DOI:
10.1039/D5TA01159K
(Perspective)
J. Mater. Chem. A, 2025,
13, 19236-19251
Recent advancements in metal–organic frameworks (MOFs) for flexible supercapacitors aimed at wearable technology
Received
12th February 2025
, Accepted 1st April 2025
First published on 3rd April 2025
Abstract
Flexible supercapacitors have made significant progress, as they can be integral to the wearable technology field due to their unique ability to allow seamless movement for the wearer. Metal–organic frameworks (MOFs) with high surface area and exceptional porous structure exhibit greater potential in developing supercapacitor electrodes and have been widely explored. Hence, integrating MOFs for flexible supercapacitor wearable technology could harness high-efficiency wearable supercapacitors to explore various flexibility forms and impact practical applications. From this perspective, we critically investigate the recent developments in MOFs and MOF-based composite materials. Advancements in flexible supercapacitors are surveyed, and their electrochemical performance is overviewed. Furthermore, their pivotal role in enabling wearable technology to reach its full potential is emphasized. Finally, the associated challenges and opportunities of flexible supercapacitors are discussed in detail, highlighting the dominant significance of low dimensional MOFs for flexible supercapacitor solutions in evolving futuristic wearable technologies.
1. Introduction
For human existence and development, energy plays a significant role and is a prerequisite for energy-storage devices.1,2 Considering the critical challenges posed by the global energy crisis, the depletion of finite fossil fuels, and the severe pollution caused by their combustion, researchers have focused on developing an alternative, cleaner, and greener source of energy.3 On this basis, in recent times, supercapacitors (SCs), batteries, and capacitors have become effective electrical energy storage devices.4 Capacitors offer high power density and low energy density, whereas batteries provide high energy density with low power density. SCs, known as electrochemical capacitors, act as a hybrid solution between these two technologies.5 They deliver high power densities of up to 10 kW kg−1, which is significantly higher than that of batteries, along with larger energy densities than conventional capacitors.6 Additionally, SCs offer the advantages of fast charging, durability, and long cycle life. The demand for these devices has increased due to the rapid growth of wearable devices, which require energy storage systems that are efficient, lightweight, and flexible, with a long-lasting lifespan. In contrast to other energy storage devices such as zinc–carbon, alkaline, lithium-ion, polymer lithium-metal, and all-polymer batteries, flexible supercapacitors (FSCs) are particularly promising.7 FSCs represent a unique combination of flexibility, tunability, lightness, and environmental friendliness, making them particularly suitable for aligning with the dynamic requirements of wearable and portable electronic devices. Beyond that, their high power density, rapid charging, and wide operating temperature (−20 to 70 °C) are considered the true enablers of the future of wearable devices.8 Recently, the growing prominence of health monitoring, alongside rapid advancements in electronic devices, has sparked interest in wearable technologies. These wearables range from implantable medical devices and bio-integrated sensors to smart fabrics, smart contact lenses, therapeutic wearables, and fitness trackers. They have become crucial to modern lifestyles and offer modified solutions to meet the needs of individual users.9 Furthermore, FSCs are safe, exhibit a long cycle life, and can be easily designed in various shapes and sizes, making them ideal for wearable applications. With all these promising features, FSCs are poised to play a key role in shaping the future of wearable technology.10 FSCs are composed of several key components that are crucial for their performance, flexibility, and overall functionality. These components include flexible electrodes, solid or gel polymer-based electrolytes, flexible substrates, current collectors, and separators, all of which are similar to those found in traditional SCs. In designing FSCs, the electrolyte also plays a crucial role, enabling SCs to achieve higher energy densities and longer cycle life while also enhancing safety. Hence, the electrolyte assessment relies on crucial elements, such as ionic conductivity and mobility.11 To enhance the cohesive functionality of FSCs, current studies are focused on the development of flexible electrode materials.12 There are various materials that exhibit important properties, including high electrochemically active surface area, excellent electrical conductivity, and stability. However, the primary objective of flexible supercapacitors is to develop materials that can withstand mechanical deformations, such as bending, twisting, folding, and stretching.13 Many materials have been explored based on this approach. Carbon-based materials, such as carbon nanotubes (CNTs), graphene, carbon aerogels, and activated carbon, exhibit high conductivity; however, they tend to have lower energy density and specific capacitance. Considering these limitations, developing materials that satisfy the specific requirements of FSCs remains a challenge. Therefore, researchers are prompted to develop materials that effectively address these limitations.
There is another class of materials known as metal–organic frameworks (MOFs), which are hybrid organic–inorganic frameworks of materials.14 MOFs are a class of porous materials made up of inorganic metal ions or clusters coordinated to organic ligands, forming a 3D network.15,16 The metal centers (such as Zn2+, Cu2+, and Fe3+) form the nodes of the framework, which is integrated by organic ligands. These ligands are organic molecules, often containing functional groups such as carboxylates and phosphonates, which connect the metal ions to form linkages that create a highly porous structure.17 MOFs exhibit an unprecedentedly high surface area, well-defined structures, exceptional porosity, high tunability, mechanical stability, and flexibility – highly desirable characteristics for FSC electrode materials.18 MOFs possessing favorable properties suitable for electrochemical energy storage applications, with their composition of metal nodes and organic linkers, allow precision over physical and chemical properties. The desired tuning can be achieved by selecting the appropriate metal ions, organic linkers, and synthesis conditions, which help maintain structural integrity and optimize electrochemical properties. To achieve mechanical flexibility, MOFs can be designed with various morphologies, such as nanosheets, nanorods, or hollow structures, which helps improve their mechanical adaptability and prevent structural collapse under different mechanical conditions. Furthermore, hybridizing MOFs with flexible carbon substrates or MXenes enhances their mechanical resilience and electrical conductivity. From an electrochemical perspective, MOFs can be tailored to provide abundant active sites for charge storage and ion diffusion, which can be achieved by incorporating bimetallic centers or redox-active ligands. This structural tuning enables the designed FSCs to maintain stable performance under mechanical deformation. Hence, the flexibility of MOFs makes them ideal for wearable and portable applications. Furthermore, MOFs not only compensate for the shortcomings of conventional materials (carbon-based materials, metal oxides, and conductive polymers) but also open up new horizons for designing high-performance energy storage devices with features such as flexibility and durability.19 However, structural instability and low electrical conductivity are two limitations of pure MOFs that limit their full potential in real-world applications. To resolve this issue, MOF-derived materials have been developed.20 This work presents a comprehensive perspective on the integration of MOFs into FSCs for wearable technology, highlighting novel advancements in material design, structural optimization, and electrochemical performance. This work explores the synergistic advantages of MOFs and their composites, including bimetallic MOFs, MOF/MXene hybrids, and MOF/carbon-based materials, to address challenges in energy density, mechanical flexibility, and long-term stability. The review highlights the role of MOFs in enabling next-generation FSCs with high surface area, tunable porosity, and enhanced redox activity, thereby paving the way for scalable and durable energy storage solutions in wearable electronics. By critically analyzing the recent developments in MOF-based FSCs, this work identifies key challenges and opportunities, providing a roadmap for future research directions in high-performance flexible energy storage systems.
2. Synthesis and electrochemical performance of MOFs
MOFs are synthesized with various approaches, and each method has an influence on their physical, chemical, and electrochemical properties. Selecting the most suitable method is crucial for optimizing the performance of MOFs to achieve high energy properties. Table 1 presents the various approaches that can be employed in the synthesis of MOFs, along with their advantages, limitations, and effects on the final product. MOF performance enhancements for the electrochemical performance of FSCs may arise due to several reasons and involve various analysis techniques to optimize performance and gain detailed insights. An instance is considered with the development of MOFs featuring various multi-metallic centers, which contribute to unique redox properties that enhance charge storage. The presence of multiple redox active sites leads to higher electrochemical performance, which can be analyzed by the cyclic voltammetry technique, and the same can be confirmed with other techniques such as X-ray photoelectron spectroscopy and In situ Raman spectroscopy. For instance, if multi-metallic ions are involved in a redox reaction, each metal ion contributes to the unique redox properties involved in charge storage enhancement. For example, NiCo-MOF's cyclic voltammetry, as shown in Fig. 1a, exhibits redox transitions for Co2+/Co3+ with the peak centered at 0 V, while the bimetallic interaction of Ni2+/Ni3+ appears as positively shifted peaks at 0.4 V and 0.16 V. This can also be verified by charge–discharge analysis as shown in Fig. 1b, where the specific capacitance is found to be increased in the potential window of 0.4 V.21 Usually, the developed electrode exhibits two charge storage conditions with a diffusion-controlled mechanism, and battery-type materials are represented by b = 0.5, and the surface-controlled process and pseudocapacitive materials are explained by b = 1. To determine the storage mechanism, the developed electrodes are subjected to various scans at selected potential windows. A related example featuring a bimetallic MOF composed of a reduced graphene oxide composite is presented. Specifically, NiCo-MOF/rGO (10 mg), referred to as NCG-10, is subjected to various scan rates, as illustrated in Fig. 1c. It is crucial to correlate the peak current with the scan rate. The corresponding peak current versus scan rate profile is shown in Fig. 1d, where the slope of the plot indicates the b value, which aids in understanding the charge storage distribution of the developed electrode. This can be done using Dunn's method or Trassitti's method, which provides a comparison of the contributions from capacitive and diffusion control characteristics at various scan rates, as shown in Fig. 1e.22 Furthermore, to analyze the charge transfer resistance and ionic diffusion at the electrodes, electrochemical impedance analysis can be carried out, as shown in Fig. 1f. The obtained output is a Nyquist plot, which is fitted to an equivalent circuit to analyze the charge transfer resistance. In the plot shown, labeled “a–e”, the monometallic MOF and bimetallic MOFs with different ratios and optimized ratios exhibited lower charge transfer resistance. Similarly, the real impedance vs. ω−1/2 plot, as shown in Fig. 1g, where the optimized material ratio exhibits a lower slope value, confirms the lower charge transfer resistance and faster ion diffusion, resulting in faster kinetics. As structural stability is a concern in developed electrode materials, the technique of multiple charge–discharge cycles is employed to assess stability, as illustrated in Fig. 1h. The inset illustrates the verification of impedance analysis through pre- and post-Nyquist plot analysis.23 By leveraging this mechanistic analysis, the electrochemical performance of MOFs can be enhanced, making them a promising electrode material for FSC applications.
Table 1 Different synthesis strategies are employed in the synthesis of MOFs, along with their advantages, limitations, and effects on the properties of MOFs for supercapacitor applications
| Synthesis method |
Advantages |
Limitations |
Effect on MOF properties on supercapacitors |
| Solvothermal |
High crystallinity, controlled morphology |
Long reaction time requires high temperature and pressure |
High surface area and porosity, good electrochemical stability |
| Hydrothermal |
Environmentally friendly, simple setup |
Limited to water-soluble precursors |
Moderate conductivity, tunable pore structure |
| Sonochemical |
Rapid synthesis, energy-efficient |
Limited scale-up potential |
Small particle size, improved conductivity |
| Microwave-assisted |
Fast reaction time, uniform heating |
Equipment cost, possible structural defects |
Enhanced surface area, good capacitance retention |
| Electrochemical |
Direct deposition on the electrode, template-free |
Limited to conductive substrates |
Improves conductivity, strong electrode adhesion |
| Mechanochemical |
Solvent-free, eco-friendly |
Hard to control the morphology |
Structural defects can enhance charge storage but may reduce stability |
| Spray drying |
Scalable, uniform particle size |
High energy input, possible pore collapse |
Spherical particles with good dispersibility |
| Template-assisted |
Tunable pore structure, precise control |
Template removal step needed |
Enhanced ion diffusion, high charge storage |
| Gel-based (sol–gel) |
Low-temperature synthesis, uniform structure |
Possible residual solvents affecting performance |
Tunable pore distribution, moderate capacitance |
 |
| | Fig. 1 (a) CV curves of Co(OH)2, Ni-MOF, and CoNi-MOF collected at a scan rate of 10 mV s−1. (b) Galvanostatic curves of Co(OH)2, Ni-MOF, and CoNi-MOF collected at a current density of 2 A g−1.21 (c) CV curves of NCG-10 at different scan rates. (d) Plot of log(scan rate) vs. log(peak current) for the NCG-10 sample. (e) Comparison of capacitive and diffusion contribution of the NCG-10 sample.22 (f) Nyquist plots, inset is the equivalent circuit. (g) Relationship between Z′ and ω−1/2 in the low-frequency region with linear fit analysis (a–e in the figure represent Co-MOF, Co/Ni-MOF-1, Co/Ni-MOF-2, Co/Ni-MOF-3, and Ni-MOF, respectively). (h) The cycle stability, inset is the impedance at 10 A g−1 before and after 5000 cycles.23 Reproduced with permission from ref. 21. (a and b) Copyright © 2018, John Wiley and Sons ref. 22. (c–e) Copyright © 2024, Elsevier, ref. 23. (f–h) Copyright © 2021, Elsevier. | |
3. Recent advances in MOF-based FSCs
MOF preparation requires organic ligands that contribute to a high surface area, conductivity, and electrochemical stability, primarily consisting of polycarboxylates, azoles, and phenolic compounds. Some ligands commonly used in the preparation of MOFs include carboxylate-based ligands, imidazolate-based ligands, phenolate-based ligands, pyridyl-based ligands, triazine-based ligands, sulfonate-based ligands, polycyclic aromatic hydrocarbon-based ligands, phosphonate-based ligands, and amino-functionalized ligands. The selection criteria for MOF ligands in supercapacitors involve higher stability, redox-active sites, porosity, and electron conductivity. FSCs, utilizing MOF-based materials, demonstrate their adaptability through integration with advanced materials. Monometallic MOFs offer high capacity but struggle with poor conductivity. In contrast, bimetallic MOFs capitalize on the synergistic effects of the various metallic active centers, enhancing both ion transport and stability. Multi-metallic MOFs may further enhance electrochemical activity by providing multiple redox-active centers, offering significant capacity and cycling stability. Furthermore, to overcome the intrinsic limitations of MOFs, composites incorporating MXene, polymer, graphene, and porous carbon-based materials have gained traction recently. These innovations demonstrate the drive towards the development of FSCs for wearable supercapacitors, targeting improved energy storage properties and enhanced mechanical durability. Table 2 shows a summary of the characteristics, advantages, and limitations of various MOFs, which are explained in further sections.
Table 2 Summary of the characteristics, advantages, and limitations of various MOFs
| MOF-based material |
Characteristics |
Advantages |
Limitations |
| Pristine MOFs |
Porous crystalline frameworks with tunable metal–ligand structures |
Large surface area, tunable porosity, multiple redox-active sites |
Poor electrical conductivity, structural fragility, low mechanical flexibility |
| Multimetallic MOFs |
Incorporation of multiple metal centers to enhance redox activity and conductivity |
Improved charge storage, higher stability, better ion diffusion |
Complex synthesis, potential phase separation, reduced flexibility |
| MOFs with MXene |
Integration of MOFs with 2D MXene nanosheets for enhanced charge transfer |
High electrical conductivity, strong mechanical stability, improved cycling performance |
Limited MXene availability, structural degradation over cycling |
| MOFs with carbon |
Hybridization with conductive carbon nanomaterials |
Enhanced electrical conductivity, mechanical robustness, better electrochemical stability |
Potential aggregation, complex fabrication, moderate charge transfer efficiency |
| MOFs with graphite derivatives |
MOFs combined with graphite-based materials to improve flexibility and stability |
High surface area, increased mechanical strength, better ionic diffusion |
Possible restacking of graphene layers, moderate conductivity |
3.1. Monometallic-oriented MOF-based FSCs
Attention has been paid to monometallic MOFs for FSCs is due to their tunable structure and excellent electrochemical properties. These MOFs, based on single metal centers, offer enhanced charge storage, and their structural flexibility and ability to accommodate various electrolytes make them promising for FSCs. A particular study by Aziz et al. explored the redox activity of Mn-MOF using 1,3,5-benzene tricarboxylic acid (H3-BTC) as an organic linker and highlighted its potential in FSC application. The choice of H3-BTC showed an enhancement in structural stability and tunable porosity, and transition metal incorporation improved the electron pathway, which facilitated better charge storage performance. The hydrothermal synthesis employed for obtaining Mn-MOF ensured stable network formation, which made an efficient candidate for practical energy storage application. The detailed synthesis procedure is shown in Fig. 2a, where the precursor and H3-BTC are mixed with dimethylformamide (DMF) and hydrothermally treated at 160 °C for 24 h to obtain the precipitate, which was further cleaned with acetone, deionized water, and ethanol, where Mn-MOF was collected by drying at 65 °C for 5 h. The 2D and 3D structure of Mn and H3-BTC is shown in Fig. 2b, and the developed material was coated on nickel foam to obtain a flexible electrode. The developed electrode, upon electrochemical analysis in alkaline electrolyte, exhibited a specific capacity of 1569.24 C g−1 at a current density of 3.6 A g−1, showing potential as a battery-type material. Furthermore, the FSCs constructed with a combination of Mn-MOF and activated carbon (AC) exhibited an energy density of 60 W h kg−1 at a power density of 2250 W kg−1. They demonstrated impressive cycling stability, with 98.57% retention after 1000 charge–discharge cycles, and achieved a coulombic efficiency of 96.97%. These results underscore the potential of FSCs for use in portable energy storage systems.24
 |
| | Fig. 2 (a) Schematic of hydrothermally synthesized Mn-MOF. (b) Structural analysis of Mn-MOF.24 (c) Schematic diagram of the narrow pore size of the Cu-TCPP membrane compared with a single Cu-TCPP layer.25 (d) Schematic illustration of a hybrid supercapacitor with Ni-MOF//AC in KOH electrolyte. (e) Digital photograph of the Ni-MOF//AC SC device.26 Reproduced with permission from ref. 24. (a and b) Copyright © 2023, American Chemical Society, ref. 25. (c) Copyright © 2023, Royal Society of Chemistry, ref. 26. (d and e) Copyright © 2023, Royal Society of Chemistry. | |
Similarly, as there are various MOFs available, Wang et al. developed micro-SCs via a quasi-solid state electrolyte-based flexible MOF membrane infused with a deep eutectic solvent (DES). They addressed the work for wearable technology with challenges of ionic conductivity, mechanical flexibility, and electrochemical stability. They highlighted the use of Cu-MOF prepared with tetrakis (4-carboxyphenyl) porphyrin (TCPP) which is more efficient than solid state electrolytes. The developed Cu-TCPP membrane had a significant enhancement in ionic conductivity through a well-ordered nanochannel, which efficiently exhibits electron transportation. The Cu-TCPP-DES membrane showed multiple advantages, such as higher surface area, pore structure tuning, and hydrophilicity, which ensure the adsorption of electrolytes. The layer comparison of Cu-TCPP is shown in Fig. 2c, where CuTCPP nanosheets synthesized from Cu2+ ions and porphyrin ligands were dispersed in a stable and translucent purple colloidal solution due to their negatively charged surface. The DES introduced with the choline chloride and 1,2-butanediol in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 maintained non-flammability and biodegradability. From the perspective of electrochemical performance, Cu-TCPP-DES showed an area-specific capacitance of 81.3 mF cm−2 at 0.1 mA cm−2. Further, the microsupercapacitor fabricated delivered an energy density of 45.17 μW h cm−2 at a power density of 8.559 mW cm−2. Moreover, performance remained consistent with different deformation, which demonstrated outstanding flexibility and reliability for wearable applications.25 Hence, this work paved the way for excellent FSCs with the leveraged MOF-based quasi-solid-state electrolyte and gave a foundation for exploring further electrolyte systems with scalable and durable FSCs. Advanced electrode material development is necessary for the efficiency enhancement of FSC performance. In a particular study by Bhosale et al., they presented Ni-MOF synthesized by a simple, cost-effective reflux condensation method with H3-BTC as the organic linker; this ensured a reduction in reaction time and obtained uniform nano-rod-like morphology. The employed plate number 1 resulted in a porous structure with a surface area of 398.4 m2 g−1. This structure demonstrated efficient electrolyte accessibility and impressive electrochemical performance, achieving a specific capacitance of up to 1956.3 F g−1 in an alkaline electrolyte solution of 2 M KOH. Furthermore, it displayed charge–discharge stability of 81.3% after 3000 cycles, with low resistance from the developed Ni-MOF electrodes, and demonstrated improved electron transport within the electrode material. Hence, Ni-MOF is an efficient FSC electrode, and when combined with AC as a negative electrode, as shown in Fig. 2d, it has the advantages of the developed FSC combination. For the practical application, the validated FSCs delivered a specific energy of 98.15 W h kg−1 at a power density of 1253.47 W kg−1, with the FSC's photographic image shown in Fig. 2e. Notably, the device performed well with stability retention of 99.29% of its initial capacitance over 3000 cycles, indicating exceptional durability.26 This developed study highlighted the potential of Ni-MOF, paving the way for its integration into wearable technologies.
6 maintained non-flammability and biodegradability. From the perspective of electrochemical performance, Cu-TCPP-DES showed an area-specific capacitance of 81.3 mF cm−2 at 0.1 mA cm−2. Further, the microsupercapacitor fabricated delivered an energy density of 45.17 μW h cm−2 at a power density of 8.559 mW cm−2. Moreover, performance remained consistent with different deformation, which demonstrated outstanding flexibility and reliability for wearable applications.25 Hence, this work paved the way for excellent FSCs with the leveraged MOF-based quasi-solid-state electrolyte and gave a foundation for exploring further electrolyte systems with scalable and durable FSCs. Advanced electrode material development is necessary for the efficiency enhancement of FSC performance. In a particular study by Bhosale et al., they presented Ni-MOF synthesized by a simple, cost-effective reflux condensation method with H3-BTC as the organic linker; this ensured a reduction in reaction time and obtained uniform nano-rod-like morphology. The employed plate number 1 resulted in a porous structure with a surface area of 398.4 m2 g−1. This structure demonstrated efficient electrolyte accessibility and impressive electrochemical performance, achieving a specific capacitance of up to 1956.3 F g−1 in an alkaline electrolyte solution of 2 M KOH. Furthermore, it displayed charge–discharge stability of 81.3% after 3000 cycles, with low resistance from the developed Ni-MOF electrodes, and demonstrated improved electron transport within the electrode material. Hence, Ni-MOF is an efficient FSC electrode, and when combined with AC as a negative electrode, as shown in Fig. 2d, it has the advantages of the developed FSC combination. For the practical application, the validated FSCs delivered a specific energy of 98.15 W h kg−1 at a power density of 1253.47 W kg−1, with the FSC's photographic image shown in Fig. 2e. Notably, the device performed well with stability retention of 99.29% of its initial capacitance over 3000 cycles, indicating exceptional durability.26 This developed study highlighted the potential of Ni-MOF, paving the way for its integration into wearable technologies.
3.2. Multimetallic-oriented MOF-based FSCs
The preparation of MOF-based materials for designing flexible electrodes is considered an effective approach to enhancing the characteristic features of FSCs. Further benefits from the stronger adhesion of the MOF-based material to the flexible electrodes ensure faster kinetics. Xu's group developed a flexible electrode that presented the in situ preparation of bimetallic cobalt–iron metal–organic frameworks (CoFe-MOF) on carbon cloth (CC), enhanced by oxygen vacancies, for use in advanced flexible asymmetric supercapacitors. This innovative material design leverages the synergistic properties of Co and Fe active sites, ultra-small nanoparticle structures, and abundant oxygen vacancies (Ov) to achieve superior electrochemical performance. The schematic diagram for the developing electrode is shown in Fig. 3a. The developed electrode with Ov-CoFe-MOF exhibited characteristic features of optimized pore structure, enhanced active site utilization, and comparatively superior redox kinetics, which was compared to different combinations such as CoM-MOF (M = Ni, Cu, Al). The developed electrodes' three-electrode electrochemical analysis was carried out in an acidic electrolyte comprising 2 M sulfuric acid (H2SO4), where Ov-CoFe-MOF on flexible carbon cloth delivered a specific capacitance of 56.94 mF cm−2 at 0.5 mA cm−2 and a wide voltage window of 1.2 V. The achieved electrochemical performance is due to material design leveraging the synergistic properties of Co and Fe active sites with abundant oxygen vacancies and smaller nanoparticle structures. For the fabrication of FSCs, NCM/CC is chosen as the negative electrode; all the prepared electrodes were immersed in a polyvinyl alcohol (PVA)-H2SO4-based gel electrolyte. Finally, the entire assembly is sandwiched with a separator and cured to form an Ov-CoFe-MOF@CC//NCM@CC FSC. The fabrication procedure for the FSC is detailed in Fig. 3b, and their bending performance is illustrated. The fabricated FSC delivered key properties, which included an energy density of 27.4 mW h cm−2, power density of 4798 mW cm−2, and flexibility under various bending conditions. As far as stability is concerned, the FSC exhibited capacity retention of 76.47% throughout 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, demonstrating exceptional stability.27 The study emphasized the benefits of integrating Ov and bimetallic active sites for redox kinetic enhancements, electrical conductivity, and active site utilization.
000 cycles, demonstrating exceptional stability.27 The study emphasized the benefits of integrating Ov and bimetallic active sites for redox kinetic enhancements, electrical conductivity, and active site utilization.
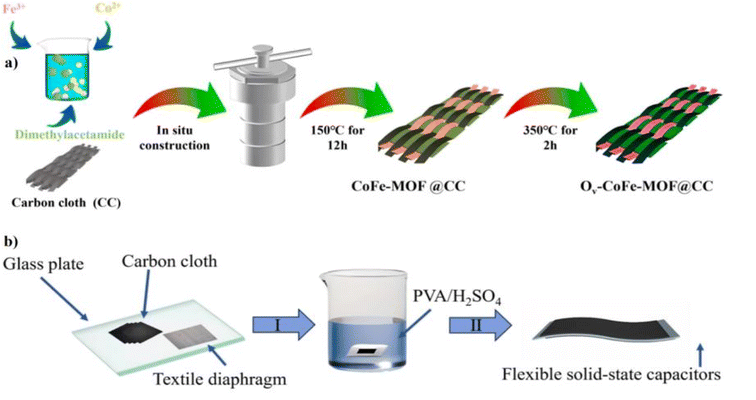 |
| | Fig. 3 (a) A schematic illustration of the synthesis process for Ov-CoFe-MOF@CC. (b) A schematic of the preparation process of the solid-state FSC.27 Reproduced with permission from ref. 27. (a and b) Copyright © 2024, Elsevier. | |
Considering the current collector's pretreatment where it could affect the electrochemical performance, Zhang's group developed an FSC with high energy storage efficiency and adaptability, and their study focused on in situ growth of CoNi0.5-MOF by utilizing the organic ligand of H3-BTC. Prior to the growth of a bimetallic MOF, carbon cloth (CC) was pretreated to enhance the surface properties and introduce oxygen functional groups, which helps in better adhesion of active materials. The bimetallic MOF with Co and Ni as metallic centers in the alkaline electrolyte of 2 M potassium hydroxide delivered a cyclic voltammogram curve, as shown in Fig. 4a, which indicated a larger area at a similar scan rate and compared with the powder material, explains that pretreated flexible and in situ grown higher specific capacity. Similarly, Fig. 4b shows the discharge curve in the voltage window of 0.4 V, where CoNi0.5-MOF grown on CC delivered a specific capacitance of 1337.5 F g−1 at 1 A g−1. In contrast, at a similar current, the specific capacitance of CoNi0.5-MOF coated on CC is found to be 578 F g−1. Additionally, as illustrated in Fig. 4c, the specific capacity discharge retention at a current density of 30 A g−1 lasted for 13 s. The calculated specific capacitance was 852 F g−1, resulting in a discharge retention of 65%. The fabrication process of the FSC involves assembling asymmetric FSC CoNi0.5-MOF/CC as the positive electrode and nitrogen-doped graphene (N-Gr) as the negative electrode with a PVA-KOH-based electrolyte and separator. As the developed electrodes were free of binders, the design ensured mechanical robustness and enhanced charge transfer efficiency. The developed FSC delivered an energy density of 61.46 W h kg−1 at a power density of 1244.56 W kg−1. It maintains stable performance under bending conditions, with capacitance slightly reduced to ∼125 F g−1 at 1 A g−1.28 This finding highlights the potential of MOF in developing flexible electrodes with a simple fabrication technique and developing advanced electrodes for advanced energy storage properties with excellent electrochemical and mechanical properties. Considering the wearable FSCs, the current collectors' stretching is the main concern, and the study led by Zha et al. explored the synthesis of NiCo-BTC nanosheets with a binder-free electrode, highlighting its potential for wearable electronics. The electrode preparation was done with in situ growth on carbon cloth using the solvothermal method with the Ni/Co/BTC molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The developed binder-free method ensured the adhesion of nanosheets onto the conductive substrate via C–C bond creation of a stable and organized structure, where it enhanced active site exposure. An electrochemical test with a three-electrode setup in alkaline electrolyte delivered a performance of NiCo-BTC with a specific capacity of 644.4C g−1 at a current density of 0.5 A g−1, significantly surpassing the capacities of single-metal MOFs like Ni-BTC and Co-BTC. The developed NiCo-BTC electrode also showed minimal resistance and enhanced ionic diffusion led by the synergistic effect of bimetallic ions and oriented nanosheet morphology. When the FSC is fabricated, the NiCo-BTC/CC electrode is paired with an activated carbon cathode, and their cyclic performance is shown in Fig. 4d. The developed activated carbon electrode exhibited electrical double-layer characteristics in the voltage range of −1.2 V to 0 V with a rectangular cyclic curve. The fabricated FSC showed the operating voltage extended until 1.8 V, as displayed in Fig. 4e, and the cyclic curve slightly showed the polarization at 2 V, limiting its working voltage to 1.8 V. The asymmetric FSC delivered an energy density of 32.4 W h kg−1 at a power density of 346 W kg−1. The device maintained 91.2% of its capacity after 8000 cycles, demonstrating exceptional cycling stability, as shown in Fig. 4f. Hence, the fabricated FSCs are viable for flexible and wearable applications and perform consistently under mechanical bending.29 This study highlighted the promise of bimetallic MOF in advanced energy storage technology, particularly in portable devices.
2. The developed binder-free method ensured the adhesion of nanosheets onto the conductive substrate via C–C bond creation of a stable and organized structure, where it enhanced active site exposure. An electrochemical test with a three-electrode setup in alkaline electrolyte delivered a performance of NiCo-BTC with a specific capacity of 644.4C g−1 at a current density of 0.5 A g−1, significantly surpassing the capacities of single-metal MOFs like Ni-BTC and Co-BTC. The developed NiCo-BTC electrode also showed minimal resistance and enhanced ionic diffusion led by the synergistic effect of bimetallic ions and oriented nanosheet morphology. When the FSC is fabricated, the NiCo-BTC/CC electrode is paired with an activated carbon cathode, and their cyclic performance is shown in Fig. 4d. The developed activated carbon electrode exhibited electrical double-layer characteristics in the voltage range of −1.2 V to 0 V with a rectangular cyclic curve. The fabricated FSC showed the operating voltage extended until 1.8 V, as displayed in Fig. 4e, and the cyclic curve slightly showed the polarization at 2 V, limiting its working voltage to 1.8 V. The asymmetric FSC delivered an energy density of 32.4 W h kg−1 at a power density of 346 W kg−1. The device maintained 91.2% of its capacity after 8000 cycles, demonstrating exceptional cycling stability, as shown in Fig. 4f. Hence, the fabricated FSCs are viable for flexible and wearable applications and perform consistently under mechanical bending.29 This study highlighted the promise of bimetallic MOF in advanced energy storage technology, particularly in portable devices.
 |
| | Fig. 4 The comparison of the electrochemical properties of CoNi0.5-MOF and CoNi0.5-MOF/CC: (a) CV curves recorded at 20 mV s−1. (b) GCD curves measured at a current density of 1 A g−1. (c) The charge–discharge behavior of CoNi0.5-MOF/CC at various current inputs.28 (d) The CV curves of activated carbon and NiCo-BTC. (e) CV curves of the FSC under different operating voltage windows ranging from 1.2 to 2.0 V. (f) The cycle life of the device.29 Reproduced with permission from ref. 28. (a–c) Royal Society of Chemistry, ref. 29. (d–f) Copyright © 2023, Elsevier. | |
3.3. MOFs/MXene-based FSCs
MXenes are a unique class of two-dimensional materials that have garnered significant interest due to their exceptional properties and potential applications, particularly in supercapacitors. Comprising transition metal carbides and nitrides, MXenes are characterized by their versatile composition and tunable surface functionalities. These materials are typically synthesized by selectively etching the “A” element from the parent MAX phase, resulting in a stacked 2D structure with the general formula Mn+1XnTx, where M represents a transition metal, X is carbon or nitrogen, and Tx denotes surface terminations such as –OH, –O, or –F. Among them, Ti3C2Tx is the most extensively studied MXene, owing to its high electrical conductivity and excellent electrochemical properties, making it highly suitable for supercapacitor applications.30 Pure MOFs may not conduct electricity well and are considered unstable, but they can be used as building blocks to create superstructures. Combining 2D MXenes with MOFs can be beneficial, as it avoids stacking, which makes the composite more stable and enhances its electrochemical performance.31 MOF/MXene composite integration leads to high surface area and exposure of redox-active sites of MOF with the excellent conductivity and flexibility of MXene. These materials enable enhanced charge storage, mechanical durability, and deformation tolerance. They are ideal for flexible supercapacitors in wearable electronics, offering high energy density, stable performance, and scalability through advanced fabrication methods. The research work by Kanthasamy et al. highlighted the integration of Cu-based metal–organic frameworks (Cu-MOF) with delaminated MXene (D-MXene) to develop high-performance supercapacitor electrodes. The study successfully demonstrated that structural tuning and synergistic interactions enhance electrochemical performance, making the composite material a promising candidate for next-generation energy storage. With this work aspect, the Cu-MOF/D-MXene-based composite enables a bendable and lightweight FSC. The developed material's mechanical robustness allowed sustainable structural integrity under deformation, which is crucial for wearable and portable electronics. The Cu MOF/D-MXene electrode exhibits a specific capacitance of 3249.92 F g−1 at 1 A g−1 in a three-electrode system by using an alkaline electrolyte. This interaction ensured excellent conductivity, low charge transfer resistance, and superior cycling stability, retaining 95% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, and the FSC with Cu MOF/D-MXene as the cathode and AC as the anode with PAV-KOH gel as the separator achieved an energy density of 77.18 W h kg−1 at a power density of 748.9 W kg−1.32 Hence, this work makes way for breakthroughs in FSCs for high-performance, durable, and adaptive energy solutions. The other research work led by Jiang et al. addressed the challenges of balancing mechanical flexibility and energy storage capacity in wearable supercapacitors through an innovative 2D/2D assembly of ultrathin NiCo-BDF-MOF and MXene sheets. Further, it highlighted the potential of scaling up to real-world applications. The composites were prepared with the combination of small-sized ultrathin MOF (SUMOF) sheets with large-sized MXene (LMX) sheets, and electrodes were prepared through a blade-coating process on pre-stretched elastomer substrates. The prepared composite exhibited electrostatic interaction between positively-charged SUMOF and negatively-charged LMX, which enabled uniform layer-by-layer assembly and displayed a wavelike structure, strengthening the flexibility of the prepared electrode. From the developed electrodes, electrochemical analysis with optimized loading of LMX (30 wt%) on SUMOF revealed excellent conductivity (3244 S cm−1) and a high specific capacitance of 1238 F g−1. The optimized composition with layered architecture showed an excellent trade-off between energy storage and mechanical strength, owing to the enhanced electron transfer and active site exposure. The schematic illustration of SUMOF/LMX architecture with 20 wt%, 30 wt%, and 40 wt% is shown in Fig. 5a–c, respectively. Lower LMX content showed ordered layer arrangements with poor mechanical performance, and with further addition of LMX to SUMOF, there was improved interaction between LMX and SUMOF, leading to fewer gaps in the composite film. With regard to the further increase in 40 wt% LMX, sufficient restacking was observed to form a robust film, which caused the electrode performance to deteriorate. The ASC device was fabricated with composite films as the anode and pure MXene as the cathode, separated by a PVA-KOH gel electrolyte. The device delivered an energy density of 27.2 W h kg−1 at a power density of 550 W kg−1 and maintained stable performance under 50% stretching and 180° bending. The fabricated ASC showed excellent mechanical and electrochemical stability under deformation, maintaining 87.4% of its initial capacitance after 10
000 cycles, and the FSC with Cu MOF/D-MXene as the cathode and AC as the anode with PAV-KOH gel as the separator achieved an energy density of 77.18 W h kg−1 at a power density of 748.9 W kg−1.32 Hence, this work makes way for breakthroughs in FSCs for high-performance, durable, and adaptive energy solutions. The other research work led by Jiang et al. addressed the challenges of balancing mechanical flexibility and energy storage capacity in wearable supercapacitors through an innovative 2D/2D assembly of ultrathin NiCo-BDF-MOF and MXene sheets. Further, it highlighted the potential of scaling up to real-world applications. The composites were prepared with the combination of small-sized ultrathin MOF (SUMOF) sheets with large-sized MXene (LMX) sheets, and electrodes were prepared through a blade-coating process on pre-stretched elastomer substrates. The prepared composite exhibited electrostatic interaction between positively-charged SUMOF and negatively-charged LMX, which enabled uniform layer-by-layer assembly and displayed a wavelike structure, strengthening the flexibility of the prepared electrode. From the developed electrodes, electrochemical analysis with optimized loading of LMX (30 wt%) on SUMOF revealed excellent conductivity (3244 S cm−1) and a high specific capacitance of 1238 F g−1. The optimized composition with layered architecture showed an excellent trade-off between energy storage and mechanical strength, owing to the enhanced electron transfer and active site exposure. The schematic illustration of SUMOF/LMX architecture with 20 wt%, 30 wt%, and 40 wt% is shown in Fig. 5a–c, respectively. Lower LMX content showed ordered layer arrangements with poor mechanical performance, and with further addition of LMX to SUMOF, there was improved interaction between LMX and SUMOF, leading to fewer gaps in the composite film. With regard to the further increase in 40 wt% LMX, sufficient restacking was observed to form a robust film, which caused the electrode performance to deteriorate. The ASC device was fabricated with composite films as the anode and pure MXene as the cathode, separated by a PVA-KOH gel electrolyte. The device delivered an energy density of 27.2 W h kg−1 at a power density of 550 W kg−1 and maintained stable performance under 50% stretching and 180° bending. The fabricated ASC showed excellent mechanical and electrochemical stability under deformation, maintaining 87.4% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, as shown in Fig. 5d. It also retained structural integrity and reliable electrochemical behavior after extensive cycling, making it a promising candidate for wearable applications.33 A work by Ji et al. presented the synthesis and electrochemical evaluation of bimetallic MOF integrated with Ti3C2Tx MXene. For electrode development, they chose a combination of two different ions and combined them with MXene. The final step in the synthesis procedure involved a hydrothermal process with the presence of metal precursors such as (Ni(NO3)2, Co(NO3)2, Cu(NO3)2) in DMF with the BTC ligand in the presence of MXene. The resulting composites exhibited distinct morphologies, with Co2+ and Cu2+ forming stronger bonds with MXene, while Ni2+ preferred ligand coordination. The detailed synthesis procedure is shown in Fig. 5e. The optimized electrode, which was a combination of NiCo-MOF/MXene, achieved a specific capacitance of 1493.6 F g−1 at 1 A g−1 in an alkaline electrolyte, and their energy storage mechanism primarily involves faradaic redox reactions of Co2+/Co3+ and Ni2+/Ni3+, along with OH− adsorption/desorption processes. Moreover, MXene's stacked layer produced a synergistic effect to provide mechanical flexibility and The study demonstrates the structural optimization and compensation effect of different metal ions, proving their impact on electrochemical performance. The FSC was fabricated using Ni1Co1@MXene as the positive electrode and activated carbon as the negative electrode, which was fabricated by employing PVA-KOH as a gel electrolyte. The device demonstrated an energy density of 73.9 W h kg−1 at a power density of 750 W kg−1. The fabricated FSC was found to be stable and retained 81% of its initial capacity even after 10
000 cycles, as shown in Fig. 5d. It also retained structural integrity and reliable electrochemical behavior after extensive cycling, making it a promising candidate for wearable applications.33 A work by Ji et al. presented the synthesis and electrochemical evaluation of bimetallic MOF integrated with Ti3C2Tx MXene. For electrode development, they chose a combination of two different ions and combined them with MXene. The final step in the synthesis procedure involved a hydrothermal process with the presence of metal precursors such as (Ni(NO3)2, Co(NO3)2, Cu(NO3)2) in DMF with the BTC ligand in the presence of MXene. The resulting composites exhibited distinct morphologies, with Co2+ and Cu2+ forming stronger bonds with MXene, while Ni2+ preferred ligand coordination. The detailed synthesis procedure is shown in Fig. 5e. The optimized electrode, which was a combination of NiCo-MOF/MXene, achieved a specific capacitance of 1493.6 F g−1 at 1 A g−1 in an alkaline electrolyte, and their energy storage mechanism primarily involves faradaic redox reactions of Co2+/Co3+ and Ni2+/Ni3+, along with OH− adsorption/desorption processes. Moreover, MXene's stacked layer produced a synergistic effect to provide mechanical flexibility and The study demonstrates the structural optimization and compensation effect of different metal ions, proving their impact on electrochemical performance. The FSC was fabricated using Ni1Co1@MXene as the positive electrode and activated carbon as the negative electrode, which was fabricated by employing PVA-KOH as a gel electrolyte. The device demonstrated an energy density of 73.9 W h kg−1 at a power density of 750 W kg−1. The fabricated FSC was found to be stable and retained 81% of its initial capacity even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, and coulombic efficiency was found to be 97%, as shown in Fig. 5f. FSC demonstration towards potential practical application was done with a series of connected FSCs, which were able to power blue and red LEDs under various bending conditions, as illustrated in Fig. 5f. This provides the flexible device's application potential. Hence, the mechanical flexibility of Ni1Co1-MOF @ MXene-based FSCs has been confirmed through various bending conditions, which makes it suitable for wearable electronics and next-generation storage devices.34
000 cycles, and coulombic efficiency was found to be 97%, as shown in Fig. 5f. FSC demonstration towards potential practical application was done with a series of connected FSCs, which were able to power blue and red LEDs under various bending conditions, as illustrated in Fig. 5f. This provides the flexible device's application potential. Hence, the mechanical flexibility of Ni1Co1-MOF @ MXene-based FSCs has been confirmed through various bending conditions, which makes it suitable for wearable electronics and next-generation storage devices.34
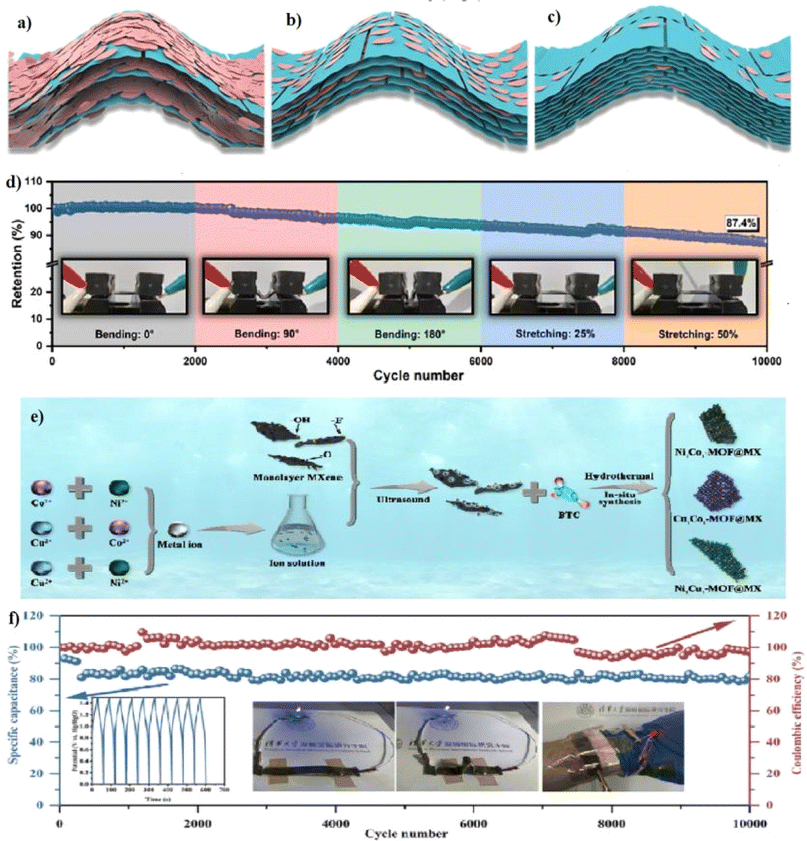 |
| | Fig. 5 (a–c) The predicted cross-sections of microstructure of SUMOF/LMX with different weight% of LMX: 20%, 30% and 40% respectively. (d) The capacitance retention of the FSCs after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles under different bending angles and stretching conditions.33 (e) The synthesis processes of MxNy-MOF@MXene composites. (f) Stability of the FSC, including constant current charge–discharge cycling data and illustrations of two devices in series lighting up blue and red LED lights.34 Reproduced with permission from ref. 33. (a–d) Copyright © 2024, John Wiley and Sons, ref. 34. (e and f) Copyright © 2024, Elsevier. 000 cycles under different bending angles and stretching conditions.33 (e) The synthesis processes of MxNy-MOF@MXene composites. (f) Stability of the FSC, including constant current charge–discharge cycling data and illustrations of two devices in series lighting up blue and red LED lights.34 Reproduced with permission from ref. 33. (a–d) Copyright © 2024, John Wiley and Sons, ref. 34. (e and f) Copyright © 2024, Elsevier. | |
3.4. MOFs/carbon-based FSCs
Carbon is considered an electrode material for electrolyte drop liquid cells due to its advantages of high electrical conductivity, a large specific surface area, and exceptional chemical stability. Although the specific capacitance of carbon compounds does not always increase directly with surface area, the correlation remains noteworthy. Factors such as electrical conductivity, pore size, and morphology play a crucial role in determining the specific capacitance of carbon materials.35 MOFs, which appear in different ligand frameworks and possess varying dimensions, can be combined with carbon-based materials to enhance their electrochemical performance. Hence, choosing the right approach is crucial for developing MOF/carbon composites, which can be achieved through a synthesis method that includes in situ synthesis, where the MOF is grown on carbon, or post-synthesis modification, where the MOF is initially synthesized and then coated or mixed with carbon materials. Amongst various types of MOF, metalloporphyrin framework promotes additional domain formation with adjacent atoms. These MOFs facilitate 2D ultrathin nanosheet-like morphology, which exhibits excellent energy storage properties. Based on this work, Diao et al. developed an FSC by designing a multi-walled CNT (MWCNT)/MOF ultrathin nanosheet composite with printable ink that delivers excellent performance. The organic ligand used in the framework was TCPP with a zinc metallic center. The integration of carboxyl-functionalized multi-walled carbon nanotubes (C-MWCNT) into MOF nanosheets improved the conductivity, mechanical strength, and structural integrity of the composite. This optimized combination achieved an electrochemical performance with a specific capacitance of 0.152 mA h g−1 at a current density of 1 A g−1. It demonstrated excellent cycling stability, retaining 89% of its performance after 5000 cycles. Fig. 6a shows the electrode composition made of the FSC, which is suitable for wearable applications, with the inset showing the MOF and MWCNT dispersion. Similarly, FSC properties were analyzed under extreme conditions, as shown in Fig. 6b and c, and upon analysis, they were found to be suitable for real-world application, delivering 98% capacitance retention. The fabricated FSC showed an energy density of 77.6 μW h cm−3 at a power density of 1206.7 μW cm−3 with excellent mechanical resilience and high performance under deformation, confirming its suitability for flexible electronics and wearable energy storage.36 The developed work demonstrated a printable approach to developing an FSC suitable for wearable applications.
 |
| | Fig. 6 (a) The composition and preparation process of the FSC, with the inset showing the dispersion of NMOF and C-NMOF. (b) The bending tests were conducted at different angles. (c) Torsion tests.36 (d) An illustration depicting the synthesis process of Cu-MOF@ACNF. (e) The flexibility characteristics of the developed FSC. (f) A practical demonstration utilizing the developed FSC.37 Reproduced with permission from ref. 36. (a–c) Copyright © 2024, Elsevier, ref. 37. (d–f) Copyright © 2023, Elsevier. | |
As there are numerous carbon sources, they can be bio-based derived and possess carbon in different dimensions. The obtained bio-based derived carbon could show the structural advantages of interconnected conductive networks, enhanced electron transfer, and prevented agglomeration. Considering this, Singh et al. prepared a Cu-MOF utilizing copper-1, 3, 5-benzene dicarboxylate (Cu3(BTC)2), which was in situ grown on Activated Carbon Nanofiber (ACNF) sheets, which inherits the advantage of lignin oxygen functionality and facilitates MOF deposition. The detailed process of preparing Cu-MOF/ACNF is illustrated in Fig. 6d, and the developed composite material in 1 M H2SO4 electrolyte delivered electrochemical properties with a specific capacitance of 303.2 F g−1 at 1 A g−1, outperforming ACNF (203.3 F g−1) and Cu-MOF alone (68.2 F g−1). Moreover, the Cu-MOF@ACNF sheet showed high cycling stability with a coulombic efficiency of 99.1% after 5000 charge–discharge cycles. Upon developing SCs in both aqueous and solid states for FSCs, the solid-state FSCs showed different flexibility, as shown in Fig. 6e, outperforming aqueous SCs with the operating voltage of 2 V delivering an energy density of 78.71 W h kg−1 at a power density of 1050 W kg−1, and maintained a 99.8% efficiency after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, confirming its long-term stability, with a practical demonstration of LEDs glowing as shown in Fig. 6f. Hence, Cu-MOF@ACNF demonstrated potential for next-generation FSCs, combining high electrochemical performance, mechanical flexibility, and sustainability.37 As MOFs can be prepared with different organic linkers, Abbas et al. developed a π–d conjugated metal–organic framework with a benzene tetramine ligand and Ni ions as metallic, active sites. To establish a freestanding film for FSCs, Ni-MOF was applied to an electrospun mat made of MXene-CNF. The material synthesis involved three steps: first, the preparation of MXene; second, the creation of the MXene-CNF mat; and finally, the in situ growth of Ni-MOF on the MXene-CNF mat. The developed material combination showed higher conductivity, enhanced charge transfer, and structural integrity with self-standing. It maintained a hydrophobic nature, and finally, the developed hybrid structure benefitted from integrating 1D MOF with 2D MXene-embedded 1D CNFs. These advantages led to a high electrochemical performance with a specific capacitance of 1076 F g−1 at 1 A g−1, capacitance retention of 40.8% at 20 A g−1, and cycling stability with 86.4% capacitance retention after 15
000 cycles, confirming its long-term stability, with a practical demonstration of LEDs glowing as shown in Fig. 6f. Hence, Cu-MOF@ACNF demonstrated potential for next-generation FSCs, combining high electrochemical performance, mechanical flexibility, and sustainability.37 As MOFs can be prepared with different organic linkers, Abbas et al. developed a π–d conjugated metal–organic framework with a benzene tetramine ligand and Ni ions as metallic, active sites. To establish a freestanding film for FSCs, Ni-MOF was applied to an electrospun mat made of MXene-CNF. The material synthesis involved three steps: first, the preparation of MXene; second, the creation of the MXene-CNF mat; and finally, the in situ growth of Ni-MOF on the MXene-CNF mat. The developed material combination showed higher conductivity, enhanced charge transfer, and structural integrity with self-standing. It maintained a hydrophobic nature, and finally, the developed hybrid structure benefitted from integrating 1D MOF with 2D MXene-embedded 1D CNFs. These advantages led to a high electrochemical performance with a specific capacitance of 1076 F g−1 at 1 A g−1, capacitance retention of 40.8% at 20 A g−1, and cycling stability with 86.4% capacitance retention after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Additionally, the FSC with the device configuration of MOF@MXene-CNF as the positive electrode, activated carbon as the negative electrode, and PVA/KOH gel electrolyte showed an operating voltage of 1.4 V with an energy density of 45.7 W h kg−1 at a power density of 719 W kg−1 and at various bending shapes the cyclic voltammogram curves remained similar.38 Hence, this kind of material configuration is highlighted by integrating a 1D–2D–1D hybrid strategy for next-generation flexible energy storage devices, offering high performance, stability, and practical applicability.
000 cycles. Additionally, the FSC with the device configuration of MOF@MXene-CNF as the positive electrode, activated carbon as the negative electrode, and PVA/KOH gel electrolyte showed an operating voltage of 1.4 V with an energy density of 45.7 W h kg−1 at a power density of 719 W kg−1 and at various bending shapes the cyclic voltammogram curves remained similar.38 Hence, this kind of material configuration is highlighted by integrating a 1D–2D–1D hybrid strategy for next-generation flexible energy storage devices, offering high performance, stability, and practical applicability.
3.5. MOF/graphite and its derivative based FSCs
Graphite derivatives such as graphene, reduced graphene oxide (rGO), and graphite oxide (GO) could play a role in the enhancement of MOF electrochemical performance with their higher conductivity, larger surface area, and porous structure facilitating ionic diffusion, which is owing to higher energy density and specific capacity. Furthermore, reinforcing graphite derivatives could enhance the chemical stability and durability of the developed electrode even under harsh environmental conditions. Overall, these composites result in high electrochemical properties, which makes them an excellent candidate for FSCs. Considering these benefits, Erçarıkc et al. developed MnCo-MOF with 2-methylimidazole as the organic linker and modified it on a flexible 3D graphene (3DG) sponge electrode suitable for wearable applications. The synthesis process involved developing MnCo-MOF, dispersing it with GO, and addition of sodium dodecyl sulfate and ascorbic acid. Finally, the mixture was subjected to thermal foaming, freezing (−18 °C), drying (90 °C), and annealing (350 °C) to form a porous and flexible MnCo-MOF/3DG electrode. The digital photographs of the flexible electrodes showing their light weight and flexibility are demonstrated in Fig. 7a and b. The developed electrode possessed the advantages of high surface area, porosity, mechanical flexibility and electrochemical performance, delivering a high specific capacitance of 4086 F g−1 at 1 A g−1 in alkaline electrolyte. Also, the MnCo-MOF/3DG electrode exhibited a rate capability of 85% at a high current of 40 A g−1. It showed cycling stability with 83% capacitance retention even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, which makes it an excellent electrode for FSCs. Additionally, the FSC fabricated with the configuration of MnCo-MOF/3DG as the positive electrode, 3DG as the negative electrode, and PVA-KOH as the gel electrolyte showed an operating voltage of 1.5 V and maintained FSC performance even at different bending angles of 180°, 90°, and 45° as shown in Fig. 7c, which make it ideal for wearable electronics. These results revealed that the developed FSCs can be used in practical SC applications with high durability and flexibility, which showed energy and power densities of 198.5 W h kg−1 and 5823 W kg−1, respectively, as shown in Fig. 7d. Hence, the robustness and mechanical stability of the developed MnCo-MOF/3DG electrodes make them a promising candidate for next-generation FSCs.39
000 cycles, which makes it an excellent electrode for FSCs. Additionally, the FSC fabricated with the configuration of MnCo-MOF/3DG as the positive electrode, 3DG as the negative electrode, and PVA-KOH as the gel electrolyte showed an operating voltage of 1.5 V and maintained FSC performance even at different bending angles of 180°, 90°, and 45° as shown in Fig. 7c, which make it ideal for wearable electronics. These results revealed that the developed FSCs can be used in practical SC applications with high durability and flexibility, which showed energy and power densities of 198.5 W h kg−1 and 5823 W kg−1, respectively, as shown in Fig. 7d. Hence, the robustness and mechanical stability of the developed MnCo-MOF/3DG electrodes make them a promising candidate for next-generation FSCs.39
 |
| | Fig. 7 (a and b) Digital camera photographs of a lightweight and flexible MnCo-MOF/3DG. (c) The bending curves of MnCo-MOF/3DG//3DG at 180°, 120°, 90°, 45°, and 15°. (d) Ragone plots for MnCo-MOF/3DG//3DG.39 (e) The detailed synthesis process used to obtain OHNM and rOHNM-AGs. (f) A schematic illustration of electron/ion transfer through the active constituent of rOHNM-AGs.40 (g) The morphological illustration of Ni-BTC-MOF@GO.41 Reproduced with permission from ref. 39. (a–d) Copyright © 2023, Royal Society of Chemistry, ref. 40. (e and f) Copyright © 2023, Elsevier. ref. 41. (g) The work is openly licensed with CC By 4.0. | |
As the flexible electrode can be prepared with various methods, preparing with aerogel brings added advantages of stability and durability. Hence with this consideration, Zhong et al. prepared open-hollow nickel-based MOF (OHNM) microspheres integrated with rGO and further processed to a 3D porous aerogel electrode (rOHNM-AGs) to obtain a flexible electrode. The detailed synthesis process is shown in Fig. 7e, where synthesis was done with polyvinylpyrrolidone (PVP) which is used to control the morphology and H3-BTC is used as a ligand to obtain Ni-MOF. Finally, the prepared OHNM is mixed with rGO to form a hydrogel and freeze-dried to form rOHNM-AGs. The developed material possessed the advantage of enhanced electrochemical performance with an electron/ion transport mechanism, as shown in Fig. 7f. The enhanced electrochemical properties were due to the designed 3D porous architecture and created an extensive eccentric contact, providing a higher electron/ion transport rate for shuttling for the electrolyte. Additionally, OHNM wrapped in a layered graphene structure stabilizes the open hollow structure and avoids collapse. This could make mechanical completeness in the electrochemical course and make the electrode exhibit excellent electrochemical performance. This led to a gain in specific capacitance of 1644 F g−1 at 1 A g−1 with higher rate capability, where it maintained high capacitance at elevated current density, which shows its potential for high-powered FSC application. With the FSC configuration made of rOHNM-AGs as the positive electrode, AC as the negative electrode, and PVA/KOH gel electrolyte, the energy density and power density were found to be 36.4 W h kg−1 and 800 W kg−1, respectively. The fabricated FSCs' mechanical flexibility was assessed with bending at multiple angles, where it maintained stable performance, which showed its potential in wearable applications. Hence, open-hollow Ni-MOF with rGO aerogels presented themselves as candidates for advanced FSCs for next-generation wearable energy storage devices.40 Similarly, considering the poor intrinsic capacity performance of MOF, Chen et al. developed an FSC by integrating Ni-BTC MOF@graphene oxide (Ni-BTC@GO), where the study emphasized enhanced electrical conductivity and electrochemical stability. In the in situ synthesis of Ni-BTC@GO, the composite obtained with a morphological illustration as shown in Fig. 7g showed excellent conductivity, mechanical flexibility and high surface area which clearly mitigates the GO aggregation and the electron pathway is enhanced, which led to excellent electrochemical performance with a specific capacitance of 1199 F g−1 at 1 A g−1 in alkaline electrolyte. Furthermore, the asymmetric FSC delivered an energy density of 40.89 W h kg−1 at a power density of 800 W kg−1. The long cycling stability was found to be 84.47% even after 5000 cycles and stable electrochemical performance was retained under different deformation. Hence, making a successful study by addressing the limitations of MOFs through GO integration paves the way for FSCs with scalability.41 Furthermore, Table 3 presents the FSC properties of a few recent literature studies.
Table 3 FSC properties of recent MOFs
| FSC structure |
Specific capacitance |
Energy density |
Power density |
Stability |
Ref. |
| Ni-MOF‖AC |
195.76 F g−1@2 mA cm−2 |
79.5 W h kg−1 |
1015.3 W kg−1 |
99% (3000 cycles) |
26
|
| Co-MOF‖AC |
110 F g−1@0.5 A g−1 |
34.4 W h kg−1 |
375.3 W kg−1 |
120% (5000 cycles) |
42
|
| NiCo-MOF‖AC |
80.3 F g−1 at 0.5 A g−1 |
28.5 W h kg−1 |
400.5 W kg−1 |
95.4% (5000 cycles) |
43
|
| Ni/Mn-MOF‖AC |
1055 F g−1@0.5 A g−1 |
47.49 W h kg−1 |
453.6 W kg−1 |
81.6% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
44
|
| Ag incorporated symmetric NiCo-MOF |
215.24 F g−1@0.5 A g−1 |
72.55 W h kg−1 |
408.61 W kg−1 |
85.9% (8000 cycles) |
45
|
| Ce/Ni-MOF‖GO |
298.8 mF cm−2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) @ 3 mA cm−2 @ 3 mA cm−2 |
120 μW h cm−2 |
2.5 mW cm−2 |
93% (5000 cycles) |
46
|
| ZnCo-MOF modified symmetric graphene sponge |
302 F g−1@1.0 A g−1 |
108 W h kg−1 |
5037 W kg−1 |
80% (7500 cycles) |
18
|
| rGO@Cd-MOF//AC |
220C g−1 @ 2 A g−1 |
78.69 W h kg−1 |
1282 W kg−1 |
94% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
47
|
| Ni/Co-MOF@MXene‖AC |
351.3 F g−1@ 0.5 A g−1 |
98.1 W h kg−1 |
600 W kg−1 |
92% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
48
|
| MXene@NiCo-MOF‖AC |
121 F g−1@2 A g−1 |
40.23 W h kg−1 |
1495.07 W kg−1 |
84% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
49
|
| Ni1Co1-MOF@MX‖AC |
236.7 F g−1 at 1.0 A g−1 |
73.9 W h kg−1 |
750 W kg−1 |
80% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
34
|
| CoNi0.5-MOF/CC‖graphene |
160.6 F g−1 at 1.0 A g−1 |
61.46 W h kg−1 |
1244.56 W kg−1 |
88% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
28
|
| Nb-MOF@CNF‖CNF |
272.1 F g−1@0.25 A g−1 |
64.24 W h kg−1 |
252.5 W kg−1 |
98.3% (4000 cycles) |
50
|
| CoNi-MOF/graphene NF‖Graphene NF |
146 F g−1@1 A g−1 |
52.2 W h kg−1 |
799.6 W h kg−1 |
90% (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
51
|
| Symmetric RuCo-MOF/ACF |
5125.4 mF cm−2@1 mA cm−2 |
10.66 W h cm−2 |
27.6 W cm−2 |
94% (6000 cycles) |
52
|
4. Prospects and outlook
FSCs emerged as pivotal energy storage devices in the advancement of wearable technologies due to their superior capacitance properties, fast charge–discharge capability, and robust flexibility. Considering these advantages, the development of FSCs and their integration with wearable technology present various challenges and opportunities that are critical for SC performance and widespread adoption. With the challenges of MOF limitation, the selection of an appropriate electrode material is key to FSC performance. Below are the challenges that warrant more concern and their prospects.
4.1. Scalability
Although MOFs have garnered significant attention for their potential in FSCs, their scalability remains a challenge. Traditional synthesis methods face a backlash due to their time-consuming nature and ineffectiveness for large-scale production. Alternative methods, such as mechanochemical, spray drying, and electrochemical approaches, offer promising pathways for the mass production of MOFs. These techniques reduce synthesis duration and improve yield, which makes MOF-based materials commercially viable for FSCs. The other major issue is the MOF's structural integrity and its integration on flexible substrates. Hence, to address this, the composite preparation strategy facilitates the maintenance of both mechanical flexibility and structural stability. Additional advancements, such as 3D printing and roll-to-roll processing of MOF films, further enhance the scalability of MOFs for real-world applications.
4.2. Durability
The long-term stability of MOFs in FSCs is crucial for their practical use. Although MOFs possess high porosity and tunable surface area, their inherent brittleness and sensitivity to moisture can limit their durability. Hybridizing MOFs with carbon-based materials could enhance mechanical flexibility and cycling stability. During charge–discharge cycles, MOFs may undergo structural degradation, which ultimately reduces their long-term retention of capacitance. Hence, to mitigate structural degradation in the long run, strategies such as metal doping, surface functionalization, and the use of a polymer binder help reinforce the MOF structure and improve its mechanical integrity, ultimately enhancing electrochemical performance. Also, research on self-healing materials and stretchable MOF composites can further enhance durability for wearable and flexible electronics.
4.3. Cost-effectiveness
Despite the MOF's outstanding electrochemical properties, which favor energy storage applications, the cost of its precursor and the complexity of its synthesis approach limit widespread adoption. The expensive organic linkers and metal precursors, along with an intensive energy-consuming synthesis process, increase the production cost. To make MOFs cost-effective, exploring low-cost precursors, such as aluminum and iron-based MOFs, is crucial. Additionally, a green synthesis approach using bio-materials or waste-derived organic linkers should be investigated. Additionally, the mechanochemical synthesis approach requires further investigation for scale-up applications, as it eliminates the need for solvents and high-temperature treatments. Hence, with these factors optimized, the MOF cost can be significantly reduced, making them more competitive with carbon-based materials for energy storage systems.
4.4. Electrolyte
The electrolyte choice plays a crucial role in determining the electrochemical performance of FSCs. Gel electrolytes offer promising solutions for FSCs due to their stability and ability to maintain ionic conductivity, which surpasses that of liquid electrolytes. They reduce the risk of leakage, which makes them ideal for wearable electronics and stretchable devices. However, they may experience dehydration over time, which can lead to performance hindrances. Other types of electrolytes, such as solid-state electrolytes, have excellent safety, mechanical strength, and flexibility, making them suitable for energy storage devices. They eliminate leakage issues and offer excellent thermal stability. However, they still lag behind in ionic conductivity and exhibit higher interfacial resistance, which limits charge transport and reduces the capability of energy storage devices. Hence, to address this, solid electrolytes combining polymers and ionic liquids are to be explored. Furthermore, liquid electrolytes remain superior in ionic conductivity, which enables high charge and discharge rates. Aqueous electrolytes offer cost-effectiveness, whereas organic and ionic liquid electrolytes provide a wider voltage window, thereby improving energy density. However, their issues with leakage and the integration of flexible substrates limit their application in FSCs. Developing hybrid and gel-based ionic liquid electrolytes could bridge this gap, offering both high performance and flexibility.
4.5. Application prospects of MOF-based FSCs
MOF-based FSCs have a wide range of applications due to their energy storage capabilities and mechanical flexibility. The key areas include wearable electronics used in smart textiles, health monitoring devices, and electronic skins, where flexibility is crucial, as well as portable energy storage devices, such as foldable phones, flexible displays, and next-generation devices that require adaptable power solutions. Other applications, such as those in the automotive and aerospace industries, require lightweight and high-performance energy storage for electric vehicles, drones, and space technology.
5. Conclusion and perspective
MOFs are emerging as a promising area of research for FSCs due to their high surface area, tunable porosity, and superior electrochemical performance. FSCs are extensively utilized in wearable technology due to their lightweight nature, mechanical flexibility, and high power density. However, challenges such as limited conductivity, structural instability, and scalability hinder their widespread application. Recent developments have demonstrated how MOF-based composites, such as those incorporating MXene, carbon-based materials, and multi-metallic centers, can overcome intrinsic limitations like poor conductivity and structural instability. However, scalability remains a challenge due to the time-consuming and cost-intensive synthesis methods traditionally used for MOFs. Alternative synthesis techniques, including mechanochemical, spray drying, and electrochemical deposition, offer scalable and cost-effective solutions. Additionally, hybridizing MOFs with flexible substrates and advanced fabrication methods, such as 3D printing and roll-to-roll processing, can further improve their integration into real-world applications. Furthermore, hybridizing MOFs with flexible substrates and utilizing advanced fabrication techniques can further enhance their integration into real-world applications.
Wearable supercapacitors require materials that not only store energy efficiently but also maintain stable performance under mechanical deformation. The structural advantage of MOFs enables faster electrolyte penetration, improving the kinetics of FSCs. Additionally, the conductive nature of specific MOFs further facilitates rapid charge transfer, thereby enhancing both energy storage and power delivery capabilities. These properties make MOFs an ideal choice for developing next-generation flexible energy storage devices with high electrochemical stability and fast charge/discharge rates. Furthermore, the versatility of MOFs allows design precision to achieve optimal properties for wearable supercapacitors. By modifying metal centers, organic linkers, and synthesis conditions, MOFs can be tailored to enhance mechanical flexibility, conductivity, and electrochemical stability. Hybridizing MOFs with conductive carbon materials, such as graphene or MXene, further enhances their mechanical robustness and electrical performance. As research advances, the integration of conductive MOFs into flexible and stretchable energy storage systems is expected to drive the development of high-performance FSCs for contributing to the needs of society.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as a part of this review.
Author contributions
Sanath Kumar: conceptualization, investigation, writing – original draft. Jhansirani Kesavan: investigation, writing – original draft. Yen-Pei Fu: funding acquisition, investigation, writing – review & editing, supervision, project administration.
Conflicts of interest
There are no conflicts to declare.
Abbreviations
| AC | activated carbon |
| ACNF | activated carbon nanofiber |
| CNF | carbon nanofiber |
| CNTs | carbon nanotubes |
| Cu-TCPP | copper-tetrakis (4-carboxyphenyl) porphyrin |
| DES | deep eutectic solvent |
| DMF | dimethylformamide |
| FSCs | flexible supercapacitors |
| H3-BTC | 1,3,5-benzenetricarboxylate |
| LMX | large-sized MXene |
| MOFs | metal–organic frameworks |
| NCG-10 | NiCo-MOF/reduced graphene oxide composite |
| NiCo-BTC | nickel-cobalt-benzene tricarboxylate |
| NiCo-MOF | nickel-cobalt metal–organic framework |
| OHNM | open-hollow nickel-based MOF |
| PVA | polyvinyl alcohol |
| rGO | reduced graphene oxide |
| rOHNM-AGs | reduced open-hollow nickel MOF aerogels |
| SCs | supercapacitors |
| SUMOF | small-sized ultrathin MOF |
| TCPP | tetrakis (4-carboxyphenyl) porphyrin |
Acknowledgements
The authors would like to thank the National Science and Technology Council of Taiwan for financially supporting this research under contract number NSTC 112-2221-E-259-MY3.
References
- Z. O. U. Caineng, M. A. Feng, P. A. N. Songqi, L. I. N. Minjie, G. Zhang, B. Xiong, W. Ying, Y. Liang and Y. Zhi, Pet. Explor. Dev., 2022, 49, 468–488 CrossRef.
- P. Thakkar, S. Khatri, D. Dobariya, D. Patel, B. Dey and A. K. Singh, J. Energy Storage, 2024, 81, 110452 CrossRef.
- D. A. Elalfy, E. Gouda, M. F. Kotb, V. Bureš and B. E. Sedhom, Energy Strategy Rev., 2024, 54, 101482 CrossRef.
- S. Shalini, T. B. Naveen, D. Durgalakshmi, S. Balakumar and R. A. Rakkesh, J. Energy Storage, 2024, 86, 111260 CrossRef.
- J. Cherusseri, D. Pandey, K. S. Kumar, J. Thomas and L. Zhai, Nanoscale, 2020, 12, 17649–17662 RSC.
- K. Keum, J. W. Kim, S. Y. Hong, J. G. Son, S. Lee and J. S. Ha, Adv. Mater., 2020, 32, 2002180 CrossRef CAS PubMed.
- J. Yan, T. Liu, X. Liu, Y. Yan and Y. Huang, Coord. Chem. Rev., 2022, 452, 214300 CrossRef CAS.
- N. Khalil, N. Nadeem, M. Zahid, Z. A. Rehan and U. Zubair, Synth. Met., 2024, 301, 117521 CrossRef CAS.
- C. V. V. M. Gopi, R. Vinodh, S. Sambasivam, I. M. Obaidat and H.-J. Kim, J. Energy Storage, 2020, 27, 101035 CrossRef.
- A. He, J. He, L. Cao, J. Chen, B. Cheng, R. Ma, Y. Ding, G. Yang and F. Yi, Adv. Mater. Technol., 2024, 2301931 CrossRef CAS.
- Y. Anil Kumar, S. Vignesh, T. Ramachandran, A. M. Fouda, H. H. Hegazy, M. Moniruzzaman and T. Hwan Oh, J. Ind. Eng. Chem., 2025, 145, 191–215 CrossRef CAS.
- B. H. Xiao, K. Xiao, J. X. Li, C. F. Xiao, S. Cao and Z. Q. Liu, Chem. Sci., 2024, 15, 11229–11266 RSC.
- B. Li, M. Yu, Z. Li, C. Yu, H. Wang and Q. Li, Adv. Funct. Mater., 2022, 32, 2201166 CrossRef CAS.
- C. Liu, J. Wang, J. Wan and C. Yu, Coord. Chem. Rev., 2021, 432, 213743 CrossRef CAS.
- J. Chi, Q. Li, L. Wei, R. Shi, X. Liu, Q. Zhang, K. Liu, Z. Li, Z. Xiao and L. Wang, Adv. Funct. Mater., 2025, 35, 2413546 CrossRef CAS.
- Z. A. Sandhu, M. A. Raza, N. S. Awwad, H. A. Ibrahium, U. Farwa, S. Ashraf, A. Dildar, E. Fatima, S. Ashraf and F. Ali, Mater. Adv., 2023, 5, 30–50 RSC.
- Y. Ji, W. Li, Y. You and G. Xu, Chem. Eng. J., 2024, 496, 154009 CrossRef CAS.
- E. Erçarıkcı, K. Dağcı Kıranşan and E. Topçu, Energy Fuels, 2022, 36, 1735–1745 CrossRef.
- T. Kshetri, D. D. Khumujam, T. I. Singh, Y. S. Lee, N. H. Kim and J. H. Lee, Chem. Eng. J., 2022, 437, 135338 CrossRef CAS.
- A. Shahzad, F. Ahmad, S. Atiq, M. Saleem, O. Munir, M. A. Khan, S. M. Bin Arif, Q. U. Ain, S. Sarwar and M. Asim, J. Energy Storage, 2024, 87, 111447 CrossRef.
- T. Deng, Y. Lu, W. Zhang, M. Sui, X. Shi, D. Wang and W. Zheng, Adv. Energy Mater., 2018, 8, 1870030 CrossRef.
- A. D. Salunkhe, P. S. Pawar, P. K. Pagare and A. P. Torane, Electrochim. Acta, 2024, 477, 143745 CrossRef CAS.
- H. Chen, Y. Huo, K. Cai and Y. Teng, Synth. Met., 2021, 276, 116761 CrossRef CAS.
- U. Aziz, M. Z. Iqbal, S. Aftab, S. M. Wabaidur and M. J. Iqbal, Energy Fuels, 2023, 37, 6248–6256 CrossRef CAS.
- X. Wang, Y. Wang, Y. Kang, B. Yao and X. Peng, Nanoscale, 2023, 15, 15626–15634 RSC.
- R. Bhosale, S. Bhosale, P. Kumbhar, D. Narale, R. Ghaware, C. Jambhale and S. Kolekar, New J. Chem., 2023, 47, 6749–6758 RSC.
- J. Xu, S. Chen, N. Han, Z. Chen, Y. Jing, Y. Zhang and P. Bing, Electrochim. Acta, 2024, 507, 145141 CrossRef CAS.
- W. Zhang, Z. Cao, Y. Li, R. Li, Y. Zheng, P. Su and X. Guo, Nanoscale, 2024, 16, 9516–9524 RSC.
- X. Zha, L. Shi and Y. Yang, J. Energy Storage, 2023, 60, 106578 CrossRef.
- Y. A. Kumar, R. M. N. Kalla, T. Ramachandran, A. M. Fouda, H. H. Hegazy, M. Moniruzzaman and J. Lee, J. Ind. Eng. Chem., 2025, 145, 216–233 CrossRef CAS.
- Y. A. Kumar, G. R. Reddy, T. Ramachandran, D. K. Kulurumotlakatla, H. S. M. Abd-Rabboh, A. A. A. Hafez, S. S. Rao and S. W. Joo, J. Energy Storage, 2024, 80, 110303 CrossRef.
- S. Kanthasamy, T. Doulassiramane, R. Padmanaban and S. Thangavelu, ACS Appl. Energy Mater., 2024, 7, 9525–9542 CrossRef CAS.
- M. Jiang, D. Jiang, X. Cao, J. Wang, Y. Sun, M. Zhang and J. Liu, Adv. Funct. Mater., 2024, 34, 2312692 CrossRef CAS.
- Y. Ji, W. Li, Y. You and G. Xu, Colloids Surf., A, 2024, 699, 134680 CrossRef CAS.
- Y. A. Kumar, J. K. Alagarasan, T. Ramachandran, M. Rezeq, M. A. Bajaber, A. A. Alalwiat, M. Moniruzzaman and M. Lee, J. Energy Storage, 2024, 86, 111119 CrossRef.
- B. Diao, C. Cong, F. Sun, H. Li, H. Zhang, X. Wang, M. Jin, S. Lim, X. Li and S. H. Kim, Chem. Eng. J., 2024, 500, 156993 CrossRef CAS.
- M. Singh, A. Gupta, P. Saharan, C. Kumar, S. Sundriyal, R. Padhye, T. Daeneke, N. R. Choudhary and S. R. Dhakate, J. Energy Storage, 2023, 67, 107617 CrossRef.
- Z. Abbas and S. M. Mobin, J. Mater. Chem. A, 2024, 12, 32937–32946 RSC.
- E. Erçarıkcı, E. Topçu and K. D. Kıranşan, J. Mater. Chem. C, 2023, 11, 9593–9601 RSC.
- H. Zong, A. Zhang, J. Dong, Y. He, H. Fu, H. Guo, F. Liu, J. Xu and J. Liu, Chem. Eng. J., 2023, 475, 146088 CrossRef CAS.
- T. Chen, T. Shen, Y. Wang, Z. Yu, W. Zhang, Y. Zhang, Z. Ouyang, Q. Cai, Y. Ji and S. Wang, ACS Omega, 2023, 8, 10888–10898 CrossRef CAS PubMed.
- H. Zhang, J. Wang, Y. Sun, X. Zhang, H. Yang and B. Lin, J. Alloys Compd., 2021, 879, 160423 CrossRef CAS.
- Y. Du, R. Liang, J. Wu, Y. Ye, S. Chen, J. Yuan, J. Chen and P. Xiao, RSC Adv., 2022, 12, 5910–5918 RSC.
- B. Li, R. Zhao, H. Yue, Y. Zhang, S. Lu, W. Shang, Z. Zhang and Y. Wen, J. Alloys Compd., 2025, 179340 CrossRef CAS.
- C. Chu, W. Zhang, X. Yan, Y. Yan, J. Pan, Z. Shahnavaz and J. M. Moradian, J. Mater. Chem. C, 2025, 13, 743–757 RSC.
- J. Liu, S. Lu, L. Wang, F. Xie, W. Shang and Y. Wen, J. Energy Storage, 2023, 68, 107885 CrossRef.
- U. Zahid and F. Kashif, Energy Fuels, 2025, 39(5), 2821–2833 CrossRef CAS.
- L. Yue, L. Chen, X. Wang, D. Lu, W. Zhou, D. Shen, Q. Yang, S. Xiao and Y. Li, Chem. Eng. J., 2023, 451, 138687 CrossRef CAS.
- J. Wei, F. Hu, Y. Pan, C. Lv, L. Bian and Q. Ouyang, Chem. Eng. J., 2024, 481, 148793 CrossRef CAS.
- M. W. Ahmad, A. Choudhury, B. Dey, S. Anand, A. K. A. Al Saidi, G. H. Lee and D.-J. Yang, J. Energy Storage, 2022, 55, 105484 CrossRef.
- N. Hussain, Z. Abbas, K. Nabeela and S. M. Mobin, J. Mater. Chem. A, 2024, 12, 17642–17650 RSC.
- T. Mohammadi, M. G. Hosseini, Z. Sadeghi, E. Pastor and K. Wang, J. Energy Storage, 2025, 110, 115288 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  *
*


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 maintained non-flammability and biodegradability. From the perspective of electrochemical performance, Cu-TCPP-DES showed an area-specific capacitance of 81.3 mF cm−2 at 0.1 mA cm−2. Further, the microsupercapacitor fabricated delivered an energy density of 45.17 μW h cm−2 at a power density of 8.559 mW cm−2. Moreover, performance remained consistent with different deformation, which demonstrated outstanding flexibility and reliability for wearable applications.25 Hence, this work paved the way for excellent FSCs with the leveraged MOF-based quasi-solid-state electrolyte and gave a foundation for exploring further electrolyte systems with scalable and durable FSCs. Advanced electrode material development is necessary for the efficiency enhancement of FSC performance. In a particular study by Bhosale et al., they presented Ni-MOF synthesized by a simple, cost-effective reflux condensation method with H3-BTC as the organic linker; this ensured a reduction in reaction time and obtained uniform nano-rod-like morphology. The employed plate number 1 resulted in a porous structure with a surface area of 398.4 m2 g−1. This structure demonstrated efficient electrolyte accessibility and impressive electrochemical performance, achieving a specific capacitance of up to 1956.3 F g−1 in an alkaline electrolyte solution of 2 M KOH. Furthermore, it displayed charge–discharge stability of 81.3% after 3000 cycles, with low resistance from the developed Ni-MOF electrodes, and demonstrated improved electron transport within the electrode material. Hence, Ni-MOF is an efficient FSC electrode, and when combined with AC as a negative electrode, as shown in Fig. 2d, it has the advantages of the developed FSC combination. For the practical application, the validated FSCs delivered a specific energy of 98.15 W h kg−1 at a power density of 1253.47 W kg−1, with the FSC's photographic image shown in Fig. 2e. Notably, the device performed well with stability retention of 99.29% of its initial capacitance over 3000 cycles, indicating exceptional durability.26 This developed study highlighted the potential of Ni-MOF, paving the way for its integration into wearable technologies.
6 maintained non-flammability and biodegradability. From the perspective of electrochemical performance, Cu-TCPP-DES showed an area-specific capacitance of 81.3 mF cm−2 at 0.1 mA cm−2. Further, the microsupercapacitor fabricated delivered an energy density of 45.17 μW h cm−2 at a power density of 8.559 mW cm−2. Moreover, performance remained consistent with different deformation, which demonstrated outstanding flexibility and reliability for wearable applications.25 Hence, this work paved the way for excellent FSCs with the leveraged MOF-based quasi-solid-state electrolyte and gave a foundation for exploring further electrolyte systems with scalable and durable FSCs. Advanced electrode material development is necessary for the efficiency enhancement of FSC performance. In a particular study by Bhosale et al., they presented Ni-MOF synthesized by a simple, cost-effective reflux condensation method with H3-BTC as the organic linker; this ensured a reduction in reaction time and obtained uniform nano-rod-like morphology. The employed plate number 1 resulted in a porous structure with a surface area of 398.4 m2 g−1. This structure demonstrated efficient electrolyte accessibility and impressive electrochemical performance, achieving a specific capacitance of up to 1956.3 F g−1 in an alkaline electrolyte solution of 2 M KOH. Furthermore, it displayed charge–discharge stability of 81.3% after 3000 cycles, with low resistance from the developed Ni-MOF electrodes, and demonstrated improved electron transport within the electrode material. Hence, Ni-MOF is an efficient FSC electrode, and when combined with AC as a negative electrode, as shown in Fig. 2d, it has the advantages of the developed FSC combination. For the practical application, the validated FSCs delivered a specific energy of 98.15 W h kg−1 at a power density of 1253.47 W kg−1, with the FSC's photographic image shown in Fig. 2e. Notably, the device performed well with stability retention of 99.29% of its initial capacitance over 3000 cycles, indicating exceptional durability.26 This developed study highlighted the potential of Ni-MOF, paving the way for its integration into wearable technologies.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, demonstrating exceptional stability.27 The study emphasized the benefits of integrating Ov and bimetallic active sites for redox kinetic enhancements, electrical conductivity, and active site utilization.
000 cycles, demonstrating exceptional stability.27 The study emphasized the benefits of integrating Ov and bimetallic active sites for redox kinetic enhancements, electrical conductivity, and active site utilization.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The developed binder-free method ensured the adhesion of nanosheets onto the conductive substrate via C–C bond creation of a stable and organized structure, where it enhanced active site exposure. An electrochemical test with a three-electrode setup in alkaline electrolyte delivered a performance of NiCo-BTC with a specific capacity of 644.4C g−1 at a current density of 0.5 A g−1, significantly surpassing the capacities of single-metal MOFs like Ni-BTC and Co-BTC. The developed NiCo-BTC electrode also showed minimal resistance and enhanced ionic diffusion led by the synergistic effect of bimetallic ions and oriented nanosheet morphology. When the FSC is fabricated, the NiCo-BTC/CC electrode is paired with an activated carbon cathode, and their cyclic performance is shown in Fig. 4d. The developed activated carbon electrode exhibited electrical double-layer characteristics in the voltage range of −1.2 V to 0 V with a rectangular cyclic curve. The fabricated FSC showed the operating voltage extended until 1.8 V, as displayed in Fig. 4e, and the cyclic curve slightly showed the polarization at 2 V, limiting its working voltage to 1.8 V. The asymmetric FSC delivered an energy density of 32.4 W h kg−1 at a power density of 346 W kg−1. The device maintained 91.2% of its capacity after 8000 cycles, demonstrating exceptional cycling stability, as shown in Fig. 4f. Hence, the fabricated FSCs are viable for flexible and wearable applications and perform consistently under mechanical bending.29 This study highlighted the promise of bimetallic MOF in advanced energy storage technology, particularly in portable devices.
2. The developed binder-free method ensured the adhesion of nanosheets onto the conductive substrate via C–C bond creation of a stable and organized structure, where it enhanced active site exposure. An electrochemical test with a three-electrode setup in alkaline electrolyte delivered a performance of NiCo-BTC with a specific capacity of 644.4C g−1 at a current density of 0.5 A g−1, significantly surpassing the capacities of single-metal MOFs like Ni-BTC and Co-BTC. The developed NiCo-BTC electrode also showed minimal resistance and enhanced ionic diffusion led by the synergistic effect of bimetallic ions and oriented nanosheet morphology. When the FSC is fabricated, the NiCo-BTC/CC electrode is paired with an activated carbon cathode, and their cyclic performance is shown in Fig. 4d. The developed activated carbon electrode exhibited electrical double-layer characteristics in the voltage range of −1.2 V to 0 V with a rectangular cyclic curve. The fabricated FSC showed the operating voltage extended until 1.8 V, as displayed in Fig. 4e, and the cyclic curve slightly showed the polarization at 2 V, limiting its working voltage to 1.8 V. The asymmetric FSC delivered an energy density of 32.4 W h kg−1 at a power density of 346 W kg−1. The device maintained 91.2% of its capacity after 8000 cycles, demonstrating exceptional cycling stability, as shown in Fig. 4f. Hence, the fabricated FSCs are viable for flexible and wearable applications and perform consistently under mechanical bending.29 This study highlighted the promise of bimetallic MOF in advanced energy storage technology, particularly in portable devices.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, and the FSC with Cu MOF/D-MXene as the cathode and AC as the anode with PAV-KOH gel as the separator achieved an energy density of 77.18 W h kg−1 at a power density of 748.9 W kg−1.32 Hence, this work makes way for breakthroughs in FSCs for high-performance, durable, and adaptive energy solutions. The other research work led by Jiang et al. addressed the challenges of balancing mechanical flexibility and energy storage capacity in wearable supercapacitors through an innovative 2D/2D assembly of ultrathin NiCo-BDF-MOF and MXene sheets. Further, it highlighted the potential of scaling up to real-world applications. The composites were prepared with the combination of small-sized ultrathin MOF (SUMOF) sheets with large-sized MXene (LMX) sheets, and electrodes were prepared through a blade-coating process on pre-stretched elastomer substrates. The prepared composite exhibited electrostatic interaction between positively-charged SUMOF and negatively-charged LMX, which enabled uniform layer-by-layer assembly and displayed a wavelike structure, strengthening the flexibility of the prepared electrode. From the developed electrodes, electrochemical analysis with optimized loading of LMX (30 wt%) on SUMOF revealed excellent conductivity (3244 S cm−1) and a high specific capacitance of 1238 F g−1. The optimized composition with layered architecture showed an excellent trade-off between energy storage and mechanical strength, owing to the enhanced electron transfer and active site exposure. The schematic illustration of SUMOF/LMX architecture with 20 wt%, 30 wt%, and 40 wt% is shown in Fig. 5a–c, respectively. Lower LMX content showed ordered layer arrangements with poor mechanical performance, and with further addition of LMX to SUMOF, there was improved interaction between LMX and SUMOF, leading to fewer gaps in the composite film. With regard to the further increase in 40 wt% LMX, sufficient restacking was observed to form a robust film, which caused the electrode performance to deteriorate. The ASC device was fabricated with composite films as the anode and pure MXene as the cathode, separated by a PVA-KOH gel electrolyte. The device delivered an energy density of 27.2 W h kg−1 at a power density of 550 W kg−1 and maintained stable performance under 50% stretching and 180° bending. The fabricated ASC showed excellent mechanical and electrochemical stability under deformation, maintaining 87.4% of its initial capacitance after 10
000 cycles, and the FSC with Cu MOF/D-MXene as the cathode and AC as the anode with PAV-KOH gel as the separator achieved an energy density of 77.18 W h kg−1 at a power density of 748.9 W kg−1.32 Hence, this work makes way for breakthroughs in FSCs for high-performance, durable, and adaptive energy solutions. The other research work led by Jiang et al. addressed the challenges of balancing mechanical flexibility and energy storage capacity in wearable supercapacitors through an innovative 2D/2D assembly of ultrathin NiCo-BDF-MOF and MXene sheets. Further, it highlighted the potential of scaling up to real-world applications. The composites were prepared with the combination of small-sized ultrathin MOF (SUMOF) sheets with large-sized MXene (LMX) sheets, and electrodes were prepared through a blade-coating process on pre-stretched elastomer substrates. The prepared composite exhibited electrostatic interaction between positively-charged SUMOF and negatively-charged LMX, which enabled uniform layer-by-layer assembly and displayed a wavelike structure, strengthening the flexibility of the prepared electrode. From the developed electrodes, electrochemical analysis with optimized loading of LMX (30 wt%) on SUMOF revealed excellent conductivity (3244 S cm−1) and a high specific capacitance of 1238 F g−1. The optimized composition with layered architecture showed an excellent trade-off between energy storage and mechanical strength, owing to the enhanced electron transfer and active site exposure. The schematic illustration of SUMOF/LMX architecture with 20 wt%, 30 wt%, and 40 wt% is shown in Fig. 5a–c, respectively. Lower LMX content showed ordered layer arrangements with poor mechanical performance, and with further addition of LMX to SUMOF, there was improved interaction between LMX and SUMOF, leading to fewer gaps in the composite film. With regard to the further increase in 40 wt% LMX, sufficient restacking was observed to form a robust film, which caused the electrode performance to deteriorate. The ASC device was fabricated with composite films as the anode and pure MXene as the cathode, separated by a PVA-KOH gel electrolyte. The device delivered an energy density of 27.2 W h kg−1 at a power density of 550 W kg−1 and maintained stable performance under 50% stretching and 180° bending. The fabricated ASC showed excellent mechanical and electrochemical stability under deformation, maintaining 87.4% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, as shown in Fig. 5d. It also retained structural integrity and reliable electrochemical behavior after extensive cycling, making it a promising candidate for wearable applications.33 A work by Ji et al. presented the synthesis and electrochemical evaluation of bimetallic MOF integrated with Ti3C2Tx MXene. For electrode development, they chose a combination of two different ions and combined them with MXene. The final step in the synthesis procedure involved a hydrothermal process with the presence of metal precursors such as (Ni(NO3)2, Co(NO3)2, Cu(NO3)2) in DMF with the BTC ligand in the presence of MXene. The resulting composites exhibited distinct morphologies, with Co2+ and Cu2+ forming stronger bonds with MXene, while Ni2+ preferred ligand coordination. The detailed synthesis procedure is shown in Fig. 5e. The optimized electrode, which was a combination of NiCo-MOF/MXene, achieved a specific capacitance of 1493.6 F g−1 at 1 A g−1 in an alkaline electrolyte, and their energy storage mechanism primarily involves faradaic redox reactions of Co2+/Co3+ and Ni2+/Ni3+, along with OH− adsorption/desorption processes. Moreover, MXene's stacked layer produced a synergistic effect to provide mechanical flexibility and The study demonstrates the structural optimization and compensation effect of different metal ions, proving their impact on electrochemical performance. The FSC was fabricated using Ni1Co1@MXene as the positive electrode and activated carbon as the negative electrode, which was fabricated by employing PVA-KOH as a gel electrolyte. The device demonstrated an energy density of 73.9 W h kg−1 at a power density of 750 W kg−1. The fabricated FSC was found to be stable and retained 81% of its initial capacity even after 10
000 cycles, as shown in Fig. 5d. It also retained structural integrity and reliable electrochemical behavior after extensive cycling, making it a promising candidate for wearable applications.33 A work by Ji et al. presented the synthesis and electrochemical evaluation of bimetallic MOF integrated with Ti3C2Tx MXene. For electrode development, they chose a combination of two different ions and combined them with MXene. The final step in the synthesis procedure involved a hydrothermal process with the presence of metal precursors such as (Ni(NO3)2, Co(NO3)2, Cu(NO3)2) in DMF with the BTC ligand in the presence of MXene. The resulting composites exhibited distinct morphologies, with Co2+ and Cu2+ forming stronger bonds with MXene, while Ni2+ preferred ligand coordination. The detailed synthesis procedure is shown in Fig. 5e. The optimized electrode, which was a combination of NiCo-MOF/MXene, achieved a specific capacitance of 1493.6 F g−1 at 1 A g−1 in an alkaline electrolyte, and their energy storage mechanism primarily involves faradaic redox reactions of Co2+/Co3+ and Ni2+/Ni3+, along with OH− adsorption/desorption processes. Moreover, MXene's stacked layer produced a synergistic effect to provide mechanical flexibility and The study demonstrates the structural optimization and compensation effect of different metal ions, proving their impact on electrochemical performance. The FSC was fabricated using Ni1Co1@MXene as the positive electrode and activated carbon as the negative electrode, which was fabricated by employing PVA-KOH as a gel electrolyte. The device demonstrated an energy density of 73.9 W h kg−1 at a power density of 750 W kg−1. The fabricated FSC was found to be stable and retained 81% of its initial capacity even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, and coulombic efficiency was found to be 97%, as shown in Fig. 5f. FSC demonstration towards potential practical application was done with a series of connected FSCs, which were able to power blue and red LEDs under various bending conditions, as illustrated in Fig. 5f. This provides the flexible device's application potential. Hence, the mechanical flexibility of Ni1Co1-MOF @ MXene-based FSCs has been confirmed through various bending conditions, which makes it suitable for wearable electronics and next-generation storage devices.34
000 cycles, and coulombic efficiency was found to be 97%, as shown in Fig. 5f. FSC demonstration towards potential practical application was done with a series of connected FSCs, which were able to power blue and red LEDs under various bending conditions, as illustrated in Fig. 5f. This provides the flexible device's application potential. Hence, the mechanical flexibility of Ni1Co1-MOF @ MXene-based FSCs has been confirmed through various bending conditions, which makes it suitable for wearable electronics and next-generation storage devices.34

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles under different bending angles and stretching conditions.33 (e) The synthesis processes of MxNy-MOF@MXene composites. (f) Stability of the FSC, including constant current charge–discharge cycling data and illustrations of two devices in series lighting up blue and red LED lights.34 Reproduced with permission from ref. 33. (a–d) Copyright © 2024, John Wiley and Sons, ref. 34. (e and f) Copyright © 2024, Elsevier.
000 cycles under different bending angles and stretching conditions.33 (e) The synthesis processes of MxNy-MOF@MXene composites. (f) Stability of the FSC, including constant current charge–discharge cycling data and illustrations of two devices in series lighting up blue and red LED lights.34 Reproduced with permission from ref. 33. (a–d) Copyright © 2024, John Wiley and Sons, ref. 34. (e and f) Copyright © 2024, Elsevier.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, confirming its long-term stability, with a practical demonstration of LEDs glowing as shown in Fig. 6f. Hence, Cu-MOF@ACNF demonstrated potential for next-generation FSCs, combining high electrochemical performance, mechanical flexibility, and sustainability.37 As MOFs can be prepared with different organic linkers, Abbas et al. developed a π–d conjugated metal–organic framework with a benzene tetramine ligand and Ni ions as metallic, active sites. To establish a freestanding film for FSCs, Ni-MOF was applied to an electrospun mat made of MXene-CNF. The material synthesis involved three steps: first, the preparation of MXene; second, the creation of the MXene-CNF mat; and finally, the in situ growth of Ni-MOF on the MXene-CNF mat. The developed material combination showed higher conductivity, enhanced charge transfer, and structural integrity with self-standing. It maintained a hydrophobic nature, and finally, the developed hybrid structure benefitted from integrating 1D MOF with 2D MXene-embedded 1D CNFs. These advantages led to a high electrochemical performance with a specific capacitance of 1076 F g−1 at 1 A g−1, capacitance retention of 40.8% at 20 A g−1, and cycling stability with 86.4% capacitance retention after 15
000 cycles, confirming its long-term stability, with a practical demonstration of LEDs glowing as shown in Fig. 6f. Hence, Cu-MOF@ACNF demonstrated potential for next-generation FSCs, combining high electrochemical performance, mechanical flexibility, and sustainability.37 As MOFs can be prepared with different organic linkers, Abbas et al. developed a π–d conjugated metal–organic framework with a benzene tetramine ligand and Ni ions as metallic, active sites. To establish a freestanding film for FSCs, Ni-MOF was applied to an electrospun mat made of MXene-CNF. The material synthesis involved three steps: first, the preparation of MXene; second, the creation of the MXene-CNF mat; and finally, the in situ growth of Ni-MOF on the MXene-CNF mat. The developed material combination showed higher conductivity, enhanced charge transfer, and structural integrity with self-standing. It maintained a hydrophobic nature, and finally, the developed hybrid structure benefitted from integrating 1D MOF with 2D MXene-embedded 1D CNFs. These advantages led to a high electrochemical performance with a specific capacitance of 1076 F g−1 at 1 A g−1, capacitance retention of 40.8% at 20 A g−1, and cycling stability with 86.4% capacitance retention after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Additionally, the FSC with the device configuration of MOF@MXene-CNF as the positive electrode, activated carbon as the negative electrode, and PVA/KOH gel electrolyte showed an operating voltage of 1.4 V with an energy density of 45.7 W h kg−1 at a power density of 719 W kg−1 and at various bending shapes the cyclic voltammogram curves remained similar.38 Hence, this kind of material configuration is highlighted by integrating a 1D–2D–1D hybrid strategy for next-generation flexible energy storage devices, offering high performance, stability, and practical applicability.
000 cycles. Additionally, the FSC with the device configuration of MOF@MXene-CNF as the positive electrode, activated carbon as the negative electrode, and PVA/KOH gel electrolyte showed an operating voltage of 1.4 V with an energy density of 45.7 W h kg−1 at a power density of 719 W kg−1 and at various bending shapes the cyclic voltammogram curves remained similar.38 Hence, this kind of material configuration is highlighted by integrating a 1D–2D–1D hybrid strategy for next-generation flexible energy storage devices, offering high performance, stability, and practical applicability.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, which makes it an excellent electrode for FSCs. Additionally, the FSC fabricated with the configuration of MnCo-MOF/3DG as the positive electrode, 3DG as the negative electrode, and PVA-KOH as the gel electrolyte showed an operating voltage of 1.5 V and maintained FSC performance even at different bending angles of 180°, 90°, and 45° as shown in Fig. 7c, which make it ideal for wearable electronics. These results revealed that the developed FSCs can be used in practical SC applications with high durability and flexibility, which showed energy and power densities of 198.5 W h kg−1 and 5823 W kg−1, respectively, as shown in Fig. 7d. Hence, the robustness and mechanical stability of the developed MnCo-MOF/3DG electrodes make them a promising candidate for next-generation FSCs.39
000 cycles, which makes it an excellent electrode for FSCs. Additionally, the FSC fabricated with the configuration of MnCo-MOF/3DG as the positive electrode, 3DG as the negative electrode, and PVA-KOH as the gel electrolyte showed an operating voltage of 1.5 V and maintained FSC performance even at different bending angles of 180°, 90°, and 45° as shown in Fig. 7c, which make it ideal for wearable electronics. These results revealed that the developed FSCs can be used in practical SC applications with high durability and flexibility, which showed energy and power densities of 198.5 W h kg−1 and 5823 W kg−1, respectively, as shown in Fig. 7d. Hence, the robustness and mechanical stability of the developed MnCo-MOF/3DG electrodes make them a promising candidate for next-generation FSCs.39

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles)
000 cycles)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) @ 3 mA cm−2
@ 3 mA cm−2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles)
000 cycles)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles)
000 cycles)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles)
000 cycles)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles)
000 cycles)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles)
000 cycles)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles)
000 cycles)