Open Access Article
Open Access ArticleDomestic groundwater wells in Appalachia show evidence of low-dose, complex mixtures of legacy pollutants†
Nicolette A.
Bugher
 *a,
Boya
Xiong
*a,
Boya
Xiong
 ad,
Runako I.
Gentles
ad,
Runako I.
Gentles
 a,
Lukas D.
Glist
a,
Lukas D.
Glist
 a,
Helen G.
Siegel
a,
Helen G.
Siegel
 b,
Nicholaus P.
Johnson
b,
Nicholaus P.
Johnson
 c,
Cassandra J.
Clark
c,
Cassandra J.
Clark
 c,
Nicole C.
Deziel
c,
Nicole C.
Deziel
 c,
James E.
Saiers
c,
James E.
Saiers
 b and
Desiree L.
Plata
b and
Desiree L.
Plata
 *a
*a
aMassachusetts Institute of Technology, Department of Civil and Environmental Engineering, Parsons Laboratory, 15 Vassar Street, Cambridge, Massachusetts 02139, USA. E-mail: nbugher@mit.edu; dplata@mit.edu
bYale School of the Environment, Environmental Science Center, 21 Sachem Street, New Haven, Connecticut 06511, USA
cYale School of Public Health, Department of Environmental Health Sciences, 60 College St., New Haven, Connecticut 06510, USA
dUniversity of Minnesota, Department of Civil, Environmental, and Geo-Engineering, 500 Pillsbury Drive S.E., Minneapolis, MN 55455, USA
First published on 6th November 2024
Abstract
Lack of water quality data for private drinking water sources prevents robust evaluation of exposure risk for communities co-located with historically contaminated sites and ongoing industrial activity. Areas of the Appalachian region of the United States (i.e., Pennsylvania, Ohio and West Virginia) contain extensive hydraulic fracturing activity, as well as other extractive and industrial technologies, in close proximity to communities reliant on private drinking water sources, creating concern over potential groundwater contamination. In this study, we characterized volatile organic compound (VOC) occurrence at 307 private groundwater well sites within Pennsylvania, Ohio, and West Virginia. The majority (97%) of water samples contained at least one VOC, while the average number of VOCs detected at a given site was 5 ± 3. The majority of individual VOC concentrations fell below applicable U.S. Environmental Protection Agency (EPA) Maximum Contamination Levels (MCLs), except for chloroform (MCL of 80 μg L−1; n = 1 at 98 μg L−1), 1,2-dibromoethane (MCL of 0.05 μg L−1; n = 3 ranging from 0.05 to 0.35 μg L−1), and 1,2-dibromo-3-chloropropane (MCL of 0.2 μg L−1; n = 7 ranging from 0.20 to 0.58 μg L−1). To evaluate well susceptibility to VOCs from industrial activity, distance to hydraulic fracturing site was used to assess correlations with contaminant occurrences. Proximity to closest hydraulic fracturing well-site revealed no statistically significant linear relationships with either individual VOC concentrations, or frequency of VOC detections. Evaluation of other known industrial contamination sites (e.g., US EPA Superfund sites) revealed elevated levels of three VOCs (chloroform, toluene, benzene) in groundwaters within 10 km of those Superfund sites in West Virginia and Ohio, illuminating possible point source influence. Lack of correlation between VOC concentrations and proximity to specific point sources indicates complex geochemical processes governing trace VOC contamination of private drinking water sources. While individual concentrations of VOCs fell well below recommended human health levels, the low dose exposure to multiple VOCs occurring in drinking supplies for Appalachian communities was noted, highlighting the importance of groundwater well monitoring.
Environmental significanceDomestic groundwater wells in the Appalachian region represent understudied and vulnerable drinking water sources, especially due to the dense co-location of industrial activity in the area (i.e., hydraulic fracturing and legacy industries). The results of this study show elevated detection frequencies of organic industrial chemicals compared to previous national averages, and co-occurrence of multiple, low-level volatile organic compounds in drinking water. The results of this study underscore the need for toxicity assessment methods focusing on multi-contaminant exposures at environmentally relevant levels. This work provides critical, health-relevant data on chemical exposures in primarily unregulated drinking water supplies. |
Introduction
An estimated 43 million people (13% of the United States population) rely on private drinking water sources that remain predominantly unmonitored and unregulated.1 Domestic wells are excluded from the routine monitoring and regulation afforded to public water systems under the Safe Drinking Water Act, which ensures compliance with maximum contamination levels (MCLs) for regulated contaminants. The distributed nature of private drinking water in the United States, absence of routine monitoring, and lack of federal- and state-level regulations on private water sources create potential risk for consumers reliant on private drinking water resources to be exposed to contaminants unknowingly and over long periods of time.A particularly vulnerable consumer group is that of the Appalachian region of the United States, where industrial activity has long been co-located with high rates of domestic well water usage. Specifically, Pennsylvania has the largest population (3.47 million people and 27% of the state's population) using domestic wells of any state, with 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new groundwater wells constructed annually.1,2 Ohio and West Virginia also have dense domestic well reliance at 16% and 21% of the state population,1 respectively; these all exceed the estimated national proportion (13%) of private well users. These domestic wells are not subjected to the same routine monitoring as municipal drinking water supplies. Importantly, Pennsylvania and West Virginia lack state-level domestic water quality regulation entirely,3 while Ohio offers limited protection (e.g., requires testing at sale and new well construction).4
000 new groundwater wells constructed annually.1,2 Ohio and West Virginia also have dense domestic well reliance at 16% and 21% of the state population,1 respectively; these all exceed the estimated national proportion (13%) of private well users. These domestic wells are not subjected to the same routine monitoring as municipal drinking water supplies. Importantly, Pennsylvania and West Virginia lack state-level domestic water quality regulation entirely,3 while Ohio offers limited protection (e.g., requires testing at sale and new well construction).4
The co-location of these water supplies with unconventional oil and gas (UOG) activities, such as horizontal drilling with hydraulic fracturing (HDHF), has raised public concern over the potential impact on groundwater quality. In these Appalachian states, the number of hydraulic fracturing wells has rapidly increased over the past two decades, including over 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new HDHF wells drilled in West Virginia, Pennsylvania, and Ohio since 2000.5 Hydraulic fracturing activities are exempt from the Clean Water Act for underground injection of chemicals related to fossil energy recovery as part of the “Underground Injection Clause” of the 2005 US Energy Act, and this lack of regulations on chemical utilization exacerbates concerns regarding HDHF fluid additives, hydrocarbons, and other organic environmental contaminants entering groundwater aquifers that supply local communities reliant on private drinking water sources.6–8
000 new HDHF wells drilled in West Virginia, Pennsylvania, and Ohio since 2000.5 Hydraulic fracturing activities are exempt from the Clean Water Act for underground injection of chemicals related to fossil energy recovery as part of the “Underground Injection Clause” of the 2005 US Energy Act, and this lack of regulations on chemical utilization exacerbates concerns regarding HDHF fluid additives, hydrocarbons, and other organic environmental contaminants entering groundwater aquifers that supply local communities reliant on private drinking water sources.6–8
Multiple industrial contaminants, specifically volatile organic compounds (VOCs), have been detected in flowback or produced waters and have been found in domestic wells9–11 following accidental release, fugitive gas contamination, surface spills, and faulty storage.8,9,12,13 (Note that flowback and produced waters are subject to the Clean Water Act, but management of their disposal tends to depend on state-level regulations). Additionally, Appalachia has a history of other industrial activities; decreased water quality of private wells has been linked to proximity to pollution sources and industrial activities.2,9,12–14 Industries of the areas (e.g., coal extraction, chemical production) and Superfund Sites on the U.S. EPA National Priority Lists (NPL) with active and historic groundwater contamination15 create potentially compounding sources of organic compound contamination and transformation products. Organic compounds used in HDHF activities and their transformation products10,16,17 are often common to many other industrial processes,18 making it difficult to discern source from chemical identity alone.
Previous studies that have investigated private well quality and hydraulic fracturing activity primarily targeted inorganic contaminants and few to no organic contaminants; few have conducted broad-spectrum analyses on mixtures of organic contaminants or chemicals used for underground injection.19,20 Importantly, even waters with no individual contaminant concentration exceedances of a human health benchmark may still pose risks to human health from combined contaminant concentrations and simultaneous exposures.21 Inadequate chemical mixture occurrence monitoring may be credited to the practical challenges associated with measurement but is necessary for toxicologic study on realistic environmental exposures.21–23 In addition to the weaknesses of single or few-compound assessments for exposure risk analysis, the lack of human health benchmarks for many industrial contaminants impede effective impact evaluation for contaminant occurrences. To address health impacts of individual organic contaminants detected in public drinking water, metrics like EPA MCLs are used as human-health benchmarks for regulatory purposes of individual contaminants. Enforceable health-based standards exist for less than 70 chemical contaminants in drinking water as regulated by the US EPA,24 yet over 1200 compounds with known chemical structures are used in HDHF processes11 and over 3400 unique substances have been identified at other legacy industrial sites (e.g., Superfund sites).25 Crucially, none of these compounds are studied in the ways in which one might be exposed to them: in low doses, at chronic levels, and in mixtures. These have been suggested for both improved toxicological assessments of and exposure potential to hydraulic fracturing fluid releases.23,26,27
This study evaluated domestic groundwater for the potential occurrence of organic compounds with known human health effects disclosed for use in hydraulic fracturing.28 This analysis adds newly sampled and analyzed data from Appalachian areas with hydraulic fracturing activity (e.g., Ohio and West Virginia) to previous studies, which focused on Pennsylvania waters, inorganic components, or health indicators.29–31 We measured regional groundwater quality via individual VOCs and gasoline-range organic (GRO) compounds from three states (Pennsylvania, Ohio, and West Virginia) in the Appalachian Region to probe organic contaminant exposure risk of domestic wells near hydraulic fracturing sites. Additionally, though this study primarily focuses on HDHF activities, we evaluated exposure risk of alternative potential industrial inputs in the study areas, specifically Superfund sites with legacies of VOC contamination. Though occurring less frequently than hydraulic fracturing (i.e., five within sampling all areas), these sites found on the EPA National Priorities List offer environmental contaminant occurrences and release disclosures to compare to potential VOC occurrences in drinking water samples. We explored proximity to two types of potential pollution sites (HDHF well pad or NPL Superfund site) as well as geochemical indicators of groundwater type to probe influences on individual compound occurrence and levels. This study evaluates impacts on groundwater quality across states with varying geology, environmental regulation, and legacy pollution to address potential concerns for public health and inform federal and state regulation on hydraulic fracturing activities. Further, this work provides previously unavailable information on organic water quality of predominantly unmonitored and unregulated water supplies and highlights the importance of developing an understanding of chronic exposure to low doses of chemical mixtures.
Methods
Study area
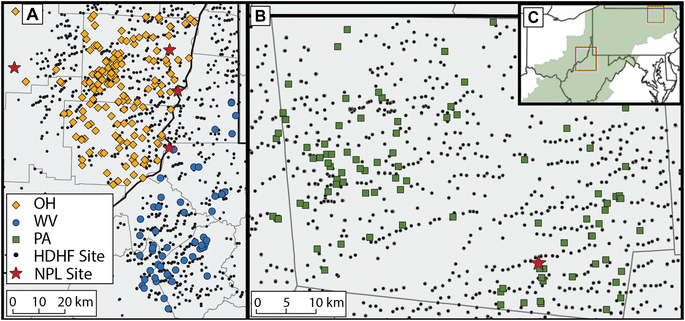
A total of 311 groundwater sources were sampled in Pennsylvania, Ohio, and West Virginia between 2018 and 2020 (Fig. 1). Samples from Pennsylvania (n = 94 sites; n = 89 VOC measurements) were primarily collected in Bradford, PA, as described in Xiong et al. 2022.29 In Ohio, sampling locations (n = 162) were primarily located in Belmont and Monroe Counties. In West Virginia, samples (n = 56) were collected in Ritchie, Doddridge, Tyler, Marshall and Wetzel counties in the northeastern region bordering Ohio. All sampling sites are within regions that contain UOG activities. Study participant recruitment was done with a large-scale recruitment effort described in Clark et al. 2022 and Xiong et al. 2022.29,30Water sample collection
Groundwater samples, taken at the wellhead and upstream of all water treatment systems, were purged until pH, temperature, dissolved oxygen, and specific conductance readings stabilized, and were collected in method-specific containers, as described previously.31–33 Briefly, samples for VOC and GRO analysis were taken in triplicate, pre-combusted, 40 mL sealed glass vials with 1 mL of 50% v/v hydrochloric acid and stored on ice or kept at 4 °C until analysis, following U.S. Geological Survey (USGS) standards.34 Samples were analyzed within two months following collection. Note that this followed EPA Method 8015C, EPA Method 3510C, and the USGS Field Manual for water collection and analysis. Field blanks were collected once per day using 18 M Ohm milli-Q water (with a total organic carbon reduction unit) from the analysis laboratory transported to the field in 4 L, pre-combusted amber jars. Laboratory blanks were analyzed daily during the period of analysis from laboratory 18 M Ohm milli-Q water (with a total organic carbon reduction unit). Authentic standards were used to calibrate the instrument daily. When extractions were conducted, surrogate authentic standards were carried through the entire extraction procedure. Sample selection and collection is detailed in Siegel et al.31 2022. Briefly, samples taken from Pennsylvania between August and September of 2018 were analyzed between September and October 2018; those taken from Ohio between June and August of 2019 were analyzed between June and August of 2019. Similarly, those taken from West Virginia in October of 2020 were analyzed between November and December 2020.Organic compound analysis
Quantification of VOC (n = 59) and GRO compounds (defined as organic compounds with volatilities spanning nC6–nC10) utilized purge and trap extraction (Teledyne Tekmar) paired with gas chromatography and flame ionization detection (GC-FID; Agilent 7890B). Authentic standards were used to confirm compound identities and calibrate the instrument following EPA-method specific requirements.32,33 GRO concentrations were quantified via integration of all compounds eluting between two characteristic compound elution retention times (1-methylpentane and 1,2,4-trimethylbenzene). Integration and analysis for VOC and GRO data was performed in ChemStation (Agilent C.01.08). The limit of detection (LOD) for each compound or group of compounds was determined based on signal-to-noise ratio equivalent to a minimum area of 0.2 pA-minutes and daily 6-point calibration curves. Duplicate calibration standards were analyzed for reproducibility and accepted if percent error was no more than 10%. LOD varied by compound and were primarily less than 0.1 ppb (Table #S1).† Daily laboratory (sampled day of analysis) and field blanks (sampled day of collection) were used for quality control; field blanks from Pennsylvania (n = 31), Ohio (n = 72), and WV (n = 22) were analyzed in tandem with samples. Note that two VOCs coeluted in seven instances within the chemical calibration standard mixture; in these cases, reported means reflect the sum of these coeluting constituents (Table #S1).† Of the 59 target compounds, methylene chloride was routinely detected in laboratory and field blanks; the upper confidence limit (UCL) adjusted limit of detection was similar sample concentrations (See ESI for UCL adjusted LOD determination).† Therefore, all detections of methylene chloride were rejected, and sample contamination bias could not be ruled out. VOCs that had detection frequencies above 10% in blanks were interrogated for potential contamination bias (n = 5; see ESI for further information†) and VOCs that were not detected in the majority of field and laboratory blanks were considered free of contamination bias.Water typing of domestic well samples utilized organic chemical analysis performed for this study and was supplemented by inorganic data for chloride and bromide collected previously in Siegel et al.31 2022 for the sample sites investigated here.
Calculation of spatial metrics
Spatial metric calculations utilized the location of conventional and unconventional production wells from the Pennsylvania Spatial Data Access well database, West Virginia Department of Environmental Protection Oil and Gas Well database, and Ohio Department of Natural Resources Oil and Gas Well database up to the year of sampling. Distances to nearest well were calculated in R (R Statistical Software; R Core Team, 2023) with base tools using exact coordinates of sampling sites and the closest oil and gas well and tested for correlation with chemical concentration using Spearman's Rank Order Correlation in Ohio and West Virginia, supplemented by the analysis in Pennsylvania by Xiong et al. 2022.29Locations of Superfund sites within 20 km of groundwater sampling sites included in the study were gathered from the EPA's National Priority List (NPL) and screened for target analyte release listed within public disclosures (using EPA Geospatial data set from Superfund NPL and the Superfund Enterprise Management System (SEMS)). Of 138 total current Superfund sites within the three states, screening analysis using ArcGIS Online (Esri Inc., Redlands, CA, USA) provided 4 sites within 10 km of Ohio and West Virginia sampling areas and a single site within 10 km of Pennsylvania sampling areas where a compound of interest was disclosed or detected at the site. Distance to superfund site calculations utilized ArcGIS and publicly available NPL data provided by the US EPA.
Results and discussion
Evaluation of individual VOC and mixture occurrences
At least one VOC was detected in 97% of sample (298 of 307) sites. Individual VOC concentrations in drinking water samples were typically low (below 1 μg L−1), with only 3.6% (n = 11) of samples containing a VOC concentration exceeding an EPA MCL. Seven occurrences of 1,2-dibromo-3-chloropropane exceeded the 0.2 μg L−1 limit, ranging from 0.20 to 0.58 μg L−1. For three homes, 1,2-dibromoethane concentrations exceeded the 0.05 μg L−1 limit and ranged from 0.05 to 0.35 μg L−1. Chloroform concentrations exceeded the total trihalomethane (TTHM) limit of 80 μg L−1 in one home at 98 μg L−1. Notably, though only 11 samples had a VOC exceeding an EPA MCL, only 25 of 59 target analytes have applicable human health benchmarks (Table S1†).Furthermore, multi-compound detections (two or more VOCs) were found in 89% (272 of 307) of sampled homes. MCLs for mixtures are rarely provided except in cases where health guidelines on the chemical class were explicitly desired. Such is the case with total trihalomethanes (TTHMs, defined as chloroform, bromodichloromethane, bromoform, and dibromochloromethane at 80 μg L−1); this regulation was promulgated in 1998 for public water supply systems. The approach to regulate mixtures is being discussed or implemented for other classes of related chemical structures (e.g., perfluorinated alkylated substances (PFAs)35 and haloacetic acid groups (HAAs)) under the presumption that compounds with related structures may exhibit similar toxicological profiles. This recognizes that explicit measurement of each individual compound within a class can be challenging and/or benefit from a common analytical method, and that a single regulation will simplify the burden of both passing the legislation and eventual compliance. While this regulatory measure is appealing for these reasons, it is does not address exposures across classes of chemicals. Furthermore, the effect of low-dose exposure to chemical mixtures is poorly understood in toxicology. Nevertheless, it may be a common, influential, and relevant exposure scenario for drinking waters. Therefore, the descriptive strength of using individual contaminant EPA MCLs as a metric for water quality in this study is weakened by both the lack of federal regulations on more than half (34 of 59) of the target analytes and the unknown health impacts of simultaneous exposure to multiple compounds.
This survey of 59 VOCs revealed that the majority of groundwater wells (n = 272) contain more than one contaminant at detectable levels (Fig. 2). Some individual homes (n = 16) had a relatively high number (e.g., greater than 10 out of 50 tested) of compounds detected, where the maximum was 22 unique VOCs detected. Of the VOCs targeted in this study, more volatile compounds (i.e., those with lower boiling points like vinyl chloride and chloromethane) generally had higher rates of occurrences, but the most frequently detected compounds varied by state (discussed below).
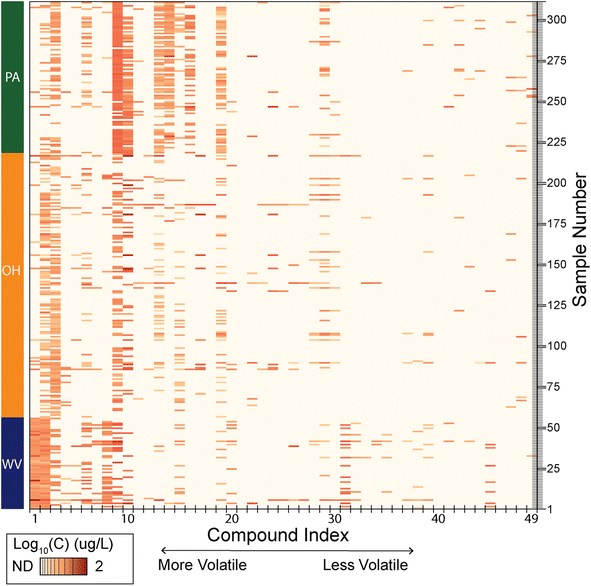 | ||
| Fig. 2 Local variation in compound occurrence in domestic groundwater wells. Due to the broad range in detected concentrations, (0.00001 to 100 μg L−1) logarithm values are used to display concentration intensity. Compound index reflects the chemical identity ordered by retention time and listed in the ESI.† Sample number indicates individual sampling sites and are grouped by state (WV: Sample # 1–56; OH: Sample # 57–218; PA: Sample # 219–311). | ||
VOCs are rarely measured in domestic well drinking water supplies, and broad-spectrum analyses like these are rarely available. These analyses suggest that chemical mixtures, where more than one contaminant is present at low levels, are common in domestic groundwaters in Appalachia. Chemical mixtures may have an underappreciated impact on human health and studies identifying exposure to chemical mixtures, including that of HDHF wastewater, suggest adverse health effects at concentrations expected in the environment (i.e., mixtures of independent concentrations at the μg L−1 level measured here and up to higher concentrations at the mg L−1 level).26,27 A deeper understanding of public health risk associated with exposure to low-dose mixtures of chemicals is needed but is currently intractable as a result of the traditional focus on exposure to single, pure compounds.23,36 To be clear, these studies are critical to inform understanding and remain highly valued; the shortcoming is that they may not represent realistic exposure scenarios. To overcome this shortcoming, it would be useful to have a qualitative description of common chemical clustering (i.e., co-occurring compounds), have a quantitative description of concentration ranges of exposure and to determine if these are universal, regionally specified, or stochastic in nature.
In national groundwater studies (n = 1537 wells representing public and domestic primary groundwater aquifers sampled between 2013 and 2019, n = 1208 domestic wells sampled between 1985 and 2001, and n = 1255 domestic drinking water wells sampled between 1992 and 1999), detection frequencies of 55 to 85 VOCs were between 36 and 50%, much lower than reported here.36–38 Specifically, a study by Bexfield et al. 2022,37 which included a similar range of individual compound detection limits, range of detected concentrations (between 0.002 μg L−1 and 224 μg L−1), and sample handling to this study, reported at least one VOC detected in 38% of wells (577 of 1537) and two or more in only 20% of wells (310 of 1537). To ensure this disparity was not simply a consequence of our improved detection limits, we applied the higher LODs for individual VOCs (0.1 ppb)37 to the measurements collected here and found the detection frequencies of at least one VOC in West Virginia and Ohio decreased to 80% and 49% (from 100 and 94%), respectively, while Pennsylvania remained unchanged (100%). Thus, the persistent and elevated VOC detections across sampling sites were not simply a consequence of enhanced detection capabilities. It is possible that this could be the result of the sheer number of samples collected, the number and identify of VOCs measured, and spatiotemporal variability; alternatively, there could be categorically more impacted waters across Appalachia.
Halogenated solvents were most the frequently detected chemical class in all sampling locations, in agreement with national assessments of VOC detection frequencies in drinking water sources.36–39 In the Appalachian region, notable frequently detected compounds in Pennsylvania (Table 1) include chloroform (76% detection frequency) and trichloroethylene (TCE; 75% detection frequency), two of the most frequently detected organic compounds in groundwater supplies since the 1980s.37,38 Chloroform was the most frequently detected VOC in the national assessment of 55 VOCs from roughly 3500 public and domestic wells between 1991 and 2010 (18% of sampled domestic wells39) and national assessment of 85 VOCs from 1547 public supply wells between 2013 and 2019 (25% of sampled municipal waters37), markedly lower than the overall rate of chloroform detection observed here (36%). Note that chloroform detection frequencies in West Virginia and Ohio were 7 and 21%, respectively. TCE detection frequency was notably high in Pennsylvania (75% detection), but nearly absent in Ohio and West Virginia (each 2%). TCE is a common breakdown product of tetrachloroethylene (PCE), as are cis- and trans-1,2-dichloroethene (cis-1,2-DCE & trans-1,2-DCE), and vinyl chloride; all of which were detected in this region as well. All groundwaters sampled in West Virginia contained vinyl chloride and chloromethane at detectable albeit low levels (see below for discussion). Vinyl chloride was less frequently detected Ohio (57%) and Pennsylvania (26%), as was chloromethane (7 and 3% in Ohio and Pennsylvania, respectively). (Note: utilizing UCL LODs determined for vinyl chloride and chloromethane in West Virginia resulted in lowered detection frequencies of 25 and 50% respectively. See ESI for UCL LOD determination.† Vinyl chloride detection frequency in Ohio decreases to 11% if using the UCL determined LOD.) Intermediate dehalogenation products (cis-1,2-DCE and trans-1,2-DCE) had higher frequencies of detection in West Virginia compared to other sampling areas (cis-1,2-DCE in West Virginia with 63% detection, Ohio with 4%, and Pennsylvania with 1%) (trans-1,2-DCE in West Virginia with 34%, Ohio with 9%, and Pennsylvania with 17%). Both cis-1,2-DCE and trans-1,2-DCE co-eluted with additional compounds (2,2-DCP with cis-1,2-DCE and 1,1-DCE with trans-1,2-DCE) which prevents interrogation of individual compound concentrations in our samples. Nevertheless, there is evidence of TCE presence and potential transformation products in shallow drinking water supply aquifers with variable detection frequencies at the local level. Most recently, Bexfield et al. 2022,37 found positive rates of detection of “solvents” (characterized as the concentration sum of 30 compounds) in less than 20% drinking water aquifers sampled nationally from 2013 to 2019 and trihalomethanes (characterized as the concentration sum of 4 disinfection byproducts (DBPs)) in less than 10%.37 As with differences in VOC detections by state, the increased halogenated solvent detection rates here could be a consequence of multiple factors (discussed above) or may indicate greater effects of local anthropogenic or industrial activity at time of sampling and potential chemical transformations (i.e., TCE degradation) compared to previous national assessments.
| VOC | WV (n = 56) | OH (n = 162) | PA (n = 89) | Total (n = 307) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| a Sum of co-eluting compounds. | ||||||||
| Bromochloromethane | 20 | 36 | 75 | 46 | 86 | 97 | 181 | 59 |
| Bromomethane | 21 | 38 | 109 | 67 | 50 | 56 | 180 | 59 |
| Vinyl chloride | 56 | 100 | 93 | 57 | 23 | 26 | 172 | 56 |
| Chloroform | 7 | 13 | 35 | 22 | 68 | 76 | 110 | 36 |
| 1,2-Dichloroethane & benzenea | 3 | 5 | 39 | 24 | 62 | 70 | 104 | 34 |
| Toluene | 6 | 11 | 33 | 20 | 57 | 64 | 96 | 31 |
| Chloromethane | 56 | 100 | 12 | 7 | 3 | 3 | 71 | 23 |
| Trichloroethene | 1 | 2 | 4 | 2 | 67 | 75 | 72 | 23 |
| Dibromomethane | 0 | 0 | 3 | 2 | 40 | 45 | 43 | 14 |
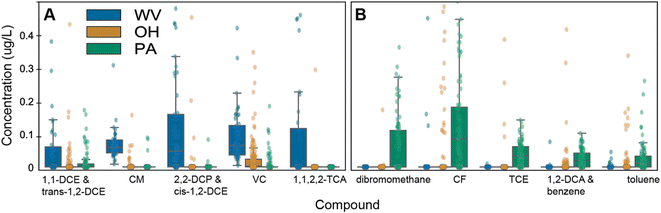
The concentration and types of elevated halogenated VOCs in groundwaters varied by state (Fig. 3). In particular, West Virginia had a predominance of chlorinated VOC concentrations, followed by Ohio, and then Pennsylvania (Fig. 3a). Chloromethane (ranging from 0.008 ± 0.01 to 65.4 ± 2.0 μg L−1) and vinyl chloride concentrations (ranging from 0.005 ± 0.02 to 1.80 ± 0.02 μg L−1) were detected in all tested West Virginia groundwaters at elevated levels compared to Ohio (ranges: ND to 1.44 ± 0.16 and ND to 0.64 ± 0.04 μg L−1, respectively) and Pennsylvania (ranges: ND to 0.09 ± 0.07 and ND to 0.18 ± 0.05 μg L−1, respectively). West Virginia also contained elevated levels of known TCE degradation products, including cis-1,2-dichloroethylene (DCE) (range: ND to 0.8 ± 0.5 μg L−1), trans-1,2-DCE (range: ND to 2.5 ± 0.4 μg L−1), and 1,1,2,2-tetrachloroethene (range: ND to 0.31 ± 0.002 μg L−1), relative to concentrations of those compounds in Ohio and Pennsylvania. Chlorinated VOCs have multiple potential industrial sources, including surface spills from manufacturing, use in industrial processes, groundwater recharge of water treated with chlorination, or the spraying of hydraulic fracturing produced waters40 (i.e., for dust control, where such waters have been shown to contain halomethanes10).
In Pennsylvania, there was a prevalence of a variety of halogenated methanes, including dibromomethane and chloroform, as well as common “solvent” chemicals (TCE, 1,2-dichloroethane (1,2 DCA), benzene, and toluene) (Fig. 3a). First, a brominated VOC, dibromomethane, had elevated concentrations in Pennsylvania (ND to 2.1 ± 0.98 μg L−1; 43% detection frequency) compared to West Virginia (not detected) and Ohio (ND to 1 ± 2 μg L−1; <1% detection frequency). Notably, other brominated VOCs were frequently detected in Pennsylvania (i.e., bromochloromethane and bromomethane, detection frequency 97% and 56% with concentrations of ND to 1.18 ± 0.17 and ND to 0.3 ± 0.5 μg L−1, respectively), though not significantly elevated compared to Ohio or West Virginia. For chlorinated compounds, TCE concentrations in Pennsylvania (up to 4.1 ± 0.15 μg L−1) were elevated relative to Ohio and West Virginia (ND to 0.390 ± 0.008 μg L−1 and ND to 0.01 ± 0.02 μg L−1, respectively), as were concentrations of chloroform in Pennsylvania (ranging from ND to 0.63 ± 0.12 μg L−1) compared to West Virginia (ranging from ND to 13 ± 2 μg L−1) and Ohio (ranging from ND to 98.4 ± 1.1 μg L−1); note, Ohio had one detection exceeding the EPA MCL for chloroform at 98.4 μg L−1. In addition to these halogenated VOCS, Pennsylvania samples contained two BTEX compounds, toluene and the sum of 1,2-DCA and benzene, more frequently (64 and 70%) and at concentrations (ND to 0.26 ± 0.05 μg L−1 and ND to 0.10 ± 0.01 μg L−1) exceeding Ohio (20% detection, ND to 2.7 ± 2.4 μg L−1 and 24% detection, ND to 2.73 ± 0.05 μg L−1, respectively) and West Virginia (11% detection, ND to 0.05 ± 0.08 μg L−1 and 5% detection, ND to 2.6 ± 0.3 μg L−1, respectively) (Table 1).
Halogenated solvents are common groundwater water contaminants, as they have many potential and common sources. First, chloroform, chloromethane, vinyl chloride, and TCE were commonly used solvents from the early 1900s to today and are legacy pollutants commonly found at the EPA's Superfund sites. As of 2019, the EPA documented TCE occurrences at 1051 of 1854 current or former National Priority List (NPL) sites.41 Contemporarily, chlorinated VOCs can form via drinking water disinfection that relies on chlorination (i.e., forming DBPs42) and groundwater recharge of treated water can represent a source of these VOCs to groundwater systems. Some of the most commonly occurring compounds (Table 1) like chloromethane and particular trihalomethanes (i.e., chloroform, bromochloromethane, and bromodichloromethane) have natural (i.e., biogenic) formation pathways and anthropogenic inputs outside of HDHF activities.42,44,45 These compounds are regulated under the EPA and identified as a hazardous waste irrespective of source. In contrast, others are thought to be purely anthropogenic (i.e., vinyl chloride)46 or are considered overwhelmingly anthropogenic in origin (i.e., trichloroethene, benzene).41,47 Additionally, some halogenated VOCs are thought to form through a combination of geochemistry and industrial additives, which could indicate formation from a nexus of natural and anthropogenic pathways.48
While there are many sources of chlorinated VOCs, brominated VOCs have fewer sources to drinking waters and can suggest an influence of brine composition. Brominated VOCs can form during disinfection processes if chemical oxidants co-occur in the presence of a bromine source, such as bromide. Drinking water disinfection processes may give rise to bromomethane in brine-impacted waters, where the brine was derived from acid mine drainage.49,50 Road spraying or groundwater recharge of brine-impacted fluids may both be expected to reflect higher levels of brominated and iodinated compounds51,52 relative to chlorinated, solvent-like molecules. Within the study region, West Virginia law prohibits road spraying of brine from unconventional oil and gas activities, but Ohio permits it from conventional oil and gas sources state-wide53 and Pennsylvania allowed it until a temporary moratorium for further investigation began in 2018.50
Fossil-fueled electricity generation may provide additional bromine sources that contribute to halogenation of VOCs. For example, coal fired power plants employ bromine as a mercury scrubber and may be a bromide source in the environment54,55 through atmospheric source terms or waste management of recovered solids. Bradford County, Pennsylvania does not have any coal fired power plants, but does have natural gas (n = 7) or biogas power plants, which should not contribute to the prevalence of brominated VOCs there. Counties sampled in West Virginia primarily utilize hydro (n = 1), natural gas (n = 1) and coal (n = 1) power plants, and sampled Ohio counties contain natural gas (n = 2) and petroleum (n = 1) power plants.56 Brominated VOCs were more commonly detected in Pennsylvania (i.e., dibromoethane) compared to West Virginia and Ohio, though two Br-VOCs, bromochloromethane and bromomethane, had detection frequences above 35% in all three states. Evaluating the explicit contribution of this source term cannot be executed within the experimental framework of this project (due to a lack of atmospheric modeling and parametric data collection) but is worth evaluating in future study. Here, we note that natural abundance of bromine isotopes and dual isotope systems57 could provide incredible value, as the variable sources may have distinct initial isotopic composition and subsequent fractionation.58
Brominated VOCs can form during disinfection processes when brines are mobilized from hydraulic fracturing (i.e., brine discharges impacted drinking water treatment intake).51,55 Also, brominated and iodinated VOCs have been identified directly in HDHF wastewater.10 Their formation can result from process additives (i.e., oxidants) reacting with saline formation brines to form these reactive halogen species.16 These transformation products include trihalomethanes and brominated products,8,10,51,52 which were observed in simulated HDHF formation fluids and dominated due to the favorable formation of reactive bromine species coupled with their reactivity. Thus, the appearance of brominated VOCs in Pennsylvania could be indicative of brine impacted fluids (related or unrelated to HDHF), whereas the prevalence of chlorinated VOCs in West Virginia could potentially be the consequence of legacy pollutants, chlorination derived DBPs, UOG activities, or some combination thereof.
Influence of water type on VOC occurrences
While brine composition can influence the appearance of particular transformation products, these reactive halides are a small fraction of the total available halogen pool; as a result, halide salts are typically thought of as non-reactive tracers. Geochemical evidence from northeastern Pennsylvania suggests groundwaters with naturally occurring elevated salinity are brine-impacted through cross-formational pathways that pre-date HDHF activities,59 and others have used subsurface brine signatures to independently trace fluid transport pathways to understand organic compound contamination sources.12 These brine signatures are classically segmented into water types defined previously59 and used here: briefly, type A and B waters were defined by low salinity (Cl concentrations <20 mg L−1), while type C and D waters contained Cl concentrations above 20 mg L−1, delineated by Br-to-Cl ratios (Type C: Br/Cl < 0.001 and Type D: Br/Cl > 0.001, where Br/Cl > 0.001 is consistent with Appalachian brine). Type D further contains low Na-to-Cl ratios (Na/Cl < 5). Siegel et al.31 2022 previously characterized the inorganic composition of the drinking water samples in this study; the bromide and chloride concentration data is used here. In this study, the majority of water samples were type A and B (n = 231), whereas fewer were classified as type C (n = 27) and D (n = 48). Four water samples matched type D waters Br-to-Cl ratios but contained Na-to-Cl ratios above 5 and were labelled unclassified.Across all three states, particular halomethanes in Type D waters (i.e., those with potential brine signatures) exceeded concentrations in Type A, B, and C waters. For instance, concentrations of bromochloromethane and chloroform (Fig. 4c) in Type D waters exceeded that of Type C waters (p-values: 0.02 and 0.0008, respectively; Mann Whitney U). Additionally, chloromethane concentrations in Type D waters exceeded Type A, B (p-value: 0.0001) and C water types (p-value: 0.044) (Fig. 4a). This is consistent with a possible brine-derived halomethane formation pathway. As discussed above, both chloroform and dibromomethane concentrations were elevated in Pennsylvania compared to Ohio and West Virginia. Concentrations of chloroform in Type D waters exceeded that of Type C for samples in Pennsylvania (p-value: 0.0025; Mann Whitney U), but not of Type A and B, suggesting the chloroform in Pennsylvania may be brine associated. Concentrations of dibromomethane in Type D Pennsylvania waters (n = 4) did not exceed that of Types A and B (n = 36) or Type C waters (n = 0). This indicates dibromomethane is not solely derived via reactions in deep formation brines (i.e., Type D water), but instead can form in surface-derived, fresher input waters. That is, multiple paths and sources may be responsible for the chemical mixtures observed in this study. Similarly, for some chlorinated vinyl, ethyl and benzene compounds, concentrations in Type D waters exceeded that of A, B or C type waters. Specifically, vinyl chloride concentrations in Type D waters exceeded Type A and B (p-values: 0.002; Mann Whitney U) and C water types (p-value: 0.021; Mann Whitney U) (Fig. 4b). Concentrations in Type D waters also exceeded Type C waters for the sum of 1,2-dichloroethane and benzene (p-values: 0.011; Mann Whitney U), suggesting that these compounds may have been brine-associated. Note that brine-associations are not necessarily restricted to subsurface transport but can be surface-derived where road spreading activities and surface discharges of produced waters are permitted.
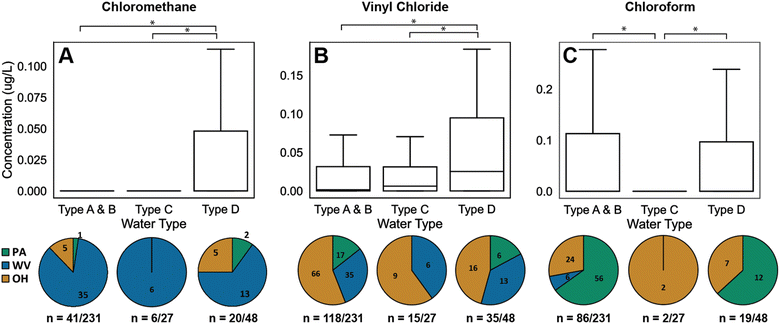 | ||
| Fig. 4 Concentration distributions of three halogenated VOCs: (a) chloromethane, (b) vinyl chloride, and (c) chloroform in water types A & B, C, and D of all three Appalachian states. Starred water type pairs indicate statistically significant differences in concentrations between water types (i.e., p-value <0.05, Mann Whitney U). Pie charts represent distribution of positive detection by water type in each sampling state. Number of samples with concentrations above limit of detections are listed for each water type. Compound specific limits of detections are found in Table #SI1.† | ||
Specific halogenated VOC occurrence in Type D water suggests that chlorinated VOCs may be forming from brine interaction in subsurface groundwaters as opposed to industrial solvent leaks. First, it has been previously illustrated that Type D waters dominate in topographical lows and show elevated levels of methane. This suggests that deep brine migration pathways may carry and accumulate methane in these regions.60,61 Light volatile organic compounds have high diffusivities and may be traveling via similar mechanisms in the subsurface. Further, methane in the presence of naturally occurring brines augmented with oxidants (industrial and natural oxidants) can promote the formation of halogenated methane species, halogenated alkanes, and other organic molecules.10,11,47,48 A similar process of formation and transport over long timescales may be occurring here. Here, we note that the population density is similar across the study region, and urban activities (e.g., road traffic, leaking underground storage facilities, or dry cleaners) are not likely to be the source of these chemical contaminants, consistent with previous results.12,29
Conversely, the occurrence of brominated organic compounds that may be attributed to potential brine interactions59 and common in Pennsylvania, do not exclusively co-occur with potentially brine derived waters. Instead, extensive experimentation confirms the possibility of brominated VOCs to form from brine-impacted drinking water and wastewater treatment processes. Thus, the brominated VOCs observed here could result from brine-derived groundwaters that are treated and reintroduced to aquifers,55 brine-impacted water treatment fluids,51,52 or other surface-derived activities.
Hydraulic fracturing and Superfund site well proximity vulnerability analysis
To evaluate the potential influence of UOG activities and Superfund sites on the groundwater chemical composition, we explored distance-associations commonly used in epidemiologic studies. Average distance between sample site and closest HDHF site was shortest in Pennsylvania (1.2 ± 0.1 km) but was only 2.0 ± 0.1 km in both Ohio and West Virginia. Notably, all sample sites were within 7.4 km of a HDHF site in across all three states. There was no significant linear relationship between detection frequency nor individual VOC concentration and proximity to closest HDHF site for any VOC within all three states (Spearman Rank Order Analysis ESI Fig. 2 and ESI Fig. 3),† consistent with findings from Xiong et al. 2022 in Pennsylvania using a subset of these data.29 (Additional exposure odds ratio analysis is available in Clark et al. 2022 (ref. 30)). Similarly, correlation analysis of GRO concentrations with respect to linear distance to HDHF site showed no significant relationships in Ohio and West Virginia, corroborating findings in Pennsylvania alone29 (ESI Fig. 1†). These add further support to previous findings that distance to closest HDHF site alone is not adequate for the assessment of private drinking water vulnerability in the Appalachian region. Nevertheless, distance to nearest well is used as a common epidemiologic indicator of exposure risk and serves as the basis for many well permitting criteria to date (e.g., set back distances62).To explore well vulnerability associated with distance to locations of historic industrial activity and contamination, we examined the occurrence of specific VOCs in groundwater wells near EPA Superfund sites. In Pennsylvania, one Superfund location had 9 groundwater wells with a 10 km radius; in West Virginia and Ohio, four Superfund locations were identified with groundwater wells (n = 21) within a 10 km radius. (Note that Ohio and West Virginia were grouped due to their proximity, where the shortest Superfund-groundwater well distance may have traversed state lines). All five relevant Superfund sites reported more than one contaminant release matching compounds most frequently detected in our study (Table 2). Compared to HDHF sites, Superfund locations were sparser in density and on average farther from private drinking well sites in all three states. The average VOC detection frequency in West Virginia and Ohio within a 10 km radius of a Superfund site (5 ± 6) was not significantly different from the average positive detections in samples considered for HDHF proximity assessment (5 ± 3). Similarly, average detection frequency of VOCs in wells within 10 km of Bell Landfill in Pennsylvania was 6 ± 3 and not significantly different compared to 6 ± 3 average frequency in samples considered for HDHF proximity assessment. Wells within a radius of 10 km from a positive contaminant release disclosure of a specific VOC (chloroform, toluene, or benzene) had elevated concentrations in West Virginia and Ohio (p-value <0.05, Mann Whitney U) as compared to those groundwater wells outside the 10 km boundary. Note that these distances are very large for groundwater transport phenomena but may be adequate given potential chemical persistence and long timescale since release. Accordingly, disclosures of chlorinated VOCs as legacy pollutants at Superfund sites in combination with the water typing analysis illuminating potential halomethane occurrence in brine-impacted (Type D) waters underscores the multiple potential pathways for contaminant introduction to drinking water sources. We note that linear distance alone was not a strong predictor of exposure risk from legacy industrial contaminant sites even with positive contaminant release disclosure (see ESI for further discussion),† as anticipated by knowledge of more complex hydrologic transport pathways.63,64
| VOC | Site name and location | ||||
|---|---|---|---|---|---|
| Ormet (Monroe, OH) | Fultz (Guernsey, OH) | Hanlin (Marshall, WV) | Buckeye (Belmont, OH) | Bell (Bradford, PA) | |
| Number of wells within 10 km radius | 6 | 2 | 5 | 8 | 9 |
| Average distance to sampling sites (km) | 4.5 ± 1.4 | 7.2 ± 0 | 5.0 ± 2.0 | 6.0 ± 1.5 | 4.8 ± 2.5 |
| Average VOC detections | 6 ± 7 | 10 ± 10 | 3 ± 4 | 4 ± 4 | 6 ± 3 |
| Vinyl chloride | ✗ | ✗ | |||
| Chloroform | ✗ | ✗ | |||
| Trichloroethene (TCE) | ✗ | ✗ | ✗ | ||
| Tetrachloroethene (PCE) | ✗ | ||||
| Benzene | ✗ | ✗ | ✗ | ||
| Toluene | ✗ | ✗ | ✗ | ✗ | |
Conclusions
This study characterized a suite of organic compounds present in private drinking water wells in areas of heavy HDHF activity and legacy industries. The findings of this study provide a valuable resource for assessing water quality, well vulnerability, and potential organic compound inputs including legacy industrial sites, HDHF sites, and brine interaction. Here, we show very few drinking waters in exceedance of national EPA MCLs for compounds with applicable human health benchmarks, as well as a lack of linear relationship between proximity to closest HDHF site and both VOC concentration and detection frequency. This may be somewhat surprising giving the density of industrial activity in these regions and suggests that systematic groundwater contamination did not result from intense hydraulic fracturing in this region for over 10 years. Of the compounds detected, we found higher frequencies of organic contaminants in West Virginia, Ohio, and Pennsylvania than reported in previous assessments of national groundwater quality.37 Additionally, specific halogenated VOCs (i.e., vinyl chloride and chloromethane) occur in highly saline waters. The presence of chloromethane may suggest halogenation from brine interaction as a dominant source for these trace contaminants; no pathway for vinyl chloride formation under these conditions currently exists, though formation through dichlorination of halogenated solvents (i.e., tetrachloroethylene) is well established.46 In contrast, other halogenated organic compounds (i.e., dibromomethane, bromochloromethane, and bromomethane, all of which contain bromine) displayed limited correlation to brine formation signatures (Type D waters), suggesting compounding sources of halogenation in drinking water supply aquifers (e.g., surface spills of high salinity wastewater or non-point source industrial inputs). Further study of potential brine-derived halogenated VOC occurrence in drinking water is necessary to elucidate dominant formation pathways and evaluate exposure risk to domestic drinking water consumers.The findings of this study indicate widespread, trace-level co-occurrences of organic compounds that have known individual adverse health effects and may have long-term consequences for residents in rural communities. This is underscored by the evidence of multiple-compound exposure and local variability of water composition. Existing literature has reinforced the need for chronic, low-dose chemical mixture toxicological assessments as the cumulative health impact of environmentally-relevant organic compound co-exposures (n > 2) at trace levels remains uncertain.37,65 Adding new independent data of compound co-occurrence has the potential to inform co-exposure assessments of compounds present at trace-levels and are directly relevant to the communities studied. This broad-spectrum organic compound water composition analysis shows evidence of potential multi-compound exposures to rural communities in the northeastern Appalachian region.
While these findings illustrate that individual organic compound exposure is typically occurring at low levels, it is only one facet of water quality. Nutrients, health-relevant metals, and microorganisms contribute to consumer exposure portfolios and the integrated risk. Previous inorganic analysis of the sampling area found elevated levels of arsenic and nitrate in 10% of drinking waters analyzed in this study.31 Further, an independent analysis of Bradford County in 2016 (ref. 66) sampling 72 domestic wells identified 49.3% of samples in exceedance of MCLs for total coliform bacteria, and 8.5% contained concerning levels of Escherichia coli. In that study, 2.8% of samples had arsenic exceedances. Clune et al. further emphasize that N and P loads can be concerning in PA surface waters,67 and that there may be a need to re-evaluate variations in state-to-state levels to ensure protection of public health. In addition to these relevant consumer risks, current toxicity assessment methods focus on single compounds and may undervalue potential effects (i.e., additive or antagonistic) of multi-contaminant presence on consumer health. Assessing multi-compound mixtures spanning many known contaminant classes or use groups (e.g., solvents, disinfection byproducts, gasoline or diesel hydrocarbons) is critical to understand the overall health implications of the complex “exposome” or portfolio of industrial chemicals present in this unmonitored and unregulated domestic drinking water supply.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
N. B. conceptualized the project, curated data, conducted formal analysis, investigated, developed methodology, supervised junior researchers, validated, visualized and wrote the original draft of the manuscript. D. P., J. S., and N. D. conceptualized, supervised, acquired funding, administered the project, and participated in reviewing and editing. R. G. participated in lab analysis and quality control. H. S., B. X., N. J., and C. C. all participated in field collection, methodology development, lab analysis, quality control, and formal analysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported in part by the National Priority Research Project under Assistance agreement no. CR839249 awarded by the U.S. Environmental Protection Agency to Yale University. The publication has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication. NB was supported by the National Science Foundation Graduate Research Fellowship under grant no. 1745302. Dall-E 2 AI Image Generator was used in the creation of the Table of Contents image with modifications from N. Bugher.References
- C. A. Dieter, M. A. Maupin, R. R. Caldwell, M. A. Harris, T. I. Ivahnenko, J. K. Lovelace, N. L. Barber and K. S. Linsey, Estimated Use of Water in the United States in 2015, U.S. Geological Survey Circular, 2018 vol. 1441, p. 65, DOI:10.3133/cir1441.
- B. R. Swistock, S. Clemens, W. E. Sharpe and S. Rummel, Water quality and management of private drinking water wells in Pennsylvania, J. Environ. Health, 2013, 75(6), 60 CAS.
- K. Bowen, T. Krishna, L. Backer, K. Hodgins, L. A. Waller and M. O. Gribble, State-Level Policies Concerning Private Wells in the United States, Water Policy, 2019, 21(2), 428–435, DOI:10.2166/wp.2019.205.
- Ohio Rev. Code § 3701.344, 2016, available at, https://codes.ohio.gov/ohio-revised-code/section-3701.344 Search PubMed.
- F. T. Alliance and E. JackonFracking Waste in the Appalachian Basin – A Story Map, 2021 Search PubMed.
- J. L. Schnoor, Shale Gas and Hydrofracturing, Environ. Sci. Technol., 2012, 46(9), 4686, DOI:10.1021/es3011767.
- Kargbo 2010 Natural Gas Plays in the Marcellus Shale: Challenges and Potential Opportunities, https://pubs.acs.org/doi/pdf/10.1021/es903811p Search PubMed.
- A. Vengosh, R. B. Jackson, N. Warner, T. H. Darrah and A. Kondash, A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States, Environ. Sci. Technol., 2014, 48(15), 8334–8348, DOI:10.1021/es405118y.
- S. A. Gross, H. J. Avens, A. M. Banducci, J. Sahmel, J. M. Panko and B. E. Tvermoes, Journal of the Air & Waste Management Association Analysis of BTEX groundwater concentrations from surface spills associated with hydraulic fracturing operations. Analysis of BTEX groundwater concentrations from surface spills associated with hydraulic, J. Air Waste Manage. Assoc., 2013, 63(4), 424–432, DOI:10.1080/10962247.2012.759166.
- K. Hoelzer, A. J. Sumner, O. Karatum, R. K. Nelson, B. D. Drollette, M. P. O’connor, E. L. D’ambro, J. G. Getzinger, P. L. Ferguson, C. M. Reddy, M. Elsner and D. L. Plata, Indications of Transformation Products from Hydraulic Fracturing Additives in Shale-Gas Wastewater, Environ. Sci. Technol., 2016, 50(15), 8036–8048, DOI:10.1021/acs.est.6b00430.
- A. J. Sumner and D. L. Plata, A geospatially resolved database of hydraulic fracturing wells for chemical transformation assessment, Environ. Sci.: Processes Impacts, 2020, 22, 945–955, 10.1039/c9em00505f.
- B. D. Drollette, K. Hoelzer, N. R. Warner, T. H. Darrah, O. Karatum, M. P. O'Connor, R. K. Nelson, L. A. Fernandez, C. M. Reddy, A. Vengosh, R. B. Jackson, M. Elsner and D. L. Plata, Elevated levels of diesel range organic compounds in groundwater near Marcellus gas operations are derived from surface activities, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(43), 13184–13189, DOI:10.1073/pnas.1511474112.
- S. G. Osborn, A. Vengosh, N. R. Warner and R. B. Jackson, Methane contamination of drinking water accompanying gas-well drilling and hydraulic fracturing, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(20), 8172–8176, DOI:10.1073/pnas.1100682108.
- X. C. Hu, D. Q. Andrews, A. B. Lindstrom, T. A. Bruton, L. A. Schaider, P. Grandjean, R. Lohmann, C. C. Carignan, A. Blum, S. A. Balan, C. P. Higgins and E. M. Sunderland, Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants, Environ. Sci. Technol. Lett., 2016, 3(10), 344–350 CrossRef CAS PubMed.
- United States Environmental Protection Agency, National Priorities List and Superfund Alternative Approach Sites, 2021, date accessed 1/20/2022 Search PubMed.
- A. J. Sumner and D. L. Plata, Halogenation Chemistry of Hydraulic Fracturing Additives under Highly Saline Simulated Subsurface Conditions, Environ. Sci. Technol., 2018, 52(16), 9097–9107, DOI:10.1021/acs.est.8b01591.
- J. L. Luek, P. Schmitt-Kopplin, P. J. Mouser, W. T. Petty, S. D. Richardson and M. Gonsior, Halogenated Organic Compounds Identified in Hydraulic Fracturing Wastewaters Using Ultrahigh Resolution Mass Spectrometry, Environ. Sci. Technol., 2017, 51(10), 5377–5385, DOI:10.1021/acs.est.6b06213.
- L. W. Canter and D. A. Sabatini, Contamination of public ground water supplies by Superfund sites, Int. J. Environ. Stud., 1994, 46(1), 35–37, DOI:10.1080/00207239408710909.
- Z. L. Hildenbrand, D. D. Carlton, B. E. Fontenot, J. M. Meik, J. L. Walton, J. T. Taylor, J. B. Thacker, S. Korlie, C. P. Shelor, D. Henderson, A. F. Kadjo, C. E. Roelke, P. F. Hudak, T. Burton, H. S. Rifai and K. A. Schug, A Comprehensive Analysis of Groundwater Quality in The Barnett Shale Region, Environ. Sci. Technol., 2015, 49(13), 8254–8262, DOI:10.1021/acs.est.5b01526.
- J. L. Luek and M. Gonsior, Organic compounds in hydraulic fracturing fluids and wastewaters: a review, Water Res., 2017, 123, 536–548, DOI:10.1016/j.watres.2017.07.012.
- P. L. Toccalino, J. E. Norman and J. C. Scott, Chemical mixtures in untreated water from public-supply wells in the U.S.-occurrence, composition, and potential toxicity, Sci. Total Environ., 2012, 431, 262–270, DOI:10.1016/j.scitotenv.2012.05.044.
- L. K. Teuschler and J. E. Simmons, Approaching DBP toxicity as a mixtures problem, J. - Am. Water Works Assoc., 2003, 95, 131–138, DOI:10.1002/j.1551-8833.2003.tb10393.x.
- D. O. Carpenter, K. Arcaro and D. C. Spink, Understanding the human health effects of chemical mixtures, Environ. Health Perspect., 2002, 110(1), 25–42, DOI:10.1289/EHP.02110S125.
- National Primary Drinking Water Regulations; Synthetic Organic Chemicals and Inorganic Chemicals, Final Rule, 57 Fed. Reg., 31776-31849, 1992 Search PubMed.
- ATSDR, Support Document to the 2022 Substance Priority List (Candidates for Toxicological Profiles), Agency for Toxic Substances and Disease Registry, Atlanta, GA, 2022, https://www.atsdr.cdc.gov/spl/resources/ATSDR-2022-SPL-Support-Document-508.pdf Search PubMed.
- S. A. Sapouckey, C. D. Kassotis, S. C. Nagel and L. N. Vandenberg, Prenatal Exposure to Unconventional Oil and Gas Operation Chemical Mixtures Altered Mammary Gland Development in Adult Female Mice, Endocrinology, 2018, 159(3), 1277–1289, DOI:10.1210/en.2017-00866.
- C. D. Kassotis, K. C. Klemp, D. C. Vu, C.-H. Lin, C.-X. Meng, C. L. Besch-Williford, L. Pinatti, R. Thomas Zoeller, E. Z. Drobnis, V. D. Balise, C. J. Isiguzo, M. A. Williams, D. E. Tillitt and S. C. Nagel, Endocrine-Disrupting Activity of Hydraulic Fracturing Chemicals and Adverse Health Outcomes After Prenatal Exposure in Male Mice, Endocrinology, 2015, 156(12), 4458–4473, DOI:10.1210/en.2015-1375.
- E. G. Elliott, A. S. Ettinger, B. P. Leaderer, M. B. Bracken and N. C. Deziel, A systematic evaluation of chemicals in hydraulic-fracturing fluids and wastewater for reproductive and developmental toxicity, J. Exposure Sci. Environ. Epidemiol., 2017, 27(1), 90–99, DOI:10.1038/jes.2015.81.
- B. Xiong, M. A. Soriano, K. M. Gutchess, N. Hoffman, C. J. Clark, H. G. Siegel, G. Andrew, D. de Vera, Y. Li, R. J. Brenneis, A. J. Cox, E. C. Ryan, A. J. Sumner, N. C. Deziel, J. E. Saiers and D. L. Plata, Groundwaters in Northeastern Pennsylvania near intense hydraulic fracturing activities exhibit few organic chemical impacts, Environ. Sci.: Processes Impacts, 2022, 24, 252–264, 10.1039/d1em00124h.
- C. J. Clark, B. Xiong, M. A. Soriano, K. Gutchess, H. G. Siegel, E. C. Ryan, N. P. Johnson, K. Cassell, E. G. Elliott, Y. Li, A. J. Cox, N. Bugher, L. Glist, R. J. Brenneis, K. M. Sorrentino, J. Plano, X. Ma, J. L. Warren, D. L. Plata and N. C. Deziel, Assessing Unconventional Oil and Gas Exposure in the Appalachian Basin: Comparison of Exposure Surrogates and Residential Drinking Water Measurements, Environ. Sci. Technol., 2022, 2022, 1091–1103, DOI:10.1021/acs.est.1c05081.
- H. G. Siegel, M. A. Soriano, C. J. Clark, N. P. Johnson, H. G. Wulsin, N. C. Deziel, D. L. Plata, T. H. Darrah and J. E. Saiers, Natural and Anthropogenic Processes Affecting Domestic Groundwater Quality within the Northwestern Appalachian Basin, Environ. Sci. Technol., 2022, 56(19), 13761–13773, DOI:10.1021/acs.est.2c04011.
- USEPA, EPA Method 8015C: Nonhalogenated Organics by Gas Chromatography, 2007 Search PubMed.
- USEPA, EPA Method 3510C: Separatory Funnel Liquid-Liquid Extraction, 1996 Search PubMed.
- USGS, National Field Manual for the Collection of Water-Quality Data, 2018 Search PubMed.
- USEPA, Drinking Water Contaminant Candidate List 5-Final. 87 FR 68060, 2022 Search PubMed.
- P. J. Squillace, J. C. Scott, M. J. Moran, B. T. Nolan and D. W. Kolpin, VOCs, Pesticides, Nitrate, and Their Mixtures in Groundwater Used for Drinking Water in the United States, Environ. Sci. Technol., 2002, 36(9), 1923–1930, DOI:10.1021/es015591n.
- L. M. Bexfield, K. Belitz, M. S. Fram and B. D. Lindsey, Volatile organic compounds in groundwater used for public supply across the United States: Occurrence, explanatory factors, and human-health context, Sci. Total Environ., 2022, 827, 154313, DOI:10.1016/j.scitotenv.2022.154313.
- J. S. Zogorski, J. M. Carter, T. Ivahnenko, W. W. Lapham, M. J. Moran, B. L. Rowe, P. J. Squillace and P. L. Toccalino, Volatile Organic Compounds in the Nation’s Ground Water and Drinking-Water Supply Wells, Geol. Surv. Circ., 2006, 1292, 101, DOI:10.3133/cir1292.
- L. A. DeSimone, P. B. McMahon and M. R. Rosen, The quality of our Nation's waters—Water quality in Principal Aquifers of the United States, 1991–2010, Geol. Surv. Circ., 2014, 1360, 151, DOI:10.3133/cir1360.
- G. J. Getzinger, M. P. O'Connor, K. Hoelzer, B. D. Drollette, O. Karatum, M. A. Deshusses, P. L. Ferguson, M. Elsner and D. L. Plata, Natural Gas Residual Fluids: Sources, Endpoints, and Organic Chemical Composition after Centralized Waste Treatment in Pennsylvania, Environ. Sci. Technol., 2015, 49(14), 8347–8355 CrossRef CAS PubMed.
- ATSDR, Toxicological Profile for Trichloroethylene, 2019 Search PubMed.
- M. Deinzer, F. Schaumburg and E. Klein, Environmental health sciences center task force review on halogenated organics in drinking water, Environ. Health Perspect., 1978, 24, 209–239, DOI:10.1289/ehp.7824209.
- EPA, Superfund Site Information, https://cumulis.epa.gov/supercpad/CurSites/srchsites.cfm, accessed 2/25/2022.
- F. Laturnus, F. R. Lauritsen and C. Grøn, Chloroform in a pristine aquifer system: Toward an evidence of biogenic origin, Water Resour. Res., 2000, 36(10), 2999–3009, DOI:10.1029/2000WR900194.
- ATSDR, Toxicological Profile for Chloromethane, 2023 Search PubMed.
- ATSDR, Toxicological Profile for Vinyl Chlorides, 2024 Search PubMed.
- J. L. Luek, P. Schmitt-Kopplin, P. J. Mouser, W. T. Petty, S. D. Richardson and M. Gonsior, Halogenated Organic Compounds Identified in Hydraulic Fracturing Wastewaters Using Ultrahigh Resolution Mass Spectrometry, Environ. Sci. Technol., 2017, 51(10), 5377–5385, DOI:10.1021/acs.est.6b06213.
- A. J. Sumner and D. L. Plata, Oxidative Breakers Can Stimulate Halogenation and Competitive Oxidation in Guar-Gelled Hydraulic Fracturing Fluids, Environ. Sci. Technol., 2019, 53(14), 8216–8226, DOI:10.1021/acs.est.9b01896.
- C. Kolb, K. D. Good and J. M. VanBriesen, Modeling Trihalomethane Increases Associated with Source Water Bromide Contributed by Coal-Fired Power Plants in the Monongahela River Basin, Environ. Sci. Technol., 2020, 54(2), 726–734, DOI:10.1021/acs.est.9b01544.
- W. Burgos, N. Warner, X. Liu, E. Chase, A. Kearney, J. Farnan, A. Eck and H. Ismail, Evaluation of Environmental Impacts from Dust Suppressants Used on Gravel Roads, Pennsylvania Department of Environmental Protection, Office of Oil and Gas, Harrisburg, PA, 2022 Search PubMed.
- K. M. Parker, T. Zeng, J. Harkness, A. Vengosh and W. A. Mitch, Enhanced formation of disinfection byproducts in shale gas wastewater-impacted drinking water supplies, Environ. Sci. Technol., 2014, 48(19), 11161–11169, DOI:10.1021/es5028184.
- J. Wilson and J. Vanbriesen, Source water changes and energy extraction activities in the Monongahela River, 2009–2012, Environ. Sci. Technol., 2013, 47, 12575–12582 CrossRef CAS PubMed.
- Ohio Rev. Code § 1509.226, 2013, available at https://codes.ohio.gov/ohio-revised-code/section-1509.226 Search PubMed.
- B. H. Lee, F. D. Lopez-Hilfiker, J. C. Schroder, P. Campuzano-Jost, J. L. Jimenez and E. E. McDuffie, et al.). Airborne observations of reactive inorganic chlorine and bromine species in the exhaust of coal-fired power plants, J. Geophys. Res.: Atmos., 2018, 123, 225–237, DOI:10.1029/2018JD029284.
- K. D. Good and J. M. VanBriesen, Current and Potential Future Bromide Loads from Coal-Fired Power Plants in the Allegheny River Basin and Their Effects on Downstream Concentrations, Environ. Sci. Technol., 2016, 50(17), 9078–9088, DOI:10.1021/acs.est.6b01770.
- U.S. Energy Information Administration, EIA-860, Annual Electric Generator Report; EIA-860M, Monthly Update to the Annual Electric Generator Report; and EIA-923, Power Plant Operations Report, 2020, date accessed September 4 2024 Search PubMed.
- T. B. Hofstetter, R. Bakkour and D. Buchner, et al.) Perspectives of compound-specific isotope analysis of organic contaminants for assessing environmental fate and managing chemical pollution, Nat. Water, 2024, 2, 14–30, DOI:10.1038/s44221-023-00176-4.
- B. Redder and E. W. Boyer, Drinking Water Quality of private wells in Pennsylvania, in ACS Fall Meeting 2023, ACS, 2023 Search PubMed.
- N. R. Warner, R. B. Jackson, T. H. Darrah, S. G. Osborn, A. Down, K. Zhao, A. White and A. Vengosh, Geochemical evidence for possible natural migration of Marcellus Formation brine to shallow aquifers in Pennsylvania, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(30), 11961–11966, DOI:10.1073/pnas.1121181109.
- Y. Li, N. A. Thelemaque, H. G. Siegel, C. J. Clark, E. C. Ryan, R. J. Brenneis, K. M. Gutchess, M. A. Soriano, B. Xiong, N. C. Deziel, J. E. Saiers and D. L. Plata, Groundwater Methane in Northeastern Pennsylvania Attributable to Thermogenic Sources and Hydrogeomorphologic Migration Pathways, Environ. Sci. Technol., 2019, 55(24), 16413–16422, DOI:10.1021/acs.est.1c05272.
- P. M. Heisig and T.-M. Scott, Occurrence of Methane in Groundwater of South-Central New York State, 2012-Systematic Evaluation of a Glaciated Region by Hydrogeologic Setting Scientific Investigations Report, 2013, pp. 2013–5190, DOI:10.3133/sir20135190.
- C. Lewis, L. H. Greiner and D. R. Brown, Setback distances for unconventional oil and gas development: Delphi study results, PloS One, 2018, 13(8), e0202462, DOI:10.1371/journal.pone.0202462.
- M. A. Soriano Jr, H. G. Siegel, K. M. Gutchess, C. J. Clark, Y. Li and B. Xiong, et al.). Evaluating domestic well vulnerability to contamination from unconventional oil and gas development sites, Water Resour. Res., 2020, 56, e2020WR028005, DOI:10.1029/2020WR028005MCL and HBSL data: https://water.usgs.gov/water-resources/hbsl/.
- M. A. Soriano, H. G. Siegel, N. P. Johnson, K. M. Gutchess, B. Xiong, Y. Li, C. J. Clark, D. L. Plata, N. C. Deziel and J. E. Saiers, Assessment of groundwater well vulnerability to contamination through physics-informed machine learning, Environ. Res. Lett., 2021, 16(8), 084013, DOI:10.1088/1748-9326/ac10e0.
- D. H. Lee and D. R. Jacobs Jr, New approaches to cope with possible harms of low-dose environmental chemicals, J. Epidemiol. Community Health, 2019, 73(3), 193–197, DOI:10.1136/jech-2018-210920.
- J. W. Clune and C. A. Cravotta III, Drinking Water Health Standards Comparison and Chemical Analysis of Groundwater for 72 Domestic Wells in Bradford County, Pennsylvania, 2016 (Ver 1.2, May 30, 2019), U.S. Geological Survey Scientific Investigations Report 2018–5170, 2019, p. 66, DOI:10.3133/sir20185170.
- J. W. Clune, J. Kent Crawford and E. W. Boyer, Nitrogen and Phosphorus Concentration Thresholds toward Establishing Water Quality Criteria for Pennsylvania, USA, Water, 2020, 12(12), 3550, DOI:10.3390/w12123550.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00364k |
| This journal is © The Royal Society of Chemistry 2024 |