A prefilled, ready-to-use electrophoresis based lab-on-a-chip device for monitoring lithium in blood†
Arjan
Floris
a,
Steven
Staal
a,
Stefan
Lenk
a,
Erik
Staijen
a,
Dietrich
Kohlheyer‡
b,
Jan
Eijkel
b and
Albert
van den Berg
b
aMedimate BV, Enschede, The Netherlands
bBIOS/the Lab on a Chip group, MESA+ Institute for Nanotechnology, Twente University, The Netherlands
First published on 7th June 2010
Abstract
We present the Medimate MultireaderR, the first point-of-care lab on a chip device that is based on capillary electrophoresis. It employs disposable pre-filled microfluidic chips with closed electrode reservoirs and a single sample opening. Several technological innovations allow operation with closed reservoirs, which is essential for reliable point-of-care operation. The chips are inserted into a hand-held analyzer. In the present application, the device is used to measure the lithium concentration in blood. Lithium is quantified by conductivity detection after separation from other blood ions. Measurements in patients show good accuracy and precision, and there is no difference between the results obtained by skilled and non-skilled operators. This point-of-care device shows great promise as a platform for the determination of ionic substances in diagnostics or environmental analysis.
Introduction
The advent of point-of care (POC) testing in the 1980s has led to a revolution in clinical medicine and patient care.1 Clinical analyses can now be performed by nurses at the bedside or in the emergency department by non-laboratory trained personnel. A typical system for bedside use is for example the i-StatR, introduced in 1992, which combines mechanical fluid handling with disposable cartridges for example for the determination of various inorganic cations by ion-selective electrodes and glucose by amperometry.2 Before the introduction of the i-Stat, a revolution in patient home care had already taken place by the introduction of blood glucose monitors. In the 1990s the development of POC devices received a new impulse by the use of micromachining technologies that allowed greater control in the manipulation of liquids on the microscale.3,4 Since then a number of handheld POC diagnostic devices have been developed that combine microfluidic methods for sample and fluid manipulation with for example optomagnetic immunosensing (Philips handheld diagnostics) or fluorescence immunosensing (Biosite TriageR cardiac panel).5,6One of the earliest microfluidics technologies employed for chemical analysis on chip was electrophoresis, reported in 1993 by Harrison and Manz for the separation of amino acids.7 Their electrokinetic platform was subsequently commercially developed into apparatus for the clinical and forensic laboratory by companies such as Caliper Life Sciences and Agilent Technologies. Perhaps surprisingly, electrophoretic separation has up to now not been employed in POC devices. The reason for this in our view is that the method does not lend itself easily to operation by untrained personnel, since it generally involves manipulation of sample and buffer by pipeting steps and the manipulation of high-voltage electrodes. Further cumbersome points are the control of electroosmotic flow and the integration of detection. To address these points we have developed a pre-filled microfluidic chip with closed reservoirs which forms the basis of the first electrophoresis-based POC device suitable for use by untrained operators which we present here. The device is a further development of the chip reported by Vrouwe et al.8,9,10 It is based on electrophoretic separation and conductivity detection and has a single opening for sample introduction. Operator actions needed are solely the deposition of a sample drop (capillary or venous blood or serum) at the sample opening, closing the chip cartridge and inserting it into a handheld analyzer. Essential for the use of closed electrode reservoirs have been several technological innovations in the present chip such as the on-chip inclusion of an expansion gas bubble and the use of large electrode reservoirs. The chip and its holder are disposable to prevent problems and risks associated with cleaning and to minimize the number of operator actions.
As a first application of this new platform we demonstrate the determination of the lithium concentration in serum and capillary blood obtained by a finger-stick. Determination of the lithium concentration is relevant for the monitoring and treatment of patients with bipolar disorder, who are treated with oral lithium which has a small therapeutic window. Approximately 1–2% of the population suffers from this disease and in the Netherlands 20–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 patients currently use oral lithium therapy.11 The clinical benefit of a POC determination of lithium could be an increasing independence for the patients, who can use the outcome to personally adjust their oral lithium therapy, if needed in consultation with their physician. Many methods exist for the determination of lithium in blood, most importantly flame emission spectrometry, ion selective electrode (ISE) measurement and colorimetry.12 Several of these methods are well amenable to the lab on a chip format, such as colorimetry and the ISE. We however decided to develop the electrophoretic POC platform presented here because of its general usefulness for the determination of ionic substances in medical diagnostics and environmental monitoring.
000 patients currently use oral lithium therapy.11 The clinical benefit of a POC determination of lithium could be an increasing independence for the patients, who can use the outcome to personally adjust their oral lithium therapy, if needed in consultation with their physician. Many methods exist for the determination of lithium in blood, most importantly flame emission spectrometry, ion selective electrode (ISE) measurement and colorimetry.12 Several of these methods are well amenable to the lab on a chip format, such as colorimetry and the ISE. We however decided to develop the electrophoretic POC platform presented here because of its general usefulness for the determination of ionic substances in medical diagnostics and environmental monitoring.
Measurement procedure and microfluidic chip
Fig. 1 illustrates the measurement procedure. A disposable plastic cartridge containing the microfluidic chip is taken from a blister pack, the cartridge is opened and a seal is removed from the sample opening of the microfluidic chip. A drop of sample (e.g. serum or capillary blood) is then deposited at this opening. Subsequently the cartridge is closed and inserted into the handheld analyzer (Multireader), after which the measurement is started. Closure of the cartridge prevents sample contamination during the measurement. | ||
| Fig. 1 (1) A cartridge containing a pre-filled microfluidic chip; (2) the cartridge is opened, the chip seal is removed and a drop of blood is deposited at the sample opening; (3) the cartridge is inserted in the Multireader and the measurement is started. | ||
Fig. 2 shows a photograph of the glass microfluidic chip as well as a layout of the chip with the different functional structures indicated. The chip is prefilled with electrolyte solution and has three openings (shown at the top): one to a channel for sample conductivity measurement (E), one to an evaporation channel (2) and one (1) to the separation channel. After application of a sample drop at these openings, sample fills the channel leading towards the electrodes E by capillary forces, where the sample conductivity is measured. This channel is open at both sides to allow complete capillary filling. Simultaneously the measurement procedure starts by applying an electrical field between high-voltage (HV) electrodes A and B. The injection channel, including the double-T, fills with sample cations in order of mobility by moving boundary electrophoresis (MBE).13 Blood cells do not enter the channel due to their negative charge, providing a convenient sample preparation method.10 Because the conductivity of the filling solution is smaller than the conductivity of blood, the ions are injected at lower concentrations in the double T channel than their concentrations in the blood sample (destacking).9 The injection voltage is applied for a time sufficient to reach a constant concentration of the lithium ions in the double T injector. Subsequently an electrical field is applied between electrodes C and D, separating the cations by capillary (zone) electrophoresis (CE). Separated bands of cations are detected by the conductivity electrodes 6. Because the electrode reservoirs are sealed, no net solution transport by electroosmosis occurs during injection or separation. The lithium concentration in the sample is calculated by application of a software algorithm on a number of measured variables, details of which are given in the experimental section.
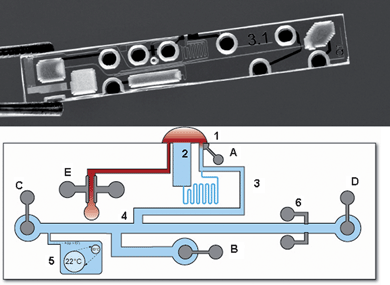 | ||
| Fig. 2 Top: photograph of the microfluidic chip; bottom: Schematic indication of the different functional units (the exact locations slightly differ from the photograph for the sake of clarity). (1) Sample opening with applied sample droplet; (2) evaporation reservoir; (3) injection channel for injection of cations by moving boundary electrophoresis; (4) double-T injector; (5) reservoir with gas bubble for liquid expansion control; (6) conductivity detection electrodes; (A, B) high-voltage injection anode and cathode; (C,D) high-voltage separation anode and cathode; (E) Electrodes for the conductometric determination of the sample conductivity. | ||
Technological innovations for electrophoresis in a sealed chip
For the first time an entirely closed system is demonstrated for electrophoretic separation. In the past hydrodynamically closed systems have been used, but here open electrode reservoirs were connected to a closed channel system by semipermeable membranes.14,15 For POC analysis however also the electrode reservoirs have to be sealed. Such a prefilled microfluidic chip with a single opening solely for sample introduction and sealed electrode reservoirs is highly convenient since the operator does not need to manipulate solutions. When this chip is placed in a cartridge and inserted into a handheld measurement device, user contact with the high voltage electrodes necessary for electrophoretic separation is also avoided. Before such a platform however can be used a number of technological hurdles have to be surmounted, as will now be discussed.From the moment the seal is removed from the sample inlet at the start of the measurement, solution evaporates from the closed channel structure, causing rapid retraction of the liquid meniscus into the sample channel. When subsequently a sample droplet is applied, it will as a result be separated from the filling solution by an air bubble. This problem has been solved by inclusion of a second evaporation channel parallel to the sample inlet channel with an opening of larger cross-sectional area (see Fig. 3), which is closed and opened by the same seal as the sample opening. The wider cross-sectional area of this channel will cause a lower Laplace pressure (capillary pressure) at its opening, with the result that the solution meniscus will recede here first when evaporation occurs and the meniscus at the sample opening will stay in place.16,17 The evaporation channel has been designed to allow at least one minute for sample application. Fig. 3A shows the two channels directly after removing the seal and Fig. 3B ten minutes after removing the seal in a room with a relative humidity of 30% and a temperature of 23 °C. Clearly the meniscus in the (right-hand side) injection channel is pinned while the meniscus in the evaporation channel recedes.
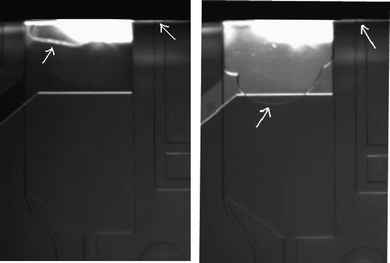 | ||
| Fig. 3 (A) Evaporation channel (left) and injection channel (right) just after removing the chip seal and (B) 10 min after removing the seal. The white arrows indicate the menisci in both channels. The top one-third of the evaporation channel in these photographs is occupied by an electrode which is used to diagnose proper channel filling in the manufacturing phase. | ||
The use of moving boundary electrophoresis for injection of the sample ions in the present case involves destacking, essentially diluting the ions from the (higher) concentration in the sample to a (lower) concentration in the injection channel.9 The extent of destacking will be determined by the difference between the (unknown) conductivity of the sample and the (known) conductivity of the filling solution. The sample conductivity must therefore be determined, for which purpose a reservoir is filled with the sample solution by capillary forces, and the conductivity is measured in contact mode by two metal electrodes (Fig. 2, E).17
The liquid volume in the electrode reservoirs is several orders of magnitude larger than the volume in the channels, which causes a problem when thermal expansion occurs in the sealed chips. When small temperature increases occur during storage, expansion of the solution in the chips cause a pressure increase that drives solution out past the seal. When subsequently temperature and pressure decrease, the seal elastically deforms and leaves an underpressure in the chip. Removal of the seal from a chip with underpressure causes the solution meniscus to retract into the injection channel, leaving a long air plug at the sample opening. In order to prevent this problem, an extra reservoir with a bubble of inert gas (approximately 0.5 μL in volume) was integrated in the chip (Fig. 2 number 5).18 In the experimental section some details on the procedure are given. Fig. 4A shows the theoretical pressure inside the chip as a function of temperature for four different bubble sizes (defined as percentage of the total volume in the chip), as well as measured values for a bubble volume of 2.5%. Fig. 4B shows photographs of the bubble reservoir at temperatures of 22 and 70 °C, demonstrating the decrease in bubble size with increasing temperature. As a result of the theoretical analysis it was decided to create bubbles with a volume of 5% of the volume in the chip, so that only moderate changes occur in the pressure inside the chip (less than 3 bar for a temperature increase from 20 °C to 70 °C), which are insufficient to drive the filling solution past the seal.
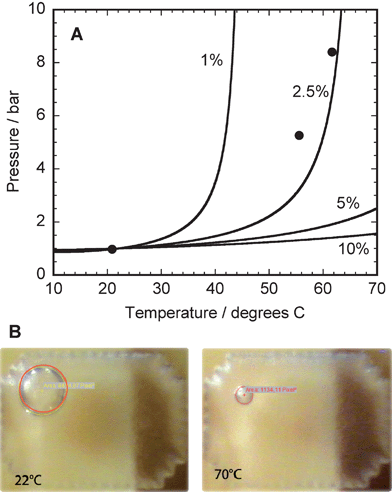 | ||
| Fig. 4 (A) Theoretical dependence of the intra-chip pressure on the temperature for four different bubble dimensions (defined as volume percentage of the total intra-chip volume). The black circles indicate measurement data for a bubble with a 2.5% volume. (B) Photomicrographs of the bubble reservoir inside the chip at 22 °C and 70 °C. The gas bubble is indicated by the red circle. | ||
A further consequence of the sealing of the chip is that hydrogen and oxygen gas that is generated by electrolysis during current application will not be able to escape to the atmosphere.19 The gases will therefore dissolve in the reservoir liquid until the solution becomes saturated, which will occur above a certain threshold of time-integrated current. After that moment, gas bubbles will form which will cause undesirable solution transport. This problem was solved by creating powder-blasted electrode reservoirs of a volume sufficiently large to dissolve all the gas evolved during a measurement.
Experimental
Analyzer
As shown in Fig. 1, the system is composed of two main parts, a hand-held analyzer (Medimate Multireader) and a disposable cartridge containing a microfluidic chip. The analyzer is powered by a 12 V DC power source. When the cartridge is inserted in the handheld analyzer, spring-loaded connectors connect the electronics to electrode pads on the microfluidic chip. The analyzer contains a high voltage (HV) power supply with switching electronics to independently address four electrodes, as well as electronics for AC conductivity detection (CD), electronics for the AC conductometric determination of the blood sample conductivity, electronics for data processing and an output screen. The HV and CD systems are electrically separated to prevent the flow of current between them.18 Precautions have also been taken to minimize capacitive coupling between HV and CD to limit transient currents through the conductivity detection electrodes when the HV supply is switched.Microfluidic chip
Fig. 2 shows the layout of the fluidic and electrical connections on the chip. The function of the different structures has been explained in the previous section. Chips were manufactured in glass by Micronit BV. In one wafer the (shallow) fluidic channels and (deep) electrode reservoirs, as well as the through holes were made. All channels were wet etched to a depth of 10 μm and a top width of 50 μm. Electrode reservoirs, the bubble reservoir and through-holes were made by powder blasting. On a second wafer all on-chip electrical connections and electrodes (for conductivity measurement and HV application) were manufactured by thin layer deposition of platinum on the channel wafer on an adhesion layer. Both wafers were subsequently fusion bonded and diced.Filling solution
Chips are filled with a specifically designed buffer solution. Vacuum filling is employed to fill the closed channel system through the single inlet opening. For this procedure the chip is placed in a container which is evacuated. By tuning the evacuation time and employing precisely calculated dimensions for the channel section that leads to the bubble reservoir, a well controlled amount of gas could be left in this reservoir while the remainder of the chip is sufficiently evacuated. Subsequent introduction of buffer solution in the container then leads to complete filling of all channels and reservoirs but for a gas bubble in the bubble reservoir. The size of this bubble proved to be quite reproducible, indicating a fairly constant channel height in the chips. The bubble was found not to decrease in size in time, indicating negligible influence of gas dissolution. Chips are finally sealed with a removable seal and immobilized in plastic cartridges.High voltage protocol
After insertion of the cartridge into the analyzer, the measurement protocol is started. An injection voltage and separation voltage are then applied for a specified time. The applied fields are comparable to the ones previously published.9Conductivity detection
For conductivity detection platinum conductivity electrodes are employed that are in direct contact with the solution.20 Geometry and location of the electrodes with respect to channel and reservoirs have been optimized to reduce electrolysis and minimize inter-chip variance of the detection signal. The conductivity is measured by applying an AC voltage signal at a specified frequency. Identical electronics has recently been applied in a device for sperm cell counting and more details on the conductivity measurement procedure can be found in ref. 21.Data processing and determination of the lithium concentration
Software in the analyzer first checks whether the measured output signal of the conductivity electrodes fulfills a number of criteria, e.g. concerning the baseline drift. If one of these criteria is not met, the measurement is declared invalid and the results are discarded. This protocol means to ensure fail-safe operation. The lithium concentration is subsequently calculated by applying an algorithm which was derived by performing a multivariate regression analysis on a large set of lithium measurements by the Multireader and the IL943 flame spectrometer measurements in samples obtained at the same time from the same patient. The input variables for this regression were for the Multireader a.o. the area under the curve of the lithium and sodium peaks (sodium by this procedure functions as an internal standard), the retention time of the sodium peak and the sample conductivity. The input variable for the IL943 flame spectrometer was the lithium concentration determined by this device. The algorithm used for the data displayed in Table 1 and Fig. 6 (see later), where Multireader serum measurements are compared with IL943 serum measurements, was derived from a regression for serum samples with lithium concentrations from 0 to 4.0 mM. The algorithm used for the data displayed in Fig. 7 (see later) and Table 2, where Multireader measurements in capillary blood are compared with IL943 measurements in serum, were derived from a regression for samples of serum (0–4.0 mM) and capillary and venous blood measured by the Multireader and samples of serum prepared from venous blood measured by the IL943.Clinical measurement protocols
The Multireader was calibrated against the IL943 flame photometer, which is the golden standard for lithium measurement. The IL943 was calibrated using the IL Standard IL0003331350 (140 mM sodium, 5 mM potassium and 1 mM lithium) and IL Standard IL 0009756400 (1.5 mM caesium).To calibrate the Multireader, a first set of measurements was performed. Measurement data on serum samples were obtained with the Multireader and the lithium concentration in the same samples was measured with the flame photometer. A total of 610 measurements were performed over a period of 4 months by multiple operators employing five Multireader devices, making on average 24 determinations per serum sample. Of these, 15 outliers were identified which were not included in the determination of the standard deviation. Six of these outliers were accompanied by an abnormal shape of the electropherogram, enabling future software detection for fail-safe operation. Nine outliers have not been correlated with a phenomenon. To investigate a larger concentration range relevant for intoxication cases, two samples were spiked with higher concentrations of lithium. The calibration procedure involved performing a multivariate regression analysis as described in the previous paragraph, which was then used to calculate the lithium concentration in the samples. These data are shown in Table 1 and Fig. 5.
In a second series of experiments (Table 2 and Fig. 6), lithium concentration values obtained with the Multireader for both venous and capillary blood were compared with values obtained for serum from the same patients using the IL943. From ten patients receiving lithium therapy, 6 tubes of venous blood were taken at the start (t < 5 min) and end (100 min < t < 120 min) of the experiment. During the experiment (5 < t < 120 min), 8 finger sticks were applied, each finger stick producing 5 drops of blood. Four finger sticks were applied by a professional and four by the patient. Lithium levels were measured with the Multireader in the venous blood from one tube obtained at the start and end of the procedure (five measurements per tube within 10 min). Lithium was also measured with the IL943 in serum prepared from the other five tubes obtained at the start and end of the procedure. In every finger-stick droplet the lithium concentration was determined with the Multireader. Data were generated by one single operator using five Multireaders. IL943 data were generated using the standard procedure of the hospital by one operator and one device. The algorithm used for derivation of the lithium concentration was obtained by a multivariate regression on measurements of lithium in serum, venous and capillary blood (Multireader) and lithium in serum of the same patient (IL943).
Using the data from the second series of experiments it was also investigated whether there was a difference in lithium concentration determined by a skilled professional or by the patient because in the course of each experiment 20 lithium measurements were performed by a skilled professional and 20 measurements by the patient.
Results and discussion
Fig. 5 shows a section of two superimposed electropherograms obtained from patient serum with different lithium concentrations. In each electropherogram two peaks can be distinguished, a large peak corresponding to sodium and a small peak corresponding to lithium. The observed baseline drift is attributed to the gradual heating of the solution during the measurement which increases the conductivity, and also to transient capacitive charging of the detection electrodes when the separation voltage is applied. As mentioned in the experimental section, the lithium concentration is derived by an algorithm which includes the area under the curve of the lithium and sodium peaks, the retention time of the sodium peak and the sample conductivity.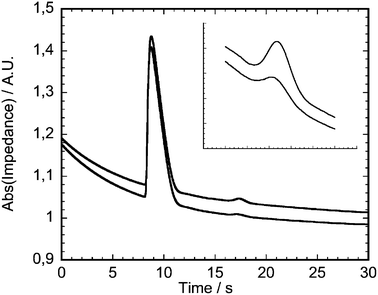 | ||
| Fig. 5 Two superimposed electropherograms of lithium determinations in serum. Lithium concentrations 0.73 mmol/L and 1.47 mmol/L. The two peaks from left to right represent sodium and lithium. The inset for comparison shows the lithium peaks enlarged and with reduced offset. | ||
Measurements in serum
The quality of the data can be characterized by the imprecision (the spread in the data) and the accuracy (the difference between the mean and the true value) of the measurements. Table 1 gives data on the imprecision of the lithium serum measurements using the Multireader. The standard deviation given is the standard deviation of the residuals from the mean value obtained for each sample. Data were obtained over a period of 4 months by multiple operators using five Multireader devices. To include data for lithium concentrations in the toxic region above 1 mM, two serum samples were spiked with lithium. It can be seen that the standard deviation at all concentration ranges is equal to approximately 0.04 mM. As a consequence the coefficient of variation decreases with increasing lithium concentration. For comparison, the imprecision of the (single) flame photometer in the investigated concentration range was found to be about 0.012 mM in the present measurement series.| Conc. range, mM | n, each conc. range | Medimate Multireader | |
|---|---|---|---|
| Standard dev. of residuals, mM | CV % | ||
| 0.21–0.4 | 4 | 0.026 | 8.6 |
| 0.41–0.60 | 61 | 0.041 | 8.2 |
| 0.61–0.80 | 86 | 0.037 | 5.2 |
| 0.81–1.00 | 428 | 0.041 | 4.5 |
| 1.5 | 18 | 0.067 | 4.4 |
| 2.0 | 7 | 0.042 | 2.1 |
| 4.0 | 6 | 0.064 | 1.6 |
The accuracy of the Multireader data was established by comparison with the golden standard, the IL943 flame photometer. A panel of clinical psychiatrists, Dutch healthcare organizations and the patient organization for patients with bipolar disorder formulated as targets for clinical efficiency that the device should be able to measure between 0.4 and 4 mM with for 95% of the cases an accuracy of 0.1 mM or 10% relative to the IL943 values. Fig. 6 plots the Multireader measurements against flame photometer measurements on the same serum samples. Good linearity was observed over the entire concentration range, with a regression coefficient R = 0.955. In the entire measurement range (0.2–1.0 mM) the Multireader data remained within the predefined specifications (95% of the data accurate within 0.1 mM or 10% with as reference the values obtained with the IL943). The inset of Fig. 5 shows the data for the measurement range from 0–4 mM by including the samples that were spiked with lithium. A linear regression analysis on these data gives the relation Li(Multireader) = −0.027 + 1.03*Li(IL943) with a regression coefficient R = 0.993. For lithium concentrations below 0.2 mM, the software algorithm for the concentration determination at the moment overestimates the lithium concentrations. If such a value was measured, the device display therefore presently is programmed to read ‘concentration below 0.2 mM’. Corrections for the algorithm are planned to address this point.
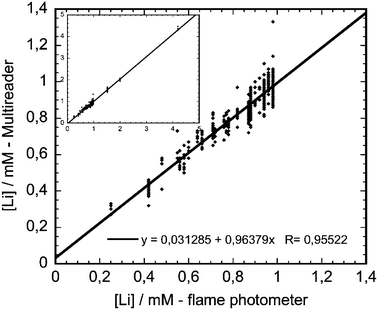 | ||
| Fig. 6 Comparison of Multireader measurements with flame photometer measurements, both in serum. The inset shows a second comparison, where lithium concentrations are included up to 4 mM, obtained from spiked samples. | ||
Measurements in capillary blood versus measurements in serum
The Multireader is ultimately designed for use by the patient at home, in which case capillary blood will be obtained by the patient himself with a finger stick procedure. It is therefore important to establish how the lithium concentrations determined in capillary blood compare to those determined in serum. Table 2 presents the imprecision of measurement data on capillary blood with the device. The average standard deviation from the residual is 0.043, which is comparable to the value obtained in serum measurements (average standard deviation 0.040, Table 1). The Multireader results for capillary blood were furthermore compared to the flame photometer results for serum prepared from venous blood and to Multireader results for venous blood of the same patients. Fig. 7 presents the data obtained, whereby the capillary blood value represents a mean of 20 determinations. It can be seen that all values obtained for the capillary blood lithium fall within 10% of the values obtained for the serum lithium with the flame photometer with the exception of patient 1 where the lithium concentration was too low to be accurately determined with the Multireader (see remark in the previous paragraph). Pharmacokinetic effects on the lithium concentration level can also be distinguished, e.g. for patient 7 where a clear concentration decrease is observed during the approximately 100 min of this assay.| Conc. range, mM | n, each conc. range | Medimate Multireader | |
|---|---|---|---|
| Standard dev. of the residuals, mM | CV % | ||
| 0.21–0.4 | 115 | 0.036 | 13 |
| 0.41–0.60 | 51 | 0.036 | 8.3 |
| 0.61–0.80 | 53 | 0.053 | 7.1 |
| 0.81–1.00 | 31 | 0.067 | 7.4 |
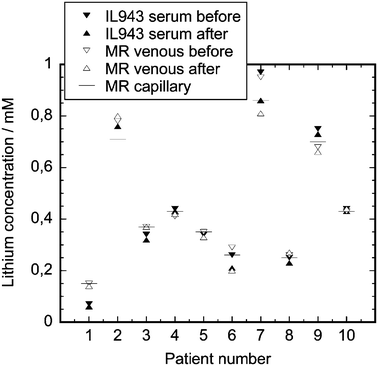 | ||
| Fig. 7 Results of a measurement series on 10 patients, where first lithium was determined in serum by the flame photometer (IL943 serum before) and in venous blood by the Multireader (MR venous before), then over the course of approximately 100 min lithium was measured in capillary blood (average of 40 measurements is presented as MR capillary), and finally lithium was determined in serum (IL943 serum after) and in venous blood (MR venous after). | ||
For the determination of the lithium concentration values in capillary blood, a software algorithm was used derived from a multivariate regression analysis on both the serum and capillary blood measurements. Application of this algorithm resulted in a slope of 0.83 for the regression line of capillary blood lithium (Multireader) against serum lithium (flame photometer), a slope significantly different from 1. This result seems to indicate that the lithium concentration in capillary blood is significantly lower than in serum. At present the cause for this has yet to be established. A possible explanation is that the difference in conductivity between whole blood and serum causes a relative decrease of the amount of lithium which is injected from capillary blood when compared to serum. Another possibility is that some erythrocyte lysis occurs during injection. This however seems less likely since the lithium concentration in erythrocytes is approximately equal to the lithium concentration in plasma.22 In view of the fact that the imprecision for the measurements in serum and capillary blood however is comparable, determination of lithium in capillary blood seems well possible by a future adjustment of the software algorithm.
Untrained versus trained operators
To establish whether the Multireader can be successfully operated by untrained personnel, the lithium concentration levels for ten patients in capillary blood were determined by both the patients themselves and skilled operators. The data of this analysis are represented in Table 3 and indicate that there is no difference in the lithium values determined by patients or professional operators. The Multireader is therefore suitable for its intended use by the patients at home.| Source | Number of determinations | Mean of the residuals | Standard deviation of the residuals |
|---|---|---|---|
| a One-way anova: No difference between patient and professional (P = 0.993). | |||
| Patient | 126 | +0.010 | 0.074 |
| Professional | 125 | +0.010 | 0.063 |
Reliability and shelf life
The Multireader is equipped with software to ensure fail-safe operation by discarding measurements on the basis of a number of characteristics of the electropherogram. At present this procedure leads to a considerable failure rate. To make the device user-friendly and to limit operation costs, a primary aim is to reduce the failure rate to below 5%. The shelf life of the pre-filled chips at present is about 1 month and is limited by deterioration of the filling solution.Speed of assay
The duration of an assay is 3.5 min from the moment of insertion of the cartridge. In comparison, a clinical cito determination of the lithium concentration in the hospital typically takes one hour, mainly due to the logistics of transport and communication. Generally, lithium measurements take one or two days. The Multireader therefore enables much more rapid POC determinations.Discussion
At present the Medimate Multireader is being field tested in a number of psychiatric wards and clinical laboratories. Simultaneously improvements are being made in precision and accuracy. In a second stage the device will be tested by patients at home. It is expected that after release on the market the cost of a single lithium assay will be comparable to that of the standard flame photometer assay in the clinical laboratory. The assay cost is mainly determined by the manufacturing cost of the disposable chip, not by its materials. To minimize this cost, the chip area and the amount of process steps were minimized. The use of the device will have considerable advantages over the laboratory assay, mainly because of the simple infrastructure needed and the convenience for the patient. Patients who monitor their oral lithium medication with the Multireader will no longer be dependent on the vicinity of a hospital, increasing their mobility. The speed with which the result is obtained will furthermore enable a rapid reaction in case dangerously high lithium concentrations are measured.Future developments
The Multireader can in principle be employed for many different POC applications, under the condition that suitable protocols can be developed. In a first approach these will concern ionic substances, though the electrokinetic platform can be adjusted to determine non-ionic substances by e.g. MEKC. Protocols are presently being developed for the determination in blood of phosphate, sodium, potassium, L-Dopa and creatinine, and of sodium in urine. A large veterinarian field test is also ongoing to determine the concentrations of calcium and magnesium in blood of cows to diagnose milk fever, by Blue4Green BV (Enschede, the Netherlands). Results on this test will be reported shortly.Acknowledgements
The Stichting Technische Wetenschappen is gratefully acknowledged for a first and second phase valorization grant. Achmea and SenterNovem are acknowledged for their financial support. Gertjan van der Sluijsveer is thanked for his critical reading of the manuscript. The Medisch Spectrum Twente (Cees Doelman and Tony Knuijf) and Pieternel Kölling are gratefully acknowledged for their organizational support.References
- U. R. Jahn and H. van Aken, Near-patient testing-point-of-care or point of costs and convenience?, Br. J. Anaesth., 2003, 90, 425–427 CrossRef CAS.
- K. A. Erickson and P. Wilding, Evaluation of a Novel Point-of-Care System, the i-STAT Portable Clinical Analyzer, Clin. Chem., 1993, 39, 283–287 CAS.
- A. J. Tüdos, G. A. J. Besselink and R. B. M. Schasfoort, Trends in miniaturized total analysis systems for point-of-care testing in clinical chemistry, Lab Chip, 2001, 1, 83–95 RSC.
- S. K. Sia and L. J. Kricka, Microfluidics and point-of-care testing, Lab Chip, 2008, 8, 1982–1983 RSC.
- D. M. Bruls, T. H. Evers, J. A. H. Kahlman, P. J. W. van Lankvelt, M. Ovsyanko, E. G. M. Pelssers, J. J. H. B. Schleipen, F. K. de Theije, C. A. Verschuren, T. van der Wijk, J. B. A. van Zon, W. U. Dittmer, A. H. J. Immink, J. H. Nieuwenhuis and M. W. J. Prins, Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles, Lab Chip, 2009, 9, 3504–3510 RSC.
- F. S. Apple, R. H. Christenson, R. Valdes, A. J. Andriak, A. Berg, S.-H. Duh, Y.-J. Feng, S. A. Jortani, N. A. Johnson, B. Koplen, K. Mascotti and A. H. B. Wu, Simultaneous Rapid Measurement of Whole Blood Myoglobin, Creatine Kinase MB, and Cardiac Troponin I by the Triage Cardiac Panel for Detection of Myocardial Infarction, Clin. Chem., 1999, 45, 2199–205.
- D. J. Harrison, K. Fluri, K. Seiler, Z. Fan, C. S. Effenhauser and A. Manz, Micromachining a Miniaturized Capillary Electrophoresis-Based Chemical Analysis System on a Chip, Science, 1993, 261, 895–897 CrossRef CAS.
- E. X. Vrouwe, R. Luttge and A. van den Berg, Direct measurement of lithium in whole blood using microchip capillary electrophoresis with integrated conductivity detection, Electrophoresis, 2004, 25, 1660–1667 CrossRef CAS.
- E. X. Vrouwe, R. Luttge, W. Olthuis and A. van den Berg, Microchip analysis of lithium in blood using moving boundary electrophoresis and zone electrophoresis, Electrophoresis, 2005, 26, 3032–3042 CrossRef CAS.
- E. X. Vrouwe, R. Luttge, I. Vermes and A. van den Berg, Microchip Capillary Electrophoresis for Point-of-Care Analysis of Lithium, Clin. Chem., 2007, 53, 117–123 CAS.
- I. Wilting, P. C. Souverein, W. A. Nolen, A. C. G. Egberts and E. R. Heerdink, Changes in outpatient lithium treatment in the Netherlands during 1996–2005, J. Affective Disord., 2008, 111, 94–99 CrossRef CAS.
- M. Aliasgharpour and H. Hagani, Evaluation of lithium determination in three analyzers: flame emission, flame atomic absorption spectroscopy and ion selective electrode, North American Journal of Medical Sciences, 2009, 1, 244–246 Search PubMed.
- J. L. Beckers and P. Boček, Sample stacking in capillary zone electrophoresis: principles, advantages and limitations, Electrophoresis, 2000, 21, 2747–2767 CrossRef CAS.
- Th.P. E. M. Verheggen, A. C. Schoots and F. M. Everaerts, Feasibility of capillary zone electrophoresis with suppression of electroendosmotic flow in completely closed systems, J. Chromatogr., A, 1990, 503, 245–255 CrossRef CAS.
- D. Kaniansky, M. Masár, R. Bodor, M. Žúborová, E. Ölvecká, M. Jöhnck and B. Stanislawski, Electrophoretic separations on chips with hydrodynamically closed separation systems, Electrophoresis, 2003, 24, 2208–2227 CrossRef CAS.
- J. C. T. Eijkel and A. van den Berg, Young 4ever—the use of capillarity for passive flow handling in lab on a chip devices, Lab Chip, 2006, 6, 1405–1408 RSC.
- European patent application EP2150815.
- European patent application filed.
- European patent application EP2089699.
- R. M. Guijt, E. Baltussen, G. van der Steen, R. B. M. Schasfoort, S. Schlautmann, H. A. H. Billiet, J. Frank, G. W. K. van Dedem and A. van den Berg, New approaches for fabrication of microfluidic capillary electrophoresis devices with on-chip conductivity detection, Electrophoresis, 2001, 22, 235–241 CrossRef CAS.
- L. I. Segerink, A. J. Sprenkels, P. M. ter Braak, I. Vermes and A. van den Berg, On-chip determination of spermatozoa concentration using electrical impedance measurements, Lab Chip, 2010, 10, 1018–1024 RSC.
- G. H. Hisayasu, J. L. Cohen and R. W. Nelson, Determination of plasma and erythrocyte lithium concentrations by atomic-absorption spectrophotometry, Clin. Chem., 1977, 23, 41–45 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary movie. See DOI: 10.1039/c003899g |
| ‡ Present address: Forschungszentrum Jülich GmbH, Germany. |
| This journal is © The Royal Society of Chemistry 2010 |
