Rapid mechanochemical synthesis of metal–organic frameworks using exogenous organic base†
Zihao
Wang
 ,
Zongzhe
Li
,
Marcus
Ng
and
Phillip J.
Milner
,
Zongzhe
Li
,
Marcus
Ng
and
Phillip J.
Milner
 *
*
Department of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14853, USA. E-mail: pjm347@cornell.edu
First published on 29th April 2020
Abstract
Metal–organic frameworks (MOFs) bearing coordinatively unsaturated metal centers, exemplified by the MOF-74 family of frameworks, are promising for applications ranging from gas separations and storage to Lewis acid catalysis. However, the scalable synthesis of MOF-74 analogues remains a significant challenge. Recently, mechanochemistry has emerged as a sustainable strategy for the preparation of MOFs in the solid state with minimal solvent waste. Mechanochemical methods typically rely on metal salts bearing basic anions to deprotonate the conjugate acid of the organic linker and a small amount of organic solvent or water to facilitate liquid assisted grinding. Here, we demonstrate that the liquid exogenous organic base Hünig's base (N,N-diisopropylethylamine) can fulfill both roles, enabling the mechanochemical synthesis of M2(dobdc) analogues (M = Mg, Mn, Co, Ni, Cu, Zn; dobdc4− = 2,5-dioxidobenzene-1,4-dicarboxylate) using metal nitrate salts in only 5 minutes at room temperature. Importantly, we demonstrate that this straightforward method can be generalized to prepare the isomeric framework Mg2(m-dobdc) (m-dobdc4− = 2,4-dioxidobenzene-1,5-dicarboxylate) and the expanded framework Mg2(dobpdc) (dobpdc4− = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) under solvent-free conditions for the first time. The MOFs prepared using this method possess high crystallinities and surface areas, with the Mg2(m-dobdc) prepared herein representing the first reported permanently porous variant of this framework. This new sustainable mechanochemical synthesis of MOF-74 analogues should enable their preparation on a large scale for industrial applications.
Introduction
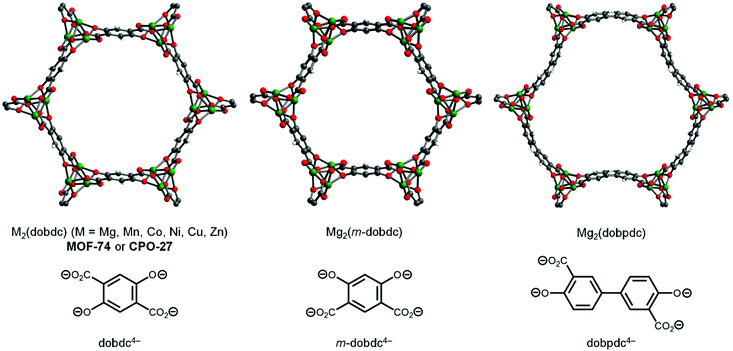
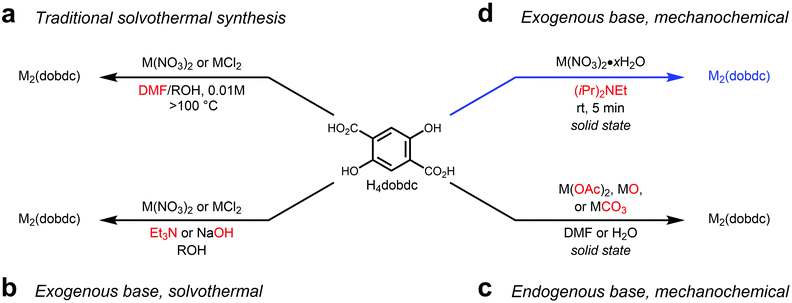
Metal–organic frameworks (MOFs) are a class of porous, crystalline solids constructed from polytopic organic “linkers” and inorganic “secondary building units” (SBUs) with myriad potential applications ranging from chemical separations to catalysis.1,2 Of these, MOFs bearing coordinatively unsaturated or open metal centers are particularly intriguing due to their ability to strongly polarize and bind guest molecules.3 In addition, MOFs with open metal centers are promising heterogeneous Lewis acid catalysts.4,5 Among open metal site MOFs, the MOF-74 or CPO-27 family stands out in terms of structural tunability and density of open metal sites (Fig. 1).6 Following the initial report of the synthesis of Zn2(dobdc) (dobdc4− = 2,5-dioxidobenzene-1,4-dicarboxylate) in 2005,6 several isostructural metal analogues, including Mg,7 Mn,8 Co,9 Ni,10 and Cu11,12 congeners, as well as isomeric13 and expanded14–21 variants, have been reported (Fig. 1). Due to their hexagonal one-dimensional channels lined with a high density of five-coordinate M2+ centers, activated MOF-74 variants hold numerous records for gas storage capacities at low pressures, including for CO2 and H2.12,22 In addition, MOF-74 analogues demonstrate promising catalytic activity for Lewis acid-mediated23,24 and C–H oxidation reactions.17,25 Given the promise of MOF-74 analogues for industrial applications, sustainable methods for their preparation on scale are desirable.26,27As with many MOFs, MOF-74 analogues are typically prepared under ultra-dilute solvothermal conditions using amide solvents such as N,N-dimethylformamide (DMF) (Fig. 2a).28,29 The role of the amide solvent is to decompose at high temperatures (>100 °C) to produce amines (e.g. N,N-dimethylamine) that deprotonate the conjugate acid of the linker prior to MOF self-assembly. While these solvothermal syntheses produce highly crystalline frameworks,12 they employ solvents that are undesirable on industrial scale29 and result in significant generation of organic waste. In addition, a lack of control over the strength of the base and the rate of its addition limits the inherent synthetic tunability of these methods. This is important because parameters such as pH and temperature can have a significant effect on framework crystallinity and polymorph formation, as has been observed previously for MOF-74 analogues.30–34 Recently, it has been shown that the base can be decoupled from the reaction solvent by instead employing metal salts bearing basic counteranions (e.g. acetate) under solvothermal conditions (not shown).35–40 More generally, it is possible to completely separate the base from the MOF precursors or solvent by utilizing exogenous organic41 or inorganic42,43 bases to prepare MOF-74 frameworks (Fig. 2b). Although these methods allow more synthetic control over framework formation, they still lead to significant solvent waste. Therefore, while it is possible to avoid the use of amide solvents during the preparation of select MOF-74 analogues, the generation of significant waste limits the sustainability of these methods. In addition, isomeric and expanded MOF-74 analogues are still widely prepared using DMF-based solvothermal approaches (Fig. 1).
Recently, mechanochemical syntheses of MOFs in the solid state have emerged as promising alternatives to solvothermal methods because they avoid the generation of significant solvent waste.44–47 In particular, liquid assisted grinding (LAG), in which precursors solids are mechanically ground together in the presence of a small amount of solvent, has been shown to enable MOF synthesis on large scale.47,48 Select MOF-74 frameworks can be prepared by LAG using basic metal precursors, albeit with variable crystallinities and surface areas (Fig. 2c).49,50 Building upon this precedent, we hypothesized that we could mechanochemically synthesize MOF-74 frameworks using a liquid exogenous base, such as Hünig's base (N,N-diisopropylethylamine), to completely decouple the base from the metal precursor (Fig. 2d).49,50 In these reactions, Hünig's base would play a dual role as both the base to enable MOF formation as well as the liquid to assist in mechanical grinding. Herein, we demonstrate that LAG with Hünig's base does in fact allow for the preparation of high-quality MOF-74 frameworks in a scalable and sustainable manner. In addition, we show that this strategy can be readily translated to prepare isomeric and expanded MOF-74 analogues in the solid state for the first time.
Results and discussion
We began exploring the mechanochemical synthesis of MOF-74 analogues with exogenous base by simply grinding an excess of Mg(NO3)2·6H2O, the most commonly used metal precursor to prepare Mg-based MOF-74 analogues,7,13,14,19 and H4dobdc together for 5 min at room temperature using a mortar and pestle (see ESI or ESI section 2† for details). Unsurprisingly due to the lack of a sufficiently strong base, Mg2(dobdc) did not form under these conditions. However, the addition of 4.4 equiv. of Hünig's base to the solid mixture led to the clean formation of nanocrystalline Mg2(dobdc), as confirmed by powder X-ray diffraction (Fig. 3, red curve; see ESI section 3† for details). Hünig's base was chosen because it possesses similar basicity (pKb = 3.2) to dimethylamine (pKb = 3.3) and triethylamine (pKb = 3.2), which have previously been used to prepare MOF-74 analogues,6,41 but is significantly less coordinating and volatile. This result confirms that the combination of organic linker, metal salt, and amine base is sufficient to prepare Mg2(dobdc) in the solid state.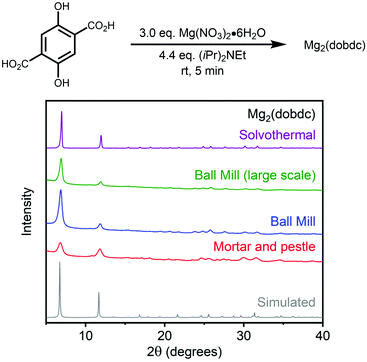 | ||
| Fig. 3 Powder X-ray diffraction (PXRD) patterns (λ = 1.5406 Å) of Mg2(dobdc) prepared under mechanochemical conditions with a mortar and pestle (red) or ball mill (blue, green) and Mg2(dobdc) prepared under standard solvothermal conditions (purple).52 The simulated pattern based on the previously reported single-crystal X-ray diffraction structure of the isostructural framework Zn2(dobdc) is included for reference (gray).53 | ||
In order to standardize our mechanochemical synthesis, we employed a ball mill to prepare Mg2(dobdc) with more consistent grinding (see ESI section 2† for details).51 Using tungsten carbide vessels and balls (ϕ = 2.0 mm) and a milling rate of 600 rpm, we successfully prepared Mg2(dobdc) on 2 mmol scale (∼0.5 g) at room temperature in only 5 min, as confirmed by powder X-ray diffraction and IR spectroscopy (Fig. 3, blue curve). Notably, the crystallinity of Mg2(dobdc) synthesized using a ball mill was superior to that prepared using a mortar and pestle. We were able to further scale up this simple method to prepare Mg2(dobdc) on 8 mmol (∼2.0 g) scale with similar results (Fig. 3, green curve). It should be noted that grinding times longer than 5 min led to a significant reduction in crystallinity, indicating that reaction time likely has a significant impact on the observed crystallite size. As a result, a standard reaction time of 5 min was used for subsequent experiments.
To evaluate whether mechanochemical synthesis produces MOF of comparable porosity to material prepared under solvothermal conditions,12,52 we soaked the large-scale samples of Mg2(dobdc) in methanol at 65 °C, changing the solvent every day for three days. Digestion of the samples using DCl (35 wt% in D2O) in DMSO-d6 and analysis by 1H NMR confirmed that soaking in methanol is sufficient to remove residual Hünig's base and ammonium salts from the framework pores (ESI Fig. S27†). Next, these samples were desolvated at 180 °C under high vacuum to remove residual methanol. Importantly, the measured N2 adsorption isotherms of these materials at 77 K are comparable to reference material prepared under solvothermal conditions (Fig. 4).52 In particular, the Langmuir surface areas of Mg2(dobdc) prepared on 2 mmol scale (1852 ± 53 m2 g−1, blue circles) and 8 mmol scale (1992 ± 49 m2 g−1, green triangles) using a ball mill are similar to MOF prepared under solvothermal conditions (1914 m2 g−1, purple squares).52 These results are summarized in Table 1 and confirm that high-quality Mg2(dobdc) can be prepared by mechanochemical synthesis using exogenous organic base.
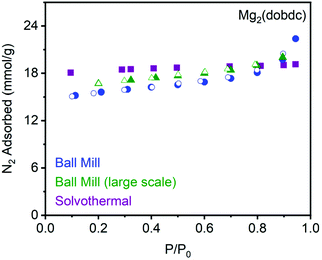 | ||
| Fig. 4 Comparison of the 77 K N2 adsorption (filled) and desorption (open) isotherms of Mg2(dobdc) prepared under mechanochemical conditions (blue circles, green triangles) and standard solvothermal conditions (purple squares).5 | ||
| Framework | Average crystalline domain sizea (nm) | Langmuir surface areab (m2 g−1) | Solvothermal langmuir surface areab (m2 g−1) |
|---|---|---|---|
| a Determined by the Scherrer equation using the first PXRD reflection. b Determined from N2 adsorption isotherm at 77 K. c From solvothermal synthesis.52 d Framework oxidized in air. e Prepared using 2,6-lutidine as base in place of Hünig's base. | |||
| Mg2(dobdc) | 21 (51)c | 1992 ± 49 | 1914 |
| Mn2(dobdc) | 28 | 385 ± 14d | 179712 |
| Co2(dobdc) | 23 | 1334 ± 51 | 143812 |
| Ni2(dobdc) | 13 | 1281 ± 48 | 157412 |
| Cu2(dobdc) | 39 | 1115 ± 6 | 151512 |
| Zn2(dobdc) | 21 (15)e | 1204 ± 16 | 127712 |
| Mg2(m-dobdc) | 22 | 1793 ± 50 | Nonporous54 |
| Mg2(dobpdc) | 10 | 3137 ± 71 | 378014 |
We next evaluated the generality of this mechanochemical strategy towards the synthesis of other M2(dobdc) congeners (see ESI sections 4–8 and 12† for details). Gratifyingly, the corresponding M2(dobdc) (M = Mn, Co, Ni, Cu, Zn) frameworks could be prepared on ∼0.5 g scale following the same procedure outlined above, as confirmed by powder X-ray diffraction (Fig. 5) and Infrared spectroscopy (ESI sections 4–8†). Qualitative analysis of these powder X-ray diffraction patterns using the Scherrer equation (K = 0.89, λ = 1.5406 Å) suggests that the average crystalline domain size of all prepared MOFs are in the nanocrystalline regime38,41 and smaller than MOF prepared under solvothermal conditions (Table 1).52 Soaking the as-synthesized M2(dobdc) frameworks in methanol followed by desolvation under vacuum at 180 °C allowed us to evaluate their 77 K N2 surface areas (Table 1; see ESI sections 4–8† for details). Similar to the Mg congener, Co2(dobdc) and Zn2(dobdc) produced under mechanochemical conditions possess Langmuir surface areas close to those reported for frameworks prepared under solvothermal conditions (Table 1).12 Although the Langmuir surface area of Ni2(dobdc) is slightly lower than expected, nanocrystalline samples of MOF-74 variants have previously been shown to have low surface areas due to a higher prevalence of defects.38 Similarly, the unexpectedly low Langmuir surface area of Cu2(dobdc) may arise due to the presence of defects or amorphous contaminants, in line with the known difficulty of preparing high surface area samples of this framework.12 Unfortunately, the porosity of Mn2(dobdc) prepared herein was quite low, likely due its oxidative degradation in air upon filtration. Nonetheless, these results confirm that of M2(dobdc) variants generally can be prepared using this mechanochemical strategy.
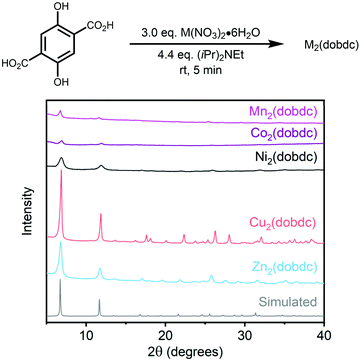 | ||
| Fig. 5 Powder X-ray diffraction patterns (λ = 1.5406 Å) of M2(dobdc) (M = Mn, Co, Ni, Cu, Zn) prepared under mechanochemical conditions. The simulated pattern based on the previously reported single-crystal X-ray diffraction structure of the framework Zn2(dobdc) is included for reference.53 | ||
While the mechanochemical synthesis of selected M2(dobdc) variants has been previously described using basic metal salts,49,50 the solid-state synthesis of related M2(m-dobdc) (m-dobdc4− = 2,4- dioxidobenzene-1,5-dicarboxylate) and M2(dobpdc) (dobpdc4− = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) frameworks (Fig. 1) has not, to the best of our knowledge, been reported to date. The sustainable synthesis of these frameworks is desirable because they are promising for H2 storage22 and CO2 capture,21 respectively. Therefore, we further extended our methodology by preparing Mg2(m-dobdc) and Mg2(dobpdc) in a ball mill using Hünig's base (see ESI sections 9 and 10† for details). In both cases, the successful formation of these frameworks was confirmed by powder X-ray diffraction (Fig. 6) and Infrared spectroscopy (ESI sections 9 and 10†). Our preliminary results suggest that Co2(dobpdc) and Zn2(dobpdc) can also be prepared under these conditions (ESI Fig. S28 and 29†). Using the Scherrer equation, we confirmed that the average crystalline domain size of Mg2(m-dobdc) is similar to that of Mg2(dobdc), which is understandable given their structural similarity (Table 1). In contrast, smaller crystallites of Mg2(dobpdc) were obtained. Consistent with our findings for Ni2(dobdc), the 77 K N2 Langmuir surface area of Mg2(dobpdc) prepared herein is lower than that for material prepared under solvothermal conditions (Table 1 and ESI Fig. S26†).14 Importantly, the Mg2(m-dobdc) synthesized herein was found to be highly porous (1793 ± 50 m2 g−1), with a surface area similar to that of the isomeric framework Mg2(dobdc) (Table 1 and ESI Fig. S23†). This is in contrast the material prepared under solvothermal conditions, which was previously reported to be non-porous due to the difficulty of removing residual DMF from the pores.21,54 To the best of our knowledge, this is the first report of the permanent porosity of Mg2(m-dobdc), further expanding the limited pool of Mg-based frameworks with coordinatively unsaturated metal centers.55 Overall, LAG with Hünig's base appears to be a general protocol for the synthesis of MOF-74 analogues.
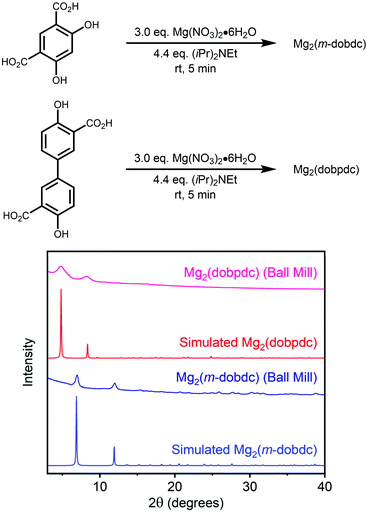 | ||
| Fig. 6 Powder X-ray diffraction patterns (λ = 1.5406 Å) of Mg2(m-dobdc) (blue) and Mg2(dobpdc) (red) prepared under mechanochemical conditions. The simulated patterns based on the previously reported single-crystal X-ray diffraction structures of the isostructural frameworks Co2(m-dobdc)54 and Zn2(dobpdc)56 are included for reference. | ||
To gain preliminary mechanistic insight into the role of organic base in MOF formation, we explored mechanochemical synthesis using different nitrogen bases (Fig. 7; see ESI section 8† for details). For these studies, Zn2(dobdc) was chosen because its mechanism of formation under mechanochemical conditions has previously been shown to proceed by step-wise deprotonation of the carboxylates of the linker followed by the less acidic phenols.50 As with Mg2(dobdc), no MOF formation was observed in the absence of base. Interestingly, when the weak organic base pyridine (pKb = 8.8) was employed for LAG, Zn2(dobdc) was not observed. This is likely because pyridine is insufficiently basic to deprotonate the phenols of the organic linker, although we cannot rule out that the coordinating nature of pyridine also plays a role in inhibiting MOF formation. Consistently, when the stronger and more sterically-hindered organic base 2,6-lutidine (pKb = 7.3) was used instead, we were able to successfully prepare Zn2(dobdc). However, the use of even more basic Hünig's base (pKb = 3.2) led to more crystalline material. This is likely because Hünig's base is sufficiently basic to deprotonate both the carboxylic acid (pKa1 ≈ 3.0) and phenol (pKa2 ≈ 13.8) of the linker to some degree. These results confirm that base of sufficient strength to deprotonate both the phenol and carboxylic acid of the linker are required to form MOF, suggesting that the strength of the base is a potential handle for controlling MOF formation. Further studies are underway in our laboratory to explore the role of organic base in MOF formation.
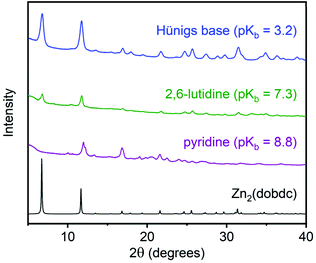 | ||
| Fig. 7 Powder X-ray diffraction patterns (λ = 1.5406 Å) of Zn2(dobdc) prepared under mechanochemical conditions with different organic bases. The simulated pattern based on the previously reported single-crystal X-ray diffraction structure of Zn2(dobdc) is included for reference.53 | ||
Conclusions
We report here the development of a new mechanochemical synthesis of MOF-74 variants using Hünig's base as both the liquid for LAG and the base required to facilitate MOF formation. This mechanochemical method is general, enables access to a range of crystalline frameworks, and increases the available methods for the sustainable synthesis of MOFs.57 Importantly, this mechanochemical method was employed to bypass the use of DMF and prepare highly porous Mg2(m-dobdc) for the first time. Therefore, this new strategy should prove beneficial for the scalable synthesis of both known and new porous MOF-74 variants. Current efforts in our laboratory are focused on exploring the mechanism of this novel LAG strategy and extending it to the synthesis of other industrially relevant MOFs.Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
The authors acknowledge Cornell University for financial support of this work. Z. W. thanks Nankai University and Z. L. thanks Beihang University for additional financial support. The authors thank Prof. Francis DiSalvo (Cornell University) for the use of the ball mill in his laboratory. The powder X-ray diffraction pattern of Co2(dobpdc) was collected using the mail-in service at beamline 11-BM of the Advanced Photon Source. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. 1H NMR data were collected on a Bruker INOVA 500 MHz spectrometer that was purchased with support from the National Science Foundation (CHE-1531632). We thank Dr Arjun Halder (Cornell University) for assistance with the preparation of this manuscript.Notes and references
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444–1230444 CrossRef PubMed.
- C. H. Hendon, A. J. Rieth, M. D. Korzyński and M. Dincă, ACS Cent. Sci., 2017, 3, 554–563 CrossRef CAS PubMed.
- J. N. Hall and P. Bollini, React. Chem. Eng., 2019, 4, 207–222 RSC.
- A. Yadav and P. Kanoo, Chem. – Asian J., 2019, 14, 3531–3551 CrossRef CAS PubMed.
- Z. Hu and D. Zhao, CrystEngComm, 2017, 19, 4066–4081 RSC.
- N. L. Rosi, J. Kim, M. Eddaoudi, B. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504–1518 CrossRef CAS PubMed.
- S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef CAS PubMed.
- W. Zhou, H. Wu and T. Yildirim, J. Am. Chem. Soc., 2008, 130, 15268–15269 CrossRef CAS PubMed.
- P. D. C. Dietzel, Y. Morita, R. Blom and H. Fjellvåg, Angew. Chem., Int. Ed., 2005, 44, 6354–6358 CrossRef CAS PubMed.
- P. D. C. Dietzel, B. Panella, M. Hirscher, R. Blom and H. Fjellvåg, Chem. Commun., 2006, 959 RSC.
- R. Sanz, F. Martínez, G. Orcajo, L. Wojtas and D. Briones, Dalton Trans., 2013, 42, 2392–2398 RSC.
- W. L. Queen, M. R. Hudson, E. D. Bloch, J. A. Mason, M. I. Gonzalez, J. S. Lee, D. Gygi, J. D. Howe, K. Lee and T. A. Darwish, Chem. Sci., 2014, 5, 4569–4581 RSC.
- M. T. Kapelewski, S. J. Geier, M. R. Hudson, D. Stück, J. A. Mason, J. N. Nelson, D. J. Xiao, Z. Hulvey, E. Gilmour, S. A. FitzGerald, M. Head-Gordon, C. M. Brown and J. R. Long, J. Am. Chem. Soc., 2014, 136, 12119–12129 CrossRef CAS PubMed.
- P. J. Milner, J. D. Martell, R. L. Siegelman, D. Gygi, S. C. Weston and J. R. Long, Chem. Sci., 2018, 9, 160–174 RSC.
- S. Peng, B. Bie, Y. Sun, M. Liu, H. Cong, W. Zhou, Y. Xia, H. Tang, H. Deng and X. Zhou, Nat. Commun., 2018, 9, 1293 CrossRef PubMed.
- J. Zheng, R. S. Vemuri, L. Estevez, P. K. Koech, T. Varga, D. M. Camaioni, T. A. Blake, B. P. McGrail and R. K. Motkuri, J. Am. Chem. Soc., 2017, 139, 10601–10604 CrossRef CAS PubMed.
- D. J. Xiao, J. Oktawiec, P. J. Milner and J. R. Long, J. Am. Chem. Soc., 2016, 138, 14371–14379 CrossRef CAS PubMed.
- D. J. Levine, T. Runčevski, M. T. Kapelewski, B. K. Keitz, J. Oktawiec, D. A. Reed, J. A. Mason, H. Z. H. Jiang, K. A. Colwell, C. M. Legendre, S. A. FitzGerald and J. R. Long, J. Am. Chem. Soc., 2016, 138, 10143–10150 CrossRef CAS.
- T. M. McDonald, J. A. Mason, X. Kong, E. D. Bloch, D. Gygi, A. Dani, V. Crocellà, F. Giordanino, S. O. Odoh, W. S. Drisdell, B. Vlaisavljevich, A. L. Dzubak, R. Poloni, S. K. Schnell, N. Planas, K. Lee, T. Pascal, L. F. Wan, D. Prendergast, J. B. Neaton, B. Smit, J. B. Kortright, L. Gagliardi, S. Bordiga, J. A. Reimer and J. R. Long, Nature, 2015, 519, 303–308 CrossRef CAS PubMed.
- H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gandara, A. C. Whalley, Z. Liu, S. Asahina, H. Kazumori, M. O'Keeffe, O. Terasaki, J. F. Stoddart and O. M. Yaghi, Science, 2012, 336, 1018–1023 CrossRef CAS.
- T. M. McDonald, W. R. Lee, J. A. Mason, B. M. Wiers, C. S. Hong and J. R. Long, J. Am. Chem. Soc., 2012, 134, 7056–7065 CrossRef CAS PubMed.
- M. T. Kapelewski, T. Runčevski, J. D. Tarver, H. Z. H. Jiang, K. E. Hurst, P. A. Parilla, A. Ayala, T. Gennett, S. A. FitzGerald, C. M. Brown and J. R. Long, Chem. Mater., 2018, 30, 8179–8189 CrossRef CAS PubMed.
- G. Calleja, R. Sanz, G. Orcajo, D. Briones, P. Leo and F. Martínez, Catal. Today, 2014, 227, 130–137 CrossRef CAS.
- H.-F. Yao, Y. Yang, H. Liu, F.-G. Xi and E.-Q. Gao, J. Mol. Catal. A: Chem., 2014, 394, 57–65 CrossRef CAS.
- D. J. Xiao, E. D. Bloch, J. A. Mason, W. L. Queen, M. R. Hudson, N. Planas, J. Borycz, A. L. Dzubak, P. Verma, K. Lee, F. Bonino, V. Crocellà, J. Yano, S. Bordiga, D. G. Truhlar, L. Gagliardi, C. M. Brown and J. R. Long, Nat. Chem., 2014, 6, 590–595 CrossRef CAS PubMed.
- D. DeSantis, J. A. Mason, B. D. James, C. Houchins, J. R. Long and M. Veenstra, Energy Fuels, 2017, 31, 2024–2032 CrossRef CAS.
- P. A. Julien, C. Mottillo and T. Friščić, Green Chem., 2017, 19, 2729–2747 RSC.
- Y. Sun and H.-C. Zhou, Sci. Technol. Adv. Mater., 2015, 16, 054202 CrossRef.
- Y.-R. Lee, J. Kim and W.-S. Ahn, Korean J. Chem. Eng., 2013, 30, 1667–1680 CrossRef CAS.
- D. Kim and A. Coskun, Angew. Chem., Int. Ed., 2017, 56, 5071–5076 CrossRef CAS.
- F. Luo, C. Yan, L. Dang, R. Krishna, W. Zhou, H. Wu, X. Dong, Y. Han, T.-L. Hu, M. O'Keeffe, L. Wang, M. Luo, R.-B. Lin and B. Chen, J. Am. Chem. Soc., 2016, 138, 5678–5684 CrossRef CAS.
- S. E. Henkelis, L. J. McCormick, D. B. Cordes, A. M. Z. Slawin and R. E. Morris, Inorg. Chem. Commun., 2016, 65, 21–23 CrossRef CAS.
- A. Douvali, G. S. Papaefstathiou, M. P. Gullo, A. Barbieri, A. C. Tsipis, C. D. Malliakas, M. G. Kanatzidis, I. Papadas, G. S. Armatas, A. G. Hatzidimitriou, T. Lazarides and M. J. Manos, Inorg. Chem., 2015, 54, 5813–5826 CrossRef CAS PubMed.
- P. D. C. Dietzel, R. Blom and H. Fjellvåg, Eur. J. Inorg. Chem., 2008, 2008, 3624–3632 CrossRef.
- R. L. Siegelman, P. J. Milner, A. C. Forse, J.-H. Lee, K. A. Colwell, J. B. Neaton, J. A. Reimer, S. C. Weston and J. R. Long, J. Am. Chem. Soc., 2019, 141, 13171–13186 CrossRef CAS PubMed.
- L. Maserati, S. M. Meckler, J. E. Bachman, J. R. Long and B. A. Helms, Nano Lett., 2017, 17, 6828–6832 CrossRef CAS PubMed.
- L. Garzón-Tovar, A. Carné-Sánchez, C. Carbonell, I. Imaz and D. Maspoch, J. Mater. Chem. A, 2015, 3, 20819–20826 RSC.
- M. Díaz-García, Á. Mayoral, I. Díaz and M. Sánchez-Sánchez, Cryst. Growth Des., 2014, 14, 2479–2487 CrossRef.
- S. Cadot, L. Veyre, D. Luneau, D. Farrusseng and E. Alessandra Quadrelli, J. Mater. Chem. A, 2014, 2, 17757–17763 RSC.
- D. J. Tranchemontagne, J. R. Hunt and O. M. Yaghi, Tetrahedron, 2008, 64, 8553–8557 CrossRef CAS.
- J. E. Bachman, Z. P. Smith, T. Li, T. Xu and J. R. Long, Nat. Mater., 2016, 15, 845–849 CrossRef CAS PubMed.
- S. M. Vornholt, S. E. Henkelis and R. E. Morris, Dalton Trans., 2017, 46, 8298–8303 RSC.
- M. Sánchez-Sánchez, N. Getachew, K. Díaz, M. Díaz-García, Y. Chebude and I. Díaz, Green Chem., 2015, 17, 1500–1509 RSC.
- D. Chen, J. Zhao, P. Zhang and S. Dai, Polyhedron, 2019, 162, 59–64 CrossRef CAS.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- M. Klimakow, P. Klobes, A. F. Thünemann, K. Rademann and F. Emmerling, Chem. Mater., 2010, 22, 5216–5221 CrossRef CAS.
- T. Friščić, J. Mater. Chem., 2010, 20, 7599 RSC.
- P. J. Beldon, L. Fábián, R. S. Stein, A. Thirumurugan, A. K. Cheetham and T. Friščić, Angew. Chem., Int. Ed., 2010, 49, 9640–9643 CrossRef CAS PubMed.
- G. Ayoub, B. Karadeniz, A. J. Howarth, O. K. Farha, I. Đilović, L. S. Germann, R. E. Dinnebier, K. Užarević and T. Friščić, Chem. Mater., 2019, 31, 5494–5501 CrossRef CAS.
- P. A. Julien, K. Užarević, A. D. Katsenis, S. A. J. Kimber, T. Wang, O. K. Farha, Y. Zhang, J. Casaban, L. S. Germann, M. Etter, R. E. Dinnebier, S. L. James, I. Halasz and T. Friščić, J. Am. Chem. Soc., 2016, 138, 2929–2932 CrossRef CAS PubMed.
- J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC.
- M. L. Aubrey, R. Ameloot, B. M. Wiers and J. R. Long, Energy Environ. Sci., 2014, 7, 667 RSC.
- P. D. C. Dietzel, R. E. Johnsen, R. Blom and H. Fjellvåg, Chem. – Eur. J., 2008, 14, 2389–2397 CrossRef CAS PubMed.
- J. E. Bachman, M. T. Kapelewski, D. A. Reed, M. I. Gonzalez and J. R. Long, J. Am. Chem. Soc., 2017, 139, 15363–15370 CrossRef CAS PubMed.
- D. Banerjee, H. Wang, B. J. Deibert and J. Li, in The Chemistry of Metal–Organic Frameworks: Synthesis, Characterization, and Applications, ed. S. Kaskel, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2016, pp. 73–103 Search PubMed.
- R. L. Siegelman, T. M. McDonald, M. I. Gonzalez, J. D. Martell, P. J. Milner, J. A. Mason, A. H. Berger, A. S. Bhown and J. R. Long, J. Am. Chem. Soc., 2017, 139, 10526–10538 CrossRef CAS PubMed.
- P. Li, F.-F. Cheng, W.-W. Xiong and Q. Zhang, Inorg. Chem. Front., 2018, 11, 2693–2708 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0dt01240h |
| This journal is © The Royal Society of Chemistry 2020 |