NIR AIE luminogens for primary and metastasis tumor imaging and tracking applications†
Yujiao
Zhu‡
a,
Yuhang
Zeng‡
b,
Huimin
Liu
a,
Yuting
Yin
b,
Bin
Chen
 *a and
Rong
Hu
*a and
Rong
Hu
 *b
*b
aSchool of Material Science and Chemical Engineering, Ningbo University, Ningbo, Zhejiang 315211, China. E-mail: chenbin2@nbu.edu.cn
bSchool of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China. E-mail: hurong@usc.edu.cn
First published on 17th December 2024
Abstract
Modern lifestyle changes, including irregular diets and late-night activities, have contributed to a significant rise in cancer rates, particularly among younger demographics, highlighting the pressing need for early detection and treatment. Fluorescence imaging techniques play a crucial role in tumor diagnosis, yet traditional organic fluorescent materials suffer from limitations such as poor photostability and fluorescence quenching in aggregates. This paper introduces the design and synthesis of four aggregation-induced emission (AIE) molecules with near-infrared I emission, which are aimed at overcoming fluorescence quenching in the molecular aggregation state. The photophysical properties of these molecules (BTA-TT, BTA-TTM, BTA-FT, and BTA-FTM) were investigated and they exhibited TICT and AIE behaviors in varying water fractions, along with notably large Stokes shifts. In vitro imaging of the four molecules successfully imaged lysosomes within 4T1 cells and they displayed minimal dark toxicity. Moreover, these AIEgens exhibited excellent anti-photobleaching properties, which were superior to those of commercial dyes. In addition, BTA-FTM nanoparticles coated with PEG-2000 showed biosafety and enabled tumor imaging in mice for 59 hours, revealing the tumor metastases in the heart and lungs of mice. This research contributes to the development of novel near-infrared molecules for advanced diagnostic applications.
Introduction
In light of societal advancements and evolving lifestyles, cancer has emerged as a significant health concern affecting humans.1–4 Currently, various treatment modalities are available for tumors, including immunotherapy,5,6 targeted radiotherapy,7 and surgical tumor resection.8 Among these methods, surgical resection is a common treatment for patients with tumors. During surgical procedures, imaging technology serves as a valuable diagnostic and therapeutic tool.9 Presently, ultrasonography (US),10 positron emission tomography (PET),11 magnetic resonance imaging (MRI),12 and computed tomography (CT)13 are frequently utilized in the imaging field. However, these imaging modalities have several disadvantages, including low resolution, high costs, and potential adverse effects on the human body. Fluorescence imaging offers several advantages, such as high sensitivity, multicolor labelling capability, strong signal intensity, and low experimental costs.14–17 Compared to visible light imaging, near-infrared fluorescence imaging can effectively resolve biological background fluorescence, minimize light scattering, reduce light-induced damage to cells, and facilitate biological imaging.18–22 Currently, near-infrared fluorescence probes are primarily categorized into organic and inorganic compounds. Inorganic materials, such as semiconductor nanocrystals and quantum dots, are associated with potential toxicity due to heavy metals.23–26 In contrast, organic near-infrared probes exhibit diverse structures, low biological toxicity, and biodegradability.27,28 Conventional fluorophores, however, are often quenched in the aggregation state due to their rigid planar structures, which promotes strong π–π stacking interactions.29–31 Examples include fluorescein, rhodamine, coumarin, and BODIPY.32–35 Fluorescence quenching in the aggregation state presents a significant challenge for the application of organic probes in bioimaging. Surprisingly, since Professor Tang's group coined the concept of aggregation-induced emission (AIE) in 2001, it has garnered considerable attention over the past two decades in biological fields.36 Therefore, designing near-infrared organic probes with AIE characteristics represents an effective strategy to address the issue of fluorescence quenching in the aggregation state.37In this study, we synthesized near-infrared (NIR) probes with aggregation-induced emission (AIE) characteristics, specifically BTA-TT, BTA-TTM, BTA-FT and BTA-FTM. These D–π–A type molecular structures were constructed via the Knoevenagel reaction, utilizing furan or thiophene as the π bridge, benzo[d]thiazole-2-acetonitrile as the acceptor, and triphenylamine or 4-methoxy-N-(4-methoxyphenyl)-N-phenylaniline as the donor. We investigated the photophysical properties of these compounds, which exhibited both twisted intramolecular charge transfer (TICT) and AIE characteristics in varying water fractions. Additionally, we demonstrated the biological imaging capabilities of these new molecules, which localize in lysosomes and exhibit negligible dark toxicity, favorable biocompatibility, and high photostability. Furthermore, BTA-FTM nanoparticles (NPs) coated with PEG-2000 showed excellent fluorescence emission in mice bearing 4T1 tumors for a duration of 59 hours. Meanwhile, BTA-FTM NPs revealed the tumor metastases in the heart and lungs of mice.
Results and discussion
Synthesis, characterization and photophysical properties
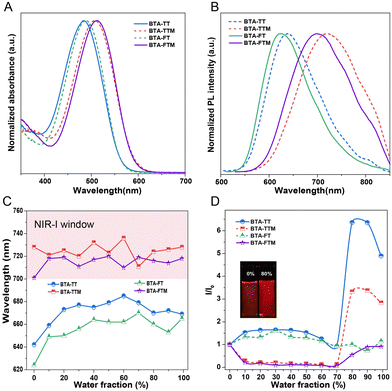
The molecular structures of BTA-TT, BTA-TTM, BTA-FT and BTA-FTM are illustrated in Scheme 1. The detailed synthesis procedures and the characterization results of their structures obtained utilizing NMR and high-resolution mass spectrometry are provided in the ESI,† specifically in Fig. S1–S8 (ESI†). To validate the feasibility of the molecular design, the photophysical properties of BTA-TT, BTA-TTM, BTA-FT and BTA-FTM were assessed using UV-Vis and photoluminescence spectroscopy.The UV-Vis spectra of the four molecules in tetrahydrofuran (THF) solution are shown in Fig. 1A. The maximum absorption peaks of BTA-TT and BTA-FT were observed at 484 and 489 nm, respectively. In contrast, the maximum absorption peaks of BTA-TTM and BTA-FTM, which contain the 4-methoxy-N-(4-methoxyphenyl)-N-phenylaniline group, were located at 508 and 512 nm, resulting in red shifts of 24 and 23 nm, respectively. This observation indicates that the stronger electron-donating ability of the 4-methoxy-N-(4-methoxyphenyl)-N-phenylaniline group increases the electron cloud density of the molecules, effectively reducing the absorption energy band and leading to a red shift in the absorption wavelength. As illustrated in Fig. 1B, the emission peaks of BTA-TT, BTA-TTM, BTA-FT, and BTA-FTM in THF were observed at 640, 709, 625, and 702 nm, respectively. Correspondingly, their Stokes shifts were observed at 156, 201, 136, and 190 nm, respectively, which are advantageous for bioimaging and diagnosis.
We explored the photophysical properties of these new molecules with D–π–A structures in mixed THF/H2O solutions with varying proportions. The relationship of emission wavelength and the water content is shown in Fig. 1C. Clearly, the emission wavelengths of BTA-TTM and BTA-FTM fall within the near-infrared I (NIR-I) window, while those of BTA-TT and BTA-FT do not. Such results should be attributed to the 4-methoxy-N-(4-methoxyphenyl)-N-phenylaniline group with more electron-rich characteristics. For example, as the water content of BTA-TT increased to 60%, the emission wavelength was red-shifted by 37 nm, due to the increase in solvent polarity inducing TICT. However, when the water fraction further increased (≥70%), the emission peak slightly blue-shifted. This may be attributed to the decrease in molecular conjugation in the crowded aggregation state. Regarding the emission intensity, it slightly decreased before 80% water content. While the water content reached 80%, the fluorescence emission intensity increased sharply, which was 6.5-fold higher than that in THF solution. This phenomenon due to the intermolecular interactions in BTA-TT aggregates in water restricted the molecular motions and enhanced the proportion of radiative decay in the excited state. Thus, the AIE of BAT-TT was active in a high water fraction. For other molecules, there were similar photophysical properties to BAT-TT, with both TICT and AIE being active with increasing water fraction. These results demonstrated that the design of D–π–A molecules was effective, not only achieving emission in the near-infrared I, but also suppressing fluorescence quenching in the aggregation state.
Single-crystal structural analysis
To gain further insight into the effect of the molecular structures on the fluorescence emission of BTA-TTM and BTA-FT, their single crystals were grown in DCM/EtOH and analyzed by X-ray diffraction. The crystal data and the collection of parameters are summarized in Table S1 (ESI†). From the single-crystal structural analysis results of BTA-TTM and BTA-FT shown in Fig. 2, it can be seen from the side view that benzo[d]thiazole-2-acetonitrile and the thiophene or furan ring were nearly coplanar in their connection. The twist angle between the furan ring and its adjacent groups was found to be only 1.44° (Fig. 2B), a value significantly smaller than that observed for the corresponding thiophene ring (18.68°). This observation suggested that compounds incorporating furan rings exhibit good conjugation compared to those with thiophene rings. Specifically, the absorption wavelength of the compound featuring a furan ring showed a slight red-shift of 4–5 nm relative to its thiophene counterpart. Additionally, the torsion angle of two phenyl groups in BTA-TTM was 70.5° and 81.75°, which effectively increased steric hindrance and impeded strong intermolecular interactions. Furthermore, numerous weak interactions such as CN⋯H (2.582 Å), N⋯H (2.649 Å), S⋯O (3.157 Å) and CH⋯π (2.847, 2.853, 2.863, and 2.894 Å) effectively suppressed molecular motions in the aggregation state and enabled the dissipation of excited-state energy through radiation transition. Similar to BTA-TTM, BTA-FT also has twist angles of two phenyl groups (30.08° and 62.89°), meanwhile multiple H⋯H (2.372 Å), CN⋯H (2.546 Å), N⋯H (2.629 Å), and CH⋯π (2.803, 2.890, and 3.351 Å) interactions exist in the crystal lattice.Theoretical calculations
Density functional theory (DFT) calculations were conducted to gain a deeper understanding of the molecular structures and electronic properties using the B3LYP method. As shown in Fig. 3, the electron cloud density of the highest occupied molecular orbital (HOMO) was primarily concentrated in the thiophene (or furan) units and the triphenylamine units (or 4-methoxy-N-(4-methoxyphenyl)-N-phenylaniline units). In contrast, the electron cloud density of the lowest unoccupied molecular orbital (LUMO) was predominantly located on the 2-benzothiazoleacetonitrile components. The energy gaps of BTA-TTM and BAT-FTM were 2.44 and 2.47 eV, respectively, which were smaller than those of BAT-TT and BAT-FT (2.59 and 2.6 eV). The enhancement of electron donation significantly elevated the HOMO energy level of molecules. Consequently, this alteration leads to a decrease in the energy band gap and a redshift of the emission wavelength, aligning with the above photophysical properties.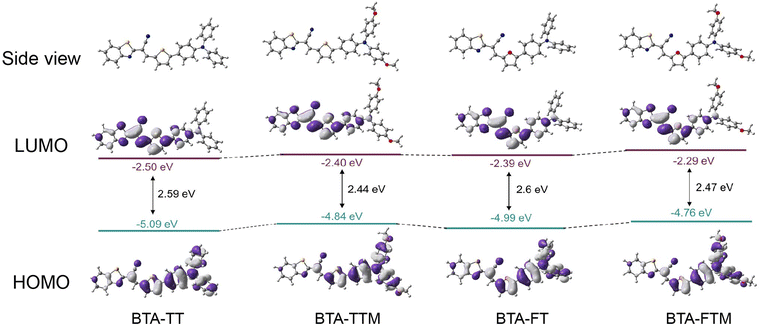 | ||
| Fig. 3 The distributions of HOMO–LUMO levels, in addition to the energy levels of BTA-TT, BTA-TTM, BTA-FT and BTA-FTM were presented herein. | ||
In vitro bioimaging
Given the desirable photophysical properties, we first investigated the cellular imaging applications of the developed AIEgens, and 4T1 cells were selected as the representatives. Initially, we investigated the cytotoxicity by co-incubating four AIEgens with 4T1 cells. The results demonstrated the treatment of these AIEgens presented a faint effect on the growth of 4T1 cells with the concentrations ranging from 1 to 128 μM, indicating their good biocompatibility. Furthermore, good biosafety could also be achieved under light irradiation (Fig. S12, ESI†). Then, we studied their bioimaging behavior. As shown in Fig. 4A, these molecules could cross the cell membrane efficiently, and further colocalization analysis revealed that the developed AIEgens showed good colocalization with Lysotracker. Taking BTA-FTM as an example, after coincubation with Lysotracker blue, a co-localization coefficient of 0.76 was obtained for BTA-FTM-treated 4T1 cells, indicating the enrichment in lysosome. Moreover, linear analysis was also performed to verify the localization of BTA-FTM (Fig. 4B), and the perfect merging of BTA-FTM and Lysotracker blue could be observed, indicating the perfect targeting ability of BTA-FTM towards lysosome. In addition, a similar phenomenon was also observed in 4T1 cells incubated with BTA-TT, BTA-TTM, BTA-FT, respectively, revealing their good lysosome targeting behavior (Fig. S15, ESI†). Additionally, the photostability property of the dye is crucial for imaging applications. As illustrated in Fig. 4C, upon continuous irradiation with a 405 nm laser at 20% power for 5 minutes, the fluorescence intensity of Lysotracker blue diminished significantly to 63% of its initial value, whereas the emission intensity of all AIEgens persisted above 80%. Notably, BTA-FT exhibited the highest photostability, maintaining an emission intensity of 93% after laser irradiation. These results suggested that the developed AIEgens possess superior photostability compared to commercial dyes, which may be attributed to the incorporation of the CN group that enabled enhanced photostability.38 Thus, the developed AIEgens not only exhibit good biocompatibility but also perfect lysosome targeting ability, showing high potential for bioimaging applications.In vivo imaging ability
Based on the outstanding in vitro bioimaging ability, we further evaluated the in vivo imaging ability. Herein, BTA-FTM was selected as the representative luminogen. A mouse model was constructed by subcutaneously injecting 4 T1 cells bilaterally into the buttocks of female BALB/c mice. As shown in Fig. 5A, nanoparticles were administered to mice bearing 4T1 tumors via intravenous injection. Due to the enhanced permeability and retention (EPR) effect, a bright emission of BTA-FTM could be detected, demonstrating a significant accumulation of particles at the solid tumor. Moreover, the fluorescence signal at the tumor site could be observed after 12 hours, which remained about 71% even 59 hours after the intravenous injection (Fig. 5B). These findings suggested that the nanoparticles show a long term retention effect, which was beneficial for in vivo imaging and monitoring applications. Additionally, the biosafety of the nanoparticles is a critical consideration. After 48 hours post-injection, we investigated the function of kidneys and the liver by evaluating the expression level of biomarkers in mice with different treatments. The expression values for ALB, ALP, and UREA in mice without any treatment were evaluated to be 35.13 g L−1, 62.79 U L−1, and 7.13 mmol L−1, respectively, meanwhile, the similar phenomena were observed for BTA-FTM NP treated-mice with the values of 35.01 g L−1, 64.29 U L−1, and 7.76 mmol L−1 for ALB, ALP, and UREA, respectively (Fig. S15, ESI†). These findings indicated that the liver and kidney functions of the experimental mice were normal, further demonstrating good biocompatibility of the nanoparticles. We also investigated the distribution of nanoparticles in the main organs of heart, liver, spleen, lungs, and kidneys, and tumors of the mice after seven days post-injection. Fig. 5C shows bright fluorescence observed in both the tumor and liver. It should be noted that a considerable signal could also be detected in the heart and lungs. To elucidate the reasons for the accumulation of nanoparticles in different organs, we performed H&E staining on the tumor, heart, liver, spleen, lungs, and kidneys. The results revealed metastatic tumors in the heart and lungs, while no lesions were observed in other organs. This confirmed that the fluorescence emission in the heart and lungs was attributable to the presence of metastatic tumors, whereas there were no metastatic tumors detected in the spleen and kidneys. These results indicated that BTA-FTM nanoparticles can accumulate at the tumor site through the EPR effect, facilitate long-term imaging, exhibit good biocompatibility, and trace tumor metastasis.Experimental
Synthetic procedures
Preparation of BTA-FTM NPs
BTA-FTM (1.0 mg) and DSPE-PEG2000 (2.0 mg) were fully dissolved in THF (1.0 mL). The mixture was quickly injected into 9.0 mL of deionized water using an ultrasonic crusher with 40% power for 2 min, which was stirred in fume hood for two days. The crude NPs were further filtered through a membrane filter (diameter = 200 nm) for further usage.Cell cultures
4T1 cells were cultured in DMEM medium containing 10% FBS and antibiotics (100 units per mL penicillin and 100 μg mL−1 streptomycin) in a humidified incubator with 5% CO2 at 37 °C.Cell imaging
4T1 cells were grown overnight in a cell culture dish for 24 h. The cells were further imaged using a CLSM using different combinations of excitation wavelengths for each dye.MTT assays
4T1 cells were seeded in a 96-well flat-bottomed microplate. Cells treated with BTA-TTM were incubated with concentrations ranging from 0 to 128 μM at 37 °C under a 5% CO2 atmosphere for 12 h. The microplate was irradiated at 450 nm (light dosage = 40 mW cm−2) for 20 min, while another microplate was kept in the dark for 20 min. After the treatment, the cell was incubated for 20 h. MTT in PBS (10 μL, 5 mg mL−1) was added to each well. The microplate was incubated at 37 °C under a 5% CO2 atmosphere for another 4 h. The growth medium was then removed, and DMSO (100 μL) was added to each well. The absorbance of the solutions at 520 nm was measured with a Powerwave XS MQX200R microplate spectrophotometer (BioTek Instruments Inc., Winooski, VT). For the MTT assays involving BTA-TT, BTA-FT and BTA-FTM, the procedure was similar.Live cell confocal imaging
4T1 cells were grown in a confocal imaging dish at 37 °C. After incubation with medium containing 12.5 μM BTA-TTM for 2 h, the medium was removed and washed with PBS (1 mL × 3). The cells were then incubated with Lyso-tracker blue (200 nM) in a growth medium at 37 °C under a 5% CO2 atmosphere for 30 min. The medium was then removed, and the cell layer was gently washed with PBS (1 mL × 3). The excitation wavelength of Lyso-tracker blue was 405 nm. Pearson's correlation coefficients (PCC) were determined using the program ImageJ. For the confocal imaging involving BTA-TT, BTA-FT and BTA-FTM, the procedure was similar.Efficacy of BTA-FTM-NPs in vivo
Female BALB/c mice (5 weeks old) were purchased from China Boryxin Biotechnology Co. (Hunan, China). All animal procedures have been approved by the Animal Ethics Committee of The University of South China. The mice were subcutaneously injected with 4T1 cells (1 × 107 cells) in PBS buffer. When the tumor size was about ∼5 to 7 mm, the 4T1 cell-bearing mice were injected with BTA-FTM-NPs.Conclusions
In summary, four NIR-I fluorescence imaging AIE agents (BTA-TT, BTA-TTM, BTA-FT, and BTA-FTM) with a donor-π-acceptor (D–π–A) structure were designed and synthesized. The absorption peaks of BTA-TTM and BTA-FTM in THF were found at 508 and 512 nm, respectively, while their emission peaks were observed at 709 and 702 nm, respectively. Due to the D–π–A structure and highly twisted donor groups, these four NIR-I molecules exhibited both TICT and AIE behaviors as the water fraction increased. Moreover, single crystal analysis demonstrated that the highly twisted donor groups, TPA and 4-methoxy-N-(4-methoxyphenyl)-N-phenylaniline, effectively inhibit intermolecular π–π interactions. Concurrently, multiple weak interactions suppress intermolecular motions, resulting in bright emission in the aggregated state. Additionally, theoretical calculations revealed the electron cloud distribution of the molecules' LUMO and HOMO, along with their energy gaps. In vitro experiments demonstrated that all AIEgens exhibited minimal dark toxicity and the ability to target lysosomal localization. Furthermore, these AIEgens possessed robust photobleaching resistance compared to commercial Lyso-tracker blue. Among them, BTA-FTM nanoparticles can image tumors for up to 59 hours and successfully trace metastatic tumors of mice in the heart and lungs. Additionally, the nanoparticles exhibited excellent biocompatibility due to negligible damage to the liver and kidneys of mice. The development of these new NIR-I window molecules provides a promising platform for surgical tumor removal and the tracking of tumor metastasis.Author contributions
Y. Zhu synthesized materials, performed all measurements, and wrote the manuscript. H. Liu analysed the photophysical properties and single crystal data. Y. Zeng and Y. Yin performed cell imaging and tumor imaging in vivo. B. Chen and R. Hu provided intellectual input and revised the manuscript. All the authors discussed the project and analysed the results.Data availability
The data supporting this article are available within the article and the ESI.† The crystallographic data for BTA-TTM and BTA-FT have been deposited at CCDC with deposition numbers 2378963 and 2378964, respectively.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the National Natural Science Foundation of China (22205120), YongJiang Talent Introduction Programme and Hunan Provincial Natural Science Foundation (2024RC3206).Notes and references
- J. Dai, H. Xue, D. Chen, X. Lou, F. Xia and S. Wang, Aggregation-induced emission luminogens for assisted cancer surgery, Coord. Chem. Rev., 2022, 464, 214552 CrossRef CAS.
- K. Xue, Y. Zhao, S. Sun, Y. Li, J. Liang and Z. Qi, NIR-AIEgens nanospheres featuring high-fidelity dynamic lipid droplet targeting, expediting ferroptosis to annihilating tumor in hypoxia, Chem. Eng. J., 2023, 470, 144125 CrossRef CAS.
- Y. Liu, Y. Li, X. Luo, P. Luo, Z. Han, Q. Peng, K. Li, H. Hou and S. Q. Zang, AIE ligand-based silver clusters used for ethion detection, Mater. Chem. Front., 2021, 5, 7982–7986 RSC.
- N. Niu, Y. Yu, Z. Zhang, M. Kang, L. Wang, Z. Zhao, D. Wang and B. Z. Tang, A cell membrane-targeting AIE photosensitizer as a necroptosis inducer for boosting cancer theranostics, Chem. Sci., 2022, 13, 5929–5937 RSC.
- M. Wang, D. Yan, M. Wang, Q. Wu, R. Song, Y. Huang, J. Rao, D. Wang, F. Zhou and B. Z. Tang, A Versatile 980 nm Absorbing Aggregation-Induced Emission Luminogen for NIR-II Imaging-Guided Synergistic Photo-Immunotherapy Against Advanced Pancreatic Cancer, Adv. Funct. Mater., 2022, 32, 2205371 CrossRef CAS.
- K. Yu, B. Ye, H. Yang, X. Xu, Z. Mao, Q. Zhang, M. Tian, H. Zhang, H. Zhang and Q. He, A Mitochondria-Targeted NIR-II AIEgen Induced Pyroptosis for Enhanced Tumor Immunotherapy, Adv. Healthc. Mater, 2023, 12, 2301693 CrossRef CAS.
- L. Huang, D. Qing, S. Zhao, X. Wu, K. Yang, X. Ren, X. Zheng, M. Lan, J. Ye, L. Zeng and G. Niu, Acceptor-donor-acceptor structured deep-red AIE photosensitizer: Lysosome-specific targeting, in vivo long-term imaging, and effective photodynamic therapy, Chem. Eng. J., 2022, 430, 132638 CrossRef CAS.
- H. Zou, S. Gan, H. Shen, B. He, Z. Zheng, J. Li, J. C. Huang, L. Zheng, B. Z. Tang and J. Zhang, A novel drug susceptibility testing AIEgen with spatiotemporal resolved progress-reporting characteristic for therapy of drug-resistant tumor, Mater. Today, 2022, 61, 117–128 CrossRef CAS.
- W. He, Z. Zhang, Y. Luo, R. T. K. Kwok, Z. Zhao and B. Z. Tang, Recent advances of aggregation-induced emission materials for fluorescence image-guided surgery, Biomaterials, 2022, 288, 121709 CrossRef CAS PubMed.
- H. Huang, A. Ali, Y. Liu, H. Xie, S. Ullah, S. Roy, Z. Song, B. Guo and J. Xu, Advances in image-guided drug delivery for antibacterial therapy, Adv. Drug Delivery Rev., 2023, 192, 114634 CrossRef CAS.
- Y. Chen, S. Wang and F. Zhang, Near-infrared luminescence high-contrast in vivo biomedical imaging, Nat. Rev. Bioeng., 2023, 1, 60–78 CrossRef CAS.
- J. Lazovic, E. Goering, A. M. Wild, P. Schutzendube, A. Shiva, J. Loffler, G. Winter and M. Sitti, Nanodiamond-Enhanced Magnetic Resonance Imaging, Adv. Mater., 2024, 36, e2310109 CrossRef.
- M. Yin, Y. Chen, X. Liu, S. Tian, L. Zhao, Y. Bai, H. Wang, J. Lin, D. Jiang, Z. Lei, F. Meng, D. Tian and L. Luo, Targeted Computed Tomography Visualization and Healing of Inflammatory Bowel Disease by Orally Delivered Bacterial-Flagella-Inspired Polydiiododiacetylene Nanofibers, ACS Nano, 2023, 17, 3873–3888 CrossRef CAS PubMed.
- S. Yang, H. Yu, J. Liu, L. Ma, Z. Hou, J. Ma, M. Z. Miao, R. T. K. Kwok, J. Sun, H. H. Y. Sung, I. D. Williams, J. W. Y. Lam, X. Liu and B. Z. Tang, Integrating Anion-pi(+) Interaction and Crowded Conformation to Develop Multifunctional NIR AIEgen for Effective Tumor Theranostics via Hippo-YAP Pathway, ACS Nano, 2023, 17, 21182–21194 CrossRef PubMed.
- M. Liang, L. Liu, Y. Sun, J. Li, L. E. Zhang, X. Jiang and W. Wu, Furan-modified thiadiazolo quinoxaline as an electron acceptor for constructing second near-infrared aggregation-induced emission fluorophores for beyond 1300 nm fluorescence/photoacoustic imaging and photothermal therapy, Aggregate, 2024, 5, e458 CrossRef CAS.
- D. Li, X. Deng, Z. Xu, D. Wang, G. Xu, P. Zhang, P. Qiu, W. Xie, D. Wang, B. Z. Tang and K. Wang, Molecular Engineering of NIR-II AIE Luminogen Excited at 1700 nm for Ultradeep Intravital Brain Two-Photon Fluorescence Imaging, Adv. Funct. Mater., 2023, 33, 2303967 CrossRef CAS.
- S. Song, Y. Zhao, M. Kang, Z. Zhang, Q. Wu, S. Fu, Y. Li, H. Wen, D. Wang and B. Z. Tang, Side-Chain Engineering of Aggregation-Induced Emission Molecules for Boosting Cancer Phototheranostics, Adv. Funct. Mater., 2021, 31, 2107545 CrossRef CAS.
- C. Liu, X. Wang, J. Liu, Q. Yue, S. Chen, J. W. Y. Lam, L. Luo and B. Z. Tang, Near-Infrared AIE Dots with Chemiluminescence for Deep-Tissue Imaging, Adv. Mater., 2020, 32, 2004685 CrossRef CAS PubMed.
- L. Liu, X. Wang, L. J. Wang, L. Guo, Y. Li, B. Bai, F. Fu, H. Lu and X. Zhao, One-for-All Phototheranostic Agent Based on Aggregation-Induced Emission Characteristics for Multimodal Imaging-Guided Synergistic Photodynamic/Photothermal Cancer Therapy, ACS Appl. Mater. Interfaces, 2021, 13, 19668–19678 CrossRef CAS.
- G. Yuan, C. Lv, J. Liang, X. Zhong, Y. Li, J. He, A. Zhao, L. Li, Y. Shao, X. Zhang, S. Wang, Y. Cheng and H. He, Molecular Engineering of Efficient Singlet Oxygen Generators with Near-Infrared AIE Features for Mitochondrial Targeted Photodynamic Therapy, Adv. Funct. Mater., 2021, 31, 2104026 CrossRef CAS.
- J. Wang, Y. Liu, M. Morsch, Y. Lu, P. Shangguan, L. Han, Z. Wang, X. Chen, C. Song, S. Liu, B. Shi and B. Z. Tang, Brain-Targeted Aggregation-Induced-Emission Nanoparticles with Near-Infrared Imaging at 1550 nm Boosts Orthotopic Glioblastoma Theranostics, Adv. Mater., 2022, 34, 2106082 CrossRef CAS.
- H. Shen, B. Wu, Q. Zhang, J. Ni, M. Liang, Y. Liu, X.-F. Zang, S. Wang, Y.-Y. Quan, X. Ye and Z.-S. Huang, Acceptor/π-bridge planarization and donor rotation manipulation for designing an NIR-II AIEgen with high photothermal conversion efficiency to enhance cancer phototherapy, Chem. Eng. J., 2023, 468, 143726 CrossRef CAS.
- J. Li, Z. Feng, X. Yu, D. Wu, T. Wu and J. Qian, Aggregation-induced emission fluorophores towards the second near-infrared optical windows with suppressed imaging background, Coord. Chem. Rev., 2022, 472, 214792 CrossRef CAS.
- Y. Wang, B. Xia, Q. Huang, T. Luo, Y. Zhang, P. Timashev, W. Guo, F. Li and X. J. Liang, Practicable Applications of Aggregation-Induced Emission with Biomedical Perspective, Adv. Healthc. Mater., 2021, 10, 2100945 CrossRef CAS.
- Z. Zheng, H. Zhang, H. Cao, J. Gong, M. He, X. Gou, T. Yang, P. Wei, J. Qian, W. Xi and B. Z. Tang, Intra- and Intermolecular Synergistic Engineering of Aggregation-Induced Emission Luminogens to Boost Three-Photon Absorption for Through-Skull Brain Imaging, ACS Nano, 2022, 16, 6444–6454 CrossRef CAS.
- D. Xu, J. Ge, Y. An, S. Bai, Z. Wang, S. Wu, Q. Dai, Z. Lu and G. Liu, Molecular Engineering of NIR-II/IIb Emitting AIEgen for Multimodal Imaging-Guided Photo-Immunotherapy, Small, 2023, 19, 2300859 CrossRef CAS.
- L. Wang, J. Qi, K. Zhang, Z. Zhuang, K. Ding, X. Chen, H. Shan, D. Ding, A. Qin and B. Z. Tang, Multifunctional nanomicelles constructedviaan aggregation and de-aggregation strategy for magnetic resonance/NIR II fluorescence imaging-guided type I photodynamic therapy, Mater. Chem. Front., 2023, 7, 3657–3667 RSC.
- K. W. Lee, Y. Gao, W. C. Wei, J. H. Tan, Y. Wan, Z. Feng, Y. Zhang, Y. Liu, X. Zheng, C. Cao, H. Chen, P. Wang, S. Li, K. T. Wong and C. S. Lee, Anti-Quenching NIR-II J-Aggregates of Benzo[c]thiophene Fluorophore for Highly Efficient Bioimaging and Phototheranostics, Adv. Mater., 2023, 35, 2211632 CrossRef CAS.
- H. Wang, Q. Li, P. Alam, H. Bai, V. Bhalla, M. R. Bryce, M. Cao, C. Chen, S. Chen, X. Chen, Y. Chen, Z. Chen, D. Dang, D. Ding, S. Ding, Y. Duo, M. Gao, W. He, X. He, X. Hong, Y. Hong, J. J. Hu, R. Hu, X. Huang, T. D. James, X. Jiang, G. I. Konishi, R. T. K. Kwok, J. W. Y. Lam, C. Li, H. Li, K. Li, N. Li, W. J. Li, Y. Li, X. J. Liang, Y. Liang, B. Liu, G. Liu, X. Liu, X. Lou, X. Y. Lou, L. Luo, P. R. McGonigal, Z. W. Mao, G. Niu, T. C. Owyong, A. Pucci, J. Qian, A. Qin, Z. Qiu, A. L. Rogach, B. Situ, K. Tanaka, Y. Tang, B. Wang, D. Wang, J. Wang, W. Wang, W. X. Wang, W. J. Wang, X. Wang, Y. F. Wang, S. Wu, Y. Wu, Y. Xiong, R. Xu, C. Yan, S. Yan, H. B. Yang, L. L. Yang, M. Yang, Y. W. Yang, J. Yoon, S. Q. Zang, J. Zhang, P. Zhang, T. Zhang, X. Zhang, X. Zhang, N. Zhao, Z. Zhao, J. Zheng, L. Zheng, Z. Zheng, M. Q. Zhu, W. H. Zhu, H. Zou and B. Z. Tang, Aggregation-Induced Emission (AIE), Life and Health, ACS Nano, 2023, 17, 14347–14405 CrossRef CAS PubMed.
- S. Wang, Y. Liao, Z. Wu, Y. Peng, Y. Liu, Y. Chen, L. Shao, Z. Zeng and Y. Liu, A lysosomes and mitochondria dual-targeting AIE-active NIR photosensitizer: Constructing amphiphilic structure for enhanced antitumor activity and two-photon imaging, Mater. Today Bio, 2023, 21, 100721 CrossRef CAS.
- G. Aiping, W. Qingqing, W. Huijuan, Z. Jun-Wei and C. Xinhua, Research progress on AIE cyanostilbene-based self-assembly gels: Design, regulation and applications, Coord. Chem. Rev., 2022, 471, 214753 CrossRef.
- H. Ma, R. Li, H. Meng, M. Tian, X. Zhang, Y. Liu, L. Li, J. Yuan and Y. Wei, A Versatile Theranostic Nanoplatform with Aggregation-Induced Emission Properties: Fluorescence Monitoring, Cellular Organelle Targeting, and Image-Guided Photodynamic Therapy, Small, 2023, 19, 2204778 CrossRef CAS.
- M. M. S. Lee, E. Y. Yu, J. H. C. Chau, J. W. Y. Lam, R. T. K. Kwok, D. Wang and B. Z. Tang, Inspiration from nature: BioAIEgens for biomedical and sensing applications, Biomaterials, 2022, 288, 121712 CrossRef CAS.
- R. Zhang, X. Huang, C. Chen, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, AIEgen for cancer discrimination, Mater. Sci. Eng. R, 2021, 146, 100649 CrossRef.
- C. Ding and T. Ren, Near infrared fluorescent probes for detecting and imaging active small molecules, Coord. Chem. Rev., 2023, 482, 215080 CrossRef CAS.
- X. Yang, X. Wang, X. Zhang, J. Zhang, J. W. Y. Lam, H. Sun, J. Yang, Y. Liang and B. Z. Tang, Donor-Acceptor Modulating of Ionic AIE Photosensitizers for Enhanced ROS Generation and NIR-II Emission, Adv. Mater., 2024, 36, 2402182 CrossRef CAS.
- X. Wang, X. Yang, G. Jiang, Z. Hu, T. Liao, G. Wang, X. Zhang, X. He, J. Zhang, J. Zhang, W. Cao, K. Zhang, J. W. Y. Lam, J. Sun, H. Sun, Y. Liang and B. Z. Tang, Unlocking the NIR-II AIEgen for High Brightness through Intramolecular Electrostatic Locking, Angew. Chem., Int. Ed., 2024, 63, e202404142 CrossRef CAS.
- N. I. Shank, H. H. Pham, A. S. Waggoner and B. A. Armitage, Twisted Cyanines: A Non-Planar Fluorogenic Dye with Superior Photostability and its Use in a Protein-Based Fluoromodule, J. Am. Chem. Soc., 2012, 135, 242–251 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2378963 and 2378964. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qm00943f |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2025 |