Excimer emission and magnetoluminescence of radical-based zinc(II) complexes doped in host crystals†
Shun
Kimura
ab,
Shojiro
Kimura
c,
Hiroshi
Nishihara
*bd and
Tetsuro
Kusamoto
 *a
*a
aInstitute for Molecular Science, 5-1 Higashiyama, Myodaiji, Okazaki, Aichi 444-8787, Japan. E-mail: kusamoto@ims.ac.jp
bDepartment of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan
cInstitute for Materials Research, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, 980-8577, Japan
dResearch Center for Science and Technology, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba 278-8510, Japan. E-mail: nisihara@rs.tus.ac.jp
First published on 1st September 2020
Abstract
A ZnII complex based on a luminescent organic radical was doped into host molecular crystals. The 5, 10, and 20 wt%-doped crystals showed excimer emissions and their luminescent behaviours were significantly modulated by an external magnetic field. These are the first examples showing excimer emissions and magnetic-field–sensitive luminescent properties for complexes based on luminescent radicals. The excimer species contributing to magnetoluminescence was determined by analyzing the emission spectra and their magnetic-field dependencies. These results suggest the general nature of magnetic field effects on the luminescence of radicals as well as the importance of the type of interaction between radicals.
Luminescent organic molecules have been developed for wide-ranging applications, including organic light-emitting diodes (OLEDs)1 and bioimaging.2 Recently, luminescent radicals have attracted much attention3–6 because of their unusual characteristics, such as long-wavelength emissions, the absence of heavy-atom effect, and the high electron–photon conversion efficiency of OLEDs. These properties arise from the unique spin states of radical molecules with an unpaired electron, so that controlling the spin state is the key to new photochemical and photophysical properties, which are difficult to realize with conventional closed-shell luminophores. We recently reported that the (3,5-dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radical (PyBTM)6b doped into (3,5-dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methane (αH-PyBTM) molecular crystals7,8 exhibits a new luminescent property for organic radicals that stems from the interplay between spin and luminescence. Crystals containing 10 wt% PyBTM displayed PyBTM monomer- and PyBTM excimer-centred emissions and magnetic-field–sensitive luminescence, namely, ‘magnetoluminescence’. These studies suggested that changes in spin multiplicities of aggregated radicals contributed to the magnetic-field effect (MFE). However, magnetoluminescence of stable radicals has to date been observed for only a few pure organic luminescent radicals. Therefore, the scope of candidate complexes showing magnetoluminescence should be expanded to provide new photofunctions in organic radicals. In particular, the development of metal–radical complexes that exhibit magnetoluminescence is a promising approach because of the ease of controlling the molecular and electronic structures through molecular design.
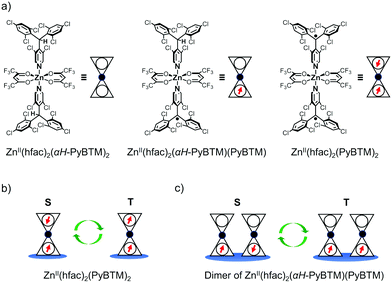
In this study, we prepared ZnII(hfac)2(αH-PyBTM)2 (where hfac is hexafluoroacetylacetonato; Fig. 1a) crystals in which αH-PyBTM was substituted with PyBTM, and investigated their luminescent properties at different radical concentrations and under an applied magnetic field. The 5, 10, and 20 wt%-substituted crystals are the first reports of luminescent radical-coordinated metal complexes displaying excimer emissions. Their luminescent behaviours were modulated substantially by an external magnetic field, suggesting that magnetic-field–sensitive emission properties may be common, even in metal complexes with luminescent radicals. Two types of spin multiplicity changes, which would contribute to the MFE, were assumed to occur in this system: intramolecular changes in ZnII(hfac)2(PyBTM)2 (Fig. 1b) and intermolecular changes in the ZnII(hfac)2(αH-PyBTM)(PyBTM) dimer (Fig. 1c). Here we discuss which of these changes contributed to the magnetoluminescence.
ZnII(hfac)2(αH-PyBTM)2 was synthesized and characterized by the procedure described in the ESI.† A single-crystal X-ray diffraction study revealed the crystal structure of ZnII(hfac)2(αH-PyBTM)2 with the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Fig. 2). The unit cell contains two crystallographically independent ZnII(hfac)2(αH-PyBTM)2 molecules with almost identical structures. The ZnII(hfac)2(αH-PyBTM)2 crystals were isostructural with ZnII(hfac)2(PyBTM)2 crystals9 except for the positions of the central carbon atoms of two (αH-)PyBTMs, which were sp3-hybridized and disordered into two positions in the former crystals while sp2-hybridized in the latter. In one of the two crystallographically independent molecules, the trifluoromethyl groups in the hfac ligands were also disordered in two positions.
(Fig. 2). The unit cell contains two crystallographically independent ZnII(hfac)2(αH-PyBTM)2 molecules with almost identical structures. The ZnII(hfac)2(αH-PyBTM)2 crystals were isostructural with ZnII(hfac)2(PyBTM)2 crystals9 except for the positions of the central carbon atoms of two (αH-)PyBTMs, which were sp3-hybridized and disordered into two positions in the former crystals while sp2-hybridized in the latter. In one of the two crystallographically independent molecules, the trifluoromethyl groups in the hfac ligands were also disordered in two positions.
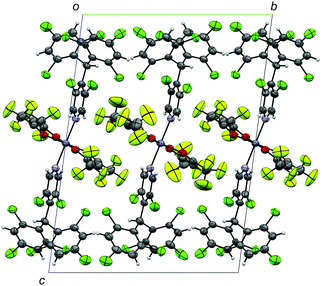 | ||
| Fig. 2 Crystal structure of ZnII(hfac)2(αH-PyBTM)2 viewed along the a-axis. Disorders of the trifluoromethyl groups and central carbon atoms of αH-PyBTM are omitted for clarity. | ||
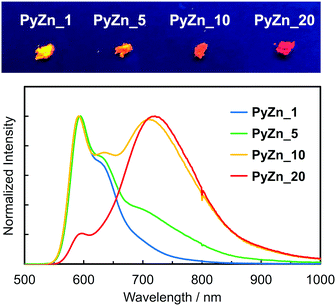
PyBTM-substituted ZnII(hfac)2(αH-PyBTM)2 with various PyBTM concentrations (1, 5, 10, and 20 wt% with respect to the sum of αH-PyBTM and PyBTM in the crystals) were prepared as follows. PyBTM, αH-PyBTM, and ZnII(hfac)2 were dissolved in dry dichloromethane. The solvent was allowed to evaporate slowly under dark and ambient conditions and the PyBTM-doped ZnII(hfac)2(αH-PyBTM)2 (PyZn_R, where R indicates the concentration (wt%) of PyBTM) crystals were washed with dry hexane. Powder X-ray diffraction (PXRD) revealed the uniformity of the crystal structures of PyZn_R (Fig. S3, ESI†). The PXRD patterns of PyZn_1, PyZn_5, PyZn_10, and PyZn_20 were similar to those of ZnII(hfac)2(αH-PyBTM)2 and ZnII(hfac)2(PyBTM)2. These results suggest that αH-PyBTM was substituted for PyBTM while maintaining the original crystal structure. We assumed that the ZnII(hfac)2(αH-PyBTM)2, ZnII(hfac)2(αH-PyBTM)(PyBTM), and ZnII(hfac)2(PyBTM)2 species were randomly mixed in the crystals (Fig. 1a).
The luminescent behaviours of PyZn_R depended strongly on the radical concentration. PyZn_1, with the lowest radical concentration, had a maximum emission wavelength, λem, of 595 nm upon excitation at λex = 370 nm (Fig. 3). Considering the low concentration of PyBTM, the emission was derived from ZnII(hfac)2(αH-PyBTM)(PyBTM), the complex with coordinating one radical. This spectral shape was similar to that of 1 wt% PyBTM-doped αH-PyBTM (λem = 563 nm).7 This bathochromic shift in emission wavelength was caused by coordination to the positively charged zinc ion, as observed in the AuI–PyBTM complex; the coordination was expected to lower the energy level of the β-singly occupied molecular orbital, decreasing the emission energy.6b,c,10 As the radical concentration increased, a new emission band at around λem = 725 nm appeared and emission at 595 nm was suppressed. These trends are similar to those reported in the previous studies,7,11 and the new long-wavelength emission band was attributed to the excimer. The excimer character was confirmed by measuring the excitation spectra of PyZn_10 at monomer- and excimer-centred emission maximum wavelengths (λem = 595 and 725 nm, respectively). These spectra were similar (Fig. S4, ESI†), suggesting that the long-wavelength emission was derived from the excimer formed after photoexcitation.12 Although ZnII(hfac)2(αH-PyBTM)2 and the ZnII(hfac)2(αH-PyBTM)(PyBTM) dimer were possible candidates for the excimer, the species from which the excimer emission originated could not be identified because they were randomly mixed throughout the samples. We could not obtain the emission spectrum of isolated ZnII(hfac)2(PyBTM)2 because of the dissociation of PyBTM, which occurred in solution9 and during grinding with a solid matrix (Fig. S5, ESI†).
The photophysical properties of PyZn_R are summarized in Table S1 (ESI†). PyZn_1 had the highest luminescent quantum yield, ϕem, of 18%; as the radical concentration increased, the excimer emission appeared and the ϕem value of PyZn_R decreased to 5.2% for PyZn_20. This is explained by concentration quenching in the crystals, with the quantum yield of excimer emission being lower than that of monomer emission. The emission lifetime at the excimer-centred emission wavelengths for PyZn_5, PyZn_10, and PyZn_20 was longer than that at the monomer-centred emission wavelengths and the emission decay could be fitted with a single exponential curve (Fig. S6, ESI†). This indicates that the excimer was a single component, and thus either ZnII(hfac)2(αH-PyBTM)2 or the ZnII(hfac)2(αH-PyBTM)(PyBTM) dimer was emissive.
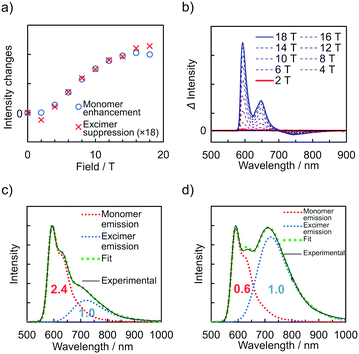
The emission spectra of PyZn_R under an external magnetic field at 4.2 K were measured to investigate the MFE on the luminescent behaviour. Fig. 4a shows the emission spectra of PyZn_1, which displayed only monomer-centred emission, under applied magnetic fields of 0, 5, 10, 15, and 18 T. The spectra were not affected by these magnetic fields, suggesting an absence of MFE. This is consistent with our previous report, in which isolated PyBTM did not show an MFE because of its luminescent character being based on a doublet–doublet transition.7 In contrast, the emissions of samples with higher radical concentrations, PyZn_5, PyZn_10, and PyZn_20, which displayed monomer- and excimer-centred emissions, were clearly modulated by an external magnetic field (Fig. 4b–d). As the applied magnetic field increased, the monomer-centred emission at λem = 595 nm was strongly enhanced and the excimer-centred emission at λem = 740 nm was slightly suppressed. This is the first-reported example of an MFE observed for luminescent metal complexes with a coordinated luminescent radical. Fig. 5a and Fig. S7 (ESI†) show the intensity changes at monomer and excimer emission wavelengths for PyZn_5, PyZn_10, and PyZn_20. Different emission spectra of PyZn_10 are shown in Fig. 5b. The magnitudes of the intensity changes at the monomer- and excimer-centred emission wavelengths were related for each sample, and the increased monomer intensities were much larger than the decreased excimer emission intensities of PyZn_5, PyZn_10, and PyZn_20 (21, 18, and 28 times, respectively). The monomer excited state thus had a much higher quantum yield than the excimer excited state.
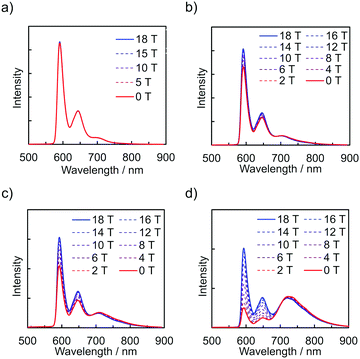 | ||
| Fig. 4 Emission spectra of (a) PyZn_1, (b) PyZn_5, (c) PyZn_10, and (d) PyZn_20 at 4.2 K under a magnetic field. | ||
In this system, ZnII(hfac)2(PyBTM)2 and the ZnII(hfac)2(αH-PyBTM)(PyBTM) dimer were both potential contributors to the excimer emissions and the magnetoluminescence. As suggested by the decay curves of the excimer emissions, which could be fitted with single components, the excimer emissive species was either one of the two species. To determine the excimer emissive species, the following three points were considered. First, the abundance ratio of ZnII(hfac)2(PyBTM)2 was statistically much smaller than that of ZnII(hfac)2(αH-PyBTM)(PyBTM) in the crystals of PyZn_5 and PyZn_10 (1/38 and 1/18, respectively). Second, the MFE on the emission spectra suggest that the emission quantum yield of the monomer excited state was much higher than that of the excimer excited state (Fig. 5a and Fig. S7, ESI†). Third, the contribution of excimer emission (Iexc) was comparable to that of the monomer emission (Imono) in PyZn_5 and PyZn_10 (Imono![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Iexc = 2.4
Iexc = 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 and 0.6
1.0 and 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, respectively; Fig. 5c and d).13 If the excimer species were ZnII(hfac)2(PyBTM)2, Iexc should be much smaller than Imono for PyZn_5 and PyZn_1014 according to the first and second points above, but this would contradict the third point. Therefore, we concluded that the excimer emissive species in this system was not ZnII(hfac)2(PyBTM)2, but the ZnII(hfac)2(αH-PyBTM)(PyBTM) dimer. Considering the similarity between emission behaviours of PyZn_R under a magnetic field and that of PyBTM-doped αH-PyBTM, a similar mechanism for the MFE was expected, where the magnetic field modulates the spin multiplicity changes of the dimer in both the ground and excited states (Fig. S8, ESI†).7,8
1.0, respectively; Fig. 5c and d).13 If the excimer species were ZnII(hfac)2(PyBTM)2, Iexc should be much smaller than Imono for PyZn_5 and PyZn_1014 according to the first and second points above, but this would contradict the third point. Therefore, we concluded that the excimer emissive species in this system was not ZnII(hfac)2(PyBTM)2, but the ZnII(hfac)2(αH-PyBTM)(PyBTM) dimer. Considering the similarity between emission behaviours of PyZn_R under a magnetic field and that of PyBTM-doped αH-PyBTM, a similar mechanism for the MFE was expected, where the magnetic field modulates the spin multiplicity changes of the dimer in both the ground and excited states (Fig. S8, ESI†).7,8
In conclusion, the luminescent behaviours of PyBTM-substituted ZnII(hfac)2(αH-PyBTM)2 crystals, PyZn_R, depend on the radical concentration and external magnetic field. PyZn_1 displayed emission from the monomer ZnII(hfac)2(αH-PyBTM)(PyBTM) and this emission was not magnetic-field–sensitive. In contrast, PyZn_5, PyZn_10, and PyZn_20 displayed both monomer and excimer emissions, which were modulated strongly by the magnetic field. These are the first-reported examples of excimer emission and magnetoluminescence in luminescent radical-coordinated metal complexes. Considering their emission properties and MFE behaviours, the excimer emissive species was identified not as ZnII(hfac)2(PyBTM)2 but as the ZnII(hfac)2(αH-PyBTM)(PyBTM) dimer. These results suggest the general nature of MFEs on the luminescence of radicals15 as well as the importance of the type of interaction between radicals. Because complexation could modulate the inter/intramolecular interaction of spins, the development of MFEs on luminescent radical-ligated complexes would be a promising strategy, and this research surely is an important step in developing new photofunctions based on the interplay between spin and luminescence.
The present study was supported by the JST CREST (Grant Number JPMJCR15F2) and JSPS KAKENHI (Grant Numbers JP20H02759, JP19K22197, JP17H04870, JP19H05460, and JP26220801). T. K. is grateful to the Iketani Science and Technology Foundation and the Kato Foundation for the Promotion of Science for financial support. S. K. acknowledges MERIT (Material Education program for the future leaders in Research, Industry, and Technology) in the MEXT Leading Graduate School Doctoral Program. Emission spectra measurements under a magnetic field were performed at the High Field Laboratory for Superconducting Materials, Institute for Materials Research, Tohoku University (Project No. 18H0018 and 19H0052). This work was partly supported by the Nanotechnology Platform Program (Molecule and Material Synthesis) of the MEXT, Japan.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) C. W. Tang, S. A. Vanslyke and C. H. Chen, Appl. Phys. Lett., 1989, 65, 3610–3616 CAS; (b) M. A. Baldo, D. F. O’brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151–154 CrossRef CAS; (c) A. Endo, M. Ogasawara, A. Takahashi, D. Yokoyama, Y. Kato and C. Adachi, Adv. Mater., 2009, 21, 4802–4806 CrossRef CAS PubMed; (d) H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- (a) E. D. Cosco, J. R. Caram, O. T. Bruns, D. Franke, R. A. Day, E. P. Farr, M. G. Bawendi and E. M. Sletten, Angew. Chem., Int. Ed., 2017, 56, 13126–13129 CrossRef CAS PubMed; (b) A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2016, 15, 235–242 CrossRef CAS PubMed.
- (a) V. Gamero, D. Velasco, S. Latorre, F. López-Calahorra, E. Brillas and L. Juliá, Tetrahedron Lett., 2006, 47, 2305–2309 CrossRef CAS; (b) A. Heckmann, S. Du, J. Pauli, M. Margraf, J. Ko, D. Stich, C. Lambert, I. Fischer and U. Resch-genger, J. Phys. Chem. C, 2009, 113, 20958–20966 CrossRef CAS.
- (a) X. Ai, E. W. Evans, S. Dong, A. J. Gillett, H. Guo, Y. Chen, T. J. H. Hele, R. H. Friend and F. Li, Nature, 2018, 563, 536–540 CrossRef CAS PubMed; (b) H. Guo, Q. Peng, X. K. Chen, Q. Gu, S. Dong, E. W. Evans, A. J. Gillett, X. Ai, M. Zhang, D. Credgington, V. Coropceanu, R. H. Friend, J. L. Brédas and F. Li, Nat. Mater., 2019, 18, 977–984 CrossRef CAS PubMed; (c) Q. Peng, A. Obolda, M. Zhang and F. Li, Angew. Chem., Int. Ed., 2015, 54, 7091–7095 CrossRef CAS PubMed; (d) A. Obolda, X. Ai, M. Zhang and F. Li, ACS Appl. Mater. Interfaces, 2016, 8, 35472–35478 CrossRef CAS PubMed; (e) Q. Gu, A. Abdurahman, R. H. Friend and F. Li, J. Phys. Chem. Lett., 2020, 11, 5638–5642 CrossRef CAS PubMed; (f) A. Abdurahman, T. J. H. Hele, Q. Gu, J. Zhang, Q. Peng, M. Zhang, R. H. Friend, F. Li and E. W. Evans, Nat. Mater., 2020 DOI:10.1038/s41563-020-0705-9.
- (a) Y. Beldjoudi, M. Nascimento, Y. J. Cho, H. Yu, H. Aziz, D. Tonouchi, K. Eguchi, M. M. Matsushita, K. Awaga, I. O. Osorio-roman, C. P. Constantinides and J. M. Rawson, J. Am. Chem. Soc., 2018, 140, 6260–6270 CrossRef CAS PubMed; (b) P. Mayorga Burrezo, V. G. Jiménez, D. Blasi, I. Ratera, A. G. Campaña and J. Veciana, Angew. Chem., Int. Ed., 2019, 58, 16282–16288 CrossRef CAS PubMed; (c) P. Mayorga-Burrezo, V. G. Jiménez, D. Blasi, T. Parella, I. Ratera, A. G. Campaña and J. Veciana, Chem. – Eur. J., 2020, 26, 3776–3781 CrossRef CAS PubMed; (d) K. An, G. Xie, S. Gong, Z. Chen, X. Zhou, F. Ni and C. Yang, Sci. China: Chem., 2020, 63, 1214–1220 CrossRef CAS.
- (a) Y. Hattori, S. Kimura, T. Kusamoto, H. Maeda and H. Nishihara, Chem. Commun., 2018, 54, 615–618 RSC; (b) Y. Hattori, T. Kusamoto and H. Nishihara, Angew. Chem., Int. Ed., 2014, 53, 11845–11848 CrossRef CAS PubMed; (c) Y. Hattori, T. Kusamoto and H. Nishihara, Angew. Chem., Int. Ed., 2015, 54, 3731–3734 CrossRef CAS PubMed.
- S. Kimura, T. Kusamoto, S. Kimura, K. Kato, Y. Teki and H. Nishihara, Angew. Chem., Int. Ed., 2018, 57, 12711–12715 CrossRef CAS PubMed.
- K. Kato, S. Kimura, T. Kusamoto, H. Nishihara and Y. Teki, Angew. Chem., Int. Ed., 2019, 58, 2606–2611 CrossRef CAS PubMed.
- T. Kusamoto, Y. Hattori, A. Tanushi and H. Nishihara, Inorg. Chem., 2015, 54, 4186–4188 CrossRef CAS PubMed.
- (a) Y. Hattori, T. Kusamoto, T. Sato and H. Nishihara, Chem. Commun., 2016, 52, 13393–13396 RSC; (b) Y. Ogino, T. Kusamoto, Y. Hattori, M. Shimada, M. Tsuchiya, Y. Yamanoi, E. Nishibori, K. Sugimoto and H. Nishihara, Inorg. Chem., 2017, 56, 3909–3915 CrossRef CAS PubMed.
- D. Blasi, D. M. Nikolaidou, F. Terenziani, I. Ratera and J. Veciana, Phys. Chem. Chem. Phys., 2017, 19, 9313–9319 RSC.
- If ZnII(hfac)2(PyBTM)2 was preferentially formed and the crystals consisted of a mixture of ZnII(hfac)2(αH-PyBTM)2 and ZnII(hfac)2(PyBTM)2, the excimer emission could not emerge as the radical concentrations increased. Therefore, these results confirmed that αH-PyBTM in the ZnII(hfac)2(αH-PyBTM)2 crystals was randomly substituted with PyBTM.
- The contributions of the emission components mean the peak areas of emission bands and were estimated by Gaussian deconvolutions. The details are shown in the ESI†.
- The exciton transfer from monomer emissive states to excimer emissive states can be negligible because emission decays of PyZn_10 at 900 nm, where excimer emission components were dominant, did not show slow rising time (Fig. S6d, ESI†).
- Similarity in the magnetoluminescence behaviours between the previous reports and the present system indicates that the MFE observed is not compound-specific but general for luminescent doublet molecules.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supplementary figures. CCDC 2008148. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc04830e |
| This journal is © The Royal Society of Chemistry 2020 |