Xue-Qing Mou, Xiang-Yu Chen, Gong Chen and Gang He
Chem. Commun., 2018,54, 515-518
DOI:
10.1039/C7CC08897C,
Communication
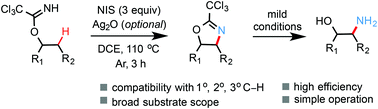
A new radical-mediated intramolecular β-C(sp3)–H amidation reaction of O-alkyl trichloro- or arylimidates is reported. Various oxazolines were efficiently prepared from easily accessible alcohol starting materials. The trichloro-oxazoline products can be hydrolyzed under mild conditions to give valuable 1,2-amino alcohols. This amidation reaction exhibits a broad substrate scope and good functional group tolerance, and offers a powerful means for the C(sp3)–H functionalization of alcohols. Mechanistic studies suggest that a sequence of 1,5-HAT of an imidate radical, iodination and cyclization might be operative.