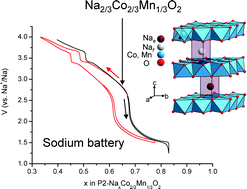
Manganese substituted sodium cobaltate, Na2/3Co2/3Mn1/3O2, with a layered hexagonal structure (P2-type) was obtained by a co-precipitation method followed by a heat treatment at 950 °C. Powder X-ray diffraction analysis revealed that the phase is pure in the absence of long-range ordering of Co and Mn ions in the slab or Na+ and vacancy in the interslab space. The oxidation states of the transition metal ions were studied by magnetic susceptibility measurements, electron paramagnetic resonance (ESR) and 23Na magic angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy. The charge compensation is achieved by the stabilization of low-spin Co3+ and Mn4+ ions. The capability of Na2/3Co2/3Mn1/3O2 to intercalate and deintercalate Na+ reversibly was tested in electrochemical sodium cells. It appears that the P2 structure is maintained during cycling, the cell parameter evolution versus the sodium amount is given. From the features of the cycling curve the formation of an ordered phase for the Na0.5Co2/3Mn1/3O2 composition is expected.