Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advances in optical and electrochemical techniques for biomedical imaging
Yi-Tao
Long
a and
Thomas J.
Meade
b
aNanjing University, China
bNorthwestern University, USA
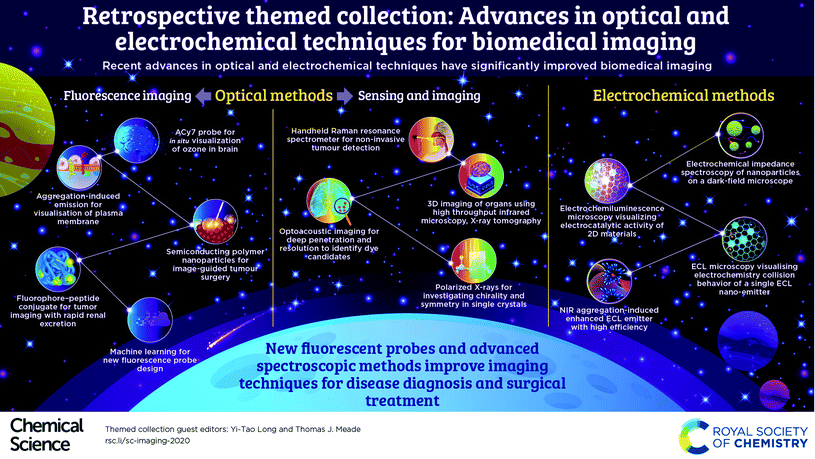
Optical methods serve as widely practicable techniques for biological imaging. Fluorescence in the NIR region (>700 nm) is superior in biomedical sensing due to the high penetration depths and low autofluorescence interference. To make this technique more applicable for in vivo detection, novel biocompatible fluorescent probes with high selectivity and sensitivity are urgently needed in this field. This motivation drives various new strategies in molecular design.
For example, a small molecule NIR fluorescent probe, ACy7, was designed for the in situ visualization of ozone in the brains of mice, which incorporates a Cy7-like molecule as the precursor of the fluorophore and 3-butenyl as the recognition group.5 The plasma membrane could be specifically imaged by a water-soluble near-infrared (NIR)-emissive fluorescent molecule with aggregation-induced emission (AIE).6 This fluorescent “light-up” probe allows a short staining period (at the second-level) with a wash-free process. Further, a fluorophore–peptide conjugate was developed for tumor imaging in the “transparent” near-infrared II (NIR-II) window with rapid renal excretion and low off-target tissue exposure.7 Meanwhile, a semiconducting polymer nanoparticle is designed for efficient NIR-II image-guided tumor surgery by using multiple pathological models.8 These methods promote clinical translation in disease diagnosis and surgical treatment. We are pleased to see that machine learning has been involved in predicting material properties,9 and could be expected to facilitate the design of novel fluorescence probes.
In addition, new highly sensitive optical methods have been developed for sensing and imaging. For example, a unique hand-held surface enhanced spatially offset resonance Raman spectroscopy (SESORRS) has been reported. This technique has the ability to yield enhanced Raman signals at a far greater sub-surface level for non-invasive detection of cancerous tumors.10 To achieve 3D chemical imaging of a complete organ, a high throughput infrared microscopy method was established by combining X-ray tomography and subsequent data analysis.11 Moreover, optoacoustic imaging has been proven to be a new sensing method capable of deep penetration with a higher spatial resolution compared to fluorescence imaging. In addition, a library screening approach was utilized to identify and evaluate the available dyes for multi-spectral optoacoustic tomography (MSOT) imaging.12 Regarding the study of enantiomers, polarized X-rays provide an original approach for the investigation of chirality and symmetry in single crystals. This flexible technique based on an anion exchange strategy was proposed for the enantiomeric resolution of an extended metal atom chain (EMAC).13
To promote the fundamental understanding of single entities, electrochemistry shows remarkable advances in the development of various electrochemical microcopy techniques. Specifically, the electrochemical impedance spectroscopy of individual nanoparticles was imaged on a dark-field microscope with an optical-to-electrochemical conversion.14 Accordingly, the electrocatalytic activity of 2D materials could be imaged directly by electrochemiluminescence microscopy (ECL), presenting nonuniform ECL distribution at single particles.15 ECL microscopy could facilitate the imaging and study of electrochemistry collision behavior of single ECL nano-emitters.16 Since the design of new ECL emitters has attracted growing interest, a new near-infrared aggregation-induced enhanced ECL emitter has been developed that shows high ECL efficiency and excellent biocompatibility.17 The thriving research on nanoscale electrochemistry will likely accelerate the exploration of new chemistry through single entities.
As guest editors of this themed collection, we thank all the authors for their outstanding contributions. We have brought together high-quality articles on the theme of imaging, especially optical and electrochemical imaging, in this themed collection. We hope researchers from various fields will enjoy examining this themed collection.
References
- F. Ding, Y. Zhan, X. Lu and Y. Sun, Chem. Sci., 2018, 9, 4370–4380 RSC
.
- R. Chouket, A. Pellissier-Tanon, A. Lemarchand, A. Espagne, T. Le Saux and L. Jullien, Chem. Sci., 2020, 11, 2882–2887 RSC
.
- A. I. Pérez-Jiménez, D. Lyu, Z. Lu, G. Liu and B. Ren, Chem. Sci., 2020, 11, 4563–4577 RSC
.
- T.-E. Lin, S. Rapino, H. H. Girault and A. Lesch, Chem. Sci., 2018, 9, 4546–4554 RSC
.
- P. Li, J. Wang, X. Wang, Q. Ding, X. Bai, Y. Zhang, D. Su, W. Zhang, W. Zhang and B. Tang, Chem. Sci., 2019, 10, 2805–2810 RSC
.
- D. Wang, H. Su, R. T. K. Kwok, X. Hu, H. Zou, Q. Luo, M. M. S. Lee, W. Xu, J. W. Y. Lam and B. Z. Tang, Chem. Sci., 2018, 9, 3685–3693 RSC
.
- R. Tian, H. Ma, Q. Yang, H. Wan, S. Zhu, S. Chandra, H. Sun, D. O. Kiesewetter, G. Niu, Y. Liang and X. Chen, Chem. Sci., 2019, 10, 326–332 RSC
.
- K. Shou, Y. Tang, H. Chen, S. Chen, L. Zhang, A. Zhang, Q. Fan, A. Yu and Z. Cheng, Chem. Sci., 2018, 9, 3105–3110 RSC
.
- X. Zheng, P. Zheng and R.-Z. Zhang, Chem. Sci., 2018, 9, 8426–8432 RSC
.
- F. Nicolson, L. E. Jamieson, S. Mabbott, K. Plakas, N. C. Shand, M. R. Detty, D. Graham and K. Faulds, Chem. Sci., 2018, 9, 3788–3792 RSC
.
- A. Ogunleke, B. Recur, H. Balacey, H.-H. Chen, M. Delugin, Y. Hwu, S. Javerzat and C. Petibois, Chem. Sci., 2018, 9, 189–198 RSC
.
- S. Roberts, C. Andreou, C. Choi, P. Donabedian, M. Jayaraman, E. C. Pratt, J. Tang, C. Pérez-Medina, M. J. de la Cruz, W. J. M. Mulder, J. Grimm, M. Kircher and T. Reiner, Chem. Sci., 2018, 9, 5646–5657 RSC
.
- A. Srinivasan, M. Cortijo, V. Bulicanu, A. Naim, R. Clérac, P. Sainctavit, A. Rogalev, F. Wilhelm, P. Rosa and E. A. Hillard, Chem. Sci., 2018, 9, 1136–1143 RSC
.
- T. Liu, M. Li, Y. Wang, Y. Fang and W. Wang, Chem. Sci., 2018, 9, 4424–4429 RSC
.
- M.-M. Chen, W. Zhao, M.-J. Zhu, X.-L. Li, C.-H. Xu, H.-Y. Chen and J.-J. Xu, Chem. Sci., 2019, 10, 4141–4147 RSC
.
- C. Ma, W. Wu, L. Li, S. Wu, J. Zhang, Z. Chen and J.-J. Zhu, Chem. Sci., 2018, 9, 6167–6175 RSC
.
- J.-L. Liu, J.-Q. Zhang, Z.-L. Tang, Y. Zhuo, Y.-Q. Chai and R. Yuan, Chem. Sci., 2019, 10, 4497–4501 RSC
.
| This journal is © The Royal Society of Chemistry 2020 |