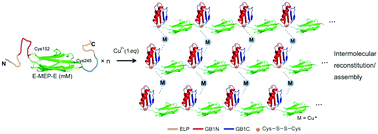
An inducible protein assembly system is desirable for developing high-order biomolecular architectures with dynamic properties. Here we demonstrate the creation of molecular networks with distinct stress-relaxation behavior using metal-induced protein assembly—a process that involves the folding and reconstitution of a pair of split IgG-binding GB1 proteins. In addition, metal–ligand coordination within the protein networks exerted great influence over their viscoelastic properties. The resulting protein networks are self-healable, amenable to biochemical decoration via SpyTag/SpyCatcher chemistry, and compatible with 3D culture of fibroblasts. This study points to a simple and robust strategy for designing recombinant protein hydrogels with tunable biochemical and mechanical properties.