Photoinduced NO release of [Fe2(μ-SL)2(NO)4] complexes and their protein adducts: insights from structure, cytotoxicity, and photodynamic studies†
Wenjun
Gong
 a,
Yating
Pang
a,
Yating
Pang
 a,
Chenyu
Wang
a,
Chenyu
Wang
 b,
Wenming
Wang
b,
Wenming
Wang
 a and
Hongfei
Wang
a and
Hongfei
Wang
 *a
*a
aKey Laboratory of Chemical Biology and Molecular Engineering of Education Ministry, Institute of Molecular Science, Shanxi University, Taiyuan 030006, China. E-mail: wanghf@sxu.edu.cn
bSchool of Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu 210009, China
First published on 6th March 2025
Abstract
The dinitrosyl iron complex (DNIC) is a ubiquitous functional brick often used as a vehicle for the NO radical and chelatable iron pool, with a key role in chemical and biomedical applications. In this work, four DNICs modified with different thiolate bridging ligands (–SL) were synthesized. These complexes were characterized by 1H-NMR, electrospray ionization mass spectrometry, and X-ray crystal diffraction. Photoinduced NO release from the complexes in solution was further explored by time-resolved infrared and electron paramagnetic resonance (EPR) spectroscopy. The cluster complexes showed relatively lower cytotoxicity against the normal human liver cell line HL-7702 and higher cytotoxicity against human cervical cancer cells, with a selectivity index of 2.4–4.8. The cluster complexes with different L groups had different rates of NO release and protein binding constants. Moreover, NO released upon photoirradiation entered living cells, which were successfully imaged using an NO-selective fluorescent probe. The ferritin complex adducts further enhanced the cytotoxicity and cellular fluorescence intensity of NO, and the crystal structure revealed the coordination of the Fe(SL)2 unit with Cys102 of ferritin. This study provides insight into the photodynamic mechanism of nitrosyl iron–sulfur clusters to understand their physiological activity.
1 Introduction
Iron–sulfur (Fe–S) clusters are key inorganic cofactors in various functional proteins and enzymes that function in many biological processes, such as the respiratory chain, the photosystem, and the regulation of gene expression.1–6 The function of proteins containing Fe–S clusters in biological systems is highly regulated through the assembly and conversion of these clusters.7–13 Protein-bound iron–sulfur clusters can react with nitric oxide (NO) to undergo iron nitrosylation and cluster transition. The nitrosylation of iron–sulfur clusters typically results in protein-bound iron nitrosyl species such as dinitrosyl iron complexes.14–25Dinuclear iron nitrosyl complexes (DINCs), such as [Fe2S2(NO)4]2−, can be generated in situ by directly reacting NO with proteins containing Fe–S clusters. DINCs are not only a crucial intermediate for the regulation of protein function but are also used as carriers to deliver nitric oxide to physiological targets on demand.26–33 [Fe2S2(NO)4]2− is water-soluble but can readily decompose. Thus, nitrosyl complexes with relative stability and appropriate hydrophobicity to cross the cell membrane are highly expected. The preparation of [Fe2(μ-SL)2(NO)4] with unique esters tailored to have pendant L-groups can provide complexes with defined biological and photochemical properties.
Nitric oxide is a signaling molecule with important functions in various physiological and pathological processes such as vasodilation, inflammation, neurotransmission, immune response and cell apoptosis.34–40 Various metal nitrosyl complexes, including Cr, Co, Mn, Fe and Ru complexes, have been synthesized to study their properties for photoinduced NO release.41–51 Fe is one of the most common and abundant transition metals in biological systems. As a result, the nitrosyl Fe complex has received considerable attention due to its high biocompatibility, adjustable reactivity and modest photosensitivity under physiological conditions.52–56 In the presence of thiol binding ligands (–SL) in DNICs, typically characterized by high π-donor activity, the transfer of electron density from the sulfur atoms to the iron–dinitrosyl groups neutralizes the charge on nitrosonium cations. This prevents their hydrolysis, ensuring the relative stability of the corresponding DNICs.
The dinuclear iron cluster [Fe2(μ-SL)2(NO)4] can act as a vehicle for the NO. However, the dynamic reactions and reactivities for these complexes need to be further explored. In this work, DNICs with different thiolate bridging ligands (–SL) were synthesized and characterized by 1H-NMR, electrospray ionization mass spectrometry (ESI-MS), and X-ray crystal diffraction. The process and mechanism of photoinduced NO release in solution were investigated using time-resolved infrared (IR) and electron paramagnetic resonance spectra (EPR), and the stabilities of the [Fe2(μ-SL)2(NO)4] complexes were compared. NO released into living cells upon photoirradiation was monitored and imaged using a selective fluorescent probe. Furthermore, the interaction of the four cluster complexes with recombinant human heavy-chain ferritin (rHuHF) was studied by fluorescence spectroscopy, and the crystal structure of the protein complex adduct was determined. The cytotoxicities of the protein complex adducts were compared, and the potential of the protein as a drug carrier for NO release was examined. This study provides insights into the photodynamic mechanism of nitrosyl iron–sulfur clusters for their applications in biomedicine.
2 Results and discussion
2.1 Synthesis and characterization of the complexes
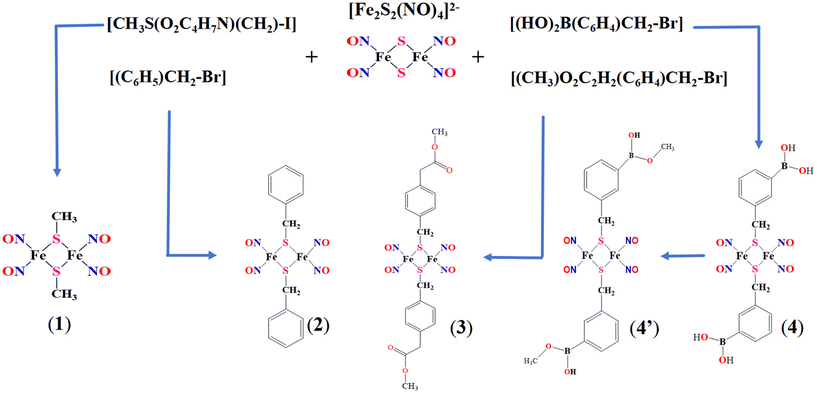
Nitrosyl iron–sulfur complexes 1, 2, 3 and 4 were synthesized by reacting (Me4N)2[Fe2S2(NO)4] with iodomethylmethionine, benzyl bromide, methyl 2-(4-(bromomethyl)phenyl)acetate and 3-(bromomethyl)phenylboronic acid, respectively. The synthesis route is shown in Scheme 1, and the detailed steps are described in the Methods section. Complexes 1–4 were separated and purified by column chromatography and then characterized using 1H-NMR spectroscopy and ESI-MS spectroscopy. The 1H-NMR and ESI-MS spectra are shown in the ESI (Fig. S1 and S2,† respectively). The 1H-NMR spectral profiles of the complex present the expected ligand peaks, integration values, and multiplicities, while the molecular weight obtained based on the MS spectra matched the calculated value.When iodomethylmethionine was used as the starting reactant for complex 1, only the methyl group was transferred and modified on the bridging sulfur atom. The separated reaction product for complex 4 contained a phenylboronic acid group (Fig. S1d and S2d†). However, an additional methoxyl group was observed after crystallization with CH3OH as the solvent. The chemical shifts and molecular weight of the additional methoxyl group in the crystallized complex 4 (4′) were respectively confirmed by 1H-NMR and ESI-MS spectra (Fig. S1e and S2e†). The crystals of complexes 1, 2, 3 and 4′ obtained by the slow evaporation of the CH2Cl2/CH3OH solvent were structurally identified by single-crystal X-ray diffraction. The solvents possibly affect the stability and crystal packing of the complexes, and therefore the structures of complexes 1 and 2 were compared with those of the reported work with similar substitute groups using different synthesis routes and crystallized solutions.57–59 Overall, the key bond lengths of Fe–N and NO bonds and bond angles of Fe–N–O are similar, and are in the range of 1.664–1.670 Å, 1.155–1.169 Å and 167.6–172.4°, respectively. The ORTEP diagram illustrating the crystal structures of these complexes is shown in Fig. 1. As expected, 1–4 were identified as nitrosyl iron–sulfur cluster complexes with different modified ligands.
The cluster of nitrosyl iron–sulfur complexes is bilaterally symmetric, with the ligand modified on the sulfur atom. Two iron atoms and two sulfur atoms form a rhombic plane, and four nitric oxides are distributed almost perpendicular to this rhombic plane. The averaged bond angles of the {Fe–NO} group in complexes 1, 2, 3 and 4′, about 170.4, 168.6, 172.2 and 170.4 degrees, respectively, were nearly linear. The averaged bond lengths corresponding to the NO group were 1.160 Å, respectively, and the results were consistent with the characteristics of the Fe–NO species.60–62 The X-ray crystallographic data, including atomic coordinates and equivalent isotropic temperature factors for the complexes, were deposited at the Cambridge Crystallographic Data Centre. The space group for complexes 2, 3, and 4′ corresponded to monoclinic systems, while complex 1 adopted a triclinic system. The crystallographic data and structure refinements of the complexes are summarized in Table S1,† with detailed information about the bond lengths and angles of the molecular structure presented in Tables S2–S5.† As the quantity of crystals for 4′ is limited, the bioactivities for complexes 1–4 were studied in the following investigation.
2.2 Photoinduced NO release from complexes 1 to 4
The photodynamic reaction of the four [Fe2(μ-SL)2(NO)4] complexes was firstly monitored by IR spectroscopy. The evolution of the IR spectra in DMSO was recorded as a function of the light irradiation time. The FT-IR spectra of complexes 1–4 ranging from 1700 to 1800 cm−1 are shown in Fig. 2. The two peaks in the range of 1740–1780 cm−1 were assigned to the stretching vibrations of the NO group in the Fe–NO complexes. The two IR peaks of NO for the [Fe2S2(NO)4]2− complex are at 1655 and 1635 cm−1;63 modifications with different thiolate bridging ligands (1–4) lead to a 100 cm−1 red shift for [Fe2(μ-SL)2(NO)4]. The time-resolved spectra showed that the peak corresponding to the NO group decreased gradually with increasing irradiation time. This suggests that the dissociation of the complex and NO release from the Fe–NO complexes were triggered by light irradiation. It is different from that of [Fe2S2(NO)4]2−, which converts into the [Fe4S3(NO)7]− cluster in solution; it is indicated by the absence of two IR peaks at 1655 and 1635 cm−1, and the formation of three new IR peaks at 1797, 1743 and 1706 cm−1, respectively. | ||
| Fig. 2 Time-resolved infrared spectra of (a–d) complexes 1–4 upon 420 nm irradiation at 100 mW cm−2. | ||
For [Fe2(μ-SL)2(NO)4], it is electroneutral and relatively stable, the amount of released NO can be controlled with photo irradiation and irradiation time. The rate of NO release varied between complexes 1–4, suggesting that the substituted group of the cluster complex affected the release of NO. In addition, the stability of the four complexes was monitored in PBS buffer containing 5% DMSO (Fig. S3†). The UV-visible spectra of the complexes were recorded continuously in 7 days. A little reduction in the intensity of absorption was observed on the first day, which is possibly related to its solubility in water, and then it almost remained unaltered in the following days.
The photodynamic process and photo induced release of NO from complexes 1–4 were further studied by utilizing EPR spectra. As shown in Fig. 3, under dark conditions, only very weak EPR signal of the nitrosyl iron complex was detected in the absence of Fe(MGD)2. After adding Fe(MGD)2, the weak EPR peak of the released NO free radical with g = 2.039 and AN = 12.80 G was observed.64,65 Upon irradiation with a mercury lamp, the intensity of the triple hyperfine splitting characteristic peak of tripped NO showed a quick enhancement with the increase of illumination time. The data indicated the photodynamic activity of the [Fe2(μ-SL)2(NO)4] complexes and further confirm the photo induced release of NO from the complexes. The rate of NO release from complexes 1 and 2 is faster than that of complexes 3 and 4, which is consistent with the inference from time resolved FTIR spectra.
2.3 Cellular cytotoxicity assay
The antitumor and antineuroinflammatory effects of nitrosyl iron–sulfur complexes have been reported.55,66 In this study, the in vitro cytotoxicity and photocytotoxicity of complexes 1–4 at different concentrations were tested by the CCK-8 assay. Human cervical cancer (HeLa) cells were incubated with the four complexes either in the dark or under photoirradiation before incubation. These complexes inhibit the growth of tumor cells and evinced relatively low cytotoxicity and genotoxicity in non-tumor cells. The cell viability profiles and half-maximal inhibitory concentration (IC50) values of complexes 1–4 are respectively shown in Fig. S4† and Table 1. The IC50 values for complexes 1–4 against HeLa cells in the absence of irradiation were 9.32, 6.66, 7.31 and 6.04 μM, respectively, which decreased to 7.41, 4.85, 5.27 and 4.77 μM, respectively, under photoirradiation. The IC50 values of all four complexes decreased after illumination, and the complexes exhibited photodynamic properties. The complexes with aromatic benzene substituted groups showed relatively lower IC50 values than that of complex 1 with the methyl group.| [Fe2(SL)2(NO)4] | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Data were obtained from three independent measurements. | |||||
| HeLa | Light (−) | 9.32 ± 0.12 | 6.66 ± 0.42 | 7.31 ± 0.45 | 6.04 ± 0.36 |
| Light (+) | 7.41 ± 0.35 | 4.85 ± 0.36 | 5.27 ± 0.35 | 4.77 ± 0.42 | |
| HL-7702 | Light (−) | 28.36 ± 0.68 | 15.98 ± 0.74 | 23.21 ± 0.64 | 18.66 ± 0.75 |
| Light (+) | 29.94 ± 0.52 | 16.86 ± 0.66 | 25.40 ± 0.75 | 19.99 ± 0.88 | |
| SI | Light (−) | 3.04 | 2.40 | 3.18 | 3.09 |
| Light (+) | 4.04 | 3.47 | 4.82 | 4.19 | |
The in vitro cytotoxicity and photocytotoxicity of the complexes against non-tumor human HL-7702 cells were also tested to evaluate their selectivity. The cell viability profiles and IC50 values of complexes 1–4 are respectively shown in Fig. S5† and Table 1. The IC50 values for complexes 1–4 against HL-7702 cells showed no obvious change when illuminated. The complexes generally showed lower cytotoxicity against normal human liver cell line HL-7702 and higher cytotoxicity against HeLa cells, with a selectivity index in the range of 2.4–4.8.
The cell cycle determines cell proliferation and regulates complex processes that determine cellular growth and division. Thus, numerous metal anticancer drugs have been demonstrated to arrest the cell cycle at a specific phase.67,68 The mechanism of cell death was investigated by flow cytometry with Annexin V-FITC/PI double staining. Compared with the control group, upon treatment with the four complexes at respective IC50 values for 24 h, the percentage of cells in the S phase increased by 5.1%, 10.9%, 6.2% and 11.0%, respectively (Fig. 4). In contrast, the percentage of cells in the S phase under irradiation increased by 22.2%, 27.6%, 26.8% and 27.4%, respectively. Complexes 1–4 caused cell cycle arrest at the S phase in HeLa cells, accompanied by a lower percentage of cells in the G1/G2 phase, in a light-enhanced manner.
 | ||
| Fig. 4 Cell cycle distribution of HeLa cells after 24 h treatment with complexes 1–4 at their respective IC50 values. | ||
Apoptosis, also known as type I programmed cell death, is a tightly regulated process of cell death used to maintain a stable internal environment. Abnormalities in the regulation of cell death are characteristic of neoplastic disease. Compelling functional studies support the utilization of apoptotic programmed cell death as a natural approach for cancer treatment.69 The effects of complexes 1–4 on HeLa cell apoptosis were evaluated by flow cytometry. The HeLa cells were distributed by flow cytometry into four quadrants (Fig. 5). The rate of apoptosis in the control group (early and late apoptotic) was 12.02%. The apoptosis rate of the HeLa cells significantly increased following treatment with complexes at their IC50 values. When exposed to light, the percentage of apoptosis in the experimental group (early apoptotic and late apoptotic/necrotic) increased from 19.06%, 26.69%, 18.27% and 16.82% to 33.12%, 33.40%, 43.85% and 45.00%, respectively. The results demonstrated the light-promoted apoptosis of HeLa cells induced by complexes 1–4 at similar concentrations.
 | ||
| Fig. 5 Apoptosis detection of HeLa cells by Annexin V-FITC/PI double-staining after 24 h treatment with complexes 1–4 at their respective IC50 values. | ||
2.4 Binding with ferritin and cytotoxicity
Ferritin, a common iron storage protein, has emerged as a promising drug delivery vehicle due to its unique architecture and excellent biocompatibility. Human ferritin has been used as a drug delivery vehicle due to its special cage structure and intrinsic tumor-targeting property. The fluorescence spectra were further used to study the interactions of the nitrosyl iron–sulfur complexes with ferritin. rHuHF has a characteristic fluorescence spectrum with a maximum emission at approximately 330 nm, attributed to the chromophore residue. The fluorescence titration spectra of the rHuHF solution with complexes 1–4 at 298 K are shown in Fig. 6. The fluorescence quenching of ferritin occurred as the concentration of the complexes increased, indicating molecular interactions and the binding of nitrosyl iron–sulfur with ferritin. The binding constant (Kb) and the number of average binding sites (n) were calculated according to the Stern–Volmer equations (1) and (2).70,71 The Kb values of complexes 1–4 binding to rHuHF at 298 K were 1.86 × 106, 5.67 × 106, 1.26 × 106 and 8.16 × 106 M−1, respectively, while the n values were 1.30, 1.41, 1.30 and 1.47, respectively. Therefore, at least one binding site was present in the ferritin structure.The interaction and dynamic process of the cluster complex with ferritin were further investigated. The structure of ferritin and the cluster complex 4 adduct were determined at a resolution of 2.4 Å. The structure of the complex 4 adduct is shown in Fig. 7, and the crystallographic data and refinement statistics are presented in Table 2. The coordinate of the structure was deposited at the Protein data bank with PDB ID: 9LNY.
| Parameters | Data |
|---|---|
| a R merge = ∑h∑l|Iih − 〈Ih〉|/∑h∑I〈Ih〉, where 〈Ih〉 is the mean of the observations Iih of reflection h. b R work = ∑(||Fp(obs)| − |Fp(calc.)||)/∑|Fp(obs)|, Rfree is an R factor for a pre-selected subset (5%) of reflections not included in refinement. c Numbers in parentheses are the corresponding values for the highest resolution shell. | |
| a (Å) | 187.26 |
| b (Å) | 187.26 |
| c (Å) | 187.26 |
| α, β, γ (°) | 90, 90, 90 |
| Space group | F432 |
| Wavelength used (Å) | 0.9785 |
| Resolution (Å) | 46.81–2.40 |
| No. of all reflections | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 177 177![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 739 739 |
| No. of unique reflections | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943 943 |
| Completeness (%) | 99.9 (100)c |
| Average I/σ(I) | 36.57 (6.46) |
| R merge (%) | 17.4 |
| No. of reflections used (σ(F) > 0) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 (1110) 540 (1110) |
| R work (%) | 18.70 |
| R free (%) | 24.24 |
| R.M.S.D. bond distance (Å) | 0.019 |
| R.M.S.D. bond angle (°) | 1.64 |
| Average B-factor (Å2) | 31.15 |
| No. of protein atoms | 1569 |
| No. of solvent atoms | 140 |
| Rotamer outliers (%) | 3.29 |
| Ramachandran plot | |
| Res. in favored regions (%) | 97.60 |
| Res. in generously allowed region (%) | 2.40 |
| Res. in disallowed region (%) | 0.00 |
The crystal structure revealed that complex 4 decomposed to the [(HO)2B(C6H4)CH2SFe(NO)2] motif, and one [(HO)2B(C6H4)CH2SFe(NO)2] unit bound with Cys102 in the groove on the rHuHF surface. The Fe atom is coordinated with two NO molecules and two S atoms, one from the –SL ligand and the other from Cys102 on α-Helix3 of rHuHF. The distances between the Fe and coordinated S atoms in Cys102 and the ligand are 2.52 and 2.09 Å, respectively. The bond angles for Fe–S–C (S and C are the atoms of BOOH) and S–Fe–S (S is the atom of BOOH and Cys102) are 92.63° and 96.13°, respectively.
The human serum albumin (HSA) as endogenous protein vehicles for the delivery of DNICs ([Fe2(μ-SCH2CH2OH)2(NO)4]) in the biological system was studied. HSA is one of the natural proteins in blood, and one free Cys34 is possibly available for binding with the Fe(NO)2 unit.66 The crystal structures of [Fe2S2(NO)4]2− and the rHuHF adduct were determined,72 two conformations for the –SH group of Cys102 were observed, and the occupancy for each one is 0.5. After modification with the L-ligand, possibly due to the large size of the substituted group, one conformation bound with Fe(μ-SL)(NO)2. The crystal structure of complex 4 and rHuHF indicated that the Fe(SL)(NO)2 unit bound spontaneously with Cys102 on the outer surface of ferritin. The clear density and relatively lower B factor of the [(BOOH)SFe(NO)2] unit suggested that the binding was stable. The caged 24-mer structure of rHuHF binds with 24 complexes on its outer surface, it is a more effective carrier for nitrosyl metal complexes. Photolysis of Roussin's red phenyl ester (Fe2(μ-SPh)2(NO)4) in different solvents gave rise to solvated Fe2(μ-SPh)2(NO)3 and released one NO molecule.73 The final structure of complex 4 and the ferritin adduct suggest that the intermediate Fe(SL)(NO)2 unit possibly existed in the process of decomposition.
The effects of four complexes and their ferritin adducts on the proliferation of HeLa cells were further evaluated by the CCK-8 method. As shown in Fig. S6,† when HeLa cells were treated with various concentrations of ferritin complex adducts 1–4 without irradiation, the viability of HeLa cells decreased as the concentration of the complexes increased. The IC50 values of the ferritin cluster adduct for HeLa cells in the absence of irradiation were 5.03, 4.87, 6.13 and 4.46 μM, respectively. An obvious decrease was observed compared with those of complexes alone. Under photoirradiation, these values further decreased to 4.02, 3.25, 4.75 and 3.36 μM, respectively. Irradiation with light slightly increased the cytotoxicity of the ferritin adducts. These findings provide a foundatio![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif) for the application of ferritin as a potential carrier to deliver Fe–NO cluster complexes.
for the application of ferritin as a potential carrier to deliver Fe–NO cluster complexes.
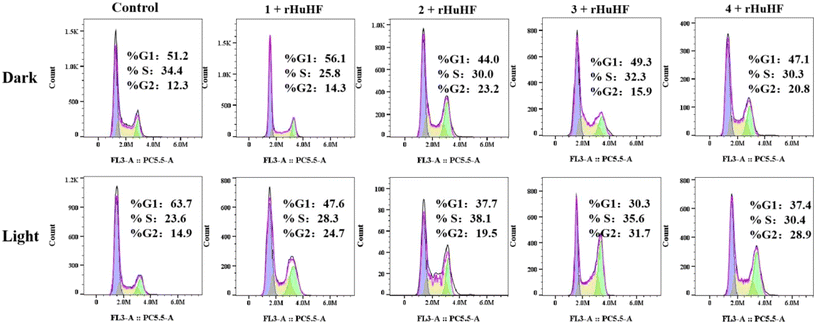
The effects of complexes 1–4 and their ferritin adducts on the cell cycle distribution of HeLa cells were evaluated by flow cytometry (Fig. 8). In the dark, reduced inhibition in the G1 and S phases and increased inhibition in the G2 phase were observed when the concentration of adducts was the same as in the control group. Compared with the dark group, upon irradiation, the proportion of HeLa cells in the G2 and S phases increased significantly, while the proportion of cells in the G1 phase decreased correspondingly. Under 420 nm light irradiation, the percentage of cells in the S phase increased by 4.70%, 14.5%, 12.0% and 6.8%, respectively; the percentage of cells in the G2 phase increased by 9.80%, 4.60%, 16.80% and 14.0%, respectively; and the proportion of cells in the G1 phase decreased. These results indicated that light treatment changed the pathway that retarded cell proliferation after the reaction of ferritin with the nitrosyl iron–sulfur complex.
 | ||
| Fig. 8 Cell cycle distribution of HeLa cells after 24 h treatment with ferritin complex (1–4) adducts at their IC50 values. | ||
The effects of the ferritin complex adducts on cell apoptosis are shown in Fig. 9. With the addition of the protein complex (1–4) adducts, the percentage of late apoptotic cells increased from 7.02% to 16.4%, 43.1%, 37.1% and 44.5%, respectively, while the percentage of early apoptotic cells did not change significantly. The apoptosis of HeLa cells was induced in the late apoptosis after the complex bound with ferritin in the dark. Under 420 nm light irradiation, the percentage of cells in early apoptosis increased from 6.20% to 34.1%, 51.2%, 44.9% and 49.6%, respectively. These results suggest that light influenced the effect of the ferritin complex adducts on HeLa cell apoptosis, inducing early apoptosis.
 | ||
| Fig. 9 Apoptosis detection of HeLa cells by Annexin V-FITC/PI double-staining after 24 h treatment with ferritin complex (1–4) adducts at their respective IC50 values. | ||
2.5 Imaging of released NO in living cells
To investigate whether NO could be released from complexes 1–4 and their corresponding ferritin complex adducts upon photoirradiation and transported into the cells, the fluorescence imaging assay for NO in living cells was performed with a confocal fluorescence microscope using DAX-J2™ Red as the NO-sensitive fluorescent probe.74 First, HeLa cells were treated with DAX-J2 alone to ensure that the probe did not interfere with the experiments. Confocal images of complexes 1–4 (Fig. 10) and their ferritin complex adducts (Fig. 11) in HeLa cells were then recorded separately under dark and light conditions. The HeLa cells showed little to no fluorescence due to endogenous NO when treated with DAX-J2 with or without light irradiation (Fig. S7†). Similar results were observed for the individual complexes 1–4 under dark conditions. However, red fluorescence was observed in HeLa cells after photoirradiation with a light-emitting diode (LED) light source at 420 nm, which showed enhancement with the increase of irradiation time.A stronger fluorescence was observed from the ferritin complex (1–4) adducts. Different ligand-modified complexes underwent photoinduced NO release at different rates, resulting in a fluorescence intensity that increased with irradiation time in the cells treated with the four complexes. In addition, HeLa cells treated with complexes 1–4 and ferritin adducts exhibited varying degrees of fluorescence enhancement with prolonged irradiation time, indicating that the ferritin complex adducts could also enter cells and undergo photoinduced NO release. The binding of ferritin with complexes 1–4 increased the release rate of NO in HeLa cells.
3 Conclusions
Four DNICs with different modified thiolate bridging ligands (–SL) were synthesized herein. Unlike the [Fe2S2(NO)4]2− complex, the modified [Fe2(SL)2(NO)4] complexes did not form larger clusters and only the release of NO was observed. Photoinduced NO release from complexes 1–4 in solution was demonstrated by time-resolved IR and EPR spectroscopy. The NO released upon photoirradiation could enter living cells, which was successfully imaged using an NO-selective fluorescent probe. The cluster complexes showed relatively higher cytotoxicity against HeLa cells and lower cytotoxicity against the normal human liver cell line HL-7702, with a higher selectivity index of 2.4–4.8.The interaction of the four cluster complexes with recombinant human heavy-chain ferritin was analyzed by fluorescence spectroscopy. The calculated binding constants (Kb) for complexes 1–4 were in the range of 1.26–8.16 × 106 M−1 with an average number of binding sites (n) of about 1.3. The crystal structure reveals that the [(HO)2B(C6H4)CH2SFe(NO)2] unit coordinated with Cys102 of ferritin. The ferritin complex adducts further enhanced the cytotoxicity and ability of NO released in cells, possibly affecting the cellular cycle and pathway to induce apoptosis. Ferritin is a potential drug carrier for nitrosyl iron–sulfur complexes used in NO release. The study provides insights into the photodynamic properties of nitrosyl iron–sulfur clusters, promoting their potential applications in the development of effective NO donors to regulate physiological and pathological processes.
4 Methods
4.1 Materials
Iodomethylmethionine, benzyl bromide, methyl 2-(4-(bromomethyl)phenyl) acetate, and 3-(bromomethyl)phenylboronic acid were purchased from Aladdin Biochemical Technology Co. Ltd (Shanghai, China). Human cervical carcinoma cells (HeLa cells) and HL-7702 (human normal liver cells) were obtained from the Shanghai Institute for Biological Sciences, the Chinese Academy of Science (China). DAX-J2™ Red (NO probe) was purchased from AAT Bioquest Inc. (Sunnyvale, CA, USA). Polyethylene glycol 3350 (PEG 3350) and glycerol were purchased from Hampton Research (Aliso Viejo, CA, USA). Human heavy-chain ferritin (rHuHF) was expressed and purified in our laboratory. Other chemicals and solvents used were obtained from locally available suppliers.4.2 Synthesis of nitrosyl iron–sulfur clusters
First, (Me4N)2[Fe2S2(NO)4] was synthesized according to previous methods and recrystallized.63,75 Next, [Fe2(μ-SL)2(NO)4] was synthesized through the alkylation of [Fe2S2(NO)4]2− with an alkyl halide. Nitrosyl iron–sulfur clusters with different ligands were then synthesized by dissolving the alkyl halide ligand (0.184 mM) in a solution of (Me4N)2[Fe2S2(NO)4] (0.04 g, 0.092 mM) with 10 mL of methanol or water. After reacting the mixture in the dark for 5 h at 25 °C, the solution was cooled and the solvent was evaporated. The resulting crude product was purified by silica gel column chromatography with CH2Cl2/CH3OH as the eluent. Four complexes 1–4 were obtained by reacting (Me4N)2[Fe2S2(NO)4] with iodomethylmethionine, benzyl bromide, methyl 2-(4-(bromomethyl)phenyl) acetate, and 3-(bromomethyl) phenylboronic acid, respectively. The 1H NMR spectra were recorded on a Bruker 600 M spectrometer, and high resolution electrospray ionization (ESI) mass spectra were recorded at the negative ion mode on a Thermo Q Exactive field MS spectrometer.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1H NMR (600 MHz, DMSO) δ 2.89 (s, 3H). ESI-MS: m/z calcd for 325.8529 [M–H+]. Found: 325.18414.
1. 1H NMR (600 MHz, DMSO) δ 2.89 (s, 3H). ESI-MS: m/z calcd for 325.8529 [M–H+]. Found: 325.18414.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1H NMR (600 MHz, DMSO) δ 8.08 (d, J = 5.2 Hz, 2H), 7.85 (d, J = 9.1 Hz, 1H), 7.76 (d, J = 7.1 Hz, 1H), 7.41 (d, J = 7.3 Hz, 1H), 7.37 (t, J = 7.4 Hz, 1H), 4.43 (s, 1H), 4.34 (s, 1H). ESI-MS: m/z calcd for 564.92206 [M–H+]. Found: 564.92186.
1. 1H NMR (600 MHz, DMSO) δ 8.08 (d, J = 5.2 Hz, 2H), 7.85 (d, J = 9.1 Hz, 1H), 7.76 (d, J = 7.1 Hz, 1H), 7.41 (d, J = 7.3 Hz, 1H), 7.37 (t, J = 7.4 Hz, 1H), 4.43 (s, 1H), 4.34 (s, 1H). ESI-MS: m/z calcd for 564.92206 [M–H+]. Found: 564.92186.
4.3 X-ray crystallography
Single crystals for X-ray analyses were prepared by dissolving complexes in a mixed solvent system consisting of methylene chloride and methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). X-ray data of the four complexes were collected on a Bruker D8 Venture diffractometer equipped with a graphite-monochromated Mo Kα radiation source (λ = 0.71073 Å) at room temperature. The crystal structures of the complexes were solved with the SHELXTL-97 software package,76 and the SADABS program was employed for empirical absorption corrections.77 Non-H atoms were refined anisotropically, while the hydrogen atoms were located at calculated positions with fixed isotropic thermal parameters and included in the structure factor calculations during the final stage of full-matrix least-squares refinement. The coordinates were deposited at the Cambridge Crystallographic Data Centre with accession numbers of 2390905 (1), 2390895 (2), 2390735 (3), and 2390745 (4′).†
1). X-ray data of the four complexes were collected on a Bruker D8 Venture diffractometer equipped with a graphite-monochromated Mo Kα radiation source (λ = 0.71073 Å) at room temperature. The crystal structures of the complexes were solved with the SHELXTL-97 software package,76 and the SADABS program was employed for empirical absorption corrections.77 Non-H atoms were refined anisotropically, while the hydrogen atoms were located at calculated positions with fixed isotropic thermal parameters and included in the structure factor calculations during the final stage of full-matrix least-squares refinement. The coordinates were deposited at the Cambridge Crystallographic Data Centre with accession numbers of 2390905 (1), 2390895 (2), 2390735 (3), and 2390745 (4′).†
The rHuHF crystals were prepared by mixing 1.5 μL of protein and 1.5 μL of reservoir solution, which contained 0.2 M Bicine (pH 9.0) and 2 M MgCl2. The crystallization was performed at 18 °C via hanging drop vapor diffusion. The complex adduct crystals suitable for diffraction were soaked in a 3 μL drop containing a 5 mM solution of complex 4 in 0.2 M Bicine and 2 M MgCl2 buffer solution at pH 9.0 for 72 h. The X-ray diffraction data were collected at the Shanghai Synchrotron Radiation Facility in China. The crystal diffraction data were processed with HKL3000 using HKL-3000 software (HKL Research, Charlottesville, VA, USA).78 The structure was solved using CCP4, Coot, and Phenix software,79–81 and structural figures were drawn using PyMOL.82
4.4 Spectral measurements
Time-resolved FT-IR was employed to monitor the photolysis of NO from complexes 1–4 on an IS50R FT-IR spectrometer (ThermoFisher Scientific). The scanning range was 1700–1800 cm−1, and the resolution was 1 cm−1. Before measurement, 60 μL of 10 mM complex solution in DMSO was injected into a liquid IR cell. The sample solution was irradiated using a Xe lamp (HSX-F300, Beijing NBeT Co.) with a 420 nm band-pass filter (100 mW cm−2).EPR spectroscopy were recorded using an EMXPLUS 10/12 paramagnetic resonance spectrometer (Bruker). The microwave power was set to 10 mW. [FeII(MGD)2] (N-methyl-D-glucamine-dithiocarbamato-iron(II)) was used as a NO radical trapper for measurement. The complexes 1–4 (10 mM) and [FeII(MGD)2] (10 mM) were dissolved in DMSO and mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio and transferred quantitatively into a quartz capillary tube to measure the spectrum under dark or light conditions. An Hg lamp (100 W) was used as an illumination source to record EPR spectra in the range of 3400–3520 G. The stock solution for the complexes and the spin trapper [FeII(MGD)2] solution was prepared freshly and the measurement was performed at once.
1 volume ratio and transferred quantitatively into a quartz capillary tube to measure the spectrum under dark or light conditions. An Hg lamp (100 W) was used as an illumination source to record EPR spectra in the range of 3400–3520 G. The stock solution for the complexes and the spin trapper [FeII(MGD)2] solution was prepared freshly and the measurement was performed at once.
4.5 In vitro cytotoxic activity
The in vitro cytotoxic activity of complexes 1–4 and their ferritin adducts in the absence and presence of light irradiation was evaluated by CCK-8 assay using human cervical cancer cells (HeLa) and non-tumor human liver cells (HL-7702). The cells were seeded in 200 μL of medium in a 96-well plate (5 × 104 cells per well) and incubated for 24 h in Dulbecco's Modified Eagle's medium, containing 10% (v/v) FBS and 1% (v/v) penicillin/streptomycin, at 37 °C under a humidified 5% CO2 atmosphere. A SpectraMax iD5 microplate reader was used to record the absorbance of each sample at 450 nm. The IC50 values were calculated with SPSS25 software. Three independent experiments were carried out to ensure the reproducibility of the results.The effects of complexes and their protein adducts on the HeLa cell cycle and apoptosis were analyzed by Beckmann Coulter flow cytometry. Cells in the logarithmic growth phase were inoculated in six-well plates, with complex media of different concentrations, and cultured for 12 h. Finally, the cells were collected and stained following the Annexin V-FITC/PI apoptosis detection assay and DNA content quantitation assay. For the irradiated group, after incubating with complexes 1–4 and their adducts for 2 h, the cells were irradiated with an LED light source (420 nm, 100 mW) for 30 min. The cells were further incubated for 24 h, and the same procedures as described above were conducted.
4.6 Fluorescence spectrometric analysis for ferritin binding
The binding of nitrosyl iron–sulfur complexes with ferritin was investigated using fluorescence quenching experiments conducted at ambient temperature. The fluorescent emission spectrum was recorded from 300 to 500 nm using a Hitachi 4600 fluorescence spectrometer. The excitation wavelength was 280 nm, and the slit widths for excitation and emission were 2.0 and 2.5 nm, respectively. Briefly, 0–25 μL of complexes 1–4 (1 × 10−3 M) was added to the ferritin (2 mL, 1.0 μM) solution at intervals of 2.5 μL, mixed, and left to react for 2 min before fluorescence spectrum determination. The binding constant and the number of binding sites were analyzed using the modified Stern–Volmer equation, where F0 and F are the fluorescence intensity of ferritin in the absence and presence of complexes 1–4, respectively, and [Q] is the molar concentration of complexes 1–4.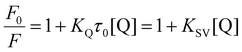 | (1) |
log(F0 − F)/F = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kb + n Kb + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[Q] log[Q] | (2) |
4.7 Cellular imaging of NO
A Zeiss LSM 880 confocal fluorescence microscope was employed to monitor the NO imaging in HeLa cells. A 561 nm laser was used as the excitation source, and the emission range was 590–700 nm. HeLa cells were seeded in confocal microscopy dishes (20 mm diameter) at a density of 7 × 103 mL−1 and incubated for 24 h at 37 °C under a humidified 5% CO2 atmosphere. The HeLa cells were washed three times with PBS and then treated with DAX-J2™ Red (5 μM) for 15 min. Subsequently, 20 μM complexes 1–4 or their ferritin complex (1–4) adducts were added and incubated for 15 min. After incubation, the HeLa cells were washed three times with PBS. The confocal fluorescence images of these cells in the absence and presence of LED light irradiation (420 nm, 100 mW, 5 or 15 min) were recorded.Author contributions
Wenjun Gong: writing – original draft, methodology, investigation, data measurement and analysis. Yating Pang: data measurement and analysis. Chenyu Wang: data measurement and analysis. Wenming Wang: X-ray diffraction data analysis. Hongfei Wang: conceptualization, writing – original draft, and writing – review and editing.Data availability
The data supporting this article have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported partially by the National Natural Science Foundation of China (No. 62075118), the Research Project of Shanxi Province (YDZJSX20231A009), the Shanxi Key Laboratory of Pharmaceutical Biotechnology, and the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi. We thank the staff from the BL18U1 and BL02U1 beamlines of the National Centre for Protein Sciences Shanghai (NCPSS) and BL17UM at the Shanghai Synchrotron Radiation Facility for assistance during data collection. We thank LetPub (https://www.letpub.com) for its linguistic assistance while preparing this manuscript.References
- B. Zhang, S. Bandyopadhyay, P. Shakamuri, S. G. Naik, B. H. Huynh, J. Couturier, N. Rouhier and M. K. Johnson, Monothiol glutaredoxins can bind linear [Fe3S4]+ and [Fe4S4]2+ clusters in addition to [Fe2S2]2+ clusters: spectroscopic characterization and functional implication, J. Am. Chem. Soc., 2013, 135, 15153–15164 CrossRef CAS PubMed.
- R. H. Holm and W. Lo, Structural Conversions of Synthetic and Protein-Bound Iron-Sulfur Clusters, Chem. Rev., 2016, 116, 13685–13713 CrossRef CAS PubMed.
- A. Villalta, B. Slour, A. Lartigue, M. Clémancey, D. Byrne, F. Chaspoul, A. Loquet, B. Guigliarelli, G. Blondin, C. Abergel and B. Burlat, Evidence for [2Fe-2S]2+ and Linear [3Fe-4S]1+ Clusters in a Unique Family of Glycine/Cysteine-Rich Fe-S Proteins from Megavirinae Giant Viruses, J. Am. Chem. Soc., 2023, 145, 2733–2738 CrossRef CAS PubMed.
- D. Pain and A. Dancis, Roles of Fe-S proteins: from cofactor synthesis to iron homeostasis to protein synthesis, Curr. Opin. Genet. Dev., 2016, 38, 45–51 CrossRef CAS PubMed.
- E. D. Badding, S. Slisantitham, D. A. Lukoyanov, B. M. Hoffman and D. L. M. Suess, Connecting the geometric and electronic structures of the nitrogenase iron-molybdenum cofactor through site-selective 57Fe labelling, Nat. Chem., 2013, 15, 658–665 CrossRef PubMed.
- R. E. Shepherd, A. C. Kreinbrink, C. L. Njimoh, S. W. Vali and P. A. Lindahl, Yeast Mitochondria Import Aqueous FeII and, When Activated for Iron-Sulfur Cluster Assembly, Export or Release Low-Molecular-Mass Iron and Also Export Iron That Incorporates into Cytosolic Proteins, J. Am. Chem. Soc., 2023, 145, 13556–13569 CrossRef CAS PubMed.
- B. Blanc, M. Clémancey, J. M. Latour, M. Fontecave and S. Ollagnier de Choudens, Molecular investigation of iron-sulfur cluster assembly scaffolds under stress, Biochemistry, 2014, 53, 7867–7869 CrossRef CAS PubMed.
- P. V. Rao and R. H. Holm, Synthetic Analogues of the Active Sites of Iron-Sulfur Proteins, Chem. Rev., 2004, 104, 527–559 CrossRef CAS PubMed.
- J. C. Crack, J. Green, A. J. Thomson and N. E. L. Brun, Iron–Sulfur clusters as biological sensors: the chemistry of reactions with molecular oxygen and nitric oxide, Acc. Chem. Res., 2014, 47, 3196–3205 CrossRef CAS PubMed.
- J. Fitzpatrick and E. Kim, Synthetic modeling chemistry of iron-sulfur clusters in nitric oxide signaling, Acc. Chem. Res., 2015, 48, 2453–2461 CrossRef CAS PubMed.
- J. C. M. Pereira, A. V. Iretskii, R. M. Han and P. C. Ford, Dinitrosyl iron complexes with cysteine. Kinetics studies of the formation and reactions of DNICs in aqueous solution, J. Am. Chem. Soc., 2015, 137, 328–336 CrossRef CAS PubMed.
- T. J. Sherbow, W. Fu, L. Z. Tao, L. N. Zakharov, R. D. Britt and M. D. Pluth, Thionitrite (SNO-) and Perthionitrite (SSNO-) are Simple Synthons for Nitrosylated Iron Sulfur Clusters, Angew. Chem., Int. Ed., 2022, 61, e202204570 CrossRef CAS PubMed.
- M. Quiroz, M. M. Lockart, M. R. Saber, S. W. Vali, L. C. Elrod, B. S. Pierce, M. B. Hall and M. Y. Darensbourg, Cooperative redox and spin activity from three redox congeners of sulfur-bridged iron nitrosyl and nickel dithiolene complexes, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2201240119 CrossRef CAS PubMed.
- J. C. Crack, L. J. Smith, M. R. Stapleton, J. Peck, N. J. Watmough, M. J. Buttner, R. S. Buxton, J. Green, V. S. Oganesyan, A. J. Thomson and N. E. Le Brun, Mechanistic Insight into the Nitrosylation of the [4Fe-4S] Cluster of WhiB-like Proteins, J. Am. Chem. Soc., 2011, 133, 1112–1121 CrossRef CAS PubMed.
- J. C. Crack, D. A. Svistunenko, J. Munnoch, A. J. Thomson, M. I. Hutchings and N. E. Le Brun, Differentiated, Promoter-specific Response of [4Fe-4S] NSLR DNA Binding to Reaction with Nitric Oxide, J. Biol. Chem., 2016, 291, 8663–8672 CrossRef CAS PubMed.
- M. C. Kennedy, W. E. Antholine and H. Beinert, An EPR investigation of the products of the reaction of cytosolic and mitochondrial aconitases with nitric oxide, J. Biol. Chem., 1997, 272, 20340–20347 CrossRef CAS PubMed.
- J. C. Crack, M. R. Stapleton, J. Green, A. J. Thomson and N. E. Le Brun, Mechanism of [4Fe-4S](Cys)4 cluster nitrosylation is conserved among NO-responsive regulators, J. Biol. Chem., 2013, 288, 11492–11502 CrossRef CAS PubMed.
- L. Castro, V. Tórtora, S. Mansilla and R. Radi, Aconitases: Non-redox Iron-Sulfur Proteins Sensitive to Reactive Species, Acc. Chem. Res., 2019, 52, 2609–2619 CrossRef CAS PubMed.
- L. A. Ekanger, P. H. Oyala, A. Moradian, M. J. Sweredoski and J. K. Barton, Nitric Oxide Modulates Endonuclease III Redox Activity by a 800 mV Negative Shift upon [Fe4S4] Cluster Nitrosylation, J. Am. Chem. Soc., 2018, 140, 11800–11810 CrossRef CAS PubMed.
- O. Karmi, H. B. Marjault, L. Pesce, P. Carloni, J. N. Onuchic, P. A. Jennings, R. Mittler and R. Nechushtai, The unique fold and lability of the [2Fe-2S] clusters of NEET proteins mediate their key functions in health and disease, J. Biol. Inorg. Chem., 2018, 23, 599–612 CrossRef CAS PubMed.
- A. P. Landry, X. W. Duan, H. Huang and H. G. Ding, Iron-sulfur proteins are the major source of protein-bound dinitrosyl iron complexes formed in Escherichia coli cells under nitric oxide stress, Free Radicals Biol. Med., 2011, 50, 1582–1590 CrossRef CAS PubMed.
- C. E. Tinberg, Z. J. Tonzetich, H. X. Wang, L. H. Do, Y. Yoda, S. P. Cramer and S. J. Lippard, Characterization of iron dinitrosyl species formed in the reaction of nitric oxide with a biological Rieske center, J. Am. Chem. Soc., 2012, 132, 18168–18176 CrossRef PubMed.
- J. C. Crack and N. E. Le Brun, Mass Spectrometric Identification of [4Fe-4S](NO)x Intermediates of Nitric Oxide Sensing by Regulatory Iron-Sulfur Cluster Proteins, Chemistry, 2019, 25, 3675–3684 CrossRef CAS PubMed.
- Y. Kim, A. Slidharan and D. L. M. Suess, The Elusive Mononitrosylated [Fe4S4] Cluster in Three Redox States, Angew. Chem., Int. Ed., 2022, 61, e202213032 CrossRef CAS PubMed.
- L. J. Li and L. L. Li, Recent Advances in Multinuclear Metal Nitrosyl Complexes, Coord. Chem. Rev., 2016, 306, 678–700 CrossRef CAS PubMed.
- C. L. Conrado, J. L. Bourassa, C. Egler, S. Wecksler and P. C. Ford, Photochemical investigation of Roussin's red salt esters: Fe2(μ-SR)2(NO)4, Inorg. Chem., 2003, 42, 2288–2293 CrossRef CAS PubMed.
- M. L. Tsai, C. C. Chen, I. J. Hsu, S. C. Ke, C. H. Hsieh, K. A. Chiang, G. H. Lee, Y. Wang, J. M. Chen, J. F. Lee and W. F. Liaw, Photochemistry of the dinitrosyl iron complex [S5Fe(NO)2]− leading to reversible formation of [S5Fe(μ-S)2FeS5]2−: spectroscopic characterization of species relevant to the nitric oxide modification and repair of [2Fe-2S] ferredoxins, Inorg. Chem., 2004, 43, 5159–5167 CrossRef CAS PubMed.
- C. H. Ke, C. H. Chen, M. L. Tsai, H. C. Wang, F. T. Tsai, Y. W. Chiang, W. C. Shih, D. S. Bohle and W. F. Liaw, {Fe(NO)2}9 Dinitrosyl Iron Complex Acting as a Vehicle for the NO Radical, J. Am. Chem. Soc., 2017, 139, 67–70 CrossRef CAS PubMed.
- T. T. Lu, Y. M. Wang, C. H. Hung, S. J. Chiou and W. F. Liaw, Bioinorganic Chemistry of the Natural [Fe(NO)2] Motif: Evolution of a Functional Model for NO-Related Biomedical Application and Revolutionary Development of a Translational Model, Inorg. Chem., 2018, 57, 12425–12443 CrossRef CAS PubMed.
- C. E. Schiewer, C. S. Müller, S. Dechert, B. Marie, J. A. Wolny, V. Schünemann and F. Meyer, Effect of Oxidation and Protonation States on [2Fe−2S] Cluster Nitrosylation Giving {Fe(NO)2}9 Dinitrosyl Iron Complexes (DNICs), Inorg. Chem., 2019, 58, 769–784 CrossRef CAS PubMed.
- M. Quiroz and M. Y. Darensbourg, Development of (NO)Fe(N2S2) as a Metallodithiolate Spin Probe Ligand: A Case Study Approach, Acc. Chem. Res., 2024, 57, 831–844 CrossRef CAS PubMed.
- W. Y. Wu, W. Y. Zheng, W. T. Chen, F. T. Tsai, M. L. Tsai, C. W. Pao, J. L. Chen and W. F. Liaw, Electronic Structure and Transformation of Dinitrosyl Iron Complexes (DNICs) Regulated by Redox Non-Innocent Imino-Substituted Phenoxide Ligand, Inorg. Chem., 2024, 63, 2431–2442 CrossRef CAS PubMed.
- A. G. Tennyson and S. J. Lippard, Generation, translocation, and action of nitric oxide in living systems, Chem. Biol., 2011, 18, 1211–1220 CrossRef CAS PubMed.
- D. Fukumura, S. Kashiwagi and R. K. Jain, The role of nitric oxide in tumour progression, Nat. Rev. Cancer, 2006, 6, 521–534 CrossRef CAS PubMed.
- D. Hirst and T. Robson, Targeting nitric oxide for cancer therapy, J. Pharm. Pharmacol., 2007, 59, 3–13 CrossRef CAS PubMed.
- Z. Miao and S. B. King, Recent advances in the chemical biology of nitroxyl (HNO) detection and generation, Nitric Oxide, 2016, 57, 1–14 CrossRef CAS PubMed.
- P. Seth, P. N. Hsieh, S. Jamal, L. Wang, S. P. Gygi, M. K. Jain, J. Coller and J. S. Stamler, Regulation of MicroRNA Machinery and Development by Interspecies S-Nitrosylation, Cell, 2019, 176, 1014–1025 CrossRef CAS PubMed.
- J. C. Pieretti, O. Rubilar, R. B. Weller, G. R. Tortella and A. B. Seabra, Nitric oxide (NO) and nanoparticles-Potential small tools for the war against COVID-19 and other human coronavirus infections, Virus Res., 2021, 291, 198202 CrossRef CAS PubMed.
- W. Fang, J. Jiang, L. Su, T. Shu, H. Liu, S. Lai, R. A. Ghiladi and J. Wang, The role of NO in COVID-19 and potential therapeutic strategies, Free Radicals Biol. Med., 2021, 163, 153–162 CrossRef CAS PubMed.
- S. Das, Kulbir, S. Ray, T. Devi, S. Ghosh, S. S. Harmalkar, S. N. Dhuri, P. Mondal and P. Kumar, Why intermolecular nitric oxide (NO) transfer ? Exploring the factors and mechanistic aspects of NO transfer reaction, Chem. Sci., 2022, 13, 1706–1714 RSC.
- P. T. Burks, A. D. Ostrowski, A. A. Mikhailovsky, E. M. Chan, P. S. Wagenknecht and P. C. Ford, Quantum dot photoluminescence quenching by Cr(III) complexes. Photosensitized reactions and evidence for a FRET mechanism, J. Am. Chem. Soc., 2012, 134, 13266–13275 CrossRef CAS PubMed.
- P. J. Huang, J. V. Garcia, A. Fenwick, G. Wu and P. C. Ford, Nitric Oxide Uncaging from a Hydrophobic Chromium(III) PhotoNORM: Visible and Near-Infrared Photochemistry in Biocompatible Polymer Disks, ACS Omega, 2019, 4, 9181–9187 CrossRef CAS PubMed.
- R. Mazumdar, B. Mondal, S. Saha, B. Samanta and B. Mondal, Reaction of a {Co(NO)}8 complex with superoxide: Formation of a six coordinated [CoII(NO)(O2−)] species followed by peroxynitrite intermediate, J. Inorg. Biochem., 2022, 228, 111698 CrossRef CAS PubMed.
- X. Q. Zhu, Q. Li, W. F. Hao and J. P. Cheng, Dissociation energies and charge distribution of the Co-NO bond for nitrosyl-alpha, beta, gamma, delta-tetraphenylporphinatocobalt(II) and nitrosyl-alpha, beta, gamma, delta-tetraphenylporphinatocobalt(III) in benzonitrile solution, J. Am. Chem. Soc., 2002, 124, 9887–9893 CrossRef CAS PubMed.
- A. K. Patra, R. Afshar, M. M. Olmstead and P. K. Mascharak, The first non-heme iron(III) complex with a ligated carboxamido group that exhibits photolability of a bound NO ligand, Angew. Chem., Int. Ed., 2002, 41, 2512–2515 CrossRef CAS PubMed.
- K. Ghosh, A. A. Eroy-Reveles, B. Avila, T. R. Holman, M. M. Olmstead and P. K. Mascharak, Reactions of NO with Mn(II) and Mn(III) centers coordinated to carboxamido nitrogen: synthesis of a manganese nitrosyl with photolabile NO, Inorg. Chem., 2004, 43, 2988–2997 CrossRef CAS PubMed.
- P. K. Mascharak, Nitric oxide delivery platforms derived from a photoactivatable Mn(II) nitrosyl complex: Entry to photopharmacology, J. Inorg. Biochem., 2022, 231, 111804 CrossRef CAS PubMed.
- I. Stepanenko, M. Zalibera, D. Schaniel, J. Telser and V. B. Arion, Ruthenium-nitrosyl complexes as NO-releasing molecules, potential anticancer drugs, and photoswitches based on linkage isomerism, Dalton Trans., 2022, 51, 5367–5393 RSC.
- A. J. Gomes, E. M. Espreafico and E. Tfouni, trans-[Ru(NO)Cl(cyclam)](PF6)2 and [Ru(NO)(Hedta)] incorporated in PLGA nanoparticles for the delivery of nitric oxide to B16-F10 cells: cytotoxicity and phototoxicity, Mol. Pharm., 2013, 10, 3544–3554 CrossRef CAS PubMed.
- K. Nakanishi, T. Koshiyama, S. Iba and M. Ohba, Lipophilic ruthenium salen complexes: incorporation into liposome bilayers and photoinduced release of nitric oxide, Dalton Trans., 2015, 44, 14200–14203 RSC.
- Y. T. Yu, S. W. Shi, Y. Wang, Q. L. Zhang, S. H. Gao, S. P. Yang and J. G. Liu, A Ruthenium Nitrosyl-Functionalized Magnetic Nanoplatform with Near-Infrared Light-Controlled Nitric Oxide Delivery and Photothermal Effect for Enhanced Antitumor and Antibacterial Therapy, ACS Appl. Mater. Interfaces, 2020, 12, 312–321 CrossRef CAS PubMed.
- A. K. Patra, J. M. Rowland, D. S. Marlin, E. Bill, M. M. Olmstead and P. K. Mascharak, Iron nitrosyls of a pentadentate ligand containing a single carboxamide group: syntheses, structures, electronic properties, and photolability of NO, Inorg. Chem., 2003, 42, 6812–6823 CrossRef CAS PubMed.
- T. C. Harrop, Z. J. Tonzetich, E. Reisner and S. J. Lippard, Reactions of synthetic [2Fe-2S] and [4Fe-4S] clusters with nitric oxide and nitrosothiols, J. Am. Chem. Soc., 2008, 130, 15602–15610 CrossRef CAS PubMed.
- K. B. Shumaev, O. V. Kosmachevskaya, E. I. Nasybullina, E. K. Ruuge, E. I. Kalenikova and A. F. Topunov, Histidine-Bound Dinitrosyl Iron Complexes: Antioxidant and Antiradical Properties, Int. J. Mol. Sci., 2023, 24, 17236 CrossRef CAS PubMed.
- W. H. Chuang, Y. T. Chou, Y. H. Chen, T. H. Kuo, W. F. Liaw, T. T. Lu, C. F. Kao and Y. M. Wang, Neuroprotective Effect of NO-Delivery Dinitrosyl Iron Complexes (DNICs) on Amyloid Pathology in the Alzheimer's Disease Cell Model, ACS Chem. Neurosci., 2023, 14, 2922–2934 CrossRef CAS PubMed.
- L. Mühlhaus, L. B. Gee, R. D. Ribson, T. S. Chan, U. P. Apfel and T. T. Lu, Ligand Control of Dinitrosyl Iron Complexes for Selective Superoxide-Mediated Nitric Oxide Monooxygenation and Superoxide-Dioxygen Interconversion, J. Am. Chem. Soc., 2023, 145, 20389–20402 CrossRef PubMed.
- C. Glidewell, M. E. Harman, M. B. Hursthouse, I. L. Johnson and M. Motevalli, The conformation of Roussin esters: crystal and molecular structures of bis(methanethiolato)bis(dinitrosyliron), bis(pentanethiolato)bis(dinitrosyliron), and bis(2-methyl-propane-2-thiolato)bis(dinitrosyliron), J. Chem. Res., 1988, 212, 1676 Search PubMed.
- T. C. Harrop, D. Song and S. J. Lippard, Reactivity pathways for nitric oxide and nitrosonium with iron complexes in biologically relevant sulfur coordination spheres, J. Inorg. Biochem., 2007, 101, 1730–1738 CrossRef CAS PubMed.
- P. B. Davidovich, V. V. Gurzhiy, N. A. Sanina, A. V. Shchukarev, A. V. Garabadzhiu and A. N. Belyaev, Synthesis and structure of dinitrosyl iron complexes with secondary thiolate bridging ligands [Fe2(μ-SCHR2)2(NO)4], R=Me, Ph, Polyhedron, 2015, 90, 197–201 CrossRef CAS.
- R. Wang, M. A. Camacho-Fernandez, W. Xu, J. Zhang and L. Li, Neutral and reduced Roussin's red salt ester [Fe2(μ-RS)2(NO)4] (R = n-Pr, t-Bu, 6-methyl-2-pyridyl and 4,6-dimethyl-2-pyrimidyl): synthesis, X-ray crystal structures, spectroscopic, electrochemical and density functional theoretical, Dalton Trans., 2009, 5, 777–786 RSC.
- T. T. Lu, S. H. Lai, Y. W. Li, I. J. Hsu, L. Y. Jang, J. F. Lee, I. C. Chen and W. F. Liaw, Discrimination of mononuclear and dinuclear dinitrosyl iron complexes (DNICs) by S K-edge X-ray absorption spectroscopy: insight into the electronic structure and reactivity of DNICs, Inorg. Chem., 2011, 50, 5396–5406 CrossRef CAS PubMed.
- N. Lehnert, E. Kim, H. T. Dong, J. B. Harland, A. P. Hunt, E. C. Manickas, K. M. Oakley, J. Pham, G. C. Reed and V. S. Alfaro, The Biologically Relevant Coordination Chemistry of Iron and Nitric Oxide: Electronic Structure and Reactivity, Chem. Rev., 2021, 121, 14682–14905 CrossRef CAS PubMed.
- Q. Jing, L. F. Liu, Y. Zhang, L. L. Xie, L. N. Song, W. M. Wang, Y. H. Liu, X. Zhao and H. F. Wang, Structure, photodynamic reaction and DNA photocleavage properties of a nitrosyl iron-sulfur cluster (Me4N)2[Fe2S2(NO)4]: A DFT calculation and experimental study, Spectrochim. Acta, Part A, 2020, 238, 118401 CrossRef CAS PubMed.
- S. Porasuphatana, J. Weaver, T. A. Budzichowski, P. Tsai and G. M. Rosen, Differential effect of buffer on the spin trapping of nitric oxide by iron chelates, Anal. Biochem., 2001, 298, 50–56 CrossRef CAS PubMed.
- A. F. Vanin, A. P. Poltorakov, V. D. Mikoyan, L. N. Kubrina and E. V. Faassen, Why iron-dithiocarbamates ensure detection of nitric oxide in cells and tissues, Nitric Oxide, 2006, 15, 295–311 CrossRef CAS PubMed.
- C. R. Wu, Y. D. Huang, Y. H. Hong, Y. H. Liu, M. Narwane, Y. H. Chang, T. K. Dinh, H. T. Hsieh, Y. J. Hseuh, P. C. Wu, C. W. Pao, T. S. Chan, I. J. Hsu, Y. Chen, H. C. Chen, T. Y. Chin and T. T. Lu, Endogenous Conjugation of Biomimetic Dinitrosyl Iron Complex with Protein Vehicles for Oral Delivery of Nitric Oxide to Brain and Activation of Hippocampal Neurogenesis, J. Am. Chem. Soc., 2021, 1, 998–1013 CAS.
- T. Z. Lv and G. S. Wang, Antiproliferation potential of withaferin A on human osteosarcoma cells via the inhibition of G2/M checkpoint proteins, Exp. Ther. Med., 2015, 10, 323–329 CrossRef CAS PubMed.
- Y. Xu, Y. P. Ching, Y. Zhou, J. F. Chiu, F. Chen and Q. Y. He, Multiple pathways were involved in tubeimoside-1-induced cytotoxicity of HeLa cells, J. Proteomics, 2011, 75, 491–501 CrossRef CAS PubMed.
- M. Li, D. Tang, T. Yang, D. Qian and R. Xu, Apoptosis Triggering, an Important Way for Natural Products From Herbal Medicines to Treat Pancreatic Cancers, Front. Pharmacol., 2022, 12, 796300 CrossRef PubMed.
- J. Shao, Q. Zhang, J. Wei, Z. Yuchi, P. Cao, S. Q. Li, S. Wang, J. Y. Xu, S. Yang, Y. Zhang, J. X. Wei and J. L. Tian, Synthesis, crystal structures, anticancer activities and molecular docking studies of novel thiazolidinone Cu(II) and Fe(III) complexes targeting lysosomes: special emphasis on their binding to DNA/BSA, Dalton Trans., 2021, 50, 13387–13398 RSC.
- Y. Zhang, A. Ho, J. Yue, L. Kong, Z. Zhou, X. Wu, F. Yang and H. Liang, Structural basis and anticancer properties of ruthenium-based drug complexed with human serum albumin, Eur. J. Med. Chem., 2014, 86, 449–455 CrossRef CAS PubMed.
- W. J. Gong, T. Wu, Y. H. Liu, S. X. Jiao, W. M. Wang, W. J. Yan, Y. Q. Li, Y. H. Liu, Y. Zhang and H. F. Wang, Insight into the photodynamic mechanism and protein binding of a nitrosyl iron-sulfur [Fe2S2(NO)4]2− cluster, Spectrochim. Acta, Part A, 2024, 320, 124603 CrossRef CAS PubMed.
- Y. J. Yu, T. W. Chiou, J. S. K. Yu and L. K. Chu, Time-resolved Infrared Characterization on the Photolysis of Roussin's Red Phenyl Ester in Different Solvents, J. Photochem. Photobiol., A, 2021, 406, 113032 CrossRef CAS.
- M. Z. Huang, Y. J. Yang, X. W. Liu, Z. Qin and J. Y. Li, Aspirin eugenol ester attenuates oxidative injury of vascular endothelial cells by regulating NOS and Nrf2 signalling pathways, Br. J. Pharmacol., 2019, 176, 906–918 CrossRef CAS PubMed.
- X. T. Lin, A. Zheng, S. H. Lin, J. L. Huang and J. X. Lu, The crystal and molecular structure of a typical red roussin salt (Me4N)2[Fe2S2(NO)4], J. Struct. Chem., 1982, 1, 79–88 CAS.
- G. M. Sheldrick, SHELXS-97, Program for the Solution of Crystal Structures, University of Gottingen, 1997 Search PubMed.
- APEX2, SADABS, and SAINT-Plus. Bruker AXS Inc., Madison, Wisconsing, USA, 2007 Search PubMed.
- W. Minor, M. Cymborowski, Z. Otwinowski and M. Chruszcz, HKL-3000: the integration of data reduction and structure solution - from diffraction images to an initial model in minutes, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2006, 62, 859–866 CrossRef PubMed.
- V. B. Chen, W. B. Arendall, J. J. Headd, D. A. Keedy, R. M. Immormino, G. J. Kapral, L. W. Murray, J. S. Richardson and D. C. Richardson, MolProbity: all-atom structure validation for macromolecular crystallography, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66, 12–21 CrossRef CAS PubMed.
- P. Emsley and K. Cowtan, Coot: model-building tools for molecular graphics, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2004, 60, 2126–2132 CrossRef PubMed.
- P. D. Adams, R. W. Grosse-Kunstleve, L. W. Hung, T. R. Ioerger, A. J. McCoy, N. W. Moriarty, R. J. Read, J. C. Sacchettini, N. K. Sauter and T. C. Terwilliger, PHENIX: building new software for automated crystallographic structure determination, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2002, 58, 1948–1954 CrossRef PubMed.
- W. L. DeLano, The PyMOL Molecular Graphics System, DeLano Scientifific, San Carlos. CA, 2002 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2390905, 2390895, 2390735 and 2390745. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5qi00255a |
| This journal is © the Partner Organisations 2025 |