Isolation and structure determination of two new nosiheptide-type compounds provide insights into the function of the cytochrome P450 oxygenase NocV in nocathiacin biosynthesis†
Xuebing
Bai‡
a,
Heng
Guo‡
b,
Dandan
Chen‡
b,
Qian
Yang
 *b,
Jiang
Tao
*ac and
Wen
Liu
*b,
Jiang
Tao
*ac and
Wen
Liu
 *bd
*bd
aDepartment of General Dentistry, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, 639 Zhizaoju Road, Shanghai 200011, China. E-mail: taojiang_doctor@hotmail.com; Tel: +86 -21-23271699-5202
bState Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence on Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: yangqian117@sioc.ac.cn; wliu@mail.sioc.ac.cn; Fax: +86-21-64166128; Tel: +86-21-54925539 Tel: +86-21-54925111
cNational Clinical Research Center for Oral Diseases, Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology, Shanghai, China
dHuzhou Center of Bio-Synthetic Innovation, 1366 Hongfeng Road, Huzhou 313000, China
First published on 7th January 2020
Abstract
Thiopeptides, which are a class of sulfur-rich, ribosomally synthesized and post-translationally modified peptides (RiPPs), have great potential in the treatment of diseases caused by oral pathogens. Nocathiacin I (NOC-I) and nosiheptide (NOS) are two structurally similar thiopeptide members that feature an indolic side ring. In the structure of NOC-I, this side ring is further rigidified through the formation of an ether linkage; however, the related biosynthetic process remains poorly understood. Here, we report that NocV, a cytochrome P450 protein found to be unique in the biosynthetic pathway of NOC-I, is responsible for the establishment of the intramolecular ether linkage through two oxidation steps. This observation benefited from the heterologous overexpression of the gene nocV in an engineered Streptomyces strain producing the bicyclic NOS intermediate NOS1260, and subsequent isolation and structure characterization of two functionalized products. The product NOS-V1 contains a new hydroxyl group at Cα of the residue Glu6, in contrast with the other product NOS-V2, in which this hydroxyl group is further coupled with the C4 methyl group of the indolic moiety to form an ether linkage. These findings provide insights into the catalytic logic of NocV in the biosynthesis of NOC-I, during which this cytochrome P450 protein appears to act in tandem on two positions in NOS1260 by selectively hydroxylating Glu6 and then oxidatively coupling the indolic moiety. Rigidifying the side ring via ether bond formation has a positive impact on the antibacterial properties of NOS-type thiopeptides, evidenced by the improved activity of NOC-V2 against various tested oral pathogens compared with NOS1260.
Introduction
Thiopeptides are a class of sulfur-rich, ribosomally synthesized and post-translationally modified peptides (RiPPs), characterized by a macrocyclic framework that contains a six-membered heterocycle domain central to multiple azoles and dehydroamino acids.1 Similar to many other RiPPs, a thiopeptide originates from a precursor peptide consisting of an N-terminal leader peptide (LP) and a C-terminal core peptide (CP). The CP undergoes a series of post-translational modifications (PTMs) in a manner either dependent or independent of the LP to produce mature products.2 Most members of thiopeptides exhibit potent activity against drug-resistant Gram-positive bacteria; however, poor water solubility and low bioavailability limit their clinical use.3 Previously, we reported the promising activities of the thiopeptide members thiostrepton (TSR) and siomycin (SIO), as well as their analogs, against oral pathogens, indicating the potential application of thiopeptides for the treatment of human oral diseases.4Nocathiacin I (NOC-I) and nosiheptide (NOS) are two thiopeptides with an indolic moiety appended to the characteristic macrocyclic framework through two ester linkages.5–7 NOC-I shares an overall similarity with NOS in the molecular skeleton but is more functionalized. As a unique glycosylated thiopeptide, NOC-I shows good water solubility, which is largely attributed to glycosylation of the Glu6 γ-hydroxyl with a rare sugar moiety.8 Besides, the aglycone of NOC-I bears additional structural modifications, such as O-methylation of the residue Thr4, N-hydroxylation of the indolic moiety, and rigid constraint of the indole-possessing side ring via an ether bond. Comparative analysis of the noc and nos biosynthetic gene clusters (BGCs) shows significant similarity in their gene sequence and arrangement,9,10 suggesting great similarity in the biosynthesis of NOC-I and NOS. Apart from homologous genes, the noc BGC contains several additional genes that may be involved in PTMs to afford the specific functionalization of NOC-I (Fig. 1A). Systematic studies have revealed in depth the biosynthetic pathway of NOS,11–16 including the LP-independent hydroxylation steps catalyzed by two cytochrome P450 oxygenases, NosB and NosC.17 In addition to the NosB and NosC homologs NocB and NocC, three other P450 oxygenases, NocT, NocU, and NocV, are supposed to be functional in multiple oxidative routes towards the maturation of NOC-I (Fig. 1B). Unfortunately, the NOC-producing strain Nocardia sp. is resistant to available genetic manipulation approaches,9 making the biosynthetic studies of NOC-I largely delayed. Given the high similarity of the molecular skeleton of NOS and NOC-I, the unclear biosynthetic pathway of NOC-I can be elucidated via heterologous expression of the noc genes in the NOS-producing strain Streptomyces actuosus. Here, we apply an NOS biosynthetic machinery to explore the function of the P450 oxygenase NocV and evaluate the antibacterial activities of the resulting NOS framework-containing analogs against oral pathogens.
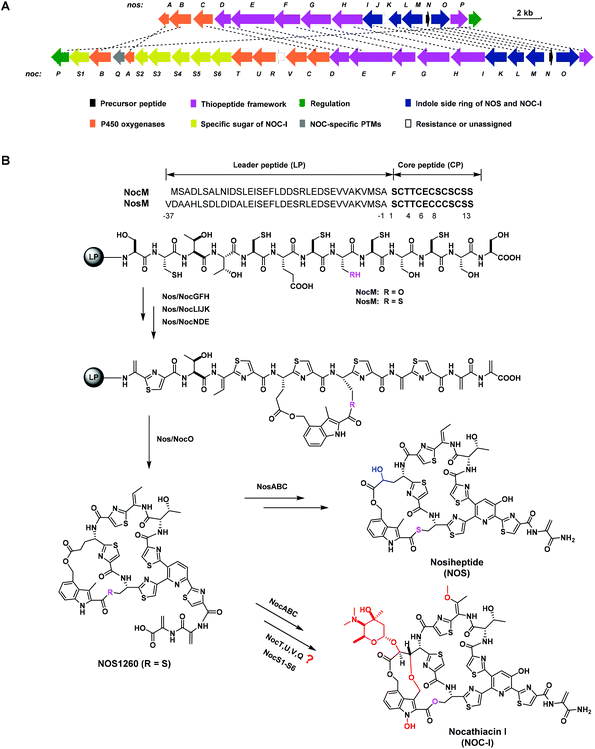 | ||
| Fig. 1 BGCs and biosynthetic pathways of NOC-I and NOS. (A) Organization of the noc BGC in comparison with the nos BGC.9,10 The deduced functions of biosynthetic genes are labeled in pattern, and the sequence homologies are indicated by a dashed line for each pair. (B) Proposed biosynthetic pathway of NOC-I in comparison to that of NOS.11–17 Precursor peptides of NocM for NOC-I and NosM for NOS go through ordered PTMs either dependent or independent of the LPs. The structural differences are colored red (for NOC-I) or blue (for NOS). | ||
Results and discussion
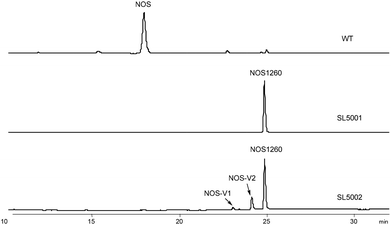
Considering the multi-substrate specificity of NosB and NosC in conjunction with NosA in the maturation of NOS,17 we constructed a Streptomyces actuosus mutant strain, SL5001, in which the contiguous genes nosA, nosB, and nosC were deleted simultaneously. In SL5001, the PTMs performed during NOS biosynthesis can be simplified, leading to accumulation of a key intermediate, NOS1260 (Fig. 2). NOS1260 is the first intermediate possessing the NOS-type thiopeptide framework after LP release. Structure characterization of NOS1260 confirmed the presence of the unoxygenated Glu6 and six-membered heterocycle core, as well as the bis-dehydroalanine (Dha) tail.To investigate the in vivo function of NocV, we heterologously expressed nocV in the engineered NOS biosynthetic system. A plasmid consisting of two copies of nocV was introduced into the engineered S. actuosus strain SL5001 via intergeneric conjugation. The resulting recombinant strain, SL5002, was capable of producing two new compounds, nosiheptide V1 (NOS-V1) and nosiheptide V2 (NOS-V2), based on HPLC analysis (Fig. 2). Both compounds showed UV absorption patterns similar to that of NOS1260, indicating that they were NOS-derived analogs (Fig. S1†). HR-ESI-MS ion data further established the molecular formulas of these two compounds as C54H45N13O13S6 (m/z 1276.1652 [M + H]+, calcd 1276.1657) and C54H44N13O13S6 (m/z 1274.1493 [M + H]+, calcd 1274.1500), suggesting that NOS-V1 and NOS-V2 were generated via introduction of a hydroxyl and an oxygen atom into NOS1260, respectively (Fig. S2 and S5†).
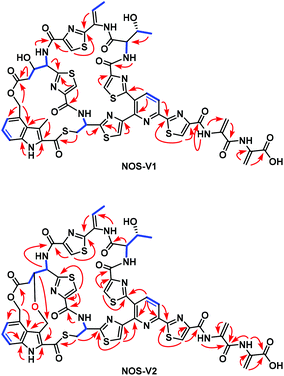
The recombinant strain SL5002 was subjected to large-scale fermentation to accumulate target products for structure elucidation. A total of 5.6 mg NOS-V1, 11.1 mg NOS-V2, and 46.2 mg NOS1260 were isolated to high purity and subjected to 1D and 2D NMR (1H and 13C NMR, 1H–1H COSY, HSQC, HMBC, and ROESY) experiments (Fig. S4 and S7†). In comparison to the NMR spectra of NOS1260, the 1D and 2D NMR spectra of NOS-V1 clearly revealed the presence of an additional hydroxyl group: (i) the H2(δH 2.38)–H3(δH 4.46)–H4(δH 5.28) spin–spin coupling system in the residue Glu6; (ii) the HMBC correlations from H-2 (δH 2.38) to C-1 (δC 169.4), C-3 (δC 68.2) and C-4 (δC 54.1); and (iii) the 1H–1H coupling constant 3JH-3/H-4 (9.0 Hz) and the H-3/H3-3′ (Ind) ROESY correlation indicated that HO-3 (Glu6) was α-oriented. Meanwhile, careful analysis of the NMR spectra of NOS-V2 revealed that the structural features of NOS-V2 were similar to those of NOS1260, differing only in the oxygenation in Glu6 C-3 (δC 78.8 and δH 4.06) and the 2,4-dimethylindolic acid (DMIA) moiety C-3′ [δC 64.0 and δH 4.77 (Ha), 4.06 (Hb)], resulting in formation of an oxygen ether bond. The findings were demonstrated by HMBC correlations from H-3 (δH 4.06, Glu6) to C-3′ (δC 64.0, indole). Therefore, the chemical structures of NOS-V1 and NOS-V2 were elucidated, featuring a hydroxyl group of Glu6 at the α-position and an ether bond between Glu6 and the indole 3-methyl, respectively (Fig. 3, S3, S6 and Table S3, S4†).
The structure characterization of both NOS-V1 and NOS-V2 supported the notion that NocV acts as a cytochrome P450 oxygenase that catalyzes the two-step oxidation of Glu6 and the indolic moiety to form a hydroxyl group and an ether bond. However, the catalytic logic of NocV in NOC biosynthesis remained unclear. We thus overexpressed and purified NocV along with another cytochrome P450 protein, NocB (Fig. S8†), whose counterpart in NOS biosynthesis has been characterized for in vitro assays.17 NocV and NocB, in the C-terminally 6× His-tagged form, showed typical characteristics of cytochrome P450 proteins in CO binding difference spectra (Fig. S9†). Although three different electron transfer systems were tested, the in vitro activity of NocV could not be detected, using either NOS1260 or NOS-V1 as the substrate (Fig. S10†). As a reference, NocB was capable of catalyzing the hydroxylation of Glu6 at Cγ, using NOS1260, NOS-V1, and NOS-V2 as the substrates (Fig. S10†). These results suggest that NocV might not match the provided electron transfer systems; however, the possibility that NOS1260 and NOS-V1 are not the real substrates of NocV cannot be excluded at this time. NocV may catalyze the oxidation reaction in an LP-dependent manner that occurs in the early stage of PTMs. Based on existing precedents of cytochrome P450 proteins, such as those in the biosynthesis of aureothin,18,19 platensimycin,20 and paspalicine,21 we proposed an enzymatic mechanism for the NocV-catalyzed ether bond formation. First, NocV functions as a hydroxylase to oxygenate the residue Glu6 in a stereoselective manner. Then, NocV abstracts a hydrogen atom from the C4 indole methyl group, giving rise to a carbocation. Eventually, NOS-V2 is produced via a cyclization by coupling the Glu6 α-hydroxyl and the indole carbocation. Alternatively, the ring closure might occur through nucleophilic substitution via a bis-hydroxylated intermediate (Fig. 4).
NOC-I exhibits significantly higher biological activity than NOS.8 This is undoubtedly attributed to the glycosylation modification. Meanwhile, it may also be due to the contribution of the side-ring ether bond that endows molecular rigidity. NOS-V1, NOS-V2, NOS, and NOS1260 were thus evaluated for their activities against a panel of oral pathogens, including Gram-positive cariogenic bacteria (Streptococcus mutans, Lactobacillus acidophilus, Actinomyces viscosus, Enterococcus faecalis) and Gram-negative periodontal bacteria (Fusobacterium nucleatum, Porphyromonas gingivalis) (Table 1). Compared with NOS1260, NOS-V2 showed markedly improved activity against Streptococcus mutans, Actinomyces viscosus, Enterococcus faecalis, and Porphyromonas gingivalis with significantly reduced minimum inhibitory concentration (MIC) values. NOS-V2 presents a side-ring ether bond that distinguished it from NOS1260, indicating that the increase in molecular rigidity has a positive impact on the biological activity of NOC-I. Among the tested compounds, NOS exhibited the best antibacterial activity in most cases, suggesting that the PTMs in NOS biosynthesis, including hydroxylation of the NOS framework and removal of one Dha tail, were of great significance for biological activity.
| Oral pathogen | MIC (μg mL−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| NOS1260 | NOS-V1 | NOS-V2 | NOS | NaF | CHX | LVX | MCC | |
| Streptococcus mutans UA159 | 0.052 | 0.065 | 0.039 | 0.009 | 3000 | 1.66 | n.d. | n.d. |
| Lactobacillus acidophilus ATCC 4356 | 0.026 | 0.104 | 0.032 | 0.032 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.5 | n.d. | n.d. |
| Actinomyces viscosus ATCC 19246 | 0.039 | 0.104 | 0.005 | 0.001 | 4000 | 1.25 | n.d. | n.d. |
| Enterococcus faecalis ATCC 29212 | 0.312 | 0.729 | 0.032 | 0.104 | >12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5 | n.d. | n.d. |
| Fusobacterium nucleatum ATCC 25286 | 10 | 10 | 10 | 5 | n.d. | n.d. | 1.25 | 0.13 |
| Porphyromonas gingivalis ATCC 33277 | 0.208 | 0.417 | 0.078 | 0.020 | n.d. | n.d. | 0.208 | 0.13 |
Conclusions
Benefiting from the high similarity of the NOC and NOS biosynthetic machinery,9,10 we studied the in vivo catalytic function of the cytochrome P450 oxygenase NocV in an engineered NOS-producing strain. Structure elucidation of the resultant products revealed the generation of two new compounds, NOS-V1 and NOS-V2, with a hydroxyl group and an ether bond in the side ring of the NOS framework, respectively. Although the in vitro enzymatic activity assays did not determine the real substrate of NocV, we can speculate that NocV catalyzes a two-step oxidation of Glu6 and the indolic moiety. NOS-V2, containing an ether bond in the side ring, showed better activity against oral pathogens than its parental molecule, indicating the importance of molecular rigidity in the biological activities of natural products. Studies on NOC biosynthesis have been greatly limited due to the resistance of the producing strain to the available genetic manipulation approaches.9 Here, we revealed the catalytic function of NocV, filling in a blank in NOC biosynthetic research. Development of thiopeptides into oral medicines could effectively avoid problems such as poor water solubility and low bioavailability. Therefore, advances in biosynthetic studies of NOC-I will promote application of thiopeptides as dental drugs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by grants from the National Key Research and Development Program of China (grant no. 2018YFA0901903), the National Natural Science Foundation of China (81974495, 31430005, 21750004, and 21520102004), Chinese Academy of Sciences (QYZDJ-SSW-SLH037 and XDB20020200), Science and Technology Commission of Shanghai Municipality (17JC1405100), Youth Innovation Promotion Association of the Chinese Academy of Sciences (2017303), and K. C. Wong Education Foundation.References
- P. G. Arnison, M. J. Bibb, G. Bierbaum, A. A. Bowers, T. S. Bugni, G. Bulaj, J. A. Camarero, D. J. Campopiano, G. L. Challis, J. Clardy, P. D. Cotter, D. J. Craik, M. Dawson, E. Dittmann, S. Donadio, P. C. Dorrestein, K.-D. Entian, M. A. Fischbach, J. S. Garavelli, U. Goeransson, C. W. Gruber, D. H. Haft, T. K. Hemscheidt, C. Hertweck, C. Hill, A. R. Horswill, M. Jaspars, W. L. Kelly, J. P. Klinman, O. P. Kuipers, A. J. Link, W. Liu, M. A. Marahiel, D. A. Mitchell, G. N. Moll, B. S. Moore, R. Mueller, S. K. Nair, I. F. Nes, G. E. Norris, B. M. Olivera, H. Onaka, M. L. Patchett, J. Piel, M. J. T. Reaney, S. Rebuffat, R. P. Ross, H.-G. Sahl, E. W. Schmidt, M. E. Selsted, K. Severinov, B. Shen, K. Sivonen, L. Smith, T. Stein, R. D. Suessmuth, J. R. Tagg, G.-L. Tang, A. W. Truman, J. C. Vederas, C. T. Walsh, J. D. Walton, S. C. Wenzel, J. M. Willey and W. A. van der Donk, Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature, Nat. Prod. Rep., 2013, 30, 108–160 RSC.
- Q. Zhang and W. Liu, Biosynthesis of thiopeptide antibiotics and their pathway engineering, Nat. Prod. Rep., 2013, 30, 218–226 RSC.
- X. Just-Baringo, F. Albericio and M. Alvarez, Thiopeptide Engineering: A Multidisciplinary Effort towards Future Drugs, Angew. Chem., Int. Ed., 2014, 53, 6602–6616 CrossRef CAS PubMed.
- J. Wang, Z. Lin, X. Bai, J. Tao and W. Liu, Optimal design of thiostrepton-derived thiopeptide antibiotics and their potential application against oral pathogens, Org. Chem. Front., 2019, 6, 1194–1199 RSC.
- T. Sasaki, T. Otani, H. Matsumoto, N. Unemi, M. Hamada, T. Takeuchi and M. Hori, MJ347-81F4 A & B, Novel Antibiotics from Amycolatopsis, sp.: Taxonomic Characteristics, Fermentation, and Antimicrobial Activity, J. Antibiot., 1998, 51, 715–721 CrossRef CAS PubMed.
- F. Benazet, M. Cartier, J. Florent, C. Godard, G. Jung, J. Lunel, D. Mancy, C. Pascal, J. Renaut, P. Tarridec, J. Theilleux, R. Tissier, M. Dubost and L. Ninet, Nosiheptide, a sulfur-containing peptide antibiotic isolated from Streptomyces actuosus, 40037, Experientia, 1980, 36, 414–416 CrossRef CAS PubMed.
- J. E. Leet, W. Y. Li, H. A. Ax, J. A. Matson, S. Huang, R. Huang, J. L. Cantone, D. Drexler, R. A. Dalterio and K. S. Lam, Nocathiacins, New Thiazolyl Peptide Antibiotics from Nocardia sp. II. Isolation, Characterization, and Structure Determination, J. Antibiot., 2003, 56, 232–242 CrossRef CAS PubMed.
- W. Y. Li, J. E. Leet, H. A. Ax, D. R. Gustavson, D. M. Brown, L. Turner, K. Brown, J. Clark, H. Yang, J. Fung-Tomc and K. S. Lam, Nocathiacins, New Thiazolyl Peptide Antibiotics from Nocardia sp. I. Taxonomy, Fermentation and Biological Activities, J. Antibiot., 2003, 56, 226–231 CrossRef CAS PubMed.
- Y. Ding, Y. Yu, H. Pan, H. Guo, Y. Li and W. Liu, Moving posttranslational modifications forward to biosynthesize the glycosylated thiopeptide nocathiacin I in Nocardia sp. ATCC202099, Mol. Biosyst., 2010, 6, 1180–1185 RSC.
- Y. Yu, L. Duan, Q. Zhang, R. Liao, Y. Ding, H. Pan, E. Wendt-Pienkowski, G. Tang, B. Shen and W. Liu, Nosiheptide Biosynthesis Featuring a Unique Indole Side Ring Formation on the Characteristic Thiopeptide Framework, ACS Chem. Biol., 2009, 4, 855–864 CrossRef CAS PubMed.
- Y. Yu, H. Guo, Q. Zhang, L. Duan, Y. Ding, R. Liao, C. Lei, B. Shen and W. Liu, NosA Catalyzing Carboxyl-Terminal Amide Formation in Nosiheptide Maturation via an Enamine Dealkylation on the Serine-Extended Precursor Peptide, J. Am. Chem. Soc., 2010, 132, 16324–16326 CrossRef CAS PubMed.
- G. Sicoli, J.-M. Mouesca, L. Zeppieri, P. Amara, L. Martin, A.-L. Barra, J. C. Fontecilla-Camps, S. Gambarelli and Y. Nicolet, Fine-tuning of a radical-based reaction by radical S-adenosyl-L-methionine tryptophan lyase, Science, 2016, 351, 1320–1323 CrossRef CAS PubMed.
- Q. Zhang, Y. Li, D. Chen, Y. Yu, L. Duan, B. Shen and W. Liu, Radical-mediated enzymatic carbon chain fragmentation-recombination, Nat. Chem. Biol., 2011, 7, 154–160 CrossRef CAS PubMed.
- J. W. LaMattina, B. Wang, E. D. Badding, L. K. Gadsby, T. L. Grove and S. J. Booker, NosN, a Radical S-Adenosylmethionine Methylase, Catalyzes Both C1 Transfer and Formation of the Ester Linkage of the Side-Ring System during the Biosynthesis of Nosiheptide, J. Am. Chem. Soc., 2017, 139, 17438–17445 CrossRef CAS PubMed.
- Y. Qiu, Y. Du, F. Zhang, R. Liao, S. Zhou, C. Peng, Y. Guo and W. Liu, Thiolation Protein-Based Transfer of Indolyl to a Ribosomally Synthesized Polythiazolyl Peptide Intermediate during the Biosynthesis of the Side-Ring System of Nosiheptide, J. Am. Chem. Soc., 2017, 139, 18186–18189 CrossRef CAS PubMed.
- Y. Qiu, Y. Du, S. Wang, S. Zhou, Y. Guo and W. Liu, Radical S-Adenosylmethionine Protein NosN Forms the Side Ring System of Nosiheptide by Functionalizing the Polythiazolyl Peptide S-Conjugated Indolic Moiety, Org. Lett., 2019, 21, 1502–1505 CrossRef CAS PubMed.
- W. Liu, Y. Xue, M. Ma, S. Wang, N. Liu and Y. Chen, Multiple Oxidative Routes towards the Maturation of Nosiheptide, ChemBioChem, 2013, 14, 1544–1547 CrossRef CAS PubMed.
- J. He, M. Muller and C. Hertweck, Formation of the Aureothin Tetrahydrofuran Ring by a Bifunctional Cytochrome P450 Monooxygenase, J. Am. Chem. Soc., 2004, 126, 16742–16743 CrossRef CAS PubMed.
- G. Zocher, M. E. A. Richter, U. Mueller and C. Hertweck, Structural Fine-Tuning of a Multifunctional Cytochrome P450 Monooxygenase, J. Am. Chem. Soc., 2011, 133, 2292–2302 CrossRef CAS PubMed.
- J. D. Rudolf, L.-B. Dong, K. Manoogian and B. Shen, Biosynthetic Origin of the Ether Ring in Platensimycin, J. Am. Chem. Soc., 2016, 138, 16711–16721 CrossRef CAS PubMed.
- M. J. Nicholson, A. Koulman, B. J. Monahan, B. L. Pritchard, G. A. Payne and B. Scott, Identification of Two Aflatrem Biosynthesis Gene Loci in Aspergillus flavus and Metabolic Engineering of Penicillium paxilli, To Elucidate Their Function, Appl. Environ. Microbiol., 2009, 75, 7469–7481 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qo01328h |
| ‡ These authors equally contributed to this work. |
| This journal is © the Partner Organisations 2020 |



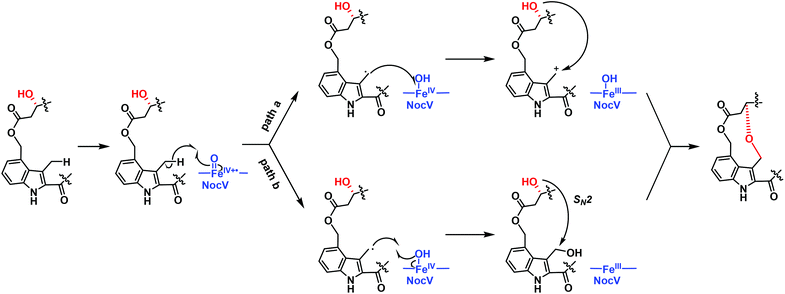
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O radical cation to form an allyl radical and an FeIV–OH species. Through path a, the FeIV–OH species reduced the allyl radical to an allyl cation, which was then captured by the hydroxyl group and formed the ether bond. Through path b, the allyl radical abstracted the hydroxyl radical to form a dihydroxyl group intermediate and an FeIII species. The α-OH substituted the newly formed hydroxyl to construct the ether bond.
O radical cation to form an allyl radical and an FeIV–OH species. Through path a, the FeIV–OH species reduced the allyl radical to an allyl cation, which was then captured by the hydroxyl group and formed the ether bond. Through path b, the allyl radical abstracted the hydroxyl radical to form a dihydroxyl group intermediate and an FeIII species. The α-OH substituted the newly formed hydroxyl to construct the ether bond.