Open Access Article
Open Access ArticlePhototransduction in a supramolecular cascade: a mimic for essential features of the vision process†
Jialei
Chen-Wu
a,
Patrícia
Máximo
 b,
Patricia
Remón
b,
Patricia
Remón
 a,
A. Jorge
Parola
a,
A. Jorge
Parola
 b,
Nuno
Basílio
b,
Nuno
Basílio
 b and
Uwe
Pischel
b and
Uwe
Pischel
 *a
*a
aCIQSO – Centre for Research in Sustainable Chemistry and Department of Chemistry, University of Huelva, Campus de El Carmen s/n, E-21071, Huelva, Spain. E-mail: uwe.pischel@diq.uhu.es; Tel: +34 959 21 99 82
bLaboratorio Associado para a Química Verde (LAQV), Rede de Química e Tecnologia (REQUIMTE), Departamento de Química, Faculdade de Ciências e Tecnología, Universidade NOVA de Lisboa, 2829-516, Caparica, Portugal
First published on 24th February 2023
Abstract
The tailored design of a light-triggered supramolecular cascade results in an artificial machinery that assimilates the transduction of photons into chemical communication and the final release of a neurotransmitter. This is reminiscent of key steps in the natural vision process.
The vision process is an intricate and complex example of chemical signal cascading, triggered by light as an external stimulus. In a nutshell, photons are used by 11-cis retinal, resulting in its conversion into all-trans retinal.1 This transformation is accompanied by structural changes of rhodopsin, which subsequently triggers a series of concatenated chemical signaling events. Ultimately this leads to a depletion of cGMP and the closing down of Na+ ion channels, which results in an attenuation of the dark current and the hyperpolarization of the photoreceptor cells.2 The retina is further composed of other cells that are intercommunicated in a circuit, thereby enabling the gift of vision in all its facets: three-dimensional vision, color perception, adaption to light intensity conditions, etc. Among these cellular components are amacrine cells,2 a diverse group of interneurons in the retina, which are responsible for the modulation between bipolar and ganglion cells.
Dopamine is essential for the regulation of light-adapted vision and photoreceptor coupling in the retina. This neurotransmitter is released by dopaminergic amacrine cells, following a light-intensity-modulated circadian rhythm (see Fig. 1).3 Within this context we sought to realize an artificial light-driven host–guest system4,5 that would mimic the following features: light-induced structural changes that lead to a cascade of supramolecular events,6–16 culminating in the final release of dopamine.
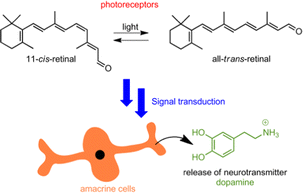 | ||
| Fig. 1 Representation of the photoisomerization of 11-cis-retinal and signal transduction, triggering dopamine release from amacrine cells. | ||
We decided to design our system based on the host–guest chemistry of cucurbit[n]urils (CBn).17–19 These water-soluble macrocycles feature a hydrophobic inner cavity with low polarizability and carbonyl-rimed portals. Hence, guests that can exert hydrophobic effects and ion–dipole interactions lead to the formation of highly stable complexes, which often reach affinities in the subnanomolar range.17,19,20 Depending on the size and shape of the guest and the resulting packing in the complex, a highly selective binding to different CBn homologues (n = 7 and 8 in our case) can be observed. This may lead to affinity differentiations of up to 5–7 orders of magnitude. Taking advantage of the high-affinity binding, CBn has been applied in bio-relevant applications,21,22 drug delivery,23 functional materials,24 and sensing,25–28 among others.
Light as an external stimulus of supramolecular assemblies is very attractive due to the possibility of spatiotemporal control.29–39 This has been explored as well in the context of CBn chemistry.40–43 Among other photoswitches, dithienylethenes (DTEs)44–46 have been proven very useful47–51 and will also play a crucial role in the present work.
The design approach towards the envisioned light-controlled supramolecular cascade is outlined in Fig. 2. The initial state of the five-component mixture is expected to obey the rules of thermodynamically controlled self-sorting. The DTE photoswitch in its non-activated form (1o) should not occupy any of the host macrocycles, while the mediator guest is bound by CB8 and dopamine (DA) is allocated inside the CB7 cavity. On photoactivation of the switch a competitor is formed (1c) that displaces the mediator from CB8, which then competes with DA for the CB7 macrocycle. This would result in the effective release of the neurotransmitter. Importantly, the activated photoswitch should not bypass the mediator function and displace dopamine directly from CB7.
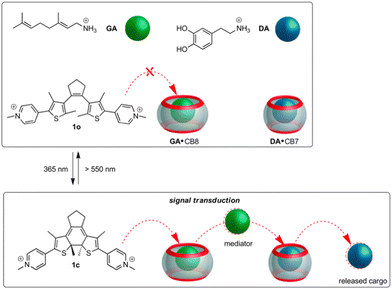 | ||
| Fig. 2 Design of the light-controlled supramolecular cascade in a self-sorted five-component mixture. | ||
As can be expected for such an intricate situation, a judicious choice of the guests is indispensable (see Table 1). The mediator should bind much stronger to CB8 than to CB7, but still have a CB7-affinity that at least equals that of DA (KDA·CB7 = 1.1 × 105 M−1).16 We identified geranylamine (GA) to fit this bill conveniently, being a subnanomolar-affinity binder for CB8 and a micromolar-affinity binder for CB7 (KGA·CB8/KGA·CB7ca. 5000);49,52 see Table 1. The DTE photoswitch should show a large CB8-affinity differentiation for its activated and non-activated forms,51 while being a poor binder for CB7 in any of its forms. To our satisfaction, switch 1 in both forms (1o and 1c) shows very low binding (ca. 103 M−1) with CB7; see Table 1. However, for CB8 high-affinity binding at the subnanomolar level is observed for 1c, while 1o binds ca. 4000 times less strong.51 The absolute CB8-binding affinity of 1c is even somewhat higher than that of GA; see Table 1. Based on these data a light-induced cascade should be feasible.
| Complex | K/M−1 |
|---|---|
| a Taken from ref. 16. b Taken from ref. 52. c Taken from ref. 49. d Measured in this work by 1H NMR titration (see ESI). e Taken from ref. 51. | |
| DA·CB7a | 1.1 × 105 |
| DA·CB8a | 4.2 × 104 |
| GA·CB7b | 3.2 × 106 |
| GA·CB8c | 1.4 × 1010 |
| 1o·CB7d | 1.0 × 103 |
| 1o·CB8e | 6.5 × 106 |
| 1c·CB7d | 1.0 × 103 |
| 1c·CB8e | 2.2 × 1010 |
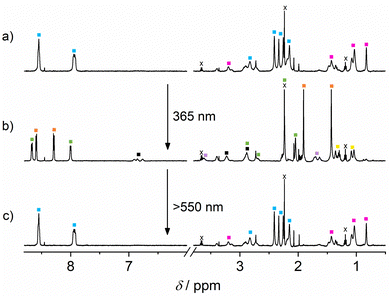
In the following steps, we sought to obtain experimental confirmation for this premise. 1H NMR spectroscopy is the method of choice to follow the distinct situations of the guests being unbound or complexed by one of the two CB homologues. When mixing equimolar amounts (200 μM each in D2O at pD 5.4) of guests GA and DA and the hosts CB7 and CB8 a clear-cut self-sorting situation is encountered (see ESI†). As predicted from the guest affinities for each host (see Table 1), GA is complexed exclusively by CB8, while DA is bound by CB7. This is for example indicated by typical upfield shifts of the methyl protons of GA.49 Noteworthily, the 1H NMR signature of GA when complexed by CB7 or CB8 is well differentiated and can be unambiguously assigned to the latter situation (see the spectra in ESI†). The proton signals of DA broaden to such an extent that they are not visible in the CB7 complex.16 The next question that arises is whether GA can displace DA from its CB7 complex. When adding 200 μM GA to the DA·CB7 complex (also at 200 μM) the appearance of free DA, signalled by the typical aromatic proton signals at 6.8–6.9 ppm, is detected (see ESI†). Also the characteristic signature of the GA·CB7 complex appears. However, some GA (about 50%) remains non-complexed. In the following we evaluated whether the DTE in either form (1o or 1c) would displace GA from its CB8 complex. For this, a solution (D2O, pD 5.4) with 300 μM 1o and 200 μM GA·CB8 was prepared. Gratifyingly 1o does not displace GA. This is expected, due to the four orders of magnitude smaller binding constant of 1o with the macrocycle (see Table 1). However, when isomerising 1o into 1c by irradiation with 365 nm light (Φ1o→1c = 0.04; quantitative conversion),51 competition is observed which results in an efficient release of GA from the CB8 macrocycle (ca. 90%); see the ESI.†
Having gathered compelling experimental proof for the stepwise competitive displacement of GA from CB8 (by means of the action of photogenerated 1c) and DA from CB7 (by means of the action of GA), we proceeded to the complete cascade experiment in the five-component mixture ([1o] = 300 μM, [DA] = [GA] = [CB7] = [CB8] = 200 μM). As anticipated, in this system a very clear self-sorting applies: GA is encountered in CB8, DA in CB7, and 1o is not complexed by any of the macrocycles (see Fig. 3a). In agreement, only the proton signals of GA in CB8 are observed. The aromatic signals of DA are not visible at all, as expected due to its complexation by CB7. The DTE signals correspond to non-complexed open form 1o, indicating that this form of the switch does not occupy the CB8 cavity. On photoisomerization (365 nm light), forming 1c, GA is found in CB7 (about 90% of the GA is competitively displaced from CB8 by 1c), 1c in CB8 (beside some amount of free 1c, due to the excess concentration of the photoswitch) and the aromatic 1H NMR signals of released DA are clearly seen (see Fig. 3b). By comparison of the integrated 1H NMR signals of released DA and the total amount of DA (released by adding 500 μM 3-aminoadamant-1-ol)19 it can be estimated that ca. 50% of DA is released in the light-induced cascade.53 On shining visible light (>550 nm) on the 1c form, 1o is formed back quantitatively (Φ1c→1o = 0.001)51 and consequently the initial situation is restored (see Fig. 3c). The 1H NMR spectra before 365 nm irradiation and after irradiation with visible light are practically indistinguishable (Fig. 3a and c).
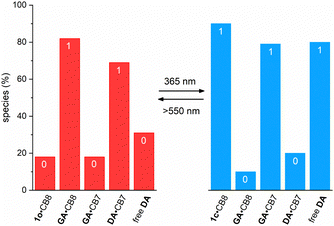
The simulation of the distribution of the constituents of the cascade before and after irradiation with UV light provides further insights into the correct function of the cascade; see Fig. 4. This prediction is based on the coupled thermodynamic equilibria of the involved host–guest complexes (see ESI†). Before irradiation about 82% of all CB8 are occupied by GA and 69% of all CB7 capsules have DA as a guest. On 365 nm light irradiation, the ring-closed DTE 1c is formed and now about 90% of all CB8 have this one as a guest. Consequently, the released GA competes with DA for the CB7 cavity and 79% of all CB7 have the former as a guest, while only 20% retain DA. This means that about 80% of all DA is non-bound after irradiation, compared to 31% before irradiation. This corresponds to about 50% light-induced release of DA, being in excellent agreement with the experiment (see above).
It should be stressed that the mediator function of GA is strictly necessary to release DA from CB7, because neither form of 1, open or closed, could displace DA directly from CB7. This is because both 1o and 1c have binding constants with CB7 that are two orders of magnitude lower than that of DA. The impossibility to release DA by means of competitive displacement with 1c was demonstrated by a corresponding 1H NMR experiment (see ESI†). All observed signals in the three-component mixture ([1] = 300 μM; [DA] = [CB7] = 200 μM) correspond perfectly to those of non-bonded DTE (either 1o or 1c). In addition, no NMR signals of free DA were observed after the light-induced conversion of 1o into 1c.
In summary, we report a light-triggered supramolecular cascade that transduces a photonic signal into the release of dopamine. The cascade builds on the principles of thermodynamic self-sorting. On conversion of a DTE phototrigger a different equilibrium situation is created, which translates into downstream cascading and the final release of the neurotransmitter. These characteristics are also found in the vision process, mimicked by the herein described system. This may be of interest for (supra)molecular information processing,54–57 where stimuli-dependent concentration patterns may be of importance,49,58 or as light-dependent actuators.
This work was supported by the Spanish Ministerio de Ciencia e Innovación (grant PID2020-119992GB-I00 for U.P.), the Consejería de Economía, Conocimiento, Empresas y Universidad/Junta de Andalucía (grant P18-FR-4080 for U.P.), the Portuguese Fundação para a Ciência e a Tecnologia – FCT/MCTES (grant CEECIND/00466/2017 for N.B.), and by LAQV-REQUIMTE (through UIDB/50006/2020 and UIDP/50006/2020).
Conflicts of interest
There are no conflicts to declare.References
- R. S. H. Liu and A. E. Asato, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 259–263 CrossRef CAS PubMed.
- Y. Shichida and T. Matsuyama, Philos. Trans. R. Soc., B, 2009, 364, 2881–2895 CrossRef CAS PubMed.
- T. Munteanu, K. J. Noronha, A. C. Leung, S. Pan, J. A. Lucas and T. M. Schmidt, eLife, 2018, 7, e39866 CrossRef PubMed.
- D.-H. Qu, Q.-C. Wang, Q.-W. Zhang, X. Ma and H. Tian, Chem. Rev., 2015, 115, 7543–7588 CrossRef CAS PubMed.
- A. Díaz-Moscoso and P. Ballester, Chem. Commun., 2017, 53, 4635–4652 RSC.
- D. Ray, J. T. Foy, R. P. Hughes and I. Aprahamian, Nat. Chem., 2012, 4, 757–762 CrossRef CAS PubMed.
- S. Ma, M. M. J. Smulders, Y. R. Hristova, J. K. Clegg, T. K. Ronson, S. Zarra and J. R. Nitschke, J. Am. Chem. Soc., 2013, 135, 5678–5684 CrossRef CAS PubMed.
- A. G. Salles Jr., S. Zarra, R. M. Turner and J. R. Nitschke, J. Am. Chem. Soc., 2013, 135, 19143–19146 CrossRef PubMed.
- M. Schmittel, Chem. Commun., 2015, 51, 14956–14968 RSC.
- G. Ashkenasy, T. M. Hermans, S. Otto and A. F. Taylor, Chem. Soc. Rev., 2017, 46, 2543–2554 RSC.
- M. J. Langton, F. Keymeulen, M. Ciaccia, N. H. Williams and C. A. Hunter, Nat. Chem., 2017, 9, 426–430 CrossRef CAS PubMed.
- A. Llopis-Lorente, P. Díez, A. Sánchez, M. D. Marcos, F. Sancenón, P. Martínez-Ruiz, R. Villalonga and R. Martínez-Máñez, Nat. Commun., 2017, 8, 15511–15517 CrossRef CAS PubMed.
- B. S. Pilgrim, D. A. Roberts, T. G. Lohr, T. K. Ronson and J. R. Nitschke, Nat. Chem., 2017, 9, 1276–1281 CrossRef CAS.
- J. S. Park, J. Park, Y. J. Yang, T. T. Tran, I. S. Kim and J. L. Sessler, J. Am. Chem. Soc., 2018, 140, 7598–7604 CrossRef CAS PubMed.
- Y.-C. Liu, W. M. Nau and A. Hennig, Chem. Commun., 2019, 55, 14123–14126 RSC.
- P. Remón, D. González, M. A. Romero, N. Basílio and U. Pischel, Chem. Commun., 2020, 56, 3737–3740 RSC.
- S. Liu, C. Ruspic, P. Mukhopadhyay, S. Chakrabarti, P. Y. Zavalij and L. Isaacs, J. Am. Chem. Soc., 2005, 127, 15959–15967 CrossRef CAS PubMed.
- K. I. Assaf and W. M. Nau, Chem. Soc. Rev., 2015, 44, 394–418 RSC.
- S. J. Barrow, S. Kasera, M. J. Rowland, J. del Barrio and O. A. Scherman, Chem. Rev., 2015, 115, 12320–12406 CrossRef CAS PubMed.
- L. P. Cao, M. Šekutor, P. Y. Zavalij, K. Mlinarić-Majerski, R. Glaser and L. Isaacs, Angew. Chem., Int. Ed., 2014, 53, 988–993 CrossRef CAS PubMed.
- J. M. Chinai, A. B. Taylor, L. M. Ryno, N. D. Hargreaves, C. A. Morris, P. J. Hart and A. R. Urbach, J. Am. Chem. Soc., 2011, 133, 8810–8813 CrossRef CAS PubMed.
- D.-W. Lee, K. M. Park, M. Banerjee, S. H. Ha, T. Lee, K. Suh, S. Paul, H. Jung, J. Kim, N. Selvapalam, S. H. Ryu and K. Kim, Nat. Chem., 2011, 3, 154–159 CrossRef CAS PubMed.
- I. Ghosh and W. M. Nau, Adv. Drug Delivery Rev., 2012, 64, 764–783 CrossRef CAS PubMed.
- Y. Ahn, Y. Jang, N. Selvapalam, G. Yun and K. Kim, Angew. Chem., Int. Ed., 2013, 52, 3140–3144 CrossRef CAS PubMed.
- F. Biedermann and W. M. Nau, Angew. Chem., Int. Ed., 2014, 53, 5694–5699 CrossRef CAS PubMed.
- S. Zhang, K. I. Assaf, C. Huang, A. Hennig and W. M. Nau, Chem. Commun., 2019, 55, 671–674 RSC.
- C. M. Hu, T. Jochmann, P. Chakraborty, M. Neumaier, P. A. Levkin, M. M. Kappes and F. Biedermann, J. Am. Chem. Soc., 2022, 144, 13084–13095 CrossRef CAS PubMed.
- A. Prabodh, S. Sinn and F. Biedermann, Chem. Commun., 2022, 58, 13947–13950 RSC.
- M. L. Pellizzaro, K. A. Houton and A. J. Wilson, Chem. Sci., 2013, 4, 1825–1829 RSC.
- M. Han, R. Michel, B. He, Y.-S. Chen, D. Stalke, M. John and G. H. Clever, Angew. Chem., Int. Ed., 2013, 52, 1319–1323 CrossRef CAS PubMed.
- D. V. Berdnikova, T. M. Aliyeu, T. Paululat, Y. V. Fedorov, O. A. Fedorova and H. Ihmels, Chem. Commun., 2015, 51, 4906–4909 RSC.
- G. Yu, W. Yu, Z. Mao, C. Gao and F. Huang, Small, 2015, 11, 919–925 CrossRef CAS PubMed.
- L. Stricker, E.-C. Fritz, M. Peterlechner, N. L. Doltsinis and B. J. Ravoo, J. Am. Chem. Soc., 2016, 138, 4547–4554 CrossRef CAS PubMed.
- S. T. J. Ryan, J. del Barrio, R. Suardíaz, D. F. Ryan, E. Rosta and O. A. Scherman, Angew. Chem., Int. Ed., 2016, 55, 16096–16100 CrossRef CAS PubMed.
- A. Tron, I. Pianet, A. Martinez-Cuezva, J. H. R. Tucker, L. Pisciottani, M. Alajarin, J. Berna and N. D. McClenaghan, Org. Lett., 2017, 19, 154–157 CrossRef CAS PubMed.
- X. D. Chi, W. L. Cen, J. A. Queenan, L. L. Long, V. M. Lynch, N. M. Khashab and J. L. Sessler, J. Am. Chem. Soc., 2019, 141, 6468–6472 CrossRef CAS PubMed.
- R. J. Li, J. J. Holstein, W. G. Hiller, J. Andréasson and G. H. Clever, J. Am. Chem. Soc., 2019, 141, 2097–2103 CrossRef CAS PubMed.
- A. Ducrot, A. Tron, R. Bofinger, I. Sanz Beguer, J.-L. Pozzo and N. D. McClenaghan, Beilstein J. Org. Chem., 2019, 15, 2801–2811 CrossRef CAS PubMed.
- M. Canton, A. B. Grommet, L. Pesce, J. Germen, S. Li, Y. Diskin-Posner, A. Credi, G. M. Pavan, J. Andréasson and R. Klajn, J. Am. Chem. Soc., 2020, 142, 14557–14565 CrossRef CAS PubMed.
- F. Tian, D. Jiao, F. Biedermann and O. A. Scherman, Nat. Commun., 2012, 3, 1207 CrossRef PubMed.
- N. Basílio and U. Pischel, Chem. – Eur. J., 2016, 22, 15208–15211 CrossRef PubMed.
- J. del Barrio, S. T. J. Ryan, P. G. Jambrina, E. Rosta and O. A. Scherman, J. Am. Chem. Soc., 2016, 138, 5745–5748 CrossRef CAS PubMed.
- M. A. Romero, R. J. Fernandes, A. J. Moro, N. Basílio and U. Pischel, Chem. Commun., 2018, 54, 13335–13338 RSC.
- M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- M. Herder, B. M. Schmidt, L. Grubert, M. Pätzel, J. Schwarz and S. Hecht, J. Am. Chem. Soc., 2015, 137, 2738–2747 CrossRef CAS PubMed.
- J. Zhang and H. Tian, Adv. Opt. Mater., 2018, 6, 1701278 CrossRef.
- G. Liu, Y.-M. Zhang, C. Wang and Y. Liu, Chem. – Eur. J., 2017, 23, 14425–14429 CrossRef CAS PubMed.
- P. Ferreira, B. Ventura, A. Barbieri, J. P. Da Silva, C. A. T. Laia, A. J. Parola and N. Basílio, Chem. – Eur. J., 2019, 25, 3477–3482 CrossRef CAS PubMed.
- P. Remón, D. González, S. M. Li, N. Basílio, J. Andréasson and U. Pischel, Chem. Commun., 2019, 55, 4335–4338 RSC.
- D. Kim, A. Aktalay, N. Jensen, K. Uno, M. L. Bossi, V. N. Belov and S. W. Hell, J. Am. Chem. Soc., 2022, 144, 14235–14247 CrossRef CAS PubMed.
- P. Máximo, M. Colaço, S. R. Pauleta, P. J. Costa, U. Pischel, A. J. Parola and N. Basílio, Org. Chem. Front., 2022, 9, 4238–4249 RSC.
- M. A. Romero, N. Basílio, A. J. Moro, M. Domingues, J. A. González-Delgado, J. F. Arteaga and U. Pischel, Chem. – Eur. J., 2017, 23, 13105–13111 CrossRef CAS PubMed.
- It should be noted that the 1H NMR signals of the released dopamine are rather broad. Therefore, the error of the stated release percentage is relatively large and conservatively estimated as ca. 15%.
- A. P. de Silva and S. Uchiyama, Nat. Nanotechnol., 2007, 2, 399–410 CrossRef CAS PubMed.
- J. Kärnbratt, M. Hammarson, S. Li, H. L. Anderson, B. Albinsson and J. Andréasson, Angew. Chem., Int. Ed., 2010, 49, 1854–1857 CrossRef PubMed.
- J. Andréasson and U. Pischel, Chem. Soc. Rev., 2015, 44, 1053–1069 RSC.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018, 47, 2228–2248 RSC.
- A. Ghosh, I. Paul and M. Schmittel, J. Am. Chem. Soc., 2019, 141, 18954–18957 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General methods and materials, additional NMR data for the individual competitive displacement events, NMR titrations of both forms of 1 with CB7, and formalism for speciation simulation. See DOI: https://doi.org/10.1039/d3cc00384a |
| This journal is © The Royal Society of Chemistry 2023 |