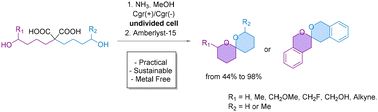
Spiroketals are important structural motifs found in natural products, pharmaceuticals, and agrochemicals. However, their synthesis often requires hazardous reagents and harsh conditions, limiting their accessibility. Here, we present eSpiro, a novel electrosynthetic method for the efficient and sustainable synthesis of spiroketals via anodic oxidation of malonic acids. This approach offers a metal- and mercury-free alternative to conventional acid-catalysed or transition metal-mediated cyclisations. The reaction proceeds through a sequential Hofer-Moest decarboxylation, followed by Brønsted acid-mediated cyclisation, achieving high yields with broad functional group tolerance. We further explore the reaction scope and demonstrate its scalability, achieving up to 98% yield in batch. Additionally, we investigate a flow electrolysis setup, highlighting key challenges such as substrate stability, in-line solvent system switch and gas evolution, and also demonstrating preliminary success in integrating electrochemical oxidation with downstream acid-catalysed cyclisation. This work provides a practical and eco-friendly route to spiroketals, with potential for industrial applications in organic synthesis.