH)2@GF107 | • Facilitated Zn2+ transport | 2 M ZnSO4 | Zn‖I2 |
| • Accelerated desolvation of Zn2+ | 3400 h (5 mA cm−2; 1 mAh cm−2) | 3600 cycles | |
| • Suppressed polyiodide transfer | 1 C; 72.3% | ||
| UiO-66-NH2@GF108 | • Zn2+ desolvation sieves | 2 M ZnSO4 (+0.2 M MnSO4) | Zn‖MnO2 |
| • Facilitated Zn2+ migration | 2000 h (2 mA cm−2; 2 mAh cm−2) | 1000 cycles | |
| • Uniform distribution of Zn2+ | 1 A g−1; 95.1% | ||
| Gra@GF109 | • Inhibition of dendrite growth | 1 M Zn (OTf)2 | Zn‖NVO |
| • Prevention of V ion diffusion | 4800 h (3 mA cm−2; 1 mAh cm−2) | 800 cycles | |
| 1 A g−1; 88.1% | |||
| BTO@GF110 | • Accelerated Zn2+ transport | 2 M ZnSO4 (+0.2 M MnSO4) | Zn‖MnO2 |
| • Homogenization of ion transport | 1600 h (10 mA cm−2; 2.5 mAh cm−2) | 1800 cycles | |
| 1 A g−1; — | |||
| GFNs-PVDF@GF115 | • Homogenization of Zn2+ transport | 2 M ZnSO4 | Zn‖MnO2 |
| • Suppression of SO42− flux | 1800 h (1 mA cm−2; 1 mAh cm−2) | 100 cycles | |
| • Inhibition of Zn dendrite | 0.2 A g−1; 90% | ||
| NDs@GF116 | • Regulation of separator pore structure | 2 M ZnSO4 (+0.2 M MnSO4) | Zn‖MnO2 |
| • Control of Zn2+ transport properties | 1800 h (5 mA cm−2; 1 mAh cm−2) | 1000 cycles | |
| 1 A g−1; — | |||
| Cu(II)/PAD@GF117 | • Improved water absorption capability | 2 M ZnSO4 | Zn‖V2O5 |
| • Optimized ion transport pathways | 1800 h (1 mA cm−2; 1 mAh cm−2) | 1000 cycles | |
| 2 A g−1; — | |||
| SA@GF118 | • Lower Zn2+ desolvation barrier | 2 M ZnSO4 | Zn‖MnO2 |
| • No concentration polarization | 1230 h (1 mA cm−2; 1 mAh cm−2) | 500 cycles | |
| • Enhanced Zn2+ transport efficiency | 1 A g−1; 70.4% | ||
| SnF2@GF119 | • Homogenization of Zn2+ flux | 2 M ZnSO4 | Zn‖MnO2 |
| • Suppression of byproduct formation | 200 cycles | ||
| 1400 h (1 mA cm−2; 1 mAh cm−2) | 1 A g−1; 80% | ||
| 1000 h (5 mA cm−2; 1 mAh cm−2) | |||
| ZLB@GF120 | • Uniform electric field | 2 M ZnSO4 | Zn‖MnO2 |
| • Uniform Zn2+ deposition | 3200 h (10 mA cm−2; 10 mAh cm−2) | 2000 cycles | |
| 0.5 A g−1; 76.7% | |||
To support the practical deployment of these strategies, a deeper understanding of their long-term performance and electrode compatibility is essential. In this context, we provide a critical evaluation of key considerations for functional layer coatings and asymmetric separator designs: (a) chemical and electrochemical stability under cycling. Functional layers (e.g., metal oxides, polymers, or 2D materials) and asymmetric separators often show promising initial performance but may undergo structural degradation or chemical transformation over prolonged cycling. For instance:
Zincophilic or zincophobic coatings may lose functionality due to side reactions with Zn2+ or electrolyte decomposition products. Polymeric coatings (e.g., PEO, PTFE) may swell, dissolve, or degrade in mildly acidic/neutral electrolytes, especially over hundreds of cycles. Metal oxide layers (e.g., TiO2, Al2O3) can experience cracking or delamination under repeated volume expansion/contraction at the Zn anode. We emphasize the need for accelerated aging tests, such as high-rate or high-temperature cycling, to simulate realistic degradation pathways and better assess durability. (b) Interfacial compatibility with both electrodes. While asymmetric separators are tailored to optimize interfacial behavior on both the anode and cathode sides, mismatched chemical environments pose risks. On the anode side, functional layers must accommodate Zn plating/stripping dynamics without impeding ion transport or triggering dendrite growth. On the cathode side, coatings must tolerate oxidative environments and avoid dissolution or passivation effects that would impede redox kinetics. Importantly, some materials (e.g., redox-active interlayers, or organic functional groups) can unintentionally catalyze parasitic reactions or shuttle effects if not properly confined or stabilized. (c) Mechanical and dimensional stability. Separator coatings are also subjected to physical stresses such as swelling/deswelling and electrode volume changes, all of which can lead to mechanical failure or interfacial delamination. Strategies like polymer crosslinking, gradient interface design, and in situ polymerization are increasingly applied to enhance mechanical integrity, though their long-term reliability requires further validation. (d) Scalability and manufacturability.
Lastly, while many interfacial designs are effective at the laboratory scale, their practical application may be constrained by synthesis complexity, cost, or scalability. For example, solution casting of ultrathin layers can suffer from reproducibility issues and limited throughput. To facilitate real-world implementation, scalable manufacturing methods such as spray-coating, roll-to-roll lamination, and electrospinning should be prioritized. These considerations underscore the importance of integrating materials design with durability testing and scalable processing to bridge the gap between laboratory demonstrations and commercial viability.
It is important to note that while separator and electrode coating technologies often employ similar materials—such as metal–organic frameworks (MOFs), carbon nanotubes (CNTs), MXene, conductive polymers, and transition metal compounds—their functional roles and operating environments are fundamentally different. Both strategies are essential for advancing high-performance AZBs, but they involve distinct design principles and mechanisms of action.
Despite their differences, these coatings share a common goal: to stabilize the electrochemical environment and enhance overall battery performance. They contribute to suppressing Zn dendrite growth, promoting uniform Zn2+ distribution and deposition, and reducing parasitic side reactions such as hydrogen evolution and corrosion. These enhancements are particularly vital in aqueous systems, where challenges like water decomposition and metal dissolution can severely impact cycle life and Coulombic efficiency.
Separator coatings are applied to the surface of commercial or custom-designed separators—typically porous polymeric or glass fiber membranes—that serve as physical barriers between the anode and cathode. The coating layer is designed to perform several important functions beyond the general roles mentioned above. First and foremost, separator coatings regulate ion transport by modulating the local electric field and controlling Zn2+ flux. This helps to avoid the formation of high-concentration gradients that can lead to uneven plating and dendrite nucleation. Secondly, separator coatings play a critical role in inhibiting shuttle effects. For example, in Zn–iodine or Zn–organic batteries, soluble redox-active species such as polyiodides or small organic molecules can migrate between the electrodes, causing capacity loss and self-discharge. Coatings with adsorption capabilities—such as those based on MOFs, metal oxides, or nitrogen-doped carbon—can trap these shuttle species and reduce crossover, thereby improving cycling stability. Thirdly, separator coatings reinforce the mechanical integrity and chemical stability of the separator itself. In aggressive electrochemical environments, uncoated separators may degrade, lose dimensional stability, or become perforated by Zn dendrites. Functional coatings can serve as protective barriers, increasing the separator's resistance to chemical attack and enhancing its ability to block dendrite penetration.
On the other hand, electrode coatings are typically applied directly to the surface of the Zn anode (or, to the cathode) and are designed to interface directly with the electrolyte during charge–discharge cycles. These coatings often focus on improving electronic conductivity, particularly in cases where the Zn surface becomes passivated by insulating by-products. Conductive materials such as CNTs, graphene, or MXene can help to establish an electronically conductive network across the anode surface, ensuring uniform current distribution and reducing localized current hotspots that accelerate dendrite growth. Moreover, electrode coatings help to guide the nucleation and growth of Zn during plating. By engineering a surface with appropriate zincophilicity—using materials like ZnO, Sn, or organic functional groups—it is possible to lower the nucleation barrier and achieve more uniform Zn deposition. This reduces the likelihood of uncontrolled dendrite formation and promotes a dense Zn layert.
Electrode coatings can also act as artificial solid–electrolyte interfaces (SEIs), especially in mildly acidic or neutral aqueous electrolytes where traditional SEI layers are either unstable or nonexistent. These engineered interfaces serve to regulate interfacial ion transport, suppress side reactions such as hydrogen evolution, and prolong the lifetime of the Zn anode. Compared to separator coatings, electrode coatings face harsher chemical and electrochemical conditions, including direct exposure to redox reactions and fluctuating pH levels at the electrode surface.
While the materials used in separator and electrode coatings often overlap, their performance metrics and evaluation methods are quite different. Separator coatings are usually assessed based on ionic conductivity, transference number, permeability to shuttle species, and dendrite-blocking ability. Electrode coatings, on the other hand, are evaluated based on their ability to reduce overpotential, lower the nucleation voltage, improve Coulombic efficiency, and extend cycle life.
In conclusion, both separator and electrode coatings are essential tools in overcoming the inherent challenges of AZBs. While they may employ similar materials, their functional purposes, physical locations in the cell, and operational demands differ markedly. Separator coatings primarily modulate ion transport and protect the separator from chemical/mechanical failure, while electrode coatings directly stabilize the anode interface and control the dynamics of Zn deposition. For next-generation AZBs, a synergistic design that integrates both coating strategies could offer a comprehensive solution to long-standing issues such as dendrite formation, low efficiency, and limited lifespan.
5. Novel separators
In addition to the previously mentioned materials, in recent years, several novel separator materials have been researched and applied. These separators not only focus on the optimization of a single electrode but also emphasize the synergistic regulation of both the anode and cathode performance, thereby significantly improving the full cells cycle stability. Here, a “Novel Separator” refers to separators that are synthesized from scratch using new or hybrid materials without relying on commercial substrates, often involving new membrane formation processes such as filtration, casting, or in situ polymerization.5.1 Polymer-based separators
Polymer materials show great potential for use in separators for AZBs due to their excellent mechanical strength, flexibility, and chemical stability. They can effectively separate the anode and cathode, preventing Zn dendrites from piercing through and causing short circuits. In addition, by adjusting their structure and functional groups, polymer materials can help guide the movement of Zn2+, suppress dendrite growth, and reduce side reactions, thereby improving the battery's cycling stability and safety. Polymers are also easy to process and can be combined with inorganic fillers or conductive polymers to create multifunctional composite separators. Moreover, polymer materials are made from widely available raw materials, have mature manufacturing processes, and are relatively low in cost, making them suitable for large-scale production and commercial applications. As a result, they have become a key focus in the development of high-performance separators for AZBs.Yuan et al.121 developed a simple dip-coating method to uniformly composite a polycationic polymer composed of poly(diallyl dimethyl ammonium) (PDDA) cations and bis(trifluoromethanesulfonyl)imide (TFSI) anions onto a GF separator (Fig. 9a and b). The PDDA cations strongly adsorb polyiodide anions, effectively inhibiting the shuttle effect of polyiodides (Fig. 9c). Moreover, the hydrophobicity of the PT@GF separator allows it to closely contact the Zn anode, forming a water-deficient electrical double-layer (EDL) interface, reducing corrosion of Zn and suppressing HER (Fig. 9d). Simultaneously, the polycation evenly regulates the electric field distribution at the anode surface, inhibiting the tip effect and effectively suppressing the growth of Zn dendrites (Fig. 9e). Thanks to this optimized design, batteries using the PT@GF separator exhibit excellent long-cycle stability.
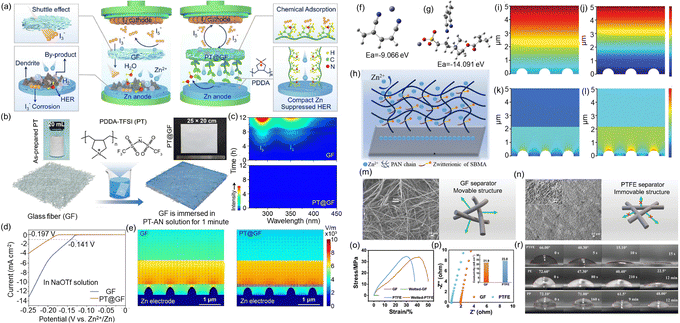 | ||
| Fig. 9 (a) Schematic representation of Zn–I2 batteries assembled with commercial GF and PT@GF separators. (b) Diagram depicting the preparation process of the PT@GF separator, with inset images showing the as-prepared PT powder (left) and the large-scale PT@GF separator (right). (c) UV-Vis spectra of 1 mM I3− solutions with GF and PT@GF soaking at various time intervals. (d) LSV curves for Zn‖Ti cells using GF and PT@GF separators with NaOTf aqueous solution. (e) Simulations illustrating the electric field distribution on Zn electrodes with GF and PT@GF separators during Zn2+ plating. Reproduced with permission.121 Copyright 2025, Elsevier. (f) and (g) DFT models showing Zn2+ binding with PAN and PAN@SBMA. (h) Schematic of Zn2+ plating behavior through the PAN@SBMA separator. (i) and (j) Zn2+ concentration distribution in cells with PAN@SBMA and PAN separators. (k) and (l) Electric field distribution in cells with PAN@SBMA and PAN separators. Reproduced with permission.122 Copyright 2024, Wiley-VCH. (m) and (n) SEM images and schematic structures of GF and PTFE separators. (o) Stress–strain curves of GF and PTFE. (p) Ionic conductivity of GF and PTFE separators. (r) Contact angles of PTFE, PE, and PP separators. Reproduced with permission.123 Copyright 2024, Wiley-VCH. | ||
Huang et al.122 designed a modified PAN (polyacrylonitrile) nanofiber separator using electrospinning technology, with PAN and a zwitterionic surfactant (sulfobetaine methacrylate, SBMA) as the starting materials (Fig. 9f–h). The SBMA, which contains sulfonic acid groups (–SO3−), can change the physical properties of the precursor solution and influence the electric field during the electrospinning process. Moreover, it helps regulate the distribution of Zn2+ by reducing concentration polarization caused by polar groups on the Zn surface (Fig. 9i–l). This leads to a more uniform electric field and smoother ion movement. This work presents a simple and effective approach to designing a PAN-based functional separator with a high ion transference number, which can guide Zn2+ deposition and improve the performance of AZBs. Yang et al.123 demonstrated that a commercially available hydrophilic polytetrafluoroethylene (PTFE) membrane can be used as a separator to significantly extend the cycling life of Zn anodes. Compared with traditional brittle GF separators, the wetted PTFE membrane shows higher mechanical strength (a stress of 34.3 MPa at 41.4% strain) (Fig. 9o) and good hydrophilicity (Fig. 9r), both of which help suppress the growth of Zn dendrites. It was found that the uniform and robust porous structure of the membrane promotes even Zn2+ distribution and achieves a high Zn2+ transference number (0.81), which ensures stable and reversible Zn plating/stripping.
5.2 COF-based separators
The framework of covalent organic frameworks (COFs) is built from various organic linkers. COFs combine porosity with structural precision, multifunctionality, and tunability, making them an excellent platform for designing materials with targeted properties. COFs uniquely combine high porosity with structural precision, versatility, and tunability, making them an excellent platform for designing controllable materials with targeted properties.To simultaneously address critical issues like Zn dendrite growth and electrolyte-cathode interface instability, Xu et al.124 proposed a quasi-single-ion conductive separator based on COFs. This separator uses Zn2+-substituted COF-SO3Zn0.5 (referred to as COF-Zn), where the abundant sulfonate groups provide efficient Zn2+ conductivity (Fig. 10a). The researchers employed bacterial cellulose (BC) as a binder, physically mixing it with COF-Zn and preparing a thin and flexible separator via a simple filtration process. This separator not only exhibits quasi-single-ion conductivity but also selectively promotes Zn2+ migration, effectively suppressing dendrite growth. The COF-Zn separator, only 25 μm thick, combined with BC's good hydrophilicity, allows the battery to operate stably with as little as 20 μL mg−1 of electrolyte, reducing cathode dissolution and improving energy density. Experiments showed that the Zn‖Zn symmetrical batteries with COF-Zn separators cycled for over 2900 hours at 0.2 mA cm−2, demonstrating extremely high stability (Fig. 10b).
 | ||
| Fig. 10 (a) Diagram showing small-molecule-based Zn‖PTO batteries with different separators. (b) Cycling performance comparison for COF-Zn and GF separators. Reproduced with permission.124 Copyright 2025, Wiley-VCH. | ||
5.3 Nafion-based separators
Nafion is a cation-exchange membrane known for its excellent ion selectivity, thanks to its sulfonic acid groups. It is widely used as a separator in flow batteries and, due to its flexibility and good electrochemical properties, is also commonly employed as a solid-state electrolyte. Ghosh et al.125 prepared a Zn2+-doped Nafion ionomer membrane (3 M-Nafion) via an electrochemical deposition method (Fig. 11a). In this membrane, Zn2+ coordinate with the –SO3− groups, enhancing both the Zn2+ transference number and the overall ionic conductivity. Additionally, the 3 M-Nafion membrane exhibits lower activation energy, smaller Zn plating/stripping voltage gaps, more uniform Zn2+ deposition, and effective suppression of dendrite growth.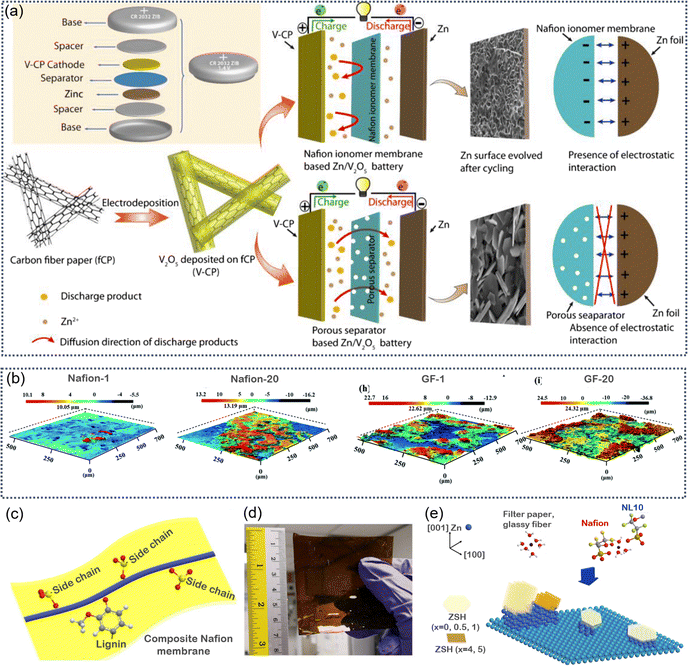 | ||
| Fig. 11 (a) Diagram showing different Zn/V2O5 cell setups with Zn2+-integrated Nafion ionomer and a regular porous membrane separator. Reproduced with permission.125 Copyright 2019, Wiley-VCH. (b) 3D surface images of Zn–Nafion and GF separators after the 1st cycle and 20th cycle. Reproduced with permission.126 Copyright 2021, Royal Society of Chemistry. (c) Design diagram of composite biomass waste lignin@Nafion membranes. (d) Photo of a 7 × 7 cm lignin@Nafion membrane. (e) Illustration showing how the membranes affect the Zn metal surface. The interaction between SO3− groups in Nafion and Zn2+ changes Zn2+ coordination from its form in the electrolyte, leading to different growth patterns of Zn deposits and ZHS byproducts. Reproduced with permission.127 Copyright 2019, Wiley-VCH. | ||
Notably, Nafion membranes not only regulate the Zn plating/stripping behavior at the anode but also significantly influence the interaction between certain cathode materials and the electrolyte. Wu et al.126 developed a Zn–Nafion composite membrane by exchanging Na+ with Zn2+. This membrane helps create a uniform electric field at the Zn anode and reduces concentration gradients in the electrolyte, effectively suppressing dendrite formation (Fig. 11b). Moreover, the strong affinity between the Zn–Nafion membrane and the cathode material enhances proton (H+) participation during H+/Zn2+ co-insertion reactions while minimizing side reactions. The membrane also facilitates the transformation of the by-product Zn4SO4(OH)4·nH2O into a dense solid protective layer, preventing the dissolution of cathode active materials. As a result, the battery shows improved energy density and capacity retention. After 150 cycles, the battery using the Zn–Nafion membrane retains 85.7% of its capacity, compared to just 44.8% with a conventional separator. Importantly, the Zn–Nafion membrane is also reusable, making it economically advantageous.
To further enhance membrane performance, some researchers have introduced multifunctional groups into the Nafion structure to increase ion transport pathways and add new functionalities such as improved wettability and stronger Zn2+ affinity. Yuan et al.127 fabricated a lignin@Nafion composite membrane by integrating lignin into Nafion (Fig. 11c and d). Rich in –SO3− groups, the composite membrane strongly coordinates with Zn2+, reconstructing ion transport channels and guiding Zn2+ to deposit preferentially on the (002) crystal plane. This suppresses the vertical growth of Zn sulfate by-products (ZHS), thereby preventing short circuits. The presence of lignin also increases ion transport, improves conductivity, reduces cost, and promotes lateral distribution of by-products along the electrode surface while favoring Zn deposition on the (100) crystal plane (Fig. 11e). Under the same conditions, batteries with lignin@Nafion separators deliver 1.2 times the capacity of conventional batteries.
In summary, Nafion membranes help achieve uniform Zn2+ transport, regulate electrolyte pH, and prevent the dissolution of cathode materials, thereby improving battery cycling stability. However, the high cost and limited economic efficiency of Nafion remain barriers to its widespread commercialization. Future research should focus on improving sustainability and reducing costs to support its broader application in energy storage systems.
5.4 Cellulose-based separators
Cellulose-based separators have shown great potential in the modification of AZBs, as demonstrated by their widespread application across various battery systems. In recent years, significant progress has been made in using cellulose-related materials to expand the application scope of AZBs. As a polymer composed of repeating units, cellulose offers several outstanding properties, including environmental friendliness, recyclability, low cost for large-scale production, strong resistance to swelling, and the ability to introduce functional groups through molecular design. These advantages have made cellulose-based separators a subject of growing interest and extensive research in the field of AZBs.Yang et al.128 developed a multifunctional separator composed of nanocellulose coated with in situ hydrolyzed Zr4+ species (Zr-CNF). Due to the crosslinking effect of Zr4+ and its ability to disrupt hydrogen bonding in cellulose through hydrolysis, the resulting separator exhibited excellent anti-swelling properties and stable porous structure. The Zr-CNF material was synthesized using a straightforward ion-coordination and hydrolysis-precipitation method (Fig. 12a).
 | ||
| Fig. 12 (a) Diagram showing how the Zr-CNF separator is made. (b) Cycling performance of different Zn‖Zn symmetric cells at 1 mA cm−2 and 1 mAh cm−2. (c) Voltage curves of Zn‖Cu half cells with Zr-CNF separator at 2 mA cm−2 and 2 mAh cm−2. Reproduced with permission.128 Copyright 2023, Wiley-VCH. (d) Process for making FACNF separators. (e) Photos of FACNF separators in different states: curling, folding, kneading, and recovering. Reproduced with permission.129 Copyright 2024, Wiley-VCH. (f) Illustration of how the plasma membrane controls selective ion flow. (g) Illustration showing how the BC/V separator controls ion and solvent transport in water-based electrolytes. (h) Coulombic efficiency of Zn‖Cu asymmetric cells at 1 mA cm−2 and 1 mAh cm−2. (i) Cycling stability of Zn|Zn cells at 2.926 mA cm−2 and 2.926 mAh cm−2 (50% depth of discharge). Reproduced with permission.130 Copyright 2025, Elsevier. | ||
During battery operation, the separator maintained uniform Zn2+ ion flux, high ionic conductivity, and a favorable Zn2+ transference number. In addition, the amorphous Zr–O coating on the surface induced a uniform directional electric field around the separator via Maxwell–Wagner polarization. This promoted faster Zn2+ ion transport, reduced the nucleation overpotential, and facilitated uniform Zn deposition. As a result, the anti-swelling Zr-CNF separator effectively stabilized the Zn anode. The battery using this separator demonstrated a long cycle life of over 1600 hours with a stable and flat voltage plateau, indicating excellent Zn plating/stripping performance (Fig. 12b). Moreover, CE tests revealed that the cell with a traditional TCNF separator short-circuited after only 250 cycles, while the cell with the Zr-CNF separator maintained stable cycling for over 450 cycles with an impressive average CE of 99.7% (Fig. 12c).
Yang et al.129 developed a low-cost and multifunctional fly ash/cellulose composite separator (FACNF) using a simple solution-casting method. Fig. 12d schematically illustrates the fabrication process of the cost-effective FACNF separator. Recycled fly ash microspheres and cellulose nanofiber dispersion were mixed in deionized water, cast onto a glass substrate, and then dehydrated overnight. As shown in the digital photograph (Fig. 12d), the resulting FACNF separator has a large area of 22 cm × 14 cm. The prepared separator was found to promote uniform Zn deposition along the (002) crystal plane, enabling long-term cycling stability of the Zn anode. Experimental results showed that the addition of fly ash improved ionic conductivity (5.76 mS cm−1), lowered the energy barrier for hydrated Zn2+ desolvation, and suppressed hydrogen evolution and surface corrosion on the Zn anode. Moreover, theoretical calculations revealed that, compared to Zn(100) and Zn(101) crystal planes, fly ash particles exhibit a lower chemical binding energy with the Zn(002) surface. This preferential interaction promotes the deposition of Zn along the (002) orientation, thereby effectively mitigating the formation and growth of Zn dendrites.
Li et al.130 developed a lightweight and mechanically strong separator made from bacterial cellulose and vermiculite. As shown in Fig. 12e and f, this highly flexible membrane helps selective ion transport through enzyme-controlled ion channels. The separator works through a combined effect, improving the selective movement of Zn2+ and speeding up desolvation, while trapping active water molecules inside its structure. These features help Zn2+ spread evenly and create a stable, low-water environment at the electrode surface. Because of this, the separator allows the Zn anode to last over 7000 hours (3500 cycles) with an average Coulombic efficiency of 99.77% (Fig. 12g). In Zn‖Zn cells with 50% depth of discharge, the BC/V separator kept stable cycling for more than 1100 hours (550 cycles), maintaining a nucleation overpotential below 10 mV (Fig. 12h).
6. Large-scale separator production
Given the application of AZBs in grid-scale energy storage, the development of scalable and efficient separator fabrication methods is of critical importance. To address this, Wang et al.131 designed a dendrite-resistant separator using a simple and scalable hot-pressing strategy (Fig. 13a). In this approach, a mixture of metal–organic framework (MOF) precursors—including metal salts, organic ligands, and a fluxing agent—is uniformly distributed onto a nonwoven fiber matrix, followed by a 30-minute hot-pressing step. During this process, MOF crystals form in situ via coordination reactions, coating the fiber surface uniformly. These MOF structures provide abundant nitrogen-containing functional groups and a high specific surface area (190.8 m2 g−1), which enhance Zn2+ adsorption and facilitate efficient ion transport. The resulting hot-pressed separator (HTS) demonstrates both excellent electrochemical performance and strong potential for large-scale manufacturing. To further validate its scalability, a wide-area HTS membrane (32 cm × 28 cm) was successfully fabricated using this one-step process, highlighting its promise for practical grid-scale applications. | ||
| Fig. 13 (a) Fabrication process of HTS using a one-step hot-press strategy, resulting in a Zn-based HTS product. Reproduced with permission.131 Copyright 2025, Springer Nature. (b) Schematic representation of the preparation method for the PP-g-AA separator. Reproduced with permission.133 Copyright 2024, Wiley-VCH. | ||
Zhu et al.132 developed a hydrophilic, radiation-grafted polypropylene (PP) separator with a thickness of only 25 μm, suitable for large-scale production at rates reaching millions of square meters per day. As shown in Fig. 13b, the fabrication process begins with electron beam (E-beam) pre-irradiation of a porous PP separator, generating peroxide (˙OOH) and hydroxyl (˙OH) radicals. The activated PP matrix is then immersed in an acrylic acid (AA) monomer solution of varying concentrations, initiating a grafting reaction to form a polyacrylic acid-modified separator. Subsequent ultrasonication removes unreacted monomers and homopolymers. The resulting PP-g-AA membrane features a dual-domain structure: a hydrophobic PP backbone that effectively resists water intrusion and suppresses parasitic reactions, and hydrophilic polyacrylic acid grafts that act as dynamic Zn2+ transport pathways. This architecture enables precise modulation of interfacial electric fields and Zn2+ concentration gradients, facilitating uniform Zn2+ flux and dendrite-free Zn deposition.
7. Summary and outlook
AZBs have emerged as promising candidates for grid-scale energy storage because of their safety, cost-effectiveness, and high theoretical capacity. However, challenges such as Zn dendrite, HER, anode corrosion, and cathode material dissolution limit their widespread use. The separator plays a key role in regulating ion transport, reducing side reactions, and improving Zn deposition uniformity. Recent advancements in separator materials and modification strategies show great potential in overcoming these challenges.This review systematically categorizes separator modifications based on their location—anode side, cathode side, and full-separator modifications—and discusses key strategies such as ion-selective layers, interfacial engineering, and composite functional membranes. These modifications have been shown to improve Zn2+ flux regulation, suppress dendrite growth, and enhance long-term cycling stability. Emerging materials like COFs, MOFs, and inorganic–organic hybrid separators have also been highlighted for their potential in optimizing battery performance.
The review provides a comprehensive analysis of the mechanisms underlying these modifications, offering theoretical insights and design principles for the development of next-generation AZB separators. It also emphasizes the importance of balancing various performance parameters, such as porosity, mechanical strength, and chemical stability, to achieve optimal separator performance.
However, existing separators still face challenges in ion selectivity, dendrite suppression, interface stability, and cost control, limiting the battery's lifespan and energy density. In the future, separator design should focus on thinness, high ion selectivity, enhanced interface stability, improved corrosion resistance, and low-cost scalable manufacturing to further enhance the overall performance of AZBs.
7.1 Key factors for future separator design
Future separator design should focus on several key factors to meet the demands of high-performance AZBs. First, ion selectivity and transport regulation are essential, requiring high Zn2+ conductivity while effectively blocking anions or undesired species such as polyiodides. Second, to mitigate dendrite formation, separators must help homogenize the electric field and ion flux. Third, chemical and electrochemical stability in mildly acidic or neutral electrolytes is crucial to prevent degradation and ensure long-term performance. Additionally, mechanical strength and flexibility are needed to withstand volume changes and mechanical stress during cycling. Finally, the materials and fabrication methods should offer scalability and cost-effectiveness to facilitate large-scale commercial application.7.2 Major practical challenges
Future separator development also faces several critical challenges. Ensuring compatibility with high-loading electrodes is essential, as many laboratory-scale separators struggle to maintain performance under practical conditions. Long-term durability is another concern, as functional coatings may degrade or delaminate during extended cycling. Additionally, the complexity of functionalization, such as multilayer or hybrid structures, can introduce manufacturing difficulties and reproducibility issues. Lastly, there are inherent trade-offs between key properties—for example, improving ion selectivity may reduce ionic conductivity—making it difficult to achieve an optimally balanced separator design.7.3 Lightweight design to increase energy density
The thickness of the separator directly affects the battery's volumetric energy density and ion transport resistance. Commercialized AZBs typically use GF or polyolefin separators, but these separators are relatively thick (>30 μm), which hinders Zn2+ transport and reduces the battery's energy density. Therefore, future efforts should focus on developing thinner separators with a thickness of less than 20 μm while ensuring mechanical strength and electrochemical stability. For instance, nanocellulose separators, ultra-thin composite-coated separators, or interface-enhanced layered structures could be employed to reduce thickness while maintaining high ion transport capabilities. Indeed, while reducing separator thickness is a proven strategy to enhance the gravimetric and volumetric energy density of AZBs, it introduces mechanical integrity, puncture resistance, and dimensional stability that must be carefully considered for practical applications, especially in large-format, flexible, or high-power systems.7.4 High ion selectivity to improve Zn2+ transport efficiency
Current separators exhibit poor selectivity between Zn2+ and other anions, leading to increased side reactions and shortened battery life. In the future, single-ion conductors or gradient ion distribution separators should be developed to regulate Zn2+ migration pathways through chemical modification. For example, separator materials based on COFs and MOFs scan provide highly ordered Zn2+ transport channels, improving ion screening ability. Additionally, a gradient Zn2+ concentration control strategy could be used to form a Zn2+-rich region at the anode and a Zn2+-deficient region at the cathode, optimizing Zn2+ deposition behavior and enhancing battery stability.7.5 Enhanced interface stability to suppress dendrite growth
The formation of Zn dendrites is one of the most significant issues during the operation of AZBs, leading to separator puncture, short circuits, and capacity degradation. Therefore, future separators should have the ability to regulate interfaces, stabilizing Zn2+ deposition and reducing dendrite growth. For example:• functional layer modification: coating the separator surface with oxides (ZnO, CuO), two-dimensional materials (MXene, g-C3N4), or polymer coatings can evenly distribute Zn2+ nucleation sites and improve the reversibility of plating/stripping.
• Asymmetric separator design: a functional layer that anchors Zn2+, such as BaTiO3 nanoparticles or nitrogen-containing functional groups, can be constructed on the side near the Zn anode, promoting uniform deposition and reducing the risk of local Zn2+ oversaturation.
7.6 Improved corrosion resistance and hydrogen evolution suppression
The HER takes place during the charging and discharging processes, resulting in the depletion of the electrolyte and the corrosion of the Zn anode. Future strategies could involve modifying the separator with amphiphilic molecules, fluorinated polymer coatings, or superhydrophobic/hydrophilic interface designs to optimize separator performance. For example:• hydrophilic–hydrophobic gradient separators: maintaining hydrophobicity near the Zn anode side to reduce water infiltration, while enhancing hydrophilicity at the electrode/electrolyte interface to promote uniform Zn2+ distribution.
7.7 Low-cost and large-scale manufacturing
The commercialization of future AZBs requires separators with low cost and scalable manufacturing capabilities. For example:• Spray-curing technology: this method can quickly fabricate large-area functionalized separators, improving production efficiency.
• Radiation-grafted polymerization: high-selectivity functional separators can be continuously fabricated on industrial production lines.
• Hot-press compositing: a simple hot-pressing process can be used to manufacture nanocoated separators, reducing preparation costs and improving mechanical strength.
The future development of AZB separators requires continuous optimization in terms of thinness, ion selectivity, interface stability, corrosion resistance, and cost control. Through rational material design and advanced manufacturing processes, the battery's cycle life, rate performance, and energy density can be significantly enhanced, providing more reliable solutions for grid-scale energy storage and high-power applications in AZBs.
Conflicts of interest
The authors declare no conflict of interest.Data availability
This review does not include any primary research results, software, or code, nor does it involve the generation or analysis of new data.Acknowledgements
C. Z. acknowledges the support from the National Key R&D Program of China under Project 2019YFA0705104, the grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. R5019-22, Project No. CityU PDFS2122-1S05, and Project No. CityU 11214023), the Talent Recruitment Project of Guangdong Province (no. 2019QN01C883), the Shenzhen Science and Technology Innovation Project (JCYJ20220818102402004) and HIT-CityU Joint Laboratory on Zinc-based Batteries. H. L. acknowledges the support from the Shenzhen Science, Technology and Innovation Commission Basic Research Project (Grant No. JCYJ20240813153125033).References
- A. Chel and G. Kaushik, Alexandria Eng. J., 2018, 57, 655–669 CrossRef.
- P. A. Østergaard, N. Duic, Y. Noorollahi and S. Kalogirou, Renewable Energy, 2022, 199, 1145–1152 CrossRef.
- P. A. Østergaard, N. Duic, Y. Noorollahi, H. Mikulcic and S. Kalogirou, Renewable Energy, 2020, 146, 2430–2437 CrossRef.
- S. Tabrizian, Sustainable Dev., 2019, 27, 537–544 CrossRef.
- M. Yavari and I. M. Bohreghi, Appl. Energy, 2025, 377, 124654 CrossRef.
- C. Cao, Y. Zhong and Z. Shao, Chin. J. Chem., 2023, 41, 1119–1141 CrossRef CAS.
- H. Cavers, P. Molaiyan, M. Abdollahifar, U. Lassi and A. Kwade, Adv. Energy Mater., 2022, 12, 2200147 CrossRef CAS.
- R. Gond, W. van Ekeren, R. Mogensen, A. J. Naylor and R. Younesi, Mater. Horiz., 2021, 8, 2913–2928 RSC.
- S. Hosamane, N. Kottam and A. Chalil Suresh, Wiley Interdiscip. Rev.: Energy Environ., 2025, 14, e544 CAS.
- Y. K. Liu, C. Z. Zhao, J. Du, X. Q. Zhang, A. B. Chen and Q. Zhang, Small, 2023, 19, e2205315 CrossRef PubMed.
- H. Lv, X. Chu, Y. Zhang, Q. Liu, F. Wu and D. Mu, Mater. Today, 2024, 78, 181–208 CrossRef CAS.
- R. Tao, Y. Gu, Z. Du, X. Lyu and J. Li, Nat. Rev. Clean Technol., 2025, 1, 116–131 CrossRef.
- X. Tian, Y. Yi, B. Fang, P. Yang, T. Wang, P. Liu, L. Qu, M. Li and S. Zhang, Chem. Mater., 2020, 32, 9821–9848 CrossRef CAS.
- T. Zhou, R. Huang, Q. Lu, P. Liu, L. Hu, K. Zhang, P. Bai, R. Xu, X. Cao, Z. Sun, S. Duan, R. Liu, Y. Qin, X. Sun, Y. Zhang, Y. Li, Y. Yan, M. Liu and X. Wang, Energy Storage Mater., 2024, 72, 103689 CrossRef.
- P. Li, H. Kim, J. Ming, H.-G. Jung, I. Belharouak and Y.-K. Sun, eScience, 2021, 1, 3–12 CrossRef.
- Y. Zheng, Y. Shen, J. Guo, J. Li, J. Wang, D. Ning, Y. Liu, Y. Huang, Y. Tang, Y. Deng, H. Yan and H. Shao, Nano Res. Energy, 2024, 3, e9120118 CrossRef.
- J. J. Roy, D. M. Phuong, V. Verma, R. Chaudhary, M. Carboni, D. Meyer, B. Cao and M. Srinivasan, Carbon Energy, 2024, 6, e492 CrossRef CAS.
- Y. Wan, B. Huang, W. Liu, D. Chao, Y. Wang and W. Li, Adv. Mater., 2024, 36, e2404574 CrossRef PubMed.
- S. Gao, Z. Zhu, H. Fang, K. Feng, J. Zhong, M. Hou, Y. Guo, F. Li, W. Zhang, Z. Ma and F. Li, Adv. Mater., 2024, 36, e2311523 CrossRef PubMed.
- B. Zhong, C. Liu, D. Xiong, J. Cai, J. Li, D. Li, Z. Cao, B. Song, W. Deng, H. Peng, H. Hou, G. Zou and X. Ji, ACS Nano, 2024, 18, 16468–16488 CrossRef CAS PubMed.
- S. Li, Y. Shang, X. Ren, A. Zhao, N. Chen, L. Li, F. Wu and R. Chen, ACS Nano, 2025, 19, 16584–16596 CrossRef CAS PubMed.
- A. Zhou, J. Zhang, M. Chen, X. Li, S. Li, J. Ma, T. Lv, X. Zhu, G. Feng and L. Suo, J. Am. Chem. Soc., 2025, 147, 11811–11820 CrossRef CAS PubMed.
- U. Ali, B. Liu, H. Jia, Y. Li, Y. Li, Y. Hao, L. Zhang, S. Xing, L. Li and C. Wang, Small, 2024, 20, e2305866 CrossRef PubMed.
- L. Li, S. Jia, Y. Shi, C. Wang, H. Qiu, Y. Ji, M. Cao and D. Zhang, Inorg. Chem. Front., 2024, 11, 2246–2259 RSC.
- Y. N. Liu, J. L. Yang, Z. Y. Gu, X. Y. Zhang, Y. Liu, M. Y. Su, X. L. Zhang, I. V. Zatovsky, K. Li, J. M. Cao and X. L. Wu, Angew. Chem., Int. Ed., 2024, 63, e202316925 CrossRef CAS PubMed.
- R. Wang, S. Zhang, S. Peng, Y. Tong and X. Hu, Carbon Neutrality, 2024, 3, 6 Search PubMed.
- H. Wu, J. Hao, Y. Jiang, Y. Jiao, J. Liu, X. Xu, K. Davey, C. Wang and S. Z. Qiao, Nat. Commun., 2024, 15, 575 CrossRef CAS PubMed.
- M. Xia, J. Zhou and B. Lu, Adv. Energy Mater., 2025, 15, 2404032 CrossRef CAS.
- F. Dong, B. Yang, X. Zhang, Z. Yang, S. Wang, Z. Hou and P. Chen, ACS Appl. Mater. Interfaces, 2025, 17, 1332–1340 CrossRef CAS PubMed.
- Q. Guo, S. Han, Y. Lu, R. Xiao, J. Li, Q. Hao, X. Rong, S. Weng, Y. Niu, F. Ding, Y. Yang, H. Xia, X. Wang, F. Xie, L. Zhou, X. Hou, H. Li, X. Huang, L. Chen and Y. S. Hu, Nat. Commun., 2025, 16, 4707 CrossRef PubMed.
- I. Jeong, S. Kim, Y. Kim, C. Kim, J. Kang, J. H. Ha, Y. Cho, S. J. Kang, J. Ryu, J. W. Han and S. Park, Adv. Mater., 2025, 37, e2412652 CrossRef PubMed.
- G. Fang, J. Zhou, A. Pan and S. Liang, ACS Energy Lett., 2018, 3, 2480–2501 CrossRef CAS.
- Y. H. Lee, Y. Jeoun, J. H. Kim, J. Shim, K. S. Ahn, S. H. Yu and Y. E. Sung, Adv. Funct. Mater., 2024, 34, 2310884 CrossRef CAS.
- Y. Lv, Y. Xiao, L. Ma, C. Zhi and S. Chen, Adv. Mater., 2022, 34, e2106409 CrossRef PubMed.
- X. Shi, J. Xie, J. Wang, S. Xie, Z. Yang and X. Lu, Nat. Commun., 2024, 15, 302 CrossRef CAS PubMed.
- J. Wei, P. Zhang, J. Sun, Y. Liu, F. Li, H. Xu, R. Ye, Z. Tie, L. Sun and Z. Jin, Chem. Soc. Rev., 2024, 53, 10335–10369 RSC.
- T. Wu, C. Hu, Q. Zhang, Z. Yang, G. Jin, Y. Li, Y. Tang, H. Li and H. Wang, Adv. Funct. Mater., 2024, 34, 2315716 CrossRef CAS.
- J. Zhu, Z. Tie, S. Bi and Z. Niu, Angew. Chem., Int. Ed., 2024, 63, e202403712 CrossRef CAS PubMed.
- Y. Shen, P. Fu, J. Liu, K. Sun, H. Wen, P. Liu, H. Lv, T. Gu, X. Yang and L. Chen, Nano Res. Energy, 2024, 3, e9120115 CrossRef.
- J. Li, A. Azizi, S. Zhou, S. Liu, C. Han, Z. Chang, A. Pan and G. Cao, eScience, 2025, 5 DOI:10.1016/j.esci.2024.100294.
- L. Deng, X. Xie, W. Song, A. Pan, G. Cao, S. Liang and G. Fang, Chem. Eng. J., 2024, 488, 151104 CrossRef CAS.
- Q. Dong, Q. Nian, X. Luo, J. Fan, J. Jiang, Z. Cui, D. Ruan and X. Ren, Sci. China: Chem., 2024, 68, 526–535 CrossRef.
- X. Luo, Q. Nian, Q. Dong, D. Ruan, Z. Cui, Z. Wang, B. Q. Xiong and X. Ren, Adv. Energy Mater., 2025, 15, 2403187 CrossRef CAS.
- Q. Nian, X. Luo, D. Ruan, Y. Li, B. Q. Xiong, Z. Cui, Z. Wang, Q. Dong, J. Fan, J. Jiang, J. Ma, Z. Ma, D. Wang and X. Ren, Nat. Commun., 2024, 15, 4303 CrossRef CAS PubMed.
- X. Yang, Z. Dong, G. Weng, Y. Su, J. Huang, H. Chai, Y. Zhang, K. Wu, J. B. Baek, J. Sun, D. Chao, H. Liu, S. Dou and C. Wu, Adv. Energy Mater., 2024, 14, 2401293 CrossRef CAS.
- W. Zhang, W. Qi, K. Yang, Y. Hu, F. Jiang, W. Liu, L. Du, Z. Yan and J. Sun, Energy Storage Mater., 2024, 71, 103616 CrossRef.
- W. Du, E. H. Ang, Y. Yang, Y. Zhang, M. Ye and C. C. Li, Energy Environ. Sci., 2020, 13, 3330–3360 RSC.
- J. Hao, X. Li, X. Zeng, D. Li, J. Mao and Z. Guo, Energy Environ. Sci., 2020, 13, 3917–3949 RSC.
- B. Li, X. Zhang, T. Wang, Z. He, B. Lu, S. Liang and J. Zhou, Nanomicro Lett, 2021, 14, 6 Search PubMed.
- A. Naveed, A. Ali, T. Rasheed, X. Wang, P. Ye, X. Li, Y. Zhou, S. Mingru and Y. Liu, J. Power Sources, 2022, 525, 231122 CrossRef CAS.
- R. Qin, Y. Wang, L. Yao, L. Yang, Q. Zhao, S. Ding, L. Liu and F. Pan, Nano Energy, 2022, 98, 107333 CrossRef CAS.
- J. Zheng, Z. Huang, F. Ming, Y. Zeng, B. Wei, Q. Jiang, Z. Qi, Z. Wang and H. Liang, Small, 2022, 18, e2200006 CrossRef PubMed.
- C. Zhu, P. Li, G. Xu, H. Cheng and G. Gao, Coord. Chem. Rev., 2023, 485, 215142 CrossRef CAS.
- A. Xia, X. Pu, Y. Tao, H. Liu and Y. Wang, Appl. Surf. Sci., 2019, 481, 852–859 CrossRef CAS.
- Y. Zeng, X. Zhang, R. Qin, X. Liu, P. Fang, D. Zheng, Y. Tong and X. Lu, Adv. Mater., 2019, 31, e1903675 CrossRef PubMed.
- Y. Guo, C. Liu, L. Xu, K. Huang, H. Wu, W. Cai and Y. Zhang, Energy Mater., 2022, 2, 200032 CrossRef CAS.
- Y. Li, Y.-F. Guo, Z.-X. Li, P.-F. Wang, Y. Xie and T.-F. Yi, Energy Storage Mater., 2024, 67, 103300 CrossRef.
- C. Mao, Y. Chang, X. Zhao, X. Dong, Y. Geng, N. Zhang, L. Dai, X. Wu, L. Wang and Z. He, J. Energy Chem., 2022, 75, 135–153 CrossRef CAS.
- C. Choi, J. B. Park, J. H. Park, S. Yu and D.-W. Kim, Chem. Eng. J., 2023, 456, 141015 CrossRef CAS.
- X. Hu, J. Borowiec, Y. Zhu, X. Liu, R. Wu, A. M. Ganose, I. P. Parkin and B. D. Boruah, Small, 2024, 20, e2306827 CrossRef PubMed.
- G.-Q. Liu, B. Fu, Z.-X. Liu, L.-Y. Li, S.-Q. Liang and G.-Z. Fang, Rare Met., 2024, 43, 5005–5016 CrossRef CAS.
- Y. Meng, M. Wang, J. Xu, K. Xu, K. Zhang, Z. Xie, Z. Zhu, W. Wang, P. Gao, X. Li and W. Chen, Angew. Chem., Int. Ed., 2023, 62, e202308454 CrossRef CAS PubMed.
- H. Zhang, G. Zhu, J. Lu, Y. Hou, Y. Bao, P. Liu and Y. Zhang, Small, 2025, 21, 2408848 CrossRef CAS PubMed.
- P. Xue, C. Guo, W. Gong, Y. Chen, X. Chen, X. Li, J. Yang, Q. Zhang, K. Davey, K. Zhu, J. Mao and Z. Guo, Angew. Chem., Int. Ed., 2025, 64, e202500295 CrossRef CAS PubMed.
- B. Li, K. Yang, J. Ma, P. Shi, L. Chen, C. Chen, X. Hong, X. Cheng, M. C. Tang, Y. B. He and F. Kang, Angew. Chem., Int. Ed., 2022, 61, e202212587 CrossRef CAS PubMed.
- Y. Chai, X. Xie, Z. He, G. Guo, P. Wang, Z. Xing, B. Lu, S. Liang, Y. Tang and J. Zhou, Chem. Sci., 2022, 13, 11656–11665 RSC.
- Y. Du, Y. Feng, R. Li, Z. Peng, X. Yao, S. Duan, S. Liu, S. C. Jun, J. Zhu, L. Dai, Q. Yang, L. Wang and Z. He, Small, 2024, 20, e2307848 CrossRef PubMed.
- X. Zheng, Z. Liu, J. Sun, R. Luo, K. Xu, M. Si, J. Kang, Y. Yuan, S. Liu, T. Ahmad, T. Jiang, N. Chen, M. Wang, Y. Xu, M. Chuai, Z. Zhu, Q. Peng, Y. Meng, K. Zhang, W. Wang and W. Chen, Nat. Commun., 2023, 14, 76 CrossRef CAS PubMed.
- M. Zhou, C. Fu, L. Qin, Q. Ran, S. Guo, G. Fang, X. Lang, Q. Jiang and S. Liang, Energy Storage Mater., 2022, 52, 161–168 CrossRef.
- Q. Ma, W. Song, X. Zhang, N. Yang, B. Wu, C. Zheng, M. Fujishige, K. Takeuchi, M. Endo, J. Niu and F. Wang, Adv. Funct. Mater., 2025, 35, 2422159 CrossRef CAS.
- J. Cao, Y. Jin, H. Wu, Y. Yue, D. Zhang, D. Luo, L. Zhang, J. Qin and X. Yang, Adv. Energy Mater., 2025, 15, 2403175 CrossRef CAS.
- Y. Ding, X. Zhang, T. Wang, B. Lu, Z. Zeng, Y. Tang, J. Zhou and S. Liang, Energy Storage Mater., 2023, 62, 102949 Search PubMed.
- Z. Hu, F. Zhang, Y. Zhao, H. Wang, Y. Huang, F. Wu, R. Chen and L. Li, Adv. Mater., 2022, 34, e2203104 CrossRef PubMed.
- S. Wang, S. Wang, Z. Wei, Y. Wang, D. Zhang, Z. Chen and C. Zhi, Nat. Commun., 2025, 16, 1800 CrossRef CAS PubMed.
- L. Cao, D. Li, E. Hu, J. Xu, T. Deng, L. Ma, Y. Wang, X. Q. Yang and C. Wang, J. Am. Chem. Soc., 2020, 142, 21404–21409 CrossRef CAS PubMed.
- F. Wang, O. Borodin, T. Gao, X. Fan, W. Sun, F. Han, A. Faraone, J. A. Dura, K. Xu and C. Wang, Nat. Mater., 2018, 17, 543–549 CrossRef CAS PubMed.
- P. Chen, X. Lin, B. Yang, Y. Gao, Y. Xiao, L. Li, H. Zhang, L. Li, Z. Zheng, J. Wang and S. Chou, Adv. Funct. Mater., 2024, 34, 2409368 CrossRef CAS.
- J. Chen, M. Chen, H. Ma, W. Zhou and X. Xu, Energy Rev., 2022, 1, 100005 Search PubMed.
- B. Li, Y. Zeng, W. Zhang, B. Lu, Q. Yang, J. Zhou and Z. He, Sci. Bull., 2024, 69, 688–703 Search PubMed.
- L. Li, S. Jia, Z. Cheng and C. Zhang, ChemSusChem, 2023, 16, e202202330 CrossRef CAS PubMed.
- Y. Qin, P. Liu, Q. Zhang, Q. Wang, D. Sun, Y. Tang, Y. Ren and H. Wang, Small, 2020, 16, 2003106 Search PubMed.
- M.-Y. Wang, R.-B. Huang, J.-F. Xiong, J.-H. Tian, J.-F. Li and Z.-Q. Tian, Acta Phys.-Chim. Sin., 2024, 40, 2307017 CrossRef.
- Y. Zong, H. He, Y. Wang, M. Wu, X. Ren, Z. Bai, N. Wang, X. Ning and S. X. Dou, Adv. Energy Mater., 2023, 13, 2300403 Search PubMed.
- H. Zhou, J. Gu, Y. Wei, W. Zhang, J. Kang, J.-Q. Huang, B. Zhang, C. Hu and X. Lin, J. Power Sources, 2023, 558, 232649 CrossRef CAS.
- L. Li, M. Sun, B. Hao, W. Chen, C. Zhu, L. Zhang, X. Shen, X. Zhou, J. Zhou, C. Yan, X. Liu and T. Qian, J. Phys. Chem. Lett., 2024, 15, 380–390 CrossRef CAS PubMed.
- R. Fazaeli, H. Aliyan, Z. Huang, Y. Wang and Y. Li, ACS Omega, 2025, 10, 3228–3261 CrossRef CAS PubMed.
- J. Zhu, M. Yanilmaz, K. Fu, C. Chen, Y. Lu, Y. Ge, D. Kim and X. Zhang, J. Membr. Sci., 2016, 504, 89–96 CrossRef CAS.
- C. Xu, B. Li, H. Du and F. Kang, Angew. Chem., Int. Ed., 2012, 51, 933–935 CrossRef CAS PubMed.
- M. H. Alfaruqi, V. Mathew, J. Gim, S. Kim, J. Song, J. P. Baboo, S. H. Choi and J. Kim, Chem. Mater., 2015, 27, 3609–3620 CrossRef CAS.
- L. Kang, M. Cui, F. Jiang, Y. Gao, H. Luo, J. Liu, W. Liang and C. Zhi, Adv. Energy Mater., 2018, 8, 1801090 CrossRef.
- Q. Zhao, W. Huang, Z. Luo, L. Liu, Y. Lu, Y. Li, L. Li, J. Hu, H. Ma and J. Chen, Sci. Adv., 2018, 4, eaao1761 CrossRef PubMed.
- N. Zhang, F. Cheng, Y. Liu, Q. Zhao, K. Lei, C. Chen, X. Liu and J. Chen, J. Am. Chem. Soc., 2016, 138, 12894–12901 CrossRef CAS PubMed.
- F. Wu, F. Du, P. Ruan, G. Cai, Y. Chen, X. Yin, L. Ma, R. Yin, W. Shi, W. Liu, J. Zhou and X. Cao, J. Mater. Chem. A, 2023, 11, 11254–11263 RSC.
- S. Liu, Q. Han, C. He, Z. Xu, P. Huang, L. Cai, H. Chen, H. Zheng, Y. Zhou, M. Wang, H. Tian, W. Q. Han and H. Ying, ACS Nano, 2024, 18, 25880–25892 CrossRef CAS PubMed.
- C. Li, Z. Sun, T. Yang, L. Yu, N. Wei, Z. Tian, J. Cai, J. Lv, Y. Shao, M. H. Rummeli, J. Sun and Z. Liu, Adv. Mater., 2020, 32, e2003425 CrossRef PubMed.
- Y. Su, B. Liu, Q. Zhang, J. Peng, C. Wei, S. Li, W. Li, Z. Xue, X. Yang and J. Sun, Adv. Funct. Mater., 2022, 32, 2204306 CrossRef CAS.
- P. Jiang, Q. Du, C. Lei, C. Xu, T. Liu, X. He and X. Liang, Chem. Sci., 2024, 15, 3357–3364 RSC.
- Y. Wang, X. Jin, J. Xiong, Q. Zhu, Q. Li, R. Wang, J. Li, Y. Fan, Y. Zhao and X. Sun, Adv. Mater., 2024, 36, e2404093 CrossRef PubMed.
- L. Zhang, J. Huang, H. Guo, L. Ge, Z. Tian, M. Zhang, J. Wang, G. He, T. Liu, J. Hofkens, D. J. L. Brett and F. Lai, Adv. Energy Mater., 2023, 13, 2203790 CrossRef CAS.
- Y. Zhao, X. Chen, W. Guo and C. Zha, Carbon Neutralization, 2024, 3, 918–949 CrossRef CAS.
- H. Bi, D. Tian, Z. Zhao, Q. Yang, Y. Yuan, R. Zhang, L. Ai, X. Wang and J. Qiu, Adv. Funct. Mater., 2025, 35, 2423115 CrossRef CAS.
- J. Yang, Q. Dai, S. Hou, C. Han and L. Zhao, Adv. Mater., 2025, 37, 2418258 CrossRef CAS PubMed.
- Y. Kang, G. Chen, H. Hua, M. Zhang, J. Yang, P. Lin, H. Yang, Z. Lv, Q. Wu, J. Zhao and Y. Yang, Angew. Chem., Int. Ed., 2023, 62, e202300418 CrossRef CAS PubMed.
- F. Bu, Z. Sun, W. Zhou, Y. Zhang, Y. Chen, B. Ma, X. Liu, P. Liang, C. Zhong, R. Zhao, H. Li, L. Wang, T. Zhang, B. Wang, Z. Zhao, J. Zhang, W. Li, Y. S. Ibrahim, Y. Hassan, A. Elzatahry, D. Chao and D. Zhao, J. Am. Chem. Soc., 2023, 145, 24284–24293 CrossRef CAS PubMed.
- Y. Tan, D. Chen, T. Yao, Y. Zhang, C. Miao, H. Yang, Y. Wang, L. Li, V. Kotsiubynskyi, W. Han and L. Shen, Adv. Sci., 2024, 11, e2407410 CrossRef PubMed.
- Y. Song, P. Ruan, C. Mao, Y. Chang, L. Wang, L. Dai, P. Zhou, B. Lu, J. Zhou and Z. He, Nano Micro. Lett., 2022, 14, 218 CrossRef CAS PubMed.
- P. Yang, K. Zhang, S. Liu, W. Zhuang, Z. Shao, K. Zhu, L. Lin, G. Guo, W. Wang, Q. Zhang and Y. Yao, Adv. Funct. Mater., 2024, 34, 2410712 CrossRef CAS.
- H. Ma, J. Yu, M. Chen, X. Han, J. Chen, B. Liu and S. Shi, Adv. Funct. Mater., 2023, 33, 2307384 CrossRef CAS.
- P. Tian, Y. Gao, S. Huang, Y. Cao, Z. Liu, Y. Huo, M. Li, X. Gu and L. Wu, Adv. Energy Mater., 2024, 14, 2401830 CrossRef CAS.
- Y. Liang, D. Ma, N. Zhao, Y. Wang, M. Yang, J. Ruan, G. Yang, H. Mi, C. He and P. Zhang, Adv. Funct. Mater., 2022, 32, 2112936 CrossRef CAS.
- Z. Zhao, Y. Zhang, H. Zhang, X. Shi, H. Zhao, J. Liu, J. Liu and L. Li, Nano Lett., 2025, 25, 7483–7490 CrossRef CAS PubMed.
- Y. Kang, G. Chen, H. Hua, M. Zhang, J. Yang, P. Lin, H. Yang, Z. Lv, Q. Wu and J. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202300418 CrossRef CAS PubMed.
- L. Deng, K. Sun, J. Liu, Z. Li, J. Cao and S. Liao, Molecules, 2024, 29, 3147 CrossRef CAS PubMed.
- Z. Sun, J. Zhang, X. Jiao and Z. Li, Modified Separator with Biomass-Derived Carbon for Dendrite-Free and Boosting Cycling Stability of Zinc-Ion Battery, Available at SSRN: DOI:10.2139/ssrn.4879884.
- B. Wu, P. Wang, H. Yang, Y. Liang, W. Ni, G. Xu, X. Wei and L. Yang, J. Power Sources, 2023, 580, 233323 CrossRef CAS.
- Q. Zhang, L. Wan, X. Gao, S. Cheng, N. Gao, C. J. Carmalt, Y. Dai, G. He and H. Li, ACS Appl. Mater. Interfaces, 2024, 16, 69388–69397 CrossRef CAS PubMed.
- F. Ma, K. Xie, S. Wu, C. Zhang, X. Liao and Q. Wang, Batteries, 2023, 9, 387 CrossRef CAS.
- Q. Gou, H. Luo, L. Qu, F. Yu, K. Wang, S. Zhang, Z. Luogu, B. Zhang, Y. Zheng and B. Song, J. Energy Chem., 2025, 101, 191–200 CrossRef CAS.
- H. Zhang, H. Yang, B. Wu, Y. Liang, W. Ni, G. Xu and L. Yang, Ionics, 2024, 30, 237–246 CrossRef CAS.
- B. Cao, Q. Qu, B. Jiang, T. Zou, S. Zhen, P. Wang, K. Li, J. Zhang, H. Guo and T. Zhang, J. Mater. Chem. A, 2024, 12, 31185–31194 RSC.
- W. Yuan, X. Qu, Y. Wang, X. Li, X. Ru, D. Jia, L. Zhao, Y. Hou, J. Shen, Z. Shen and N. Zhang, Energy Storage Mater., 2025, 76, 104130 CrossRef.
- L. Cheng, W. Li, M. Li, S. Zhou, J. Yang, W. Ren, L. Chen, Y. Huang, S. Yu and J. Wei, Adv. Funct. Mater., 2024, 34, 2408863 CrossRef CAS.
- G. Wu, R. Zhu, W. Yang, Y. Yang, J. Okagaki, Z. Lu, J. Sun, H. Yang and E. Yoo, Adv. Funct. Mater., 2024, 34, 2316619 CrossRef CAS.
- J. Xu, Y. Yang, Q. Dai, Z. Zheng, Y. Cao, Y. Cheng, B. Peng, L. Ma and Y. Wang, Angew. Chem., Int. Ed., 2025, 64, e202423118 CrossRef CAS PubMed.
- M. Ghosh, V. Vijayakumar and S. Kurungot, Energy Technol., 2019, 7, 1900442 CrossRef.
- B. Wu, Y. Wu, Z. Lu, J. Zhang, N. Han, Y. Wang, X.-M. Li, M. Lin and L. Zeng, J. Mater. Chem. A, 2021, 9, 4734–4743 RSC.
- D. Yuan, W. Manalastas, Jr., L. Zhang, J. J. Chan, S. Meng, Y. Chen and M. Srinivasan, ChemSusChem, 2019, 12, 4889–4900 CrossRef CAS PubMed.
- S. Yang, Y. Zhang, Y. Zhang, J. Deng, N. Chen, S. Xie, Y. Ma and Z. Wang, Adv. Funct. Mater., 2023, 33, 2304280 CrossRef CAS.
- C. Yang, P. Woottapanit, Y. Yue, S. Geng, J. Cao, X. Zhang, G. He and J. Qin, Small, 2024, 20, e2311203 CrossRef PubMed.
- T. Li, Y. Gong, H. Yang, Y. Liu, X. Dong, Y. Xu, H. Ma, C. Wei, S. Zhang, F. Huang, M. Yang and T. Lin, Energy Storage Mater., 2025, 79, 104311 CrossRef.
- D. Wang, S. Hu, T. Li, C. Chang, S. Li, S. Guo, H. Li, Q. Liu, J. Gong, J. Zhou and C. Han, Nat. Commun., 2025, 16, 259 CrossRef CAS PubMed.
- X. Zhu, Z. Xu, T. Zhang, J. Zhang, Y. Guo, M. Shan, K. Wang, T. Shi, G. Cui and F. Wang, Adv. Funct. Mater., 2024, 34, 2407262 CrossRef CAS.
- X. Zhu, Z. Xu, T. Zhang, J. Zhang, Y. Guo, M. Shan, K. Wang, T. Shi, G. Cui, F. Wang, G. Xu and M. Zhu, Adv. Funct. Mater., 2024, 34, 2407262 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |