Boosting fluorescence efficiency of NIR-II dyes for multifunctional fluorescence imaging via hydrogen bonding†
Liangyu
Zheng
a,
Weidan
Na
*b,
Fan
Gao
 a and
Changjin
Ou
a and
Changjin
Ou
 *a
*a
aInstitute of Advanced Materials and Flexible Electronics (IAMFE), School of Chemistry and Materials Science, Nanjing University of Information Science and Technology, Nanjing, JiangSu 210044, China. E-mail: ocj1987@163.com
bCollege of Chemistry and Chemical Engineering, Xuzhou University of Technology, Xuzhou, JiangSu 221111, China. E-mail: wdna@xzit.edu.cn
First published on 26th March 2025
Abstract
Donor–acceptor–donor (D–A–D) type fluorophores with a planar conformation hold great promise for second near-infrared (NIR-II) fluorescence imaging due to their enhanced light absorption and red-shifted absorption/emission peaks. However, achieving high fluorescence efficiency remains challenging because of severe fluorescence quenching in the aggregate state. Herein, five 6,7-diphenyl-[1,2,5]thiadiazoloquinoxaline-based NIR-II dyes (TP-TQ1, TP-OH, TP-OMe, TP-F and TP-Acr) were synthesized by modifying the acceptor core with various substituents to create planar π-conjugated D–A–D structures. We systematically investigated the influence of the substituent effect on the dye's band gap, molecular conformation, absorption/emission wavelengths, fluorescence efficiency, and aggregation behaviors. The results indicate that hydroxyl-substituted TP-OH nanoparticles (NPs) possess the highest NIR absorption ability and fluorescence brightness. This is attributed to intermolecular hydrogen bonding, which effectively suppresses π–π stacking. Furthermore, the steric hindrance of substituents plays a prominent role in limiting the intramolecular potential energy. In vivo experiments demonstrated the potential of TP-OH NPs as NIR-II fluorescent contrast agents for gastrointestinal tract imaging, vascular imaging, and navigation of lymph node dissection. These findings suggest that hydrogen bonding functionalization on the acceptor offers a valuable strategy for significantly enhancing the NIR-II fluorescence performance of planarized D–A–D fluorophores.
Introduction
Fluorescence imaging technology is versatile technology widely used in monitoring physiological and pathological processes, as well as in imaging-guided surgery and therapeutics due to its advantages of non-invasive nature, low radiation, minimal toxicity and real-time feedback.1–5 Compared with fluorescence imaging in the visible and near-infrared I regions, second near-infrared (NIR-II, 1000–1700 nm) fluorescence imaging offers several advantages, including reduced autofluorescence and suppressed photon scattering. This makes it an ideal tool for clinical diagnosis and surgical navigation, providing high resolution and deep tissue penetration.6–11 Organic NIR-II fluorophores have attracted considerable research interest due to low biotoxicity, high biocompatibility, rapid metabolism, and easy modifiability.3,11–13 However, typical cyanine and (aza)BODIPY dyes often suffer from low quantum yield (QY), small Stokes shifts, short emission wavelengths, and chemical instability.5,14–19 Consequently, researchers have focused on designing and synthesizing NIR-II fluorescent dyes with a D–A–D skeleton based on benzothiadiazole and its derivatives as acceptors, and a diverse array of NIR-II dyes have been established for biomedical structural imaging and functional imaging, and biosensing probes.20–35The imaging quality of fluorescent dyes is influenced by factors such as their maximum absorption/emission wavelengths, QY, and molar absorption coefficient (ε).36–39 Studies have demonstrated that molecular structure planarization can extend the absorption/emission wavelengths and enhance photon absorption.40–42 Nevertheless, those planar-structured D–A–D type fluorophores were subjected to severe aggregation-caused quenching effects (ACQ), leading to low QY in the aggregated state due to intermolecular π–π stacking.31,43–46 Therefore, exploring the structure–property relationships is a vital approach to improving the QY of dyes with planar π-conjugated systems. While previous research has primarily concentrated on donor engineering, the influence of the acceptor core on NIR-II dyes is equally crucial.47–51 Given the huge challenges in structure modification of benzobisthiadiazole and selenadiazolo[3,4-f]benzo[c][1,2,5]thiadiazole, the thiadiazoloquinoxaline (TQ) acceptor unit offers significant potential for modification, allowing for the study of how acceptor modification impacts the performance of fluorescent dyes. Based on the TQ unit, both extending the conjugation of the acceptor and inserting heterocycles into rigid planar cores can enhance the electron-delocalization effect, shorten the bandgap, and promote bathochromic shifts.52–54 Moreover, incorporating electron-withdrawing groups into the TQ unit could affect the pathway of non-radiative energy transition by adjusting molecular planarity, consequently enhancing fluorescence performance or photothermal effects.55–57 Besides, some TQ-based fluorophore acceptors have been modified with alkoxy groups to shield the core from polar environments, decreasing the non-radiative energy transfer from the excited state to the surrounding water molecules.57–59 Thus, the electronic structure and supramolecular interactions are of importance to promote the emission performance of NIR-II dyes. Therefore, exploring the effect of substituents on acceptors from the perspective of dye fluorescence properties holds profound significance for improving the fluorescence performance of fluorophores with planar structures.
Previously, we developed a NIR-II dye, TP-TQ1, based on 6,7-diphenyl-[1,2,5]thiadiazoloquinoxaline utilizing a molecular planarization strategy, and the resulting TP-TQ1 nanoparticles exhibited strong absorption in the NIR region and a high QY, rendering this dye suitable for fluorescence imaging-guided phototherapy.60 It is well established that the photophysical properties of organic fluorophores are influenced by molecular aggregation behaviors, which is highly susceptible to supramolecular interactions. Herein, to further explore the substituent effect on photophysical properties of the NIR-II dye, using TP-TQ1 as the model molecule, four novel NIR-II fluorophores named TP-OH, TP-OMe, TP-F, and TP-Acr were synthesized by introducing hydroxyl, methoxy, fluoro, and acrylate groups into the benzene ring of 6,7-diphenyl-[1,2,5]thiadiazoloquinoxaline (Scheme 1). Theoretical calculations indicate that all designed fluorophores possess quasi-planar molecular conformations with dihedral angles less than 20°, and the energy gaps are narrowed to ∼1.4 eV. The dyes exhibited significant differences in absorption and emission spectra, and the maximum absorption peaks of TP-OH, TP-OMe, TP-F, and TP-Acr NPs were located at 843 nm, 861 nm, 911 nm, and 877 nm, respectively. Thanks to intermolecular hydrogen bonding suppressing π–π stacking, TP-OH NPs exhibited a high relative QY of up to 1.05% and bright NIR-II fluorescence (ε × QY: ∼305 M−1 cm−1), along with good stability. In vivo experiments evidenced that TP-OH NPs possessed the potential to serve as multifunctional contrast agents for angiography, lymphatic imaging and gastrointestinal imaging (Scheme 1).
Results and discussion
Molecular design and synthesis
Our previous work established that the 6,7-diphenyl-[1,2,5]thiadiazoloquinoxaline unit (TQ1) is an effective acceptor for constructing NIR-II fluorophores, with TP-TQ1 NPs exhibiting a large ε value and highly bright fluorescence.60 However, because of the ACQ effect via intermolecular π–π stacking in the condensed phase, it is necessary to improve the fluorescence efficiency of TQ-based dyes. To further clarify the substituent effect of acceptors on dye performance, we synthesized four new NIR-II dyes (TP-OH, TP-OMe, TP-F, and TP-Acr, Fig. 1a) by introducing different substituents (–F, –OH, –OMe, and acryloyloxy) at the para positions of the two phenyl rings on TQ1. The synthetic routes for these dyes are presented in Scheme S1 (ESI†). Initially, dinitro-containing compound 1 underwent nitro-reduction using zinc powder as the reducing agent, yielding diamino-substituted compound 2. Subsequently, dehydration condensation between compound 2 and substituted benzils produced TP-OH, TP-OMe, and TP-F. Finally, TP-OH was subjected to an esterification reaction with acryloyl chloride to synthesize TP-Acr. The chemical structures of the target compounds were confirmed by nuclear magnetic resonance and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Fig. S1–S13, ESI†).Theoretical calculations
To gain detailed insights into the ground-state molecular conformation of the fluorophores, density functional theory (DFT) calculations were performed using the Gaussian 09 program at the B3LYP/6-31G(d,p) basis set. The resulting dihedral angles between donors and acceptor were 19.8°/11.0° for TP-F, 14.8°/18.5° for TP-Acr, 16.3°/14.3° for TP-OH, and 16.5°/16.1° for TP-OMe (Fig. 1b). Compared to TP-TQ1 (4.0°/4.0°), the introduction of substituents on the acceptor slightly disrupted the planarity of the molecule, while the four dyes still maintained a quasi-planar conformation.60 The corresponding highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO) are depicted in Fig. 1c. The HOMO delocalized over the whole π-conjugated backbones for four NIR-II dyes, while the LUMO mainly located on the acceptor cores, the spatial separation of HOMO and LUMO suggests a strong intramolecular charge-transfer effect within these dyes. Furthermore, the modification of the benzene ring with electron-withdrawing groups induced delocalization of the LUMO over the acceptor unit, resulting in a slight decrease of the LUMO from −3.165 eV for TP-TQ1 to −3.241 eV for TP-F. In contrast, electron-donating groups led to an increase of the LUMO to −3.171 eV for TP-Acr, −3.094 eV for TP-OH, and −3.067 eV for TP-OMe. Consequently, the energy gaps were 1.384, 1.394, 1.429 and 1.442 eV for TP-F, TP-Acr, TP-OH and TP-OMe, respectively. The relatively small change in the energy gap among these dyes (<0.06 eV) suggests that substituent modification has a limited effect on absorption and emission in the molecular state.Photophysical properties of NIR-II dyes
To endow the dyes with good water solubility, TP-OH, TP-OMe, TP-F and TP-Acr were formulated into water-dispersible NPs by the nanoprecipitation method using amphiphilic copolymer Pluronic F127 as an encapsulation matrix. Dynamic light scattering results revealed that the average particle diameters of TP-OH, TP-OMe, TP-F and TP-Acr NPs were 206.1, 189.2, 192.4 and 150.4 nm, respectively (Fig. S14, ESI†). Transmission electron microscopy showed that TP-OH NPs are spherical structures (Fig. S15, ESI†).Absorption and fluorescence spectra were recorded to investigate the photophysical properties of the dyes. As shown in Fig. 2a and b and summarized in Table 1, the absorption maxima of TP-OH, TP-OMe, TP-F, TP-Acr and TP-TQ1 in toluene were 842, 838, 857, 849 and 858 nm with a molar extinction coefficients (ε) of 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622, 31
622, 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198, 21
198, 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 838, 9055 and 26
838, 9055 and 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 409 L mol−1 cm−1, respectively. As expected, incorporating electron-donating groups into the acceptor resulted in a blue shift of the dyes' maximum absorption peaks in toluene. The maximum absorption peaks were at 843, 861, 911, 877 and 877 nm for TP-OH, TP-OMe, TP-F, TP-Acr and TP-TQ1 NPs, respectively. From solution to nanoaggregate, the absorption spectra of all dyes except for TP-OH exhibited a significant red shift. This was mainly because the hydrogen bonds between TP-OH molecules suppressed intermolecular π–π stacking, while other dyes exhibited severe aggregation phenomena. The nature of the noncovalent interaction in the dimer of TP-OH molecules was studied using the independent gradient model (IGM) method using Multiwfn software, the visualization of IGM and orbitals was achieved by visual molecular dynamics. As illustrated in Fig. 2c, theoretical calculations clearly indicated the presence of hydrogen bonds between the two TP-OH monomers. In addition, the ε values decreased to 29
409 L mol−1 cm−1, respectively. As expected, incorporating electron-donating groups into the acceptor resulted in a blue shift of the dyes' maximum absorption peaks in toluene. The maximum absorption peaks were at 843, 861, 911, 877 and 877 nm for TP-OH, TP-OMe, TP-F, TP-Acr and TP-TQ1 NPs, respectively. From solution to nanoaggregate, the absorption spectra of all dyes except for TP-OH exhibited a significant red shift. This was mainly because the hydrogen bonds between TP-OH molecules suppressed intermolecular π–π stacking, while other dyes exhibited severe aggregation phenomena. The nature of the noncovalent interaction in the dimer of TP-OH molecules was studied using the independent gradient model (IGM) method using Multiwfn software, the visualization of IGM and orbitals was achieved by visual molecular dynamics. As illustrated in Fig. 2c, theoretical calculations clearly indicated the presence of hydrogen bonds between the two TP-OH monomers. In addition, the ε values decreased to 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 077, 16
077, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497, 18
497, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515, 5696 and 13
515, 5696 and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 072 L mol−1 cm−1 for TP-OH, TP-OMe, TP-F, TP-Acr and TP-TQ1 NPs, respectively.
072 L mol−1 cm−1 for TP-OH, TP-OMe, TP-F, TP-Acr and TP-TQ1 NPs, respectively.
| Entry | λ abs,max | λ em,max | Stokes shift | ε | QYd | ε × QY |
|---|---|---|---|---|---|---|
| (nm) | (nm) | (nm) | (L mol−1 cm−1) | (%) | (L mol−1 cm−1) | |
| a λ abs,max is the maximum absorption. b λ em,max is the maximum emission. c ε is molar extinction coefficient at the maximum absorption. d Quantum yield. | ||||||
| TP-OH | 842 | 1012 | 170 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622 622 |
8.1 | 2804 |
| TP-OMe | 838 | 989 | 151 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198 198 |
8.4 | 2621 |
| TP-F | 857 | 1026 | 169 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 838 838 |
7.2 | 1572 |
| TP-Acr | 849 | 1003 | 154 | 9055 | 10.2 | 924 |
| TP-TQ1 | 858 | 1003 | 145 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 409 409 |
8.0 | 2113 |
| TP-OH NPs | 843 | 1029 | 186 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 077 077 |
1.05 | 305 |
| TP-OMe NPs | 861 | 1016 | 155 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497 497 |
0.62 | 103 |
| TP-F NPs | 911 | 1029 | 118 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 515 |
0.17 | 31 |
| TP-Acr NPs | 877 | 996 | 119 | 5696 | 1.47 | 83 |
| TP-TQ1 NPs | 877 | 1032 | 155 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 072 072 |
0.50 | 65 |
Benefiting from their co-planar conformation and strong intramolecular charge transfer effect, all dyes displayed NIR-II emission in toluene solution and NPs (Fig. 2d and e). In toluene, the emission maxima of TP-OH, TP-OMe, TP-F, TP-Acr and TP-TQ1 were 1012, 989, 1026, 1003 and 1003 nm, respectively. Similarly, the emission profile peaked at 1029, 1016, 1029, 996 and 1032 nm for TP-OH, TP-OMe, TP-F, TP-Acr and TP-TQ1 NPs, respectively. Notably, TP-OH exhibited the largest Stokes shift in both toluene and aggregated state, resulting in less spectral overlap and thus reducing cross-talk between its excitation and emission spectra.
As shown in Fig. S16–S19 (ESI†), the relative QYs were calculated to be 8.1%, 8.4%, 7.2%, 10.2% and 8.0% for TP-OH, TP-OMe, TP-F, TP-Acr and TP-TQ1, respectively, using a NIR-II fluorophore FT-BBT (QY = 19% in toluene) as the standard.60 The QY values of the fluorophores in toluene is roughly similar, indicating that the D–A skeleton is the key factor determining the QY in the non-aggregated state. Additionally, the large size of substituents can restrict intramolecular rotation of the fluorophore and improve fluorescence efficiency in the non-aggregated state (Fig. 2f). Impressively, TP-OH and TP-Acr NPs still maintained a relatively high QYs of 1.05% and 1.47%, respectively, in the nano-aggregated state, significantly higher than those of TP-F (0.17%), TP-OMe (0.62%), and TP-TQ1 NPs (0.50%). The high QY of TP-OH NPs can be attributed to the hydrogen bonds formed by the hydroxyl groups, while the high QY of TP-Acr NPs is due to the introduction of an acrylate group with larger steric hindrance. Both hydrogen bonding and steric hindrance can alleviate the π–π stacking between molecules, thereby weakening the ACQ effect. Furthermore, TP-OH NPs exhibited superior fluorescence brightness compared to other NPs at high concentrations under both 808 nm and 980 nm excitation (Fig. S20, ESI†). As listed in Table S1 (ESI†), TP-TQ1 NPs displayed the highest QY and strong NIR absorption ability among the TQ-based small molecule NIR-II dyes with the maximum absorption peak >800 nm. The result of ε × QY for TP-OH NPs was 4.7 fold higher than that of TP-TQ1 NPs (Table 1), suggesting that TP-OH NPs have superior NIR-II fluorescence capability.
Stability and photothermal properties
As illustrated in Fig. 3a and b, at the same molar concentration (0.1 μmol mL−1), TP-OH NPs and Indo cyanine green (ICG) aqueous solutions were irradiated with an 808 nm laser at 0.5 W cm−2 for varying durations (0, 2, 4, 6, and 8 min). The fluorescence intensity of ICG diminished rapidly as illumination time increased, whereas TP-OH NPs displayed robust photostability, maintaining a stable fluorescence signal. Moreover, the fluorescence intensity of TP-OH NPs remained quietly stable in neutral and mildly alkaline environments, and displayed good acid degradation resistance (Fig. 3c and Fig. S21, ESI†), indicating that TP-OH NPs could function as an imaging agent for tissues, disease diagnosis and digestive system. As shown in Fig. 3d, the temperature difference (ΔT) of TP-OH NPs remained almost unchanged after five laser on–off cycles, indicating that TP-OH NPs had excellent resistance to photo-bleaching. To further evaluate the influence of acceptor substituents on photothermal properties, the photothermal effects of four types of NPs were measured under 808 nm laser irradiation. Studies have demonstrated that hydrogen bonds within the material break under NIR light irradiation and recombine once the light is swithced off.61,62 These processes involve the absorption and release of energy. Therefore, TP-OH NPs exhibited a slower heat release rate and significantly lower photothermal conversion efficiency compared to other NPs (Fig. S22 and S23, ESI†).Biocompatibility
In the light of strong NIR absorption and high fluorescence brightness of TP-OH NPs, in vivo NIR-II fluorescence imaging was performed under 808 nm laser irradiation. Good biosafety is crucial for a contrast agent used in in vivo imaging. MTT assays were conducted to evaluate the cytotoxicity and biocompatibility of TP-OH NPs towards 4T1 cells. As indicated in Fig. 3e, the cell viability still remained at approximately 0.86 when the culture concentration of TP-OH NPs was 90 μg mL−1, indicating low cytotoxicity of the NPs. To intuitively appraise the in vitro biosafety of TP-OH NPs, live (green) and dead (red) cells were co-stained by propidium iodide (PI) and calcein AM. As presented in Fig. 3f, 4T1 cells maintained a high survival rate under the treatment of PBS and TP-OH NPs. These results demonstrated that TP-OH NPs are biocompatible and suitable for in vivo imaging experiments.In vivo NIR-II fluorescence imaging
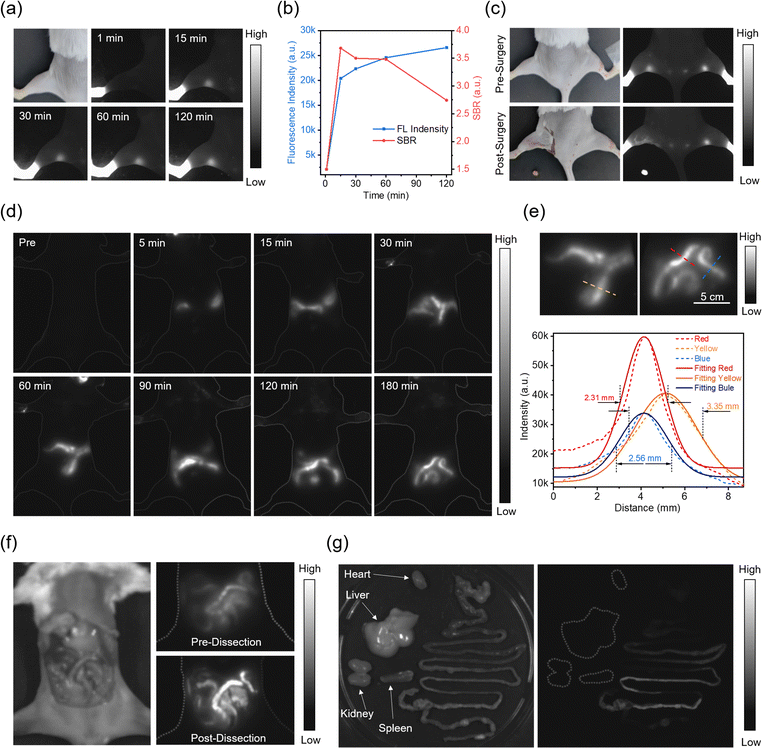
Accurate visualization of vasculature is essential for diagnosing and treating cardiovascular diseases.63,64 Inspired by their excellent fluorescent performance, TP-OH NPs (2 mg mL−1, 150 μL) were used for fluorescence angiography in nude mice. As illustrated in Fig. 4a and b, the abdominal and hindlimb vasculature could be seen clearly after intravenous injection. The full width at half maximum (FWHM) for these vessels was calculated to be 0.670, 0.882, and 0.629 mm, respectively, and the signal-to-background ratio (SBR) was 1.22, 1.19, and 1.41, respectively. Furthermore, TP-OH NPs still displayed higher imaging resolution than TP-TQ1 NPs under the same experimental conditions, even with lower excitation power fluence (Fig. 4c).60Early tumor diagnosis is of significant importance for improving the cure rate of cancer.65–67 The accumulation of TP-OH NPs in 4T1 tumor-bearing BALB/c mice was monitored. Fig. 4d and e present NIR-II fluorescence images and corresponding quantitative analysis of tumor-bearing mice within 36 h after intravenous injection. The fluorescence intensity at the tumor site increased rapidly within 18 hours, followed by a gradual stabilization of the upward trend. The SBR increased to 1.9 at 30 h post-injection, and the tumor edges became clearly distinguishable, aiding in tumor localization and guiding surgical resection. Ex vivo NIR-II fluorescence imaging and quantitative analysis of major organs and tumor showed that TP-OH NPs were primarily distributed to the liver and spleen (Fig. 4f and g), indicating that NPs accumulated mainly in the reticuloendothelial system. The main metabolic pathway for NPs was liver metabolism, with clearance by the mononuclear phagocyte system. Furthermore, the excised tumor exhibited a moderate fluorescence signal intensity, demonstrating that NPs can effectively accumulate at the tumor site. As illustrated in Fig. S24 (ESI†), the absence of obvious histological damage in the major organs and tumors of sacrificed mice 36 hours after injection with NPs indicated that TP-OH NPs had good biocompatibility. These in vivo experiments demonstrate that TP-OH NPs possess excellent NIR-II fluorescence vascular imaging capabilities and biosafety, exhibiting good passive tumor targeting via enhanced permeability and retention effects.
Since lymphatic vessels are important pathways for tumor cell metastasis, sentinel lymph node biopsy is extremely important for detecting tumor metastasis and guiding therapeutic decisions.68–71 Therefore, imaging-guided lymph node removal surgery holds remarkable significance in clinical research. To investigate the lymphatic imaging performance of TP-OH NPs, BALB/c mice were depilated and then intradermally injected with NPs (40 μL, 0.15 μmol mL−1) into the footpad. As shown in Fig. 5a and b, the fluorescence signals in the mouse's popliteal lymph nodes increased sharply and peaked at 15 min post-injection, and then gradually slowed down. Interestingly, the lymph node displayed a high SBR of 3.7. Due to the precise localization of popliteal lymph nodes by TP-OH NPs, accurate excision of the lymph nodes was achieved, guided by the NIR-II fluorescence imaging. The fluorescence at the site of the sacrificed mouse's popliteal lymph node disappeared, while the excised tissue emitted bright fluorescence (Fig. 5c). This result further demonstrates that TP-OH NPs could completely outline the boundaries of popliteal lymph nodes and possess the capability to guide surgery through fluorescence imaging.
The high incidence of gastrointestinal diseases, such as colorectal polyps and digestive tract tumors, leads to a significant increase in the demand for precise gastrointestinal imaging technology.72,73 Compared with injection, oral administration represents a safer method of drug delivery. Owing to the good stability of TP-OH NPs in acidic environments, in vivo NIR-II imaging performance of NPs in the gastrointestinal system of BALB/c mice via oral administration was further evaluated. To avoid interference from food, the mice were fasted for 24 hours. Moderate fluorescence appeared in the stomach and liver of the mouse after oral administration (2 mg mL−1, 0.75 mL/10 g), indicating that some NPs crossed the gastric mucosa and were metabolized through the liver (Fig. 5d). Within 180 min after administration, as the digestive activity progresses, the fluorescence signal continuously moved towards the abdomen of the mouse, with the ileum and cecum becoming successively visible. As shown in Fig. 5e, intestinal structures were visualized with a width as narrow as 2.31 mm, and the SBR at the selected red, yellow, and blue lines were 3.91, 3.86, and 2.79, respectively. Benefiting from the NIR absorption and NIR-II emission of TP-OH NPs, the fluorescence signal can well penetrate through skin and fat structures under non-invasive conditions, accurately reflecting the distribution of the NPs in the intestines inside the abdominal cavity and delineating the outline of the intestines (Fig. 5f). As shown in Fig. 5g, the sacrificed mouse's intestine emitted significant fluorescence, while other ex vivo organs did not show any fluorescence signal, indicating that NPs are mainly excreted through the digestive system. Additionally, compared to previously reported NIR-II contrast agents used for gastrointestinal imaging, TP-OH NPs reached the cecum within 2 hours, exhibiting a faster clearance rate.74–77 Collectively, the above results evidence that TP-OH NPs could be used as an NIR-II fluorescence imaging contrast agent for the vascular system, lymph nodes, tumors and gastrointestinal imaging with excellent resolution.
Conclusions
In summary, five D–A–D NIR-II dyes with quasi-planar conformation have been developed to uncover the structure–property relationships. Our results indicate that the substituents have a limited effect on charge transfer and photophysical properties in solution, while they have a significant impact on absorption/emission spectra and QY in the aggregate state via regulating the intermolecular supramolecular interactions. Consequently, the hydroxyl-functionalized dye TP-OH NPs possess high NIR absorption and long-wavelength emission with a relative QY of 1.05% due to intermolecular hydrogen bonding alleviating the ACQ effect. More importantly, in vivo fluorescence imaging results indicate that TP-OH NPs can serve as a multifunctional NIR-II fluorescent imaging agent for angiography, lymphatic imaging, surgical guidance, and gastrointestinal imaging. Overall, D–A–D structured NIR-II fluorophores with coplanar molecular structures can achieve outstanding fluorescence imaging performance via rationally tailoring chemical structures and aggregate behaviors.Data availability
The raw data supporting the conclusions of this article are included in the article/ESI;† further inquiries can be directed to the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by NNSF of China (52102349), the open research fund of the State Key Laboratory of Organic Electronics and Information Displays and the Startup Foundation for Introducing Talent of NUIST (2021r089). We kindly appreciate Dr Yu Cai for the help with in vivo experiments at the Zhejiang Provincial People's Hospital.References
- S. Diao, J. L. Blackburn, G. Hong, A. L. Antaris, J. Chang, J. Z. Wu, B. Zhang, K. Cheng, C. J. Kuo and H. Dai, Fluorescence Imaging In Vivo at Wavelengths beyond 1500 nm, Angew. Chem., Int. Ed., 2015, 54, 14758–14762 CrossRef CAS PubMed.
- S. Yoon, S. Y. Cheon, S. Park, D. Lee, Y. Lee, S. Han, M. Kim and H. Koo, Recent advances in optical imaging through deep tissue: imaging probes and techniques, Biomater. Res., 2022, 26, 57 CrossRef PubMed.
- J. Mu, M. Xiao, Y. Shi, X. Geng, H. Li, Y. Yin and X. Chen, The Chemistry of Organic Contrast Agents in the NIR-II Window, Angew. Chem., Int. Ed., 2022, 61, e202114722 CrossRef CAS PubMed.
- E. Hemmer, A. Benayas, F. Légaré and F. Vetrone, Exploiting the biological windows: current perspectives on fluorescent bioprobes emitting above 1000 nm, Nanoscale Horiz., 2016, 1, 168–184 RSC.
- L. Li, X. Dong, J. Li and J. Wei, A short review on NIR-II organic small molecule dyes, Dyes Pigm., 2020, 183, 108756 CrossRef CAS.
- V. Ntziachristos, Going deeper than microscopy: the optical imaging frontier in biology, Nat. Meth., 2010, 7, 603–614 CAS.
- A. Cios, M. Ciepielak, Ł. Szymański, A. Lewicka, S. Cierniak, W. Stankiewicz, M. Mendrycka and S. Lewicki, Effect of Different Wavelengths of Laser Irradiation on the Skin Cells, Int. J. Mol. Sci., 2021, 22, 2437 CrossRef CAS PubMed.
- C. Ash, M. Dubec, K. Donne and T. Bashford, Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods, Lasers Med. Sci., 2017, 32, 1909–1918 CrossRef PubMed.
- S. He, J. Song, J. Qu and Z. Cheng, Crucial breakthrough of second near-infrared biological window fluorophores: design and synthesis toward multimodal imaging and theranostics, Chem. Soc. Rev., 2018, 47, 4258–4278 RSC.
- Y. Tang, F. Pei, X. Lu, Q. Fan and W. Huang, Recent Advances on Activatable NIR-II Fluorescence Probes for Biomedical Imaging, Adv. Opt. Mater., 2019, 7, 1900917 CrossRef CAS.
- H. Dai, Q. Shen, J. Shao, W. Wang, F. Gao and X. Dong, Small Molecular NIR-II Fluorophores for Cancer Phototheranostics, Innovation, 2021, 2, 100082 CAS.
- C. Yin, X. Li, Y. Wang, Y. Liang, S. Zhou, P. Zhao, C.-S. Lee, Q. Fan and W. Huang, Organic Semiconducting Macromolecular Dyes for NIR-II Photoacoustic Imaging and Photothermal Therapy, Adv. Funct. Mater., 2021, 31, 2104650 CrossRef CAS.
- X. Liu, B. Yu, Y. Shen and H. Cong, Design of NIR-II high performance organic small molecule fluorescent probes and summary of their biomedical applications, Coord. Chem. Rev., 2022, 468, 214609 CrossRef CAS.
- E. D. Cosco, J. R. Caram, O. T. Bruns, D. Franke, R. A. Day, E. P. Farr, M. G. Bawendi and E. M. Sletten, Flavylium Polymethine Fluorophores for Near- and Shortwave Infrared Imaging, Angew. Chem., Int. Ed., 2017, 56, 13126–13129 CrossRef CAS PubMed.
- S. Zhu, Z. Hu, R. Tian, B. C. Yung, Q. Yang, S. Zhao, D. O. Kiesewetter, G. Niu, H. Sun, A. L. Antaris and X. Chen, Repurposing Cyanine NIR-I Dyes Accelerates Clinical Translation of Near-Infrared-II (NIR-II) Bioimaging, Adv. Mater., 2018, 30, 1802546 CrossRef PubMed.
- Q. Zheng and L. D. Lavis, Development of photostable fluorophores for molecular imaging, Curr. Opin. Chem. Biol., 2017, 39, 32–38 CrossRef CAS PubMed.
- A. P. Gorka and M. J. Schnermann, Harnessing cyanine photooxidation: from slowing photobleaching to near-IR uncaging, Curr. Opin. Chem. Biol., 2016, 33, 117–125 CrossRef CAS PubMed.
- Z. Jiang, C. Zhang, X. Wang, M. Yan, Z. Ling, Y. Chen and Z. Liu, A Borondifluoride-Complex-Based Photothermal Agent with an 80% Photothermal Conversion Efficiency for Photothermal Therapy in the NIR-II Window, Angew. Chem., Int. Ed., 2021, 60, 22376–22384 CrossRef CAS PubMed.
- L. Bai, P. Sun, Y. Liu, H. Zhang, W. Hu, W. Zhang, Z. Liu, Q. Fan, L. Li and W. Huang, Novel aza-BODIPY based small molecular NIR-II fluorophores for in vivo imaging, Chem. Commun., 2019, 55, 10920–10923 RSC.
- Y. Feng, S. Zhu, A. L. Antaris, H. Chen, Y. Xiao, X. Lu, L. Jiang, S. Diao, K. Yu, Y. Wang, S. Herraiz, J. Yue, X. Hong, G. Hong, Z. Cheng, H. Dai and A. J. Hsueh, Live imaging of follicle stimulating hormone receptors in gonads and bones using near infrared II fluorophore, Chem. Sci., 2017, 8, 3703–3711 RSC.
- Y. Xu, Y. Zhang, J. Li, J. An, C. Li, S. Bai, A. Sharma, G. Deng, J. S. Kim and Y. Sun, NIR-II emissive multifunctional AIEgen with single laser-activated synergistic photodynamic/photothermal therapy of cancers and pathogens, Biomaterials, 2020, 259, 120315 CrossRef CAS PubMed.
- H. Wan, H. Ma, S. Zhu, F. Wang, Y. Tian, R. Ma, Q. Yang, Z. Hu, T. Zhu, W. Wang, Z. Ma, M. Zhang, Y. Zhong, H. Sun, Y. Liang and H. Dai, Developing a Bright NIR-II Fluorophore with Fast Renal Excretion and Its Application in Molecular Imaging of Immune Checkpoint PD-L1, Adv. Funct. Mater., 2018, 28, 1804956 CrossRef PubMed.
- J. Wang, Y. Liu, M. Morsch, Y. Lu, P. Shangguan, L. Han, Z. Wang, X. Chen, C. Song, S. Liu, B. Shi and B. Z. Tang, Brain-Targeted Aggregation-Induced-Emission Nanoparticles with Near-Infrared Imaging at 1550 nm Boosts Orthotopic Glioblastoma Theranostics, Adv. Mater., 2022, 34, 2106082 CrossRef CAS PubMed.
- Y. Lai, Y. Dang, Q. Sun, J. Pan, H. Yu, W. Zhang and Z. Xu, Design of an activatable NIR-II nanoprobe for the in vivo elucidation of Alzheimer's disease-related variations in methylglyoxal concentrations, Chem. Sci., 2022, 13, 12511–12518 RSC.
- S. Chen, B. Sun, H. Miao, G. Wang, P. Sun, J. Li, W. Wang, Q. Fan and W. Huang, NIR-II Dye-Based Multifunctional Telechelic Glycopolymers for NIR-IIa Fluorescence Imaging-Guided Stimuli-Responsive Chemo-Photothermal Combination Therapy, ACS Mater. Lett., 2020, 2, 174–183 CrossRef CAS.
- J. Cui, F. Zhang, D. Yan, T. Han, L. Wang, D. Wang and B. Z. Tang, “Trojan Horse” Phototheranostics: Fine-Engineering NIR-II AIEgen Camouflaged by Cancer Cell Membrane for Homologous-Targeting Multimodal Imaging-Guided Phototherapy, Adv. Mater., 2023, 35, 2302639 CrossRef CAS.
- H. Lou, A. Ji, C. Qu, S. Duan, H. Liu, H. Chen and Z. Cheng, A novel NIR-II nanoprobe for precision imaging of micro-meter sized tumor metastases of multi-organs and skin flap, Chem. Eng. J., 2022, 449, 137848 CrossRef CAS.
- X.-D. Zhang, H. Wang, A. L. Antaris, L. Li, S. Diao, R. Ma, A. Nguyen, G. Hong, Z. Ma, J. Wang, S. Zhu, J. M. Castellano, T. Wyss-Coray, Y. Liang, J. Luo and H. Dai, Traumatic Brain Injury Imaging in the Second Near-Infrared Window with a Molecular Fluorophore, Adv. Mater., 2016, 28, 6872–6879 CrossRef CAS PubMed.
- W. Wang, Q. Yang, Y. Du, X. Zhou, X. Du, Q. Wu, L. Lin, Y. Song, F. Li, C. Yang and W. Tan, Metabolic Labeling of Peptidoglycan with NIR-II Dye Enables In Vivo Imaging of Gut Microbiota, Angew. Chem., Int. Ed., 2020, 59, 2628–2633 CrossRef CAS PubMed.
- S. Liu, C. Chen, Y. Li, H. Zhang, J. Liu, R. Wang, S. T. H. Wong, J. W. Y. Lam, D. Ding and B. Z. Tang, Constitutional Isomerization Enables Bright NIR-II AIEgen for Brain-Inflammation Imaging, Adv. Funct. Mater., 2020, 30, 1908125 CrossRef CAS.
- D. Wu, S. Liu, J. Zhou, R. Chen, Y. Wang, Z. Feng, H. Lin, J. Qian, B. Z. Tang and X. Cai, Organic Dots with Large π-Conjugated Planar for Cholangiography beyond 1500 nm in Rabbits: A Non-Radioactive Strategy, ACS Nano, 2021, 15, 5011–5022 CrossRef CAS PubMed.
- L. Zhang, C. Liu, S. Zhou, R. Wang, Q. Fan, D. Liu, W. Wu and X. Jiang, Improving Quantum Yield of a NIR-II Dye by Phenylazo Group, Adv. Healthcare Mater., 2020, 9, 1901470 CrossRef CAS.
- D. Wei, Y. Yu, Y. Huang, Y. Jiang, Y. Zhao, Z. Nie, F. Wang, W. Ma, Z. Yu, Y. Huang, X.-D. Zhang, Z.-Q. Liu, X. Zhang and H. Xiao, A Near-Infrared-II Polymer with Tandem Fluorophores Demonstrates Superior Biodegradability for Simultaneous Drug Tracking and Treatment Efficacy Feedback, ACS Nano, 2021, 15, 5428–5438 CrossRef CAS PubMed.
- J. Q. Zhang, X. Y. Xu, S. Q. Cao and Y. Q. Zou, Structures and Applications of NIR-II AIEgens Containing Benzobisthiadiazole Derivatives, Eur. J. Org. Chem., 2022, e202201131 CrossRef CAS.
- Z. Hu, L. Feng and P. Yang, 2,1,3-Benzothiadiazole Derivative Small Molecule Fluorophores for NIR-II Bioimaging, Adv. Funct. Mater., 2024, 34, 2310818 CrossRef CAS.
- Y. Chen, S. Chen, H. Yu, Y. Wang, M. Cui, P. Wang, P. Sun and M. Ji, D–A Type NIR-II Organic Molecules: Strategies for the Enhancement Fluorescence Brightness and Applications in NIR-II Fluorescence Imaging-Navigated Photothermal Therapy, Adv. Healthcare Mater., 2022, 11, 2201158 CrossRef CAS PubMed.
- Y. Chen, H. Yu, Y. Wang, P. Sun, Q. Fan and M. Ji, Thiadiazoloquinoxaline derivative-based NIR-II organic molecules for NIR-II fluorescence imaging and photothermal therapy, Biomater. Sci., 2022, 10, 2772–2788 RSC.
- Z. Lei and F. Zhang, Molecular Engineering of NIR-II Fluorophores for Improved Biomedical Detection, Angew. Chem., Int. Ed., 2021, 60, 16294–16308 CrossRef CAS PubMed.
- X. Feng, L. Wei, Y. Liu, X. Chen and R. Tian, Orchestrated Strategies for Developing Fluorophores for NIR-II Imaging, Adv. Healthcare Mater., 2023, 12, 2300537 CrossRef CAS PubMed.
- G. Qian, B. Dai, M. Luo, D. Yu, J. Zhan, Z. Zhang, D. Ma and Z. Y. Wang, Band Gap Tunable, Donor−Acceptor−Donor Charge-Transfer Heteroquinoid-Based Chromophores: Near Infrared Photoluminescence and Electroluminescence, Chem. Mater., 2008, 20, 6208–6216 CrossRef CAS.
- S. Liu, H. Ou, Y. Li, H. Zhang, J. Liu, X. Lu, R. T. K. Kwok, J. W. Y. Lam, D. Ding and B. Z. Tang, Planar and Twisted Molecular Structure Leads to the High Brightness of Semiconducting Polymer Nanoparticles for NIR-IIa Fluorescence Imaging, J. Am. Chem. Soc., 2020, 142, 15146–15156 CrossRef CAS PubMed.
- S. Liu, R. Chen, J. Zhang, Y. Li, M. He, X. Fan, H. Zhang, X. Lu, R. T. K. Kwok, H. Lin, J. W. Y. Lam, J. Qian and B. Z. Tang, Incorporation of Planar Blocks into Twisted Skeletons: Boosting Brightness of Fluorophores for Bioimaging beyond 1500 Nanometer, ACS Nano, 2020, 14, 14228–14239 CrossRef CAS PubMed.
- S. Liu, X. Zhou, H. Zhang, H. Ou, J. W. Y. Lam, Y. Liu, L. Shi, D. Ding and B. Z. Tang, Molecular Motion in Aggregates: Manipulating TICT for Boosting Photothermal Theranostics, J. Am. Chem. Soc., 2019, 141, 5359–5368 CrossRef CAS PubMed.
- Y. Li, Y. Liu, Q. Li, X. Zeng, T. Tian, W. Zhou, Y. Cui, X. Wang, X. Cheng, Q. Ding, X. Wang, J. Wu, H. Deng, Y. Li, X. Meng, Z. Deng, X. Hong and Y. Xiao, Novel NIR-II organic fluorophores for bioimaging beyond 1550 nm, Chem. Sci., 2020, 11, 2621–2626 RSC.
- Z. Cheng, T. Zhang, W. Wang, Q. Shen, Y. Hong, J. Shao, X. Xie, Z. Fei and X. Dong, D–A–D structured selenadiazolesbenzothiadiazole-based near-infrared dye for enhanced photoacoustic imaging and photothermal cancer therapy, Chin. Chem. Lett., 2021, 32, 1580–1585 CrossRef CAS.
- S. Chen, Y. Pan, K. Chen, P. Chen, Q. Shen, P. Sun, W. Hu and Q. Fan, Increasing Molecular Planarity through Donor/Side-Chain Engineering for Improved NIR-IIa Fluorescence Imaging and NIR-II Photothermal Therapy under 1064 nm, Angew. Chem., Int. Ed., 2023, 62, e202215372 CrossRef CAS PubMed.
- D. Yan, W. Xie, J. Zhang, L. Wang, D. Wang and B. Z. Tang, Donor/π-Bridge Manipulation for Constructing a Stable NIR-II Aggregation-Induced Emission Luminogen with Balanced Phototheranostic Performance**, Angew. Chem., Int. Ed., 2021, 60, 26769–26776 CrossRef CAS PubMed.
- Q. Yang, Z. Hu, S. Zhu, R. Ma, H. Ma, Z. Ma, H. Wan, T. Zhu, Z. Jiang, W. Liu, L. Jiao, H. Sun, Y. Liang and H. Dai, Donor Engineering for NIR-II Molecular Fluorophores with Enhanced Fluorescent Performance, J. Am. Chem. Soc., 2018, 140, 1715–1724 CrossRef CAS PubMed.
- S. Li, C. Yin, R. Wang, Q. Fan, W. Wu and X. Jiang, Second Near-Infrared Aggregation-Induced Emission Fluorophores with Phenothiazine Derivatives as the Donor and 6,7-Diphenyl-[1,2,5]Thiadiazolo[3,4-g]Quinoxaline as the Acceptor for In Vivo Imaging, ACS Appl. Mater. Interfaces, 2020, 12, 20281–20286 CrossRef CAS PubMed.
- K. He, S. Chen, Y. Chen, J. Li, P. Sun, X. Lu, Q. Fan and W. Huang, Water-Soluble Donor–Acceptor–Donor-Based Fluorophore for High-Resolution NIR-II Fluorescence Imaging Applications, ACS Appl. Polym. Mater., 2021, 3, 3238–3246 CrossRef CAS.
- S. Li, T. Cheng, C. Yin, S. Zhou, Q. Fan, W. Wu and X. Jiang, Phenothiazine versus Phenoxazine: Structural Effects on the Photophysical Properties of NIR-II AIE Fluorophores, ACS Appl. Mater. Interfaces, 2020, 12, 43466–43473 CrossRef CAS PubMed.
- Y. Li, M. Zha, T. Kang, C. Li, X. Wu, S. Wang, S.-B. Lu, Y.-S. Lee, Y.-R. Wu, J.-S. Ni and K. Li, Promoted NIR-II Fluorescence by Heteroatom-Inserted Rigid-Planar Cores for Monitoring Cell Therapy of Acute Lung Injury, Small, 2022, 18, 2105362 CrossRef CAS PubMed.
- Y. Li, M. Zha, G. Yang, S. Wang, J.-S. Ni and K. Li, NIR-II Fluorescent Brightness Promoted by “Ring Fusion” for the Detection of Intestinal Inflammation, Chem. Eur. J., 2021, 27, 13085–13091 CrossRef CAS PubMed.
- A. Ji, H. Lou, C. Qu, W. Lu, Y. Hao, J. Li, Y. Wu, T. Chang, H. Chen and Z. Cheng, Acceptor engineering for NIR-II dyes with high photochemical and biomedical performance, Nat. Commun., 2022, 13, 3815 CrossRef CAS PubMed.
- Y. Luo, Y. Lan, L. Peng, T. Qiu, Q. Li, Q. Hou, C. Liu, J. Zhang, Y.-P. Cai and S. Liu, Acrylate-Substituted Thiadiazoloquinoxaline Yields Ultralow Band Gap (0.56 eV) Conjugated Polymers for Efficient Photoacoustic Imaging, ACS Appl. Polym. Mater., 2021, 3, 3247–3253 CrossRef CAS.
- M. Zha, X. Lin, J.-S. Ni, Y. Li, Y. Zhang, X. Zhang, L. Wang and K. Li, An Ester-Substituted Semiconducting Polymer with Efficient Nonradiative Decay Enhances NIR-II Photoacoustic Performance for Monitoring of Tumor Growth, Angew. Chem., Int. Ed., 2020, 59, 23268–23276 CrossRef CAS PubMed.
- Y. Liu, J. Liu, D. Chen, X. Wang, Z. Zhang, Y. Yang, L. Jiang, W. Qi, Z. Ye, S. He, Q. Liu, L. Xi, Y. Zou and C. Wu, Fluorination Enhances NIR-II Fluorescence of Polymer Dots for Quantitative Brain Tumor Imaging, Angew. Chem., Int. Ed., 2020, 59, 21049–21057 CrossRef CAS PubMed.
- Y. Liu, J. Liu, D. Chen, X. Wang, Z. Liu, H. Liu, L. Jiang, C. Wu and Y. Zou, Quinoxaline-Based Semiconducting Polymer Dots for in Vivo NIR-II Fluorescence Imaging, Macromolecules, 2019, 52, 5735–5740 CrossRef CAS.
- S.-i Kato, K. Watanabe, M. Tamura, M. Ueno, M. Nitani, Y. Ie, Y. Aso, T. Yamanobe, H. Uehara and Y. Nakamura, Tetraalkoxyphenanthrene-Fused Thiadiazoloquinoxalines: Synthesis, Electronic, Optical, and Electrochemical Properties, and Self-Assembly, J. Org. Chem., 2017, 82, 3132–3143 CrossRef CAS PubMed.
- L. Zheng, Z. Zhao, C. Xue, L. An, W. Na, F. Gao, J. Shao and C. Ou, Planar-structured thiadiazoloquinoxaline-based NIR-II dye for tumor phototheranostics, J. Mater. Chem. B, 2024, 12, 4197–4207 RSC.
- J. Sun, J. Zhang, L. Zhao, S. Wan, B. Wu, C. Ma, J. Li, F. Wang, X. Xing, D. Chen, H. Zhang and K. Liu, Contribution of Hydrogen-Bond Nanoarchitectonics to Switchable Photothermal-Mechanical Properties of Bioinorganic Fibers, CCS Chem., 2023, 5, 1242–1250 CrossRef CAS.
- Y. Zhang, Y. Cui, M. Li, K. Cui, R. Li, W. Xie, L. Liu and Z. Xiao, DNA-assembled visible nanodandelions with explosive hydrogen-bond breakage achieving uniform intra-tumor distribution (UITD)-guided photothermal therapy, Biomaterials, 2022, 282, 121381 CrossRef CAS PubMed.
- Z. Wang, X. Wang, J.-B. Wan, F. Xu, N. Zhao and M. Chen, Optical Imaging in the Second Near Infrared Window for Vascular Bioimaging, Small, 2021, 17, 2103780 CrossRef CAS PubMed.
- P. M. Ridker and N. R. Cook, Statins: new American guidelines for prevention of cardiovascular disease, Lancet, 2013, 382, 1762–1765 CrossRef PubMed.
- I. I. Wistuba, J. G. Gelovani, J. J. Jacoby, S. E. Davis and R. S. Herbst, Methodological and practical challenges for personalized cancer therapies, Nat. Rev. Clin. Oncol., 2011, 8, 135–141 CrossRef CAS PubMed.
- J. Tseng, J. Miller, X. Feng, A. Gangi, J. Gong and M. Burch, Racial differences in tumor biology and treatment of gastric cancer in the United States, J. Clin. Oncol., 2021, 39, 174 Search PubMed.
- O. Elemento, C. Leslie, J. Lundin and G. Tourassi, Artificial intelligence in cancer research, diagnosis and therapy, Nat. Rev. Cancer, 2021, 21, 747–752 CrossRef CAS PubMed.
- L. Philp, H. Chan, M. Rouzbahman, M. Overchuk, J. Chen, G. Zheng and M. Q. Bernardini, Use of Porphysomes to detect primary tumour, lymph node metastases, intra-abdominal metastases and as a tool for image-guided lymphadenectomy: proof of concept in endometrial cancer, Theranostics, 2019, 9, 2727–2738 CrossRef CAS PubMed.
- E. R. Pereira, D. Kedrin, G. Seano, O. Gautier, E. F. J. Meijer, D. Jones, S.-M. Chin, S. Kitahara, E. M. Bouta, J. Chang, E. Beech, H.-S. Jeong, M. C. Carroll, A. G. Taghian and T. P. Padera, Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice, Science, 2018, 359, 1403–1407 CrossRef CAS PubMed.
- D. Jones, Z. Wang, I. X. Chen, S. Zhang, R. Banerji, P.-J. Lei, H. Zhou, V. Xiao, C. Kwong, J. W. M. van Wijnbergen, E. R. Pereira, B. J. Vakoc, P. Huang, H. T. Nia and T. P. Padera, Solid stress impairs lymphocyte infiltration into lymph-node metastases, Nat. Biomed. Eng., 2021, 5, 1426–1436 CrossRef CAS PubMed.
- M. Frumovitz, M. Plante, P. S. Lee, S. Sandadi, J. F. Lilja, P. F. Escobar, L. T. Gien, D. L. Urbauer and N. R. Abu-Rustum, Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): a randomised, phase 3, multicentre, non-inferiority trial, Lancet Oncol., 2018, 19, 1394–1403 CrossRef PubMed.
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, Ca-Cancer J. Clin., 2021, 71, 209–249 CrossRef PubMed.
- K. J. Maloy and F. Powrie, Intestinal homeostasis and its breakdown in inflammatory bowel disease, Nature, 2011, 474, 298–306 CrossRef CAS PubMed.
- W. Wang, Y. Kong, J. Jiang, Q. Xie, Y. Huang, G. Li, D. Wu, H. Zheng, M. Gao, S. Xu, Y. Pan, W. Li, R. Ma, M. X. Wu, X. Li, H. Zuilhof, X. Cai and R. Li, Engineering the Protein Corona Structure on Gold Nanoclusters Enables Red-Shifted Emissions in the Second Near-infrared Window for Gastrointestinal Imaging, Angew. Chem., Int. Ed., 2020, 59, 22431–22435 CrossRef CAS PubMed.
- Y. Liu, Y. Yuzhen, T. Tian, W. Wang, J. Nong, X. Qiao, F. Xu, J. Gao and X. Hong, Novel CD-MOF NIR-II fluorophores for gastric ulcer imaging, Chin. Chem. Lett., 2021, 32, 3061–3065 CrossRef CAS.
- T. Li, K. Cao, X. Yang, Y. Liu, X. Wang, F. Wu, G. Chen and Q. Wang, An oral ratiometric NIR-II fluorescent probe for reliable monitoring of gastrointestinal diseases in vivo, Biomaterials, 2023, 293, 121956 CrossRef CAS PubMed.
- L.-L. Yang, W. Zhao, Z.-Y. Liu, M. Ren, J. Kong, X. Zong, M.-Y. Luo, B. Tang, J. Xie, D.-W. Pang and A.-A. Liu, Acid-Resistant Near-Infrared II Ag2Se Quantum Dots for Gastrointestinal Imaging, Anal. Chem., 2023, 95, 15540–15548 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures of TP-OH, TP-F, TP-OMe and TP-Acr, NMR spectra, MALTI-TOF-MS, DLS data, TEM, absorption and fluorescence spectra, cell culture, and in vivo treatment data. See DOI: https://doi.org/10.1039/d5qm00060b |
| This journal is © the Partner Organisations 2025 |