Evaluating solvothermal and mechanochemical routes towards the metal–organic framework Mg2(m-dobdc)†
Elena Y.
Chen‡
 ,
Ruth M.
Mandel‡
,
Ruth M.
Mandel‡
 and
Phillip J.
Milner
and
Phillip J.
Milner
 *
*
Department of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14850, USA. E-mail: pjm347@cornell.edu
First published on 30th June 2022
Abstract
Metal–organic frameworks bearing coordinatively unsaturated Mg(II) sites are promising materials for gas storage, chemical separations, and drug delivery due to their low molecular weights and lack of toxicity. However, there remains a limited number of such MOFs reported in the literature. Herein, we investigate the gas sorption properties of the understudied framework Mg2(m-dobdc) (dobdc4− = 4,6-dioxido-1,3-benzenedicarboxylate) synthesized under both solvothermal and mechanochemical conditions. Both materials are found to be permanently porous, as confirmed by 77 K N2 adsorption measurements. In particular, Mg2(m-dobdc) synthesized under mechanochemical conditions using exogenous organic base displays one of the highest capacities reported to date (6.14 mmol g−1) for CO2 capture in a porous solid under simulated coal flue gas conditions (150 mbar, 40 °C). As such, mechanochemically synthesized Mg2(m-dobdc) represents a promising new framework for applications requiring high gas adsorption capacities in a porous solid.
Introduction
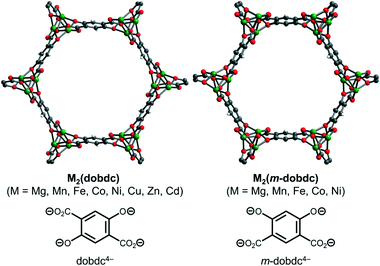
Metal–organic frameworks (MOFs) are porous, crystalline extended solids that consist of inorganic metal nodes or secondary building units (SBUs) bridged by polytopic organic linkers.1 Their uniquely modular structures coupled with high internal surface areas have enabled numerous applications in drug delivery, catalysis, chemical separations, and gas storage.2–5 Frameworks containing coordinatively unsaturated metal centers, also known as open-metal site MOFs, have been extensively studied due to their ability to strongly interact with guest molecules.6 In particular, the canonical M2(dobdc) (M = Mg, Mn, Fe, Co, Ni, Cu, Zn, Cd; dobdc4− = 2,5-dioxidobenzene-1,4-dicarboxylate), MOF-74, or CPO-27 family of frameworks features hexagonal one-dimensional channels decorated with a high density of exposed M(II) centers (Fig. 1, left).7 Among the reported isostructural metal variants, the Mg analogue, Mg2(dobdc), is particularly promising due to its low cost, lack of toxicity, and high gravimetric and volumetric adsorption capacities for a range of adsorbates.8–12 As such, the identification of new porous frameworks bearing accessible Mg(II) sites is highly desirable.A closely related family of frameworks, M2(m-dobdc) (M = Mg, Mn, Fe, Co, Ni; m-dobdc4− = 4,6-dioxido-1,3-benzenedicarboxylate), have been reported to possess an even higher density of exposed cationic sites than MOF-74 materials due to slight differences in the ligand field around the metal center.13 However, Mg2(m-dobdc) prepared under solvothermal conditions was initially reported to be non-porous due to difficulties associated with removing coordinating solvents such as N,N-dimethylformamide (DMF) or methanol (MeOH) from the framework pores.13 Although Mg2(m-dobdc) was later reported to be porous when prepared under mechanochemical conditions,14 much about its intrinsic gas sorption properties, such as the accessibility of the Mg(II) sites to guest molecules,8 remains relatively unknown.
Herein, we systematically investigate the synthesis and gas sorption properties of fully desolvated Mg2(m-dobdc). Careful washing and activation of Mg2(m-dobdc) prepared under traditional solvothermal (ST) conditions, termed Mg2(m-dobdc)-ST, enables access to a material with a similar 77 K N2 Brunauer–Emmett–Teller (BET) surface area as closely related Mg2(dobdc). In addition, we report an improved mechanochemical (MC) method to reliably prepare Mg2(m-dobdc)-MC using exogenous organic base.14 Beyond the inherent advantages in scalability and waste minimization offered by mechanochemical syntheses,15–17 Mg2(m-dobdc)-MC exhibits higher gas adsorption capacities than Mg2(m-dobdc)-ST. Indeed, at 150 mbar of CO2 and 40 °C, conditions relevant to CO2 capture from coal flue gas,18 Mg2(m-dobdc)-MC exhibits an higher CO2 uptake (6.14 mmol g−1) than even Mg2(dobdc) (5.28 mmol g−1).12,19 This represents one of the highest capacities reported to date for CO2 capture in a porous solid under simulated coal flue gas conditions.20 Overall, our findings suggest that Mg2(m-dobdc) prepared under mechanochemical conditions represents a promising and scalable alternative to ubiquitous Mg2(dobdc) for applications in chemical separations, gas storage, and beyond.
Results and discussion
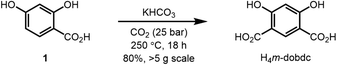
We commenced our studies into the synthesis and gas sorption properties of Mg2(m-dobdc) by optimizing the synthesis of H4m-dobdc (Fig. 2). In our hands, the previously reported synthesis of H4m-dobdc from resorcinol via the Kolbe–Schmitt reaction was only modestly reproducible, often yielding the monocarboxylic acid 1 instead of H4m-dobdc (Fig. 2). Two modifications to the standard preparation were identified to make the synthesis of H4m-dobdc more reliable (see ESI† section 2 for details). First, starting from commercially available 1 in place of resorcinol improved the reproducibility of the reaction, likely because only a single carboxylation reaction must take place to yield H4m-dobdc.21 Second, the reaction temperature (250 °C) was found to be a critical parameter and best monitored using an internal thermocouple placed directly in the solvent-free reaction mixture. With these modifications in place, we were able to reliably synthesize H4m-dobdc on >5 g scale in a single batch.The previously reported small-scale solvothermal synthesis of Mg2(m-dobdc) employed Mg(NO3)2·6H2O as the Mg precursor in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMF
1 DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH at 120 °C.13 In order to identify the optimal solvothermal conditions for preparing Mg2(m-dobdc), several combinations of amide (DMF or N,N-dimethylacetamide) and alcohol (MeOH, ethanol, H2O) solvents were evaluated (ESI† Table S1, see ESI† section 3 for details). Other methods previously reported for the preparation of Mg2(dobdc), such as employing Mg(OAc)2·4H2O as a basic Mg precursor, were tested as well.22–24 Characterization of the produced solids by powder X-ray diffraction (PXRD)25 validated that combining H4m-dobdc and Mg(NO3)2·6H2O in 1
MeOH at 120 °C.13 In order to identify the optimal solvothermal conditions for preparing Mg2(m-dobdc), several combinations of amide (DMF or N,N-dimethylacetamide) and alcohol (MeOH, ethanol, H2O) solvents were evaluated (ESI† Table S1, see ESI† section 3 for details). Other methods previously reported for the preparation of Mg2(dobdc), such as employing Mg(OAc)2·4H2O as a basic Mg precursor, were tested as well.22–24 Characterization of the produced solids by powder X-ray diffraction (PXRD)25 validated that combining H4m-dobdc and Mg(NO3)2·6H2O in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMF
1 DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (0.03 M) at 120 °C for 48 h was optimal to yield highly crystalline Mg2(m-dobdc)-ST (Fig. 3, ESI† Fig. S5). This synthesis can be readily scaled to produce Mg2(m-dobdc)-ST on at least 0.5 g scale (Fig. 3, ESI† Fig. S6) and is reproducible as well (ESI† Fig. S17). Soaking the resulting MOF in DMF at 120 °C to remove residual starting materials, then in MeOH at 60 °C to remove DMF, and then in acetone at room temperature to remove MeOH, was sufficient to remove coordinating solvents and soluble impurities from Mg2(m-dobdc)-ST, as confirmed by acid digestion and analysis of the resulting solution by 1H NMR (ESI† Fig. S13). Unfortunately, higher reaction concentrations (>0.1 M) led to impure materials (not shown), limiting the scalability of this solvothermal method.26
MeOH (0.03 M) at 120 °C for 48 h was optimal to yield highly crystalline Mg2(m-dobdc)-ST (Fig. 3, ESI† Fig. S5). This synthesis can be readily scaled to produce Mg2(m-dobdc)-ST on at least 0.5 g scale (Fig. 3, ESI† Fig. S6) and is reproducible as well (ESI† Fig. S17). Soaking the resulting MOF in DMF at 120 °C to remove residual starting materials, then in MeOH at 60 °C to remove DMF, and then in acetone at room temperature to remove MeOH, was sufficient to remove coordinating solvents and soluble impurities from Mg2(m-dobdc)-ST, as confirmed by acid digestion and analysis of the resulting solution by 1H NMR (ESI† Fig. S13). Unfortunately, higher reaction concentrations (>0.1 M) led to impure materials (not shown), limiting the scalability of this solvothermal method.26
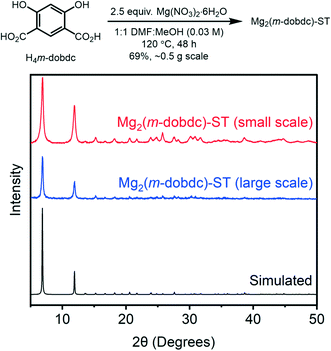 | ||
| Fig. 3 PXRD (λ = 1.5406 Å) patterns of Mg2(m-dobdc)-ST synthesized on small and large scale under solvothermal conditions. The simulated pattern based on the previously reported single-crystal X-ray diffraction structure of the isostructural framework Co2(m-dobdc) is included for reference.25 | ||
Having optimized the solvothermal synthesis of Mg2(m-dobdc), we set out to improve the mechanochemical synthesis of this material for comparison. Previously, we reported the mechanochemical synthesis of Mg2(dobdc) using N,N-diisopropylethylamine (Hünig's base) as both the base required to deprotonate the linker precursor and as the liquid to facilitate liquid-assisted grinding in a planetary ball mill.14 This method could be generalized to the synthesis of porous Mg2(m-dobdc) with modest crystallinity. We hypothesized that careful optimization of the Mg precursor (Mg(NO3)2·6H2O, Mg(OAc)2·4H2O, or MgO), base (Hünig's base, Et3N, or 2,6-lutidine), and grinding time (1, 5, or 10 min) would enable the synthesis of Mg2(m-dobdc)-MC with maximum crystallinity and porosity (see ESI† section 4 for details). Indeed, the combination of Mg(NO3)2·6H2O, Et3N, and 5–10 min grinding time was optimal to yield crystalline Mg2(m-dobdc)-MC (Fig. 4, ESI† Fig. S18 and S19). This method could be reproducibly carried out with 10 min of grinding at 600 rpm to produce Mg2(m-dobdc)-MC on 0.5 g scale in excellent yield (Fig. 3, ESI† Fig. S20 and S31). Notably, this mechanochemical synthesis bypasses the use of toxic DMF,27 representing a green alternative to the solvothermal synthesis of Mg2(m-dobdc)-ST. No MOF was obtained with 2,6-lutidine, likely because it is not basic enough to fully deprotonate H4(m-dobdc).
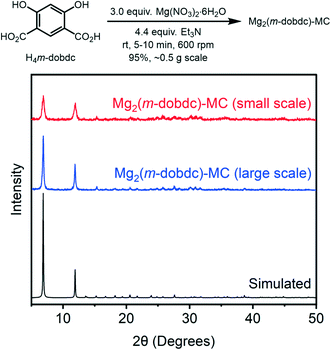 | ||
| Fig. 4 PXRD (λ = 1.5406 Å) patterns of Mg2(m-dobdc)-MC synthesized on small and large scale under mechanochemical conditions. The simulated pattern based on the previously reported single-crystal X-ray diffraction structure of the isostructural framework Co2(m-dobdc) is included for reference.25 | ||
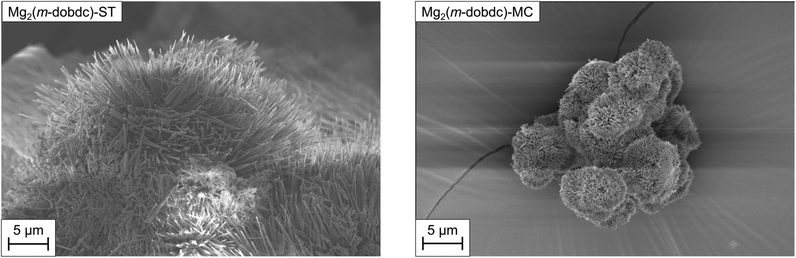
With optimized samples of Mg2(m-dobdc)-ST and Mg2(m-dobdc)-MC in hand, we compared their crystallite morphologies (Fig. 5) and porosities (Table 1) in order to understand how synthesis procedure affects the physical properties of Mg2(m-dobdc). Characterization of Mg2(m-dobdc)-ST by scanning electron microscopy (SEM) revealed that it is composed of crystalline needles >5 μm in length on average (Fig. 5 left, ESI† Fig. S12). A needle-like morphology for Mg2(m-dobdc)-ST is consistent with that previously reported for single crystals of the isostructural framework Co2(m-dobdc).25,28 Similarly, Mg2(m-dobdc)-MC is comprised of needles <1 μm in length (Fig. 5, right, ESI† Fig. S26). The smaller crystallites for mechanochemically synthesized Mg2(m-dobdc)-MC likely arise due to rapid deprotonation of H4m-dobdc by triethylamine during the reaction.14,29
Careful activation of Mg2(m-dobdc)-ST and Mg2(m-dobdc)-MC under high vacuum (<10 μbar) at 180 °C for at least 24 h was sufficient to fully remove solvent molecules from both frameworks. Their porosities were assessed by collecting 77 K N2 adsorption isotherms (ESI† Fig. S9 and S23). As expected, Mg2(m-dobdc)-ST and Mg2(m-dobdc)-MC are both microporous, with BET and Langmuir surface areas comparable to those reported for related Mg2(dobdc) (Table 1).10,12 The BET surface area of Mg2(m-dobdc)-MC (1653 ± 2 m2 g−1) is somewhat higher than that of Mg2(m-dobdc)-ST (1556 ± 2), reflecting a greater degree of accessible pores and/or the presence of insoluble, amorphous impurities in the latter material. We note that heating Mg2(m-dobdc) with ramp rates faster than 1 °C min−1 or to temperatures greater than 180 °C consistently led to lower surface areas, likely due to partial pore collapse. Nonetheless, these findings confirm that highly porous and crystalline Mg2(m-dobdc) can be readily prepared under both solvothermal and mechanochemical conditions.
Given the microporosity of both Mg2(m-dobdc) samples and the high capacities of closely related Mg2(dobdc) for a range of adsorbates,10,12 we assessed the potential suitability of Mg2(m-dobdc)-ST and Mg2(m-dobdc)-MC for gas capture applications using CO2 scrubbing from coal flue gas as a representative separation. As such, CO2 adsorption and desorption isotherms at 30 °C, 40 °C, and 50 °C and N2 adsorption and desorption isotherms at 40 °C were collected for Mg2(m-dobdc)-ST (Fig. 6a, ESI† Fig. S14 and S15) and Mg2(m-dobdc)-MC (Fig. 6b, ESI† Fig. S28 and S29). In all cases, gas sorption was found to be completely reversible. Both materials exhibit steep uptake at low CO2 pressures, indicative of strong interaction of CO2 with exposed Mg(II) sites.12 The maximum CO2 uptakes for both materials at 30 °C and 1 bar of CO2 are 6.50 mmol g−1 for Mg2(m-dobdc)-ST and 8.69 mmol g−1 for Mg2(m-dobdc)-MC; the latter value is similar to that predicted for binding one CO2 per Mg(II) site in this material (8.24 mmol g−1). The higher CO2 capacity of Mg2(m-dobdc)-MC than Mg2(m-dobdc)-ST is consistent with its higher 77 K N2 BET surface area and suggests that the mechanochemically synthesized MOF has more accessible Mg(II) sites.
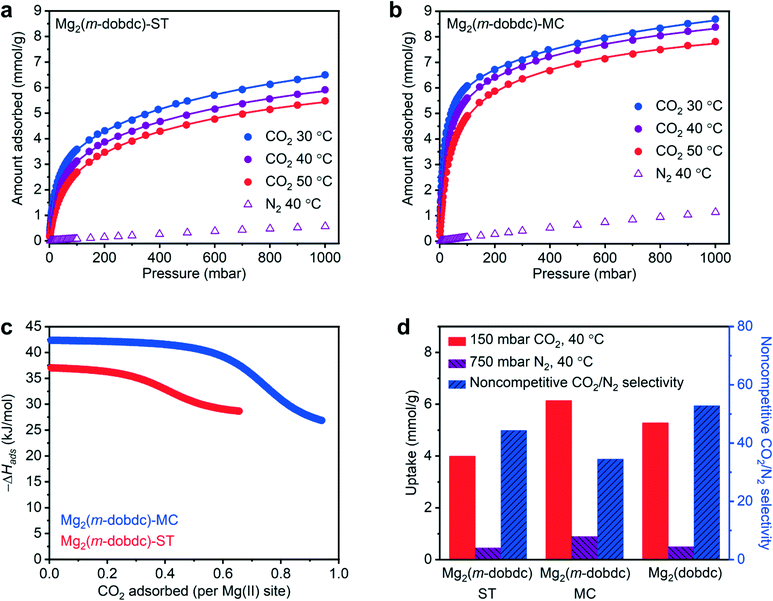 | ||
| Fig. 6 30 °C, 40 °C, and 50 °C CO2 and 40 °C N2 adsorption isotherms in a) Mg2(m-dobdc)-ST and b) Mg2(m-dobdc)-MC. The lines correspond to individual fits to the dual-site Langmuir model. A data point was considered equilibrated when <0.01% pressure change occurred over a 30 s interval. c) Enthalpy of adsorption (−ΔHads) values for Mg2(m-dobdc)-ST and Mg2(m-dobdc)-MC determined using simultaneous fits to dual-site Langmuir models. d) Summary of CO2 and N2 uptake values and non-competitive CO2/N2 selectivities relevant to CO2 capture from coal flue gas for Mg2(m-dobdc)-ST and Mg2(m-dobdc)-MC. The corresponding values for Mg2(dobdc) reported in the literature are included for reference.19 | ||
To confirm that CO2 binding in Mg2(m-dobdc) materials likely occurs at coordinatively unsaturated Mg(II) sites, the CO2 adsorption isotherms were fit using dual-site Langmuir models both independently and simultaneously (ESI† Tables S2 and S4). The independent dual-site Langmuir model fits are included in Fig. 6a and b and represent good fits to the experimental data. Using the Clausius–Clapeyron relationship, the differential enthalpies of adsorption (−ΔHads) as a function of CO2 loading were calculated (Fig. 6c, ESI† Fig. S16 and S30). The −ΔHads values at low coverage are comparable for Mg2(m-dobdc)-MC (42 kJ mol−1) and Mg2(m-dobdc)-ST (37 kJ mol−1) and are similar to those previously reported for CO2 adsorption in MOFs bearing accessible Mg(II) sites as well (38–43 kJ mol−1).10,30 The −ΔHads plots support that Mg2(m-dobdc)-MC contains more accessible Mg(II) sites than Mg2(m-dobdc)-ST, as the strong binding of CO2 drop offs at higher loadings in this material (∼0.7 CO2 per Mg vs. ∼0.4 CO2 per Mg in Mg2(m-dobdc)-ST).
Among MOFs, Mg2(dobdc) possesses one of the highest reported CO2 capacities (5.28 mmol g−1) under conditions relevant to CO2 capture from coal flue gas (150 mbar, 40 °C) (Fig. 6d).10,18,19 Coupled with minimal uptake of N2 at 750 mbar and 40 °C (0.50 mmol g−1),19 this high CO2 uptake at low pressures makes Mg2(dobdc) a promising material for CO2/N2 separations. While the CO2 uptake of Mg2(m-dobdc)-ST at 150 mbar and 40 °C (3.99 mmol g−1) is less than that reported for Mg2(dobdc), likely due to its dearth of accessible Mg(II) sites, Mg2(m-dobdc)-MC exhibits a higher capacity for CO2 (6.14 mmol g−1) than Mg2(dobdc) under these conditions (Fig. 6d). Previous studies have suggested that the metal centers of M2(m-dobdc) MOFs are slightly more Lewis acidic than those of M2(dobdc) MOFs,13 which may account for the enhanced CO2 uptake at low pressures in Mg2(m-dobdc)-MC. Consistently, the N2 capacity of Mg2(m-dobdc)-MC at 750 mbar and 40 °C (0.89 mmol g−1) is higher than that reported for Mg2(dobdc) as well. Given the unclear suitability of calculating selectivities in open metal site MOFs using ideal adsorbed solution theory (IAST),31 we elected to calculate non-competitive CO2/N2 selectivities under conditions relevant to coal flue gas capture for Mg2(m-dobdc)-ST, Mg2(m-dobdc)-MC, and Mg2(dobdc) instead (Fig. 6d). The non-competitive CO2/N2 selectivity calculated for Mg2(dobdc) is the highest (53),19 followed by Mg2(m-dobdc)-ST (44), and then Mg2(m-dobdc)-MC (35). The diminished non-competitive CO2/N2 selectivity for Mg2(m-dobdc)-MC is due to the higher uptake of N2 in this material. Nonetheless, these findings support that Mg2(m-dobdc)-MC is competitive with the widely studied MOF Mg2(dobdc) for this representative separation. Further, the superior gas sorption performance of Mg2(m-dobdc)-MC over Mg2(m-dobdc)-ST indicates that mechanochemical methods may be preferable for the scalable synthesis of this framework.
Conclusions
Owing to their low cost and high gravimetric gas storage capacities, MOFs bearing high densities of coordinatively unsaturated Mg(II) centers are highly sought after. We have demonstrated that Mg2(m-dobdc) synthesized under mechanochemical conditions is a promising new Mg-based MOF due to its strong binding and high capacity for CO2 at low pressures. Notably, the work presented herein represents a rare example in which a mechanochemically synthesized MOF displays superior gas sorption properties compared to material synthesized under traditional solvothermal conditions.14–17 Moving forward, mechanochemical methods will prove to be a valuable alternative to solvothermal syntheses for the preparation of high-quality MOFs for applications in gas storage, chemical separations, and drug delivery.Author contributions
P. J. M. and R. M. M. conceived the project. E. Y. C. and R. M. M. carried out all experiments. The manuscript was written through the contributions of all authors, and all authors approved of the final version.Conflicts of interest
P. J. M. is listed as an inventor on several patents related to the application of MOFs for gas capture.Acknowledgements
The development of new methods for the synthesis of Mg-based MOFs was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM138165. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The characterization of the CO2 adsorption properties of Mg2(m-dobdc) was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under award number DE-SC0021000. This work made use of the Cornell Center for Materials Research Shared Facilities, which are supported through the NSF MRSEC program (DMR-1719875). Some of the 1H NMR data in this work were collected on a Bruker INOVA 500 MHz spectrometer that was purchased with support from the NSF (CHE-1531632). We thank Cornell University for providing a Summer Experience Grant to E. Y. C.Notes and references
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- Y. Sun, L. Zheng, Y. Yang, X. Qian, T. Fu, X. Li, Z. Yang, H. Yan, C. Cui and W. Tan, Nano-Micro Lett., 2020, 12, 103 CrossRef CAS PubMed.
- A. Bavykina, N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez and J. Gascon, Chem. Rev., 2020, 120, 8468–8535 CrossRef CAS PubMed.
- H. Li, K. Wang, Y. Sun, C. T. Lollar, J. Li and H.-C. Zhou, Mater. Today, 2018, 21, 108–121 CrossRef CAS.
- C. A. Trickett, A. Helal, B. A. Al-Maythalony, Z. H. Yamani, K. E. Cordova and O. M. Yaghi, Nat. Rev. Mater., 2017, 2, 17045 CrossRef CAS.
- Ü. Kökçam-Demir, A. Goldman, L. Esrafili, M. Gharib, A. Morsali, O. Weingart and C. Janiak, Chem. Soc. Rev., 2020, 49, 2751–2798 RSC.
- N. L. Rosi, J. Kim, M. Eddaoudi, B. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504–1518 CrossRef CAS PubMed.
- M. E. Zick, J.-H. Lee, M. I. Gonzalez, E. O. Velasquez, A. A. Uliana, J. Kim, J. R. Long and P. J. Milner, J. Am. Chem. Soc., 2021, 143, 1948–1958 CrossRef CAS PubMed.
- À. Ruyra, A. Yazdi, J. Espín, A. Carné-Sánchez, N. Roher, J. Lorenzo, I. Imaz and D. Maspoch, Chem. – Eur. J., 2015, 21, 2508–2518 CrossRef PubMed.
- W. L. Queen, M. R. Hudson, E. D. Bloch, J. A. Mason, M. I. Gonzalez, J. S. Lee, D. Gygi, J. D. Howe, K. Lee and T. A. Darwish, Chem. Sci., 2014, 5, 4569–4581 RSC.
- T. Grant Glover, G. W. Peterson, B. J. Schindler, D. Britt and O. Yaghi, Chem. Eng. Sci., 2011, 66, 163–170 CrossRef CAS.
- S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef CAS PubMed.
- M. T. Kapelewski, S. J. Geier, M. R. Hudson, D. Stück, J. A. Mason, J. N. Nelson, D. J. Xiao, Z. Hulvey, E. Gilmour, S. A. FitzGerald, M. Head-Gordon, C. M. Brown and J. R. Long, J. Am. Chem. Soc., 2014, 136, 12119–12129 CrossRef CAS PubMed.
- Z. Wang, Z. Li, M. Ng and P. J. Milner, Dalton Trans., 2020, 49, 16238–16244 RSC.
- D. Chen, J. Zhao, P. Zhang and S. Dai, Polyhedron, 2019, 162, 59–64 CrossRef CAS.
- J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC.
- T. Friščić, in Encyclopedia of Inorganic and Bioinorganic Chemistry, John Wiley & Sons, Ltd, 2014, pp. 1–19 Search PubMed.
- E. J. Granite and H. W. Pennline, Ind. Eng. Chem. Res., 2002, 41, 5470–5476 CrossRef CAS.
- J. A. Mason, K. Sumida, Z. R. Herm, R. Krishna and J. R. Long, Energy Environ. Sci., 2011, 4, 3030 RSC.
- A. Modak and S. Jana, Microporous Mesoporous Mater., 2019, 276, 107–132 CrossRef CAS.
- Y. Sadamitsu, A. Okumura, K. Saito and T. Yamada, Chem. Commun., 2019, 55, 9837–9840 RSC.
- K. A. Colwell, M. N. Jackson, R. M. Torres-Gavosto, S. Jawahery, B. Vlaisavljevich, J. M. Falkowski, B. Smit, S. C. Weston and J. R. Long, J. Am. Chem. Soc., 2021, 143, 5044–5052 CrossRef CAS PubMed.
- L. Garzón-Tovar, A. Carné-Sánchez, C. Carbonell, I. Imaz and D. Maspoch, J. Mater. Chem. A, 2015, 3, 20819–20826 RSC.
- D. J. Tranchemontagne, J. R. Hunt and O. M. Yaghi, Tetrahedron, 2008, 64, 8553–8557 CrossRef CAS.
- J. E. Bachman, M. T. Kapelewski, D. A. Reed, M. I. Gonzalez and J. R. Long, J. Am. Chem. Soc., 2017, 139, 15363–15370 CrossRef CAS PubMed.
- D. R. Du Bois, K. R. Wright, M. K. Bellas, N. Wiesner and A. J. Matzger, Inorg. Chem., 2022, 61, 4550–4554 CrossRef CAS PubMed.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef.
- M. I. Gonzalez, M. T. Kapelewski, E. D. Bloch, P. J. Milner, D. A. Reed, M. R. Hudson, J. A. Mason, G. Barin, C. M. Brown and J. R. Long, J. Am. Chem. Soc., 2018, 140, 3412–3422 CrossRef CAS PubMed.
- G. Ayoub, B. Karadeniz, A. J. Howarth, O. K. Farha, I. Đilović, L. S. Germann, R. E. Dinnebier, K. Užarević and T. Friščić, Chem. Mater., 2019, 31, 5494–5501 CrossRef CAS.
- P. J. Milner, J. D. Martell, R. L. Siegelman, D. Gygi, S. C. Weston and J. R. Long, Chem. Sci., 2018, 9, 160–174 RSC.
- R. Krishna and J. M. van Baten, ACS Omega, 2021, 6, 15499–15513 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: All procedures and characterization data. See DOI: https://doi.org/10.1039/d2ce00739h |
| ‡ Contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |