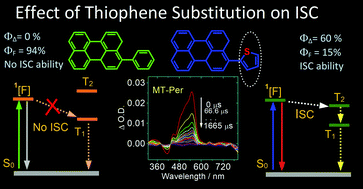
The effect of thienyl substitution on the intersystem crossing (ISC) of a few arenes was studied using steady state and time-resolved transient absorption and emission spectroscopies, as well as DFT/TDDFT computations. We found that the phenyl and thienyl substituents generally induce red-shifted absorptions for the chromophores, and the DFT/TDDFT computations show that the red-shifted absorption and emission are due to the increased HOMO and the reduced LUMO energy levels. Nanosecond transient absorption spectra indicate the formation of a triplet state, the triplet state lifetime is up to 282 μs, and the singlet oxygen quantum yields (ΦΔ) are up to 60%. DFT/TDDFT computations indicate that introducing the thienyl substituent alters the relative singlet/triplet excited state energy levels, and the energy level-matched S1/T2 states are responsible for the enhanced ISC of the thienyl compounds. This information is useful for the design of heavy atom-free triplet photosensitizers and for the study of the fundamental photochemistry of organic compounds.