Synthesis and design of terbium-activated CaWO4 nanocrystals for fluorescence sensing of alkaline phosphatase and bioimaging applications†
Terefe Tafese
Bezuneh
 abf,
Xin
Li
ab,
Tongtong
Kou
ab,
Fuchun
Nan
ab,
Zilong
Wu
ab,
Lanbo
Shen
c,
Xinyuan
Xia
d,
Bin
Li
*c,
Liang
Wang
abf,
Xin
Li
ab,
Tongtong
Kou
ab,
Fuchun
Nan
ab,
Zilong
Wu
ab,
Lanbo
Shen
c,
Xinyuan
Xia
d,
Bin
Li
*c,
Liang
Wang
 *abe and
William W.
Yu
*abe and
William W.
Yu
 *ab
*ab
aSchool of Chemistry and Chemical Engineering, Ministry of Education Key Laboratory of Special Functional Aggregated Materials, Shandong Key Laboratory of Advanced Organosilicon Materials and Technologies, Shandong University, Jinan 250100, China. E-mail: liangwang2023@sdu.edu.cn; wyu6000@gmail.com
bShandong Provincial Key Laboratory for Science of Material Creation and Energy Conversion, Science Center for Material Creation and Energy Conversion, Shandong University, Qingdao 266237, China
cResearch Center of Translational Medicine, Central Hospital Affiliated to Shandong First Medical University, Jinan 250013, China. E-mail: qingquan0615@163.com
dCollege of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Jinan 250014, China
eSchool of Integrated Circuits, Shandong University, Jinan 250100, China
fDepartment of Chemistry, College of Natural Sciences, Arba Minch University, P. O. Box 21, Arba Minch, Ethiopia
First published on 19th May 2025
Abstract
Calcium tungstate nanocrystals CaWO4 NCs represent an important family of inorganic oxides and are well-known for their rare-earth (RE) sensitizing properties. Although RE-doped CaWO4 NCs have been extensively used in the optical field, their applications as a fluorescent probe is yet to be explored. In this report, inspired by their excellent luminescence properties, we demonstrate the potential of terbium-activated CaWO4 NCs as a fluorescent probe for sensing and biological imaging applications. In our study, we successfully synthesized nano-sized water dispersible PEG coated Tb–CaWO4 NCs via a facile one-step hydrothermal route. Next, the potential of PEG–Tb–CaWO4 NCs as a fluorescent sensing probe was evaluated by employing a “signal-off–on” sensing strategy for the detection of a key disease biomarker (alkaline phosphatase, ALP). The proposed detection system realized sensitive detection of ALP with a low limit of detection of 0.5 U L−1. Moreover, the cell imaging potential of PEG–Tb–CaWO4 NCs was investigated using HeLa cells. PEG–Tb–CaWO4 NCs were internalized by HeLa cells through endocytosis and showed characteristic bright green emission, demonstrating the cell imaging potential of the as-synthesized NCs. Also, the viability study confirmed the very low cell cytotoxicity of PEG–Tb–CaWO4 NCs to HeLa cells. Overall, we highlighted the potential of PEG–Tb–CaWO4 NCs as a promising fluorescence probe for sensing and biological imaging applications.
1. Introduction
In the past decade, rare-earth activated nanoparticles (RE-NPs) have received extensive attention in fluorescence sensing applications.1,2 This is due to their unique features and exceptional optical properties including tunable emission wavelengths, excellent photostability, large anti-Stokes shifts, and long lifetimes (milliseconds to microseconds).3–5 Another interesting characteristic of RE-NPs is the host–lattice sensitization, where fluorescence occurs due to the resonant energy transfer from a host-sensitizer to a RE-activator dopant such as terbium (Tb3+), europium (Eu3+), ytterbium (Yb3+), gadolinium (Gd3+), or cerium (Ce3+), whose relaxation produces unique activator-dependent emission.6 Since RE-ions are poor in direct light absorption,7,8 their luminescence efficiency highly depends on the efficiency of photon absorption by the host-sensitizer and resonance energy transfer to the luminescence center. Hence, careful selection of the host material is essential in designing RE-NPs with desired optical properties.Among the wide range of RE-host materials, CaWO4 NCs have proven to be appealing due to their excellent optical, chemical, and physical properties.9,10 CaWO4 NCs are built from Ca2+ ions and [WO4]2− luminescent groups with a coordination number of eight for Ca2+ and four for W6+.11 They are also known for their self-activation properties, large quantum yields, and strong absorption in the UV-visible region.10 Moreover, new optical properties of CaWO4 NCs can be obtained by doping with RE-metal ions. RE-doped CaWO4 NCs show characteristic luminescence of RE-ions due to the efficient energy transfer from the host [WO4]2− group to the RE-luminescent center. Owing to their intriguing luminescent properties, RE-CaWO4 NCs have been extensively studied for application in optical devices.12
Although RE-CaWO4 NCs exhibit excellent optical properties and high chemical stability, their potential as a fluorescent sensing probe has rarely been reported. This might be related to the following major impediments. Firstly, traditional RE-CaWO4 NC synthesis procedure usually involves solid-state annealing at extremely high temperatures (>500 °C), which makes it energy-intensive.13–16 Secondly, the conventional RE-CaWO4 NC synthesis procedure mainly results in large (micron-size) and agglomerated nanocrystals with the lack of surface tunability16 and hence they are not ideal for biological applications. Thirdly, CaWO4 NCs show poor solubility in aqueous and physiological solutions and this further limits their solution-based sensing applications.17,18 Hence, the above listed problems need to be resolved for enabling the practicality of this material.
In this work, we for the first time demonstrated the potential of Tb–CaWO4 NCs as a fluorescent probe for sensing and biological imaging applications. Scheme 1 presents the synthesis procedure and applications of Tb–CaWO4 NCs. In our synthesis procedure, nano-sized Tb–CaWO4 NCs were synthesized by employing a facile one-step hydrothermal method at mild temperature (140 °C). Also, to increase the aqueous solubility of Tb–CaWO4 NCs, a biocompatible hydrophilic polymer (polyethylene glycol, PEG-400) was used as a surface coating ligand. Next, the potential of PEG–Tb–CaWO4 NCs as a fluorescent probe was examined by designing a “signal-off–on” sensing strategy for detecting the ALP biomarker. ALP is widely distributed in various organs of the human body and its abnormal level in serum could be associated with various diseases.19,20 Hence, the development of a sensitive and selective ALP detection method helps diagnosis of many diseases. For this purpose, our PEG–Tb–CaWO4-based probe system showed excellent sensitivity in both buffer and real serum samples. Moreover, the cell imaging potential of PEG–Tb–CaWO4 NCs was investigated using HeLa cells. HeLa cells internalized PEG–Tb–CaWO4 NCs through endocytosis and showed characteristic bright green emission, proving the cell imaging capability of PEG–Tb–CaWO4 NCs. The viability study also confirmed the very low cell cytotoxicity of PEG–Tb–CaWO4 NCs to HeLa cells.
 | ||
| Scheme 1 Schematic diagram showing (A) synthesis and (B) fluorescence sensing and cell imaging applications of PEG–Tb–CaWO4 NCs. | ||
2. Experimental section
2.1. Chemicals and reagents
Tris-hydrochloride buffer (Tris–HCl), L-ascorbic acid, and L-ascorbic acid 2-phosphate tri-sodium salt (AAP) were purchased from Aladdin Reagent Co., Ltd (Shanghai, China). Polyethylene glycol (MW, 400), bovine serum albumin (BSA), human serum albumin (HSA), alkaline phosphatase from bovine intestinal mucosa (ALP), sodium chloride (99.99%), sodium tungstate dehydrate (99.9%), and terbium(III) nitrate hexahydrate (99.9%) were purchased from Sigma-Aldrich Co., Ltd (Shanghai, China). Ammonium cerium(IV) sulfate dehydrate (99.5%), calcium chloride (99.9%), glucose oxidase (GOx), cysteine (Cys), and glutathione (GSH) were provided by Macklin Biochemical Technology Co., Ltd (Shanghai, China). Milli-Q water was used to prepare aqueous solutions. All reagents used were of analytical grade and used without any further purification.2.2. Instruments
UV-visible absorption spectra were recorded using a UV-Vis spectrometer (UV-3600i, Shimadzu, Japan). X-ray diffraction patterns were acquired using an X-ray diffractometer (D/Max 2400 X-ray powder diffractometer, Cu Kα radiation). Fluorescence measurements were performed on a Shimadzu RF-6000 fluorescence spectrophotometer. High resolution transmission electron microscopy (HR-TEM) images and energy-dispersive spectra (EDS) were recorded on a JEOL JEM-2100F field-emission high-resolution transmission electron microscope. The surface chemical composition of the nanomaterial was analyzed using X-ray photoelectron spectrophotometry (XPS, Thermo Scientific K-Alpha). Thermal gravimetric analysis was performed using a thermogravimetric analyzer (TGA, TG 209 F3). A Nicolet Nexus 670 FTIR spectrometer was used to obtain Fourier transform infrared spectra. The fluorescence lifetime was obtained on an FLS1000 fluorescence spectrometer.2.3. Synthesis of PEG–Tb–CaWO4 NCs
In a typical synthesis procedure, aqueous solutions of CaCl2 (1 mL, 0.173 mmol) and terbium nitrate (0.2 mL, 0.026 mmol) were mixed with 10 mL of polyethylene glycol and stirred for 5 min at room temperature. Then, an aqueous solution of sodium tungstate (1 mL, 0.199 mmol) was quickly injected into the above mixture and vigorously stirred for another 5 min. The mixture was then transferred into a 20 mL Teflon-lined autoclave and then heated in an oven for 5 h at 140 °C. After cooling to room temperature, the white-milky product was collected by centrifugation at 3500 rpm for 5 min. Then, through consecutive re-dispersion in water (via sonication for 5 min) and centrifugation, the as-obtained product was washed several times (at least 3 times) to remove unreacted substances and impurities. Finally, the purified product was re-dispersed in water via sonication and occasional shaking for 10 minutes and stored as a 5 mg mL−1 stock solution.2.4. Detection of ALP
In a typical procedure, 54 μL of AAP (10 mM) and various concentrations of ALP were mixed with 100 μL of Tris–HCl buffer solution (10 mM, pH 8.5) and incubated for 60 min at 37 °C. Then, 88 μL of aqueous solution of Ce4+ ions (10 mM) was added into the mixture and stored for another 5 min. Subsequently, 60 μL of PEG–Tb–CaWO4 NCs (5 mg mL−1) was added and the final volume was made to 3 mL with ultrapure water. After storing the mixture for another 5 min at room temperature, the fluorescence spectra were measured at excitation and emission wavelengths of 262 nm and 546 nm, respectively. The same procedure was applied to detect ALP in 10-fold diluted serum samples spiked with various amounts of ALP.2.5. In vitro cytotoxicity assay
The cytotoxicity of PEG–Tb–CaWO4 NCs was evaluated by the methyl thiazolyl tetrazolium (MTT) reduction assay using HeLa cells cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS at 37 °C and 5% CO2 in a humidified atmosphere. In a typical procedure, HeLa cells were cultured at 5000 cells per well in a 96-well plate for 12 h. Subsequently, cells were incubated with various concentrations of Tb–CaWO4 NCs in a 5% CO2 atmosphere at a constant temperature of 37 °C for 24 h. Finally, cell viabilities were determined by CCK-8 according to the manufacturer's protocol.2.6. Biological imaging of HeLa cells
HeLa cells were cultured in humidified 5% CO2 for 24 h with DMEM supplemented with 10% FBS. Then, the cultured HeLa cells were transferred to a four-well chambered slide and further incubated for 24 h. Next, the adherent cells were incubated with 100 μg mL−1 PEG–Tb–CaWO4 NCs for 24 h. After washing the adherent cells (3 times) with fresh PBS solution, the cells were observed under a confocal laser scanning microscope (CLSM).3. Results and discussion
3.1. Synthesis and characterization of Tb–CaWO4 NCs
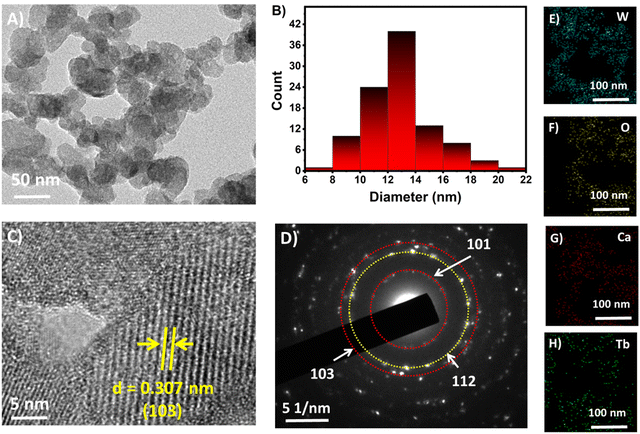
In this study, a solvothermal synthesis approach was employed to prepare PEG coated Tb–CaWO4 NCs. During the synthesis approach, the effect of some of the experimental factors was optimized to obtain the desired product. For instance, Fig. S1A (ESI†) presents the effect of synthesis temperature on the fluorescence intensity of PEG–Tb–CaWO4 NCs. The PEG–Tb–CaWO4 NCs with higher fluorescence intensity were obtained at a heating temperature of 140 °C. Beyond this temperature, the fluorescence intensity started declining, which could be due to cross-relaxation among Tb3+ ions resulting from the agglomeration of particles at higher temperature.21,22 Also, the PEG–Tb–CaWO4 NCs synthesized at lower temperature appeared as a milky white dispersion, while those prepared at higher temperature appeared dark brown (Fig. S1A, inset, ESI†). This could be due to decomposition of surface PEG molecules at higher temperature, which could also result in a decrease in the fluorescence emission intensity of the as-synthesized PEG–Tb–CaWO4 NCs.23,24 Hence, a synthesis temperature of 140 °C was used to prepare the NCs as it enabled the highest fluorescence emission intensity. Next, the effect of synthesis time was optimized. As presented in Fig. S1B (ESI†), a synthesis time of 5 h gave the highest florescence intensity and it was taken as an optimum value. The effect of %Tb3+ ion doping on photoluminescence response was studied and the results are presented in Fig. S2A (ESI†). Based on the fluorescence intensity response, 15% doping showed the highest fluorescence response and it was taken as an optimum. Moreover, the effect of various amounts of PEG in the reaction mixture on the colloidal stability of Tb–CaWO4 NCs was studied. As presented in Fig. S2B (ESI†), Tb–CaWO4 NCs showed higher colloidal stability when the amount of PEG was higher than 50%.Next, the morphology and particle size of the as-synthesized PEG–Tb–CaWO4 NCs were characterized by HR-TEM. The PEG–Tb–CaWO4 NCs showed well-dispersed quasi-spherical particles (Fig. 1A) and size distribution in the range of 6 to 22 nm with an average size of 13 nm (Fig. 1B). Also, the HR-TEM image in Fig. 1C illustrates the lattice fringes with a characteristic spacing of the lattice surface of 0.307 nm corresponding to the (103) crystallographic plane of PEG–Tb–CaWO4 NCs (JCPDS Card No. 41-1431). Fig. 1D presents the selected area electron diffraction (SAED) image of PEG–Tb–CaWO4 NCs. The SAED image reveals dense diffraction patterns arranged into rings forming continuous concentric circles. This indicates the polycrystalline nature of the as-synthesized PEG–Tb–CaWO4 NCs, which is also in good agreement with HR-TEM results (Fig. S3, ESI†). Moreover, the elemental mapping results (Fig. 1E–H) show the presence of calcium, terbium, tungsten and oxygen. More importantly, the results confirmed the successful doping of terbium ions into the crystal structure of CaWO4 NCs. The successful doping of Tb3+ ions was further proved by the presence of a Tb-peak in the EDS spectra (Fig. S4, ESI†).
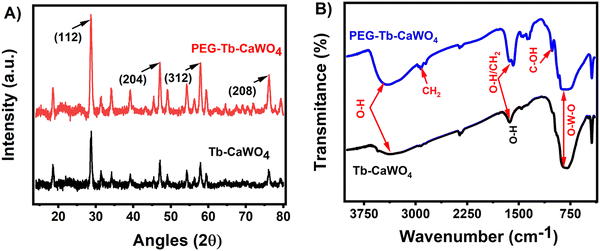
To understand the crystal structure of the as-synthesized PEG–Tb–CaWO4 NCs, X-ray diffraction patterns were recorded. As presented in Fig. S5 (ESI†), PEG–Tb–CaWO4 NCs exhibit diffraction patterns similar to the standard data of CaWO4 NCs (JCPDS Card No. 41-1431).25 Both uncoated and PEG coated Tb–CaWO4 NCs (Fig. 2A) showed identical diffraction patterns with four intense diffraction peaks at 2θ values of 28.7, 47.1, 57.8, and 76.1°, which, respectively, belong to the (112), (204), (312) and (208) planes (JCPDS no. 41-1431).25 From the results, it can be concluded that both the doping of Tb3+ ions and surface coating with PEG molecules had no or little effect on the crystal nature of CaWO4 NCs.
Furthermore, the FT-IR, XPS, and TGA measurements were performed to understand the surface chemical composition of Tb–CaWO4 NCs. In the FT-IR spectra (Fig. 2B), both uncoated and PEG coated Tb–CaWO4 NCs showed strong absorption bands at about 800 cm−1, characteristic of the anti-symmetric stretching vibrations of O–W–O within the WO4 tetrahedron.26 The strong absorption bands at about 3600–3000 cm−1 could be assigned to the O–H stretching vibration from PEG molecules in coated Tb–CaWO4 NCs or physically adsorbed water molecules in uncoated Tb–CaWO4 NCs. The coating of PEG molecules on the surface of Tb–CaWO4 NCs was confirmed by the appearance of additional peaks of CH2 stretching (2910 cm−1), CH2 bending/scissoring (1595 cm−1), and C–OH stretching (1016 cm−1) vibrations.27Fig. 3A–F shows the XPS spectra of the as-synthesized PEG–Tb–CaWO4 NCs. The survey XPS results confirmed the presence of calcium (Ca), tungsten (W), terbium (Tb), carbon (C), and oxygen (O) elements. More importantly, the appearance of the carbon peak proved the PEG surface coating on PEG–Tb–CaWO4 NCs. Also, the two peaks at 287.68 and 284.73 eV in the deconvoluted C 1s spectrum (Fig. 3E) can be assigned to the C–O and C–C/C–H of surface PEG molecules,28,29 respectively. Moreover, the presence of a C–O peak (531.6 eV) in the high resolution XPS spectrum of O 1s (Fig. 3F) confirmed the presence of PEG molecules on the surface of PEG coated Tb–CaWO4 NCs. Finally, TGA analysis was performed to further confirm the coating of PEG molecules on the surface of PEG–Tb–CaWO4 NCs. As presented in Fig. S6 (ESI†), due to the decomposition of surface PEG molecules, the PEG coated Tb–CaWO4 NCs exhibited a higher decomposition rate than that of uncoated Tb–CaWO4 NCs. This result, along with FT-IR and XPS results, strongly confirmed the coating of PEG molecules on PEG–Tb–CaWO4 NCs. The presence of PEG molecules on the surface of PEG–Tb–CaWO4 NCs significantly improved the colloidal stability, while uncoated PEG–Tb–CaWO4 NCs aggregated within a few minutes of dispersion (Fig. S7, ESI†).
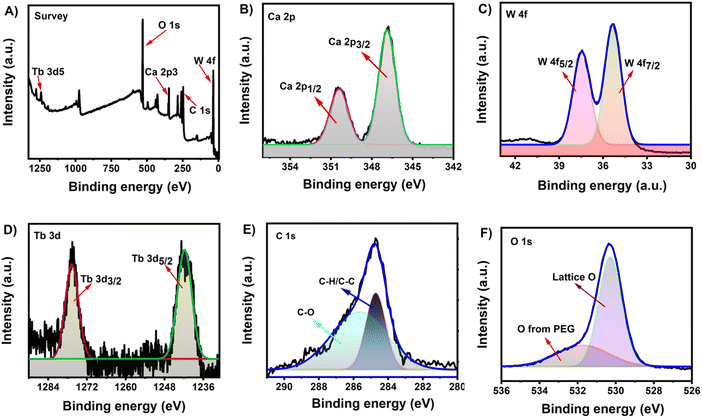 | ||
| Fig. 3 (A) XPS survey spectrum of PEG–Tb–CaWO4 NCs. High resolution spectra of (B) Ca 2p, (C) W 4f, (D) Tb 3d, (E) C 1s, and (F) O 1s. | ||
3.2. Optical properties of PEG–Tb–CaWO4 NCs
The UV-visible absorption spectra of CaWO4 and PEG–Tb–CaWO4 NCs are presented in Fig. 4A. The CaWO4 NCs showed a maximum absorption peak at 246 nm, which is attributed to the O-to-W charge transfer transition within the [WO4]2− group.30,31 PEG–Tb–CaWO4 NCs showed a maximum absorbance at 254 nm with an additional broad absorption peak in the wavelength range of 260–370 nm, which could be attributed to the f–f electronic transitions of Tb3+ ions.32 It can be noted that the absorption edge of PEG–Tb–CaWO4 NCs red-shifted by about 8 nm in comparison to CaWO4 NCs. This could be due to the occurrence of energy transfer from the host [WO4]2− group to Tb3+ ions as has been reported elsewhere.32,33 The band gaps of CaWO4 and PEG–Tb–CaWO4 NCs computed according to the Tauc model34 were 4.5 and 4.2 eV (Fig. 4B), respectively.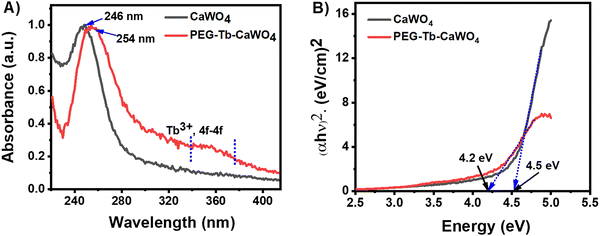 | ||
| Fig. 4 (A) UV-visible absorption spectra of CaWO4 and PEG–Tb–CaWO4 NCs. (B) Corresponding spectra showing the Tauc plot curve for calculating the energy band gap. | ||
Fig. 5A depicts the possible excitation, emission, and energy transfer processes in CaWO4 and PEG–Tb–CaWO4 NCs. The photoluminescence emission spectrum of undoped CaWO4 consists of a strong and broad band from 300 to 600 nm, with the maximum emission at 415 nm (Fig. S8, ESI†). This is due to O-to-W charge transfer transitions within the [WO4]2− group.35,36 The blue luminescence of solid CaWO4 NCs was also demonstrated using UV-light at 254 nm (Fig. S8, inset, ESI†) and the chromaticity coordinates of x = 0.1472 and y = 0.0413 (Fig. S9A, ESI†). Upon the introduction of Tb3+ ions, the luminescence properties of CaWO4 NCs changed due to the energy transfer from the [WO4]2− group to luminescent Tb3+ ions as schematically shown in Fig. 5A and B. This was proved by the presence of a strong O-to-W charge transfer transition band in the excitation spectrum of PEG–Tb–CaWO4 NCs at an emission wavelength of 547 nm (Fig. 5C).37 In the photoluminescence emission spectrum of PEG–Tb–CaWO4 NCs, the wide and broad band of the host CaWO4 almost disappeared with the appearance of sharp and narrow bands and characteristic green luminescence in both solid powder and aqueous dispersion (Fig. 5D and insets). The emission spectrum of PEG–Tb–CaWO4 NCs consists of four distinct bands at wavelengths of 470 (5D4 → 7F6), 546 (5D4 → 7F5), 588 (5D4 → 7F4), and 622 nm (5D4 → 7F3),38 among which the 5D4 → 7F5 transition dominated and resulted in a characteristic vibrant green luminescence. Also, the calculated chromaticity coordinates (x = 0.3192, y = 0.5908) were in the green region of the CIE diagram (Fig. S9B, ESI†).
3.3. Stability of PEG–Tb–CaWO4 NCs
The stability of a nanomaterial is a key determining factor for its practical sensing applications. In this regard, the luminescence stability of PEG–Tb–CaWO4 NCs against ionic concentration, storage and pH was examined. From the fluorescence intensity response (Fig. S10A, ESI†), it can be observed that PEG–Tb–CaWO4 NCs demonstrated excellent stability against various ionic concentrations. Also, the fluorescence intensity of PEG–Tb–CaWO4 NCs showed excellent storage stability (Fig. S10B, ESI†). Moreover, as we were intended to employ PEG–Tb–CaWO4 NCs as a fluorescence probe for the detection of ALP enzyme, which requires alkaline conditions, its fluorescence emission stability in alkaline medium was also investigated. Interestingly, the excellent fluorescence intensity of PEG–Tb–CaWO4 NCs in the pH range from 7.0 to 9.5 proved its potential for application in alkaline medium (Fig. S10C, ESI†).3.4. Designing a PEG–Tb–CaWO4 NC based sensor for ALP detection
To test the fluorescence sensing potential of PEG–Tb–CaWO4 NCs, a “signal-off–on” sensing strategy was proposed to detect ALP (Scheme 2A). In the detection system, Ce4+ ions were employed as an effective fluorescence signal quencher. From Fig. S11 (ESI†), the fluorescence emission of the PEG–Tb–CaWO4 probe significantly decreased with increasing concentrations of Ce4+ ions, demonstrating their effectiveness as a fluorescence quencher. However, as presented in Scheme 2B, ALP can dephosphorylate its substrate AAP into AA and phosphate ions. The in situ generated AA undergoes a redox reaction with Ce4+ to produce Ce3+ ions (eqn (S1), ESI†),38 which then combine with phosphate ions to form a non-quenching CePO4 precipitate, hence resulting in fluorescence recovery. | ||
| Scheme 2 Schematic diagram showing (A) fluorescence responses with and without target ALP and (B) possible reactions in the detection system. | ||
Fig. 6A and the inset present the quenched and recovered fluorescence emission of the detection system. To understand the fluorescence quenching process in the PEG–Tb–CaWO4/Ce4+ system, the involvement of various possible mechanisms was examined. First, the involvement of static quenching was investigated. One of the rapid identification techniques for static quenching is a change in the absorption spectrum of the fluorophore due to the formation of a fluorophore–quencher complex.39,40 However, there is no noticeable change in the absorption of PEG–Tb–CaWO4 after the addition of Ce4+ ions (Fig. S12, ESI†) and hence the involvement of static quenching can be ruled out. But it should be noted that the UV-visible absorption spectrum of Ce4+ ions significantly overlapped with the excitation spectrum of PEG–Tb–CaWO4 NCs (Fig. S13, ESI†). This suggests the involvement of energy transfer (FRET) or inner filter effect (IFE) quenching, which leads to disruption of energy transfer from [WO4]2− to Tb3+ ions and hence results in fluorescence quenching. To identify which one occurred, we measured the lifetimes of the PEG–Tb–CaWO4 NCs with and without the addition of Ce4+ ions (Fig. 6B). For the IFE, the quencher did not cause a change in average lifetime (τ0/τ = 1), while the lifetime was expected to change in the resonance energy transfer process (τ0/τ ≠ 1).41 From the lifetime measurement results, the average lifetimes of PEG–Tb–CaWO4 and PEG–Tb–CaWO4/Ce4+ were found to be 1.14 and 0.98 ms, respectively. The result indicated that the addition of Ce4+ did not lead to a significant change in the lifetime of PEG–Tb–CaWO4 NCs, and hence the quenching mechanism could be the IFE rather than FRET.
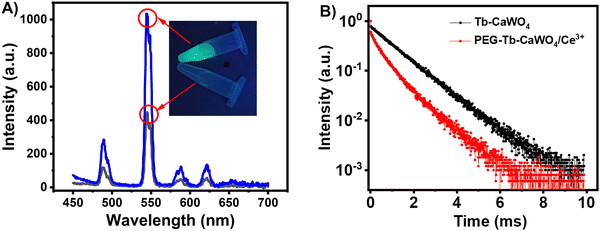 | ||
| Fig. 6 (A) Fluorescence responses of the detection system with and without target ALP. (B) Fluorescence decay lifetime curves of PEG–Tb–CaWO4 and Tb–CaWO4/Ce4+ (τav = 1.14 and 0.93 ms, respectively). | ||
3.5. Analytical performance of the PEG–Tb–CaWO4 NC based sensor
To obtain the best analytical performance, the effects of various experimental conditions such as pH, the concentration of AAP, enzymatic reaction time, and the concentration of Ce4+ ions were optimized. As alkaline phosphatase enzyme performs well in alkaline conditions,42 a pH higher than 7.0 was adopted. Also, a pH > 9.0 was not considered since Ce4+ displayed low quenching efficiency beyond this pH (Fig. S14A, ESI†). Moreover, ALP exhibited higher activity at a pH of 8.5 (Fig. S14B, ESI†) and thus this pH was used for the following experiments. In addition, the detection system showed better fluorescence recovery at an AAP concentration of 180 μM and an enzymatic reaction time of 60 min (Fig. S14 C and D, ESI†). Moreover, the amounts of PEG–Tb–CaWO4 NCs were optimized and 100 μg mL−1 was taken as an optimum (Fig. S14E, ESI†). Lastly, as 260 μM of Ce4+ ions (1.625 times higher) were required to completely oxidize 160 μM of AA (Fig. S15, ESI†), and assuming complete conversion of 180 μM of AAP into AA, 292 μM of Ce4+ ions (180 × 1.625) was considered as an optimum concentration.Next, the analytical performance of the proposed sensor for the detection of ALP was evaluated. As presented in Fig. 7A, the fluorescence intensity of the detection system gradually increased with an increase in ALP concentration in the range from 6 to 70 U L−1. By plotting the fluorescence intensity ratio (F/F0) versus the concentration of ALP, the linear response range from 6 to 40 U L−1 with a linear fitting equation of F/F0 = 0.27 + 0.13[ALP] (U L−1) could be obtained (Fig. 7B). The LOD (3σ rule) was calculated to be 0.5 U L−1. Considering the normal range of ALP (40–120 U L−1) in adults,43 the proposed detection procedure is sensitive enough to detect low concentrations of ALP. Moreover, the analytical performance of the proposed detection system was compared with previous approaches (Table S1, ESI†). It can be seen that the proposed detection system exhibited a lower or comparable LOD with these methods. This proves the capability of the proposed fluorescence method for the detection of ALP.
 | ||
| Fig. 7 (A) Fluorescence response of the detection system with the addition of various concentrations of ALP. (B) Corresponding linear range. | ||
3.6. Selectivity and anti-interference test
The selectivity of the detection system was evaluated by recording its fluorescence response toward various potentially interfering biomolecules and commonly coexisting cations and anions. The tested biomolecules include amylase, bovine serum albumin (BSA), glucose oxidase (GOx), glutathione (GSH), human serum albumin (HAS), cysteine (Cyst), histidine (His), sulfatase, trypsin (Try), Na+, K+, Ca2+, Fe3+, Cl−, HCO3−, CO32−, SO42−, and NO3−. As presented in Fig. 8A, only the introduction of ALP caused significant fluorescence recovery, while other biomolecules and enzymes hardly caused changes in the fluorescence of the detection system. Also, the anti-interference performance of the detection system (Fig. 8B) was proved as the coexistence of these biomolecules and enzymes did not significantly affect the fluorescence response of the detection method. In conclusion, the detection platform exhibits excellent selectivity and anti-interference performance.3.7. Detection of ALP in serum samples
The applicability of the proposed method for the detection of ALP in a real biological matrix was tested by using serum samples. Considering the similarity in the components of mammals (including humans) and easy availability,44 fetal bovine serum (FBS) was used to test the practical applicability of the as-proposed method for ALP detection. As presented in Table S2 (ESI†), the recoveries of the spiked ALP ranged from 83.9% to 107.7% with a RSD of 1.5% to 2.1%. The results demonstrate the potential applicability of the proposed method for the detection of ALP in real and complex biological samples.3.8. Cytotoxicity and cell imaging applications
We assessed the cytotoxicity of the PEG–Tb–CaWO4 NCs by examining their effect on the cell viability of HeLa cells using the MTT assay. It can be seen from Fig. S16 (ESI†) that increasing the concentration of PEG–Tb–CaWO4 NCs did not affect the cell viability of HeLa cells. Even at a relatively high dose of PEG–Tb–CaWO4 NCs (400 μg mL−1), the cell viability still remained above 90%, demonstrating the very low cytotoxicity of the NCs. The observed non-toxicity of PEG–Tb–CaWO4 NCs could be attributed to the good biocompatibility of PEG to the biological system45 and the minimal probability of ion leakage from the PEG–Tb–CaWO4 NCs in the cells.18,46 Based on these results, it can be inferred that PEG–Tb–CaWO4 NCs are nontoxic to live cells and can be used for in vivo biological applications.Furthermore, HeLa cells were employed to investigate the biological imaging potential of PEG–Tb–CaWO4 NCs (Fig. 9). From the green channel of CLSM images, no fluorescence signal can be detected from the control group (Fig. 9B), while a bright green fluorescence can be observed from the PEG–Tb–CaWO4 NC treated cells (Fig. 9D). This indicates effective cellular uptake of PEG–Tb–CaWO4 NCs by the HeLa cells. Also, the green fluorescence emission was observed in the cytoplasmic area, while no luminescence was observed at the nucleus, suggesting that PEG–Tb–CaWO4 NCs were internalized through endocytosis and diffused into the cytoplasm.47,48 Hence, based on the viability test and cell imaging results, it can be concluded that PEG–Tb–CaWO4 NCs exhibited low cytotoxicity and could be considered as a potential probe for in vivo biological applications.
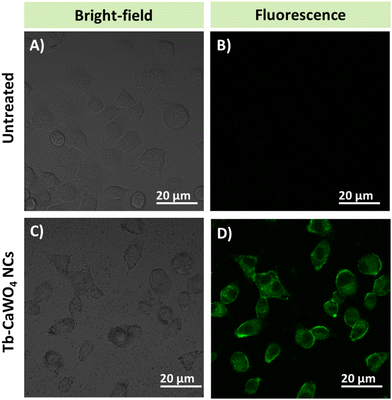 | ||
| Fig. 9 CLSM images of (A) and (B) untreated and (C) and (D) PEG–Tb–CaWO4 NC treated HeLa cells upon 488 nm laser irradiation. | ||
4. Conclusions
Unlike conventional solid-state methods, which require annealing at extremely high temperatures and result in micron-sized NCs, we successfully synthesized nano-sized PEG–Tb–CaWO4 NCs with excellent luminescence properties at a mild temperature (140 °C). In addition, coating with the PEG ligand significantly improved the colloidal stability of Tb–CaWO4 NCs in aqueous and biological solutions. Furthermore, for the first time, we demonstrated the sensing and biological imaging applications of PEG–Tb–CaWO4 NCs. To this end, a fluorescence “signal-off–on” sensing strategy was developed to detect a useful disease biomarker, ALP. The as-constructed sensor exhibited excellent sensitivity for the detection of ALP in both buffer solution as well as serum samples. Moreover, the cell imaging potential of PEG–Tb–CaWO4 NCs was demonstrated by the bright green luminescence observed in HeLa cells incubated with the NCs. The viability study also confirmed the very low cell cytotoxicity of PEG–Tb–CaWO4 NCs to HeLa cells. Overall, in this study, we demonstrated and highlighted the potential of PEG–Tb–CaWO4 NCs as a promising probe for sensing and biological applications.Author contributions
Terefe Tafese Bezuneh contributed to the investigation involving synthesis, characterization, visualization, and writing original draft, review, and editing of the manuscript. Xin Li, Tongtong Kou, Fuchun Nan, Zilong Wu, and Lanbo Shen supported various characterization studies and investigation. Xinyuan Xia provided funding acquisition and resources. Bin Li was responsible for funding acquisition, supervision and resources. Liang Wang was responsible for project administration, conceptualization, and supervision. William W. Yu conceptualized this work and was responsible for supervision, project administration, funding acquisition, and review and editing of the manuscript.Data availability
All experimental details and data supporting the findings of this article are available within the paper and also included in the ESI.†Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was financially supported by the Natural Science Foundation of China (62374103), the Jinan Central Hospital (1190022050), the Taishan Scholar Foundation of Shandong Province (tsqn2023120051105), the Shandong Postdoctoral Innovation Project (SDCX-ZG-202400274), and Shandong Provincial Natural Science Foundation (ZR2022QH069, ZR2020MB083, and ZR2023QE322).References
- S. Y. Choi, S. H. Baek, S.-J. Chang, Y. Song, R. Rafique, K. T. Lee and T. J. Park, Synthesis of upconversion nanoparticles conjugated with graphene oxide quantum dots and their use against cancer cell imaging and photodynamic therapy, Biosens. Bioelectron., 2017, 93, 267–273 CrossRef CAS PubMed.
- D. Su, X. Zhao, X. Yan, X. Han, Z. Zhu, C. Wang, X. Jia, F. Liu, P. Sun, X. Liu and G. Lu, Background-free sensing platform for on-site detection of carbamate pesticide through upconversion nanoparticles-based hydrogel suit, Biosens. Bioelectron., 2021, 194, 113598 CrossRef CAS PubMed.
- Z. Yi, Z. Luo, X. Qin, Q. Chen and X. Liu, Lanthanide-activated nanoparticles: a toolbox for bioimaging, therapeutics, and neuromodulation, Acc. Chem. Res., 2020, 53, 2692–2704 CrossRef CAS PubMed.
- Q.-X. Wang, S.-F. Xue, Z.-H. Chen, S.-H. Ma, S. Zhang, G. Shi and M. Zhang, Dual lanthanide-doped complexes: the development of a time-resolved ratiometric fluorescent probe for anthrax biomarker and a paper-based visual sensor, Biosens. Bioelectron., 2017, 94, 388–393 CrossRef CAS PubMed.
- D. Kang, S. Lee, H. Shin, J. Pyun and J. Lee, An efficient NIR-to-NIR signal-based LRET system for homogeneous competitive immunoassay, Biosens. Bioelectron., 2020, 150, 111921 CrossRef CAS PubMed.
- R. Marin and D. Jaque, Doping Lanthanide Ions in Colloidal Semiconductor Nanocrystals for Brighter Photoluminescence, Chem. Rev., 2021, 121, 1425–1462 CrossRef CAS PubMed.
- R. Xiong, D. Mara, J. Liu, R. Van Deun and K. E. Borbas, Excitation- and Emission-Wavelength-Based Multiplex Spectroscopy Using Red-Absorbing Near-Infrared-Emitting Lanthanide Complexes, J. Am. Chem. Soc., 2018, 140, 10975–10979 CrossRef CAS PubMed.
- F. Ahmed, M. Muzammal Hussain, W. Ullah Khan and H. Xiong, Exploring recent advancements and future prospects on coordination self-assembly of the regulated lanthanide-doped luminescent supramolecular hydrogels, Coord. Chem. Rev., 2024, 499, 215486 CrossRef CAS.
- J. Janbua, J. Mayamae, S. Wirunchit, R. Baitahe and N. Vittayakorn, Directed synthesis, growth process and optical properties of monodispersed CaWO4 microspheres via a sonochemical route, RSC Adv., 2015, 5, 19893–19899 RSC.
- R. Gonçalves, M. Godinho, A. Marques, M. Santos, I. Rosa, E. Longo, M. S. Li, J. Sa and L. Cavalcante, Structure, morphology, and optical properties of (Ca1−3xEu2x)WO4 microcrystals, Electron. Mater. Lett., 2015, 11, 193–197 CrossRef.
- Y. Tian, B. Chen, H. Yu, R. Hua, X. Li, J. Sun, L. Cheng, H. Zhong, J. Zhang, Y. Zheng, T. Yu and L. Huang, Controllable synthesis and luminescent properties of three-dimensional nanostructured CaWO4:Tb3+ microspheres, J. Colloid Interface Sci., 2011, 360, 586–592 CrossRef CAS PubMed.
- S. Wang, H. Gao, G. Sun, Y. Li, Y. Wang, H. Liu, C. Chen and Y. Liang, Structure characterization, optical and photoluminescence properties of scheelite-type CaWO4 nanophosphors: Effects of calcination temperature and carbon skeleton, Opt. Mater., 2020, 99, 109562 CrossRef CAS.
- A. M. Kaczmarek and R. Van Deun, Rare earth tungstate and molybdate compounds – from 0D to 3D architectures, Chem. Soc. Rev., 2013, 42, 8835–8848 RSC.
- H. P. Barbosa, I. G. N. Silva, M. C. F. C. Felinto, E. E. S. Teotonio, O. L. Malta and H. F. Brito, Photoluminescence of single-phased white light emission materials based on simultaneous Tb3+, Eu3+ and Dy3+ doping in CaWO4 matrix, J. Alloys Compd., 2017, 696, 820–827 CrossRef CAS.
- M. V. Nazarov, D. Y. Jeon, J. H. Kang, E. J. Popovici, L. E. Muresan, M. V. Zamoryanskaya and B. S. Tsukerblat, Luminescence properties of europium–terbium double activated calcium tungstate phosphor, Solid State Commun., 2004, 131, 307–311 CrossRef CAS.
- V. Dabre and K. S. J. Dhoble, Thermoluminescence glow curve analysis of Eu3+ activated CaWO4 phosphor, Adv. Mater. Lett., 2013, 4, 921–926 CrossRef.
- J. Lee, S. Choi, K. H. Kim, H. G. Heng, S. E. Torregrosa-Allen, B. S. Ramsey, B. D. Elzey and Y.-Y. Won, Nontoxic Formulations of Scintillation Nanocrystals for Use as X-ray Computed Tomography Contrast Agents, Bioconjugate Chem., 2017, 28, 171–182 CrossRef CAS PubMed.
- J. Lee, N. J. Rancilio, J. M. Poulson and Y.-Y. Won, Block Copolymer-Encapsulated CaWO4 Nanoparticles: Synthesis, Formulation, and Characterization, ACS Appl. Mater. Interfaces, 2016, 8, 8608–8619 CrossRef CAS PubMed.
- F. Ma, W.-J. Liu, L. Liang, B. Tang and C.-Y. Zhang, Sensitive detection of alkaline phosphatase by dephosphorylation-initiated transcription reaction-mediated dual signal amplification, Chem. Commun., 2018, 54, 2413–2416 RSC.
- T. Xiao, J. Sun, J. Zhao, S. Wang, G. Liu and X. Yang, FRET Effect between Fluorescent Polydopamine Nanoparticles and MnO2 Nanosheets and Its Application for Sensitive Sensing of Alkaline Phosphatase, ACS Appl. Mater. Interfaces, 2018, 10, 6560–6569 CrossRef CAS PubMed.
- O. Lehmann, K. Kömpe and M. Haase, Synthesis of Eu3+-doped core and core/shell nanoparticles and direct spectroscopic identification of dopant sites at the surface and in the interior of the particles, J. Am. Chem. Soc., 2004, 126, 14935–14942 CrossRef CAS PubMed.
- K. Kömpe, H. Borchert, J. Storz, A. Lobo, S. Adam, T. Möller and M. Haase, Green-emitting CePO4: Tb/LaPO4 core–shell nanoparticles with 70% photoluminescence quantum yield, Angew. Chem., Int. Ed., 2003, 42, 5513–5516 CrossRef PubMed.
- B. Chen, J. R. G. Evans and S. Holding, Decomposition of poly(ethylene glycol) in nanocomposites, J. Appl. Polym. Sci., 2004, 94, 548–552 CrossRef CAS.
- S. Han, C. Kim and D. Kwon, Thermal/oxidative degradation and stabilization of polyethylene glycol, Polymer, 1997, 38, 317–323 CrossRef CAS.
- J. Liao, B. Qiu, H. Wen and W. You, Photoluminescence green in microspheres of CaWO4:Tb3+ processed in conventional hydrothermal, Opt. Mater., 2009, 31, 1513–1516 CrossRef CAS.
- A. J. Peter and I. B. Shameem Banu, Synthesis and luminescent properties of Tb3+ activated AWO4 based (A = Ca and Sr) efficient green emitting phosphors, J. Mater. Sci.: Mater. Electron., 2014, 25, 2771–2779 CrossRef CAS.
- J. Kluczka, G. Dudek, A. Kazek-Kęsik and M. Gnus, Chitosan Hydrogel Beads Supported with Ceria for Boron Removal, Int. J. Mol. Sci., 2019, 20, 1567 CrossRef CAS PubMed.
- X. She, D. Yang, D. Jing, F. Yuan, W. Yang, L. Guo and Y. Che, Nitrogen-doped one-dimensional (1D) macroporous carbonaceous nanotube arrays and their application in electrocatalytic oxygen reduction reactions, Nanoscale, 2014, 6, 11057–11061 RSC.
- J. Y. Kim, T. Kim, J. W. Suk, H. Chou, J. H. Jang, J. H. Lee, I. N. Kholmanov, D. Akinwande and R. S. Ruoff, Enhanced dielectric performance in polymer composite films with carbon nanotube–reduced graphene oxide hybrid filler, Small, 2014, 10, 3405–3411 CrossRef CAS PubMed.
- M. J. Treadaway and R. C. Powell, Luminescence of calcium tungstate crystals, J. Chem. Phys., 1974, 61, 4003–4011 CrossRef CAS.
- T. George, S. Joseph, A. T. Sunny and S. Mathew, Fascinating morphologies of lead tungstate nanostructures by chimie douce approach, J. Nanopart. Res., 2008, 10, 567–575 CrossRef CAS.
- S. Kuriakose, H. Hitha, A. Jose, M. John and T. Varghese, Studies on the effect of Tb doping on the structural and optical properties of La2(WO4)3 nanoparticles, Mater. Today: Proc., 2023 DOI:10.1016/j.matpr.2023.11.132.
- S. Schwung, D. Rytz, B. Heying, U. C. Rodewald, O. Niehaus, D. Enseling, T. Jüstel and R. Pöttgen, The crystal structure and luminescence quenching of poly-and single-crystalline KYW2O8:Tb3+, J. Lumin., 2015, 166, 289–294 CrossRef CAS.
- M. Liu, L. Zheng, J. Deng, J. Gao, K. Su, X. Sheng, J. He, D. Feng, L. Guo and C. Chen, Construction of Ag nanoparticle decorated AgBr/BiVO4 shell/core structure plasmonic photocatalysts towards high photocatalytic elimination of contaminations under visible light, J. Alloys Compd., 2023, 931, 167584 CrossRef CAS.
- S. K. Gupta, K. Sudarshan, P. Ghosh, K. Sanyal, A. Srivastava, A. Arya, P. Pujari and R. Kadam, Luminescence of undoped and Eu3+ doped nanocrystalline SrWO4 scheelite: time resolved fluorescence complimented by DFT and positron annihilation spectroscopic studies, RSC Adv., 2016, 6, 3792–3805 RSC.
- B. Ding, C. Han, L. Zheng, J. Zhang, R. Wang and Z. Tang, Tuning oxygen vacancy photoluminescence in monoclinic Y2WO6 by selectively occupying yttrium sites using lanthanum, Sci. Rep., 2015, 5, 9443 CrossRef PubMed.
- J. Liao, B. Qiu, H. Wen, J. Chen and W. You, Hydrothermal synthesis and photoluminescence of SrWO4:Tb3+ novel green phosphor, Mater. Res. Bull., 2009, 44, 1863–1866 CrossRef CAS.
- N. K. Sahu, N. Shanta Singh, R. S. Ningthoujam and D. Bahadur, Ce3+-Sensitized GdPO4:Tb3+ Nanorods: An Investigation on Energy Transfer, Luminescence Switching, and Quantum Yield, ACS Photonics, 2014, 1, 337–346 CrossRef CAS.
- W. Zhai, C. Wang, P. Yu, Y. Wang and L. Mao, Single-Layer MnO2 Nanosheets Suppressed Fluorescence of 7-Hydroxycoumarin: Mechanistic Study and Application for Sensitive Sensing of Ascorbic Acid in Vivo, Anal. Chem., 2014, 86, 12206–12213 CrossRef CAS PubMed.
- H. Wang, C. Zheng, T. Dong, K. Liu, H. Han and J. Liang, Wavelength Dependence of Fluorescence Quenching of CdTe Quantum Dots by Gold Nanoclusters, J. Phys. Chem. C, 2013, 117, 3011–3018 CrossRef CAS.
- J. R. Lakowicz and B. R. Masters, Principles of fluorescence spectroscopy, J. Biomed. Opt., 2008, 13, 029901 CrossRef.
- Y. Zeng, J.-Q. Ren, S.-K. Wang, J.-M. Mai, B. Qu, Y. Zhang, A.-G. Shen and J.-M. Hu, Rapid and Reliable Detection of Alkaline Phosphatase by a Hot Spots Amplification Strategy Based on Well-Controlled Assembly on Single Nanoparticle, ACS Appl. Mater. Interfaces, 2017, 9, 29547–29553 CrossRef CAS PubMed.
- T.-U. Hausamen, R. Helger, W. Rick and W. Gross, Optimal conditions for the determination of serum alkaline phosphatase by a new kinetic method, Clin. Chim. Acta, 1967, 15, 241–245 CrossRef CAS.
- Z. Zhang, Z. Chen, S. Wang, F. Cheng and L. Chen, Iodine-Mediated Etching of Gold Nanorods for Plasmonic ELISA Based on Colorimetric Detection of Alkaline Phosphatase, ACS Appl. Mater. Interfaces, 2015, 7, 27639–27645 CrossRef CAS PubMed.
- K. Liu, X. Liu, Q. Zeng, Y. Zhang, L. Tu, T. Liu, X. Kong, Y. Wang, F. Cao, S. A. G. Lambrechts, M. C. G. Aalders and H. Zhang, Covalently Assembled NIR Nanoplatform for Simultaneous Fluorescence Imaging and Photodynamic Therapy of Cancer Cells, ACS Nano, 2012, 6, 4054–4062 CrossRef CAS PubMed.
- A. L. Gomes Cornélio, L. P. Salles, M. Campos da Paz, J. A. Cirelli, J. M. Guerreiro-Tanomaru and M. Tanomaru Filho, Cytotoxicity of Portland Cement with Different Radiopacifying Agents: A Cell Death Study, J. Endodontics, 2011, 37, 203–210 CrossRef PubMed.
- S. Zhang, H. Gao and G. Bao, Physical Principles of Nanoparticle Cellular Endocytosis, ACS Nano, 2015, 9, 8655–8671 CrossRef CAS PubMed.
- H. Li, X. Yan, S. Qiao, G. Lu and X. Su, Yellow-Emissive Carbon Dot-Based Optical Sensing Platforms: Cell Imaging and Analytical Applications for Biocatalytic Reactions, ACS Appl. Mater. Interfaces, 2018, 10, 7737–7744 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5qm00122f |
| This journal is © the Partner Organisations 2025 |