Bioinspired colloidal crystal hydrogel pressure sensors with Janus wettability for uterus cervical canal tension perception†
Yufei
Chen‡
a,
Yuan
Zhou‡
b,
Lihao
Zhang
a,
Yue
Cao
a,
Sunlong
Li
a,
Weipeng
Lu
a,
Zheng
Mao
a,
Zhiwei
Jiang
a,
Ying
Wang
a,
Cihui
Liu
 *a and
Qian
Dong
*cde
*a and
Qian
Dong
*cde
aCenter for Future Optoelectronic Functional Materials, School of Computer and Electronic Information/School of Artificial Intelligence, Nanjing Normal University, Nanjing 210023, China. E-mail: cihui@njnu.edu.cn
bDepartment of Critical Care Medicine, Shanghai General Hospital, Shanghai Jiaotong University, School of Medicine, 650 Xinsongjiang Rd, Shanghai 201620, China
cDepartment of Obstetrics and Gynecology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China. E-mail: dongqian95fy@126.com
dShanghai Key Laboratory of Gynecologic Oncology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China
eState Key Laboratory of Systems Medicine for Cancer, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China
First published on 7th August 2024
Abstract
The pursuit of flexible, sensitive, and cost-effective pressure sensors plays a pivotal role in medical diagnostics, particularly in the domain of cervical health monitoring. However, significant challenges remain in the economical production of flexible piezoresistive materials and the integration of microstructures aimed at enhancing sensor sensitivity. This urge highlights the use of innovative, stable hydrogel films that demonstrate robust adherence to soft biological tissues, thereby enabling prolonged bio-signal monitoring. In this study, we introduce an innovative integration of a flexible pressure electrical signal sensor with structural color hydrogel scaffolds. This integration leverages the tunability of the inverse opal structure to fine-tune the scaffold's adherence to the endocervical wall under varying environmental conditions and to amplify the sensitivity of pressure measurements. Our findings indicate that this novel approach holds promise for substantial enhancements in the manufacturing and functional capabilities of cervical pressure sensors, potentially revolutionizing personalized medical treatments and improving patient monitoring.
1. Introduction
The quest for flexible, sensitive, and cost-effective pressure sensors has captured the attention of scientific and medical communities, particularly in the realm of cervical pressure-sensing stents.1–6 These scaffolds are designed with the purpose of detecting cervical pressure changes, thereby facilitating personalized health monitoring and improved medical treatment for women.7–9 Overcoming the challenge of achieving affordable and facile fabrication of flexible piezoresistive materials for these scaffolds remains a prominent obstacle.10–13Recent advancements in piezoresistive sensors, including those used in cardiovascular and intestinal applications, have garnered substantial scientific recognition within the field of medical scaffolds.14–17 Metals and polymers are commonly employed materials for piezoresistive sensor scaffolds.3,18,19 An ideal scaffold material for cervical tension sensors should exhibit three key characteristics: (1) strong adhesion to the inner cervix wall,20 (2) appropriate material toughness that can be adjusted to maintain cervical patency,21 and (3) easy removal from the implantation site to minimize patient discomfort.22,23 Consequently, the pursuit of cost-effective and straightforward manufacturing of flexible piezoresistive sensor materials persists as a significant challenge.24–26
Stable hydrogel films have shown promise for constructing flexible medical scaffolds capable of self-adhering to soft biological tissues, enabling continuous, long-term bio-signal monitoring.27,28 Nevertheless, the creation of pressure sensor scaffolds that can reliably adhere to human organs and sustain functionality for prolonged health monitoring remains a formidable task.29,30
Concurrently, the incorporation of microstructures plays a pivotal role in designing flexible devices to enhance signal sensing sensitivity.4,31–35 Recent research has demonstrated the effectiveness of microstructures or patterned polydimethylsiloxanes (PDMS), characterized by high elasticity and biocompatibility, in increasing the sensitivity of resistive sensors.5,6 However, the intricate design of these microstructures and the high cost associated with their conductive materials impose limitations on their widespread adoption. Furthermore, the intrinsic qualities of microstructure geometry make parameter adjustments challenging.36 Consequently, there is a pressing need for an economical and straightforward fabrication approach to produce highly sensitive pressure sensors with customizable microstructure surfaces suitable for deployment across a wide range of pressures, particularly within the human body.37–39
In response to this challenge, we have developed a flexible pressure electrical signal sensor integrated with structural color hydrogel scaffolds. The hydrogel sensor is flexible, sensitive and cost-effective. This integration enables adjustment of hydrogel scaffold adherence to the endocervical wall based on parameters such as humidity and temperature. The effect of pressure and different temperatures on the adhesion of hydrogel scaffolds is shown in Fig. S1–S3 (ESI†). Simultaneously, we have investigated variations in the sensitivity of the pressure sensor through the tunability of the inverse opal structure. This innovative and practical strategy holds the potential for significant advancements in the field of cervical pressure monitoring.
2. Results and discussion
Ensuring effective implantation and reliable sensor adhesion at the cervix is of utmost importance. Considering this, the present study draws inspiration from the lotus leaf's remarkable attributes (Fig. 1A), characterized by its dual hydrophilic and self-cleaning properties on both upper and lower surfaces. Consequently, this research employs a binary synergistic approach, combining hydrophobic and hydrophilic characteristics, to craft cervical pressure sensors with Janus wettability and functional Janus hydrogel scaffolds endowed with adjustable structural colors. The fundamental principles and critical attributes of a multi-functional cervical pressure-sensing stent, encompassing stress detection and selective adhesion capabilities, are elucidated in Fig. 1. The sensing catheter is engineered with a flexible structure designed to accommodate substantial deformations, such as longitudinal pressure and bending, thus mitigating potential risks associated with invasive medical interventions (Fig. 1B). The components of the cervical pressure sensor, as delineated in Fig. 1C, are an elastic, hollow, cylindrical polydimethylsiloxane (PDMS) substrate, four flexible pressure sensors for electrical signal transmission, and a P(NiPAAm-bis-AA) hydrogel film scaffold featuring Janus properties and structural color alteration on the lower surface. The P(NiPAAm-bis-AA) hydrogel film is deemed an optimal material for the fabrication of pliable medical scaffolds, primarily owing to its inverse-opal surface contact angle of 24°, signifying its hydrophilic nature. This flexible scaffold exhibits a propensity to adhere autonomously to soft biological tissues, facilitating continuous and long-term monitoring of biosignals. In contrast, PDMS, characterized by a surface contact angle of 125°, is inherently hydrophobic, endowing it with exceptional resistance to contamination and self-cleaning properties. The outstanding Janus characteristics of the sensor system ensure secure adhesion of the catheter body to the endocervical wall without causing harm to human tissue and minimizing the risk of slippage. Furthermore, the sensor system's capacity for continuous biosignal monitoring over extended durations guarantees the accurate detection of physiological events, all while minimizing patient discomfort and irritation. In light of these attributes, the combination of these features positions cervical pressure-sensing stents as a promising medical device with potential applications across a spectrum of clinical scenarios.The block diagram illustrating the operational framework of the sensor system is presented in Fig. 1D. Upon insertion of the sensor into the cervix, it exhibits the capability of dynamically modulating its adhesion state in response to varying humidity levels, thereby influencing the output signal. The sensor encompasses a pressure-sensitive unit designed for the measurement of pressure fluctuations within the cervical region. An analog representation of the cervical pressure, acquired by the flexible pressure sensor, is transmitted externally through an insulated, pliable wire. Additionally, a dual voltage system comprising reverse and forward voltage is utilized to energize a power amplification circuit. This circuit, in turn, yields an amplified electrical signal representing the pressure in the cervix. Simultaneously, as illustrated in Fig. 1E, it is noteworthy that regions of the cervical tissue where cancerous lesions are present tend to exhibit reduced moisture levels and a coarser texture. Consequently, the hydrogel scaffold is susceptible to deformations owing to fluctuations in the surrounding humidity. This, in turn, leads to alterations in the particle interstice configuration on the surface of the reverse spectral structure of the P(NiPAAm-bis-AA) hydrogel. The outcome of this process is the modification of the scaffold's color, resulting in the generation of a chromatic signal.
A comprehensive elucidation of the manufacturing process for flexible pressure sensors based on colloidal crystals hydrogel films can be found in Fig. 2A. Initially, the rGO/P(NiPAAm-bis-AA) precursor solution was meticulously prepared and stored under controlled conditions, characterized by low temperature and absence of light. Subsequently, the pre-gel solution was introduced between two hydrogel sheets, one being unadorned and the other featuring an interlaced electrode array design. Before integration, the PDMS surface underwent plasma treatment to ensure intimate contact with the pre-gel solution, facilitating robust bonding. Capillary forces then facilitated the infiltration of the pre-gel solution into the pores of the interleaved electrode pattern, followed by polymerization under ultraviolet (UV) irradiation, yielding a consolidated composite hydrogel. Capitalizing on the malleability and the elasticity of the hydrogel, the resultant sensing device manifested remarkable flexibility. To mitigate invasive discomfort, the sensing stent was meticulously crafted to be as diminutive and slender as possible, measuring 3.5 mm in width, 5 mm in length, and a mere 0.1 mm in thickness (Fig. 2B). This design objective aimed to minimize patient discomfort during the examination. Furthermore, a scanning electron microscopy image illustrating the PDMS under the overlay can be found in Fig. 2C.
The underlying principle governing the sensor's operation hinges upon the pressure-responsive interaction between the P(NiPAAm-bis-AA) composite hydrogel and the interleaved electrode array. When a hydrogel pressure sensor is subjected to external pressure, the P(NiPAAm-bis-AA) in it compresses or bends, the internal pores and mesh structure of the hydrogel changes, and the ions and molecules inside are redistributed, resulting in a change in the electrical properties between the electrode arrays, and subsequently a change in the conductivity of the hydrogel. The interleaved electrode array detects the change in conductivity and converts it into an electrical signal, so the resistance value obtained from the measurement also changes. As depicted in Fig. 2D, the three-dimensional conductive network established by the composite hydrogel and the electrode arrays exhibited stability and preserved optimal electrical conductivity, as evidenced by the unchanging resistance value. However, the application of pressure induced partial deformation of the sensor's sensitive unit, with the degree of deformation increasing in proportion to the applied pressure. This effect translated into enhanced contact between the composite hydrogel and the electrode arrays, consequently resulting in the elevation of the resistance value. Essentially, the conductive network, constituted by the composite hydrogel and the electrode array, can be conceptualized as a variable resistor (Rn), with the resistance value contingent upon the extent of network contact. As illustrated in Fig. 2E, the application of external pressure, leading to the deformation of the sensitive cells, led to an increase in ΔR between the conductive networks. Upon the release of pressure, the conductive network reverted to its initial configuration, and the resistance value returned to its original state.
A flexible pressure sensor was affixed to the surface of a polydimethylsiloxane (PDMS) hollow conduit characterized by an elastic thickness of 3 mm. The relative resistance change (ΔR/R) associated with the sensor's response to varying pressure levels was measured and calculated when the sensor was placed on different substrates. The pressure sensor has a response threshold of 0.5 kPa and the sensitivity of the pressure sensor is approximately 0.025 kPa−1. The results, depicted in Fig. 3A, reveals notable distinctions in the sensor's sensitivity when compared to a glass substrate. Specifically, at a pressure change of approximately 4 kPa, the sensor's sensitivity was enhanced by a factor of approximately 16 in comparison to its performance on a glass substrate, and this enhancement escalated to a factor of approximately 60 as the pressure reached about 8 kPa. Moreover, an exploration of the response and recovery time of the flexible pressure sensor in the presence of external pressure was undertaken to characterize the pressure-sensitive response features of the sensor. Fig. 3B and C illustrates the instantaneous resistance variations during the application and removal of an 8 kPa pressure load. The real-time step-response curves, presented in the same figures, serve as a reflection of the flexible pressure sensor's exceptional response and recovery times, demonstrating its consistent and rapid responsiveness to pressure fluctuations. The response and recovery times were determined to be 10 ms when external pressure was applied and removed, respectively.
To assess the repeatability, reliability, and stability of the flexible pressure sensor, cyclic pressure at 9 kPa was recurrently applied to the sensor to achieve 100% deformation. This process revealed that ΔR/R increased from 0 to 1.42% as the sensor reached 100% deformation. Furthermore, it was observed that the resistance stabilized upon the application of pressure and maintained remarkable consistency over multiple cycles, affirming the sensor's precision in medium-pressure range sensing, as displayed in Fig. 3D. In Fig. 3E, a load of 9 kPa is repeatedly applied 5000 times at 1 Hz, and ΔR/R varies from 0 to about 1.4% during the pressure increase, and returns to the initial state almost immediately after the pressure is released. The amplitude and waveform of each response consistently maintained a high degree of uniformity at each instance, with response variations kept within a 20% margin.
Furthermore, the sensor's response frequency was subjected to examination (Fig. 3F), revealing its capability to accurately discern load changes at different frequencies as the pressing frequency gradually increased from 0.2 Hz to 0.5 Hz and 1 Hz. This capacity to precisely detect load variations across distinct frequencies underscores the flexible pressure sensor's outstanding reliability and stability, providing a solid foundation for its application in intracervical environments.
To visually capture pressure measurements utilizing this flexible sensing system, a custom-made setup, depicted in Fig. 4A, was devised. This setup comprises stepper motors, pressure sensors, voltage amplification components, and an output display. The stepper motor guarantees a consistent and controlled application of external pressure, ranging from 0 to 10 kPa, thereby replicating the internal pressure conditions within the cervix. The pressure sensors, affixed to a flexible polydimethylsiloxane (PDMS) catheter using PDMS as an adhesive and evenly spaced at 90° intervals, are responsible for capturing pressure data. These sensors detect alterations in resistance signals, which are subsequently conveyed through copper wires to the voltage amplification module. This module serves to enhance and convert the subtle resistance variations into voltage values, subsequently exhibited as electrical signals on a monitor. The configuration of the voltage amplification circuit can be observed in Fig. 4D. Notably, this amplification can be customized to increase or decrease the ΔR/R signal by rotating the knob, with the potential to attain an amplification factor of up to 50.
Following the adjustment of amplification, an exploration of the correlation between pressure and voltage output was conducted, as demonstrated in Fig. 4B. The outcomes underscored a conspicuous alignment between the observed relationship and the pressure-sensor resistance relationship, indicating the intuitive ability of the sensing system to provide real-time assessment of pressure magnitude through voltage monitoring. It is pertinent to mention that in this study, a fixed supply voltage of 10 V, with 10-times voltage amplification, was utilized. Subsequently, a systematic variation in the magnitude of the relative resistance load value was performed, and the bidirectional pulse signals of the system were plotted over a range of relative resistance values (0.1%, 0.3%, 0.5%, 0.7%, and 0.9%), as illustrated in Fig. 4C. The figure reflects a sampling rate of 500 Hz, underscoring the system's remarkable ability to sample the ΔR/R signal effectively.
Four flexible pressure sensors were integrated into the catheter, enabling pressure sensing from four distinct directions. Simultaneous activation of the four voltage output channels on the sensors was executed to simulate the pressure conditions at the cervix. The voltage outputs of all four channels were diligently monitored over a 15-minute duration, as depicted in Fig. 4E. This approach elevates the precision of pressure detection at the cervix and facilitates continuous monitoring of physiological activity by the other three flex sensors in the case of sensor malfunction. Furthermore, the exceptional durability of the flexible pressure sensors was demonstrated at a pressure of 5 kPa and a frequency of 1 Hz, as delineated in Fig. 4F. Voltage change curves were recorded every 1000 cycles, with data logged for 200 cycles at a time. This investigation revealed minimal changes in the voltage amplitude and consistent sensitivity, affirming the system's robust stability and reliability for long-term pressure monitoring. The performance of the hydrogel sensor is compared with the previous works as shown in Fig. S4 (ESI†).
The creation of hydrogel film scaffolds was imperative to facilitate on-demand sensor adhesion at the cervical site. To construct the graphene hybrid-structured, colorized hydrogel on a counter-patterned hydrogel film, a template sacrifice method was employed (Fig. S5 and S6, ESI†). The formation of colloidal crystal templates necessitated the self-assembly of monodisperse silica nanoparticles on glass slides, culminating in the generation of densely packed arrays through evaporation, consequently yielding tightly stacked arrays with a hexagonal organization, as revealed in scanning electron microscopy (SEM) pictures (Fig. S7, ESI†). Following this, the P(NiPAAm-bis-AA) precursor solution, prepared and stored in a controlled, light-restricted environment (Fig. 2A), was extracted, and the pre-gel solution was introduced into the template. The liquid exhibited capillary action as it permeated the interconnected nanopores among the adjacent nanoparticles. Upon exposure to UV irradiation, the liquid underwent polymerization, resulting in the formation of a solidified composite hydrogel. By subsequently etching the silica template, the freestanding hydrogel film acquired an inverse opal structure and a periodic pore arrangement (Fig. S8, ESI†). This composite hydrogel membrane exhibits a unique optical attribute referred to as the photonic band gap (PBG), resulting from the periodic arrangement of its internal structure. When illuminated by incident light, light propagation experiences interference from the nanopores, causing a color shift induced by reflection. Typically, the inverse-opal structure of such hydrogel membranes generates vibrant structural colors when the irradiating light wavelength falls within the visible spectrum. For light incident perpendicular to the surface, the position of the reflection peak can be determined through the Bragg formula:
| λ = 1.633dnaverage. | (1) |
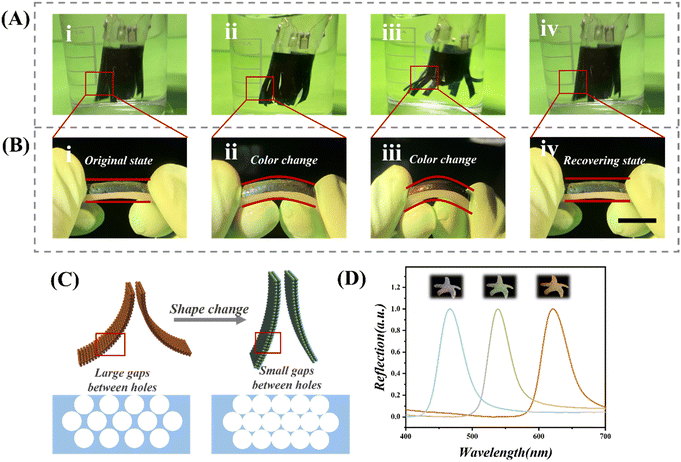
Through a series of experiments, we observed that the hydrogel scaffold displayed notable resilience while retaining a degree of softness and elasticity when the piezoresistive sensor was immersed in a liquid and subjected to repeated shaking (Fig. 5A). Furthermore, Fig. 5B illustrates the attachment of this composite hydrogel film to a wet and flexible catheter for the analysis of the optical response of the P(NiPAAm-bis-AA) hydrogel film to deformation. When subjected to mechanical forces, the composite hydrogel film undergoes deformation, leading to modifications in its internal nanostructure and, consequently, a shift in its color. In its unaltered state, the composite hydrogel film presents a blue appearance. As the film experiences slight bending, its color progressively transitions to green. With further bending angles, the hue shifts towards orange. Upon full relaxation of the deformation, the hydrogel's color reverts to its original blue state. On the one hand the change in color can help to locate specific pressure points and areas of stress. Points or areas with a significant change in color indicate greater pressure, which is important for cervical testing. The color change provides immediate visual feedback on the state of the hydrogel scaffold. This can be particularly useful during the implantation process to ensure proper placement and to monitor the scaffold's interaction with the cervical tissue. On the other hand, the color change can be used to quantitatively measure pressure based on the correspondence between the color change and the force applied. In addition to this, the color change can be used to monitor pressure changes over time, allowing healthcare professionals to monitor the pressure changes in the cervix at different points in time in real time. As the hydrogel scaffold bends or deforms under pressure, the inverse opal structure within the hydrogel changes, leading to a shift in the reflection peak wavelength and a corresponding color change. This visual cue indicates that the scaffold is responding to pressure or deformation. The schematic diagram of hydrogel film deformation is shown in Fig. 5C, when the film is bent, the distance between neighboring nanoparticle holes increases, and the wavelength of the reflection peak increases with d according to formula (1), as shown in Fig. 5D, the wavelength of the reflection peak of the film changes from 466 nm to 620 nm as the film bending angle increases.
This paper explores the investigation of a catheterized pressure sensor distinguished by its remarkable elastic properties. As illustrated in Fig. 6A, this sensor displays deformability in response to external bending forces or applied pressure, a pivotal attribute for conducting invasive measurements within the human body. More specifically, we have integrated our pressure sensor into a catheter, positioning it within the cervix for detection. The sensor's body is constructed using polydimethylsiloxane (PDMS), a material characterized by its exceptional softness and flexibility. While traversing the cervix, the composite hydrogel scaffold on the catheter closely conforms to the moist and elastic cervical wall. Even slight deformations in the cervical canal trigger discernible changes in the relative resistance of the flexible sensors on the catheter, resulting in significant shifts in the electrical signal output from the sensing system. This adaptability and sensitivity render the sensor exceptionally precise for pressure measurements within the cervix. We also investigated how temperature and acidity affected the composite hydrogel scaffold at the cervical location. As illustrated in Fig. S9 (ESI†), four portions of the composite hydrogel film of the same size and shape were intercepted and placed in an aqueous citric acid solution at pH 3–5, with the liquid temperature adjusted to 37 °C and left to stand for one week. The structure, size, and color of the composite hydrogel film did not change considerably in the cervical environment, indicating that the composite hydrogel scaffold was stable.
To recreate the surface environment of the human cervix, we used the pig colon as the surface material. Because the porcine colon has elastic and wetting qualities that are similar to those of human tissue, it was selected as a good modeling material. We employed 3D printing technology as the foundation for our modeling in order to guarantee the precision and dependability of our investigations. First, we used medical imaging methods to obtain 3D structural data of the human cervix. After being cleaned up and optimized, these data were loaded into 3D modeling programs to create models. We produced a 3D printed model that resembled the structure and form of the cervix by modifying the model's size, shape, and features. In order to make sure it fits snugly and replicates the wettability and flexibility of the cervix's surface—which was prepared in order to create an artificial cervix—we then coated the 3D printed model with pig colon. In order to accomplish this, we made sure the pig colon complied with the experimental specifications by pre-treating it with procedures like cleaning, sterilization, and cutting.
P(NiPAAm-bis-AA) hydrogels exhibit strong adhesion to the moist endocervical wall due to the humid environment in the human body, allowing the sensors to efficiently adhere to the healthy cervical wall and are less prone to ectasia. On the surface of a moist artificial cervix, we tested the adhesion of a catheter-based pressure transducer decorated with a composite hydrogel scaffold. As illustrated in Movie S1 (ESI†), the sensor was placed in a moist artificial cervix and the model was shaken up and down without the sensor becoming ectopic or displaced. A citric acid solution with a pH of 3 was utilized to replicate the passage of biological fluids on the surface of the cervix, as seen in Movie S2 (ESI†), and neither the hydrogel scaffold nor the catheter became ectopic or dislodged when the solution dripped. This shows that long-term pressure monitoring at the cervical location is possible when the catheter-based pressure sensor has the composite hydrogel scaffold securely attached to it and the cervix is in good health.
The water content and elasticity of the cervical tissue, however, gradually decrease in the case of cervical lesions, which presents a problem for the composite hydrogel scaffold's consistent adherence. The composite hydrogel scaffold firmly adheres to the surface of the wet tissue when the artificial cervix is wet, as illustrated in Fig. 6B. However, when the artificial cervix is dry, the scaffold is unable to achieve effective adhesion, making the PDMS catheter for measuring the pressure signal susceptible to anisotropy, which causes variations in the pressure output signal's amplitude and frequency. We carried out cyclic adhesion and peeling tests for the adhesion of hydrogel film scaffolds to the surface of the artificial cervix under different humidity conditions, and a total of 20 cycles were performed (Fig. S10, ESI†). The experimental results showed that the adhesion of the hydrogel film scaffold could be stabilized at a level of about 22 kPa when the surface of the artificial cervix was in the wet state, while the adhesion decreased to about 6.5 kPa when the surface of the artificial cervix was relatively dry. The transducer's test findings in cervical contexts with different levels of health are simulated in Fig. 6C. Catheter-based pressure transducers provide a convenient method for monitoring changes within the cervix and converting them into electrical signals.
In summary, the catheterized pressure sensor amalgamates remarkable elastic properties, flexibility, and sensitivity, rendering it highly suitable for real-time monitoring within the cervix. It offers valuable insights into the cervix's health status and is poised to provide robust support for future medical diagnosis and treatment.
3. Conclusion
In this study, we explored the development of a catheterized pressure sensor with exceptional elastic properties, aiming to address the challenges associated with implantation and reliable sensor adhesion within the cervix. Drawing inspiration from the lotus leaf's dual hydrophilic and self-cleaning attributes, we have employed a binary synergistic approach, combining hydrophobic and hydrophilic characteristics, to create cervical pressure sensors with Janus wettability and functional Janus hydrogel scaffolds capable of adjustable structural color changes. The operational framework of this multi-functional cervical pressure-sensing stent, which encompasses stress detection and selective adhesion capabilities, has been elaborated.4. Experimental section
Materials
The hydrogel was produced using acrylic acid (Sigma-Aldrich) and N-isopropylacrylamide (Sigma-Aldrich). N,N′-Methylene bisacrylamide (BIS, Sigma-Aldrich) and 2-hydroxy-2-methyl-1-phenyl-propanone (HMPP) were employed as a crosslinking agent and a heat initiator, respectively. The Aladdin Industrial Corporation provided hydrofluoric acid (HF). Nanjing XFNANO Materials Tech supplied reduced graphene oxide (rGO) aqueous solutions. Poly(vinylpyrrolidone) was used as the surfactant in the rGO aqueous solutions. All studies utilized water filtered with a Milli-Q Plus 185 water purification system (Millipore, Bedford, MA, USA) with a resistivity greater than 18 MΩ cm.Preparation of the P(NiPAAm-bis-AA) inverse opal film
In order to prepare hydrogel films with an anti-opal structure, we first needed to formulate a rGO/P(NiPAAm-bis-AA) pre-gel solution. In this process, the monomer P(NiPAAm-bis-AA) was mixed with the cross-linking agent BIS in a weight ratio of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to ensure that the final concentration of the solution reached 30% (w/w). Subsequently, we added the photoinitiator HMPP to the mixture at a concentration of 1% (v/v) to facilitate the subsequent polymerisation reaction. Next, rGO solution at a concentration of 2 mg mL−1 was added to the pre-gelled rGO/P(NiPAAm-bis-AA) solution and sonicated at room temperature. The purpose of this step was to ensure that rGO was uniformly dispersed in the pre-gelled solution, thus forming a stable composite system. In addition, we note that the colour of hydrogel films with anti-opal structures is affected by the observation angle. In order to accurately record the peak reflections of the hybrid-structured colour films, we should take measurements from a fixed vertical viewing position to ensure the consistency and reliability of the data.
1 to ensure that the final concentration of the solution reached 30% (w/w). Subsequently, we added the photoinitiator HMPP to the mixture at a concentration of 1% (v/v) to facilitate the subsequent polymerisation reaction. Next, rGO solution at a concentration of 2 mg mL−1 was added to the pre-gelled rGO/P(NiPAAm-bis-AA) solution and sonicated at room temperature. The purpose of this step was to ensure that rGO was uniformly dispersed in the pre-gelled solution, thus forming a stable composite system. In addition, we note that the colour of hydrogel films with anti-opal structures is affected by the observation angle. In order to accurately record the peak reflections of the hybrid-structured colour films, we should take measurements from a fixed vertical viewing position to ensure the consistency and reliability of the data.
Sensor fabrication
A custom-made shadow mask facilitated the deposition of interdigitated Ti/Au electrodes, which had a thickness range of 3–30 nm, onto PDMS substrates measuring 30 × 27 mm2. The process utilized an electric beam evaporator (model Intlvac Nanochrome II, operating at 10 kV). The electrodes were spaced approximately 0.1 mm apart, and each interdigitated electrode had a width of 0.5 mm. To facilitate external connections, two 10 × 10 mm2 contact pads were integrated at the extremities of the electrode array. Subsequently, both the PDMS base coated with the electrode layer and the top layer of the uncoated PDMS support were subjected to a thin layer of oxygen plasma treatment, employing a Harrick Plasma Cleaner PDC-001. The rGO/P(NiPAAm-bis-AA) precursor solution was prepared and carefully stored in a cold, dark environment. The pre-gel solution was then injected between two PDMS sheets, one blank and one with an interleaved electrode array design. Due to capillary forces, the pre-gel solution penetrated into the pores of the interleaved electrode pattern and then polymerized under UV irradiation to form a solidified composite hydrogel.Assembly of hydrogel scaffolds on the PDMS catheter
The prepolymer gel (Sylgard 184 Silicone Elastomer Base) and the cross linker (Sylgard 184 Silicone Elastomer Curing Agent) were mixed at a weight ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was placed in a cylindrical plastic mold with an outer diameter of 15 mm and an inner diameter of 10 mm, and it was cured in a 65 °C oven for 8 hours. After curing, the 2.5 mm thick PDMS catheter was removed from the mold and cut into 40 mm length. The PDMS catheter was treated with thin oxygen plasma (Harrick Plasma Cleaner PDC-001) for 3 minutes to create hydrophilic surface functional groups on the catheter before bonding the P(NiPAAm-bis-AA) inverse-opal layer. The PDMS substrates were then submerged in the TMSPMA solution for 3 minutes to silanize them. The TMSPMA solution was made by dissolving 1 mL of TMSPMA (Sigma-Aldrich, 440159) in 200 mL of ethanol and then adding 6 mL of acetic acid (10% (v/v) in water) while gently stirring. Following surface functionalization, the PDMS substrate was completely washed with ethanol and dried under a nitrogen gas stream. Under UV irradiation, the pre-gel solution was employed as the “glue” to connect the dual-responsive graphene hybrid structural color hydrogel film to the functionalized PDMS catheter.
1. The mixture was placed in a cylindrical plastic mold with an outer diameter of 15 mm and an inner diameter of 10 mm, and it was cured in a 65 °C oven for 8 hours. After curing, the 2.5 mm thick PDMS catheter was removed from the mold and cut into 40 mm length. The PDMS catheter was treated with thin oxygen plasma (Harrick Plasma Cleaner PDC-001) for 3 minutes to create hydrophilic surface functional groups on the catheter before bonding the P(NiPAAm-bis-AA) inverse-opal layer. The PDMS substrates were then submerged in the TMSPMA solution for 3 minutes to silanize them. The TMSPMA solution was made by dissolving 1 mL of TMSPMA (Sigma-Aldrich, 440159) in 200 mL of ethanol and then adding 6 mL of acetic acid (10% (v/v) in water) while gently stirring. Following surface functionalization, the PDMS substrate was completely washed with ethanol and dried under a nitrogen gas stream. Under UV irradiation, the pre-gel solution was employed as the “glue” to connect the dual-responsive graphene hybrid structural color hydrogel film to the functionalized PDMS catheter.
Preparation of an artificial cervix
Firstly, we used medical imaging techniques to obtain 3D structural data of the human cervix. These data were processed, optimised and imported into 3D max software for further processing and optimisation. We microstructured the surface of the uterine cervix model to ensure the adhesion of the model to biological tissues. We then exported the designed cervix model to the obj file format and 3D printed it using a cost-effective high-precision resin as the printing material. The printing device we used was a modified REMP3D light-curing printer. After printing, we sanded, cleaned and inspected the printed cervix model to ensure its quality and accuracy. Next, we treated the cervical model with plasma for 3 minutes to form hydrophilic surface functional groups on its surface. The model was then immersed in TMSPMA solution for 3 minutes. After completing surface functionalisation, we thoroughly cleaned the model with ethanol and dried it. Next, we removed an appropriate amount of tissue samples from fresh porcine colon and cleaned them thoroughly. We cut the porcine colon into rectangular shapes and immersed it in an aqueous citric acid solution with a pH of 3 for 2 hours to mimic the acidic environment of the human cervical region. Finally, we covered the treated porcine colon tissue section over the cervix model, ensuring that it fits tightly and was able to mimic the wettability and elasticity of the cervical surface. In this way, we prepared the artificial cervix.Author contributions
Yufei Chen and Yuan Zhou contributed equally to this work. Cihui Liu and Qian Dong conceived the idea and designed the experiment; Lihao Zhang, Yufei Chen, Yue Cao and Zhiwei Jiang conducted experiments and wrote the paper. Sunlong Li, Weipeng Lu, Zheng Mao and Ying Wang analyzed the data. Yufei Chen, Yuan Zhou, Cihui Liu and Qian Dong took part in the discussion. All authors contributed to the preparation of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grants 21802074) and the funding from Shanghai Jiao Tong University.References
- L. Rosalia, C. Ozturk, J. Coll-Font, Y. Fan, Y. Nagata, M. Singh, D. Goswami, A. Mauskapf, S. Chen, R. A. Eder, E. M. Goffer, J. H. Kim, S. Yurista, B. P. Bonner, A. N. Foster, R. A. Levine, E. R. Edelman, M. Panagia, J. L. Guerrero, E. T. Roche and C. T. Nguyen, A soft robotic sleeve mimicking the haemodynamics and biomechanics of left ventricular pressure overload and aortic stenosis, Nat. Biomed. Eng., 2022, 6, 1134–1147 CrossRef PubMed.
- K. Nan, S. Babaee, W. W. Chan, J. L. P. Kuosmanen, V. R. Feig, Y. Luo, S. S. Srinivasan, C. M. Patterson, A. M. Jebran and G. Traverso, Low-cost gastrointestinal manometry via silicone–liquid-metal pressure transducers resembling a quipu, Nat. Biomed. Eng., 2022, 6, 1092–1104 CrossRef CAS PubMed.
- M. Wang, H. Zhang, H. Wu, S. Ma, L. Ren, Y. Liang, C. Liu and Z. Han, Bioinspired flexible piezoresistive sensor for high-sensitivity detection of broad pressure range, Bio-Des. Manuf., 2022, 6(3), 243–254 CrossRef.
- X. Liu, S. Tian, S. Xu, W. Lu, C. Zhong, Y. Long, Y. Ma, K. Yang, L. Zhang and J. Yang, A pressure-resistant zwitterionic skin sensor for domestic real-time monitoring and pro-healing of pressure injury, Biosens. Bioelectron., 2022, 214, 114528 CrossRef CAS PubMed.
- K. Meng, X. Xiao, Z. Liu, S. Shen, T. Tat, Z. Wang, C. Lu, W. Ding, X. He, J. Yang and J. Chen, Kirigami-Inspired Pressure Sensors for Wearable Dynamic Cardiovascular Monitoring, Adv. Mater., 2022, e2202478 CrossRef PubMed.
- Y. Ni, J. Huang, S. Li, X. Dong, T. Zhu, W. Cai, Z. Chen and Y. Lai, Robust Superhydrophobic rGO/PPy/PDMS Coatings on a Polyurethane Sponge for Underwater Pressure and Temperature Sensing, ACS Appl. Mater. Interfaces, 2021, 13(44), 53271–53281 CrossRef CAS PubMed.
- F. He, X. You, W. Wang, T. Bai, G. Xue and M. Ye, Recent Progress in Flexible Microstructural Pressure Sensors toward Human-Machine Interaction and Healthcare Applications, Small Methods, 2021, 5(3), e2001041 CrossRef PubMed.
- H. Ouyang, Z. Li, M. Gu, Y. Hu, L. Xu, D. Jiang, S. Cheng, Y. Zou, Y. Deng, B. Shi, W. Hua, Y. Fan, Z. Li, Z. Wang and A. Bioresorbable, Dynamic Pressure Sensor for Cardiovascular Postoperative Care, Adv. Mater., 2021, 33(39), e2102302 CrossRef PubMed.
- C. H. Zhang, B. Zhang, H. Y. Ma, Z. Li, X. Xiao, Y. H. Zhang, X. Y. Cui, C. M. Yu, M. Y. Cao and L. Jiang, Bioinspired Pressure-Tolerant Asymmetric Slippery Surface for Continuous Self-Transport of Gas Bubbles in Aqueous Environment, ACS Nano, 2018, 12(2), 2048–2055 CrossRef CAS PubMed.
- S. Li, Y. Zeng, W. Hou, W. Wan, J. Zhang, Y. Wang, X. Du and Z. Gu, Photo-responsive photonic hydrogel: in situ manipulation and monitoring of cell scaffold stiffness, Mater. Horiz., 2020, 7(11), 2944–2950 RSC.
- T. Yin, R. Du, Y. Wang, J. Huang, S. Ge, Y. Huang, Y. Tan, Q. Liu, Z. Chen, H. Feng, J. Du, Y. Wang and G. Wang, Two-stage degradation and novel functional endothelium characteristics of a 3-D printed bioresorbable scaffold, Bioact. Mater, 2022, 10, 378–396 CAS.
- X. Wang, Y. Yu, C. Yang, C. Shao, K. Shi, L. Shang, F. Ye and Y. Zhao, Microfluidic 3D Printing Responsive Scaffolds with Biomimetic Enrichment Channels for Bone Regeneration, Adv. Funct. Mater., 2021, 31(7), 2105190 CrossRef CAS.
- L. Siebert, E. Luna-Cerón, L. E. García-Rivera, J. Oh, J. Jang, D. A. Rosas-Gómez, M. D. Pérez-Gómez, G. Maschkowitz, H. Fickenscher, D. Oceguera-Cuevas, C. G. Holguín-León, B. Byambaa, M. A. Hussain, E. Enciso-Martínez, M. Cho, Y. Lee, N. Sobahi, A. Hasan, D. P. Orgill, Y. K. Mishra, R. Adelung, E. Lee and S. R. Shin, Light-Controlled Growth Factors Release on Tetrapodal ZnO-Incorporated 3D-Printed Hydrogels for Developing Smart Wound Scaffold, Adv. Funct. Mater., 2021, 31(22), 2007555 CrossRef CAS PubMed.
- Z. Jiang, Y. Chen, M. Xu, W. Shao, L. Zhang and C. Liu, Bioinspired Superwettable Catheters with Tunable Structural Color for Efficient Drug Release Monitoring, Adv. Mater. Interfaces, 2023, 10(8), 2202047 CrossRef CAS.
- M. Xu, Z. Xu, Z. Jiang, W. Shao, L. Zhang, Y. Chen, Z. Dong, C. Liu, W. Zhang and X. Wan, Biomimicking integrates peristome surface of Nepenthes alata onto biliary stents tips, Chem. Eng. J., 2023, 454, 140064 CrossRef CAS.
- Y. He, Q. Li, P. Chen, Q. Duan, J. Zhan, X. Cai, L. Wang, H. Hou and X. Qiu, A smart adhesive Janus hydrogel for non-invasive cardiac repair and tissue adhesion prevention, Nat. Commun., 2022, 13(1), 7666 CrossRef CAS PubMed.
- B. Li, Z. Xie, Q. Wang, X. Chen, Q. Liu, W. Wang, Y. Shen, J. Liu, A. Li, Y. Li, G. Zhang, J. Liu, D. Zhang, C. Liu, S. Wang, Y. Xie, Z. Zhang and J. Ding, Biodegradable polymeric occluder for closure of atrial septal defect with interventional treatment of cardiovascular disease, Biomaterials, 2021, 274, 120851 CrossRef CAS PubMed.
- C. Y. Yan, J. X. Wang, W. B. Kang, M. Q. Cui, X. Wang, C. Y. Foo, K. J. Chee and P. S. Lee, Highly Stretchable Piezoresistive Graphene-Nanocellulose Nanopaper for Strain Sensors, Adv. Mater., 2014, 26(13), 2022–2027 CrossRef CAS PubMed.
- J. Zhao, G. Wang, R. Yang, X. Lu, M. Cheng, C. He, G. Xie, J. Meng, D. Shi and G. Zhang, Tunable Piezoresistivity of Nanographene Films for Strain Sensing, ACS Nano, 2015, 9(2), 1622–1629 CrossRef CAS PubMed.
- J. Du, Z. Nie, H. Yu, J. Xu, L. Xu and Q. Chen, Protective cleaning of Chinese paper artworks with strong hydrogels: An interfacial adhesion perspective, Sci. China: Technol. Sci., 2023, 66, 2681–2695 CrossRef.
- Y. Chen, P. Gao, L. Huang, X. Tan, N. Zhou, T. Yang, H. Qiu, X. Dai, S. Michael, Q. Tu, N. Huang, Z. Guo, J. Zhou, Z. Yang and H. Wu, A tough nitric oxide-eluting hydrogel coating suppresses neointimal hyperplasia on vascular stent, Nat. Commun., 2021, 12(1), 7079 CrossRef CAS PubMed.
- L. H. Zhang, Y. F. Chen, Y. Cao, S. L. Li, W. P. Lu, W. Cao, J. L. Zhu, W. T. Bao, M. Shao, Z. X. Gan, Y. S. Di, F. J. Xing, X. Li, L. Zhang and C. H. Liu, Bioinspired hierarchical colloidal crystal paper with Janus wettability for oil/water separation and heavy metal ion removal, Nanoscale, 2023, 15(29), 12212–12219 RSC.
- T. Kang, D. X. Oh, J. Heo, H.-K. Lee, S. Choy, C. J. Hawker and D. S. Hwang, Formation, Removal, and Reformation of Surface Coatings on Various Metal Oxide Surfaces Inspired by Mussel Adhesives, ACS Appl. Mater. Interfaces, 2015, 7(44), 24656–24662 CrossRef CAS PubMed.
- M. Li, J. Hao, H. Bai, X. Wang, Z. Li and M. Cao, On-Chip Liquid Manipulation via a Flexible Dual-Layered Channel Possessing Hydrophilic/Hydrophobic Dichotomy, ACS Appl. Mater. Interfaces, 2023, 15(15), 19773–19782 CrossRef CAS PubMed.
- D. C. Wang, H. Y. Yu, L. Jiang, D. Qi, X. Zhang, L. Chen, W. Lv, W. Xu and K. C. Tam, Flexible, anti-damage, and non-contact sensing electronic skin implanted with MWCNT to block public pathogens contact infection, Nano Res., 2021, 1–10 Search PubMed.
- A. B. Patil, Z. Meng, R. Wu, L. Ma, Z. Xu, C. Shi, W. Qiu, Q. Liu, Y. Zhang, Y. Lin, N. Lin and X. Y. Liu, Tailoring the Meso-Structure of Gold Nanoparticles in Keratin-Based Activated Carbon Toward High-Performance Flexible Sensor, Nanomicro Lett., 2020, 12(1), 117 CAS.
- J. Li, J. Li, J. Sun, S. Feng and Z. Wang, Biological and Engineered Topological Droplet Rectifiers, Adv. Mater., 2019, 31(14), e1806501 CrossRef PubMed.
- H. Zhang, K. C. S. Ly, X. Liu, Z. Chen, M. Yan, Z. Wu, X. Wang, Y. Zheng, H. Zhou and T. Fan, Biologically inspired flexible photonic films for efficient passive radiative cooling, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(26), 14657–14666 CrossRef PubMed.
- S. Gong, X. Zhang, X. A. Nguyen, Q. Shi, F. Lin, S. Chauhan, Z. Ge and W. Cheng, Hierarchically resistive skins as specific and multimetric on-throat wearable biosensors, Nat. Nanotechnol., 2023, 18(8), 889–897 CrossRef CAS PubMed.
- W. Zhou, P. Xiao and T. Chen, Carbon-Based Janus Films toward Flexible Sensors, Soft Actuators, and Beyond, Acc. Mater. Res., 2023, 4(4), 334–347 CrossRef CAS.
- Y. Fang, Y. Zou, J. Xu, G. Chen, Y. Zhou, W. Deng, X. Zhao, M. Roustaei, T. K. Hsiai and J. Chen, Ambulatory Cardiovascular Monitoring Via a Machine-Learning-Assisted Textile Triboelectric Sensor, Adv. Mater., 2021, 33(41), e2104178 CrossRef PubMed.
- D. Li, T. Cui, J. Jian, J. Yan, J. Xu, X. Li, Z. Li, A. Yan, Z. Chen, W. Shao, Z. Tang, Z. Xu, G. Wu, H. Liu, Y. Yang and T. L. Ren, Lantern-Inspired On-Skin Helical Interconnects for Epidermal Electronic Sensors, Adv. Funct. Mater., 2023, 33, 2213335 CrossRef CAS.
- J. Lin, R. Fu, X. Zhong, P. Yu, G. Tan, W. Li, H. Zhang, Y. Li, L. Zhou and C. Ning, Wearable sensors and devices for real-time cardiovascular disease monitoring, Cell Rep. Phys. Sci., 2021, 2(8), 100541 CrossRef.
- B. B. Gao, Z. Z. He, B. F. He and Z. Z. Gu, Wearable eye health monitoring sensors based on peacock tail-inspired inverse opal carbon, Sens. Actuators, B, 2019, 288, 734–741 CrossRef CAS.
- X. Liu, X. Zhang, Q. Chen, Y. Pan, C. Liu and C. Shen, A simple superhydrophobic/superhydrophilic Janus-paper with enhanced biocompatibility by PDMS and candle soot coating for actuator, Chem. Eng. J., 2021, 406, 126532 CrossRef CAS.
- X. Li, J. Liu, R. Qu, W. Zhang, Y. Liu, H. Zhai, Y. Wei, H. Hu and L. Feng, Universal and tunable liquid-liquid separation by nanoparticle-embedded gating membranes based on a self-defined interfacial parameter, Nat. Commun., 2021, 12(1), 80 CrossRef CAS PubMed.
- W. Shi, H. Bai, Y. Tian, X. Wang, Z. Li, X. Zhu, Y. Tian and M. Cao, Designing Versatile Superhydrophilic Structures via an Alginate-Based Hydrophilic Plasticene, Micromachines, 2023, 14(5), 962 CrossRef PubMed.
- C. Zhang, X. Xiao, Y. Zhang, Z. Liu, X. Xiao, A. Nashalian, X. Wang, M. Cao, X. He, J. Chen, L. Jiang and C. Yu, Bioinspired Anisotropic Slippery Cilia for Stiffness-Controllable Bubble Transport, ACS Nano, 2022, 16(6), 9348–9358 CrossRef CAS PubMed.
- Y. L. Jiao, C. Z. Li, X. D. Lv, Y. Y. Zhang, S. Z. Wu, C. Chen, Y. L. Hu, J. W. Li, D. Wu and J. R. Chu, In situ tunable bubble wettability with fast response induced by solution surface tension, J. Mater. Chem. A, 2018, 6(42), 20878–20886 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01220h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |