Engineering a versatile nanoscale COF-based multienzyme loading strategy to enhance cancer therapy†
Wen-Xiu
Ren‡
a,
Qing-Yun
Huang‡
a,
Jie
Feng
 *a,
Yue-Lu
Tian
a,
Ya-Ni
Wu
a,
Seeram
Ramakrishna
*a,
Yue-Lu
Tian
a,
Ya-Ni
Wu
a,
Seeram
Ramakrishna
 b and
Yu-Bin
Dong
b and
Yu-Bin
Dong
 a
a
aCollege of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Shandong Normal University, Jinan, 250014, China. E-mail: jiefeng@sdnu.edu.cn
bCentre for Nanotechnology and Sustainability, Department of Mechanical Engineering, National University of Singapore, 117574, Singapore
First published on 4th March 2025
Abstract
Herein, a simple and efficient in situ assembly approach for immobilizing enzymes within nanoscale COFs under ambient conditions is presented. This approach is versatile and can be applied to immobilize a wide range of enzymes. More importantly, a dual-enzyme-loaded COF is synthesized using this in situ assembly method, followed by coating it with MnO2 to create GOx&HRP@COF@MnO2. Of note, GOx&HRP@COF@MnO2 exhibits remarkable therapeutic efficacy, which can be attributed to the synergistic effect of the Mn2+-induced Fenton-like reaction and the cascade enzyme-catalyzed reaction mediated by GOx and HRP. Its significant therapeutic efficacy was validated through both in vitro and in vivo experiments.
Introduction
As an important class of biomacromolecules with catalytic abilities, enzymes play a crucial role in our daily lives.1 However, their practical application is often limited due to intrinsic vulnerabilities, such as their instability in high-temperature, organic, acidic, alkaline, metal ion-containing, and trypsin digestive environments.2 Enzyme immobilization, including surface attachment, covalent linkage, and pore entrapment approaches, has been recognized as a powerful tool to protect and enhance enzyme performance.3–5 In contrast to these immobilization methods, the in situ enzyme encapsulation in the aqueous-phase at room temperature is rare but of great significance. The in situ enzyme encapsulation can overcome the physical compression or leaking, allowing for orderly enzyme loading in a self-assembled manner.6 However, the in situ synthesis of enzyme@carrier under mild conditions remains a big challenge. Most reported carriers for in situ enzyme immobilization are primarily limited to several types of amorphous or nonporous materials, including organic microparticles, sol–gel matrices, hydrogels, and mesoporous silicas.7–10 So, the development of new kinds of highly porous carriers, especially with ordered and defined structures, is imperative and significant because they are expected to be more powerful for mass transfer.Covalent organic frameworks (COFs)11 have great potential to be porous carriers for enzyme immobilization due to their high surface area, tunable pore size, and ease of modification. However, their synthesis is usually carried out under harsh conditions,12 which significantly hinders their enzyme loading via an in situ approach. So far, only a handful of COF-based in situ enzyme immobilizations have been reported.13,14 For instance, Chen and Yang groups independently developed an approach for the in situ assembly of enzymes with COFs under mild conditions. On the other hand, most of the reported COFs are obtained in the micrometer level, severely limiting their biomedical applications.15,16 In biomedical fields, especially in drug delivery systems and in vivo imaging, materials need to interact with biological entities at a microscopic scale.17,18 Micrometer-sized COFs are usually bulky in size, indispersible in water, and have limited loading capacity for drugs, limiting cellular uptake and intracellular bioavailability.19 Moreover, if their size is too large they can be inhaled or embedded into small blood vessels, causing blood vessels to become blocked and causing nanoparticles to accumulate in the body, which increases the risk of an immune response.20,21 In contrast, nanosized COFs not only possess the properties of traditional nanoparticles, such as good dispersion, small volume and high bioavailability, but also retain the crystallinity, porous features and tunable mechanical properties of COF materials. Therefore, these properties make nano-COFs ideal drug carriers and nanomedicines for drug delivery and tumor therapy, endowing therapeutic agents with high loading efficiency, prolonged half-life time, enhanced cell uptake efficiency and cancer tissue enrichments, and minimal side effects.22
In practical applications, the collaboration of two or more enzymes is often necessary to achieve enhanced functionality. The fabrication of multi-enzyme architectures has gained significant attention and holds tremendous promise in the fields of biocatalysis and biomedicine.23,24 For example, the incorporation of glucose oxidase (GOx) and horseradish peroxidase (HRP) enzymes exemplifies a standard cascade reaction model within a biologically significant framework. GOx efficiently facilitates the conversion of glucose into gluconic acid while simultaneously producing hydrogen peroxide (H2O2) as a byproduct.25 This H2O2, in turn, serves as a substrate for horseradish peroxidase (HRP). Recently, this dual-enzyme system has gained extensive application when combined with various nanomaterials in the field of cancer treatment.26
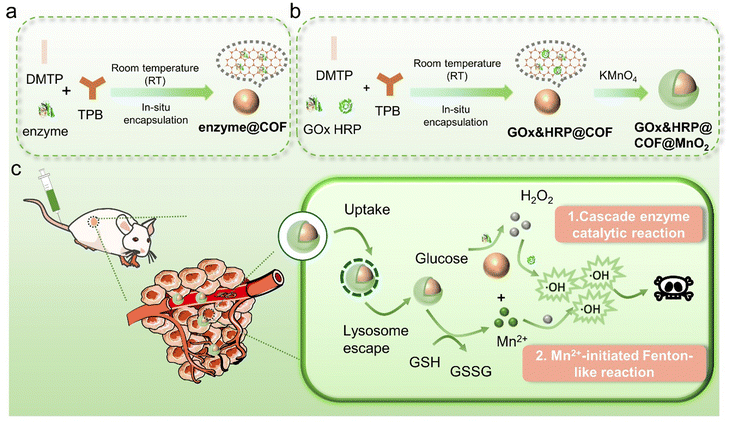
In this study, we reported a simple and efficient approach for fabrication of a nanoscale enzyme@COF using in situ assembly of enzyme with nanosized COFs in aqueous solution at room temperature. By assembly of 1,3,5-tris(4-aminophenyl) benzene (TPB) and 2,5-dimethoxyterephthalaldehyde (DMTP) with the target enzymes, a series of nanoscale enzyme@COF materials (enzyme = ribonuclease A, pancreatin, GOx, lipase, lysozyme, HRP, trypsin, and urease) were successfully prepared under ambient conditions (water and room temperature) (Scheme 1). The robust catalytic activity demonstrated by enzyme@COF materials, along with their stability across different pH conditions, suggests their potential as intracellular catalytic agents. Also, we synthesized a dual-enzyme-loaded COF using this in situ assembly method, followed by coating it with MnO2 to create GOx&HRP@COF@MnO2. Once this multifunctional hybrid nanoagent enters the cell, the MnO2 shell consumes GSH, leading to the production of Mn2+. Simultaneously, the encapsulated GOx promotes the endogenous glucose oxidation to afford H2O2 and gluconic acid. These generated compounds, however, are the raw materials for HRP-mediated cascade enzyme catalysis and the Mn2+-initiated Fenton-like reaction. Ultimately, this cascade reaction leads to the production of more cytotoxic ˙OH radicals, consequently, greatly enhancing the therapeutic efficacy against cancer.
Results and discussion
Fabrication of enzyme@COF
Due to the intrinsic nature of enzymes, the safe and efficient in situ enzyme loading usually requires that the carriers can be synthesized in water at ambient temperature. In addition, for meeting the application requirements in nanomedicine, the size of carriers should be within the nanoscale range. After screening, we found that the nanoscale COF (Fig. S1, ESI†)27–29 could be readily prepared in water at room temperature with the aid of glacial acetic acid. Moreover, the obtained COF particles are at the nanoscale level (diameter of ∼188 nm), which is further confirmed by the scanning electron microscopy (SEM) image (Fig. 1a) and dynamic light scattering (DLS) analysis (Fig. 1b). Of note, this COF from the aqueous phase is highly crystalline and features an AA-stacked 2D network, which was the same as the COF previously reported by us and it was then prepared in an organic phase with the aid of polyvinylpyrrolidone (PVP).27,28To demonstrate the proof-of-concept, we employed HRP as a representative enzyme to illustrate the enzyme@COF synthesis. The successful construction of HRP@COF was well confirmed through powder X-ray diffraction (PXRD) analysis. As shown in Fig. 1c, the structure and crystallinity of COF were well maintained after the in situ HRP loading (COF: 2θ = 2.71, 4.72, 5.52, 7.32, 9.64, and 25.30°, HRP@COF: 2θ = 2.71, 4.73, 5.53, 7.34, 9.67, and 25.28°). Additionally, the characteristic absorption band for HRP at 2939 cm−1 appeared in the Fourier transform infrared spectroscopy (FTIR) of HRP@COF further confirming the successful HRP encapsulation (Fig. 1d). Energy-dispersive X-ray spectroscopy (EDS) mapping demonstrated the presence of iron (Fe) within HRP@COF, corresponding to the iron ion found in HRP's heme group (Fe(III) in the ground state and involvement in highly oxidized intermediates during catalytic reactions) (Fig. 1e), providing additional evidence for the enzyme loading. The porosity of the HRP@COF was also evaluated via N2 adsorption–desorption measurement at 77 K (Fig. 1f). According to the Brunauer–Emmett–Teller (BET) model, the BET specific surface area (SBET) of the HRP@COF was significantly lower than that of COF (47.32 vs. 470.87 cm3 g−1) due to the HRP loading.
To investigate the distribution of HRP within the COF matrix, fluorescent molecular rhodamine B (RhB) was utilized to label the HRP by synthesis of RhB–HRP@COF (Fig. 1g). As is shown, confocal laser scanning microscopy (CLSM) revealed that the RhB–HRP (red) species evenly distributed in the COF matrix. The maximum HRP loading amount in HRP@COF was up to 23.1 wt% (determined by the fluorescence standard curve assay of enzyme concentration before and after the reaction) (Fig. S2, ESI†), which is well consistent with the result obtained from thermogravimetric analysis (TGA) (Fig. 1h). The loading efficiency surpassed the highest reported HRP encapsulation system (14.4 wt%).30 These compelling evidences demonstrated that the COF-based in situ enzyme loading strategy is a powerful and efficient approach for HRP loading within COFs.
To demonstrate the generality of the synthetic strategy, the in situ enzyme loading approach was applied to various enzymes. To our delight, this method is versatile and all the selected enzymes, including ribonuclease A, lipase, lysozyme, GOx, trypsin, pancreatin, and urease, were successfully incorporated into COFs under the same conditions. The successful enzyme immobilization was unequivocally confirmed by comprehensive characterization techniques, including PXRD (Fig. 1i), zeta potentials (Fig. 1j), TEM (Fig. S3, ESI†), SEM (Fig. S4, ESI†), FT-IR (Fig. S5, ESI†), N2 sorption isotherm (Fig. S6, ESI†), and TGA curves (Fig. S7, ESI†). The maximum enzyme loading amounts in COF were in the range of 23.1–39.3 wt% (Table S1, ESI†). Compared with other reported COF-based enzyme immobilization methods,31 higher enzyme loading was observed herein.
Catalytic activities of enzyme@COF
As we know, enzyme immobilization often results in their activity loss due to the denaturation or mass transfer limitation within the solid carriers. To demonstrate the effectiveness of the in situ self-assembly approach, we chose GOx@COF and HRP@COF as the representative examples to evaluate their catalytic activity.For GOx@COF, the GOx-mediated glucose decomposition was selected as the model reaction to examine its biocatalytic activity. It is known that GOx can convert glucose into gluconic acid and produces H2O2 at the same time (Fig. 2a). The experimental results showed that a noticeable pH decrease caused by the generated gluconic acid was observed (Fig. 2b). Meanwhile, the generated H2O2 was also detected using a H2O2 testing kit, indicating that the GOx activity was well maintained after encapsulation by COF (Fig. 2c and d). The immobilized GOx showed a comparable catalytic activity to that of free enzyme (Fig. 2e). The recyclability of GOx@COF was also examined, and it could be reused for at least five times, giving a relative activity of ca. 82% at the fifth catalytic run (Fig. 2f). So, GOx@COF is a typical heterogeneous catalyst with excellent reusability.
For HRP@COF, its catalytic activity was evaluated by monitoring the oxidation of the chromogenic substrate 3,3,5,5-tetramethylbenzidine (TMB) at a wavelength of 652 nm, which can produce a detectable blue colour in the presence of H2O2 (Fig. 2g). After incubation of HRP@COF with H2O2 (0.1 mM) and TMB (1 mM) in PBS at 37 °C for 12 min, a significant increase in absorbance at 652 nm was observed. In contrast, the absorbance remained nearly constant over time in the control groups (TMB and TMB + H2O2 groups, Fig. 2h). Additionally, the catalytic activity of HRP@COF was concentration-dependent (Fig. 2i), and its catalytic activity was nearly identical to that of the free HRP enzyme (Fig. 2j). Also, HRP@COF is stable and could be reused, and its relative catalytic activity was up to ca. 81% after five runs (Fig. 2k).
For comparing the catalytic efficiency between natural enzymes and nanozymes, a systematic study on the enzyme catalysis kinetics of HRP@COF and GOx@COF was also carried out. The maximum reaction velocity (Vmax) and Michaelis–Menten constant (Km), which are key parameters for evaluating the catalytic ability of nanozymes, were obtained through steady-state catalytic kinetics tests.32 For both HRP@COF and GOx@COF, their calculated Km and Vmax values closely resembled those of their respective natural enzymes. HRP@COF had a Vmax of 8.73 × 10−8 M s−1 and Km of 3.19 mM, similar to natural horseradish peroxidase (Vmax = 8.56 × 10−8 M s−1; Km = 3.05 mM),33 while GOx@COF had a Vmax of 2.77 × 10−8 M s−1 and Km of 3.99 mM, comparable to natural glucose oxidase (Vmax = 2.64 × 10−8 M s−1; Km = 3.70 mM).34 This indicates that both HRP@COF and GOx@COF exhibit ultrafast affinities and catalytic kinetics towards their respective substrates, as shown in Fig. S8, ESI.† Overall, these results clearly demonstrate that the inherent catalytic properties of enzymes are well preserved within the COF matrix.
The protective effect of the COF framework was then evaluated by measuring the enzyme catalytic activity under various harsh conditions (Fig. S9, ESI†). For comparison, two additional enzyme-loaded systems were synthesized: HRP@ZIF-8 (Fig. S10, ESI†)28 and HRP-COF (Fig. S11, ESI†). The results suggest that HRP@COF maintained a higher relative activity even when exposed to adverse conditions such as trypsin exposure, high temperature, and organic solvent. This enhanced performance is likely attributed to the inherent protective properties of the COF framework, akin to MOF, as similar relative activity results were also observed in HRP@ZIF-8.
We also assessed the stability of GOx@COF (Fig. S12, ESI†) and HRP@COF (Fig. S13 and S14, ESI†) under acidic conditions using PBS solutions with pH levels of 5.0, 6.0, and 7.4. These results emphasize the significant stability of HRP@COF under physiological conditions, providing a solid foundation for future biological applications involving COFs.
Preparation and characterization of GOx&HRP@COF@MnO2
Encouraged by the above results, we proceeded to explore the dual enzyme (GOx and HRP)-loaded COF catalytic system for the cascade reaction. GOx&HRP@COF was synthesized by combining TPB with a DMTP aqueous solution containing GOx and HRP via in situ assembly (Fig. 3a). The successful synthesis of the dual-enzyme co-immobilized GOx&HRP@COF was confirmed by a series of characterization, including zeta potential (Fig. 3b), SEM (Fig. 3c), TEM (Fig. 3c), PXRD (Fig. 3d), FT-IR (Fig. 3e), XPS (Fig. 3f), and N2 sorption isotherm (Fig. 3g). Its catalytic performance was then examined (Fig. S15, ESI†). It was shown that an enhanced absorption intensity at 652 nm of the glucose/TMB aqueous solution was observed with the increase of GOx&HRP@COF concentration. This concentration-dependent process was accompanied by a deepening blue color (Fig. S16, ESI†). This indicated glucose was first oxidized to H2O2 by GOx, and then HRP promoted a H2O2-mediated TMB oxidation to provide the blue oxidation product of TMBox.To prove the potential application of GOx&HRP@COF in cancer treatment, it was further treated with potassium permanganate (KMnO4) under ultrasonic conditions to generate GOx&HRP@COF@MnO2 (Fig. 3a, the detailed synthetic process is shown in the ESI†).35 After coating with MnO2, its zeta potential (Fig. 3b) shifted from −20.4 to −17.2 mV, and the particle size increased from 300 to 320 nm accompanied by surface roughening (Fig. 3c), but its PXRD pattern was intact (Fig. 3d). Additionally, the characteristic absorption band for Mn–O at 471 cm−1 appeared in the FTIR (Fig. 3e), which provided further evidence for the formation of the MnO2 shell. XPS analysis (Fig. 3f and Fig. S17, ESI†) revealed that the binding energies of Mn 2p1/2 and Mn 2p3/2 are 654.3 and 642.8 eV, respectively, indicating that the Mn species in GOx&HRP@COF@MnO2 is tetravalent.36 The surface area of GOx&HRP@COF@MnO2 was determined to be ca. 78.63 cm3 g−1 on the basis of the BET model (Fig. 3g).
FITC-labelled GOx and RhB-labelled HRP were used for enzyme labelling, and fluorescence spectrophotometry (Fig. S18, ESI†) revealed loading capacities of 8.61% for GOx and 7.26% for HRP within GOx&HRP@COF@MnO2. These findings closely matched the results obtained from TGA analysis (Fig. S19, ESI†). For comparison, we also synthesized COF@MnO2 systems (Fig. S20, ESI†).37
MnO2 is known for its ability to oxidize GSH, and the resulting Mn2+ can further catalyze the Fenton-like reaction to convert H2O2 into hydroxyl radicals (˙OH) in acid microenvironments.38 It was different from the GOx&HRP@COF case, the GSH solution exhibited a reduced fluorescence at 525 nm after incubating with FITC-labeled GOx&HRP@COF@MnO2 (Fig. 3h), likely due to the fluorescence shielding effect of the MnO2 shell.39 However, the fluorescence significantly recovered after incubation with GSH (0.5 mM), indicating that the reaction between MnO2 and GSH occurred. Notably, the fluorescent maximum was achieved in pH 5.0 GSH solution after 15 min (Fig. 3i), suggesting that the MnO2 shell was almost completely decomposed by GSH under the given conditions. Then, the solution was subjected to incubation with TMB and glucose. With the increase of GOx&HRP@COF@MnO2 concentration, the absorption intensity at 652 nm increased with a deepening of the blue color (Fig. 3j), suggesting ˙OH generation. Of note, GOx&HRP@COF@MnO2 displayed a greater ˙OH generating ability than those of COF@MnO2 and GOx&HRP@COF under the given conditions (Fig. 3k and Fig. S21 ESI†). This result clearly demonstrates the synergistic effect of the integrated GOx and HRP enzymes with the MnO2 shell in the cascade catalytic process. These findings were further corroborated by the MB detection method (Fig. S22, ESI†).
In vitro antitumor efficacy of Gox&HRP@COF@MnO2
For biological application, the relevant aspects have been comprehensively investigated to ensure the stability and safety of GOx&HRP@COF@MnO2 particles in biological systems. For the evaluation of colloidal stability, especially under physiological conditions, the stability of GOx&HRP@COF@MnO2 in PBS and blood dispersions was examined. The Tyndall effect, which is a reliable indicator of colloidal stability, was still clearly observable in both the PBS and blood dispersions of GOx&HRP@COF@MnO2 even after 24 hours (Fig. S23, ESI†). This result strongly indicates the good colloidal stability of the particles under physiological-like conditions, suggesting their potential for in vivo applications. Encouraged by the excellent catalytic performance of GOx&HRP@COF@MnO2, we further investigated its antitumor activity at the cellular level. As shown in Fig. 4a, a noticeable green fluorescence of FITC-GOx&HRP@COF@MnO2 appeared in cellular cytoplasm as the incubation time increased, indicating the successful endocytosis of FITC-GOx&HRP@COF@MnO2 by 4T1 cells. Quantitative analysis by ImageJ software showed that the cellular uptake efficiency of FITC-GOx&HRP@COF@MnO2 provided a 4.7-fold increase in fluorescence within 4 h compared to the control group without any treatment.To clarify the intracellular distribution of GOx&HRP@COF@MnO2, co-localization experiments were conducted using FITC-labelled GOx&HRP@COF@MnO2 and lysosome/mitochondria specific fluorescent indicators in 4T1 cells. As is shown in Fig. 4b, the green fluorescence of FITC-labelled GOx&HRP@COF@MnO2 was well overlaid with the red fluorescence of the lysosome tracker under 2 h incubation, indicating the rapid endocytosis of GOx&HRP@COF@MnO2. However, the colocation degree decreased at 4 h, suggesting that GOx&HRP@COF@MnO2 escaped from the lysosomes. Furthermore, intracellular mitochondrial localization images revealed a notable overlap between the red fluorescence of mitochondria and the green fluorescence of GOx&HRP@COF@MnO2 after co-incubation for 4 h, implying its continuous intracellular diffusion behavior (Fig. 4c). Then, the underlying mechanisms of the mitochondrial targeting properties of GOx&HRP@COF@MnO2 have been explored. Research indicates that the mitochondrial membrane potential of cancer cells is significantly higher than that of normal cells (approximately −220 mV vs. approximately −140 mV).40 This implies that mitochondrial-targeting groups with positive charge and lipophilicity can be preferentially enriched in the mitochondria of cancer cells.41 Consequently, the change in the zeta potential of GOx&HRP@COF@MnO2 over time in the presence of GSH has been measured (Fig. S24, ESI†). These results demonstrate that the generation of cationic Mn2+ endows GOx&HRP@COF@MnO2 with a large, dispersed positive charge, enabling effective mitochondrial targeting.
The excellent biocompatibility of GOx&HRP@COF@MnO2 was confirmed using the methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay (Fig. S25, ESI†). The results showed that the cell viability of HL-7702 cells, a normal cell line with low-expressed GSH, remained above 80% even after incubating with GOx&HRP@COF@MnO2 at a high concentration of 400 μg mL−1. However, when 4T1 cells with over-expressed GSH were incubated with different concentrations of GOx&HRP@COF@MnO2, significant cell death was observed. For example, 400 μg mL−1 of GOx&HRP@COF@MnO2 induced up to 80% cell death.
The cytotoxicity of GOx&HRP@COF@MnO2 prompted us to further study its mechanism of killing cancer cells, and DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)) was used to assess changes of the intracellular GSH levels under various treatments. After treatment with COF@MnO2 and GOx&HRP@COF@MnO2, the intracellular GSH content was significantly reduced by 0.37-fold and 0.41-fold, respectively, as compared with that in the control group (Fig. S26, ESI†). This observation suggests that the MnO2 component could be oxidated by GSH within 4T1 cells, resulting in the depletion of GSH. Additionally, the intracellular pH alteration was also tracked by a cytoplasmic pH fluorescence probe (BCECF, AM). After treatment with GOx&HRP@COF, a noticeable green fluorescence decrease was observed in cells (Fig. 4d), likely due to the generation of gluconic acid resulting from the enzymatic oxidation of endogenous glucose by GOx within GOx&HRP@COF. Interestingly, an 0.74-fold fluorescence decrease of the GOx&HRP@COF@MnO2 group was observed compared to that of the GOx&HRP@COF group. This may be attributed to the simultaneous decomposition of H2O2 by HRP and Mn2+, which, in turn, accelerated the oxidation rate of endogenous glucose by GOx.
Intracellular ROS generation was assessed using 2′,7′-dichlorofluorescein diacetate (DCFH-DA) as a fluorescent indicator.42 As shown in Fig. 4e, the green fluorescence intensity of the GOx&HRP@COF@MnO2 group was 1.48-fold and 1.05-fold stronger than COF@MnO2 and GOx&HRP@COF groups. This meant that GOx&HRP@COF@MnO2 was more efficient in intracellular ROS production, which is apparently caused by the synergistic effect between the Mn2+-induced Fenton-like reaction and the GOx/HRP-catalyzed cascade reaction. Indeed, MTT assays (Fig. S25, ESI†) showed that the cell death was up to ca. 80% for the GOx&HRP@COF@MnO2-treated group. In contrast, the cell death caused by COF@MnO2 and GOx&HRP@COF were only ca. 50% and 65%, respectively. These results collectively demonstrate the superior therapeutic efficacy of GOx&HRP@COF@MnO2, highlighting the advantages of cascade catalysis in cancer treatment.
Considering that mitochondrial function is closely related to ROS levels, we then assessed the mitochondrial membrane potential (MMP) using the fluorescent dye JC-1.43 In healthy mitochondria, JC-1 accumulated in the mitochondrial matrix, forming a polymer that emits strong red fluorescence when mitochondrial membrane potential collapsed, it existed as a cytoplasmic monomer, emitting green fluorescence. The results showed that the green fluorescence intensity in the GOx&HRP@COF@MnO2 group was 1.25-fold, 1.34-fold, and 1.54-fold higher than those in the GOx&HRP@COF, COF@MnO2, and PBS groups, respectively, while the red fluorescence intensity in the same group was 0.28-fold, 0.59-fold, and 0.64-fold lower (Fig. 4f), indicating the most severe mitochondrial dysfunction induced by GOx&HRP@COF@MnO2. Additionally, the decrease in MMP could lead to the downregulation of intracellular adenosine triphosphate (ATP) production, ultimately resulting in apoptosis. So, we further detected the change of intracellular ATP after different treatments using an ATP kit. As expected, the lowest ATP content was detected in the GOx&HRP@COF@MnO2 group (Fig. S27, ESI†).
In vivo antitumor efficacy
For investigating the in vivo anti-tumor effect, we conducted experiments on 4T1 tumor-bearing mice, dividing them randomly into five groups: Group I (PBS), Group II (COF), Group III (COF@MnO2), Group IV (GOx&HRP@COF), and Group V (GOx&HRP@COF@MnO2) (Fig. 5a). As shown in Fig. 5b and c, the tumor volume increased rapidly over time in the PBS and COF treatment groups. Partial suppression was observed in the COF@MnO2 and GOx&HRP@COF-treated groups. For the GOx&HRP@COF@MnO2 treatment group, however, tumor growth was significantly suppressed. This remarkable outcome can be attributed to the synergistic effect of the Mn2+-induced Fenton-like reaction and the cascade enzyme-catalysed reaction mediated by GOx and HRP, thereby enhancing the therapeutic efficacy of GOx&HRP@COF@MnO2. The trend in tumor weight within various groups further confirmed these results, with the GOx&HRP@COF@MnO2 group demonstrating the lowest average tumor weight (Fig. 5d). Additionally, the GOx&HRP@COF@MnO2 group demonstrated the longest survival time in comparison to the other groups, benefiting from its optimal therapeutic effectiveness (Fig. 5e). The results of tumor images stained with hematoxylin and eosin (H&E) in different mouse groups, as depicted in Fig. 5f, provide further support for the efficacy of GOx&HRP@COF@MnO2 as a highly effective tumor therapeutic agent. In the GOx&HRP@COF@MnO2 group, the most pronounced cell nucleus shrinkage and cytoplasmic leakage were observed.The GOx&HRP@COF@MnO2 not only successfully inhibited tumor growth but also kept the mice's body weight consistently stable throughout the entire treatment (Fig. S28, ESI†), suggesting its minimal toxicity to the mice. Additionally, the results of H&E staining did not reveal any significant tissue damage or inflammatory reactions in the major organs of the mice (Fig. S29, ESI†). The biosafety was further confirmed through a hemolysis test (Fig. S30, ESI†). Based on these comprehensive biocompatibility evaluations, the Mn content at 6.18% did not seem to have a negative impact on the biocompatibility of GOx&HRP@COF@MnO2 in the biological systems investigated (Fig. S31, ESI†). These findings collectively suggested that GOx&HRP@COF@MnO2 holds significant clinical potential as an efficient therapeutic agent.
Conclusions
In summary, we introduce a straightforward and highly effective method for immobilizing enzymes within nanoscale COFs through a simple in situ assembly process carried out under ambient conditions. This versatile approach can be utilized for immobilizing a wide range of enzymes. Furthermore, the effectiveness of this in situ assembly method in simultaneously immobilizing two enzymes is demonstrated. To prove this concept, representative enzymes GOx and HRP were encapsulated within the COF, resulting in the formation of GOx&HRP@COF, followed by a study of their cascade catalytic effect at the cellular level. To enhance its applicability in tumor therapy, MnO2 was introduced on the surface of GOx&HRP@COF to create GOx&HRP@COF@MnO2, and its significant therapeutic efficacy was validated through both in vitro and in vivo experiments.Author contributions
Wen-Xiu Ren and Qing-Yun Huang executed the experiments and conducted a comprehensive analysis of the data. Jie Feng was responsible for funding acquisition, writing – review & editing. Yue-Lu Tian was responsible for investigation. Ya-Ni Wu was responsible for investigation. Seeram Ramakrishna was responsible for supervision. Yu-Bin Dong was responsible for funding acquisition and supervision.Data availability
All the data supporting this article have been included in the main text and the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the NSFC (Grant No. 22371172 and 21971153), the Major Basic Research Projects of Shandong Provincial Natural Science Foundation (No. ZR2020ZD32), and the Taishan Scholars Climbing Program of Shandong Province, the Natural Science Foundation of Shandong Province (No. ZR202102280580). Feng J is thankful for the financial support from the China Scholarship Council (202108370157).References
- L. Zhang, H. Wang and X. Qu, Biosystem-Inspired Engineering of Nanozymes for Biomedical Applications, Adv. Mater., 2023, 36, 2211147 CrossRef PubMed.
- Y. Su, F. Wu, Q. Song, M. Wu, M. Mohammadniaei, T. Zhang, B. Liu, S. Wu, M. Zhang, A. Li and J. Shen, Dual Enzyme-Mimic Nanozyme Based on Single-atom Construction Strategy for Photothermal-Augmented Nanocatalytic Therapy in the Second Near-Infrared Biowindow, Biomaterials, 2022, 281, 121325 CrossRef CAS PubMed.
- S. Huang, G. Chen and G. Ouyang, Confining Enzymes in Porous Organic Frameworks: from Synthetic Strategy and Characterization to Healthcare Applications, Chem. Soc. Rev., 2022, 51, 6824–6863 RSC.
- X. Pei, Z. Luo, L. Qiao, Q. Xiao, P. Zhang, A. Wang and R. A. Sheldon, Putting Precision and Elegance in Enzyme Immobilisation with Bio-Orthogonal Chemistry, Chem. Soc. Rev., 2022, 51, 7281–7304 RSC.
- V. Aggarwal, S. Solanki and B. D. Malhotra, Applications of Metal–Organic Framework-Based Bioelectrodes, Chem. Sci., 2022, 13, 8727–8743 RSC.
- A. Chatterjee, A. Reja, S. Pal and D. Das, Systems Chemistry of Peptide-Assemblies for Biochemical Transformations, Chem. Soc. Rev., 2022, 51, 3047–3070 RSC.
- J. H. Kim, D. W. Kang, H. Yun, M. Kang, N. Singh, J. S. Kim and C. S. Hong, Correction: Post-Synthetic Modifications in Porous Organic Polymers for Biomedical and Related Applications, Chem. Soc. Rev., 2022, 51, 6864 RSC.
- L. Dong, C. Huang, B. Zhao, G. Hu, Y. Huang, X. Zhang, X. Hu, Y. Wang, X. Sun, W. Qian and G. Luo, A pH/Enzyme Dual Responsive PMB Spatiotemporal Release Hydrogel Promoting Chronic Wound Repair, J. Nanobiotechnol., 2023, 21, 213 CrossRef CAS PubMed.
- J. P. Gonçalves, D. Promlok, T. Ivanov, S. Tao, T. Rheinberger, S.-M. Jo, Y. Yu, R. Graf, M. Wagner, D. Crespy, F. R. Wurm, L. C. D. Silva, S. Jiang and K. Landfester, Confining the Sol-Gel Reaction at the Water/Oil Interface: Creating Compartmentalized Enzymatic Nano-Organelles for Artificial Cells, Angew. Chem., Int. Ed., 2023, 62, e202216966 CrossRef PubMed.
- M. Liu, L. Chen, Z. Zhao, M. Liu, T. Zhao, Y. Ma, Q. Zhou, Y. S. Ibrahim, A. A. Elzatahry, X. Li and D. Zhao, Enzyme-Based Mesoporous Nanomotors with Near-Infrared Optical Brakes, J. Am. Chem. Soc., 2022, 144, 3892–3901 CrossRef CAS PubMed.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O’Keeffe, A. J. Matzger and O. M. Yaghi, Porous and Crystalline, Covalent Organic Frameworks, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Covalent Organic Frameworks: Design, Synthesis, and Functions, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- Y. Zheng, S. Zhang, J. Guo, R. Shi, J. Yu, K. Li, N. Li, Z. Zhang and Y. Chen, Green and Scalable Fabrication of High-Performance Biocatalysts Using Covalent Organic Frameworks as Enzyme Carriers, Angew. Chem., Int. Ed., 2022, 61, e202208744 CrossRef CAS PubMed.
- H. Chao, Z. Zhou, W. He, M. Li, X. Yuan, P. Su, J. Song and Y. Yang, Template-Free in Situ Encapsulation of Enzymes in Hollow Covalent Organic Framework Capsules for the Electrochemical Analysis of Biomarkers, ACS Appl. Mater. Interfaces, 2022, 14, 20641–20651 CrossRef CAS PubMed.
- Q. Guan, G.-B. Wang, L.-L. Zhou, W.-Y. Li and Y.-B. Dong, Nanoscale Covalent Organic Frameworks as Theranostic Platforms for Oncotherapy: Synthesis, Functionalization, and Applications, Nanoscale Adv., 2020, 2, 3656–3733 RSC.
- S. Khalil, M. D. Meyer, A. Alazmi, M. H. K. Samani, P.-C. Huang, M. Barnes, A. B. Marciel and R. Verduzcoo, Enabling Solution Processable COFs through Suppression of Precipitation during Solvothermal Synthesis, ACS Nano, 2022, 16, 20964–20974 CrossRef CAS PubMed.
- A. C. Daly, L. Riley, T. Segura and J. A. Burdick, Hydrogel Microparticles for Biomedical Applications, Nat. Rev. Mater., 2020, 5, 20–43 CrossRef CAS PubMed.
- S. Gou, G. Wang, Y. Zou, W. Geng, T. He, Z. Qin, L. Che, Q. Feng and K. Cai, Non-Pore Dependent and MMP-9 Responsive Gelatin/Silk Fibroin Composite Microparticles as Universal Delivery Platform for Inhaled Treatment of Lung Cancer, Adv. Mater., 2023, 35, 2303718 CrossRef CAS PubMed.
- W. Zhang, S. Xiang, Y. Han, H. Wang, Y. Deng, P. Bian, Y. Bando, D. Golberg and Q. Weng, Phospholipid-Inspired Alkoxylation Induces Crystallization and Cellular Uptake of Luminescent COF Nanocarriers, Biomaterials, 2024, 306, 122503 CrossRef CAS PubMed.
- N. Singh, J. Kim, J. Kim, K. Lee, Z. Zunbul, I. Lee, E. Kim, S.-G. Chi and J. S. Kim, Covalent Organic Framework Nanomedicines: Biocompatibility for Advanced Nanocarriers and Cancer Theranostics Applications, Bioact. Mater., 2023, 21, 358–380 Search PubMed.
- M. Xu, Y. Qi, G. Liu, Y. Song, X. Jiang and B. Du, Size-Dependent In Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance, ACS Nano, 2023, 17, 20825–20849 CrossRef PubMed.
- L. Zhang, S. Wang, Y. Zhou, C. Wang, X.-Z. Zhang and H. Deng, Covalent Organic Frameworks as Favorable Constructs for Photodynamic Therapy, Angew. Chem., Int. Ed., 2019, 58, 14213–14218 CrossRef CAS PubMed.
- P. Lin, H. Dinh, Y. Morita, E. Nakata and T. Morii, Dynamic Assembly of Cascade Enzymes by the Shape Transformation of a DNA Scaffold, Adv. Funct. Mater., 2023, 33, 2215023 CrossRef CAS.
- C. Yang, W. Liu, S. Chen, X. Zong, P. Yuan, X. Chen, X. Li, Y. Li, W. Xue and J. Dai, MOF-Immobilized Two-in-One Engineered Enzymes Enhancing Activity of Biocatalytic Cascade for Tumor Therapy, Adv. Healthcare Mater., 2023, 12, 2203035 CrossRef CAS PubMed.
- J. Ye, J. Lu and D. Wen, Engineering Carbon Nanomaterials Toward High-Efficiency Bioelectrocatalysis for Enzymatic Biofuel Cells: a Review, Mater. Chem. Front., 2023, 7, 5806–5825 RSC.
- E. R. Moody, R. Obexer, F. Nickl, R. Spiess and S. L. Lovelock, An Enzyme Cascade Enables Production of Therapeutic Oligonucleotides in a Single Operation, Science, 2023, 380, 1150–1154 CrossRef CAS PubMed.
- H. Xu, J. Gao and D. Jiang, Stable, Crystalline, Porous, Covalent Organic Frameworks as a Platform for Chiral Organocatalysts, Nat. Chem., 2015, 7, 905–912 CrossRef CAS PubMed.
- J. Feng, W.-X. Ren, F. Kong, C. Zhang and Y.-B. Dong, Nanoscale Covalent Organic Framework for the Low-Temperature Treatment of Tumor Growth and Lung metastasis, Sci. China Mater., 2022, 65, 1122–1133 CrossRef CAS.
- Q. Guan, L.-L. Zhou, Y.-N. Li, W.-Y. Li, S. Wang, C. Song and Y.-B. Dong, Nanoscale Covalent Organic Framework for Combinatorial Antitumor Photodynamic and Photothermal Therapy, ACS Nano, 2019, 13, 13304–13316 CrossRef CAS PubMed.
- G. Chen, S. Huang, X. Kou, S. Wei, S. Huang, S. Jiang, J. Shen, F. Zhu and G. Ouyang, A Convenient and Versatile Amino-Acid-Boosted Biomimetic Strategy for the Nondestructive Encapsulation of Biomacromolecules within Metal–Organic Frameworks, Angew. Chem., Int. Ed., 2019, 58, 1463–1467 CrossRef CAS PubMed.
- Y. Zhang, C. Xing, Z. Mu, Z. Niu, X. Feng, Y. Zhang and B. Wang, Harnessing Self-Repairing and Crystallization Processes for Effective Enzyme Encapsulation in Covalent Organic Frameworks, J. Am. Chem. Soc., 2023, 145, 13469–13475 CrossRef CAS PubMed.
- Z. Li, F. Liu, C. Chen, Y. Jiang, P. Ni, N. Song, Y. Hu, S. Xi, M. Liang and Y. Lu, Regulating the N Coordination Environment of Co Single-Atom Nanozymes for Highly Efficient Oxidase Mimics, Nano Lett., 2023, 23, 1505 CrossRef CAS PubMed.
- Y. Qiu, B. Yuan, H. Mi, J.-H. Lee, S.-W. Chou and Y.-K. Peng, An Atomic Insight into the Confusion on the Activity of Fe3O4 Nanoparticles as Peroxidase Mimetics and Their Comparison with Horseradish Peroxidase, J. Phys. Chem. Lett., 2022, 13, 8872–8878 CrossRef CAS PubMed.
- Y. Wang, X. Cao, S. Jiang, L. Gao, X. Han, J. Qu, X. Jiang, G. Liu and Y. Qu, Engineering the Substrate Preference of Glucose Oxidase for the Enzymatic Oxidation of Xylose, Green Chem., 2024, 26, 4851–4859 RSC.
- J. Hu, F. Wang, F. Liu, W. Sun, Q. Jiang, Y. Liu, Y. Zhao and X. Liu, Effective Nanotherapeutic Approach for Metastatic Breast Cancer Treatment by Supplemental Oxygenation and Imaging-Guided Phototherapy, Nano Res., 2020, 13, 1111–1121 CrossRef CAS.
- Y. Wu, D. Li, F. Zhou, H. Liang, Y. Liu, W. Hou, Q. Yuan, X. Zhang and W. Tan, Versatile in Situ Synthesis of MnO2 Nanolayers on Upconversion Nanoparticles and Their Application in Activatable Fluorescence and MRI Imaging, Chem. Sci., 2018, 9, 5427–5434 RSC.
- Z. Lu, S. Bai, Y. Jiang, S. Wu, D. Xu, Y. Chen, Y. Lan, Y. An, J. Mao, X. Liu and G. Liu, Porphyrin-Based Covalent Organic Framework for Imaging Guided Cancer Combinatorial Immuno-Sonodynamic Therapy, Adv. Funct. Mater., 2022, 32, 2207749 CrossRef CAS.
- S. Tang, L. Zhou, H. He, L. Cui, Z. Ren, Y. Tai, Z. Xie, Y. Cao, D. Meng, Q. Liu, Y. Wu, J. Jiang and X. Zhou, MnO2-Melittin Nanoparticles Serve as an Effective Anti-Tumor Immunotherapy by Enhancing Systemic Immune Response, Biomaterials, 2022, 288, 121706 CrossRef CAS PubMed.
- Y. Liu, C. S. Gong, L. Lin, Z. Zhou, Y. Liu, Z. Yang, Z. Shen, G. Yu, Z. Wang, S. Wang, Y. Ma, W. Fan, L. He, G. Niu, Y. Dai and X. Chen, Core-Shell Metal-Organic Frameworks with Fluorescence Switch to Trigger an Enhanced Photodynamic Therapy, Theranostics, 2019, 9, 2791–2799 CrossRef CAS PubMed.
- V. R. Fantin, J. St-Pierre and P. Leder, Attenuation of LDH-A Expression Uncovers a Link between Glycolysis, Mitochondrial Physiology, and Tumor Maintenance, Cancer Cell, 2006, 9, 425–434 CrossRef CAS PubMed.
- W. Zhen, Y. Fan, T. Germanas, L. Tillman, J. Li, A. L. Blenko, R. R. Weichselbaum and W. Lin, Digitonin-Loaded Nanoscale Metal-Organic Framework for Mitochondria-Targeted Radiotherapy-Radiodynamic Therapy and Disulfidptosis, Adv. Mater., 2024, 2405494 CrossRef PubMed.
- J. Huang, G. Deng, S. Wang, T. Zhao, Q. Chen, Y. Yang, Y. Yang, J. Zhang, Y. Nan, Z. Liu, K. Cao, Q. Huang and K. Ai, A NIR-II Photoactivatable “ROS Bomb” with High-Density Cu2O-Supported MoS2 Nanoflowers for Anticancer Therapy, Adv. Sci., 2023, 10, 2302208 CrossRef CAS PubMed.
- X. Pan, S. Cui, S. Fan, C. Cao, Y. Jiao, Y. Fu, J. Niu, S. Lin, Y. Zhu and Y. Liu, Au-Based Conjugated Microporous Polymers for Combined Photodynamic and Radiation Therapy in Cancer Treatment, Mater. Chem. Front., 2024, 8, 3925–3934 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm01129e |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2025 |