Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A perspective on ecologically relevant plant-UV research and its practical application
T. Matthew
Robson†
 *a,
Pedro J.
Aphalo
*a,
Pedro J.
Aphalo
 a,
Agnieszka Katarzyna
Banaś
a,
Agnieszka Katarzyna
Banaś
 b,
Paul W.
Barnes
b,
Paul W.
Barnes
 c,
Craig C.
Brelsford
c,
Craig C.
Brelsford
 a,
Gareth I.
Jenkins
a,
Gareth I.
Jenkins
 d,
Titta K.
Kotilainen
d,
Titta K.
Kotilainen
 ae,
Justyna
Łabuz
ae,
Justyna
Łabuz
 f,
Javier
Martínez-Abaigar
f,
Javier
Martínez-Abaigar
 g,
Luis O.
Morales
g,
Luis O.
Morales
 ah,
Susanne
Neugart
ah,
Susanne
Neugart
 i,
Marta
Pieristè
i,
Marta
Pieristè
 aj,
Neha
Rai
aj,
Neha
Rai
 a,
Filip
Vandenbussche
a,
Filip
Vandenbussche
 k and
Marcel A. K.
Jansen
k and
Marcel A. K.
Jansen
 l
l
aOrganismal and Evolutionary Biology, Viikki Plant Science Centre (ViPS), University of Helsinki, Finland. E-mail: matthew.robson@helsinki.fi
bDepartment of Plant Biotechnology, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, Gronostajowa 7, 30-387, Krakow, Poland
cDepartment of Biological Sciences and Environment Program, Loyola University New Orleans, New Orleans, USA
dInstitute of Molecular Cell and Systems Biology, University of Glasgow, Glasgow, UK
eNatural Resources Institute Finland (Luke) Turku, Finland
fLaboratory of Photobiology, Malopolska Centre of Biotechnology, Jagiellonian University, Gronostajowa 7A, Krakow, 30-387, Poland
gFaculty of Science and Technology, University of La Rioja, Madre de Dios 53, 26006 Logroño, La Rioja, Spain
hSchool of Science & Technology, Örebro Life Science Center, Örebro University, SE-70182 Örebro, Sweden
iLeibniz-Institute of Vegetable and Ornamental Crops, Theodor-Echtermeyer-Weg 1, 14979 Grossbeeren, Germany
jNormandie Université, UNIROUEN, Ecodiv URA/EA1293, IRSTEA, FR Scale CNRS 3730, Rouen, France
kLaboratory of Functional Plant Biology, Department of Biology, Faculty of Sciences, Ghent University, KL Ledeganckstraat 35, B-9000 Ghent, Belgium
lSchool of Biological Earth, and Environmental Sciences, University College Cork, Cork, Ireland
First published on 17th January 2019
Abstract
Plants perceive ultraviolet-B (UV-B) radiation through the UV-B photoreceptor UV RESISTANCE LOCUS 8 (UVR8), and initiate regulatory responses via associated signalling networks, gene expression and metabolic pathways. Various regulatory adaptations to UV-B radiation enable plants to harvest information about fluctuations in UV-B irradiance and spectral composition in natural environments, and to defend themselves against UV-B exposure. Given that UVR8 is present across plant organs and tissues, knowledge of the systemic signalling involved in its activation and function throughout the plant is important for understanding the context of specific responses. Fine-scale understanding of both UV-B irradiance and perception within tissues and cells requires improved application of knowledge about UV-attenuation in leaves and canopies, warranting greater consideration when designing experiments. In this context, reciprocal crosstalk among photoreceptor-induced pathways also needs to be considered, as this appears to produce particularly complex patterns of physiological and morphological response. Through crosstalk, plant responses to UV-B radiation go beyond simply UV-protection or amelioration of damage, but may give cross-protection over a suite of environmental stressors. Overall, there is emerging knowledge showing how information captured by UVR8 is used to regulate molecular and physiological processes, although understanding of upscaling to higher levels of organisation, i.e. organisms, canopies and communities remains poor. Achieving this will require further studies using model plant species beyond Arabidopsis, and that represent a broad range of functional types. More attention should also be given to plants in natural environments in all their complexity, as such studies are needed to acquire an improved understanding of the impact of climate change in the context of plant-UV responses. Furthermore, broadening the scope of experiments into the regulation of plant-UV responses will facilitate the application of UV radiation in commercial plant production. By considering the progress made in plant-UV research, this perspective highlights prescient topics in plant-UV photobiology where future research efforts can profitably be focussed. This perspective also emphasises burgeoning interdisciplinary links that will assist in understanding of UV-B effects across organisational scales and gaps in knowledge that need to be filled so as to achieve an integrated vision of plant responses to UV-radiation.
(a) Introduction
The ultraviolet (UV) region of the solar spectrum can be divided into UV-C (100–280 nm), UV-B (280–315 nm) and UV-A (315–400 nm) radiation, of which only UV-A and UV-B at wavelengths greater than 290 nm reach the biosphere.1 Solar UV-A radiation contributes a fairly constant fraction of global radiation at ground level, equivalent to approximately 5% of the photons in photosynthetically active radiation (400–700 nm, PAR). In contrast, UV-B radiation is a minor and highly variable fraction of the photons in incident solar radiation, equivalent to no more than 0.33% of the photons in PAR.2 However, UV-B photons are the most energetic reaching the Earth's surface, and thus most able to break chemical bonds and rearrange molecular structures. Correctly pinpointing the effects of such a small fraction of incoming solar radiation has always been challenging, as has been the interpretation of UV-B effects in the context of the rest of the solar spectrum. Approaches to quantification of UV-B radiation and to the study of its effects on plants have been refined over the last 30 years, with intense research efforts following the discovery of the Antarctic ozone (O3) hole. As a result, real progress has been made in understanding the conditions under which UV-B can be a stressor, as well as the mechanism of UV-B perception. In contrast, there has been much less research on the effects of solar UV-A radiation, which is not affected by stratospheric O3 depletion.Understanding of how plants perceive UV-B radiation as an informational cue made a leap forward with the identification of a UV-B-specific photoreceptor, UV RESISTANCE LOCUS 8 (UVR8).3 This in turn has boosted efforts to describe UV-B-dependent cell-signalling networks, and the role of UV-B radiation in the regulation of metabolic pathways and associated physiological responses. However, slower progress has been made towards understanding the basis of responses to UV-B radiation at higher levels of organisation within the plant and in scaling up to plant populations and communities. This is partially due to the complex interactions of UV-B responses with those to other cues and signals concurrently present in natural and anthropogenic habitats. In this perspective, we set out to find links between different responses within and across levels of organisation and highlight those gaps in knowledge that need to be filled so as to achieve an integrated vision of plant responses to UV-radiation.
| There remain many unanswered questions concerning plant responses to UV-B radiation, of which the most crucial is: how can knowledge of the described mechanisms of response be integrated into a coherent conceptual model explaining the roles of UV-B in adaptation and acclimation of plants? And what contribution do these responses make to the effects of UV-B radiation on both vegetation composition and ecosystem function? |
The reader is first taken back through the evolution of plant UV responses to set the context of how plants overcame the challenge of being exposed to UV radiation. This leads us to explore the evolutionary conservation of UVR8 and associated signalling elements among plants, comparing its function and its localisation within the plant among different phyla. This highlights the need to go beyond testing UV-B responses in standard model organisms under controlled conditions, by exploring UV-B responses in species with more complex and varied anatomical, structural and morphological, physiological and life history attributes. Furthermore, crosstalk between UV-B induced signalling pathways and pathways governed by other environmental stimuli is explored and likewise alternative mechanisms of UV-B detection and response. The potential ecological implications of these UV-B responses are considered when they are scaled up to natural environments where plants are exposed to dynamic patterns of UV-B radiation as part of the solar spectrum. Finally, the opportunities created by recent innovations in lighting technologies are discussed. These optimise the control of environmental conditions for applications in commercial plant production, and likewise for the study of molecular responses to UV-B radiation, by bringing them closer to natural light environments. Initially, this may make it more challenging to isolate UV-B responses, but creating more-relevant conditions will improve our understanding of the contribution of UV-B radiation as a part of the solar spectrum in shaping the adaptation and acclimation of plants in nature.
(b) Understanding adaptation to UV radiation over evolutionary time scales is key to interpreting current and potential future responses
Incident solar UV radiation at ground level has changed dramatically since the beginning of life on Earth. Approximately 3800 MY ago, early forms of microbial life appeared in the oceans, where these could avoid extreme climatic conditions. This aquatic environment provided a degree of shelter from UV irradiances which are estimated to have been 1000-fold greater than at present.4 At around 2900 MY ago, the first oxygenic photosynthesizers (cyanobacteria) appeared. In the absence of a stratospheric O3 layer, these microorganisms were protected from UV radiation through: (a) other UV attenuators in the atmosphere (aerosols, methane-derived organic haze) and the water column (organic matter, iron-silica-rich precipitates); (b) living in protected habitats, such as sediment pores, endolithic habitats or deep water; and (c) physiological mechanisms, such as DNA repair, antioxidant systems and UV-screening through UV-absorbing compounds.5Around 2300 MY ago, the oxygen (O2) content of the air had increased greatly, enabling the formation of the stratospheric O3 layer.6 O3 is formed in the stratosphere by the photochemical cleavage of O2 by UV-C radiation, and the subsequent combination of an O2 free radical atom with a ground state O2 molecule. This process blocks the most energetic UV-C radiation from entering the biosphere, while stratospheric O3 strongly absorbs UV-B radiation but not UV-A radiation. The O3 layer was fully formed around 1500 MY ago and, since then, organisms in the biosphere have had to cope with a mixture of UV-A (around 95% of the total UV) and UV-B radiation, but not UV-C radiation.
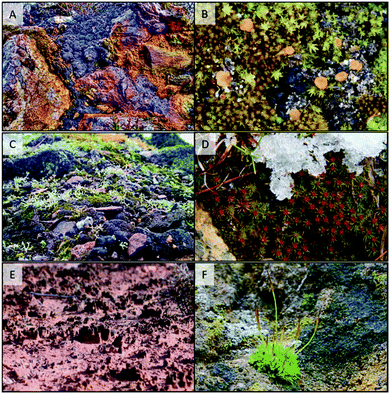
It is surprising, given the early origin and rapid evolution of life in water ∼3800 MY ago (only 500 MY after the planet's formation), that the water-to-land transition and subsequent land colonisation did not occur until the Ordovician period, just ∼450 MY ago.7 This long time span gives an indication of the tremendous evolutionary challenges to overcome during this process. Probably, the first forms of terrestrial life were cryptogamic crusts not very different in their structure and function to those present today in similarly harsh environments.8,9 The components of these crusts may have included cyanobacterial colonies, unicellular to filamentous green algae, fungi, and lichens, perhaps the first symbiotic association on land (Fig. 1). The environmental pressures limiting land colonisation by photosynthetic cryptogamic organisms were high solar radiation (both photosynthetic and UV), low availability of water and mineral nutrients, high and fluctuating temperatures, high O2 concentrations, and even gravity itself. Other limitations would have been related to sexual reproduction and dispersal that previously required water. The challenge for plants colonising land would have been coping with all of these factors simultaneously. Land colonisation did not occur until the stratospheric O3 layer had fully developed, suggesting that UV radiation was a limiting factor.10 However, it was not likely to have been the critical constraint for land colonisation.5 For example, stromatolites in intertidal zones were exposed to full ambient UV radiation before the presence of stratospheric O3, and were able to survive, probably taking advantage of the physiological mechanisms mentioned above for UV protection.
| Plant UV-B responses are underpinned by a complex mixture of UV-perception and responses inherited from green algal forebears, and more modern physiological and reproductive innovations to cope with UV radiation and interacting environmental pressures. |
Bryophytes (mosses, liverworts and hornworts) evolved as the first “true” plants (embryophytes), and formed part of the cryptogamic crusts (Fig. 1). Structurally simple bryophytes, and later-evolving more structurally complex lineages (tracheophytes: pteridophytes and seed plants), adapted to high UV exposures by using a number of protective mechanisms the origins of which were already present in their ancestors. For instance, UV-B perception through UVR8, and the regulatory and metabolic pathways underpinning the mechanisms of plant UV-B response, were all inherited from green algal ancestors.11,12 The evolution of UV-absorbing compounds exemplifies a similar pattern of evolution. Scytonemin from cyanobacteria and mycosporine-like amino acids (MAAs) from both cyanobacteria and other eukaryotic algal lineages were two early-evolving classes of UV-absorbing compounds. It has been proposed that phenolic compounds, and especially flavonoids, replaced MAAs in embryophytes, constituting a specific innovation allowing adaptation to conditions on land.13 However, diverse algal lineages including cyanobacteria can synthesize phenolic compounds (scytonemin itself is partially derived from phenolic subunits), although they might have acted as antioxidants or developmental regulators rather than UV screens.14,15
Embryophytes developed subtle innovations in otherwise ancestral processes and structures, including in secondary metabolism, UV-B perception, cell wall composition, cellular compartmentalisation of UV-absorbing compounds, and the protection of gametes and embryos by a layer of protective, sterile cells. These innovations are thought to have contributed to UV adaptation12,16–18 and sometimes also facilitated adaptation to other interacting environmental pressures, promoting tolerance to multiple stressors. For example, typical tracheophyte structures such as leaf hairs, waxes, thick cuticles and epidermis, can not only reduce UV penetration and damage, but also contribute to improved water use efficiency and temperature regulation. Thus, early in plant evolution, a strong element of crosstalk existed between UV and drought-protection responses, a synergy that is important in the context of the climate change experienced by modern plants.
Individual plant species have evolved their own “global” strategies of response to multiple environmental pressures, including UV radiation, resulting in a highly diverse biosphere in its species composition and ecosystem function. Our knowledge of how photosynthetic organisms have adapted to past UV irradiances feeds into understanding of how they respond to the full range of present-day exposures to UV radiation.
(c) Plant UV-B exposure involves more than just the incident irradiance
Photosynthesis entails that green plants require sunlight to live, and are thus unavoidably exposed to solar UV-radiation. Many studies have quantified plant UV-exposure, including local and regional UV-B-regimes and links with altitude, latitude, and weather conditions.19,20 Yet, very few consider the fine-scale UV exposure patterns experienced by plants within a canopy, cells within leaves, or organelles within cells; although the physical phenomena involved are well understood and can be modelled.21,22 These UV-exposure patterns depend, not only on the incident solar irradiance at the top of the canopy and its relative UV-fraction, but also on the absorption, reflection and scattering of radiation in the atmosphere, canopy, at the leaf surface and within leaves themselves. Thus, the precise UV exposure of cells, tissues and even individual leaves can diverge dramatically from that in the atmosphere, or at the top of the canopy.Plant architecture and leaf morphology are important when estimating UV-exposure. The influence of UV-B radiation on plant and leaf architecture has been widely studied,23,24 but the opposite, i.e. the effect of plant architecture and leaf morphology on the transmittance of UV radiation within the canopy, is still poorly understood. The interception of direct sunlight by forest canopies typically produces shade that is less depleted in UV than PAR at ground level.25,26 However, rapid measurements of irradiance are required to resolve the mixture of shade and sunflecks, differing in their UV/PAR ratios, composing the under-canopy environment.27 UV-B radiation has been well established to enhance plant branching, cause leaf thickening and/or to impede stem elongation.24 Yet, it remains unknown how these architectural changes will, in turn, affect local UV irradiance.
To assess the effects of UV radiation on plant tissues, it is essential to consider not only UV radiation incident on the leaf surfaces, but also how much and how deeply it penetrates. Both reflection and absorption can attenuate the amount of UV radiation reaching the mesophyll, while reflection at the cuticle or by trichomes can protect epidermal cells. At cell walls and other internal surfaces, multiple reflections in different directions result in scattering which lengthens the path of photons within plant tissues. Absorption of PAR and UV radiation typically accounts for a much larger proportion of attenuation of the incident irradiance than reflection,28 and depends on the leaf structure and UV-shielding capacity, which is achieved principally by screening pigments deposited in the vacuole of epidermal cells. Nevertheless, cuticular wax deposition and/or changes in its composition induced by UV-B exposure, can increase leaf UV reflectance.29 Similarly, UV-B exposure can induce trichome formation in some Arabidopsis thaliana (Arabidopsis) accessions, and this is sometimes associated with increased shielding, and decreased UV-sensitivity.30,31
| It is not known to what extent UV-induced changes in plant architecture and leaf optical properties affect local UV-regimes within a canopy. |
It has been suggested that the epidermis may present an uneven barrier to UV radiation, both because epidermal cells are not of uniform size and because the cell walls of many higher plant species have high UV transmittance.32,33 Open stomata could constitute a further entry route for UV irradiance in to the leaf. While general trends of UV irradiance versus leaf depth have been established,34 our understanding of fine-scale leaf penetration of PAR, never mind UV radiation, remains sketchy.19,35 To explore the fate of UV radiation within leaves and obtain a better understanding of UV-attenuation at this scale, there is exciting potential to exploit the available mutants of model plants with altered leaf anatomy; cell size and shape; number and size of chloroplasts, and vacuole morphology. Another option may be to study leaf optics by exploiting local variations in pigments found in cultivated and model plants that have variegated leaves, such as some Coleus varieties or Arabidopsis immutans (im) and variegated2 (var2) mutants.36 Assessing concurrent UV penetration and UV-induced damage within tissues can contribute to a better understanding of local versus systemic plant UV-responses.
Plants in natural environments are exposed to higher irradiances of blue light than UV radiation. Thus, it is very important to understand how the effects of UV radiation interact with blue-light-induced responses in plants. Chloroplasts37 and nuclei38 move within the cell to avoid exposure to excess blue light.39 These responses are controlled by the UV-A-radiation/blue-light photoreceptor phototropin2 and cause the chloroplasts and nuclei to position themselves parallel to the direction of incident light.40–42 The nuclear avoidance response has been shown to be associated with reduced UV-induced nuclear DNA damage.38 Differential positioning of chloroplasts in low and high light changes leaf optical properties in a wavelength-dependent manner, producing large differences in blue and red light absorption efficiency within the leaves of mutant plants with impaired chloroplast movements compared to wild-type (WT) plants.43 However, the relative importance of chloroplast positioning for plant UV responses, and indeed chloroplast UV protection, e.g. from organellar DNA damage, remains to be established. Nevertheless, the radiation-dependent movements of the nucleus and chloroplasts provide additional evidence that underlines the importance of using sufficiently high PAR and blue light levels to generate meaningful data on the impacts of UV-radiation on plants.
(d) Stress-induction and information acquisition are intertwined components of plant UV-B responses
When considering plant UV photobiology, just as with visible radiation, both stress-induction and information acquisition are important and often intertwined components of the plant response. As a consequence, plant UV responses are complex, and vary according to the balance between stress and regulatory signalling. In turn, this balance strongly depends on the amount and spectral composition of radiation that plants are exposed to, as well as other aspects of environment and condition of the plant. In general, high doses (see definition in ref. 44) or relatively short UV-B wavelengths (<300 nm) or both together cause plants stress, while low doses and longer UV-wavelengths within a realistic context resembling “natural” sunlight are more likely to predominantly cause regulatory signalling. This is not surprising, as plants have evolved over millions of years in conditions where the shortest UV-B wavelengths were not present, while acute high doses of UV-B radiation occur sporadically and are limited to a few environments and times of day and year. In natural conditions, when high UV-B exposures occur they are always preceded and followed by lower doses of UV-B radiation and periods of exposure to UV-A and visible radiation, which make a crucial contribution to plant acclimation and recovery.Clear cut categorisation of plant UV-responses as either stress-inducing or regulatory is overly simplistic. A modest degree of stress has long been known to cause acclimation, a regulatory response that helps the plant adjust to new or even future “stressful” conditions. Such a “state of good stress” is known as eustress, and is associated with the upregulation of various defence responses, including antioxidant defences, UV-screening and altered morphological characteristics of organs and organisms.44 The distress response is associated with excessive stress which causes aggravated cellular damage. In practice, the distinction between eustress and distress is not always obvious, and activation of regulatory responses can be seen under both modest and more-severe exposure conditions. The length of UV exposure time can also affect the distinction between distress and eustress, as short term distress may evolve into eustress whereby the plant recovers into an acclimated individual.44
In plants, UV induces changes in, amongst others, gene-expression, enzyme activity, and plant morphology and physiology. Upregulation of plant antioxidant defences can be detected under very low UV-B irradiance as well as under strong stress-inducing conditions where oxidative damage is apparent. Exposure to UV-B is also associated with shorter, more compact morphology, the functional role of which is not entirely clear. However, the best documented UV-induced changes are alterations in the secondary metabolite profile. Many studies have reported increases in concentrations of individual phenylpropanoids, such as hydroxycinnamates and flavonoids, in UV-B exposed plants, and these changes are usually thought to increase antioxidant capacity and UV-screening.44–46 In natural sunlight, however, increases in total flavonoid and phenolic acid concentrations in response to UV-B exposure are consistently reported but in most cases so small (5–15%)47 as to be of questionable significance in enhancing optical screening and antioxidant capacity.
The physiological functions and ecological roles of specific flavonoids are not yet well understood, making it difficult to assign a functional role to the changes in flavonoid composition that sometimes occur in response to UV and high exposure to visible radiation. For example, UV-B exposure typically causes increases in the quercetin to kaempferol ratio in those species that contain them, and this has been hypothesised to relate to the relatively strong antioxidant activity of the penta-hydroxylated quercetins.48,49 Furthermore, nearly all flavonoids are glycosylated, a modification traditionally explained as a strategy to reduce the toxicity of aglycones.45 However, it is becoming increasingly clear that UV-B, UV-A and visible radiation induce highly specific changes in glycosylation patterns, sometimes involving induction of specific transferases.48 For example, UV-B exposed Arabidopsis leaves tend to accumulate 7-rhamnosylated flavonoids.50 The function of such specific glycosylation patterns remains to be established, but possible roles relate to the stability and reactivity of glycosylated compounds.
The preceding discussion centres on the role of phenolic compounds in protection from UV-B radiation. However, many functions and activities of these compounds are unrelated to UV-B protection (e.g.ref. 46, 51, and 52). As such, responses caused by UV-B radiation cannot be assumed to promote UV-protection, as plants may be utilising UV-B radiation as a cue to pre-empt or acclimate to other co-occurring changes in their environment.53
The role of UV radiation in driving the accumulation, and changes in composition, of other groups of secondary metabolites such as terpenes and alkaloids, has been much less frequently studied than flavonoids, possibly because their role in UV-B protection is not well defined. Nevertheless, studies in controlled conditions have found UV-B radiation to induce terpene biosynthesis and accumulation of terpenoids,54 as well as indole alkaloids;55 although the environmental relevance of such observations needs to be established.
| In future research, awareness of the possibility that protection against UV-B might be triggered by exposure to visible light, and that changes in the composition of secondary metabolites triggered by UV-B and UV-A radiation are not necessarily related to UV-B protection will be crucial for the design of unbiased experimental designs and for meaningful interpretation of observed responses. |
In plant cells, DNA is present in the nucleus, chloroplasts and mitochondria, and can be exposed to the UV radiation that penetrates into plant tissues. DNA-damage can be induced directly as a result of absorption of UV photons and by UV-driven production of reactive oxygen species (ROS). Exposure of DNA to UV-photons can generate two major types of DNA lesion, i.e. dimers between adjacent pyrimidines. Cyclobutane pyrimidine dimers (CPDs) are induced by both UV-B and UV-A radiation, and are more common than 6-4 photoproducts (6-4 PPs) that result only from the action of UV-B radiation, but may isomerise under UV-A radiation into Dewar photolesions.56 In plants, CPDs and 6-4 PPs are repaired mainly in a blue/UV-A-specific manner via photoreactivation by photolyases. Photolyases specific to CPDs and 6-4 PPs from rice and Arabidopsis, respectively, have been found in nuclei, chloroplasts and mitochondria.57,58 Past research has focused mainly on nuclear DNA lesions, at the expense of organellar DNA damage and photoreactivation. Additionally, chloroplast movements,59 often triggered by blue light, play an unquantified role in preventing UV exposure and damage to chloroplast DNA, as was discussed in section c.
Many physiological responses can be initiated following DNA damage. However, data are scarce on signalling cascades triggered specifically by UV-induced pyrimidine dimers in plants. ATAXIA-TELANGIECTASIA MUTATED (ATM) and ATM AND RAD3-RELATED (ATR) kinases mediating the DNA damage response are involved in CPD-dependent inhibition of hypocotyl growth in etiolated Arabidopsis seedlings.60 In addition, photolyases which may function in dimer recognition, even in darkness, and which promote the splitting of bonds between neighbouring pyrimidines under blue light/UV-A radiation,61 might also function as receptors for UV-damaged DNA.62 After dimer binding, in either light or darkness, photolyases may activate specific signalling pathways or act as docking platforms for other proteins involved in DNA repair. The latter possibility is supported by some evidence that photolyases could assist the nucleotide excision repair (NER) pathway63 – requiring further study.
Beyond damage to DNA, UV-B irradiation can lead to lipid peroxidation and changes in the composition of cell membranes. One of the best-known outcomes is a decrease in monogalactosyldiglyceride (MGDG) content in chloroplast membranes.64 Lower MGDG content in seedlings leads to disruption of chloroplast biogenesis, formation of thylakoids, chlorophyll accumulation and photosynthetic electron transport.65 In addition to their structural role, lipids can act as signalling molecules, and could therefore, along with ROS, be candidates for the initiation of some UVR8-independent UV-B effects. Phospholipid signalling can be induced in the nuclei of mammalian cells by UV-B radiation,66 but to date only indirect evidence suggests their involvement in plant responses to UV-B radiation. Gene expression in the phosphatidylinositol signalling pathway is up-regulated by UV-B radiation67 and the UV-B LIGHT INSENSITIVE 3 (ULI3) protein, which has a putative diacylglycerol-binding domain, is essential for inhibition of hypocotyl growth under UV-B radiation.68 Surprisingly, there has been little follow-up research into the role of this protein since 2002 when it was first described. Thus, the role of phospholipid signalling, the associated involvement of ULI3, as well as the role of phospholipid peroxidation in plant responses to UV-B radiation remain to be studied.
Studies in controlled environments have revealed that exposure to high UV-B irradiance increases ROS production in the chloroplasts, mitochondria, peroxisomes and on the extracellular side of the plasma membrane causing distress.44 This high-dose UV-B-response is independent of UVR8-mediated signalling. To scavenge ROS in every cell compartment, plants use both enzymatic and non-enzymatic mechanisms that under optimal growth conditions keep ROS concentration low.69 When stress is imposed, the balance between ROS production and scavenging is disturbed, leading to oxidative damage to DNA, lipids, proteins and, in specific cases, a hypersensitive response leading to cell death.70 Although often associated with stress, ROS are also ubiquitous key signalling molecules regulating physiological processes.71 The expression of several genes involved in photosynthesis (such as Lhcb and psbA) in response to UV-B radiation is modulated by ROS signalling.72 ROS have also been identified as an important component of retrograde signalling from organelles to the nucleus;73 however this role has not yet been studied with respect to UV-radiation exposure. Finally, the redox state of some of the cellular key antioxidants, such as ascorbate and glutathione, has been implicated in cellular signalling and cell cycle control.74
In contrast to the distress produced by high UV-treatments, the role of the low-levels of ROS found in plants receiving realistic doses of UV-B radiation is not well understood. The main reason for this gap in knowledge is that currently available ROS-detection methods lack the necessary sensitivity at low ROS concentrations (reviewed in ref. 70). Most published studies on ROS production under UV-B radiation were performed on plants first grown under controlled conditions without any UV-B radiation and later suddenly exposed to an acute UV-B dose. Clearly, the results of studies of this sort are difficult to interpret with respect to natural environmental conditions where UV-B radiation is present throughout a plant's life cycle and fluctuates along with other environmental factors such as high PAR, drought and high temperature, changing the dynamics of ROS generation and quenching. At a smaller scale, in vitro and simple model systems such as isolated thylakoid membranes or leaf organelles have been used to study ROS, which may help to gain a mechanistic understanding of their role but do not scale up to the whole plant level.
In the future it would be beneficial to develop methods allowing the study of the low-level ROS generated by the UV-B exposure conditions usually found in the natural environment. This should include identification of the different molecules involved in ROS signalling along with assessment of their importance in regulating gene expression in response to realistic UV-B irradiance. Building on this, the role in ROS signalling and quenching of other environmental factors that can interact with UV-B radiation needs to be considered.
(e) UV-B perception and signalling through UVR8: a conserved mechanism of UV protection in plants
The presence of photoreceptors in plants allows the direct perception of visible and UV radiation, initiating signalling that leads to the regulation of gene expression even in the absence of stress. Of the known photoreceptors, UVR8 is the most important for sensing of UV-B radiation.A major objective in trying to understand the molecular basis of UV responses in an ecological context is to determine the role of UVR8, both in diverse plant species and in different natural environments. The discovery that UVR8 regulates the expression of a substantial set of genes and consequently mediates a range of metabolic, morphogenic and physiological responses to UV-B radiation in Arabidopsis has been a significant advance.75,76 However, research needs to be extended to other species and to natural growth conditions. A key feature of UVR8, that underlines its importance in understanding how diverse plant species respond to UV-B radiation, is that its structure and mechanism of action appear to be highly conserved,3,77–79 from unicellular green algae, through bryophytes, to angiosperms. An apparent exception are the red algae, which seem to lack UVR8, and uncertainty remains over the presence of UVR8 in gymnosperms and particular aquatic species. Nevertheless, given its wide distribution, UVR8 is evidently important in disparate groups of plants.
UVR8 is a 7-bladed β-propeller protein that can form a dimer through electrostatic interactions between charged amino acids at the dimerisation surface.77,78 Comparison of UVR8 sequences among plant species reveals that these charged amino acids are strongly conserved.77–79 UV-B exposure leads to monomerisation of UVR8 through breakage of these interactions, and the consequent initiation of signal transduction.3,77,78 The absorption of UV-B radiation by UVR8 and the photoreception mechanism rely on the presence of specific tryptophan amino acids in its structure. Arabidopsis UVR8 has 14 tryptophans, and the number and location of these in the protein are highly conserved between diverse species.3,77–79 The function of UVR8 in mediating UV-B responses involves interaction of its monomer with other proteins, and a vital site for these interactions is the C-terminal region of the protein.80 Again, critical amino acids in this region are highly conserved.
It appears that the evolutionary conservation of the UVR8 structure is matched by the conservation of the downstream regulatory process. Monomeric UVR8 initiates signal transduction through interaction with the CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) protein,3 and sequence orthologues of COP1 are found throughout the plant kingdom. In addition, the REPRESSOR OF UV-B PHOTOMORPHOGENESIS (RUP) proteins, which interact with UVR8 to promote dimerization,81 are found in a wide range of species. Experimental evidence for the conservation of UVR8 function has been obtained in studies with bryophytes and the green alga, Chlamydomonas reinhardtii, where CrUVR8 undergoes UV-B induced monomerisation and interacts with CrCOP1 to initiate gene expression responses promoting acclimation and protecting against photoinhibition.82,83 The moss Physcomitrella patens has two UVR8 genes encoding very similar proteins that monomerise in response to UV-B radiation, whereas transcripts of the single Marchantia polymorpha UVR8 gene are differentially spliced to produce proteins that have a reduced propensity to form dimers.12 However, the UVR8 proteins of both species are functional and complement the Arabidopsis uvr8-1 mutant. It will be interesting to examine whether species with more than one UVR8 gene are able to form heterodimers between different protein isoforms.
The fundamental mechanism of UVR8 action is likely to be very similar in diverse species; however, it is not clear whether UVR8 regulates the same range of responses in different species, mainly because so few species have been studied. To date, UVR8 has been shown to play a key role in protection against damage by excessive UV-B radiation, in that it mediates UV-B stimulated expression of genes involved in flavonoid biosynthesis, DNA repair, antioxidant activity and other protective processes.84,85 This role is likely to be important across a wide range of species. In addition, Arabidopsis UVR8 mediates a number of morphogenic and physiological responses, principally through interaction with other signalling pathways.75 Among the most important interactions is its involvement in regulating auxin biosynthesis, which underpins UVR8 action in phototropism and in impairing shade avoidance and thermomorphogenic responses.86–88 Recently, it has been shown that chloroplast development in tomato fruits is regulated by UV-B radiation in a UVR8-dependent manner.89 While some of these aspects of UVR8 action have been found in angiosperms other than Arabidopsis, we have little idea of what responses UVR8 may regulate in other phyla and what underlying mechanisms may be involved. A complicating factor is that, in contrast to Arabidopsis, many plant species have at least two UVR8 genes, raising the possibility of differential spatial, temporal and environmental regulation of expression, and differences in function as is the case for the other families of plant photoreceptors.
A further aspect to consider when examining UVR8 function in diverse species is its localisation, especially considering that UV-B penetration into sub-epidermal tissues is limited.32 Research with Arabidopsis shows that UVR8 is present throughout the life of the plant and is expressed in different organs and tissues.3 A study where the UVR8 promoter was fused to a yellow fluorescent protein (YFP) in Arabidopsis revealed that UVR8 was most strongly expressed in the epidermis of seedling cotyledons. This is consistent with its importance in regulating protective gene expression, with less UVR8 expression in mesophyll cells, and none detected in vascular tissue.90 Epidermal UVR8 was shown to be involved in mediating the suppression of cotyledon expansion and hypocotyl growth by UV-B, as well as flavonoid biosynthesis. In mesophyll cells, UVR8 also regulated flavonoid biosynthesis and hypocotyl growth.90 The action of UVR8 in regulating gene expression is largely cell-autonomous and will therefore be related to the amount of UVR8 in any particular cell and the extent of UV-B penetration into the leaf,90 as was discussed in section c. It will be interesting to discover whether similar localisation patterns and spatial functions of UVR8 are found at later developmental stages and in other species.
| It is becoming increasingly evident that UVR8 functions through interaction with other signalling pathways and it is important to discover the interplay between UV-B and other signalling pathways in species adapted to different environments. |
To date, little research has been undertaken to examine UVR8 function in natural environments, where there is additional complexity in the action and regulation of the photoreceptor. UVR8 is found in a photo-equilibrium between dimer and monomer forms in plants growing under a realistic photoperiod. Environmental fluctuations in temperature91 and transient sunflecks92 can affect this balance (section c). The expression and activity of RUP proteins provides a potential mechanism for non-UV-B stimuli such as temperature to modulate UVR8 signalling.75 It is becoming increasingly evident that UVR8 functions through interaction with other signalling pathways and it is important to discover the interplay between UV-B and other signalling pathways in species adapted to different environments. For instance, at high altitudes elevated levels of UV-B are usually accompanied by lower temperatures and in some regions reduced water availability, so potential interactions between the corresponding signalling pathways may be ecologically significant. Furthermore, UVR8 inhibits the phytochrome-regulated response to avoid vegetational shading, found in shade intolerant species, by regulating auxin and gibberellic acid signalling,86 and it will be interesting to see whether this mechanism operates in species with different degrees of shade tolerance. In an experiment where Arabidopsis plants were transferred outside, many of the same genes were regulated by UVR8 in sunlight and in experiments in growth cabinets,93 but there were notable differences and evidence for crosstalk between different photoreception systems, as discussed further below (section e).
A final point to consider is whether there are additional UV-B photoreceptors. Arabidopsis uvr8 null mutants retain a residual response to UV-B, even at low fluence rates, so the involvement of another photoreceptor is conceivable. It is interesting that the peak of the UVR8 action spectrum in vivo differs from that of UVR8 dimer-to-monomer conversion,94 and while there are several reasonable explanations for this discrepancy (see section h), the possibility that plants possess an additional UV-B photoreceptor, perhaps dependent on the presence of UVR8 or co-acting with UVR8, needs to be considered. Other known photoreceptors, which are able to absorb UV-B wavelengths, or flavin-binding proteins that absorb short wavelength UV-A, could also potentially interact with UVR8 to mediate responses, and such photoreceptor interactions are discussed further below (section f). One can speculate on the possible nature of an additional putative UV-B photoreceptor,95 but at present there is no direct evidence for such a molecule in Arabidopsis or any other species.
(f) Signalling networks integrate UVR8 into sunlight perception
Interactions in photoperception can originate both through signalling crosstalk downstream of photoreceptors and through physical interactions between photoreceptor molecules. These two mechanisms can co-exist. However, physical interactions between photoreceptors can be expected to consistently affect all signalling downstream of the given photoreceptors, while crosstalk in signalling at multiple points further downstream could lead to multiple patterns of interaction, possibly each one revealed by the expression of a distinct set of genes.53 Furthermore, signalling downstream of a photoreceptor can also regulate the expression of genes for components of other signalling pathways and even of genes encoding other photoreceptors.For simplicity of presentation, signalling pathways are often depicted as linear. However, components of one pathway may directly control components of another pathway, leading to a response overlap. Signalling components may even be shared between multiple signalling pathways leading to either competition for substrate or to redundancy in upstream signalling. Interactions have been found at different points along signalling pathways from perception through to responses96 such as regulation of common outputs, determining hormone activity and/or the phenotype.97,98 Hence, simple pathways are in reality part of a huge interdependent signalling network that integrates inputs and outputs in a “many-to-many” fashion.
The role of crosstalk in light-induced signalling can be assessed in experiments whereby mutants deficient in one or more photoreceptor are exposed to different regions of the spectrum. Large scale factorial experiments can be designed to reveal interactions between signalling pathways downstream of specific photoreceptors. Such experiments using polychromatic radiation can reveal photoreceptor functions across the wavelengths in the solar spectrum, which can differ from those observed under monochromatic UV radiation.
| UV-B induced signalling cascades are part of a huge interdependent signalling network that integrates inputs and outputs in a “many-to-many” fashion. At present, neither the modes of interaction, nor the outcomes for plant growth and development are understood. |
Ample evidence exists for crosstalk between the signalling pathways associated with distinct photoreceptors, including between the phytochromes (receptors for red-and-far-red light) and the UV-A/blue light photoreceptors, cryptochromes and phototropins.88,99–101 UVR8-signalling integrates the sensing of UV-B with that of longer wavelengths by other photoreceptors. For example, the phenotypic outcome of UVR8 signalling, visible as the inhibition of cell and organ expansion and an increase in photoprotective pigment biosynthesis is to some extent shared with other photoreceptors including cryptochromes, phototropins and phytochromes,88,93,102 yet little is known about potential interactions between UVR8 and these photoreceptors.
A broad view of photoreceptor signalling networks can be acquired by the analysis of transcriptome data obtained in factorial experiments. This approach has shown that UV-B radiation induces flavonoid biosynthesis genes through UVR8,103 just as UV-A radiation/blue light does via cryptochromes.99 The same can be said for expression of CPD photolyase transcripts.104 It can be expected that in solar radiation the full arsenal of these UV, blue and red/far-red photoreceptor systems operate in a coordinated manner to drive the synthesis of various photoprotective pigments. A question that remains today is how this occurs mechanistically. Although the absorption spectrum of blue light photoreceptors often extends far into the UV, even into the UV-B region,105–107 there is little information on their function and possible interactions with UVR8-signalling in UV-B region of the spectrum. Furthermore, phototropins and UVR8 can induce similar responses such as asymmetric growth resulting in the orientation of organs towards a UV-B source.87
There are additional possible modes of interaction beyond protein–protein interactions between photoreceptors and downstream crosstalk. A current working model is based on a common signalling component shared among photoreceptors. One central component of light signalling is COP1 which connects signalling pathways operating over a wide range of wavelengths, encompassing phytochromes, cryptochromes and UVR8.108,109 COP1 was first characterised based on loss-of-function mutants displaying photomorphogenic traits when grown in darkness.110 Subsequently, the associated protein was found to function in signalling dependent on phytochromes,111 cryptochromes112,113 and UVR8,85,114,115 and as part of an E3 ubiquitin ligase complex that mediates the degradation of positive regulators of photosignalling. COP1 regulates the breakdown of several transcription factors that promote photomorphogenesis. These include some that are spectrally specific: for instance; LONG HYPOCOTYL IN FAR-RED 1 (HFR1) and LONG AFTER FAR-RED LIGHT 1 (LAF1) appear to be associated with far-red light and thus with PhyA signalling; while ELONGATED HYPOCOTYL 5 (HY5) and HY5-HOMOLOG (HYH) are associated with blue light and UV-B radiation signalling, through cryptochrome and UVR8 respectively.116,117 Furthermore, HY5 and HYH are associated with far-red light through PhyA and PhyB respectively.118–120
Beyond their common signalling components, light signalling pathways affect hormonal pathways, which also interact with each other, thus regulating plant growth and architecture.98,121 For instance, the well-studied shade avoidance syndrome that is driven by phytochromes and cryptochromes, also involves auxins, gibberellins, brassinosteroids, strigolactones, karrikins and ethylene as the central targets for elongation control.121–123 The principal regulators for stem extension are the PHYTOCHROME INTERACTING FACTORS (PIFs). Both phytochromes and cryptochromes interact with PIFs.124,125 PIF targets include auxin biosynthesis and signalling genes, which again suggest possible common control points with pathways other than that of phytochromes. Recent efforts have been able to integrate the function of UVR8 in the shade avoidance network,86 showing HY5, PIFs and DELLAs to function as a control point. PIFs are the drivers of auxin biosynthesis and DELLAs are inhibitors of gibberellin signalling, so both have crucial roles in all the light-regulated stem-elongation processes described to date. However, it remains to be seen how these pathways intertwine with those pathways that regulate differential growth during phototropism. Phototropin-controlled auxin transport and UVR8-regulated aspects of auxin signalling are likely to share some components,87 but the mechanisms by which these processes are integrated remain to be explained. A better understanding of how UVR8, and by extension other photoreceptors, regulate auxin in growth responses will help unravel how light determines plant architecture. Furthermore, UVR8 has recently been shown to interact directly with the BRI1-EMS-SUPPRESSOR1 (BES1) and BES1-INTERACTING MYC-LIKE 1 (BIM1) transcription factors involved in brassinosteroid signalling,126 and future studies will reveal the significance of these interactions in the regulation of extension growth by UVR8.
(g) Plant signalling and systemic responses to UV-B radiation
Most plant responses to UV-B radiation have been assessed in the aerial parts of plants, where UVR8 has been shown to mediate the acclimation to UV-B through photomorphogenesis and the accumulation of phenolic compounds.75 In contrast, relatively little is known about below-ground responses to UV-B radiation. Plant photoreceptors, including cryptochromes, phytochromes, and phototropins are present in most plant tissues including root apices.3 Furthermore, roots express UVR8 and root-specific UV-B sensing proteins (reviewed by ref. 127 and 128). Studies on root-located UVR8 have typically been done by exposing the root system to direct light in experiments that create conditions from which it is difficult to draw inferences about responses in natural environments. Even though plant below-ground responses to UV radiation remain poorly understood, recent research suggests a role for UVR8 in root responses to UV-B radiation.129 Multiple routes for UV-responses in roots are thought possible, such as shoot-to-root transmission of mobile signalling messengers (like phytohormones, sucrose, RNAs and proteins)130 or even direct sensing of light which may be channelled through plant stems over short distances into root tissue near the soil surface.131,132 These exciting findings highlight an opportunity to establish new research directions studying the interaction between above- and below-ground UV signalling within the plant.| Future work needs to critically analyse UV-mediated local and systemic signalling, including the interactions between above- and below-ground UV responses, which remain poorly understood. |
Below-ground responses to UV-B radiation could have meaningful consequences for below-ground ecosystems. For instance, a drought-sensitive mutant of Arabidopsis which also overexpressed UVR8 was able to partially ameliorate the effects of water and salt stress in the presence of UV-B radiation. The underlying mechanism involved reduced cell expansion resulting in a compact morphology with diminished water loss.133 This suggests that UVR8 in roots may be implicated in drought and salinity tolerance. Furthermore, ROS generated by UV-B radiation may play a role in negative phototropism by roots.128 UV-B signalling in roots can potentially also control plant phenotype, and specifically the relative allocation of biomass to roots.134 Changes in the quantity and quality of root exudates135 have been reported as a consequence of UV-B radiation exposure, and root exudates regulate the species composition and function of microbial communities living in close association with roots.136 Thus, below ground UV-B responses need to be studied in more detail to elucidate potential consequences for root, rhizosphere and ecosystem function.
(h) The varying spectrum of sunlight and photoreceptor function
As described above, interactions between signalling pathways allow the responses mediated by different photoreceptors to be both fine-tuned and coordinated. Researchers often try to understand photomorphogenesis based on the study of individual photoreceptors or regions of the spectrum, thus ignoring interactive effects. In reality, photomorphogenesis depends on the whole spectrum that plants are exposed to through the day.92,101 Thus, changes in the spectral composition of sunlight during the course of the day, and the gradual increase and decrease of irradiance at the start and the end of the day, are likely to have a strong influence on the responses elicited. As plants have evolved under such diurnal changes in illumination, it is to be expected that they have adapted to tolerate exposure to UV radiation and to make use of environmental UV cues as sources of information under these diurnally changing conditions.Photoreceptors have broad absorption spectra, meaning that it is not necessarily functionally significant that the maximum spectral absorbance of UVR8 in vitro is at 280 nm,77 in fact a shorter wavelength than any present in the solar spectrum at ground level. The breadth of the UVR8 absorption spectrum extends through the UV-B region and into the UV-A, allowing photons of these wavelength to drive excitation to varying extents. What a given photoreceptor senses depends both on its inherent optical properties and on the spectrum of the radiation it is exposed to. This raises multiple questions, some more easily tractable than others: (1) what spectral photon irradiance are photoreceptor molecules exposed to within the plant? The spectrum of radiation inside the leaf will be different from that of the incident radiation on its surface (as described in section c). (2) How does the absorption spectrum in-planta differ from that measured in vitro? The absorption spectra of molecules depend to some extent on the properties of the medium with which they are in contact, the solvent in the case of in vitro measurements, and the cell structures and cytoplasm in vivo. (3) What action spectrum can be expected as the result of combining our knowledge of points (1) and (2)?
Currently the best we can do, to predict the relative numbers of photons of different wavelengths absorbed in vivo by a photoreceptor, is to combine the spectrum of the radiation a plant or leaf is exposed to with its spectral absorbance measured in vitro for a photoreceptor. This is far from ideal and can provide only a rough approximation to what actually happens within the leaf. For instance, the absorption maximum of UVR8 at 280 nm in vitro tells very little about its function in plants growing in sunlight. The only published absorption spectrum for UVR8 covers the UV-C and UV-B regions, and extends less than 20 nm into the UV-A region.77 The convolution of this spectrum with a typical spectrum for sunlight at ground level shows that the maximum predicted rate of photon absorption (photons m−2 s−2 nm−1) is at or near the UV-A extreme of the available data for UVR8. We can conclude from this that UVR8 is likely to play an important role in UV-A perception in sunlight.
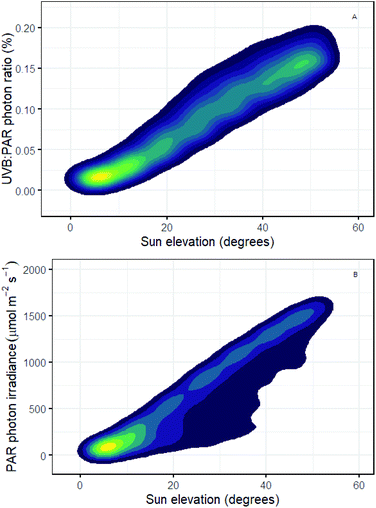
It is well known that the daily course of incident solar UV-B irradiance differs from that of PAR. The role of the balance between UV-B and UV-A, blue and PAR exposure in determining UV-B tolerance is also well documented but the mechanisms involved are not yet fully understood.137 Using simulations for Helsinki, we see that with increasing solar elevation the UV-B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAR photon ratio increases reaching on average 0.18% at the sun's maximal elevation (solar noon) compared with very low values (<0.02%) at low solar elevations (i.e., dawn and dusk; Fig. 2A). While some plants have a diurnal rhythm in epidermal transmittance which moderates UV-B exposure,19 we know little about how diurnal variation in UV-B
PAR photon ratio increases reaching on average 0.18% at the sun's maximal elevation (solar noon) compared with very low values (<0.02%) at low solar elevations (i.e., dawn and dusk; Fig. 2A). While some plants have a diurnal rhythm in epidermal transmittance which moderates UV-B exposure,19 we know little about how diurnal variation in UV-B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAR ratio affects both plant tolerance to UV-B exposure and regulatory responses dependent on UV-B perception. Plant-UV-B experiments in controlled environments often use unrealistically high UV-B
PAR ratio affects both plant tolerance to UV-B exposure and regulatory responses dependent on UV-B perception. Plant-UV-B experiments in controlled environments often use unrealistically high UV-B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAR ratios making it difficult to infer how UV-B responses might be modulated diurnally outdoors. For example, if we assume 0.2% as a reasonable value for UV-B
PAR ratios making it difficult to infer how UV-B responses might be modulated diurnally outdoors. For example, if we assume 0.2% as a reasonable value for UV-B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAR (Fig. 2A), then experiments using a UV-B photon irradiance of 3 μmol m−2 s−1 would require a PAR photon irradiance of 1800 μmol m−2 s−1 to achieve this ratio. Few growth rooms can provide such a high PAR irradiance. A more commonly reported PAR irradiance of 200 μmol m−2 s−1 will give a UV-B
PAR (Fig. 2A), then experiments using a UV-B photon irradiance of 3 μmol m−2 s−1 would require a PAR photon irradiance of 1800 μmol m−2 s−1 to achieve this ratio. Few growth rooms can provide such a high PAR irradiance. A more commonly reported PAR irradiance of 200 μmol m−2 s−1 will give a UV-B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAR ratio of 1.5% when used in combination with 3 μmol m−2 s−1 of UV-B radiation. This ratio is 7.5 times more than the maximum UV-B
PAR ratio of 1.5% when used in combination with 3 μmol m−2 s−1 of UV-B radiation. This ratio is 7.5 times more than the maximum UV-B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAR ratio outdoors in Helsinki at solar noon in mid-summer.2 Conversely, a PAR irradiance, of 200 μmol m−2 s−1 would accurately reflect the irradiance under a clear sky at a solar elevation angle of just 10 degrees in Helsinki. However, under these conditions UV-B radiation is equivalent to only 0.025% of PAR (Fig. 2B). This illustrates that despite efforts to improve experimental the design, many experiments continue to be based on unrealistic UV-B treatments, even when the UV-B irradiance treatments used are no higher than the maximal UV-B irradiances outdoors.
PAR ratio outdoors in Helsinki at solar noon in mid-summer.2 Conversely, a PAR irradiance, of 200 μmol m−2 s−1 would accurately reflect the irradiance under a clear sky at a solar elevation angle of just 10 degrees in Helsinki. However, under these conditions UV-B radiation is equivalent to only 0.025% of PAR (Fig. 2B). This illustrates that despite efforts to improve experimental the design, many experiments continue to be based on unrealistic UV-B treatments, even when the UV-B irradiance treatments used are no higher than the maximal UV-B irradiances outdoors.
| Currently the best we can do, to predict the relative numbers of photons of different wavelengths absorbed in vivo by a photoreceptor, is to combine the spectrum of the radiation a plant is exposed to with the spectral absorbance measured in vitro for a photoreceptor. This is far from ideal and can provide only a rough approximation to what actually happens within the leaf. |
In experiments using filters to modify the solar spectrum and/or using supplemental UV-B radiation with modulated systems, the shape of the daily course of illumination will track that of natural sunlight, allowing for a proportional enrichment UV-B radiation at midday and depletion at the beginning and end of the day.138 On the other hand, most experiments done in controlled environments use a daily course of UV-B exposure that will drastically differ from that in sunlight and even from that in other controlled environment experiments. Consequently, it should come as no surprise that results obtained in indoor systems are not necessarily environmentally relevant and can be difficult to interpret, even though such studies are vitally important for elucidation of response mechanisms. Furthermore, the daily course of the changes in solar radiation is often paralleled by diurnal patterns in other environmental variables such as temperature. Thus, when designing future experiments on regulation and acclimation, it is vital to consider the environmental context, as this may mask changes in the features of interest and/or make interpretation impossible, especially where the experimental context differs from that of a natural or agricultural environment.
(i) Does UV-B radiation have a role in regulating circadian clocks and phenology?
The relatively large seasonal changes in UV-B irradiance compared with those in other regions of the spectrum (Fig. 2) can potentially hold information that could be used as a phenological cue by plants. Known relationships between UVR8 and the shade avoidance syndrome86 might suggest a role for UV-B radiation in the timing of leaf developmental phases. Identifying consistent shifts in the timing of phenological events in response to UV-B radiation will help us to interpret if and how plants are utilising changing patterns of UV-B in nature, and to define a role of UV-B radiation distinct from that of other parts of the spectrum. However, most ecological studies to date have reported only weak and inconsistent patterns of delay or advance in phenology caused by UV-B radiation (reviewed for flowering139 and tree phenology140).Most research into mechanisms underlying the role of spectral cues in phenological processes has focused on the regulation of flowering time. It has been proposed that downstream expression of HY5 and HYH, regulated by UVR8, may mediate the regulation of POLYCOMB REPRESSIVE COMPLEX 2 (PRC2), and ultimately FLOWERING LOCUS T (FT).141 This hypothesis could be tested by following the flowering response of hy5 and hyh mutants to UV-B radiation. However, any UV-B-mediated delay of flowering time needs to be considered in the context of multiple photoreceptors responding to received spectral irradiance as a whole. In this respect, HY5 and COP1 may form a central node for light-signal integration via cryptochromes, phytochromes and UVR8 in Arabidopsis.142 UVR8 is also implicated in the photoperiodic control of flowering in Arabidopsis, whereby a mutation in RUP2 removes the long-day control of flowering in the presence of UV-B radiation.143
Phenological research can be placed in a broader context through its study in a diverse range of plant species.144 In particular, a better understanding of the mechanisms underpinning seasonal phenological events can be gained through experiments with mutant tree species. For instance, phenological cycles over several years could be studied in a mutant of Betula platyphylla over-expressing the UVR8 homologue BpUVR8,89 which responds to UV-B radiation and is involved in abscisic acid (ABA) signalling. Research into tree phenology, exemplifies how molecular-genetic research can be successfully scaled-up to ecologically relevant processes across different plant populations and species (reviewed in ref. 145). In Populus tremuloides, FT regulates bud burst and bud set as well as flowering, and genome sequences homologous to Arabidopsis UVR8 have been identified.146 In a field experiment with Populus tremula, UVR8-mediated ABA-signalling with FT allowed UV-B radiation to advance bud set.147
Autumn leaf senescence and bud-set phenology are relatively neglected subjects of climate change research.149 In addition to its effect on bud set, UV-B radiation has also been shown to advance autumnal leaf senescence in Fagus sylvatica.150 Not only does early autumn leaf senescence have the potential to create asynchrony with other organisms such as herbivores, but it also impacts global carbon assimilation and leaf-litter decomposition. Key questions remain unanswered regarding phenological responses to UV-B radiation, including how great a contribution is made by patterns of UV-B radiation in comparison to other environmental variables such as temperature and photoperiod? Furthermore, the question arises, why does UV-B radiation advance bud burst and flowering in some plant species, but delay phenology in others?151,152 Is it possible that different species have evolved to respond differently to UV cues? Phenology is potentially critical for survival and fitness of plants, so answering these questions is important for understanding the interactions of UV-B and climate change parameters (Fig. 3).
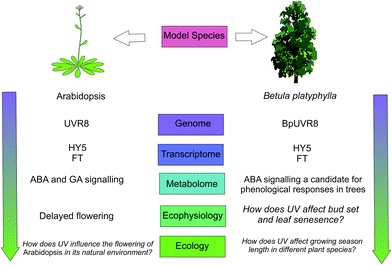 | ||
| Fig. 3 A schematic showing how plant phenological responses to UV-B radiation could up-scale through levels of organisation. Evidence for a coherent framework scaling through these levels is given in ref. 86, 141, 144, 146–148 and those questions which remain to be resolved are in italics. | ||
(j) From model studies to the real world
In previous sections we have highlighted new discoveries and breakthroughs in research into the response of plants to UV-B radiation at the molecular, biochemical, cellular and physiological scales. However, there are no clear channels for this knowledge to be extrapolated from model species to other species, from controlled experimental conditions to complex field environments, and from single plants to higher organisational levels (populations and communities). Exposure to UV-B radiation in natural environments is typically highly dynamic, governed by strong temporal fluctuations (daily and seasonal) and geographic gradients (with elevation and latitude) in UV-B irradiance. However, the overwhelming majority of studies underpinning our knowledge of UV-B radiation have used stable irradiance treatments, or unnatural UV-spectra and/or artificial exposure kinetics whereby plants which were grown without any UV-B are subsequently challenged with such radiation. This makes extrapolation of findings to environmentally relevant conditions complicated and potentially unreliable. A particularly problematic gap in our understanding of plant UV-responses is our uncertainty as to how results obtained using “low PAR” growth chambers, can be realistically scaled up to highly dynamic, high PAR outdoor environments. Ecological trait-based experiments to examine the role that UV plays under natural conditions may provide a mechanistic link between UV-B radiation and plant traits. This approach involves assessment of how plant traits, or suites of traits reflecting an ecological strategy, tend to respond within the established limits of their plasticity in a given environment. For instance, an experiment could test the responses of several different populations or species that segregate along a continuum of conceptual “trait space” (in one, two or multiple dimensions), when subject to a gradient of ecologically relevant UV-B exposure. However, to date such experiments are still largely lacking.In the natural world, the effects of highly variable solar UV irradiances are further modified by other environmental parameters. This is particularly evident from long-term UV-B supplementation or filtration experiments, where plant communities and ecosystems have been found to respond inconsistently to solar UV-B radiation over multiple years (e.g.ref. 153–155). Likewise, UV-absorbing compounds in the leaves of trees and crop plants routinely co-vary with UV irradiance and other environment variables over the course of their development or with seasonal environmental variation.156–158 Even on a much shorter timescale, plants may modulate their epidermal UV transmittance through the day, as a strategy to reduce UV exposure in the leaf mesophyll at midday when irradiance is at its highest.159 At present, the controls on temporal variation in plant UV-responses are poorly understood, further hampering scaling between the laboratory and environmentally-relevant conditions.
| There are no clear channels for the knowledge of basic plant UV-responses to be extrapolated from model species to others, from controlled experimental conditions to complex environments, and from single plants to higher organisational levels (communities and populations). |
Plants are able to perceive many aspects of their environment, from changes in resource availability to the presence of other organisms, competitors and pathogens. Moreover, plants have the capability to communicate with each other and other organisms.160–162 An important question is whether UV affects plant-plant communication. Plant-plant communication has been best detailed in the case of herbivory, when the emission of VOCs is enhanced. Interestingly, UV-B and UV-A radiation have also been reported to enhance VOC emission.163 Moreover, UV-B radiation is required for the production of specific floral volatiles needed to attract pollinators in some orchid species.164,165 The mechanism (and consequences) of this response remain to be established, however it is possible that the response is mediated by ROS, produced in response to UV-B radiation, in a scenario analogous to herbivory damage.166 This outcome would also be consistent with reports of UV-B-enhanced plant defence against herbivory operating via UVR8.167–169 If verified, it is conceivable that plants could use this mechanism to systemically transmit information about their UV environment.
(k) Applications of UV in commercial plant production and crop breeding
Ongoing climate change is leading to changes in temperature, drought prevalence and UV-B radiation that consequently affect plant growth and development.170,171 These climatic shifts constitute a threat to global food security, requiring growers to adjust their crop selection and plant breeders to tailor cultivars suited to new climates. Selection for traits that include resistance to high UV radiation, or the cross-protection produced by a strong response to UV-B radiation, is required for those environments where climate change is altering weather patterns to produce drier sunnier conditions, e.g. in the Andean Altiplano and parts of the Mediterranean region. Plant biotechnology provides well proven approaches to improve breeding programmes and the selection of cultivars for commercial production: for instance using quantitative trait loci (QTL) mapping for genetic markers conferring shared resistance to drought and UV-B radiation is a promising approach for maize.172 Innovative approaches to crop transformation, such as CRISPR/Cas9 combined with rapid nucleotide sequencing techniques, further facilitate targeted breeding, but require identification of specific target loci.| There is a lack of knowledge about the potential to exploit plant UV-responses in breeding programmes that focus on climate change resilience. |
New technologies based on the detection of changes in leaf optical properties are being exploited by UV research159 but are also useful in commercial plant production. The utility of such devices has been greatest in vineyards where small differences in flavonoids at harvest can have large effects on quality and price of the end product, which makes detailed non-destructive analysis of optical properties particularly worthwhile.173 To this end, handheld devices and remote sensing of flavonoids has allowed fine-scale spatial differentiation of berry quality.174–176 If farmers are convinced of the cost-benefits of such surveys of pigments to guide fertiliser application, harvest time and to assess quality, they are likely to herald a revolution in remote optical-sensors. These can be used to feed information into management decision-making systems on crop pigment responses to UV-B radiation as one component of comprehensive monitoring of the environment and crop.177,178
While plant-UV research has increasingly focused on the regulatory responses of plants to UV-B radiation, a common misconception remains among commercial plant producers that UV-B radiation has overwhelmingly damaging effects. This suspicion represents a barrier to the application of supplemental UV-B radiation as a tool to aid commercial growers. Nevertheless, exposure to solar UV-B radiation has been shown to aid the development and improve the nutritional value of fruit and vegetables.179 Furthermore, the application of post-harvest UV-B radiation can improve the value of these foods through more desirable colour, aroma and flavour, as well as higher flavonoid content.180 Research is ongoing to test if UV-B radiation can be used to affect plant metabolite composition to improve the nutritional value of crops,181 which if feasible would have considerable economic potential in the food industry. Growers could use UV-B radiation as part of an integrated management strategy: to enhance UV-induced plant defence that can deter pests and inhibit pathogens or through the direct action of UV-B radiation on these organisms182–185 and to restrict stem elongation producing compact plants for retail.186,187 Development of these applications of UV-B radiation, has the potential to improve the economic and environmental efficiency of commercial plant production, and provide energy savings from judicious application of solar UV-B radiation and/or supplemental UV lighting to replace other management practices.
| The development of UV-emitting LEDs is giving growers unprecedented scope to manipulate the spectrum that crops are exposed to. However, there is still a lack of data on the wavelength dependency of many plant UV-responses. |
A variety of different approaches to manipulate UV-B radiation can be used in glasshouses and controlled environments. In fully enclosed facilities, such as those used in vertical farming, UV radiation will need to be generated artificially. In the past, UV-fluorescent tubes were the only option available but now lighting fixtures using UV-emitting LEDs are starting to be commercialised. These allow far greater control than before of the spectral composition of radiation emitted within the UV-region. As advances in research reveal more about the spectral regions over which photoreceptor-mediated responses operate, it will become feasible for commercial growers to tailor their LED lighting to specifically match the regions of interest, assuming that we can scale knowledge of mechanisms to whole plant responses. Multiple types of LEDs each with narrow emission spectra can be combined in a lighting fixture to match a particular spectral requirement. Alternatively, a variety of LEDs with more-comprehensive coverage of the spectrum could be installed with the option for the grower to select which LEDs are on and off to produce their different desired spectra depending on the crop or time of year.
More effective use of solar UV radiation in plant production represents an alternative approach to UV lighting in order to exploit beneficial plant-UV regulatory responses in both horticultural and agricultural settings. UV-transmitting cladding materials are now widely available, including both plastic screens and films, and glass designed to selectively transmit part or all of the UV region of sunlight to the plants inside polytunnels and greenhouses.188 Although, typically more expensive than the standard plastics or glass used, this represents the most cost-effective way to expose crop plants grown under cover to solar UV-radiation. Eventually, the value of using UV radiation in commercial plant production is likely to depend on the crop and cultivar grown and the geographical location of the facilities. A key objective for applied researchers in UV photobiology should be the development and provision of light recipes for specific applications of UV radiation on a case-by-case basis to fit recommendations to particular scenarios.188
(l) Discussion and conclusions
Understanding of plant UV-B responses has markedly improved over the last few decades. The discovery of the UV-B photoreceptor UVR8, as well as downstream signalling elements and target genes, in combination with detailed characterisation of physiological and metabolic plant responses has resulted in in-depth knowledge of UV-B-induced acclimation. These plant UV-B responses are underpinned by a complex mixture of ancestral UV-perception and response pathways, combined with more recent innovations in plant secondary metabolism, cell-wall composition, and compartmentalisation of UV-absorbing compounds. The evolutionary conservation of plant UV-B responses implies their functional importance. Yet, there is much to consider if we are to understand how UVR8 functions in diverse plant species in an ecological context. It will be important to determine whether a given plant species has multiple UVR8 genes, whether these are differentially expressed, the localisation of UVR8, and functional responses, most likely involving interactions with other pathways that may differ between species adapted to particular environments. While studies with Arabidopsis have highlighted numerous functions of UVR8,75 it is not known how widely these functions are conserved and what additional responses may be present in species other than Arabidopsis and other model species. A complication is that some plant species appear to have at least two UVR8 genes, so it is conceivable that they may possess UVR8 proteins with different patterns of expression and potentially different functions.To understand spatial, temporal and environmental regulation of plants’ UV-B responses, it is imperative that a much better understanding is acquired about UV-exposure of plants, organs, and cells. Currently, it is not well understood how plant architecture, leaf structure, trichome density and wax deposition affect local UV-regimes within a canopy or within a plant. Even less is known about how UV-B-induced changes in these same morphological parameters affect UV-B exposure. In contrast, there is good evidence that UV-screening pigments in the cuticle and epidermis can decrease UV-B penetration in underlying tissues. Yet, although UV-B radiation has been well documented to induce UV-screening pigments, there is also increasing awareness of the possibility that protection against UV-B radiation can be triggered by exposure to, for example, visible light. Indeed, changes in the total concentrations of secondary metabolites triggered by UV-B and UV-A radiation are not necessarily substantial and/or significant under environmentally relevant conditions. This in turn triggers questions about the functional importance of UVR8 in plants growing in natural environments. Studies in Arabidopsis show that UVR8-mediated responses are not exclusively linked to plant UV-B protection and affect a number of morphological and physiological processes. It is already becoming increasingly evident that UVR8 functions through interaction with other signalling pathways and it is important to extend our understanding of the interplay between UV-B- and other signalling pathways in species adapted to different environments, in order to understand the full scope of the function of UVR8 and of UV-B sensing.
There are no clear routes to extrapolate our knowledge of basic plant UV-responses from model species to others, from controlled experimental conditions to complex environments, and from single plants to higher organisational levels (communities and populations). There are often large differences between plant UV-responses under controlled environments and under field conditions. Furthermore, very few links have been drawn between UV-B-induced regulation of gene expression, whole-plant performance and predicted fitness. Thus, while the evolutionary conservation of UVR8 implies a key role for UV-B sensing, studies of plant UV-B responses under environmentally relevant conditions do not necessarily back this up with evidence of significant UV-B impacts on plants. It is anticipated that the increased use of molecular methods in outdoor studies, in combination with more traditional ecophysiological and growth analysis, will fill this knowledge void. Furthermore, there is substantial scope for indoor studies to be more environmentally relevant through the use of appropriate light-sources.
Glasshouse and growth room studies have already shown substantial UV-B impacts on plants, some of which are commercially desirable, such as the increase in specific secondary metabolites, a more stocky architecture and pest and disease resistance. Despite such commercially interesting impacts, slow transfer of knowledge means the potential to exploit plant UV-responses in a horticultural setting is still underutilised. This lack of biological knowledge contrasts with the rapid development of UV-emitting LEDs, which are giving growers unprecedented scope to manipulate the crops exposure spectrum. Thus, it can be anticipated that in the next decade there will be opportunities for highly detailed mapping of spectral UV-responses in a wide variety of crop species. By integrating such spectral mapping with detailed molecular expression profiling, as well as traditional biochemical and physiological analysis of plant growth, a comprehensive understanding of plant UV-responses is within reach.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This perspective piece was conceived by participants at the UV4Plants 2nd Network Meeting in Bled, April 2018 and builds on discussions arising with the participants in that meeting. Financial support was provided by: the Academy of Finland decision 304519 and 266523 to TMR; Ministerio de Economía y Competitividad of Spain (MINECO) and Fondo Europeo de Desarrollo Regional (FEDER) Project CGL2014-54127-P to JM-A; the Polish National Science Centre grants UMO-2016/22/E/NZ3/00326 to AKB and UMO-2017/25/B/NZ3/01080 to JŁ; bursaries from UV4Plants and the Doctoral Programme in Plant Science of the University of Helsinki to CCB and NR; Deutsche Forschungsgemeinschaft (DFG) project 359552155 to SN; the Region Haute-Normandie GRR-TERA SCALE, UFOSE Project to MP; Ghent University and The Research Foundation Flanders (FWO, G000515N) to FV; and Science Foundation Ireland project 16-IA-4418 to MAKJ.References
- L. O. Björn, UV4Plants Bulletin, 2015, vol. 1, pp. 17–18 Search PubMed.
- P. J. Aphalo, UV4Plants Bulletin, 2018, vol. 2018, pp. 45–56 Search PubMed.
- L. Rizzini, J. J. Favory, C. Cloix, D. Faggionato, A. O'Hara, E. Kaiserli, R. Baumeister, E. Schäfer, F. Nagy, G. I. Jenkins and R. Ulm, Science, 2011, 332, 103–106 CrossRef CAS PubMed.
- F. Westall, C. E. J. De Ronde, G. Southam, N. Grassineau, M. Colas, C. S. Cockell and H. Lammer, Philos. Trans. R. Soc., B, 2006, 361, 1857–1875 CrossRef CAS PubMed.
- C. S. Cockell and J. A. Raven, Philos. Trans. R. Soc., A, 2007, 365, 1889–1901 CrossRef CAS PubMed.
- E. Nisbet and C. M. R. Fowler, Chin. Sci. Bull., 2011, 56, 4–13 CrossRef CAS.
- L. O. Björn, in Stratospheric ozone depletion: the effects of enhanced UV-B radiation on terrestrial ecosystems, ed. J. Rozema, Backhuys Publishers, Leiden, 1999, pp. 21–37 Search PubMed.
- J. Belnap, S. L. Philips, S. D. Flint, J. Money and M. M. Caldwell, Global Change Biol., 2008, 14, 670–686 CrossRef.
- D. Edwards, L. Cherns and J. A. Raven, Palaeontology, 2015, 58, 803–837 CrossRef.
- A. M. Mloszewska, D. B. Cole, N. J. Planavsky, A. Kappler, D. S. Whitford, G. W. Owttrim and K. O. Konhauser, Nat. Commun., 2018, 9, 3088 CrossRef PubMed.
- S. A. Rensing, Curr. Opin. Plant Biol., 2018, 42, 49–54 CrossRef CAS PubMed.
- G. Soriano, C. Cloix, M. Heilmann, E. Núñez-Olivera, J. Martínez-Abaigar and G. I. Jenkins, New Phytol., 2018, 217, 151–162 CrossRef CAS PubMed.
- C. S. Cockell and J. Knowland, Biol. Rev., 1999, 74, 311–345 CrossRef CAS PubMed.
- A. Mouradov and G. Spangenberg, Front. Plant Sci., 2014, 5, 620 CrossRef PubMed.
- R. P. Rastogi, R. P. Sinha, S. H. Moh, T. K. Lee, S. Kottuparambil, Y. J. Kim, J. S. Rhee, E. M. Choi, M. T. Brown, D. P. Hader and T. Han, J. Photochem. Photobiol., B, 2014, 141, 154–169 CrossRef CAS PubMed.
- K. J. Niklas, E. D. Cobb and A. J. Matas, J. Exp. Bot., 2017, 68, 5261–5269 CrossRef CAS PubMed.
- Y. Carrington, J. Guo, C. H. Le, A. Fillo, J. Kwon, L. T. Tran and J. Ehlting, Plant J., 2018, 95, 823–833 CrossRef CAS PubMed.
- L. Monforte, G. Soriano, E. Núñez-Olivera and J. Martínez-Abaigar, Funct. Ecol., 2018, 32, 882–893 CrossRef.
- P. W. Barnes, T. M. Robson, M. A. Tobler, I. N. Bottger and S. D. Flint, in Plant UV Biology, ed. B. Jordan, CABI Publishers, 2017, ch. 6, pp. 72–89 Search PubMed.
- J. F. Bornman, P. W. Barnes, T. M. Robson, S. A. Robinson, M. A. K. Jansen, C. L. Ballaré and S. D. Flint, Photochem. Photobiol. Sci., 2019, 10.1039/c8pp90061b.
- E. H. DeLucia, T. A. Day and T. C. Vogelman, Plant, Cell Environ., 1992, 15, 921–929 CrossRef.
- P. W. Barnes, A. R. Kersting, S. D. Flint, W. Beyschlag and R. J. Ryel, Physiol. Plant., 2013, 149, 200–213 CrossRef CAS PubMed.
- T. M. Robson, K. Klem, O. Urban and M. A. Jansen, Plant, Cell Environ., 2015, 38, 856–866 CrossRef CAS PubMed.
- M. A. K. Jansen, T. M. Robson, K. Klem and O. Urban, in Plant UV Biology, ed. B. Jordan, CABI Publishers, 2017, ch. 5, pp. 58–71 Search PubMed.
- S. D. Flint and M. M. Caldwell, Global Change Biol., 1998, 4, 863–870 CrossRef.
- R. H. Grant, K. Apostol and W. Gao, Agric. For. Meteorol., 2005, 132, 28–43 CrossRef.
- S. M. Hartikainen, A. Jach, A. Grané and T. M. Robson, Ecol. Evol., 2018, 8, 10206–10218 CrossRef PubMed.
- T. A. Day, Oecologia, 1993, 95, 542–550 CrossRef CAS PubMed.
- S. Fukuda, A. Satoh, H. Kasahara, H. Matsuyama and Y. Takeuchi, J. Plant Res., 2008, 121, 179–189 CrossRef CAS PubMed.
- A. Yan, J. Pan, L. An, Y. Gan and H. Feng, J. Photochem. Photobiol., B, 2012, 113, 29–35 CrossRef CAS PubMed.
- Y. Manetas, New Phytol., 2003, 158, 503–508 CrossRef.
- M. M. Caldwell, R. Robberecht and S. D. Flint, Physiol. Plant., 1983, 58, 445–450 CrossRef CAS.
- P. W. Barnes, S. D. Flint, R. J. Ryel, M. A. Tobler, A. E. Barkley and J. J. Wargent, Plant Physiol. Biochem., 2015, 93, 94–100 CrossRef CAS PubMed.
- V. Liakoura, J. F. Bornman and G. Karabourniotis, Physiol. Plant., 2003, 117, 33–43 CrossRef CAS.
- J. M. Earles, G. Théroux-Rancourt, M. E. Gilbert, A. J. McElrone and C. R. Brodersen, Plant Physiol., 2017, 174, 1082–1096 CrossRef CAS PubMed.
- A. Putarjunan, X. Liu, T. Nolan, F. Yu and S. Rodermel, Photosynth. Res., 2013, 116, 437–453 CrossRef CAS PubMed.
- A. K. Banaś, C. Aggarwal, J. Łabuz, O. Sztatelman and H. Gabryś, J. Exp. Bot., 2012, 63, 1559–1574 CrossRef PubMed.
- K. Iwabuchi, J. Hidema, K. Tamura, S. Takagi and I. Hara-Nishimura, Plant Physiol., 2016, 170, 678–685 CrossRef CAS PubMed.
- M. Kasahara, T. Kagawa, K. Oikawa, N. Suetsugu, M. Miyao and M. Wada, Nature, 2002, 420, 829–832 CrossRef CAS PubMed.
- T. Kagawa, T. Sakai, N. Suetsugu, K. Oikawa, S. Ishiguro, T. Kato, S. Tabata, K. Okada and M. Wada, Science, 2001, 291, 2138–2141 CrossRef CAS PubMed.
- J. A. Jarillo, H. Gabryś, J. Capel, J. M. Alonso, J. R. Ecker and A. R. Cashmore, Nature, 2001, 410, 952–954 CrossRef CAS PubMed.
- K. Iwabuchi, R. Minamino and S. Takagi, Plant Physiol., 2010, 152, 1309–1319 CrossRef CAS PubMed.
- P. A. Davis, S. Caylor, C. W. Whippo and R. P. Hangarter, Plant, Cell Environ., 2011, 34, 2047–2059 CrossRef CAS PubMed.
- É. Hideg, M. A. K. Jansen and Å. Strid, Trends Plant Sci., 2013, 18, 107–115 CrossRef PubMed.
- G. Agati, C. Brunetti, M. Di Ferdinando, F. Ferrini, S. Pollastri and M. Tattini, Plant Physiol. Biochem., 2013, 72, 35–45 CrossRef CAS PubMed.
- C. Brunetti, A. Fini, F. Sebastiani, A. Gori and M. Tattini, Front. Plant Sci., 2018, 9, 1042 CrossRef PubMed.
- A. Coffey and M. A. K. Jansen, Plant Physiol. Biochem., 2018, 134, 64–72 CrossRef PubMed.
- K. Csepregi, S. Neugart, M. Schreiner and É. Hideg, Molecules, 2016, 21, E208 CrossRef PubMed.
- K. Csepregi, A. Coffey, N. Cunningham, E. Prinsen, É. Hideg and M. A. K. Jansen, Environ. Exp. Bot., 2017, 140, 19–25 CrossRef CAS.
- K. Hectors, S. Van Oevelen, J. Geuns, Y. Guisez, M. A. Jansen and E. Prinsen, Physiol. Plant., 2014, 152, 219–230 CrossRef CAS PubMed.
- R. Nakabayashi, K. Yonekura-Sakakibara, K. Urano, M. Suzuki, Y. Yamada, T. Nishizawa, F. Matsuda, M. Kojima, H. Sakakibara, K. Shinozaki, A. J. Michael, T. Tohge, M. Yamazaki and K. Saito, Plant J., 2014, 77, 367–379 CrossRef CAS PubMed.
- P. Singla and N. Garg, in Mycorrhiza - Function, Diversity, State of the Art, ed. A. Varma, R. Prasad and N. Tuteja, Springer, 2017, pp. 133–176 Search PubMed.
- M. A. K. Jansen, W. Bilger, É. Hideg, Å. Strid, U. P. W. Participants and O. Urban, Plant Physiol. Biochem., 2018, 134, 1–8 CrossRef PubMed.
- H. Liu, X. Cao, X. Liu, R. Xin, J. Wang, J. Gao, B. Wu, L. Gao, C. Xu, B. Zhang, D. Grierson and K. Chen, Plant, Cell Environ., 2017, 40, 2261–2275 CrossRef CAS PubMed.
- C. Gao, B. Yang, D. Zhang, M. Chen and J. Tian, BMC Plant Biol., 2016, 16, 231 CrossRef PubMed.
- A. P. Schuch, N. C. Moreno, N. J. Schuch, C. F. M. Menck and C. C. M. Garcia, Free Radicals Biol. Med., 2017, 107, 110–124 CrossRef CAS PubMed.
- M. Takahashi, M. Teranishi, H. Ishida, J. Kawasaki, A. Takeuchi, T. Yamaya, M. Watanabe, A. Makino and J. Hidema, Plant J., 2011, 66, 433–442 CrossRef CAS PubMed.
- A. K. Banaś, P. Hermanowicz, O. Sztatelman, J. Łabuz, C. Aggarwal, P. Zgłobicki, D. Jagiełło-Flasińska and W. Strzałka, Plant Cell Physiol., 2018, 59, 44–57 CrossRef PubMed.
- S. G. Kong and M. Wada, J. Plant Res., 2016, 129, 159–166 CrossRef CAS PubMed.
- J. J. Biever, D. Brinkman and G. Gardner, J. Exp. Bot., 2014, 65, 2949–2961 CrossRef CAS PubMed.
- G. B. Sancar, F. W. Smith and A. Sancar, Biochemistry, 1985, 24, 1849–1855 CrossRef CAS PubMed.
- M. Zhang, L. Wang and D. Zhong, Photochem. Photobiol., 2017, 93, 78–92 CrossRef CAS PubMed.
- G. B. Sancar and F. W. Smith, Mol. Cell. Biol., 1989, 9, 4767–4776 CrossRef CAS PubMed.
- S. Predieri, H. A. Norman, D. T. Krizek, P. Pillai, R. M. Mirecki and R. H. Zimmerman, Environ. Exp. Bot., 1995, 35, 151–160 CrossRef CAS.
- S. Fujii, K. Kobayashi, Y. Nakamura and H. Wada, Plant Physiol., 2014, 166, 1436–1449 CrossRef PubMed.
- O. Bossi, M. Gartsbein, M. Leitges, T. Kuroki, S. Grossman and T. Tennenbaum, J. Cell. Biochem., 2008, 105, 194–207 CrossRef CAS PubMed.
- M. Y. Yoon, M. Y. Kim, S. Shim, K. D. Kim, J. Ha, J. H. Shin, S. Kang and S. H. Lee, Front. Plant Sci., 2016, 7, 1917 Search PubMed.
- C. Suesslin and H. Frohnmeyer, Plant J., 2003, 33, 591–601 CrossRef CAS PubMed.
- C. Waszczak, M. Carmody and J. Kangasjärvi, Annu. Rev. Plant Biol., 2018, 69, 209–236 CrossRef CAS PubMed.
- G. Czégény, A. Mátai and É. Hideg, Plant Sci., 2016, 248, 57–63 CrossRef PubMed.
- R. Mittler, Trends Plant Sci., 2017, 22, 11–19 CrossRef CAS PubMed.
- S. A. H. Mackerness, B. R. Jordan and B. Thomas, in Plants and UV-B: Responses to environmental change, ed. P. Lumsden, Cambridge University Press, 1997, pp. 113–134 Search PubMed.
- T. Crawford, N. Lehotai and Å. Strand, J. Exp. Bot., 2018, 69, 2783–2795 CrossRef PubMed.
- G. Potters, N. Horemans and M. A. K. Jansen, Plant Physiol. Biochem., 2010, 48, 292–300 CrossRef CAS PubMed.
- G. I. Jenkins, Plant, Cell Environ., 2017, 40, 2544–2557 CrossRef CAS PubMed.
- R. H. Yin and R. Ulm, Curr. Opin. Plant Biol., 2017, 37, 42–48 CrossRef CAS PubMed.
- J. M. Christie, A. S. Arvai, K. J. Baxter, M. Heilmann, A. J. Pratt, A. O'Hara, S. M. Kelly, M. Hothorn, B. O. Smith, K. Hitomi, G. I. Jenkins and E. D. Getzoff, Science, 2012, 335, 1492–1496 CrossRef CAS PubMed.
- D. Wu, Q. Hu, Z. Yan, W. Chen, C. Yan, X. Huang, J. Zhang, P. Yang, H. Deng, J. Wang, X. Deng and Y. Shi, Nature, 2012, 484, 214–219 CrossRef PubMed.
- M. B. Fernández, V. Tossi, L. Lamattina and R. Cassia, Front. Plant Sci., 2016, 7, 1698 Search PubMed.
- C. Cloix, E. Kaiserli, M. Heilmann, K. J. Baxter, B. A. Brown, A. O'Hara, B. O. Smith, J. M. Christie and G. I. Jenkins, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 16366–16370 CrossRef CAS PubMed.
- M. Heijde and R. Ulm, Proc. Natl. Acad. Sci. USA, 2013, 110, 1113–1118 CrossRef CAS PubMed.
- G. Allorent, L. Lefebvre-Legendre, R. Chappuis, M. Kuntz, T. B. Truong, K. K. Niyogi, R. Ulm and M. Goldschmidt-Clermont, Proc. Natl. Acad. Sci. USA, 2016, 113, 14864–14869 CrossRef CAS PubMed.
- K. Tilbrook, M. Dubois, C. D. Crocco, R. Yin, R. Chappuis, G. Allorent, E. Schmid-Siegert, M. Goldschmidt-Clermont and R. Ulm, Plant Cell, 2016, 28, 966–983 CAS.
- B. A. Brown, C. Cloix, G. H. Jiang, E. Kaiserli, P. Herzyk, D. J. Kliebenstein and G. I. Jenkins, Proc. Natl. Acad. Sci. USA, 2005, 102, 18225–18230 CrossRef CAS PubMed.
- J. J. Favory, A. Stec, H. Gruber, L. Rizzini, A. Oravecz, M. Funk, A. Albert, C. Cloix, G. I. Jenkins, E. J. Oakeley, H. K. Seidlitz, F. Nagy and R. Ulm, EMBO J., 2009, 28, 591–601 CrossRef CAS PubMed.
- S. Hayes, C. N. Velanis, G. I. Jenkins and K. A. Franklin, Proc. Natl. Acad. Sci. USA, 2014, 111, 11894–11899 CrossRef CAS PubMed.
- F. Vandenbussche, K. Tilbrook, A. C. Fierro, K. Marchal, D. Poelman, D. Van Der Straeten and R. Ulm, Mol. Plant, 2014, 7, 1041–1052 CrossRef CAS PubMed.
- S. Hayes, A. Sharma, D. P. Fraser, M. Trevisan, C. K. Cragg-Barber, E. Tavridou, C. Fankhauser, G. I. Jenkins and K. A. Franklin, Curr. Biol., 2017, 27, 120–127 CrossRef CAS PubMed.
- X. Li, M. Ma, W. Shao, H. Wang, R. Fan, X. Chen, X. Wang, Y. Zhan and F. Zeng, Plant Sci., 2018, 274, 294–308 CrossRef CAS PubMed.
- P. Bernula, C. D. Crocco, A. B. Arongaus, R. Ulm, F. Nagy and A. Viczian, Plant, Cell Environ., 2017, 40, 1104–1114 CrossRef CAS PubMed.
- K. M. Findlay and G. I. Jenkins, Plant, Cell Environ., 2016, 39, 1706–1714 CrossRef CAS PubMed.
- V. Moriconi, M. Binkert, C. Costigliolo, R. Sellaro, R. Ulm and J. J. Casal, Plant Physiol., 2018, 177, 75–81 CAS.
- L. O. Morales, M. Brosche, J. Vainonen, G. I. Jenkins, J. J. Wargent, N. Sipari, A. Strid, A. V. Lindfors, R. Tegelberg and P. J. Aphalo, Plant Physiol., 2013, 161, 744–759 CrossRef CAS PubMed.
- L. A. Diaz-Ramos, A. O'Hara, S. Kanagarajan, D. Farkas, A. Strid and G. I. Jenkins, Photochem. Photobiol. Sci., 2018, 17, 1108–1117 RSC.
- J. Takeda, R. Nakata, H. Ueno, A. Murakami, M. Iseki and M. Watanabe, Photochem. Photobiol., 2014, 90, 1043–1049 CAS.
- A. Goyal, B. Szarzynska and C. Fankhauser, Trends Plant Sci., 2013, 18, 393–401 CrossRef CAS PubMed.
- B. Van de Poel, D. Smet and D. Van Der Straeten, Plant Physiol., 2005, 169, 61–72 CrossRef PubMed.
- F. Vandenbussche, J. P. Verbelen and D. Van Der Straeten, Bioessays, 2005, 27, 275–284 CrossRef CAS PubMed.
- H. K. Wade, T. N. Bibikova, W. J. Valentine and G. I. Jenkins, Plant J., 2001, 25, 675–685 CrossRef CAS PubMed.
- C. Kami, S. Lorrain, P. Hornitschek and C. Fankhauser, Curr. Top. Dev. Biol., 2010, 91, 29–66 CAS.
- J. J. Casal, Annu. Rev. Plant Biol., 2013, 64, 403–427 CrossRef CAS PubMed.
- C. Brelsford, L. O. Morales, J. Nezval, T. K. Kotilainen, S. M. Hartikainen, P. J. Aphalo and T. M. Robson, Physiol. Plant., 2018 DOI:10.1111/ppl.12749.
- D. J. Kliebenstein, J. E. Lim, L. G. Landry and R. L. Last, Plant Physiol., 2002, 130, 234–243 CrossRef CAS PubMed.
- N. Li, M. Teranishi, H. Yamaguchi, T. Matsushita, M. K. Watahiki, T. Tsuge, S. S. Li and J. Hidema, Plant Cell Physiol., 2015, 56, 2014–2023 CrossRef CAS PubMed.
- G. Whitelam, Curr. Biol., 1995, 5, 1351–1353 CrossRef CAS PubMed.
- W. R. Briggs and J. M. Christie, Trends Plant Sci., 2002, 7, 204–210 CrossRef CAS PubMed.
- C. A. Dodson, P. J. Hore and M. I. Wallace, Trends Biochem. Sci., 2013, 38, 435–446 CrossRef CAS PubMed.
- R. Podolec and R. Ulm, Curr. Opin. Plant Biol., 2018, 45, 18–25 CrossRef CAS PubMed.
- U. Hoecker, Curr. Opin. Plant Biol., 2017, 37, 63–69 CrossRef CAS PubMed.
- X. W. Deng and P. H. Quail, Plant J., 1992, 2, 83–95 CrossRef CAS.
- X. D. Lu, C. M. Zhou, P. B. Xu, Q. Luo, H. L. Lian and H. Q. Yang, Mol. Plant, 2015, 8, 467–478 CrossRef CAS PubMed.
- B. Liu, Z. Zuo, H. Liu, X. Liu and C. Lin, Genes Dev., 2011, 25, 1029–1034 CrossRef CAS PubMed.
- H. L. Lian, S. B. He, Y. C. Zhang, D. M. Zhu, J. Y. Zhang, K. P. Jia, S. X. Sun, L. Li and H. Q. Yang, Genes Dev., 2011, 25, 1023–1028 CrossRef CAS PubMed.
- X. Huang, P. Yang, X. Ouyang, L. Chen and X. W. Deng, PLoS Genet., 2014, 10, e1004218 CrossRef PubMed.
- R. H. Yin, M. Y. Skvortsova, S. Loubery and R. Ulm, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E4415–E4422 CrossRef CAS PubMed.
- M. Holm, L. G. Ma, L. J. Qu and X. W. Deng, Genes Dev., 2002, 16, 1247–1259 CrossRef CAS PubMed.
- R. Ulm, A. Baumann, A. Oravecz, Z. Mate, E. Adam, E. J. Oakeley, E. Schafer and F. Nagy, Proc. Natl. Acad. Sci. USA, 2004, 101, 1397–1402 CrossRef CAS PubMed.
- M. Desai and J. Hu, Plant Physiol., 2008, 146, 1117–1127 CrossRef CAS PubMed.
- R. Sellaro, U. Hoecker, M. Yanovsky, J. Chory and J. J. Casal, Curr. Biol., 2009, 19, 1216–1220 CrossRef CAS PubMed.
- I. C. Jang, R. Henriques and N. H. Chua, Plant, Cell Physiol., 2013, 54, 907–916 CrossRef CAS PubMed.
- F. Vandenbussche, R. Pierik, F. F. Millenaar, L. A. Voesenek and D. Van Der Straeten, Curr. Opin. Plant Biol., 2005, 8, 462–468 CrossRef CAS PubMed.
- R. Pierik, T. Djakovic-Petrovic, D. H. Keuskamp, M. de Wit and L. A. Voesenek, Plant Physiol., 2009, 149, 1701–1712 CrossRef CAS PubMed.
- C. Yang and L. Li, Front. Plant Sci., 2017, 8, 1527 CrossRef PubMed.
- U. V. Pedmale, S. C. Huang, M. Zander, B. J. Cole, J. Hetzel, K. Ljung, P. A. B. Reis, P. Sridevi, K. Nito, J. R. Nery, J. R. Ecker and J. Chory, Cell, 2016, 164, 233–245 CrossRef CAS PubMed.
- V. N. Pham, P. K. Kathare and E. Huq, Plant Physiol., 2018, 176, 1025–1038 CrossRef CAS PubMed.
- T. Liang, S. Mei, C. Shi, Y. Yang, Y. Peng, L. Ma, F. Wang, X. Li, X. Huang, Y. Yin and H. Liu, Dev. Cell, 2018, 44, 512–523 CrossRef CAS PubMed.
- K. Yokawa, R. Fasano, T. Kagenishi and F. Baluška, Front. Plant Sci., 2014, 5, 718 Search PubMed.
- K. Yokawa and F. Baluska, Phytochemistry, 2015, 112, 80–83 CrossRef CAS PubMed.
- K. van Gelderen, C. Kang and R. Pierik, Plant Physiol., 2018, 176, 1049–1060 CrossRef CAS PubMed.
- J. Wan, P. Zhang, R. Wang, L. Sun, W. Wang, H. Zhou and J. Xu, Front. Plant Sci., 2018, 9, 618 CrossRef PubMed.
- Q. Sun, K. Yoda and H. Suzuki, J. Exp. Bot., 2005, 56, 191–203 CAS.
- H. J. Lee, Y. J. Park, J. H. Ha, I. T. Baldwin and C. M. Park, Trends Plant Sci., 2017, 22, 803–812 CrossRef CAS PubMed.
- R. Fasano, N. Gonzalez, A. Tosco, F. Dal Piaz, T. Docim, R. Serrano, S. Grillo, A. Leone and D. Inzé, Mol. Plant, 2014, 7, 773–791 CrossRef CAS PubMed.
- R. Rinnan, A. M. Nerg, P. Ahtoniemi, H. Suokanerva, T. Holopainen, E. Kyrö and E. Bååth, Global Change Biol., 2008, 14, 925–937 CrossRef.
- M. M. Caldwell, J. F. Bornman, C. L. Ballare, S. D. Flint and G. Kulandaivelu, Photochem. Photobiol. Sci., 2007, 6, 252–266 RSC.
- D. V. Badri and J. M. Vivanco, Plant, Cell Environ., 2009, 32, 666–681 CrossRef CAS.
- D. T. Krizek, Photochem. Photobiol., 2004, 79, 307–315 CrossRef CAS PubMed.
- P. J. Aphalo, A. Albert, A. R. McLeod, A. Heikkilä, I. Gömez, F. Lopez Figueroa, T. M. Robson and Å. Strid, in Beyond the Visible: a handbook of best practice in plant UV photobiology, ed. P. J. Aphalo, A. Albert, L. O. Björn, A. R. McLeod, T. M. Robson and E. Rosenqvist, University of Helsinki, Department of Biosciences, Helsinki, 2012, ch. 2, pp. 35–70 Search PubMed.
- L. Llorens, F. R. Badenes-Pérez, R. Julkunen-Tiitto, C. Zidorn, A. Fereres and M. A. K. Jansen, Perspect. Plant Ecol. Evol. Syst., 2015, 17, 243–254 CrossRef.
- C. C. Brelsford, L. Nybakken, T. K. Kotilainen and T. M. Robson, OSF Preprints, 2019, DOI:10.31219/osf.io/8q5wz.
- M. Dotto, M. S. Gómez, M. S. Soto and P. Casati, Plant, Cell Environ., 2018, 41, 1394–1406 CrossRef CAS PubMed.
- C. M. M. Gommers and S. Hayes, Plant Physiol., 2018, 177, 437–438 CrossRef CAS PubMed.
- A. B. Arongaus, S. Chen, M. Pireyre, N. Glockner, V. C. Galvao, A. Albert, J. B. Winkler, C. Fankhauser, K. Harter and R. Ulm, Genes Dev., 2018, 32, 1332–1343 CrossRef CAS PubMed.
- R. J. Oakenfull and S. J. Davis, Urban Ecosyst., 2017, 9, 243–257 Search PubMed.
- I. Chuine and J. Régnière, Annu. Rev. Ecol. Evol. Syst., 2017, 48, 159–182 CrossRef.
- B. E. Frewen, T. H. Chen, G. T. Howe, J. Davis, A. Rohde, W. Boerjan and H. D. Bradshaw Jr., Genetics, 2000, 154, 837–845 CAS.
- C. Stromme, U. Sivadasan, K. Nissinen, A. Lavola, T. Randriamanana, R. Julkunen-Tiitto and L. Nybakken, Plant Physiol. Biochem., 2019, 134, 31–39 CrossRef CAS PubMed.
- H. Li, Y. Li, H. Deng, X. Sun, A. Wang, X. Tang, Y. Gao, N. Zhang, L. Wang, S. Yang, Y. Liu and S. Wang, Sci. Rep., 2018, 8, 6097 CrossRef PubMed.
- A. S. Gallinat, R. B. Primack and D. L. Wagner, Trends Ecol. Evol., 2015, 30, 169–176 CrossRef PubMed.
- J. Zeuthen, T. N. Mikkelsen, G. Paludan-Müller and H. Ro-Poulsen, Physiol. Plant., 1997, 100, 281–290 CrossRef CAS.
- K. Neil and J. Wu, Urban Ecosyst., 2006, 9, 243–257 CrossRef.
- U. Sivadasan, T. Randriamanana, C. Chenhao, V. Virjamo, L. Nybakken and R. Julkunen-Tiitto, Ecol. Evol., 2017, 7, 7998–8007 CrossRef PubMed.
- T. M. Robson, V. A. Pancotto, C. L. Ballaré, S. E. Sala, A. L. Scopel and M. M. Caldwell, New Phytol., 2003, 160, 379–389 CrossRef.
- T. V. Callaghan, L. O. Bjorn, Y. Chernov, I. F. S. Chapin, T. R. Christensen, B. Huntley, R. A. Ims, D. Jolly, M. Johansson, S. Jonasson, N. Matveyeva, N. Panikov, W. C. Oechel, G. R. Shaver, J. Elster, I. S. Jonsdottir, K. Laine, K. Taulavuori, E. Taulavuori and C. Zockler, Ambio, 2004, 33, 418–435 CrossRef PubMed.
- A. Hyyryläinen, M. Turunen, P. Rautio and S. Huttunen, Perspect. Plant Ecol. Evol. Syst., 2018, 33, 1–8 CrossRef.
- T. Kotilainen, R. Tegelberg, R. Julkunen-Tiitto, A. Lindfors, R. B. O'Hara and P. J. Aphalo, Physiol. Plant., 2010, 140, 297–309 CAS.
- A. Hyyryläinen, P. Rautio, M. Turunen and S. Huttunen, Springer Plus, 2015, 4, 478 CrossRef PubMed.
- M. A. Del-Castillo-Alonso, M. P. Diago, R. Tomás-Las-Heras, L. Monforte, G. Soriano, J. Martínez-Abaigar and E. Núñez-Olivera, Plant Physiol. Biochem., 2016, 109, 374–386 CrossRef CAS PubMed.
- P. W. Barnes, M. A. Tobler, K. Keefover-Ring, S. D. Flint, A. E. Barkeley, R. J. Ryel and R. L. Lindroth, Plant, Cell Environ., 2016, 39, 222–230 CrossRef CAS PubMed.
- R. Karban, Ecol. Lett., 2008, 11, 727–739 CrossRef PubMed.
- M. Heil and R. Karban, Trends Ecol. Evol., 2010, 25, 137–144 CrossRef PubMed.
- B. M. Delory, P. Delaplace, M. L. Fauconnier and P. Du Jardin, Plant Soil, 2016, 402, 1–26 CrossRef CAS.
- M. M. Maja, A. Kasurinen, T. Holopainen, R. Julkunen-Tiitto and J. K. Holopainen, Sci. Total Environ., 2016, 547, 39–47 CrossRef CAS PubMed.
- V. Falara, R. Amarasinghe, J. Poldy, E. Pichersky, R. A. Barrow and R. Peakall, Ann. Bot., 2013, 111, 21–30 CrossRef CAS PubMed.
- R. Amarasinghe, J. Poldy, Y. Matsuba, R. A. Barrow, J. M. Hemmi, E. Pichersky and R. Peakall, Ann. Bot., 2015, 115, 693–703 CrossRef CAS PubMed.
- T. Piofczyk, G. Jeena and A. Pecinka, Plant Physiol. Biochem., 2015, 93, 34–43 CrossRef CAS PubMed.
- C. L. Ballaré, C. A. Mazza, A. T. Austin and R. Pierik, Plant Physiol., 2012, 160, 145–155 CrossRef PubMed.
- C. A. Mazza, P. I. Gimenez, A. G. Kantolic and C. L. Ballare, Physiol. Plant., 2013, 147, 307–315 CrossRef CAS PubMed.
- C. L. Ballaré, Annu. Rev. Plant Biol., 2014, 65, 335–363 CrossRef PubMed.
- C. E. Williamson, R. G. Zepp, R. M. Lucas, S. Madronich, A. T. Austin, C. L. Ballaré, M. Norval, B. Sulzberger, A. F. Bais, R. L. McKenzie, S. A. Robinson, D. P. Häder, N. D. Paul and J. F. Bornman, Nat. Clim. Change, 2014, 4, 434–441 CrossRef.
- A. F. Bais, R. M. Lucas, J. F. Bornman, C. E. Williamson, B. Sulzberger, A. T. Austin, S. R. Wilson, A. L. Andrady, G. Bernhard, R. L. McKenzie, P. J. Aucamp, S. Madronich, R. E. Neale, S. Yazar, A. R. Young, F. R. de Gruijl, M. Norval, Y. Takizawa, P. W. Barnes, T. M. Robson, S. A. Robinson, C. L. Ballare, S. D. Flint, P. J. Neale, S. Hylander, K. C. Rose, S. A. Wangberg, D. P. Hader, R. C. Worrest, R. G. Zepp, N. D. Paul, R. M. Cory, K. R. Solomon, J. Longstreth, K. K. Pandey, H. H. Redhwi, A. Torikai and A. M. Heikkila, Photochem. Photobiol. Sci., 2018, 17, 127–179 RSC.
- G. B. Makumburage, H. L. Richbourg, K. D. LaTorre, A. Capps, C. Chen and A. E. Stapleton, G3 (Bethesda), 2013, 3, 2195–2204 CrossRef PubMed.
- B. R. Jordan, in UV radiation and plant life, ed. B. Jordan, CABI, 2017, ch. 10 Search PubMed.
- N. Ben Ghozlen, Z. G. Cerovic, C. Germain, S. Toutain and G. Latouche, Sensors, 2010, 10, 10040–10068 CrossRef PubMed.
- G. Agati, W. Bilger and Z. Cerovic, in Sensor Based Quality Assessment Systems for Fruits and Vegetables, ed. B. Kuswandi and M. Siddiqui, Academic Press, 2018 Search PubMed.
- G. Agati, K. Soudani, L. Tuccio, E. Fierini, N. Ben Ghozlen, E. M. Fadaili, A. Romani and Z. G. Cerovic, J. Agric. Food Chem., 2018, 66, 5778–5789 CrossRef CAS PubMed.
- J. Li and R. K. Chan, Opt. Lett., 2010, 35, 3330–3332 CrossRef PubMed.
- T. Adão, E. Peres, L. Pádua, J. Hruška, J. J. Sousa and R. Morais, Proceedings of Small Unmanned Aerial Systems for Environmental Research, Vila Real, Portugal, 5th edn, 2017, pp. 28–30 Search PubMed.
- S. Neugart and M. Schreiner, Sci. Hortic., 2018, 234, 370–381 CrossRef CAS.
- M. Schreiner, M. Korn, M. Stenger, L. Holzgreve and M. Altmann, Sci. Hortic., 2013, 163, 63–69 CrossRef.
- C. Scattino, A. Castagna, S. Neugart, H. M. Chan, M. Schreiner, C. H. Crisosto, P. Tonutti and A. Ranieri, Food Chem., 2014, 163, 51–60 CrossRef CAS PubMed.
- Y. Antignus, Adv. Virus Res., 2014, 90, 1–33 Search PubMed.
- M. Raviv and Y. Antignus, Photochem. Photobiol., 2004, 79, 219–226 CrossRef CAS PubMed.
- A. Suthaparan, K. A. Solhaug, N. Bjugstad, H. R. Gislerød, D. M. Gadoury and A. Stensvand, Plant Dis., 2016, 100, 1643–1650 CrossRef CAS PubMed.
- S. Hodge, J. Bennett, C. N. Merfield and R. W. Hofmann, N. Z. J. Crop Hortic. Sci., 2019, 47, 48–62 CrossRef CAS.
- J. J. Wargent, in UV radiation and Plant Life, ed. B. R. Jordan, CABI, 2017, ch. 11, p. 162 Search PubMed.
- D. P. Fraser, A. Sharma, T. Fletcher, S. Budge, C. Moncrieff, A. N. Dodd and K. A. Franklin, Sci. Rep., 2017, 7, 17758 CrossRef PubMed.
- T. Kotilainen, T. M. Robson and R. Hernández, PLoS One, 2018, 13, e0199628 CrossRef PubMed.
Footnote |
| † Authors in alphabetical order apart from the first and last authors. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2019 |