Juan García de la Concepción, Martín Ávalos, Pedro Cintas, José L. Jiménez and Mark E. Light
Org. Biomol. Chem., 2018,16, 4778-4783
DOI:
10.1039/C8OB01195H,
Communication
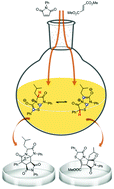
A mesoionic bicycle, easily synthesized from a proteinogenic amino acid, L-leucine, behaves as both thiazolium-olate and diazolium-olate dipoles, as unveiled by its dipolar cycloadditions. This chameleonic reactivity has been thoroughly interpreted by dissecting the mechanistic landscape aided by the distortion–interaction model.