Dattatraya H. Dethe and Manmohan Shukla
Chem. Commun., 2021,57, 10644-10646
DOI:
10.1039/D1CC04739F,
Communication
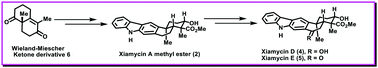
The enantioselective first total syntheses of marine pentacyclic indolosesquiterpenoids xiamycins D (4) and E (5) have been described for the first time to the best of our knowledge. The synthetic approach was designed to feature functionalization of enantiopure Wieland–Miescher ketone, Michael addition followed by Heck-type annulation/aromatization, regioselective sp3(C–H) activation, benzylic oxidation and diastereoselective reduction.