Rolling circle amplification/transcription-based nanotechnology for efficient delivery of nucleic acid drugs
Xun You†
a,
Qingxuan Zeng†a,
Tianshuang Xia†a,
Xiaocui Guo*a,
Chi Yao *a and
Dayong Yang
*a and
Dayong Yang *ab
*ab
aState Key Laboratory of Synthetic Biology, Frontiers Science Center for Synthetic Biology, Key Laboratory of Systems Bioengineering (MOE), School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300350, P. R. China. E-mail: dayongyang@fudan.edu.cn; chi.yao@tju.edu.cn; guoxc@tju.edu.cn
bDepartment of Chemistry, State Key Laboratory of Molecular Engineering of Polymers, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, College of Chemistry and Materials, Fudan University, Shanghai, 200438, P. R. China
First published on 8th July 2025
Abstract
Rolling circle amplification/transcription (RCA/RCT) nanotechnology offers a breakthrough platform for nucleic acid drug delivery, leveraging enzymatically produced ultra-long, programmable nucleic acid chains to engineer multifunctional nanostructures. In this review, we give an overview of RCA/RCT-based nanocarriers for nucleic acid drug delivery, systematically summarizing their key design aspects: (1) nanoization strategies through biomineralization, electrostatic compression, nanomaterial-assisted assembly and base pairing/entanglement; (2) drug loading approaches via design on template, complementary base pairing and electrostatic binding; (3) targeting modalities including aptamers, proteins, polymers and small molecule ligands; and (4) controlled release mechanisms responsive to endogenous/exogenous enzymes and intracellular microenvironments. We showcase their significant therapeutic advances in gene therapy, immunotherapy, and combination therapy. This overview provides critical insights for developing next-generation RCA/RCT delivery platforms to address pressing biomedical needs.
1. Introduction
Nucleic acid drugs are a class of therapeutic agents composed of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), or nucleic acid–protein complexes.1 As a transformative modality in precision medicine, these drugs have rapidly advanced by leveraging mechanisms such as gene repression, gene editing, gene replacement and target protein binding to achieve targeted therapeutic effects.2 The developed nucleic acid drugs broadly encompass three categories: (1) Nucleic acid-targeting nucleic acid drugs. This class comprises therapeutic agents that modulate protein expression through direct interaction with nucleic acids, either by promoting or by inhibiting translation processes.3 The key representatives include siRNA, miRNA and ASOs for target mRNA silencing, and a clustered regularly interspaced short palindromic repeat-associated (CRISPR–Cas) system for genomic DNA editing.4–7 (2) Protein-targeting nucleic acid drugs. This category operates through direct protein interaction rather than genetic regulation, represented by aptamers and immune agonists.8–10 Aptamers demonstrate antibody-like binding specificity to target proteins, enabling precise molecular recognition for blocking the lesion signaling pathway.11 Immune agonists represented by the cytosine–phosphate–guanosine (CpG) motif can activate the Toll-like receptor 9 (TLR9) signaling pathway for immunotherapy.12 (3) Protein-expressing nucleic acid drugs. This class serves as transient templates for in vivo protein production, including mRNA and plasmid DNA, offering a versatile platform for various clinical applications.13Despite their significant therapeutic promise, nucleic acid drugs face major delivery hurdles that stem from their fundamental biochemical properties.14,15 As naturally occurring biomolecules, nucleic acid drugs are highly susceptible to rapid enzymatic degradation by nucleases in biological fluids, severely compromising their stability and bioavailability.16 Furthermore, their polyanionic nature and large molecular size create substantial barriers to cellular uptake, resulting in poor membrane permeability and inefficient intracellular delivery.17,18 These combined challenges currently represent the primary obstacles limiting the clinical translation of nucleic acid therapeutics, driving ongoing research into novel delivery platforms. Various delivery platforms have been developed to address these challenges, including viral vectors, lipid nanoparticles (LNPs), polymeric nanoparticles, peptide-based formulations, and engineered biomaterials (e.g., exosomes).19–26 However, these systems still face critical challenges, including limited drug-loading capacity, off-target effects, uncontrollable drug release, and suboptimal biocompatibility. For example, while LNPs have been successfully employed in mRNA vaccines, their lack of tissue and cell specificity remains a major hurdle.19 Despite significant advancements, achieving an optimal balance between delivery efficiency, stability, and biocompatibility continues to be a central challenge in nucleic acid delivery platform development.
Currently, DNA nanotechnology has been employed to construct a diverse array of nucleic acid-based nanomaterials, which demonstrate significant application potential and research value in the biomedical field.27–30 Notably, as biomolecules of the same class as nucleic acid drugs, nucleic acid-based nanomaterials exhibit distinct advantages in the delivery of nucleic acid drugs.31 First, the programmable sequences of nucleic acid molecules allow for the customized design and synthesis of oligonucleotide drug sequences or guide RNA sequences for CRISPR/Cas systems, which can be directly integrated into the building blocks of nucleic acid materials or loaded via base complementary pairing, achieving efficient drug loading.32,33 Second, specific nucleic acid sequences can respond to intracellular microenvironments (e.g., enzymes, pH, metal ions), endowing nucleic acid materials with intelligent responsiveness for controlled drug release.34–40 For instance, the abundance of specific nucleases in cells can cleave particular nucleic acid sequences or structures, facilitating the controlled release of nucleic acid-based drugs.41,42 Moreover, they excel in specific recognition and targeted delivery via aptamers or modular conjugation of biological ligands (e.g., antibodies, peptides) that enable receptor-mediated cellular uptake.43,44 With the advancement of DNA nanotechnology, various precise nucleic acid assembly strategies have been established for constructing functional nucleic acid-based nanocarriers for nucleic acid drug delivery.45–52
Rolling circle amplification (RCA) for DNA production and rolling circle transcription (RCT) for RNA generation are two innovative isothermal enzymatic techniques that mimic natural nucleic acid replication and transcription processes.53,54 These two powerful methods utilize circular DNA templates along with specialized polymerases—Phi29 DNA polymerase for RCA and T7 RNA polymerase for RCT—to generate ultra-long single-stranded DNA or RNA (ssDNA/ssRNA) molecules featuring precisely controlled periodic repeats in vitro.55,56 The exceptional capability of RCA/RCT to produce nucleic acid chains with programmable lengths and sequences has established these techniques as preferred approaches for constructing sophisticated DNA/RNA-based nanocarriers.57–60 Through rational template design and integration with functional materials, the resulting ultra-long ssDNA/ssRNA can self-assemble into diverse nanostructures with tailored functionalities, serving as ideal carriers for nucleic acid drugs.61 This review prioritizes RCA for its core strengths: scalable production of linear therapeutic DNA constructs with high payload capacity and sequence-defined functional domains (e.g., aptamers, siRNA templates, CRISPR/Cas). Applications demanding intricate 3D spatial organization (e.g., nanorobotics, multi-enzyme nanocomplexes) may benefit more from origami-based approaches. This selection of RCA represents a deliberate technological compromise, prioritizing high-throughput scalability and therapeutic sequence flexibility over ultra-precise nanoscale structural control.
In this review, we systematically overview the emerging field of RCA/RCT-based nanotechnology for nucleic acid drug delivery (Scheme 1). We first elucidate four fundamental nano-architectural design strategies for RCA/RCT-derived carriers. The discussion then progresses to a critical analysis of three core technological aspects, including nucleic acid drug loading strategies, precision targeting strategies, and drug-controlled release strategies, which are fundamental to achieving efficient drug delivery. Finally, we showcase their advances in therapeutics including gene therapy, immunotherapy and combination therapy, providing insights into both current achievements and future development directions.
2. RCA/RCT-based nanotechnology
Nanocarriers engineered through RCA and RCT have emerged as versatile platforms for biomedical applications, leveraging their programmable design, high payload capacity, and tunable physicochemical properties.62–66 The synthesis strategy of RCA/RCT nanocarriers offers distinct advantages: (i) scalability—enzyme-driven amplification allows rapid, cost-effective production; (ii) modularity—sequence customization enables precise control over size, stability, and targeting ligands; and (iii) multifunctionality—simultaneous delivery of diverse payloads for combinatorial therapy.2.1. RCA and RCT
The Andrew Fire group firstly proposed the concept of RCA in 1995, and the RCT model was established by the Eric T. Kool team in 1995.67 A typical RCA/RCT reaction mainly requires four components: (i) a linear DNA template; (ii) a DNA initiator strand (called a linear DNA primer) for RCA or a T7 promoter for RCT, which are partially complementary to the DNA template; (iii) DNA polymerase (e.g., Phi29 DNA polymerase) or RNA polymerase (e.g., T7 RNA polymerase); and (iv) deoxyribonucleotide triphosphates (dNTPs) or nucleotide triphosphates (NTPs).62 On the basis of a circular DNA template constructed via primer-assisted looping of a linear DNA template, the primer/promoter is extended through the action of DNA/RNA polymerase with strand displacement activity, resulting in ultra-long ssDNA or ultra-long ssRNA with hundreds of tandem repeats, and the length of the RCA/RCT product can be controlled by adjusting the reaction time, circular DNA template concentration, dNTP/NTP concentration, or polymerase concentration (Fig. 1).2.2. Nanoization of RCA/RCT products
By leveraging the programmability of nucleic acids and the scalability of enzymatic reactions, RCA and RCT offer a versatile platform for the bottom-up fabrication of nanostructures with tailored functionalities. In this section, we systematically summarized the nanoization strategies of RCA/RCT products into the following four categories: biomineralization, electrostatic compression, nanomaterial-assisted assembly, and base pairing/physical entanglement (Fig. 2).3. Nucleic acid drug loading strategies
In this section, we summarize the loading strategies of nucleic acid drugs onto the constructed nanocarriers by the following three methods: (i) design on template (e.g., DNAzyme, ASO, crRNA, and siRNA); (ii) complementary base pairing (e.g., siRNA, ribonucleoprotein (RNP), and ASO); and (iii) electrostatic binding (e.g., acid-degradable mask coated RNP) (Fig. 3). | ||
| Fig. 3 Schematic diagram of RCA/RCT-based strategies for nucleic acid drug loading, including design on template, complementary base pairing and electrostatic binding. | ||
3.1. Design on template
Owing to the sequence-specific programmability of DNA/RNA, customizing the sequence of the circular template allows for the inclusion of multiple nucleic acid drugs, like CpG adjuvant sequences, ASO sequences, DNAzyme sequences, siRNA sequences, miRNA sequences, sgRNA sequences, and crRNA sequences, within the ultra-long ssDNA/ssRNA. In detail, the complementary sequences for the abovementioned nucleic acid drugs can be designed on the template, with subsequent amplification via RCA/RCT. Additionally, both RCA and RCT are performed at constant and moderate temperatures. As a result, the nucleic acid drugs designed on the template can be efficiently amplified via RCA/RCT in a short timeframe. Incorporating functional modules onto the ssDNA chain offers a straightforward and efficient loading approach. On this basis, Yao et al.69 designed an ssDNA chain incorporating two distinct DNAzymes via RCA, creating a DNA-based nanocomplex with a cascading DNAzyme system for enhanced gene therapy efficacy. Li et al.42 constructed a circular template integrating complementary sequences for the DNAzyme, and other functional motifs, enabling the production of ultra-long ssDNA chains with multiple functional modules through RCA, resulting in the efficient loading of nucleic acid drugs. For RNA-based nucleic acid drugs, Guo et al.70 developed an RNA nanocarrier for efficient delivery of siRNA. Specifically, ultra-long ssRNAs including two kinds of siRNA targeting both CD47 and SIRPα were synthesized by RCT. Moreover, Ha et al.82 developed a polymeric CRISPR/Cas9 system utilizing poly-ribonucleoprotein (RNP) nanoparticles via RCT, which was composed of polymeric nucleic acid drugs (sgRNA and siRNA), to enhance delivery efficiency. Despite its high integration efficiency, the approach of designing nucleic acid drugs on a DNA template is restricted to integrating length-limited DNA/RNA sequences.3.2. Complementary base pairing
Leveraging complementary base pairing to embed nucleic acid drugs into RCA/RCT-derived nanocarriers is another widely used strategy in the field of nucleic acid drug delivery, especially for integrating RNA drugs such as siRNA, miRNA, and Cas9/sgRNA RNPs into DNA-based nanocarriers, alternatively, integrating DNA drugs such as ASO and DNAzymes into RNA-based nanocarriers. Specifically, the nucleic acid drug sequences must be pre-configured with an anchoring segment to support subsequent base pairing. By designing sequences identical to the anchoring segment on the DNA templates, RCA/RCT can be employed to synthesize ultra-long ssDNA/ssRNA that carries a large number of complementary sequences. Based on the Watson–Crick complementary pairing principle, the desired nucleic acid drugs can be precisely loaded into the DNA/RNA nanocarriers. To enable targeted cancer gene therapy, researchers now often incorporate small RNA therapeutics and the widely adopted CRISPR/Cas system into DNA/RNA nanocarriers using complementary base pairing. Li et al.42 utilized RCA to produce extended ssDNA strands incorporating numerous sgRNA-binding sequences. By leveraging complementary base pairing, these strands efficiently assembled Cas9/sgRNA RNPs, resulting in a functional gene-editing system. Zhang et al.83 engineered an RCA-generated nanostructure that carried siRNA through base-pairing complementarity, resulting in gene silencing for cancer treatment. Sun et al.74 synthesized an RCA-based DNA nanocarrier to efficiently load Cas12a/crRNA RNPs using a complementary base pairing strategy and successfully achieved downregulation of serum cholesterol levels. The strategy of nucleic acid drug loading through base complementary pairing is also applicable to RCT-based RNA nanocarriers. Han et al.78 synthesized two fully complementary ultra-long ssRNAs by complementary RCT (cRCT) to achieve efficient loading of nucleic acid drug siRNAs. Regarding the integration strategy relying on complementary base pairing, it should be noted that the complementary duplex region must have appropriate Gibbs free energy to ensure efficient loading of the necessary modules.3.3. Electrostatic binding
Due to the abundance of negatively charged phosphate groups in the nucleic acid backbone, the RCA/RCT-produced ultra-long ssDNA/ssRNA carries a strong negative charge. As a result, cationic polymers can be loaded into RCA/RCT-derived nanocarriers via electrostatic interactions. For example, Fan et al.84 designed a positively charged acid-degradable nanocapsule to encapsulate Cas13a/crRNA to achieve controllable regulation of Cas13a activity from the spatial dimension and electrostatically anchored the nanocapsule to the surface of RNA nanocarriers synthesized by RCT to achieve efficient delivery of nucleic acid drugs for gene therapy. This loading mode protects the biological activity of Cas13a protein and effectively prevents the degradation of crRNA in the blood circulation, which is a potential nucleic acid drug loading strategy. However, the realization of nucleic acid drug loading via electrostatic interactions faces several limitations, including poor stability under physiological conditions (e.g., serum protein adsorption, enzymatic degradation, and competitive displacement by anions), low loading efficiency for large nucleic acids, and potential cytotoxicity due to excessive cationic charge density. Additionally, premature release in circulation and insufficient endosomal escape further hinder therapeutic efficacy.4. Target strategies
RCA/RCT-based DNA/RNA nanocarriers represent a class of modular and programmable biopolymers, whose structural versatility enables the precise integration of diverse targeting moieties through rational sequence design, thereby achieving cell- or tissue-specific delivery.85 In this section, we systematically categorized four functional targeting units compatible with RCA/RCT-based nanocarriers: aptamers (e.g., AS1411 and EpCAM), proteins (e.g., antibodies or engineered peptides with natural binding capabilities), polymers (e.g., HA and PEI, functionalized for multivalent interactions), and small-molecule ligands (e.g., folic acid).74,81,86,87 These units can be incorporated into nanocarriers to establish precise targeting frameworks, offering tailored solutions for therapeutic and diagnostic applications (Fig. 4).4.1. Aptamer-mediated targeting
Aptamers, a type of nucleic acid sequence, can specifically bind to target molecules, which are obtained through the in vitro selection process (SELEX) and exhibit high affinity and specificity.88 Aptamers can be designed to recognize a variety of targets, including small molecules, proteins, cells, and even viruses.89 Compared with biological antibodies, aptamers offer numerous advantages, including high specificity, ease of synthesis and modification, a wide target range, small molecular weight, low immunogenicity, and favorable biocompatibility.90 Poor targeting efficacy and off-target toxicity are major issues associated with current chemotherapy approaches for the treatment of cancer.85 RCA/RCT technology can encode and control the sequence composition, repeating units, and secondary structure of aptamers (e.g., hairpins and G-quadruplexes) by designing specific circular templates, enhancing the specificity of target binding, and generating tandem and repeat aptamers that can form multivalent binding interfaces and significantly improve affinity for targeted molecules.91Zhao et al.66 constructed a Poly-Sgc8-Aptamer-Drug system using RCA and demonstrated that Poly-Sgc8-Aptamer-Drug is more effective than monovalent drugs in targeting and killing leukemia cells due to enhanced binding affinity (∼40-fold higher) and cellular internalization through multivalent effects. Tan et al.43 have engineered bioinspired, size-controllable, self-degradable cancer-targeting DNA nanoflowers via the incorporation of an artificial sandwich base by RCA. The aptamer sgc8 in DNA nanoflowers could recognize the target protein tyrosine kinase 7. Zhang et al.92 designed an RCA-based DNA nanosponge (DNS) structure, which contains tandem binding sites to an miRNA of interest, for competitive inhibition of miRNA function in cells. The synthesized DNSs with multivalent MUC1 aptamers can specifically target MUC1-overexpressing cells with enhanced binding affinity and cell internalization ability. Zhao et al.68 constructed doxorubicin (Dox)-delivery nanocarriers containing AS1411 aptamers through RCA for efficient delivery and enhanced therapy. Xu et al.81 created a nanosponge therapeutic medication system (AS1411@antimiR-21@Dox) by RCT, resulting in accomplished targeted delivery to tumor cells. Pei et al.93 hybridized polymerized RNAi microspheres synthesized by RCT with cholesterol-modified DNA and AS1411 aptamers by base pairing to produce self-assembled and tumor-targeted RNAi nanospheres. Li et al.94 developed a nano-drug delivery system by fabricating an RNA nanocarrier with a special porous, compact and spherical nanostructure. The porous RNA nanospheres were prepared through RCT and a unique supramolecule-mediated approach. The RCT generated tandem and repeated sequences containing the aptamer of EpCAM for targeted delivery and plenty of siRNA for gene silencing of EpCAM. Wang et al.83 designed RCA-based DNA nano-assembly with multivalent aptamers specifically targeting B16, achieving efficient delivery of siRNA and Dox. With the continuous development of new materials and technologies, aptamers can be further optimized to improve target accuracy and reduce off-target effects, offering great potential for precision medicine and personalized treatment.
4.2. Protein-mediated targeting
Protein-mediated cell targeting is a powerful strategy that utilizes specific protein interactions to direct therapeutic agents, imaging probes, or drug delivery systems to particular cells or tissues.95 This approach leverages naturally occurring protein–ligand interactions, such as antibody–antigen binding, receptor–ligand recognition, or engineered protein scaffolds, to achieve precise cellular localization. Key examples include monoclonal antibodies targeting cancer cell surface markers (e.g., HER2 or CD20) and viral vector proteins engineered for tissue-specific tropism.96 Advances in protein engineering, such as the development of bispecific antibodies or nanobodies, have further enhanced targeting accuracy and efficiency. By minimizing off-target effects and improving therapeutic efficacy, protein-mediated targeting holds great promise for precision medicine, immunotherapy, and advanced drug delivery systems.97 Antibody–drug conjugates (ADCs), enabled by breakthroughs in linker technology, revolutionize cancer treatment by precisely delivering cytotoxic payloads to malignant cells.98 Roh et al.87 constructed a new polymeric siRNA nanoparticle based on RCT, which is functionalized with chemical conjugation of the HER2 antibody for specifically receptor-mediated delivery to cancer cells. Roh et al.99 constructed a novel polymeric siRNA nanoparticle by RCT, which had two targeting ligands, namely, the anti-CD19 antibody and the anti-CD20 antibody, the RCT nanocarrier could be targeted into “hard-to-transfect” hematologic cancer cells.Electrostatic interactions, coordination interactions and chemical bonds provide the possibility for the binding of antibodies and nucleic acid strands. These antibody engineering strategies provide methods for the development of targeted nano-carrier systems based on RCA/RCT. Therefore, through rational design of nucleic acid carriers, researchers can strategically incorporate functional proteins/peptides with programmable binding modalities and spatial configurations. This versatility enables precise control over nucleic acid drug delivery, allowing for tissue-specific targeting while minimizing off-target effects.
4.3. Polymer-mediated targeting
Polymers, with their tunable architectures and multifunctional capabilities, are emerging as versatile aptamer-like platforms for precision targeting in biomedical and materials sciences. Polymer-mediated cell targeting represents an effective strategy in biomedicine, leveraging functionalized polymers to deliver therapeutic agents with high specificity to desired cells or tissues. A notable example is the use of hyaluronic acid (HA)-coated nanocarriers to target CD44-overexpressing cancer cells.100 By using RCA technology, Kim et al.101 synthesized DNA nanoballs with ASO-complementary sequences for the delivery of dual ASOs. The dual ASO-loaded DNA nanoballs were then condensed with cationic peptides and coated with HA to achieve CD44 receptor-mediated cell specific delivery for gene target therapy. Roh et al.99 constructed a novel polymeric siRNA nanoparticle by RCT for specific receptor-mediated delivery of cancer cells. By coating polymeric siRNA spheres with HA, the RCT nanocarrier could be targeted into “hard-to-transfect” hematologic cancer cells.In addition, Lee et al.102 proposed a newly designed RCT method for synthesis of polymeric siRNA nanoflowers, referred to as RCT and annealing-generated polymeric siRNA (RAPSI). RAPSI was further complexed with thiol-modified glycol chitosan (tGC), which showed enhanced tumor-homing efficacy. The resultant RAPSI/tGC nanoparticles specifically localized in tumor tissue. Gu et al.74 constructed a DNA nanoclew-based carrier by RCA for delivery of Cas12a/crRNA RNPs to regulate serum cholesterol levels. By modifying the polyethyleneimine (PEI) layer with galactose (Gal) and 2,3-dimethylmaleic anhydride (DM), the polymer Gal-PEI-DM was obtained, which could deliver nanoparticles into hepatocytes by binding to sialic acid glycoprotein receptors specifically expressed on the surface of hepatocytes.
4.4. Small molecule ligand-mediated targeting
Small molecules (<1 kDa) offer advantages such as excellent chemical stability, controllable synthesis, and superior tissue penetration capabilities. By leveraging high-affinity, small molecule ligands that selectively bind to cell-surface biomarkers (e.g., receptors or transporters), small molecule ligand-mediated targeting enables the directed accumulation of therapeutic or imaging agents at diseased sites while minimizing off-target effects.103–105 Folate receptor (FR) is a glycosylphosphatidylinositol-linked membrane glycoprotein mediating endocytosis of folate and its derivatives.106,107 Folic acid (FA) is a classic ligand for targeting FR-positive cancers.108,109 Zhang et al.110 developed a co-drug delivery system for targeting cancer therapy based on magnetic RNA nanoflowers (RNA NF). FA modified MNP/RNA NF was used as a targeting nanocarrier with excellent biocompatibility to overcome the non-selectivity of MNP/RNA NF. Ahn et al.103 reported an RNA/DNA hybrid with precise targeting capability, which was composed of RNAs containing multiple tandem copies of hairpins via RCT, DNA–cholesterol (DNA–Chol) and folate–DNA (FA–DNA) with targeted function conjugates. Ju et al.111 constructed a DNA nanomachine (DNM) by alternately hybridizing two pairs of DNA/RNA hybrids to a DNA scaffold generated by RCA for highly efficient in situ siRNA assembly in living cells. The negatively charged DNM could form a stable nanocomposite with cationic FA modified polyethylenimine to facilitate target cell-specific delivery and assist the endosomal escape of DNM into the cytoplasm. By leveraging structural optimization and combinatorial screening, small-molecule aptamers can selectively recognize diverse targets, including proteins, nucleic acids, and cellular metabolites, enabling applications in drug delivery, diagnostics, and therapeutics. Their ability to mimic natural interactions while minimizing off-target effects underscores their potential as next-generation targeting tools in precision medicine.5. Controlled release strategies for nucleic acid drugs
Controlled release of nucleic acid drugs plays a pivotal role in enhancing therapeutic efficacy while minimizing adverse effects. Notably, tumor cells exhibit a distinct biochemical landscape characterized by microenvironmental hallmarks such as acidic pH, elevated reactive oxygen species (ROS), dysregulated glutathione (GSH) homeostasis, and enzyme overexpression.112 Nanocarriers constructed via RCA/RCT technology can be designed as dynamically responsive platforms that sense tumor-specific biochemical cues, initiating spatiotemporally controlled release of therapeutic nucleic acids. In this section, we systematically discuss stimulus-responsive nanocarriers tailored to three key triggers: endogenous enzymes, the intracellular microenvironment, and exogenous enzymes (Fig. 5).41,575.1. Endogenous enzyme-responsive release
Endogenous enzymes overexpressed in diseased tissues can cleave specific substrates on nanocarriers, enabling enzyme-triggered nucleic acid drug release.113 Hammond et al.57 synthesized RCT-derived RNA microsponge aggregates as delivery vehicles for siRNA. After being internalized by cells, these microsponges were cleaved into siRNA with a length of about 21 nt by the ribonuclease Dicer (a multidomain ribonuclease III). Moreover, RNase H is a sequence-nonspecific endonuclease that cleaves RNA strands in RNA/DNA hybrids, which is highly expressed in tumor cells.114 Song et al.41 reported an RCA-based multifunctional DNA/UCNP complex that enables the co-delivery of Cas9 RNPs, hemin, and protoporphyrin (PP) for synergistic photodynamic therapy. Upon the cellular uptake of the nanoparticle complex, RNase H digested the RNA component of the DNA–RNA complex, releasing Cas9 RNPs for gene editing. Thermostable flap endonuclease 1 (FEN1) enzyme is a structurally responsive nuclease that is highly expressed in tumor cells.115 Singh et al.116 found that FEN1 expression was significantly elevated in breast cancer, uterine cancer, kidney cancer, ovarian cancer, and colon cancer tissues, and the protein level of FEN1 was almost always increased in all tumors. FEN1 enzyme possesses 5′ flap endonuclease activity, gap-dependent endonuclease activity, and 5′ exonuclease activity. The structures of FEN1 responsiveness mainly include Y-shaped DNA structures, bubble structures, nick structures, gap structures, blunt-ended double-stranded DNA, and Holliday junctions.117,118 Therefore, during RCA/RCT, these structures can be constructed by designing corresponding sequences in the circular DNA templates, enabling the successful construction of FEN1-responsive nanocarriers.5.2. Intracellular microenvironment-responsive release
Tumor microenvironment (TME)-responsive release leverages the unique pathological features of the TME—such as acidic pH, GSH, and ATP—to enable smart, stimuli-responsive drug delivery systems.119 The pH gradient serves as a critical biochemical hallmark in oncology, with tumor cores (∼6.5–6.8) and endosomal/lysosomal compartments (∼4.3–6.8) exhibiting significantly lower pH values compared to normal physiological environments (pH 7.4).120,121 This intrinsic pH differential serves as an exploitable trigger for engineering stimuli-responsive nanocarriers such as conformational switching through i-motif quadruplex structural transitions for controlled release of drugs.122 Du et al.123 developed drug-loaded targeted DNA nanoflowers via RCA, co-encapsulating a chemotherapeutic agent and an oxygen-generating drug to enhance chemo-sonodynamic therapy for lung cancer. The acidic microenvironment of the tumor can disintegrate the magnesium pyrophosphate skeleton of the DNA nanoflowers to achieve effective controlled release of the drug, enhancing the therapeutic effect of chemotherapy synergistic with sonodynamic therapy. Li et al.124 developed pH-responsive multifunctional DNA nanomicelles as delivery vehicles for the chemo–gene combination therapy of anaplastic large cell lymphoma (ALCL). Due to the pH-responsive mechanism dependent on the Hoogsteen interactions for triplex-helix molecular switch, the release of Dox and siRNA was facilitated to enhance chemosensitivity in ALCL K299 cells. Zhang et al.92 designed an RCA-based DNA nanosponge (DNS) structure, which collapsed under acidic conditions to trigger the release of Dox for chemotherapy due to the dissolution of co-assembled magnesium pyrophosphate after endocytosis.The elevated intracellular GSH levels in cancer cells provide a unique opportunity for targeted drug delivery.125 By designing drug carriers or prodrugs that undergo GSH-triggered activation or degradation, therapeutic agents can be selectively released within tumor microenvironments. Yao et al.69 reported a DNA nanocomplex (DNC–ZMF) synthesized through RCA, containing cascade DNAzymes and promoter-like Zn–Mn-ferrite (ZMF) for combined gene/chemo-dynamic treatment of cancers. The promoter-like ZMF decomposed by consuming intertumoral H+ and GSH, then released metal ions to initiate the subsequent circuits of combination therapy. Furthermore, the elevated production of hydrogen peroxide (H2O2) in cancer cells serves as a distinctive biochemical marker for designing tumor-specific drug delivery systems.126 Tan et al.43 reported self-degradable, cancer-targeted DNA nanoflowers synthesized by adding metal-containing artificial analogues (ferrocene, Fc) to RCA-based DNA strands. Through the Fenton reaction, the introduced Fc facilitates the self-degradation of DNA nanoflowers in the presence of H2O2, facilitating the release of drugs. Moreover, controlled release of nucleic acid drugs can be achieved by utilizing high concentrations of ATP in cells. Lv et al.127 constructed a smart DNA-based nanosystem containing ATP aptamer that was able to respond to ATP for realizing efficient mRNA transfection in macrophages. We envisioned that the ATP aptamer can be designed on the template of RCA/RCT, conferring ATP responsiveness to RCA/RCT-based nanocarriers.
5.3. Exogenous enzyme-responsive release
When endogenous enzyme expression in tumors and their microenvironment proves insufficient to activate drug release, externally controlled enzymes provide a precise solution for triggering nucleic acid drug delivery at the tumor site. Gu et al.86 designed a bioinspired cocoon-like anticancer drug delivery system, consisting of a deoxyribonuclease (DNase)-degradable DNA nanoclew (NCl) synthesized by RCA that was embedded with an acid-responsive DNase I nanocapsule (NCa). In an acidic environment, the activity of DNase I was activated through the acid-triggered shedding of the polymeric shell of the NCa, resulting in the self-degradation of the NCl and promoting the release of Dox for treatment. Gu et al.128 developed an RCA-based DNA nanocarrier (DNC) containing repeating CpG sequences interspersed with HhaI restriction enzyme cleavage sites, enabling enzymatic digestion of the DNC. The HhaI enzyme was encapsulated within triglycerol monostearate (TGMS) and conjugated to the DNC. Under the inflammatory conditions present in the wound site of a tumor resection incision, TGMS undergoes enzymatic degradation, leading to the release of HhaI for the cleavage of DNC to achieve successful release of drugs. Song et al.42 reported a proton-activatable RCA-based DNA nanosystem (denoted as H-DNC) to achieve the co-delivery of Cas9/sgRNA RNPs and DNAzyme. The acid-degradable polymer-coated HhaI enzyme was assembled on the surface of the nanocarrier. After the H-DNC was internalized into cancer cells via a lysosome mediated pathway, in the lysosomal acidic environment, the polymer coating on HhaI was degraded, triggering the release of HhaI, resulting in the release of Cas9/sgRNA RNPs and DNAzyme to achieve combined gene therapy.6. Applications
RCA/RCT-based nanocarriers have demonstrated remarkable potential in nucleic acid drug delivery due to their exceptional ability to encapsulate and protect therapeutic nucleic acids, ensuring stable transport in vivo and precise targeting of diseased cells.129,130 These carriers, leveraging the programmable structural design and molecular recognition properties of DNA/RNA, address critical challenges in conventional cancer therapies—such as gene therapy, PDT, chemodynamic therapy, and immunotherapy—including low delivery efficiency and poor biocompatibility.57,86,131–133 By enabling tailored drug delivery systems and gene-editing tools, nucleic acid nanotechnology offers innovative solutions to overcome tumor resistance and achieve synergistic multi-mechanism therapy. In this section, we summarize the applications of RCR/RCT based nanocarriers in the therapeutics field, including gene therapy, immunotherapy and combination therapy.6.1. Gene therapy
As a critical branch of precision medicine, the clinical translation of gene therapy has long been hindered by insufficient targeting specificity of delivery systems, poor stability of nucleic acid drugs, and the lack of multi-gene regulatory strategies.134 Developing efficient and controllable multifunctional delivery platforms to achieve synergistic tumor-specific gene silencing and editing represents a promising therapeutic strategy.RCT enables the delivery of therapeutic RNA as an integrated carrier-cargo system. Guo et al.135 developed RNA nanospheres via RCT to encapsulate millions of anti-miR21 sequences. These nanospheres were functionalized with dehydroascorbic acid (DHA) to target tumor cells overexpressing glucose transporter 1 (GLUT1). In the high GSH tumor microenvironment, the nanospheres disassembled, subsequently releasing anti-miR21 sequences that were processed by Dicer (Fig. 6(A)). This resulted in a 60% downregulation of oncogenic miR21 (Fig. 6(B)) and upregulation of tumor suppressors phosphatase and tensin homolog deleted on chromosome ten (PTEN) and programmed cell death protein 4 (PDCD4) at the protein level (Fig. 6(C)). In a breast cancer model, the targeted nanoparticles significantly inhibited tumor growth in 70% volume reduction (Fig. 6(D)), induced apoptosis by elevated cleaved caspase-3, and suppressed proliferation by reduced Ki67 expression. While single-gene targeting strategies have shown progress, the heterogeneity of malignant tumors and multi-pathway activation demand more sophisticated interventions.
 | ||
| Fig. 6 RCA/RCT-based nanocarriers for gene therapy. (A) Schematic illustration of the preparation, compression, and therapeutic delivery of the anti-miRNA21 pompon. (B) miR21 gene down-regulation in the MDA-MB-231 cell line. (C) Western blot analysis of PDCD4 and PTEN. (D) Tumor growth curve over the course of 5 injections.135 Copyright 2019, Elsevier Ltd. All rights reserved. (E) Schematic illustration of the preparation for the co-delivery of Cas9/sgRNA and DNAzyme by the RCA technique. (F) Schematic illustration of the cancer therapeutic process of proton-activatable nanocarriers. (G) Western blotting analysis of PLK1 and EGR-1 proteins in MCF-7 cells with different treatments. H-DSCS: DNAzyme and sgRNA were replaced with scramble sequences in H-DNC; H-DECS: DNAzyme was designed for EGR-1 silencing, and sgRNA was replaced with scramble sequences; H-DSCE: DNAzyme was replaced with scramble sequences, and sgRNA was designed for PLK1 gene recognition; H-DECE: DNAzyme was designed for EGR-1 silencing, and sgRNA was designed for PLK1 gene recognition. (H) Apoptosis analysis of MCF-7 cells treated with different drug formulations using flow cytometry with the Annexin V-FITC/PI assay. (I) The average tumor weight in groups treated with different drug formulations.42 Copyright 2022, Wiley-VCH GmbH. | ||
Our group advanced the field by integrating gene editing and silencing technologies, enhancing the precision and synergy of delivery systems. Li et al.42 synthesized ultra-long ssDNA scaffolds via RCA, incorporating sgRNA recognition sequences, DNAzyme sequences, and HhaI enzyme cleavage sites. Mn2+ was used to compress the DNA scaffolds into nanoparticles (DNC), which were coated with acid-degradable polymer (PGDA)-encapsulated HhaI enzymes (GHhaI) to form a proton-responsive system (H-DNC) (Fig. 6(E)). In the acidic lysosomal environment, PGDA degradation triggered HhaI release, cleaving the DNA scaffold to liberate Cas9/sgRNA complexes and DNAzymes. These components synergistically regulated polo-like kinase 1 (PLK1) and early growth responsive gene-1 (EGR-1) genes (Fig. 6(F)). In vitro experiments demonstrated a 75.26% apoptosis rate in MCF-7 cells (Fig. 6(H)), while in vivo studies revealed efficient tumor targeting in breast cancer models, achieving an 85% tumor suppression rate without significant toxicity (Fig. 6(I)).
6.2. Immunotherapy
The combination between nucleic acid drug delivery and immunotherapy lies in precise targeting and immune modulation. Encoding antigens with mRNA, silencing immune-inhibiting factors with siRNA, or enhancing immune-stimulating motifs can directly boost the antitumor immune response. Meanwhile, intelligent delivery systems, such as targeted nanoparticles and stimuli-responsive carriers, ensure accurate drug delivery to immune cells and spatiotemporally controlled release.However, cancer immunotherapy remains constrained by inefficient antigen presentation, immunosuppressive microenvironments, and limited activation of single signaling pathways. The following studies address these challenges through multi-targeted delivery and integrated signaling pathway activation to remodel antitumor immunity. Zhu et al.79 developed self-assembled DNA–RNA nanocapsules (iDR-NCs) co-loaded with Adpgk (a neoantigen derived from MC38 tumor mutation, ASMTNRELM → ASMTNMELM), Stat3 shRNA (targeting the immunosuppressive STAT3 signaling pathway), and CpG ODN (a TLR9 agonist), achieving triple synergistic therapeutic effects (Fig. 7(A)). CpG ODNs activated TLR9 to enhance antigen presentation by dendritic cells (DCs), improving the delivery efficiency of Adpgk and activating antigen-presenting cells (APCs). Stat3 shRNA alleviated immunosuppression in APCs via RNA interference. In the MC38 colon adenocarcinoma model, iDR-NC/Adpgk-treated mice showed a 5-fold reduction in lung metastasis weight compared to the CpG+ Adpgk group (Fig. 7(C)). After 21 days, the Adpgk-specific CD8+ T cell counts increased 8-fold (Fig. 7(D) and (E)), and after 49 days, the proportion of Adpgk-specific central memory T cells (CD44+CD62Lhigh) rose significantly with upregulated PD-1 (Fig. 7(F)).
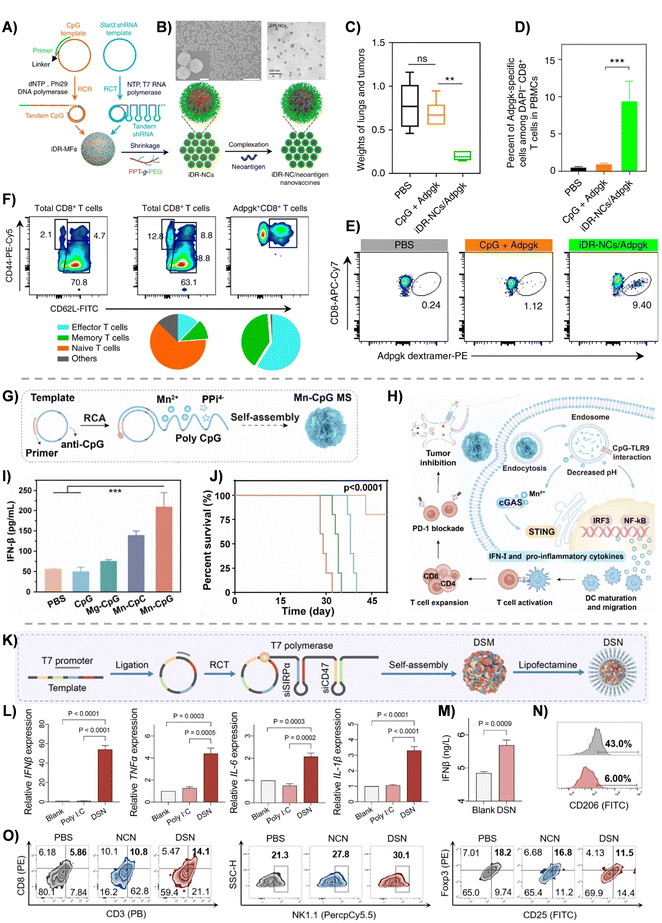 | ||
Fig. 7 RCA/RCT-based nanocarriers for immunotherapy. (A) Schematics of iDR-NC/neoantigen nanovaccines for synergistic tumor immunotherapy. Concurrent RCR and RCT in the same solution generated tandem CpG and Stat3 shRNA, which were self-assembled into intertwining DNA–RNA MFs. (B) SEM images showing the morphologies of CpG-shRNA MFs generated after combined RCR/RCT for 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h. TEM images showing the morphologies of iDR-NCs shrunk by PPT-g-(PEG)6. (C) Representative weights of the lungs and tumors from as-treated mice on day 40 post tumor inoculation. (D) Percent of Adpgk-specific cells among ASMTNMELM-specific CD8 T cells among live (DAPI−) CD8+ cells in peripheral blood mononuclear cells (PBMCs). (E) Representative flow cytometry of DNPI− CD8+ T cells in PBMCs. (F) Upper: representative flow cytometry plots showing CD8+ T cell effector/effector memory/central memory phenotypes in PBMCs of naive mice and mice vaccinated with iDR-NC/Adpgk on day 49; lower: quantification of the fractions of CD8+ T cell populations in mice vaccinated with iDR-NC/Adpgk. iDR-NC/Adpgk induced substantial central memory (CD44+CD62Lhigh) CD8+ T cells, especially memory Adpgk+CD8+ T cells compared with age-matched naive mice.79 Copyright 2017, Nature Publishing Group. (G) Schematic illustration of the construction process of Mn-CpG MS. (H) Schematic illustration of the drug self-delivery system for synergistic innate immune-stimulation via co-activating STING and TLR9 pathways. (I) Relative mRNA expression of the cGAS–STING axis in cells treated with Mn-CpG for 24 h. TEM images showing the morphologies of iDR-NCs shrunk by PPT-g-(PEG)6. (C) Representative weights of the lungs and tumors from as-treated mice on day 40 post tumor inoculation. (D) Percent of Adpgk-specific cells among ASMTNMELM-specific CD8 T cells among live (DAPI−) CD8+ cells in peripheral blood mononuclear cells (PBMCs). (E) Representative flow cytometry of DNPI− CD8+ T cells in PBMCs. (F) Upper: representative flow cytometry plots showing CD8+ T cell effector/effector memory/central memory phenotypes in PBMCs of naive mice and mice vaccinated with iDR-NC/Adpgk on day 49; lower: quantification of the fractions of CD8+ T cell populations in mice vaccinated with iDR-NC/Adpgk. iDR-NC/Adpgk induced substantial central memory (CD44+CD62Lhigh) CD8+ T cells, especially memory Adpgk+CD8+ T cells compared with age-matched naive mice.79 Copyright 2017, Nature Publishing Group. (G) Schematic illustration of the construction process of Mn-CpG MS. (H) Schematic illustration of the drug self-delivery system for synergistic innate immune-stimulation via co-activating STING and TLR9 pathways. (I) Relative mRNA expression of the cGAS–STING axis in cells treated with Mn-CpG for 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h. (J) Survival curves of tumor-bearing mice after various post-surgical treatments.136 Copyright 2023, Elsevier Ltd. All rights reserved. (K) Schematic illustration of the preparation procedure of DSN. (L) RT-qPCR analysis of the mRNA level of IFNβ, TNFα, IL-6 and IL-1β in THP-1 cells treated with poly I: h. (J) Survival curves of tumor-bearing mice after various post-surgical treatments.136 Copyright 2023, Elsevier Ltd. All rights reserved. (K) Schematic illustration of the preparation procedure of DSN. (L) RT-qPCR analysis of the mRNA level of IFNβ, TNFα, IL-6 and IL-1β in THP-1 cells treated with poly I:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C or DSN for 24 h. (M) ELISA quantification of IFNβ in the supernatant after incubating bone marrow-derived dendritic cells (BMDCs) with DSN for 24 h. (N) Flow cytometric analysis of CD206 expression on M2-like bone marrow-derived macrophages (BMDMs) after treatment with DSN for 24 h. (O) Flow cytometric analysis of other immune cells, including the infiltration of CD8 T cells, NKs and Tregs in the tumors.70 Copyright 2024, Wiley-VCH GmbH. C or DSN for 24 h. (M) ELISA quantification of IFNβ in the supernatant after incubating bone marrow-derived dendritic cells (BMDCs) with DSN for 24 h. (N) Flow cytometric analysis of CD206 expression on M2-like bone marrow-derived macrophages (BMDMs) after treatment with DSN for 24 h. (O) Flow cytometric analysis of other immune cells, including the infiltration of CD8 T cells, NKs and Tregs in the tumors.70 Copyright 2024, Wiley-VCH GmbH. | ||
Despite progress in antigen targeting and T cell activation, the limited breadth of innate immune responses remains a barrier. Yu et al.136 integrated STING and TLR9 pathway activation through a biomineralization strategy, enabling dual-engine activation of innate/adaptive immunity and synergizing with immune checkpoint blockade to amplify systemic antitumor immunity. They constructed a biomineralized DNA sponge (Mn-CpG) via RCA, co-loading the STING agonist Mn2+ and TLR9 agonist CpG ODNs (Fig. 7(G)). Mn2+ activated the cGAS-STING pathway to induce type I interferon (IFN-I) production, while CpG triggered TLR9 to facilitate APC maturation and co-stimulatory molecule expression (Fig. 7(H)). Mn-CpG treatment could significantly promote the IFN-β production of immune cells (Fig. 7(I)). In a 4T1 breast cancer model, Mn-CpG monotherapy inhibited primary tumor growth by 90.2% and suppressed lung metastasis. When combined with anti-PD-1 checkpoint blockade, Mn-CpG completely eradicated primary and distant tumors, significantly prolonging survival (Fig. 7(J)).
Xu et al.70 further advanced RNA self-assembly technology by generating long-strand RNA via RCT to activate the RIG-I/MDA5 pathway, while integrating dual siRNA (targeting CD47 and SIRPα) to block the “don’t eat me” signals (Fig. 7(K)). This approach not only strongly activated RIG-I/MDA5 signaling but also effectively inhibited the CD47–SIRPα checkpoint, thereby enhancing APC phagocytic activity. It also promoted the cross-priming of effector T cells and activated anti-tumor immune responses. In the study, the dual-functional RNA nanostructure (DSN) activated the RIG-I/MDA5 pathway, upregulated IRF3/NF-κB phosphorylation, and increased IFN-β and IL-6 secretion 3–5 times compared to the control group (Fig. 7(L)). It also facilitated DC maturation and repolarized tumor-associated macrophages (TAMs) toward the antitumor M1 phenotype (Fig. 7(M)), reducing CD206+ macrophages from 43% to 6% (Fig. 7(N)). By silencing CD47 and SIRPα through RNA interference, DSN effectively suppressed the CD47–SIRPα immune checkpoint and enhanced APC phagocytosis of tumor cells. In a bilateral CT26 tumor model, DSN inhibited the growth of both primary and distant tumors, reducing tumor volume by 60% compared to controls. Additionally, it remodeled the immunosuppressive tumor microenvironment (TIME), increasing CD8+ T cell infiltration 2.4 times, reducing Treg cells by 37%, and boosting NK cell proportion to 30.1% (Fig. 7(O)).
6.3. Combination therapy
In oncology, monotherapy often falls short due to the disease's complexity and heterogeneity.137 Combining nucleic acid drugs with other treatments like chemotherapy, phototherapy, or immune checkpoint inhibitors has emerged as a promising strategy. This combination not only enhances tumor cell destruction but also overcomes drug resistance and immune evasion, significantly improving therapeutic outcomes.138RCT can transcribe large quantities of RNA molecules, such as siRNA, miRNA, and mRNA, which are used to regulate gene expression.139 Yu et al.81 used RCT to create an RNA nanosponge containing AS1411 aptamers and anti-miR-21 sequences, loaded with Dox. The AS1411 aptamer targets colorectal cancer cells, while anti-miR-21 inhibits oncogene expression and counteracts Dox-induced miR-21 upregulation. Dox intercalates into DNA base pairs, causing damage and apoptosis (Fig. 8(A)). In contrast to the 1.61% rate in the control group, the apoptosis rate considerably increased to 3.39% and 8.03% in the Dox and FND groups, respectively (Fig. 8(B)). The FND induced G2/M cell cycle arrest in 77.16% of cells (Fig. 8(C)).
 | ||
| Fig. 8 RCA/RCT-based nanocarriers for combination therapy. (A) Schematic illustration of the FND preparation principle and application. (B) Flow cytometry detection of cell apoptosis induced by FND. (C) Flow cytometry detection of cell G2/M cycle arrest induced by FND.81 Copyright 2023, Nature Publishing Group. (D) Schematic illustration of an RCA-generated ZMF nanoflower coupled with DNAzyme for gene/chemo-dynamic therapy. (E) Western blotting analysis with antibodies against EGR-1 and β-actin. (F) Average ratio of fluorescence changes in intracellular ˙OH detected by coumarin and O2 detection by [Ru(dpp)3]2+Cl2.69 Copyright 2021, Wiley-VCH GmbH. (G) Schematic illustration of the preparation of multifunctional RCR-CC-ZnO NSs and the stimulus-responsive cascade activation of DNAzyme for efficient gene silencing and drug delivery in cancer cells. (H) PAGE analysis of DNAzyme-mediated substrate cleavage under different concentrations of Zn2+. (I) Western blot analysis of intracellular survivin protein expression of differently treated MCF-7 cells. (J) Live/dead staining of MCF-7 cells after different treatments. (a) PBS, (b) RCR NSs, (c) CC, (d) ZnO NPs, (e) RCR-CC-ZnO NSs, (f) RCR (rMT)-CC-ZnO NSs, (g) RCR-ZnO NSs, and (h) RCR (tMT)-CC-ZnO NSs. Scale bar = 100 μm.141 Copyright 2021, Wiley-VCH GmbH. (K) Schematic illustration of the molecular design and preparation of UCND. (L) Schematic illustration of the preparation of UCND and cellular uptake of UNCE and the process of synergistic photodynamic therapy. (M) CLSM images of the ROS level in MCF-7 cells. (N) The average growth profiles of tumors in different treatment groups.41 Copyright 2023, Wiley-VCH GmbH. | ||
DNAzyme-based gene therapy shows great promise but faces challenges in delivery efficiency and conditional activation.140 Yao et al.69 used RCA to synthesize ultralong DNA strands with AS1411 aptamers and cascade DNA enzymes, combined with ZMF to release metal ions and generate ROS. DNAzymes sequentially cleave DNA strands and EGR-1 mRNA, inhibiting tumor proliferation. ZMF releases Zn2+, Mn2+, and Fe2+, generating ROS via the Fenton reaction (Fig. 8(D)). Results showed that DNC–ZMF downregulated EGR-1 protein expression by 66% and induced 54.6% late apoptosis in MCF-7 cells (Fig. 8(E)). The fluorescence intensity of [Ru(dpp)3]2+Cl2 decreased, suggesting O2 generation in the DNC–ZMF treated cells. The fluorescence intensity of 7-hydroxycoumarin increased during the incubation period, suggesting that free radicals were generated in the DNC–ZMF-treated tumor cells and accumulated over the 6-h incubation period (Fig. 8(F)). In a breast cancer model, DNC–ZMF achieved a 70.4% tumor suppression rate without systemic toxicity. Similarly, Wang et al.141 used RCA to assemble DNAzyme nanocapsules with therapeutic DNAzymes and self-cleaving DNAzymes for sustained tumor-specific activation. ZnO nanoparticles released Zn2+ in the acidic tumor environment, activating DNAzymes. The nanocapsule surface was modified with MUC-1 aptamers for specific tumor cell recognition. The therapeutic DNAzyme silenced survivin mRNA to relieve apoptosis inhibition, while pro-apoptotic cytochrome c protein (CC) activated the mitochondrial apoptosis pathway (Fig. 8(G)). In vitro, tDNAzyme significantly reduced survivin mRNA and protein levels (Fig. 8(H)). Nanocapsules induced caspase-3 activation and 29.4% apoptosis by Annexin V/PI staining (Fig. 8(J)). In MCF-7 xenograft models, targeted nanocapsules markedly reduced tumor growth by 5-fold.
While the chemodynamic and gene silencing combination is effective, tumor hypoxia and ROS scavenging mechanisms remain limitations. PDT, a non-invasive cancer treatment, involves light-activated photosensitizers (PS) to generate ROS, causing oxidative damage and apoptosis in cancer cells. Song et al.41 combined PDT with CRISPR gene editing to enhance precision. They synthesized ultra-long ssDNA via RCA, incorporating sgRNA recognition sequences and G-quadruplexes, combined with UCNPs and PP (Fig. 8(K)). UCNPs convert 980 nm near-infrared light to 409 nm visible light to activate PP, generating singlet oxygen (1O2) for PDT. CRISPR–Cas9 knockout of the antioxidant regulator Nrf2 reduced the ROS clearance capacity of cancer cells. G-quadruplex/hemin mimicked the HRP enzyme, catalyzing H2O2 to O2 and alleviating tumor hypoxia (Fig. 8(L)). Results showed that UCND groups had higher ROS levels than controls (Fig. 8(M)), with Nrf2 protein expression downregulated by 54%. Gene editing combined with PDT increased 1O2 accumulation and reduced mitochondrial membrane potential. In a breast cancer model, UCND achieved 86.32% tumor inhibition without organ toxicity (Fig. 8(N)).
7. Conclusion and outlook
In this review, we present an overview of two nucleic acid-based nanotechnologies, namely RCA and RCT, for nucleic acid drug delivery, which are powerful molecular techniques derived from natural biological processes. On this basis, we first conclude four fundamental nanoization strategies for RCA/RCT-derived carriers and then systematically summarize their three core technological aspects, including nucleic acid drug loading strategies, precision targeting strategies, and drug-controlled release strategies, which are fundamental to achieving efficient drug delivery. With appropriate molecular design and various mechanisms of action, such as biomineralization, electrostatic interactions, base complementary pairing, and hydrophobic effects, RCA/RCT-based ultra-long ssDNA/ssRNA can be self-assembled into diverse nanocarriers such as DNA/RNA nanostructures, DNA/RNA nanoparticles, DNA/RNA nanocomplexes and DNA–RNA nanocomposites. RCA/RCT-based nanotechnology enables extensive integration of nucleic acid drugs into DNA/RNA-based nanocarriers through three key strategies: by designing them into RCA/RCT-derived ssDNA or ssRNA, functional DNA/RNA ODNs—DNAzymes, ASOs, siRNA, and crRNA/sgRNA—can be effectively incorporated; the functional DNA/RNA ODNs above can also be integrated via complementary base pairing; moreover, nucleic acid drugs encapsulated in a positively charged shell can be integrated through electrostatic interactions. An optimal drug carrier must possess precise targeting capabilities. At present, RCA/RCT-based DNA/RNA nanocarriers are commonly functionalized with nucleic acid aptamers, proteins, polymers, and small molecule ligands to achieve targeted delivery. Another excellent quality of a good nucleic acid drug nanocarrier is that it can protect its cargo from degradation in a highly controlled manner until it is triggered by certain signals. Endogenous enzymes, exogenous enzymes and the intracellular microenvironment are all important examples of stimuli for responsive RCA/RCT-based DNA/RNA nanocarriers. Currently, RCA/RCT-derived nanocarriers have been developed as advanced delivery platforms for nucleic acid therapeutics, with applications in gene therapy, immunotherapy, and combination treatment strategies.Despite the considerable promise of RCA/RCT-based nanotechnology for nucleic acid delivery, critical challenges remain to be addressed to enable successful clinical translation: (1) improving the in vivo stability – while these systems demonstrate superior stability compared to free nucleic acids, they remain vulnerable to nuclease degradation in biological environments, necessitating additional stabilization approaches such as chemical modifications or protective coatings; (2) prolonging the accumulation time in target tissues – achieving specific delivery and long-term accumulation in target tissues requires further optimization of targeting ligands and understanding of nanoparticle–biological interactions; (3) developing more smart responsive systems – future designs may incorporate more sophisticated stimuli-responsive elements that enable precise spatial and temporal control over drug release; (4) improving immune compatibility. While RCA/RCT systems are less immunogenic than viral vectors, their repetitive nucleic acid structures may still activate innate immune pathways (e.g., TLR or STING signaling). Strategies to mitigate immune recognition—such as incorporating immunosuppressive motifs or stealth coatings—could broaden their therapeutic applicability.
In summary, RCA/RCT-based nanotechnology offers a versatile and powerful platform for nucleic acid drug delivery, uniquely combining the programmability of nucleic acids with the precision of enzymatic synthesis. This approach has shown remarkable potential in overcoming critical delivery challenges, including payload capacity limitations, tissue-specific targeting, and spatiotemporal control of therapeutic release. With continued advancements in DNA nanotechnology, RCA/RCT-based delivery platforms are poised to expand their therapeutic scope, enabling efficient delivery of diverse nucleic acid payloads—including mRNA vaccines, plasmid DNA, and emerging genomic medicines. Future developments in RCA/RCT-based nanocarriers will focus on these directions: (1) innovative nanocarrier design: developing dynamically responsive hybrid vectors to enhance drug loading capacity and targeting precision; (2) intelligent delivery optimization: implementing logic-gated release mechanisms and barrier-penetration strategies to achieve spatiotemporally controlled drug delivery; (3) enhanced safety: reducing off-target risks and long-term toxicity through immunogenicity modulation and advanced toxicity control technologies. This evolution will position RCA/RCT systems as indispensable tools for advancing both nucleic acid therapeutics and precision medicine paradigms. Looking forward, integrating staple-strand assisted folding strategies—akin to DNA origami—with RCA products could bridge this technological gap, enabling programmable 3D architectures while maintaining RCA's advantages in scalable production. Such hybrid approaches may substantially enhance the structural precision and clinical potential of next-generation DNA nanocarriers.
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 22225505, 22322407, 22174097, and W2412018). Dayong Yang thanks Fudan University Ruiqing Education Funding.References
- J. Belgrad, H. H. Fakih and A. Khvorova, Nucleic Acid Ther., 2024, 34, 52–72 CrossRef CAS PubMed.
- S. Naeem, J. Zhang, Y. Zhang and Y. Wang, Mol. Ther.–Nucleic Acids, 2025, 36, 102440 CrossRef CAS PubMed.
- X. Sun, S. Setrerrahmane, C. Li, J. Hu and H. Xu, Signal Transduction Targeted Ther., 2024, 9, 316 CrossRef PubMed.
- Z.-G. Lu, J. Shen, J. Yang, J.-W. Wang, R.-C. Zhao, T.-L. Zhang, J. Guo and X. Zhang, Signal Transduction Targeted Ther., 2023, 8, 39 CrossRef CAS PubMed.
- M. A. Havens and M. L. Hastings, Nucleic Acids Res., 2016, 44, 6549–6563 CrossRef PubMed.
- A. Wittrup and J. Lieberman, Nat. Rev. Genet., 2015, 16, 543–552 CrossRef CAS PubMed.
- D. Bhatnagar, S. Ladhe and D. Kumar, Mol. Neurobiol., 2023, 60, 5954–5974 CrossRef CAS PubMed.
- A. M. Costello, M.-A. Elizondo-Riojas, X. Li, D. E. Volk, A. K. Pillai and H. Wang, Molecules, 2021, 26, 6525 CrossRef CAS PubMed.
- W. Ren, S. Duan, C. Dai, C. Xie, L. Jiang and Y. Shi, Molecules, 2023, 28, 3500 CrossRef CAS PubMed.
- T. Hermann and D. J. Patel, Science, 2000, 287, 820–825 CrossRef CAS PubMed.
- J. E. Rosenberg, R. M. Bambury, E. M. Van Allen, H. A. Drabkin, P. N. Lara, Jr., A. L. Harzstark, N. Wagle, R. A. Figlin, G. W. Smith, L. A. Garraway, T. Choueiri, F. Erlandsson and D. A. Laber, Invest. New Drugs, 2014, 32, 178–187 CrossRef CAS PubMed.
- Y. Huang, X. Liu, J. Zhu, Z. Chen, L. Yu, X. Huang, C. Dong, J. Li, H. Zhou, Y. Yang and W. Tan, J. Am. Chem. Soc., 2024, 146, 13805–13816 CrossRef CAS PubMed.
- J. A. Wolff, R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani and P. L. Felgner, Science, 1990, 247, 1465–1468 CrossRef CAS PubMed.
- M. E. Davis, J. E. Zuckerman, C. H. J. Choi, D. Seligson, A. Tolcher, C. A. Alabi, Y. Yen, J. D. Heidel and A. Ribas, Nature, 2010, 464, 1067–1070 CrossRef CAS PubMed.
- B. Kim, J. H. Park and M. J. Sailor, Adv. Mater., 2019, 31, e1903637 CrossRef PubMed.
- J. A. Kulkarni, D. Witzigmann, S. B. Thomson, S. Chen, B. R. Leavitt, P. R. Cullis and R. van der Meel, Nat. Nanotechnol., 2021, 16, 630–643 CrossRef CAS PubMed.
- G. W. Liu, E. B. Guzman, N. Menon and R. S. Langer, Pharm. Res., 2023, 40, 3–25 CrossRef CAS PubMed.
- G. J. Doherty and H. T. McMahon, Annu. Rev. Biochem., 2009, 78, 857–902 CrossRef CAS PubMed.
- S. W. Tse, K. McKinney, W. Walker, M. Nguyen, J. Iacovelli, C. Small, K. Hopson, T. Zaks and E. Huang, Mol. Ther., 2021, 29, 2227–2238 CrossRef CAS PubMed.
- Y. Liu, K. Tiruthani, M. Wang, X. Zhou, N. Qiu, Y. Xiong, C. V. Pecot, R. Liu and L. Huang, Nanoscale Horiz., 2021, 6, 319–329 RSC.
- M. Qiu, Y. Tang, J. Chen, R. Muriph, Z. Ye, C. Huang, J. Evans, E. P. Henske and Q. Xu, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2116271119 CrossRef CAS PubMed.
- N. N. Parayath, B. V. Hong, G. G. Mackenzie and M. M. Amiji, Nanomedicine, 2021, 16, 2291–2303 CrossRef CAS PubMed.
- Q. Zhang, Y. Guo, L. Zhu, X. Liu, J. Yang, Y. Li, X. Zhu and C. Zhang, Biomater. Sci., 2021, 9, 4755–4764 RSC.
- J. Karlsson, S. Y. Tzeng, S. Hemmati, K. M. Luly, O. Choi, Y. Rui, D. R. Wilson, K. L. Kozielski, A. Quiñones-Hinojosa and J. J. Green, Adv. Funct. Mater., 2021, 31, 2009768 CrossRef CAS PubMed.
- L. Zhao, C. Gu, Y. Gan, L. Shao, H. Chen and H. Zhu, J. Controlled Release, 2020, 318, 1–15 CrossRef CAS PubMed.
- M. Li, Q. Wang, X. Zhang, N. Yan and X. Li, Cancer Cell Int., 2020, 20, 574 CrossRef CAS PubMed.
- Y. Tian, J. R. Lhermitte, L. Bai, T. Vo, H. L. Xin, H. Li, R. Li, M. Fukuto, K. G. Yager, J. S. Kahn, Y. Xiong, B. Minevich, S. K. Kumar and O. Gang, Nat. Mater., 2020, 19, 789–796 CrossRef CAS PubMed.
- Y. Zhao, X. Dai, F. Wang, X. Zhang, C. Fan and X. Liu, Nano Today, 2019, 26, 123–148 CrossRef CAS.
- M. M. F. A. Baig, W. L. Dissanayaka and C. Zhang, Int. J. Biol. Macromol., 2021, 188, 657–669 CrossRef CAS PubMed.
- H. Lu, T. Hailin, X. Yi and J. Wang, Langmuir, 2020, 36, 10708–10714 CrossRef CAS PubMed.
- L. Fang, C. Shi, Y. Wang, Z. Xiong and Y. Wang, J. Nanobiotechnol., 2023, 21, 290 CrossRef CAS PubMed.
- Y. Huang, Z. Chen, H. Huang, S. Ding and M. Zhang, RSC Adv., 2025, 15, 6208–6230 RSC.
- S. Sun, H. Yang, Z. Wu, S. Zhang, J. Xu and P. Shi, Chem. Commun., 2024, 60, 3098–3117 RSC.
- W. Zhang, F. Wang, Y. Wang, J. Wang, Y. Yu, S. Guo, R. Chen and D. Zhou, J. Controlled Release, 2016, 232, 9–19 CrossRef CAS PubMed.
- H. Zhao, X. Yuan, J. Yu, Y. Huang, C. Shao, F. Xiao, L. Lin, Y. Li and L. Tian, ACS Appl. Mater. Interfaces, 2018, 10, 15418–15427 CrossRef CAS PubMed.
- H.-Q. Yu, D.-H. Zhang, X.-B. Gu, D. Miyoshi and N. Sugimoto, Angew. Chem., Int. Ed., 2008, 47, 9034–9038 CrossRef CAS PubMed.
- L. Zhu, P. Kate and V. P. Torchilin, ACS Nano, 2012, 6, 3491–3498 CrossRef CAS PubMed.
- J. Li, C. Zheng, S. Cansiz, C. Wu, J. Xu, C. Cui, Y. Liu, W. Hou, Y. Wang, L. Zhang, I. T. Teng, H.-H. Yang and W. Tan, J. Am. Chem. Soc., 2015, 137, 1412–1415 CrossRef CAS PubMed.
- F. Perche, S. Biswas, T. Wang, L. Zhu and V. P. Torchilin, Angew. Chem., Int. Ed., 2014, 53, 3362–3366 CrossRef CAS PubMed.
- X. Zhuang, X. Ma, X. Xue, Q. Jiang, L. Song, L. Dai, C. Zhang, S. Jin, K. Yang, B. Ding, P. C. Wang and X.-J. Liang, ACS Nano, 2016, 10, 3486–3495 CrossRef CAS PubMed.
- N. Song, X. Fan, X. Guo, J. Tang, H. Li, R. Tao, F. Li, J. Li, D. Yang, C. Yao and P. Liu, Adv. Mater., 2024, 36, 2309534 CrossRef CAS PubMed.
- F. Li, N. Song, Y. Dong, S. Li, L. Li, Y. Liu, Z. Li and D. Yang, Angew. Chem., Int. Ed., 2022, 61, e202116569 CrossRef CAS PubMed.
- L. Zhang, R. Abdullah, X. Hu, H. Bai, H. Fan, L. He, H. Liang, J. Zou, Y. Liu, Y. Sun, X. Zhang and W. Tan, J. Am. Chem. Soc., 2019, 141, 4282–4290 CrossRef CAS PubMed.
- S. Paul, M. F. Konig, D. M. Pardoll, C. Bettegowda, N. Papadopoulos, K. M. Wright, S. B. Gabelli, M. Ho, A. van Elsas and S. Zhou, Nat. Rev. Cancer, 2024, 24, 399–426 CrossRef CAS PubMed.
- X. Gong, H. Wang, R. Li, K. Tan, J. Wei, J. Wang, C. Hong, J. Shang, X. Liu, J. Liu and F. Wang, Nat. Commun., 2021, 12, 3953 CrossRef CAS PubMed.
- V. Linko, A. Ora and M. A. Kostiainen, Trends Biotechnol., 2015, 33, 586–594 CrossRef CAS PubMed.
- Y. Guo, S. Li, Z. Tong, J. Tang, R. Zhang, Z. Lv, N. Song, D. Yang and C. Yao, J. Am. Chem. Soc., 2023, 145, 23859–23873 CrossRef CAS PubMed.
- Y. Hu, S. Gao, H. Lu, S. Tan, F. Chen, Y. Ke and J. Y. Ying, Nano Lett., 2023, 23, 9778–9787 CrossRef CAS PubMed.
- J. Han, Y. Cui, F. Li, Z. Gu and D. Yang, Nano Today, 2021, 39, 101160 CrossRef CAS.
- J. Han, Y. Cui, Z. Gu and D. Yang, Biomaterials, 2021, 273, 120846 CrossRef CAS PubMed.
- Y. Dong, F. Li, Z. Lv, S. Li, M. Yuan, N. Song, J. Liu and D. Yang, Angew. Chem., Int. Ed., 2022, 61, e202207770 CrossRef CAS PubMed.
- Z. Lv, Z. Li, S. Zou, P. Li, N. Song, R. Zhang, M. Xu, M. Liu, F. Li, J. Li, P. Liu, C. Yao and D. Yang, Adv. Funct. Mater., 2024, 34, 2311069 CrossRef CAS.
- M. G. Mohsen and E. T. Kool, Acc. Chem. Res., 2016, 49, 2540–2550 CrossRef CAS PubMed.
- M. M. Ali, F. Li, Z. Zhang, K. Zhang, D. K. Kang, J. A. Ankrum, X. C. Le and W. Zhao, Chem. Soc. Rev., 2014, 43, 3324–3341 RSC.
- J. Nelson, Y. Cai, T. Giesler, J. Farchaus, S. Sundaram, M. Ortiz-Rivera, L. Hosta, P. Hewitt, J. Mamone, C. Palaniappan and C. Fuller, Biotechniques, 2002, 32, 44–47 CrossRef PubMed.
- N. Abe, K. Matsumoto, M. Nishihara, Y. Nakano, A. Shibata, H. Maruyama, S. Shuto, A. Matsuda, M. Yoshida, Y. Ito and H. Abe, Sci. Rep., 2015, 5, 16435 CrossRef CAS PubMed.
- J. B. Lee, J. Hong, D. K. Bonner, Z. Poon and P. T. Hammond, Nat. Mater., 2012, 11, 316–322 CrossRef CAS PubMed.
- P. Guo, Mol. Ther.–Nucleic Acids, 2012, 1, e36 CrossRef PubMed.
- A. A. Seyhan, A. V. Vlassov and B. H. Johnston, Oligonucleotides, 2006, 16, 353–363 CrossRef CAS PubMed.
- G. Zhu, G. M. Lynn, O. Jacobson, K. Chen, Y. Liu, H. Zhang, Y. Ma, F. Zhang, R. Tian, Q. Ni, S. Cheng, Z. Wang, N. Lu, B. C. Yung, Z. Wang, L. Lang, X. Fu, A. Jin, I. D. Weiss, H. Vishwasrao, G. Niu, H. Shroff, D. M. Klinman, R. A. Seder and X. Chen, Nat. Commun., 2017, 8, 1954 CrossRef PubMed.
- C. Yao, R. Zhang, J. Tang and D. Yang, Nat. Protoc., 2021, 16, 5460–5483 CrossRef CAS PubMed.
- R. R. Garafutdinov, A. R. Sakhabutdinova, A. R. Gilvanov and A. V. Chemeris, Russ. J. Bioorg. Chem., 2021, 47, 1172–1189 CrossRef CAS PubMed.
- Y. Li, M. Qian, Y. Cheng and X. Qiu, Colloids Surf., B, 2025, 248, 114486 CrossRef CAS PubMed.
- R. Deng, L. Tang, Q. Tian, Y. Wang, L. Lin and J. Li, Angew. Chem., Int. Ed., 2014, 53, 2389–2393 CrossRef CAS PubMed.
- K. Ren, Y. Xu, Y. Liu, M. Yang and H. Ju, ACS Nano, 2018, 12, 263–271 CrossRef CAS PubMed.
- Z. Zhang, M. M. Ali, M. A. Eckert, D.-K. Kang, Y. Y. Chen, L. S. Sender, D. A. Fruman and W. Zhao, Biomaterials, 2013, 34, 9728–9735 CrossRef CAS PubMed.
- A. Fire and S. Q. Xu, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 4641–4645 CrossRef CAS PubMed.
- H. Zhao, J. Lv, F. Li, Z. Zhang, C. Zhang, Z. Gu and D. Yang, Biomaterials, 2021, 268, 120591 CrossRef CAS PubMed.
- C. Yao, H. Qi, X. Jia, Y. Xu, Z. Tong, Z. Gu and D. Yang, Angew. Chem., Int. Ed., 2022, 61, e202113619 CrossRef CAS PubMed.
- X. Xu, S. Li, W. Yu, S. Yao, H. Fan and Z. Guo, Angew. Chem., Int. Ed., 2024, 63, e202318544 CrossRef CAS PubMed.
- N. Fan, X. Bian, M. Li, J. Chen, H. Wu, Q. Peng, H. Bai, W. Cheng, L. Kong, S. Ding, S. Li and W. Cheng, Sci. Adv., 2022, 8, eabn7382 CrossRef CAS PubMed.
- S. Yang, Y. Cheng, M. Liu, J. Tang, S. Li, Y. Huang, X. Kou, C. Yao and D. Yang, Nano Today, 2024, 56, 102224 CrossRef CAS.
- W. Sun, W. Ji, J. M. Hall, Q. Hu, C. Wang, C. L. Beisel and Z. Gu, Angew. Chem., Int. Ed., 2015, 54, 12029–12033 CrossRef CAS PubMed.
- W. Sun, J. Wang, Q. Hu, X. Zhou, A. Khademhosseini and Z. Gu, Sci. Adv., 2020, 6, eaba2983 CrossRef CAS PubMed.
- H. Zhao, L. Li, F. Li, C. Liu, M. Huang, J. Li, F. Gao, X. Ruan and D. Yang, Adv. Mater., 2022, 34, e2109920 CrossRef PubMed.
- J. Yu, J. Li, S. Zhai, L. Lin, K. Wang, B. Tang, H. Meng and L. Tian, ACS Appl. Bio Mater., 2019, 2, 5204–5215 CrossRef CAS PubMed.
- H. Nam, S. H. Ku, H. Y. Yoon, K. Kim, I. C. Kwon, S. H. Kim and J. B. Lee, Adv. Ther., 2019, 2, 1900014 CrossRef.
- H. Han, D. Kim, Y. Jang, M. Seo, K. Kim, J. B. Lee and H. Kim, J. Controlled Release, 2020, 322, 346–356 CrossRef CAS PubMed.
- G. Zhu, L. Mei, H. D. Vishwasrao, O. Jacobson, Z. Wang, Y. Liu, B. C. Yung, X. Fu, A. Jin, G. Niu, Q. Wang, F. Zhang, H. Shroff and X. Chen, Nat. Commun., 2017, 8, 1482 CrossRef PubMed.
- Y. Park, H. Kim and J. B. Lee, ACS Biomater. Sci. Eng., 2016, 2, 616–624 CrossRef CAS PubMed.
- L. Zhu, J. Yuhan, H. Yu, B. Zhang, L. Zhu, X. He, K. Huang and W. Xu, J. Nanobiotechnol., 2023, 21, 182 CrossRef CAS PubMed.
- J. S. Ha, J. S. Lee, J. Jeong, H. Kim, J. Byun, S. A. Kim, H. J. Lee, H. S. Chung, J. B. Lee and D.-R. Ahn, J. Controlled Release, 2017, 250, 27–35 CrossRef CAS PubMed.
- Z. Zhang, D. Xu, J. Wang, R. Zhang, H. Du, T. Zhou, X. Wang and F. Wang, Biomacromolecules, 2023, 24, 439–448 CrossRef CAS PubMed.
- N. Fan, X. Bian, M. Li, J. Chen, H. Wu, Q. Peng, H. Bai, W. Cheng, L. Kong, S. Ding, S. Li and W. Cheng, Sci. Adv., 2022, 8, eabn7382 CrossRef CAS PubMed.
- C. Li, Y. Wang, P.-F. Li and Q. Fu, Acta Biomater., 2023, 160, 1–13 CrossRef CAS PubMed.
- W. Sun, T. Jiang, Y. Lu, M. Reiff, R. Mo and Z. Gu, J. Am. Chem. Soc., 2014, 136, 14722–14725 CrossRef CAS PubMed.
- T. Kim, H. N. Hyun, R. Heo, K. Nam, K. Yang, Y. M. Kim, Y. S. Lee, J. Y. An, J. H. Park, K. Y. Choi and Y. H. Roh, Chem. Commun., 2020, 56, 6624–6627 RSC.
- A. D. Ellington and J. W. Szostak, Nature, 1990, 346, 818–822 CrossRef CAS PubMed.
- Y. Chen, Z. Guo, J. Li, K. Wang and J. Huang, Coord. Chem. Rev., 2025, 535, 216673 CrossRef CAS.
- J. Zhou and J. Rossi, Nat. Rev. Drug Discovery, 2017, 16, 181–202 CrossRef CAS PubMed.
- K. Chen, Y. Zhang, L. Zhu, H. Chu, X. Shao, C. Asakiya, K. Huang and W. Xu, J. Controlled Release, 2022, 341, 869–891 CrossRef CAS PubMed.
- K. Zhang, J. Liu, Q. Song, X. Yang, D. Wang, W. Liu, J. Shi and Z. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 46604–46613 CrossRef CAS PubMed.
- H. Cheng, S. Hong, Z. Wang, N. Sun, T. Wang, Y. Zhang, H. Chen and R. Pei, J. Mater. Chem. B, 2018, 6, 4638–4644 RSC.
- X. Chen, T. Chen, L. Zhang, Z. Wang, Q. Zhou, T. Huang, C. Ge, H. Xu, M. Zhu, F. Zhao, M. Yao, H. Tian, H. Li, X. Zhu and J. Li, Biomaterials, 2020, 261, 120304 CrossRef CAS PubMed.
- H. Su, G. Rong, L. Li and Y. Cheng, Adv. Drug Delivery Rev., 2024, 212, 115387 CrossRef CAS PubMed.
- S. Jin, Y. Sun, X. Liang, X. Gu, J. Ning, Y. Xu, S. Chen and L. Pan, Signal Transduction Targeted Ther., 2022, 7, 39 CrossRef CAS PubMed.
- C. Klein, U. Brinkmann, J. M. Reichert and R. E. Kontermann, Nat. Rev. Drug Discovery, 2024, 23, 301–319 CrossRef CAS PubMed.
- R. Wang, B. Hu, Z. Pan, C. Mo, X. Zhao, G. Liu, P. Hou, Q. Cui, Z. Xu, W. Wang, Z. Yu, L. Zhao, M. He, Y. Wang, C. Fu, M. Wei and L. Yu, J. Hematol. Oncol., 2025, 18, 51 CrossRef PubMed.
- E. Kwak, T. Kim, K. Yang, Y. M. Kim, H. S. Han, K. H. Park, K. Y. Choi and Y. H. Roh, Biomacromolecules, 2022, 23, 2255–2263 CrossRef CAS PubMed.
- L. Wang, Y. Wang, J. Hao and S. Dong, Biomacromolecules, 2017, 18, 1029–1038 CrossRef CAS PubMed.
- M.-G. Kim, J. Y. Park, G. Shim, H.-G. Choi and Y.-K. Oh, Biomaterials, 2015, 62, 155–163 CrossRef CAS PubMed.
- J. H. Lee, S. H. Ku, M. J. Kim, S. J. Lee, H. C. Kim, K. Kim, S. H. Kim and I. C. Kwon, J. Controlled Release, 2017, 263, 29–38 CrossRef CAS PubMed.
- M. Jang, J. H. Kim, H. Y. Nam, I. C. Kwon and H. J. Ahn, Nat. Commun., 2015, 6, 7930 CrossRef CAS PubMed.
- Y. Wang, X. Shang, J. Liu and Y. Guo, Talanta, 2018, 176, 652–658 CrossRef CAS PubMed.
- H. Lee, A. K. R. Lytton-Jean, Y. Chen, K. T. Love, A. I. Park, E. D. Karagiannis, A. Sehgal, W. Querbes, C. S. Zurenko, M. Jayaraman, C. G. Peng, K. Charisse, A. Borodovsky, M. Manoharan, J. S. Donahoe, J. Truelove, M. Nahrendorf, R. Langer and D. G. Anderson, Nat. Nanotechnol., 2012, 7, 389–393 CrossRef CAS PubMed.
- C. P. Leamon and P. S. Low, Drug Discovery Today, 2001, 6, 44–51 CrossRef CAS PubMed.
- J. T. Douglas, B. E. Rogers, M. E. Rosenfeld, S. I. Michael, M. Feng and D. T. Curiel, Nat. Biotechnol., 1996, 14, 1574–1578 CrossRef CAS PubMed.
- O. Young, N. Ngo, L. Lin, L. Stanbery, J. F. Creeden, D. Hamouda and J. Nemunaitis, Curr. Probl. Cancer, 2023, 47, 100917 CrossRef PubMed.
- M. Scaranti, E. Cojocaru, S. Banerjee and U. Banerji, Nat. Rev. Clin. Oncol., 2020, 17, 349–359 CrossRef PubMed.
- Y. Guo, S. Li, Y. Wang and S. Zhang, Anal. Chem., 2017, 89, 2267–2274 CrossRef CAS PubMed.
- K. Ren, Y. Zhang, X. Zhang, Y. Liu, M. Yang and H. Ju, ACS Nano, 2018, 12, 10797–10806 CrossRef CAS PubMed.
- K. E. de Visser and J. A. Joyce, Cancer Cell, 2023, 41, 374–403 CrossRef CAS PubMed.
- Y. Wei, J. Lv, S. Zhu, S. Wang, J. Su and C. Xu, Drug Discovery Today, 2024, 29, 104014 CrossRef CAS PubMed.
- C. Yi, J. Yang, T. Zhang, S. Xie, W. Li, L. Qin and D. Chen, Clin. Transl. Oncol., 2023, 25, 2569–2586 CrossRef CAS PubMed.
- S. E. Tsutakawa, M. J. Thompson, A. S. Arvai, A. J. Neil, S. J. Shaw, S. I. Algasaier, J. C. Kim, L. D. Finger, E. Jardine, V. J. B. Gotham, A. H. Sarker, M. Z. Her, F. Rashid, S. M. Hamdan, S. M. Mirkin, J. A. Grasby and J. A. Tainer, Nat. Commun., 2017, 8, 15855 CrossRef CAS PubMed.
- P. Singh, M. Yang, H. Dai, D. Yu, Q. Huang, W. Tan, K. H. Kernstine, D. Lin and B. Shen, Mol. Cancer Res., 2008, 6, 1710–1717 CrossRef CAS PubMed.
- H. Xu, R. Shi, W. Han, J. Cheng, X. Xu, K. Cheng, L. Wang, B. Tian, L. Zheng, B. Shen, Y. Hua and Y. Zhao, Nucleic Acids Res., 2018, 46, 11315–11325 CrossRef CAS PubMed.
- R. Liu, J. Qiu, L. D. Finger, L. Zheng and B. Shen, Nucleic Acids Res., 2006, 34, 1772–1784 CrossRef CAS PubMed.
- S. Uthaman, K. M. Huh and I. K. Park, Biomater. Res., 2018, 22, 22 CrossRef PubMed.
- R. J. Lee, S. Wang and P. S. Low, Biochim. Biophys. Acta, Mol. Cell Res., 1996, 1312, 237–242 CrossRef PubMed.
- L. E. Gerweck and K. Seetharaman, Cancer Res., 1996, 56, 1194–1198 CAS.
- E. Cheng, Y. Xing, P. Chen, Y. Yang, Y. Sun, D. Zhou, L. Xu, Q. Fan and D. Liu, Angew. Chem., Int. Ed., 2009, 48, 7660–7663 CrossRef CAS PubMed.
- H. Liao, Y. Cao, C. Hu, S. Shen, Z. Zhang, D. Li and Y. Du, Mater. Today Bio, 2024, 25, 101005 CrossRef CAS PubMed.
- Y. Li, S. Yue, J. Cao, C. Zhu, Y. Wang, X. Hai, W. Song and S. Bi, Theranostics, 2020, 10, 8250–8263 CrossRef CAS PubMed.
- L. Kennedy, J. K. Sandhu, M. E. Harper and M. Cuperlovic-Culf, Biomolecules, 2020, 10, 1429 CrossRef CAS PubMed.
- C. Lennicke, J. Rahn, R. Lichtenfels, L. A. Wessjohann and B. Seliger, Cell Commun. Signaling, 2015, 13, 39 CrossRef PubMed.
- Z. Lv, M. Huang, J. Yang, P. Li, L. Chang, Q. Tang, X. Chen, S. Wang, C. Yao, P. Liu and D. Yang, Adv. Mater., 2023, 35, 2300823 CrossRef CAS PubMed.
- C. Wang, W. Sun, G. Wright, A. Z. Wang and Z. Gu, Adv. Mater., 2016, 28, 8912–8920 CrossRef CAS PubMed.
- L. Zhu, J. Luo and K. Ren, J. Mater. Chem. B, 2023, 11, 261–279 RSC.
- A. L. Buddolla and S. Kim, Colloids Surf., B, 2018, 172, 315–322 CrossRef CAS PubMed.
- Y. Jin, H. Wang, X. Li, H. Zhu, D. Sun, X. Sun, H. Liu, Z. Zhang, L. Cao, C. Gao, H. Wang, X.-J. Liang, J. Zhang and X. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 26832–26841 CrossRef CAS PubMed.
- X. Huo, Z. Zhang, H. Du, R. Zhang, J. Zheng, T. Zhou, X. Wang, G. Zhang and F. Wang, ACS Appl. Polym. Mater., 2024, 6, 10936–10950 CrossRef CAS.
- R. Zhang, Z. Lv, L. Chang, J. Wang, J. Tang, Z. Wang, S. Li, J. Guo, C. Yao and D. Yang, Adv. Funct. Mater., 2024, 34, 2401563 CrossRef CAS.
- D. B. Kohn, Y. Y. Chen and M. J. Spencer, Gene Ther., 2023, 30, 738–746 CrossRef CAS PubMed.
- Q. Guo, C. Li, W. Zhou, X. Chen, Y. Zhang, Y. Lu, Y. Zhang, Q. Chen, D. Liang, T. Sun and C. Jiang, Acta Pharm. Sin. B, 2019, 9, 832–842 CrossRef PubMed.
- W. Yu, Y. Chen, M. Yan, Z. Xu, J. Sun, Y. Shen, D. Song, F. Wang and X. Liu, Nano Today, 2023, 52, 101996 CrossRef CAS.
- J. M. Vancoppenolle, S. N. Koole, J. F. O’Mahony, N. Franzen, J. A. Burgers, V. P. Retèl and W. H. van Harten, Drug Discovery Today, 2023, 28, 103620 CrossRef CAS PubMed.
- S. G. Huayamares, D. Loughrey, H. Kim, J. E. Dahlman and E. J. Sorscher, Nat. Rev. Clin. Oncol., 2024, 21, 407–427 CrossRef CAS PubMed.
- S. Yue, Y. Li, Z. Qiao, W. Song and S. Bi, Trends Biotechnol., 2021, 39, 1160–1172 CrossRef CAS PubMed.
- W. Huo, X. Li, B. Wang, H. Zhang, J. Zhang, X. Yang and Y. Jin, Biophys. Rep., 2020, 6, 256–265 CrossRef CAS.
- J. Wang, S. Yu, Q. Wu, X. Gong, S. He, J. Shang, X. Liu and F. Wang, Angew. Chem., Int. Ed., 2021, 60, 10766–10774 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |





