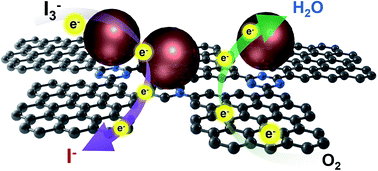
Platinum (Pt) nanoparticles were stably anchored on triazine-functionalized graphene nanoplatelets (TfGnPs), which were prepared by a two-step reaction starting from carboxylic acid- (CGnPs), acyl chloride- (AcGnPs) and amide-functionalized graphene nanoplatelets (AfGnPs). The resulting Pt nanoparticles on TfGnPs (Pt/TfGnPs) exhibited outstanding electrocatalytic activity with significantly enhanced stability compared with commercial Pt-based catalysts for the oxygen reduction reaction (ORR) in fuel cells (FCs) and the iodine reduction reaction (IRR) in dye-sensitized solar cells (DSSCs). For the ORR in FCs, the onset and half-wave potentials of Pt/TfGnPs under acidic conditions displayed greater positive shifts to 0.58 and 0.53 V, respectively, than those of the commercial Pt/C catalyst (0.57 and 0.52 V). For the IRR in DSSCs, Pt/TfGnPs displayed a reduced charge transfer resistance (Rct) of 0.13 Ω cm2 at the CE/electrolyte interface. This value was much lower than the Pt CE of 0.52 Ω cm2. More importantly, Pt/TfGnPs exhibited profoundly improved electrochemical stability in both the ORR and IRR compared to the Pt-based catalysts. The combination of extraordinarily high electrocatalytic activity with stability could be attributed to the high specific surface area (963.0 m2 g−1) and the triazine units of the TfGnPs, respectively, which provided more active sites and stably anchored the Pt nanoparticles.