Flexible TADF-based organic X-ray scintillating films for high-resolution imaging†
Haoqiang
Xu
 ,
Wenjing
Zhao
,
Xinning
Zhang
,
Jingjing
Cui
,
Zhenhua
Wang
,
Wenjing
Zhao
,
Xinning
Zhang
,
Jingjing
Cui
,
Zhenhua
Wang
 ,
Xiaowang
Liu
,
Junqing
Shi
,
Xiaowang
Liu
,
Junqing
Shi
 * and
Lei
Ji
* and
Lei
Ji
 *
*
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NWPU), 127 West Youyi Road, Xi’an 710072, China. E-mail: iamjqshi@nwpu.edu.cn; iamlji@nwpu.edu.cn
First published on 3rd December 2024
Abstract
X-ray scintillators are commonly used, with significant applications in scientific research and daily life. However, most commercial scintillators are based on expensive mechanically rigid inorganic crystalline arrays, which normally bear a long-lived afterglow that reduces the resolution in dynamic X-ray monitoring, while low X-ray absorbance and inefficient exciton utilization are deficiencies associated with traditional organic scintillators. Herein, we report a series of readily obtained pure organic single-component scintillators with halogen-enhanced X-ray absorbance, high fluorescence quantum yield, and short decay time. They can be easily prepared into flexible and transparent scintillating imaging films with a high light yield (approaching 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons MeV−1) and high resolution (above 20 line pairs per mm) that are among the highest levels for organic scintillators reported thus far.
000 photons MeV−1) and high resolution (above 20 line pairs per mm) that are among the highest levels for organic scintillators reported thus far.
1. Introduction
X-ray scintillators, which emit light upon X-ray radiation, are of great importance due to their widespread applications in diverse areas including medical imaging, nondestructive X-ray inspection, high-energy particle detectors, security screening, and astronomical discovery.1–7 During the past several decades, there has been considerable development of X-ray imaging scintillators with high X-ray excited luminescence (XEL) efficiency, and they have evolved from classic inorganic species to different new trends.8–11Conventional high-performance X-ray imaging scintillators are usually fabricated from inorganic crystals, e.g., Bi4Ge3O12 (BGO) and CsI:Tl, which have been investigated in detail and thoroughly commercialized. Given their crystalline form and component, the long-term disadvantages of these inorganic scintillators are scarce resources, ultra-high fabrication temperatures, poor moisture and light stability, poor biocompatibility, high toxicity, and easily introduced defects.12–14 These issues limit inorganic scintillators in the fulfilment of the requirements for flexible X-ray imaging and detection, e.g., flat plane detectors (FPDs). Notably, the elongated afterglow and slow luminescence decay of traditional scintillators severely affects the resolution of X-rays due to the ghosting of X-ray images in dynamic monitoring.
In contrast, organic scintillators possess natural advantages consisting of abundant resources, high mechanical flexibility, easy processing, favorable environmental adaptability, low cost, and large-area fabrication,10,15–18 which will enable their application in future flexible technologies.4 However, there has been little commercialization of organic scintillators, and their evolution has been slow due to their unsatisfactory performance and structural type limited to that of aromatic hydrocarbons.19,20 Although typical fluorescent scintillators (e.g., anthracene) have been reported since 1979, their development has been impeded for many years due to weak X-ray absorption and inefficient exciton utilization.21 These organic molecules mainly consist of light atoms, and their limited effective atomic number leads to low X-ray absorption cross-section (X-ray absorbance is proportional to the fourth power of atomic number) and subsequent poor detection sensitivity.22 In addition, these molecules are normally fluorescent dyes facing a maximum exciton utilization efficiency of 25%, as 75% of the excitons formed in charge-recombination are dark triplets.23–26 Therefore, there is an urgent demand for the development of novel high-performance organic scintillators.27,28
Although it has been found that introducing heavy atoms of halogen into organic fluorophores enhances X-ray absorption, their introduction concomitantly decreases fluorescence efficiency. Recently, organic phosphors and metal-containing materials (e.g., MOFs, metal halides, and coinage-metal clusters) have aroused research enthusiasm in X-ray scintillation due to their high exciton utilization efficiency with high photoluminescence quantum yields (PLQYs).22,29–31 However, their long decay times are detrimental for dynamic high-resolution X-ray imaging, as mentioned above.
Herein, we placed our attention on pure single-component thermally activated delayed fluorescence (TADF) chromophores as alternatives, where the light emission is stimulated by electronic transitions induced by a cascade of secondary electrons, generating many triplet excitons such as those that are produced during the electrical excitation scenario in organic light-emitting diodes (OLEDs).32–35 Since 2012, pure organic TADF chromophores have been reported32 that can theoretically achieve 100% exciton utilization through intersystem crossing (ISC) and reverse intersystem crossing (RISC), and are widely employed as emitters to construct efficient OLEDs.34
In comparison, the benefits of TADF-based pure organic scintillators are lower cost and toxicity, in addition to high light yields and resolution due to the TADF features of 100% exciton utilization, less self-absorption, and high RISC rates.36,37 Notably, the halogen effect on the TADF property is complicated and discrepant, and further exploration is desired for scintillator design. Halogen introduction is beneficial for delayed fluorescence because it strengthens spin–orbit coupling (SOC) interaction and RISC with shortened lifetimes, while there are also reports that heavy atoms cause a great decrease in emission efficiencies.38,39
High PLQY and short decay are crucial for high-performance TADF chromophores as well as imaging scintillators.40 Feasible designs decrease lifetimes and strengthen the donor and/or acceptor, as well as distort the molecular structure to reduce ΔEST and promote the RISC process.41,42 As for increasing fluorescence efficiencies, bridging D–A by aromatic ring, dispersing HOMO/LUMO orbitals, and introducing dual-emissive nuclei would be useful methods.43 Therefore, novel TADF scintillators are favored by the integration of TADF molecule design strategies into scintillator design.
In this work, we generated a design that synergically combines the X-ray absorption enhancement by many halogens (Fig. 1a),15,24,37 and the TADF performance improvement by introducing bulky substituents with large distortion. Following this thought, a series of pure organic single-component scintillators with halogen-enhanced PLQY was easily obtained via one-pot reaction. These scintillators can be easily incorporated into flexible scintillating imaging films (Fig. 1b) and generate a high light yield (approaching 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons MeV−1) and high resolution (above 20 line pairs per mm) that are at the highest levels for organic scintillators reported thus far. In detail, these molecules possess a D–π–A-type structure, severe molecular distortion, and substantial HOMO–LUMO separation with dispersed HOMOs, which ensures their TADF properties with high PLQY.
000 photons MeV−1) and high resolution (above 20 line pairs per mm) that are at the highest levels for organic scintillators reported thus far. In detail, these molecules possess a D–π–A-type structure, severe molecular distortion, and substantial HOMO–LUMO separation with dispersed HOMOs, which ensures their TADF properties with high PLQY.
 | ||
| Fig. 1 (a) Typical mechanism of TADF scintillators under X-ray exposure. (b) Process for preparing transparent flexible films. | ||
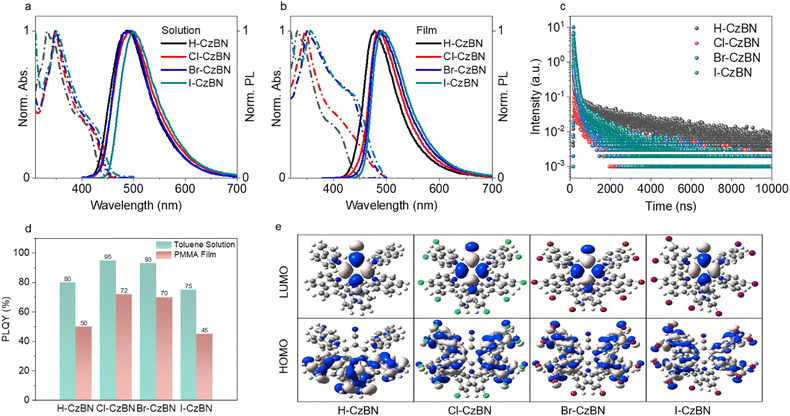
Among the scintillating films of four TADF fluorophores, Cl-CzBN and Br-CzBN showed a higher PLQY against H-CzBN, and I-CzBN exhibited the lowest detection limit of 75.4 nGy s−1 and highest light yield of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 306 photons MeV−1. The imaging screen based on I-CzBN also achieved high resolution above 20 line pairs (lp) per mm. Moreover, its resistance against high temperatures and pressures suggests that it can also be potentially applied for use in deep-sea exploration and real-time nuclear radiation detection. In sum, this work presents flexible pure organic single-component TADF-based scintillating films with high light yield and imaging resolution, low cost, and facile production and preparation, offering novel high-performance materials for FPD application.
306 photons MeV−1. The imaging screen based on I-CzBN also achieved high resolution above 20 line pairs (lp) per mm. Moreover, its resistance against high temperatures and pressures suggests that it can also be potentially applied for use in deep-sea exploration and real-time nuclear radiation detection. In sum, this work presents flexible pure organic single-component TADF-based scintillating films with high light yield and imaging resolution, low cost, and facile production and preparation, offering novel high-performance materials for FPD application.
2. Results and discussion
Following the design principles for TADF molecules, we devised a series of halogen-enhanced TADF molecules by increasing the number of carbazoles (Cz) and attached halogens to highly twist the peripheral groups against the core. H-CzBN is a previously reported TADF chromophore with large HOMO–LUMO separation, which makes it a priority choice for halogen incorporation.44 According to Zhang et al.,44H-CzBN was easily obtained via a one-step reaction with greater than 50% yield, whereas Cl-CzBN, Br-CzBN, and I-CzBN were obtained via the same reaction with different halogen-substituted carbazole reactants (Fig. S1–S6, ESI†).These compounds in a polymethyl methacrylate (PMMA) and polydimethylsiloxane (PDMS) matrix displayed excellent film-forming ability, yielding transparent and flexible thin films with high homogeneity via simple drop-cast and curing processes (Fig. 1b). These films are applicable for radioluminescence (RL) and X-ray imaging screens due to their convenient and facile processability. With the films bearing 1 wt% PMMA and PDMS, investigations of absorption spectroscopy, steady-state and time-resolved PL spectroscopy, X-ray excited RL spectroscopy, and X-ray imaging were performed. Complementary calculations on single molecules at the TD-DFT level were also performed for an in-depth understanding of their mechanism.
There was high similarity between the UV-vis and PL spectra of R-CzBN compounds, either in solutions or films (Fig. 2a and b). In the absorption spectra, the main absorption with a lower-energy shoulder covered the ultraviolet and blue ranges. As previously reported, the main absorption over 330–350 nm is attributed to the π–π* transition of the derived carbazole units and the localized charge transfer state. The low-energy shoulder peak at approximately 400 nm is related to the delocalized charge transfer state.45 In comparison, a slight redshift was observed for the halogenated molecules against H-CzBN, which increased in the order of Cl, Br, I. In the PL spectra, there was only one broad emission band over the range of 420–650 nm (Fig. S7, ESI†), which was ascribed to the charge transfer transition. Similar to the case of absorption, the emission peak at approximately 480 nm showed a slight redshift in the sequence of Cl, Br, and I substitution. Similar to analogs containing 4 Cz derivatives, these molecules also showed a small overlap between the absorption and emission. This feature suggests low self-absorption during X-ray imaging, which is critical for high-resolution scintillation (vide infra).
As shown in Fig. S8 (ESI†), the lifetimes significantly increased after purging nitrogen to remove the dissolved O2 molecules, demonstrating the role of the triplet exciton for TADF emission of the four molecules. Furthermore, there was an excellent match between these decay curves and the biexponential decay model, which further proves the TADF nature of the molecules. As displayed in Fig. S9 (ESI†), the intensity of the emission spectrum from 77 K to 297 K initially decreases and then increases, indicating that the emission of molecules in the triplets gradually changes to singlets, which shows a characteristic balance between the triplet to singlet upconversion (RISC process) of molecules and the quenching of thermal vibrational triplet states.
As calculated in the fluorescence and phosphorescence onsets (Fig. S10 and Table S1, ESI†), the ΔEST of I-CzBN was only 10 meV, which is significantly less than that of H-CzBN (140 meV), Cl-CzBN (55 meV), and Br-CzBN (75 meV). In comparison, the delayed and short fluorescence lifetime decreased from 3.1 μs and 4.8 ns for H-CzBN to 0.8 μs and 1.1 ns for I-CzBN, respectively (Fig. 2c and Table 1). These short lifetimes upon halogen substitution are ascribed to the acceleration of the ISC and RISC processes. PLQY is vitally important for the RL performance of scintillators. As shown in Fig. 2d, the PLQYs of Cl-CzBN and Br-CzBN were higher than that of H-CzBN in toluene solution and PMMA film, while the PLQY of I-CzBN showed a slight decrease compared to H-CzBN. As calculated in Table S2 (ESI†), the kRISC values of Br-CzBN (8.01 × 106 s−1) and I-CzBN (10.8 × 106 s−1) were larger than those of H-CzBN (0.29 × 106 s−1) and Cl-CzBN (0.99 × 106 s−1), which was attributed to the heavy atom effect. Such high kRISC rates and ΔEST are favorable for the TADF process.
| Compound | λ Abs (nm) | λ Em (nm) | λ RL (nm) | τ p (ns) | τ d (μs) | PLQY (%) |
|---|---|---|---|---|---|---|
| H-CzBN | 333 | 477 | 544 | 4.8 | 3.06 | 50 |
| Cl-CzBN | 348 | 487 | 507 | 4.5 | 2.88 | 72 |
| Br-CzBN | 351 | 489 | 514 | 1.9 | 1.86 | 70 |
| I-CzBN | 357 | 493 | 507 | 1.1 | 0.80 | 45 |
TD-DFT calculations demonstrated that the HOMO and LUMO are mainly distributed over the carbazole and phenylacetonitrile units, respectively (Fig. 2e). The HOMO–LUMO spatial separation is consistent among the four compounds, indicating a slight heavy-atom effect. For most TADF fluorophores, significant HOMO–LUMO separation spreading over twisted backbones is observed, which is associated with ΔEST reducing to activate TADF characteristics. As the calculated results here demonstrated, this is also the case for these four molecules. However, the details of HOMO distribution over multiple carbazoles is significantly different between H-CzBN and the halogenated carbazoles.
For H-CzBN, the HOMO is mainly situated on the carbazole groups at lower positions 2, 3, and 4. For the other R-CzBNs, the HOMO shifts to side positions 1, 2, 4, and 5. Therefore, by halogen incorporation, the HOMOs and LUMOs preserve a significant separation, and there is greater dispersion of the HOMOs over the halogenated carbazoles. As mentioned earlier, the dispersion of HOMO/LUMO would increase the HOMO–LUMO overlap to enhance the oscillator strength and thus the PLQY. As the experiments showed, the PLQYs of the halogenated molecules are larger than that of H-CzBN (Fig. 2d and Table 1), which is related to the above heavy-atom effect on HOMO dispersion.
Next, we investigated the RL behaviours of these TADF materials in the solid state. As shown in the RL spectra (Fig. 3a), it is evident that all the materials exhibited similar emission in pristine solids. Then, we prepared a series of doped films with variable proportions of PMMA and film thickness to test their scintillation performance. Evaluation of the X-ray-excited RL intensity suggested that the doping ratio of 41 wt% is the most optimal for scintillating films (Fig. S11, ESI†). We then examined the RL spectra at different thicknesses, and there is usually a gradual increase in intensity along with optimal thickness (Fig. S12, 0.1 mm for I-CzBN, ESI†). We subsequently selected BGO as a standard reference material to quantify the light yield using a relative approach (Fig. 3b). The comparison showed that these TADF molecules exhibited highly increased relative light yields ranging from a few thousand photons per MeV for H-CzBN to approximately 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons MeV−1 for Cl-CzBN, Br-CzBN, and I-CzBN (dose rate of 212.97 μGy s−1, Fig. S12 and Table S3, ESI†). The high relative light yields of I-CzBN were mainly due to its higher X-ray absorption cross-section against the others, as well as its high level of exciton utilization efficiency and PLQY, which is different from what was described in a previous similar report.37
000 photons MeV−1 for Cl-CzBN, Br-CzBN, and I-CzBN (dose rate of 212.97 μGy s−1, Fig. S12 and Table S3, ESI†). The high relative light yields of I-CzBN were mainly due to its higher X-ray absorption cross-section against the others, as well as its high level of exciton utilization efficiency and PLQY, which is different from what was described in a previous similar report.37
 | ||
| Fig. 3 (a) Normalized RL spectra of the solid R-CzBN under X-rays with a dose rate of 212.97 μGy s−1. The insets are related photographs of the scintillators under exposure to X-rays. (b) RL spectra of BGO and the four TADF scintillators at the optimal thickness. (c) The relationship between X-ray absorbances and X-ray energy.46 (d) Detection limits of the TADF scintillators. (e) Normalized RL intensity at the corresponding emission maxima of the four TADF scintillator films under continuous X-ray irradiation (dose rate, 18.6 mGy s−1). | ||
After that, we conducted X-ray absorption measurements of the four different films. As expected, the resonant absorption edges gradually increased from H-CzBN to I-CzBN, which confirmed the heavy-atom effect on the X-ray photon absorption ability of the organic chromophores (Fig. 3c). The X-ray attenuation efficiency curves indicate that, in comparison to BGO, the thicker I-CzBN would be more conducive to achieving nearly 100% X-ray absorption (Fig. S13, ESI†). The RL intensities of all the TADF chromophores demonstrated a satisfactory linear relationship relative to the X-ray dosages, with R-squared values exceeding 0.998 (Fig. 3d). The calculated detection limit (Fig. 3d and Fig. S12, S14, Fig. S15, ESI†) improved from 332.5 and 131.3 nGy s−1 for H-CzBN and Cl-CzBN to 101.2 and 75.4 nGy s−1 for Br-CzBN and I-CzBN, respectively. The detection limit obtained for the I-CzBN is two magnitudes lower than that for commonly used X-ray diagnostics (5.5 μGy s−1), demonstrating their application potential for X-ray imaging. To assess the radiation stability of the compound series, a series of tests was conducted under the maximum dose of our equipment. Under continuous irradiation with a dose rate of 18.6 mGy s−1 lasting up to 2100 seconds (in air), their luminescence intensity was maintained at over 90% (Fig. 3e), exhibiting an excellent irradiation stability at a high dose rate.
Furthermore, we performed a series of imaging tests based on these TADF scintillators to explore their practical application, using a simple imaging setup (Fig. 4a). Using I-CzBN as example, the scintillator screen shows excellent transparency (Fig. 4b). Additionally, it exhibits high stability against humidity and temperature (Fig. S16, ESI†), as well as flexibility and stretchability (Fig. S16 and S17, ESI†), which suggests it can be used for scene detection under hot and high-pressure conditions such as those found in an ocean abyss. The outline of the peanut is clearly presented on film, whereas the shell is nearly transparent (Fig. 4c). In addition, X-ray contrast imaging was applied to inspect the complex inner structure of an electronic chip, which is completely opaque to visible light (Fig. 4d). Notably, the flexible imaging was achieved by the PDMS scintillating film for a PI circuit board (Fig. 4e). More importantly, this flexible I-CzBN-based scintillating imaging screen achieved a high resolution of greater than 20 lp per mm (see Fig. 4f, the maximum of resolution card), exceeding most reported organic and commercial scintillating imaging materials. As shown in Table S4 (ESI†), the resolution and relative light yield were improved compared with values from the reported literature.37
3. Conclusions
We designed novel pure organic TADF-based scintillators by synergically enhancing the X-ray absorption as well as the PLQY, which were investigated by the use of flexible scintillating films capable of high-resolution imaging greater than 20 lp per mm. First, via one-pot reaction, we readily obtained a series of halogen-introduced TADF fluorophores, where halogen introduction led to improved TADF properties with increased PLQYs and shortened lifetimes, as well as enhanced X-ray absorption ability. The increased PLQYs of Cl-CzBN and Br-CzBN resulted from the heavy-atom effect on HOMO dispersion. Furthermore, via facile preparation, these fluorophores were fabricated into environmentally stable and transparent films for X-ray scintillating imaging.
I-CzBN achieved the best performance with a light yield of nearly 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons MeV−1 and a low detection of 75.4 nGy s−1, due to the synergic contribution of a high X-ray absorption cross-section, nearly unity exciton utilization efficiency, and high PLQY. The X-ray imaging based on I-CzBN showed a high resolution of over 20 lp per mm, surpassing most of the reported organic and inorganic scintillators. Thus, these scintillators with halogen-enhanced X-ray absorbance, high fluorescence quantum yield, and short decay time are readily obtained, pure-organic, and single-component. The films based on them are flexible, environmentally stable, inexpensive, easily fabricated, and achieve a high light yield and high image resolution that are among the highest levels when compared to previously reported organic scintillators. This work offers novel materials beyond conventional types, with possible commercialization and FPD application, and will inject fresh drive into organic scintillator research.
000 photons MeV−1 and a low detection of 75.4 nGy s−1, due to the synergic contribution of a high X-ray absorption cross-section, nearly unity exciton utilization efficiency, and high PLQY. The X-ray imaging based on I-CzBN showed a high resolution of over 20 lp per mm, surpassing most of the reported organic and inorganic scintillators. Thus, these scintillators with halogen-enhanced X-ray absorbance, high fluorescence quantum yield, and short decay time are readily obtained, pure-organic, and single-component. The films based on them are flexible, environmentally stable, inexpensive, easily fabricated, and achieve a high light yield and high image resolution that are among the highest levels when compared to previously reported organic scintillators. This work offers novel materials beyond conventional types, with possible commercialization and FPD application, and will inject fresh drive into organic scintillator research.
4. Experimental section
A detailed Experimental section can be found in the ESI.†Author contributions
Haoqiang Xu: conceptualization, investigation, methodology, and writing – original draft. Wenjing Zhao: investigation, methodology, and project administration. Xinning Zhang: data curation and formal analysis. Jingjing Cui: project administration. Zhenhua Wang: project administration. Xiaowang Liu: project administration. Junqing Shi: founding acquisition, project administration, writing – original draft and writing – review and editing. Lei Ji: founding acquisition, investigation, project administration, writing – original draft and writing – review and editing.Data availability
Data for this article is available upon request to the corresponding authors.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work is supported by the Natural Science Foundation of China (Grant No. 62004170 to J. S., no. 62174137 to L. J.), the Fundamental Research Funds for the Central Universities, and Northwestern Polytechnical University (NWPU).References
- C. Ronda, H. Wieczorek, V. Khanin and P. Rodnyi, Scintillators for medical imaging: a tutorial overview, ECS J. Solid State Sci. Technol., 2015, 5, R3121 CrossRef.
- J. H. Heo, D. H. Shin, J. K. Park, D. H. Kim, S. J. Lee and S. H. Im, High-performance next-generation perovskite nanocrystal scintillator for nondestructive X-ray imaging, Adv. Mater., 2018, 30, 1801743 CrossRef PubMed.
- Z. Lin, S. Lv, Z. Yang, J. Qiu and S. Zhou, Structured scintillators for efficient radiation detection, Adv. Sci., 2022, 9, 2102439 CrossRef CAS PubMed.
- L.-J. Xu, X. Lin, Q. He, M. Worku and B. Ma, Highly efficient eco-friendly X-ray scintillators based on an organic manganese halide, Nat. Commun., 2020, 11, 4329 CrossRef CAS PubMed.
- Q. Chen, J. Wu, X. Ou, B. Huang, J. Almutlaq, A. A. Zhumekenov, X. Guan, S. Han, L. Liang and Z. Yi, All-inorganic perovskite nanocrystal scintillators, Nature, 2018, 561, 88 CrossRef CAS PubMed.
- J. A. Posar, M. Petasecca and M. J. Griffith, A review of printable, flexible and tissue equivalent materials for ionizing radiation detection, Flexible Printed Electron., 2021, 6, 043005 CrossRef CAS.
- Y. Wang, M. Li, Z. Chai, Y. Wang and S. Wang, Perovskite scintillators for improved X-ray detection and imaging, Angew. Chem., Int. Ed., 2023, 62, e202304638 CrossRef CAS PubMed.
- M. Gandini, I. Villa, M. Beretta, C. Gotti, M. Imran, F. Carulli, E. Fantuzzi, M. Sassi, M. Zaffalon, C. Brofferio, L. Manna, L. Beverina, A. Vedda, M. Fasoli, L. Gironi and S. Brovelli, Efficient, fast and reabsorption-free perovskite nanocrystal-based sensitized plastic scintillators, Nat. Nanotechnol., 2020, 15, 462 CrossRef CAS PubMed.
- H. Zhang, Z. Yang, M. Zhou, L. Zhao, T. Jiang, H. Yang, X. Yu, J. Qiu, Y. Yang and X. Xu, Reproducible X-ray imaging with a perovskite nanocrystal scintillator embedded in a transparent amorphous network structure, Adv. Mater., 2021, 33, 2102529 CrossRef CAS PubMed.
- N. Gan, X. Zou, M. Dong, Y. Wang, X. Wang, A. Lv, Z. Song, Y. Zhang, W. Gong and Z. Zhao, Organic phosphorescent scintillation from copolymers by X-ray irradiation, Nat. Commun., 2022, 13, 3995 CrossRef CAS PubMed.
- Y. Zhou, Z. Deng, B. Wang, P. Li, L. Li, W. Han, J. Huang, W. Jia, X. Ouyang, Q. Xu and K. Ostrikov, Nanocomposite scintillation perovskite-delignified wood photonic guides for X-ray imaging, Chem. Eng. J., 2023, 471, 144431 CrossRef CAS.
- C. Tian, S. Liu, Y. Xie, L. Guo, D. Chen, Y. Liu and Z. Zhong, Study on the mechanism of afterglow in CsI: Tl and the afterglow suppression in CsI: Tl, Eu, J. Radioanal. Nucl. Chem., 2019, 320, 123–128 CrossRef CAS.
- V. M. Khanin, I. Venevtsev, K. Chernenko, T. Tukhvatulina, P. A. Rodnyi, S. Spoor, J. Boerekamp, A.-M. van Dongen, D. Buettner and H. Wieczorek, Influence of 3d transition metal impurities on garnet scintillator afterglow, Cryst. Growth Des., 2020, 20, 3007–3017 CrossRef CAS.
- Y. Li, Q.-L. Li, Y. Li, Y.-L. Yang, S.-L. Zhang, J. Zhao, J. Wan and Z. Zhang, Water-assistant ultrahigh fluorescence enhancement in perovskite polymer-encapsulated film for flexible X-ray scintillators, Chem. Eng. J., 2023, 452, 139132 CrossRef CAS.
- L. Ji, J. Shi, J. Wei, T. Yu and W. Huang, Air-Stable Organic Radicals: New-Generation Materials for Flexible Electronics?, Adv. Mater., 2020, 32, 1908015 CrossRef CAS PubMed.
- S. K. Behera, S. Y. Park and J. Gierschner, Dual emission: classes, mechanisms, and conditions, Angew. Chem., Int. Ed., 2021, 60, 22624–22638 CrossRef CAS PubMed.
- I. McCulloch, M. Chabinyc, C. Brabec, C. B. Nielsen and S. E. Watkins, Sustainability considerations for organic electronic products, Nat. Mater., 2023, 22, 1304–1310 CrossRef CAS PubMed.
- N. Tang, J. Zhou, L. Wang, M. Stolte, G. Xie, X. Wen, L. Liu, F. Würthner, J. Gierschner and Z. Xie, Anomalous deep-red luminescence of perylene black analogues with strong π–π interactions, Nat. Commun., 2023, 14, 1922 CrossRef CAS PubMed.
- R. C. Sangster and J. W. Irvine Jr, Study of organic scintillators, J. Chem. Phys., 1956, 24, 670 CrossRef CAS.
- M. Chen, L. Sun, X. Ou, H. Yang, X. Liu, H. Dong, W. Hu and X. Duan, Organic Semiconductor Single Crystals for X-ray Imaging, Adv. Mater., 2021, 33, 2104749 CrossRef CAS PubMed.
- J. B. Birks, Scintillations from Organic Crystals: Specific Fluorescence and Relative Response to Different Radiations, Proc. Phys. Soc., London, Sect. A, 1951, 64, 874 CrossRef.
- J. Perego, I. Villa, A. Pedrini, E. C. Padovani, R. Crapanzano, A. Vedda, C. Dujardin, C. X. Bezuidenhout, S. Bracco, P. E. Sozzani, A. Comotti, L. Gironi, M. Beretta, M. Salomoni, N. Kratochwil, S. Gundacker, E. Auffray, F. Meinardi and A. Monguzzi, Composite fast scintillators based on high-Z fluorescent metal–organic framework nanocrystals, Nat. Photonics, 2021, 15, 393–400 CrossRef CAS.
- L. Clinckemalie, D. Valli, M. B. J. Roeffaers, J. Hofkens, B. Pradhan and E. Debroye, Challenges and Opportunities for CsPbBr3 Perovskites in Low- and High-Energy Radiation Detection, ACS Energy Lett., 2021, 6, 1290–1314 CrossRef CAS.
- X. Wang, H. Shi, H. Ma, W. Ye, L. Song, J. Zan, X. Yao, X. Ou, G. Yang, Z. Zhao, M. Singh, C. Lin, H. Wang, W. Jia, Q. Wang, J. Zhi, C. Dong, X. Jiang, Y. Tang, X. Xie, Y. Yang, J. Wang, Q. Chen, Y. Wang, H. Yang, G. Zhang, Z. An, X. Liu and W. Huang, Organic phosphors with bright triplet excitons for efficient X-ray-excited luminescence, Nat. Photonics, 2021, 15, 187–192 CrossRef CAS.
- A. Jana, S. Cho, S. A. Patil, A. Meena, Y. Jo, V. G. Sree, Y. Park, H. Kim, H. Im and R. A. Taylor, Perovskite: Scintillators, direct detectors, and X-ray imagers, Mater. Today, 2022, 55, 110–136 CrossRef CAS.
- H. Wang, C. Peng, M. Chen, Y. Xiao, T. Zhang, X. Liu, Q. Chen, T. Yu and W. Huang, Wide-Range Color-Tunable Organic Scintillators for X-Ray Imaging Through Host–Guest Doping, Angew. Chem., Int. Ed., 2023, e202316190 Search PubMed.
- H. Chen, M. Lin, Y. Zhu, D. Zhang, J. Chen, Q. Wei, S. Yuan, Y. Liao, F. Chen, Y. Chen, M. Lin and X. Fang, Halogen-bonding boosting the high performance X-ray imaging of organic scintillators, Small, 2023, 2307277 Search PubMed.
- Y. Zhang, M. Chen, X. Wang, M. Lin, H. Wang, W. Li, F. Chen, Q. Liao, H. Chen, Q. Chen, M. Lin and H. Yang, Efficient and Fast X-Ray Luminescence in Organic Phosphors Through High-Level Triplet-Singlet Reverse Intersystem Crossing, CCS Chem., 2023, 6, 334–341 CrossRef.
- X. Wang, X. Zhang, Y. Liu and Y. Zhang, Shape-on-demand synthesis of luminescent (ETP) 2MnBr4 glass scintillator, Chem. Eng. J., 2024, 483, 149239 CrossRef CAS.
- W.-F. Wang, M.-J. Xie, P.-K. Wang, J. Lu, B.-Y. Li, M.-S. Wang, S.-H. Wang, F.-K. Zheng and G.-C. Guo, Thermally Activated Delayed Fluorescence (TADF)-active Coinage-metal Sulfide Clusters for High-resolution X-ray Imaging, Angew. Chem., Int. Ed., 2024, 63, e202318026 CrossRef CAS PubMed.
- T. Chen, Y. Xu, A. Ying, C. Yang, Q. Lin and S. Gong, Through-Space Charge-Transfer Organogold(III) Complexes Enable High-Performance X-ray Scintillation and Imaging, Angew. Chem., Int. Ed., 2024, 63, e202401833 CrossRef CAS PubMed.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- Y. Tao, K. Yuan, T. Chen, P. Xu, H. Li, R. Chen, C. Zheng, L. Zhang and W. Huang, Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics, Adv. Mater., 2014, 26, 7931–7958 CrossRef CAS PubMed.
- G. Xia, C. Qu, Y. Zhu, J. Ye, K. Ye, Z. Zhang and Y. Wang, A TADF Emitter Featuring Linearly Arranged Spiro-Donor and Spiro-Acceptor Groups: Efficient Nondoped and Doped Deep-Blue OLEDs with CIEy < 0.1, Angew. Chem., Int. Ed., 2021, 60, 9598–9603 CrossRef CAS PubMed.
- Y. Zhang, J. Wei, L. Wang, T. Huang, G. Meng, X. Wang, X. Zeng, M. Du, T. Fan, C. Yin, D. Zhang and L. Duan, Multiple Fusion Strategy for High-Performance Yellow OLEDs with Full Width at Half Maximums Down to 23 nm and External Quantum Efficiencies up to 37.4%, Adv. Mater., 2023, 35, 2209396 CrossRef CAS PubMed.
- W. Ma, Y. Su, Q. Zhang, C. Deng, L. Pasquali, W. Zhu, Y. Tian, P. Ran, Z. Chen, G. Yang, G. Liang, T. Liu, H. Zhu, P. Huang, H. Zhong, K. Wang, S. Peng, J. Xia, H. Liu, X. Liu and Y. Yang, Thermally activated delayed fluorescence (TADF) organic molecules for efficient X-ray scintillation and imaging, Nat. Mater., 2022, 21, 210–216 CrossRef CAS PubMed.
- J. X. Wang, L. Gutiérrez-Arzaluz, X. Wang, T. He, Y. Zhang, M. Eddaoudi, O. M. Bakr and O. F. Mohammed, Heavy-atom engineering of thermally activated delayed fluorophores for high-performance X-ray imaging scintillators, Nat. Photonics, 2022, 16, 869–875 CrossRef CAS.
- S. A. Alomar, J.-X. Wang, L. Gutiérrez-Arzaluz, S. Thomas, H. N. Alshareef, O. M. Bakr, M. Eddaoudi and O. F. Mohammed, TADF-Based X-ray Screens with Simultaneously Efficient Singlet and Triplet Energy Transfer for High Spatial Imaging Resolution, ACS Appl. Mater. Interfaces, 2023, 15, 34263–34271 CrossRef CAS PubMed.
- F. Masetti, U. Mazzucato and G. Galiazzo, Heavy atom effect on the luminescence of phenanthrene, J. Lumin., 1971, 4, 8–12 CrossRef CAS.
- S. Cao, C. Li, P. He, J. A. Lai, K. An, M. Zhou, P. Feng, M. Zhou and X. Tang, Ultrahigh photoluminescence quantum yield organic manganese halide scintillator for X-ray imaging, ACS Appl. Opt. Mater., 2023, 1, 623–632 CrossRef CAS.
- Y. Im, M. Kim, Y. J. Cho, J.-A. Seo, K. S. Yook and J. Y. Lee, Molecular design strategy of organic thermally activated delayed fluorescence emitters, Chem. Mater., 2017, 29, 1946–1963 CrossRef CAS.
- M. Y. Wong and E. Zysman-Colman, Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes, Adv. Mater., 2017, 29, 1605444 CrossRef PubMed.
- T. Hosokai, H. Matsuzaki, H. Nakanotani, K. Tokumaru, T. Tsutsui, A. Furube, K. Nasu, H. Nomura, M. Yahiro and C. Adachi, Evidence and mechanism of efficient thermally activated delayed fluorescence promoted by delocalized excited states, Sci. Adv., 2017, 3, e1603282 CrossRef PubMed.
- D. Zhang, M. Cai, Y. Zhang, D. Zhang and L. Duan, Sterically shielded blue thermally activated delayed fluorescence emitters with improved efficiency and stability, Mater. Horiz., 2016, 3, 145–151 RSC.
- H. Noda, X.-K. Chen, H. Nakanotani, T. Hosokai, M. Miyajima, N. Notsuka, Y. Kashima, J.-L. Brédas and C. Adachi, Critical role of intermediate electronic states for spin-flip processes in charge-transfer-type organic molecules with multiple donors and acceptors, Nat. Mater., 2019, 18, 1084–1090 CrossRef CAS PubMed.
- J. H. H. M. J. Berger, S. M. Seltzer, J. Chang, J. S. Coursey, R. Sukumar, D. S. Zucker and K. Olsen, XCOM: Photon Cross Sections Database, National Institute of Standards and Technology, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm00795f |
| This journal is © the Partner Organisations 2025 |