Development of proficient photocatalytic systems for enhanced photocatalytic reduction of carbon dioxide
Mufeedah
Muringa Kandy
 *a,
Anjana
Rajeev K
b and
Muniyandi
Sankaralingam
*a,
Anjana
Rajeev K
b and
Muniyandi
Sankaralingam
 *b
*b
aDepartment of Chemical Engineering, Institute of Chemical Technology, Mumbai 400019, India. E-mail: mufeedahmk@gmail.com
bBioinspired & Biomimetic Inorganic Chemistry Lab, Department of Chemistry, National Institute of Technology Calicut, Kozhikode, Kerala-673601, India. E-mail: msankaralingam@nitc.ac.in
First published on 17th November 2020
Abstract
Global warming due to the unrestricted release of CO2 into the atmosphere is a prevalent challenge faced by the 21st century. Scavenging atmospheric CO2 using solar energy and converting it to useful products is a dual beneficial approach to overcome this issue. In spite of considerable attractive advancements of existing research, the synthesis of a stable photocatalyst has remained a challenge, leaving it in its infant stage. The design of more advanced photocatalytic reactors equipped with solar concentrators also demands equal importance. In this review article, we summarized the recent trends of current strategies that are adopted by various researchers to intensify the rate of photocatalytic CO2 reduction. Persistent challenges in the pursuit of achieving higher photocatalytic reduction of CO2 into solar fuels are examined. The first part of this review deals with different structural engineering strategies that are adopted by various researchers to synthesize functional materials that display enhanced photocatalytic activity and stability. The current advancements in the exploration of technology for the design of an efficient photocatalytic reactor that is capable of harnessing light energy towards the photocatalyst comprise the next part of the review. Research challenges, perspectives, brief insights, and endorsements on the progress of proficient photocatalytic systems are suggested. It is expected that this review will provide a framework that would upgrade the process from a lab-scale to an industrially viable technology.
1. Introduction
The overconsumption of fossil fuels by a rapidly growing economy releases a large amount of CO2 into the atmosphere. Among the various heat-trapping gases, CO2 predominantly increases the earth's temperature leading to global warming.1,2 There have been concentrated efforts to control and scavenge the greenhouse gases from the atmosphere. Among various technologies adopted, consumption of CO2 as a valuable chemical feedstock, photocatalytic reduction of CO2, is more futuristic as it requires ambient natural conditions and responds mutually to global warming and energy crisis issues. The prominent dominance of photocatalysis, when compared to thermal reduction, is that the former is driven by visible light and the latter is activated by high temperature and electroreduction by applied voltage.3–8 Selective transformation of small molecules like CO2 into value-added products is a topic of interest as it helps in improving the economic development and satisfying the global energy demand.4The photocatalytic reduction of CO2 with water molecules is highly beneficial in dual terms as it converts inexpensive, abundant, and harmful carbon sources like CO2 to energy fuels using solar energy.8–11 The readily available solar energy makes the process of reduction of CO2 economically viable. This process has occupied a distinct position over other processes due to its excellent features like turning waste-into-wealth, renewability, and sustainability. The revolutionary effort by Halmann9 in 1978 on the photoelectrochemical reduction of CO2 was pursued by Inoue et al.10 in 1979 which brought into being many semiconductors like TiO2, CdS, ZnO, SiC, SrTiO3, and GaP that act as effective photocatalysts for the process. Researchers are therefore insisted to develop efficient photocatalysts that are capable of reducing CO2 to various fuels (H2, CO, CH4, HCHO, HCOOH and CH3OH) by consuming sunlight energy.11
The occurrence of rapid electron/hole recombination of photogenerated charge carriers drastically hinders the photocatalytic reduction process.12 Around 90% of the photogenerated charge carriers do recombine within 10 nanoseconds after illumination.13 As the search for an efficient and stable photocatalyst continues, finding a solution to this process remains in its infant stage. Numerous excellent reviews cover the design of various photocatalysts for CO2 reduction.2,14,15 In this review article, we briefly discuss the relation between proficient photocatalytic systems that involve precise engineering of photocatalysts and the design of photocatalytic reactors for an enhanced rate of photoreduction. The existing state, challenges in this area and future perspectives have been presented.
2. Mechanism of the photocatalytic process
Diminutive attention has been paid by most of the researchers towards the mechanism of photocatalytic reduction of CO2 due to the extreme complexity of the process. However, it is very important to understand its mechanism to develop photocatalysts with superior activity, selective product formation, and higher quantum yield.16,17 This multi-electron transfer process involves three major steps.18(i) Absorption of light by the photocatalyst and simultaneous production of charge carriers like electron–hole pairs in the system,
(ii) separation and transport of photoexcited charge carriers to the photocatalyst surface, and
(iii) the chemical process between photoexcited charge carriers and reactant species. This process would essentially involve adsorption, activation of CO2, and dissociation of the C–O bond.
Upon illumination of light of sufficient energy on the photocatalyst surface, which would either be equal or greater than its bandgap energy, electrons in the valence band (VB) get photoexcited to the conduction band (CB), leaving behind holes in the VB. These high energetic photogenerated charge carriers facilitate various redox reactions to take place. CO2 is an extremely stable molecule with a linear structure. The transformation of stable CO2 is a thermodynamically unfavorable uphill reaction. The feasibility of this uphill reaction by the photocatalytic process with certain photocatalysts largely depends on the CB and VB potential edge. Therefore, for an ideal photocatalyst, the conduction band potential edge should be more negative than the reduction potential of CO2 to facilitate the transfer of photo-excited electrons from the CB to CO2, enabling its reduction. As a result, under the illumination of light, the multi-electron process of CO2 reduction is thermodynamically favorable relative to its direct reduction with H2O.1,18 The process of reduction of CO2 to CO2˙ radicals is a single electron process and occurs at a high negative potential of −1.9 eV. This high energy consumption makes the process greatly unfavorable. The central C atom in CO2 is bonded to two highly electronegative oxygen atoms, and this will shift the electrons towards the oxygen atoms, leaving carbon electron deficient. This makes the carbon highly electrophilic and enables it to participate favorably in the proton assisted multi-electron reduction process of CO2 at low potential.9 A schematic illustration of a general mechanism of photocatalytic reduction of CO2 that illustrates the potential of various photocatalysts based on their CB and VB edge potential, and the proton assisted multi-electron process to produce CO/HCOOH, HCHO, CH3OH, and CH4 at various reduction potentials by transfer of 2, 4, 6 and 8 electrons, respectively is displayed in Fig. 1(a). Probable reactions and several products that would be obtained after CO2 reduction and their corresponding standard redox potentials acquired from thermodynamic data are shown in Table 1. The conversion of CO2 to valuable fuels like CH3OH and CH4 needs the transfer of six and eight electrons respectively. This is more difficult than the two-electron process of water splitting reactions to produce hydrogen fuel. The photocatalytic reduction of CO2 suffers from low conversion due to competing water splitting reactions as the transfer of more electrons depends mutually on the concentration of accessible protons in the reaction medium and the partial electron density on the photocatalyst surface.19
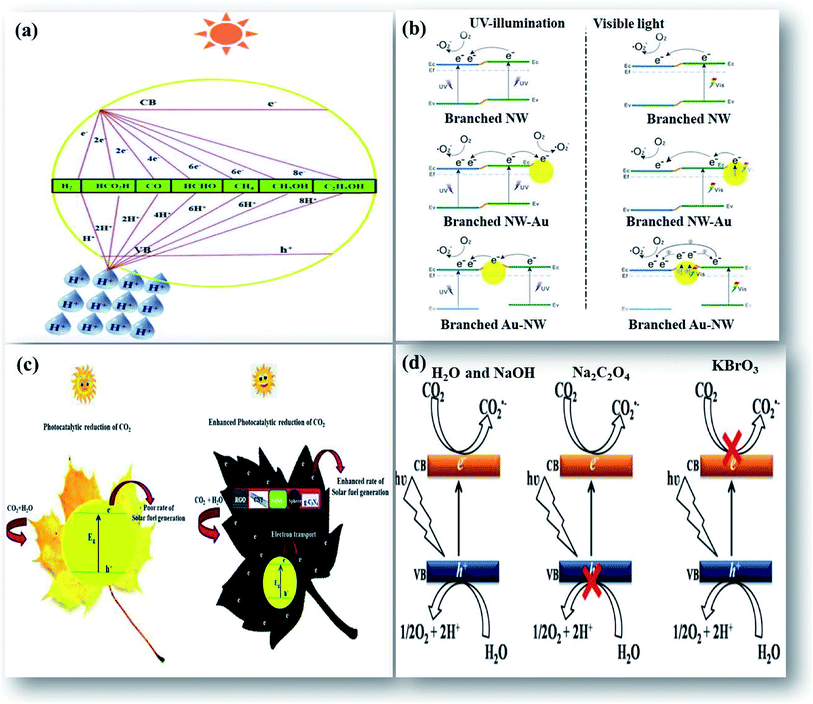 | ||
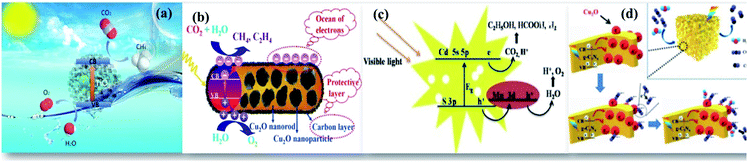
| Fig. 1 Schematic illustration of enhanced photocatalytic reduction of CO2: (a) general mechanism, reprinted with permission from ref. 1 copyright 2019, RSC publishing group, (b) Au as the plasmonic photocatalyst on TiO2, reprinted with permission from ref. 21 copyright 2017, Nature publishing group, (c) the use of carbon supports, reprinted with permission from ref. 1 copyright 2019, RSC publishing group, and (d) effect of electrolyte, reprinted with permission from ref. 27 copyright 2019, Nature publishing group. | ||
| Products | E 0 vs. NHE |
|---|---|
| O2 | 0.82 |
| H2 | −0.41 |
| HCOOH | −0.665 |
| CO | −0.521 |
| CH3OH | −0.399 |
| CH4 | −0.24 |
| C2H5OH | −0.31 |
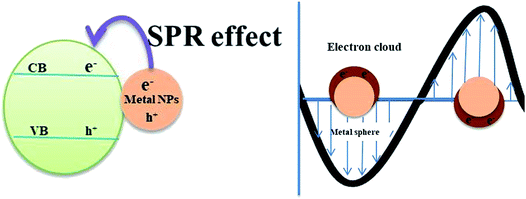
The UV active photocatalyst displays enhanced photocatalytic activity due to its wide bandgap that preferentially reduces the electron–hole recombination rate. However, as sunlight has only limited UV spectral content, the majority of UV active photocatalysts will not act as efficient semiconducting nanomaterials for solar applications, wherein the construction of heterostructures is demanded. The findings on enhanced photocatalysis by surface plasmon resonance (SPR) have displayed swift improvement and develop a potentially promising avenue that would benefit the need of the present process.20 The beneficial effects offered by the differential mechanisms of photocatalysis under UV and visible light enhance the photocatalytic activity of nanocomposites. Visible light responsive noble metals like Au and Ag enhance the photocatalytic activity of the TiO2 photocatalyst under both UV and visible light. Yu et al. have deposited Au nanoparticles at different locations on the TiO2 photocatalytic system to assess their beneficial effects. It was found that Au nanoparticles deposited at the interface of anatase/rutile TiO2 led to a remarkably enhanced photocatalytic activity under both UV and visible light. The Au-deposition at the interface increased the photoresponse under irradiation of either UV or visible light due to the beneficial effects of either electron trapping or localized surface plasmon resonance (LSPR) as shown in Fig. 1(b).21
The reaction certainly comprises numerous reaction intermediates that deteriorate the catalyst surface over time. Poudyal et al.16 have explored the ground-state surface reaction mechanism by DFT modeling for CO2 reduction over SiC and GaN photocatalysts. The modeling results were correlated with experimentally detected catalysts. The results suggested that the reactivity of the photocatalyst surface plays a role in C–O bond cleavage that promotes product formation. Isotope labeling studies are critical to identifying the carbon source due to high organic contamination that occurs.22 However, most of the research reports do not consider this important point. The large energy bandgap, as well as high electron affinity of CO2, makes it highly inactive in photocatalytic reactions. Beneficially, the adsorption of CO2 on the photocatalyst surface lowers its energy barrier after the transformation of its linear structure to a bent form exhibiting high reactivity.23 Ji et al.24 have proposed the fast-hydrogenation (FH) path and the fast-deoxygenation (FdO) path as two possible pathways on the most used TiO2 photocatalyst. They found that the presence of defective surfaces in TiO2 like oxygen vacancies can significantly lower the barrier of deoxygenation processes making it more active than the perfect TiO2 surface. The author has studied the reaction pathways at both surfaces including perfect TiO2 and the oxygen vacant defective surface such as Ti5f and the Ov paths to comprehensively obtain a deep insight.
The addition of certain boosters onto the photocatalyst surface as charge collectors like co-catalysts and sacrificial reagents in the reaction medium can significantly alter the reaction rate. Hence, it is necessary to suggest feasible photoreduction mechanisms to understand the influence of reactive species on product selectivity.25 The use of inexpensive carbon materials drives an enhanced photocatalytic reduction by acting as charge collectors that quickly accept the photoexcited electrons from the CB of the photocatalyst inhibiting their simultaneous recombination reactions. This increases the lifetime of charge carriers for an enhanced photoreduction of CO2. Apart from this beneficial effect, the active sites provided by high surface area carbon materials do also enhance the reaction rate as shown in Fig. 1(c). Previously, we (Kandy et al.) have shown that the addition of Mn2O3 to the CdS photocatalyst does simply enhance the product yield without altering the product selectivity of the CdS photocatalyst towards C2H5OH and HCOOH formation.26 Nougeria et al.27 have studied the influence of reaction media on product selectivity as shown in Fig. 1(d). The results demonstrated that the use of different electrolytes strongly influenced product selectivity like NaOH directed generation of CH4, Na2C2O4 to CO, and KBrO3 to O2 along with CuO crystalline phase changes.
In spite of unremitting research with a huge number of reports in this area, information about the comprehensive mechanism and activation steps are still lacking. Concerning the above discussions, it is well understood that the process of CO2 photoreduction is very complex, involving intricate pathways with several intermediates. Hence, it is very difficult to obtain product selectivity that obstructs the studies of the mechanism, which subsequently obstructs the photocatalyst design. Ideally, an understanding of the requirements of photocatalytic reactions that can initiate, carry forward, and regulate product distributions is the most critical part of the rational design of industrially viable solar-fuel conversion systems. Owing to the strong dependence of product formation on catalyst design and reaction conditions, surface engineering together with optimization of reaction parameters is extremely desired to obtain enhanced CO2 photoreduction systems as shown in Fig. 1.
3. Photocatalyst for CO2 reduction
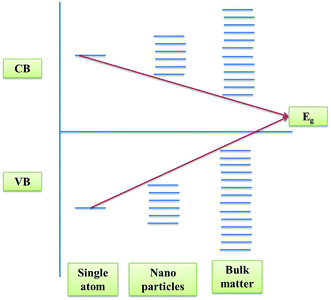
Numerous methodologies have been used to improve the performance of identified photocatalysts, like structural design to endure improved morphology and crystallinity of the photocatalyst, innovative architectures with the use of porous and conducing supports, overview of cationic and anionic doping, and introduction of point defects.24 The design of a perfect photocatalyst with enhanced photocatalytic performance and stability is the initial and vital step. The design of the bandgap of a photocatalyst is an important criterion during its synthesis. Nanomaterials apart from their high surface area also possess high bandgaps compared to their bulk materials resulting in an enhanced photocatalytic activity.28 This phenomenon of confinement of electrons is known as quantum confinement of electrons as shown in Fig. 2.3.1 Structural engineering of the photocatalyst
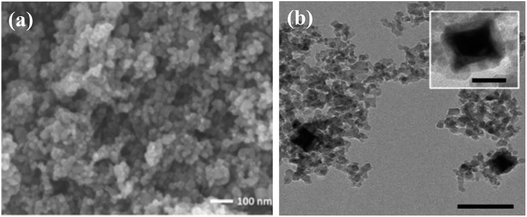
The diversity in morphological and structural properties of photocatalysts adds to their light extraction properties. Size reduction from the bulk form to nanoparticles has increased the photocatalytic efficiency due to the exceptionally improved properties of nanomaterials. Facile transport of photoexcited charge carriers to the photocatalyst surface can be achieved due to a reduction in path length. The increase in the surface area using nanomaterials with hollow and mesoporous shells offers a large number of active sites for enhanced diffusion, adsorption, and the reaction of reactant molecules leading to enhanced photocatalytic activity. Cui et al.29 after their scientific survey have reported that ordered mesoporous metal oxides displayed enhanced photocatalytic activity in comparison with non-porous metal oxides due to their improved features. Small mesoporous TiO2 nanocrystals synthesized by Zhang et al.30 as shown in Fig. 3(a) display much better photocatalytic conversion of CO2 to CH4 than commercial Degussa P25 TiO2 photocatalysts. The mesoporous TiO2 possesses huge accessible and active catalytic sites as shown in Fig. 4(a) to display an enhanced rate. Brunauer–Emmett–Teller (BET) theory explains the physical adsorption of gas molecules like N2 on a photocatalytic surface and assists an important analysis technique for the measurement of the specific surface area and porosity. The hollow nature of TiO2 spheres has aided in the integration of a cobalt complex for higher separation of charge carriers that is attributable to enhanced photoconversion. The N2 adsorption/desorption isotherm of the self-assembled hollow structured TiO2 gives a type-IV isotherm with a type H3 hysteresis loop, which indicates its mesoporous structure.31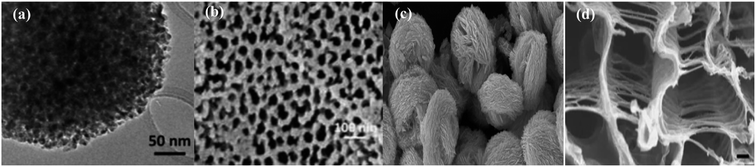 | ||
| Fig. 3 FE-SEM and TEM images of (a) mesoporous TiO2, reprinted with permission from ref. 30 copyright 2018, ACS publishing group, (b) CdS nanorods on a porous anodic alumina (PAA) support, reprinted with permission from ref. 25 copyright 2018, Elsevier publishing group, (c) ball-flower-like Bi2WO6, reprinted with permission from ref. 46 copyright 2014, Elsevier publishing group, and (d) SrTiO3 as a photocatalyst, reprinted with permission from ref. 48 copyright 2013, Nature publishing group. | ||
 | ||
| Fig. 4 Schematic illustration of enhanced photocatalytic reduction of CO2 using (a) mesoporous TiO2, reprinted with permission from ref. 30 copyright 2018, ACS publishing group, (b) carbon-coated Cu2O, reprinted with permission from ref. 33 copyright 2016, ACS publishing group, (c) CdS/Mn2O3, reprinted with permission from ref. 26 copyright 2019, Elsevier publishing group, and (d) GCN/Cu2O as a photocatalyst, reprinted with permission from ref. 47 copyright 2019, Elsevier publishing group. | ||
Intensification in light-harvesting efficiency is the key objective of the photocatalyst synthesis strategy. 1D nanostructures exhibit significantly higher photocatalytic activity due to the quantum confinement effect and interesting optical properties. Previously, it was reported that interesting 1D CdS nanorods grown on a porous support are capable of light entrapment in between the nanorods as shown in Fig. 3(b). The availability of immense light and reactant CO2 molecules near the surface has led to higher photocatalytic activity.25 Wang et al.32 have reported the significant influence of size and structure on photocatalytic reduction of CO2. The results demonstrated that the huge surface area and single crystallinity of the 1D structure of TiO2 films and the proficient electron–hole separation by the Pt NPs of nm size were attributable to this development. The thin carbon layer coated on the Cu2O photocatalyst by Yu et al.33 generates mesoporous 1D nanorods to facilitate enhanced CO2 adsorption. Significantly, the carbon-coated samples revealed better stability under visible light irradiation. The light entrapment well created in between 1D nanorods allows multiple scattering of incident light in the vicinity of the photocatalyst increasing its photocatalytic performance. This light intensive area would also be accessible for the permeation of reactants towards electron-rich catalytically active sites as shown in Fig. 4(b).
Synthesis of 2D photocatalysts in the form of nanosheets contributes greatly to enhanced CO2 reduction due to their fascinating electronic and optical properties. Extremely favorable features like high surface area assist in light-harvesting, mass transportation, surface availability to reactants, and diminishing electron–hole recombination resulting in an improved photocatalytic performance.15,34 The discovery of fascinating 2D carbon materials like graphitic carbon nitride (GCN) has opened up a new avenue of metal-free photocatalysts in the process.35 2D materials provide an excellent platform for the synthesis of various heterostructures.36 Previously, nanoporous CdS nanosheets enhance the adsorption of reactant molecules on the photocatalyst surface. Additional progress in the photocatalytic activity is achieved by uniformly loading Mn2O3 nanoparticles on CdS nanosheets as shown in Fig. 4(c).26 It was also noted that it is important for 2D nanomaterials to have an ultrathin structure to exhibit excellent light-harvesting, and facile migration of charge carriers from the bulk to the surface. Improved electronic and optical properties are achieved when multilayered materials are thinned to single or few layers. For instance, C-doped SnS2 nanoplates exhibited lower photocatalytic activity in comparison with SnS2 nanoplates due to their bigger and thicker morphology. Photoluminescence and time-resolved photoluminescence (TRP) studies have been extensively performed to study the transfer and exciton separation performance of photoexcited electrons and holes. It was observed that the two PL peaks corresponding to the recombination of charge carriers become weak after carbon doping which signifies efficient interfacial transfer in the nanocomposite photocatalytic system.37 GCN has enormous applications as a photocatalyst due to its significant optical and electronic properties. This metal devoid photocatalyst with a bandgap of 2.7 eV has been recently developed as a low cost and robust visible light active photocatalyst.38 Mao et al.39 fabricated two different kinds of GCN photocatalysts by pyrolysis of urea or the melamine substrate. The mesoporous flake-like structure of urea derived GCN leads to further effective surface adsorption, rapid photogenerated charge carrier separation, and finally enhanced photoactivity compared to the non-porous flaky melamine derived GCN. However, due to the high recombination of photogenerated charge carriers, low CO2 activation ability limits the potential use of a single-component system. Therefore, the fabrication of GCN with binary or ternary heterostructures or heterojunctions would be a highly efficient and stimulating way to progress photoreduction of CO2 to solar fuels. Accordingly, there are primarily four types of heterojunctions that include type I, type II, type III, and Z-scheme heterojunction systems.40 The assembly of GCN based heterojunctions will improve the properties of composite materials, such as magnified absorption range, separation, and migration of photogenerated charge carriers. A profusion of GCN based heterojunctions have already been well reported.41 Typically, photocatalysts like metal sulfides and metal oxides were combined with GCN to obtain a Z-scheme photocatalytic system, mimicking the natural photosynthesis, and Z-scheme photocatalysts have been proven to be extremely beneficial due to their high light-harvesting, separation of charge carriers and strong redox ability.42 The design of a Z-scheme heterojunction is an effective way to overcome the challenges faced by photocatalysts including those related to their activity and stability. Z-scheme heterostructures utilize the low valence band maximum (VBM) of one photocatalyst and high conduction band minimum (CBM) of another photocatalyst to inhibit the highly retarding recombination effect.43 Di et al.44 have constructed a direct Z-scheme heterojunction of SnS2 quantum dots on GCN. The heterostructures existing in the nanocomposite create a Schottky barrier that would further prevent electron–hole recombination giving rise to enhanced photocatalytic activity. The abundant surface active sites that are in the vicinity and in contact with reactants enable 2D materials to have superior photocatalytic performance. Apart from this, the amine groups that are anchored on the GCN surface during their hydrothermal synthesis procedure have increased the CO2 adsorption capacity of the nanocomposite.
The 3D flower-like morphology can expand light harvesting, and also assist in bringing the reactant molecules towards the TiO2 reactive sites from bulk matter.45 The 3D hierarchical ball-flower-like Bi2WO6 photocatalyst synthesized by Sun et al.46 as shown in Fig. 3(c) has exhibited enhanced performance due to enhanced crystallinity. A novel strategy for assembling 0D Cu2O photocatalysts on 3D hierarchical GCN foam nanocomposite systems as shown in Fig. 4(d) was designed by Sun et al.47 The unique porous structure and the synergistic effect between GCN foam and Cu2O speed up the adsorption and reaction process to maximize photocatalyst kinetics. The progress of artificial photosynthetic systems together with the corresponding significant structural components and reaction driving features to achieve enhanced photocatalytic CO2 reduction has been reported. Artificial photosynthetic systems are developed by mimicking both the significant structural elements and reaction structures of natural photosynthesis. This results in the construction of a well-organized mass flow network, high surface area, and exceptional 3D architecture for enhanced light harvesting. The process mimics the functionality of leaves and is responsible for the high yield of both gas phase and liquid phase products. Zhou et al.48 have designed leaf architecture 3D hierarchical perovskite titanates as shown in Fig. 3(d) to mimic the functional role of a real leaf for enhanced photocatalytic performance. The detection of the VB potential edge and CB potential edge of perovskites including SrTiO3 and CaTiO3 is critical to develop a design strategy for a 3D artificial leaf.
The precise engineering of photocatalysts with exceptional morphology has provided with unique features such as large surface area, enhanced light-harvesting, and active sites for CO2 adsorption and reaction as summarized in Table 2. This does serve as the initial and vital step in any photocatalytic process. However, it is also very important to control the size of photocatalysts and the size of mesopores that are present on their surface to encourage an enhanced process. In general, it would be ideal to establish an approach from nature to utilize synthetic prototypes. A green leaf can be assumed as a large surface solar collector with a large number of minute photocatalytic cells. The efficiency of green leaves in high photoconversion of CO2 calls for a biomimetic methodology in this area. The microscopy images of photocatalysts illuminating different shapes and schematic illustrations of their influence on photocatalytic reduction of CO2 as reported by various researchers are presented in Fig. 3 and 4 respectively. The need to fabricate efficient photocatalysts has driven the research interest for their precise structural engineering.
| Catalyst | Source of radiation | Sacrificial agent | Co-catalyst | Gas product (μmol g−1 h−1) | Liquid product (μmol g−1 h−1) | Ref. |
|---|---|---|---|---|---|---|
| CdS/Mn2O3 | Sunlight | H2O | — | — | C2H5OH (52.2), HCOOH (1392.3) | 26 |
| Mesoporous TiO2 | 300 W solar Xe arc lamp | H2O | — | CH4 (14.75) | — | 30 |
| CdS/PAA | 10 W LED lamp | H2O | — | — | CH3OH [144.5] | 25 |
| TiO2 | 400 W Xe lamp | H2O | Pt | CH4 (1361.0) | — | 32 |
| GCN | 300 W Xe lamp | 1.0 M NaOH | — | — | CH3OH, C2H5OH [6.3, 4.5] | 39 |
| Bi2WO6 | 300 W Xe arc lamp | H2O | — | CO [0.5] | — | 46 |
| GCN/Cu2O | 300 W Hg lamp | H2O | — | CO [8.2] | — | 47 |
3.2 Supported catalysts
The challenge of aggregation of nanoparticle resulting from their high surface area limits their practical use. The immobilization of photocatalysts on suitable supports that increase the dispersion density of nanoparticles improves the surface area and provides superior exposure to light, thus overcoming the challenge of nanoparticle aggregation.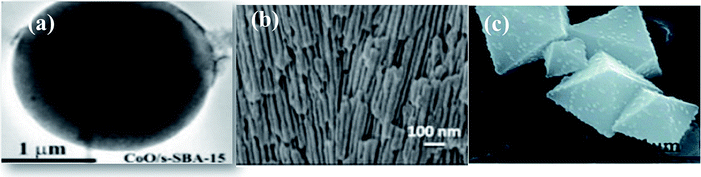 | ||
| Fig. 5 (a) TEM image of CoO/mesoporous silica, reprinted with permission from ref. 53 copyright 2019, RSC publishing group, (b) CdS/PAA,25 reprinted with permission from ref. 25 copyright 2018, Elsevier publishing group, and (c) CdS/UiO-bpy/Co, reprinted with permission from ref. 63 copyright 2018, RSC publishing group. | ||
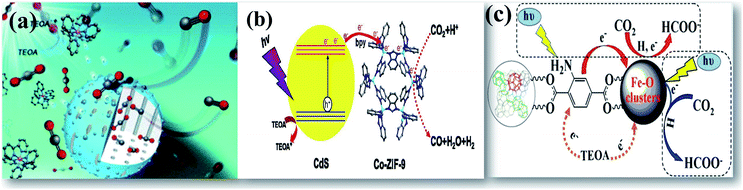 | ||
| Fig. 6 Schematic illustration of enhanced photocatalytic reduction of CO2 displayed by (a) CoO/mesoporous silica, reprinted with permission from ref. 53 copyright 2019, RSC publishing group, (b) CdS on a zeolitic framework, reprinted with permission from ref. 54 copyright 2015, Elsevier publishing group, and (c) Fe containing MOF, reprinted with permission from ref. 56 copyright 2014, ACS publishing group. | ||
Metal organic frameworks (MOFs) are a class of recently developed materials consisting of the inorganic–organic porous structure.18 Recent advancement of these materials in photocatalytic applications as photocatalysts and as supports has been encouraged due to their unique electronic band structure, tailored light absorption, high CO2 adsorption, and high surface area.55,56 Cu–TiO2 on a molecular sieve support shows increased photocatalytic performance in selective yield of oxalic acid in addition to other energy fuels.57 Crake et al.58 have optimally loaded TiO2 nanosheets on a MOF to maintain the porosity of the framework for high CO2 adsorption. The heterojunction created in the bifunctional structure increases the lifetime of charge carriers that drives an enhanced photoreduction of CO2. The use of conventional molecular sieves would, however, display less enhancement due to the large crystal size. This would limit CO2 adsorption and surface excited states, limiting the mass transfer of reactants and products.59 Wang et al.56 have shown in their studies that an amine-functionalized Fe containing MOF displayed higher photocatalytic activity due to the presence of coordination unsaturated sites and dual excitation pathways. DRS spectroscopy shows that the band in the UV region is recognized for charge transfer from oxygen to iron in an octahedral coordination environment. The other band in the visible light region can be attributed to the Fe3O clusters in MIL-101(Fe). The presence of an electron trap in MOFs would inhibit the electron–hole recombination, thereby enhancing photocatalytic reactions. Xu et al.61 have demonstrated a photoactive porphyrin-based MOF for enhanced photocatalytic reduction of CO2. Consequently, the role of cocatalysts is of great importance for CO2 activation and to avoid the formation of thermodynamically unfavorable intermediates.62 Chen et al.63 have designed a ternary nanocomposite of inorganic TiO2 semiconductors and molecular redox catalysts of cobalt through zeolitic imidazole frameworks UiO-bpy for photoreduction of CO2 as shown in Fig. 5(c). The rich active sites offered for CO2 adsorption and photoreduction after integrating inorganic semiconductors on MOFs boosting electron–hole separation encourage their potential as emerging photocatalysts.
For photocatalytic reactions, it is highly advantageous to have a hollow interior structure like a hollow core or hollow channel for enhanced light availability due to reflection or multiple scattering phenomena.31,64,65 Previously, it has been shown that unique honeycomb nanopores of PAA act as nanoreactors for the growth of CdS nanorods. CdS has been synthesized along the pore walls of PAA retaining its porous nature as shown in Fig. 5(b). These pores later assist in the adsorption of CO2 molecules and also help in trapping of light energy.25 Brunetti et al.66 have incorporated the CN–TiO2 photocatalyst inside the pores of the Nafion membrane to significantly enhance the rate of mass transfer of CO2 and enhance the accessibility of reactants and light towards the catalyst surface, an easier recovery of the catalyst for its reuse.
The use of porous supports of high surface area increases the dispersion of photocatalysts and increases CO2 and water adsorption which enable the reactant molecules to be accessible in the surrounding of the photocatalyst as summarized in Table 3. The findings to date that highlight the features and advantages of involving porous supports can be considered as nanotechnology advancement for enhanced photocatalytic reduction of CO2. The TEM images of photocatalysts on porous supports and schematic illustration of the influence of porous supports on enhanced photocatalytic performance are illustrated in Fig. 5 and 6 respectively.
| Catalyst | Source of radiation | Sacrificial agent | Co-catalyst | Gas product (μmol g−1 h−1) | Liquid product (μmol g−1 h−1) | Ref. |
|---|---|---|---|---|---|---|
| Cu2O/SiO2 | White LED lamp | H2O | Ru | CO [25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 626] 626] |
— | 53 |
| CdS/zeolitic imidazole framework | 300 W Xe arc lamp | Bipyridine, triethanol amine | — | CO [2520.0] | — | 54 |
| TiO2/HKUST-1 MOF | 1500 W Xe lamp | H2O | Cu | CH4 [0.18] | — | 60 |
| CdS/UiO-bpy | Visible light | Triethanol amine | Co | CO [235] | — | 63 |
| C3N4/TiO2/Nafion | Hg vapor pressure lamp | H2O | — | — | CH3OH [17.9], HCHO [27], C2H5OH [14.9], CH3COCH3 [1.8] | 66 |
Graphene has been recognized as an efficient platform for building nanocomposite photocatalytic systems.67 The Fermi level of RGO is lower than the CB of most of the photocatalysts permitting a swift capture of photogenerated electrons and transportation within itself. The ample availability of free photogenerated electrons over the RGO surface assists in photocatalytic CO2 reduction reactions. The photocatalyst is essentially involved in charge generation, which remains vital in the process. The RGO material gifted with a wide π–π conjugation structure has exceptional conductivity of electrons and increases dispersion due to its extended surface area.1 An improved interfacial bonding between conducting RGO and the TiO2 photocatalyst has effectively transferred photoexcited electrons of the TiO2 CB to RGO for enhanced photoreduction of CO2. The enhanced adsorption of reactant CO2 molecules on RGO based conductive supports increases the rate of photocatalytic reduction of CO2 to obtain CH4.68 These inexpensive carbon materials as supports for photocatalysts have shown higher photocatalytic performance over noble metals.69 Rambabu et al.70 have constructed a unique nanocomposite structure of GO/RGO wrapped TiO2 nanotubes for the photocatalytic reduction of CO2. The unique nanocomposite structure assisted a facile separation of photogenerated electron–hole pairs to achieve enhanced reduction of CO2 molecules that are adsorbed on the surface. Jung et al.71 have demonstrated that controlled morphologies of the macropores and mesopores on a hierarchical TiO2/3D graphene/MoS2 nanocomposite as shown in Fig. 7(a) and 8(a) play a leading role in photocatalyst performance. BET analysis of the nitrogen adsorption/desorption isotherms specified that the specific surface area and higher micropore volume of the macroporous nanocomposite are much higher than those of the non-macroporous composite. The macroporous structure as observed by morphological analysis like SEM offered by the 3D graphene aerogel forms an efficient mass transport network that increases the surface area and mechanical/chemical stability. Stumpy and high loading of RGO as a support for the CdS photocatalyst displays lower photocatalytic performance. The black-colored RGO exhibits zero bandgaps after exhibiting an entire absorption of the solar spectrum. However, this superior absorption does not generate any active charge carriers like electrons and holes for the photocatalytic reaction to take place. Overloading of graphene will hinder the absorption of light by the photocatalyst that is deposited on its surface. It was also found that an optimal loading of RGO serves as an important criterion.69,72 The rate of photocatalytic conversion of CO2 to CH3OH has been shown to be 36 times higher for the RGO–Cu2O nanorod system than pure Cu2O nanorods by Liu et al.73 after an optimal loading of 5% of RGO. Yang et al.74 have recently reported a metal-free photocatalyst by fabricating quinacridone (QA) particles on the RGO substrate. An RGO content of 2 wt% was recognized to be the optimal loading that expresses its assistance and obstructs its limitation in the enhancement of the process. Photocurrent measurements helped to compute the structure of QA and optimal loading of QA and RGO in their nanocomposite for a favorable transfer of photogenerated charge carriers.
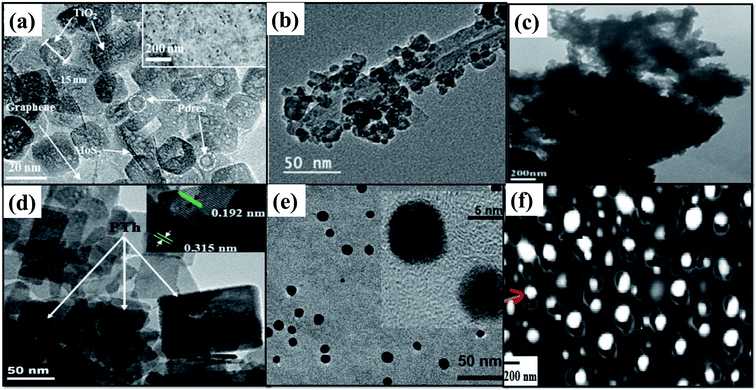 | ||
| Fig. 7 TEM image of (a) TiO2/RGO/MoS2, reprinted with permission from ref. 71 copyright 2018, ACS publishing group, (b) CNT/TiO2, reprinted with permission from ref. 78 copyright 2019, ACS publishing group (c) ZnFe2O4/TiO2/polyaniline, reprinted with permission from ref. 82 copyright 2015, Elsevier publishing group, (d) Bi2WO6/polythiophene, reprinted with permission from ref. 84 copyright 2015, Elsevier publishing group, (e) carbon nanoparticles/Au, reprinted with permission from ref. 85 copyright 2011, ACS publishing group, and (f) NiO/TiO2 on an activated carbon nanofibre, reprinted with permission from ref. 86 copyright 2017, Elsevier publishing group. | ||
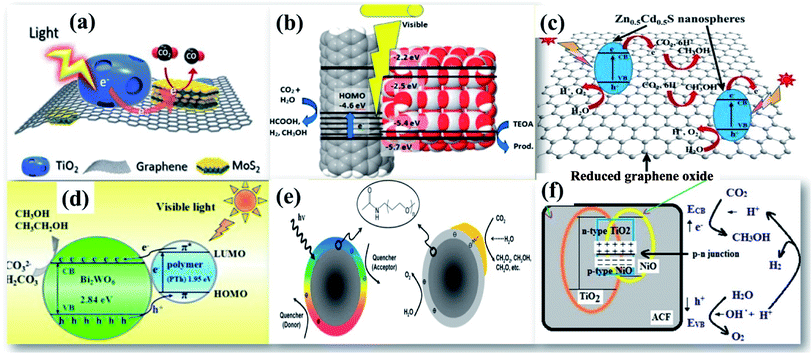 | ||
| Fig. 8 TEM image of (a) TiO2/RGO/MoS2, reprinted with permission from ref. 71 copyright 2018, ACS publishing group, (b) CNT/TiO2, reprinted with permission from ref. 78 copyright 2019, ACS publishing group, (c) Zn0.5Cd0.5S, reprinted with permission from ref. 76 copyright 2020, Elsevier publishing group, (d) Bi2WO6/polythiophene, reprinted with permission from ref. 84 copyright 2015, Elsevier publishing group, (e) carbon nanoparticles/Au, reprinted with permission from ref. 85 copyright 2011, ACS publishing group, and (f) NiO/TiO2 on an activated carbon nanofibre, reprinted with permission from ref. 86 copyright 2017, Elsevier publishing group. | ||
A tight contact between different components in the nanocomposite is highly essential to display their symbiotic effects. Olowoyo et al.75 have synthesized a self-assembled RGO/TiO2 nanocomposite for superior photocatalytic reduction of CO2. Theoretical investigations by density functional theory (DFT) calculations suggested that photocatalytic reactions continue by the mechanism of transfer of photogenerated electrons to RGO using UV light while visible light assisted CO2 reduction generates electrons in RGO and holes in TiO2 as a consequence of charge transfer photoexcitation. Despite presenting such improved performance, RGO based photocatalysts still suffer from π–π stacking interactions that lead to a high agglomeration of the photocatalyst, which hinders their use as potential catalysts for practical applications. The development of interesting materials like wrinkled, crumpled, and nanoporous graphene RGO has attracted great interest among researchers. Previously, it has been reported that CdS nanorods deposited on nanoporous RGO exhibit an enhanced photocatalytic reduction of CO2 to CH3OH with high photocatalytic stability.69 Madhusudan et al.76 have fabricated graphene interlayered between Zn0.5Cd0.5S hierarchical nanospheres as shown in Fig. 8(c) to enable a rapid charge transfer channel that shields against photocorrosion with improved photogenerated charge carrier separation.
Multi-walled carbon nanotubes (MWCNTs) serve as alternative promising carbon-based conductive supports for photocatalysts. This carbon material has effectively increased the photocatalytic performance by decreasing the bandgap energy, suppressing photogenerated electron–hole recombination, increasing the adsorption of CO2 on the photocatalyst surface, and shifting the redox potential, selectivity, and activity. The Fermi level of CNTs is −0.2 V and therefore the material retains the ability to receive photogenerated electrons from the CB of the photocatalyst and transports through its unique multiple concentric cylindrical structure after combining it with an appropriate photocatalyst.77 Olowoyo et al.78 have studied the feasibility of the high potential of the CNT/TiO2 nanocomposite under both UVA and visible light. The CNT–TiO2 interface as shown in Fig. 7(b) and 8(b) acts as an absorption site for photons with concurrent addition of electrons into the TiO2 CB. The synthesis of the Ag-MWCNT@TiO2 ternary nanocomposite by Gui et al.79 showed a 1.60 times increase in photocatalytic yield of CH4 by adding 2 wt% Ag over the undoped-MWCNT@TiO2 core–shell nanocomposite. The replacement of MWCNTs with two-dimensional graphene as a conducting support using the cobalt chlorin complex as a photocatalyst has shown lower photocatalytic activity, which further highlights the influential role of the three-dimensional assembly of MWCNTs on photocatalytic systems.80
Other conducting forms of carbon like conducting polymers, carbon nanoparticles, carbon nanodots, activated carbon spheres, and carbon fibers have also been observed to show a similar beneficial effect. Recently, Ong et al.81 have fabricated a well-contacted heterojunction interface between 2D protonated GCN and 0-dimensional carbon nanodots as shown in Fig. 7(c). The protonated GCN via electrostatic attraction favors the uniform dispersion of negatively charged carbon nanodots of 4.4 nm size. They exhibited enhanced CO2 photoreduction performance due to the effective transfer of photogenerated electrons from protonated g-C3N4 (PGCN) to conducting carbon nanodots. The low charge transfer resistance at their interface was evidenced by the minimum arc radius in the EIS Nyquist diagram.
Conducting polymers like polyaniline and dye-sensitized solar cells are less exploited in photocatalytic CO2 reduction but are more explored in photocatalytic methylene blue degradation and also to develop photo-electrochemical cells for CO2 reduction. Conductive polymers are suitable photo-support materials due to their remarkable features, like high electrical conductivity, good flexibility, controllable performance, and ease of handling. Polyaniline is a promising material for the photocatalyst support due to its high conductivity, photoresponse to ultraviolet-visible light, and also effective prevention of the recombination of photogenerated electrons and holes.82 The photocatalytic efficiency of the polyaniline–polytitanate–clay (PPTC) nanocomposite has been determined using photodegradation studies of dyes under natural sunlight.83 Various conducting polymers like polyaniline, polypyrrole, and polythiophene have been exploited by Dai et al.84 as conducting supports for the Bi2WO6 photocatalyst as shown in Fig. 7(d). The nanophotocatalyst exhibited the highest CH3OH and C2H5OH yield due to the narrow bandgap and improved charge transfer as observed from its low PL intensity. Cao et al.85 have developed an aqueous soluble carbon photocatalyst as shown in Fig. 7(e) that is capable of driving this reaction under homogenous conditions. These small carbon nanoparticles with active surface functional groups and added noble metals are highly effective in harvesting visible light and charge separation. This can create surface-confined charge carriers that promote an enhanced photocatalytic process as shown in Fig. 8(e). The activated carbon fiber support similar to other conducting carbon supports reduced the recombination of photogenerated charge carriers and increased the photocatalytic conversion rate of Ni-doped TiO2 photocatalysts as shown in Fig. 7(f) and 8(f). The catalyst was easy to separate from the reaction medium and retained excellent stability during its repeated use.86
Apart from their primary function of swift capture and circulation of photogenerated electrons, these conducting substrates also increase the photocatalytic performance of the deposited photocatalyst by providing increased surface area and enhanced CO2 adsorption and induce a visible light response. Liu et al.87 have used phenolic resin-based activated carbon spheres to impregnate the BiOBr photocatalyst. The high BET surface area calculated from N2 adsorption isotherms provides more accessible activation sites for CO2 adsorption. The higher amine-functionalized graphene/CdS nanocomposite used as a photocatalyst by Cho et al.88 shows 20 times higher photocatalytic conversion to CH4 than RGO/CdS. This can be recognized to be due to higher CO2 adsorption after amine functionalization.
The most commercially used TiO2 photocatalyst exhibits its activity in the UV range. This is only 3–5% of the solar spectrum, which limits its use in the visible region. Tan et al.89 have fabricated a visible light active RGO–TiO2 photocatalyst with enhanced visible-light photoactivity. The nanocomposite has displayed enhanced photocatalytic conversion of CO2 to CH4. The excellent optical properties displayed by graphene quantum dots drive zinc-based MOFs towards visible light photocatalytic performance with the generation of more photoinduced electron–hole pairs.90
The significant potential of conducting carbon supports as platforms for photocatalysts to drive photocatalytic reactions to an enhanced version from their conventional version of production of low photocatalytic yield is well perceived as summarized in Table 4. The conducting carbon support not only acts as a photogenerated electron capture layer but also assists in enhancing CO2 adsorption, increasing the visible light response, increasing the surface area, and retarding photocorrosion of photocatalysts. Previously, in our recent article on carbon-based photocatalysts, the beneficiary roles of carbon materials and their probable limitations are well examined.1 The TEM images of various photocatalysts on carbon supports and schematic illustration of conducting supports as electron acceptors are presented in Fig. 7 and 8.
| Catalyst | Source of radiation | Sacrificial agent | Co-catalyst | Gas product (μmol g−1 h−1) | Liquid product (μmol g−1 h−1) | Ref. |
|---|---|---|---|---|---|---|
| RGO/TiO2 | 500 W Hg lamp | H2O | — | CH4, CO (12.75, 11.93) | — | 68 |
| RGO/CdS/PAA | Sunlight | H2O | — | — | CH3OH (153.8) | 25 |
| RGO/TiO2 | 200W UV-A lamp | H2O | — | CO (380) | — | 71 |
| RGO/Cu2O | 300 W Xe arc lamp | H2O | — | — | CH3OH (17.8) | 73 |
| RGO/quinacridone | 300 W Xe arc lamp | Triethanolamine | — | CH4, CO (275, 450) | — | 74 |
| RGO/TiO2 | 8 W UV-A lamp | Triethanolamine | — | — | CH3OH (2330.0) | 75 |
| Zn0.5Cd0.5S | 300 W Xe arc lamp | NaHCO3 | CH3OH (1.96) | 76 | ||
| CNT/TiO2 | 8 W UV-A lamp | Triethanol amine | — | — | CH3OH, HCOOH (2360.0, 68.5) | 78 |
| MWCNT/TiO2 | 15 W LED lamp | H2O | Ag | CH4,C2H2 (0.91,0.048) | — | 79 |
| pGCN | 300 W Xe arc lamp | H2O | — | CH4, CO (2.92, 5.88) | — | 81 |
| Polythiophene/Bi2WO6 | 300W Xe lamp | H2O | — | — | CH3OH, C2H5OH (14.1, 5.1) | 84 |
| BiOBr/activated carbon | 300W Xe lamp | H2O | — | CO (23.74) | — | 87 |
| Amine functionalized RGO/CdS | Visible light | H2O | — | CH4 (2.84) | — | 88 |
3.2.2.1 Biomimetic support. The process of photocatalytic reduction of CO2 is often regarded as artificial photosynthesis as the process mimics the natural process of photosynthesis. Therefore, the function of a leaf needs to be imitated by the photocatalyst. Biomimetic materials are materials that are developed after procuring inspiration from nature.91 Biomimetic strategies that incorporate gentle and precise structures of natural species are developed to solve more proficiently the issues regarding photocatalysis as those schemes are sustainable.
Tseng et al.92 have duplicated the structure of Xanthosoma sagittifolium on the surface of a flexible and thermally stable polyimide surface by a nanocasting technique. Copper oxide was introduced onto the hierarchical structure by ion exchange followed by thermal treatment. The biomimetic surface enhances the adsorption of reactant molecules and light capture and also tunes the hydrophobicity of the surface. The enhanced hydrophobicity of the surface as observed in contact angle measurements increases CO production. Chen et al.93 have recently used polyimide covalent organic frameworks with tunable porosity as biomimetic surfaces. Single Ni sites were integrated into the biomimetic channels to render enhanced and selective photocatalytic reduction of CO2 to CO. The development of a biomimetic surface requires careful examination of systems and processes of nature to derive the path that runs on the least expenditure of energy. Zhou et al.94 have fabricated a ternary nanocomposite of TiO2 photocatalyst/carbon@MOF to obtain a well-integrated leaf branch structure with excellent functional roles like enhanced photocatalytic efficiency, selectivity, and stability as shown in Fig. 9.
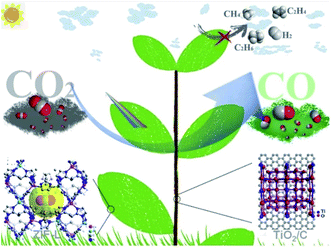 | ||
| Fig. 9 Schematic illustration of the ternary nanocomposite of TiO2 photocatalyst/carbon@MOF with a well-integrated leaf branch structure for enhanced photoreduction of CO2, reprinted with permission from ref. 94 copyright 2020, Elsevier publishing group. | ||
These advantages and distinctive properties are exploited by several researchers to fabricate bioinspired constituents to customize a sustainable technology as shown in Table 5. The extremely efficacious process of photosynthesis by nature is quite inspiring and should be the driving force to fabricate artificial leaves for artificial photosynthesis. The selection of biomimetic structures and tuning of their functional parameters should be the key to the photocatalyst design.
| Catalyst | Source of radiation | Sacrificial agent | Co-catalyst | Gas product (μmol g−1 h−1) | Liquid product (μmol g−1 h−1) | Ref. |
|---|---|---|---|---|---|---|
| Cu2O/polyimide | 14 W LED lamp | H2O | — | CO (0.008) | — | 92 |
| Ni/polyimide covalent organic framework | 300 W Xe lamp | Triethanolamine | — | CO (483.25) | — | 93 |
| TiO2/carbon@MOF | 300 W Xe lamp | H2O | — | CO (28.6) | — | 94 |
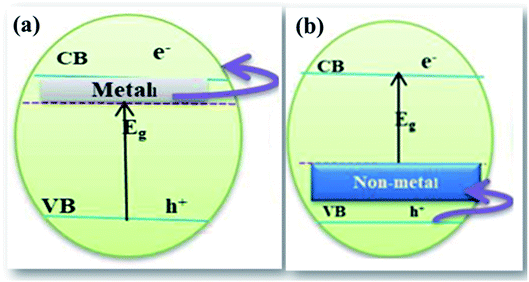 | ||
| Fig. 10 Schematic illustration of (a) cationic doping and (b) anionic doping.95 | ||
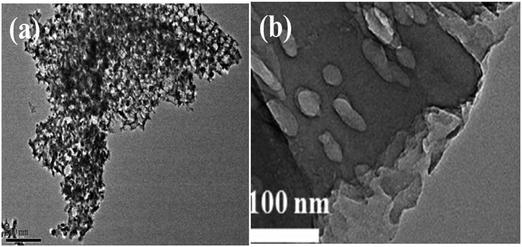 | ||
| Fig. 11 TEM images of (a) IOB–TiO2/Ni, reprinted with permission from ref. 100 copyright 2019, Elsevier publishing group and (b) S doped TiO2, reprinted with permission from ref. 111 copyright 2015, Elsevier publishing group. | ||
Anionic doping chiefly involves the addition of non-metals like N, C, and S to extend activity in the visible range. Additional energy states are formed nearer to the VB with anionic doping.108 The presence of these mid states caused by impurities enables the electrons to jump from the VB of the photocatalyst to the intra energy band of the anion that is occupied above the VB and from the impurity state to the CB. This results in the increase in the lifetime of photogenerated electrons and is accountable for enhanced activity. Tashibi et al.109 have synthesized carbon-doped TiO2 to create a heteroatomic external structure with modified physicochemical properties. Nitrogen doping on the photocatalyst increases the visible light absorbance and reduces electron–hole recombination. The ionic radius of nitrogen is similar to that of oxygen, which facilitates its substitution in the oxygen lattice of metal oxides enhancing its visible light absorption. Nitrogen-doped TiO2 extends its light absorption capability from UV light to the visible light region, making it a more promising photocatalyst. UV Vis DRS spectra display a considerable shift from UV light to the visible light region after N doping on TNTs.110 Sulfur-doped photocatalysts, similarly to N doped photocatalysts like GCN, exhibit wide bandgap energy and reduce Gibbs free energy from 1.43 eV to 1.15 eV giving rise to a visible-light response. The TEM image of S doped TiO2 is given in Fig. 11(b).111
Doping into photocatalysts would be a capable strategy for enhanced photocatalytic reduction of CO2 as summarized in Table 6. The TEM images of various dopant incorporated photocatalysts are given in Fig. 11. The effective utilization of the stimulating properties of these fascinating ingredients that include structural engineering, incorporation of porous and conducting supports, and the inclusion of dopants and point defects to form a proficient photocatalytic system would be a more favorable method. The remarkable properties exhibited by these nanocomposite structures like high surface area, visible light activity, higher photogeneration of charge carriers, and their lower recombination rates illustrate their potential to act as artificial leaves that display enhanced photoreduction of CO2. This review thus provides an insight into the rational design of well-integrated nanocomposite structures to boost photocatalytic performance via a balanced synergistic effect.
| Catalyst | Source of radiation | Sacrificial agent | Co-catalyst | Gas product (μmol g−1 h−1) | Liquid product (μmol g−1 h−1) | Ref. |
|---|---|---|---|---|---|---|
| IOB–TiO2 | 300 W Xe lamp | NaOH, Na2SO3 | Ni | CO (12.1) | — | 100 |
| N-doped TiO2 | 500 W tungsten halogen lamp | NaOH | — | — | HCOOH (1210.8) | 110 |
| S-doped GCN | 300 W simulated solar Xe arc lamp | H2O | Pt | — | CH3OH (0.38) | 111 |
 | ||
| Fig. 12 (a) SEM images of Au/Ti, reprinted with permission from ref. 117 copyright 2018, ACS publishing group. (b) TEM image of Rh/Al2O3, reprinted with permission from ref. 118 copyright 2017, Nature publishing group. | ||
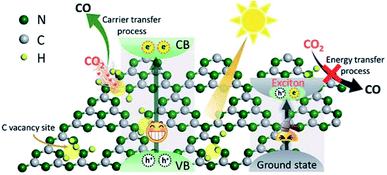 | ||
| Fig. 14 Schematic illustration of enhanced photocatalytic reduction of carbon vacancy modified GCN, reprinted with permission from ref. 126 copyright 2019, RSC publishing group. | ||
The rising trend in the engineering of photocatalysts with point defects would be a promising approach for higher interaction of reactant molecules like CO2 on the photocatalyst surface. Consequently, it is rational to study the improvement in photocatalytic yield by the introduction of atomic defects like vacant sites in crystalline lattices.
4. Reaction parameters
To efficiently utilize the merits of photocatalytic reactions, all reaction parameters including reaction media and reaction conditions must be taken care of.4.1 Reaction media
Rodrigues et al.127 have pointed out the prominence of considering reaction parameters like the solvent, electron source, proton source, and photosensitizer in the selectivity of product formation. It was observed that the proton source and the photosensitizer were found to strongly influence the selectivity of photocatalytic CO2 reduction compared to the other factors like the nature of the solvent, the sacrificial electron donor and structure of the photocatalyst. This work illustrates the need to concentrate on photocatalytic reaction conditions rather than just mere catalyst design. Typically, photocatalytic reduction of CO2 is carried out in both the liquid and gas phases. The active photocatalyst is suspended in a CO2 purged reaction medium in the liquid phase whereas CO2 is immobilized on a fixed support and moist CO2 is passed into the reactor in gas-phase reactions.The solubility of CO2 is high at basic pH compared to that under acidic and neutral conditions. In addition, OH− could efficiently scavenge holes and form OH radicals, thereby reducing the availability of holes to recombine with electrons and permitting charge separation. The soluble CO2 is readily available on the photocatalyst surface to capture electrons and follows the reduction pathway. Aqueous NaOH was found to be a suitable reaction medium for yielding both gas phase and liquid phase products due to the high solubility of CO2 in this strong base compared to Na2SO3, NH4OH and others. However, CO2 gets converted to form carbonates and bicarbonates in the presence of basic media, which are very difficult to reduce compared to CO2 itself. This problem can be encountered while carrying out reactions in the gas phase wherein CO2 is bubbled in a water bubbler saturator and passed to the photocatalytic reactor.128 It is reported by Reli et al.129 that methanol, isopropanol and aliphatic amines are good hole scavengers and allow their counter ions i.e. the photogenerated electrons to react with H+ to form hydrogen and increase the reaction yield. It has also been observed that the presence of a co-feed along with CO2 increases the photoreduction of CO2. The addition of CH4 or H2 as a co-feed with CO2 resulted in a high photoconversion of CO2.130,131 The influence of CO as a co-feed was studied by Li et al.132 during the photocatalytic reduction of CO2 in a novel twin reactor. Meng et al.133 have modulated a reaction medium that has a maximum concentration of active reaction species with an effective and stable photocatalytic reaction rate. The fluorescence data and photocatalytic activity results show that the co-catalyst significantly expands the separation of photoexcited charge carriers to enable improved CO2 reduction rate with directed product selectivity.
The water-splitting process is the major challenge as it competitively inhibits the availability of photogenerated electrons for CO2 reduction and itself produces H2 gas. The production of hydrogen fuel is a single electron reaction and is kinetically simpler in comparison to the thermodynamically less favorable multi-electron process of photoreduction of CO2. The BiVO4 photocatalyst synthesized by Liu et al.134 is utilized for selective formation of C2H5OH during the photocatalytic reduction of CO2. The photogenerated electrons are not suitable to produce hydrogen from protons and they produce only oxygen during the water-splitting process. Metalloporphyrin complexes like iron porphyrin show enhanced photocatalytic performance selectively in the conversion of CO2 compared to the competitive water splitting process.106
4.2 Effect of pressure and temperature
Mizuno et al.135 have studied the influence of CO2 pressure under aqueous conditions using TiO2 as a photocatalyst. The increase in pressure favours photocatalytic conversion due to the increase in solubility of CO2 in the reaction medium. High-pressure photoreduction of CO2 was performed by Bahadori et al.136 to improve the solubility of CO2. It has been shown that the increase in pressure increases the formation of liquid products, whereas too high pressure reduces gas-phase products. Hence, an intermediate pressure should be chosen for high photocatalytic yield process productivity. The rapid increase in the rate of product formation was observed by Kometani et al.137 as a homogeneous single-phase mixture of water and CO2 is formed under supercritical conditions.High-temperature photocatalysis has been demonstrated by Poudyal et al.138 and has been shown that a high temperature of 623 K enables the promotion of both water dissociation and C–O cleavage giving rise to enhanced CH4, CO and H2 generation. The rate of diffusion of reactant molecules and their collision increase at higher temperatures. However, it has also been observed by Mizuno et al.135 that the increase in temperature can also play a negative role in CO2 reduction simply preventing the desorption of products, making a lower number of catalytic sites available for further reaction to take place. The reactant molecules easily adsorb on the catalyst surface at lower temperatures due to the lower thermal agitation of molecules. The solubility of CO2 is 2.5 times greater when water is cooled from 25 to 0 °C. Hence, it is important to adopt an optimal operating temperature. Previously, we have observed a significant influence of reaction temperature on the photocatalytic yield. The use of the Fresnel lens has increased the temperature up to 473 K, which would heat up the photocatalyst and reactants. A high photocatalytic yield was observed under these superior reaction conditions in comparison to the conventional ambient conditions.26 The influence of reaction conditions on enhanced photocatalytic reduction of CO2 as reported by different researchers is given in Fig. 15.
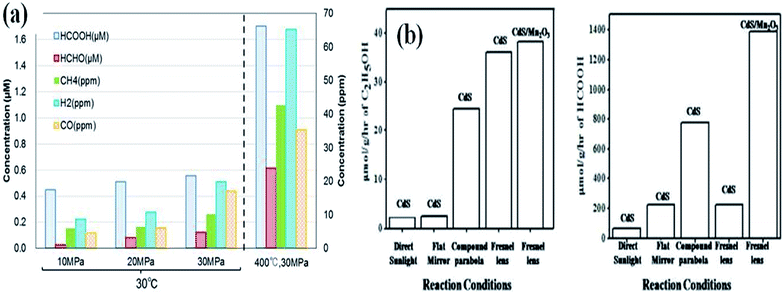 | ||
| Fig. 15 Influence of reaction conditions like (a) pressure, reprinted with permission from ref. 137 copyright 2017, Elsevier publishing group and (b) temperature on enhanced photocatalytic reduction of CO2, reprinted with permission from ref. 26 copyright 2019, Elsevier publishing group. | ||
Significant increases in product formation rate were detected with an increase in temperature and CO2 pressure in the reaction mixture. Certainly, the intensification of pressure permits an enhanced CO2 uptake in the reaction medium, by way of increasing the rate of reduction product formation.
5. Design of a photocatalytic reactor
The key parameters that govern the design of an efficient photocatalytic reactor involve an increase in the quantum yield by collecting the incident energy to the photocatalyst surface. The dependence of product yield on the reactor geometry shows a strong dependence on the reactor diameter, volume of the liquid phase, position of the lamp to the reactor, low power consumption, even light distribution, excellent quantum efficiency, and high throughput and uniform distribution of the catalyst.139The simple batch scale photoreactor designed by Kočì et al.140 was used as a fluidized bed reactor for the batch scale process. The photocatalyst was highly dispersed in a CO2 purged reaction medium. This would facilitate the reactant molecules to interact with the well-suspended photocatalyst. However, this type of reactor can be simply used to evaluate the potential photocatalytic activity and is not suitable for commercial application. To further extend the process for large scale application and industrial use, a tubular continuous flow reactor was designed by Dilla et al.141 as shown in Fig. 16(a) to improve the interaction between gaseous moist CO2 reactant molecules on the TiO2 photocatalyst surface.
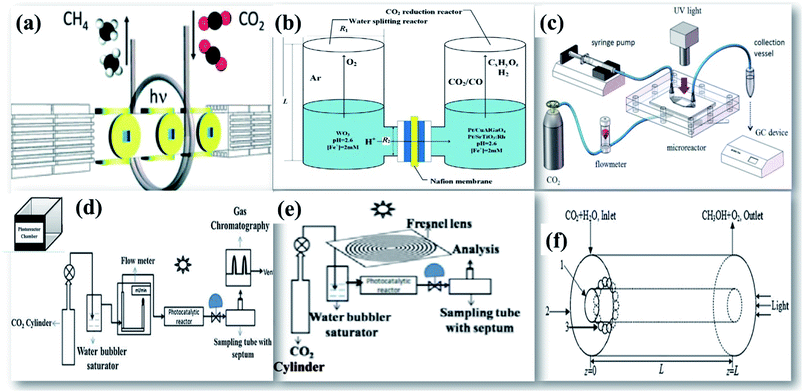 | ||
| Fig. 16 Schematic drawings of (a) a continuous photocatalytic reactor, reprinted with permission from ref. 141 copyright 2019, RSC publishing group, (b) a twin photocatalytic reactor, reprinted with permission from ref. 132 copyright 2016, Elsevier publishing group, (c) an optofluidic microreactor, reprinted with permission from ref. 147 copyright 2016, Elsevier publishing group, (d) a photoreactor with mirror reflectors, reprinted with permission from ref. 25 copyright 2018, Elsevier publishing group, (e) a solar concentrator, reprinted with permission from ref. 26 copyright 2019, Elsevier publishing group, and (f) optical fibers on the photoreactor, reprinted with permission from ref. 150 copyright 2018, Elsevier publishing group. | ||
The incorporation of various solar concentrators and reflectors over conventional reactors have developed efficient reactant geometries. It is critical to ensure that the maximum portion of the photocatalyst is illuminated and exhaustive interaction of reactants with the photocatalyst surface does occur. This broadly involves two approaches (i) to increase the catalyst surface area, and (ii) to increase the amount of light incident on the reactor. A monolith photoreactor was constructed on a continuous photocatalytic reactor by Tahir et al.142 after loading the TiO2 photocatalyst on the montmorillonite [MMT] support. The surface-coated monolith was introduced inside the cylindrical reactor. A high conversion rate with good stability was observed using the monolith photoreactor in comparison with the cell type reactor due to its high illuminated surface area to volume of the reactor, high flow rates, lower pressure drop, more catalyst filling and efficient utilization of photon energy. Novel twin reactors designed for the process by Li et al.132 comprise two separate photocatalytic chambers for photocatalytic water splitting and photocatalytic reduction of CO2 as shown in Fig. 16(b). Initial photocatalytic production of hydrogen by water splitting reactions in one chamber for its later use in CO2 hydrogenation in the next chamber has been proven to be one of the best routes as it mimics the natural photosynthesis. The 2 chambers are separated by a membrane, which is typically the Nafion membrane and loaded with either similar or different photocatalysts. This dual photocatalyst system has shown higher photocatalytic performance over a single photocatalyst system. Recent progress has been observed in the development of photocatalytic membrane reactors.66,143,144 Pomilla et al.143 have used a continuous membrane reactor for performing photoreduction of CO2 in a continuous mode. The membrane reactors have been proven to be 10 times efficient than the batch system. Sellaro et al.145 have reported the highest methanol formation rate by using Nafion based membrane reactors that provide the finest TiO2 distribution. The unique design, increase in surface area, uniform distribution of light and enhanced photon transfer promote the usage of an optofluidic reactor as shown in Fig. 16(c) for photocatalytic reduction of CO2.146,147 A high-pressure photocatalytic reactor had been fabricated by Rossetti et al.148 which can operate up to 20 bar. This would increase the solubility of CO2 and also allows working under unconventional operating conditions. Previously, a series of two photocatalytic reactors has been fabricated in such a way that the unreacted CO2 coming from the exit of the first reactor is consumed by the second reactor. The addition of one more reactor in the design has increased CO2 conversion efficiency by 60%. This further illustrates the need to have a critical design of photoreactors in the process.69
Numerous efforts have been made in the literature to increase light availability on the catalyst surface. Previously, a light-trapping photocatalytic reactor has been fabricated for large scale applications. A photocatalytic chamber was constructed with mirrors attached to its 5 faces. The top portion was exposed to light. The photoreactor is placed in such a way that light gets reflected from these mirrors to the catalyst surface as shown in Fig. 16(d). A high photoconversion of 144.5 μmol g−1 h−1 of methanol formation rate was observed using CdS on PAA support with mirror reflectors, when compared with thin sheets of Al foil as reflectors.25 Guan et al.149 have carried out the photocatalytic conversion of CO2 using Cu/ZnO/K2Ti6O13 under concentrated sunlight. The reaction temperature has exceeded 580 K after using a solar concentrator.26,69 The photocatalytic reduction using concentrated sunlight shows improvement in selectivity and yield of products. Previously, the influence of various solar reflectors and concentrators ranging from flat sheet mirror reflectors, compound parabola and Fresnel lens has been studied. It was found that the use of the Fresnel lens as a solar concentrator as shown in Fig. 16(e) has exceptionally increased the photocatalytic yield due to a very high increase in temperature up to 493 K.26 Chen et al.150 have exploited small transparent glass beads that are uniformly coated with photocatalysts for enhanced absorption of light. Higher photoreduction of CO2 to methane was obtained when the glass beads are kept nearer to the optical fiber and inlet of the reactor as shown in Fig. 16(f). Schematic drawings of various photocatalytic reactors and comparison of their photocatalytic yields discussed above are given in Fig. 16 and Table 7 respectively.
Designing of efficient reactors suitable for application studies should be of prime importance in any proposed work. However, in contradiction, most of the previously reported studies give only importance to the catalyst design. This limits the process of photocatalytic reduction of CO2 in laboratories. It should also be noticed that the natural driving process of photosynthesis is performed by a plant or a tree and not by a single leaf. The precise engineering of the photocatalyst deals with the fabrication of an artificial leaf. Various research reports are available with different functional elements. The arrangement of these artificial leaves to drive an efficient photocatalytic process RGO will be accomplished with perfect reactor design. Hence, the concept should rise from an artificial leaf to an artificial tree that lets this process to rise from the lab scale to an industrially viable technology.
6. Analysis of photocatalytic products
Photocatalytic products are typically analyzed by chromatographic techniques. Gaseous products involving H2, CO, CH4 and higher hydrocarbons like ethane, propane, ethylene, and ethane are analyzed using gas chromatography (GC) coupled with a thermal conductivity detector (TCD) and flame ionization detector (FID) detector. Liquid products like alcohol, acids and aldehydes (CH3OH, HCOOH, and HCHO) are analyzed by the high-performance liquid chromatography (HPLC) technique. However, when the reactions are carried out in the gas phase, the volatile liquid products are available in the gas phase and analyzed by the GC FID technique. In spite of these available techniques, an efficient product analysis technique with high accuracy is still deficient. The gaseous products can be accurately accounted for. However, liquid products that get adsorbed on solid photocatalysts become a major problem during analysis. This problem can be taken care of by washing the catalyst with a very low quantity of water and subjecting it to analysis. Products like CH3OH, HCOOH, and C2H2O4 are highly soluble in water and get easily leached from the surface.151It is also very important to identify the source of origin of products obtained during the analysis. GC MS techniques are carried out to accurately determine the product source. Isotopic labeling of carbon with the C-13 isotope of CO2 helps us to further authorize that the products are formed by photocatalytic reduction of CO2 and not from any other carbon source that involves organic contaminants.15,112 A detailed and precise determination of products is of critical significance for the assessment of the success of a photocatalytic process. However, limited studies have provided great attention to the analysis of reduction products. The demand for a more broad technique that forms a part of the photocatalytic reactor would be a promising approach.
7. Expression of photocatalytic performance
The outcome of the photocatalytic reaction is typically expressed in terms of the rate of product formation as the number of moles of products formed after using 1 g of catalyst for one hour of reaction, which is mol g−1 h−1. The universal expression of the photocatalytic performance in terms of the rate of product formation eases their comparison. The photocatalytic performance can be better evaluated in terms of quantum yield, turnover number, photocatalytic conversion efficiency and carbon conversion efficiency using eqn (1)–(5).25,152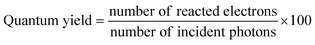 | (1) |
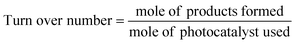 | (2) |
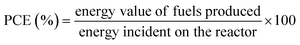 | (3) |
| Energy value of organics produced = ΔG0 × molar rate of fuels produced | (4) |
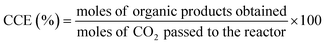 | (5) |
8. Conclusions and future perspectives
Although the dual beneficial process of photocatalytic CO2 reduction to solar fuel generation is highly valuable in principle, there is quite an extensive way for it to become an industrially valuable technology. The extensive review of current trends on photocatalytic reduction of CO2 has revealed that the precise engineering of a photocatalyst to improve its photocatalytic performance is the major part of the system. However, there are various challenges concerning low visible light absorption, immediate recombination of photoexcited charge carriers, and contact of reactant molecules on the catalyst surface. To address these issues several valuable efforts have been made in the literature. The lack of a clear understanding of the mechanism of the present process itself questions the criteria to develop an efficient photocatalyst. The involvement of more analytical techniques like time-resolved spectroscopy and computational methods like density functional theory would bring more insight into this zone. Synthesis of photocatalysts with interesting morphology ranging from 0D to 3D shapes contributes to enhanced light-harvesting towards the catalyst surface. The development of porous photocatalysts and the use of a porous support help in better dispersion of the photocatalyst with an increased surface area. The increased adsorption of reactant molecules and facile transport of charge carriers towards the photocatalyst surface increase the photocatalytic yield. The synthesis of visible light active photocatalysts with comparatively lower quantum yields is much more attractive than the synthesis of UV active photocatalysts in terms of their extension to industrial applications. This demands the replacement or tuning of highly stable and economical TiO2 photocatalysts. Metal chalcogenides like CdS exhibit enhanced visible light activity. The issue related to deactivation and photostability, however, prevents their extensive use. These challenges call for the development of nanocomposites like the use of conducting supports, introduction of defects, and doping with metallic and non-metallic ions to produce semiconductor heterostructures in the system. The use of conducting supports like graphene, MWCNTs, and polyaniline assists in enhanced capture of photoexcited electrons from the photocatalyst and their circulation within their extensive π conjugated system. Doping photocatalysts with cations and anions significantly improves their properties in terms of lower recombination rates and enhanced visible light absorption. Plasmonic photocatalysis has demonstrated rapid improvement and develops a potentially promising avenue for enhanced visible-light activity. The high photostability exhibited by these noble metal incorporated photocatalysts makes them superb materials in the process. However, the high cost of noble metals limits their use in large scale application studies. It would be highly beneficial to develop some low-cost techniques for the synthesis of platinum nanoparticles, like biosynthesis, and utilize them in the photocatalytic process. Further, the presence of surface defects also enhances the photoreaction rate when acting as sites for electron capture and reactant adsorption. Reaction parameters like reaction media, temperature, pressure, and wavelength of light also significantly influence the photocatalytic yield. However, the development of a process that operates under ambient conditions similar to those of natural photosynthesis would only be feasible and sustainable when considered on an industrial scale.The construction of an efficient photoreactor simply means increasing the surface area and quantum yield by collecting the maximum incident energy to the photocatalyst surface. Continuous photocatalytic reactors serve as potential candidates for large scale industrial use over batch scale photoreactors. The use of monolith photoreactors, novel twin reactors, and membrane photoreactors increases the quantum yield due to their unique design with enhanced surface area and higher photon transfer. The increase of light entrapment by the use of solar concentrators and optical fibres enables the transmission of intense light towards the photocatalyst surface. It is advised to develop such a process that would be suitable for large scale applications with a significant improvement in yield. Challenges like low-yield products remain unsatisfactory and limit it from being a potentially promising technology. Therefore, it would be essential to develop integrated photocatalytic systems that assemble a network of photocatalysts and duplicate the stacking pattern of thylakoids to increase their surface area and electron transport chain. Researchers need to overcome this hurdle by considering the fundamental and essential issues to implement the process as an industrially viable technology.
The development of industrially viable synthetic routes to fabricate photocatalysts possessing favourable architectures and detailed interpretation of the tuning of physicochemical properties concerning their structural design need to be a part of future scientific investigations. It is vital to progress novel photocatalysts having excellent light-harvesting ability, photocatalytic activity, and stability in terms of their reuse in industrial applications. The construction of porous and high surface area photocatalysts can improve the adsorption of CO2 and provide surface active sites. The broad knowledge of mechanistic comprehensions into the nature of active sites, adsorbing states of CO2, and reusability demands studies on the interface of photocatalysts and should be a vital part of future research. Other research efforts like identification of the source of origin like isotopic labeling need to be a critical part in the research as carbon residues and even organic solvents deposited on the photocatalyst surface during its synthesis can undergo a similar reaction and contribute to the product yield.
The advanced development of various heterostructures with exceptional electron transport properties, especially those mimic natural leaves are suitable as sustainable efficient materials for photocatalytic reduction of CO2. The natural leaf is a concerted effort of complex structural design with enormous functional components that extensively offers a sustainable technological feature. Precisely, the present process on the development of artificial leaves would reflect their efficient gas diffusion and light scattering phenomena. The biomimetic surface enhances the adsorption of reactant molecules and light capture and also tunes the hydrophobicity of the surface. The enhanced hydrophobicity of the surface prevents moisture loss. The arrangement of these artificial leaves to drive an efficient photocatalytic process will be accomplished with a perfect reactor design that resembles an artificial tree. Therefore, the concept should rise from artificial leaves to artificial trees that simply raises this process from the lab scale to an industrially viable technology.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
MS sincerely acknowledges the Department of Science and Technology for the award of the DST-Inspire Faculty research grant (IFA-17-CH286) and also the National Institute of Technology Calicut for the Faculty Research Grant.References
- M. M. Kandy, Sustainable Energy Fuels, 2020, 4, 469–484 RSC
.
- K. Li, X. An, K. H. Park, M. Khraisheh and J. Tang, Catal. Today, 2014, 224, 3–12 CrossRef CAS
.
- S. Roy, A. Cherevotan and S. C. Peter, ACS Energy Lett., 2018, 3, 1938–1966 CrossRef CAS
.
- M. Jaccob, M. Sankaralingam and N. J. Britto, Chem. Modell., 2020, 15, 131–172 Search PubMed
.
- C. Li and Z. Wang, Nanoscale Res. Lett., 2017, 12, 530 CrossRef
.
- S. Muthuramalingam, M. Sankaralingam, M. Velusamy and R. Mayilmurugan, Inorg. Chem., 2019, 58, 12975–12985 CrossRef CAS
.
- S. Sarina, E. R. Waclawik and H. Zhu, Green Chem., 2013, 15, 1814–1833 RSC
.
- S. Nahar, M. F. M. Zain, A. A. H. Kadhum, H. A. Hasan and Md. R. Hasan, Materials, 2017, 10, 629 CrossRef
.
- M. Halmann, Nature, 1978, 275, 115 CrossRef CAS
.
- T. Inoue, A. Fujishima, S. Konishi and K. Honda, Nature, 1979, 277, 637 CrossRef CAS
.
- E. V. Kondratenko, G. Mul, J. Baltrusaitis, G. O. Larrazábal and J. Pérez-Ramírez, Energy Environ. Sci., 2013, 6, 3112–3135 RSC
.
- H. Razavi-Khosroshahi, S. Mohammadzadeh, M. Hojamberdiev, S. Kitano, M. Yamauchi and M. Fuji, Dalton Trans., 2019, 48, 9284–9290 RSC
.
- N. Serpone, D. Lawless, R. Khairutdinov and E. Pelizzetti, J. Phys. Chem., 1995, 99, 16655–16661 CrossRef CAS
.
- L. Liu, S. Wang, H. Huang, Y. Zhang and T. Ma, Nano Energy, 2020, 75, 104959 CrossRef CAS
.
- Y. Izumi, Coord. Chem. Rev., 2013, 257, 171–186 CrossRef CAS
.
- S. Poudyal and S. Laursen, Catal. Sci. Technol., 2019, 9, 1048–1059 RSC
.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS
.
- S. R. Lingampalli, M. M. Ayyub and C. N. R. Rao, ACS Omega, 2017, 2, 2740–2748 CrossRef CAS
.
- X. Chang, T. Wang and J. Gong, Energy Environ. Sci., 2016, 9, 2177–2196 RSC
.
- X. Zhou, G. Liu, J. Yu and W. Fan, J. Mater. Chem., 2012, 22, 21337–21354 RSC
.
- Y. Yu, W. Wen, X.-Y. Qian, J.-B. Liu and J.-M. Wu, Sci. Rep., 2017, 7, 41253 CrossRef CAS
.
- C.-C. Yang, Y.-H. Yu, B. van der Linden, J. C. S. Wu and G. Mul, J. Am. Chem. Soc., 2010, 132, 8398–8406 CrossRef CAS
.
- E. Karamian and S. Sharifnia, J. CO2 Util., 2016, 16, 194–203 CrossRef CAS
.
- Y. Ji and Y. Luo, J. Am. Chem. Soc., 2016, 138, 15896–15902 CrossRef CAS
.
- M. M. Kandy and V. G. Gaikar, Mater. Res. Bull., 2018, 102, 440–449 CrossRef CAS
.
- M. M. Kandy and V. G. Gaikar, Renewable Energy, 2019, 139, 915–923 CrossRef CAS
.
- A. E. Nogueira, J. A. Oliveira, G. T. S. T. da Silva and C. Ribeiro, Sci. Rep., 2019, 9, 1–11 CrossRef CAS
.
- M. Thambidurai, N. Muthukumarasamy, D. Velauthapillai and C. Lee, J. Mater. Sci.: Mater. Electron., 2013, 24, 4535–4541 CrossRef CAS
.
- Y. Cui, X. Lian, L. Xu, M. Chen, B. Yang, C. Wu, W. Li, B. Huang and X. Hu, Materials, 2019, 12, 276 CrossRef CAS
.
- T. Zhang, J. Low, K. Koh, J. Yu and T. Asefa, ACS Sustainable Chem. Eng., 2018, 6, 531–540 CrossRef CAS
.
- J. Lin, X. Sun, B. Qin and T. Yu, RSC Adv., 2018, 8, 20543–20548 RSC
.
- W.-N. Wang, W.-J. An, B. Ramalingam, S. Mukherjee, D. M. Niedzwiedzki, S. Gangopadhyay and P. Biswas, J. Am. Chem. Soc., 2012, 134, 11276–11281 CrossRef CAS
.
- L. Yu, G. Li, X. Zhang, X. Ba, G. Shi, Y. Li, P. K. Wong, J. C. Yu and Y. Yu, ACS Catal., 2016, 6, 6444–6454 CrossRef CAS
.
- F. Haque, T. Daeneke, K. Kalantar-zadeh and J. Z. Ou, Nano-Micro Lett., 2017, 10, 23 CrossRef
.
- K. S. Lakhi, D.-H. Park, K. Al-Bahily, W. Cha, B. Viswanathan, J.-H. Choy and A. Vinu, Chem. Soc. Rev., 2017, 46, 72–101 RSC
.
- Y. Chen, G. Jia, Y. Hu, G. Fan, Y. H. Tsang, Z. Li and Z. Zou, Sustainable Energy Fuels, 2017, 1, 1875–1898 RSC
.
- I. Shown, S. Samireddi, Y.-C. Chang, R. Putikam, P.-H. Chang, A. Sabbah, F.-Y. Fu, W.-F. Chen, C.-I. Wu, T.-Y. Yu, P.-W. Chung, M. C. Lin, L.-C. Chen and K.-H. Chen, Nat. Commun., 2018, 9, 169 CrossRef
.
- F. Ding, D. Yang, Z. Tong, Y. Nan, Y. Wang, X. Zou and Z. Jiang, Environ. Sci.: Nano, 2017, 4, 1455–1469 RSC
.
- J. Mao, T. Peng, X. Zhang, K. Li, L. Ye and L. Zan, Catal. Sci. Technol., 2013, 3, 1253–1260 RSC
.
- M. Shen, L. Zhang and J. Shi, Nanotechnology, 2018, 29, 412001 CrossRef
.
- Z. Kong, X. Chen, W.-J. Ong, X. Zhao and N. Li, Appl. Surf. Sci., 2019, 463, 1148–1153 CrossRef CAS
.
- Q. Xu, L. Zhang, J. Yu, S. Wageh, A. A. Al-Ghamdi and M. Jaroniec, Mater. Today, 2018, 21, 1042–1063 CrossRef CAS
.
- L. Zou, H. Wang and X. Wang, ACS Sustainable Chem. Eng., 2017, 5, 303–309 CrossRef CAS
.
- T. Di, B. Zhu, B. Cheng, J. Yu and J. Xu, J. Catal., 2017, 352, 532–541 CrossRef CAS
.
- Z. He, Q. Cai, H. Fang, G. Situ, J. Qiu, S. Song and J. Chen, J. Environ. Sci., 2013, 25, 2460–2468 CrossRef CAS
.
- Z. Sun, Z. Yang, H. Liu, H. Wang and Z. Wu, Appl. Surf. Sci., 2014, 315, 360–367 CrossRef CAS
.
- Z. Sun, W. Fang, L. Zhao, H. Chen, X. He, W. Li, P. Tian and Z. Huang, Environ. Int., 2019, 130, 104898 CrossRef CAS
.
- H. Zhou, J. Guo, P. Li, T. Fan, D. Zhang and J. Ye, Sci. Rep., 2013, 3, 1–9 Search PubMed
.
- B.-J. Liu, T. Torimoto, H. Matsumoto and H. Yoneyama, J. Photochem. Photobiol., A, 1997, 108, 187–192 CrossRef CAS
.
- N. Ulagappan and H. Frei, J. Phys. Chem. A, 2000, 104, 7834–7839 CrossRef CAS
.
- K. Kočí, L. Obalová and Z. Lacný, Chem. Pap., 2008, 62, 1–9 Search PubMed
.
- K. Ikeue, H. Yamashita, M. Anpo and T. Takewaki, J. Phys. Chem. B, 2001, 105, 8350–8355 CrossRef CAS
.
- Z.-C. Fu, J. T. Moore, F. Liang and W.-F. Fu, Chem. Commun., 2019, 55, 11523–11526 RSC
.
- S. Wang and X. Wang, Appl. Catal., B, 2015, 162, 494–500 CrossRef CAS
.
- R. Li, W. Zhang and K. Zhou, Adv. Mater., 2018, 30, 1705512 CrossRef
.
- D. Wang, R. Huang, W. Liu, D. Sun and Z. Li, ACS Catal., 2014, 4, 4254–4260 CrossRef CAS
.
- B. Srinivas, B. Shubhamangala, K. Lalitha, P. A. K. Reddy, V. D. Kumari, M. Subrahmanyam and B. R. De, Photochem. Photobiol., 2011, 87, 995–1001 CrossRef CAS
.
- A. Crake, K. C. Christoforidis, A. Kafizas, S. Zafeiratos and C. Petit, Appl. Catal., B, 2017, 210, 131–140 CrossRef CAS
.
- S. Zhu, S. Liang, Y. Wang, X. Zhang, F. Li, H. Lin, Z. Zhang and X. Wang, Appl. Catal., B, 2016, 187, 11–18 CrossRef CAS
.
- B. Di Credico, M. Redaelli, M. Bellardita, M. Calamante, C. Cepek, E. Cobani, M. D'Arienzo, C. Evangelisti, M. Marelli, M. Moret, L. Palmisano and R. Scotti, Catalysts, 2018, 8, 353 CrossRef
.
- H.-Q. Xu, J. Hu, D. Wang, Z. Li, Q. Zhang, Y. Luo, S.-H. Yu and H.-L. Jiang, J. Am. Chem. Soc., 2015, 137, 13440–13443 CrossRef CAS
.
- S. Yan, S. Ouyang, H. Xu, M. Zhao, X. Zhang and J. Ye, J. Mater. Chem. A, 2016, 4, 15126–15133 RSC
.
- C. Chen, T. Wu, H. Wu, H. Liu, Q. Qian, Z. Liu, G. Yang and B. Han, Chem. Sci., 2018, 9, 8890–8894 RSC
.
- A. M. Cubillas, X. Jiang, T. G. Euser, N. Taccardi, B. J. M. Etzold, P. Wasserscheid and P. S. J. Russell, Analyst, 2017, 142, 925–929 RSC
.
- T. Zhao, Z. Liu, K. Nakata, S. Nishimoto, T. Murakami, Y. Zhao, L. Jiang and A. Fujishima, J. Mater. Chem., 2010, 20, 5095–5099 RSC
.
- A. Brunetti, F. R. Pomilla, G. Marcì, E. I. Garcia-Lopez, E. Fontananova, L. Palmisano and G. Barbieri, Appl. Catal., B, 2019, 255, 117779 CrossRef CAS
.
- M.-Q. Yang and Y.-J. Xu, Nanoscale Horiz., 2016, 1, 185–200 RSC
.
- N. Shehzad, M. Tahir, K. Johari, T. Murugesan and M. Hussain, J. Environ. Chem. Eng., 2018, 6, 6947–6957 CrossRef CAS
.
- M. M. Kandy and V. G. Gaikar, J. Nanosci. Nanotechnol., 2019, 19, 5323–5331 CrossRef CAS
.
- Y. Rambabu, U. Kumar, N. Singhal, M. Kaushal, M. Jaiswal, S. L. Jain and S. C. Roy, Appl. Surf. Sci., 2019, 485, 48–55 CrossRef CAS
.
- H. Jung, K. M. Cho, K. H. Kim, H.-W. Yoo, A. Al-Saggaf, I. Gereige and H.-T. Jung, ACS Sustainable Chem. Eng., 2018, 6, 5718–5724 CrossRef CAS
.
- J. Yu, J. Jin, B. Cheng and M. Jaroniec, J. Mater. Chem. A, 2014, 2, 3407–3416 RSC
.
- S.-H. Liu, J.-S. Lu and Y.-C. Chen, Sustain, 2018, 10, 4145 CrossRef CAS
.
- P. Yang, S. Guo, X. Yu, F. Zhang, B. Yu, H. Zhang, Y. Zhao and Z. Liu, Ind. Eng. Chem. Res., 2019, 58, 9636–9643 CrossRef CAS
.
- J. O. Olowoyo, M. Kumar, B. Singh, V. O. Oninla, J. O. Babalola, H. Valdés, A. V. Vorontsov and U. Kumar, Carbon, 2019, 147, 385–397 CrossRef CAS
.
- P. Madhusudan, S. Wageh, A. A. Al-Ghamdi, J. Zhang, B. Cheng and Y. Yu, Appl. Surf. Sci., 2020, 506, 144683 CrossRef CAS
.
- Z. Fang, S. Li, Y. Gong, W. Liao, S. Tian, C. Shan and C. He, J. Saudi Chem. Soc., 2014, 18, 299–307 CrossRef
.
- J. O. Olowoyo, M. Kumar, S. L. Jain, J. O. Babalola, A. V. Vorontsov and U. Kumar, J. Phys. Chem. C, 2019, 123, 367–378 CrossRef CAS
.
- M. M. Gui, W. M. P. Wong, S.-P. Chai and A. R. Mohamed, Chem. Eng. J., 2015, 278, 272–278 CrossRef CAS
.
- S. Aoi, K. Mase, K. Ohkubo and S. Fukuzumi, Catal. Sci. Technol., 2016, 6, 4077–4080 RSC
.
- W.-J. Ong, L. K. Putri, Y.-C. Tan, L.-L. Tan, N. Li, Y. H. Ng, X. Wen and S.-P. Chai, Nano Res., 2017, 10, 1673–1696 CrossRef CAS
.
- J. Li, Q. Xiao, L. Li, J. Shen and D. Hu, Appl. Surf. Sci., 2015, 331, 108–114 CrossRef CAS
.
- R. Tanwar and U. K. Mandal, RSC Adv., 2019, 9, 8977–8993 RSC
.
- W. Dai, H. Xu, J. Yu, X. Hu, X. Luo, X. Tu and L. Yang, Appl. Surf. Sci., 2015, 356, 173–180 CrossRef CAS
.
- L. Cao, S. Sahu, P. Anilkumar, C. E. Bunker, J. Xu, K. A. S. Fernando, P. Wang, E. A. Guliants, K. N. Tackett and Y.-P. Sun, J. Am. Chem. Soc., 2011, 133, 4754–4757 CrossRef CAS
.
- A. Sharma and B.-K. Lee, Catal. Today, 2017, 298, 158–167 CrossRef CAS
.
- K. Liu, X. Zhang, C. Zhang, G. Ren, Z. Zheng, Z. Lv and C. Fan, RSC Adv., 2019, 9, 14391–14399 RSC
.
- K. M. Cho, K. H. Kim, K. Park, C. Kim, S. Kim, A. Al-Saggaf, I. Gereige and H.-T. Jung, ACS Catal., 2017, 7, 7064–7069 CrossRef CAS
.
- L.-L. Tan, W.-J. Ong, S.-P. Chai and A. R. Mohamed, Nanoscale Res. Lett., 2013, 8, 465 CrossRef
.
- D. Wei, W. Tang, Y. Gan and X. Xu, Catal. Sci. Technol., 2020, 10, 5666–5676 RSC
.
- N. Suresh Kumar, R. Padma Suvarna, K. Chandra Babu Naidu, P. Banerjee, A. Ratnamala and H. Manjunatha, Appl. Phys. A, 2020, 126, 445 CrossRef CAS
.
- I.-H. Tseng, L.-H. Kang, P.-Y. Chang, M.-H. Tsai, J.-M. Yeh and T.-I. Yang, ACS Omega, 2019, 4, 1636–1644 CrossRef CAS
.
- X. Chen, Q. Dang, R. Sa, L. Li, L. Li, J. Bi, Z. Zhang, J. Long, Y. Yu and Z. Zou, Chem. Sci., 2020, 11, 6915–6922 RSC
.
- A. Zhou, Y. Dou, C. Zhao, J. Zhou, X.-Q. Wu and J.-R. Li, Appl. Catal., B, 2020, 264, 118519 CrossRef
.
- W.-J. Yin, H. Tang, S.-H. Wei, M. M. Al-Jassim, J. Turner and Y. Yan, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 045106 CrossRef
.
- J. C. S. Wu, I.-H. Tseng and W.-C. Chang, J. Nano Res., 2001, 3, 113–118 CrossRef CAS
.
- S. Qin, F. Xin, Y. Liu, X. Yin and W. Ma, J. Colloid Interface Sci., 2011, 356, 257–261 CrossRef CAS
.
- D. Liu, Y. Fernández, O. Ola, S. Mackintosh, M. Maroto-Valer, C. M. A. Parlett, A. F. Lee and J. C. S. Wu, Catal. Commun., 2012, 25, 78–82 CrossRef CAS
.
- M. Bellardita, A. D. Paola, E. García-López, V. Loddo, G. Marcì and L. Palmisano, Curr. Org. Chem., 2013, 17, 2440–2448 CrossRef CAS
.
- J. Ye, J. He, S. Wang, X. Zhou, Y. Zhang, G. Liu and Y. Yang, Sep. Purif. Technol., 2019, 220, 8–15 CrossRef CAS
.
- O. Ola and M. M. Maroto-Valer, Appl. Catal., A, 2015, 502, 114–121 CrossRef CAS
.
- B. M. Hockin, C. Li, N. Robertson and E. Zysman-Colman, Catal. Sci. Technol., 2019, 9, 889–915 RSC
.
- J. Huo, Y.-B. Zhang, W.-Y. Zou, X. Hu, Q. Deng and D. Chen, Catal. Sci. Technol., 2019, 9, 2716–2727 RSC
.
- E. Boutin, L. Merakeb, B. Ma, B. Boudy, M. Wang, J. Bonin, E. Anxolabéhère-Mallart and M. Robert, Chem. Soc. Rev., 2020, 49, 5772–5809 RSC
.
- H. Takeda, Y. Monma, H. Sugiyama, H. Uekusa and O. Ishitani, Front. Chem., 2019, 7, 418 CrossRef CAS
.
- S. Fukuzumi, Y.-M. Lee, H. S. Ahn and W. Nam, Chem. Sci., 2018, 9, 6017–6034 RSC
.
- N. Sharma, J. Jung, K. Ohkubo, Y.-M. Lee, M. E. El-Khouly, W. Nam and S. Fukuzumi, J. Am. Chem. Soc., 2018, 140, 8405–8409 CrossRef CAS
.
- J. O. Olowoyo, M. Kumar, S. L. Jain, S. Shen, Z. Zhou, S. S. Mao, A. V. Vorontsov and U. Kumar, Int. J. Hydrogen Energy, 2018, 43, 17682–17695 CrossRef CAS
.
- M. Tasbihi, M. Schwarze, M. Edelmannová, C. Spöri, P. Strasser and R. Schomäcker, Catal. Today, 2019, 328, 8–14 CrossRef CAS
.
- Z. Zhao, J. Fan, J. Wang and R. Li, Catal. Commun., 2012, 21, 32–37 CrossRef CAS
.
- K. Wang, Q. Li, B. Liu, B. Cheng, W. Ho and J. Yu, Appl. Catal., B, 2015, 176–177, 44–52 CAS
.
- B. Wang, W. Chen, Y. Song, G. Li, W. Wei, J. Fang and Y. Sun, Catal. Today, 2018, 311, 23–39 CrossRef CAS
.
- P. Wang, B. Huang, Y. Dai and M.-H. Whangbo, Phys. Chem. Chem. Phys., 2012, 14, 9813–9825 RSC
.
- U. Guler, V. M. Shalaev and A. Boltasseva, Mater. Today, 2015, 18, 227–237 CrossRef CAS
.
- M. R. Khan, T. W. Chuan, A. Yousuf, M. N. K. Chowdhury and C. K. Cheng, Catal. Sci. Technol., 2015, 5, 2522–2531 RSC
.
- Y. Gao, K. Qian, B. Xu, Z. Li, J. Zheng, S. Zhao, F. Ding, Y. Sun and Z. Xu, Carbon Resour. Convers., 2020, 3, 46–59 CrossRef CAS
.
- H. Zhao, X. Zheng, X. Feng and Y. Li, J. Phys. Chem. C, 2018, 122, 18949–18956 CrossRef CAS
.
- X. Zhang, X. Li, D. Zhang, N. Q. Su, W. Yang, H. O. Everitt and J. Liu, Nat. Commun., 2017, 8, 14542 CrossRef CAS
.
- S. Sun, X. Yu, Q. Yang, Z. Yang and S. Liang, Nanoscale Adv., 2019, 1, 34–63 RSC
.
- L. B. Hoch, P. Szymanski, K. K. Ghuman, L. He, K. Liao, Q. Qiao, L. M. Reyes, Y. Zhu, M. A. El-Sayed, C. V. Singh and G. A. Ozin, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E8011–E8020 CrossRef CAS
.
- X. Wang, Z. Feng, J. Shi, G. Jia, S. Shen, J. Zhou and C. Li, Phys. Chem. Chem. Phys., 2010, 12, 7083–7090 RSC
.
- H. Fujiwara, H. Hosokawa, K. Murakoshi, Y. Wada, S. Yanagida, T. Okada and H. Kobayashi, J. Phys. Chem. B, 1997, 101, 8270–8278 CrossRef CAS
.
- S. Zhu, S. Liang, Y. Wang, X. Zhang, F. Li, H. Lin, Z. Zhang and X. Wang, Appl. Catal., B, 2016, 187, 11–18 CrossRef CAS
.
- Q. Lang, W. Hu, P. Zhou, T. Huang, S. Zhong, L. Yang, J. Chen and S. Bai, Nanotechnology, 2017, 28, 484003 CrossRef
.
- T. Bora, M. T. Z. Myint, S. H. Al-Harthi and J. Dutta, RSC Adv., 2015, 5, 96670–96680 RSC
.
- M. Shen, L. Zhang, M. Wang, J. Tian, X. Jin, L. Guo, L. Wang and J. Shi, J. Mater. Chem. A, 2019, 7, 1556–1563 RSC
.
- R. R. Rodrigues, C. M. Boudreaux, E. T. Papish and J. H. Delcamp, ACS Appl. Energy Mater., 2019, 2, 37–46 CrossRef CAS
.
- A. Olivo, E. Ghedini, M. Signoretto, M. Compagnoni and I. Rossetti, Energies, 2017, 10, 1394 CrossRef
.
- M. Reli, M. Sihor, K. Koci, P. Praus, O. Kozak and L. Obalova, GeoScience Engineering, 2012, 58, 34–42 Search PubMed
.
- K. Teramura, T. Tanaka, H. Ishikawa, Y. Kohno and T. Funabiki, J. Phys. Chem. B, 2004, 108, 346–354 CrossRef CAS
.
- F. Sastre, A. V. Puga, L. Liu, A. Corma and H. García, J. Am. Chem. Soc., 2014, 136, 6798–6801 CrossRef CAS
.
- S. Li, L. Yang, O. Ola, M. Maroto-Valer, X. Du and Y. Yang, Energy Convers. Manage., 2016, 116, 184–193 CrossRef CAS
.
- X. Meng, Q. Yu, G. Liu, L. Shi, G. Zhao, H. Liu, P. Li, K. Chang, T. Kako and J. Ye, Nano Energy, 2017, 34, 524–532 CrossRef CAS
.
- Y. Liu, B. Huang, Y. Dai, X. Zhang, X. Qin, M. Jiang and M.-H. Whangbo, Catal. Commun., 2009, 11, 210–213 CrossRef CAS
.
- T. Mizuno, K. Adachi, K. Ohta and A. Saji, J. Photochem. Photobiol., A, 1996, 98, 87–90 CrossRef CAS
.
- E. Bahadori, A. Tripodi, A. Villa, C. Pirola, L. Prati, G. Ramis and I. Rossetti, Catalysts, 2018, 8, 430 CrossRef
.
- N. Kometani, S. Hirata and M. Chikada, J. Supercrit. Fluids, 2017, 120, 443–447 CrossRef CAS
.
- S. Poudyal and S. Laursen, J. Phys. Chem. C, 2018, 122, 8045–8057 CrossRef CAS
.
- T. Wang, L. Yang, X. Du and Y. Yang, Energy Convers. Manage., 2013, 65, 299–307 CrossRef CAS
.
- K. Kočì, L. Obalovà, L. Matějová, D. Plachà, Z. Lancý, J. Jirkovský and O. Šolcovà, Appl. Catal., B, 2009, 89, 494–502 CrossRef
.
- M. Dilla, A. E. Becerikli, A. Jakubowski, R. Schlögl and S. Ristig, Photochem. Photobiol. Sci., 2019, 18, 314–318 RSC
.
- M. Tahir and N. S. Amin, Chem. Eng. J., 2013, 230, 314–327 CrossRef CAS
.
- F. R. Pomilla, A. Brunetti, G. Marcì, E. I. Garcia-Lopez, E. Fontananova, L. Palmisano and G. Barbieri, ACS Sustainable Chem. Eng., 2018, 6, 8743–8753 CrossRef CAS
.
- P. Pathak, M. J. Meziani, Y. Li, L. T. Cureton and Y.-P. Sun, Chem. Commun., 2004, 10, 1234–1235 RSC
.
- M. Sellaro, M. Bellardita, A. Brunetti, E. Fontananova, L. Palmisano, E. Drioli and G. Barbieri, RSC Adv., 2016, 6, 67418–67427 RSC
.
- M. Cheng, S. Yang, R. Chen, X. Zhu, Q. Liao and Y. Huang, Int. J. Hydrogen Energy, 2017, 42, 9722–9732 CrossRef CAS
.
- X. Cheng, R. Chen, X. Zhu, Q. Liao, X. He, S. Li and L. Li, Int. J. Hydrogen Energy, 2016, 41, 2457–2465 CrossRef CAS
.
- I. Rossetti, A. Villa, M. Compagnoni, L. Prati, G. Ramis, C. Pirola, C. L. Bianchi, W. Wang and D. Wang, Catal. Sci. Technol., 2015, 5, 4481–4487 RSC
.
- G. Guan, T. Kida, T. Harada, M. Isayama and A. Yoshida, Appl. Catal., A, 2003, 249, 11–18 CrossRef CAS
.
- H. Chen, F. Chu, L. Yang, O. Ola, X. Du and Y. Yang, Appl. Energy, 2018, 230, 1403–1413 CrossRef CAS
.
- J. Hong, W. Zhang, J. Ren and R. Xu, Anal. Methods, 2013, 5, 1086–1097 RSC
.
- Y. Tamaki and O. Ishitani, ACS Catal., 2017, 7, 3394–3409 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2021 |