A review on the insights into redox-based regeneration strategies for LiFePO4 batteries
Junhui
Cai
,
Yanjuan
Li
*,
Shengnv
Xu
,
Yiran
Li
,
ZhanZhan
Wang
,
Jie
Liu
,
Shun
Yang
 * and
Xiao
Yan
* and
Xiao
Yan
 *
*
School of Chemistry & Materials Science, Jiangsu Normal University, Xuzhou, 221116, China. E-mail: yanxiao@jsnu.edu.cn
First published on 29th April 2025
Abstract
In recent years, the market share of lithium iron phosphate (LiFePO4: LFP) batteries within the power battery sector has witnessed substantial growth. In light of low-carbon initiatives and environmental sustainability, the recycling of spent LiFePO4 (SLFP) batteries, especially their regeneration, is of paramount importance for environmental protection, resource conservation, and enhancement of economic efficiency. Current literature reviews predominantly concentrate on synthesizing existing research from the perspective of regeneration methodologies. However, they insufficiently address the chemical reactions that are integral to the regeneration process, which are essential for optimizing the recycling of SLFP batteries. To address this gap in the literature, this review, for the first time, systematically compiles studies from the innovative perspective of redox reactions occurring during the regeneration of SLFP batteries. This review commences with an analysis of the economic benefits and failure mechanisms linked to the regeneration of SLFP batteries, thereby elucidating the rationale and necessity for this process. Subsequently, it delves into indirect regeneration methods based on oxidation reactions and direct regeneration technologies based on reduction reactions. Furthermore, the review underscores research dedicated to the enhancement and repurposing of SLFP battery cathodes, offering a prospective outlook on the novel trends in the recycling of SLFP battery materials. This review aspires to promote further scholarly inquiry into the regeneration of SLFP batteries.
Introduction
The advancement of societies and the expansion of economies have resulted in an increased global demand for sustainable energy.1–3 To mitigate carbon emissions and achieve carbon neutrality, nations are actively seeking alternative energy sources to supplant conventional fossil fuels.4–6 Lithium-ion batteries (LIBs) have become prevalent in powering vehicles and facilitating energy storage owing to their high capacity and extended cycle life.7–11 In 2023, the global sales of electric vehicles approached 14 million units, representing an increase of 3.5 million units from 2022, which is an annual growth of 35 per cent,12 as shown in Fig. 1a. Projections indicate that it may reach around 17 million units by 2024, accounting for more than one-fifth of global vehicle sales. As the service life of electric vehicle power batteries ranges from five to eight years, early new energy vehicle batteries have already entered the end-of-life period, and the scale of the end-of-life batteries market will continue to increase with the process of electrification. Various countries have repeatedly modified the primary categories of power batteries with the advancements in these batteries. For instance, in China, the predominant types of power batteries have transitioned from lithium iron phosphate (LFP) to ternary cathode materials (NCM) and back to LFP. Prior to 2017, LFP was predominantly installed in the domestic renewable energy vehicles.13 Since 2017, China has revised its subsidy policy for new energy vehicles to incorporate the energy density of batteries as a criterion for subsidy eligibility. Consequently, the subsidies for low-density LFP batteries have been reduced, as these batteries no longer meet the subsidy standards. In contrast, high energy density NCM batteries are being increasingly promoted. According to statistics from the Power Battery Industry Innovation Alliance, NCM batteries surpassed LFP batteries in market share after 2018, exceeding 60% in 2020. The freezing of the energy density index after 2021 resulted in the gradual development with LFP, and the proportion using this type of power battery is gradually increasing. Nowadays, LFP is the mainstream type of installed power battery. In March 2023, the installed capacity of LFP has reached 69%. Currently, the battery recycling market remains at a developmental stage similar to that of five years prior. As LFP batteries enter their decommissioning phase, they are anticipated to constitute the majority of recyclable batteries. The peak of the growth rate of the LFP battery recycling business is in the distant future. Projections reveal that the LFP power battery recycling market space could expand to a value of 56.7 billion by 2027, with the 2024–2026 period for LFP battery recycling market space representing high growth.Compared to other parts of the battery, the recycling of LFP cathode materials offers the most favorable economic viability for recycling. The main recycling methods are divided into pyrometallurgy and hydrometallurgy (Fig. 1b).14–17 The main research direction is to improve the recovery rate of lithium as much as possible.18–21 Since 2022, the price of lithium carbonate continues to be at a high level, with the recycling value more economical, so the current market for the pricing of spent LiFePO4 (SLFP) batteries basically only considers the value of lithium. Compared to NCM batteries, the current LFP recycling technology and the development of the recycling market are relatively insufficient.22 Although the proportion of iron phosphate is significantly higher compared to lithium metal, the recovery of iron phosphate from LFP is challenging, resulting in limited economic benefits. Consequently, the market has historically perceived the recycling of LFP batteries as economically unviable, leading to a relatively small number of enterprises engaging in lithium iron recycling. Compared with traditional pyrometallurgical and hydrometallurgical recycling of waste lithium iron phosphate batteries, direct recycling offers significant economic benefits,23–25 considering the costs of recycling and regeneration in terms of energy consumption, chemical reagents, environmental protection, and so on (additional Table 1). Direct regeneration usually costs much less than recycling and offers higher profits,26 which can improve the entire battery closed-loop recycling system. In summary, although LFP battery recycling presents economic potential, there are numerous challenges and bottlenecks that must be overcome. It is undeniable that considerable economic potential exists behind these challenges and bottlenecks. However, LFP batteries are currently primarily utilized in a step-by-step manner due to its stable structure and high safety advantages.27–29 LFP batteries in new energy vehicles with an attenuation of more than 20% will not be able to meet the requirements of car driving, indicating that the battery needs to be recycled. Decommissioned batteries with an attenuation interval of 20%–40% can meet the requirements of secondary use, such as in the communication base station, solar street lamps, UPS power supply and other small energy storage areas. An attenuation of more than 40% is generally taken in the way of recycling, dismantling for the sale of materials. LFP regeneration research has gradually become the current research hotspot by comprehensively considering the nature of LFPs and the economic value of recycling.30,31 Regeneration of lithium iron phosphate has a very promising future from an economic and environmentally friendly point of view. A number of scholars have conducted research in this area, and some reviews have been published. In the existing research, researchers summarize the classification or battery regeneration, such as pyrometallurgical, hydrometallurgical and other methods.32,33 However, these approaches merely serve as auxiliary means of regeneration, especially in regeneration methods. To enhance exploration and optimize the regenerating SLFP battery cathode materials, it is imperative to synthesize existing studies from a more fund amential perspective.
| Pyrometallurgical ($ per t) | Hydrometallurgical ($ per t) | Regeneration ($ per t) | |
|---|---|---|---|
| Pretreatment | ∼46 | ∼120 | ∼77 |
| Chemical agent | ∼123 | ∼460 | ∼185 |
| Energy consumption | ∼538 | ∼307 | ∼123 |
| Equipment depreciation | ∼231 | ∼185 | ∼92 |
| Environmentally friendly treatment | ∼307 | ∼230 | ∼62 |
| Total cost | ∼1290 | ∼1302 | ∼539 |
In this review, we systematically compile reported studies from the innovative perspective of the redox reactions occurring during the regeneration of SLFP batteries (Fig. 1c and d). The regeneration process can be categorized into two distinct types based on the underlying reaction mechanisms: indirect regeneration based on oxidation reactions and direct regeneration based on reduction reactions.34,35 In the indirect regeneration process, adding oxidizing agents to elevate the valence of Fe in SLFP battery cathode materials facilitates the maximal extraction of lithium and enables the effective separation of lithium and iron, resulting in the formation of compounds such as Li2CO3 and FePO4. Subsequently, these compounds are utilized in the synthesis of regenerated LiFePO4 (RLFP) cathode materials. However, the unavoidable oxidation separation process may lead to secondary environmental pollution, and the treatment process is complicated and consumes a lot of energy, resulting in higher costs. On the other hand, the direct regeneration method selects a suitable reductant to directly reduce the Fe(III) occupying the Li-site in the SLFP battery to Fe(II) to reduce the Li–Fe anti-site defects and repair its structure.36,37 Direct regeneration obviates the necessity for the leaching process, thereby enhancing its environmental sustainability and energy efficiency compared with indirect regeneration. This method can effectively regenerate and restore its original electrochemical properties, but also upgrade and modify the anode material, resulting in improved performance.38,39
Based on a comprehensive review of recent research on LFP regeneration, a promising direction for future investigations into the regeneration of SLFP batteries has been identified. This direction is crucial for advancing the understanding of the regeneration mechanism of SLFP batteries.
Failure mechanisms of LFP cathodes
Compositional failure
The major cause of capacity degradation in LFP cathode materials after long-term cycling is lithium (Li) loss which will result in inducing partial formation of lithium vacancy (Fig. 2a). Peng et al.40 demonstrated that lithium defects in the cycled LFP reached 47.1% after 6500 cycles. Furthermore, the decomposition of lithium hexafluorophosphate (LiPF6) in the electrolyte occurs under conditions of trace water or elevated temperatures and leads to the formation of hydrofluoric acid (HF). The presence of HF corrodes the surface of the LFP cathode, thereby facilitating the dissolution of iron within the material's structure. This process directly results in the degradation of cathode active material and induces structural phase change, whereby Fe ions dissolved into the electrolyte are unevenly deposited on the surface of the negative electrode and indirectly catalyse the excessive growth of SEI, leading to a loss of active lithium, attenuation of the overall battery capacity and increased impedance. Fu et al.41 investigated the electrochemical performance of LFP//graphite batteries at 25, 45 and 65 °C over 2000 cycles. Their study demonstrated that aged batteries exhibit an activation period and a linear decay period exists in the aged battery at 25 and 45 °C, while an additional accelerated decay period exists at 65 °C. The findings indicate that the principal mechanism contributing to capacity degradation in LFP//graphite batteries under conditions of temperature-accelerated aging due to elevated temperatures is the depletion of active lithium reserves. The losses of inactive lithium were classified into three distinct categories: inorganic solid-electrolyte interphase (SEI), organic SEI, and LixC6. Among these, inorganic SEI is identified as the predominant factor in capacity loss. | ||
| Fig. 2 (a–c) Degradation mechanism of LFP cathode materials. Reproduced from ref. 17, Copyright 2024, with permission from Elsevier. | ||
Structural failure
The deintercalation/intercalation mechanism in LFP cathodes involves a two-phase transition process between LFP of the rhombohedral crystal system and iron phosphate of the hexagonal crystal system. Upon completion of the charging process, the cell volume of FePO4 decreases by 6.77% compared to that of LFP.42–44 During the long-term cycling process of the battery, the recurrent expansion and contraction of the cell induces compressive stress accumulation within the particles, which subsequently leads to the formation of dislocations and other material defects.45–48 The eventual large-scale release of this compressive stress will directly cause a wide range of particles to crack, which is macroscopically manifested as a sudden decrease in the electrode's electronic conductivity. Another cause of structural failure is an antisite defect (Fe occupying Li sites) within LFP, which can have an impact on the performance of the battery. Since lithium-ion transport within LiFePO4 is one-dimensional, the presence of such defects can impede the movement of lithium ions.29,49 Furthermore, these defects can cause destabilization of the LFP structure due to the increased electrostatic repulsion introduced by the high valence state (Fig. 2b and c).Surface-interface issues
Thermally unstable decomposition byproducts of LiPF6 in the electrolyte will form a cathode electrolyte interphase (CEI) layer on the surface of LFP. In variable application environments, high-temperature cycling accelerates the increase of LiPF6 oxide byproducts in the electrolyte, resulting in cathodic interface failure.50In conclusion, the essential approach to rejuvenating LFP cathode materials involves addressing the depletion of active lithium ions and reestablishing the stable structure of LFP. Currently, the predominant restoration techniques are categorized into two primary strategies based on their regeneration methodologies: indirect regeneration and direct regeneration. The following sections provide a detailed overview of these two strategies.
Indirect regeneration based on oxidation reaction
Based on the battery discharge mechanism, Fe2+ in the LFP crystal undergo oxidation to Fe3+ by the oxidant, with the diffusion of Li+ ions out of the crystal lattice. Consequently, the introduction of an oxidant into the solution during the regeneration process facilitates the selective release of embedded Li+ ions from the LFP cathode material. This process facilitates the efficient separation and recovery of lithium and iron. Subsequently, the SLFP battery material can be regenerated by supplementing with lithium salts as a precursor. The present study focuses on identifying the suitable oxidizing agents and reaction methods for the green and efficient separation and recovery of Li and Fe. By judiciously selecting a suitable oxidizing agent, the generation of waste and pollutants in the recovery process can be minimized. Furthermore, optimizing redox reactions could reduce energy consumption and enhance recovery energy efficiency.Oxidizing agent-H2O2
Hydrogen peroxide is a common oxidizing agent used in the hydrometallurgical recovery process of LFP since it is green and inexpensive. In the selective leaching process, the addition of a precise quantity coupled with the regulation of solution pH facilitates the presence of lithium in the liquid phase as Li+. Concurrently, iron and phosphorus are sequestered as FePO4 precipitate. This methodology enables the efficient recycling of lithium and reduces energy costs and environmental pollution. H2O2 is commonly used with acids or alone.In an acidic environment, Fe2+ is oxidized to Fe3+, forming iron phosphate (FePO4), and lithium is dissolved from LFP to form Li+, which can be precipitated as lithium salt by subsequent treatment. The above obtained lithium salt and FePO4 were used to regenerate LFP:
| LiFePO4 + acid + H2O2 → Li+ + FePO4 + H2O | (1) |
| Lithium salt + FePO4 → LiFePO4. | (2) |
The selective leaching process will be shortened to one step of leaching and separation, which has received widespread attention and subsequent research. Li et al.19 was the first to propose adding hydrogen peroxide as an oxidant to assist sulfuric acid leaching, and the results showed a high selectivity and leaching rate: the lithium leaching rate reached 96.85%, while the iron leaching rate was only 0.027%.
Chen et al.51 developed a decontamination method using mixed bases to extract lithium, analyzing the effectiveness of strong and weak bases at the same pH. Initially, Li+ and FePO4 were selectively extracted from an SLFP battery using low concentrations of H2SO4 and H2O2. Then, NaOH and NH3·H2O were used to purify the extracted Li+-containing liquid, and sodium carbonate was used to precipitate Li2CO3. The RLFP using Li2CO3 (99.51%) and FePO4 as raw materials showed an initial discharge capacity of 126.7 mA h g−1 and retained 98.02% capacity after 100 cycles at 0.5 C.
Carbon coating is the most commonly used way to further enhance the electrochemical performance of RLFP.52 Fu et al.53 used the H2SO4–H2O2 system to leach LFP powder, optimized the leaching process conditions, and the leaching rate of lithium and iron elements reached 98.79% and 94.97%, respectively. Glucose was added as a carbon source in the subsequent process of RLFP, and the final LFP/C material regenerated at 700 °C had excellent electrochemical performance, with a first discharge specific capacity of 160.1 mA h g−1 at 0.1 C.
Zhou et al.54 added the SLFP powder into the combined treatment solution of NaH2PO4 and H2O2 for the oxidative leaching reaction (Fig. 3a and b). The lithium leaching rate was 98.65% and the iron leaching rate was only 0.028%, which produced Li3PO4 and FePO4. The RLFP showed excellent multiplicity performance and long-term cycling stability. To promote environmentally sustainable recycling and regeneration practices, a novel green and efficient regeneration process devoid of acids and alkalis have been developed for SLFP batteries. This process is based on an oxidative leaching reaction and has been successfully implemented to regenerate cathode materials. In the oxidative leaching process, H2O2 functions as both an oxidizing and reducing agent. Part of it is dedicated to oxidizing Fe(II), while the remainder executes the reduction reaction. Efficient leaching of lithium can be achieved by appropriately adjusting or controlling the leaching parameters, realizing the conversion of high-purity lithium carbonate and iron phosphate. Qiu et al.55 utilized this method to achieve a 97.6% leaching rate of lithium to regenerate the cathode material (Fig. 3c). The final RLFP maintained a capacity of 144 mA h g−1 at 1 C, with a capacity loss of less than 1% after 100 cycles, which provides excellent multiplication capability and long-term cycling stability. Xu et al.56 directly decomposed the positive electrode into aluminum foil a high-purity FePO4 and lithium-containing solution, in which the leaching efficiency of Li was more than 96.3% and the impurities were scarce. The RLFP had a discharge capacity of 137.1 mA h g−1 at 1 C after 250 cycles, with almost no capacity loss, showing excellent electrochemical performance and the ability to meet the requirements for secondary utilization.
 | ||
| Fig. 3 (a) Flowchart of recycling and regeneration of SLFP battery cathode powder. Reproduced from ref. 54, Copyright 2023, with permission from Elsevier. (b) Long-term performance of SLFP and RLFP batteries at 0.5 C for 200 cycles. Reproduced from ref. 54, Copyright 2023, with permission from Elsevier. (c) Schematic of the regeneration of RLFP cathode materials via hydrogen peroxide leaching. Reproduced from ref. 55, Copyright 2022, with permission from the Royal Society of Chemistry. (d) Schematic of the detailed transformation of different products for the in situ recycling of FePO4 and Li+via advanced oxidation metallurgy. Reproduced from ref. 57, Copyright 2023, with permission from Elsevier. | ||
Hydrogen peroxide also provides the intermediate oxidizing species required for the reaction. The mechanism suggests that the oxidation of Fe(II) and the release of Li+ from LFP are mainly triggered by the rapid attack of a large amount of –OH during advanced oxidation according to DFT calculations and chemical reaction analyses. The released Li+ is recycled as Li2CO3 and used as a precursor for the remanufacture of LFP together with FePO4. Chen et al.57 innovatively proposed an in situ advanced oxidative metallurgy method to selectively extract lithium from LFP by Fenton oxidation instead of the conventional metallurgical process (Fig. 3d). Li can be completely released without destroying the olive-type structure of LFP and form the FePO4 precursor. The RLFP showed excellent electrochemical performance with a first discharge capacity of 138.9 mA h g−1 at 0.5 C and a capacity retention of 93.6% after 50 cycles.
Oxidizing agent-persulfate
Persulfate (MS2O8) is a commonly used oxidizing agent for the regeneration of SLFP battery cathode materials because it is solid at room temperature and soluble in water. Persulfate has a wider range of applications compared with hydrogen peroxide. It is suitable for hydrometallurgical regeneration and can be used in traditional thermal regeneration:| 2LiFePO4 + MS2O8 = 2FePO4 + MSO4 + Li2SO4. | (3) |
Zhang et al.58 devised a thermal oxidation strategy to extract lithium from an SLFP battery using a hot phase process and synergistically controlled melt oxidation of sulfate under oxygen atmosphere. The desired conversion of LFP to soluble lithium salts and FePO4 was achieved while maintaining the initial morphology of the particles with the following reaction:
 | (4) |
Thermodynamic and DFT calculations confirmed the feasibility of lithium precipitation from molten salt. The RLFP showed excellent electrochemical performance with a discharge capacity of 136.2 mA h g−1 at 1 C and a capacity retention of 98.9% after 100 cycles.
Sun et al.59 proposed that the selective extraction and recovery of lithium from LFP cells could be achieved using only NH4S2O8 as the oxidizing and leaching agent (Fig. 4a). Over 99% of lithium was selectively extracted, while the olivine skeleton of FePO4 remains intact after oxidative leaching (Fig. 4b).
 | ||
| Fig. 4 (a) Flow chart of the recovery of Li2CO3 and FePO4 from SLFP black powder. Reproduced from ref. 59, Copyright 2023, with permission from Elsevier. (b) Schematic of the reaction between LiFePO4 and (NH4)2S2O8. Reproduced from ref. 59, Copyright 2023, with permission from Elsevier. (c) RLFP rate performance and cycling stability at 0.1 C and 0.2 C. Reproduced from ref. 59, Copyright 2023, with permission from Elsevier. (d) Cyclic performance of sample RLFP at 1 C, 2 C, and 5 C. Reproduced from ref. 60, Copyright 2022, with permission from Elsevier. | ||
This route does not include inorganic or organic acid leaching, complex pH adjustment and waste water treatment processes. After optimizing the process conditions, Li2CO3 and FePO4 can be recovered with a recovery rate of more than 96%, and the RLFP cathode has the electrochemical performance with a discharge capacity of 163.9 mA h g−1 at 0.1 C and 120 mA h g−1 at 4 C. After 100 cycles of 0.2 C, the capacity retention rate is 98.0% (Fig. 4c).
Chen et al.60 investigated and proposed a carbothermal reduction technique for the regeneration of LFP using the recycled raw materials from SLFP batteries to further improve the electrochemical performance of the RLFP, in which Li2CO3 recovered from the SLFP battery served as the lithium source, and FePO4 provided the iron and phosphorus sources. The application of a carbon coating facilitated more intimate particle contact, reduced the Li+ diffusion pathway within LFP, and enhanced the electronic and ionic conductivity. Optimal electrochemical performance was achieved with a carbon coating mass fraction of 12 wt% in the RLFP material. It has a high specific discharge capacity of 146.89 mA h g−1 at 1 C and an excellent capacity retention of 97.9% after 200 cycles. In addition, it has satisfactory capacity retention of 96.1% and 94.3% at 2 C and 5 C, respectively (Fig. 4d).
Oxidizing agent-O2
In contrast to the aforementioned methods, an alternative indirect regeneration strategy involves fire pretreatment. This approach entails the direct oxidation of Fe to iron oxide by calcining the SLFP battery materials in air, thereby completely disrupting the original structure to facilitate lithium–iron separation. Concurrently, this method effectively removes certain impurities present in the cathode materials, including PVDF, conductive carbon, and aluminum. Subsequently, SLFP battery is regenerated through the addition of a lithium source, among other components. Chen et al.61 first explored the direct thermal treatment of recycled cathode powder at different temperatures. The recycled powder could be effectively restored and reused for lithium-ion batteries after high-temperature treatment at 650 °C. The researchers further explored the reaction mechanism. Li et al.62 employed a novel approach combining pre-oxidation and a granulation-based co-coating method to regenerate a severely degraded SLFP battery. The pre-oxidation process effectively decomposed the binder and residual carbon, resulting in the conversion of lithium and iron into Li3Fe2(PO4)3 and Fe2O3, respectively. The subsequent regeneration process involved the addition of lithium carbonate to synthesize spherical LFP co-coated with carbon and Li3PO4 (Fig. 5a). The electrochemical performance of the regenerated material was comparable to that of commercial LFP. Wang et al.63 developed a direct regeneration method of LFP based on a doping strategy (Fig. 5b). Initially, the SLFP battery was oxidized in a high-temperature oxygen environment. Then, sucrose and glycine were added to prevent Fe ion migration and enhance Li+ and electron diffusion by forming a nitrogen-doped carbon coating. The RLFP cathode demonstrated excellent cycling stability, retaining 98.7% capacity after 100 cycles at 1 C and 87.9% after 500 cycles at 1 C. | ||
| Fig. 5 (a) Schematic of cooperative RLFP. Reproduced from ref. 62, Copyright 2024, with permission from the American Chemical Society. (b) Schematic of the regeneration of SLFP batteries. Reproduced from ref. 63, Copyright 2024, with permission from the Royal Society of Chemistry. | ||
Electrochemical indirect oxidation
Electrochemical indirect oxidation involves the generation of oxidizing intermediates during the electrolysis process. These oxidizing agents are either attached to electrode surfaces or dispersed in the solution, where they facilitate the oxidation of target substances. This process can occur at the anode and cathode.64 Indirect electrolysis can be achieved at positive and negative potentials that are lower than those required for direct electron transfer at the electrode through the use of a homogeneous electron transfer medium. Indirect electrolysis can be performed at reduced positive and negative potentials using a homogeneous electron transfer medium compared with the higher potentials needed for direct electron transfer at the electrode. This leads to reduced energy consumption, enhanced reaction energy efficiency, decreased electron transfer overpotential, and avoidance of direct electrolysis limitations.65 The process of indirect oxidation primarily depends on the production of potent oxidizing agents to oxidize the Fe2+ ions in LFP. The leaching process closely resembles a homogeneous reaction, facilitating complete interaction between LFP and the oxidizing agents without necessitating elevated temperatures to enhance LFP dispersion. The utilization of electrons as environmentally friendly oxidants minimizes the need for additional chemical reagents, thereby supporting the efficient recovery of lithium products in subsequent stages.Tian et al.66 developed an electrochemical system employing a boron-doped diamond (BDD) electrode with a high oxygen evolution potential as the anode and RuO2/Ti as the cathode to produce oxidizing species and enhance oxidative reaction contact for the indirect oxidation of LFP. This innovative approach facilitates efficient and rapid selective leaching of lithium and iron ions through indirect oxidation, thereby achieving selective leaching while substantially minimizing reaction time. Zhang et al.67 used a low-cost sodium chloride solution as the electrolyte, Pt as the cathode and graphite as the anode. In the electrochemical LFP regeneration reactor, the Cl−/ClO− pair that is generated electrochemically in NaCl solution is adopted as the redox mediator to break down LFP into FePO4 and Li+via the redox targeting reaction without extra chemicals. The RLFP cathode material was regenerated from recycled Li2CO3 and FePO4 and offers enhanced electrochemical performance and excellent cycling stability.
In summary, the indirect regeneration strategy for LFP relies on the oxidation reaction, which can preferentially and selectively leach lithium with high efficiency, but the leaching residue requires additional processing. Simultaneous leaching of all elements from SLFP battery materials allows for the extraction of valuable components in a single step and may result in lithium loss. Meanwhile, simultaneous leaching of all elements from SLFP battery materials allows for the extraction of valuable components in one step, which complicates the separation process and may lead to lithium loss. Consequently, indirect regeneration methods are often characterized by a prolonged recovery process and the production of substantial quantities of waste liquid. This method does not provide economic benefits, particularly for LFP materials that do not contain expensive metals (e.g., cobalt). Additionally, although the profile regeneration recovers lithium and iron, respectively, during regeneration, it remains essential to adjust the lithium-to-iron ratio appropriately to synthesize new LFP material.
This adjustment may necessitate the addition of supplementary lithium or iron sources, thereby diminishing economic efficiency. Therefore, current researchers are committed to further improving the lithium leaching efficiency, while environmental protection is an even more significant issue for them to consider. Table 2 summarizes the effect of different oxidizers on the yield of lithium iron phosphate and the performance of regenerated batteries.
| Oxidising agent | Recovery of Li (%) | Recovery of Fe (%) | Regeneration additives | Electrochemical performance | Ref. |
|---|---|---|---|---|---|
| H2SO4–H2O2 | 98.79 | 94.97 | Glucose | 160.1 mA h g−1 at 0.1 C and capacity retention of 99.7% after 100 cycles at 1 C | 53 |
| NH4S2O8 | 96 | — | Glucose | 163.9 mA h g−1 at 0.1 C, 120 mA h g−1 at 4 C and capacity retention ratio of 98.0% after 100 cycles at 0.2 C | 59 |
| NH4S2O8 | >99 | — | Glucose | 136.2 mA h g−1 at 1 C and 98.9% capacity retention after 100 cycles | 58 |
| NaH2PO and H2O2 | 98.65 | — | Glucose | 144.3 mA h g−1 at 0.5 C and 99% capacity retention after 200 cycles | 54 |
| Fe(II)–H2O2 | 99.9 | — | Glucose | 138.9 mA h g−1 at 0.5 C and 93.6% capacity retention after 50 cycles | 57 |
| H2SO4–H2O2 | 99.51 | — | — | 126.7 mA h g−1 at 0.5 C and 98.02% capacity retention after 100 cycles | 51 |
| NH4S2O8 | 97.06 | — | — | 154.2 mA h g−1 at 0.5 C and 91.0% capacity retention after 300 cycles at 1 C | 68 |
| NH4S2O8 | — | — | Glucose | 146.89 mA h g−1 at 1 C and 97.9% capacity retention after 200 cycles | 60 |
| H2O2 | 96.3 | — | Glucose | 137.1 mA h g−1 at 1 C after 250 cycles with almost no capacity loss | 56 |
| H2O2 | 97.6 | — | Citric acid | 144 mA h g−1 at 1 C with less than 1% capacity loss after 100 cycles | 55 |
| H2O2 | 99.9 | 97.5 | Glucose | 144.2, 139.0, 133.2, 125.5, and 110.5 mA h g−1 at 0.1, 0.5, 1, 2, and 5 C | 69 |
| O2 | — | — | Sucrose | 138.8 mA h g-1 at 1 C and 98.7% capacity retention after 100 cycles | 63 |
| O2 | — | — | — | 155.7 mA h g−1 at 0.1 C and it remained at 149.2 mA h g−1 after 100 cycles | 62 |
| ClO− | 99.70% | 99.15% | — | 114.6 mA h g−1 at 5 C and 94.0% capacity retention after 300 cycles | 67 |
Direct regeneration based on reduction reaction
In recent years, a strategy of direct regeneration of LFP has been proposed. This approach is informed by the failure mechanism of LFP and aims to address two critical aspects to achieve successful regeneration. First, it is necessary to restore the structure of Fe(III), which occupies the position of Li, back to Fe(II) through the addition of reducing agents. Secondly, the appropriate selection of lithium salts is necessary to replenish the deficient active lithium and restore its capacity. Among these considerations, the most crucial is the repair of the LFP structure, underscoring the importance of selecting suitable reducing agents and strategies.Inorganic reducing agents
Inorganic reductants have the advantages of lower cost and easy availability of raw materials, which are usually synergized with an auxiliary strategy to regenerate LFP. Current research focuses on inorganic reductants that are environmentally friendly, low in dosage, and functional. Jing et al.70 first used N2H4–H2O as a reductant and adopted a one-step hydrothermal method to regenerate the SLFP battery, confirming the feasibility of N2H4–H2O as a reductant for SLFP regeneration. However, researchers later used other auxiliary strategies to regenerate SLFP batteries since the hydrothermal method is hard to apply in industry. N2H4–H2O is a commonly utilized reducing agent to reduce Fe(III) back to Fe(II) and then lithium salt was added to repair lithium vacancy defects and antisite defects and consequently regenerate LFP.Song et al.71 employed N2H4–H2O as a reducing agent and LiCl as a lithium source to address lithium vacancy defects and antisite defects in SLFP batteries. This was achieved through the application of ultrasound, which facilitated the generation of localized high temperatures, high pressures, and intense shock wave jets (Fig. 6a). The RLFP has a discharge specific capacity of 135.1 mA h g−1 after 100 cycles at a current density of 1 C, with a capacity retention rate as high as 97%. The reaction product of N2H4–H2O is mainly N2, which is environmentally friendly. To enhance energy efficiency and safety, researchers revisited a low-temperature liquid lithium replenishment strategy using N2H4–H2O as a reducing agent and LiCl as a lithium source.72 This method replenishes missing Li+ ions and reduces antisite defects through annealing, restoring nearly all lost Li+ ions at 80 °C over 6 h (Fig. 6b). Subsequent annealing removed the Li+ ions and electrochemical evaluation showcased the outstanding properties of RLFP-80 °C/6 h.
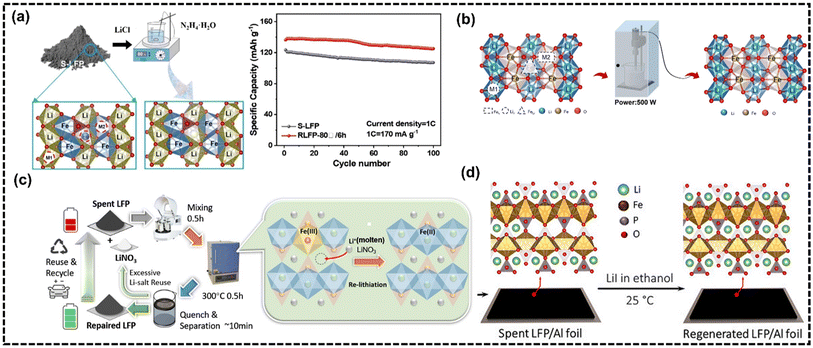 | ||
| Fig. 6 (a) LFP battery regeneration enhanced via an eco-friendly N2H4–H2O method, restoring Li ions and reducing defects and the comparison of cycle performance before and after regeneration. Reproduced from ref. 71, Copyright 2024, with permission from Elsevier. (b) Flowchart of the regeneration of an SLFP battery cathode powder. Reproduced from ref. 72, Copyright 2024, with permission from Elsevier. (c) Schematic of the SLFP battery repairing procedures. Reproduced from ref. 73, Copyright 2023, with permission from the Royal Society of Chemistry. (d) SLFP battery regenerated at room temperature via DCL. Reproduced from ref. 74, Copyright 2023, with permission from Elsevier. | ||
Wang et al.73 proposed a simple recrystallization method by reacting an SLFP battery cathode material with LiNO3 (Fig. 6c). Benefiting from the thermodynamic instability and low melting point of LiNO3 (∼250 °C), the SLFP battery was completely relithiated by heating in air at 300 °C (just below the LFP melting point (∼250 °C) of LiNO3, the SLFP battery was completely relithiated by heating in air at 300 °C (just below the LFP oxidation temperature) for 30 min. The specific capacity of the repaired LFP was restored from 134 mA h g−1 to 162 mA h g−1, with improved specific capacity and cycling performance comparable to that of commercial new LFP. However, harmful NO2 gas is generated in the process, which is not environmentally friendly enough.
Reductive lithium-containing substances as a bifunctional additive are also used to achieve Li compensation while reducing Fe3+, which has the advantages of low energy consumption and good environment. For instance, the regeneration mechanism using LiI is as follows:
| Li1−xFePO4 + xLiI = LiFePO4 + 1/2xI2 | (5) |
Ouaneche et al.74 reported that LFP can be efficiently recovered by optimizing the experimental parameters using direct lithiation of LiI in ethanol solution, which is one of the greenest and cheapest solvents, without any additional heat treatment (Fig. 6d). The RLFP has excellent electrochemical performance: the first turn capacity is 168 mA h g−1 at 0.1 C and the coulombic efficiency of 25 cycles exceeds 98%.
In addition to inorganic solids, gaseous atmospheres with reducing properties can also be effective in regenerating LFP. Sun et al.75 roasted discarded LFP cathodes in CO2 to partially remove carbon coatings, then used mechanical milling to mix the lithium source with LFP. The CO2 reacted with the carbon, creating a reducing atmosphere that converted Fe3+ to Fe2+, decreasing the Fe3+ content. The improved pretreatment method more effectively restored the crystal structure of SLFP battery cathode material, resulting in excellent electrochemical performance and cycling stability, with an initial capacity of 149.1 mA h g−1 at 0.1 C. Compared with the traditional pretreatment method, the crystal structure of the SLFP battery cathode material was repaired more completely, and the RLFP material showed good electrochemical performance and cycling stability. The initial capacity could reach 149.1 mA h g−1 at 0.1 C.
Organic reducing agents
Compared with inorganic reductants, organic reductants can effectively form a carbon coating layer during the regeneration process, which can effectively regenerate LFP, further optimize the conductive network of LFP and improve its electrochemical performance. The selection of a suitable organic reductant combined with a suitable lithium source for direct regeneration of LFP is a hotspot in current research.76,77Glucose is an extremely common and easily available organic material that is often used as a carbon source in the indirect regeneration of LFP. In the direct regeneration process, glucose can be used as a carbon source, but more importantly as a reducing agent to reduce Fe3+ to Fe2+ to achieve the repair of the structure of the SLFP battery and to achieve a uniform layer of carbon coating, which optimizes the electrical conductivity of the cathode material. The main reaction process is as follows:
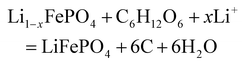 | (6) |
Han et al.78 extracted SLFP battery cathode materials from SLFP batteries using pyrolysis and flotation. They adjusted the Li to Fe ratio with lithium carbonate, used glucose as a reducing agent, and calcined the mixture at 650 °C under argon for 11 h. The regenerated LiFePO4/C achieved an initial discharge capacity of 161.88 mA h g−1 and retained 157.89 mA h g−1 after 100 cycles, demonstrating strong electrochemical and cycling performance. Ascorbic acid is a more commonly used organic reductant, with advantages of effective reducing ability and compatibility with aqueous systems. Song et al.79 directly regenerated LFP using a high-power ultrasonic liquid-phase reaction with ascorbic acid as the reducing agent and LiOH as the lithium source (Fig. 6a), where C6H6O6 was produced by the reaction of ascorbic acid without the regenerated LFP surface. Its electrochemical performance was more general since it formed an effective carbon coating.
Heteroatom participation and the construction of high-performance carbon cladding layers are usually employed in the regeneration process of organic reductants to achieve better electrochemical performance of the regenerated LFP, such that the carbon cladding layer can effectively enhance the conductivity of the cathode material and the stability of the overall structure.80–82 For example, Mao et al.83 used ascorbic acid as the reductant and LiOH as the lithium source to regenerate LFP using an ultrasonic-assisted method (Fig. 7b). The subsequent introduction of graphene regenerated from the negative electrode by electrostatic self-assembly helps to form a layered structure and conductive network for the RLFP (Fig. 7c), thus realizing the rapid ion and electron transfer of the redox reaction. When doped with 5 wt% graphene, the specific capacity of the regenerated LFP/MWrGO composites was increased to 161.4 mA h g−1, with a capacity retention of 94.9% at 0.2 C. Zhou et al.84 used the solution mixture with NaCl salt as the template for pore creation which undergoes an “ice and fire” process: ice drying helps to embed the NaCl into the glucose coating on the LFP particles, and annealing (carbonization) at 650 °C induces the conversion of glucose from an electron donor into a unique 3D porous carbon network between the RLFP nanoparticles (Fig. 7d). The reconfiguration of the porous channels provided convenient access for lithium-ion transport and electrolyte penetration. The RLFP achieved reversible capacities of 169.74 and 141.79 mA h g−1 at 0.1 C and 1 C, respectively, with a retention rate of over 95.7% at 1 C after 200 cycles.
 | ||
| Fig. 7 (a) Schematic showing the methodology of high-power sonication using ascorbic acid as the reducing agent and LiOH as the lithium source. Reproduced from ref. 79, Copyright 2024, with permission from Elsevier. (b) Microwave-hydrothermal (MWHT) regenerating process. Reproduced from ref. 83, Copyright 2021, with permission from the Royal Society of Chemistry. (c) Composition of the transition state between SLFP and MWrGO during the MWHT regenerating process. Reproduced from ref. 83, Copyright 2021, with permission from the Royal Society of Chemistry. (d) Workflow diagram of ‘ice and fire’ two-step method via template-assisted regeneration and common solid-phase roasting method. Reproduced from ref. 84, Copyright 2023, with permission from Elsevier. (e) Schematic of the direct regeneration of SLFP battery. Reproduced from ref. 85, Copyright 2022, with permission from Wiley. (f) Preparation process of R-(C + N)-LiFePO4. Reproduced from ref. 86, Copyright 2024, with permission from Elsevier. (g) Schematic of the degraded and restored crystal structures. Reproduced from ref. 85, Copyright 2023, with permission from Springer Nature. | ||
In addition to constructing a unique carbon network, heteroatom doping can effectively improve the stability of the carbon layer and enhance the ion diffusion performance. Cheng et al.85 regenerated an SLFP battery by hydrothermal treatment and sintering using ethanol as solvent and reducing agent and lithium acetate (CH3COOLi) as lithium source (Fig. 7e). The destruction of LFPs generally starts from the surface, so inhibiting the destruction of the LFP's adjacent surfaces plays a key role in preventing the LFP's destruction of LFP as a whole. A heterogeneous interface was constructed between the nitrogen-doped carbon (NC) obtained by adding polyvinylpyrrolidone (PVP) and the regenerated LFP. The cycling stability of the regenerated LFP (RSLFP@NC) was improved by introducing N atoms to regulate the position of the d-band center of Fe near the anode surface.
Melamine is a wise selection to reduce the amount of pharmaceuticals and improve the economic efficiency. The reduction of Fe3+ to Fe2+ was favored by doping using melamine with a certain reducing property. More importantly, the N atoms contained in melamine itself are doped into the carbon layer through C–N bonds, forming carbon-doped LFP composites without the need of adding additional carbon sources. Jin et al.86 regenerated an SLFP battery and repaired the battery performance by adding lithium carbonate and melamine under heat treatment conditions (Fig. 7f). The RLFP also exhibited excellent capacity retention up to 99.03% after 200 cycles and excellent multiplicative performance with a discharge capacity of 116 mA h g−1 at 5 C. The systematic study demonstrated that the nitrogen-doped carbon coating plays a crucial role in improving the performance of the RLFP cathode material.
Cheng et al.87 advanced the process by using a multifunctional organolithium salt (3,4-dihydroxybenzonitrile dilithium) that acts as a reductant, Li source, and carbon source to directly regenerate discarded LFP cathodes (Fig. 7g). The organolithium salt's functional groups bond with RLFP, lithium fills vacancies, and cyano creates a reducing atmosphere to prevent the Fe(III) phase. Additionally, salt pyrolysis forms an amorphous conductive carbon layer on the LFP particles. Based on the mechanism of LFP regeneration, the regeneration of SLFP battery cathode materials can be achieved under room temperature conditions with a suitable reducing agent and supplementation of an appropriate lithium source. Inspired by room temperature lithiation, Zhang et al.88 tried to regenerate the whole cathode directly without stripping off the cathode material, and the triethyl lithium borohydride/tetrahydrofuran solution was used as a lithium replenishment agent and a reductant to achieve the direct regeneration of cathode material. Direct regeneration of the cathode material eliminates the need for separation, regeneration, and electrode remanufacturing processes, reduces total energy consumption by 80%, and increases revenues by 53% compared with traditional direct regeneration. This method is environmentally and economically competitive and is a very interesting direction for future research.
Reducing agent-electron(e−)
Electrochemical reduction mainly relies on electrons as a reducing agent to directly reduce Fe(III) to Fe(II), which is very environmentally friendly. In this process, lithium ions in the lithium salt aqueous solution are driven to the empty space of the degraded cathode through the potential difference on the electrode, and the RLFP is subsequently obtained through a short annealing process. Huang et al.89 first put forward the targeted electrically driven lithiation, achieving low-temperature repair of lithium iron phosphate. The regenerated material has a discharge capacity of 135.2 mA h g−1 at 1 C and a capacity retention rate of 95.3% after 500 cycles, confirming the feasibility of electrochemical regeneration of lithium iron phosphate.Wu et al.90 proposed a re-lithiation approach that intercalates lithium ions into scrapped LFP in an aqueous solution system. Specifically, Fe(III) was reduced by electrons acting as a reducing agent, while Li in the lithium salt solution acted as a Li source to replenish the missing Li in the SLFP. The RLFP exhibits excellent electrochemical performance with a high discharge capacity of 134.0 mA h g−1 at 1 C. In order to further reduce the use of additives, Wang et al.91 proposed an ingenious electrochemical method with simultaneous anodic de-lithiation/cathodic lithium-embedded regeneration to regenerate an SLFP battery. The SLFP was charged/discharged in an electrolytic system, where it was used as the anode and the cathode (LixFePO4|Li2SO4|LixFePO4). The electrolysis process does not require an external Li source and the identical material of the cathode and anode could reduce the theoretical voltages for Li embedding and de-embedding, thus reducing energy consumption.
Yang et al.92 proposed a nondisassembly repair strategy for degraded cells through a lithium restoration method based on deep discharge, which can elevate the anodic potential to result in the selective oxidative decomposition and thinning of the SEI on the graphite anode. Both the electron and lithium sources required for the reductive regeneration of SLFP batteries come from the oxidative decomposition of the SEI.
Current research in direct regeneration has progressively shifted toward shortened-process methodologies characterized by low-energy-consumption profiles and enhanced environmental compatibility. Although numerous regeneration strategies employ brief annealing processes to improve crystallinity and structural stability of regenerated materials, this approach inevitably prolongs procedural complexity and escalates energy expenditure. Consequently, emerging research focuses on developing post-annealing-free regeneration protocols to optimize process efficiency. This paradigm shift necessitates stringent requirements for reducing agent selection and precise control over ambient condition during the redox-driven reconstruction phase.93 As demonstrated by Wu et al.94 in their electrochemical regeneration protocol for SLFP batteries, well-crystallized RLFP was successfully synthesized by applying a controlled electrolytic current of 5 mA, achieving structural reconstruction without requiring post-annealing treatment. Electrochemical regeneration shows that adjusting the current can regulate the crystallinity of RLFP, enhancing its electrochemical performance. However, other regeneration methods require further investigation into their mechanisms to achieve efficient, closed-loop regeneration with low energy consumption. Direct regeneration streamlines the process compared with indirect regeneration, enhancing environmental sustainability, and significantly reducing costs, yielding economic and ecological advantages. The use of suitable lithium salts and reduction additives facilitates the regeneration of SLFP batteries more rapidly and with reduced energy consumption. Current perspectives on multifunctional organic lithium salts suggest that, besides restoring lithium and structural integrity, they also contribute to the formation of an effective carbon coating layer. This approach is economically more viable than the addition of lithium source and reductant, with a very good prospect for development. Therefore, more multifunctional reducing agents and reducing processes with lower costs will be the mainstream of future development for the direct regeneration of lithium iron phosphate. Table 3 summarized the effect of different reductants and reduction method on the performance of regenerative batteries.
| Reducing agent and additives | Lithium salt | Strategy | Electrochemical performance | Ref. |
|---|---|---|---|---|
| Chitin and graphene oxide | LiOH | Repeated freezing and thawing, spray-drying | 124.8 mA h g−1 at 2 C, 117.5 mA h g−1 at 5 C, and 149.7 mA h g−1 at 0.2 C | 95 |
| CO | Li2CO3 | High-temperature calcination | 149.1 mA h g−1 at 0.1 C | 75 |
| N2H4·H2O | LiCl | Low-temperature liquid-phase and high-temperature sintering | 146.45 mA h g−1 at 1 C and 92% capacity retention after 100 cycles | 72 |
| Ascorbic acid | LiOH | High power ultrasonic reactions | 154.71 mA h g−1 at 0.1 C and 93.56% capacity retention after 200 cycles at 1 C | 79 |
| Melamine | Li2CO3 | High-temperature calcination | 168 mA h g−1, 156 mA h g−1 and 151 mA h g−1 at 0.05 C, 0.2 C and 1 C, respectively | 86 |
| N2H4·H2O | LiCl | High power ultrasonic reactions | 135.1 mA h g−1 and 97% capacity retention after 100 cycles at 1 C | 71 |
| Glucose | LiOH | Solution-based relithiation and high-temperature sintering | 169.74 and 141.79 mA h g−1 at 0.1 and 1 C, respectively and a > 95.7% retention rate at 1 C after 200 cycles | 84 |
| LiI | LiI | Direct lithiation in solution | 160 mA h g−1 at 1 C, | 74 |
| LiNO3 | LiNO3 | recrystallization | 162 mA h g−1 at 0.1 C, and 90% capacity retention after 500 cycles | 73 |
| Na2SO3 | Li2SO4 | Hydrothermal | 145.1, 142.7, 139.9, 135.9, and 129.3 mA h g−1 at 0.1,0.2, 0.5, 1, 2, and 5 C, respectively and >99% capacity retention after 100 cycles at 1 C | 96 |
| 3,4-Dihydroxybenzonitrile dilithium | 3,4-Dihydroxybenzonitrile dilithium | High-temperature calcination | 157 mA h g−1, 127, 111, and 97 mA h g−1 at 0.1, 2, 5 and 10 C, respectively | 87 |
| Glucose | Li2CO3 | Flotation process after effective pyrolysis | 161.37 mA h g−1 at 0.1 C and 97.53% capacity retention after 100 cycles at 0.2 C | 78 |
| Ascorbic acid | Li2CO3 | Spray drying and high-temperature solid-phase method | 160 mA h g−1 at 0.1 C and 80% capacity retention after 800 cycles at 1 C | 97 |
| CH3CH2OH | CH3COOLi | Hydrothermal | ∼80% capacity retention after 1000 cycles at 10 C | 85 |
| Ascorbic acid | LiOH | Microwave-reduced | 161.4 mA h g−1 at 0.2 C and 94.9% capacity retention after 100 cycles | 80 |
| Tartaric acid | LiOH | Hydrothermal | 165.9, 151.93, 145.92, 133.11 and 114.96 mA h g−1 at 0.1, 0.5, 1, 2, and 5 C, respectively | 98 |
| e− | SEI | Electrochemical | Stable cycling for 300 cycles at 1 C | 91 |
Conclusion and outlook
LFP has emerged as a significant commercial battery technology following extensive research and application. In the foreseeable future, the disposal of SLFP batteries present a critical issue requiring urgent resolution. To achieve more environmentally friendly and efficient disposal of SLFP battery cathode materials, the direct generation of SLFP battery cathode materials offers a promising approach that balances economic and environmental benefits. Consequently, the development of a strategy for LFP restoration that is capable of practical production use and higher performance remains a great challenge. These include the following:Lack of economy and practicality
The more popular organic lithium salt offers the advantage of simultaneously providing a carbon source and lithium source. However, the high cost of organic lithium salt, coupled with the potential generation of carbon dioxide during their regeneration process poses environmental concerns. These factors present significant challenges to their large-scale industrial application. Most current research remains at the laboratory stage and is not easily scalable for industrial use. Thus, future research should focus on large-scale production and exploring more efficient and universal regeneration processes.Repair of crystal structure
Indirect recycling and subsequent regeneration of LFP via high-temperature calcination produce cathode materials with enhanced crystal structure stability compared with some direct regeneration methods. These direct methods often involve low-temperature reduction for lithium replenishment and omit the annealing process, resulting in weaker crystal structure stability. Therefore, a significant challenge lies in balancing low energy consumption with the improvement in crystal structure stability. Therefore, balancing the low energy consumption and improving the stability of the crystal structure is also a major research focus.Problems of impurities
During the recycling process, impurities such as copper and aluminum in the battery need to be effectively removed to ensure the purity of the recovered material and the safety of reuse. It is imperative to develop more refined separation techniques, such as magnetic separation and froth flotation, to enhance the efficiency of the separation processes and the purity of the recovered materials. Synchronous interfacial impurity removal and lithium replenishment could shorten the closed-loop battery material recycling process. However, the battery failure and regeneration mechanisms need deeper exploration.Problems with the surface interface
The surface interface properties of battery materials are pivotal to their electrochemical performance; however, there is a paucity of research concerning LFP recycling. It is imperative for future investigations to focus on enhancing the surface interface properties of recycled materials to optimize their performance in secondary applications. Potential methodologies to achieve this enhancement may encompass surface coating, doping, or the development of specialized surface-interface structures.The chemical reaction mechanism of the regeneration process is not well understood
The intercalation/expulsion kinetics of lithium ions during regeneration are affected by many factors. Most of the existing studies rely on empirical adjustment and lack an atomic-level simulation of the phase transition mechanism of LiFePO4 → FePO4 → LiFePO4. It is difficult to form a regeneration process that can be accurately controlled.Author contributions
Junhui Cai and Yanjuan Li wrote the original draft. Yiran Li and Shengnv Xu contributed to the preparation of figures and tables. Zhanzhan Wang and Jie Liu contributed to the review of typography and language. Shun Yang and Xiao Yan contributed to the review design and supervision.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 52474439) and Natural Science Research of Jiangsu Higher Education Institutions of China (No. 24KJA480002 and 21KJA430003).References
- D. Larcher and J. M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS PubMed.
- V. Anoopkumar, B. John and T. D. Mercy, ACS Appl. Energy Mater., 2020, 3, 9478–9492 CrossRef CAS.
- H. Kim, J. Hong, K.-Y. Park, H. Kim, S.-W. Kim and K. Kang, Chem. Rev., 2014, 114, 11788–11827 CrossRef CAS PubMed.
- R. A. Kerr, Science, 1998, 281, 1128–1131 CrossRef CAS.
- S. Jenu, I. Deviatkin, A. Hentunen, M. Myllysilta, S. Viik and M. Pihlatie, J. Energy Storage, 2020, 27, 101023 CrossRef.
- T. M. Gür, Energy Environ. Sci., 2018, 11, 2696–2767 RSC.
- Y. Chen, J. Qian, X. Hu, Y. Ma, Y. Li, T. Xue, T. Yu, L. Li, F. Wu and R. Chen, Adv. Mater., 2023, 35, 2212096 CrossRef CAS PubMed.
- D. Qin, L. Wang, X. Zeng, J. Shen, F. Huang, G. Xu, M. Zhu and Z. Dai, Energy Storage Mater., 2023, 54, 498–507 CrossRef.
- B. L. Ellis, K. T. Lee and L. F. Nazar, Chem. Mater., 2010, 22, 691–714 CrossRef CAS.
- Y. Miao, P. Hynan, A. von Jouanne and A. Yokochi, Energies, 2019, 12, 1074 CrossRef CAS.
- Y. Ding, Z. P. Cano, A. Yu, J. Lu and Z. Chen, Electrochem. Energy Rev., 2019, 2, 1–28 CrossRef CAS.
- Global EV Outlook 2024.
- J. Weng and L. Peng, Mater. Chem. Phys., 2022, 282, 125997 CrossRef.
- X.-G. Yang, T. Liu and C.-Y. Wang, Nat. Energy, 2021, 6, 176–185 CrossRef CAS.
- N. Nitta, F. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef CAS.
- T. Zhao, H. Mahandra, R. Marthi, X. Ji, W. Zhao, S. Chae, M. Traversy, W. Li, F. Yu, L. Li, Y. Choi, A. Ghahreman, Z. Zhao, C. Zhang, Y. Kang, Y. Lei and Y. Song, Chem. Eng. J., 2024, 485, 149923 CrossRef CAS.
- M. Shan, C. Dang, K. Meng, Y. Cao, X. Zhu, J. Zhang, G. Xu and M. Zhu, Mater. Today, 2024, 73, 130–150 CrossRef CAS.
- T. Yan, S. Zhong, M. Zhou, X. Guo, J. Hu, F. Wang, F. Zeng and S. Zuo, Nanotechnol. Rev., 2020, 9, 1586–1593 CrossRef CAS.
- H. Li, S. Xing, Y. Liu, F. Li, H. Guo and G. Kuang, ACS Sustainable Chem. Eng., 2017, 5, 8017–8024 CrossRef CAS.
- K. Liu, S. Yang, F. Lai, Q. Li, H. Wang, T. Tao, D. Xiang and X. Zhang, Ionics, 2021, 27, 5127–5135 CrossRef CAS.
- Y. Chen, L. Wang, H. He, J. Li, Y. Li, R. Liu, D. Li and Z. He, J. Mater. Sci.: Mater. Electron., 2020, 31, 4083–4091 CrossRef CAS.
- M. Zhang, L. Wang, S. Wang, T. Ma, F. Jia and C. Zhan, Small Methods, 2023, 7, e2300125 CrossRef PubMed.
- G. Ji, J. Wang, Z. Liang, K. Jia, J. Ma, Z. Zhuang, G. Zhou and H. M. Cheng, Nat. Commun., 2023, 14, 584 CrossRef CAS PubMed.
- Y. Zou, J. Cao, H. Li, W. Wu, Y. Liang and J. Zhang, Ind. Chem. Mater., 2023, 1, 254–261 RSC.
- J. Tang, H. Qu, C. Sun, X. Xiao, H. Ji, J. Wang, J. Li, G. Ji, X. Zhang, H. M. Cheng and G. Zhou, Adv. Mater., 2025, e2420238 CrossRef PubMed.
- T. Zhao, Y. Choi, C. Wu, Z. Zhang, C. Wang, D. Liu, W. Xu, H. Huang, X. Huo, W. Zhao, Z. Zhao and W. Li, Sci. Total Environ., 2024, 957, 177748 CrossRef CAS PubMed.
- T. Zhao, W. Li, M. Traversy, Y. Choi, A. Ghahreman, Z. Zhao, C. Zhang, W. Zhao and Y. Song, J. Environ. Manage., 2024, 351, 119670 CrossRef CAS PubMed.
- T. Zhao, H. Mahandra, R. Marthi, X. Ji, W. Zhao, S. Chae, M. Traversy, W. Li, F. Yu, L. Li, Y. Choi, A. Ghahreman, Z. Zhao, C. Zhang, Y. Kang, Y. Lei and Y. Song, Chem. Eng. J., 2024, 485, 149923 CrossRef CAS.
- P. Xu, Q. Dai, H. Gao, H. Liu, M. Zhang, M. Li, Y. Chen, K. An, Y. S. Meng, P. Liu, Y. Li, J. S. Spangenberger, L. Gaines, J. Lu and Z. Chen, Joule, 2020, 4, 2609–2626 CrossRef CAS.
- S.-H. Luo, Y. Wang, Q. Liu, P. Li, Z. Wang, S. Yan and F. Teng, J. Ind. Eng. Chem., 2024, 133, 65–73 CrossRef CAS.
- D. Saju, J. Ebenezer, N. Chandran and N. Chandrasekaran, Ind. Eng. Chem. Res., 2023, 62, 11768–11783 CrossRef CAS.
- J. Yan, J. Qian, Y. Li, L. Li, F. Wu and R. Chen, Adv. Funct. Mater., 2024, 34, 495 Search PubMed.
- S.-Q. Jiang, X.-G. Li, Q. Gao, X.-J. Lyu, S. N. Akanyange, T.-T. Jiao and X.-N. Zhu, Sep. Purif. Technol., 2023, 324, 104015 Search PubMed.
- R. Malik, D. Burch, M. Bazant and G. Ceder, Nano Lett., 2010, 10, 4123–4127 CrossRef CAS PubMed.
- Y. Jin, T. Zhang and M. Zhang, Adv. Energy Mater., 2022, 12, 2201526 CrossRef CAS.
- T. Yang, D. Luo, A. Yu and Z. Chen, Adv. Mater., 2023, 35, 2203218 CrossRef CAS PubMed.
- X. Chen, S. Li, Y. Wang, Y. Jiang, X. Tan, W. Han and S. Wang, Waste Manage., 2021, 136, 67–75 CrossRef CAS PubMed.
- Q. Jing, J. Zhang, Y. Liu, W. Zhang, Y. Chen and C. Wang, ACS Sustainable Chem. Eng., 2020, 8, 17622–17628 CrossRef CAS.
- Y. Yang, Z. Liu, J. Zhang, Y. Chen and C. Wang, J. Alloys Compd., 2023, 947, 169660 CrossRef CAS.
- Y. Peng, C. Zhong, M. Ding, H. Zhang, Y. Jin, Y. Hu, Y. Liao, L. Yang, S. Wang, X. Yin, J. Liang, Y. Wei, J. Chen, J. Yan, X. Wang, Z. Gong and Y. Yang, Adv. Funct. Mater., 2024, 34, 2404495 CrossRef CAS.
- K. Fu, X. Li, K. Sun, H. Yang, L. Gong and P. Tan, J. Power Sources, 2024, 606, 234568 CrossRef CAS.
- D. Wang, X. Wu, Z. Wang and L. Chen, J. Power Sources, 2005, 140, 125–128 CrossRef CAS.
- H. Gabrisch, J. Wilcox and M. M. Doeff, Electrochem. Solid-State Lett., 2008, 11, A25 CrossRef CAS.
- R. Xu, H. Sun, L. S. de Vasconcelos and K. Zhao, J. Electrochem. Soc., 2017, 164, A3333 CrossRef CAS.
- I. Azzouz, R. Yahmadi, K. Brik and F. B. Ammar, Electr. Eng., 2022, 104, 27–43 CrossRef.
- C. R. Birkl, M. R. Roberts, E. McTurk, P. G. Bruce and D. A. Howey, J. Power Sources, 2017, 341, 373–386 CrossRef CAS.
- Y. Liu and J. Xie, J. Electrochem. Soc., 2015, 162, A2208 CrossRef CAS.
- H. Zheng, L. Chai, X. Song and V. Battaglia, Electrochim. Acta, 2012, 62, 256–262 CrossRef CAS.
- P. Liu, J. Wang, J. Hicks-Garner, E. Sherman, S. Soukiazian, M. Verbrugge, H. Tataria, J. Musser and P. Finamore, J. Electrochem. Soc., 2010, 157, A499 CrossRef CAS.
- J. Deng, G. J. Wagner and R. P. Muller, J. Electrochem. Soc., 2013, 160, A487 CrossRef CAS.
- W. L. Chen, C. Chen, H. Xiao, C. W. Chen and D. Sun, Molecules, 2023, 28, 144343 Search PubMed.
- J. Wang and X. Sun, Energy Environ. Sci., 2012, 5, 5163–5185 RSC.
- D. Fu, W. Zhou, J. Liu, S. Zeng, L. Wang, W. Liu, X. Yu and X. Liu, Sep. Purif. Technol., 2024, 342, 127069 CrossRef CAS.
- H. Zhou, Z. Luo, S. Wang, X. Ma and Z. Cao, Sep. Purif. Technol., 2023, 315, 6057–6066 Search PubMed.
- X. Qiu, B. Zhang, Y. Xu, J. Hu, W. Deng, G. Zou, H. Hou, Y. Yang, W. Sun, Y. Hu, X. Cao and X. Ji, Green Chem., 2022, 24, 2506–2515 RSC.
- Y. Xu, X. Qiu, B. Zhang, A. Di, W. Deng, G. Zou, H. Hou and X. Ji, Green Chem., 2022, 24, 7448–7457 RSC.
- X. Chen, L. Yuan, S. Yan and X. Ma, Chem. Eng. J., 2023, 471, 123742 Search PubMed.
- J. Zhang, J. Zou, D. He, W. Hu, D. Peng, Y. Li, Z. Zhao, S. Wang, P. Li, S. Su, K. Ma and X. Wang, Green Chem., 2023, 25, 6057–6066 RSC.
- F. Sun, M. Gao, W. Jiao, L. Qi, Z. He, H. Zhang, Y. Cao, D. Song and L. Zhang, J. Alloys Compd., 2023, 965, 127069 Search PubMed.
- B. Chen, M. Liu, S. Cao, G. Chen, X. Guo and X. Wang, Mater. Chem. Phys., 2022, 279, 14323–14334 Search PubMed.
- J. Chen, Q. Li, J. Song, D. Song, L. Zhang and X. Shi, Green Chem., 2016, 18, 2500–2506 RSC.
- X. Li, M. Wang, Q. Zhou, M. Ge, M. Zhang, W. Liu, Z. Shi, H. Yue, H. Zhang, Y. Yin and S.-T. Yang, ACS Mater. Lett., 2024, 6, 640–647 CrossRef CAS.
- J. Wang, S. Ji, Q. Han, F. Wang, W. Sha, D. Cheng, W. Zhang, S. Tang, Y.-C. Cao and S. Cheng, J. Mater. Chem. A, 2024, 12, 15311–15320 RSC.
- R. Fu, P.-S. Zhang, Y.-X. Jiang, L. Sun and X.-H. Sun, Chemosphere, 2023, 311, 136993 CrossRef CAS PubMed.
- G. Nair, B. Soni and M. Shah, Groundw. Sustain. Dev., 2023, 23, 100980 CrossRef.
- S. Tian, Y. Cao, L. Dong, P. Shi, R. Li, Y. Jin, Y. Tu, C. Du, Z. Ren and Z. Zhou, J. Environ. Chem. Eng., 2024, 12, 112871 CrossRef CAS.
- B. Zhang, L. He, J. Wang, Y. Liu, X. Xue, S. He, C. Zhang, Z. Zhao, L. Zhou, J. Wang and Z. L. Wang, Energy Environ. Sci., 2023, 16, 3873–3884 RSC.
- Z. Liu, C. Zhang, M. Ye, H. Li, Z. Fu, H. Zhang, G. Wang and Y. Zhang, ACS Appl. Energy Mater., 2022, 5, 14323–14334 CrossRef CAS.
- C. Yang, J.-L. Zhang, Q.-K. Jing, Y.-B. Liu, Y.-Q. Chen and C.-Y. Wang, Int. J. Miner., Metall. Mater., 2021, 28, 1478–1487 CrossRef CAS.
- Q. Jing, J. Zhang, Y. Liu, W. Zhang, Y. Chen and C. Wang, ACS Sustainable Chem. Eng., 2020, 8, 17622–17628 CrossRef CAS.
- X. Song, Y. Xu, L. Cheng, T. Ren, B. Cai, D. Yang, J. Chen, T. Liang, R. Huang, E. H. Ang, X. Liao, B. Ge and H. Xiang, J. Energy Storage, 2024, 82, 110578 CrossRef.
- T. Ren, B. Zou, B. Cai, T. Liang, J. Chen, R. Huang, D. Yang, H. Xiang, E. H. Ang and X. Song, Waste Manage., 2024, 183, 209–219 CrossRef CAS PubMed.
- Z. Wang, H. Xu, Z. Liu, M. Jin, L. Deng, S. Li and Y. Huang, J. Mater. Chem. A, 2023, 11, 9057–9065 RSC.
- T. Ouaneche, M. Courty, L. Stievano, L. Monconduit, C. Guéry, M. T. Sougrati and N. Recham, J. Power Sources, 2023, 579, 233248 CrossRef CAS.
- H. Sun, X. Li, B. Wu, K. Zhu, Y. Gao, T. Bao, H. Wu and D. Cao, Chin. Chem. Lett., 2024, 110041 Search PubMed.
- S. Hao, Y. Lv, Y. Zhang, S. Liu, Z. Tan, W. Liu, Y. Xia, W. Yin, Y. Liao, H. Ji, Y. Kong, Y. Shao, Y. Huang and L. Yuan, Energy Environ. Sci., 2025, 18, 3750–3760 RSC.
- C. Feng, Y. Cao, L. Song, B. Zhao, Q. Yang, Y. Zhang, X. Wei, G. Zhou and Y. Song, Angew. Chem., Int. Ed., 2025, 64, e202418198 CrossRef CAS PubMed.
- X. Zhong, X. Mao, W. Qin, H. Zeng, G. Zhao and J. Han, Waste Manage., 2023, 156, 236–246 CrossRef CAS PubMed.
- X. Song, B. Zou, J. Wang, T. Ren, B. Cai, B. Ge, J. Chen, T. Liang, E. H. Ang, X. Liao and H. Xiang, Mater. Today Chem., 2024, 38, e202409929 Search PubMed.
- M. Xiao, X. Fu, M. Chen, M. Ye, C. Zhu, F. Wan and X. Guo, ACS Appl. Mater. Interfaces, 2025, 17, 12199–12207 CrossRef PubMed.
- M. Xiao, X. Fu, M. Chen, M. Ye, C. Zhu, F. Wan and X. Guo, ACS Appl. Mater. Interfaces, 2025, 17, 12199–12207 CrossRef PubMed.
- Y. Cao, J. Li, D. Tang, F. Zhou, M. Yuan, Y. Zhu, C. Feng, R. Shi, X. Wei, B. Wang, Y. Song, H.-M. Cheng and G. Zhou, Adv. Mater., 2024, 36, 2414048 CrossRef CAS PubMed.
- Z. Jiang, J. Sun, P. Jia, W. Wang, Z. Song, X. Zhao and Y. Mao, Sustainable Energy Fuels, 2022, 6, 2207–2222 RSC.
- J. Sun, Z. Jiang, P. Jia, S. Li, W. Wang, Z. Song, Y. Mao, X. Zhao and B. Zhou, Waste Manage., 2023, 158, 125–135 CrossRef CAS PubMed.
- K. Jia, J. Ma, J. Wang, Z. Liang, G. Ji, Z. Piao, R. Gao, Y. Zhu, Z. Zhuang, G. Zhou and H. M. Cheng, Adv. Mater., 2023, 35, e2208034 CrossRef PubMed.
- Y. Gou, C. Qi, R. Li, X. Liu, Z. Zhou, M. Zhang, Q. Sun, L. Song and Y. Jin, Electrochim. Acta, 2024, 488, 169660 CrossRef.
- G. Ji, J. Wang, Z. Liang, K. Jia, J. Ma, Z. Zhuang, G. Zhou and H. M. Cheng, Nat. Commun., 2023, 14, 584 CrossRef CAS PubMed.
- D. Peng, X. Wang, S. Wang, B. Zhang, X. Lu, W. Hu, J. Zou, P. Li, Y. Wen and J. Zhang, Green Chem., 2022, 24, 4544–4556 RSC.
- D. Yang, Z. Fang, Y. Ji, Y. Yang, J. Hou, Z. Zhang, W. Du, X. Qi, Z. Zhu, R. Zhang, P. Hu, L. Qie and Y. Huang, Angew. Chem., Int. Ed. Engl., 2024, e202409929, DOI:10.1002/anie.202409929.
- S. Zhou, J. Du, X. Xiong, L. Liu, J. Wang, L. Fu, J. Ye, Y. Chen and Y. Wu, Green Chem., 2022, 24, 6278–6286 RSC.
- Y. Yang, J. Zhang, H. Zhang, Y. Wang, Y. Chen and C. Wang, Energy Storage Mater., 2024, 65, 103081 CrossRef.
- D. Yang, Z. Fang, Y. Ji, Y. Yang, J. Hou, Z. Zhang, W. Du, X. Qi, Z. Zhu, R. Zhang, P. Hu, L. Qie and Y. Huang, Angew. Chem., Int. Ed., 2024, 63, e202409929 CrossRef CAS PubMed.
- S. Zhou, J. Du, X. Xiong, L. Liu, J. Wang, L. Fu, J. Ye, Y. Chen and Y. Wu, Green Chem., 2022, 24, 6278–6286 RSC.
- X. Zhao, H. Chen, H. Wu, Y. Zhao and J. Luo, ACS Nano, 2024, 18, 21125–21134 CrossRef CAS PubMed.
- B. Wang, Y. Li, X. Zhu, F. Guo, D. Zhang and H. Wang, New J. Chem., 2024, 48, 10949–10960 RSC.
- Y. Yang, Z. Liu, J. Zhang, Y. Chen and C. Wang, J. Alloys Compd., 2023, 947, 169660 CrossRef CAS.
- Y. Zou, J. Cao, H. Li, W. Wu, Y. Liang and J. Zhang, Ind. Chem. Mater., 2023, 1, 254–261 RSC.
- B. Chen, M. Liu, S. Cao, H. Hu, G. Chen, X. Guo and X. Wang, J. Alloys Compd., 2022, 924, 166487 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |



