Open Access Article
Open Access ArticleFurther synthetic investigation of the general lanthanoid(III) [Ln(III)]/copper(II)/pyridine-2,6-dimethanol/carboxylate reaction system: {CuII5LnIII4} coordination clusters (Ln = Dy, Tb, Ho) and their yttrium(III) analogue†
Despina
Dermitzaki
ab,
Catherine P.
Raptopoulou
 b,
Vassilis
Psycharis
b,
Vassilis
Psycharis
 b,
Albert
Escuer
b,
Albert
Escuer
 c,
Spyros P.
Perlepes
*ad,
Julia
Mayans
c,
Spyros P.
Perlepes
*ad,
Julia
Mayans
 *e and
Theocharis C.
Stamatatos
*e and
Theocharis C.
Stamatatos
 *ad
*ad
aDepartment of Chemistry, University of Patras, 26504 Patras, Greece. E-mail: perlepes@upatras.gr; thstama@upatras.gr; Tel: +30 2610 996730 Tel: +30 2610 997732
bInstitute of Nanoscience and Nanotechnology, NCSR “Demokritos”, 15310 Aghia Paraskevi Attikis, Greece
cDepartament de Química Inorgànica i Orgànica, Secció Inorgànica and Institut de Nanociència i Nanotecnologia (IN2UB), Universitat de Barcelona, Martí i Franquès 1-11, 08028-Barcelona, Spain
dInstitute of Chemical Engineering Sciences, Foundation for Research and Technology – Hellas (FORTH/ICE – HT), Platani, P.O. Box 1414, 26504, Patras, Greece
eInstituto de Ciencia Molecular (ICMol), Universidad de Valencia, Catedrático José Beltran 2, 46980 Paterna, Spain. E-mail: julia.mayans@uv.es
First published on 25th November 2020
Abstract
In addition to previously studied {CuII3Gd6}, {CuII8Gd4}, {CuII15Ln7} and {CuII4Ln8} coordination clusters (Ln = trivalent lanthanide) containing pdm2− or Hpdm− ligands (H2pdm = pyridine-2,6-dimethanol) and ancillary carboxylate groups (RCO2−), the present work reports the synthesis and study of three new members of a fifth family of such complexes. Compounds [Cu5Ln4O2(OMe)4(NO3)4(O2CCH2But)2(pdm)4(MeOH)2] (Ln = Dy, 1; Ln = Tb, 2; Ln = Ho, 3) were prepared from the reaction of Ln(NO3)3·xH2O (x = 5, 6), CuX2·yH2O (X = ClO4, Cl, NO3; y = 6, 2 and 3, respectively), H2pdm, ButCH2CO2H and Et3N (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) in MeCN/MeOH. Rather surprisingly, the copper(II)/yttrium(III) analogue has a slightly different composition, i.e. [Cu5Y4O2(OMe)4(NO3)2(O2CCH2But)4(pdm)4(MeOH)2] (4). The structures of 1·4MeCN·1.5MeOH and 4·2MeOH were solved by single-crystal X-ray crystallography. The five CuII and four DyIII centres in 1 are held together by two μ5-O2−, four μ-MeO−, two syn,syn η1:η1:μ ButCH2CO2−, four η2:η1:η2:μ3 pdm2− (each of these groups chelates a CuII atom and simultaneously bridges two DyIII atoms through its two –CH2O− arms) and two μ-MeOH ligands. The four terminal nitrato groups each chelate (η1:η1) a DyIII centre. The five CuII atoms are co-planar (by symmetry) forming a bow-tie arrangement; the four outer CuII atoms form a rectangle with edges of 3.061(1) and 6.076(1) Å. The four DyIII centres also form a rectangle that lies above and below the plane of the CuII centres, with edges of 3.739(1) and 5.328(1) Å. The two strictly planar rectangles are almost perpendicular. Two trigonal bipyramidal μ5-O2− groups link the perpendicular Cu5 and Dy4 frameworks together. The molecule 4 has a very similar structure to that of 1, differences being the replacement of the two chelating nitrato groups of 1 by two chelating ButCH2CO2− ligands in 4 and the coordination polyhedra of the LnIII and YIII atoms (Snub diphenoids in 1 and biaugmented trigonal prisms in 4). Dc magnetic susceptibility data (χM) on analytically pure samples of 1–3, collected in the 300–2 K range, indicate that ferromagnetic exchange interactions dominate leading to large spin ground states. The χMT vs. T data for 4 suggest moderately strong antiferromagnetic CuII⋯CuII exchange interactions. Studies of the dynamic magnetic properties of the {Cu5Ln4} clusters show that 1 behaves as a SMM at zero field and 2 is a very weak field-induced SMM, while 3 exhibits only weak tails in the χ′′Mvs. T plots at various ac frequencies at zero dc field.
9) in MeCN/MeOH. Rather surprisingly, the copper(II)/yttrium(III) analogue has a slightly different composition, i.e. [Cu5Y4O2(OMe)4(NO3)2(O2CCH2But)4(pdm)4(MeOH)2] (4). The structures of 1·4MeCN·1.5MeOH and 4·2MeOH were solved by single-crystal X-ray crystallography. The five CuII and four DyIII centres in 1 are held together by two μ5-O2−, four μ-MeO−, two syn,syn η1:η1:μ ButCH2CO2−, four η2:η1:η2:μ3 pdm2− (each of these groups chelates a CuII atom and simultaneously bridges two DyIII atoms through its two –CH2O− arms) and two μ-MeOH ligands. The four terminal nitrato groups each chelate (η1:η1) a DyIII centre. The five CuII atoms are co-planar (by symmetry) forming a bow-tie arrangement; the four outer CuII atoms form a rectangle with edges of 3.061(1) and 6.076(1) Å. The four DyIII centres also form a rectangle that lies above and below the plane of the CuII centres, with edges of 3.739(1) and 5.328(1) Å. The two strictly planar rectangles are almost perpendicular. Two trigonal bipyramidal μ5-O2− groups link the perpendicular Cu5 and Dy4 frameworks together. The molecule 4 has a very similar structure to that of 1, differences being the replacement of the two chelating nitrato groups of 1 by two chelating ButCH2CO2− ligands in 4 and the coordination polyhedra of the LnIII and YIII atoms (Snub diphenoids in 1 and biaugmented trigonal prisms in 4). Dc magnetic susceptibility data (χM) on analytically pure samples of 1–3, collected in the 300–2 K range, indicate that ferromagnetic exchange interactions dominate leading to large spin ground states. The χMT vs. T data for 4 suggest moderately strong antiferromagnetic CuII⋯CuII exchange interactions. Studies of the dynamic magnetic properties of the {Cu5Ln4} clusters show that 1 behaves as a SMM at zero field and 2 is a very weak field-induced SMM, while 3 exhibits only weak tails in the χ′′Mvs. T plots at various ac frequencies at zero dc field.
Introduction
The chemistry of mixed 3d/4f-metal molecular compounds and materials is not new.1–7 The synthesis of 3d/4f-metal complexes has been a “hot” topic in molecular inorganic chemistry because of their potential applications in several fields such as luminescence,8,9 near-IR chiroptical sensors,10 non-linear optical materials,11 molecular adsorption,12 catalysis13 and various aspects of Molecular Magnetism.14–24In the beginning of the present century, there was an intense re-ignition of research interest in the synthesis of polynuclear (or polymetallic) coordination clusters containing both 3d- and 4f-metal ions. The intense activity originated from two major sources: single-molecule magnetism25,26 and molecular cooling;27,28 we briefly comment only on the former, because this area is related to the present work. The discovery in the early 1990s that well-isolated magnetic molecules containing 3d-metal ions can exhibit slow paramagnetic relaxation reminiscent of single-domain magnetic particles27–30 sparked an explosive interest in the magnetism community. The so named Single-Molecule Magnets (SMMs) were originally polynuclear 3d-metal clusters that exhibit a large overall ground-state spin value and a significant uniaxial magnetic anisotropy.25,31 Until ∼2000, the search for new examples of SMMs focused mainly on clusters containing 3d-metal ions. Since the barrier to magnetization reversal (Ueff) is related to both the overall spin and the magnitude of the anisotropy in the cluster, researchers began slowly after 2000 to investigate the incorporation of LnIII ions in such systems,32–35 since they often have high spin as well as significant anisotropy arising from strong spin–orbit contributions. In addition, the 3d–4f exchange interactions (the study of which has been pioneered by the Gatteschi and Winpenny groups) are stronger than the 4f–4f ones suppressing quantum tunneling of magnetization. Thus, much of the current polynuclear SMM research has been shifted toward 3d/4f-metal clusters and hundreds of such SMMs have been prepared and characterized.36–42 The most preferred LnIII ions for such studies are TbIII, DyIII, HoIII and ErIII, since it has been shown that mononuclear complexes containing these LnIII ions can display hysteresis loops in magnetization vs. field studies.34
From the synthetic inorganic chemistry viewpoint, methods to combine 3d- and 4f-metal ions within a coordination cluster are highly desirable. There is one strategy and one more empirical route for the synthesis of 3d/4f-metal clusters. The strategy is based on the “metal complexes as ligands” approach.43–45 Mononuclear or dinuclear 3d-metal complexes with uncoordinated O-donor groups can be used as starting materials; such complexes can be considered as “ligands” (metalloligands) and further react with the strongly oxophilic LnIII (Ln = lanthanoid) ions. Alternatively, the metalloligands can be mononuclear or dinuclear LnIII complexes with uncoordinated N-donor sites which further react with the 3d-metal ions. The route which is most often used, is based on “one-pot” procedures.43–45 These require a mixture of the appropriate 3d- and 4f-metal “salts” (usually with inorganic anions, e.g. Cl−, NO3−, ClO4−, BF4−, CF3SO3−,…) and a carefully selected organic ligand possessing distinct coordination compartments (“pockets”) for preferential binding of the 3d- and the 4f-metal ion. Sometimes the 3d-metal ions are used in the form of small clusters to ensure high nuclearity in the final heterometallic products. A variation of the “one-pot” approach is the “assisted self-assembly” when the introduction of a second suitable organic co-ligand (e.g. a simple carboxylate group) is essential to assist the self-assembly process and often to increase the nuclearity of the heterometallic cluster.36 Primary organic ligands used include polydentate Schiff bases, oximes, 2-pyridyl alcohols, amino polyalcohols, pyridylcarbonyl amines and amino acids.36–45 The Hard–Soft Acid–Base (HSAB) model plays an important role in the “one-pot” route facilitating selective heterometallic coordination.46 For example, LnIII ions are hard acids, whereas the late divalent 3d metals (e.g. CoII, NiII, CuII) are borderline acids; thus, the former can bind strongly to hard O-donors, while the latter prefer the less hard N-sites or purely soft bases.45
We have been involved in a research programme aiming to prepare, characterize and study the magnetic properties of CuII/LnIII coordination clusters. Such clusters currently attract the intense interest of the inorganic chemistry community.47–50 We have been using the “assisted self-assembly” variation of the “one-pot” approach by employing pyridine-2,6-dimethanol (H2pdm) as the primary organic ligand and simple carboxylate ions (RCO2−) as co-ligands.51,52 The anionic forms of H2pdm are well-explored ligands in transition-metal53–56 and lanthanoid(III)57–59 cluster chemistry, having yielded complexes with aesthetically pleasing structures and interesting properties. On the contrary, their use in 3d/4f-metal chemistry has been limited.51,52,60–64 The tridentate pdm2− anion is not a compartmental ligand. However, it provides a stable tridentate chelating O,N,O environment to CuII (formation of two 5-membered chelating rings), while each of the deprotonated alkoxide O atoms can further bridge a LnIII centre (Scheme 1). Following on our previous efforts, which led to {CuII15LnIII7} and {CuII4LnIII8} clusters with the pdm2−/RCO2− ligation, we report here the synthesis and study of {CuII5LnIII4} complexes (Ln = Tb, Dy, Ho) along with their {CuII5YIII4} analogue.
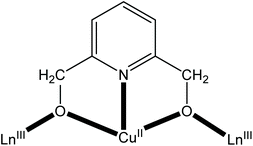 | ||
| Scheme 1 The anticipated coordination mode of the doubly deprotonated pyridine-2,6-dimethanol (pdm2−) ligand in CuII/LnIII clusters. | ||
Results and discussion
Synthetic comments and IR characterization
Most of the to-date reported 3d/4f-metal complexes containing the doubly (pdm2−) or singly (Hpdm−) form of H2pdm as ligand are CuII/LnIII clusters.51,52,63,64 A common feature of all such complexes is that they possess a secondary (ancillary) carboxylate ligand. The general CuII/LnIII/H2pdm/RCO2− reaction system is very fertile, and the identity of products has been found to depend on a number of synthetic parameters, the most important of which is the nature of R. Using rather similar reaction conditions (MeOH, MeOH/MeCN or MeOH/CH2Cl2 as solvent, Et3N as base, RCO2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2pdm reaction ratios equal to or higher than 1), our51,52 and other63,64 groups have isolated and studied four families of clusters. When R = But (i.e. the pivalate ion), the nonanuclear [Cu3Gd6(OH)(CO3)4(O2CBut)9(pdm)3(MeOH)3]64 and [Cu8Gd4(OH)8(O2CBut)8(Hpdm)8](ClO4)4
H2pdm reaction ratios equal to or higher than 1), our51,52 and other63,64 groups have isolated and studied four families of clusters. When R = But (i.e. the pivalate ion), the nonanuclear [Cu3Gd6(OH)(CO3)4(O2CBut)9(pdm)3(MeOH)3]64 and [Cu8Gd4(OH)8(O2CBut)8(Hpdm)8](ClO4)4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 complexes were prepared; for the isolation of the former, CO2 gas was bubbled through the reaction solution. For R = Ph, the cage-like clusters [Cu15Ln7(OH)6(CO3)4(O2CPh)19(pdm)9(H2pdm)3(H2O)2] (Ln = Gd, Dy) were obtained;51 the CO32− ions were derived from the fixation of atmospheric CO2 under the basic conditions. In an attempt to further investigate this general reaction system, we used the tert-butylacetate (ButCH2CO2−) ion which had not been previously employed in 3d/4f-metal cluster chemistry; the result52 was the isolation of members of the [Cu4Ln8(OH)6(NO3)2(O2CCH2But)16(pdm)4] (Ln = La, Gd, Tb, Dy) family of dodecanuclear clusters.
63 complexes were prepared; for the isolation of the former, CO2 gas was bubbled through the reaction solution. For R = Ph, the cage-like clusters [Cu15Ln7(OH)6(CO3)4(O2CPh)19(pdm)9(H2pdm)3(H2O)2] (Ln = Gd, Dy) were obtained;51 the CO32− ions were derived from the fixation of atmospheric CO2 under the basic conditions. In an attempt to further investigate this general reaction system, we used the tert-butylacetate (ButCH2CO2−) ion which had not been previously employed in 3d/4f-metal cluster chemistry; the result52 was the isolation of members of the [Cu4Ln8(OH)6(NO3)2(O2CCH2But)16(pdm)4] (Ln = La, Gd, Tb, Dy) family of dodecanuclear clusters.
In the above mentioned families of clusters that contain the doubly deprotonated pdm2− ligand, the RCO2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pdm2− ratio in the formulae of the complexes is higher than 2, i.e. 3 in the {Cu3Gd6} complex, 4 in the {Cu4Ln8} family and ∼2 in the {Cu15Ln7} clusters. We suspected that clusters with more pdm2− than RCO2− groups might be capable of existence and we set out experiments to prepare such products. Below we describe the realisation of this goal which provided access to a family of {Cu5Ln4} clusters (Ln = Tb, Dy, Ho) containing an 1
pdm2− ratio in the formulae of the complexes is higher than 2, i.e. 3 in the {Cu3Gd6} complex, 4 in the {Cu4Ln8} family and ∼2 in the {Cu15Ln7} clusters. We suspected that clusters with more pdm2− than RCO2− groups might be capable of existence and we set out experiments to prepare such products. Below we describe the realisation of this goal which provided access to a family of {Cu5Ln4} clusters (Ln = Tb, Dy, Ho) containing an 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 RCO2−
2 RCO2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pdm2− ratio.
pdm2− ratio.
The reaction of Dy(NO3)3·5H2O, Cu(ClO4)2·6H2O, H2pdm, ButCH2CO2H and Et3N in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 molar ratio in MeCN/MeOH led to a blue solution that upon storage at room temperature gave blue crystals of [Cu5Dy4O2(OMe)4(NO3)4(O2CCH2But)2(pdm)4(MeOH)2]·4MeCN·1.5MeOH (1·4MeCN·1.5MeOH) in ∼35% yield. The crystals were of X-ray quality and the structure of the cluster was solved by single-crystal X-ray crystallography. A point of interest is that the nature of the copper(II) source does not affect the product identity; employment of CuCl2·2H2O or Cu(NO3)2·3H2O gives again complex 1 in comparable yields, as evidenced by microanalyses and IR spectra. Completely analogous reactions with Tb(NO3)3·6H2O and Ho(NO3)3·5H2O, using chloride or nitrate or perchlorate copper(II) sources, led to crystals of the isomorphous complexes [Cu5Tb4O2(OMe)4(NO3)4(O2CCH2But)2(pdm)4(MeOH)2]·4MeCN·1.5MeOH (2·4MeCN·1.5MeOH) and [Cu5Ho4O2(OMe)4(NO3)4(O2CCH2But)2(pdm)4(MeOH)2]·4MeCN·1.5MeOH (3·4MeCN·1.5MeOH), respectively. The isomorphous character of the three complexes was confirmed by determining the unit cell dimensions for the {Cu5Tb4} and {Cu5Ho4} clusters (vide infra).
9 molar ratio in MeCN/MeOH led to a blue solution that upon storage at room temperature gave blue crystals of [Cu5Dy4O2(OMe)4(NO3)4(O2CCH2But)2(pdm)4(MeOH)2]·4MeCN·1.5MeOH (1·4MeCN·1.5MeOH) in ∼35% yield. The crystals were of X-ray quality and the structure of the cluster was solved by single-crystal X-ray crystallography. A point of interest is that the nature of the copper(II) source does not affect the product identity; employment of CuCl2·2H2O or Cu(NO3)2·3H2O gives again complex 1 in comparable yields, as evidenced by microanalyses and IR spectra. Completely analogous reactions with Tb(NO3)3·6H2O and Ho(NO3)3·5H2O, using chloride or nitrate or perchlorate copper(II) sources, led to crystals of the isomorphous complexes [Cu5Tb4O2(OMe)4(NO3)4(O2CCH2But)2(pdm)4(MeOH)2]·4MeCN·1.5MeOH (2·4MeCN·1.5MeOH) and [Cu5Ho4O2(OMe)4(NO3)4(O2CCH2But)2(pdm)4(MeOH)2]·4MeCN·1.5MeOH (3·4MeCN·1.5MeOH), respectively. The isomorphous character of the three complexes was confirmed by determining the unit cell dimensions for the {Cu5Tb4} and {Cu5Ho4} clusters (vide infra).
Yttrium has radii (atomic, metallic, ionic) that fall close to those of Er and Ho, and all of its chemistry is in the trivalent state.65 Hence it resembles the late lanthanides closely in its chemistry and occurs with them in nature. In the older literature in particular, it is not uncommon to find explicitly or implicitly the belief that an Y(III) complex of a given set of ligands will be isostructural with the corresponding late Ln(III) compounds. The test of this belief has been carried out for only a few complexes.66–68 Somewhat to our surprise, use of Y(NO3)3·6H2O instead of Ln(NO3)3·xH2O (Ln = Tb, Dy, Ho) in an otherwise identical reaction system, gave complex [Cu5Y4O2(OMe)4(NO3)2(O2CCH2But)4(pdm)4(MeOH)2]·2MeOH (4·2MeOH) which contains two nitrato (instead of four in 1–3) and four tert-butylacetato (instead of two in 1–3) ligands.
Description of structures
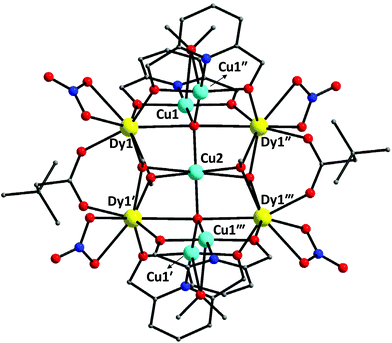
The structures of 1·4MeCN·1.5MeOH and 4·2MeOH were solved by single-crystal X-ray crystallography. Compound 1·4MeCN·1.5MeOH crystallizes in the monoclinic space group I2/m (Table S1†) with the asymmetric unit containing ¼ of the cluster [CuCu4*Dy4O2(OMe)4(NO3)4(O2CCH2But)2(pdm)4(MeOH)2] (Fig. 1 and S1†); the star (*) on the second CuII atom indicates that this cation occupies a site with different symmetry from the unstarred one. The point group symmetry is 2/m (C2h). The nonanuclear cluster molecule contains five CuII and four DyIII atoms. The core of the cluster, shown in Fig. 2, is {Cu5Dy4(μ5-O)2(μ3-OMeO−)4(μ-OMeOH)2(μ-OR′)8}6+, where R′ is the carbon/nitrogen/hydrogen containing part of pdm2−.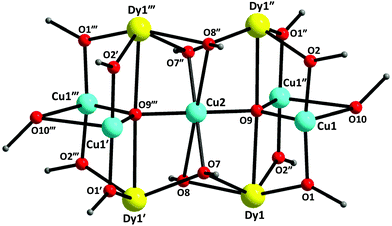 | ||
| Fig. 2 The {Cu5Dy4(μ5-O)2(μ3-OMeO−)4(μ-OMeOH)2(μ-OR′)8}6+ core of the cluster molecule 1. O9, O9′′′ represent the μ5-oxo groups; O7, O7′′, O8, O8′′ are the μ3-methoxo oxygen atoms; O10, O10′′′ denote the bridging methanol oxygen atoms; O1, O2 and their symmetry equivalents belong to the pdm2− ligands. The symmetry codes are the same with those defined in the caption of Fig. 1. | ||
Concerning the symmetry elements, the central CuII atom (Cu2) lies on 2/m site symmetry. The mirror plane possessed by the molecule is defined by Cu2, the four backbone carbon atoms (and their symmetric ones) that belong to the ButCH2CO2− ligands, and the four carbon and oxygen atoms of the methoxo groups (and their symmetry equivalents). The two-fold axis is defined by Cu2 and the two μ5-O2− atoms (O9, O9′′′).
The five CuII atoms are co-planar forming a bow-tie arrangement. The four outer CuII atoms (Cu1, Cu1′, Cu1′′, Cu1′′′) form a rectangle with edges of 3.061(1) and 6.076(1) Å; the central-to-outer Cu2–Cu(1,1′,1′′,1′′′) distance is 3.402(1) Å. The four DyIII atoms also form a rectangle (Fig. 3) that lies above and below the plane of the CuII centres, with edges of 3.739(1) and 5.328(1) Å; the diagonal of this rectangle is 6.509(1) Å. The two, strictly planar rectangles are almost perpendicular forming an angle of 89.0(1)°. The Cu⋯Dy distances between the outer CuII atoms and the DyIII centres are in the range 3.266(1)–5.778(1) Å, whereas the distance of the central CuII atom to the DyIII centres is 3.255(1) Å. Overall the metallic skeleton can be described as four face- and vertex-sharing {Cu3Dy} tetrahedral units.
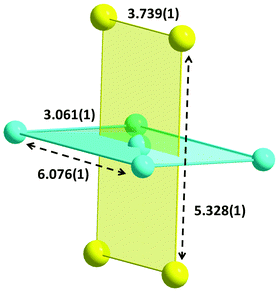 | ||
| Fig. 3 The metallic skeleton in 1·4MeCN·1.5MeOH; the numbers indicate distances in Å. Colour code: CuII, cyan; DyIII, yellow. | ||
Two trigonal bipyramidal μ5-O2− groups (O9 and O9′′′) link the perpendicular Cu5 and Dy4 frameworks together. Each of them bridges the central Cu2 atom with two CuII centres of the short edge of the Cu4 rectangle and with two DyIII centres that belong to a long edge of the Dy4 rectangle. The four methoxo groups (defined by O7, O8, O7′′ and O8′′) display a μ3 mode each bridging the central Cu2 atom with two DyIII atoms of the short edge of the Dy4 rectangle. The oxygen atoms (O10, O10′′′) of the neutral MeOH molecules bridge two CuII atoms that belong to a short edge of the Cu4 rectangle; these atoms lie on a two-fold axis of symmetry and each MeOH molecule is thus disordered over two positions. The two ButCH2CO2− groups (equivalent by symmetry) behave as syn,syn η1:η1:μ ligands, each bridging two DyIII atoms that belong to a short edge of the Dy4 rectangle. The four η2:η1:η2:μ3 pdm2− ligands each chelate one of the four outer CuII atoms forming two 5-membered chelating rings and simultaneously bridge two DyIII centres of the long edge of the Dy4 rectangle. The four terminal nitrato groups each chelate (η1:η1) one of the four DyIII atoms. The coordination modes of the ligands that are present in 1·4MeCN·1.5MeOH are summarized in Fig. 4.
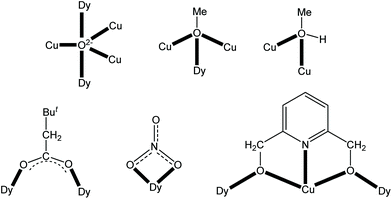 | ||
| Fig. 4 The coordination modes of all the ligands that are present in 1·4MeCN·1.5MeOH; the coordination bonds are indicated with bold lines. | ||
The central CuII atom, Cu2, is 6-coordinate and presents a Jahn–Teller elongated (4 + 2) tetragonal bipyramidal geometry with a {CuIIO6} coordination sphere. The long Cu2–O8 (and its symmetry equivalent) distance of 2.746(6) Å can be considered as a weak interaction. The four bonds in the equatorial plane are much shorter [1.922(4) and 1.985(4) Å]. The outer copper(II) (Cu1 and its symmetry equivalents) coordination geometries are described as distorted square pyramidal with the bridging MeOH oxygen atom (O10) occupying the apical position. The coordination sphere is of the {CuIIO4N} type. Analysis of the shape-determining angles using the approach of Reedijk and Addison69 yields a value of 0.19 for the trigonality index τ (τ = 0 and 1 for perfect square pyramidal and trigonal bipyramidal geometries, respectively). As expected, the axial bond [Cu1–O10 = 2.515(4) Å] is the longest, the coordination bond lengths in the basal plane being in the 1.879(4)–1.951(3) Å range. The crystallographically unique DyIII centre is 8-coordinate with a {DyIIIO8} coordination sphere and Dy–O distances in the 2.241(3)–2.664(1) Å range. To estimate the closer coordination polyhedron defined by the eight donor atoms around the DyIII centre in 1·4MeCN·1.5MeOH, a comparison of the experimental data with the theoretical values for the most common polyhedral shapes with 8 vertices was performed using the SHAPE program.70 The best fit was obtained for the Snub diphenoid JSD – 8 (CShM = 2.744), Fig. S2† (left). Since the nitrato group imposes a small bite angle, the polyhedron is distorted.
The molecules in the crystal of 1·4MeCN·1.5MeOH interact through non-classical hydrogen bonds and they are arranged in a body-centered lattice in conformity with the I2/m space group, forming channels along the a and c crystallographic axes where the lattice MeCN and MeOH molecules are residing (Fig. S3†).
The crystal structure of 4·2MeOH consists of cluster molecules [Cu5Y4O2(OMe)4(NO3)2(O2CCH2But)4(pdm)4(MeOH)2] (Fig. 5 and S4†) and lattice MeOH molecules in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio; the latter will not be further discussed. The complex crystallizes in the triclinic space group P
2 ratio; the latter will not be further discussed. The complex crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with the asymmetric unit containing half the cluster, which lies upon an inversion centre. The structure of the molecule 4 is very similar with that of 1. Again the core is {Cu5Y4(μ5-O)2(μ3-OMeO−)4(μ-OMeOH)2(μ-OR′)8}6+ (Fig. S5†). Notable differences (except the presence of four YIII centres instead of four DyIII atoms) are: (i) 1 possesses 2/m point group symmetry while 4 is centrosymmetric; (ii) two of the chelating nitrato groups of 1 have been replaced by two chelating ButCH2CO2− ligands in 4 and the composition of the two cluster molecules is thus different; and (iii) the coordination polyhedra of the two crystallographically independent YIII atoms (Y1 and Y2) in 4 can be described as biaugmented trigonal prisms (CShM = 2.825 for Y1 and 2.271 for Y2), whereas the polyhedron of the crystallographically unique DyIII centre in 1 is Snub diphenoid (Fig. S2†).
with the asymmetric unit containing half the cluster, which lies upon an inversion centre. The structure of the molecule 4 is very similar with that of 1. Again the core is {Cu5Y4(μ5-O)2(μ3-OMeO−)4(μ-OMeOH)2(μ-OR′)8}6+ (Fig. S5†). Notable differences (except the presence of four YIII centres instead of four DyIII atoms) are: (i) 1 possesses 2/m point group symmetry while 4 is centrosymmetric; (ii) two of the chelating nitrato groups of 1 have been replaced by two chelating ButCH2CO2− ligands in 4 and the composition of the two cluster molecules is thus different; and (iii) the coordination polyhedra of the two crystallographically independent YIII atoms (Y1 and Y2) in 4 can be described as biaugmented trigonal prisms (CShM = 2.825 for Y1 and 2.271 for Y2), whereas the polyhedron of the crystallographically unique DyIII centre in 1 is Snub diphenoid (Fig. S2†).
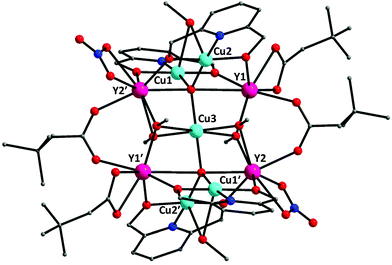 | ||
| Fig. 5 The structure of the cluster molecule [Cu5Y4O2(OMe)4(NO3)2(O2CCH2But)4(pdm)4(MeOH)2] that is present in the crystal of 4·2MeOH. Symmetry code: (′) 2 − x, 2−y, 2 − z. | ||
The edges of the Cu4 rectangle consisting of the four outer CuII atoms are 3.124(1) Å (Cu1⋯Cu2 = Cu1′⋯Cu2′) and 5.978(1) Å (Cu1⋯Cu2′ = Cu2⋯Cu1′), where prime (′) is the symmetry operation 2 − x, 2 − y, 2 − z. The edges of the Y4 rectangle are 3.766(1) Å (Y1⋯Y2 = Y1′⋯Y2′) and 5.419(1) Å (Y1⋯Y2′ = Y2⋯Y1′). The two rectangles are strictly planar by symmetry; the two planes are almost perpendicular with a dihedral angle of 89.3(1)°. As in 1, the central CuII atom (Cu2) is 6-coordinate with a {CuIIO6} coordination sphere and an elongated (4 + 2) tetragonal bipyramidal geometry. Two of its six coordination bonds are considered as long contacts [Cu3–O14 = Cu3–O14′ = 2.785(3) Å], whereas the four equatorial bonds are much shorter [Cu3–O13 = Cu3–O13′ = 1.992(2) Å and Cu3–O15 = Cu3–O15′ = 1.919(2) Å]. The coordination geometries of the outer CuII atoms are square pyramidal (τ = 0.08 for Cu1, Cu1′ and 0.04 for Cu2, Cu2′), with the bridging MeOH oxygen atom (O12 and its symmetry equivalent) occupying the apical position. As expected, the Cu1–O12 and Cu2–O12 bonds are long [Cu1–O12 = 2.563(3) Å, Cu2–O12 = 2.495(2) Å], whereas the corresponding bond distances in the basal planes lie in the range 1.884(2)–1.950(2) Å. The coordination spheres of the YIII atoms are of the {YIIIO8} type, with bond distances in the 2.243(2)–2.527(2) Å range.
The molecules in the crystal of 4·2MeOH interact through hydrogen bonds and form layers parallel to the (001) crystallographic plane (Fig. S6†). Molecules belonging to neighbouring layers further interact through van der Waals forces and are stacked along the c crystallographic axis, thus building the 3D architecture of the structure.
Complexes 1–3 are new members of the small family of 3d/4f-metal clusters containing H2pdm and its anionic forms as ligands.51,52,60–64 The previously characterized compounds are conveniently summarized in Table 1, together with diagnostic structural and magnetic information. It is clear that the nuclearity, metallic skeleton and core are all unique in the clusters of the present work. As far as the H2pdm/Hpdm−/pdm2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RCO2− ratio is concerned, compounds 1–3 contain the highest ratio by far and this has a variety of structural consequences.
RCO2− ratio is concerned, compounds 1–3 contain the highest ratio by far and this has a variety of structural consequences.
| Complexa | Coordination mode of Hpdm− and pdm2− | Metal topology | Magnetic features | Ref. |
|---|---|---|---|---|
| a Lattice solvent molecules have been omitted. b L is the dianionic ligand (6-hydroxymethylpyridin-2-yl)(6-hydroxymethylpyridin-2-ylmethoxy)methanol obtained from the in situ reaction of two H2pdm groups. c The neutral H2pdm molecules behave as η2:η1:η1:μ ligands. d Information was not provided. F = ferromagnetic; AF = antiferromagnetic. | ||||
| [CoII2Ln2(O2CBut)4(Hpdm)4] (Ln = Y, Gd, Tb, Dy, Ho) | η3:η1:μ3 | Cubane | SMM (Ln = Dy) | 61 |
| [MnIIMnIIILn2(O2CMe)6(L)(Hpdm)2](NO3)b (Ln = Gd, Dy) | η2:η1:η1:μ | Butterfly | Weak F exchange | 62 |
| [FeIII2Ln2Cl4(Hpdm)6]Cl2 (Ln = Y, Ho) | η2:η1:η1:μ, η2:η1:μ | U-shaped | AF exchange | 60 |
| [CuII4Ln8(OH)6(NO3)2(O2CCH2But)16(pdm)4] (Ln = La, Gd, Tb, Dy) | η3:η1:η1:μ3, η2:η1:η2:μ3 | Cage-like | SMM (Ln = Dy) | 52 |
| [CuII15Ln7(OH)6(CO3)4(O2CPh)19(pdm)3(H2pdm)9(H2O)2]c (Ln = Gd, Dy) | η3:η1:η2:μ4 | Cage-like | Magnetic refrigerant (Ln = Gd), SMM (Ln = Dy) | 51 and 71 |
| [CuII3Gd6(OH)(CO3)4(O2CBut)9(pdm)3(MeOH)3] | η2:η1:η2:μ3 | Tridiminished icosahedron | Magnetic refrigerant | 64 |
| [CuII8Gd4(OH)8(O2CBut)8(Hpdm)8](ClO4)4 | η3:η1:μ3 | Wheel of four corner-sharing {CuII2Gd2} cubanes | 63 | |
Compounds 1–4 also join a small group of {Mx5Ln4} and {Mx5Y4} clusters, where M is a 3d-metal and x = II–IV. The previously characterized complexes are listed in Table 2, together with their metal topology and magnetic characteristics. With the exception of the members of the [CuII5Ln4O2(OMe)4(NO3)4(O2CBut)2(Htea)4] (Ln = Gd, Tb, Dy, Ho) family (Htea2− is the dianion of triethanolamine),73–75 complexes 1–3 have a different composition and different structural features compared with those of the previously characterized complexes. The molecular structure of 1 is quite similar to the structure of the {CuII5Ln4}/Htea2− clusters. The Htea2− groups adopt the η2:η1:η2:μ3 coordination mode exhibited by the pdm2− ligand in 1 (Fig. 4). The coordination modes of the oxide, methoxide, nitrate and carboxylate ligands are exactly the same. The metallic skeletons are also very similar. These experimental observations, emphasized in Fig. S7,† indicate that the pdm2−vs. Htea2− and ButCH2CO2−vs. ButCO2− changes have little structural effect (this structural similarity is extended to 4, despite its slightly different chemical composition). However, there are three differences between the molecular structures of 1 and [CuII5Ln4O2(OMe)4(NO3)4(O2CBut)2(Htea)4]: (i) the μ-MeOH group is missing in the Htea2− clusters resulting in a square planar coordination for the outer CuII atoms and a longer distance (∼3.29 vs. ∼3.06 Å) between the CuII centres that occupy the short edges of the Cu4 rectangle; (ii) the coordination polyhedron in 1 approximates a Snub diphenoid, whereas that in the Htea2− clusters is best described as a square antiprism; and (iii) 1 possesses 2/m symmetry, while the Htea2− clusters are simply centrosymmetric with respect to the central CuII atom, i.e. that in the middle of the rectangle.
| Complexa | Metal topology | Magnetic features | Ref. |
|---|---|---|---|
| a Lattice solvent molecules have been omitted. b The ligands mdea and Hmdea are the di- and monoanions of N-methyl-diethanolamine. c The ligand Htea is the dianion of triethanolamine. d H2L is the dianionic form of a polydentate ligand synthesised by the reaction of pyridine-2,6-dicarbohydrazide and two equiv. of 6-hydroxymethylpyridine-2-carbaldehyde. e The ligand bis-C[4] is the octaanion of bis-But-calix[4]arene. F = ferromagnetic; AF = antiferromagnetic. | |||
| [MnIII4MnIVLn4O6(NO3)4(O2CBut)6(mdea)2(Hmdea)2(H2O)2]b (Ln = Y, Tb, Dy, Ho) | Two {MnIVMnIIILn2} cubanes sharing a MnIV vertex | SMMs (all) | 72 |
| [CuII5Ln4O2(OMe)4(NO3)4(O2CBut)2(Htea)4]c (Ln = Gd, Tb, Dy, Ho) | Four face- and vertex-sharing tetrahedral units | Magnetic refrigerant (Ln = Gd) SMMs (Ln = Tb, Dy, Ho) | 73–75 |
[CuII5Dy4(OH)4(SCN)8(H2L)4]Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
[3 × 3]-shaped heterometallic grid | SMM | 76 |
| [FeIII5Gd4O4(NO3)2(bis-C[4])2(DMF)8(H2O)2](OH)e | Two {FeIII2Gd2} butterflies linked by a central FeIII cation | Competing F–AF exchange interactions | 77 |
| [MII5Ln4(OMe)8(NO3)2(O2CMe)12(MeOH)6] (M/Ln = Co/Eu, Co/Gd, Ni/Eu, Ni/Dy) | Two {MII2Ln2} cubanes connected via a MII centre | Magnetic refrigerant ({CoII5Gd4}) SMMs ({CoII5Eu4} and {NiII5Dy4}) | 78 |
| [MII5Ln4(OH)2(OMe)6(NO3)4(O2CMe)10(MeOH)6] (M/Ln = Co/Dy, Ni/Gd) | Two {MII2Ln2} cubanes connected via a MII centre | Magnetic refrigerant ({NiII5Gd4}) SMM ({CoII5Dy4}) | 78 |
Magnetic studies
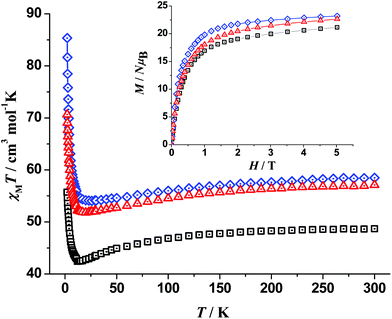
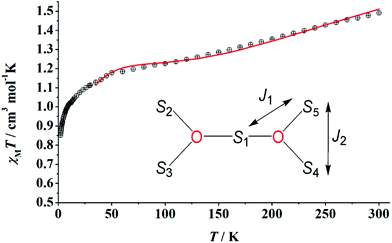
Direct current (dc) magnetic susceptibility (χM) data on well-dried and analytically pure samples of 1–3 were collected in the 300–2 K temperature range under fields of 0.3 T (300–30 K) and 0.02 T (30–2 K). The data are plotted as χMT products vs. T in Fig. 6. Compounds 1–3 have room-temperature χMT values of 58.5, 48.7 and 57.1 cm3 K mol−1, respectively. These values are in good agreement with those expected for five CuII atoms (S = 1/2, g = 2) and four DyIII atoms (S = 5/2, L = 5, 6H15/2, gJ = 4/3) [the theoretical value is 58.55 cm3 K mol−1] for 1, for five CuII atoms (S = 1/2, g = 2) and four TbIII atoms (S = 3, L = 6, 7F6, gJ = 3/2) [the theoretical value is 49.11 cm3 K mol−1] for 2, and for five CuII atoms (S = 1/2, g = 2) and four HoIII atoms (S = 2, L = 6, gJ = 5/4) [the theoretical value is 57.78 cm3 K mol−1] for 3, with the assumption that there are no interactions between the spin carriers. Upon cooling, the χMT value of 1 decreases slowly to 54.05 cm3 K mol−1 at 26 K, to 42.43 cm3 K mol−1 at 12 K for 2 and to 51.81 cm3 K mol−1 at 20 K for 3. Below these temperatures, the χMT values increase abruptly reaching the values of 85.35 (1), 55.69 (2) and 70.65 cm3 K mol−1 (3) at 2 K. The slight decrease is due to the depopulation of the mj sublevels of the ground J state of the LnIII centres (Stark sublevels) and may also be due to weak antiferromagnetic interactions between the metal ions. The large increase in χMT values at low temperatures suggests ferromagnetic interactions, with large spin ground states for the complexes. Magnetization (M) vs. external applied field (H) studies at 2 K (inset of Fig. 6) show a rapid increase of M as H increases reaching almost saturation values of 23.20, 21.17 and 22.65 NμB for 1, 2 and 3, respectively, at 5 T and 2 K, suggesting large spin ground states.The value of the χMT product for 4 at 300 K is 1.50 cm3 K mol−1, lower than the theoretical value of 1.875 cm3 K mol−1 expected for five non-interacting CuII (S = 1/2, g = 2) centres. Upon cooling, the value of the product decreases rather slowly reaching ∼1.2 cm3 K mol−1 at ∼50 K and then decreases rapidly to the value of 0.84 cm3 K mol−1 at 2 K (Fig. 7). M increases rapidly as H increases at 2 K to the value of ∼2 NμB at 5 T without reaching saturation. The diamagnetic character of YIII allowed us to fit the experimental data of the static magnetic properties taking into account only the five CuII atoms which are arranged as two vertex-sharing triangles; the CuII atoms of each triangle are bridged by a μ3-O2− group (the oxo groups are structurally μ5, Fig. S5†) resulting in two superexchange pathways, as shown in the inset of Fig. 7.
Using the program PHI,79 the data were fitted using the spin Hamiltonian given by eqn (1). The best-fit parameters in the 300–35 K range are J1 = −102(1) cm−1, J2 = −58(1) cm−1 and g = 2.24. Taking into account the large Cu–O–Cu angles [Cu1–O15–Cu2 = 110.8(1)°, Cu1–O15–Cu3 = 123.7(1)° and Cu2–O15–Cu3 = 124.6(1)°; see Fig. S5†], moderately strong antiferromagnetic CuII⋯CuII exchange interaction with |J1| > |J2| should be expected. From the inset of Fig. 7, it is clear that the ground state will be S = 3/2 if J1 dominates, whereas the ground state will be S = 1/2 if the dominating interaction is J2. Intermediate low-temperature χMT or magnetization values could be found around the frustration point J1 = 2J2; this seems to be the case here.
| \H = −2J1(S1·S2 + S1·S3 + S1·S4 + S1·S5) − 2J2(S2·S3 + S4·S5) | (1) |
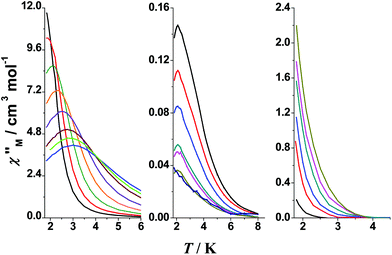
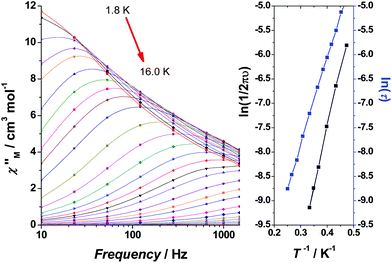
The dynamic magnetic properties of 1–3 were investigated in search of slow relaxation in the magnetization response (SMM behaviour). Preliminary measurements at the fixed, alternating current (ac) frequency of 1000 Hz and variable field revealed clear temperature dependence of the imaginary, out-of-phase component of the ac susceptibility, χ′′M, at zero field for 1, weak tails at zero field with increasing intensity up to 0.2 T of transverse field for 2 and poorly field-dependent tails for 3. According to this preliminary information, ac measurements were performed at zero dc field for 1 and 3, and under an applied field of 0.2 T for 2 (Fig. 8).
The temperature dependence of χ′′M for 1 in the 10–1488 Hz range at zero field is shown in the left part of Fig. 8; signals appear above 2 K. The value of the relaxation time τ (τ = 1/2πv) is large (7.6 × 10−3 s) at 2.1 K. The data were fitted using an Arrhenius model, τ = τ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Ueff/kBT), by using two different methods: the data from the χ′′Mvs. T plot, and the data from the χ′′Mvs. frequency (v) plot, assuming a magnetization relaxation through an Orbach process. Comparable relaxation parameters were obtained. The fit of the higher-temperature maxima in the χ′′Mvs. T plot yields Ueff = 16.7 cm−1 (∼24 K) and τ0 = 3.75 × 10−8 s, while the fit of τ from the χ′′Mvs. v plot for a wider temperature range yields Ueff = 12.2 cm−1 (17.6 K) and τ0 = 2.0 × 10−6 s, Fig. 9. The linear dependence of ln(τ) with the inverse temperature suggests the occurrence of only one relaxation process, in agreement with the Argand plot (Fig. S8†) in which only one semicircle appears.
exp(Ueff/kBT), by using two different methods: the data from the χ′′Mvs. T plot, and the data from the χ′′Mvs. frequency (v) plot, assuming a magnetization relaxation through an Orbach process. Comparable relaxation parameters were obtained. The fit of the higher-temperature maxima in the χ′′Mvs. T plot yields Ueff = 16.7 cm−1 (∼24 K) and τ0 = 3.75 × 10−8 s, while the fit of τ from the χ′′Mvs. v plot for a wider temperature range yields Ueff = 12.2 cm−1 (17.6 K) and τ0 = 2.0 × 10−6 s, Fig. 9. The linear dependence of ln(τ) with the inverse temperature suggests the occurrence of only one relaxation process, in agreement with the Argand plot (Fig. S8†) in which only one semicircle appears.
Measurements of the dynamic magnetic properties of 2 exhibited very weak out-of-phase susceptibility signals under an applied field of 0.2 T, which are poorly field-dependent and decrease for higher frequencies, Fig. 8 (middle). Maxima with negligible frequency dependence were defined for low frequencies (1.45–10 Hz). This behaviour of the ac curves indicates magnetic relaxation through a tunneling mechanism.
The magnetization dynamics of the {CuII5Ho4} cluster 3 were investigated in the 10–1500 Hz frequency range. The χ′′Mvs. T plots show only tails (Fig. 8, right), indicating that slow magnetization relaxation occurs below 2 K (the lowest-temperature limit of our setup). Because no maxima in χ′′M were observed, we were unable to determine the energy barrier Ueff and the pre-exponential factor τ0via the conventional Arrhenius plot method. Another method, established by Bartolomé et al.,80 is to assume that there is only one characteristic relaxation process of the Debye type with one energy barrier and one time constant. From eqn (2) and by plotting ln(χ′′M/χ′M) vs. 1/T, we can perform linear regressions to obtain the gradients (Ea/kB) and intercepts [ln(ωτ0)] and then extract an estimation of the activation energy and τ0; χ′M is the real, in-phase component of the ac susceptibility and ω = 2πν. These estimates for 3 are Ueff ≈ Ea = 10.0 ± 0.1 K and τ0 = 7.1(± 0.2) × 10−7 s (Fig. 10).
| ln(χ′′M/χ′M) = ln(ωτ0) + Ea/kBT | (2) |
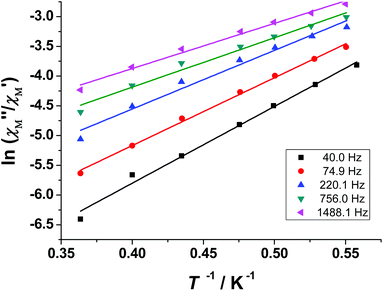 | ||
| Fig. 10 Plots of ln(χ′′M/χ′M) vs. 1/T for 3 (the temperature range is 1.8–2.75 K) at different ac frequencies; the solid lines are the best-fit curves. | ||
A comparison of the magnetic properties of 1–3 with those of the structurally similar clusters [Cu5Ln4O2(OMe)4(NO3)4(O2CBut)2(Htea)4] (Ln = Dy, Tb, Ho)74 might be useful at this point. The Htea2− clusters also have large spin ground states and exhibit slow relaxation of the magnetization, the Ueff = Ea values estimated to be 7 ± 1 K, 11.9 ± 0.8 K and 10 ± 4 K for the Dy(III), Tb(III) and Ho(III) members, respectively. The values for the Ho(III) clusters seem to be comparable, while the Ueff value for 1 is higher (at least double) than that for the CuII/DyIII/Htea2− cluster. Ab initio calculations that employ DyIII and CuII fragments of [Cu5Dy4O2(OMe)4(NO3)4(O2CBut)2(Htea)4] and the lowest Kramers levels resulting therefrom yielded74 good fits of the susceptibility vs. temperature behaviour and a corresponding set of best-fit J1 (CuII⋯DyIII), J2 (CuII central⋯CuII outer) and J3 (CuII outer⋯CuII outer) values. The first two J values correspond to ferromagnetic pathways and the third corresponds to antiferromagnetic pathways. The exchange interaction between DyIII ions is negligible due to the perpendicular arrangement of the main anisotropy axes. The main anisotropy axis of the cluster molecule is almost perpendicular to the plane defined by the four DyIII centres.
Concluding comments and perspectives
Complexes 1–3 are the first members of a new, fifth family of clusters arising from the general CuII/LnIII/H2pdm/RCO2− reaction system. By contrast with the previously reported {Cu3Gd6},64 {Cu8Gd4},63 {Cu15Ln7}51 and {Cu4Ln8}52 families which contain a RCO2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) primary ligand ratio higher than 2, in the {Cu5Ln4} complexes of the present family this ratio is 0.5 leading to a different nuclearity, topology and core. The {Cu5Ln4} clusters were prepared by simply using an excess of H2pdm (H2pdm
primary ligand ratio higher than 2, in the {Cu5Ln4} complexes of the present family this ratio is 0.5 leading to a different nuclearity, topology and core. The {Cu5Ln4} clusters were prepared by simply using an excess of H2pdm (H2pdm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RCO2− = 2
RCO2− = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the reaction mixtures. Thus, the chemical message of this work is that a detailed investigation of all synthetic parameters (here the primary to ancillary ligand reaction ratio) is more than necessary to isolate the maximum number of 3d/4f-metal products from a given reaction system. From the magnetism viewpoint, complexes 1–3 exhibit different characteristics concerning their magnetization relaxation. The DyIII-containing cluster 1 behaves as a SMM, which is the expected behaviour due to the high magnetic moment and Kramer's nature of DyIII, that ensures the degeneracy of the two lowest-lying levels and reduces the possibility of the relaxation of the magnetization via quantum tunneling.34 This is confirmed by examining the magnetic relaxation of the TbIII and HoIII analogues (2 and 3, respectively). Both are non-Kramers ions and strong tunneling is the principle magnetization relaxation making 2 a very weak field-induced SMM with ac maxima practically independent of the frequency, while 3 exhibits only weak tails in the χ′′Mvs. T plots at zero dc field.
1) in the reaction mixtures. Thus, the chemical message of this work is that a detailed investigation of all synthetic parameters (here the primary to ancillary ligand reaction ratio) is more than necessary to isolate the maximum number of 3d/4f-metal products from a given reaction system. From the magnetism viewpoint, complexes 1–3 exhibit different characteristics concerning their magnetization relaxation. The DyIII-containing cluster 1 behaves as a SMM, which is the expected behaviour due to the high magnetic moment and Kramer's nature of DyIII, that ensures the degeneracy of the two lowest-lying levels and reduces the possibility of the relaxation of the magnetization via quantum tunneling.34 This is confirmed by examining the magnetic relaxation of the TbIII and HoIII analogues (2 and 3, respectively). Both are non-Kramers ions and strong tunneling is the principle magnetization relaxation making 2 a very weak field-induced SMM with ac maxima practically independent of the frequency, while 3 exhibits only weak tails in the χ′′Mvs. T plots at zero dc field.
With the above results in mind, we continue working in the area of the chemistry and magnetism of CuII/LnIII cluster chemistry using the H2pdm/RCO2− ligand “blend”, paying more attention to the study of the influence of R on the chemical and structural identity of the products. Although CuII/LnIII/pdm2− or Hpdm− clusters with R = Ph,51 But,63,64 and ButCH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 (together with results of the present work) have been reported, there is a plethora of R groups with various electronic and steric properties that can be examined. Ongoing studies reveal new families of CuII/LnIII/pdm2− clusters with novel structures and interesting magnetic properties, proving that the general CuII/LnIII/H2pdm/RCO2− reaction system is fertile and surprising. Our results, already well advanced, will be reported soon.
52 (together with results of the present work) have been reported, there is a plethora of R groups with various electronic and steric properties that can be examined. Ongoing studies reveal new families of CuII/LnIII/pdm2− clusters with novel structures and interesting magnetic properties, proving that the general CuII/LnIII/H2pdm/RCO2− reaction system is fertile and surprising. Our results, already well advanced, will be reported soon.
Experimental section
General, physical measurements and spectroscopic studies
All manipulations were performed under aerobic conditions using materials (reagent grade) and solvents as received. Elemental analyses were performed by the University of Patras microanalytical service. FT-IR spectra (4000–400 cm−1) were recorded using a PerkinElmer spectrometer with samples prepared as KBr pellets. Direct-current (dc) and alternating-current (ac) magnetic susceptibility studies were performed at the University of Barcelona Chemistry Department on a DSM5 Quantum Design magnetometer operating at 0.3 T in the 300–30 K range and at 0.02 T in the 30–2.0 K range to avoid saturation effects. Pascal's constants were used to estimate the diamagnetic contribution, which was subtracted from the experimental susceptibility to give the molar paramagnetic susceptibility (χM).81Synthetic details
Single-crystal X-ray crystallography
Blue crystals of 1·4MeCN·1.5MeOH and 4·2MeOH were taken directly from the mother liquor and immediately cooled to −113 °C. X-ray diffraction data were collected on a Rigaku R-AXIS SPIDER Image Plate diffractometer using graphite-monochromated Mo Kα radiation. Data collection (ω-scans) and processing (cell refinement, data reduction and empirical absorption correction) were performed using the CrystalClear program package.82 The structures were solved by direct methods using SHELXS ver. 2013/183 and refined by full-matrix least-squares techniques on F2 with SHELXL ver. 2014/6.84 H atoms were either located by difference maps and refined isotropically or were introduced at calculated positions as riding on their respective bonded atoms. All non-H atoms were refined anisotropically. The SQUEEZE procedure85 was used for the analysis of the structure of the {Cu5Dy4} cluster and the estimated additional solvents in the lattice voids are 2 MeCN and 1.5 MeOH molecules per formula unit. For compound 4·2MeOH, one of the coordinated ButCH2CO2− ligands has been treated as disordered. Important crystallographic data are listed in Table S1.† Full details can be found in the CIF files.The unit cell dimensions of 2·4MeCN·1.5MeOH and 3·4MeCN·1.5MeOH were calculated from single-crystal diffraction measurements (Rigaku R-AXIS SPIDER Image Plate diffractometer, graphite-monochromated Mo Kα radiation). The dimensions clearly show that these two complexes are isomorphous with 1·4MeCN·1.5MeOH. Data are as follows: 2·4MeCN·1.5MeOH: a = 13.208(1), b = 18.964(1), c = 18.409(1) Å, α = γ = 90.0°, β = 98.45(1)°, V = 4561.10(1) Å3; 3·4MeCN·1.5MeOH: a = 13.160(1), b = 18.910(1), c = 18.488(1) Å, α = γ = 90.0°, β = 98.90(1)°, V = 4545.54(1) Å3. Both compounds (like 1·4MeCN·1.5MeOH) crystallize in the monoclinic space group I2/m.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. E. and J. M. thank the Ministerio de Economia y Competitividad, Project PGC2018-094031-B-100 and PID2019-109735GB-100 for funding. S. P. P. is grateful to the COST Action: CA15128-Molecular Spintronics (MOLSPIN) for encouraging research activities in Patras. V. P. would like to thank the special Account of the NCSR “Demokritos” for financial support concerning the operation of the X-ray facilities at INN through the internal program entitled “Structural study and characterisation of crystalline materials” (NCSR “Demokritos”, ELKE #10813).Notes and references
- C. James and P. S. Willand, J. Am. Chem. Soc., 1916, 38, 1497 CrossRef CAS.
- W. E. Bailey, R. J. Williams and W. O. Milligan, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1973, 29, 1365 CrossRef CAS.
- For example, see: A. E. Crease and P. Legzdins, J. Chem. Soc., Dalton Trans., 1973, 1501 RSC.
- J. M. Boncella and R. A. Andersen, Inorg. Chem., 1984, 23, 432 CrossRef CAS.
- O. Kahn, Molecular Magnetism, VCH Publishers, New York, USA, 1993 Search PubMed.
- Physics of Magnetic Materials, ed. J. Rauluszkiewics, H. Szymczak and H. K. Lachowicz, World Scientific, Singapore, 1985 Search PubMed.
- M. Sagawa, S. Fujimura, M. Togawa, H. Yamamoto and Y. Matsnura, J. Appl. Phys., 1984, 55, 2083 CrossRef CAS.
- For example, see: C. Edder, C. Piguet, J.-C. G. Bünzli and G. Hopfgartner, Chem. – Eur. J., 2001, 7, 3014 CrossRef CAS.
- T. Wei, S. Zhao, W. Bu, X. Lü, Y. Hui, J. Song, W.-K. Wong and R. A. Jones, Inorg. Chem. Commun., 2009, 12, 1216 CrossRef CAS.
- M. A. Subhan, T. Suzuki and J. Kaizaki, J. Chem. Soc., Dalton Trans., 2002, 1416 RSC.
- O. Margeat, P. G. Lacroix, J. P. Costes, B. Donnadieu, C. Lepetit and K. Nakatani, Inorg. Chem., 2004, 43, 4743 CrossRef CAS.
- B. Zhao, P. Cheng, X. Y. Chen, C. Cheng, W. Shi, D. Z. Liao, S. P. Yan and Z. H. Jiang, J. Am. Chem. Soc., 2004, 126, 3012 CrossRef CAS.
- E. Loukopoulos, K. Griffiths, G. R. Akien, N. Kourkoumelis, A. Abbul-Sada and G. E. Kostakis, Inorganics, 2015, 3, 448 CrossRef CAS.
- C. Benelli and D. Gatteschi, Chem. Rev., 2002, 102, 2369 CrossRef CAS.
- G. Condorelli, I. Fragala, S. Giuffrida and A. Cassol, Z. Anorg. Allg. Chem., 1975, 251, 412 Search PubMed.
- A. Bencini, C. Benelli, A. Caneschi, R. L. Carlin, A. Dei and D. Gatteschi, J. Am. Chem. Soc., 1985, 107, 8128 CrossRef CAS.
- A. Bencini, C. Benelli, A. Caneschi, A. Dei and D. Gatteschi, Inorg. Chem., 1986, 25, 572 CrossRef CAS.
- N. Matsumoto, M. Sakamoto, H. Tamaki and H. Okawa, Chem. Lett., 1990, 853 CrossRef CAS.
- O. Kahn, Struct. Bonding, 1987, 68, 69 CrossRef.
- C. Benelli, A. Caneschi, D. Gatteschi, O. Guillou and L. Pardi, Inorg. Chem., 1990, 29, 1570 Search PubMed.
- M. Andruh, I. Ramade, E. Codjovi, O. Guillou, O. Kahn and J. C. Trombe, J. Am. Chem. Soc., 1993, 115, 1822 CrossRef CAS.
- O. Kahn and O. Guillou, Res. Front. Magnetochem., 1993, 179 CAS.
- R. E. P. Winpenny, Chem. Soc. Rev., 1998, 27, 447 RSC.
- F. Hulliger, M. Landolt and H. Vetsch, J. Solid State Chem., 1976, 18, 283 CrossRef CAS.
- For an early review, see: G. Aromi and E. K. Brechin, Struct. Bonding, 2006, 122, 1 CrossRef CAS.
- R. Bagai and G. Christou, Chem. Soc. Rev., 2009, 38, 1011 RSC.
- M. Evangelisti and E. K. Brechin, Dalton Trans., 2010, 39, 4672 RSC (Dalton Perspective), and references therein.
- Y.-Z. Zheng, G.-J. Zhou, Z. Zheng and R. E. P. Winpenny, Chem. Soc. Rev., 2014, 43, 1462 RSC.
- R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141 CrossRef CAS.
- R. Sessoli, H.-L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804 CrossRef CAS.
- C. J. Milios and R. E. P. Winpenny, Struct. Bonding (Berlin), 2015, 164, 1 CAS.
- N. Ishikawa, M. Sugita, T. Ishikawa, S.-Y. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694 CrossRef CAS.
- S. T. Liddle and J. van Slageren, Chem. Soc. Rev., 2015, 44, 6655 RSC.
- D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110 CrossRef CAS.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Science, 2018, 362, 1400 CrossRef CAS.
- K. Liu, W. Shi and P. Cheng, Coord. Chem. Rev., 2015, 289–290, 74 CrossRef CAS.
- For a review, see: A. Dey, J. Acharya and V. Chandrasekhar, Chem. – Asian J., 2019, 14, 4433 CrossRef CAS.
- For a minireview, see: A. Chakraborty, J. Goura, P. Bag and V. Chandrasekhar, Eur. J. Inorg. Chem., 2019, 1180 CrossRef CAS.
- For a perspective, see: L. R. Piquer and E. C. Sañudo, Dalton Trans., 2015, 44, 8771 RSC.
- J. W. Sharples and D. Collison, Coord. Chem. Rev., 2014, 260, 1 CrossRef CAS.
- H. L. C. Feltham and S. Brooker, Coord. Chem. Rev., 2014, 276, 1 CrossRef CAS.
- Y. Peng, M. K. Singh, V. Mereacre, C. E. Anson, C. Rajaraman and A. K. Powell, Chem. Sci., 2019, 10, 5528 RSC.
- R. Sessoli and A. K. Powell, Coord. Chem. Rev., 2009, 253, 2328 CrossRef CAS.
- C. D. Polyzou, C. G. Efthymiou, A. Escuer, L. Cunha-Silva, C. Papatriantafyllopoulou and S. P. Perlepes, Pure Appl. Chem., 2013, 85, 315 CAS.
- Z. G. Lada, E. Katsoulakou and S. P. Perlepes, in Single-Molecule Magnets: Molecular Architectures and Building Blocks for Spintronics, ed. M. Holyńska, Wiley-VCH, Weinheim, Germany, 2019, pp. 245–313 Search PubMed.
- H. Li, Z.-J. Yao, D. Liu and G.-X. Jin, Coord. Chem. Rev., 2015, 293–294, 139 CrossRef CAS.
- S. K. Langley, B. Moubaraki, C. Tomasi, M. Evangelisti, E. K. Brechin and K. S. Murray, Inorg. Chem., 2014, 53, 13154 CrossRef CAS.
- J. Wu, L. Zhao, M. Guo and J. Tang, Chem. Commun., 2015, 51, 17317 RSC.
- J. Wu, L. Zhao, L. Zhang, X.-L. Li, M. Guo, A. K. Powell and J. Tang, Angew. Chem., Int. Ed., 2016, 55, 15774 Search PubMed.
- J. Wu, X.-L. Li, M. Guo, L. Zhao, Y.-Q. Zhang and J. Tang, Chem. Commun., 2018, 54, 1065 RSC.
- D. Dermitzaki, G. Lorusso, C. P. Raptopoulou, V. Psycharis, A. Escuer, M. Evangelisti, S. P. Perlepes and T. C. Stamatatos, Inorg. Chem., 2013, 52, 10235 CrossRef CAS.
- D. Dermitzaki, C. P. Raptopoulou, V. Psycharis, A. Escuer, S. P. Perlepes and T. C. Stamatatos, Inorg. Chem., 2015, 54, 7555 CrossRef CAS.
- For example, see: T. C. Stamatatos, G. C. Vlahopoulou, C. P. Raptopoulou, A. Terzis, A. Escuer and S. P. Perlepes, Inorg. Chem., 2009, 48, 4610 CrossRef CAS.
- For example, see: T. Taguchi, T. C. Stamatatos, K. A. Abboud, C. M. Jones, K. M. Poole, T. A. O'Brien and G. Christou, Inorg. Chem., 2008, 47, 4095 CrossRef CAS.
- For example, see: M. Murugesu, M. Habrych, W. Wernsdorfer, K. A. Abboud and G. Christou, J. Am. Chem. Soc., 2004, 126, 4766 CrossRef CAS.
- For example, see: K. I. Alexopoulou, C. P. Raptopoulou, V. Psycharis, A. Terzis, V. Tangoulis, T. C. Stamatatos and S. P. Perlepes, Aust. J. Chem., 2012, 65, 1608 CrossRef CAS.
- D. Alexandropoulos, L. Cunha-Silva, L. Pham, V. Bekiari, G. Christou and T. C. Stamatatos, Inorg. Chem., 2014, 53, 3220 CrossRef CAS.
- P.-P. Yang, Z. Anorg. Allg. Chem., 2011, 637, 1234 CrossRef CAS.
- P.-P. Yang, X.-F. Gao, H.-B. Song, S. Zhang, X.-L. Mei, L.-C. Li and D.-Z. Liao, Inorg. Chem., 2011, 50, 720 CrossRef CAS.
- M. Murugesu, A. Mishra, K. A. Abboud, G. Christou and W. Wernsdorfer, Polyhedron, 2006, 25, 613 CrossRef CAS.
- X.-Q. Zhao, Y. Lan, B. Zhao, P. Cheng, C. E. Anson and A. K. Powell, Dalton Trans., 2010, 39, 4911 RSC.
- T.-X. Lan, W.-S. Gao, C.-N. Chen, H.-S. Wang, W. Wang and Y.-H. Fan, New J. Chem., 2018, 42, 5798 RSC.
- T. N. Hooper, R. Inglis, G. Lorusso, J. Ujma, P. E. Barran, D. Uhrin, J. Schnack, S. Piligkos, M. Evangelisti and E. K. Brechin, Inorg. Chem., 2016, 55, 10535 CrossRef CAS.
- T. N. Hooper, R. Inglis, M. A. Palacios, G. S. Nichol, M. B. Pitak, S. J. Coles, G. Lorusso, M. Evangelisti and E. K. Brechin, Chem. Commun., 2014, 50, 3498 RSC.
- F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced Inorganic Chemistry, 6th edn, Wiley, New York, 1999, p. 1109 Search PubMed.
- W. J. Evans, J. L. Shreeve, J. W. Ziller and R. J. Doedens, Inorg. Chem., 1995, 34, 576 CrossRef CAS.
- P. C. Junk, D. L. Kepert, B. W. Skelton and A. H. White, Aust. J. Chem., 1999, 52, 497 CrossRef CAS.
- A. K. Boudalis, V. Nastopoulos, A. Terzis, C. P. Raptopoulou and S. P. Perlepes, Z. Naturforsch., 2001, 56b, 122 Search PubMed.
- A. W. Addison, T. N. Rao, J. Reedijk, J. Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC.
- M. Llunell, D. Casanova, J. Girera, P. Alemany and S. Alvarez, SHAPE, version 2.0, Barcelona, Spain, 2010 Search PubMed.
- D. Dermitzaki, C. P. Raptopoulou, V. Psycharis, A. Escuer, S. P. Perlepes and T. C. Stamatatos, Unpublished results.
- V. Mereacre, A. M. Ako, R. Clérac, W. Wernsdorfer, I. J. Hewitt, C. E. Anson and A. K. Powell, Chem. – Eur. J., 2008, 14, 3577 CrossRef CAS.
- S. K. Langley, N. F. Chilton, B. Moubaraki, T. Hooper, E. K. Brechin, M. Evangelisti and K. S. Murray, Chem. Sci., 2011, 2, 1166 RSC.
- S. K. Langley, L. Ungur, N. F. Chilton, B. Moubaraki, L. F. Chibotaru and K. S. Murray, Chem. – Eur. J., 2011, 17, 9209 CrossRef CAS.
- T. Rajeshkumar, H. V. Annadata, M. Evangelisti, S. K. Langley, N. F. Chilton, K. S. Murray and G. Rajaraman, Inorg. Chem., 2015, 54, 1661 CrossRef CAS.
- J. Wu, L. Zhao, L. Zhang, X.-L. Li, M. Guo and J. Tang, Inorg. Chem., 2016, 55, 5514 CrossRef CAS.
- M. Coletta, R. McLellan, S. Sanz, K. J. Gagnon, S. J. Teat, E. K. Brechin and S. J. Dalgarno, Chem. – Eur. J., 2017, 23, 14073 CrossRef CAS.
- F. Shao, J.-J. Zhuang, M.-G. Chen, N. Wang, H.-Y. Shi, J.-P. Tong, G. Luo, J. Tao and L.-S. Zheng, Dalton Trans., 2018, 47, 16850 RSC.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164 CrossRef CAS.
- J. Bartolomé, G. Filoti, V. Kuncser, G. Schinteie, V. Mereacre, C. E. Anson, A. K. Powell, D. Prodius and C. Turta, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 014430 CrossRef.
- G. A. Bain and J. F. Berry, J. Chem. Educ., 2008, 85, 532 CrossRef CAS.
- CrystalClear, Rigaku/MSC Inc., The Woodlands, TX, USA, 2005 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2015, 71, 3 CrossRef.
- A. L. Spek, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2015, 71, 9 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Various structural plots for 1·4MeCN·1.5MeOH and 4·2MeOH (Fig. S1–S7), Argand plots for 1 (Fig. S8), and crystallographic data for 1·4MeCN·1.5MeOH and 4·2MeOH (Table S1) and short IR discussion. CCDC 2038607 and 2038608. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0dt03582c |
| This journal is © The Royal Society of Chemistry 2021 |