Open Access Article
Open Access ArticleOrganocatalytic enantioselective conjugate addition of 2-naphthols to ortho-hydroxyphenyl substituted para-quinone methides: access to unsymmetrical triarylmethanes†
Yuyu Chenga,
Zhiqiang Fanga,
Yanwen Jiaa,
Zhongyue Lua,
Wenjun Li *b and
Pengfei Li
*b and
Pengfei Li *a
*a
aDepartment of Chemistry and Shenzhen Key Laboratory of Marine Archaea Geo-Omics, Southern University of Science and Technology, Shenzhen, Guangdong 518055, China. E-mail: lipf@sustech.edu.cn; flyli1980@gmail.com
bDepartment of Medicinal Chemistry, School of Pharmacy, Qingdao University, Qingdao, Shandong 266021, China. E-mail: liwj@qdu.edu.cn
First published on 5th August 2019
Abstract
The enantioselective conjugate addition of 2-naphthols to ortho-hydroxyphenyl substituted para-quinone methides has been achieved with the aid of a chiral phosphoric acid. Importantly, the reaction took place with excellent chemo- and regioselectivities. In addition, the protocol features a low catalyst loading, mild reaction conditions, and enables the formation of unsymmetrical triarylmethanes in good to high yields with generally high enantioselectivities.
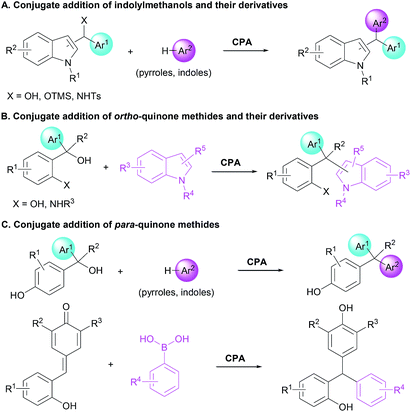
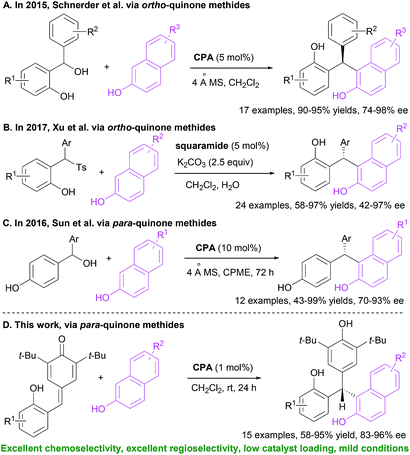
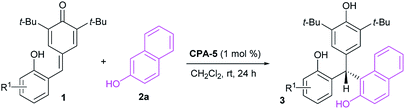
Unsymmetrical triarylmethanes, especially enantiomerically enriched triarylmethanes have been regarded as unique structural frameworks due to their remarkable significance in materials science, natural products, and medicinal chemistry.1 Accordingly, much effort has been devoted to developing catalytic synthetic methodologies for accessing these motifs,2 especially in a enantioselective fashion.3 However, besides limited examples of transition metal-mediated construction of chiral triarylmethanes,4 there are only a few organocatalytic enantioselective synthetic strategies,5–9 of which most processes focused on transformations of indolylmethanols (Scheme 1A),10 in situ generated ortho-quinone methides (o-QMs, Scheme 1B),11 and para-quinone methides (p-QMs, Scheme 1C).12 On the other hand, triarylmethanes containing the 2-naphthol moiety is a family of biologically active compounds,13 but reports on catalytic enantioselective construction of triarylmethanes bearing the 2-naphthol motif are very limited.14 In 2015, Schneider et al. realized the enantioselective construction of chiral triarylmethanes via a chiral phosphoric acid (CPA) catalyzed 1,4-addition of 2-naphthol to o-QMs generated from ortho-hydroxy benzhydrols (Scheme 2A).15 Similarly, in the presence of squaramide combined with excess base as acid scavenger, Xu et al. established an enantioselective 1,4-addition of 2-naphthols to in situ generated o-QMs from 2-[phenyl(tosyl)methyl]phenols (Scheme 2B).16 Independently, Sun et al. developed a CPA catalyzed 1,6-addition between 2-naphthols and p-QMs in situ generated from para-hydroxy benzhydrols to construct the optically active triarylmethanes bearing 2-naphthol motif (Scheme 2C).17 In spite of these elegant approaches, the organocatalytic enantioselective construction of chiral triarylmethanes still represents a challenging task. Therefore, a direct and facile synthetic strategy for this important structural motif would be highly valuable. On the basis of asymmetric additions to ortho-hydroxyphenyl substituted para-quinone methides18,19 and as a continuation of our efforts in asymmetric reactions of p-QMs,20 we report herein a direct and efficient CPA-mediated asymmetric conjugate addition of 2-naphthols to p-QMs (Scheme 2D).
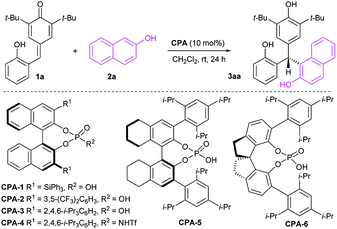
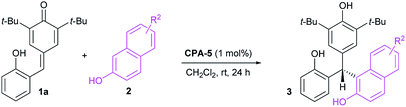
Initial investigations were carried out using a series of CPAs for the model reaction of 4-(2-hydroxybenzylidene)-2,6-di-tert-butylcyclohexa-2,5-dienone 1a with 2-naphthol 2a in dichloromethane at room temperature for 24 h. As shown in Table 1, with a catalyst loading of 10 mol%, CPA-1 mediated reaction proceeded smoothly to afford the triarylmethane 3aa in 81% yield with 5% ee (entry 1). An essential enhancement was achieved when the reaction was catalyzed by CPA-3, furnishing 3aa in 90% yield with 91% ee (entry 3). To our delight, further modification of catalyst structure led to the formation of 3aa in 95% yield with 94% ee (entry 5). With CPA-5 as the suitable catalyst, reaction media was screened (entries 7–11). Solvent was found to have a great influence on the reaction efficiency and stereoselectivity, and dichloromethane was identified as the best reaction media. Notably, decreasing the catalyst loading from 10 mol% to 1 mol%, the desired triarylmethane 3aa was still obtained in 95% yield with 94% ee when the reaction was carried out in CH2Cl2 of 1.0 mL at room temperature for 24 h (entry 12). Shorting reaction time from 24 h to 12 h, the yield of triarylmethane 3aa decreased from 95% to 83% without compromising the enantioselectivity (entry 13).
| Entry | Catalyst | Solvent | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a Unless noted, 1a (0.20 mmol), 2a (0.24 mmol), catalyst (10 mol%) in the solvent (2.0 mL) at room temperature for 24 h.b Isolated yield.c Determined by HPLC analysis using a chiral stationary phase.d CPA-5 (1 mol%), CH2Cl2 (1.0 mL).e CPA-5 (1 mol%), CH2Cl2 (1.0 mL), 12 h. | ||||
| 1 | CPA-1 | CH2Cl2 | 3aa, 81 | 5 |
| 2 | CPA-2 | CH2Cl2 | 3aa, 77 | 28 |
| 3 | CPA-3 | CH2Cl2 | 3aa, 90 | 91 |
| 4 | CPA-4 | CH2Cl2 | 3aa, 56 | 3 |
| 5 | CPA-5 | CH2Cl2 | 3aa, 95 | 94 |
| 6 | CPA-6 | CH2Cl2 | 3aa, 91 | 35 |
| 7 | CPA-5 | CHCl3 | 3aa, 90 | 88 |
| 8 | CPA-5 | EtOAc | 3aa, 70 | 69 |
| 9 | CPA-5 | Toluene | 3aa, 84 | 88 |
| 10 | CPA-5 | THF | 3aa, 59 | 10 |
| 11 | CPA-5 | MeCN | 3aa, 83 | 94 |
| 12d | CPA-5 | CH2Cl2 | 3aa, 95 | 94 |
| 13e | CPA-5 | CH2Cl2 | 3aa, 83 | 93 |
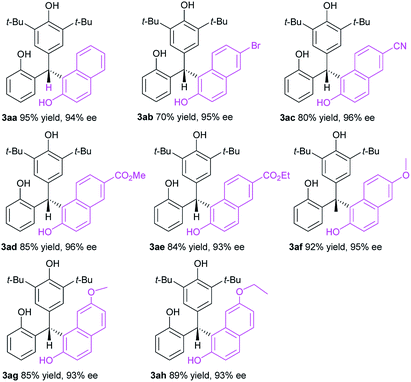
Having optimized the catalyst structure and reaction conditions, we then explored the substrate scope of this organocatalytic enantioselective transformation. Firstly, the generality of 2-naphthols component was evaluated (Table 2). Pleasingly, a wide range of 2-naphthols 2a–h reacted smoothly with p-QM 1a to afford the corresponding enantioenriched triarylmethanes 3aa–ah in high yields (70–95%) with excellent enantioselectivities (93–96%). Various different substituents, including electron-withdrawing (Br, CN, CO2Me, CO2Et) and electron-donating groups (MeO, EtO) at different positions of the aromatic ring of the 2-naphthols component were tolerated with only slight effects on the reaction efficiency and asymmetric induction. No significant electronic effects were observed for the substituents on the aromatic moiety. Confirmed by the impressive results, the organocatalytic enantioselective conjugate addition of p-QMs has been successfully extended to a variety of 2-naphthols and provided an efficient and facile access to optically active triarylmethanes.
| a Unless noted, 1a (0.20 mmol), 2 (0.24 mmol), CPA-5 (1 mol%) in CH2Cl2 (1.0 mL) at room temperature for 24 h. Products 3aa–ah were obtained in isolated yield and ee values were determined by chiral HPLC analysis. |
|---|
 |
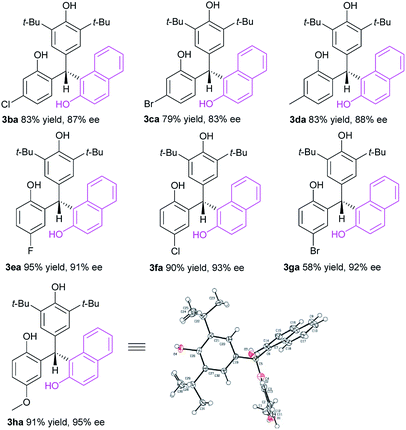
With these encouraging data in hand, we then investigated the substrate scope of p-QMs 1 in the CPA-5 catalyzed conjugate addition of 2-naphthol 2a (Table 3). It was found that this strategy was applicable to various p-QMs 1b–h bearing different types of substituents to furnish the corresponding optically active triarylmethanes 3ba–ha in generally high yields with enantioselectivities. Both electron-withdrawing (F, Cl, Br) and electron-donating groups (Me, MeO) could be introduced into different positions of the aromatic ring of p-QMs with a little effect on the reaction efficiency and stereoselectivity. The absolute configuration of 3ha was unambiguously confirmed by X-ray crystallography.21 In all, a broad scope of p-QMs has been successfully involved in the organocatalytic conjugate addition of 2-naphthols for the chemo-, regio- and enantioselective construction of chiral triarylmethanes.
| a Unless noted, 1 (0.20 mmol), 2a (0.24 mmol), CPA-5 (1 mol%) in CH2Cl2 (1.0 mL) at room temperature for 24 h. Products 3ba–ha were obtained in isolated yield and ee values were determined by chiral HPLC analysis. |
|---|
 |
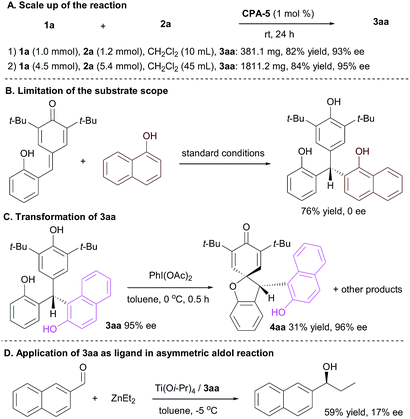
To demonstrate the robustness and utility of this synthetic strategy, the scale up of the reaction were carried out (Scheme 3A). The CPA-5 mediated conjugate reaction of p-QM 1a at 1.0 mmol proceeded well under the standard conditions to generate 3aa in 82% yield with 93% ee. When the reaction was scaled up to 4.5 mmol, the product 3aa was obtained in 84% yield with 95% ee, which indicated this protocol has the potential for a large-scale production. The reaction of 1-naphthol furnished racemic products in 76% yield under standard conditions (Scheme 3B). The transformation of 3aa was also investigated. Treated with PhI(OAc)2, product 4aa was isolated in 31% yield with 96% ee (Scheme 3C). Then employing 3aa as ligand in catalytic asymmetric aldol reaction was surveyed. The initial result indicated that the Ti(Oi-Pr)4/3aa system mediated the asymmetric aldol reaction of ZnEt2 to 2-naphthaldehyde effectively to generate adduct in 59% yield, although the enantioselectivity was low (Scheme 3D).
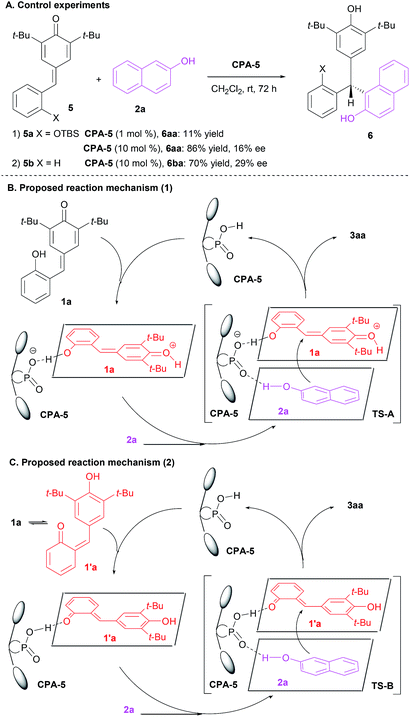
To light some insight into the reaction mechanism, control experiments were carried out (Scheme 4A). When the hydroxyl group of p-QM 1a was shielded by t-butyldimethylsilyl (TBS) group (p-QM 5a), the reaction was found to proceed quite slowly and the corresponding product 6aa was obtained in 11% yield after 72 h. The yield of 6aa could be improved to 86% when the catalyst loading was increased to 10 mol%, however, the enantioselectivity remained poor (16% ee). When the hydroxyl group was removed, p-QM 5b could also reacted smoothly with 2a to generate the adduct 6ba in 70% yield with 29% ee under the standard conditions. Consequently, it is not too hard to make the case that the free hydroxyl group of p-QM 1a played a key role in terms of the reaction efficiency and stereoselectivity. Based on these results and considering reported plausible transition state,22 a possible reaction mechanism was suggested. As shown in Scheme 4B, p-QM 1a was protonated and activated in the presence of CPA-5. Then, both p-QM 1a and 2-naphthol 2a were arranged by CPA-5 via hydrogen bond to generate the desired product 3aa in high yield with high enantioselectivity. Particularly, Li et al. reported that the isomerization energy of 1a and 1′a was 6.7 kcal mol−1, indicating that the transformation of p-QM 1a to o-QM 1′a was not difficult.19g As a result, we could not exclude the possibility that 2-hydroxyphenyl p-QM 1a isomerized initially to 6-(3,5-di-tert-butyl-4-hydroxybenzylidene)cyclohexa-2,4-dienone 1′a and then the CPA-5 activated and oriented both o-QM 1′a and 2-naphthol 2a to afford the desired adduct 3aa with high efficiency and enantioselectivity (Scheme 4C).
In conclusion, we have established the enantioselective construction of optically active triarylmethanes bearing naphthol motif via a chiral phosphoric acid mediated conjugate addition of 2-naphthols to 2-hydroxyphenyl p-QMs. A series of enantioenriched (83–96%) triarylmethanes were obtained in 58–95% yields. Moreover, transformation and application of triarylmethanes were investigated. Further modification of substrates to generate practical chiral triarylmethanes are undergoing in our lab.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from National Natural Science Foundation of China (21871128), Shenzhen Innovation of Science and Technology Commission (JCYJ20170817110526264, ZDSYS201802081843490).Notes and references
- For reviews, see:
(a) D. F. A. Duxbury, Chem. Rev., 1993, 93, 381 CrossRef CAS
; (b) M. S. Shchepinov and V. A. Korshun, Chem. Soc. Rev., 2003, 32, 170 RSC
; (c) V. Nair, S. Thomas, S. C. Mathew and K. G. Abhilash, Tetrahedron, 2006, 62, 6731 CrossRef CAS
; (d) H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC
; (e) M. Beija, C. A. M. Afonso and J. M. G. Martinbo, Chem. Soc. Rev., 2009, 38, 2410 RSC
; (f) M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Chem. Rev., 2010, 110, 2250 CrossRef CAS PubMed
.
- For reviews, see:
(a) S. Mondal and G. Panda, RSC Adv., 2014, 4, 28317 RSC
; (b) M. Nambo and C. M. Crudden, ACS Catal., 2015, 5, 4734 CrossRef CAS
.
- For a comprehensive review, see: S. Mondal, D. Roy and G. Panda, ChemCatChem, 2018, 10, 1941 CrossRef CAS
.
-
(a) B.-F. Shi, N. Maugel, Y.-H. Zhang and J.-Q. Yu, Angew. Chem., Int. Ed., 2008, 47, 4882 CrossRef CAS PubMed
; (b) L.-L. Cao, Z.-S. Ye, G.-F. Jiang and Y.-G. Zhou, Adv. Synth. Catal., 2011, 353, 3352 CrossRef CAS
; (c) Y. Lou, P. Cao, T. Jia, Y. Zhang, M. Wang and J. Liao, Angew. Chem., Int. Ed., 2015, 54, 12134 CrossRef CAS PubMed
; (d) Y. Huang and T. Hayashi, J. Am. Chem. Soc., 2015, 137, 7556 CrossRef CAS PubMed
; (e) J. Zheng, L. Lin, L. Dai, X. Yuan, X. Liu and X. Feng, Chem.–Eur. J., 2016, 22, 18254 CrossRef CAS PubMed
; (f) J. H. Kim, S. Greßies, M. Boultadakis-Arapinis, C. Daniliuc and F. Glorius, ACS Catal., 2016, 6, 7652 CrossRef CAS
.
- F.-L. Sun, M. Zeng, Q. Gu and S.-L. You, Chem.–Eur. J., 2009, 15, 8709 CrossRef CAS PubMed
.
- S. Shirakawa, K. Koga, T. Tokuda, K. Yamamoto and K. Maruoka, Angew. Chem., Int. Ed., 2014, 53, 6220 CrossRef CAS PubMed
.
- S. Lu, X. Song, S. B. Poh, H. Yang, M. W. Wong and Y. Zhao, Chem.–Eur. J., 2017, 23, 2275 CrossRef CAS PubMed
.
- Y. Zhang, S.-X. Zhang, L.-N. Fu and Q.-X. Guo, ChemCatChem, 2017, 9, 3107 CrossRef CAS
.
- A. B. Gade, P. N. Bagle, P. S. Shinde, V. Bhardwaj, S. Banerjee, A. Chande and N. T. Patil, Angew. Chem., Int. Ed., 2018, 57, 5735 CrossRef CAS PubMed
.
-
(a) F.-L. Sun, X.-J. Zheng, Q. Gu, Q.-L. He and S.-L. You, Eur. J. Org. Chem., 2010, 47 CrossRef CAS
; (b) M.-H. Zhuo, Y.-J. Jiang, Y.-S. Fan, Y. Gao, S. Liu and S. Zhang, Org. Lett., 2014, 16, 1096 CrossRef CAS PubMed
; (c) S. Qi, C.-Y. Liu, J.-Y. Ding and F.-S. Han, Chem. Commun., 2014, 50, 8605 RSC
; (d) Y.-X. Gong, Q. Wu, H.-H. Zhang, Q.-N. Zhu and F. Shi, Org. Biomol. Chem., 2015, 13, 7993 RSC
; (e) M. H. Zhuo, G.-F. Liu, S.-L. Song, D. An, J. Gao, L. Zheng and S. Zhang, Adv. Synth. Catal., 2016, 358, 808 CrossRef CAS
; (f) C. Yue, F. Na, X. Fang, Y. Cao and J. C. Antilla, Angew. Chem., Int. Ed., 2018, 57, 11004 CrossRef CAS PubMed
.
-
(a) W. Zhao, Z. Wang, B. Chu and J. Sun, Angew. Chem., Int. Ed., 2015, 54, 1910 CrossRef CAS PubMed
; (b) H.-H. Liao, A. Chatupheeraphat, C.-C. Hsiao, I. Atodiresei and M. Rueping, Angew. Chem., Int. Ed., 2015, 54, 15540 CrossRef CAS PubMed
; (c) Q. Wu, C. Ma, X.-H. Du, Y. Chen, T.-Z. Huang, X.-Q. Shi, S.-J. Tu and P.-J. Cai, Tetrahedron: Asymmetry, 2016, 27, 307 CrossRef CAS
.
-
(a) Z. Wang, Y. F. Wong and J. Sun, Angew. Chem., Int. Ed., 2015, 54, 13711 CrossRef CAS PubMed
; (b) G.-B. Huang, W.-H. Huang, J. Guo, D.-L. Xu, X.-C. Qu, P.-H. Zhai, X.-H. Zheng, J. Weng and G. Lu, Adv. Synth. Catal., 2019, 361, 1241 CrossRef CAS
; (c) J.-R. Wang, X.-L. Jiang, Q.-Q. Hang, S. Zhang, G.-J. Mei and F. Shi, J. Org. Chem., 2019, 84, 7829 CrossRef CAS PubMed
.
- S. K. Das, G. Panda, V. Chaturvedi, Y. S. Manju, A. K. Gaikwad and S. Sinha, Bioorg. Med. Chem. Lett., 2007, 17, 5586 CrossRef CAS PubMed
.
- For catalytic non-enantioselective construction of triaylmethanes bearing 2-naphthol unit, see:
(a) S. Yaragorla, P. L. Saini, P. V. Babu, A. I. Almansour and N. Arumugam, Tetrahedron Lett., 2016, 57, 2351 CrossRef CAS
; (b) R. Dada, G. Singh, A. Pareek, S. Kausar and S. Yaragorla, Tetrahedron Lett., 2016, 57, 3739 CrossRef CAS
; (c) P. Arde and R. V. Anand, RSC Adv., 2016, 6, 77111 RSC
; (d) T. Zhou, S. Li, B. Huang, C. Li, Y. Zhao, J. Chen, A. Chen, Y. Xiao, L. Liu and J. Zhang, Org. Biomol. Chem., 2017, 15, 4941 RSC
; (e) G.-P. Yang, D. Dilixiati, T. Yang, D. Liu, B. Yu and C.-W. Hu, Appl. Organomet. Chem., 2018, 32, e4450 CrossRef
; (f) G. Singh, P. Goswami and R. V. Anand, Org. Biomol. Chem., 2018, 16, 384 RSC
; (g) G.-J. Mei, S.-L. Xu, W.-Q. Zheng, C.-Y. Bian and F. Shi, J. Org. Chem., 2018, 83, 1414 CrossRef CAS PubMed
.
- S. Saha, S. K. Alamsetti and C. Schneider, Chem. Commun., 2015, 51, 1461 RSC
.
- Y. Wang, C. Zhang, H. Wang, Y. Jiang, X. Du and D. Xu, Adv. Synth. Catal., 2017, 359, 791 CrossRef CAS
.
- Y. F. Wong, Z. Wang and J. Sun, Org. Biomol. Chem., 2016, 14, 5751 RSC
.
- For reviews on asymmetric reactions of p-QMs, see:
(a) A. Parra and M. Tortosa, ChemCatChem, 2015, 7, 1524 CrossRef CAS
; (b) W. Li, X. Xu, P. Zhang and P. Li, Chem.–Asian J., 2018, 13, 2350 CrossRef CAS PubMed
.
- For examples, see:
(a) K. Zhao, Y. Zhi, T. Shu, A. Valkonen, K. Rissanen and D. Enders, Angew. Chem., Int. Ed., 2016, 55, 12104 CrossRef CAS PubMed
; (b) S. Liu, X.-C. Lan, K. Chen, W.-J. Hao, G. Li, S.-J. Tu and B. Jiang, Org. Lett., 2017, 19, 3831 CrossRef CAS PubMed
; (c) Z.-P. Zhang, K.-X. Xie, C. Yang, M. Li and X. Li, J. Org. Chem., 2018, 83, 364 CrossRef CAS PubMed
; (d) G.-J. Mei, S.-L. Xu, W.-Q. Zheng, C.-Y. Bian and F. Shi, J. Org. Chem., 2018, 83, 1414 CrossRef CAS PubMed
; (e) Y. Zhi, K. Zhao, C. von Essen, K. Rissanen and D. Enders, Org. Chem. Front., 2018, 5, 1348 RSC
; (f) J. Yan, M. Chen, H. H.-Y. Sung, I. D. Williams and J. Sun, Chem.–Asian J., 2018, 13, 2440 CrossRef CAS PubMed
; (g) Z.-P. Zhang, K.-X. Xie, C. Yang, M. Li and X. Li, J. Org. Chem., 2018, 83, 364 CrossRef CAS PubMed
; (h) Z.-P. Zhang, L. Chen, X. Li and J.-P. Cheng, J. Org. Chem., 2018, 83, 2714 CrossRef CAS PubMed
; (i) L. Liu, Z. Yuan, R. Pan, Y. Zeng, A. Lin, H. Yao and Y. Huang, Org. Chem. Front., 2018, 5, 623 RSC
; (j) F. Jiang, F.-R. Yuan, L.-W. Jin, G.-J. Mei and F. Shi, ACS Catal., 2018, 8, 10234 CrossRef CAS
; (k) G.-H. Yang, Q. Zhao, Z.-P. Zhang, H.-L. Zheng, L. Chen and X. Li, J. Org. Chem., 2019, 84, 7883 CrossRef CAS PubMed
; (l) Z. Li, W. Wang, H. Jian, W. Li, B. Dai and L. He, Chin. Chem. Lett., 2019, 30, 386 CrossRef CAS
; (m) M. Sun, C. Ma, S.-J. Zhou, S.-F. Lou, J. Xiao, Y. Jiao and F. Shi, Angew. Chem., Int. Ed., 2019, 58, 8703 CrossRef CAS PubMed
.
-
(a) L. Zhang, X. Zhou, P. Li, Z. Liu, Y. Liu, Y. Sun and W. Li, RSC Adv., 2017, 7, 39216 RSC
; (b) L. Zhang, Y. Liu, K. Liu, Z. Liu, N. He and W. Li, Org. Biomol. Chem., 2017, 15, 8743 RSC
; (c) W. Li, H. Yuan, Z. Liu, Z. Zhang, Y. Cheng and P. Li, Adv. Synth. Catal., 2018, 360, 2460 CrossRef CAS
; (d) W. Li, X. Xu, Y. Liu, H. Gao, Y. Cheng and P. Li, Org. Lett., 2018, 20, 1142 CrossRef CAS PubMed
; (e) L. Zhang, H. Yuan, W. Lin, Y. Cheng, P. Li and W. Li, Org. Lett., 2018, 20, 4970 CrossRef CAS PubMed
; (f) P. Zhang, Q. Huang, Y. Cheng, R. Li, P. Li and W. Li, Org. Lett., 2019, 21, 503 CrossRef CAS PubMed
.
- CCDC 1908826 (3ha)..
- For reviews, see:
(a) E. P. Ávila and G. W. Amarante, ChemCatChem, 2012, 4, 1713 CrossRef
; (b) M. Mahlau and B. List, Angew. Chem., Int. Ed., 2013, 52, 518 CrossRef CAS PubMed
; for recent reports, see:; (c) H. G. O. Alvim, D. L. J. Pinheiro, V. H. Carvalho-Silva, M. Fioramonte, F. C. Gozzo, W. A. da Silva, G. W. Amarante and B. A. D. Neto, J. Org. Chem., 2018, 83, 12143 CrossRef CAS PubMed
; (d) J. Zhang, P. Yu, S.-Y. Li, H. Sun, S.-H. Xiang, J. Wang, K. N. Houk and B. Tan, Science, 2018, 361, eaas8707 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1908826. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra04768a |
| This journal is © The Royal Society of Chemistry 2019 |