Side-chain engineering of nonfullerene small-molecule acceptors for organic solar cells
Zhenghui
Luo
*,
Tongle
Xu
,
Cai'e
Zhang
 and
Chuluo
Yang
and
Chuluo
Yang
 *
*
Shenzhen Key Laboratory of New Information Display and Storage Materials, College of Materials Science and Engineering, Shenzhen University, Shenzhen 518060, China. E-mail: zhhuiluo@szu.edu.cn; clyang@whu.edu.cn
First published on 12th May 2023
Abstract
Thanks to their broad absorption spectra, easily modifiable molecular energy levels and chemical structures, nonfullerene small-molecule acceptors (SMAs) have attracted significant attention in the recent decade. To date, SMAs and polymer donor-based organic solar cells (OSCs) have achieved power conversion efficiencies (PCEs) of over 19%. During this time period, side-chain engineering has emerged as an effective method for enhancing the photovoltaic efficiency of the corresponding OSCs due to its simplicity and effectiveness in optimizing the physicochemical properties of nonfullerene SMAs. In this article, side-chain engineering of nonfullerene SMAs, including A–(π)–D–(π)–A-type SMAs and A–DA1D–A-type SMAs, is summarized. The general methods for modifying the side chains of SMAs and their pivotal structure–performance relationships are combined and highlighted. The future challenges and prospects for the further side-chain optimization of the SMAs are proposed.
Broader contextSince 2015, the research field of organic solar cells (OSCs) has witnessed a significant growth of nonfullerene small-molecule acceptors (SMAs), and the power conversion efficiencies (PCE) of SMA-based binary OSCs have rapidly increased from 6% to 19%. Typically, nonfullerene SMAs consist of a central core, two or more terminal accepting units, and side chains. By manipulating central cores or terminal acceptor units, the optoelectronic properties of the molecules can be finely tuned, but the drawback is that manipulating these two parts is relatively time-consuming and complex as compared with modifying side chains. The “side-chain engineering” strategy is a direct, convenient and feasible method to optimize the chemical structures of molecules, and then effectively regulate the absorption, energy levels, solubility, electron mobility, and molecular interaction/aggregation, and therefore widely utilized in molecular design for OSCs. In this article, side-chain engineering of nonfullerene SMAs is summarized. The general methods for modifying the side chains of SMAs and their pivotal structure–performance relationships are combined and highlighted. The future challenges and prospects for the further side-chain optimization of the SMAs are proposed. |
1. Introduction
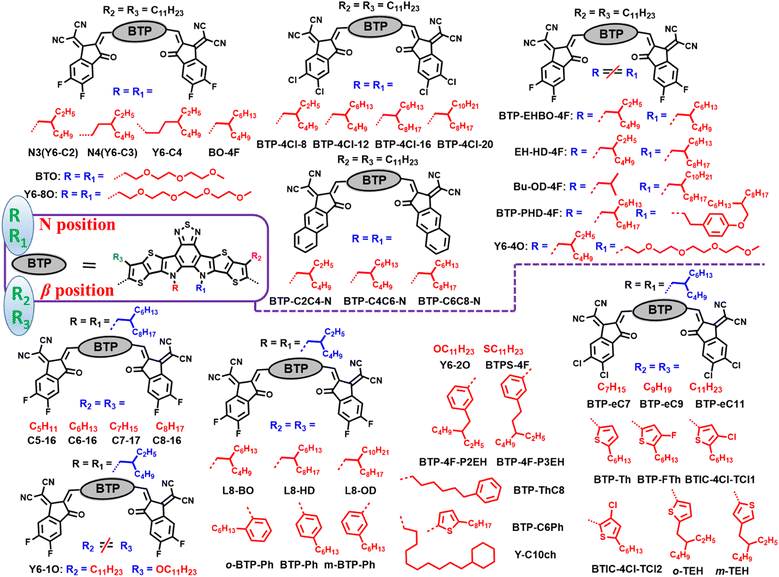
With the increasing demand for environmental friendly renewable energy, it is imperative to develop new photovoltaic (PV) technologies to meet the diverse market application needs.1–20 Bulk-heterojunction (BHJ) organic solar cells (OSCs), as a prospective PV technology, have attracted a great deal of attention due to their unique advantages, including solution processibility, color-turnability, and easy fabrication into lightweight, large-area and flexible devices.8,21–35 Thanks to material development and device innovation, BHJ OSCs have achieved rapid progress over the past three decades.36–58 The device efficiencies of OSCs have increased explosively since the emergence of fused-ring small-molecule acceptors (SMAs) in 2015.59–65 Among a myriad of nonfullerene SMAs, imides, acceptor–(π)–donor–(π)–acceptor (A–(π)–D–(π)–A)-type and A–DA1D–A-type acceptors are the most popular systems due to their excellent device performance,66–78 among which the most representative SMAs are ITIC9 and Y6,10 respectively. To date, nonfullerene SMA-based OSCs have achieved PCEs over 19%,25,62–65,79–81 which has now reached the threshold of commercial application.Typically, nonfullerene SMAs consist of a central core, two or more terminal acceptor units, and side chains that ensure sufficient solubility so that the molecules can be solution-processable.9–12 By manipulating central cores or terminal acceptor units, the optoelectronic properties of the molecules can be finely tuned, but the only drawback is that manipulating these two parts is relatively time-consuming and complex as compared with modifying side chains.72,82–85 Side-chain engineering is a direct, convenient and feasible method to optimize the chemical structures of molecules, then effectively regulate the absorption, energy levels, solubility, electron mobility, molecular interaction/aggregation, and ultimately promote the improvement of device efficiency.72,82–85 On the other hand, side-chain engineering offers an intuitive method to probe the structure–property relationship of SMAs with a subtle structural change in side chains.72,82–85 Basically, there are four main avenues to modify the side chains (Fig. 1): (1) changing the length, branched alkyl chains, branching point, and position; (2) heteroatom substitution, such as O, S, Se, Si, F, Cl and so on; (3) employing different aromatic rings, including benzene, thiophene, selenophene, thieno[3,2-b]thiophene, diphenylamine, etc.; and (4) incorporating special functional groups into side chains, such as oligo(ethylene glycol) chains, siloxane-terminated side chains, urea and carboxylic acid groups. As a matter of fact, much effort has been devoted to amending the side chains of A–(π)–D–(π)–A-type acceptors and A–DA1D–A-type acceptors, and the PCEs of OSCs based on SMAs have increased from 2% to 5% through optimizing the side chains.
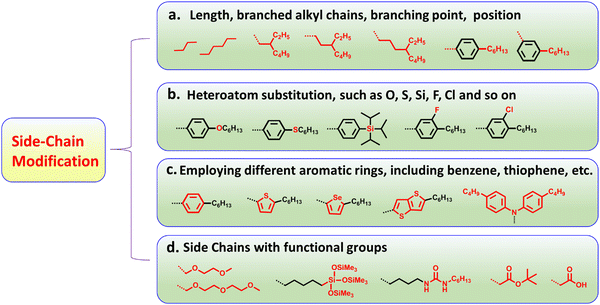 | ||
| Fig. 1 The illustration of methods for modifying the side chains of organic semiconductor materials. | ||
There have been a great deal of review articles and accounts presenting the evolution of nonfullerene SMAs on different topics, including asymmetric SMAs,18,86,87 isomerization strategy of SMAs,37 end-capping engineering on SMAs,88,89 and the influences of central cores on SMAs.5,45,90–92 However, up to now, there is still no review or account that systematically summarizes the development of side-chain engineering on nonfullerene SMAs. In light of the recent important advances in side-chain engineering of SMAs, it is necessary to briefly review the influence of side chains on the physicochemical properties and cell efficiency of SMAs in recent years. In this article, we first present the recent progress of side-chain engineering in A–(π)–D–(π)–A-type nonfullerene SMAs and A–DA1D–A-type nonfullerene SMAs. Then, some important examples that can enhance device performance via side-chain engineering will be highlighted. Finally, we will carefully discuss the future directions of side-chain engineering and provide a perspective for next generation SMAs.
2. Side-chain engineering of A–(π)–D–(π)–A-type SMAs
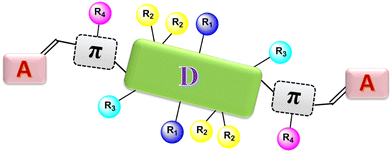
Since the invention of ITIC9 (a typical A–D–A-type SMA) developed by Zhan and co-workers, SMAs with an A–D–A architecture have received considerable attention and made great breakthroughs.93–97 In general, A–D–A-structured SMAs are made up of a central electron-donating core (D), multiple side chains and two terminal acceptor groups. Conjugated π bridges are sometimes employed to connect the central core and the two terminal units in A–D–A architecture SMAs, thus constructing A–π–D–π–A-structured SMAs. Manipulation of D, A, side chains and π bridges is a feasible way to enhance device efficiency. As shown in Fig. 2, there are typically four positions for altering the side chains of A–π–D–π–A-structured SMAs. Much effort has been devoted to modifying the side chains at these four positions, resulting in rapid progress in device efficiency.2.1. Side-chain engineering of IDTT-based SMAs
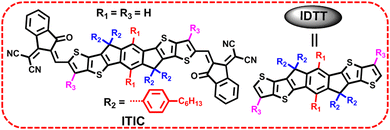
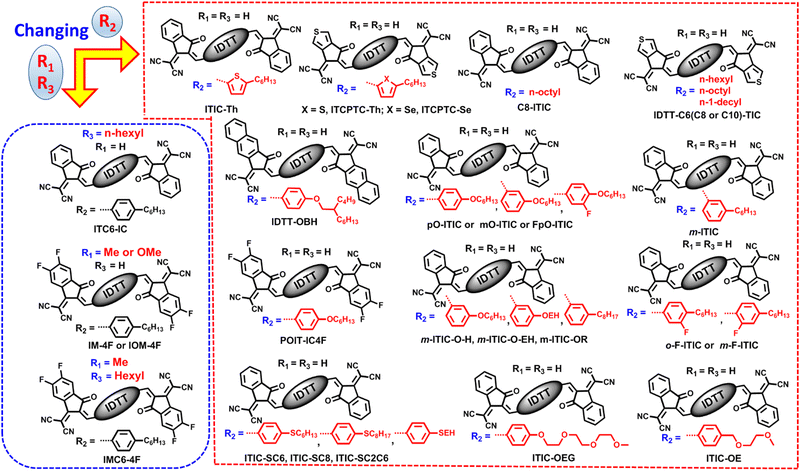
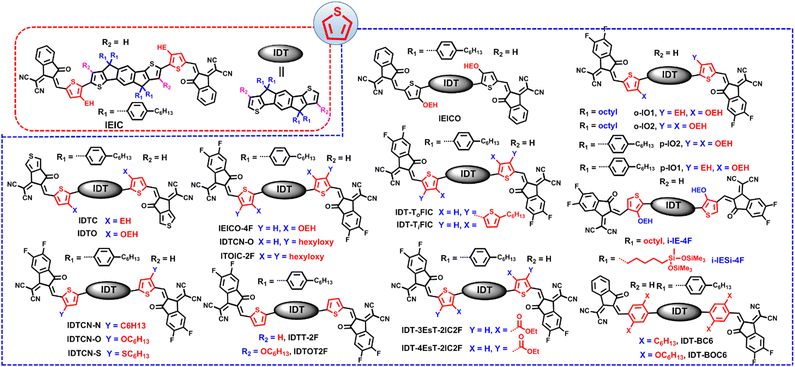
As we know, ITIC is composed of a indacenodithieno[3,2-b]thiophene (IDTT) unit flanked by four para-hexylphenyls and two end groups of 2-(3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (INCN).9 Modifying the side chains (R1 in benzene, R2 in cyclopentadiene, and R3 in thieno[3,2-b]thiophene) in IDTT of ITIC can be a useful means to trim the molecular structures (Fig. 3), which in turn enables high cell efficiency.Much effort was devoted to altering the side chains R2 in ITIC. For example, Zhan and co-works developed a SMA of ITIC-Th by replacing 4-hexylphenyl in ITIC with 2-hexylthienyl.93 In comparison with ITIC, ITIC-Th possesses enhanced intermolecular interactions ascribed to the presence of more polarized S atom. When ITIC-Th is blended with a small band-gap polymer donor PTB7-Th, it can afford a device efficiency of 8.7%, and a higher PCE of 9.6% can be obtained after pairing it with wide-band-gap donor PDBT-T1 (Table 1). Similarly, Yang et al. replaced the hexylphenyl in ITCPTC with hexylthienyl and hexylselenyl to get two SMAs, namely, ITCPTC-Se and ITCPTC-Th.94 ITCPTC-Th and ITCPTC-Se show much higher absorption coefficients than ITCPTC. Compared to ITCPTC-Th, ITCPTC-Se exhibits slightly redshifted absorption and upshifted energy levels ascribed to the σ-inductive effect of Se, but weaker crystallinity duo to the larger atomic radius of Se. OSC based ITCPTC-Th yielded a PCE of 10.61% along with an FF of 0.727, which are superior to those of ITCPTC-Se based one (PCE = 9.02%; FF = 0.683) (Fig. 4). Parallelly, Li et al. changed the alkyl substitution position on ITIC with 4-hexylphenyl substitution to 3-hexylphenyl substitution to create m-ITIC.95 Compared to ITIC, m-ITIC shows slightly blue-shifted absorption in solution but redshifted absorption in films, indicating its reinforced molecular parking. The J61:m-ITIC device presents a better PCE of 11.77% as compared to the ITIC-based device (10.57%). Moreover, the PCE remains over 8.00% when the thickness of the active layer is 350 nm.
| SMA |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (eV) a (eV) |
HOMOb/LUMOb (eV) | Donor | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from Eoptg = 1240/λonset. b Calculated from CV curves. | ||||||||
| ITIC | 1.59 | −5.48/−3.83 | PTB7-Th | 0.81 | 14.21 | 0.591 | 6.8 | 9 |
| ITIC-Th | 1.60 | −5.66/−3.93 | PDBT-T1 | 0.88 | 16.24 | 0.671 | 9.6 | 93 |
| ITCPTC-Th | 1.60 | −5.66/−3.93 | PBDB-T | 0.856 | 17.05 | 0.727 | 10.61 | 94 |
| ITCPTC-Se | 1.59 | −5.66/−3.93 | PBDB-T | 0.869 | 15.20 | 0.683 | 9.02 | 94 |
| m-ITIC | 1.58 | −5.52/−3.82 | J61 | 0.912 | 18.31 | 0.7055 | 11.77 | 95 |
| m-ITIC-OR | 1.65 | −5.65/−3.97 | HFQx-T | 0.90 | 16.15 | 0.64 | 9.13 | 96 |
| IDTT-BH | 1.54 | −5.42/−3.86 | J71 | 0.90 | 17.77 | 0.691 | 11.05 | 97 |
| IDTT-OBH | 1.57 | −5.41/−3.86 | PBDB-T | 0.87 | 17.46 | 0.720 | 10.93 | 97 |
| m-ITIC-H | 1.61 | −5.21/−3.60 | PBDB-T | 0.85 | 15.09 | 0.67 | 8.54 | 98 |
| m-ITIC-O-H | 1.60 | −5.25/−3.65 | PBDB-T | 0.85 | 15.99 | 0.70 | 9.55 | 98 |
| m-ITIC-O-EH | 1.62 | −5.25/−3.63 | PBDB-T | 0.88 | 15.88 | 0.68 | 9.77 | 98 |
| pO-ITIC | 1.61 | −5.49/−3.71 | PTB7-Th | 0.80 | 14.79 | 0.591 | 7.51 | 99 |
| mO-ITIC | 1.63 | −5.50/−3.74 | PTB7-Th | 0.80 | 14.19 | 0.601 | 7.33 | 99 |
| FpO-ITIC | 1.64 | −5.61/−3.72 | PTB7-Th | 0.78 | 12.99 | 0.567 | 6.17 | 99 |
| ITIC-SC6 | — | −5.68/−3.91 | PBDB-ST | 0.90 | 13.92 | 0.58 | 7.27 | 100 |
| ITIC-SC8 | — | −5.70/−3.90 | PBDB-ST | 0.90 | 14.43 | 0.60 | 7.79 | 100 |
| ITIC-SC2C6 | — | −5.74/−3.86 | PBDB-ST | 0.92 | 15.81 | 0.63 | 9.16 | 100 |
| o-F-ITIC | 1.58 | −5.66/−3.94 | PBDB-T | 0.918 | 18.07 | 0.6697 | 11.11 | 101 |
| m-F-ITIC | 1.62 | −5.69/−3.96 | PBDB-T | 0.883 | 15.80 | 0.6379 | 8.9 | 101 |
| C8-ITIC | — | −5.63/−3.91 | PFBDB-T | 0.94 | 19.6 | 0.72 | 13.2 | 103 |
| IDTT-C6-TIC | 1.60 | −5.55/−3.99 | PBT1-C | 0.85 | 17.0 | 0.667 | 10.0 | 104 |
| IDTT-C8-TIC | 1.59 | −5.64/−3.97 | PBT1-C | 0.88 | 20.3 | 0.746 | 13.7 | 104 |
| IDTT-C10-TIC | 1.61 | −5.71/−3.91 | PBT1-C | 0.98 | 18.1 | 0.713 | 12.7 | 104 |
| ITIC-OE | 1.57 | −5.67/−4.03 | PBDB-T | 0.85 | 14.8 | 0.67 | 8.5 | 105 |
| ITIC-OEG | 1.54 | −5.39/−3.99 | PPDT2FBT | 0.90 | 3.56 | 0.49 | 1.58 | 106 |
| ITC6-IC | 1.60 | −5.73/−3.92 | PBDB-T | 0.97 | 16.41 | 0.73 | 11.61 | 107 |
| ITIC | 1.58 | −5.68/−4.01 | PBDB-T | 0.91 | 16.27 | 0.69 | 10.21 | 107 |
| IT-4F | 1.51 | −5.74/−4.26 | PM6 | 0.85 | 20.02 | 0.7522 | 12.80 | 108 |
| IM-4F | 1.46 | −5.69/−4.19 | PM6 | 0.88 | 22.12 | 0.7279 | 14.17 | 108 |
| IOM-4F | 1.48 | −5.72/−4.27 | PM6 | 0.86 | 21.66 | 0.7200 | 13.41 | 108 |
| IMC6-4F | 1.49 | −5.71/−3.94 | PM6 | 0.90 | 22.29 | 0.7759 | 15.57 | 109 |
The alkoxyl chains have been widely utilized to survey their impact on device efficiency. For instance, Zou et al. adopted a long alkoxyl chain to replace the alkyl chain on m-ITIC to obtain m-ITIC-OR, which exhibited relatively blue-shifted absorption spectra and a downshifted LUMO value relative to m-ITIC.96 The device based on m-ITIC-OR achieved a decent efficiency of 9.30%. In addition, Chen et al. developed two SMAs of IDTT-OBH and IDTT-BH with alkoxyl chains.97 Compared with IDTT-BH, IDTT-OBH showed slightly redshifted absorption in solution but blue-shifted absorption in films. When blended with J71 and PDCBT, IDTT-BH based devices present better morphology, and thus higher PCEs than IDTT-OBH based devices; while when blended with PBDB-T, the device based on IDTT-OBH shows higher PCE than the IDTT-BH-based device. Lee and co-workers also studied the influence of alkoxyl chains on photoelectric properties and cell efficiency.98 Compared to m-ITIC-H, m-ITIC-O-EH and m-ITIC-O-H with alkoxyl side chains exhibit increased surface energy, and m-ITIC-O-EH with branched alkoxyl chains shows weaker aggregation properties in comparison to m-ITIC-O-H with linear alkoxyl side chains. The device based on m-ITIC-O-EH showed good active layer-thickness tolerance, and a high PCE of 9.68% could be achieved by the printing process in air. Gao et al. further surveyed the impacts of alkoxy chains and fluorination on cell efficiency.99 pO-ITIC with 4-hexyloxyphenyl and mO-ITIC with 3-hexyloxyphenyl demonstrated lower band gaps, enhanced absorption ability and higher LUMO energy levels relative to those of FpO-ITIC with 3-fluorine-4-hexyloxy-phenyl. As results, PTB7-Th:mO-ITIC and PTB7-Th:pO-ITIC devices present very similar PCEs of 7.51% and 7.33%, which are higher than those of device based on FpO-ITIC (6.17%).
In addition to O atoms, S atoms are often inserted into alkyl chains to optimize the molecular properties. Bo and co-workers developed three SMAs (ITIC-SC2C6, ITIC-SC8, and ITIC-SC6), changed the alkylphenyl on ITIC to alkylthiophenyl, and investigated the influence of branched chains and side-chain length on photovoltaic performance.100 ITIC-SC2C6 with branched 2-ethylhexyl chains showed a longer molecular packing distance in comparison to linear side chains molecules (ITIC-SC8 and ITIC-SC6), and ITIC-SC2C6 presented slightly larger bandgaps and downshifted energy levels. The PBDB-ST:ITIC-SC2C6 device gave the best PCE of 9.16% due to well-performing morphology.
Incorporation of an F atom into the phenyl side chains of ITIC can also be a useful for promoting cell efficiency. Yang et al. reported two acceptors, m-F-ITIC and o-F-ITIC, with different fluorination sites; in comparison with ITIC, m-F-ITIC with meta-alkyl substitution and o-F-ITIC with ortho-alkyl substitution show gradually blue-shifted absorption, downshifted energy levels, and weaker crystallinity.101 OSCs based on o-F-ITIC yielded a higher efficiency of 11.11% compared with m-F-ITIC-based ones due to the closer donor–acceptor interaction in the o-F-ITIC:PBDB-T blend. The same molecules and similar conclusions were also reported by Xin et al., that is, the device based on mF-ITIC presented a higher PCE of 9.50% with enhanced thermal stability relative to that of the oF-ITIC-based device.102
Via alkyl side-chain substitution, Heeney et al. obtained a new SMA of C8-ITIC with octyl groups. C8-ITIC delivered much redshifted absorption, higher absorption coefficients and enhanced crystallinity in comparison to ITIC.103 The PFBDB-T:C8-ITIC device showed a decent PCE of 13.2%, accompanied with a low energy loss of 0.60 eV, which are superior to those of the ITIC-based device. Additionally, Sun and co-workers investigated the influence of alkyl side-chain length on molecular packing behaviors.104 IDTT-C6-TIC with hexyl chains showed a strong molecular π–π stacking mode, and IDTT-C8-TIC with octyl chains exhibited and intermixed packing mode, whereas IDTT-C10-TIC with decyl side chains showed a non-stacking mode. Eventually, PBT1-C:IDTT-C8-TIC OSCs afforded the best PCE of 13.7% with low structural disorder and non-radiative recombination loss among the three devices.
Exploiting SMAs with high dielectric constants (εr) is a promising method to reduce the exciton binding energy and boost device efficiency. Huang et al. replaced the hexyl alkyl side chains on ITIC with oligoethylene oxide (OE) side chains to get ITIC-OE.105 Compared to ITIC, ITIC-OE showed slightly blue-shifted absorption in solution but redshifted absorption in films, a decreased surface energy, and a larger εr of 9.4. The devices based on ITIC-OE delivered a lower efficiency of 8.5% due to the weaker crystallinity of ITIC-OE and smaller microphase separation, as compared with ITIC-based devices. Also, Woo and co-workers empolyed oligoethyleneglycol (OEG) groups to replace the hexyl alkyl side chains in ITIC to afford ITIC-OEG.106 ITIC-OEG exhibited distinctly redshifted absorption, upshifted energy levels, reinforced molecular parking, and a greatly enhanced dielectric constant in comparison to ITIC. Ascribed to the high hydrophilic properties of OEG chains, ITIC-OEG presented poor miscibility with PPDT2FBT, and thus the PPDT2FBT:ITIC-OEG device exhibited a PCE of only 1.58%.
Different from modifying R2, manipulation of R1 and R3 in IDTT-based SMAs received less attention, and most of the related works were reported by Tang’ group. In 2018, they incorporated two hexyl side chains into the β position of peripheral thiophene in the central core of ITIC for conformation locking to obtain a new SMA of ITC6-IC (Fig. 5).107 According to density functional theory (DFT) calculations, ITC6-IC showed a highly unified and planar configuration because of the steric hindrance impact of the hexyl moiety. In comparison with ITIC, ITC6-IC exhibited redshifted absorption and higher absorption coefficients in solution due to the planar structure caused by conformation locking, but an upshifted LUMO value and blue-shifted absorption in films, which resulted from the weak electron-donating properties of the hexyl moiety. After pairing ITIC and ITC6-IC with PBDB-T, the device based on ITC6-IC afforded a device efficiency of 11.61%, accompanied with a high VOC of 0.97 V, performing better than the ITIC-based device (10.21%). Later, the same group focused on the central benzene moiety in IDTT-based SMAs and developed two SMAs (IOM-4F and IM-4F) by replacing H atoms with weak electron-rich methoxy or methyl units.108 Compared to IT-4F, IOM-4F and IM-4F showed redshifted absorption due to the electron-donating ability of methyl and methoxy groups. In addition, IM-4F exhibited obviously upshifted molecular energy levels relative to those of IT-4F, whereas IOM-4F presented higher crystallinity and rigid conformations due to S⋯O noncovalent interactions. Together with PM6, the devices based on PM6:IM-4F and PM6:IOM-4F achieved simultaneously enhanced JSCs and VOCs with PCEs of 14.17% and 13.41%, respectively. The higher VOC in IOM-4F and IM-4F devices is ascribed to the reduced nonradiative recombination loss, as confirmed by energy loss experiments. Furthermore, they employed a strategy of combining the weak electron-donating methyl substitution in the central phenyl unit and outer thiophene β position alkyl conformation locking to develop IMC6-4F.109 IMC6-4F presented slightly blue-shifted absorption spectra and downshifted HOMO values compared with IM-4F. Finally, PM6:IMC6-4F cells delivered a good efficiency of 15.57%, with an outstanding FF of 0.7759, a JSC of 22.29 mA cm−2 and a decent VOC of 0.90 V.
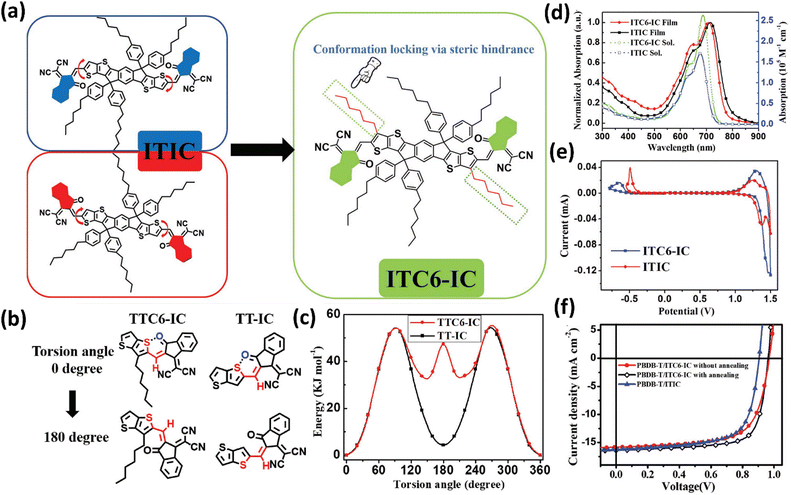 | ||
| Fig. 5 (a) The chemical structures of ITIC and ITC6-IC; (b) possible rotamers at 0° and 180° in TTC6-IC and TT-IC; (c) potential energy surface curves of TT-IC and TTC6-IC; (d) absorption spectra of ITC6-IC and ITIC; (d) CV curves of ITC6-IC and ITIC; (f) J–V curves of PBDB-T:ITC6-IC device without and with thermal annealing and PBDB-T:IT-IC device. Adapted with permission.107 Copyright 2018, Wiley-VCH. | ||
2.2. Side-chain engineering of IDT-based SMAs
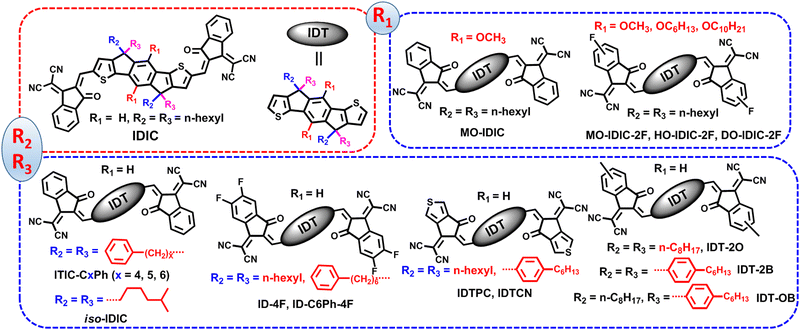
Indacenodithiophene (IDT), as an earlier developed electron-donating unit, has shown great potential in constructing high-efficiency SMAs and polymer acceptors.110–113 In this part, we will introduce side-chain engineering of IDT-based SMAs from two aspects: (a) side-chain engineering of IDT-based A–D–A type SMAs and (b) side-chain engineering of IDT-based A–π–D–π–A type SMAs.Li and co-workers introduced two methoxy groups into benzene of IDIC to afford MO-IDIC.112 The synthetic route of MO-IDIC was simplified, and the molecule displayed slightly red-shifted absorption but an up-shifted LUMO value relative to IDIC. The OSC based PTQ10:MO-IDIC yielded a PCE of 11.16% (Fig. 6). To further improve the device performance, they replaced INCN in IDIC with monofluorinated INCN and systematically optimized the length of alkoxy side chains on the benzene ring, and finally obtained four new SMAs (MO-IDIC-2F, HO-IDIC-2F and DO-IDIC-2F).113 As the length of the alkoxy side chain increased, the three SMAs (from MO-IDIC-2F, HO-IDIC-2F to DO-IDIC-2F) demonstrated gradually red-shifted absorption spectra, elevated HOMO and LUMO levels, an improved molecular order and molecular self-assembly. Consequently, the PM6:DO-IDIC-2F device with longest chains realized the highest PCE of 13.02% along with the best JSC of 19.63 mA cm−2 among the three SMA-based devices (Table 2).
| SMA |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (eV) a (eV) |
HOMOb/LUMOb (eV) | Donor | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from Eoptg = 1240/λonset. b Calculated from CV curves. | ||||||||
| IDIC | 1.62 | −5.69/−3.91 | PDBT-T1 | 0.89 | 15.05 | 0.65 | 8.71 | 111 |
| MO-IDIC | 1.60 | −5.69/−3.89 | PTQ-10 | 0.969 | 16.92 | 0.681 | 11.16 | 112 |
| IDIC-2F | 1.57 | −5.75/−3.95 | PM6 | 0.846 | 17.95 | 0.758 | 11.52 | 112 |
| MO-IDIC-2F | 1.55 | −5.80/−3.93 | PM6 | 0.843 | 18.92 | 0.767 | 12.23 | 113 |
| HO-IDIC-2F | 1.55 | −5.81/−3.91 | PM6 | 0.863 | 19.05 | 0.763 | 12.53 | 113 |
| DO-IDIC-2F | 1.54 | −5.79/−3.98 | PM6 | 0.864 | 19.63 | 0.765 | 13.02 | 113 |
| IDTCN | 1.67 | −5.91/−3.84 | PTQ10 | 0.98 | 13.9 | 0.54 | 7.4 | 114 |
| IDTPC | 1.52 | −5.98/−3.98 | PTQ10 | 0.93 | 17.5 | 0.746 | 12.2 | 114 |
| IDT-2B | 1.73 | −5.80/−3.84 | PBDB-T | 0.89 | 13.3 | 0.539 | 6.42 | 115 |
| IDT-OB | 1.66 | −5.77/−3.87 | PBDB-T | 0.88 | 16.18 | 0.711 | 10.12 | 115 |
| IDT-2O | 1.64 | −5.73/−3.85 | PBDB-T | 0.86 | 15.64 | 0.723 | 9.68 | 115 |
| IDIC-PhC6 | 1.71 | −5.72/−3.86 | PBDB-T | 0.869 | 12.2 | 0.579 | 6.14 | 116 |
| IDIC | 1.63 | −5.70/−3.92 | PM6 | 0.947 | 18.17 | 0.6988 | 12.02 | 116 |
| IDIC-C4Ph | 1.62 | −5.70/−3.93 | PM6 | 0.941 | 19.06 | 0.7832 | 14.04 | 117 |
| IDIC-C5Ph | 1.66 | −5.76/−3.89 | PM6 | 0.948 | 19.19 | 0.8002 | 14.56 | 117 |
| IDIC-C6Ph | 1.65 | −5.84/−3.86 | PM6 | 0.946 | 17.29 | 0.7683 | 12.57 | 117 |
| iso-IDIC | — | −5.84/−3.84 | PM6 | 0.961 | 18.91 | 0.7445 | 13.53 | 118 |
| IDIC | — | −5.83/−3.87 | PM6 | 0.963 | 17.94 | 0.7160 | 12.37 | 118 |
Yang and co-workers compared the effect of aryl and alkyl chains in cyclopentadiene on molecular orientation and device efficiency.114 GIWAXS experiments indicated that IDTCN with 4-hexylphenyl shows an unoriented molecular arrangement, while obvious face-to-face molecular orientation was observed in IDTPC with n-hexyl chains. The IDTPC-based device yielded higher JSC and FF, thereby better PCE (12.2%), as compared to IDTCN-based ones. The enhanced JSC and FF mainly attribute to better molecular π–π packing and promoted charge mobility.
Bo and co-workers simultaneously introduced A and B onto the same sp3-hybridized carbon atom of cyclopentadiene to develop a SMA of IDT-OB with asymmetric chains.115 In comparison with two sysmetric counterparts (IDT-2O and IDT-2B), IDT-OB exhibited improved solubility, closer molecular packing and more favorable domain size after blending with PBDB-T. Finally, the device based on IDT-OB delivered the best efficiency of 10.12%.
Li and co-workers found that IDIC shows strong crystallization and face-on orientations, and IDIC-PhC6 with 4-hexylphenyl exhibits weak crystallization but good solubility. To achive a balance between molecular aggregation and miscibility, they introduced a bulky phenyl onto the the tail of n-butyl and reported an SMA, IDIC-C4Ph.116 IDIC-C4Ph inherits the face-on orientation features of IDIC and shows moderate crystallinity as compared with IDIC and IDIC-PhC6 (Fig. 7). After blending with star polymer donor PM6, an ideal morphology with proper phase separation and good molecular crystallization characteristics were observed in the IDIC-C4Ph-based blend, which led to the highest efficiency of 14.02% as well as an outstanding FF of 0.7832 in the PM6:IDIC-C4Ph device. Then, they trimmed the length of the alkyl chains (from C4 to C6) and obtained three SMAs, namely, IDIC-C4Ph, IDIC-C5Ph, and IDIC-C6Ph.117 The single crystal results showed that a two charge-transport channel was observed in IDIC-C5Ph, contributing to the electron hopping. As the alkyl chain continue to grow, disordered molecular orientations are seen in IDIC-C6Ph. The cells based on IDIC-C5Ph realized an efficiency of 14.56% along with an impressive FF of 0.8002, outperforming those of the devices based on IDIC-C4Ph (PCE = 13.94%; FF = 0.7805) and IDIC-C6Ph (PCE = 12.57%; FF = 0.7683). Finally, the same group focused on the isomerization of alkyl chains. They replaced the linear alkyl chains in IDIC with a branched one containing an isopropyl terminal to obtain an acceptor, iso-IDIC.118 This strategy endows iso-IDIC with slightly reduced crystallinity but comparable optical and electrochemistry characteristics relative to those of IDIC. Consequently, the iso-IDIC-based device realizes a better efficiency of 13.50% because of a morphology with decreased aggregation.
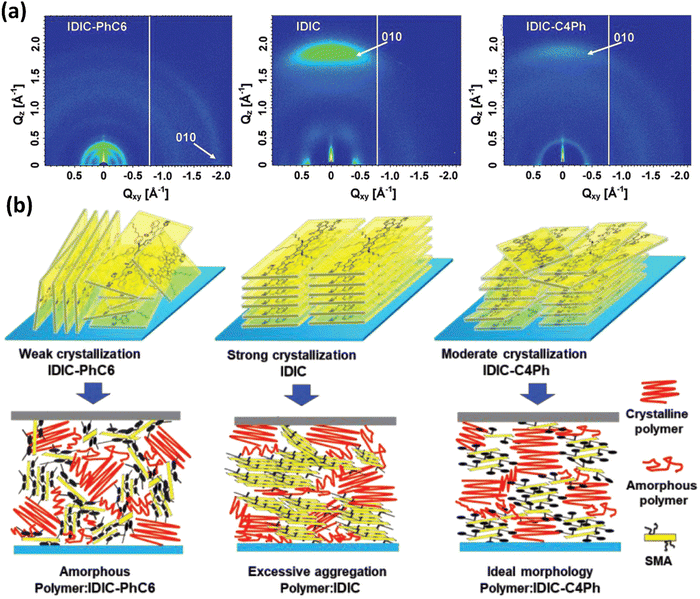 | ||
| Fig. 7 (a) GIWAXS scattering patterns of IDIC-C4Ph, IDIC, and IDIC-PhC6; (b) the schematic diagram of molecular stacking in pure IDIC-C4Ph, IDIC, and IDIC-PhC6 films and their corresponding PM6:acceptor blends. Adapted with permission.116 Copyright 2019, Wiley-VCH. | ||
In 2015, Zhan and co-workers reported an A–π–D–π–A type acceptor (IEIC) with a side chain at the inner position of the π spacer (Fig. 8), and the device based on PTB7-TH:IEIC yielded a PCE of 6.31% (Table 3), which represented the best result for SMA-based OSCs at the time.119 Then, Hou and co-workers replaced 2-ethylhexyl with an alkoxy side chain to obtain a low bandgap SMA of IEICO. IEICO showed an obvious smaller optical bandgap of 1.34 eV and enhanced molecular stacking relative to IEIC.120 In consequence, the IEICO-based device delivered a higher PCE of 8.4% compared to the IEIC-based one due to the significantly improved JSC and FF. Furthermore, Bazan et al. employed an alkyl–alkoxy-combination strategy to develop two asymmetric new acceptors (p-IO1 and o-IO1) with monoalkoxy chains.121 Compared with bisalkoxy counterparts, the monoalkoxy ones showed blue-shifted absorption spectra and higher LUMO values. Together with the polymer donor PTB7-Th, the o-IO1-based device realizes a higher PCE of 13.2%, which is mainly ascribed to the high JSC and low energy loss.
| SMA |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (eV) a (eV) |
HOMOb/LUMOb (eV) | Donor | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from Eoptg = 1240/λonset. b Calculated from CV curves. | ||||||||
| IEIC | 1.57 | −5.42/−3.82 | PTB7-Th | 0.97 | 13.5 | 0.48 | 6.31 | 119 |
| IEICO | 1.34 | −5.32/−3.95 | PBDTTT-E-T | 0.82 | 17.7 | 0.58 | 8.4 | 120 |
| p-IO1 | 1.34 | −5.46/−4.13 | PTB7-Th | 0.78 | 22.3 | 0.62 | 10.8 | 121 |
| p-IO2 | 1.28 | −5.44/−4.15 | PTB7-Th | 0.74 | 26.3 | 0.67 | 13.1 | 121 |
| o-IO1 | 1.24 | −5.44/−4.19 | PTB7-Th | 0.70 | 23.0 | 0.67 | 10.8 | 121 |
| o-IO2 | 1.20 | −5.41/−4.21 | PTB7-Th | 0.68 | 21.8 | 0.63 | 9.3 | 121 |
| IDTCN-C | 1.48 | −5.59/−3.92 | PBDB-T | 0.84 | 20.33 | 0.696 | 11.92 | 122 |
| IDTCN-O | 1.53 | −5.54/−3.80 | PBDB-T | 0.91 | 19.96 | 0.732 | 13.28 | 122 |
| IDTCN-S | 1.48 | −5.57/−3.90 | PBDB-T | 0.85 | 19.04 | 0.657 | 10.60 | 122 |
| IDTT2F | 1.46 | −5.57/−4.03 | PBDB-T | 0.81 | 18.51 | 0.59 | 8.85 | 123 |
| IDTOT2F | 1.44 | −5.54/−3.94 | PBDB-T | 0.85 | 20.87 | 0.72 | 12.79 | 123 |
| IDT-ToFIC | 1.50 | −5.55/−3.86 | PBDB-T | 0.88 | 17.79 | 0.71 | 11.09 | 124 |
| IDT-TiFIC | 1.41 | −5.57/−4.05 | PBDB-T | 0.86 | 16.97 | 0.65 | 9.46 | 124 |
| IDTC | 1.51 | −5.57/−3.96 | PBDB-T | 0.917 | 16.56 | 0.6161 | 9.35 | 125 |
| IDTO | 1.53 | −5.52/−3.84 | PBDB-T | 0.943 | 16.25 | 0.6541 | 10.02 | 125 |
| i-IE-4F | 1.51 | −5.43/−3.79 | J52 | 0.84 | 18.34 | 0.4757 | 7.34 | 126 |
| i-IESi-4F | 1.51 | −5.50/−3.71 | PBZ-2Si | 0.87 | 22.55 | 0.7403 | 14.54 | 126 |
| IDT-BC6 | 1.75 | −5.55/−3.82 | PBDB-T | 0.92 | 5.63 | 0.44 | 2.3 | 127 |
| IDT-BOC6 | 1.63 | −5.51/−3.78 | PBDB-T | 1.01 | 17.52 | 0.54 | 9.60 | 127 |
Bo and co-workers investigate the impact of the outer side chains of the π spacer in IDT-based A–π–D–π–A-type acceptors on molecular interaction and cell performance. Three SMAs with different chains (alkoxyl, alkylthio, and alkyl) were synthesized.122 Compared with IDTCN-C and IDTCN-S, IDTCN-O exhibits a higher LUMO level and stronger molecular packing, resulting in enhanced VOC and FF, and thereby a better PCE of 13.28% in PBDB-T:IDTCN-O as the cast device. The same group demonstrated that the introduction of alkoxy chains on the outermost thiophenes of IDT in IDT-based A–π–D–π–A-type acceptors is also a means to improve molecular packing and device efficiency.123 In addition, they reported two regioisomeric SMAs, namely, IDT-T-iFIC with inner 5-hexylthienyl chains and IDT-T-oFIC with outer 5-hexylthienyl chains.124 In comparison with IDT-TiFIC, the IDT-ToFIC-based device delivered an enhanced PCE of 11.09%, which is mainly due to the better morphology and lower energy loss. This isomeric method was also reported by employing a 2-ethylhexyl carboxylate group, but a different trend in PCE was observed.
When the termial unit INCNs of IEIC and IEICO were replaced with thiophene-fused end groups, two new SMAs, IDTC and IDTO, were acquired.125 These two SMAs exhibited completely different properties from those of IEIC and IEICO. IDTO with alkoxy chains displayed slightly blue-shifted absorption and an upshifted LUMO value relative to those of IDTC. The IDTO-based device showed an efficiency of 10.02%, higher than that of the IDTC-based one (PCE = 9.35%).
Chen and co-workers first empolyed siloxane-terminated chains to develop an SMA, i-IESi-4F. The siloxane-terminated units endows i-IESi-4F with good solubility and low surface energy.126 When blended with polymer donors PBZ-2Si or J52, i-IESi-4F shows better misbility than i-IE-4F with ackyl chains. In OSCs, i-IESi-4F shows a significantly higher PCE of 14.54% compared to i-IE-4F (7.34%).
Employing benzene as π spacers to construct high-performance SMAs has been proven to be a successful way. Bo and co-workers developed two electron acceptors (IDT-BOC6 and IDT-BC6) with benzene as π spacers; the difference between the two SMAs was that the side chains on the benzene were different: for IDT-BC6, it was n-hexyl, and for IDT-BOC6, it was hexyloxy.127 The conformation locking of S⋯O and O⋯H endows IDT-BOC6 with a more planar molecular structure, a narrower bandgap, emhanced charge mobility, lower energy loss compared with IDT-BC6. In OSCs with PBDB-T as a donor, IDT-BOC6 realized a PCE of 9.6%, obviously better than the IDT-BC6-based cell (2.3%).
2.3. Side-chain engineering of BDT-based SMAs
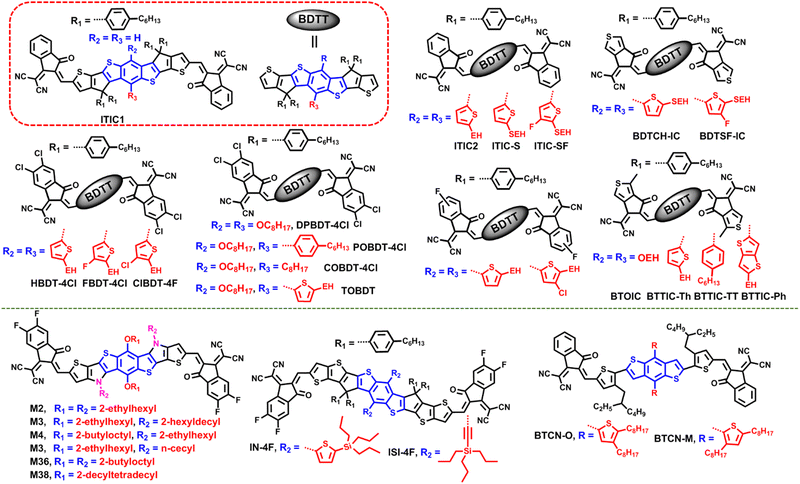
Benzo[1,2-b:4,5-b′]dithiophene (BDT) as a star scaffold has been widely applied to synthetize organic photovoltaic materials, such as small-molecular donors, polymer donors and SMAs, due to its excellent charge transport properties.128–133In 2017, Zhan et al. introduced 2D thiophene conjugated side chains onto an SMA, ITIC1 (the isomer of ITIC), to construct nonfullerene acceptor ITIC2 (Fig. 9).131 Attributed to enhanced intramolecular conjugation and intermolecular interaction, ITIC2 presents red-shifted and enhanced absorption, slightly elevated HOMO/LUMO values, and improved charge mobility compared to ITIC1. The device based on ITIC2 exhibited a much better efficiency of 11.0% compared to the ITIC1-based device (8.54%). Then, Chen et al. introduced S atoms onto the 2D side chains and synthesized ITIC-S. Compared with ITIC2, ITIC-S showed slightly blue-shifted absorption, a reduced LUMO value, a higher absorption coefficient and electron mobility, and decreased crystallinity.132 The PBDB-T-SF:ITIC-S-based device displayed a good efficiency PCE of 11.6% (Table 4). Subsequently, they introduced F atoms into 2D thiophene chains and synthesized ITIC-SF. The absorption of ITIC-SF was further blue-shifted and the energy level was further reduced.133 Meanwhile, the absorption coefficient and crystallinity were increased. Finally, the device based on ITIC-SF displayed a decent efficiency of 12.1%. Furthermore, they replaced the benzene-fused end group with a thiophene-fused end group, and synthesized BDTSF-IC and BDTCH-IC.134 Compared with BDTCH-IC, BDTSF-IC presents weaker crystallinity, which may be beneficial for forming favorable interpenetrating networks when blended with polymer donors. The PM6:BDTSF-IC device delivered an efficiency of 13.10%. In addition, Wang et al. adopted a 2D halogenated thiophene chain strategy and synthesized three nonfullerene SMAs, namely, ClBDT-4Cl FBDT-4Cl, and HBDT-4Cl.135 Compared with non-halogenated HBDT-4Cl, ClBDT-4Cl and FBDT-4Cl with substituted Cl atoms and F atoms showed slightly blue-shifted absorption, enhanced light absorption ability and reduced energy levels, but reinforced molecular packing. ClBDT-4Cl- and FBDT-4Cl-based blends displayed a PCE of 11.65% and 12.36%, respectively, performing better than the HBDT-4Cl-based one (10.35%). Similar work was also reported by Zhang's team.136
| SMA |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (eV) a (eV) |
HOMOb/LUMOb (eV) | Donor | V OC (V) | J SC (mA cm−2) | FF | PCE% | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from Eoptg = 1240/λonset. b Calculated from CV curves. | ||||||||
| ITIC1 | 1.55 | −5.48/−3.84 | FTAZ | 0.921 | 16.45 | 0.564 | 8.54 | 131 |
| ITIC2 | 1.53 | −5.43/−3.80 | FTAZ | 0.925 | 18.88 | 0.630 | 11.0 | 131 |
| ITIC-S | 1.55 | −5.50/−3.86 | PBDB-T-SF | 1.06 | 16.43 | 0.668 | 11.6 | 132 |
| ITIC-SF | 1.58 | −5.57/−3.92 | PBDB-T-SF | 1.04 | 16.85 | 0.686 | 12.1 | 133 |
| BDTCH-IC | 1.50 | −5.51/−3.89 | PM6 | 0.96 | 17.11 | 0.636 | 10.51 | 134 |
| BDTSF-IC | 1.56 | −5.58/−3.93 | PM6 | 0.90 | 20.36 | 0.7148 | 13.10 | 134 |
| HBDT-4Cl | 1.43 | −5.67/−3.90 | PM6 | 0.898 | 17.79 | 0.6476 | 10.35 | 135 |
| FBDT-4Cl | 1.45 | −5.70/−3.91 | PM6 | 0.888 | 19.83 | 0.7019 | 12.36 | 135 |
| ClBDT-4Cl | 1.44 | −5.72/−3.92 | PM6 | 0.879 | 19.02 | 0.6971 | 11.65 | 135 |
| BTC-2F | 1.53 | −5.64/−3.91 | PM6 | 0.92 | 20.3 | 0.693 | 12.9 | 136 |
| BTH-2F | 1.50 | −5.59/−3.91 | PM6 | 0.92 | 19.5 | 0.631 | 11.3 | 136 |
| BTTIC-Th | 1.467 | −5.49/−3.90 | PBDB-T | 0.902 | 19.45 | 0.736 | 12.91 | 137 |
| BTTIC-TT | 1.474 | −5.54/−3.80 | PBDB-T | 0.924 | 19.61 | 0.742 | 13.44 | 137 |
| BTTIC-Ph | 1.455 | −5.48/−3.78 | PBDB-T | 0.930 | 16.47 | 0.597 | 9.14 | 137 |
| BTOIC | 1.39 | −5.42/−3.94 | PBDB-T | 0.862 | 18.60 | 0.684 | 10.96 | 138 |
| TOBDT | 1.41 | −5.56/−3.97 | PM6 | 0.89 | 18.7 | 0.68 | 11.3 | 139 |
| DPBDT-4Cl | 1.41 | −5.62/−3.94 | PM6 | 0.90 | 19.2 | 0.66 | 11.4 | 140 |
| POBDT-4Cl | 1.39 | −5.58/−3.97 | PM6 | 0.88 | 21.0 | 0.68 | 12.6 | 140 |
| COBDT-4Cl | 1.39 | −5.57/−3.98 | PM6 | 0.87 | 21.8 | 0.71 | 13.5 | 141 |
| IN-4F | 1.38 | −5.56/−3.99 | PM6 | 0.870 | 21.8 | 0.692 | 13.0 | 141 |
| ISI-4F | 1.43 | −5.65/−4.01 | PM6 | 0.878 | 22.8 | 0.622 | 12.5 | 141 |
| BTCN-O | 1.53 | −5.59/−3.95 | PBDB-T | 0.95 | 5.03 | 0.34 | 1.62 | 142 |
| BTCN-M | 1.63 | −5.69/−3.95 | PBDB-T | 0.98 | 12.03 | 0.50 | 5.89 | 142 |
| M2 | 1.39 | −5.60/−3.96 | PM6 | 0.88 | 19.76 | 0.6384 | 11.26 | 143 |
| M4 | 1.38 | −5.61/−3.97 | PM6 | 0.88 | 23.44 | 0.7152 | 14.75 | 143 |
| M36 | 1.39 | −5.62/−3.95 | PM6 | 0.90 | 24.63 | 0.7209 | 16.00 | 144 |
| M38 | 1.47 | −5.65/−3.93 | PM6 | 0.87 | 18.28 | 0.5574 | 8.89 | 144 |
| M3 | 1.41 | −5.60/−3.85 | PM6 | 0.96 | 11.34 | 0.5196 | 5.67 | 145 |
| M32 | 1.41 | −5.69/−3.84 | PM6 | 0.91 | 24.03 | 0.7622 | 16.66 | 145 |
In 2019, Yang et al. investigated the impact of the 2D conjugated moiety on photovoltaic performance and synthesized BTTIC-Th with a thiophene moiety, BTTIC-TT with a thieno[3,2-b]thiophene moiety, and BTTIC-Ph with a benzene moiety.137 Due to the larger dihedral angle between benzene (or thieno[3,2-b]thiophene) and the ladder-type fused-ring skeleton, BTTIC-Ph and BTTIC-TT displayed an upshifted LUMO value relative to BTTIC-Th. The PM6:BTTIC-Ph blend presented different types of π–π stacking and larger domain size, which are detrimental for carrier transport and exciton dissociation. Finally, the BTTIC-TT-based device achieved the best device efficiency of 13.44% and the BTTIC-Th-based device achieved a high efficiency of 12.91%, which is obviously superior to that of the BTTIC-Ph-based device (9.14%). In addition, they compared the impact of thiophene chains and alkoxyl side chains on acceptors’ properties and device performance, and found that BTOIC with alkoxyl chains shows redshifted absorption and elevated energy levels relative to those of BTTIC, which could be ascribed to the strong electron donating ability of alkoxyl chains.138 Furthermore, BTOIC showed intense crystallinity, resulting in a rough surface morphology and oversize domain size, leading to a low PCE of 10.96%.
The asymmetric side-chain strategy is a useful way to improve cell efficiency. For instance, A. K.-Y. Jen and co-workers developed three SMAs with asymmetric side chains, namely, TOBDT with a thiophene side chain and an alkoxyl side chain, POBDT-4Cl with a phenylalkyl chain and an octyloxy chain, and COBDT-4Cl with a phenylalkyl chain and a flexible octyl chain.139,140 COBDT-4Cl showed slightly redshifted absorption and elevated energy levels, enhanced molecular packing and electron mobility compared to TOBDT and POBDT-4Cl. The PM6:COBDT-4Cl device achieved the highest efficiency of 13.5% and an Eloss of 0.52 eV.
In addition to introducing heteroatoms such as fluorine, chlorine, sulfur, etc. into the side chain, silicon atoms were also introduced into the thiophene side chain of BDT-based SMAs to tune the materials’ properties. Zhan's group developed two BDT-based SMAs of IN-4F tri(n-propyl)silylthienyl side chains and ISI-4F with tri(n-propyl)silylethynyl side chains.141 Owing to the significant contribution of the ethynyl group in HOMO and LUMO orbitals, ISI-4F presented blue-shifted absorption spectra and upshifted molecular energy levels as compared with IN-4F. The OSCs based on PM6:ISI-4F delivered PCEs of 12.5%, slightly lower than that of IN-4F-based device (13.0%) due to the reduced FF.
Swapping the position of alkyl side chains has a great impact on the acceptors’ physicochemical properties. Hou et al. added another alkyl chain to 2D thiophene side chains at different positions to modulate the steric hindrance and electron accepting and donating properties.142 In BTCN-O, the alkyl chain is attached to the 4-position, whereas in BTCN-M, the alkyl chain is introduced at the 3-position. With this subtle change, the dihedral angle between the 2D thiophene chain in BTCN-O and the BDT unit is smaller than that of BTCN-M (59° versus 70°). BTCN-O presents much redshifted absorption in films and an elevated HOMO value, and enhanced hole mobility but much lower electron mobility compared to BTCN-M. Thus, the PBDB-T:BTCN-M blend achieved a better efficiency of 5.89% compared to the PBDB-T:BTCN-O blend (1.62%), where BTCN-M shows electron accepting properties, and the BTCN-O:PC71BM blend yielded a better efficiency of 6.68% compared to BTCN-M:PC71BM (0.29%), where BTCN-O shows electron donating properties.
Side-chain engineering on M-series acceptors is of significant importance for boosting device performance, where the central core of M-series acceptors typically consists a BDT unit and two N alkyl chains.156–158 In this part, Zheng and co-worker did some nice work. First, they fixed the alkyl chain on the nitrogen as 2-ethylhexyl and changed the length of alkoxyl on the BDT unit to obtain two SMAs of M2 with 2-ethylhexyloxy and M4 with 2-butyloctyloxy.143 Despite the different side chain on BDT, two SMAs showed similar optical bandgaps and energy levels. However, the device based on PM6:M4 realized better PCE (14.75%) than the M2-based device (11.16%) because of the more balanced charge transport. Next, based on M2, they developed two new SMAs (M36 and M38) by simultaneously increasing the length of alkyl-chains on N and O, and ensuring that both alkyl chains were of the same length.144 M38 with 2-decyltetradecyl chains showed obviously blue-shifted absorption in films and broader bandgaps compared to M2 with 2-ethylhexyl chains and M36 with 2-butyloctyl chains. Compared to M2 and M38 with too short or too long side chains, M36 presented the most ordered and closest π–π stacking, which is favorable to charge transport; thus the PM6:M36 device achieved the highest PCE of 16.00%. Finally, they fixed the alkyl chain on BDT as 2-ethylhexyl and changed the alkyl chains on N to acquire two SMAs, namely, M3 and M32.145 The relation and difference between M3 and M32 in chemical structures is that these two SMAs are a pair of isomers, the alkyl chain on N of M3 is a branched chain, while for M32, it is a linear chain. Compared with M32 (edge-on molecular orientation), M3 exhibits a significantly different molecular orientation (face-on) and enhanced electron mobility. Consequently, the PM6:M3 device yielded a record efficiency of 16.6% for the A–D–A type SMA-based device.
2.4. Side-chain engineering of DTP-based SMAs
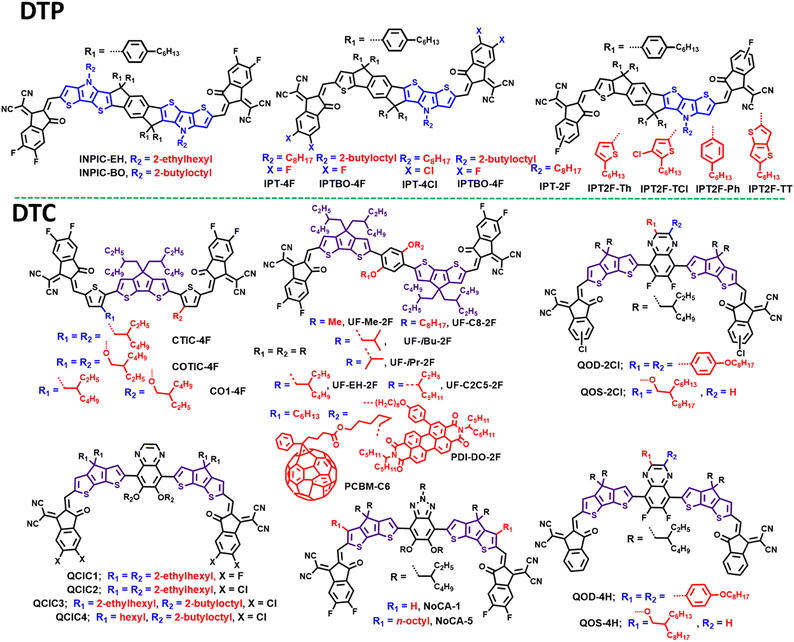
Due to the electron-donating properties of nitrogen atoms, dithieno[3,2-b:2′,3′-d]pyrrol (DTP) has widely been used as a building block to develop narrow bandgap nonfullerene SMAs with higher LUMO energy levels.146–149 In 2018, Tang and co-workers reported a DTP-based nonacyclic SMA of INPIC-4F with two linear C8 side chains which achieved a high PCE of 13.1%.146 Later, they replaced the linear chains with branched alkyl side chains (2-ethylhexyl (EH) and 2-butyloctyl (BO)) to obtain two new SMAs of INPIC-EH and INPIC-BO (Fig. 10).147 Altering the side chains in INPIC had little effect on electrochemical and optical properties. However, the INPIC-EH- and INPIC-BO-based OSCs gave obviously lower PCE (PCE = 11.9% for INPIC-EH; PCE = 11.2% for INPIC-BO) than that of INPIC-based device (Table 5), originating from the oversize phase separation in PBDB-T:INPIC-BO and PBDB-T:INPIC-EH blends.| SMA |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a(eV) a(eV) |
HOMOb/LUMOb (eV) | Donor | V OC (V) | J SC (mA cm−2) | FF | PCE% | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from Eoptg = 1240/λonset. b Calculated from CV curves. | ||||||||
| INPIC-4F | 1.39 | −5.42/−3.95 | PBDB-T | 0.852 | 21.9 | 0.694 | 13.1 | 146 |
| INPIC-EH | 773 | −5.48/−3.99 | PBDB-T | 0.837 | 20.7 | 0.684 | 11.9 | 147 |
| INPIC-BO | 776 | −5.47/−3.97 | PBDB-T | 0.839 | 20.0 | 0.668 | 11.2 | 147 |
| IPT-4F | 1.42 | −5.57/−4.08 | PM6 | 0.914 | 22.08 | 0.7415 | 14.96 | 148 |
| IPTBO-4F | 1.41 | −5.57/−4.07 | PM6 | 0.917 | 22.08 | 0.7245 | 14.67 | 149 |
| IPT-4Cl | 1.39 | −5.58/−4.11 | PM6 | 0.883 | 23.18 | 0.7037 | 14.40 | 149 |
| IPTBO-4Cl | 1.39 | −5.64/−4.08 | PM6 | 0.893 | 23.15 | 0.7257 | 15.00 | 149 |
| IPT2F-ThCl | 1.48 | −5.59/−4.00 | PBDB-T | 0.86 | 20.59 | 0.7751 | 13.74 | 150 |
| IPT2F-Th | 1.47 | −5.58/−4.03 | PBDB-T | 0.86 | 20.44 | 0.7120 | 12.52 | 151 |
| IPT2F-Ph | 1.46 | −5.57/−4.00 | PBDB-T | 0.86 | 21.18 | 0.7211 | 13.13 | 151 |
| IPT2F-TT | 1.45 | −5.60/−4.00 | PBDB-T | 0.84 | 22.24 | 0.7506 | 14.02 | 151 |
| DOC6-IC | 1.43 | −5.33/−3.72 | PBDB-T | 0.91 | 19.21 | 0.6011 | 10.52 | 152 |
| DOC8-IC | 1.39 | −5.36/−3.71 | PBDB-T | 0.92 | 17.74 | 0.5765 | 9.41 | 152 |
| DOC2C6-IC | 1.44 | −5.38/−3.73 | PBDB-T | 0.93 | 18.85 | 0.6333 | 11.10 | 152 |
| DC6-IC | 1.69 | −5.53/−3.79 | PBDB-T | 0.99 | 11.19 | 0.6221 | 6.87 | 152 |
| UF-EH-2F | 1.38 | −5.49/−4.11 | J52 | 0.79 | 24.87 | 0.69 | 13.56 | 153 |
| QOD-4H | 1.45 | −5.61/−3.77 | PBDB-T | 0.843 | 17.04 | 0.4834 | 6.94 | 154 |
| QOD-2Cl | 1.43 | −5.61/−3.81 | PBDB-T | 0.824 | 20.74 | 0.6244 | 10.67 | 154 |
| QOS-4H | 1.43 | −5.55/−3.70 | PBDB-T | 0.893 | 16.74 | 0.5355 | 8.01 | 154 |
| QOS-2Cl | 1.43 | −5.60/−3.77 | PBDB-T | 0.845 | 21.03 | 0.6870 | 12.19 | 154 |
| QCIC1 | 1.41 | −5.52/−3.80 | PBDB-T | 0.822 | 18.81 | 0.555 | 8.58 | 155 |
| QCIC2 | 1.39 | −5.53/−3.84 | PBDB-T | 0.807 | 18.65 | 0.603 | 9.09 | 155 |
| QCIC3 | 1.38 | −5.52/−3.89 | PBDB-T | 0.816 | 19.39 | 0.669 | 10.55 | 155 |
| QCIC4 | 1.35 | −5.49/−3.90 | PBDB-T | 0.780 | 19.16 | 0.638 | 9.53 | 155 |
| CTIC-4F | 1.3 | −5.4/−4.0 | PTB7-Th | 0.70 | 23.4 | 0.64 | 10.5 | 156 |
| CO1-4F | 1.2 | −5.3/−4.1 | PTB7-Th | 0.64 | 24.8 | 0.64 | 10.0 | 156 |
| COTIC-4F | 1.1 | −5.2/−4.1 | PTB7-Th | 0.57 | 20.7 | 0.61 | 7.3 | 156 |
| NoCA-1 | — | −5.34/−3.43 | J52 | 0.769 | 24.69 | 0.6169 | 11.71 | 157 |
| NoCA-5 | — | −5.30/−3.38 | J52 | 0.814 | 26.02 | 0.6996 | 14.82 | 157 |
| PDI-DO-2F | 1.44 | −5.54/−3.69 | PBDB-T | 0.89 | 20.04 | 0.6618 | 11.78 | 158 |
| PCBM-C6 | 1.43 | −5.26/−3.84 | PBDB-T | 0.86 | 20.41 | 0.7115 | 12.51 | 159 |
| PCBM-C10 | 1.41 | −5.27/−3.84 | PBDB-T | 0.87 | 21.30 | 0.7206 | 13.55 | 159 |
In 2019, Tang et al. reported an asymmetric heptacyclic core by removing the peripheral thieno[3,2-b]pyrrole unit of the central core in INPIC, and employed the new core to obtain a SMA of IPT-2F with an efficiency of 14%.148 Based on IPT-2F containing DTP, the same team developed four asymmetric SMAs (IPTBO-4F, IPT-4F, IPTBO-4Cl, and IPT-4Cl) through fine regulation of the terminal accepting units and side chains.149 As a result, IPT-4F- and IPTBO-4Cl-based devices delivered a higher and similar PCE of 15% due to the balanced molecular packing and aggregation, as compared with IPTBO-4F and IPT-4Cl. Replacing an alkyl chain with an aryl side chain is a common approach to tune the device efficiency. Then, they first incorporated 2D conjugated chains into the asymmetric IPT central core by replacing linear n-octyl in IPT-2F with 3-chloro-2-hexylthienyl to acquire a new SMA of IPT2F-TCl.150 The OSCs based on PBDB-T:IPT2F-TCl realized a decent PCE and an outstanding FF of 77.51%. The high FF can be mainly ascribed to the large electron (μe) and hole (μh) mobility, and nearly symmetric charge transport (μe/μh = 1.02) in the PBDB-T:IPT2F-TCl blend. Apart from using 3-chloro-2-hexylthienyl as side chains, they systematically compared the three asymmetric acceptors (IPT2F-TT, IPT2F-Ph and IPT2F-Th) with different N-conjugated side chains (4-hexylphenyl, 5-hexylthiophen-2-yl, and 5-hexylthieno[3,2-b]thiophen-2-yl).151 These three SMAs showed similar optical and electrochemical properties. After blending these SMAs with PBDB-T separately, IPT2F-TT containing TT side chains yielded the highest PCE of 14.02%, performing better than the devices based on phenylated IPT2F-Ph (13.11%) and thineylated IPT2F-Th (12.52%). The best PCE of the IPT2F-TT-based device should be attributed to the better morphology, including the dominated face-on orientation, proper phase separation with interpenetrating network structures.
2.5. Side-chain engineering of DTC-based SMAs
Employing dithieno[3,2-b:2',3'-d]cyclopentadiene to exploit unfused SMAs has attracted significant attention. For example, Bo and co-workers developed a series of non-fused SMAs based on a central 2,5-bis(alkyloxy)phenylene core, two DTC spacerrs, and two terminal accepting groups.152 Due to the multiple S⋯O non-covalent interactions, DOC6-IC, DOC8-IC and DOC2C6-IC showed markedly larger fluorescence quantum yields and lower band gaps relative to those of DC6-IC (Fig. 10). The OSCs based on PBDB-T:DOC2C6-IC exhibited a better PCE of 11.10%, higher than that of the device based on PBDB-T:DOC6-IC (10.52%) and PBDB-T:DOC8-IC (9.41%). Notably, these three devices gave small non-radiative recombination losses of ∼0.21 eV, obviously lower than those of the DC6-IC-based device (0.30 eV). After fluorination of DOC2C6-IC, the generated DOC2C6-2F exhibited an enhanced PCE of 13.24%. Similar work was reported by Chen and co-workers, and they also confirmed that the SMA (UF-EH-2F) with 2-ethylhexyl side chains exhibited better device performance.153Yang and co-workers synthesized four unfused SMAs (QOD-4H, QOS-4H, QOD-2Cl, and QOS-2Cl) based on DTC and quinoxaline with different terminal units and side chains.154 In particular, the branched alkyl chains in two QOS-based acceptors exhibited reduced crystallization tendency but smaller π–π stacking distances relative to those of the two QOD-based SMAs. This trend was also observed through the chlorination of QOD(s)-4H. When these four SMAs were paired with PBDB-T, the QOS-2Cl-based device achieved the highest PCE of 12.19%, resulting from the tightest molecular packing and the clearest nanofibrillar networks. Chen et al. also reported four unfused SMAs (QCIC1, QCIC2, QCIC3, and QCIC4) with the same backbone as the abovementioned QOD-4H, but different alkyl chains.155 Thanks to the more and the longer branched chains, the blend of PBDB-T and QCIC3 demonstrated the best molecular packing and an optimal domain size, which endowed the PBDB-T:QCIC3 device to with the best PCE of 10.55%, when compared to the other three group devices.
Unlike other non-fused SMAs with DTC as a π-spacer, Bazan and co-workers developed a non-fused SMA of COTIC-4F with DTC as the central core, and they studied the impact of side chains on physicochemical properties and device parameters of SMAs based on the COTIC-4F backbone.156 By progressively replacing the alkoxy side chain in COTIC-4F with alkyl groups, two new SMAs, CO1-4F and CTIC-4F, were obtained. Because of the electron-donating characteristic of alkoxy side chains, the optical band gaps gradually increased from COTIC-4F, CO1-4F, to CTIC-4F. The OSCs based on PTB7-Th: CTIC-4F and PTB7-Th:CO1-4F delivered PCEs of over 10% along with impressive JSCs of 25 mA cm−2, outperforming than the COTIC-4F-based devices.
Incorporation of an alkyl chain into the outermost aromatic ring of the central core in IDTT-based SMAs to restrict the rotation of the terminal units is an important means to boost the device efficiency. This strategy has also been proven effective in DTC-based non-fused SMAs. Huang and co-workers introduced an n-octyl group at the β position of thiophene in DTC, and developed a new SMA of NoCA-5.157 Compared with unoctylated NoCA-1, NoCA-5 demonstrated an upshifted LUMO value, enhanced crystallinity and smaller reorganization energy due to better molecular rigidity. Finally, the devices based on NoCA-5 achieved a high efficiency of 14.82%, significantly better than that of the NoCA-1-based one (11.71%).
It is feasible to develop high-performance non-fused ring electron acceptors by using large aromatic rings as side chains.158,159 For example, Bo and co-workers reported a non-fused ring SMA of PDI-DO-2F by attaching perylenediimide (PDI) units as a side chain. Compared to DO-2F with alkyl side chains on the central benzene ring, PDI-DO-2F showed improved solubility, enhanced fluorescence quantum yield and decreased crystallinity, endowing PDI-DO-2F with better PCE and lower nonradiative energy loss.158 Furthermore, they developed two non-fused ring SMAs with PCBM as the side group, namely, PCBM-C6 and PCBM-C10.159 The addition of bulky and electronically isotropic fullerene side groups to the SMAs was found to be an effective strategy to suppress severe aggregation behaviors and improve the active-layer morphology. This approach resulted in enhanced efficiencies for charge collection and exciton separation compared to the control molecule of CH3COO-C6. Consequently, the use of fullerene pendants resulted in simultaneous improvements in the VOC, JSC, and FF values of the solar cells. These enhancements led to a significantly increased PCE of 13.55% in the PCBM-C10-based device.
2.6. Side-chain engineering of oligothiophene-based non-fused SMAs
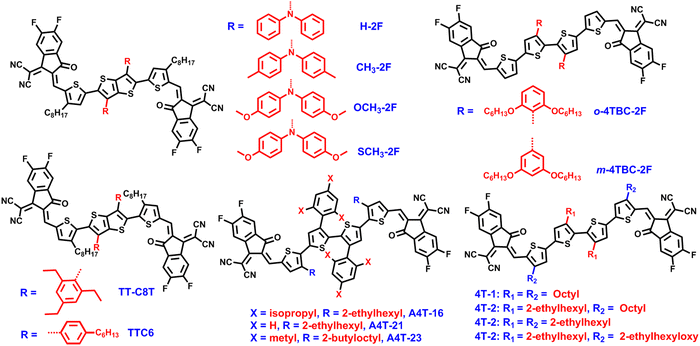
Oligothiophenes have become the mainstream backbones for the construction of non-fused SMAs.160–164 Bo and co-workers developed four tetrathiophene-based non-fused ring SMAs, namely 4T-1, 4T-2, 4T-3, and 4T-4, with different lengths of the lateral side chains (Fig. 11).160 In a dilute chlorobenzene (CB) solution, the four acceptors showed similar absorption curves. In the solid state, 4T-1 exhibited remarkably redshifted absorption spectra due to the strong molecular packing, as compared with the ones in solution, while for the other three SMAs, because of the steric hindrance of the 2-ethylhexyl chains, the red shift of the absorption spectra of the acceptors was not obvious and the intermolecular packing was not as tight as 4T-1. As a result, the PBDB-T:4T-3 device delivered a decent PCE of 10.15% (Table 6), resulting from the better compatibility between PBDB-T and 4T-3. During the same period, the same group reported two SMAs (o-4TBC-2F and m-4TBC-2F) built on tetrathiophene backbones, by altering the hexyloxy side-chain position in phenyl groups.161o-4TBC-2F containing ortho-hexyloxy-phenyl chains displayed a more planar molecular structure, narrower band gap, and better molecular packing and orientation relative than m-4TBC-2F with meta-hexyloxy-phenyl chains. The PBDB-T:o-4TBC-2F cell yielded a good efficiency of 10.26%, performing better than the device based on m-4TBC-2F (2.63%).| SMA |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a(eV) a(eV) |
HOMOb/LUMOb (eV) | Donor | V OC (V) | J SC (mA cm−2) | FF | PCE% | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from Eoptg = 1240/λonset. b Calculated from CV curves. | ||||||||
| 4T-1 | 1.47 | −5.71/−4.09 | PBDB-T | 0.84 | 12.70 | 0.5171 | 5.53 | 160 |
| 4T-2 | 1.54 | −5.85/−4.02 | PBDB-T | 0.82 | 15.68 | 0.7032 | 9.09 | 160 |
| 4T-3 | 1.52 | −5.86/−3.98 | PBDB-T | 0.81 | 17.27 | 0.7245 | 10.15 | 160 |
| 4T-4 | 1.60 | −5.85/−3.86 | PBDB-T | 0.94 | 14.27 | 0.6182 | 8.27 | 160 |
| o-4TBC-2F | 1.47 | −5.63/−4.00 | PBDB-T | 0.76 | 20.48 | 0.657 | 10.26 | 161 |
| m-4TBC-2F | 1.66 | −5.68/−4.00 | PBDB-T | 0.84 | 7.90 | 0.400 | 2.63 | 161 |
| A4T-16 | 1.45 | −5.67/−3.96 | PM6 | 0.876 | 21.8 | 0.798 | 15.2 | 162 |
| A4T-21 | 1.66 | −5.89/−3.92 | PM6 | 0.936 | 5.55 | 0.303 | 1.57 | 162 |
| A4T-23 | 1.43 | −5.63/−3.98 | PM6 | 0.870 | 21.0 | 0.568 | 10.4 | 162 |
| H-2F | 1.46 | −5.58/−4.06 | PBDB-T | 0.73 | 18.78 | 0.6299 | 8.64 | 163 |
| CH3-2F | 1.42 | −5.59/−4.01 | PBDB-T | 0.77 | 22.76 | 0.6985 | 12.28 | 163 |
| OCH3-2F | 1.33 | −5.40/−4.04 | PBDB-T | 0.74 | 16.66 | 0.6463 | 8.01 | 163 |
| SCH3-2F | 1.41 | −5.50/−4.06 | PBDB-T | 0.67 | 15.38 | 0.6494 | 6.67 | 163 |
| TTC6 | 1.73 | −5.79/−3.93 | D18 | 0.93 | 10.20 | 0.463 | 4.41 | 164 |
| TT-C8T | 1.55 | −5.77/−3.91 | D18 | 0.91 | 19.31 | 0.591 | 10.42 | 164 |
| TT-TC8 | 1.44 | −5.79/−3.90 | D18 | 0.86 | 23.06 | 0.662 | 13.13 | 164 |
Hou et al. also synthesized and reported three tetrathiophene-based non-fused ring SMAs (A4T-16, A4T-21, A4T-23) with different phenyl substituted side chains, including 2,4,6-triisopropylphenyl (Pi), 2,4,6-trimethylphenyl, and unsubstituted benzene.162 Pi endowed A4T-16 with good molecular planarity and large steric hindrance, leading to the three-dimensional charge transport, as confirmed by the single-crystal data of A4T-16. When the three SMAs were paired with PM6, the device based on A4T-16 realized a high efficiency of 15.2%, representing the best value for the fully non-fused SMA-based OSCs.
Apart from employing tetrathiophene as a backbone to develop non-fused ring SMAs, thieno[3,2-b]thiophene (TT) is also a commonly used building block to construct non-fused ring acceptors. For instance, Bo and co-workers first employed diphenylamine derivatives as side chains to prepare four non-fused SMAs, namely, H-2F, CH3-2F, OCH3-2F, and SCH3-2F.163 The incorporation of diphenylamine improved the molecular solubility, prevented the formation of oversized self-aggregates, and boosted the ICT effect. Different substituents (H, –CH3, –SCH3, and –OCH3) on diphenylamine of the four acceptors have great impact on physicochemical properties and cell efficiency (Fig. 12). Compared with the other three SMAs, methylated CH3-2F showed stronger absorption ability in a solid state, better molecular orientation and π–π stacking. Due to the strongest electron-donating ability of methoxy, OCH3-2F exhibited the lowest bandgap and shallowest HOMO energy level. A two-dimensional change transport mode was observed in CH3-2F and validated by the single-crystal data, which resulted from intermolecular non-covalent interactions of S⋯π, N⋯N, F⋯F and π⋯π. It is because of these advantages that the device based on PBDB-T:CH3-2F achieved the highest efficiency of 12.28%, higher than those of the H-2F-based device (8.64%), OCH3-2F-based device (8.01%), and SCH3-2F-based device (6.67%). The high PCE of the CH3-2F-based device is mainly ascribed to the significant face-to-face molecular orientation, suitable phase separation and low energy loss. Additionally, they compared the effects of 4-hexylphenyl and 2,4,6-triethylphenyl on the TT of two SMAs (TTC6 and TT-C8T) on the device performance, and found that 2,4,6-triethylphenyl endowed TT-C8T with good molecular planarity, higher electron mobility, more favorable molecular orientation, and better device performance.164
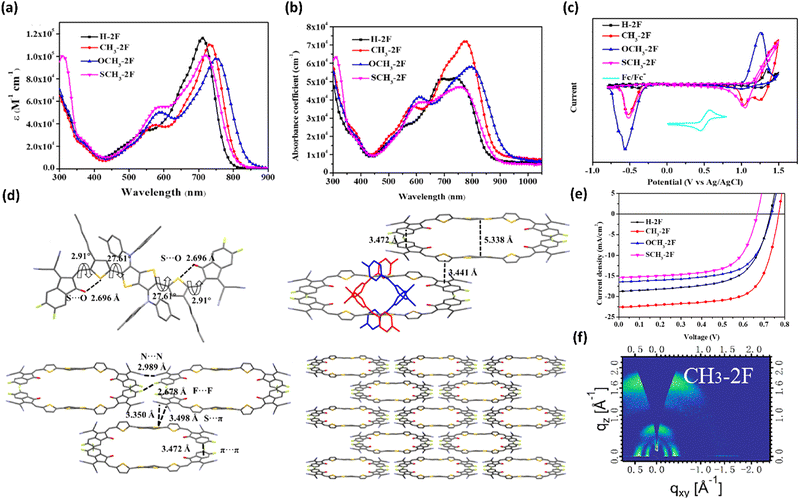 | ||
| Fig. 12 (a) Absorption spectra of H-2F, CH3-2F, OCH3-2F, and SCH3-2F in solutions; (b) Absorption spectra and CV curves (c) of H-2F, CH3-2F, OCH3-2F, and SCH3-2F in films; (d) Single-crystal structure of CH3-2F; (e) J–V curves of H-2F-, CH3-2F-, OCH3-2F-, and SCH3-2F-based devices; (f) 2D GIWAXS patterns of the neat CH3-2F acceptor. Adapted with permission.163 Copyright 2021, American Chemical Society. | ||
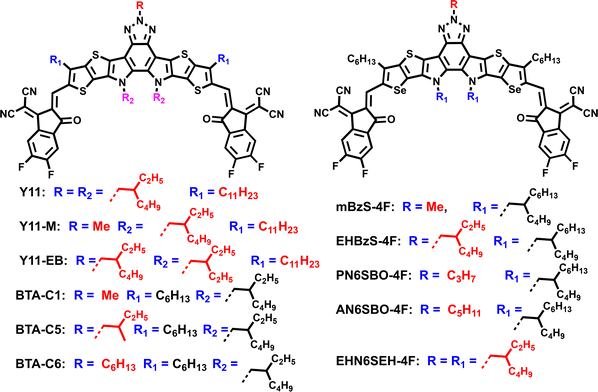
3. Side-chain engineering of A–DA1D–A-type nonfullerene SMAs
In comparison with traditional A–D–A structured SMAs, A–DA1D–A type SMAs usually demonstrate lower band gaps and smaller energy loss due to intramolecular multiple D–A interactions and high fluorescence quantum yields. The most common A–DA1D–A type SMAs include benzo[c][1,2,5]thiadiazole (BT)-, benzotriazole (BTZ)- and quinoxaline(Qx)-based ones. In this part, we mainly introduce side-chain engineering of BT- and BTZ-based SMAs.3.1. Side-chain engineering of BT-based SMAs
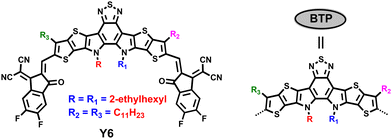
Y6,10 as a typical BT-based SMA, has four relatively independent alkyl chains: two of them are distributed at the β position of the outer thiophene, and the other two are located on the nitrogen atom of the pyrrole ring (Fig. 13). Modifying the side chains on the nitrogen atom or at the β position of the outer thiophene in A–DA1D–A type SMAs is a simple and useful way to boost device efficiency.At the N position, Zou et al. altered the N alkyl chains to linear chains and different branching position chains. N-C11 with linear chains exhibited much inferior solubility and excessive aggregation.165 N4 (Fig. 14) with 4th-position branched chains showed larger domain size and predominantly edge-on orientation when blended with PM6. N3 with 3rd-position branched chains delivered an optimum morphology, and the binary device based on N3 achieved a PCE of 15.98% (Table 7), and the PM6:N3:PC71BM ternary device achieved a PCE of 16.74%. Altering the branching point of N alkyl chains in Y6 was also reported by our group, and similar results were obtained, that is, the devices based on Y6-C2 (or N3) realized the highest PCEs as compared with Y6 and Y6-C3 (or N4).166
| SMA |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (eV) a (eV) |
HOMOb/LUMOb (eV) | Donor | V OC (V) | J SC (mA cm−2) | FF | PCE% | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from Eoptg = 1240/λonset. b Calculated from CV curves. | ||||||||
| N3 | — | — | PM6 | 0.837 | 25.81 | 0.739 | 15.98 | 166 |
| Y6 | 1.33 | −5.67/−4.08 | PM6 | 0.859 | 25.22 | 0.703 | 15.24 | 166 |
| Y6-C2 | 1.33 | −5.65/−4.09 | PM6 | 0.860 | 25.11 | 0.736 | 15.98 | 167 |
| Y6-C3 | 1.41 | −5.68/−4.07 | PM6 | 0.852 | 24.07 | 0.674 | 13.76 | 167 |
| BTP-4Cl-8 | — | −5.67/−4.11 | PBDB-TF | 0.872 | 25.2 | 0.743 | 16.3 | 168 |
| BTP-4Cl-12 | — | −5.66/−4.09 | PBDB-TF | 0.858 | 25.6 | 0.776 | 17.0 | 168 |
| BTP-4Cl-16 | — | −5.68/−4.09 | PBDB-TF | 0.862 | 24.2 | 0.748 | 15.6 | 168 |
| BTIC-BO-4Cl | 1.34 | −5.54/−4.14 | PBDB-TF | 0.85 | 25.26 | 0.7625 | 16.43 | 169 |
| BTP-C2C4-N | 1.34 | −5.49/−3.90 | PM6 | 0.93 | 18.2 | 0.571 | 10.3 | 170 |
| BTP-C4C6-N | 1.35 | −5.50/−3.90 | PM6 | 0.94 | 20.7 | 0.621 | 12.1 | 170 |
| BTP-C6C8-N | 1.35 | −5.50/−3.88 | PM6 | 0.95 | 20.2 | 0.620 | 11.9 | 170 |
| EH-HD-4F | 1.39 | −5.69/−4.04 | PM6 | 0.84 | 27.5 | 0.793 | 18.38 | 172 |
| BO-4F | 1.40 | −5.70/−4.01 | PM6 | 0.84 | 27.0 | 0.767 | 17.39 | 172 |
| Bu-OD-4F | 1.42 | −5.68/−4.01 | PM6 | 0.85 | 26.2 | 0.766 | 17.10 | 172 |
| BTP-EHBO-4F | 1.33 | −5.80/−3.97 | PM6 | 0.85 | 26.12 | 0.7578 | 16.82 | 173 |
| BTP-PHD-4F | 1.30 | −5.79/−3.95 | PM6 | 0.87 | 25.64 | 0.7134 | 15.91 | 173 |
Apart from branching position, regulating the length of branched side chains is also a main direction for optimizing the molecular structure. For example, Yao et al. finely modified the inner branched side chain length of chlorinated SMAs for superior processability. The 2-butyloctyl chains in BTP-4Cl-12 could balance the solution processability and aggregation behavior.167 The PBDB-TF:BTP-4Cl-12 device achieved a PCE of 17.0% via spin-coating process and 15.5% via blade-coating process in a 1 cm2 device. Similarly, He et al. synthesized four chlorinated SMAs with different alkyl chain lengths on the inner side chains. BTIC-HD-4Cl and BTIC-BO-4Cl exhibited good solubility in comparison to BTIC-C12-4Cl with linear alkyl chains and branched short alkyl-substituted BTIC-EH-4Cl.168 BTIC-BO-4Cl showed relatively red-shifted absorption spectra and enhanced absorption capacity and short (010) stacking distance in the PM6:acceptor blend. The cell based on BTIC-BO-4Cl presented the best PCE of 16.43%. In addition, Gao et al. tuned the branched alkyl chain length in the inner N alkyl chains with naphthalene-fused end groups.169 Compared with BTP-C4C6-N with 2-butyloctyl and BTP-C6C8-N with 2-hexyldecyl, BTP-C2C4-N with 2-ethylhexyl chains presented redshifted absorption in films and the strongest crystallinity. The PM6:BTP-C4C6-N device delivered an efficiency of 12.4%, higher than those of BTP-C6C8-N- and BTP-C2C4-N-based devices (10.3% and 11.9%, respectively). Besides, the three SMAs with naphthalene-fused end groups can help reduce the nonradiative recombination energy loss.
Introducing highly polarizable OEG groups into SMAs can increase the solubility of the resulting molecules in non-halogenated solvents. Li et al. replaced the inner alkyl side chains of Y6 with amphiphilic OEG chains to get BTO.170 BTO exhibited better solubility in non-halogenated paraxylene, higher coplanarity, and a more ordered film induced by OEG chains. BTO showed excellent compatibility with the Y6 host, and Y6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTO (1
BTO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2) exhibited reinforced crystallinity. By using this guest-assisted assembly strategy, PM6:Y6:BTO achieved a high PCE of over 16% in chlorobenzene (CB) and PX solvent, and the PCE of the PM6:Y6:BTO:PC71BM device was further increased to over 17%. Impressively, the PM6:Y6:BTO:PC71BM large-area module (36 cm2) showed a record PCE of 14.26% for OSCs with the module area >20
0.2) exhibited reinforced crystallinity. By using this guest-assisted assembly strategy, PM6:Y6:BTO achieved a high PCE of over 16% in chlorobenzene (CB) and PX solvent, and the PCE of the PM6:Y6:BTO:PC71BM device was further increased to over 17%. Impressively, the PM6:Y6:BTO:PC71BM large-area module (36 cm2) showed a record PCE of 14.26% for OSCs with the module area >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm2.
cm2.
Above, we discussed the case where the two alkyl chains on the nitrogen of BT-based SMAs are the same; when the two alkyl chains on the nitrogen are different, then a molecule with asymmetric alkyl chains is formed. Taking Y6-4O as an example, Lin et al. tailored the inner alkyl side chains of Y6 to asymmetric highly polarizable OEG and alkyl side chains to get Y6-4O.171 Compared with Y6, Y6-4O showed a larger dipole moment, higher dielectric constant, excellent solubility in halogen-free solvents, and tendency toward face-on orientation. The toluene-processed PM6:Y6-4O as-cast device delivered lower energy loss, a higher exciton dissociation rate, and a smaller bimolecular recombination ratio, resulting in an improved device efficiency of 15.2%.
Huang et al. adopted a strategy to utilize asymmetric inner side chains to boost the photovoltaic performance.172 Compared to BO-4F with symmetric 2-butylocyl chains, Bu-OD-4F with asymmetric 2-octyldodecyl and 2-butyl chains showed relatively blue-shifted absorption and poor miscibility in blends, an EH-HD-4F with asymmetric 2-hexyldecyl and 2-ethylhexyl chains showed slightly red-shifted absorption and favorable morphology in blends. The device based on EH-HD-4F achieved the best efficiency of 18.38%, which is better than that of the device based on Bu-OD-4F (17.10%) and BO-4F (17.39%). In addition, Hsu et al. adopted a strategy utilizing asymmetric inner N alkyl chains, namely, 2-ethylhexyl and 2-butyloctyl chains for BTP-EHBO-4F and 2-hexyldecyl and phenyl alkoxy chains for BTP-PHD-4F.173 In comparison with Y6, BTP-PHD-4F and BTP-EHBO-4F showed an obviously enhanced absorption coefficient and stronger molecular π–π interactions. PM6:BTP-PHD-4F and PM6:BTP-EHBO-4F devices achieved much higher PCEs of 15.91% and 16.82% compared to the PM6:Y6 device with o-xylene as green solvent.
Focusing on the β-position side chains of the outer thiophene in BT-based A–DA1D–A acceptors, many high-efficiency SMAs have emerged by modifying the outer side chains. Yao et al. investigated the impact of the outer linear side-chain length on photovoltaic performance. By gradually tailoring the alkyl chains from C11 to C9, then to C7, BTP-eC9 maintained good solubility and enhanced intermolecular ordering. BTP-eC7 exhibited lower solubility, more ordered packing, and excessive aggregation in blends.85 The device based on BTP-eC9 achieved the highest efficiency of 17.8% because of the simultaneously increased JSC and FF (Table 8). Wang et al. developed four nonfullerene acceptors (C5–16, C6–16, C7–16, and C8–16) by meticulously tailoring the outer thiophene alkyl chains (from C5–C8), investigated their aggregation and optoelectronic properties.174 With the decrease in the alkyl-chain length, the molecular energy levels were gradually downshifted and the absorption spectra almost remained unchanged. BTP-4F-C5–16 presented the strongest intermolecular π–π stacking and highest electron mobility, leading to the highest efficiency of 18.20% in the PM6:BTP-4F-C5–16 device.
| SMA |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (eV) a (eV) |
HOMOb/LUMOb (eV) | Donor | V OC (V) | J SC (mA cm−2) | FF | PCE% | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from Eoptg = 1240/λonset. b Calculated from CV curves. | ||||||||
| BTP-eC7 | — | −5.62/−4.03 | PM6 | 0.843 | 24.1 | 0.735 | 14.9 | 85 |
| BTP-eC9 | — | −5.64/−4.05 | PM6 | 0.839 | 26.2 | 0.811 | 17.8 | 85 |
| BTP-eC11 | — | −5.63/−4.04 | PM6 | 0.851 | 25.7 | 0.775 | 16.9 | 85 |
| C5-16 | — | −5.73/−3.93 | PM6 | 0.844 | 27.78 | 0.7768 | 18.20 | 174 |
| C6-16 | — | −5.72/−3.92 | PM6 | 0.847 | 27.17 | 0.7770 | 17.82 | 174 |
| C7-16 | — | −5.72/−3.92 | PM6 | 0.848 | 26.52 | 0.7621 | 17.07 | 174 |
| C8-16 | — | −5.71/−3.91 | PM6 | 0.854 | 26.20 | 0.7577 | 16.88 | 174 |
| L8-BO | 1.40 | −5.68/−3.90 | PM6 | 0.87 | 25.72 | 0.815 | 18.32 | 72 |
| L8-HD | 1.43 | −5.71/−3.90 | PM6 | 0.88 | 25.08 | 0.788 | 17.29 | 72 |
| L8-OD | 1.42 | −5.71/−3.91 | PM6 | 0.89 | 24.57 | 0.746 | 16.26 | 72 |
| BTP-C6Ph | 1.35 | −5.60/−3.94 | PM6 | 0.839 | 24.3 | 0.762 | 15.5 | 83 |
| BTP-PhC6 | 1.36 | −5.58/−3.85 | PM6 | 0.865 | 25.0 | 0.77 | 16.7 | 83 |
| o-BTP-PhC6 | 1.39 | −5.53/−3.76 | PTQ10 | 0.924 | 22.8 | 0.762 | 16.0 | 84 |
| m-BTP-PhC6 | 1.35 | −5.59/−3.86 | PTQ10 | 0.883 | 25.3 | 0.793 | 17.7 | 84 |
| p-BTP-PhC6 | 1.36 | −5.59/−3.85 | PTQ10 | 0.888 | 24.7 | 0.779 | 17.1 | 84 |
| BTP-4F-PC6 | 1.37 | −5.65/−3.90 | PBDB-T-2F | 0.855 | 25.08 | 0.8033 | 17.22 | 175 |
| BTP-4F-P2EH | 1.41 | −5.65/−3.87 | PBDB-T-2F | 0.880 | 25.85 | 0.8008 | 18.22 | 175 |
| BTP-4F-P3EH | 1.40 | −5.66/−3.89 | PBDB-T-2F | 0.861 | 26.11 | 0.7813 | 17.57 | 175 |
| BTP-Th | 1.34 | — | PTQ10 | 0.878 | 25.2 | 0.762 | 16.8 | 176 |
| BTP-FTh | 1.32 | −5.80/−4.04 | PTQ10 | 0.849 | 26.33 | 0.767 | 17.16 | 62 |
| m-TEH | 1.38 | −5.71/−3.92 | PBQ6 | 0.880 | 26.61 | 0.7903 | 18.51 | 177 |
| o-TEH | 1.39 | −5.70/−3.90 | PBQ6 | 0.882 | 26.10 | 0.7042 | 16.22 | 177 |
| BTIC-4Cl-TCl-β | 1.36 | −5.43/−3.89 | PBDB-TF | 0.86 | 24.30 | 0.7461 | 15.65 | 178 |
| BTIC-4Cl-TCl-γ | 1.35 | −5.46/−3.91 | PBDB-TF | 0.80 | 23.91 | 0.7524 | 14.35 | 178 |
| 2-ClTh | 1.41 | −5.73/−4.32 | D18-Cl | 0.857 | 26.7 | 0.756 | 17.3 | 179 |
| 3-ClTh | 1.42 | −5.71/−4.29 | D18-Cl | 0.891 | 26.9 | 0.770 | 18.5 | 179 |
| 4-ClTh | 1.44 | −5.73/−4.28 | D18-Cl | 0.924 | 24.9 | 0.727 | 16.7 | 179 |
| Y6-2O | 1.45 | −5.73/−3.76 | PM6 | 0.92 | 13.3 | 0.535 | 6.6 | 180 |
| Y6-1O | 1.43 | −5.71/−3.84 | PM6 | 0.89 | 23.2 | 0.783 | 16.1 | 180 |
| BTP1O-4Cl-C12 | 1.41 | −5.84/−3.90 | PM6 | 0.91 | 23.85 | 0.788 | 17.1 | 181 |
| BTPS-4F | 1.38 | −5.73/−3.91 | PM6 | 0.82 | 25.2 | 0.78 | 16.2 | 182 |
| BTPS-4Cl | 1.36 | −5.65/−3.93 | PM6 | 0.81 | 24.3 | 0.69 | 13.5 | 182 |
| Y-C10ch | 1.35 | −5.65/−3.93 | PM6 | 0.858 | 26.9 | 0.763 | 17.6 | 183 |
| A-C10ch | 1.38 | −5.64/−3.90 | PM6 | 0.887 | 26.5 | 0.781 | 18.4 | 183 |
Sun et al. replaced the outer linear side chains of Y6 with bulk branched side chains, and investigated the branched side-chain length effect on molecular packing.72 Compared with Y6, L8-BO (2-butyloctyl substitution), L8-HD (2-hexyldecyl substitution) and L8-OD (2-octyldodecyl substitution) showed better solubility, blue-shifted absorption, upshifted LUMO energy levels, more condensed molecular stacking, and more prominent face-on orientation. However, as the branched alkyl-chain length increased, the electron mobility decreased, and the crystallization was enhanced due to interchain interaction. L8-BO showed better structural order with three π–π stacking motifs, favorable morphology and balanced charge transport, resulting in the best PCE of 18.32% with a record FF of 81.5% and an Eloss of 0.55 eV.
In addition to utilizing alkyl side chains to replace the n-undecyl group in Y6, some side chains with aryl groups are also widely used to optimize the structure of BT-based SMAs. In this area, Zhang and co-workers did a lot of great work. First, they changed the outer linear alkyl chains to 6-phenylhexyl and 4-hexylphenyl chains to get BTP-PhC6 and BTP-C6Ph.83 BTP-PhC6 with bulk hexylphenyl chains showed larger steric hindrance, upshifted LUMO values, enhanced crystallinity and molecular packing, and smaller domain size in blends in comparison to BTP-C6Ph. The PM6:BTP-PhC6 device achieved a higher PCE of 16.7% than the BTP-C6Ph-based device (15.5%). Next, they adopted a strategy utilizing outer isomeric hexylphenyl side chains for high performance OSCs.84 Because of the steric hindrance effect, the outer side chain in o-BTP-PhC6 with ortho-substitution position presents a vertical orientation, while p-BTP-PhC6 with para-substituted chains presents a horizontal orientation, and m-BTP-PhC6 with meta-substituted chains shows a ‘‘tilted’’ orientation (Fig. 15). Compared to p-BTP-C6Ph and m-BTP-PhC6, o-BTP-PhC6 demonstrates blue-shifted absorption spectra and a shallower LUMO value because of the low degree of molecular aggregation. As a result, m-BTP-PhC6 displays the best intermolecular π–π stacking. The device based on m-BTP-C6Ph and low-cost PTQ10 achieved the best efficiency of 17.7% in comparison to PTQ10:o-BTP-C6Ph (16.0%) and PTQ10:p-BTP-C6Ph (17.1%) devices, resulting from an appropriate phase separation, improved molecular stacking and more ordered side-chain orientations. Then, they changed the outer linear m-hexylphenyl chains to γ-branched BTP-4F-P3EH and β-branched BTP-4F-P2EH.175 BTP-4F-P2EH and BTP-4F-P3EH exhibited better solubility, slightly blue-shifted absorption and upshifted LUMOs, and suitable domain size in blends compared with p-BTP-C6Ph. BTP-4F-P2EH and BTP-4F-P3EH-based devices achieve enhanced VOC due to reduced non-radiative voltage losses, resulting in superior PCEs of 18.22% and 17.57%, respectively. Additionally, they replaced the outer 4-hexylphenyl alkyl side chains of BTP-PhC6 with 5-octylthienyl (BTP-Th), and a similar PCE of 16.8% was achieved for the PTQ10:BTP-Th device.176 Recently, Li et al. adopted a strategy of using thiophene outer side chains with branched 2-ethylhexyl-substituted at α- or β-position to get two isomeric SMAs, namely o-THE and m-TEH.177 The 2-ethylhexyl substitution position has a negligible effect on their optical and electrochemical properties; however, m-TEH presents reinforced molecular π–π stacking and enhanced charge mobility due to smaller steric hindrance. The PBQ6:m-TEH device achieved a better efficiency of 18.51% compared to the o-THE-based device (16.22%).
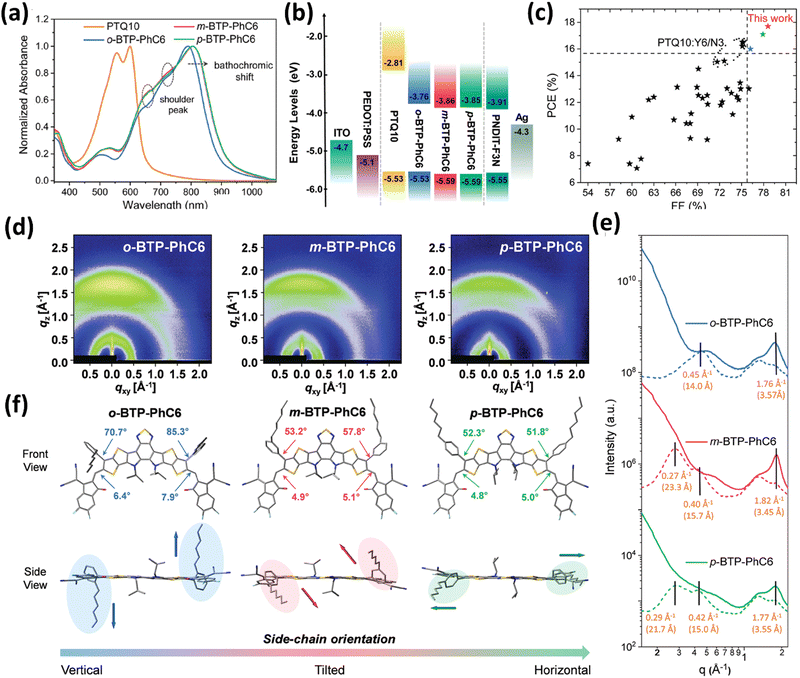 | ||
| Fig. 15 (a) Normalized UV-vis absorption spectra and (b) energy level diagram of o-BTP-C6Ph, m-BTP-C6Ph and p-BTP-C6Ph; (c) comparisons of PCE and FF for PTQ10-based OSCs; (d) 2D GIWAXS patterns, (e) corresponding 1D scattering profiles and (f) DFT calculations of o-BTP-C6Ph, m-BTP-C6Ph and p-BTP-C6Ph. Adapted with permission.84 Copyright 2021, Royal Society of Chemistry. | ||
Introducing a halogen into thiophene side chains of SMAs is an important means to enhance intermolecular forces and improve photovoltaic performance. Peng et al. synthesized BTP-FTh with β-fluorination of outer thiophene side chains.62 Compared with non-fluorinated BTP-Th, BTP-FTh exhibited slightly redshifted absorption, higher absorption coefficients, downshifted energy levels, higher crystallinity and electron mobility, thereby resulting in a higher PCE of 17.16% in the PTQ10:BTP-FTh device. He et al. investigated the chlorination effect in outer thiophene side chains. Compared with non-chlorinated BTIC-4Cl-T, BTIC-4Cl-TCl-β with chlorination at β position displayed obviously blue-shifted absorption, and BTIC-4Cl-TCl-γ with chlorination at γ position presented slightly blue-shifted absorption.178 Chlorination had little impact on energy levels. Additionally, the single crystal structure indicates that BTIC-4Cl-TCl-β exhibits a twisty skeleton due to large steric hindrance effects, resulting in the presence of only J-aggregation in the BTIC-4Cl-TCl-β quasi-3D network. BTIC-4Cl-TCl-γ can form both J-aggregation and H–aggregation 3D networks due to a highly planar structure. BTIC-4Cl-TCl-β-based cells achieved a PCE of 15.65% and the BTIC-4Cl-TCl-γ-based device achieved a PCE of 14.35%, which are much better than that of the BTIC-4Cl-T-based device (10.86%). Furthermore, Jen and co-workers reported three isomeric SMAs by changing the position of chlorine and 2-butyloctyl in thienyl side chains, namely, 2-ClTh, 3-ClTh, and 4-ClTh.179
The 3-ClTh isomer showed balanced end- and side-group torsion angles relative to those of 2-ClTh and 4-ClTh, which endowed 3-ClTh with a higher PCE of 18.5% along with a lower energy loss of 0.528 V.
Heteroatoms, such as O and S, were incorporated into the outer side chains of BT-based A–DA1D–A type SMAs. For example, Yan et al. modified the two outer side chains to get Y6-2O with alkoxy chains and Y6-1O with asymmetric alkoxy and alkyl chains.180 Because of the conformational-locking role of the alkoxy chains, Y6-2O showed poor solubility and excessive aggregation. Compared with Y6, alkoxy chains endow Y6-1O and Y6-2O relatively blue-shifted absorption spectra, elevated LUMO values and almost unchanged HOMO energy levels. The PM6:Y6-1O device delivered a high efficiency of 16.1%, and the PM6:Y6-1O:PC71BM ternary device showed a PCE of 17.6%. Based on the outer asymmetric alkoxy and alkyl side chains and chlorine-substitution strategies, the same group adopted inner side chain engineering to improve photovoltaic performance.181 BTP1O-4Cl-C12 exhibited good solubility and favorable morphology characterization, and the PM6:BTP1O-4Cl-C12 blend achieved an outstanding efficiency of 17.1%. Furthermore, they replaced the outer alkyl chains of Y6 with alkylthio chains to get BTPS-4F and BTPS-4Cl.182 BTPS-4Cl showed red-shifted absorption and enhanced absorption ability, upshifted HOMO values, but weaker molecular π–π packing in comparison to BTPS-4F. The PM6:BTPS-4F device delivered a higher PCE of 16.2% than the PM6:BTPS-4Cl device (13.5%).
Cycloalkyl–alkyl chains were used to develop symmetric/asymmetric SMAs, namely, Y-C10ch and A-C10ch. Compared to L8-BO, the cyclohexyldecyl chain in Y-C10ch and A-C10ch leads to a more planar backbone and tighter 3D network stacking, resulting in the better domain purity. As a result, the asymmetric A-C10ch achieved the best PCE of 18.4% due to its balanced VOC, JSC and FF values.183
3.2. Side-chain engineering of BTA-based SMAs
In comparison with BT-based SMAs, benzo[2,1,3]triazole (BTA)-based SMAs have one more side chain due to the introduction of an additional nitrogen atom. Modifying the N alkyl chain of BTA plays a vital role in promoting cell efficiency. Zou et al. replaced the 2-ethylhexyl chains in the BTA unit with methyl groups for Y11-M and the 2-ethylhexyl chains in the pyrrole ring with 2-ethylbutyl chains for Y11-EB.184 In comparison with Y11, Y11-EB and Y11-M exhibited intense absorption in the wavelength range from 450 to 850 nm and narrow bandgaps due to smaller steric hindrance (Fig. 16). PM6:Y11-EB and PM6:Y11-M devices achieved better PCEs of 17.15% and 16.64% (Table 9), respectively. They also systematically investigated the impact of alkyl side chain length in the BTA unit of BTA-C8 (Y18).185 BTA-C1 and BTA-C6 with linear chains showed lower solubility and excessive aggregation. BTA-C5 with shorter 2-methylbutyl branched chains presented suitable solubility and enhanced crystallinity. The PCEs of the four devices were over 16%, and PM6:BTA-C5 achieved the highest PCE of 17.11%.| SMA |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (eV) a (eV) |
HOMOb/LUMOb (eV) | Donor | V OC (V) | J SC (mA cm−2) | FF | PCE% | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from Eoptg = 1240/λonset. b Calculated from CV curves. | ||||||||
| Y11 | 1.30 | −5.65/−3.90 | PM6 | 0.87 | 23.69 | 0.720 | 14.85 | 184 |
| Y11-M | 1.30 | −5.60/−3.96 | PM6 | 0.86 | 25.54 | 0.7615 | 16.64 | 184 |
| Y11-EB | 1.30 | −5.59/−3.95 | PM6 | 0.88 | 26.20 | 0.7473 | 17.15 | 184 |
| BTA-C1 | — | −5.64/−3.99 | PM6 | 0.844 | 24.75 | 0.7762 | 16.21 | 185 |
| BTA-C5 | — | −5.66/−4.00 | PM6 | 0.847 | 26.51 | 0.7619 | 17.11 | 185 |
| BTA-C6 | — | −5.65/−3.97 | PM6 | 0.851 | 25.20 | 0.7568 | 16.23 | 185 |
| BTA-C8 | — | −5.66/−3.96 | PM6 | 0.837 | 26.18 | 0.7567 | 16.59 | 185 |
| mBzS-4F | 1.25 | −5.60/−3.90 | PM6 | 0.804 | 27.72 | 0.7635 | 17.02 | 186 |
| EHBzS-4F | 1.24 | −5.61/−3.86 | PM6 | 0.825 | 27.58 | 0.7007 | 15.94 | 186 |
| PN6SBO-4F | 1.28 | −5.63/−3.87 | PM6 | 0.825 | 23.12 | 0.6665 | 12.73 | 187 |
| AN6SBO-4F | 1.27 | −5.60/−3.88 | PM6 | 0.822 | 16.06 | 0.6298 | 8.32 | 187 |
| EHN6SEH-4F | 1.29 | −5.59/−3.89 | PM6 | 0.809 | 28.83 | 0.7464 | 17.48 | 187 |
K.-Y. Jen exploited a series of Se-substituted BTA-based SMAs, and systematically investigated the effects of alkyl chains on nitrogen of BTA-based SMAs on molecular packing and device performance. First, they replaced the 2-ethylhexyl in the BTA unit of selenophene-fused EHBzS-4F with a methyl group to get mBzS-4F. Compared with EHBzS-4F, mBzS-4F delivered intense absorption in the 300–900 nm region in films, downshifted LUMO values, stronger π–π interaction and favorable crystallinity. The device based on mBzS-4F achieved an efficiency of 17.02% as well as a high JSC (27.72 mA cm−2), which are better than those of the EHBzS-4F-based device (PCE = 15.94% and JSC = 27.58 mA cm−2).186 Furthermore, they replaced 2-butyloctyl in EHBzS-4F with 2-ethylhexyl to develop EHN6SEH-4F, and a better PCE of 17.48% was achieved, originating from the effective charge-transporting behaviors in 3D networks.187
4. Conclusion and outlook
Side-chain engineering is of significant importance for developing high-performance nonfullerene SMAs in the field of OSCs. Side chains of SMAs not only act as soluble groups, but also have an important influence on interchain/intermolecular stacking properties and the photoelectric efficiency of the resulting SMAs. To date, binary OSCs based on newly reported SMAs via side chain optimization have achieved PCEs of over 18%. Despite this good progress, some aspects require further research, such as the design and synthesis of novel small-molecule electron acceptors with some specific side chains, and the systematic investigation of the relationship among structure, morphology, performance, and stability. For future development of side-chain engineering in SMAs, the following research guidelines are worth considering from the perspective of molecular design.(1) Developing SMAs with hydrogen-bond-assisted molecular packing via aryl side chains. Very recently, our group demonstrated that BTP-PhC6 with 3-hexylphenyl side chains shows hydrogen-bond-assisted 3D network packing; however, there is no intermolecular hydrogen bond in the 3D crystal stacking of Y6 (Fig. 17). Compared with Y6, hydrogen bonds endow BTP-PhC6 with enhanced π⋯π stacking between two close end groups and larger electronic coupling, thus resulting in better FF and PCE in BTP-PhC6-based devices.82 With Y6 and BTP-PhC6 as parent acceptors, we developed an asymmetric acceptor (BTP-PhC6-C11) by replacing one side chain on thiophene of Y6 with 3-hexylphenyl in BTP-PhC6. Thanks to the synergistic impact of small torsion angles (originated from Y6) and hydrogen-bond interactions (originated from BTP-PhC6), the asymmetric acceptor exhibited the closest π⋯π packing between two end groups and a more obvious (001) diffraction signal in the IP direction, contributing to the charge transport along molecular backbones of the asymmetric acceptor. Finally, the OSCs based on the asymmetric acceptor BTP-PhC6-C11 yielded a distinguished efficiency of 18.33%.
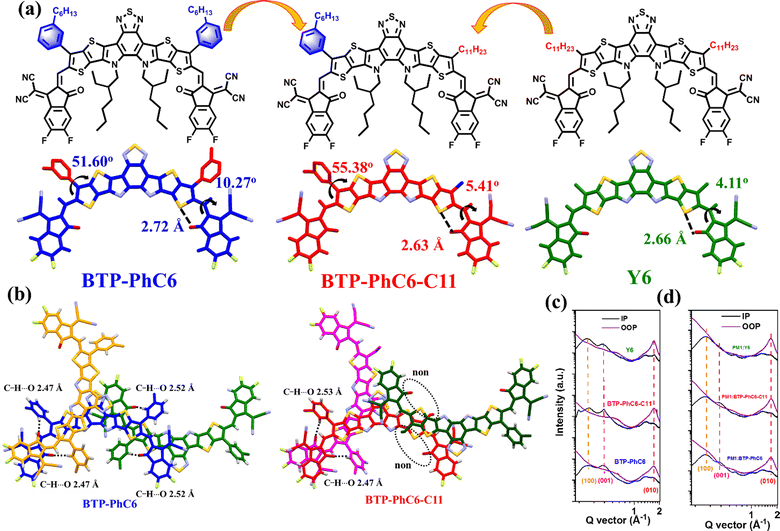 | ||
| Fig. 17 (a) Molecular and monomolecular single crystallographic structures of asymmetric BTP-PhC6-C11, and symmetric Y6, and BTP-PhC6; (b) hydrogen bonding interactions of C_H⋯O in BTP-PhC6-C11 and BTP-PhC6; the IP and OOP line-cut profiles of (c) neat BTP-PhC6-C11, Y6, BTP-PhC6 films and (d) PM1:acceptor blends. Adapted with permission.82 Copyright 2022, Royal Society of Chemistry. | ||
(2) Developing SMAs with nitrogen-containing side chains. The nitrogen atom in the diphenylamine side chains can form an N⋯S conformational lock with the S atom on the adjacent thiophene, which can improve the planarity of the molecule, thereby increasing the electron mobility of the acceptors. Therefore, choosing suitable nitrogen-containing side chains is a good method to boost the photovoltaic performance.
(3) Developing SMAs containing functional groups (hydroxyl, acetoxy groups, carboxyl, etc.) in side chains. He et al. reported a polymer donor NTI-OAc with acetoxy groups as the terminal units of the side chains.188 NTI-OAc-based quasiplanar heterojunction OSCs realized high device efficiency (16.53%) and quite superior device stability.
(4) Developing SMAs with more π–π packing motifs. Optimizing side chains can increase the number of π–π packing motifs in the resulting SMAs, creating additional charge-hopping pathways, thereby resulting in better molecular packing in films and enhanced charge mobility.
(5) Developing non-fused-ring SMAs with bulky side chains. The bulky side chains can usually make the central core of non-fused-ring SMAs smoother due to the effect of steric hindrance, which is beneficial to the electron transport and the intermolecular packing between two central cores. While for fused-ring SMAs, large steric side chains are unfavorable for molecular stacking between two end groups.
(6) Developing SMAs with a suitable length of alkyl side chains. When the alkyl chains are too short, the SMAs can have poor solubility and be challenging to process in solution. On the other hand, if the alkyl chains are too long, the material can be prone to crystallization, leading to significant phase separation between the donor and the acceptor components.72 Recently, Tang and co-workers found that longer side chains in SMAs are beneficial in reducing non-radiative recombination loss and increasing the VOC of the device without affecting the dissociation efficiency of the excitons, implying the importance of longer side chains in developing high-performance SMAs.189 Therefore, it is essential to find a balance in the length of the alkyl chains to optimize the material's solubility and crystallization/aggregation behavior.
Overall, side-chain engineering is a useful, important and convenient approach to optimize the chemical structure of small-molecule acceptors, so as to realize organic photovoltaic devices with high efficiency and good stability. We believe that evolving SMAs through side-chain engineering, together with innovations in donor materials, device process optimization, interface selection, and packaging technology improvements, will bring us closer to the commercialization of organic solar cells.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work described in this paper was supported by the Shenzhen Science and Technology Program (ZDSYS20210623091813040 and RCBS20221008093225021).References
- P. Cheng and X. Zhan, Chem. Soc. Rev., 2016, 45, 2544 RSC.
- J. Zhang, H. S. Tan, X. Guo, A. Facchetti and H. Yan, Nat. Energy, 2018, 3, 720 CrossRef CAS.
- X. Wan, C. Li, M. Zhang and Y. Chen, Chem. Soc. Rev., 2020, 49, 2828 RSC.
- Y. Lin and X. Zhan, Acc. Chem. Res., 2016, 49, 175 CrossRef CAS PubMed.
- J. Wang, P. Xue, Y. Jiang, Y. Huo and X. Zhan, Nat. Rev. Chem., 2022, 6, 614 CrossRef PubMed.
- G. Zhang, F. R. Lin, F. Qi, T. Heumüller, A. Distler, H.-J. Egelhaaf, N. Li, P. C. Y. Chow, C. J. Brabec, A. K.-Y. Jen and H.-L. Yip, Chem. Rev., 2022, 122, 14180 CrossRef CAS PubMed.
- J. Hou, O. Inganas, R. H. Friend and F. Gao, Nat. Mater., 2018, 17, 119 CrossRef CAS PubMed.
- L. Ye, H. Hu, M. Ghasemi, T. Wang, B. A. Collins, J.-H. Kim, K. Jiang, J. H. Carpenter, H. Li, Z. Li, T. McAfee, J. Zhao, X. Chen, J. L. Y. Lai, T. Ma, J.-L. Bredas, H. Yan and H. Ade, Nat. Mater., 2018, 17, 253 CrossRef CAS PubMed.
- Y. Lin, J. Wang, Z.-G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, Y. Li and Y. Zou, Joule, 2019, 3, 1140 CrossRef CAS.
- J. Yuan, T. Huang, P. Cheng, Y. Zou, H. Zhang, J. L. Yang, S.-Y. Chang, Z. Zhang, W. Huang, R. Wang, D. Meng, F. Gao and Y. Yang, Nat. Commun., 2019, 10, 570 CrossRef CAS PubMed.
- Y. Li, Acc. Chem. Res., 2012, 45, 723 CrossRef CAS PubMed.
- T. Xu, J. Lv, K. Yang, Y. He, Q. Yang, H. Chen, Q. Chen, Z. Liao, Z. Kan, T. Duan, K. Sun, J. Ouyang and S. Lu, Energy Environ. Sci., 2021, 14, 5366 RSC.
- Y. Liu, B. Liu, C.-Q. Ma, F. Huang, G. Feng, H. Chen, J. Hou, L. Yan, Q. Wei, Q. Luo, Q. Bao, W. Ma, W. Liu, W. Li, X. Wan, X. Hu, Y. Han, Y. Li, Y. Zhou, Y. Zou, Y. Chen, Y. Li, Y. Chen, Z. Tang, Z. Hu, Z.-G. Zhang and Z. Bo, Sci. China Chem., 2022, 65, 224 CrossRef CAS.
- Q. Bai, Q. Liang, H. Li, H. Sun, X. Guo and L. Niu, Aggregate, 2022, 3, e281 CAS.
- C.-Y. Liao, Y. Chen, C.-C. Lee, G. Wang, N.-W. Teng, C.-H. Lee, W.-L. Li, Y.-K. Chen, C.-H. Li, H.-L. Ho, P. H.-S. Tan, B. Wang, Y.-C. Huang, R. M. Young, M. R. Wasielewski, T. J. Marks, Y.-M. Chang and A. Facchetti, Joule, 2020, 4, 189 CrossRef CAS.
- S. Li, L. Zhan, Y. Jin, G. Zhou, T.-K. Lau, R. Qin, M. Shi, C.-Z. Li, H. Zhu, X. Lu, F. Zhang and H. Chen, Adv. Mater., 2020, 32, 2001160 CrossRef CAS PubMed.
- Y. Zhang, Y. Ji, Y. Zhang, W. Zhang, H. Bai, M. Du, H. Wu, Q. Guo and E. Zhou, Adv. Funct. Mater., 2022, 32, 2205115 CrossRef CAS.
- B. Li, X. Zhang, Z. Wu, J. Yang, B. Liu, Q. Liao, J. Wang, K. Feng, R. Chen, H. Y. Woo, F. Ye, L. Niu, X. Guo and H. Sun, Sci. China Chem, 2022, 65, 1157 CrossRef CAS.
- Z. Luo, R. Ma, J. Yu, H. Liu, T. Liu, F. Ni, J. Hu, Y. Zou, A. Zeng, C.-J. Su, U.-S. Jeng, X. Lu, F. Gao, C. Yang and H. Yan, Natl. Sci. Rev., 2022, 9, nwac076 CrossRef CAS PubMed.
- Z. Xiao, S. Yang, Z. Yang, J. Yang, H. L. Yip, F. Zhang, F. He, T. Wang, J. Wang, Y. Yuan, H. Yang, M. Wang and L. Ding, Adv. Mater., 2019, 31, 1804790 CrossRef CAS PubMed.
- X. Gu, J. Gao, Z. Han, Y. Shi, Y. Wei, Y. Zhang, Q. Peng, Z. Wei, X. Zhang and H. Huang, Chin. J. Polym. Sci., 2023, 41, 556 CrossRef CAS.
- L. Meng, Y. Zhang, X. Wan, C. Li, X. Zhang, Y. Wang, X. Ke, Z. Xiao, L. Ding, R. Xia, H.-L. Yip, Y. Cao and Y. Chen, Science, 2018, 361, 1094 CrossRef CAS PubMed.
- R. Ma, C. Yan, J. Yu, T. Liu, H. Liu, Y. Li, J. Chen, Z. Luo, B. Tang, X. Lu, G. Li and H. Yan, ACS Energy Lett, 2022, 7, 2547 CrossRef CAS.
- W. Gao, F. Qi, Z. Peng, F. R. Lin, K. Jiang, C. Zhong, W. Kaminsky, Z. Guan, C. S. Lee, T. J. Marks, H. Ade and A. K. Jen, Adv. Mater., 2022, 34, 2202089 CrossRef CAS PubMed.
- B. Fan, D. Zhang, M. Li, W. Zhong, Z. Zeng, L. Ying, F. Huang and Y. Cao, Sci. China: Chem., 2019, 62, 746 CrossRef CAS.
- Z. Luo, T. Liu, J. Oh, R. Ma, J. Miao, F. Ni, G. Zhang, R. Sun, C. Zhang, Z. Chen, Y. Zou, J. Min, C. Yang, H. Yan and C. Yang, Adv. Funct. Mater., 2022, 32, 2203200 CrossRef CAS.
- H. B. Naveed and W. Ma, Joule, 2018, 2, 621 CrossRef CAS.
- O. V. Mikhnenko, P. W. M. Blom and T.-Q. Nguyen, Energy Environ. Sci., 2015, 8, 1867 RSC.
- H. Lai, Q. Zhao, Z. Chen, H. Chen, P. Chao, Y. Zhu, Y. Lang, N. Zhen, D. Mo, Y. Zhang and F. He, Joule, 2020, 4, 688 CrossRef CAS.
- R. Ma, C. Yan, P. W.-K. Fong, J. Yu, H. Liu, J. Yin, J. Huang, X. Lu, H. Yan and G. Li, Energy Environ. Sci., 2022, 15, 2479 RSC.
- Z. Zhou, S. Xu, J. Song, Y. Jin, Q. Yue, Y. Qian, F. Liu, F. Zhang and X. Zhu, Nat. Energy, 2018, 3, 952 CrossRef CAS.
- Z. Zhang, Y. Li, G. Cai, Y. Zhang, X. Lu and Y. Lin, J. Am. Chem. Soc., 2020, 142, 18741 CrossRef CAS PubMed.
- N. Gasparini, M. Salvador, S. Strohm, T. Heumueller, I. Levchuk, A. Wadsworth, J. H. Bannock, J. C. de Mello, H. J. Egelhaaf, D. Baran, I. McCulloch and C. J. Brabec, Adv. Energy Mater, 2017, 7, 1700770 CrossRef.
- Y. Jiang, X. Dong, L. Sun, T. Liu, F. Qin, C. Xie, P. Jiang, L. Hu, X. Lu, X. Zhou, W. Meng, N. Li, C. J. Brabec and Y. Zhou, Nat. Energy, 2022, 7, 352 CrossRef CAS.
- R. Ma, K. Zhou, Y. Sun, T. Liu, Y. Kan, Y. Xiao, T. A. D. Peña, Y. Li, X. Zou, Z. Xing, Z. Luo, K. S. Wong, X. Lu, L. Ye, H. Yan and K. Gao, Matter, 2022, 5, 725 CrossRef CAS.
- Z. Luo, T. Liu, H. Yan, Y. Zou and C. Yang, Adv. Funct. Mater., 2020, 30, 2004477 CrossRef CAS.
- C. Yan, J. Qin, Y. Wang, G. Li and P. Cheng, Adv. Energy Mater, 2022, 12, 2201087 CrossRef CAS.
- Q. Fan, R. Ma, W. Su, Q. Zhu, Z. Luo, K. Chen, Y. Tang, F. Lin, Y. Li, H. Yan, C. Yang, A. K. Jen and W. Ma, Carbon Energy, 2022, 5, e267 Search PubMed.
- Z. Luo, T. Liu, R. Ma, Y. Xiao, L. Zhan, G. Zhang, H. Sun, F. Ni, G. Chai, J. Wang, C. Zhong, Y. Zou, X. Guo, X. Lu, H. Chen, H. Yan and C. Yang, Adv. Mater., 2020, 32, 2005942 CrossRef CAS PubMed.
- Y. Li, J. D. Lin, X. Che, Y. Qu, F. Liu, L. S. Liao and S. R. Forrest, J. Am. Chem. Soc., 2017, 139, 17114 CrossRef CAS PubMed.
- L. Yan, H. Zhang, Q. An, M. Jiang, A. Mahmood, M. H. Jee, H.-R. Bai, H.-F. Zhi, S. Zhang, H. Y. Woo and J.-L. Wang, Angew. Chem., Int. Ed., 2022, 134, e202209454 Search PubMed.
- H. Sun, X. Guo and A. Facchetti, Chem, 2020, 6, 1310 CAS.
- Z. Luo, R. Ma, Z. Chen, Y. Xiao, G. Zhang, T. Liu, R. Sun, Q. Zhan, Y. Zou, C. Zhong, Y. Chen, H. Sun, G. Chai, K. Chen, X. Guo, J. Min, X. Lu, C. Yang and H. Yan, Adv. Energy Mater., 2020, 10, 2002649 CrossRef CAS.
- C. Yan, S. Barlow, Z. Wang, H. Yan, A. K. Y. Jen, S. R. Marder and X. Zhan, Nat. Rev. Mater, 2018, 3, 18003 CrossRef CAS.
- G. Zhang, J. Zhao, P. C. Y. Chow, K. Jiang, J. Zhang, Z. Zhu, J. Zhang, F. Huang and H. Yan, Chem. Rev., 2018, 118, 3447 CrossRef CAS PubMed.
- X. Li, F. Pan, C. Sun, M. Zhang, Z. Wang, J. Du, J. Wang, M. Xiao, L. Xue, Z. G. Zhang, C. Zhang, F. Liu and Y. Li, Nat. Commun., 2019, 10, 519 CrossRef CAS PubMed.
- Z. Luo, T. Liu, Y. Wang, G. Zhang, R. Sun, Z. Chen, C. Zhong, J. Wu, Y. Chen, M. Zhang, Y. Li and C. Yang, Adv. Energy Mater., 2019, 9, 1900041 CrossRef.
- V. Coropceanu, X.-K. Chen, T. Wang, Z. Zheng and J.-L. Brédas, Nat. Rev. Mater., 2019, 4, 689 CrossRef.
- G. J. Hedley, A. Ruseckas and I. D. Samuel, Chem. Rev., 2017, 117, 796 CrossRef CAS PubMed.
- K. Feng, H. Guo, J. Wang, Y. Shi, Z. Wu, M. Su, X. Zhang, J. H. Son, H. Y. Woo and X. Guo, J. Am. Chem. Soc., 2021, 143, 1539 CrossRef CAS PubMed.
- J. Yan, X. Rodrıguez-Martınez, D. Pearce, H. Douglas, D. Bili, M. Azzouzi, F. Eisner, A. Virbule, E. Rezasoltani, V. Belova, B. Dorling, S. Few, A. A. Szumska, X. Hou, G. Zhang, H. Lap Yip, M. Campoy-Quiles and J. Nelson, Energy Environ. Sci., 2022, 15, 2958 RSC.
- Y. Shi, Y. Chang, K. Lu, Z. Chen, J. Zhang, Y. Yan, D. Qiu, Y. Liu, M. A. Adil, W. Ma, X. Hao, L. Zhu and Z. Wei, Nat. Commun., 2022, 13, 3256 CrossRef CAS PubMed.
- T. Xu, J. Lv, Z. Chen, Z. Luo, G. Zhang, H. Liu, H. Huang, D. Hu, X. Lu, S. Lu and C. Yang, Adv. Funct. Mater., 2022, 33, 2210549 CrossRef.
- H. Lai, H. Chen, J. Zhou, J. Qu, P. Chao, T. Liu, X. Chang, N. Zheng, Z. Xie and F. He, iScience, 2019, 17, 302 CrossRef CAS PubMed.
- C. Lee, S. Lee, G. U. Kim, W. Lee and B. J. Kim, Chem. Rev., 2019, 119, 8028 CrossRef CAS PubMed.
- X. Xu, Y. Li and Q. Peng, Adv. Mater., 2022, 34, 2107476 CrossRef CAS PubMed.
- J. Xu, S. B. Jo, X. Chen, G. Zhou, M. Zhang, X. Shi, F. Lin, L. Zhu, T. Hao, K. Gao, Y. Zou, X. Su, W. Feng, A. K.-Y. Jen, Y. Zhang and F. Liu, Adv. Mater., 2022, 34, 2108317 CrossRef CAS PubMed.
- K.-N. Zhang, X.-Y. Du, Z.-H. Chen, T. Wang, Z.-Q. Yang, H. Yin, Y. Yang, W. Qin and X.-T. Hao, Adv. Energy Mater., 2022, 12, 2103371 CrossRef CAS.
- M. Jiang, H.-F. Zhi, B. Zhang, C. Yang, A. Mahmood, M. Zhang, H. Y. Woo, F. Zhang, J.-L. Wang and Q. An, ACS Energy Lett., 2023, 8, 1058 CrossRef CAS.
- A. Classen, C. L. Chochos, L. Lüer, V. G. Gregoriou, J. Wortmann, A. Osvet, K. Forberich, I. McCulloch, T. Heumüller and C. J. Brabec, Nat. Energy, 2020, 5, 711 CrossRef CAS.
- K. Chong, X. Xu, H. Meng, J. Xue, L. Yu, W. Ma and Q. Peng, Adv. Mater., 2022, 34, 2109516 CrossRef CAS PubMed.
- Y. Cui, Y. Xu, H. Yao, P. Bi, L. Hong, J. Zhang, Y. Zu, T. Zhang, J. Qin, J. Ren, Z. Chen, C. He, X. Hao, Z. Wei and J. Hou, Adv. Mater., 2021, 33, 2102420 CrossRef CAS PubMed.
- L. Zhu, M. Zhang, J. Xu, C. Li, J. Yan, G. Zhou, W. Zhong, T. Hao, J. Song, X. Xue, Z. Zhou, R. Zeng, H. Zhu, C.-C. Chen, R. C. I. MacKenzie, Y. Zou, J. Nelson, Y. Zhang, Y. Sun and F. Liu, Nat. Mater., 2022, 21, 656 CrossRef CAS PubMed.
- C. He, Y. Pan, Y. Ouyang, Q. Shen, Y. Gao, K. Yan, J. Fang, Y. Chen, C.-Q. Ma, J. Min, C. Zhang, L. Zuo and H. Chen, Energy Environ. Sci., 2022, 15, 2537 RSC.
- L. Zuo, S. B. Jo, Y. Li, Y. Meng, R. J. Stoddard, Y. Liu, F. Lin, X. Shi, F. Liu, H. W. Hillhouse, D. S. Ginger, H. Chen and A. K. Y. Jen, Nat. Nanotechnol., 2022, 17, 53 CrossRef CAS PubMed.
- F. Eisner and J. Nelson, Joule, 2021, 5, 1319 CrossRef.
- M. Moser, A. Wadsworth, N. Gasparini and I. McCulloch, Adv. Energy Mater., 2021, 11, 2100056 CrossRef CAS.
- G. Bernardo, T. Lopes, D. G. Lidzey and A. Mendes, Adv. Energy Mater., 2021, 11, 2100342 CrossRef CAS.
- T. Liu, K. Zhou, R. Ma, L. Zhang, C. Huang, Z. Luo, H. Zhu, S. Yao, C. Yang, B. Zou and L. Ye, Aggregate, 2023, 4, e308 Search PubMed.
- T. Wang, G. Kupgan and J.-L. Brédas, Trends Chem., 2020, 2, 535 CrossRef CAS.
- C. Li, J. Zhou, J. Song, J. Xu, H. Zhang, X. Zhang, J. Guo, L. Zhu, D. Wei, G. Han, J. Min, Y. Zhang, Z. Xie, Y. Yi, H. Yan, F. Gao, F. Liu and Y. Sun, Nat. Energy, 2021, 6, 605 CrossRef CAS.
- L. Zhan, S. Li, Y. Li, R. Sun, J. Min, Y. Chen, J. Fang, C.-Q. Ma, G. Zhou, H. Zhu, L. Zuo, H. Qiu, S. Yin and H. Chen, Adv. Energy Mater., 2022, 12, 2201076 CrossRef CAS.
- Z. Chen, X. Chen, Z. Jia, G. Zhou, J. Xu, Y. Wu, X. Xia, X. Li, X. Zhang, C. Deng, Y. Zhang, X. Lu, W. Liu, C. Zhang, Y. Yang and H. Zhu, Joule, 2021, 5, 1832 CrossRef CAS.
- X. Xia, T.-K. Lau, X. Guo, Y. Li, M. Qin, K. Liu, Z. Chen, X. Zhan, Y. Xiao, P. F. Chan, H. Liu, L. Xu, G. Cai, N. Li, H. Zhu, G. Li, Y. Zhu, T. Zhu, X. Zhan, X.-L. Wang and X. Lu, Nat. Commun., 2021, 12, 6226 CrossRef CAS PubMed.
- T. P. Chaney, A. J. Levin, S. A. Schneider and M. F. Toney, Mater. Horiz., 2022, 9, 43 RSC.
- S. Liu, J. Yuan, W. Deng, M. Luo, Y. Xie, Q. Liang, Y. Zou, Z. He, H. Wu and Y. Cao, Nat. Photonics, 2020, 14, 300 CrossRef CAS.
- T. Liu, R. Ma, Z. Luo, Y. Guo, G. Zhang, Y. Xiao, T. Yang, Y. Chen, G. Li, Y. Yi, X. Lu, H. Yan and B. Tang, Energy Environ. Sci., 2020, 13, 2115 RSC.
- L. Zhan, S. Yin, Y. Li, S. Li, T. Chen, R. Sun, J. Min, G. Zhou, H. Zhu, Y. Chen, J. Fang, C.-Q. Ma, X. Xia, X. Lu, H. Qiu, W. Fu and H. Chen, Adv. Mater., 2022, 34, 2206269 CrossRef CAS PubMed.
- R. Sun, Y. Wu, X. Yang, Y. Gao, Z. Chen, K. Li, J. Qiao, T. Wang, J. Guo, C. Liu, X. Hao, H. Zhu and J. Min, Adv. Mater., 2022, 34, 2110147 CrossRef CAS PubMed.
- Y. Wei, Z. Chen, G. Lu, N. Yu, C. Li, J. Gao, X. Gu, X. Hao, G. Lu, Z. Tang, J. Zhang, Z. Wei, X. Zhang and H. Huang, Adv. Mater., 2022, 34, 2204718 CrossRef CAS PubMed.
- Z. Luo, Y. Gao, H. Lai, Y. Li, Z. Wu, Z. Chen, R. Sun, J. Ren, C. Zhang, F. He, H. Woo, J. Min and C. Yang, Energy Environ. Sci., 2022, 15, 4601 RSC.
- G. Chai, Y. Chang, Z. Peng, Y. Jia, X. Zou, D. Yu, H. Yu, Y. Chen, P. C. Y. Chow, K. S. Wong, J. Zhang, H. Ade, L. Yang and C. Zhan, Nano Energy, 2020, 76, 105807 CrossRef.
- G. Chai, Y. Chang, J. Zhang, X. Xu, L. Yu, X. Zou, X. Li, Y. Chen, S. Luo, B. Liu, F. Bai, Z. Luo, H. Yu, J. Liang, T. Liu, K. S. Wong, H. Zhou, Q. Peng and H. Yan, Energy Environ. Sci., 2021, 14, 3469 RSC.
- Y. Cui, H. Yao, J. Zhang, K. Xian, T. Zhang, L. Hong, Y. Wang, Y. Xu, K. Ma, C. An, C. He, Z. Wei, F. Gao and J. Hou, Adv. Mater., 2020, 32, 1908205 CrossRef CAS PubMed.
- C. Li, H. Fu, T. Xia and Y. Sun, Adv. Energy Mater., 2019, 9, 1900999 CrossRef.
- D. Li, C. Sun, T. Yan, J. Yuan and Y. Zou, ACS Cent. Sci., 2021, 7, 1787 CrossRef CAS PubMed.
- J. Huang, H. Tang, C. Yan and G. Li, Cell Rep. Phys. Sci., 2021, 2, 100292 CrossRef CAS.
- S. Suman and S. P. Singh, J. Mater. Chem. A, 2019, 7, 22701 RSC.
- Z.-Q. Jiang, T.-T. Wang, F.-P. Wu, J.-D. Lin and L.-S. Liao, J. Mater. Chem. A, 2018, 6, 17256 RSC.
- F. Shen, J. Xu, X. Li and C. Zhan, J. Mater. Chem. A, 2018, 6, 15433 RSC.
- W. Liu, X. Xu, J. Yuan, M. Leclerc, Y. Zou and Y. Li, ACS Energy Lett., 2021, 6, 598 CrossRef CAS.
- Y. Lin, F. Zhao, Q. He, L. Huo, Y. Wu, T. C. Parker, W. Ma, Y. Sun, C. Wang, D. Zhu, A. J. Heeger, S. R. Marder and X. Zhan, J. Am. Chem. Soc., 2016, 138, 4955 CrossRef CAS PubMed.
- W. Gao, Q. An, R. Ming, D. Xie, K. Wu, Z. Luo, Y. Zou, F. Zhang and C. Yang, Adv. Funct. Mater., 2017, 27, 1702194 CrossRef.
- Y. Yang, Z. G. Zhang, H. Bin, S. Chen, L. Gao, L. Xue, C. Yang and Y. Li, J. Am. Chem. Soc., 2016, 138, 15011 CrossRef CAS PubMed.
- Z. Zhang, L. Feng, S. Xu, Y. Liu, H. Peng, Z. G. Zhang, Y. Li and Y. Zou, Adv. Sci., 2017, 4, 1700152 CrossRef PubMed.
- M. Chang, Y. Wang, Y.-Q.-Q. Yi, X. Ke, X. Wan, C. Li and Y. Chen, J. Mater. Chem. A, 2018, 6, 8586 RSC.
- S. Lee, K. H. Park, J. H. Lee, H. Back, M. J. Sung, J. Lee, J. Kim, H. Kim, Y. H. Kim, S. K. Kwon and K. Lee, Adv. Energy Mater., 2019, 9, 1900044 CrossRef.
- B. Zhao, W. Wang, J. Xin, H. Wu, H. Liu, Z. Guo, Z. Cong, W. Ma and C. Gao, ACS Sustainable Chem. Eng., 2018, 6, 2177 CrossRef CAS.
- C. Zhang, S. Feng, Y. Liu, R. Hou, Z. Zhang, X. Xu, Y. Wu and Z. Bo, ACS Appl. Mater. Interfaces, 2017, 9, 33906 CrossRef CAS PubMed.
- J. Lee, E. M. Go, S. Dharmapurikar, J. Xu, S. M. Lee, M. Jeong, K. C. Lee, J. Oh, Y. Cho, C. Zhang, M. Xiao, S. K. Kwak and C. Yang, J. Mater. Chem. A, 2019, 7, 18468 RSC.
- Y. Xin, G. Zeng, J. OuYang, X. Zhao and X. Yang, J. Mater. Chem. C, 2019, 7, 9513 RSC.
- Z. Fei, F. D. Eisner, X. Jiao, M. Azzouzi, J. A. Rohr, Y. Han, M. Shahid, A. S. R. Chesman, C. D. Easton, C. R. McNeill, T. D. Anthopoulos, J. Nelson and M. Heeney, Adv. Mater., 2018, 30, 1705209 CrossRef PubMed.
- L. Ye, K. Weng, J. Xu, X. Du, S. Chandrabose, K. Chen, J. Zhou, G. Han, S. Tan, Z. Xie, Y. Yi, N. Li, F. Liu, J. M. Hodgkiss, C. J. Brabec and Y. Sun, Nat. Commun., 2020, 11, 6005 CrossRef CAS PubMed.
- X. Liu, B. Xie, C. Duan, Z. Wang, B. Fan, K. Zhang, B. Lin, F. J. M. Colberts, W. Ma, R. A. J. Janssen, F. Huang and Y. Cao, J. Mater. Chem. A, 2018, 6, 395 RSC.
- B. Jang, C. Lee, Y. W. Lee, D. Kim, M. A. Uddin, F. S. Kim, B. J. Kim and H. Y. Woo, Chin. J. Chem., 2018, 36, 199 CrossRef CAS.
- Z. Zhang, J. Yu, X. Yin, Z. Hu, Y. Jiang, J. Sun, J. Zhou, F. Zhang, T. P. Russell, F. Liu and W. Tang, Adv. Funct. Mater., 2018, 28, 1705095 CrossRef.
- Z. Zhang, H. Wang, J. Yu, R. Sun, J. Xu, L. Yang, R. Geng, J. Cao, F. Du, J. Min, F. Liu and W. Tang, Chem. Mater., 2020, 32, 1297 CrossRef CAS.
- Z. Zhang, S. Guang, J. Yu, H. Wang, J. Cao, F. Du, X. Wang and W. Tang, Sci. Bull., 2020, 65, 1533 CrossRef CAS PubMed.
- K. Feng, J. Huang, X. Zhang, Z. Wu, S. Shi, L. Thomsen, Y. Tian, H. Y. Woo, C. R. McNeill and X. Guo, Adv. Mater., 2020, 32, 2001476 CrossRef CAS PubMed.
- Y. Lin, Q. He, F. Zhao, L. Huo, J. Mai, X. Lu, C. J. Su, T. Li, J. Wang, J. Zhu, Y. Sun, C. Wang and X. Zhan, J. Am. Chem. Soc., 2016, 138, 2973 CrossRef CAS PubMed.
- X. Li, F. Pan, C. Sun, M. Zhang, Z. Wang, J. Du, J. Wang, M. Xiao, L. Xue, Z. G. Zhang, C. Zhang, F. Liu and Y. Li, Nat. Commun., 2019, 10, 519 CrossRef CAS PubMed.
- X. Li, H. Huang, I. Angunawela, J. Zhou, J. Du, A. Liebman-Pelaez, C. Zhu, Z. Zhang, L. Meng, Z. Xie, H. Ade and Y. Li, Adv. Funct. Mater., 2019, 30, 1906855 CrossRef.
- Z. Luo, C. Sun, S. Chen, Z.-G. Zhang, K. Wu, B. Qiu, C. Yang, Y. Li and C. Yang, Adv. Energy Mater., 2018, 8, 1800856 CrossRef.
- S. Feng, C. Zhang, Y. Liu, Z. Bi, Z. Zhang, X. Xu, W. Ma and Z. Bo, Adv. Mater., 2017, 29, 1703527 CrossRef PubMed.
- Y. Li, N. Zheng, L. Yu, S. Wen, C. Gao, M. Sun and R. Yang, Adv. Mater., 2019, 31, 1807832 CrossRef PubMed.
- Y. Li, L. Yu, L. Chen, C. Han, H. Jiang, Z. Liu, N. Zheng, J. Wang, M. Sun, R. Yang and X. Bao, Innovation, 2021, 2, 100090 Search PubMed.
- C. Han, H. Jiang, P. Wang, L. Yu, J. Wang, J. Han, W. Shen, N. Zheng, S. Wen, Y. Li and X. Bao, Mater. Chem. Front., 2021, 5, 3050 RSC.
- Y. Lin, Z.-G. Zhang, H. Bai, J. Wang, Y. Yao, Y. Li, D. Zhu and X. Zhan, Energy Environ. Sci., 2015, 8, 610 RSC.
- H. Yao, Y. Chen, Y. Qin, R. Yu, Y. Cui, B. Yang, S. Li, K. Zhang and J. Hou, Adv. Mater., 2016, 28, 8283 CrossRef CAS PubMed.
- J. Lee, S. Song, J. Huang, Z. Du, H. Lee, Z. Zhu, S.-J. Ko, T.-Q. Nguyen, J. Y. Kim, K. Cho and G. C. Bazan, ACS Mater. Lett., 2020, 2, 395 CrossRef CAS.
- Y. Liu, M. Li, J. Yang, W. Xue, S. Feng, J. Song, Z. Tang, W. Ma and Z. Bo, Adv. Energy Mater., 2019, 9, 1901280 CrossRef.
- Y. Liu, M. Li, X. Zhou, Q.-Q. Jia, S. Feng, P. Jiang, X. Xu, W. Ma, H.-B. Li and Z. Bo, ACS Energy Lett., 2018, 3, 1832 CrossRef CAS.
- S. Ming, C. Zhang, P. Jiang, Q. Jiang, Z. Ma, J. Song and Z. Bo, ACS Appl. Mater. Interfaces, 2019, 11, 19444 CrossRef CAS PubMed.
- Z. Luo, Y. Zhao, Z. G. Zhang, G. Li, K. Wu, D. Xie, W. Gao, Y. Li and C. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 34146 CrossRef CAS PubMed.
- R. Qiu, Z. Wu, S. Li, H. Jiang, Q. Wang, Y. Chen, X. Liu, L. Zhang and J. Chen, Sci. China Chem., 2021, 64, 1208 CrossRef CAS.
- Y. Liu, Z. Zhang, S. Feng, M. Li, L. Wu, R. Hou, X. Xu, X. Chen and Z. Bo, J. Am. Chem. Soc., 2017, 139, 3356 CrossRef CAS PubMed.
- Z.-G. Zhang and Y. Li, Sci. China Chem., 2014, 58, 192 CrossRef.
- Y. Li, H. Chen, H. Lai, X. lai, T. Rehman, Y. Zhu, Y. Wang, Q. Wu and F. He, ACS Mater. Lett., 2022, 4, 1322 CrossRef CAS.
- Y. Xu, H. Yao, L. Ma, J. Wang and J. Hou, Rep. Prog. Phys., 2020, 83, 082601 CrossRef CAS PubMed.
- J. Wang, W. Wang, X. Wang, Y. Wu, Q. Zhang, C. Yan, W. Ma, W. You and X. Zhan, Adv. Mater., 2017, 29, 1702125 CrossRef PubMed.
- Y. Zhang, Y. Wang, T. Yang, T. Liu, Y. Xiao, X. Lu, H. Yan, Z. Yuan, Y. Chen and Y. Li, ACS Appl. Mater. Interfaces, 2019, 11, 32218 CrossRef CAS PubMed.
- Y. Zhang, L. Shi, T. Yang, T. Liu, Y. Xiao, X. Lu, H. Yan, Z. Yuan, Y. Chen and Y. Li, J. Mater. Chem. A, 2019, 7, 26351 RSC.
- Y. Zhang, Y. Cho, L. Zhong, Y. Wang, B. Huang, Z. Yuan, C. Yang, Y. Chen and Y. Li, Sol. RRL, 2020, 4, 2000071 CrossRef CAS.
- G.-Z. Yuan, H. Fan, S.-S. Wan, Z. Jiang, Y.-Q. Liu, K.-K. Liu, H.-R. Bai, X. Zhu and J.-L. Wang, J. Mater. Chem. A, 2019, 7, 20274 RSC.
- G. Li, J. Wu, J. Fang, X. Guo, L. Zhu, F. Liu, M. Zhang and Y. Li, Chin. J. Chem., 2020, 38, 697 CrossRef CAS.
- T. Liu, W. Gao, Y. Wang, T. Yang, R. Ma, G. Zhang, C. Zhong, W. Ma, H. Yan and C. Yang, Adv. Funct. Mater., 2019, 29, 1902155 CrossRef.
- W. Gao, T. Liu, R. Ming, Z. Luo, K. Wu, L. Zhang, J. Xin, D. Xie, G. Zhang, W. Ma, H. Yan and C. Yang, Adv. Funct. Mater., 2018, 28, 1803128 CrossRef.
- B. Kan, X. Chen, K. Gao, M. Zhang, F. Lin, X. Peng, F. Liu and A. K. Y. Jen, Nano Energy, 2020, 67, 104209 CrossRef CAS.
- X. Chen, B. Kan, Y. Kan, M. Zhang, S. B. Jo, K. Gao, F. Lin, F. Liu, X. Peng, Y. Cao and A. K. Y. Jen, Adv. Funct. Mater., 2020, 30, 1909535 CrossRef CAS.
- D. Su, M.-A. Pan, Z. Liu, T.-K. Lau, X. Li, F. Shen, S. Huo, X. Lu, A. Xu, H. Yan and C. Zhan, Chem. Mater., 2019, 31, 8908 CrossRef CAS.
- D. Liu, L. Yang, Y. Wu, X. Wang, Y. Zeng, G. Han, H. Yao, S. Li, S. Zhang, Y. Zhang, Y. Yi, C. He, W. Ma and J. Hou, Chem. Mater., 2018, 30, 619 CrossRef CAS.
- D. Cai, J. Zhang, J.-Y. Wang, Y. Ma, S. Wan, P. Wang, Z. Wei and Q. Zheng, J. Mater. Chem. A, 2020, 8, 24543 RSC.
- Y. Ma, D. Cai, S. Wan, P. Yin, P. Wang, W. Lin and Q. Zheng, Natl. Sci. Rev., 2020, 7, 1886 CrossRef CAS PubMed.
- Y. Ma, M. Zhang, S. Wan, P. Yin, P. Wang, D. Cai, F. Liu and Q. Zheng, Joule, 2021, 5, 197 CrossRef CAS.
- J. Sun, X. Ma, Z. Zhang, J. Yu, J. Zhou, X. Yin, L. Yang, R. Geng, R. Zhu, F. Zhang and W. Tang, Adv. Mater., 2018, 30, 1707150 CrossRef PubMed.
- H. Feng, X. Song, M. Zhang, J. Yu, Z. Zhang, R. Geng, L. Yang, F. Liu, D. Baran and W. Tang, Mater. Chem. Front., 2019, 3, 702 RSC.
- L. Yang, X. Song, J. Yu, H. Wang, Z. Zhang, R. Geng, J. Cao, D. Baran and W. Tang, J. Mater. Chem. A, 2019, 7, 22279 RSC.
- L. Yang, Z. Hu, Z. Zhang, J. Cao, H. Wang, J. Yu, F. Zhang and W. Tang, J. Mater. Chem. A, 2020, 8, 5458 RSC.
- J. Cao, S. Qu, L. Yang, H. Wang, J. Yu and W. Tang, Sci. Bull., 2020, 65, 1876 CrossRef CAS PubMed.
- J. Cao, H. Wang, S. Qu, J. Yu, L. Yang, Z. Zhang, F. Du and W. Tang, Adv. Funct. Mater., 2020, 30, 2006141 CrossRef CAS.
- H. Huang, Q. Guo, S. Feng, C. Zhang, Z. Bi, W. Xue, J. Yang, J. Song, C. Li, X. Xu, Z. Tang, W. Ma and Z. Bo, Nat. Commun., 2019, 10, 3038 CrossRef PubMed.
- M. Chang, L. Meng, Y. Wang, X. Ke, Y.-Q.-Q. Yi, N. Zheng, W. Zheng, Z. Xie, M. Zhang, Y. Yi, H. Zhang, X. Wan, C. Li and Y. Chen, Chem. Mater., 2020, 32, 2593 CrossRef CAS.
- J. Huang, C.-Y. Gao, X.-H. Fan, X. Zhu and L.-M. Yang, Energy Technol., 2021, 10, 2100912 CrossRef.
- S. Ye, S. Chen, S. Li, Y. Pan, X. Xia, W. Fu, L. Zuo, X. Lu, M. Shi and H. Chen, ChemSusChem, 2021, 14, 3599 CrossRef CAS PubMed.
- J. Lee, S.-J. Ko, H. Lee, J. Huang, Z. Zhu, M. Seifrid, J. Vollbrecht, V. V. Brus, A. Karki, H. Wang, K. Cho, T.-Q. Nguyen and G. C. Bazan, ACS Energy Lett., 2019, 4, 1401 CrossRef CAS.
- X. Zhang, C. Li, L. Qin, H. Chen, J. Yu, Y. Wei, X. Liu, J. Zhang, Z. Wei, F. Gao, Q. Peng and H. Huang, Angew. Chem., Int. Ed., 2021, 60, 17720 CrossRef CAS PubMed.
- H. Huang, Q. Chen, Q. Guo, L. Wang, B. Wu, X. Xu, W. Ma, Z. Tang, C. Li and Z. Bo, Dyes Pigm., 2023, 210, 111033 CrossRef CAS.
- Y. Zhou, M. Li, S. Shen, J. Wang, R. Zheng, H. Lu, Y. Liu, Z. Ma, J. Song and Z. Bo, ACS Appl. Mater. Interfaces, 2021, 13, 1603 CrossRef CAS PubMed.
- Y. Zhou, M. Li, H. Lu, H. Jin, X. Wang, Y. Zhang, S. Shen, Z. Ma, J. Song and Z. Bo, Adv. Funct. Mater., 2021, 31, 2101742 CrossRef CAS.
- Y. N. Chen, M. Li, Y. Wang, J. Wang, M. Zhang, Y. Zhou, J. Yang, Y. Liu, F. Liu, Z. Tang, Q. Bao and Z. Bo, Angew. Chem., Int. Ed., 2020, 59, 22714 CrossRef CAS PubMed.
- L. Ma, S. Zhang, J. Zhu, J. Wang, J. Ren, J. Zhang and J. Hou, Nat. Commun., 2021, 12, 5093 CrossRef CAS PubMed.
- X. Wang, H. Lu, J. Zhou, X. Xu, C. Zhang, H. Huang, J. Song, Y. Liu, X. Xu, Z. Xie, Z. Tang and Z. Bo, ACS Appl. Mater. Interfaces, 2021, 13, 39652 CrossRef CAS PubMed.
- H. Lu, X. Wang, H. Wang, A. Zhang, X. Zheng, N. Yu, Z. Tang, X. Xu, Y. Liu, Y.-N. Chen and Z. Bo, Sci. China Chem., 2022, 65, 594 CrossRef CAS.
- K. Jiang, Q. Wei, J. Y. L. Lai, Z. Peng, H. K. Kim, J. Yuan, L. Ye, H. Ade, Y. Zou and H. Yan, Joule, 2019, 3, 3020 CrossRef CAS.
- Z. Luo, R. Sun, C. Zhong, T. Liu, G. Zhang, Y. Zou, X. Jiao, J. Min and C. Yang, Sci. China Chem., 2020, 63, 361 CrossRef CAS.
- Y. Cui, H. Yao, L. Hong, T. Zhang, Y. Tang, B. Lin, K. Xian, B. Gao, C. An, P. Bi, W. Ma and J. Hou, Natl. Sci. Rev., 2020, 7, 1239 CrossRef CAS PubMed.
- D. Mo, H. Chen, J. Zhou, N. Tang, L. Han, Y. Zhu, P. Chao, H. Lai, Z. Xie and F. He, J. Mater. Chem. A, 2020, 8, 8903 RSC.
- J. Pan, Y. Shi, J. Yu, H. Zhang, Y. Liu, J. Zhang, F. Gao, X. Yu, K. Lu and Z. Wei, ACS Appl. Mater. Interfaces, 2021, 13, 22531 CrossRef CAS PubMed.
- H. Chen, R. Zhang, X. Chen, G. Zeng, L. Kobera, S. Abbrent, B. Zhang, W. Chen, G. Xu, J. Oh, S.-H. Kang, S. Chen, C. Yang, J. Brus, J. Hou, F. Gao, Y. Li and Y. Li, Nat. Energy, 2021, 6, 1045 CrossRef CAS.
- T. Li, K. Wang, G. Cai, Y. Li, H. Liu, Y. Jia, Z. Zhang, X. Lu, Y. Yang and Y. Lin, JACS Au, 2021, 1, 1733 CrossRef CAS PubMed.
- S. Chen, L. Feng, T. Jia, J. Jing, Z. Hu, K. Zhang and F. Huang, Sci. China Chem., 2021, 64, 1192 CrossRef CAS.
- Y. J. Su, H. Nie, C. F. Chang, S. C. Huang, Y. H. Huang, T. W. Chen, K. K. Hsu, T. Y. Lee, H. M. Shih, C. W. Ko, J. T. Chen and C. S. Hsu, ACS Appl. Mater. Interfaces, 2021, 13, 59043 CrossRef CAS PubMed.
- L. Wang, C. Guo, X. Zhang, S. Cheng, D. Li, J. Cai, C. Chen, Y. Fu, J. Zhou, H. Qin, D. Liu and T. Wang, Chem. Mater., 2021, 33, 8854 CrossRef CAS.
- J. Zhang, F. Bai, I. Angunawela, X. Xu, S. Luo, C. Li, G. Chai, H. Yu, Y. Chen, H. Hu, Z. Ma, H. Ade and H. Yan, Adv. Energy Mater., 2021, 11, 2102596 CrossRef CAS.
- Y. Chang, J. Zhang, Y. Chen, G. Chai, X. Xu, L. Yu, R. Ma, H. Yu, T. Liu, P. Liu, Q. Peng and H. Yan, Adv. Energy Mater., 2021, 11, 2100079 CrossRef CAS.
- X. Kong, C. Zhu, J. Zhang, L. Meng, S. Qin, J. Zhang, J. Li, Z. Wei and Y. Li, Energy Environ. Sci., 2022, 15, 2011 RSC.
- P. Tan, L. Liu, Z. Y. Chen, H. Lai, Y. Zhu, H. Chen, N. Zheng, Y. Zhang and F. He, Adv. Funct. Mater., 2021, 31, 2106524 CrossRef CAS.
- B. Fan, W. Gao, R. Zhang, W. Kaminsky, F. R. Lin, X. Xia, Q. Fan, Y. Li, Y. An, Y. Wu, M. Liu, X. Lu, W. J. Li, H.-L. Yip, F. Gao and A. K.-Y. Jen, J. Am. Chem. Soc., 2023, 145, 5909 CrossRef CAS PubMed.
- Y. Chen, F. Bai, Z. Peng, L. Zhu, J. Zhang, X. Zou, Y. Qin, H. K. Kim, J. Yuan, L. K. Ma, J. Zhang, H. Yu, P. C. Y. Chow, F. Huang, Y. Zou, H. Ade, F. Liu and H. Yan, Adv. Energy Mater., 2020, 11, 2003141 CrossRef.
- Y. Chen, R. Ma, T. Liu, Y. Xiao, H. K. Kim, J. Zhang, C. Ma, H. Sun, F. Bai, X. Guo, K. S. Wong, X. Lu and H. Yan, Adv. Energy Mater., 2021, 11, 2003777 CrossRef CAS.
- A. M. H. Cheung, H. Yu, S. Luo, Z. Wang, Z. Qi, W. Zhou, L. Arunagiri, Y. Chang, H. Yao, H. Ade and H. Yan, J. Mater. Chem. A, 2020, 8, 23239 RSC.
- C. Xiao, X. Wang, T. Zhong, R. Zhou, X. Zheng, Y. Liu, T. Hu, Y. Luo, F. Sun, B. Xiao, Z. Liu, C. Yang and R. Yang, Adv. Sci., 2023, 10, 2206580 CrossRef CAS PubMed.
- Z. Li, C. Zhu, J. Yuan, L. Zhou, W. Liu, X. Xia, J. Hong, H. Chen, Q. Wei, X. Lu, Y. Li and Y. Zou, J. Energy Chem., 2022, 65, 173 CrossRef CAS.
- H. Chen, X. Xia, J. Yuan, Q. Wei, W. Liu, Z. Li, C. Zhu, X. Wang, H. Guan, X. Lu, Y. Li and Y. Zou, ACS Appl. Mater. Interfaces, 2021, 13, 36053 CrossRef CAS PubMed.
- F. Qi, K. Jiang, F. Lin, Z. Wu, H. Zhang, W. Gao, Y. Li, Z. Cai, H. Y. Woo, Z. Zhu and A. K. Y. Jen, ACS Energy Lett., 2020, 6, 9 CrossRef.
- F. Qi, L. O. Jones, K. Jiang, S. H. Jang, W. Kaminsky, J. Oh, H. Zhang, Z. Cai, C. Yang, K. L. Kohlstedt, G. C. Schatz, F. R. Lin, T. J. Marks and A. K. Jen, Mater. Horiz., 2022, 9, 403 RSC.
- Y. Li, H. Chen, H. Lai, X. lai, T. Rehman, Y. Zhu, Y. Wang, Q. Wu and F. He, ACS Mater. Lett., 2022, 4, 1322 CrossRef CAS.
- J. Wang, X. Jiang, H. Wu, G. Feng, H. Wu, J. Li, Y. Yi, X. Feng, Z. Ma, W. Li, K. Vandewal and Z. Tang, Nat. Commun., 2021, 12, 6679 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |