Open Access Article
Open Access ArticleMagnetically recoverable catalysts for efficient multicomponent synthesis of organosulfur compounds
Fadhil Faez Seadabc,
Vicky Jaind,
Anjan Kumar e,
Rekha M. M.f,
Mayank Kundlasg,
Sofia Guptah,
Mukesh Kumarii,
Mosstafa Kazemi
e,
Rekha M. M.f,
Mayank Kundlasg,
Sofia Guptah,
Mukesh Kumarii,
Mosstafa Kazemi
 *j and
Ramin Javahershenas
*j and
Ramin Javahershenas
 *k
*k
aDepartment of Dentistry, College of Dentistry, The Islamic University, Najaf, Iraq
bDepartment of Medical Analysis, Medical Laboratory Technique College, The Islamic University of Al Diwaniyah, Al Diwaniyah, Iraq
cDepartment of Medical Analysis, Medical Laboratory Technique College, The Islamic University of Babylon, Babylon, Iraq
dMarwadi University Research Center, Department of Chemistry, Faculty of Science, Marwadi University, Rajkot-360003, Gujarat, India
eDepartment of Electronics and Communication Engineering, GLA University, Mathura-281406, India
fDepartment of Chemistry and Biochemistry, School of Sciences, JAIN (Deemed to Be University), Bangalore, Karnataka, India
gCentre for Research Impact & Outcome, Chitkara University Institute of Engineering and Technology, Chitkara University, Rajpura, 140401, Punjab, India
hDepartment of Chemistry, Chandigarh Engineering College, Chandigarh Group of Colleges-Jhanjeri, Mohali 140307, Punjab, India
iDepartment of Applied Sciences-Chemistry, NIMS Institute of Engineering & Technology, NIMS University Rajasthan, Jaipur, India
jYoung Researchers and Elite Club, Tehran Branch, Islamic Azad University, Tehran, Iran. E-mail: mosstafakazemi@gmail.com
kOrganic Development, Chemistry Faculty, Urmia University, Urmia, Iran. E-mail: jshbco@yahoo.com
First published on 6th February 2025
Abstract
This manuscript introduces a groundbreaking study on the development and application of magnetically recoverable catalysts for the efficient multicomponent synthesis of organosulfur compounds. Capitalizing on the unique advantages of magnetic recovery, these catalysts streamline the synthesis process, offering an innovative solution that marries efficiency with environmental sustainability. By facilitating the multicomponent reaction of key precursors in the presence of sulfur sources, the catalysts enable the straightforward synthesis of various valuable organosulfur compounds, crucial in numerous pharmaceutical, agricultural, and material science applications. Key findings demonstrate a significant enhancement in reaction yields and selectivity and the remarkable ease with which the catalysts can be recovered and reused, thereby reducing both waste and operational costs. Magnetic catalysts, often based on magnetic iron nanoparticles, facilitate rapid and efficient reactions under mild conditions, offering superior atom economy, reduced solvent use, and the potential for scalable processes. Additionally, magnetically separating the catalysts from the reaction mixture enables multiple recycling cycles, reducing waste and operational costs. The review also discusses the mechanistic insights, challenges, and recent advancements in this field alongside future directions for developing more robust and versatile magnetic catalytic systems. This research embodies a significant step forward in the field of catalysis, highlighting the potential of magnetically recoverable catalysts to revolutionize the synthesis of complex molecules. Future perspectives discussed in the manuscript focus on expanding the scope of these catalysts to broader applications, optimizing catalyst design for enhanced performance, and further aligning chemical synthesis processes with the principles of green chemistry. This review covers the literature from 2010 to the end of 2024, and it encompasses the different one-pot protocols for synthesizing various heterocyclic organosulfur compounds based on magnetically recoverable catalysts.
1. Introduction
1.1. Catalysis
Multicomponent synthesis (MCS) has emerged as a transformative approach in organic synthesis, whereby multiple reactants are combined to create a single product through a one-pot reaction. This innovative methodology presents several advantages over conventional synthetic strategies. Primarily, it significantly minimizes the need for purification and isolation steps, which are often time-consuming and resource-intensive. By allowing the integration of various reactants into a single reaction vessel, MCS streamlines the overall synthesis process, saving both time and materials.1–3 Moreover, this synthesis approach promotes more excellent atom economy, a key principle in sustainable chemistry that emphasizes the efficient use of atoms in chemical processes. By maximizing the incorporation of reactants into the final product, MCS reduces waste generation, making it a more environmentally friendly option. These characteristics align closely with the principles of green chemistry, which aim to improve the sustainability of chemical processes by reducing toxicity and environmental impact.4,5A critical element of multicomponent reactions (MCRs) is catalysis, which plays a vital role in facilitating the formation of complex organosulfur compounds. Catalysis enhances reaction rates and selectivity, allowing reactions to occur under milder conditions that are often more efficient and safer than traditional methods. The integration of catalysis into MCRs marks a significant evolution in synthetic chemistry, as it empowers chemists to generate diverse and intricate molecular architectures with high efficiency.6 Recent advancements in the field underscore the utility of metal catalysts, such as rhodium and palladium—that have demonstrated exceptional effectiveness in promoting reactions involving sulfur-containing substrates. These catalysts are adept at influencing various reaction pathways and selectivity, thus enhancing the overall efficiency of the synthesis process. Their ability to operate under mild conditions is particularly noteworthy, as traditional synthetic methods frequently require harsh environments—high temperatures or extreme pressures—that may lead to undesirable side reactions or degradation of sensitive substrates.7
Furthermore, the strategic development of these metal catalysts has addressed many challenges historically associated with organic synthesis, thereby enabling the selective formation of desired products. The recent trend toward using sustainable and catalytic approaches reflects a broader commitment within the chemical community to innovate while minimizing the ecological footprint of synthetic processes.8 Additionally, ongoing research continues to explore new catalytic systems and methodologies that will expand the repertoire of available tools for chemists, further enhancing the versatility and applicability of MCRs. Through these innovations, multicomponent synthesis is poised for ongoing growth, providing exciting opportunities for developing novel materials, pharmaceuticals, and other valuable chemical entities.9,10
Catalysts are fundamental players in the realm of chemical reactions, functioning as crucial agents that significantly enhance the rate at which these reactions occur without undergoing any permanent change themselves. This unique ability to facilitate reactions without being consumed is what sets catalysts apart from other reactants.11 Catalysts operate by providing an alternative reaction pathway, which possesses a dramatically lower activation energy compared to the standard uncatalyzed process. Activation energy is the energy barrier that must be overcome for reactants to be converted into products, and by lowering this barrier, catalysts enable reactions to proceed more readily.12–14 This means that at any given temperature, a larger proportion of the reactant molecules can achieve the energy necessary to reach the transition state, the critical point at which reactants can transform into products. Consequently, this increased accessibility to the transition state results in higher reaction rates, allowing for a more efficient conversion of starting materials into desired products.15,16
The significance of catalysts in chemistry cannot be overstated. Their essential role is evident across a vast array of industrial processes, where they expedite reactions and enhance process efficiency.17,18 In various sectors, including petrochemicals, pharmaceuticals, and materials science, catalysts contribute to synthesizing a wide range of substances, from everyday items like plastics and fuels to more complex compounds used in drug development.19 Catalysts play a crucial role in conserving energy by enabling reactions to occur more rapidly or at reduced temperatures. This is particularly important in global sustainability efforts, as reducing energy consumption directly correlates with decreased environmental impact. Additionally, using catalysts leads to substantial cost savings in industrial operations.20 By allowing for more efficient reactions, companies can optimize resource use, minimize waste, and achieve greater throughput, all of which translate into lower production costs and enhanced profitability. The influence of catalysts extends beyond mere economic benefits; they are pivotal in driving innovation and efficiency within the field of chemical manufacturing. The ongoing development and optimization of new catalytic processes open doors to novel reactions and applications, often allowing chemists to explore previously untenable synthetic pathways.21
Catalysts play a transformative role in green chemistry, fundamentally altering chemical reaction approaches to foster more environmentally sustainable methods.22 This shift toward sustainability is crucial in addressing the environmental challenges faced by the chemical industry, where traditional processes often result in excessive waste and pollution. By facilitating reactions that generate significantly less waste and capitalize on renewable resources, catalysts embody the core principles of sustainability, promoting processes that are efficient and low in environmental impact.23–25 In nature, enzymes serve as natural catalysts indispensable for life. These remarkable proteins catalyze an array of complex biochemical reactions essential for the functioning of living organisms, such as digestion, metabolism, and DNA replication.26 Enzymes excel in their specificity and efficiency, showcasing nature's ability to fine-tune reactions in a way that supports life. This inherent ability to accelerate reactions is crucial for maintaining vital biological processes, demonstrating the importance of catalysts beyond the confines of the laboratory.27,28
Another essential characteristic of catalysts is their selectivity, which refers to their capacity to direct chemical reactions toward the formation of specific desired products while minimizing the production of unwanted byproducts.29 This selectivity is particularly valuable in the pharmaceutical industry, where the demand for high-purity substances is paramount for producing effective medications. High selectivity ensures that the desired therapeutic compounds are generated in reliable quantities and reduces the cost and complexity associated with purifying reaction mixtures, which can significantly enhance the overall efficiency of drug development processes.30 Furthermore, catalysts are at the forefront of groundbreaking research in renewable energy. They play a pivotal role in developing innovative technologies, such as solar fuels, where catalysts facilitate the conversion of sunlight into energy-storing molecules, mimicking photosynthesis. This area holds immense potential as a sustainable energy source, directly contributing to the quest for cleaner energy alternatives.31 Additionally, catalysts are instrumental in pioneering processes to convert carbon dioxide—a significant greenhouse gas—into beneficial chemicals and fuels, effectively recycling emissions into valuable products.32 By enabling these transformations, catalysts not only enhance efficiency in energy production but also play a crucial role in efforts to combat climate change and minimize our carbon footprint.33 The positive impact of catalysts on sustainable practices extends to various fields, including waste treatment, sustainable agriculture, and the production of biodegradable materials. Their ability to streamline reactions while conserving resources makes them integral to developing more sustainable processes that align with global environmental goals.
The primary objective of this review manuscript is to explore and critically analyze the recent advancements in the field of magnetically recoverable catalysts engineered explicitly for the efficient multicomponent synthesis of organosulfur compounds. To achieve this, the following key objectives have been established:
• Comprehensive overview: provide a detailed overview of the current state of research in magnetically recoverable catalysts, highlighting their structural designs, functional principles, and material compositions that facilitate their application in synthesizing organosulfur compounds.
• Synthesis strategies: examine the various synthetic strategies and reaction pathways employed in multicomponent reactions involving magnetically recoverable catalysts, emphasizing how these methods enhance reaction efficiency, selectivity, and yield compared to traditional catalytic approaches.
• Environmental and economic benefits: evaluate the environmental and economic advantages offered by these catalysts, including their contribution to greener synthesis processes through recyclability, reduced energy consumption, and diminished chemical waste.
• Challenges and limitations: identify the current challenges, limitations, and technical barriers associated with using magnetically recoverable catalysts in organosulfur compound synthesis, providing a balanced perspective on areas requiring further research and development.
• Future directions: propose future research directions and innovative approaches to overcome existing challenges, enhance catalytic performance, and broaden the applicability of these catalysts in diverse chemical synthesis processes.
• The review aims to synthesize current knowledge, inspire continued innovation in the field, and reinforce the significance of magnetically recoverable catalysts in developing sustainable and efficient chemical synthesis methodologies.
In summary, catalysts are vital in accelerating chemical reactions, enhancing selectivity, saving energy, and enabling sustainable industrial processes. Their use spans across various sectors, making them indispensable tools in modern chemistry and industry. The ongoing research and development of new and improved catalysts continue to open up possibilities for future innovations and advancements in science and technology.
1.2. Magnetic catalysts
Magnetic catalysts have emerged as a groundbreaking advancement in the realm of green chemistry and modern catalyst science, presenting a sustainable and environmentally-friendly strategy for facilitating chemical reactions (Fig. 1).34,35 Typically constructed from metal complexes that are meticulously supported on magnetic nanoparticles, these innovative catalysts offer the unique advantage of being easily separable from the reaction mixture.36 By applying an external magnetic field, the catalysts can be swiftly removed without the need for traditional separation methods, such as filtration or centrifugation.37,38 This simplified separation process not only significantly diminishes the consumption of solvents and energy but also substantially reduces waste generation, making it a key player in promoting sustainable practices.39 The design and operation of magnetic catalysts align seamlessly with the central tenets of green chemistry, which prioritize minimizing environmental impact while enhancing safety for both the processes involved and the products produced.40,41 Through this integration of efficiency and sustainability, magnetic catalysts are paving the way for a more responsible approach to chemical synthesis and industrial applications.42The reusability of magnetic catalysts presents a crucial advantage in contemporary chemistry, as these innovative materials can be effectively recovered and employed multiple times without experiencing a significant decline in their catalytic activity.43 This characteristic not only enhances cost-effectiveness but also promotes resource conservation in chemical processes.44 In the realm of modern catalyst science, the design and synthesis of magnetic catalysts are meticulously crafted to elevate their catalytic performance, stability, and selectivity.45–48 Cutting-edge advancements in material science have paved the way for the development of magnetic nanocatalysts, which possess tailored surface properties and functionalities.49 These properties can be precisely adjusted to optimize their effectiveness for specific reactions, thereby broadening the potential applications of magnetic catalysts across a diverse array of chemical transformations.50,51
Moreover, magnetic catalysts facilitate the development of novel reaction pathways and mechanisms, potentially leading to more efficient and less energy-intensive processes.52 For instance, the magnetic properties of these catalysts can influence reaction kinetics and dynamics, offering a unique control over the reaction by simply applying an external magnetic field.53 This aspect opens up new possibilities for catalysis, such as magnetic field-driven technologies that can change the catalytic output or enable the use of magnetic fluidized-bed reactors for improved mass and heat transfer.54 In the pharmaceutical industry, magnetic catalysts have been instrumental in the synthesis of valuable compounds with significant medicinal applications.55 The ability to perform reactions under milder conditions and with higher yields is particularly beneficial for the production of pharmaceuticals, where purity and efficacy are paramount.56 The environmental benefits of magnetic catalysts are also noteworthy, as they support the shift towards greener processes in the chemical industry.57 For example, they have been used to enhance hydrogen production and water splitting, which are critical for the development of clean energy technologies.58
The versatility and efficiency of magnetic catalysts are not limited to organic synthesis but also extend to material science, where they play a crucial role in the production of dyes, plasticizers, and fiber-reinforced composite materials.59,60 Their application in creating vulcanization accelerators for the rubber industry further exemplifies their industrial significance. In summary, magnetic catalysts represent a transformative approach in green chemistry and modern catalyst science, offering a harmonious blend of environmental stewardship, economic viability, and scientific innovation.
1.3. Magnetic catalysts in comparison with conventional catalysts
Magnetic nanocatalysts offer several advantages over conventional catalysts, making them increasingly popular in various chemical processes. Here are some key benefits:✓ Ease of separation: magnetic nanocatalysts can be easily separated from reaction mixtures using an external magnetic field. This eliminates the need for filtration or centrifugation, making the process cleaner and more efficient.61
✓ Reusability: due to their magnetic properties, magnetic catalysts can be recovered and reused multiple times without significant loss of activity. This enhances their cost-effectiveness and sustainability.35
✓ High surface area: magnetic nanocatalysts have a high specific surface area, which increases the number of active sites available for reactions. This leads to higher catalytic activity and efficiency compared to conventional catalysts.62
✓ Enhanced selectivity: the high surface area and the ability to functionalize the surface of magnetic nanocatalysts allow for better control over reaction pathways, improving the selectivity of the desired products.63
✓ Environmental benefits: magnetic separation avoids the use of additional chemicals and solvents, reducing the environmental impact of the catalytic process. This makes the process greener and more sustainable.64
✓ Versatility: magnetic nanocatalysts can be used in a wide range of reactions, including biofuel production, organic synthesis, and environmental remediation.65
In summary, magnetic nano catalysts offer improved efficiency, selectivity, and reusability, along with environmental benefits over conventional catalysts.66 These properties make them an attractive option for modern chemical processes, driving the continuous advancements in catalysis and green chemistry.67 The ongoing development of magnetic nanocatalysts is expected to further enhance their performance and application scope, solidifying their role in the future of catalytic science.
1.4. Methods for the construction of magnetic catalysts
There are several methods for preparing magnetic catalysts, each with its own advantages and applications. Here are some of the most common methods:✓ Co-precipitation: this is one of the simplest and most widely used methods. It involves the simultaneous precipitation of magnetic nanoparticles from a solution containing metal salts, usually under basic conditions. This method is cost-effective and can produce large quantities of nanoparticles.68
✓ Thermal decomposition: this method involves the decomposition of organometallic compounds at high temperatures in the presence of surfactants. It allows for precise control over the size and shape of the nanoparticles, resulting in highly uniform particles.69
✓ Hydrothermal and solvothermal methods: these methods use high-temperature and high-pressure conditions to synthesize nanoparticles in aqueous or non-aqueous solutions. They are particularly useful for producing well-crystallized and highly pure nanoparticles.63
✓ Sol–gel method: this involves the transition of a system from a liquid “sol” (mostly colloidal) into a solid “gel” phase. It is versatile and can be used to produce a wide range of materials, including magnetic nanoparticles.39
✓ Microemulsion: this method uses microemulsions as nanoreactors to control the size and shape of the nanoparticles. It is particularly useful for producing monodisperse nanoparticles with narrow size distributions.32
✓ Ball milling: this mechanical method involves grinding bulk materials into fine powders using a ball mill. It is a straightforward and scalable method, though it may result in a broad size distribution of particles.70
✓ Biological methods: these involve the use of biological organisms or biomolecules to synthesize nanoparticles. This eco-friendly approach can produce nanoparticles with unique properties, though it is still under extensive research.42
The preparation of magnetic catalysts involves various methods, each offering specific advantages in terms of control over particle size, composition, stability, and catalytic activity.71,72 The choice of method depends on the desired properties of the final catalyst, the type of reaction, and the scale of production.73 Regardless of the method, magnetic catalysts are particularly advantageous due to their ability to be easily separated and recycled, making them ideal for many catalytic processes, including environmental and industrial applications.
1.5. Multicomponent reactions
Multicomponent reactions (MCRs) are a powerful and increasingly important class of reactions in modern chemistry, particularly in the context of green chemistry.74 These reactions involve the simultaneous combination of three or more reactants to form a complex product, typically in a single reaction vessel and under relatively simple conditions.75 Here are several reasons why MCRs are beneficial:✓ Fewer steps: instead of performing multiple individual reactions to form a desired product, MCRs allow chemists to assemble complex molecules in a single step. This reduces the number of synthetic steps, simplifying workflows and saving both time and resources.77
✓ Simplified purification: fewer steps often mean fewer intermediate compounds, leading to simpler purification processes, which further reduces costs and environmental impact.79
✓ Safer processes: MCRs can sometimes replace hazardous reagents or energy-intensive processes with safer alternatives. Additionally, the use of fewer solvents reduces the potential for harmful solvent waste and emissions.75
✓ Reduced use of toxic reagents: the efficiency of MCRs can enable the use of more benign reagents, thereby minimizing the generation of harmful byproducts and reducing environmental impact.82
In summary, multicomponent reactions are a cornerstone of modern synthetic chemistry, offering multiple benefits that align well with the principles of green chemistry. They provide more efficient, cost-effective, and environmentally friendly synthetic routes, reduce waste, and enable the synthesis of complex molecules. As a result, MCRs are crucial not only in academic research but also in the development of sustainable industrial processes.
1.6. Organosulfur compounds
Organosulfur compounds are a broad class of compounds that contain carbon (C) and sulfur (S) atoms bonded together, and they have significant medicinal, biological, therapeutic, and industrial importance due to their unique chemical properties.86–89 These compounds are present in many natural substances and have a wide range of applications in various fields.✓ Antibiotics: many organosulfur compounds are essential in the development of antibiotics. A classic example is penicillin, where the sulfur atom plays a crucial role in its antibacterial activity by inhibiting bacterial cell wall synthesis.86,91
✓ Anticancer agents: compounds like sulforaphane, found in cruciferous vegetables (such as broccoli), have shown promising anticancer properties by inducing phase II detoxifying enzymes and inhibiting carcinogenic activity. Other organosulfur compounds, like diallyl disulfide (from garlic), have also demonstrated potential anticancer effects in preclinical studies.92,93
✓ Antioxidant activity: some organosulfur compounds possess antioxidant properties, which can help protect cells from oxidative stress. These include compounds like alpha-lipoic acid, which is involved in mitochondrial energy production and acts as a powerful antioxidant.94,95
✓ Antiviral and antimicrobial: garlic-derived organosulfur compounds, such as allicin, have been shown to possess broad-spectrum antimicrobial and antiviral activities. These compounds are thought to inhibit the growth of pathogens through their ability to modify the thiol groups in microbial enzymes and proteins.96,97
✓ Cardiovascular health: organosulfur compounds, particularly from garlic (ajoene), can have cardiovascular benefits by reducing blood pressure, lowering cholesterol, and improving circulation.98
✓ Amino acids and proteins: certain amino acids that contain sulfur, such as cysteine and methionine, are essential for protein synthesis, enzyme function, and cellular metabolism.99,100 These sulfur-containing amino acids are involved in the formation of disulfide bonds that stabilize protein structures.101
✓ Vitamins: thiamine (vitamin B1) and biotin (vitamin B7) are examples of vitamins that contain sulfur atoms in their structures. These vitamins are important for energy metabolism and various enzymatic processes in the body.102
✓ Enzyme co-factors: several important coenzymes like coenzyme A and lipoic acid contain sulfur. These molecules are involved in key metabolic processes, such as the citric acid cycle and fatty acid metabolism.103
✓ Detoxification: organosulfur compounds, especially glutathione (a tripeptide composed of glutamine, cysteine, and glycine), play a critical role in cellular detoxification.104 Glutathione is a key antioxidant and detoxifier, helping neutralize reactive oxygen species and other harmful molecules in the body.
✓ Anti-inflammatory: organosulfur compounds like diallyl sulfide (from garlic) have anti-inflammatory effects and are thought to modulate the immune response. They may help reduce inflammation in conditions such as arthritis.105,106
✓ Antidiabetic: certain organosulfur compounds show potential in managing diabetes. For example, sulforaphane has been studied for its ability to improve insulin sensitivity and reduce blood glucose levels.107
✓ Neurological health: organosulfur compounds, particularly alpha-lipoic acid, have neuroprotective effects. Lipoic acid has been used as a supplement for managing neurodegenerative diseases like Alzheimer's disease due to its ability to reduce oxidative stress and improve mitochondrial function.108
✓ Detoxification: compounds such as sulforaphane and cysteine (often in the form of N-acetylcysteine) are used therapeutically to support detoxification processes in the liver and improve overall cellular health.109
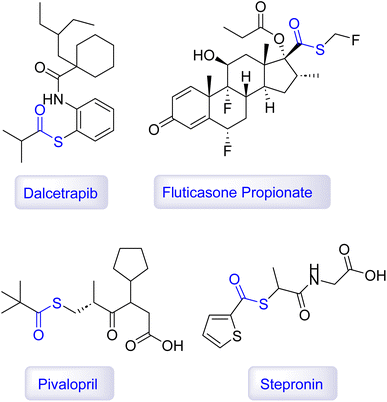
Overall, the multifaceted importance of aryl sulfides underscores their value in advancing health and industrial processes (Fig. 2).110–115
✓ Petrochemical industry: in the oil and gas industry, sulfur is a significant byproduct. Organosulfur compounds, such as thiols and sulfides, are found in crude oil and natural gas and need to be removed during the refining process due to their undesirable odors and their role in causing corrosion. Sulfur compounds are also used in the production of hydrogen sulfide (H2S) and sulfuric acid (H2SO4), both of which are crucial in a wide range of chemical processes.116,117
✓ Agriculture: organosulfur compounds, such as dimethyl disulfide and methyl isothiocyanate, are used as fumigants and pesticides. These compounds are especially effective at controlling pests and fungi in agricultural settings.118
✓ Rubber industry: in the production of synthetic rubber, sulfur is used in a process called vulcanization. Sulfur is used to crosslink the rubber molecules, making the material more durable, elastic, and heat-resistant.119
✓ Food industry: some organosulfur compounds, such as those in garlic and onions (e.g., allyl sulfides), are used to flavor food and are known for their health benefits. Additionally, sulfur-containing preservatives like sulfur dioxide are used to prevent spoilage in dried fruits, wines, and other food products.120
✓ Cosmetic industry: organosulfur compounds are also utilized in cosmetics for their antioxidant, antimicrobial, and anti-aging properties. For example, sulfur is used in topical treatments for skin conditions like acne and seborrheic dermatitis.121
In summary, organosulfur compounds are an essential class of chemicals with a wide range of applications across various fields, from medicine to industry. Their biological and medicinal properties, including anticancer, antimicrobial, and antioxidant activities, make them valuable in therapeutic contexts. Additionally, their utility in industrial processes like petroleum refining, rubber production, and agriculture highlights their versatility and importance in daily life. As research continues, it is likely that new uses and benefits of organosulfur compounds will emerge, further cementing their significance in modern science and industry.122–125
2. Magnetic catalysts in the multi-component preparation of organosulfur compounds
The field of chemical synthesis is perpetually evolving, with significant emphasis on enhancing catalytic processes' efficiency, selectivity, and sustainability. Magnetically recoverable catalysts have emerged as a pivotal innovation in this realm, particularly for the multicomponent synthesis of organosulfur compounds. These catalysts combine the reactive versatility required for complex chemical transformations with magnetic recovery's environmental and operational benefits.71–75Magnetically recoverable catalysts are designed to offer a straightforward, economical, and eco-friendly approach to chemical synthesis. These catalysts typically consist of a magnetic core material, such as iron oxide (Fe3O4), functionalized with various catalytic active sites. The core–shell structure enables the catalyst to participate actively in the reaction to form organosulfur compounds while allowing for an easy magnetic separation process post-reaction, thereby eliminating the need for filtration or centrifugation.76–80
Key advantages in multicomponent syntheses:
• Enhanced catalytic activity: magnetic catalysts often display superior catalytic activity due to the high surface area and easy accessibility of active sites on the magnetic nanoparticles.
• Selectivity and yield: these catalysts facilitate improved selectivity and higher yields in multicomponent reactions due to their tailored surface chemistry, which can be engineered to favor specific reaction pathways.
• Operational efficiency: these catalysts' magnetic properties allow for quick and easy recovery using an external magnetic field, significantly reducing downtime between batches and minimizing catalyst loss.
• Reusability: one of the standout features of magnetically recoverable catalysts is their reusability, which significantly reduces cost and environmental impact by reducing chemical waste and the need for fresh catalysts (Fig. 3).
The use of magnetic catalysts in the multicomponent synthesis of organosulfur compounds is a promising approach that combines the efficiency of MCRs with the benefits of magnetically separable catalysts. This method improves sustainability, reduces environmental impact, and offers practical advantages in terms of catalyst recovery and reuse. By leveraging the unique properties of magnetic materials, researchers can optimize reaction conditions and broaden the scope of organosulfur compound synthesis. Continuing our research on magnetic catalysts, this review explores the advantages of employing magnetic catalysts in the preparation of multicomponent organosulfur compounds, emphasizing their enhanced catalytic activity, ease of separation, and environmental sustainability. The advantages and disadvantages of using magnetic catalysts can be summarized in Fig. 4.75–85
2.1. Environmental implications of magnetically recoverable nanocatalysts
Integrating environmentally sustainable practices within chemical synthesis is increasingly important in contemporary research. Magnetically recoverable nanocatalysts, particularly in synthesizing organosulfur compounds through multicomponent reactions, exemplify a forward-thinking approach to merging catalytic efficiency with environmental stewardship. These innovative catalysts streamline synthetic processes and significantly mitigate the ecological footprint associated with traditional chemical manufacturing.40–45Magnetically recoverable nanocatalysts are engineered with magnetic properties that facilitate easy separation and recovery using a magnetic field, thus dramatically reducing waste and eliminating the need for chemical solvents or extensive energy inputs typically used in catalyst recovery. This feature is pivotal in minimizing the environmental impact traditionally associated with catalyst filtration, washing, reduction of hazardous waste, energy conservation, and disposal processes.46–50
Research demonstrates that magnetically recoverable nanocatalysts sustain high catalytic activity and promote greener reaction environments. For instance, in synthesizing thiophenes and thiazoles, which are important organosulfur compounds, magnetic nanocatalysts have been shown to facilitate reactions efficiently at room temperature, thereby conserving energy that would otherwise be needed for heating. Furthermore, these catalysts can often operate in water or other green solvents, which are significantly less toxic and more biodegradable than the organic solvents typically used in such syntheses. This reduces VOC emissions and decreases the risk of environmental contamination associated with solvent use.51–56
Magnetically recoverable nanocatalysts represent a significant breakthrough in green chemistry, particularly for synthesizing organosulfur compounds through multicomponent reactions. By reducing waste, conserving energy, and facilitating the use of greener solvents, these catalysts contribute positively to environmental conservation efforts. Ongoing research and development will be key to overcoming existing challenges and unlocking the full potential of these catalysts in sustainable industrial chemistry.57–60
2.2. Challenges directions
(1) Catalyst stability and degradation: one of the primary concerns with magnetically recoverable catalysts is their stability under typical reaction conditions. Repeated magnetic recovery and exposure to reactive environments can degrade the catalyst, reducing its effectiveness over time. Research is required to develop more robust materials to withstand the mechanical and chemical stresses of multiple reaction cycles.(2) Magnetic saturation and aggregation: due to magnetic attraction, magnetized nanoparticles tend to aggregate, which can decrease the surface area available for reactions and, consequently, reduce catalytic activity. Addressing issues of nanoparticle aggregation and ensuring uniform dispersion during the reaction is crucial for maintaining the high efficiency of these catalysts.
(3) Leaching of active species: leaching metal or active sites from the catalyst surface into the reaction medium is a significant challenge. It diminishes the catalyst's efficacy and contaminates the product, complicating purification processes. Innovative anchoring strategies and stronger chemical bonds between the active sites and magnetic support are needed.115–120
2.3. Future directions
(1) Advanced material engineering: research into new materials that can enhance the durability and reusability of magnetically recoverable catalysts is essential. Exploring alloys or composite materials that resist corrosion and physical wear could extend the life and efficiency of these catalysts.(2) Innovative catalyst systems: developing ‘smart’ catalysts that can adjust their activity in response to environmental changes or specific reaction stages could revolutionize their application in complex syntheses. Incorporating stimuli-responsive materials or molecular switches could allow for more precise control over the catalytic process.
(3) Scale-up and commercialization: scaling up the production and use of magnetically recoverable catalysts from laboratory to industrial scale remains challenging. Studies focused on the engineering aspects of catalyst manufacture, including the design of large-scale magnetic separation systems and continuous flow reactors fitted with magnetic recovery units, will be critical.
(4) Environmental impact assessment: comprehensive life-cycle assessments are necessary to evaluate the environmental impacts associated with the production, use, and disposal of magnetically recoverable catalysts. These studies will help identify potential environmental risks and guide the development of practices to mitigate them.
(5) Integration with renewable energy sources: using magnetically recoverable catalysts with renewable energy sources in chemical syntheses could further enhance their environmental profile. Research into systems that utilize solar or wind energy to drive reactions catalyzed by these advanced materials could set new benchmarks for sustainability in the chemical industry.115–120
2.4. MCRs preparation of sulfides
The three-component synthesis of aryl sulfides from a sulfur source using magnetic catalysts is a fascinating area of research in organic chemistry, particularly due to the importance of aryl sulfides in pharmaceuticals and materials science.126–130 This process typically involves the use of a magnetic nanocatalyst, which facilitates the formation of the C–S bond through a coupling reaction.131 The use of magnetic catalysts offers several advantages, including easy separation by an external magnetic field and recyclability, which makes the process more environmentally friendly and cost-effective. Additionally, the presence of a base can enhance the catalytic activity and suppress side reactions, leading to higher yields of the desired aryl sulfides. This method represents a significant advancement in the field of synthetic chemistry, providing a more efficient route to synthesize valuable sulfur-containing compounds (Fig. 5).In a pioneering study, Chang and his research group unveiled a novel catalyst system utilizing Fe3O4-supported 3-amino-4-mercaptobenzoic acid copper complex (Fe3O4@AMBA-CuI) magnetically recoverable catalyst [MRC-1] (Fig. 4).132 This innovative catalyst proved to be both effective and magnetically recoverable, facilitating the synthesis of a wide array of heteroaryl-aryl and di-heteroaryl sulfides. The method involves reacting various heteroaryl halides with aryl or heteroaryl boronic acids, utilizing elemental sulfur (S8) as the source of sulfur, all under environmentally-friendly conditions. The catalytic efficiency of this system is particularly noteworthy, as it successfully accommodated a diverse range of heteroaryl substrates, including challenging compounds like benzothiazole, benzoxazole, benzimidazole, oxadiazole, benzofuran, and imidazo[1,2-a]pyridine. Remarkably, the desired diaryl and di-heteroaryl sulfides were synthesized in high yields, showcasing the utility and versatility of this approach. While the products synthesized—heteroaryl-aryl and di-heteroaryl sulfides—are known compounds with previous reports, the scale and variety achieved in this methodology are unprecedented and have not been documented by any other synthetic routes (Scheme 1). This achievement not only highlights the effectiveness of the catalyst but also contributes significantly to the field of organic synthesis. Reusability tests confirmed that the Fe3O4@AMBA-CuI catalyst [MRC-1] (Fig. 4) has high catalytic activity despite being reused 6 times. To assess the stability and performance of the Fe3O4@AMBA-CuI nanocatalyst, various techniques such as VSM and ICP-OES techniques were employed. These analyses demonstrated that even after six cycles of use, the catalyst retained its magnetic properties and overall stability, which is crucial for practical applications.
 | ||
| Scheme 1 Fe3O4@AMBA-CuI (MRC-1) catalyzed synthesis of heteroaryl-aryl and di-heteroaryl sulfides from S8. | ||
The three-component synthesis of aryl sulfides through C–H bond sulfonylation is a significant advancement in organic chemistry, allowing for the creation of sulfide compounds via a direct and selective functionalization process.133 This method typically involves the reaction of an aromatic precursor with a sulfur dioxide surrogate and a reagent that facilitates the formation of the C–S bond.134 The process is advantageous due to its step and atom economy, often proceeding under mild conditions, which is crucial for maintaining the integrity of sensitive molecules.135 Recent developments have even enabled asymmetric sulfonylation, providing access to chiral sulfones with high regio- and enantioselectivity.
In a research publication authored by Saleh Shafik and his research team, the use of CuFe2O4 nanoparticles as a highly effective nanocatalyst for the synthesis of 2-thio-benzothiazoles and 2-thioaryl-benzoxazoles in ChCl–urea was detailed. The size distribution of CuFe2O4 particles was assessed using TEM analysis, revealing an average nanoparticle diameter of 15 nm. The results clearly demonstrate that the CuFe2O4 NPs/ChCl–urea system, in the presence of KOAc, serves as a versatile and effective catalytic platform for the one-pot three-component coupling reaction involving benzoxazoles or benzothiazoles with aryl iodides and S8 as the sulfur donor.136 It is important to note that substituents on the aromatic ring do not significantly influence the reaction progress. Following this mechanism, the initial reaction between the aryl halide and the catalyst leads to the formation of an intermediate. Subsequently, the addition of S8 produces another intermediate (Scheme 2). In the next step, this intermediate reacts with either benzoxazole or benzothiazole in the presence of a base, yielding an additional intermediate. Finally, the compound is synthesized from this intermediate through a reductive elimination step. Reusability tests confirmed that the CuFe2O4 catalyst [MRC-2] has high catalytic activity despite being reused 9 times.
 | ||
| Scheme 2 CuFe2O4 NPs (MRC-2) catalyzed synthesis of benzothiazole or benzoxazole-sulfide aryls from S8. | ||
The Fe3O4@ABA-aniline-CuI nanocomposite operates as a highly effective and reusable nanocatalyst, playing an essential role in the production of benzothiazole-sulfide compounds including both aryls and heteroaryls. This innovative material demonstrates remarkable catalytic activity, making it a valuable tool in organic synthesis processes. The Fe3O4@ABA-aniline-CuI nanocomposite [MRC-1] (Fig. 4) was expertly characterized using a range of advanced techniques. SEM and TEM images convincingly demonstrated that the particles are spherical and optimally sized, measuring around 12 to 25 nanometers.137 The incorporation of both the catalyst and the base was crucial for successfully synthesizing the desired product. In a one-pot, three-component reaction, 2-iodoaniline was reactively combined with carbon disulfide and a variety of aryl or heteroaryl iodides. This reaction was efficiently catalyzed by the Fe3O4@ABA-aniline-CuI nanomaterial, while potassium acetate (KOAc) acted as the base, with the reaction occurring in PEG-400 serving as the solvent (refer to Scheme 3 for a visual representation). This approach not only streamlines the synthesis process but also enhances overall efficiency and yield. Furthermore, Shen and his research team proposed a detailed tentative mechanism for the synthesis of benzothiazole scaffolds that integrate aryl and heteroaryl sulfides, elucidated clearly in Scheme 3 to provide deeper insight into the reaction pathway. Reusability tests confirmed that the Fe3O4@ABA-aniline-CuI catalyst [MRC-3] has high catalytic activity despite being reused 7 times.
 | ||
| Scheme 3 Fe3O4@ABA-aniline-CuI nanomaterial (MRC-3) catalyzed preparation of benzothiazole sulfides from CS2. | ||
Recently, Chen made a significant advancement in green chemistry by developing an innovative, eco-friendly magnetic nanocatalyst, designated as Fe3O4@SiO2-ABHA-CuCl nanocomposite (MRC-4) (Fig. 4). This cutting-edge catalyst facilitates the efficient synthesis of diaryl sulfides infused with intricate imidazo[1,2-a]pyridine scaffolds, showcasing its potential in organic transformations.138 The synthesis of this remarkable catalyst involved the strategic immobilization of copper(II) chloride (CuCl) onto magnetic Fe3O4 nanoparticles, which were artfully modified using 4-amino-3-hydroxybenzoic acid. Characterization techniques revealed that the resulting nanoparticles exhibited a captivating spherical morphology, with sizes ranging from 15 to 30 nanometers, which is ideal for maximizing surface area and catalytic efficiency. In practical applications, the catalyst demonstrated outstanding catalytic activity by promoting the sulfenylation of C–H bonds in imidazopyridines, utilizing a green solvent for the reaction, identified as PEG-400 (Scheme 4). This not only underscores the catalyst's effectiveness but also highlights its alignment with sustainable practices in chemical synthesis. Notably, substrates decorated with electron-donating groups, such as iodobenzene and phenyl groups in the derivatives of 2-phenylimidazo[1,2-a]pyridine, yielded particularly higher reaction rates, emphasizing the importance of these functional groups in enhancing reactivity. Reusability tests confirmed that the Fe3O4@SiO2-ABHA-CuCl catalyst [MRC-4] has high catalytic activity despite being reused 8 times. A comprehensive analysis after repeated use confirmed that the catalyst not only retained its magnetic properties but also its structural integrity, suggesting that it can be effectively employed in continuous processes without compromising its function.
 | ||
| Scheme 4 Fe3O4@SiO2-ABHA-CuCl nanomaterial (MRC-4) catalyzed synthesis of diaryl sulfides infused with intricate imidazo[1,2-a]pyridine scaffolds from S8. | ||
In a compelling and efficient discovery, Chang and Wang introduced a novel catalyst for the formation of carbon–sulfur (C–S) bonds. This catalyst denoted as Fe3O4@BTH-Pyr-CuCl nanomaterial (MRC-5) (Fig. 4), is composed of copper(I) chloride immobilized on magnetic nanoparticles that have been modified with a unique benzothiazole-pyrimidine ligand.139 The innovative design allows for the catalyst to be magnetically recovered following its use in reactions involving a specific set of heterocyclic compounds in combination with aryl iodides, as well as sulfur and selenium reagents. The structural characteristics of the Fe3O4@BTH-Pyr-CuCl nanocatalyst were thoroughly analyzed using a range of advanced techniques, including FT-IR, SEM, TEM, EDX, elemental mapping, TGA, XRD, VSM and ICP-OES techniques. Significantly, the recycling tests demonstrated that the Fe3O4@BTH-Pyr-CuCl nanocatalyst maintained its effectiveness through six consecutive uses, exhibiting only a minimal decrease in catalytic activity. This method stands out in comparison to other reported methodologies for C–S coupling of heterocycles due to several advantages: it employs an environmentally friendly solvent, achieves high product yields, utilizes a catalyst that can be easily separated and reused, and facilitates reactions within a significantly shorter time frame. Reusability tests confirmed that the Fe3O4@BTH-Pyr-CuCl catalyst [MRC-5] has high catalytic activity despite being reused 6 times (Scheme 5).
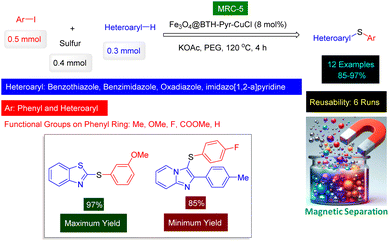 | ||
| Scheme 5 Fe3O4@BTH-Pyr-CuCl nanomaterial (MRC-5) catalyzed synthesis of heteroaryl-aryl sulfides from sulfur. | ||
2.5. MCRs synthesis of thioesters
Thioesters play a crucial role in various biological and medical applications due to their involvement in fundamental biochemical processes.140 In the realm of medicinal chemistry, thioesters are known for their therapeutic potential, exhibiting a range of biological activities including antibacterial, antioxidant, anticancer, anti-inflammatory, anti-Alzheimer, antituberculosis, and antimalarial properties (Fig. 6).141,142 These compounds serve as intermediates in metabolic pathways, particularly in the synthesis and degradation of fatty acids and steroids, such as in the formation of acetyl-CoA, a key molecule in energy production and biosynthesis.143 The carbonyl reaction is integral to the synthesis of thioesters, providing a reactive site for nucleophilic attack.144 This reactivity is harnessed in the preparation of thioesters, where the carbonyl carbon of a carboxylic acid reacts with a thiol group.145 The increased reactivity of the carbonyl group in thioesters compared to oxygen nucleophiles allows for the formation of amides, which are pivotal in peptide synthesis through native chemical ligation methods.146 This enhanced reactivity also facilitates the synthesis of thioesters from aldehydes and thiols, a process that is critical for the production of biologically active molecules and polymers with applications ranging from bioconjugates to biodegradable materials. The versatility and reactivity of thioesters underscore their importance in both biological systems and synthetic chemistry, making them valuable targets for drug development and material science.147To synthesize thioester derivatives, Wei's research group developed a nickel magnetic catalyst [Fe3O4@BA/Pyrim-carboxamide-NiCl2] and examined its catalytic performance in three-component reactions involving aryl iodides, aryl or benzyl phenols or thiols, and Cr(CO)6 as the carbonyl source.138 Initially, Fe3O4 nanoparticles were surface modified using 4-(aminomethyl)benzoic acid to create the magnetic nickel catalyst, followed by aminolysis through the reaction of the amine with pyrimidine-2-carbonyl chloride, which resulted in the formation of the magnetic Fe3O4@BA/Pyrim-carboxamide ligand; the subsequent addition of nickel led to the successful synthesis of the Fe3O4@BA/Pyrim-carboxamide-NiCl2 catalyst [MRC-6] (Fig. 7). The Vibrating Sample Magnetometry (VSM) analysis clearly indicated that the Fe3O4@BA/Pyrim-carboxamide-NiCl2 catalyst possesses significant magnetic potential. The structure and functional groups of the Fe3O4@BA/Pyrim-carboxamide-NiCl2 catalyst [MRC-6] were confirmed through X-ray Diffraction (XRD) and Fourier Transform Infrared (FT-IR) analyses. The authors performed a range of experiments to optimize the conditions for the synthesis of esters and thioesters, finding that both the type of base and the amount of catalyst were critical to the optimization process. By evaluating different solvents, the authors discovered that the ChCl–urea solvent was more effective than others for synthesizing ester and thioester derivatives. Reactions involving aryl iodides, aryl or benzyl thiols, and Cr(CO)6 as the carbonyl source were catalyzed by the Fe3O4@BA/Pyrim-carboxamide-NiCl2 nanocomposite in the presence of KOAc, resulting in various thioester derivatives being synthesized with high yields in ChCl–urea under mild conditions and air. Based on recycling tests, the Fe3O4@BA/Pyrim-carboxamide-NiCl2 catalyst [MRC-6] was successfully separated via magnetic decantation and could be reused up to eight times without a decrease in its catalytic efficiency (Scheme 6).148
To synthesize thioester derivatives, Khalili's research team introduced an intriguing and practical method that involves the reaction of methylarene derivatives with aryl or alkyl thiols, utilizing t-BuOOH and employing rGO/Fe3O4–CuO nanocomposite as an effective and recyclable catalyst.143 The catalyst rGO/Fe3O4–CuO [MRC-7] (Fig. 6) was synthesized through a multi-step procedure that involved coating graphene with Fe3O4 nanoparticles and then attaching copper oxide onto its surface. The structure and morphology of the rGO/Fe3O4–CuO catalyst were thoroughly confirmed via XRD and TEM analyses. To optimize the reaction, the influence of the catalyst quantity and solvent was investigated. As illustrated in Scheme 7, ten thioester derivatives were produced with relatively favorable yields in an aqueous medium under mild reaction conditions. The reaction between toluene derivatives and t-BuOOH in the presence of the rGO/Fe3O4–CuO catalyst generated a carbonyl radical. During the reaction with aryl and alkyl thiols in the presence of the rGO/Fe3O4–CuO catalyst, a sulfide–copper intermediate was formed; this intermediate then entered the reaction cycle, leading to the synthesis of the desired thioester products with good yields. Recycling tests indicated that the rGO/Fe3O4–CuO catalyst [MRC-7] could be separated via magnetic decantation and reused six times without a decline in its catalytic performance.
2.6. MCRs synthesis of thiazoles
Thiazole ring compounds represent a vital class of heterocyclic compounds that are increasingly recognized in medicinal chemistry for their remarkable range of biological activities, making them important candidates for drug development and therapeutic applications.149 The thiazole ring, a five-membered structure containing both sulfur and nitrogen atoms, is a key feature in many pharmacologically active molecules.150 These compounds exhibit a broad spectrum of therapeutic properties, including antimicrobial, antifungal, antiprotozoal, and antitumor activities.151 For instance, thiazole derivatives have been found to be effective in treating bacterial infections, with some acting as potent inhibitors of bacterial growth.152 Additionally, thiazole rings are present in several antiretroviral drugs, which are crucial in the management of HIV/AIDS.153 In the realm of cancer treatment, certain thiazole compounds have demonstrated antineoplastic properties, making them valuable in the development of new anticancer drugs.154 Moreover, the thiazole ring is a component of vitamin B1 (thiamine), which is essential for carbohydrate metabolism and normal neural function.155 The versatility of thiazole compounds in drug design is further highlighted by their inclusion in drugs with anti-inflammatory and neuroprotective effects, showcasing their potential in treating a variety of diseases and conditions.156,157 Several bioactive examples of thiazole molecules are shown in Fig. 8.158Magnetic catalysts are transforming the landscape of thiazole derivative synthesis, offering a sustainable and efficient approach to synthesis.153 These catalysts typically consist of magnetic nanoparticles that can be easily separated from the reaction mixture using an external magnet, thus simplifying the purification process and reducing waste.72 For instance, iron oxide magnetic nanoparticles have been employed as a reusable catalyst for the synthesis of various thiazole derivatives, enhancing the green chemistry aspect of these reactions.72 The use of such catalysts aligns with the principles of green chemistry, aiming to minimize environmental impact while maintaining high efficiency and product yield.
The innovative research team of Bodke has pioneered an effective and streamlined methodology for the one-pot three-component synthesis of isatin-thiazole derivatives. By employing a catalytic amount of biogenic Fe2O3 nanoparticles [MRC-8], this approach not only enhances efficiency but also underscores the remarkable potential of using biogenic materials in modern chemical synthesis. This development represents a significant advancement in the field, promising to simplify complex reactions and improve overall yields.157 A variety of spectroscopic techniques, such as SEM, FT-IR, TGA, XRD, TEM, and VSM analyses, were employed to confirm the structure of Fe2O3 nanoparticles. A one-pot, three-component reaction combining different isatins, thiosemicarbazide, and various phenacyl bromides was promoted by Fe2O3 nanoparticles under reflux conditions in ethanol, leading to the successful synthesis of isatin-thiazole products with high yields (Scheme 8). TEM images of the Fe2O3 nanoparticles showed that the particles are spherical and fall within the nanometer size range, with some degree of aggregation noted. Tests for reusability demonstrated that the Fe2O3 catalyst [MRC-8] maintained its high catalytic activity even after being used 5 times.
 | ||
| Scheme 8 Fe2O3 nanoparticles (MRC-8) catalyzed preparation of isatin-thiazole derivatives from thiosemicarbazide. | ||
In a research study, Hangirgekar et al. described a comprehensive and effective method for a one-pot three-component reaction involving tosylates, aryl aldehydes, and thiosemicarbazide to create a collection of hydrazinyl thiazoles, utilizing Fe2O3 nanoparticles [MRC-8] as a nanomagnetic catalyst.152 This study examined the effects of varying Fe2O3 nanoparticle quantities and solvent types to optimize reaction conditions. Characterization techniques including XRD, SEM, VSM, HR-TEM, EDX, and FT-IR were utilized to analyze the structural properties of the Fe2O3 nanoparticles.We also performed a one-pot three-component reaction using tosylates, aryl aldehydes, and thiosemicarbazide, with Fe2O3 nanoparticles as the catalyst in water (see Scheme 3). Reusability tests confirmed that the Fe3O4@dopamine-PO-CuBr2 catalyst [MRC-9] has high catalytic activity despite being reused 7 times (Scheme 9).
 | ||
| Scheme 9 Fe2O3 nanoparticles (MRC-9) catalyzed preparation of hydrazinyl thiazole derivatives from thiosemicarbazide. | ||
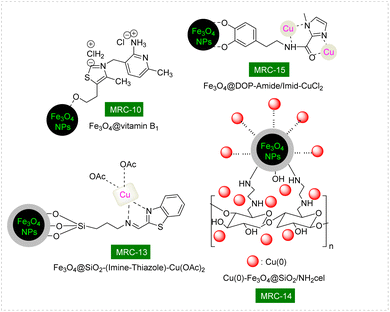
A novel and environmentally friendly approach have been developed for the synthesis of biologically active trisubstituted 1,3-thiazole derivatives. This methodology utilizes a one-pot, three-component reaction involving aryl glyoxal monohydrate, cyclic 1,3-dicarbonyl compounds, and thioamides. Central to this innovative process is the use of a unique catalyst: vitamin B1 that is immobilized on the surface of magnetic Fe3O4 nanoparticles, referred to as [Fe3O4@vitamin B1]. This method not only enhances the efficiency of the reaction but also allows for the convenient recovery of the catalyst using a magnetic field, making it a significant advancement in synthetic chemistry as reported by researchers Shaterian and Molaei.156 The formation of the Fe3O4@vitamin B1 nanocatalyst [MRC-10] (Fig. 9) was confirmed through EDX and FT-IR techniques. As shown in Scheme 10, one-pot three-component reactions involving aryl glyoxal monohydrate, cyclic 1,3-dicarbonyls, and thioamides were conducted in an aqueous medium under mild conditions, resulting in the successful synthesis of trisubstituted 1,3-thiazole products with moderate to high yields. Reusability tests confirmed that the Fe3O4@vitamin B1 catalyst [MRC-10] has high catalytic activity despite being reused 6 times.
 | ||
| Scheme 10 Fe3O4@vitamin B1 nanomaterial (MRC-10) catalyzed preparation of trisubstituted 1,3-thiazole derivatives from thioamides. | ||
In a research paper authored by Saleh Shafik and Jasim Elaibi, a novel method for synthesizing thiazole derivatives has been introduced, characterized by its efficiency, cost-effectiveness, and simplicity.159 As shown in Scheme 11, this approach employs a one-pot three-component reaction involving ethyl chloroacetate, thiosemicarbazide, and various acetophenone derivatives, all facilitated by the use of Fe3O4@CeO2 nanocatalyst [MRC-11]. The research details the preparation of Fe3O4@CeO2 magnetic nanoparticles through a hydrothermal method, resulting in nanoparticles with an average size of approximately 25 nm. These nanoparticles were thoroughly characterized using FT-IR and XRD techniques. The FT-IR analysis confirmed the presence of specific functional groups associated with each oxide, while the XRD results validated the successful synthesis of the nanocatalyst by identifying distinct peaks corresponding to the oxides involved. One of the standout features of this synthetic method is its high efficiency, yielding thiazole products in a remarkably short time frame of just 40 minutes. Moreover, the process is notable for its ease of execution, mild reaction conditions, and outstanding performance, making it an appealing option for researchers in the field. The integration of mechanization further enhances the practicality of this approach, positioning it as a valuable contribution to the synthesis of thiazole derivatives. Reusability tests confirmed that the Fe3O4@CeO2 catalyst [MRC-11] has high catalytic activity despite being reused 7 times.
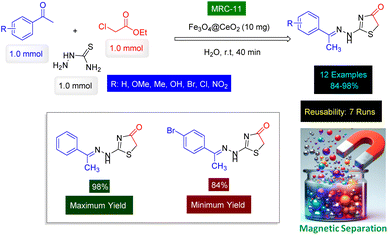 | ||
| Scheme 11 Fe3O4@CeO2 nanomaterial (MRC-11) catalyzed preparation of thiazole derivatives from thiosemicarbazide. | ||
In an important study conducted by Mokhtari and Naseri, a sophisticated and highly effective method was unveiled for synthesizing 2-(thiazol-2-yl)-4,5-dihydropyridazine-3-(2H)-one derivatives through a novel multicomponent cyclization process.160 This innovative synthetic pathway combines three key reactants: ketoacid, thiosemicarbazide, and phenacyl bromide, which together form the foundation of the desired compounds. The reaction is significantly enhanced by the use of a specialized catalyst, ZnS–ZnFe2O4 [MRC-12], known for its heterogeneous and recoverable properties, all while utilizing ethanol as a solvent under controlled reflux conditions (Scheme 12). This research is characterized by several noteworthy features that demonstrate its potential: efficient direct synthesis, intriguing structural complexity, rapid reaction times, mild and green reaction conditions, impressive efficiency. Overall, Mokhtari and Naseri's findings present a comprehensive and promising strategy for producing complex organic molecules, setting the stage for future explorations in therapeutic applications and contributing significantly to advancements in the field of organic chemistry. Reusability tests confirmed that the ZnS–ZnFe2O4 catalyst [MRC-12] has high catalytic activity despite being reused 5 times.
 | ||
| Scheme 12 ZnS–ZnFe2O4 nanomaterial (MRC-12) catalyzed preparation of pyridazinone-thiazole derivatives from thiosemicarbazide. | ||
2.7. MCRs synthesis of benzothiazoles
Benzothiazole compounds represent an important category of heterocyclic compounds that have attracted interest due to their broad biological and pharmaceutical characteristics.161 These compounds exhibit a wide range of biological activities, including antimicrobial, anticancer, antifungal, anthelmintic, anti-diabetic, and amyloid imaging agents, making them crucial in drug development and therapeutic applications.162,163 The synthesis of benzothiazole derivatives is of great scientific interest as they are found in bioorganic and medicinal chemistry with applications in drug discovery.164 They are also used industrially as antioxidants and vulcanization accelerators.165 The diverse biological activities of benzothiazole compounds, such as their use in radioactive amyloid imaging agents and as anticancer agents, underscore their importance in the synthesis of new pharmacological products with potential benefits in treating various diseases.166 The structure of several bioactive benzothiazole molecules is shown in Fig. 10.167,168Magnetic catalysts offer several advantages in the synthesis of benzothiazole compounds. They are easy to synthesize and provide high catalytic activity, which can significantly reduce the reaction time.169 Additionally, their magnetic properties allow for easy separation from the reaction mixture, enhancing the convenience of the process.170 The reusability of magnetic catalysts also contributes to a more sustainable and cost-effective synthesis, as they can be recovered and reused multiple times without significant loss of activity.170 Furthermore, the large surface area of these catalysts facilitates better interaction with reactants, leading to higher yields and more efficient reactions.
Patra and his team meticulously described the innovative construction of copper acetate (Cu(OAc)2) that was effectively immobilized onto the surface of specially modified magnetic nanoparticles featuring an imine-thiazole ligand. The resulting composite, identified as [Fe3O4@SiO2-(imine-thiazole)-Cu(OAc)2], functions as a magnetically recoverable catalyst for copper. This catalyst facilitates the synthesis of 2-substituted benzothiazoles via a streamlined one-pot three-component reaction. This reaction involves 2-iodoanilines, benzyl chlorides, and a sulfur source, all carried out in the presence of K2CO3 as the base, while utilizing dimethyl sulfoxide (DMSO) under controlled thermal conditions.171 TGA and VSM investigations revealed that the Fe3O4@SiO2-(imine-thiazole)-Cu(OAc)2 nanomaterial [MRC-12] (Fig. 8) exhibits remarkable stability and magnetic properties. The attempted synthesis involving a template reaction with 2-iodoaniline, benzyl chloride, and sulfur (S8) was unsuccessful when either the catalyst or base was omitted, indicating the essential role of these components in the reaction. Building upon the findings detailed in this methodology, a diverse array of 2-substituted benzothiazole products was successfully generated, with a total of 24 distinct examples demonstrating yields ranging from high to excellent, all achieved under mild reaction conditions (as illustrated in Scheme 13). Reusability tests confirmed that the Fe3O4@SiO2-(imine-thiazole)-Cu(OAc)2 catalyst [MRC-13] has high catalytic activity despite being reused 8 times.
 | ||
| Scheme 13 Fe3O4@SiO2-(imine-thiazole)-Cu(OAc)2 nanomaterial (MRC-13) catalyzed preparation of 2-substituted benzothiazole derivatives from S8. | ||
Paul et al. successfully synthesized a promising category of 2-substituted benzothiazoles with high yields through an innovative one-pot, three-component reaction. This reaction involved the combination of 2-iodoaniline, various aldehydes, and thiourea, all carried out in refluxing water. The remarkable aspect of their approach was the use of copper nanoparticles, which were supported on an ethylene diamine functionalized inorganic/organic composite known as [Cu(0)-Fe3O4@SiO2/NH2cel]. This composite served as a nanomagnetic recoverable catalyst, providing both efficiency and ease of recovery at the completion of the reaction. The process is illustrated in Scheme 14, showcasing the effectiveness of this catalytic system in facilitating the desired chemical transformations.161 The study focused on investigating the type of catalyst to determine the optimal conditions for the reaction. Additionally, the quantity of the Cu(0)-Fe3O4@SiO2/NH2cel nanocatalyst [MRC-14] (Fig. 8) was carefully assessed to achieve the best results. TEM and SEM images revealed that the average size of the Cu(0) nanoparticles was approximately 9 nm. This measurement closely aligned with the values obtained through XRD analysis, indicating a strong consistency between the different characterization methods used. Reusability tests confirmed that the Cu(0)-Fe3O4@SiO2/NH2cel catalyst [MRC-14] has high catalytic activity despite being reused 6 times.
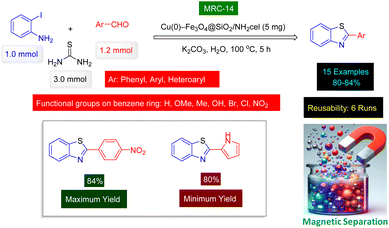 | ||
| Scheme 14 Cu(0)-Fe3O4@SiO2/NH2cel nanomaterial (MRC-14) catalyzed preparation of 2-aryl benzothiazole derivatives from thiourea. | ||
In a remarkable synthetic endeavor, Zhang and his research team discovered an innovative catalytic system utilizing a Fe3O4@DOP-amide/Imid-CuCl2 nanocomposite.172 This system operates in the presence of potassium acetate (KOAc) within a ChCl–urea solvent environment, making it an eco-friendly and highly effective method for producing 2-aryl benzothiazoles. The process involves a one-pot, three-component reaction, integrating 2-iodoaniline, aromatic aldehydes, and thiourea as the sulfur source. This catalytic approach has proven to be versatile, successfully yielding a wide array of 2-aryl benzothiazoles with high to excellent efficiency. When evaluated against other reported catalysts and magnetic nanocatalysts, the Fe3O4@DOP-amide/Imid-CuCl2 nanocatalyst [MRC-15] (Fig. 8) stands out due to several key characteristics. Notably, it features the clever design of a magnetic ligand achieved through an ammonolysis reaction. Additionally, it demonstrates impressive magnetic properties, which facilitate easy separation and recovery. The nanocatalyst showcases remarkable catalytic activity in the synthesis of various benzothiazole derivatives, while also emphasizing its reusability and high stability throughout multiple catalytic cycles. This combination of attributes underscores the potential of this nanocomposite in advancing sustainable and efficient chemical synthesis. Reusability tests confirmed that the Fe3O4@DOP-amide/Imid-CuCl2 catalyst [MRC-15] has high catalytic activity despite being reused 5 times. The techniques of SEM, VSM, BET, and ICP-OES demonstrated that the Fe3O4@DOP-amide/Imid-CuCl2 catalyst, after being reused 8 times, exhibited significant stability, as its magnetic characteristics, structure, and surface properties were comparable to those of the fresh catalyst (Scheme 15).
 | ||
| Scheme 15 Fe3O4@DOP-amide/Imid-CuCl2 nanomaterial (MRC-15) catalyzed preparation of 2-aryl benzothiazole derivatives from thiourea. | ||
2.8. MCRs synthesis of sulfonamides
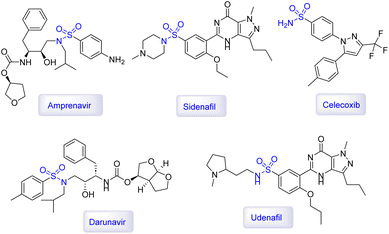
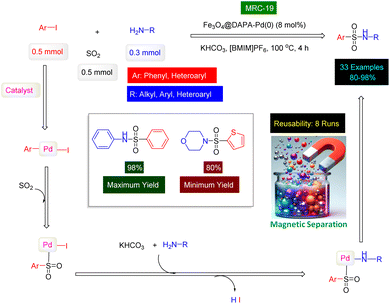
Sulfonamides are a class of synthetic antimicrobial agents that have played a significant role in treating bacterial infections due to their ability to inhibit the synthesis of folic acid in bacteria, which is essential for their growth and replication.173,174 They are structural analogs of para-aminobenzoic acid (PABA) and competitively inhibit the enzyme dihydropteroate synthase in the folate synthesis pathway.175 They are known for their ability to inhibit enzymes like carbonic anhydrase and dihydropteroate synthetase, which makes them effective in treating various disease states including diuresis, hypoglycemia, thyroiditis, inflammation, and glaucoma.176–178 This disruption of folate production is crucial as it leads to bacterial cell death. In medicine, sulfonamides are used to treat a variety of bacterial infections, including urinary tract infections, respiratory infections, and certain types of diarrhea.179 The structure of several bioactive examples of sulfonamide molecules are shown in Fig. 11.180–182 Chemically, the sulfonamide group (–SO2NH2) imparts certain properties to these compounds, such as solubility and the ability to interact with biological targets, which can be modified by substituting different groups onto the molecule to enhance its therapeutic index.183,184 The preparation of sulfonamides has been revolutionized by the use of magnetic catalysts, which offer a sustainable and efficient approach to synthesizing these compounds.185 These catalysts facilitate one-pot, multi-component reactions under eco-friendly conditions, and their magnetic properties allow for easy separation and reuse, thus reducing waste and improving reaction efficiency.186 The use of such catalysts is a testament to the advancements in green chemistry, providing a more environmentally friendly and cost-effective method for producing sulfonamides, which continue to be vital in the pharmaceutical industry.186In 2023, a paper was published in which Chang's research group used copper(II) bromide immobilized on Fe3O4 nanoparticles modified by dopamine and styrene epoxide as a magnetic reusable catalyst [Fe3O4@dopamine-PO-CuBr2] to prepare N-aryl sulfonamide derivatives.187 The authors first covered the surface of Fe3O4 nanoparticles with dopamine, then by the aminolysis of styrene epoxide, an attractive magnetic ligand was made to stabilize the CuBr2 (Fig. 12) [MRC-16]. VSM analysis showed that the Fe3O4@dopamine-PO-CuBr2 catalyst has high magnetic properties, and TGA analysis confirmed the thermal stability of the Fe3O4@dopamine-PO-CuBr2 catalyst. After conducting optimization experiments on the selected model reaction, the three-component reactions of phenyl boronic acids with aniline derivatives in the presence of DABSO as the SO2 source was successfully catalyzed by Fe3O4@dopamine-PO-CuBr2 nanocomposite in the presence of KOAc in DMSO under thermal conditions for 8 h. As seen in Scheme 16, a category of the N-aryl sulfonamide products (21 examples) was synthesized with high efficiencies (76–97%) under the standardized conditions. Reusability tests confirmed that the Fe3O4@dopamine-PO-CuBr2 catalyst [MRC-16] has high catalytic activity despite being reused 8 times.
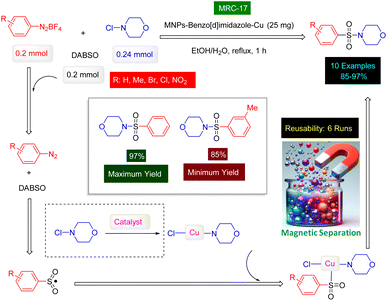
The innovative research team headed by Shams has successfully devised a highly effective and environmentally sustainable technique for synthesizing a range of sulfonamide derivatives, utilizing MNPs-benzo[d]imidazole-Cu [MRC 17] (Fig. 11) as a magnetically separable catalyst.188 The MNPs-benzo[d]imidazole-Cu nanocatalyst was synthesized through a four-step process, which included the silica protection of Fe3O4 nanoparticles, functionalization with (3-aminopropyl)trimethoxysilane (APTMS), modification with (1-H-benzo[d]imidazol-2-yl)methanamine, and coordination with copper(II) chloride. Characterization by XRD analysis confirmed the stability and alignment of the catalyst's structure with previously reported magnetic nanoparticles. XPS and FT-IR spectroscopy further validated the bond formation between the ligand and the copper complex. The resulting nanocomposite was utilized for the synthesis of sulfonamide derivatives through a one-pot, three-component reaction involving aryldiazonium tetrafluoroborate, DABCO(SO2)2, and N-chloroamines in an aqueous medium under mild conditions, as illustrated in Scheme 17. The synthesis of sulfonamide products presents a remarkable opportunity, significantly enhanced by the choice of catalyst and solvent. In carefully controlled conditions, our team successfully produced a diverse range of ten sulfonamides, achieving astonishing yields between 88% and 97%. Our innovative MNPs-benzo[d]imidazole-Cu catalyst demonstrated impressive reusability; when tested with 4-(phenylsulfonyl)morpholine as the model substrate, it retained its catalytic efficiency across six consecutive cycles, showcasing its exceptional durability and effectiveness. Moreover, we have proposed a comprehensive mechanistic pathway that elucidates the formation of sulfonamides through the strategic reactions of aryldiazonium tetrafluoroborate, DABCO(SO2)2, and N-chloroamines-facilitated seamlessly by the cutting-edge MNPs-benzo[d]imidazole-Cu nanocomposite (MRC-17). This is clearly outlined in Scheme 17, emphasizing the sophistication and potential of our approach.
Naderi's research team has made a significant advancement by presenting an innovative and flexible method for synthesizing sulfonamides. This approach utilizes a palladium complex anchored to the surface of magnetic nanoparticles, which are enhanced with arginine, providing an exceptionally efficient and magnetically recoverable catalyst.100 The magnetic Fe3O4 nanoparticles were expertly coated with arginine, resulting in the innovative Fe3O4@arginine nano ligand. This was followed by the strategic attachment of the Pd(0) complex on its surface, culminating in the advanced Fe3O4@arginine-Pd(0) nanocatalyst [MRC-18] (Fig. 11), designed to enhance catalytic efficiency. The bonding of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups with the palladium complex was verified through FT-IR spectroscopy. In this study, thermogravimetric analysis (TGA) was employed to confirm the successful attachment of functional groups on the surface of the magnetic nanoparticles, demonstrating the effectiveness of the immobilization process. The research further explored the potential of the Fe3O4@arginine-Pd(0) nanocomposite, referred to as MRC-18, as a catalyst for one-pot three-component reactions involving bromo benzenes, anilines, and potassium persulfate (K2S2O5). Conducted in n-hexane under mild conditions, these reactions resulted in the efficient synthesis of various sulfonamide derivatives, showcasing an impressive yield range of 88% to 95% across ten different examples within a remarkably short reaction time of less than one hour (as depicted in Scheme 18). To assess the practical applicability of the Fe3O4@arginine-Pd(0) catalyst (MRC-18), the researchers conducted tests to evaluate its reusability. The synthesis of N-phenylbenzene sulfonamide was selected as a model reaction for this purpose. Remarkably, the recovered catalyst retained its catalytic efficacy across eight consecutive cycles, illustrating its robustness and efficiency. Furthermore, the research group put forth a plausible mechanistic pathway to elucidate how sulfonamides are formed through the one-pot three-component reactions catalyzed by MRC-18. This proposed mechanism is carefully illustrated in Scheme 18, providing a comprehensive understanding of the reaction dynamics involved.
N groups with the palladium complex was verified through FT-IR spectroscopy. In this study, thermogravimetric analysis (TGA) was employed to confirm the successful attachment of functional groups on the surface of the magnetic nanoparticles, demonstrating the effectiveness of the immobilization process. The research further explored the potential of the Fe3O4@arginine-Pd(0) nanocomposite, referred to as MRC-18, as a catalyst for one-pot three-component reactions involving bromo benzenes, anilines, and potassium persulfate (K2S2O5). Conducted in n-hexane under mild conditions, these reactions resulted in the efficient synthesis of various sulfonamide derivatives, showcasing an impressive yield range of 88% to 95% across ten different examples within a remarkably short reaction time of less than one hour (as depicted in Scheme 18). To assess the practical applicability of the Fe3O4@arginine-Pd(0) catalyst (MRC-18), the researchers conducted tests to evaluate its reusability. The synthesis of N-phenylbenzene sulfonamide was selected as a model reaction for this purpose. Remarkably, the recovered catalyst retained its catalytic efficacy across eight consecutive cycles, illustrating its robustness and efficiency. Furthermore, the research group put forth a plausible mechanistic pathway to elucidate how sulfonamides are formed through the one-pot three-component reactions catalyzed by MRC-18. This proposed mechanism is carefully illustrated in Scheme 18, providing a comprehensive understanding of the reaction dynamics involved.
Jin has developed an innovative magnetic palladium (Pd) catalyst, designated as Fe3O4@DAPA-Pd(0), which facilitates the synthesis of a wide variety of N-aryl and alkyl sulfonamides. This catalyst is created by immobilizing a Pd(0) complex onto iron oxide (Fe3O4) nanoparticles, which have been coated with 2,3-diaminopropionic acid, enhancing the stability and effectiveness of the catalyst in various reactions. In his research, Jin thoroughly examined the catalytic performance of this system in a one-pot, three-component reaction process. This process involves a combination of aryl or heteroaryl iodides, amines, and sulfur dioxide (SO2), all while employing potassium bicarbonate (KHCO3) as a base within an ionic liquid solvent. This approach not only streamlines the synthetic pathway but also showcases the practical applications of the catalyst in producing valuable sulfonamide compounds efficiently.189 The TEM and XRD analyses provided strong evidence confirming that the particles are situated within the nanometer range, measuring approximately 10 to 25 nanometers. Additionally, the VSM analysis revealed that the Fe3O4@DAPA-Pd(0) catalyst, referred to as MRC-19 (Fig. 11), demonstrates exceptional magnetic properties both before it is recovered and after. As depicted in Scheme 19, a diverse array of aryl and heteroaryl halides was meticulously reacted with a selection of aryl, heteroaryl, and alkyl amines. This careful approach resulted in the successful synthesis of sulfonamide products, achieving high yields in the process. The recyclability of the Fe3O4@DAPA-Pd(0) catalyst, designated as [MRC-19], was thoroughly investigated in the context of synthesizing sulfonamides. The results demonstrated that the recovered catalyst maintained its catalytic efficiency effectively, allowing it to be reused for up to eight consecutive cycles without any noticeable decline in performance. To illustrate the process, a proposed synthetic pathway for the formation of sulfonamides is presented. This pathway involves the reaction of aryl or heteroaryl iodides with amines and sulfur dioxide (SO2), all facilitated by the Fe3O4@DAPA-Pd(0) nanomaterial (MRC-19) in the presence of potassium bicarbonate (KHCO3). This mechanism is visually represented in Scheme 19, highlighting the intricate interactions that enable the successful synthesis of sulfonamides.
In a different methodological framework, Saleh-Shafik and Saif meticulously documented the development of a palladium (Pd) complex that is immobilized on nanoparticles consisting of a core of iron oxide (Fe3O4) coated with silica (SiO2). These nanoparticles were further modified with picolylamine, resulting in the innovative formation of a magnetic catalyst, designated as [Fe3O4@SiO2-picolylamine-Pd] (referred to as MRC-20) (Fig. 11). This newly synthesized magnetic catalyst demonstrates significant potential for the efficient production of a specific class of sulfonamides, showcasing its application in catalytic processes.190 The Fe3O4@SiO2-picolylamine-Pd catalyst (MRC-20) was developed using a straightforward method with readily available materials. The construction of the Fe3O4@SiO2-picolylamine-Pd catalyst [MRC-20] (Fig. 11) was verified through FT-IR, TGA, EDX, BET, and ICP-OES analyses. The Fe3O4@SiO2-picolylamine-Pd catalyst (MRC-20) effectively catalyzed one-pot three-component reactions involving anilines, phenylboronic acids, and DABSO in the presence of K2CO3 in DMF under thermal conditions, resulting in high yields of the corresponding sulfonamide products (Scheme 20). The reutilization of the Fe3O4@SiO2-picolylamine-Pd catalyst (MRC-20) was investigated using N-phenylbenzene sulfonamide as the model substrate, and the recovered catalyst maintained its catalytic efficiency over six consecutive cycles.
2.9. MCRs synthesis of diaryl sulfones
Sulfones are a class of organic compounds that have found extensive applications across various fields due to their unique chemical properties.191 In the medical and pharmaceutical industries, sulfones are utilized for their antimicrobial properties, particularly in the treatment of leprosy and tuberculosis.192 The introduction of sulfonic acid groups into biomaterials through sulfonation enhances their properties, making them suitable for use in drug delivery systems, tissue engineering, and regenerative medicine.193 This chemical modification can improve biomaterial interactions with cells, promoting cell adhesion, proliferation, and differentiation. In the biological realm, sulfones serve as intermediates in the synthesis of complex molecules, including those used in medicinal chemistry.194 Sulfones are a crucial class of compounds in the medical field, with dapsone being a prominent example. Dapsone has been used as an antibiotic to treat various bacterial infections, including leprosy, dermatitis herpetiformis, and tuberculosis. It also played a role in the treatment of pneumocystis pneumonia (PCP), particularly in patients with compromised immune systems.195 Another significant application of sulfones in medicine is in the treatment of inflammatory diseases. For instance, sulfasalazine is used in managing rheumatoid arthritis and ulcerative colitis, due to its anti-inflammatory properties.196 These examples highlight the importance of sulfones in developing treatments that improve the quality of life for patients with infectious and chronic inflammatory diseases.197 The structure several bioactive examples of sulfone molecules is shown in Fig. 13.198,199Their role in chemical synthesis is also significant; they act as intermediates in various reactions, such as the Ramberg–Bäcklund reaction, which is useful in the construction of biologically active molecules or functional materials.200 Industrially, sulfones are employed in the creation of polymers and agrochemicals, where their ability to function as electron-withdrawing substituents or leaving groups is particularly valuable.201 The versatility of sulfones is further exemplified by their use in the synthesis of sulfonimidates, which are important intermediates for accessing other organosulfur compounds. Overall, the applications of sulfones are diverse and continue to expand as research advances in these areas.
Magnetic catalysts have emerged as a significant tool in the synthesis of sulfones, offering a sustainable and efficient alternative to traditional methods. These catalysts are particularly advantageous due to their ease of separation using an external magnetic field, which simplifies the purification process and minimizes waste. In the context of sulfone synthesis, magnetic catalysts can facilitate various reactions, including the oxidation of sulfides, the coupling of sulfinates, and C–H bond activation. These advancements reflect the ongoing efforts to develop more sustainable and environmentally friendly approaches in organic synthesis, aligning with the broader goals of green chemistry. Overall, the integration of magnetic catalysts in sulfone synthesis aligns with the principles of green chemistry, aiming to minimize waste and reduce the environmental footprint of chemical processes.
In an innovative research endeavor led by Mustafa and his research team, a novel nanocomposite, Fe3O4@DABA-PA-CuBr2, was successfully developed using a straightforward and cost-effective approach.202 This innovative method effectively immobilizes copper(II) bromide onto the surface of magnetic nanoparticles, which have been expertly modified with 3,4-diaminobenzoic acid and picolinic acid. This strategic enhancement significantly boosts the binding capabilities and functional properties of these nanoparticles, making them a powerful solution for a variety of applications. The study highlights the use of deep eutectic solvents (DESs), which are recognized as green and environmentally friendly alternatives, serving as both catalysts and solvents in various chemical reactions. The application of the Fe3O4@DABA-PA-CuBr2 nanocatalyst [MRC-21] (Fig. 14) in a ChCl–urea solvent system at a reaction temperature of 100 °C demonstrated an appealing and sustainable catalytic solution. This system facilitated the efficient one-pot three-component coupling reaction of aryl iodides, aryl boronic acids, and DABSO-acting as a source of sulfur dioxide-leading to the synthesis of a diverse array of diaryl sulfones. The results underscore the potential of this catalytic system in advancing green chemistry methodologies. Reusability tests confirmed that the Fe3O4@DABA-PA-CuBr2 catalyst [MRC-21] has high catalytic activity despite being reused 8 times. The analysis conducted using VSM, TEM, and ICP-OES techniques on the reused Fe3O4@DABA-PA-CuBr2 catalyst after eight catalytic cycles indicated that the fundamental properties of the catalyst remained remarkably stable. Specifically, the structural integrity, magnetic characteristics, morphology, and particle dimensions demonstrated minimal alterations, suggesting that the catalyst retains its effectiveness and stability over repeated uses (Scheme 21).
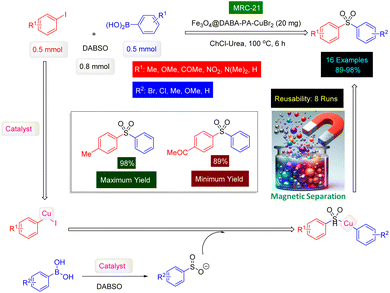 | ||
| Scheme 21 Fe3O4@DABA-PA-CuBr2 nanomaterial (MRC-21) catalyzed preparation of diaryl sulfone derivatives from DABSO. | ||
Fakri Mustafa and his team have made a groundbreaking advancement in catalysis with the creation of a magnetically recoverable copper catalyst. Discover an innovative catalyst designed through the precise immobilization of Cu(OAc)2 onto the surface of silica-coated magnetic Fe3O4 nanoparticles (Fe3O4@SiO2). This advanced approach combines cutting-edge materials science with exceptional catalytic properties, making it a game-changer in the field.203 These nanoparticles are expertly functionalized with amine and thiol groups, enhancing their ability to support the copper species effectively. The resulting Fe3O4@SiO2-imine/Thio-Cu(II) nanocomposite [MRC-22] (Fig. 13) has been thoroughly characterized using a suite of advanced techniques such as FT-IR spectroscopy, SEM, EDX, TEM, XRD, VSM, ASS, and ICP-OES. The findings reveal that this nanocomposite exhibits remarkable catalytic activity in the synthesis of biologically active diaryl sulfones, showcasing its potential in real-world applications. This method offers numerous benefits: the catalyst can be easily separated using an external magnetic field, resulting in efficient processing. Moreover, it yields excellent results in terms of product yield and reaction time, while utilizing a non-toxic metal catalyst. These features make the Fe3O4@SiO2-imine/Thio-Cu(II) nanocomposite an attractive and practical option for synthesizing diaryl sulfones, promising to enhance the efficiency and sustainability of chemical processes in various applications. Reusability tests confirmed that the Fe3O4@SiO2-imine/Thio-Cu(II) catalyst [MRC-1] has high catalytic activity despite being reused 8 times (Scheme 22).
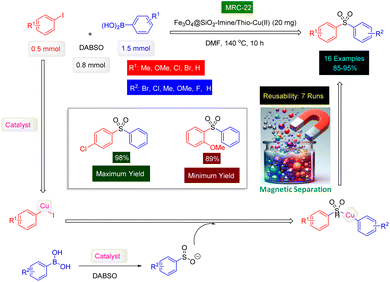 | ||
| Scheme 22 Fe3O4@SiO2-imine/Thio-Cu(II) nanomaterial (MRC-22) catalyzed preparation of diaryl sulfone derivatives from DABSO. | ||
To facilitate the preparation of diaryl sulfones, a novel copper(I) complex immobilized on magnetic Fe3O4 nanoparticles, referred to as Fe3O4@BBI-CuBr, was developed by Mustafa and his research team.204 This innovative nanomagnetic catalyst serves as both a green and highly efficient option for the sulfonylative-Suzuki–Miyaura cross-coupling reaction, which combines aryl iodides with arylboronic acids in the presence of DABSO as a source of sulfur dioxide. The characterization of the synthesized Fe3O4@BBI-CuBr nanocatalyst [MRC-23] (Scheme 23) involved a range of advanced spectroscopic techniques, confirming its composition and structural integrity. Employing this methodology, the team successfully synthesized a variety of diaryl sulfones, achieving notable efficiencies, ranging from good to excellent yields. The reactions were conducted under environmentally friendly and mild conditions, enhancing the sustainability of the process. One of the standout features of the Fe3O4@BBI-CuBr catalyst is its magnetic property, which allows for its easy separation from the reaction mixture using a magnet. This convenient separation method also enables the catalyst to be reused multiple times, making the process more economical and efficient. Additionally, when heterocyclic compounds were utilized as substrates, the resulting products were obtained with acceptable yields, demonstrating the versatility of this catalytic system in different reaction contexts. Reusability tests confirmed that the Fe3O4@BBI-CuBr catalyst [MRC-23] has high catalytic activity despite being reused 7 times. The results obtained from the XRD, TEM, and ICP-OES techniques reveal that the reused catalyst demonstrates remarkable stability and retains its catalytic activity even after being utilized in seven consecutive cycles (Scheme 23).
 | ||
| Scheme 23 Fe3O4@BBI-CuBr nanomaterial (MRC-23) catalyzed preparation of diaryl sulfone derivatives from DABSO. | ||
3. Summary and outlook
This study has extensively explored the use of magnetically recoverable catalysts in the multicomponent synthesis of organosulfur compounds, presenting a significant advancement in the field of catalysis. The employment of these catalysts has demonstrated an enhancement in the efficiency and selectivity of the reactions and a substantial reduction in environmental impact through the ease of catalyst recovery and reuse. Our findings underscore the effective integration of magnetic recovery technology with robust catalytic performance, providing a sustainable approach to complex synthesis processes. The magnetic properties of these catalysts allow for easy separation and recycling, significantly reducing catalyst loss and improving the overall efficiency and sustainability of the process. Additionally, magnetic catalysts often exhibit high selectivity and catalytic activity, improving reaction rates and product yields. Their versatility in various reaction conditions, combined with the ability to facilitate green chemistry practices such as solvent-free reactions, further enhances their appeal. The implications of this research for the catalysis community are profound. By leveraging the unique properties of magnetically recoverable catalysts, researchers can streamline the synthesis of organosulfur compounds, which are critical in various industrial applications, including pharmaceuticals, agrochemicals, and materials science. Recovering and reusing catalysts without significant losses in activity or selectivity presents a cost-effective and environmentally friendly alternative to traditional methods, which often involve tedious purification steps and generate considerable waste. The ability to manipulate magnetic catalysts with external magnetic fields also opens new possibilities for reaction control and optimization. While challenges remain in terms of scalability and the development of more robust magnetic materials, the progress made in recent years highlights the potential of these catalysts to revolutionize the synthesis of organosulfur compounds. Continued research and innovation in this field will likely lead to even more efficient, cost-effective, and environmentally friendly synthetic strategies, positioning magnetic catalysts as a key tool in the future of organosulfur chemistry.3.1. Advantageous of the use of magnetic catalysts
✓ Ease of recovery and reusability: magnetic catalysts can be easily separated from reaction mixtures using an external magnetic field. This simplifies the recovery process and allows the catalysts to be reused multiple times without significant loss of activity.✓ Enhanced catalytic performance: magnetic catalysts often exhibit high surface area and unique electronic properties, which can enhance their catalytic activity and selectivity in various chemical reactions.
✓ Versatility: magnetic catalysts can be used in a wide range of reactions, including oxidation, reduction, and condensation reactions. They are particularly useful in complex organic syntheses and industrial processes.
3.2. Importance in green chemistry
✓ Reduction of waste: the ability to recover and reuse magnetic catalysts reduces the amount of waste generated in chemical processes. This aligns with the green chemistry principle of minimizing waste production.✓ Energy efficiency: magnetic catalysts can often operate under milder conditions (lower temperatures and pressures), which reduces energy consumption and makes the processes more sustainable.
✓ Reduced use of hazardous substances: the use of magnetic catalysts can eliminate the need for hazardous solvents and reagents, further reducing the environmental impact of chemical processes.
3.3. Modern catalyst science
✓ Nanotechnology integration: the development of magnetic nanocatalysts involves advanced nanotechnology techniques, which allow for precise control over the size, shape, and composition of the catalysts. This leads to improved performance and new catalytic properties.✓ Innovative reactor designs: magnetic catalysts enable the design of novel reactors, such as magnetic fluidized-bed reactors, which can enhance reaction efficiency and scalability.
✓ Mechanistic insights: research into magnetic catalysts provides valuable insights into reaction mechanisms, helping scientists to design more efficient and selective catalysts for various applications.
3.4. Future research directions
Looking ahead, the field of magnetically recoverable catalysts holds vast potential for further exploration and innovation. Future research could focus on several promising areas:✓ Catalyst optimization: there is ongoing scope to enhance the design and functionality of magnetically recoverable catalysts. Research could focus on optimizing the composition, structure, and surface properties to increase catalytic activity, stability, and selectivity for a broader range of reactions.
✓ Expansion of substrate scope: exploring the use of magnetically recoverable catalysts in reactions involving a wider variety of substrates would be invaluable. It would expand the applicability of these catalysts in synthesizing a more diverse array of chemical products.
✓ Mechanistic studies: detailed mechanistic studies could provide deeper insights into the interactions at the molecular level between the catalyst and reactants. Understanding these interactions could lead to the rational design of catalysts tailored for specific reactions.
✓ Integration with flow chemistry: combining magnetically recoverable catalysts with continuous flow processes could revolutionize industrial-scale chemical synthesis. This integration promises to enhance the efficiency of chemical processes, reducing both time and resource expenditure.
✓ Environmental impact assessment: while these catalysts are designed for reusability and reduced waste, systematic studies assessing their long-term environmental impact are crucial. Research could focus on lifecycle analysis and the potential ecological footprint of the synthesis processes utilizing these catalysts.
By continuing to investigate these areas, the field of magnetically recoverable catalysts will not only refine its own techniques and applications but also significantly contribute to the broader discipline of sustainable chemical synthesis. The profound utility and versatile applications of these catalysts invite a collaborative, interdisciplinary approach to research, promising exciting developments in the synthesis of complex molecules and the advancement of green chemistry principles. The use of magnetic catalysts in the multicomponent synthesis of organosulfur compounds offers a compelling perspective on the future of green and efficient chemical processes. In conclusion, while there are hurdles to address, the potential of magnetic catalysts in the synthesis of organosulfur compounds is vast. With continued advancements in materials science, catalyst design, and reaction engineering, magnetic catalysts could become a cornerstone of sustainable and efficient synthetic methodologies, making significant contributions to both green chemistry and industrial applications.
Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Author contributions
Mostofa Kazemi was responsible for the supervision, conceptualization, and design of the study. Ramin Javahershenas played a pivotal role in overseeing the research project, providing strategic direction, guidance, and insight throughout the course of the study. Mosstafa Kazemi was responsible for defining the key objectives, designing the study methodology, and maintaining the project timeline. Fadhil Faez Sead and Vicky Jain were responsible for the investigation and searching of articles. Anjan Kumar, Rekha M. M., and Mayank Kundlas played an instrumental role in the drafting of the manuscript, while Sofia Gupta and Mukesh Kumari articulated the findings from the research and presented the data in a clear, prepared figures. Mosstafa Kazemi and Ramin Javahershenas conceived the idea, analyzed the data, and wrote the original draft. They also reviewed, edited, and proofread the draft. All authors contributed to the discussion and editing of the manuscript. The final version of the manuscript was read, reviewed, and approved by all authors.Conflicts of interest
The authors declare no conflict of interest.References
- S. Imeni, A. Makarem and R. Javahershenas, Asian J. Org. Chem., 2023, 12, e202300303 CrossRef CAS.
- R. Javahershenas and S. Nikzat, Ultrason. Sonochem., 2024, 102, 106741 CrossRef CAS PubMed.
- R. Javahershenas and S. Nikzat, RSC Adv., 2023, 13, 16619 RSC.
- R. Javahershenas, J. Han, M. Kazemi and P. J. Jervis, ChemistrySelect, 2024, 9, e202401496 CrossRef CAS.
- R. Javahershenas, J. Han, M. Kazemi and P. J. Jervis, ChemistryOpen, 2024, 13, e202400185 CrossRef CAS PubMed.
- R. Javahershenas, V. A. Soloshonok, K. D. Klika and P. J. Jervis, Carbon Lett., 2024 DOI:10.1007/s42823-024-00818-x.
- H. Zhang, Z. Zong, S. Lei, S. Srinivas, J. Sun, Y. Feng, M. Huang and Y. Feng, Adv. Sci., 2019, 3, 1900034 CrossRef.
- R. L. Mohlala, T. J. Rashamuse and E. M. Coyanis, Front. Chem., 2024, 12, 1469677 CrossRef CAS PubMed.
- B. A. D. Neto, R. O. Rocha and M. O. Rodrigues, Molecules, 2022, 27, 132 CrossRef CAS PubMed.
- P. S. G. Nunes, H. D. A. Vidala and A. G. Corrêa, Org. Biomol. Chem., 2020, 18, 7751 RSC.
- B. Xin, C. Jia and X. Li, Curr. Org. Chem., 2015, 20, 616 CrossRef.
- N. Basavegowda, K. Mishra and Y. R. Lee, New J. Chem., 2015, 39, 972 RSC.
- T. Biglari and A. Jafarzadeh, Biol. Mol. Chem., 2024, 1, 94 Search PubMed.
- M. A. Mustafa and A. Younes, Nanomater. Chem., 2024, 1, 120 Search PubMed.
- N. Mahato, P. Agarwal, D. Mohapatra, M. Sinha, A. Dhyani, B. Pathak, M. K. Tripathi and S. Angaiah, Processes, 2021, 9, 1544 CrossRef CAS.
- N. F. A., H. K. Aboud, H. A. Al-Bahrani and M. Kazemi, Polycyclic Aromat. Compd., 2024, 44, 4932–4978 CrossRef.
- H. Zangeneh, S. A. Mousavi and P. Eskandari, Mater. Sci. Semicond. Process., 2022, 140, 106383 CrossRef CAS.
- M. Ghobadi, P. Q. Pourmoghaddam and M. Kazemi, Synth. Commun., 2020, 50, 3717 CrossRef CAS.
- P. Du, W. Ran, C. Wang, L. Luo and W. Li, Adv. Mater. Interfaces, 2021, 8, 2100749 CrossRef CAS.
- J. Hou and M. Kazemi, Res. Chem. Intermed., 2024, 50, 1713 CrossRef CAS.
- P. Chandra, ChemistrySelect, 2021, 6, 10274 CrossRef CAS.
- Y. Zhao, ACS Appl. Nano Mater., 2020, 3, 4917 CrossRef CAS.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743 RSC.
- S. Jiang, RSC Adv., 2023, 13, 34273 RSC.
- M. Naderi and M. Farhadi, Biol. Mol. Chem., 2024, 2, 69 Search PubMed.
- S. Mondal, S. Samanta and A. Hajra, Adv. Synth. Catal., 2018, 360, 1026 CrossRef CAS.
- S. Awasthi, S. K. Pandey, E. Arunan and C. Srivastava, J. Mater. Chem. B, 2021, 9, 228 RSC.
- A. Arabmarkadeh, R. Javahershenas and M. Kazemi, Synth. Commun., 2021, 51, 880 CAS.
- W. Sun, N. E. Sahin, D. Sun, X. Wu, C. Munoz, J. Thakare, T. Aulich, J. Zhang, X. Hou, N. Oncel, D. Pierce and J. X. Zhao, ACS Appl. Mater. Interfaces, 2023, 15, 1115 CrossRef CAS.
- S. Kanithan, N. Arun Vignesh, K. M. Katubi, P. S. Subudhi, E. Yanmaz, J. A. Dhanraj, N. S. Alsaiari, k. M. Abualnaja, M. Sukumar, M. Sundararajan, S. Baskar, S. Sahu and C. S. Dash, J. Mol. Struct., 2022, 1265, 133289 CrossRef CAS.
- G. D. Fanou, M. Traore, B. K. Yao and S. Kone, Environ. Sci. Pollut. Res., 2021, 28, 21326 CrossRef CAS PubMed.
- M. Alighardashi, A. Assadi, F. Kazemi, Z. Zand and M. R. Mehrasbi, Int. J. Environ. Anal. Chem., 2024, 104, 5538–5551 CrossRef CAS.
- S. Sultana, G. Borah and P. K. Gogoi, Appl. Organomet. Chem., 2019, 33, e4595 CrossRef.
- E. Bertolucci, A. M. R. Galletti, C. Antonetti and A. Pucci, IEEE International Instrumentation and Measurement Technology Conference Proceedings, 2015, p. 1492 Search PubMed.
- M. Ghobadi, M. Kargar Razi, R. Javahershenas and M. Kazemi, Synth. Commun., 2021, 51, 647 CrossRef CAS.
- W. Li, J. Yan, W. Xu and L. Y. Zhang, RSC Adv., 2023, 13, 28964 RSC.
- T. H. V. Kumar, J. Rajendran, R. Atchudan, S. Arya, M. Govindasamy, M. A. Habila and A. K. Sundramoorthy, Environ. Res., 2023, 238, 117193 CrossRef CAS.
- M. Kazemi, Synth. Commun., 2020, 50, 1409 CrossRef CAS.
- A. Wang, M. Ding, Y. Cai, L. Wang, Y. Guo, Y. Guo and W. Zhan, Environ. Sci. Technol., 2024, 58, 20300 CrossRef CAS PubMed.
- I. Khan, K. Saeed and I. Khan, Arabian J. Chem., 2019, 12, 908 CrossRef CAS.
- M. Kazemi and M. Ghobadi, Nanotechnol. Rev., 2017, 6, 549 CAS.
- J. Rajendran, J. Hazard. Mater., 2023, 449, 130979 CrossRef CAS PubMed.
- A. M. Abu-Dief and S. M. Abdel-Fatah, Beni-Suef University Journal of Basic and Applied Sciences, 2018, 7, 55 CrossRef.
- Q. Zhang, X. Yang and J. Guan, ACS Appl. Nano Mater., 2019, 2, 4681 CrossRef CAS.
- J. Govan and Y. Gun'ko, Nanomaterials, 2014, 4, 222 CrossRef PubMed.
- M. Ghobadi, P. Pourmoghaddam Qhazvini, M. Eslami and M. Kazemi, Synth. Commun., 2021, 51, 325 CrossRef CAS.
- M. Kazemi, M. Ghobadi and A. Mirzaie, Nanotechnol. Rev., 2018, 7, 43 CrossRef CAS.
- J. Singh, Nanomater. Chem., 2023, 1, 58 Search PubMed.
- C. Sappino, L. Primitivo, M. De Angelis and V. Abeti, ACS Omega, 2019, 4, 21809 CrossRef CAS PubMed.
- J. Dadashi, M. Khaleghian, B. Mirtamizdoust, A. Golzary and S. J. Rouhani, Crystals, 2022, 12, 862 CrossRef CAS.
- M. Abedi, M. Hosseini, A. Arabmarkadeh and M. Kazemi, Synth. Commun., 2021, 51, 835–855 CAS.
- N. Chidhambaram, S. J. J. Kay, S. Priyadharshini and V. Ramesh, Catalysts, 2023, 13, 440 CrossRef CAS.
- R. Eisavi and F. Ahmadi, Sci. Rep., 2022, 12, 11939 CrossRef CAS PubMed.
- S. Sheikh, M. A. Nasseri, A. Allahresani and R. S. Varma, Sci. Rep., 2022, 12, 17986 CrossRef CAS PubMed.
- A. Moslehi and M. Zarei, New J. Chem., 2019, 43, 12690 RSC.
- Y. Riadi, M. Kadhim, S. Jawad Shoja and B. K. Hameed, Synth. Commun., 2022, 52, 875 CrossRef CAS.
- P. Rai and D. Gupta, Synth. Commun., 2021, 51, 3059 CrossRef CAS.
- R. Hudson, Y. Feng, R. S. Varma and A. Moores, Green Chem., 2014, 16, 4493 RSC.
- M. Ma, P. Hou, P. Zhang, H. Zhong and B. Peng, Appl. Catal., A, 2020, 602, 117709 CrossRef CAS.
- Z. Moghadasi and M. Jalali, Nanomater. Chem., 2023, 1, 94 Search PubMed.
- N. Wang, Z. Zhang, Y. Zhang, X. Xu and Q. Guan, Sep. Purif. Technol., 2025, 355, 129566 CrossRef CAS.
- D. K. Yi, S. S. Lee and J. Y. Ying, Chem. Mater., 2006, 18, 2459 CrossRef CAS.
- D. Wang, C. Deraedt, J. Ruiz and D. Astruc, Acc. Chem. Res., 2015, 48, 1871 CrossRef CAS PubMed.
- Z. Hua, H. Song, C. Zhou, Q. Xin, F. Zhou, W. Fan, S. Liu, X. Zhang, C. Zheng, Y. Yang and X. Gao, Chem. Eng. J., 2023, 473, 145106 CrossRef CAS.
- B. Jaleh, A. Khalilipour, S. Habibi and M. Kameli, J. Mater. Sci.: Mater. Electron., 2017, 28, 4974 CrossRef CAS.
- J. Hou and M. Kazemi, Res. Chem. Intermed., 2024, 50, 1845 CrossRef CAS.
- P. Hu and M. Kazemi, J. Coord. Chem., 2024 DOI:10.1080/00958972.2024.2378041.
- M. P. Conte, J. K. Sahoo, Y. M. Abul-Haija and G. L. Rosair, ACS Appl. Mater. Interfaces, 2018, 10, 3069 CrossRef CAS.
- F. Zhou, Q. Xin, Y. Fu, Z. Hua, Y. Dong, M. Ran, H. Song, S. Liu, R. Qu, Y. Yang, X. Zhang, C. Zheng and X. Gao, Chem. Eng. J., 2023, 464, 142471 CrossRef CAS.
- K.-G. Liu, Z. Sharifzadeh, F. Rouhani and J. Yang, Coord. Chem. Rev., 2021, 436, 213827 CrossRef CAS.
- E. Doustkhah, M. Heidarizadeh, S. Rostamnia and A. Shokri, Mater. Lett., 2018, 216, 139 CrossRef CAS.
- J. Hou and M. Kazemi, Mini-Rev. Org. Chem., 2024 DOI:10.2174/0118756298296678240402080951.
- A. Baghban, M. Heidarizadeh, E. Doustkhah and M. T. Ghaemi, Int. J. Biol. Macromol., 2017, 103, 1194 CrossRef CAS.
- S. G. Pharande, Synthesis, 2021, 53, 418 CrossRef CAS.
- M.-N. Chen, L.-P. Mo, Z.-S. Cui and Z.-H. Zhang, Curr. Opin. Green Sustainable Chem., 2019, 15, 27 CrossRef.
- S. Ghosh and K. Biswas, RSC Adv., 2021, 11, 2047 RSC.
- A. Ramazani and A. Reza Kazemizadeh, Curr. Org. Chem., 2011, 15, 3986 CrossRef CAS.
- D. Becerra, R. Abonia and J.-C. Castillo, Molecules, 2022, 27, 4723 CrossRef CAS.
- M. Lakshman and R. Barrett, J. Synth. Chem., 2024, 3, 121 Search PubMed.
- K. Zolfaghari, M. Panahi and Z. Omidi, J. Synth. Chem., 2024, 3, 49 Search PubMed.
- E. M. de Marigorta, J. M. d. L. Santos, A. M. Ochoa de Retana, J. Vicario and F. Palacios, Beilstein J. Org. Chem., 2019, 15, 1065 CrossRef CAS PubMed.
- B. H. Rotstein, S. Zaretsky, V. Rai and A. K. Yudin, Chem. Rev., 2014, 114, 8323 CrossRef CAS PubMed.
- P. Brandão, A. J. Burke and M. Pineiro, Eur. J. Org. Chem., 2020, 2020, 5501 CrossRef.
- A. Delawari and I. Fatehi, J. Synth. Chem., 2024, 3, 163 Search PubMed.
- M. Datta, ChemistrySelect, 2021, 6, 187 CrossRef CAS.
- S. Petropoulos, F. Di Gioia and G. Ntatsi, Curr. Pharm. Des., 2017, 23, 2850 CrossRef CAS.
- R. J. Reddy and A. H. Kumari, RSC Adv., 2021, 11, 9130 RSC.
- N. Amri and T. Wirth, Chem. Rec., 2021, 21, 2526 CrossRef CAS.
- L. Chen, A. N. Fajer, Z. Yessimbekov, M. Kazemi and M. Mohammadi, J. Sulfur Chem., 2019, 40, 451 CrossRef CAS.
- Z. Xu, Z. Qiu, Q. Liu and P. Wang, Nat. Commun., 2018, 9, 3713 CrossRef CAS PubMed.
- N. Miękus, K. Marszałek, M. Podlacha and P. Nowak, Molecules, 2020, 25, 3804 CrossRef.
- M. C. Egbujor, M. Petrosino, K. Zuhra and L. Saso, Antioxidants, 2022, 11, 1255 CrossRef CAS.
- M. A. Vazquez-Prieto and R. M. Miatello, Mol. Aspects Med., 2010, 31, 540 CrossRef CAS PubMed.
- X. Gu and Y. Z. Zhu, Expert Rev. Clin. Pharmacol., 2011, 4, 123 CrossRef CAS PubMed.
- O. Sagdic and F. Tornuk, in Dietary Phytochemicals and Microbes, Springer Netherlands, Dordrecht, 2012, p. 127 Search PubMed.
- M. F. Mahomoodally, N. Nabee and N. Baureek, in Antioxidants Effects in Health, Elsevier, 2022, pp. 417–426 Search PubMed.
- S. Gupta and B. Shroff, J. Synth. Chem., 2024, 3, 61 Search PubMed.
- A. Tizazu and A. Nebi, Biomed. J. Sci. Tech. Res., 2024 DOI:10.22541/au.172982344.49462320/v1.
- A. M. P. Walag, O. Ahmed, J. Jeevanandam and G. Johnson, in Functional Foods and Nutraceuticals, Springer International Publishing, Cham, 2020, pp. 445–472 Search PubMed.
- A. Naderi and Z. Jalali, Biol. Mol. Chem., 2024, 2, 33 Search PubMed.
- M. Iciek, I. Kwiecień and L. Włodek, Environ. Mol. Mutagen., 2009, 50, 247 CrossRef CAS PubMed.
- A. Francioso, A. B. Conrado, L. Mosca and M. Fontana, Oxid. Med. Cell. Longevity, 2020 DOI:10.1155/2020/8294158.
- A. Kamel and M. Saleh, Stud. Nat. Prod. Chem., 2000, 23, 455 CAS.
- A. Amrani, Annu. Rev. Earth Planet. Sci., 2014, 42, 733 CrossRef CAS.
- V. P. Osipova and N. T. Berberova, Russ. J. Coord. Chem., 2023, 49, S196 CrossRef CAS.
- R. Ghorbani and L. Saeedi, Biol. Mol. Chem., 2024, 2, 45 Search PubMed.
- K. M. Sadek, N. A. Shib, E. S. Taher, F. Rashed, M. Shukry, G. A. Atia, N. Taymour, M. El-Nablaway, A. M. Ibrahim, M. M. Ramadan, A. Abdelkader, M. Abdo, I. Imbrea, E. Pet, L. S. Ali and A. Abdeen, Front. Pharmacol, 2024, 15, 1412245 CrossRef CAS PubMed.
- H. Zhu, V. Dronamraju, W. Xie and S. S. More, Med. Chem. Res., 2021, 30, 305 CrossRef CAS PubMed.
- A. Milito, M. Brancaccio, G. D'Argenio and I. Castellano, Cells, 2019, 8, 1356 CrossRef CAS PubMed.
- D.-L. Chen, Y. Sun, M. Chen and X. Chen, Org. Lett., 2019, 21, 3986 CrossRef CAS.
- C. Ravi, D. Chandra Mohan and S. Adimurthy, Org. Biomol. Chem., 2016, 14, 2282 RSC.
- X. Yin, Q. Zhang and Q. Zeng, Organics, 2023, 4, 173 CrossRef CAS.
- S. M. Allin, W. R. Bowman, R. Karim and S. S. Rahman, Tetrahedron, 2006, 62, 4306 CrossRef CAS.
- E. D. Weil, Phosphorus, Sulfur Silicon Relat. Elem., 1991, 59, 31 CrossRef.
- V. Polshettiwar and M. P. Kaushik, J. Sulfur Chem., 2006, 27, 353 CrossRef CAS.
- C. Lamberth, H. Walter, F. M. Kessabi and A. M. Abdallah, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 1225 CrossRef CAS.
- N. K. Lyapina, Russ. Chem. Rev., 1982, 51, 189 CrossRef.
- C.-L. Lee and P. Brimblecombe, Earth-Sci. Rev., 2016, 160, 1 CrossRef CAS.
- M. A. Marcinkowska and H. H. Jeleń, Molecules, 2022, 27, 6116 CrossRef CAS PubMed.
- M. B. Marakalala, E. M. Mmutlane and H. H. Kinfe, Beilstein J. Org. Chem., 2018, 14, 1668 CrossRef CAS PubMed.
- L. Wang, M. Chen and J. Zhang, Org. Chem. Front., 2019, 6, 32 RSC.
- C. C. Eichman and J. P. Stambuli, Molecules, 2011, 16, 590 CrossRef CAS PubMed.
- J. K. Park and S. Lee, J. Org. Chem., 2021, 86, 13790 CrossRef CAS PubMed.
- J. Rafique, G. Farias, S. Saba and A. Ali, Dyes Pigm., 2020, 180, 108519 CrossRef CAS.
- Z.-H. Fu, H.-D. Tian, S.-F. Ni, J. S. Wright, M. Li, L.-R. Wen and L.-B. Zhang, Green Chem., 2022, 24, 4772 RSC.
- S. Pradhan, S. Patel and I. Chatterjee, Chem. Commun., 2020, 56, 5054 RSC.
- K. Takagi, Chem. Lett., 1987, 16, 2221 CrossRef.
- X. Hao, D. Feng, H. Chen and Y. Huang, Chem.–Eur. J., 2023, 29, e202302119 CrossRef CAS PubMed.
- B. Azizi, M. R. Poor Heravi, Z. Hossaini and A. H. Hajizadeh, RSC Adv., 2021, 11, 13138 RSC.
- F. Xiao, S. Chen, C. Li and Z. Zhang, Adv. Synth. Catal., 2016, 358, 3881 CrossRef CAS.
- A. Rostami, A. Rostami, A. Ghaderi and F. Allahyari, Synthesis, 2017, 49, 5025 CrossRef CAS.
- Y. Fang, S. Chen and L.-Y. Chang, RSC Adv., 2024, 14, 812 RSC.
- K. Zhou, J. Zhang, L. Lai and W. Zhao, Chem. Commun., 2018, 54, 7459 RSC.
- G. Qiu, K. Zhou and J. Wu, Chem. Commun., 2018, 54, 12561 RSC.
- C. Guo, X. Wang, Q. Ding and J. Wu, J. Org. Chem., 2024, 89, 9672 CrossRef CAS PubMed.
- F. Al-Dolaimy, D. Yahaia Alhameedi, F. A. Rasen, H. A. Lafta, N. K. Abed and A. H. Alawadi, Polycyclic Aromat. Compd., 2023, 44, 6681 CrossRef.
- M. Sun, W. Liu, W. Wu, Y. Zhang and H. Wang, RSC Adv., 2023, 13, 20351 RSC.
- L. Chen, J. Sulfur Chem., 2024, 45, 981 CrossRef CAS.
- K. Wang and L.-Y. Chang, J. Iran. Chem. Soc., 2024, 21, 1547 CrossRef CAS.
- V. Hirschbeck, P. H. Gehrtz and I. Fleischer, Chem.–Eur. J., 2018, 24, 2854 CrossRef CAS.
- N. Zhou, L. Shen, Z. Dong and X. Yang, Catalysts, 2018, 8, 249 CrossRef.
- W. Dan, H. Deng, J. Chen and Q. Zhu, Tetrahedron, 2010, 66, 7384 CrossRef CAS.
- M. Rousta, D. Khalili, E. Ebrahimi and A. Khoy, Catal. Lett., 2024, 154, 5439 CrossRef.
- K. Fuchibe, I. Mukohara, A. Yamada and M. Shibata, Org. Lett., 2022, 24, 169 CrossRef CAS PubMed.
- A. S. El-Azab and A. A.-M. Abdel-Aziz, Phosphorus, Sulfur Silicon Relat. Elem., 2012, 187, 1046 CrossRef CAS.
- X. Wang and Z. Dong, Eur. J. Org. Chem., 2022, 2022, e202200452 CrossRef CAS.
- N. Iranpoor, H. Firouzabadi, E. Etemadi-Davan and M. Zeynali, New J. Chem., 2015, 39, 6445 RSC.
- C. Yan, Q. Wei, Q. Chen and L. Zhang, J. Mol. Struct., 2024, 1317, 138853 CrossRef CAS.
- Y.-B. Yu, H.-L. Chen, L.-Y. Wang and Y.-Q. Liu, Molecules, 2009, 14, 4858 CrossRef CAS PubMed.
- S. H. Ali and A. R. Sayed, Synth. Commun., 2021, 51, 670–700 CrossRef CAS.
- T. Ji, J. Ye, L. Tang and T. Mandal, Synth. Commun., 2021, 51, 1636 CAS.
- R. Gurav, S. K. Surve, S. Babar, P. Choudhari, D. Patil, V. More, S. Sankpala and S. Hangirgekar, Org. Biomol. Chem., 2020, 18, 4575–4582 RSC.
- R. Ghorbani-Vaghei, S. Alavinia, Z. Merati and V. Izadkhah, Appl. Organomet. Chem., 2018, 32, e4038 CrossRef.
- Z.-J. Wang, W.-T. Chen, C. He, J. Li, L. Zhang and Q. Liu, Tetrahedron, 2020, 76, 130953 CrossRef CAS.
- H. Zandieh, J. Mokhtari and K. Larijani, Catal. Lett., 2023, 153, 3527 CrossRef CAS.
- H. R. Shaterian and P. Molaei, Appl. Organomet. Chem., 2019, 33, e4964 CrossRef.
- A. K. G. Chandrappa, Y. D. R. Bodke, N. Obaih and S. Hamzad, Chem. Data Collect., 2022, 42, 100964 CrossRef CAS.
- S. Khan, H. Ullah, M. Taha, A. Ahmed, H. Kim and M. Hassan, Molecules, 2023, 28, 559 CrossRef CAS PubMed.
- M.-S. Shafik and H. Jasim Elaibi, Nanomater. Chem., 2024, 2, 51 Search PubMed.
- M. Mokhtari and B. Naseri, Nanomater. Chem., 2024, 2, 66 Search PubMed.
- M. Bhardwaj, B. Jamwal and S. Paul, Catal. Lett., 2016, 146, 629 CrossRef CAS.
- R. G. Kalkhambkar and K. K. Laali, Tetrahedron Lett., 2012, 53, 4212 CrossRef CAS.
- Y. Sun, H. Jiang, W. Wu, Z. Yang and F. Li, Org. Lett., 2013, 15, 1598 CrossRef CAS PubMed.
- E. A. Jaseer, D. J. C. Prasad, A. Dandapat and G. Sekar, Tetrahedron Lett., 2010, 51, 5009 CrossRef CAS.
- Q. Xing, Y. Ma, H. Xie, J. Liu and L. Zhang, J. Org. Chem., 2019, 84, 1238 CrossRef CAS PubMed.
- P. Chander Sharma, D. Sharma, A. Sharma, V. Gupta, R. Kumar and M. Singh, Appl. Mater. Today, 2020, 20, 100783 CrossRef.
- R. R. Putta, S. Chun, S. H. Choi, K. H. Kim and J. Y. Lee, J. Org. Chem., 2020, 85, 15396 CrossRef CAS PubMed.
- P. Ghosh, B. Ganguly, E. Perl and S. Das, Tetrahedron Lett., 2017, 58, 2751 CrossRef CAS.
- Z. Hameed Mahmood, Y. Riadi, H. A. Hammoodi, A. Ali and R. Karim, Polycyclic Aromat. Compd., 2023, 43, 3687 CrossRef CAS.
- K. Azizi, M. Karimi and A. Heydari, Tetrahedron Lett., 2015, 56, 812 CrossRef CAS.
- I. Patra, M. M. Kadhim, H. H. Kzar, H. H. Kzar, Y. Zhao, J. Tan and Q. Wan, J. Sulfur Chem., 2023, 44, 217 CrossRef CAS.
- S. Dang, Y. Hu, S. Zhai and L.-Y. Zhang, Res. Chem. Intermed., 2024, 50, 4275 CrossRef CAS.
- M. Khalaj, M. Ghazanfarpour-Darjani, M. R. Talei Bavil Olyai and S. F. Shamami, J. Sulfur Chem., 2016, 37, 211 CrossRef CAS.
- I. Ahmed, H. J. Lee and S. H. Jhung, Chem. Eng. J., 2022, 437, 135386 CrossRef CAS.
- Q. Lei, J. Zhong, S.-F. Chen, Y. Wang, L. Liu and X. Xu, Environ. Res., 2023, 235, 116570 CrossRef CAS PubMed.
- S. E. Gatarz, O. M. Griffiths, H. A. Esteves, M. J. Smith and T. L. Brown, J. Org. Chem., 2024, 89, 1898 CrossRef CAS PubMed.
- N. Gök, A. Akıncıoğlu, E. Erümit Binici, S. Yıldırım, H. Doğan and K. Özdemir, Arch. Pharm., 2021, 354, 2000491 CrossRef PubMed.
- H. Wu, X. Chen, N. Sun and A. Sanchez-Mendoza, Synth. Commun., 2021, 51, 2287 CrossRef CAS.
- K. Chen, W. Chen, B. Han, Y. Zhao, X. Li and Y. Liu, Org. Lett., 2020, 22, 1841 CrossRef CAS PubMed.
- J. H. Bormio Nunes, D. Hideki Nakahata, P. P. Corbi and R. E. Ferraz de Paiva, Coord. Chem. Rev., 2023, 490, 215228 CrossRef CAS.
- Z. Luo, R. Spinney, Z. Wei, M. Huang, T. Zhang and L. Sun, ACS ES&T Water, 2021, 1, 2339 Search PubMed.
- Y. Wan, G. Fang, H. Chen, J. Wu, S. Liu and Q. Li, Eur. J. Med. Chem., 2021, 226, 113837 CrossRef CAS PubMed.
- A. Ovung and J. Bhattacharyya, Biophys. Rev., 2021, 13, 259 CrossRef CAS PubMed.
- Y. Liu, Q. Pan, X. Hu, Z. Wang, J. Zhou and H. Ding, Org. Lett., 2021, 23, 3975 CrossRef CAS.
- M. J. Tilby, D. F. Dewez, L. R. E. Pantaine, J. L. Hardy and T. C. Fox, ACS Catal., 2022, 12, 6060 CrossRef CAS PubMed.
- C. Song and M. Kazemi, Mol. Diversity, 2024 DOI:10.1007/s11030-024-11030-4.
- W. Wei, Z. Y. Xia and L.-Y. Chang, Catal. Lett., 2024, 154, 1495 CrossRef CAS.
- Y. M. Shams and S. Al Malak, J. Synth. Chem., 2023, 2, 227 Search PubMed.
- X. Jin, J. Inorg. Organomet. Polym. Mater., 2024, 34, 5132–5153 CrossRef CAS.
- M.-S. Shafik and S. Sami, J. Synth. Chem., 2024, 3, 74 Search PubMed.
- Y.-C. Xiao and F.-E. Chen, Expert Opin. Drug Discovery, 2024, 19, 239 CrossRef CAS PubMed.
- R. Ahmadi and S. Emami, Eur. J. Med. Chem., 2022, 234, 114255 CrossRef CAS PubMed.
- Y. Li, S. Chen, M. Wang and X. Jiang, Angew. Chem., Int. Ed., 2020, 59, 8907 CrossRef CAS PubMed.
- U. Lücking, Chem.–Eur. J., 2022, 28, e202201993, DOI:10.1002/chem.202201993.
- Y. Huang, J. Li, H. Chen, P. Wang, G. Liu and Z. Zhang, Chem. Rec., 2021, 21, 1216 CrossRef CAS PubMed.
- Y. Han, K. Xing, J. Zhang, L. Wang, M. Li and F. Zhang, Eur. J. Med. Chem., 2021, 209, 112885 CrossRef CAS PubMed.
- C. Zhu, Y. Cai and H. Jiang, Org. Chem. Front., 2021, 8, 5574 RSC.
- E. Azzi, A. Lanfranco, R. Moro, S. Rossi, A. Bianchi and L. Ferrari, Synthesis, 2021, 53, 3440 CrossRef CAS.
- S. Swarnkar, M. Y. Ansari and A. Kumar, Org. Lett., 2021, 23, 1163 CrossRef CAS PubMed.
- E. Wojaczyńska and J. Wojaczyński, Chem. Rev., 2020, 120, 4578 CrossRef.
- W.-H. Lu, D. Yang, G.-Q. Wang, T. Li, X. Wu and Y. Zhou, Org. Biomol. Chem., 2023, 21, 2822 RSC.
- A. Kamel, A. H. Khalaf, A. H. D. Al-Khafaji, A. H. D. Al-Khafaji, J. Ali, M. Mustafa and T. Ahmed, Polycyclic Aromat. Compd., 2023 DOI:10.1080/10406638.2023.2212103.
- W. K. Abdelbasset, A. M. Mohsen, M. M. Kadhim, F. Zhao, S. Liu and J. Zhang, Polycyclic Aromat. Compd., 2023, 43, 4032 CrossRef CAS.
- M. H. Mahdi, R. Thabit, M. S. Mutlaq, F. Alajeeli, A. H. Dawood, B. M. Ridha, A. H. Alsalamy and H. S. Mustafa, React. Kinet., Mech. Catal., 2024, 137, 209–229 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |