Ultrasound defect sensitive mechanochromic material with blue-shifted emission for the detection of Cu2+ in Alzheimer's disease cells†
Aayoosh
Singh
 a,
Pranjalee
Yadav
a,
Pranjalee
Yadav
 a,
Amit K.
Singh
a,
Rupen
Tamang
b,
Biplob
Koch
a,
Amit K.
Singh
a,
Rupen
Tamang
b,
Biplob
Koch
 b and
Vinod P.
Singh
b and
Vinod P.
Singh
 *a
*a
aDepartment of Chemistry, Institute of Science, Banaras Hindu University, Varanasi-221005, India. E-mail: singhvp@bhu.ac.in
bGenotoxicology and Cancer Biology Laboratory, Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi-221005, India
First published on 28th March 2025
Abstract
The mechanistic investigation and design of ultra-sensitive smart materials with multi-stimuli responsive properties are attracting much interest due to their utilization in several areas concurrently, such as mechanochromic and acidochromic materials, defect sensors, and chemosensors for analytes. Herein, the tailoring of an external stimuli-responsive novel coumarin-based luminogen (CFH) exhibiting green emission (λem = 515 nm) in the liquid state and red emission (λem = 698 nm) in the solid state opens up new avenues for the design of near-infrared emitting coumarin-based materials. CFH is an aggregation-induced enhanced emission (AIEE)-active material exhibiting solvatochromism and viscochromism. The weakly emissive crystals of CFH showed a relatively rare blue-shifted (48 nm) enhanced emission (3-fold) upon grinding. Fluorescence microscopy demonstrated that defect areas on the crystal surface become extremely emissive, indicating a “turn-on” defect-sensitive mechanochromism, susceptible to impact, friction, sculpting and ultrasonic vibrations. Solid CFH displayed acidochromic properties with extraordinary reversibility when exposed to trifluoroacetic acid (TFA)/triethylamine (TEA) vapour, displaying an on–off–on emission. Furthermore, CFH demonstrated “on–off” fluorescence responses to Cu2+ in water, exhibiting a detection limit (LOD) of 1.1 nM and Stern–Volmer constant (Ksv) of 2.84 × 106 M−1. The Job's plot and SC-XRD demonstrated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry for the CFH–Cu2+ complex. Leveraging this fluorescence response, portable test kit devices were developed for the detection of Cu2+. Bioimaging was carried out to examine the quenching of the probe with accumulated Cu2+ in SH-SY5Y model cells for neurodegenerative disorders compared to HEK-293 cells, suggesting that CFH can also be used for the intracellular sensing of Cu2+ in Alzheimer's disease (AD) cells.
1 binding stoichiometry for the CFH–Cu2+ complex. Leveraging this fluorescence response, portable test kit devices were developed for the detection of Cu2+. Bioimaging was carried out to examine the quenching of the probe with accumulated Cu2+ in SH-SY5Y model cells for neurodegenerative disorders compared to HEK-293 cells, suggesting that CFH can also be used for the intracellular sensing of Cu2+ in Alzheimer's disease (AD) cells.
Introduction
Currently, the scientific community is witnessing a huge acceleration in the advancement of organic fluorophores with multifarious properties in the solid and liquid states owing to their diverse applications. In this context, materials exhibiting a reversible change in luminescence properties when exposed to environmental stimuli such as pressure, ultrasound, light,1 temperature,2 solvent and pH3 find a wide variety of applications. In the class of smart materials, when mechanical stimulations like shearing, grinding and compressing lead to changes in luminescence properties (viz., intensity and wavelength), such materials are called mechanochromic luminescent (MCL) materials. These materials are exploited in tamper-proof packaging, force or pressure sensor design, maintenance and safety of materials, optical storage, pressure-sensitive paints, anti-counterfeiting applications, etc.4 The majority of reported MCL materials display either bathochromic shift or decreased emission in response to mechanical force. The presence of both hypsochromic shift and emission enhancement in a single MCL material is scarce and has rarely been reported.5–7 The development of MCL materials is hindered due to the undesirable aggregation-caused quenching (ACQ) phenomenon, which suppresses emission in the solid state. To overcome this hurdle, a unique phenomenon of aggregation-induced emission (AIE) has been discovered.8 AIE active fluorophores are weakly emissive in good solvents (often organic solvents) but show strong emission in poor solvents (often water/buffers) due to molecular aggregation. AIE provides distinct benefits over standard aggregation-caused quenching (ACQ) due to the ‘turn-on’ fluorescence behaviour upon aggregation. As a result, AIE active fluorophores have recently been used in bioimaging applications,9,10 phototheranostic agents,11 chemosensors,12 biosensors,13 organic field-effect transistors (OFETs), organic light-emitting diodes (OLEDs),14 solar cells and laser dyes.15 In addition to mechanical stress, other stimuli-responsive properties such as viscochromism, solvatochromism, reversible acidochromism, and ultrasound and defect-sensitive emission endow a material with numerous important applications such as acid–base sensing,16 data storage,17 and the measurement of intracellular viscosity18 and properties of lipid membranes.19 However, to develop biomedical and high-tech applications, fluorophores with strong emission in the red/near-infrared regions are highly desired. However, most of the developed AIE/AIEE fluorophores emit blue or green light.20 Red/near-infrared emission can be achieved by increasing the conformational planarity and introducing electron donor (D) and acceptor (A) duos, which cause intramolecular charge transfer (ICT). In this regard, coumarins are the best fit due to their low toxicity, biomedical applications and remarkable luminescence properties like high quantum yield as a result of their intrinsic charge transfer properties.21Copper actively participates in several biological and physiological processes and plays an important role in living systems, as it is an integral part of several enzymes such as mitochondrial cytochrome c oxidase, superoxide dismutase and dopamine β-monooxygenase.22 The acceptable range for Cu2+ ions in drinking water is suggested to be between 15–25 mM, according to the United States Environmental Protection Agency (USEPA) and World Health Organization (WHO). However, when left unchecked, Cu2+ can produce neurotoxic reactive oxygen species, resulting in liver damage, renal problems, Wilson's and Alzheimer's disease,23 oxidative stress and ultimately neuronal death.24 It is also toxic to fish, fungus and algae, thereby affecting the biodiversity of aquatic ecosystems as well.25 Alzheimer's disease (AD) is a widespread neurodegenerative condition characterized by rapidly changing personality, and declining memory, cognitive function, and social interactions. Metal ions, specifically Zn2+ and Cu2+, are found to have notable involvement in the progression of AD.26 These ions bind with amyloid β and initiate its nucleation and aggregation, thus leading to the buildup of senile plaques in the brain.27 The inclusion of residues His6, His13, and His14 at the N-terminus of the amyloid β gives it a significantly high affinity for Cu2+.28 Thus, preventing Cu2+ from incorporating into amyloid β is crucial for effectively slowing down the advancement of AD. Consequently, there is a great deal of demand for analytical instruments capable of detecting Cu2+ ions in fluid and physiological environments with significant sensitivity and selectivity.
In the majority of previous studies, organic solvents with relatively high toxicity have been used as the sensing medium, which should be avoided.29 It is generally observed that most mechanochromic materials show a decrease in emission intensity upon applying pressure which reduces their performance. Shen et al. have recently reported a mechanochromic material with AIEE and Cu2+ sensing, in which the emission intensity declines upon grinding.30 Li et al. have designed pyrene-based probes which exhibit mechanochromism and near-infrared emission.31 Although some past studies have significantly contributed to the advancement of AIE and mechanochromic materials, there is still the possibility to explore the mechanistic perspective of their supramolecular interactions and design strategies. Furthermore, the influence of various environmental factors like viscosity, solvent, acid and ultrasonic vibrations on their photophysical properties has not been fully explored. Taking these points into consideration, we have developed an efficient and smart red-emitting fluorescent material (E)-N′-(1-(7-(diethylamino)-2-oxo-2H-chromen-3-yl)ethylidene)furan-2-carbohydrazide (CFH), with multifarious properties and applications in both solid and liquid states in an aqueous medium.
CFH demonstrates excellent reversible MCL and acidochromic properties. To our surprise, we noticed that the crystals of CFH are weakly emissive with bright emission in the defect region. This prompted us to investigate the change in emission on exposure to ultrasonic vibrations. In the liquid state, CFH exhibits AIE, solvatochromism, viscochromism and Cu2+-sensing properties. In addition, CFH is also utilized in live-cell imaging in both HEK-293 cells and SH-SY5Y (AD) model cells. The fabrication of test kit devices and logic gates enhances the quality of the present work. To the best of our knowledge, there is no previous single report on a probe with the aforementioned multi-responsive properties and applications with in-depth analysis.
Experimental
Synthesis of (E)-N′-(1-(7-(diethylamino)-2-oxo-2H-chromen-3-yl)ethylidene)furan-2-carbohydrazide (CFH)
The starting material for the probe synthesis, 3-acetyl-7-(diethylamino)-2H-chromen-2-one was made according to the standard method.32 1 mmol solution of 3-acetyl-7-(diethylamino)-2H-chromen-2-one was prepared by dissolving 0.259 g of the compound in 20 mL of ethanol, followed by the addition of a few drops of glacial acetic acid. In this solution, 20 mL of ethanolic solution of furan-2-carbohydrazide (1 mmol, 0.126 g) was added dropwise. The reaction mixture was refluxed with stirring in a round-bottom flask for 3 h and the progress of the reaction was monitored using TLC. The resulting clear solution was slowly evaporated at room temperature to get the product. This was recrystallized in ethanol to obtain red-coloured single crystals suitable for X-ray crystallography. Analytical data: red crystals, yield 84%. M.P. 183 °C, HRMS calculated for C20H21N3O4: m/z [M+] 367.1532, found m/z [M + H]+ 368.1709, [M + Na]+ 390.1532; IR (KBr, cm−1): ν(NH) 3110, ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)lactone
O)lactone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ring 1719, ν(C
ring 1719, ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)amide 1683, ν(C
O)amide 1683, ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) 1604; 1H-NMR (500 MHz, DMSO-D6): δ 10.47 (1H, –NH), 8.02 (1H, Ar–H), 7.88 (1H, Ar–H), 7.52 (1H, Ar–H), 7.34 (1H, Ar–H), 6.69 (1H, Ar–H), 6.64 (1H, Ar–H), 6.50 (1H, Ar–H), 3.39 (4H, >CH2), 2.24 (3H, –CH3), 1.08 (6H, –CH3); 13C-NMR (126 MHz, DMSO-D6): δ 160.54 (>C
N) 1604; 1H-NMR (500 MHz, DMSO-D6): δ 10.47 (1H, –NH), 8.02 (1H, Ar–H), 7.88 (1H, Ar–H), 7.52 (1H, Ar–H), 7.34 (1H, Ar–H), 6.69 (1H, Ar–H), 6.64 (1H, Ar–H), 6.50 (1H, Ar–H), 3.39 (4H, >CH2), 2.24 (3H, –CH3), 1.08 (6H, –CH3); 13C-NMR (126 MHz, DMSO-D6): δ 160.54 (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oamide), 157.08 (>C
Oamide), 157.08 (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Olactone
Olactone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ring), 151.68 (>C
ring), 151.68 (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 146.40, 142.96, 130.95, 118.70, 112.49, 112.32, 110.00, 108.18, 96.91, 96.65 (aromatic carbons), 44.70 (–CH2), 16.60 (–CH3), 12.87 (–CH3).
N), 146.40, 142.96, 130.95, 118.70, 112.49, 112.32, 110.00, 108.18, 96.91, 96.65 (aromatic carbons), 44.70 (–CH2), 16.60 (–CH3), 12.87 (–CH3).
Synthesis of the CFH–Cu2+ complex
20 mL of ethanolic solution of Cu(NO3)2·3H2O (1 mmol, 0.241 g) were taken in a round-bottom flask and 20 mL (1 mmol, 0.367 g) of ethanolic solution of CFH were added dropwise. The whole solution was stirred using a magnetic stirrer continuously for 2 h at room temperature. A brown precipitate thus formed was filtered and washed several times with ethanol and finally with diethyl ether and vacuum-dried over anhydrous CaCl2. To obtain a single crystal of the complex, NH4PF6 was added to the saturated solution of the CFH–Cu2+ complex in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (1
MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), which stabilised the cationic complex and brown block-shaped single crystals of appropriate size were produced by slow evaporation. Analytical data: brown crystals, yield: 81%. M.P. >300 °C. HRMS calculated for C20H22CuN3O5: m/z [M+] 447.0855, found m/z [M]+ 447.1632. IR (KBr, cm−1): ν(OH) 3436, ν(C
9), which stabilised the cationic complex and brown block-shaped single crystals of appropriate size were produced by slow evaporation. Analytical data: brown crystals, yield: 81%. M.P. >300 °C. HRMS calculated for C20H22CuN3O5: m/z [M+] 447.0855, found m/z [M]+ 447.1632. IR (KBr, cm−1): ν(OH) 3436, ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)lactone
O)lactone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ring 1653, ν(>C
ring 1653, ν(>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)1 1602, ν(>C
N)1 1602, ν(>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) 1571, ν(C–O−) 1376.
N) 1571, ν(C–O−) 1376.
The synthetic procedures for the CFH and CFH–Cu2+ are shown in Scheme 1.
Results and discussion
Structural characterization of CFH
Red-coloured fluorescent crystals of (E)-N′-(1-(7-(diethylamino)-2-oxo-2H-chromen-3-yl)ethylidene)furan-2-carbohydrazide (CFH) were easily synthesized in good yield. The structure and purity of the compound were confirmed using IR, 1H-NMR, 13C-NMR, and HRMS (ESI,† Fig. S1–S4), and further established by X-ray crystallography.The asymmetric unit of CFH crystallizes in a monoclinic crystal system (a = 14.9321(4) Å, b = 13.0338(3) Å and c = 30.6182(8) Å) with a P21/c space group (ESI,† Tables S1 and S2, CCDC no. 2331258). The P21/c space group is a centrosymmetric space group in which ‘P’ signifies the primitive lattice type, ‘21’ indicates the screw axis along the b-axis and the ‘c’ glide plane is perpendicular to the b-axis and translation along the c-direction. The molecular structure (ORTEP) with 30% probability is shown in Fig. 1a. The CFH molecule exhibits an E configuration around the >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. C(7)–N(2), C(5)–O(2), and C(16)–O(3) have bond distances of 1.297(3) Å, 1.237(3) Å, and 1.221(2) Å, as reported for double bonds.33 The bond lengths of N(2)–N(1) and N(1)–C(5) are 1.359(3) Å and 1.338(3), respectively, which are somewhat less than the single bond distance (1.411 Å), suggesting partial double bond character.34
N bond. C(7)–N(2), C(5)–O(2), and C(16)–O(3) have bond distances of 1.297(3) Å, 1.237(3) Å, and 1.221(2) Å, as reported for double bonds.33 The bond lengths of N(2)–N(1) and N(1)–C(5) are 1.359(3) Å and 1.338(3), respectively, which are somewhat less than the single bond distance (1.411 Å), suggesting partial double bond character.34
 | ||
| Fig. 1 (a) ORTEP view of CFH at the 30% probability level; (b) Hirshfeld surface analysis mapped over dnorm; and (c) 2D fingerprint plot of CFH. | ||
Hirshfeld surface analysis effectively illustrates the role of various weaker interactions in the crystal framework. A transparent framework is used to showcase the molecular surfaces of CFH that allow the aromatic moieties to be clearly visualised. The Hirshfeld surfaces are represented over dnorm (0.185 to 2.857 Å), di (0.988 to 3.512 Å), de (0.989 to 3.580 Å), shape index (−1.00 to 1.00 Å) and curvedness (−4.00 to 4.00 Å) (ESI,† Fig. S5a). The C–H⋯O and O⋯C–H hydrogen bonding interactions are confirmed from the red-shaded area over the dnorm surface as shown in Fig. 1b. The red-blue triangle shape, like a bow-tie structure in the shape index diagram, suggests π⋯π stacking. The 2D fingerprint plot demonstrates the fraction of different intermolecular interactions (Fig. 1c). The percentages of C–C, C–H, C–N, C–O, H–H, N–H and O–H interactions are 5.4%, 12.6%, 4.7%, 1.4%, 45.2%, 1.0% and 27.8% of the entire Hirshfeld surfaces (ESI,† Fig. S5b).
Photo-physical studies
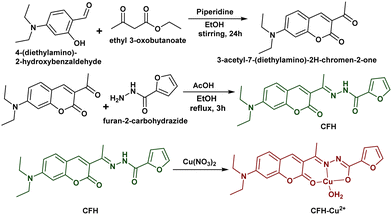
The UV-Vis and fluorescence spectra of CFH were recorded in solvents (THF, CHCl3, CH3CN, EtOH, DMF, DMSO and H2O) of various polarities. CFH exhibits two absorption maxima in the ranges 415–434 nm (λmax1) and 499–494 nm (λmax2), due to the π–π* transition and ICT process, respectively (ESI,† Fig. S6).35 In the emission spectra, λmax at 489 nm attributed to an ICT emission demonstrated a significant change with solvent polarity ranging from THF to water (Fig. 2a). The CFH exhibits a positive solvatochromism with a bathochromic shift of 26 nm upon switching from non-polar to polar solvents.36 Bathochromic shift from 489 to 515 nm with increasing solvent polarity validates the ICT from the N,N-diethylamino group (donor) to the furan group (acceptor). In the DFT-optimized structure of CFH, electron density in the HOMO and LUMO is localised near N,N-diethylamino and furan regions, respectively (ESI,† Fig. S7). Thus, theoretical calculations also reveal an intramolecular charge transfer (ICT) trend from the N,N-diethylamino to the furan moiety.Viscochromism
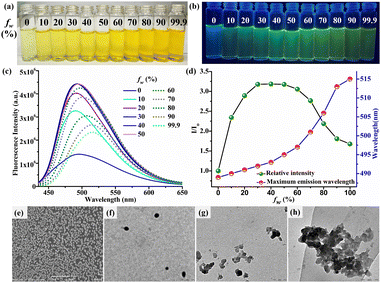
To explore the behaviour of CFH in environments with variable viscosities, ethanol-glycerol solutions with varying glycerol concentrations were used. The UV-Vis spectra of CFH (1 μM) in various glycerol fractions (fgly) ranging from 0% to 99.9% do not exhibit any specific characteristics (ESI,† Fig. S8). However, when the glycerol fraction is increased from 0 to 99.9%, the fluorescence spectra witness a four-fold enhancement (Fig. 2b). The increase in calculated emission efficiency (ϕ) from 2.80 × 10−4 (fgly = 50%) to 14.30 × 10−4 (fgly = 99.9%) indicates a notable enhancement in emission with viscosity. Fluorescence lifetime enhancement from 0.85 ns (fgly = 50%) to 1.72 ns (fgly = 99.9%) is consistent with increased emission intensity in a highly viscous medium (ESI,† Fig. S9). Table S4 (ESI†) summarises the calculated radiative (Kr) and non-radioactive (Knr) rate constants. This behaviour can be explained by changes in the radiative and non-radiative decay paths. In the presence of effective intramolecular rotation, return to the ground state without fluorescence emission was preferred.37 When the intramolecular rotation around the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond is limited or restricted (RIR) with an increase in viscosity, the nonradioactive decay process slows down. This leads to increased fluorescence emission in a viscous medium. These findings showed that CFH is a useful molecular rotor that can be used for health monitoring and early biomedical diagnosis.38
N bond is limited or restricted (RIR) with an increase in viscosity, the nonradioactive decay process slows down. This leads to increased fluorescence emission in a viscous medium. These findings showed that CFH is a useful molecular rotor that can be used for health monitoring and early biomedical diagnosis.38
Aggregation-induced enhanced emission (AIEE)
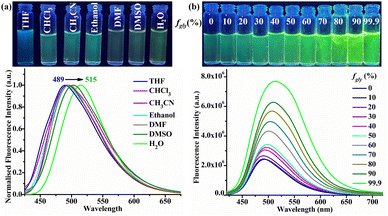
The observed bright green emission in viscous solvents such as glycerol provides evidence that CFH has the AIEE property. The AIEE behaviour of CFH (1 μM) is studied in a THF solution with varying water fractions (fw) from 0% to 99.9% (v/v). In the UV-Vis spectrum, the change in spectral profile is attributed to an increase in polarity with increasing water fraction (ESI,† Fig. S10 and Fig. 3a). However, when photoexcited at 400 nm, CFH solution in THF (fw = 0%) exhibits a weak fluorescence at 489 nm. The fluorescence intensity at fw = 50% is intensified by around 3.3 times as compared to pure THF solution, showing the AIEE phenomenon (Fig. 3b–d). The intramolecular motions are hindered due to space constraints in the aggregates, resulting in increased emission. Conversely, the emission of CFH is red-shifted from 495 to 515 nm with the gradual addition of water from fw = 60% to 99.9%. The increase in solvent polarity strengthens the ICT process and causes the red shift. In contrast to many other AIEE/AIE systems, increasing the water fraction reduces the emission intensity after fw = 60% owing to the crystallization of CFH. The emission intensity is still 2 times higher than that in pure THF.DLS investigations of CFH at different water fractions indicated mean diameters of 168.1 nm (fw = 0%) and 596.6 nm (fw = 50%), justifying the aggregate formation (ESI,† Fig. S11a and b). A further reduction of particle size to 197.6 nm (fw = 99.9%) suggests the development of bigger aggregates that separate out from the solution phase (ESI,† Fig. S11c).39,40 The calculated emission efficiency of CFH at different water fractions (fw = 0%, ϕ = 2.07 × 10−4, fw = 50%, ϕ = 5.80 × 10−4 and fw = 99%, ϕ = 3.92 × 10−4) supports the AIEE phenomenon. The fluorescence average lifetime (τ) increases from 0.46 ns (fw = 0%) to 0.71 ns (fw = 50%) and then decreases to 0.52 ns (fw = 99.9%) (ESI,† Fig. S12). The radiative rate constant (Kr) and non-radiative rate constant (Knr) were computed using fluorescence decay parameters and quantum yield calculations and are summarised in Table S5 (ESI†). In the aggregated state, intramolecular rotation is limited, preventing the non-radiative decay route and allowing the molecules to remain excited for a longer period of time.41
SEM morphology in THF reveals that CFH is structured in a regular open stomata pattern that is responsible for its weaker emission (Fig. 3e). To better understand the process of emission change with water fraction, TEM analyses were performed, which revealed dispersed fluorescent organic nanoparticles (FONs) at fw = 0% (Fig. 3f), which formed smaller nano-aggregates at fw = 50% (Fig. 3g) and then accumulated to form larger nano-aggregates at fw = 99.9% (Fig. 3h).
Mechanochromism (piezochromism, defect-sensitive emission and ultrasound-sensitive emission)
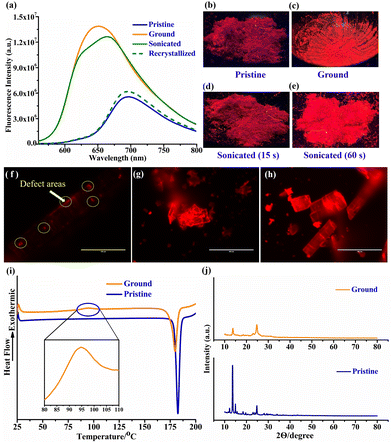
AIEE predicts that CFH may exhibit intriguing solid-state emission properties. A bathochromic shift in emission colour and a drop in fluorescence intensity are often visible when mechanical force is applied. However, it is unusual to find mechanochromic luminogens that exhibit a blue shift together with a turn-on capability. Herein, the crystals of CFH that were successfully isolated showed a weak red fluorescence with an emission peak centred at 698 nm (Fig. 4a). The diethylamine substitution on the coumarin ring increases the donor strength, thus providing a strong donor (D), and the furan ring serves as a strong acceptor (A). The planar arrangement of the D–A group causes a weak red fluorescence in the solid, whereas the bright green fluorescence in solution is due to its weak interaction and dispersed nature. As a result of grinding and striking, the crystals unexpectedly transformed into a relatively bright emissive orange solid with a blue-shifted peak centred at 650 nm. Photographs captured under UV light vividly demonstrate the fluorescence change (Fig. 4b and c). Interestingly, the recrystallization of ground CFH was accompanied by a change in fluorescence from orange to red when the solid was exposed to organic solvents such as CH2Cl2, CHCl3 and MeOH. In light of the fact that CFH is sensitive to defects, an ultrasound attack could be another approach to causing defects. Thus, we propose that CFH should also possess ultrasound-induced emission properties. In order to validate this concept, CFH microcrystals are exposed to ultrasound (frequency = 40 KHz and intensity = 100 W cm−2) for periods of 15 and 60 s (Fig. 4d and e). Remarkably, the defects appear to increase over time. To examine the impact of applied mechanical stress on the surface of CFH crystals, pictures are captured using an inverted fluorescence microscope at a scale of 100 μm. The pristine CFH crystals exhibit a few orange emissive dots (Fig. 4f). These spots are the result of defects that were generated during the processing of the crystal. Interestingly, when the pristine crystal is struck with tweezers, orange emission appears all over its surface (Fig. 4g). The findings indicate that the edge of the defect region begins to exhibit much higher emission in comparison to the core section of the crystal. Under an inverted fluorescence microscope, strongly emissive crystals caused by ultrasonification were clearly visible (Fig. 4h). Hence, the defects are introduced onto the surface of CFH crystals through the use of ultrasound. Consequently, the emission change of CFH, which might be a typical ratio-metric turn-on mechanochromic luminogen, can be induced by mechanical force, which can generate defects in the crystal's structure.The above results demonstrate that CFH crystals are weakly emissive, but the defect area on the crystal displays clear fluorescence with a blue shift. Several measurements on the pristine and ground CFH crystals were conducted to obtain an understanding of the process behind the mechanochromism. In differential scanning calorimetry (DSC), there is a single endothermic peak observed at 183 °C for pristine CFH crystals, which corresponds to their melting temperature (Fig. 4i). Conversely, at 95 °C, the ground sample undergoes an exothermic process before melting at 180 °C. A phase-to-phase transition to the metastable state during grinding may be responsible for the newly observed exothermic peak.42 The powder X-ray diffraction (PXRD) analysis shows strong diffraction peaks for pristine CFH crystals, indicative of a microcrystalline and well-ordered structure (Fig. 4j). The reduction in diffraction peak intensity indicates an amorphous form of CFH with disrupted molecule stacking after grinding.43
Investigations into the crystal structure and packing configuration of CFH are useful for understanding the influence of molecular packing on the emission behaviour. While most of the AIEE/AIE molecules exhibit twisted conformations in the crystalline state, CFH adopts an almost planar conformation in the crystal lattice with an inter-planar angle of 7.07° (Fig. 5a). The (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)amide in CFH displays strong non-classical intermolecular hydrogen bonds [O(2)⋯H–C(11) = 2.672 Å and O(2)⋯H–C(12) = 2.591 Å] with coumarin protons of the neighbouring molecule (Fig. 5b). The CFH molecules are closely packed with an interlayer spacing of around 4.926 Å (Fig. 5c). This distance is still greater than the standard π–π stacking distance (i.e., 3.5 Å). Conventional π–π stacking typically inhibits fluorescence; however, due to loose π–π interactions and the planar nature of CFH, the molecule is weakly emissive.44 The donor–acceptor (DA) effect causes the electron-withdrawing furan ring to point to the electron-donating diethylamine unit, resulting in a typical head-to-tail packing configuration for CFH (Fig. 5d). The molecular packing forms a block-shaped stacked array of CFH along the b-axis (Fig. 5e), and the molecules are arrayed in a 1D column through intermolecular H-bonding with molecules in the adjacent column as highlighted by the red circles in Fig. 5f. This 1D H-bonded interaction with the neighbouring column promotes the decay of excited species by non-radiative routes, resulting in weak emission in the crystalline state.45 Both the stacking procedure and the fluorescence properties of bulk samples in various phases are therefore considerably affected by the molecular design.
O)amide in CFH displays strong non-classical intermolecular hydrogen bonds [O(2)⋯H–C(11) = 2.672 Å and O(2)⋯H–C(12) = 2.591 Å] with coumarin protons of the neighbouring molecule (Fig. 5b). The CFH molecules are closely packed with an interlayer spacing of around 4.926 Å (Fig. 5c). This distance is still greater than the standard π–π stacking distance (i.e., 3.5 Å). Conventional π–π stacking typically inhibits fluorescence; however, due to loose π–π interactions and the planar nature of CFH, the molecule is weakly emissive.44 The donor–acceptor (DA) effect causes the electron-withdrawing furan ring to point to the electron-donating diethylamine unit, resulting in a typical head-to-tail packing configuration for CFH (Fig. 5d). The molecular packing forms a block-shaped stacked array of CFH along the b-axis (Fig. 5e), and the molecules are arrayed in a 1D column through intermolecular H-bonding with molecules in the adjacent column as highlighted by the red circles in Fig. 5f. This 1D H-bonded interaction with the neighbouring column promotes the decay of excited species by non-radiative routes, resulting in weak emission in the crystalline state.45 Both the stacking procedure and the fluorescence properties of bulk samples in various phases are therefore considerably affected by the molecular design.
Fluorescence spectra of CFH at different concentrations in THF were collected to demonstrate the stacking phenomenon. The fluorescence maximum (λem) of CFH (1 μM) in THF solution exhibited a red shift from 489 to 549 nm as the concentration increased from 1 μM to 5000 μM (ESI,† Fig. S13). The concentration-dependent red shift of CFH in THF may be due to the stronger hydrogen bonding interactions with the neighbouring column.46 The extent of hydrogen bonding increases with an increase in the concentration of CFH. Thus, red-shifted fluorescence of pristine CFH is due to the charge transfer interactions and extensive intermolecular hydrogen bonding with molecules in adjacent columns, which is in contrast to the amorphous state.45,47 The disruption of hydrogen bonding interactions by a mechanical stimulation results in a disordered, twisted conformation, which in turn limits the amount of non-radiative dissipation and shifts the emission to lower wavelength.48 As a result, the ground sample exhibits a blue-shifted emission from weak red to intense orange emission, making it relatively more emissive than pristine CFH crystals.
Acidochromic (ACL) behaviour
The Schiff base skeleton, which comprises an acyl hydrazone containing Lewis basic nitrogen, renders CFH susceptible to protonation in the presence of certain proton sources. Furthermore, subsequent proton removal may be achieved by base exposure and hence its acid sensing or acidochromic luminescence (ACL) behaviour has been investigated in the solid state. Upon exposure to trifluoroacetic acid (TFA) vapours for 10 s, weak red fluorescence of CFH (pristine) was completely quenched. Furthermore, when the TFA treated sample was exposed to triethylamine (TEA) vapours for 30 s, the weak red fluorescence of CFH (pristine) was restored (Fig. 6a). The pristine CFH crystals have a noticeable emission peak at 698 nm. After exposure to TFA vapour for 10 s, the sample becomes non-emissive. However, after being fumed with TEA vapour for 30 s, the sample deprotonates and becomes strongly emissive (Fig. 6b).To investigate the mechanism of ACL, the effect of reversibility features was examined in the solution state. When TFA (1 equiv.) was added to CFH (1 μM) solution in CH3CN, a colour change was seen under visible light and contrasting fluorescence quenching under UV light (Fig. 6c). This was subsequently reversed with the addition of TEA (1 equiv.). Thus, the absorption and emission spectra of CFH (1 μM) were analysed in CH3CN upon addition of TFA/TEA (1 equiv.). Two characteristic peaks were found in the absorption spectra at 411 and 491 nm (Fig. 6d). When TFA was added, a strong single band at 487 nm appeared, which was ascribed to the strengthening of the ICT process. With the addition of TEA, the entire process was reversible. Similarly, the emission peak for CFH in CH3CN at 495 nm was quenched upon addition of TFA (Fig. 6e). The complete recovery of the strong emission of CFH observed upon further treatment with TEA demonstrates highly reversible ACL.
Furthermore, to evaluate the reversible protonation process and locate the site of protonation, 1H NMR titration of CFH in DMSO-d6 was carried out by sequentially adding TFA and TEA (ESI,† Fig. S14). The addition of TFA resulted in a downfield shift of the –NH proton signal from δ 10.45 to 11.33 due to the change in chemical environment by protonation.45 Upon further addition of TEA, the –NH proton signal shifts upfield to δ 10.45, suggesting the regeneration of neutral CFH due to the deprotonation process. Similarly, the hydrogen signals in the range of 7.0–8.0 ppm exhibit a consistent pattern when exposed to TFA and TEA. Thus, acid stimulation causes protonation of CFH, which increases the ICT effect. Molecules with significant ICT effects have low oscillator strength between excited and ground states, resulting in fluorescence quenching via a non-radiative decay mechanism.46
Anti-counterfeiting and vapour-sensing application
The reversible MCL and ACL behaviours of CFH encouraged us to fabricate rewritable and vapour-sensing fluorescent platforms, respectively (Fig. 7a). The reversible ratio-metric turn-on MCL behaviour of CFH during grinding/recrystallization has been utilised in the construction of a rewritable fluorescent platform. A non-fluorescent silica plate was coated with CFH paste by applying pressure and drying for 10 min. Under UV light, the silica plate (as prepared) showed strong orange fluorescence, signifying a ground amorphous state of CFH. Using DCM ink, desired inscriptions (letters/numerals) were inscribed on the prepared plate. The strongly orange emissive plate starts displaying a weaker red emission owing to crystallisation (i.e., pristine state) over the written area. Furthermore, the written inscription can be erased by applying pressure and can be used again. This reversible cycle was illustrated by writing “COF” on the prepared plates and erasing it with pressure (Fig. 7b). In addition, the reversible ACL behaviour of CFH upon exposure to TFA/TEA has been utilised in fabricating vapour-sensing fluorescent plates. Desired letters/numerals were inscribed on the plate with CFH paste and air-dried for 10 min. As prepared (ground) sensing plates are engraved with strong orange emissive letters “COF”, which totally quench when exposed to TFA vapour (Fig. 7c). Similarly, the DCM fumed weakly red emissive plate also quenches upon exposure to TFA vapour. The inscription became readable due to the weak fluorescence of CFH (pristine) when exposed to TEA vapour. Thus, the CFH-coated plate was established as an effective platform capable of anti-counterfeiting and detecting vapour across several cycles in a reversible manner.Fluorescence response of CFH with metal ions
The high sensitivity and selectivity of a probe toward an analyte of interest, as compared to other potentially competing species present in the sample, are crucial for its practical application. The selectivity of the probe CFH (1 μM) in a range of metal ions (Na+, Ag+, Ca2+, Cd2+, Co2+, Ni2+, Cu2+, Zn2+, Ba2+, Pb2+, Hg2+, Al3+, Cr3+, Fe3+, and Bi3+) was investigated in water. The UV-Vis spectra of CFH exhibited two absorption bands at 434 and 495 nm attributed to the π–π* and n–π* transitions, respectively. There is no significant change observed in the absorption spectrum upon the addition of metal ions (1 equiv.) except for Cu2+ (ESI,† Fig. S15). Upon addition of Cu2+ ions (1 equiv.), CFH displays a new band with an absorption maximum centred at 480 nm. The concurrent addition of Cu2+ (0–1 equiv.) resulted in a gradual appearance of a new absorption band at 480 nm with a distinct isosbestic point at 435 nm, suggesting the formation of a new host–guest species. (ESI,† Fig. S16).Additionally, fluorescence spectra were recorded to evaluate the selective recognition behaviour of CFH towards Cu2+ in water. The emission spectra of CFH (1 μM) exhibited an emission band at 515 nm. Except for Cu2+, the aforementioned metal cations (20 equiv.) had an insignificant effect on the fluorescence spectra of CFH. The addition of Cu2+ induced an apparent fluorescence change from bright green to non-fluorescent, resulting in the ‘turn-off’ quenching of fluorescence (Fig. 8a). Additionally, the fact that various anionic salts of Cu2+ produce the same absorption and emission pattern towards CFH shows that the counter-anion had no discernible influence on the process of Cu2+ recognition (ESI,† Fig. S17).
To explore the possibility of using probe CFH as a practical ion-selective fluorescent probe for Cu2+, fluorescence interference experiments were conducted in water (Fig. 8b). Upon the addition of 20 equiv. of other metal ions, no significant change in the emission intensity of CFH was observed, except for with Cu2+ (1 equiv.). When 1 equiv. of Cu2+ was added, the emission intensities of the CFH solution containing 20 equiv. of other metal ions were quenched significantly more than when Cu2+ was added alone. Thus, the interference study indicates that CFH exhibits exceptional sensitivity and selectivity towards Cu2+, even when there is an abundance of other competing cations.
To obtain more insight into the sensing capabilities of CFH (1 μM) toward Cu2+, a detailed fluorescence titration investigation was carried out (Fig. 8c). The gradual addition of Cu2+ (0–2 equiv.) results in a concurrent decrease of the emission peak at 515 nm. The fluorescence intensity reached a plateau after the addition of 1 equiv. of Cu2+ ions, indicating the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between CFH and Cu2+ (ESI,† Fig. S18). A Job's plot constructed on the basis of the fluorescence spectra shows an inflection point close to 0.5, demonstrating 1
1 complex between CFH and Cu2+ (ESI,† Fig. S18). A Job's plot constructed on the basis of the fluorescence spectra shows an inflection point close to 0.5, demonstrating 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between CFH and Cu2+ (ESI,† Fig. S19). Using a Benesi–Hildebrand plot based on 1
1 binding stoichiometry between CFH and Cu2+ (ESI,† Fig. S19). Using a Benesi–Hildebrand plot based on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry, the binding constant was found to be 1.13 × 106 M−1 (R2 = 0.9916) (ESI,† Fig. S20). The limit of detection (LOD) of CFH was calculated to be 1.1 × 10−9 M from the linear plot between the emission intensity of CFH and Cu2+ ion concentration [Cu2+] in the range of 4.99 × 10−8 M to 6.50 × 10−7 M (R2 = 0.9893) (ESI,† Fig. S21). This nano-molar LOD qualifies CFH as a promising candidate for the sensitive detection of Cu2+. The Stern–Volmer constant (Ksv) was calculated from a plot of I0/I vs. quencher concentration [Cu2+]. The resulting plot possesses good linearity in the range of 4.99 × 10−8 M to 4.10 × 10−7 M (R2 = 0.9994) (ESI,† Fig. S22). An excellent Ksv value of 2.837 × 106 M−1 suggests high sensitivity and better interaction between Cu2+ and CFH.
1 binding stoichiometry, the binding constant was found to be 1.13 × 106 M−1 (R2 = 0.9916) (ESI,† Fig. S20). The limit of detection (LOD) of CFH was calculated to be 1.1 × 10−9 M from the linear plot between the emission intensity of CFH and Cu2+ ion concentration [Cu2+] in the range of 4.99 × 10−8 M to 6.50 × 10−7 M (R2 = 0.9893) (ESI,† Fig. S21). This nano-molar LOD qualifies CFH as a promising candidate for the sensitive detection of Cu2+. The Stern–Volmer constant (Ksv) was calculated from a plot of I0/I vs. quencher concentration [Cu2+]. The resulting plot possesses good linearity in the range of 4.99 × 10−8 M to 4.10 × 10−7 M (R2 = 0.9994) (ESI,† Fig. S22). An excellent Ksv value of 2.837 × 106 M−1 suggests high sensitivity and better interaction between Cu2+ and CFH.
The calculated emission efficiency (ϕ) and fluorescence average lifetime (τ) of CFH in water were found to be ϕ = 3.92 × 10−4 and τ = 0.52 ns, decreasing to ϕ = 0.10 × 10−4 and τ = 0.35 ns for the CFH–Cu2+ complex, respectively, supporting the quenching phenomenon (ESI,† Fig. S23 and Table S5). The calculated Kr and Knr values demonstrate the non-radiative decay domination for the CFH–Cu2+ complex. These results indicate that the probe CFH exhibits sensitive and selective quantification for Cu2+ by fluorescence “turn-off” behaviour.
The effect of pH on the emission spectra of CFH was monitored to check the applicability of CFH over a wide range of pH. The emission intensity of CFH was measured as a function of pH in the absence and presence of Cu2+. The CFH–Cu2+ does not show any noteworthy emission at any pH, but a remarkable increase in emission intensity of CFH is visible from pH 3 to 11 (ESI,† Fig. S24). This implies that CFH can be effectively utilized for the recognition of Cu2+ in a pH range of 3 to 11.
The reversibility of the binding between CFH and Cu2+ was examined in order to assess the reusability and reversibility of CFH. As discussed, the introduction of Cu2+ to the CFH solution switches off the green fluorescence due to the formation of the CFH–Cu2+ complex. When EDTA was added to the CFH–Cu2+ solution, Cu2+ was removed through the formation of EDTA–Cu2+, which resulted in the recovery of the green fluorescence with a peak at 515 nm (Fig. 8d). At least 5 repetitions of this cycle are possible (ESI,† Fig. S25). Therefore, by adding EDTA solution to the medium, the CFH response to the Cu2+ ions can be reversed.
Plausible mechanism and mode of interaction
The 1H-NMR spectrum of CFH upon the addition of 1 equivalent of Cu2+ has been recorded in DMSO-d6 (ESI,† Fig. S26). It is evident that the >NH signal at 10.47 ppm in CFH completely vanishes with the addition of Cu2+, implying its deprotonation and involvement in the chelation process. The IR spectrum of CFH–Cu2+ exhibits the downshifting of ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)lactone
O)lactone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ring and ν(C
ring and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) from 1719 cm−1 and 1604 cm−1 to 1653 cm−1 and 1571 cm−1, respectively (ESI,† Fig. S27). The ν(>N–H) and ν(C
N) from 1719 cm−1 and 1604 cm−1 to 1653 cm−1 and 1571 cm−1, respectively (ESI,† Fig. S27). The ν(>N–H) and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)amide bands disappear completely, and a new ν(C–O−) band appears at 1376 cm−1.49 The occurrence of a new ν(>C
O)amide bands disappear completely, and a new ν(C–O−) band appears at 1376 cm−1.49 The occurrence of a new ν(>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)1 band at 1602 cm−1 further suggests the enolization of (C
N)1 band at 1602 cm−1 further suggests the enolization of (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)amide and subsequent deprotonation of the >N–H group. Thus, the IR spectra clearly indicate the bonding interactions through azomethine-N, carbonylate-O and lactone-O with Cu2+. A band at 3436 cm−1 in the complex is due to ν(OH) of coordinated water. HRMS data for CFH–Cu2+ display a peak at 447.1228 m/z corresponding to [CFH + Cu2+ + (H2O)–H]+ (ESI,† Fig. S28).
O)amide and subsequent deprotonation of the >N–H group. Thus, the IR spectra clearly indicate the bonding interactions through azomethine-N, carbonylate-O and lactone-O with Cu2+. A band at 3436 cm−1 in the complex is due to ν(OH) of coordinated water. HRMS data for CFH–Cu2+ display a peak at 447.1228 m/z corresponding to [CFH + Cu2+ + (H2O)–H]+ (ESI,† Fig. S28).
Furthermore, to comprehend the binding interaction, the molecular structures of CFH (ESI,† Fig. S7) and CFH–Cu2+ (Fig. 9a) have been optimized using density functional theory (DFT). The lowest unoccupied molecular orbital (LUMO) is stabilized from (−2.097 eV) in CFH to (−2.263 eV) in CFH–Cu2+. The decrease in the energy band gap from CFH (3.461 eV) to CFH–Cu2+ (2.138 eV) suggests the increased stabilization of CFH upon coordination with Cu2+.
TEM analysis has been carried out to investigate the morphological changes of CFH upon complexation with Cu2+. The TEM morphology at fw = 99.9% shows that CFH accumulates to form larger nano-aggregates (Fig. 3h) with sizes of around 197.6 nm (ESI,† Fig. S11c). The inclusion of Cu2+ disassembles the nano-aggregates and causes significant quenching (Fig. 9b and c). These findings are further corroborated by DLS analysis, which demonstrates that the average particle size of the aggregates reduces from 197.6 nm to 92.89 nm upon Cu2+ addition (ESI,† Fig. S29).
In addition to the aforementioned spectroscopic investigations, single crystal X-ray diffraction analysis has also been performed to further characterize the CFH–Cu2+. The complex crystallizes in a triclinic crystal system with the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (a = 8.0746(10) Å, b = 9.8592(10) Å and c = 15.685(3) Å; α = 94.4180(10)°, β = 90.3060(10)° and γ = 92.1680(10)°) (ESI,† Tables S1 and S3, CCDC no. 2339921). The SC-XRD analysis of CFH–Cu2+ indicated that it consists of a mononuclear [Cu(CFH)(H2O)]+ cation with a PF6− anion and a H-bonded water molecule in the asymmetric unit (ESI,† Fig. S30a). The ORTEP view of CFH–Cu2+ is shown in Fig. 9d. The Cu2+ is coordinated by a tridentate ligand CFH using azomethine-N, carbonylate-O and lactone-O. The average bond lengths in the complex, O2–C5 = 1.293(5), N2–N1 = 1.394(4), N2–C7 = 1.304(5) and O3–C16 = 1.245(4) Å, are greater than those of the corresponding distances in the CFH ligand, indicating significant delocalization of charge upon chelation. Cu2+ coordinates with azomethine-N (Cu–N2, 1.938(3) Å), carbonylate-O (Cu–O2, 1.908(3) Å) and lactone-O (Cu–O3, 1.934(3) Å) of CFH and an O atom of water (Cu–O5, 1.977(3) Å) (ESI,† Fig. S30b). Cu2+ exhibits bond angles N2–Cu–O2 = 82.31(12)°; N2–Cu–O3 = 92.35(12)°; O5–Cu–O3 = 91.24(13); and O5–Cu–O2 = 94.69(14)°. All of the bond lengths and angles in CFH–Cu2+ are within the range of previously reported similar compounds.50,51 An intermolecular H-bond (O2⋯H6–O6, 2.05 Å) between carbonylate-O and the hydrogen atom of lattice water is also observed (ESI,† Fig. S30c). The complex is slightly more distorted than CFH, as the inter-planar angle increases from 7.07° to 15.55°, suggesting a distorted square planar geometry (ESI,† Fig. S30d). The head-to-tail arrangement of adjacent CFH–Cu2+ molecules leads to the formation of a block-like stacked array (ESI,† Fig. S30e and f).
space group (a = 8.0746(10) Å, b = 9.8592(10) Å and c = 15.685(3) Å; α = 94.4180(10)°, β = 90.3060(10)° and γ = 92.1680(10)°) (ESI,† Tables S1 and S3, CCDC no. 2339921). The SC-XRD analysis of CFH–Cu2+ indicated that it consists of a mononuclear [Cu(CFH)(H2O)]+ cation with a PF6− anion and a H-bonded water molecule in the asymmetric unit (ESI,† Fig. S30a). The ORTEP view of CFH–Cu2+ is shown in Fig. 9d. The Cu2+ is coordinated by a tridentate ligand CFH using azomethine-N, carbonylate-O and lactone-O. The average bond lengths in the complex, O2–C5 = 1.293(5), N2–N1 = 1.394(4), N2–C7 = 1.304(5) and O3–C16 = 1.245(4) Å, are greater than those of the corresponding distances in the CFH ligand, indicating significant delocalization of charge upon chelation. Cu2+ coordinates with azomethine-N (Cu–N2, 1.938(3) Å), carbonylate-O (Cu–O2, 1.908(3) Å) and lactone-O (Cu–O3, 1.934(3) Å) of CFH and an O atom of water (Cu–O5, 1.977(3) Å) (ESI,† Fig. S30b). Cu2+ exhibits bond angles N2–Cu–O2 = 82.31(12)°; N2–Cu–O3 = 92.35(12)°; O5–Cu–O3 = 91.24(13); and O5–Cu–O2 = 94.69(14)°. All of the bond lengths and angles in CFH–Cu2+ are within the range of previously reported similar compounds.50,51 An intermolecular H-bond (O2⋯H6–O6, 2.05 Å) between carbonylate-O and the hydrogen atom of lattice water is also observed (ESI,† Fig. S30c). The complex is slightly more distorted than CFH, as the inter-planar angle increases from 7.07° to 15.55°, suggesting a distorted square planar geometry (ESI,† Fig. S30d). The head-to-tail arrangement of adjacent CFH–Cu2+ molecules leads to the formation of a block-like stacked array (ESI,† Fig. S30e and f).
The presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and photo-induced electron transfer (PET) from the aldimine nitrogen to the excited state of the coumarin fluorophore leads to the non-fluorescent nature of the probe. However, CFH solution in water is emissive (λem = 515 nm), mainly due to the presence of a methyl group across the C
N isomerization and photo-induced electron transfer (PET) from the aldimine nitrogen to the excited state of the coumarin fluorophore leads to the non-fluorescent nature of the probe. However, CFH solution in water is emissive (λem = 515 nm), mainly due to the presence of a methyl group across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, which is capable of adjusting and tuning the conformational change around the amine moiety.43 With the addition of Cu2+, the decrease in fluorescence intensity is primarily ascribed to the paramagnetic quenching effect of Cu2+ (Fig. 9e).52 As Cu2+ possesses a partially filled d-subshell, it strongly suppresses the emission of CFH owing to a photo-induced metal-to-fluorophore electron or energy transfer process.53 Due to the high thermodynamic affinity of Cu2+ for N- or O-based ligands, a rapid metal-to-ligand binding takes place.54
N bond, which is capable of adjusting and tuning the conformational change around the amine moiety.43 With the addition of Cu2+, the decrease in fluorescence intensity is primarily ascribed to the paramagnetic quenching effect of Cu2+ (Fig. 9e).52 As Cu2+ possesses a partially filled d-subshell, it strongly suppresses the emission of CFH owing to a photo-induced metal-to-fluorophore electron or energy transfer process.53 Due to the high thermodynamic affinity of Cu2+ for N- or O-based ligands, a rapid metal-to-ligand binding takes place.54
Logic gate
The “on–off” fluorescence response of a chemosensor to metal ions, as well as the role of transition metal ions in molecular switch devices, has increased the interest in molecules capable of executing logic operations.55 The sensing behaviour of CFH was studied with molecular logic functions of AND, OR and NOT gates. The probe CFH emits fluorescence at 515 nm, which is quenched by Cu2+via the formation of a CFH–Cu2+ complex. The removal of Cu2+ from the CFH–Cu2+ complex upon the addition of EDTA results in the recovery of fluorescence emission at 515 nm. An IMPLICATION logic gate was built by taking Cu2+ and EDTA as inputs and the fluorescence emission intensity at 515 nm as an output. The output is “OFF” in the presence of Cu2+ alone, while all other combinations result in the output being “ON” (ESI,† Fig. S31).Test kit construction for the determination of Cu2+
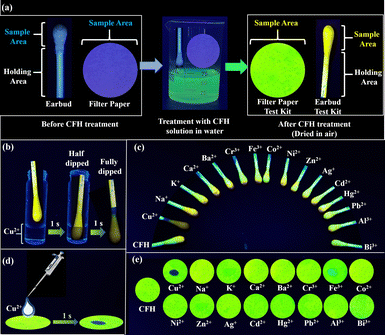
The fabrication of a test kit using CFH offers a fast, easy and realistic method for the determination of Cu2+. The steps for constructing a test kit from earbuds and filter paper are illustrated and explained in the ESI† and schematized in Fig. 10a. The CFH-adsorbed earbuds were submerged in a Cu2+ solution, as shown schematically in Fig. 10b. As the CFH–Cu2+ complex was formed, the green fluorescence (on state) of the earbuds was turned off, whereas it was unaffected with other metal ions (Fig. 10c). Similarly, adding Cu2+ ions with a pipette turned off the green fluorescence of CFH adsorbed filter paper (Fig. 10d). Only Cu2+, among the aforementioned metal ions, was able to turn off the fluorescence of CFH adsorbed earbuds or filter paper (Fig. 10e). The green fluorescence of CFH was shown to be strongly suppressed upon the addition of Cu2+ ions in the solid state.Toxicity assay
The viability of SH-SY5Y and HEK-293 cells was observed to be biocompatible upon treatment with increasing concentrations of probe CFH. Increased concentrations of CFH showed lower toxicity to the SH-SY5Y cell line as well as the normal HEK-293 cell line as presented in Fig. 11. The results demonstrate that even at a concentration of 25 μM, cell viability was greater than 80% for both the SH-SY5Y and HEK-293 cells. Therefore, these data suggest that the probe CFH, when complexed with Cu2+, bears higher biocompatibility with lower toxicity to neuronal and normal cells, suggesting a potential probe that can be used for in vitro cell imaging, live cell imaging and cell comparative studies.Intracellular uptake
The intracellular uptake of the fluorescent probe CFH was studied in SH-SY5Y and HEK-293 cells using an inverted fluorescence microscope. The probe CFH exhibits fluorescent nature and emits fluorescence in the green channel when observed under the fluorescence microscope. However, the fluorescent nature of the probe CFH is reduced in the presence of Cu2+. Several studies have demonstrated that the bioaccumulation of metals is high in the case of neurodegenerative cells;38 therefore, to focus on the presence of Cu2+ inside the cells, the fluorescent probe CFH has been formulated. When entrapped inside the cells, the probe gets quenched due to the presence of Cu2+. The results obtained through fluorescence imaging (Fig. 12a) in HEK-293 cells show the intense fluorescence starting from the initial concentration, which intensified with increasing concentration of CFH. However, HEK-293 cells treated initially with Cu2+ exhibit the quenching of the fluorescence of probe CFH due to the presence of Cu2+ inside the cells (Fig. 12b). Similar quenching of the fluorescence due to Cu2+/metal accumulation in SH-SY5Y was obtained when the neurodegenerative cells SH-SY5Y were treated with probe CFH (Fig. 12c), suggesting that CFH chelates and extracts the Cu2+ ions present in SH-SY5Y cells.56,57 Therefore, these results suggest that the CFH can be an efficient probe for cell analysis and bioimaging as well as live cell imaging.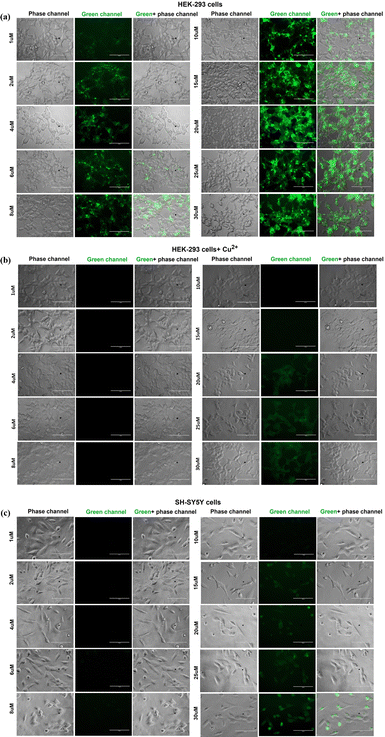 | ||
| Fig. 12 Images representing the intracellular uptake of the probe CFH at different concentrations in cell lines (a) normal HEK-293, (b) normal HEK-293 + Cu2+ and (c) SH-SY5Y. | ||
Comparison with previous reports
An exhaustive comparison with previously reported works encompassing multifarious properties and applications in solid, liquid and biological systems has been tabulated (ESI,† Table S6). It is clearly evident that none of the works cover such a thorough investigation of numerous properties, viz. MCL, ACL, solvatochromism, viscochromism and AIEE, and applications, viz. defect-sensitive emission, ultrasound-induced emission, vapour phase sensing, anticounterfeiting, Cu2+ sensing in pure water, reversibility with EDTA, logic gate, test kit device, live cell imaging and neuroprotective therapy for Alzheimer disease. Additionally, the present work contributes to our fundamental understanding of molecular modification and paves the way for the development of multifunctional optical materials for security, trace inspection, rewritable platforms and sensor devices via a cost-effective manufacturing process.Conclusions
A novel multi-stimuli responsive coumarin functionalized furoyl hydrazone (CFH) was synthesised and the smart switching of luminosity properties in the solid and solution states was described in detail. CFH exhibited solvatochromism with bathochromic shift in polar solvents. The probe showed excellent viscochromism attributed to restricted intramolecular rotation (RIR). CFH exhibited AIEE characteristics with 3.3-fold enhanced emission at around fw = 50%. It displayed excellent mechanochromic luminescence (MCL) with rare blue-shift enhanced emission from weakly emissive red to highly emissive orange after grinding, providing mechanistic insight into the mechanochromic behaviour of the tuning of the blue shift or red shift in the emission of the fluorophore. Defect regions of the CFH crystals become highly orange emissive, thereby making the behaviour ultrasound-induced defect sensitive. CFH also displayed reversible acidochromic behaviour in both solid and solution states when exposed to TFA/TEA vapours. It may be effectively applied as a stimuli-responsive luminous material for press jet printing, vapour sensing and multi-level decryption to improve its candidacy in security, anti-theft and information inspection applications. In addition to the aforementioned properties, CFH successfully discriminated Cu2+ from a pool of metal ions in water at the nanomolar level. It has been effectively utilized in designing molecular logic gates and constructing test kit devices. Furthermore, its biological application in the intracellular detection of Cu2+ in HEK-293 has been demonstrated. CFH was also employed in chelating and extracting excess Cu2+ accumulated in neurodegenerative SH-SY5Y cells. Thus, this work provides a unique insight into the tailoring of future designs of a “smart material” with multifarious applications owing to its multi-regulatory properties depending on the state of the material.Author contributions
Aayoosh Singh: conceptualization, investigation, methodology, formal analysis, software, visualization, writing – original draft. Pranjalee Yadav: investigation, methodology, writing, review and editing. Amit Kumar Singh: investigation, formal analysis, data validation. Rupen Tamang: investigation, formal analysis, writing biological section. Biplob Koch: resources, funding acquisition, writing, review and editing biological section. Vinod Prasad Singh: conceptualization, supervision, data validation, resources, visualization, funding acquisition, writing, review and editing.Data availability
The data supporting this article have been included in the main article and ESI.† Crystallographic data for compounds CFH and CFH–Cu2+ have been deposited at the CCDC under 2331258 and 2339921, respectively.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. Singh thanks the University Grants Commission, New Delhi, for the UGC Research Fellowship (R/Dev./IX-Sch. (BHU Res. Sch.)2021-22/37605). The author, P. Yadav, thanks the CSIR, New Delhi, India, for the award of CSIR-RA [09/0013(18503)/2024-EMR-I]. The author, V. P. Singh, is thankful to Banaras Hindu University for a grant under the IoE Scheme (Dev. Scheme No. 6031). The author, B. Koch, acknowledges Banaras Hindu University for providing funds under the IoE Scheme (File No. R/Dev/D/IoE/Incentive/2021-22/32449).Notes and references
- F. Xu and B. L. Feringa, Photoresponsive Supramolecular Polymers: From Light-Controlled Small Molecules to Smart Materials, Adv. Mater., 2023, 35, 2204413 CAS.
- K. Zhang, X. Zhou, S. Li, L. Zhao, W. Hu, A. Cai, Y. Zeng, Q. Wang, M. Wu, G. Li, J. Liu, H. Ji, Y. Qin and L. Wu, A General Strategy for Developing Ultrasensitive “Transistor-Like” Thermochromic Fluorescent Materials for Multilevel Information Encryption, Adv. Mater., 2023, 35, 2305472 CAS.
- H. Nawaz, S. Chen, X. Zhang, X. Li, T. You, J. Zhang and F. Xu, Cellulose-Based Fluorescent Material for Extreme pH Sensing and Smart Printing Applications, ACS Nano, 2023, 17, 3996–4008 CAS.
- Z. Y. Sun, Y. Li, M. Wu, W. He, Y. Yuan, Y. Cao and Y. Chen, A Rhodamine-Spiropyran Conjugate Empowering Tunable Mechanochromism in Polymers under Multiple Stimuli, Angew. Chem., Int. Ed., 2024, 63, e202411629 CAS.
- J. Zou, Y. Fang, Y. Shen, Y. Xia, K. Wang, C. Zhang and Y. Zhang, Piezochromic Tetracoordinate Boron Complex: Blue-Shifted and Enhanced Luminescence, Angew. Chem., Int. Ed., 2022, 61, e202207426 CAS.
- J. Fang, X. Yu, Y. Liu, Y. Yusran, Y. Wang, V. Valtchev, S. Qiu, B. Zou and Q. Fang, Piezofluorochromism in Covalent Organic Frameworks: Pressure-induced Emission Enhancement and Blue-Shifted Emission, Angew. Chem., Int. Ed., 2024, 63, e202409099 CAS.
- H. Liu, Y. Gu, Y. Dai, K. Wang, S. Zhang, G. Chen, B. Zou and B. Yang, Pressure-Induced Blue-Shifted and Enhanced Emission: A Cooperative Effect between Aggregation-Induced Emission and Energy-Transfer Suppression, J. Am. Chem. Soc., 2020, 142, 1153–1158 CAS.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Aggregation-induced emission, Chem. Soc. Rev., 2011, 40, 5361–5388 CAS.
- R. Xu, P. Zhang, Q. Shen, Y. Zhou, Z. Wang, Y. Xu, L. Meng, D. Dang and B. Z. Tang, AIE nanocrystals: Emerging nanolights with ultra-high brightness for biological application, Coord. Chem. Rev., 2023, 477, 214944 CrossRef CAS.
- J. J. Morsby, Z. Zhang, A. Burchett, M. Datta and B. D. Smith, Ratiometric near-infrared fluorescent probe for nitroreductase activity enables 3D imaging of hypoxic cells within intact tumor spheroids, Chem. Sci., 2024, 15, 3633–3639 RSC.
- S. Song, Y. Zhao, M. Kang, F. Zhang, Q. Wu, N. Niu, H. Yang, H. Wen, S. Fu, X. Li, Z. Zhang, B. Z. Tang and D. Wang, An NIR-II Excitable AIE Small Molecule with Multimodal Phototheranostic Features for Orthotopic Breast Cancer Treatment, Adv. Mater., 2024, 36, 2309748 CrossRef CAS PubMed.
- F. Y. Ye, M. Hu and Y. S. Zheng, Advances and challenges of metal ions sensors based on AIE effect, Coord. Chem. Rev., 2023, 493, 215328 CrossRef CAS.
- X. Jian, G. Jiang and J. Wang, Recent advances of aggregation-induced emission luminogens for point-of-care biosensing systems, Chem. Commun., 2024, 60, 8484–8496 RSC.
- P. Sudhakar, A. K. Gupta, D. B. Cordes and E. Z. Colman, Thermally activated delayed fluorescence emitters showing wide-range near-infrared piezochromism and their use in deep-red OLEDs, Chem. Sci., 2024, 15, 545–554 CAS.
- H. Wang, Q. Li, P. Alam, H. Bai, V. Bhalla, M. R. Bryce, M. Cao, C. Chen, S. Chen, X. Chen, Y. Chen, Z. Chen, D. Dang, D. Ding, S. Ding, Y. Duo, M. Gao, W. He, X. He, X. Hong, Y. Hong, J. J. Hu, R. Hu, X. Huang, T. D. James, X. Jiang, G. I. Konishi, R. T. K. Kwok, J. W. Y. Lam, C. Li, H. Li, K. Li, N. Li, W. J. Li, Y. Li, X. J. Liang, Y. Liang, B. Liu, G. Liu, X. Liu, X. Lou, X. Y. Lou, L. Luo, P. R. McGonigal, Z. W. Mao, G. Niu, T. C. Owyong, A. Pucci, J. Qian, A. Qin, Z. Qiu, A. L. Rogach, B. Situ, K. Tanaka, Y. Tang, B. Wang, D. Wang, J. Wang, W. Wang, W. X. Wang, W. J. Wang, X. Wang, Y. F. Wang, S. Wu, Y. Wu, Y. Xiong, R. Xu, C. Yan, S. Yan, H. B. Yang, L. L. Yang, M. Yang, Y. W. Yang, J. Yoon, S. Q. Zang, J. Zhang, P. Zhang, T. Zhang, X. Zhang, X. Zhang, N. Zhao, Z. Zhao, J. Zheng, L. Zheng, Z. Zheng, M. Q. Zhu, W. H. Zhu, H. Zou and B. Z. Tang, Aggregation-Induced Emission (AIE), Life and Health, ACS Nano, 2023, 17, 14347–14405 CAS.
- L. D. Thai, J. A. Kammerer, H. Mutlu and C. Barner-Kowollik, Photo- and halochromism of spiropyran-based main-chain polymers, Chem. Sci., 2024, 15, 3687–3697 CAS.
- X. Zhang, Y. Liu, W. Wei, L. Gao, Y. Duan, H. Han and T. A. Han, A defect-induced emission material with turn-on mechanoresponsive luminescence serving as a data storage, Dyes Pigm., 2022, 197, 109916 CAS.
- P. Yadav, S. Gond, A. Shekher, S. C. Gupta, U. P. Singh and V. P. Singh, A multifunctional basic pH indicator probe for distinguishable detection of Co2+, Cu2+ and Zn2+ with its utility in mitotracking and monitoring cytoplasmic viscosity in apoptotic cells, Dalton Trans., 2022, 51, 6927–6935 CAS.
- M. N. Bongiovanni, J. Godet, M. H. Horrocks, L. Tosatto, A. R. Carr, D. C. Wirthensohn, R. T. Ranasinghe, J. E. Lee, A. Ponjavic, J. V. Fritz, C. M. Dobson, D. Klenerman and S. F. Lee, Multi-dimensional super-resolution imaging enables surface hydrophobicity mapping, Nat. Commun., 2016, 7, 13544 CAS.
- Y. Y. Chen, X. M. Jiang, G. F. Gong, H. Yao, Y. M. Zhang, T. B. Wei and Q. Lin, Pillararene-based AIEgens: research progress and appealing applications, Chem. Commun., 2021, 57, 284–301 CAS.
- S. Das, H. K. Indurthi, P. Saha and D. K. Sharma, Coumarin-based fluorescent probes for the detection of ions, biomolecules and biochemical species responsible for diseases, Dyes Pigm., 2024, 228, 112257 CAS.
- H. Tapiero, D. M. Townsend and K. D. Tew, Trace Elements in Human Physiology and Pathology. Copper, Biomed. Pharmacother., 2003, 57, 386–398 CAS.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Copper Homeostasis and Neurodegenerative Disorders (Alzheimer's, Prion, and Parkinson's Diseases and Amyotrophic Lateral Sclerosis), Chem. Rev., 2006, 106, 1995–2044 CAS.
- Z. Hong, J. Zhong, D. Ding, S. Gong, L. Zhang, S. Zhao, X. C. Shen, H. Liang and F. P. A. Huang, A Cu(I)-based Fenton-like agent inducing mitochondrial damage for photo-assisted enhanced chemodynamic therapy, Dalton Trans., 2023, 52, 6187–6193 RSC.
- W. Gui and W. X. Wang, Copper redox state in cells and aquatic organisms: Implication for toxicity, J. Hazard. Mater., 2024, 476, 135039 CrossRef CAS PubMed.
- S. G. Ratés, M. S. García-Ayllón, N. Falgàs, S. A. Brangman, M. M. Esiri, C. W. Coen and S. A. Greenfield, Evidence for a novel neuronal mechanism driving Alzheimer's disease, upstream of amyloid, Alzheimer's Dementia, 2024, 20, 5027–5034 CrossRef PubMed.
- S. K. Singh, V. Balendra, A. A. Obaid, J. Esposto, M. A. Tikhonova, N. K. Gautam and B. Poeggeler, Copper-mediated β-amyloid toxicity and Its chelation therapy in Alzheimer's disease, Metallomics, 2022, 14, mfac018 CrossRef PubMed.
- A. Abelein, Metal binding of Alzheimer's amyloid-β and Its effect on peptide self-assembly, Acc. Chem. Res., 2023, 56, 2653–2663 CrossRef CAS PubMed.
- K. Duraimurugan, M. Harikrishnan, J. Madhavan, A. Siva, S. J. Lee, J. Theerthagiri and M. Y. Choi, Anthracene-based fluorescent probe: Synthesis, characterization, aggregation-induced emission, mechanochromism, and sensing of nitroaromatics in aqueous media, Environ. Res., 2021, 194, 110741 CrossRef CAS PubMed.
- L. Shen, C. J. Yu, H. F. Xie, N. Xu, H. Xu, Y. L. Huang, C. Redshaw, X. Feng and Q. L. Zhang, Naphthaldehyde-based Schiff base dyes: aggregation-induced emission and high-contrast reversible mechanochromic luminescence, Mater. Chem. Front., 2022, 6, 2491–2498 RSC.
- H. L. Li, Z. M. Xue, G. Yang, F. Meng, H. T. Lin, W. X. Zhao, S. H. Chen and C. Z. Wang, Pyrene-based, red-emitting, aggregation-induced emission luminogens: from structural construction to anti-counterfeiting applications, Mater. Chem. Front., 2025, 9, 318–324 RSC.
- S. Lee, K. Sivakumar, W. S. Shin, F. Xie and Q. Wang, Synthesis and anti-angiogenesis activity of coumarin derivatives, Bioorg. Med. Chem. Lett., 2006, 16, 4596–4599 CrossRef CAS PubMed.
- S. Gond, P. Yadav, A. Singh, S. Garai, A. Shekher, S. C. Gupta and V. P. Singh, A colorimetric and ‘OFF–ON’ fluorometric chemosensor based on a rhodamine-pyrazole derivative for the detection of Al3+, Fe3+ and Cr3+ metal ions, and its intracellular application, Org. Biomol. Chem., 2023, 21, 4482–4490 RSC.
- P. Yadav, R. Kumar, S. Srikrishna, A. K. Pandey, L. H. Choudhury, C. Upadhyay and V. P. Singh, A reversible and efficient probe for dual mode recognition of Al3+ and Cu2+ with logic gate behaviour: Crystal structure, theoretical and in vivo bio-imaging investigations, Spectrochim. Acta, Part A, 2022, 267, 120552 CrossRef CAS PubMed.
- G. Xiao, Y. J. Ma, Z. Qi, X. Fang, T. Chen and D. Yan, A flexible ligand and halogen engineering enable one phosphor-based full-color persistent luminescence in hybrid perovskitoids, Chem. Sci., 2024, 15, 3625–3632 RSC.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: Together We Shine, United We Soar!, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- F. N. C. Vaz, B. L. Fermino, M. V. L. Haskel, J. Wouk, G. B. L. de Freitas, R. Fabbri, E. Montagna, J. B. T. Rocha and J. S. Bonini, The Relationship Between Copper, Iron, and Selenium Levels and Alzheimer Disease, Biol. Trace Elem. Res., 2018, 181, 185–191 CrossRef CAS PubMed.
- M. M. Sreejaya, V. M. Pillai, A. Ayesha, M. Baby, M. Bera and M. Gangopadhyay, Mechanistic analysis of viscosity-sensitive fluorescent probes for applications in diabetes detection, J. Mater. Chem. B, 2024, 12, 2917–2937 RSC.
- S. K. Padhan, S. N. Sahu, N. Murmu, S. Mahapatra and M. K. Dalai, Ultrasensitive detection of aqueous Cu2+ ions by a coumarin-salicylidene based AIEgen, Mater. Chem. Front., 2019, 3, 2437 RSC.
- M. Yang, Y. Zhang, W. Zhu, H. Wang, J. Huang, L. Cheng, H. Zhou, J. Wua and Y. Tiana, Difunctional chemosensor for Cu(II) and Zn(II) based on Schiff base modified anthryl derivative with aggregation-induced emission enhancement and piezochromic characteristics, J. Mater. Chem. C, 2015, 3, 1994–2002 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Aggregation-induced emission: phenomenon, mechanism and applications, Chem. Commun., 2009, 4332–4353 RSC.
- Z. Ding, T. Lu, C. Bi, B. Li, S. T. Zhang, W. Xu and S. A. Jiang, A multifunctional material with distinct mechanochromic and piezochromic properties: π-stacking in play, Mater. Chem. Front., 2022, 6, 86–93 RSC.
- C. Wang, B. Xu, M. Li, Z. Chi, Y. Xie, Q. Li and Z. A. Li, A stable tetraphenylethene derivative: aggregation-induced emission, different crystalline polymorphs, and totally different mechanoluminescence properties, Mater. Horiz., 2016, 3, 220–225 CAS.
- Y. Gu, Z. Zhao, H. Su, P. Zhang, J. Liu, G. Niu, S. Li, Z. Wang, R. T. K. Kwok, X. L. Ni, J. Sun, A. Qin, J. W. Y. Lam and B. Z. Tang, Exploration of biocompatible AIEgens from natural resources, Chem. Sci., 2018, 9, 6497–6502 Search PubMed.
- C. Dou, L. Han, S. Zhao, H. Zhang and Y. Wang, Multi-Stimuli-Responsive Fluorescence Switching of a Donor-Acceptor π-Conjugated Compound, J. Phys. Chem. Lett., 2011, 2, 666–670 CrossRef CAS.
- H. Yu, W. Ren, H. Lu, Y. Liang and Q. Wang, Synthesis and piezochromic luminescence study of a coumarin hydrozone compound, Chem. Commun., 2016, 52, 7387–7389 RSC.
- F. Paquin, J. Rivnay, A. Salleo, N. Stingelin and C. Silva, A triphenylamine-based benzoxazole derivative as a high-contrast piezofluorochromic material induced by protonation, Chem. Commun., 2014, 30, 2569–2571 Search PubMed.
- W. Zhong, J. Zhang, Y. Lin, S. Li, Y. Yang, W. J. Wang, C. Si, F. E. Kühn, Z. Zhao, X. M. Cai and B. Z. Tang, Multi-site isomerization of synergistically regulated stimuli-responsive AIE materials toward multi-level decryption, Chem. Sci., 2024, 15, 3920–3927 RSC.
- P. Yadav, A. K. Singh, C. Upadhyay and V. P. Singh, Photoluminescence behaviour of a stimuli responsive Schiff base: Aggregation induced emission and piezochromism, Dyes Pigm., 2019, 160, 731–739 CrossRef CAS.
- A. Singh, P. Yadav, S. Singh, P. Kumar, S. Srikrishna and V. P. Singh, A multifunctional coumarin-based probe for distinguishable detection of Cu2+ and Zn2+: Its piezochromic, viscochromic and AIE behavior with real sample analysis and bio-imaging applications, J. Mater. Chem. C, 2023, 11, 13056–13066 RSC.
- J. P. Costes, C. Duhayon and L. Vendier, Synthesis, Structural Characterization, and Magnetic Properties of a Copper-Gadolinium Complex Derived from a Hydroxybenzohydrazide Ligand, Inorg. Chem., 2014, 53, 2181–2187 CrossRef CAS PubMed.
- S. Anbu, A. Paul, K. Surendranath, N. S. Solaiman and A. J. L. Pombeiro, A benzimidazole-based new fluorogenic differential/sequential chemosensor for Cu2+, Zn2+, CN−, P2O74−, DNA, Its live-cell imaging and pyrosequencing applications, Sens. Actuators, B, 2021, 337, 129785 CrossRef CAS.
- G. Huang, R. Wen, Z. Wang, B. S. Li and B. Z. Tang, Novel chiral aggregation induced emission molecules: self-assembly, circularly polarized luminescence and copper(II) ion detection, Mater. Chem. Front., 2018, 2, 1884–1892 RSC.
- R. Krämer, Fluorescent Chemosensors for Cu2+ ions: Fast, Selective, and Highly Sensitive, Angew. Chem., Int. Ed., 1998, 37, 772–773 CrossRef.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Molecular logic gates: the past, present and future, Chem. Soc. Rev., 2018, 47, 2228–2248 CAS.
- Y. Liu, M. Nguyen, A. Robert and B. Meunier, Metal Ions in Alzheimer's Disease: A Key Role or Not?, Acc. Chem. Res., 2019, 52, 2026–2035 CrossRef CAS PubMed.
- C. Esmieu, D. Guettas, A. Conte-Daban, L. Sabater, P. Faller and C. Hureau, Copper-Targeting Approaches in Alzheimer's Disease: How to Improve the Fallouts Obtained from in Vitro Studies, Inorg. Chem., 2019, 58, 13509–13527 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods, measurements, calculations, characterization, supplementary figures, comparative account of multifunctional properties, and crystal structure files (CIF). CCDC 2331258 and 2339921. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5qm00203f |
| This journal is © the Partner Organisations 2025 |