Organelle-targeting activity-based hemicyanine derivatives for enhanced and selective type-I photodynamic therapy under hypoxia conditions†
Musa
Dirak‡
 a,
Ayca
Saymaz‡
a,
Alperen
Acari‡
b,
Yunus
Akkoc
ce,
Haluk Samet
Kocak
d,
Cansu M.
Yenici
a,
Ayca
Saymaz‡
a,
Alperen
Acari‡
b,
Yunus
Akkoc
ce,
Haluk Samet
Kocak
d,
Cansu M.
Yenici
 a,
Devrim
Gozuacik
ce,
Hande
Gunduz
*af and
Safacan
Kolemen
a,
Devrim
Gozuacik
ce,
Hande
Gunduz
*af and
Safacan
Kolemen
 *a
*a
aDepartment of Chemistry, Koç University, 34450 Istanbul, Turkey. E-mail: hagunduz@ku.edu.tr; skolemen@ku.edu.tr
bCellular and Molecular Medicine Graduate Program, Koç University, 34450 Istanbul, Turkey
cKoç University Research Center for Translational Medicine (KUTTAM), 34450 Istanbul, Turkey
dBiomedical Science and Engineering Graduate Program, Koç University, 34450 Istanbul, Turkey
eDepartment of Medical Biology, School of Medicine, Koç University, 34450 Istanbul, Turkey
fNanofabrication and Nanocharacterization Center for Scientific and Technological Advanced Research, Koç University, 34450 Istanbul, Turkey
First published on 31st December 2024
Abstract
Type-I photosensitizers (PSs) have attracted great attention in recent years as they minimally rely on the tissue oxygen (3O2) to generate highly cytotoxic reactive oxygen species (ROS) in the scope of photodynamic therapy (PDT). Thus, they hold great promise for effective treatment of hypoxic cancer cells, which is a challenging task for type-II PSs. However, compared to conventional type-II PSs, the number of cancer cell selective type-I PSs is quite low. Thus, there is still a need for type-I PSs that can induce photocytotoxicity only in cancer cells without causing damage to normal tissues even under light irradiation. Additionally, targeting PSs to specific organelles has lately appeared to be a promising approach to improve the therapeutic outcome of PDT. Although a few examples of organelle-targeted type-I PS cores have emerged recently, activity-based and organelle-targeted type-I PSs have remained scarce. In this study, we report two organelle-targeted and hydrogen sulfide (H2S) responsive type-I PSs (HEHM and HEH) based on a highly modular and easily accessible heavy atom decorated hemicyanine core. HEHM localizes to mitochondria due to its cationic structure, whereas HEH targets endoplasmic reticulum (ER) as it bears an ER-targeting sulfonamide moiety, and it marks the first example of an activity-based and ER-targeted type-I PS based on a hemicyanine core. Both PSs can be selectively activated in neuroblastoma cells (SH-SY5Y) upon reacting with high levels of endogenous H2S and induce similar photocytotoxicity through a type-I PDT mechanism under both normoxic (20% O2) and hypoxic (1% O2) conditions. HEHM is shown to cause PDT-induced mitochondria stress, while HEH triggers ER stress upon LED irradiation (640 nm, 66.7 mW cm−2). Additionally, HEH is shown to induce immunogenic cell death (ICD) followed by PDT action. In contrast, negligible ROS generation and cell death are observed in normal cells, which is a critical and challenging task for any type of therapeutic modality. They also allow fluorescence imaging of cancer cells due to their emissive nature, suggesting that they function as phototheranostic agents. This study introduces a rational approach to develop new generation activity-based and organelle-targeted type-I PDT agents towards effective and selective treatment of hypoxic tumors.
Introduction
Photodynamic therapy (PDT), which involves the generation of reactive oxygen species (ROS) through the interaction of a photosensitizer (PS) and light, is a highly attractive treatment method for several forms of cancer.1–7 The unique advantage of PDT is more pronounced when it is compared to conventional therapies such as radiotherapy and chemotherapy, which are mostly immunosuppressive. In contrast, PDT is known to stimulate the host immune system against cancer cells and accordingly it can be easily combined with immunotherapy.8 In addition, PDT offers minimal invasiveness, high spatial selectivity, opportunities for repeated applications without having severe side effects and initiating tumor resistance.9 Therefore, it has appeared to be an approved modality to be used in cancer treatment.Photochemical processes that result in ROS generation are divided into two major mechanisms (Fig. S1, ESI†). In a typical type-II mechanism, a PS is irradiated using an external light source, and it is excited to a singlet excited state (S1), which is followed by intersystem crossing (ISC) to a triplet excited state (T1). Then, the triplet PS transfers its energy to molecular oxygen (3O2) to generate highly reactive and cytotoxic singlet oxygen (1O2) (Fig. S1, ESI†). Accordingly, the type-II mechanism heavily relies on tissue oxygen (3O2), which is known to be depleted in most of the hypoxic solid tumors. To this end, type-II PDT appears to be a self-limiting approach, especially under hypoxic conditions. In the case of the type-I mechanism, the triplet PS transfers electrons/hydrogen from biological substrates to generate free radicals. Then, these radicals react either with water or 3O2 to yield a superoxide radical anion (O2˙−). Later, a highly cytotoxic hydroxyl radical (OH˙) is generated in the presence of a superoxide dismutase (SOD) enzyme through biological cascade reactions (Fig. S1, ESI†). Therefore, tissue oxygen dependence is minimal during the type-I PDT action, which makes it a highly promising candidate for hypoxic tumor treatment.
Type-I PSs can address some critical problems of type-II PSs; however, there are still significant challenges in the clinical translation of PDT such as limited tissue penetration depth of irradiation light, cancer cell selectivity, and low water solubility of the first-generation photosensitizers.10 The limited penetration problem of the light arises from the fact that UV-vis light is absorbed and/or scattered by biomolecules.11 This can be partially solved by using red or near-IR (NIR) light to alleviate the absorption of biomolecules, allowing light to penetrate deeper tissues.12 Additionally, new generation PDT agents offer improved cancer cell selectivity, which ensures cytotoxicity only in the tumor region without harming healthy tissues even under light irradiation. One way to implement such selectivity is to design activity-based photosensitizers (aPSs), which bear protecting groups that can be sensitive to pH, small biomolecules (e.g. biothiols, ROS and reactive nitrogen species (RNS)), or enzymes abundant in the tumor microenvironment.13,14 Upon removal of these protecting groups by tumor-associated triggers, the PS turns on its ROS generation and fluorescence signal, if any, and enables therapeutic action and imaging at the same time. Thus, water soluble type-I aPSs that can operate within the therapeutic window (650–900 nm) are highly sought to treat cancer cells selectively.
The number of small molecule organic type-I PSs is low compared to type-II PDT agents. This can be widely attributed to the unclear structure–activity relationship of the PS cores. To date, several dicyanochromene, phenalenone, and benzophenothiazine derivatives along with aggregation-induced emission (AIE) active agents have been reported as type-I PSs.10,15,16 However, activity-based type-I PS designs remained scarce as most of the type-I PS cores do not offer suitable modification sides towards the development of aPSs. Hemicyanine is an attractive subclass of the cyanine family that can be utilized both for imaging and PDT applications as it offers NIR absorption/emission, high water solubility, and low dark toxicity.17 Additionally, it can be easily converted to an activity-based agent by modulating the intramolecular charge transfer (ICT) process. Parent hemicyanine is highly emissive and exhibits a very low ISC efficiency.18 Therefore, it is a poor PS. We and others showed that incorporation of heavy atoms (such as bromine, iodine or selenium) on the core structure improves the ISC yield of hemicyanines by enhancing the spin–orbit coupling (SOC) and eventually they can generate 1O2 upon light excitation through the type-II mechanism.19–22 However, especially for the brominated hemicyanines 1O2 quantum yields are still quite low despite their high therapeutic efficacy in cell cultures.20 This suggests that an alternative type of ROS might be generated upon light irradiation. While we were investigating the type-I potential of hemicyanines in the scope of this study, Miao and coworkers very recently showed that both brominated and iodinated hemicyanine derivatives generate superoxide and hydroxyl radicals effectively through the type-I mechanism.23,24 They also converted their meta-brominated hemicyanine core to alkaline phosphatase (ALP) and aminopeptidase N (APN) responsive aPSs by simply caging the phenolic group of the hemicyanine with appropriate masking units.23,24 Thus, brominated hemicyanines appeared to be a highly promising activity-based type-I PS, which also have adequate fluorescence quantum yields to serve as phototheranostic agents.
Along with activity-based PS designs, it is also highly beneficial to localize short-lived ROS in critical components of cancer cells participating in vital metabolic functions to increase the lethal effect of PDT.25 To this end, targeting organelles is a promising approach. Accordingly, a variety of organelle-targeted PSs localizing mitochondria, ER, lysosome, Golgi apparatus, and nuclei have been developed in recent years.26 Among these organelles, mitochondria have attracted special attention as they play critical roles in ATP production, and cell apoptosis.27 The localization of PSs to mitochondria is mostly accomplished by using cationic molecules due to the negative transmembrane potential of mitochondria.26 In this direction, numerous mitochondria-targeted PSs, which are mostly following the type-II mechanism, have been reported.28 In addition to mitochondria, endoplasmic reticulum (ER) holds great promise as a target in PDT studies because of its vital roles in the regulation of protein synthesis, folding and modification, cellular lipid synthesis, intracellular signaling, and calcium ion (Ca2+) storage and balance.29 Therefore, disturbing the function of ER may lead effective cell death as in the case of mitochondria. The generated ROS in PDT triggers ER-stress and increases the amount of intracellular Ca2+ ions, which eventually cause cell death.30 In the design of ER-targeting imaging and therapeutic agents, glibenclamide analogues, methyl sulfonamides, and some specific peptides like pardaxin peptides have been utilized so far.25 Several ER-targeted PSs have been already reported in the literature, which are based on phthalocyanine (Pc) derivatives, iridium complexes, inorganic/organic nanoparticles, and boron dipyrromethene (BODIPY) derivatives.25,26,31,32 Most recently, a paper was published by Guo and co-workers, in which a benzophenothiazine derivative as a type-I PS was modified with a ER targeting unit methyl sulfonamide group to treat cancer cells under hypoxic conditions.33 Additionally, Zhao et al. showed that their phosphindole oxide-based type-I PS can localize to ER.34 However, studies on activity-based PDT agents targeting ER organelle are very rare and there is only one example, which involves a type-I PS.35
Herein, we have introduced two organelle-targeted and hydrogen sulfide (H2S)-responsive type-I PSs (HEHM and HEH) based on the ortho-brominated hemicyanine skeleton for selective and effective treatment of cancer cells (Fig. 1). H2S is an essential gasotransmitter, which takes critical roles in signaling pathways and regular physiological processes. It is synthesized by certain enzymes such as cystathionine β-synthase and cystathionine γ-lyase.36 The activity of these enzymes is known to be elevated in cancer cells, which eventually increases the H2S concentration in cancer cells. Accordingly, H2S has attracted great attention in the design of cancer cell selective activity-based imaging and therapeutic agents over the years.37HEHM and HEH carry the same H2S-responsive 2,4-dinitrophenyl masking group,38 which quenches the photocytotoxicity and NIR fluorescence signal of the parent hemicyanine as a result of the blocked ICT process. The hemicyanine core in both designs also bears a heavy bromine to boost the ROS generation capacity. The bromine is located at the ortho position of the phenolic hydroxyl group to increase the phenolate concentration at pH 7.4 by decreasing the pKa upon H2S-induced activation. Furthermore, it is known that ortho-brominated hemicyanines can serve as a type-I PS.23,24 The cationic nature of HEHM makes it a mitochondria targeted PS (Fig. 1). On the other hand, HEH is further modified with a methyl sulfonamide unit at the indolenine ring through versatile cyanine chemistry to obtain an ER localizing type-I PS (Fig. 1). Therefore, both HEHM and HEH are getting activated with high levels of H2S in cancer cells upon a nucleophilic aromatic substitution reaction (SNAr) but turn on their photocytotoxicity in different organelles, either in mitochondria or in ER (Fig. 1). Photoactive brominated hemicyanine cores (HC-1 and H) (Fig. 1) that are released upon activation are also fluorescent enough to enable visualization of H2S activity in two organelles.
 | ||
| Fig. 1 Molecular structures and the activation of organelle-targeted and H2S-responsive PSs HEHM and HEH. | ||
Results and discussion
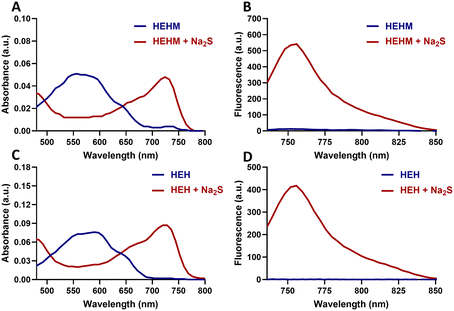
Synthesis of HEHM and HEH is depicted in Scheme S1 (ESI†). The brominated hemicyanine (HC-1) core was obtained by following the procedure reported in our previous study.39 Then, it reacted with commercially available 2,4-dinitrofluorobenzene to mask the phenolic moiety. For the synthesis of the ER targeting H2S responsive probe, HEH, compound (1) (Scheme S1, ESI†) was synthesized according to previous study reported by Ye and co-workers.40 Later, the retro Knoevenagel reaction between (1) and 4-bromo resorcinol was performed, which was followed by deprotection of the Boc group to give compound (4) (Scheme S1, ESI†). Finally, the ER directing sulfonamide unit was attached on compound (4) to yield HEH. Both HEHM and HEH were characterized by 1H, 13C NMR and high-resolution mass spectrometry (HR-MS).After completing the synthesis of target compounds, the photophysical properties of HEH and HEHM were investigated in DMSO (2% PBS, pH 7.4). Both PSs displayed a broad absorption peak laying from 500 nm to 650 nm in their caged form with no detectable fluorescence signals as expected from a masked hemicyanine core. Addition of Na2S resulted in a dramatic red shift in the absorption signal of the both PSs and a new peak at 726 nm appeared (Fig. 2A, C and Fig. S2A, C, ESI†). In the case of fluorescence measurements, a strong and very rapid turn-on response was noted at 754 nm (λex = 726 nm) after both agents reacted with Na2S (50 μM) (Fig. 2B and D). After reacting with Na2S, fluorescence quantum yields for HEHM and HEH were measured in DMSO (2% PBS, pH 7.4) as 22.3% and 16.7%, respectively. These results clearly show that HEHM and HEH can be activated with H2S and emit a strong NIR fluorescence signal, which is highly critical for bioimaging studies. Treating PSs with increasing concentrations of Na2S (1–50 μM) resulted in a linear increase in the emission signals of both PSs at 754 nm (Fig. S2B and D, ESI†) and the detection limits were calculated as 3.93 μM and 2.27 μM for HEHM and HEH, respectively. The selectivity of the PSs was assessed by detecting their fluorescence response against potentially interfering analytes such as biothiols (glutathione (GSH), cysteine (Cys)), inorganic salts (Na2SO3, KCl, Na2S2O3, CuSO4, KI, KBr, NaF, NaNO2, SCN−, NH4OAc, MgCl2, KNO3, K2CO3, and CaCl2), and amino acid (Ala). The concentration of the GSH was set to 5 mM, while the concentration of other analytes was adjusted to 1 mM. The PSs did not exhibit a notable response in the presence of competing analytes, and only HEH displayed a small amount of response towards Cys (Fig. S3, ESI†). It is important to note that the concentration of Cys (1 mM) was significantly greater than that of Na2S (50 μM) in the selectivity assay.
 | ||
| Fig. 2 Photophysical properties of HEHM and HEH. Absorption and fluorescence spectra of (A) and (B) HEHM (10 μM) and (C) and (D) HEH (10 μM) in the absence or presence of Na2S (50 μM). λex = 726 nm. | ||
The photophysical properties of photoactive products that were released after the reaction between H2S and HEHM/HEH were also investigated. The photoactive product of HEHM, namely compound HC-1, was obtained as an intermediate during the synthesis of HEHM. It was characterized by acquiring the 1H NMR spectrum (Fig. S4, ESI†). Its absorption and fluorescence spectra were recorded in an aqueous solution (PBS, 1% DMSO, pH 7.4) and in DMSO (2% PBS, pH 7.4) (Fig. S5, ESI†). HC-1 exhibited strong absorption and emission signals centered at 694 and 715 nm, respectively, in the aqueous solution. In DMSO, signals were slightly red shifted and maximum intensities were recorded at 710 and 740 nm, respectively. The photoactive product of HEH, which is named as compound H, was obtained by treating HEH with Na2S in acetonitrile. The resulting compound H was purified through preparative TLC and characterized by 1H NMR and HR-MS (Fig. S6, ESI†). After obtaining compound H, photophysical measurements were conducted. H displayed absorption and emission maxima at 696 and 716 nm, respectively, in PBS (1% DMSO, pH 7.4) (Fig. S7, ESI†). On the other side, absorption and emission maxima were reported at 732 and 754 nm, respectively, in DMSO (Fig. S7, ESI†).
The ROS generation capacity of the PSs was first investigated by utilizing commercially available indicators under LED light irradiation (640 nm, 66.7 mW cm−2) after activating them with H2S. Singlet oxygen sensor green (SOSG) and dihydroethidium (DHE) were used to detect 1O2 and O2˙−, respectively. In the presence of superoxide radical anions, DHE is oxidized to ethidium. Following this oxidation, it intercalates into DNA or RNA and subsequently becomes emissive upon irradiation.41 As shown in Fig. 3, up to a 15-fold increase was detected in the emission signal of DHE at 576 nm upon 120 seconds light excitation, while only 1.2-fold enhancement was observed in the SOSG fluorescence at 528 nm under identical conditions. It was also shown that light treatment did not cause any turn on response in both SOSG and DHE signals in the absence of PSs (Fig. 3). These results suggest that both HEH and HEHM predominantly serve as type-I PSs upon H2S-induced activation. Furthermore, the 1O2 generation capacity of Na2S-treated HEHM and HEH was evaluated by using another water soluble 1O2 selective trap molecule, 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA) in PBS (1% DMSO, pH 7.4) (Fig. S8, ESI†). Again, no significant decrease was observed in the absorption signal of ABDA at 380 nm upon light irradiation, supporting that these PSs are acting as a type-I PS. On the other side, when the same experiment was repeated with methylene blue as a positive control, a clear 1O2 generation was observed as evidenced from the decrease in the absorption signal of ABDA at 380 nm (Fig. S8, ESI†).
Later, we checked the ROS generation capacity of the photoactive product HC-1 by utilizing SOSG and ABDA for 1O2 and DHE for O2˙− in PBS (1% DMSO, pH 7.4) (Fig. S9, ESI†). In the case of SOSG, a maximum 1.15-fold increase was detected in the SOSG fluorescence signal at 530 nm, while almost no change was noted in the absorption signal of ABDA at 380 nm, once more suggesting that HC-1 is a very poor 1O2 generator. When we repeated the same experiment with DHE, a dramatic increase was reported in DHE fluorescence at 587 nm upon light irradiation. Similarly, ROS generation ability of photoactive H was analyzed by utilizing SOSG, ABDA and DHE. A significant change was only detected in the case of the DHE signal upon light irradiation, suggesting that the H core also acts as a type-I PS (Fig. S10, ESI†). All these results are in good correlation with the ROS detection experiments that are performed with H2S-treated HEHM/HEH.
Activation of both PSs was also proved by HR-MS analyses. To this end, HEHM and HEH were treated with Na2S and then the mass spectra were recorded. In the case of HEHM, a peak at 462.1068 m/z was observed, which belongs to the active brominated hemicyanine core, HC-1 (calculated as 462.1063 [M+]) (Fig. S11A, ESI†). When HEH was reacted with Na2S, a signal at 659.1574 m/z appeared, indicating the formation of the uncaged ER-targeted brominated hemicyanine core, H (calculated as 659.1574 [M+]) (Fig. S11B, ESI†). These results confirmed the release of the 2,4-dinitrophenyl masking group as a result of the H2S-induced SNAr reaction. Furthermore, LC-QTOF-MS measurements were conducted by using HEHM to analyze the time-dependent consumption of HEHM and formation of the active core HC-1. The release of HC-1 and the consumption of HEHM were shown to be very rapid and the reaction between HEHM and Na2S was completed approximately in 4 minutes (Fig. S12, ESI†).
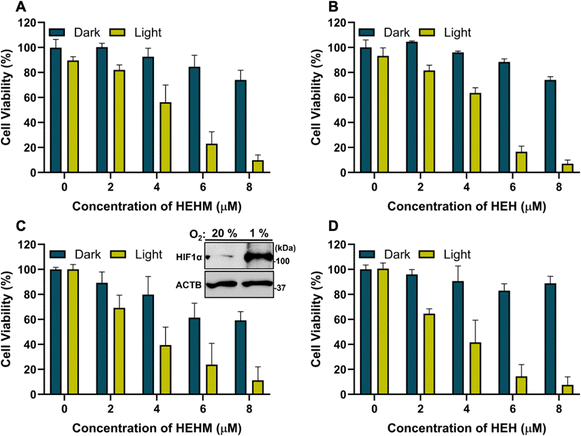
Next, we wanted to evaluate the cytotoxicity of PSs in normal Vero (kidney epithelial) and H2S-rich SH-SY5Y (human neuroblastoma) cells.42 Initially, we investigated the dark toxicity of the PSs to demonstrate their biocompatibility. Both HEH and HEHM were determined to be non-toxic in the absence of light within the concentration range of 1–8 μM in Vero cells (Fig. S13, ESI†). Upon 640 nm LED light (66.7 mW cm−2) irradiation for 10 minutes, PSs remained non-toxic in Vero cells as they could not be activated by H2S but cause damage to SH-SY5Y cells in a dose dependent manner (Fig. 4A, B and Fig. S13, ESI†) under normoxic (20% O2) conditions. A small amount of dark toxicity was observed in SH-SY5Y neuroblastoma cells; cell viability dropped to 80% at the highest concentration (Fig. 4A and B). At an 8 μM dose, almost complete cell death was reported with both PSs under light irradiation (Fig. 4A and B). Remarkably, a dose dependent cell death was also detected under hypoxic conditions (1% O2) in HEHM/HEH-treated SH-SY5Y cells upon light irradiation, showcasing the success of type-I PSs under severe hypoxia conditions (Fig. 4C and D). In the dark, HEHM displayed slightly higher toxicity at higher doses compared to HEH. Formation of the hypoxic conditions was confirmed by checking the HIF1α expression after incubating SH-SY5Y cells in a hypoxia incubator for 3 hours (Fig. 4C inset).43
Thus, both PSs were shown to induce selective photocytotoxicity towards cancer cells, while staying in their off state in normal cells even under light irradiation. This is a unique advantage of activity-based PSs, which is highly critical to eliminate severe side effects.
To further support the cancer cell selective PDT action, the intracellular ROS generation capacity of the PSs was assessed using DCF-DA (2,7-dichlorodihydrofluorescein diacetate), a very well-known non-specific ROS sensor. Firstly, we evaluated the ROS generation in Vero cells. After HEH or HEHM treatment, cells were incubated with DCF-DA and either left in the dark or put under 640 nm light exposure. No fluorescence in the green channel was detected in both groups (Fig. S14, ESI†), indicating that the PSs remained in their caged form, which is in very good agreement with the cell viability results. When the same procedure was applied to SH-SY5Y cells, similarly, no green fluorescence was detected under dark conditions (Fig. S15, ESI†). In contrast, strong green fluorescence of the activated sensor was observed in SH-SY5Y cells after light exposure, confirming light-induced ROS generation of the uncaged PSs (Fig. S15, ESI†).
Later, we specifically checked the intracellular 1O2, O2˙− and OH˙ generation by using SOSG, DHE and hydroxyphenyl fluorescein (HPF), respectively, in SH-SY5Y cells (Fig. 5). As expected, the SOSG signal was very weak due to the low 1O2 generation capacity of the PSs. In contrast, a strong DHE signal was observed indicating effective O2˙− production upon light excitation. Importantly, the characteristic green fluorescence signal of HPF was also detected in both HEH and HEHM incubated cells, suggesting intracellular conversion of O2˙− to highly cytotoxic OH˙, which is necessary to get a high therapeutic outcome. Under dark conditions, none of the ROS sensors displayed a remarkable fluorescence signal.
We also demonstrated the selective activation of the PSs with H2S using confocal microscopy (Fig. 6). A remarkable red emission was detected when SH-SY5Y neuroblastoma cells were treated with HEH or HEHM as PSs were activated by the endogenous H2S. However, the emission recorded from the red channel was significantly reduced when cells were pre-treated with ZnCl2, a known H2S quencher.44 On the other hand, when cells were incubated with exogenous NaHS, an approximately 2.1-fold increase was recorded in the fluorescence signal indicating that the H2S level was increased as expected. These results showed that both PSs can detect the fluctuations in the intracellular H2S level, and they are suitable for theranostic applications.
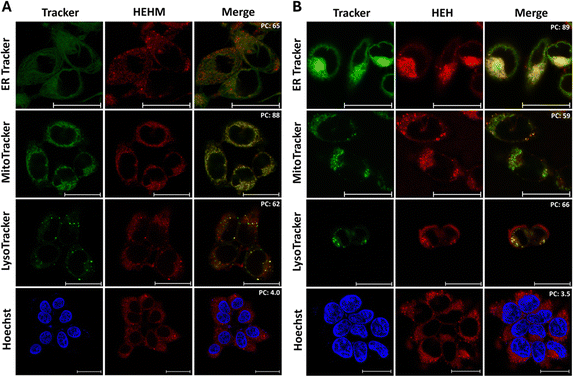
Subcellular localization of the PSs was investigated using commercially available MitoTracker Green, ER-Tracker Green, LysoTracker Green and nucleus stain Hoechst. As previously mentioned, the indole ring of the hemicyanine core was modified to target different subcellular organelles. Since HEHM bears only a positively charged indole ring without any additional unit, it is expected to localize in mitochondria, while HEH is expected to localize in ER with the help of the ER-targeting sulfonamide moiety. To prove our hypothesis, colocalization experiments were conducted on SH-SY5Y cells treated with HEHM or HEH. As shown in Fig. 7A, HEHM specifically targeted mitochondria with a high Pearson coefficient (PC) of 0.88. In the case of HEH, the red signal originating from the PS perfectly overlapped with the ER-Tracker Green with a PC of 0.89 (Fig. 7B). As opposed to this, fluorescence signals of HEHM and HEH displayed poorer overlay with other trackers (Fig. 7). Additionally, both PSs exhibited a very low level of colocalization with the nucleus stain (Fig. 7).
Afterward, a JC-1 dye was utilized to assess the changes in mitochondrial membrane potential upon the PDT action in HEHM-treated SH-SY5Y cells (Fig. 8). JC-1 emits green emission in its monomeric state, while a red emission is observed when it forms J-aggregates. The green emission indicates disrupted membrane potential, which is a clear sign of damaged mitochondria. On the other side, formation of aggregates and consequent red emission suggests healthy mitochondria with intact membrane potential. A strong green emission was observed only in light treated cells as a result of PDT-induced mitochondrial stress (Fig. 8).
The ER is the main organelle responsible for the protein synthesis and maintenance of proteostasis. Accumulation of unfolded and misfolded proteins in the ER causes ER stress. Upon formation of ER stress, PERK multimerizes and autophosphorylates itself leading to the inactivation of EIF2A by phosphorylating EIF2A.45 Persistent ER stress further causes specific translation of ATF4 and activates the C/EBP-homologous protein (CHOP) promoter, which controls the target gene expression and may trigger apoptotic cell death.46 Formation of ER stress was initially investigated using confocal microscopy. To this end, a thioflavin T (ThT) dye, an emerging viscosity probe known to intensify its fluorescence upon binding to unfolded protein aggregates, was used.47–50 As expected, the green emission of ThT was significantly higher compared to control groups, reflecting impaired protein folding in the ER due to locally generated ROS (Fig. S16, ESI†). Later, transcription levels of ER stress-related genes ATF4, DDIT3, ERN1 and ATF6 were analyzed by the qRT-PCR after irradiating HEH-incubated SH-SY5Y cells (Fig. 9). Following PDT, significant ATF4 and DDIT3 mRNA elevation was observed in HEH-treated cells but not under control conditions (Fig. 9A and B). However, we did not observe a prominent alteration in mRNA levels of other ER stress markers including ATF6 and ERN1 under this condition (Fig. S17A and B, ESI†).
We further dissected the EIF2AK3/EIF2A pathway in which ATF4 and DDIT3 were downstream effectors (Fig. 9C, D and Fig. S19, ESI†). We evaluated the phosphorylation status of both EIF2AK3 and EIF2A proteins in our model. We observed that ER stress following PDT in HEH-treated cells was regulated by the consecutive phosphorylation of EIF2AK3 and its target protein EIF2A (Fig. 9C). In addition, our data revealed an increase in the level of CHOP. Elevation of ATF4 and CHOP could facilitate the expression of ER stress-associated genes and may control apoptosis.51 Therefore, we perform immunoblotting to check the alteration of well-known apoptotic markers PARP and caspase-3 in this context. Cleavage of both PARP and caspase-3 was increased indicating apoptotic cell death in SH-SY5Y cells following PDT (Fig. 9C and Fig. S19, ESI†). Of note, we also realized that immunological cell death markers IL1β and HMGB1 were increased with HEH treatment in SH-SY5Y cells upon PDT (Fig. S18, ESI†).
Conclusions
In summary, we have developed two H2S-responsive and organelle-targeted type-I PSs based on an ortho-brominated hemicyanine core to treat cancer cells in a selective and effective manner. Both PSs were selectively activated upon reacting with H2S and efficiently generated type-I radicals (O2˙− and OH˙) after 640 LED irradiation. The therapeutic potential of the PSs was investigated via cell culture studies in detail. They exhibited selective ROS generation in SH-SY5Y cancer cells with low IC50 values. Moreover, the effective therapeutic action of both PSs was preserved even under severe hypoxic conditions. The mitochondria stress induced by HEHM was monitored using confocal microscopy. On the other side, HEH caused ER stress as evidenced from the qPCR and immunoblot analyses in addition to imaging studies conducted with confocal microscopy. Our results suggest that HEH facilitates ER stress and triggers cell death through the EIF2AK3/EIF2A/ATF4/CHOP pathway upon PDT in SH-SY5Y neuroblastoma cells. Additionally, we showed that HEH can trigger ICD, which is a unique advantage of ER-targeted PSs. Both PSs can also be used to image H2S-rich cancer as two competing pathways namely fluorescence and ISC are both active in excited bromo-hemicyanines. We anticipate that this study can pave the way for multi-featured type-I PS designs, which can be used to treat a wide variety of different cancer cells under hypoxia conditions.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is partially supported by the Scientific and Technological Research Council of Turkey (TUBITAK), Grant 121Z662. SK thanks the Turkish Academy of Sciences, Outstanding Young Scientists Program (TUBA-GEBIP) for the financial support. The authors acknowledge the use of the services and facilities of n2STAR-Koç University Nanofabrication and Nano-characterization Center for Scientific and Technological Advanced Research. The authors gratefully acknowledge the use of the services and facilities of the Koç University Research Center for Translational Medicine (KUTTAM), funded by the Presidency of Türkiye, Head of Strategy and Budget.References
- X. Li, N. Kwon, T. Guo, Z. Liu and J. Yoon, Innovative strategies for hypoxic-tumor photodynamic therapy, Angew. Chem., Int. Ed., 2018, 57, 11522–11531 CrossRef CAS.
- X. Li, D. Lee, J. D. Huang and J. Yoon, Phthalocyanine-assembled nanodots as photosensitizers for highly efficient type I photoreactions in photodynamic therapy, Angew. Chem., Int. Ed., 2018, 57, 9885–9890 CrossRef CAS.
- Y. Y. Zhao, X. Zhang, Y. Xu, Z. Chen, B. Hwang, H. Kim, H. Liu, X. Li and J. Yoon, A Renal Clearable Nano-Assembly with Förster Resonance Energy Transfer Amplified Superoxide Radical and Heat Generation to Overcome Hypoxia Resistance in Phototherapeutics, Angew. Chem., Int. Ed., 2024, e202411514 CAS.
- L. Li, Y. Liao, S. Fu, Z. Chen, T. Zhao, L. Fang and X. Li, Efficient hydroxyl radical generation of an activatable phthalocyanine photosensitizer: oligomer higher than monomer and nanoaggregate, Chem. Sci., 2024, 15, 10980–10988 RSC.
- Y.-Y. Zhao, L. Zhang, Z. Chen, B.-Y. Zheng, M. Ke, X. Li and J.-D. Huang, Nanostructured phthalocyanine assemblies with efficient synergistic effect of type I photoreaction and photothermal action to overcome tumor hypoxia in photodynamic therapy, J. Am. Chem. Soc., 2021, 143, 13980–13989 CrossRef CAS PubMed.
- R. Wang, X. Li and J. Yoon, Organelle-targeted photosensitizers for precision photodynamic therapy, ACS Appl. Mater. Interfaces, 2021, 13, 19543–19571 CrossRef CAS.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Clinical development and potential of photothermal and photodynamic therapies for cancer, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef.
- T. C. Pham, V.-N. Nguyen, Y. Choi, S. Lee and J. Yoon, Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy, Chem. Rev., 2021, 121, 13454–13619 CrossRef CAS.
- J. Du, T. Shi, S. Long, P. Chen, W. Sun, J. Fan and X. Peng, Enhanced photodynamic therapy for overcoming tumor hypoxia: From microenvironment regulation to photosensitizer innovation, Coord. Chem. Rev., 2021, 427, 213604 CrossRef CAS.
- D. Chen, Q. Xu, W. Wang, J. Shao, W. Huang and X. Dong, Type I photosensitizers revitalizing photodynamic oncotherapy, Small, 2021, 17, 2006742 CrossRef CAS PubMed.
- K. Deng, C. Li, S. Huang, B. Xing, D. Jin, Q. Zeng, Z. Hou and J. Lin, Recent progress in near infrared light triggered photodynamic therapy, Small, 2017, 13, 1702299 CrossRef.
- P. C. A. Swamy, G. Sivaraman, R. N. Priyanka, S. O. Raja, K. Ponnuvel, J. Shanmugpriya and A. Gulyani, Near Infrared (NIR) absorbing dyes as promising photosensitizer for photo dynamic therapy, Coord. Chem. Rev., 2020, 411, 213233 CrossRef.
- J. F. Lovell, T. W. Liu, J. Chen and G. Zheng, Activatable photosensitizers for imaging and therapy, Chem. Rev., 2010, 110, 2839–2857 CrossRef CAS.
- S. Zeng, Z. Guo, Y. Hao, Y. S. Kafuti, Z. Yang, Q. Yao, J. Wang, X. Peng and H. Li, Tumor-microenvironment-activatable organic phototheranostic agents for cancer therapy, Coord. Chem. Rev., 2024, 509, 215786 CrossRef CAS.
- Y.-Y. Wang, Y.-C. Liu, H. Sun and D.-S. Guo, Type I photodynamic therapy by organic–inorganic hybrid materials: From strategies to applications, Coord. Chem. Rev., 2019, 395, 46–62 CrossRef CAS.
- Y. Wan, L. H. Fu, C. Li, J. Lin and P. Huang, Conquering the hypoxia limitation for photodynamic therapy, Adv. Mater., 2021, 33, 2103978 CrossRef CAS.
- K. Bilici, S. Cetin, E. Celikbas, H. Yagci Acar and S. Kolemen, Recent advances in cyanine-based phototherapy agents, Front. Chem., 2021, 9, 707876 CrossRef CAS.
- H. Li, H. Kim, F. Xu, J. Han, Q. Yao, J. Wang, K. Pu, X. Peng and J. Yoon, Activity-based NIR fluorescent probes based on the versatile hemicyanine scaffold: design strategy, biomedical applications, and outlook, Chem. Soc. Rev., 2022, 51, 1795–1835 RSC.
- M. Santra, M. Owens, G. Birch and M. Bradley, Near-infrared-emitting hemicyanines and their photodynamic killing of cancer cells, ACS Appl. Bio Mater., 2021, 4, 8503–8508 CrossRef CAS PubMed.
- B. Arslan, K. Bilici, G. Demirci, T. Almammadov, M. Khan, A. Sennaroglu, H. Y. Acar and S. Kolemen, A leucine aminopeptidase activatable photosensitizer for cancer cell selective photodynamic therapy action, Dyes Pigm., 2021, 195, 109735 CrossRef CAS.
- Y. Wang, C. Zhang, L. Hao, X. Huo, L. Dou, Y. Xie, J. Wang, X. Peng and H. Li, Using single atom substitution strategy for constructing a novel Se-substituted hemicyanine dye with hydroxyl group as an ideal activatable photosensitizer dye scaffold, Dyes Pigm., 2024, 224, 112026 CrossRef CAS.
- S. Yao, Y. Chen, W. Ding, F. Xu, Z. Liu, Y. Li, Y. Wu, S. Li, W. He and Z. Guo, Single-atom engineering of hemicyanine and its amphiphilic derivative for optimized near infrared phototheranostics, Chem. Sci., 2023, 14, 1234–1243 RSC.
- M. Zhao, Y. Zhang, J. Miao, H. Zhou, Y. Jiang, Y. Zhang, M. Miao, W. Chen, W. Xing and Q. Li, An Activatable Phototheranostic Probe for Anti-hypoxic Type I Photodynamic-and Immuno-Therapy of Cancer, Adv. Mater., 2024, 36, 2305243 CrossRef CAS.
- Y. Zhang, M. Zhao, J. Miao, W. Gu, J. Zhu, B. Cheng, Q. Li and Q. Miao, Hemicyanine-based type I photosensitizers for antihypoxic activatable photodynamic therapy, ACS Mater. Lett., 2023, 5, 3058–3067 CrossRef CAS.
- R. Wang, X. Li and J. Yoon, Organelle-targeted photosensitizers for precision photodynamic therapy, ACS Appl. Mater. Interfaces, 2021, 13, 19543–19571 CrossRef CAS PubMed.
- M. Dirak, C. M. Yenici and S. Kolemen, Recent advances in organelle-targeted organic photosensitizers for efficient photodynamic therapy, Coord. Chem. Rev., 2024, 506, 215710 CrossRef CAS.
- H. Vakifahmetoglu-Norberg, A. T. Ouchida and E. Norberg, The role of mitochondria in metabolism and cell death, Biochem. Biophys. Res. Commun., 2017, 482, 426–431 CrossRef CAS.
- M. D. Yaqoob, L. Xu, C. Li, M. M. L. Leong and D. D. Xu, Targeting mitochondria for cancer photodynamic therapy, Photodiagn. Photodyn. Ther., 2022, 38, 102830 CrossRef CAS.
- E. Sammels, J. B. Parys, L. Missiaen, H. De Smedt and G. Bultynck, Intracellular Ca2+ storage in health and disease: a dynamic equilibrium, Cell Calcium, 2010, 47, 297–314 CrossRef CAS PubMed.
- I. Moserova and J. Kralova, Role of ER stress response in photodynamic therapy: ROS generated in different subcellular compartments trigger diverse cell death pathways, PLoS One, 2012, 7, e32972 CrossRef CAS.
- Y. Liu, H.-R. Jia, X. Han and F.-G. Wu, Endoplasmic reticulum-targeting nanomedicines for cancer therapy, Smart Mater. Med., 2021, 2, 334–349 Search PubMed.
- Y. Shi, S. Wang, J. Wu, X. Jin and J. You, Pharmaceutical strategies for endoplasmic reticulum-targeting and their prospects of application, J. Controlled Release, 2021, 329, 337–352 CrossRef CAS PubMed.
- S. Li, Y. Chen, Y. Wu, S. Yao, H. Yuan, Y. Tan, F. Qi, W. He and Z. Guo, An endoplasmic reticulum targeting type I photosensitizer for effective photodynamic therapy against hypoxic tumor cells, Chem. – Eur. J., 2022, 28, e202202680 CrossRef CAS PubMed.
- Z. Zhuang, J. Dai, M. Yu, J. Li, P. Shen, R. Hu, X. Lou, Z. Zhao and B. Z. Tang, Type I photosensitizers based on phosphindole oxide for photodynamic therapy: apoptosis and autophagy induced by endoplasmic reticulum stress, Chem. Sci., 2020, 11, 3405–3417 RSC.
- J. Zhang, Y. Zhang, H. Zhang, W. Zhai, X. Shi and C. Li, A hypoxia-activatable theranostic agent with intrinsic endoplasmic reticulum affinity and type-I photosensitivity, J. Mater. Chem. B, 2023, 11, 4102–4110 RSC.
- H. Ding, J. Chang, F. He, S. Gai and P. Yang, Hydrogen sulfide: an emerging precision strategy for gas therapy, Adv. Healthcare Mater., 2022, 11, 2101984 CrossRef CAS.
- M. Dirak, S. E. Turan and S. Kolemen, Hydrogen Sulfide Responsive Phototherapy Agents: Design Strategies and Biological Applications, ACS Bio Med. Chem. Au, 2023, 3, 305–321 CrossRef CAS PubMed.
- V. S. Lin, W. Chen, M. Xian and C. J. Chang, Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems, Chem. Soc. Rev., 2015, 44, 4596–4618 RSC.
- H. Gunduz, K. Bilici, S. Cetin, A. Muti, A. Sennaroglu, H. Y. Acar and S. Kolemen, Dual laser activatable brominated hemicyanine as a highly efficient and photostable multimodal phototherapy agent, J. Photochem. Photobiol., B, 2021, 217, 112171 CrossRef CAS PubMed.
- Z. Luo, Z. Huang, K. Li, Y. Sun, J. Lin, D. Ye and H.-Y. Chen, Targeted delivery of a γ-glutamyl transpeptidase activatable near-infrared-fluorescent probe for selective cancer imaging, Anal. Chem., 2018, 90, 2875–2883 CrossRef CAS.
- K.-X. Teng, L.-Y. Niu, N. Xie and Q.-Z. Yang, Supramolecular photodynamic agents for simultaneous oxidation of NADH and generation of superoxide radical, Nat. Commun., 2022, 13, 6179 CrossRef CAS PubMed.
- M. Dirak, D. Kepil, T. Almammadov, Z. Elmazoglu, S. Cetin, N. Ozogul, G. Gunbas and S. Kolemen, Selective monitoring and treatment of neuroblastoma cells with hydrogen sulfide activatable phototheranostic agent, Dyes Pigm., 2023, 210, 111011 CrossRef CAS.
- M. Pérez-Mato, A. Dopico-López, Y. Akkoc, S. López-Amoedo, C. Correa-Paz, M. Candamo-Lourido, R. Iglesias-Rey, E. López-Arias, A. Bugallo-Casal and A. da Silva-Candal, Preclinical validation of human recombinant glutamate-oxaloacetate transaminase for the treatment of acute ischemic stroke, iScience, 2024, 27, 111108 CrossRef.
- Y. Zhang, J. Fang, S. Ye, Y. Zhao, A. Wang, Q. Mao, C. Cui, Y. Feng, J. Li and S. Li, A hydrogen sulphide-responsive and depleting nanoplatform for cancer photodynamic therapy, Nat. Commun., 2022, 13, 1685 CrossRef CAS.
- K. Ma, K. M. Vattem and R. C. Wek, Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress, J. Biol. Chem., 2002, 277, 18728–18735 CrossRef CAS.
- H. P. Harding, I. Novoa, Y. Zhang, H. Zeng, R. Wek, M. Schapira and D. Ron, Regulated translation initiation controls stress-induced gene expression in mammalian cells, Mol. Cell, 2000, 6, 1099–1108 CrossRef CAS.
- P. Verwilst, K. Kim, K. Sunwoo, H.-R. Kim, C. Kang and J. S. Kim, Revealing protein aggregates under thapsigargin-induced ER stress using an ER-targeted thioflavin, ACS Sens., 2019, 4, 2858–2863 CrossRef CAS PubMed.
- P. K. Singh, M. Kumbhakar, H. Pal and S. Nath, Viscosity effect on the ultrafast bond twisting dynamics in an amyloid fibril sensor: thioflavin-T, J. Phys. Chem. B, 2010, 114, 5920–5927 CrossRef CAS PubMed.
- L. S. Wolfe, M. F. Calabrese, A. Nath, D. V. Blaho, A. D. Miranker and Y. Xiong, Protein-induced photophysical changes to the amyloid indicator dye thioflavin T, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 16863–16868 CrossRef CAS.
- V. I. Stsiapura, A. A. Maskevich, V. A. Kuzmitsky, V. N. Uversky, I. M. Kuznetsova and K. K. Turoverov, Thioflavin T as a molecular rotor: fluorescent properties of thioflavin T in solvents with different viscosity, J. Phys. Chem. B, 2008, 112, 15893–15902 CrossRef CAS PubMed.
- J. Han, S. H. Back, J. Hur, Y.-H. Lin, R. Gildersleeve, J. Shan, C. L. Yuan, D. Krokowski, S. Wang and M. Hatzoglou, ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death, Nat. Cell Biol., 2013, 15, 481–490 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm00744a |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2025 |