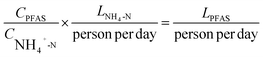Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploring the variability of PFAS in urban sewage: a comparison of emissions in commercial versus municipal urban areas†
Krlovic
N.
 *a,
Saracevic
E.
*a,
Saracevic
E.
 a,
Derx
J.
a,
Derx
J.
 bc,
Gundacker
C.
bc,
Gundacker
C.
 d,
Krampe
J.
d,
Krampe
J.
 a,
Kreuzinger
N.
a,
Kreuzinger
N.
 a,
Zessner
M.
a,
Zessner
M.
 ab and
Zoboli
O.
ab and
Zoboli
O.
 a
a
aTU Wien, Institute for Water Quality and Resource Management, Vienna, Austria. E-mail: nikola.krlovic@tuwien.ac.at
bInteruniversity Cooperation Centre for Water and Health (ICC Water & Health), Vienna, Austria
cTU Wien, Institute of Hydraulic Engineering and Water Resources Management, Vienna, Austria
dMedical University of Vienna, Center for Pathobiochemistry and Genetics, Institute of Medical Genetics, Vienna, Austria
First published on 13th September 2024
Abstract
Per- and polyfluoroalkyl substances (PFAS) are recognized for their persistence and ubiquitous occurrence in different environmental compartments. Conventional wastewater treatment plants (WWTPs) cannot effectively remove PFAS from wastewater, and a better understanding of the occurrence and sources of PFAS in this medium would enable effective source abatement. We compared sewage from urban areas exhibiting differentiating characteristics with respect to activities in their catchments. These included a sewer that serves primarily a municipal area, with no commercial activities involving PFAS emissions being identified, another sewer with a strong influence of commercial activities potentially related to PFAS emissions, and the influent of the whole city sewage network. The year-long monitoring campaign consisted of flow-proportional, monthly composite samples and targeted analysis of 29 PFAS compounds. Principal component analysis was used to investigate the relationships between selected PFAS and standard water quality parameters such as ammonium, a known tracer of urine and thus of typical municipal wastewater. Notable findings were seen for PFOS and 6:2 FTS, whose concentrations were most negatively correlated with ammonium. Ammonium concentration data allowed for a normalized per-person median load calculation, which resulted in loads of the observed PFAS ranging from below 0.4 up to 4.7 μg per person per day. Both the commercial area sewer and the city influent exhibited significantly higher (p < 0.05) median loads (>0.9 μg per person per day) in the case of 6:2 FTS and PFOS, compared to the municipal sewer (<0.6 μg per person per day). No statistically significant difference was found for other compounds, such as PFBA, PFHxA, PFOA, and PFHxS. We argue that this approach demonstrates that PFAS can differ in speciation and quantity within an urban wastewater setting, and consideration of both municipal and commercial activities is needed for a proper understanding of sources and emission pathways within the urban environment.
Environmental significancePer- and Polyfluoroalkyl Substances (PFAS) are a group of persistent pollutants, which occur in a variety of environmental compartments. Identifying the main sources of these substances will assist stakeholders in making informed decisions on future management initiatives. The wide array of PFAS applications makes it imperative to consider all the potential emission points. The results of this work demonstrate the occurrence, magnitude, and discrepancy of different PFAS within urban sewage, comparing the wastewater treatment plant (WWTP) influent and two characteristically distinct sewers (industry-intensive versus non-industrial) among each other. Such an intercomparison allows for a clearer overview of PFAS occurrence, and drives further identification efforts of PFAS emitters within a municipal sewage system. |
1 Introduction
Per- and polyfluoroalkyl substances are anthropogenic organic compounds, well known for their environmental persistence1–3 and potential detrimental health effects.4 PFAS are ubiquitously used in many products and processes,5 and are globally present in a variety of anthropogenic and environmental compartments, with wastewater being one of them.6,7 The treated effluent of wastewater treatment plants (WWTP) usually contains PFAS as well, due to this group of chemicals' rather poor removal efficiency.8 This makes WWTPs a significant PFAS emission pathway in the aquatic environment.9Researched emission sources of PFAS into the urban wastewater system include consumer product use and disposal,10–12 stormwater runoff,13,14 aqueous film forming foam (AFFF) use,15 and atmospheric deposition.16 However, a potential yet hardly quantified PFAS contributor to urban wastewater can be found in the form of commercial activities within city boundaries. For the purpose of this paper, we define “commercial” activities as industrial processes that occur within an urban environment and use the municipal sewage system. Based on the rather diverse applications of PFAS that have been noted, one should not ignore these commercial activities that occur within an urban environment. Albeit not contributing to the same intensity as the heavier industry facilities with their own treatment systems,17 commercial activities may be an additional source of PFAS which ultimately reaches urban wastewater.18
Although there is ample PFAS-centric research that addresses the aspect of urban wastewater,19–21 it usually focuses on the occurrence and behavior of PFAS during the WWTP treatment. Therefore, knowledge concerning the incidence of these chemicals in the steps prior, specifically in the sewers themselves, is largely lacking.18 Nevertheless, since WWTPs exhibit a rather poor performance in the removal of PFAS,8 identifying, assessing, and estimating the PFAS situation in the sewers, and taking the necessary precautions in limiting pollutant emission could be considered important steps in the mitigation of environmental risk arising from these chemicals.22
Krlovic et al.23 developed a modelling framework to estimate the loads of PFAS, based on release from consumer products and municipal diffuse inputs. The results indicated that for certain PFAS, such input considerations were not sufficient to explain their occurrence in the context of a typical urban wastewater setting. This means that other, not yet quantified activities could account for the remainder of the load. The goal of this paper was to compare two sewers and the WWTP influent of a large, economically active, central European city. The urban catchments of the selected sewers are distinct in terms of population density and ongoing activities. One sewer area is defined as exclusively affected by residential, touristic, and third-sector activities, with no known commercial emitters that could be attributed to PFAS. The other exhibits a strong effect of commercial activities of an industrial nature, some suspected to contribute to the overall PFAS load in the municipal wastewater, based on a prior systematic review of PFAS sources.5 A significantly higher per-person load in commercial sewage would indicate an industry-specific contribution of PFAS, in addition to expected consumer and diffuse inputs, as the latter two are assumed to occur ubiquitously throughout the sewage system. Conversely, a lack of significant difference would mean that no specific phenomena contribute to the overall load of the urban wastewater. Such a dichotomy would allow for the identification of additional contributions of PFAS into the wastewater, as in the case of commercial activities. Influent of the city's WWTP was compared to the two aforementioned sewers, in order to establish a clearer insight into the occurrence and mass loads of PFAS within the boundary of the city overall.
2 Materials and methods
2.1 Study area and population estimations
The studied area is a large, commercially active city that has four main sewers leading to one WWTP. The two selected sewers are identified as sewers “M” and “C”, standing for municipal and commercial, respectively. In addition, for comparative purposes, the municipal influent to the WWTP was sampled as well. With respect to the share that the two sewers contribute to the city influent, they are nearly similar, with sewer M accounting for 10.3% and sewer C for 13.3% of the overall average wastewater flow of the city. While no registered PFAS-relevant industrial entities were considered to be potential PFAS emitters in sewer M, several entities in sewer C were identified. This was done by comparing the potential activities noted in the works of Glüge and colleagues5 and in the CHEMSEC database24 to the description of the operational focal points of business entities in the sewer C area. The activities associated with the city were identified through the database of the city's chamber of commerce (Table 1). AFFFs, although found to be a significant contributor of PFAS to urban wastewater of certain cities,6,18 are not considered in the analysis, since no firefighting training sites are located within the study area.| Potential PFAS-related operations of commerce in sewer C (according to Glüge et al.5 2020 and CHEMSEC 2023) |
|---|
| Chemical industry |
| Coatings, paints and varnishes manufacturing |
| Wire, fabric and screen manufacturing |
| Production of coatings, plastic, glass and rubber |
| Production of various chemical products |
| Manufacturing of plastics and molds |
| Chemical manufacturing |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Textile and upholstery industry |
| Shoe manufacturing |
| Manufacturing of upholstery |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Paper industry |
| Packaging material finishing and finishing |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Building materials industry |
| Production of various building elements |
| Production of metal, wood and plastic building materials |
According to cadastral records from 2022, the areas of the city that correspond to sewers M and C have had population numbers that correspond to 10% and 12% of the total population, respectively. However, given that the city in question is an important location in the context of economic, educational, and tourism activities in central Europe, the number of actual people is likely to differ from the cadastral values. Especially in the municipal area, a higher number of people is expected to operate for work, educational, or recreational purposes on a daily basis, in addition to the registered inhabitants. Such additional influxes of people are not expected to occur in the sewer C catchment, except for the individuals who go there for work-related purposes. Therefore, estimating the mass loads of PFAS, specifically in the dimension of per capita loads, and examining the actual source of PFAS emissions requires adjusting the population values to a realistic magnitude of human presence. For this purpose, a population adjustment was done based on the concentration of ammonium (NH4-N) measured at the same sampling points used in the PFAS survey. This approach was applied previously when assessing population-dependent loads of other substances, such as narcotics,25 owing to the fact that NH4-N mainly occurs in wastewater due to urea hydrolysis, and is thus a marker of urine.26 Hence, an average population equivalent (PE) of 8 grams NH4-N per individual per day was taken from previous work,25 which allowed for the calculation of normalized loads LPFAS [μg] according to the following equation:
2.2 Sample preparation, analysis and quality control
For PFAS analysis, daily composite samples with 1 L of volume were taken from the three observed areas. The number of total samples per month ranged from 3 to 5, depending on the availability of local authorities to provide samples on a weekly basis. The obtained daily composite samples were kept refrigerated at 4 °C until the end of the month, when an overview of wastewater discharges was made available. A flow-proportional, 1 L monthly composite sample was created by taking all the daily samples within the given month and combining them together. The entire sampling campaign lasted for a year, from January 2022 until the end of December 2022, resulting in twelve monthly, flow-proportional samples for each sewer, as well as for the WWTP influent.After flow-proportional mixing, chemical analysis was undertaken. Prior to the monitoring campaign, a pre-selection procedure was conducted, in order to determine which PFAS compounds could be reliably analyzed. In these pre-monitoring trials, PFPeS, PFPeA, and PFOSA had rather poor recovery rates, and a decision was made to omit these substances for the duration of the monitoring in order to allocate resources to compounds with reliable analytical results. In the end, 29 compounds were selected for analysis (ESI 1.1†). Reusable items, such as bottles, tubes and syringes were rinsed with acid, base and water prior to use. The samples were then enriched by spiking 100 mL of the aliquot with an internal standard solution and then extracted via manual solid phase extraction (SPE). Two internal standard solutions were used for spiking, with Extracted Internal Standard (EIS) used before, and Non-extracted Internal Standard (NIS) after the SPE. For quantification, an external calibration was used, with at least five different methanol-diluted concentrations. Reference PFAS compounds in ultra-pure water (1 ng L−1) were used as positive control samples. The quality control and analysis protocol were made in accordance with the EPA 1633 norm.27 The details regarding the analytical procedures, instrumentation and materials used, and quality control are presented in the ESI 2–3.† Conventional water-quality parameters, namely Chemical Oxygen Demand (COD) and ammonium (NH4-N) were measured on-site for every single sample before mixing a monthly composite by the responsible authority in charge of the sewage and influent management, and results were made available for further use.
2.3 Statistical analysis
PFAS concentration data in this study was found to be skewed, resulting in a log-normal distribution. This is especially true for instances where there was a large number of censored values, i.e. below limit of quantification (LOQ). As such, the median value was chosen for further observation, comparison and interpretation of the PFAS analysis results.28 Omitting or replacing censored data with fixed values, such as LOQ or LOQ/2 should be avoided whenever possible, in order to limit bias of the overall dataset.29 For this reason, a statistical approach that enables processing datasets containing censored values in a robust way was used, namely the regression on order statistics (ROS) method.30 The ROS method was implemented by using the NADA31 package in the R programming environment.32 This enabled a quantitative approach in the statistical assessment of the concentration results. However, ROS does not provide single imputed values for specific censored data points, but delivers statistical estimates for the whole dataset by considering hypothetical distributions based on the occurrence of non-censored values. ROS in its true meaning could thus not be applied when calculating the ammonium-based per person load, which requires both values to be known at each specific time. Instead, in the case of ammonium-based load estimation, the censored values were replaced with the average of the range of values below LOQ imputed via the ROS method.The Kruskal–Wallis test33 was used to identify whether there was a significant difference among the three observed regions for each substance examined. This was followed by a posthoc approach, the Mann–Whitney U test,34 allowing for an in-depth inspection of areas monitored. Because the adjusted load estimation was calculated with two values, namely PFAS and ammonium concentrations, and the former exhibited censored values, there was a predisposition for an occurrence of Type 1 statistical error. To avoid this, a Bonferroni correction35 was applied to the post hoc results, and the adjusted p-values were used for the final determination of statistical significance. For the comparisons of concentrations, no adjustment was made since there was no interrelation of parameters nor multivariate statistical issues that might impact the analysis.
Originally, ROS-imputed values are considered tenuous, when more than 80% of the instances of the dataset is censored.31 However, this study has rather small sample sizes in its analysis, increasing the risk of questionable interpretation. Overview of the samples post-ROS has unraveled that the imputed values tend to be more reliable with four or more values above LOQ. Therefore, to present more robust results, the minimal threshold for statistical interpretation was set at 75% of censored values. Where one of the three examined areas had censored values above this threshold, the area was omitted from statistical analysis, and the remaining areas were tested with the Mann–Whitney U-test amongst themselves.
In order to examine the interrelation between PFAS and the two sewer-specific characteristics (NH4-N and COD), Principal Component Analysis (PCA) was conducted. As in the case of ammonium-based per-person PFAS loads, censored values were replaced with an average value of the range imputed via the ROS method. In an effort to reduce bias, only the most frequently occurring PFAS were selected for the PCA analysis, and the influent results were omitted so as not to disturb the purpose of the evaluation, which was the dichotomy examination between two sewers.
3 Results
3.1 Annual survey of PFAS concentrations
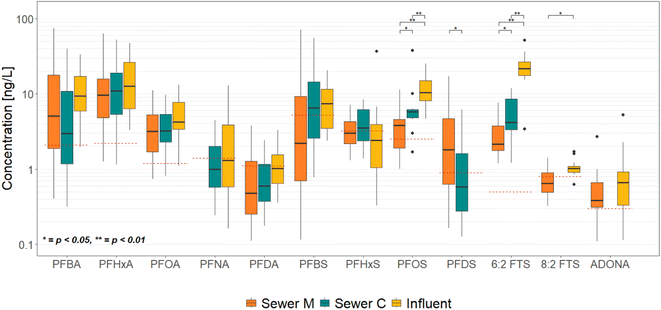
Concentrations of PFAS that were present above LOQ in more than four instances throughout the year are represented graphically in the form of a boxplot in Fig. 1. Out of 29 initially considered PFAS compounds, 12 are presented in this analysis, having more than 4 values above LOQ in at least two sewers. The full list of measured values for all analyzed PFAS is reported in ESI.†For PFCAs, the concentrations ranged from less than 0.1 up to 75 ng L−1, with the highest median concentration for the group being that of PFHxA at 12.7 ng L−1. For PFSAs, the range was from 0.1 to 72 ng L−1, with the highest median value noted for PFOS at 10.5 ng L−1. The highest overall median value was noted in 6:2 FTS, a fluorotelomer compound, at 21.8 ng L−1.
Statistical differences (unadjusted Kruskal–Wallis, p < 0.05) were found for PFOS, PFDS, 6:2 FTS and 8:2 FTS, and are presented in Fig. 1. When observing pairwise comparisons, PFOS and 6:2 FTS concentrations were significantly lower in the municipal sewer than in the commercial one. For these two compounds, the influent median concentrations were significantly higher than what was found in either sewer C or sewer M. 8:2 FTS concentrations were significantly higher in the influent than in sewer M, while the sewer C was omitted from investigation owing to a high number (>75%) of censored values. PFDS concentrations were higher in sewer M than in sewer C, whereas the influent was omitted.
3.2 Principal component analysis
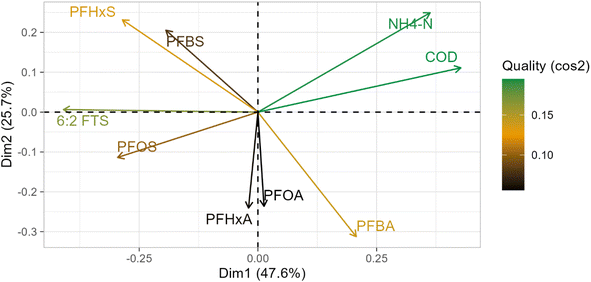
The PCA conducted for the most frequently detected PFAS is presented in Fig. 2, whereas the correlation matrix obtained via Kendall's Tau is presented in ESI.† The first principal component (PC 1) explained 47.6% variability, whereas the second (PC 2) explained 25.7%, cumulatively accounting for 73.3% of the total variance. PC 1 has high positive loadings for both NH4-N and COD, with a strong correlation between them (p < 0.05, R = 0.56). This indicates that the main drivers of variance are the contrasting types of land use and activities in the observed sub catchments, confirmed by the varying concentrations of these two parameters. 6:2 FTS and PFOS were the only two compounds where there was linear divergence and, therefore, an implied negative correlation with these two parameters. Significant negative correlation (p < 0.05) was observed between 6:2 FTS and NH4-N (R = 0.31), as well as between PFOS and COD (R = 0.30). The other chemicals, albeit grouped together, were near the 90° angle from these parameters, with the sulfonic group of PFAS (PFHxS and PFBS) occupying a contrasting quadrant compared to the carboxylic group of PFAS (PFHxA, PFOA, and PFBA), indicating that the parameters NH4-N and COD could not adequately explain the dynamics of the two groups in this assessment. This was further confirmed by the lack of statistically significant correlation.3.3 Ammonium-adjusted PFAS loads
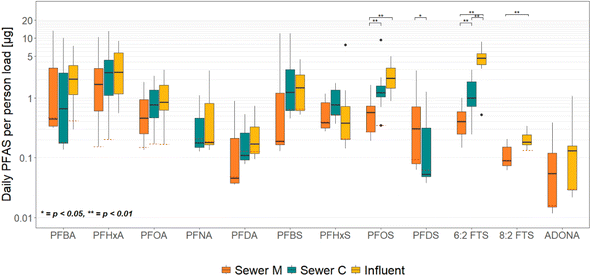
The average concentration of NH4-N throughout the sampling campaign was 52.4 and 35.7 mg L−1 for municipal and commercial sewers, respectively. For the influent, the average NH4-N concentration was 41.1 mg L−1, which is in between the values of the sewers. Back-calculating the actual active population in the catchment area of each sewer from such values leads to an estimation of a 60% increase in people operating in the municipal area and a 20% increase in the commercial area on average, compared to the original cadastral population data. The monthly averages, based on the days the wastewater was sampled for PFAS, are shown in ESI.†The ammonium-adjusted PFAS daily per capita loads for the year 2022 are presented in Fig. 3. All the chemicals that were selected for representation during the assessment of concentrations in Section 3.1 were used for the estimation of loads as well. The median, 25th, and 75th percentiles of the represented loads are presented in ESI.†
The overall per-person adjusted PFAS loads ranged from slightly above 0.01 up to 13.2 g per person per day. The highest load of the PFCA compounds was noted for PFHxA in the influent (2.7 g per person per day), whereas influent PFOS had the highest median from the PFSA group (2.15 g per person per day). 6:2 FTS found in the influent had the overall highest median load at 4.6 g per person per day.
The statistical results between the concentrations and the loads coincide to a large extent, with few differences noticed (Table 2). PFOS was not significantly different in terms of median per person load between the commercial sewer and the influent, whereas both of them were significantly higher compared to the municipal sewer. This is a different outcome from the statistical results of concentrations covered beforehand, where all three sewers were significantly different from each other. The pairwise comparison for 6:2 FTS loads, on the other hand, showed that all three monitored areas were significantly different from each other, with municipal sewer again exhibiting the lowest loads per person, and the influent the highest one. In the case of 8:2 FTS, the influent load was significantly higher when compared to the municipal values. PFDS loads were the same in terms of differences as in the concentration assessment, with the municipal sewer having higher values than the commercial. The quantitative depiction of the 25th, 75th, and 50th percentile, with respect to the most frequently detected PFAS, is demonstrated in ESI.†
| Substance | # Of uncensored values | # Of uncensored values | # Of uncensored values | Statistical difference, loads (p < 0.05) | Statistical difference, concentrations (p < 0.05) |
|---|---|---|---|---|---|
| sewer M | sewer C | Influent | |||
| PFBA | 5 | 8 | 11 | — | — |
| PFHxA | 11 | 11 | 12 | — | — |
| PFOA | 9 | 11 | 11 | — | — |
| PFNA | 3* | 4 | 4 | — | — |
| PFDA | 4 | 4 | 6 | — | — |
| PFBS | 5 | 6 | 9 | — | — |
| PFHxS | 5 | 7 | 7 | — | — |
| PFOS | 8 | 11 | 12 | M < C, M < influent | M < C, M < influent, C < influent |
| PFDS | 7 | 4 | 3* | M > C | M > C |
| 6:2 FTS | 12 | 12 | 12 | M < C, M < influent, C < influent | M < C, M < influent, C < influent |
| 8:2 FTS | 5 | 2* | 11 | M < influent | M < influent |
| ADONA | 8 | 2* | 8 | — | — |
4 Discussion
Our findings indicate that certain PFAS exhibit polarizing divergence within the urban wastewater system, based on their intended manufacturing and utilization purpose. PCA results have shown that PFOS and 6:2 FTS were linearly diverging from the ammonium and COD concentrations. PFOS and 6:2 FTS also exhibited a significantly higher population-specific load in sewer C and the total city influent, when compared to sewer M. These findings indicate that these two PFAS are likely to be heavily influenced by commercial activity occurring within the city. For the remainder of the examined PFAS, this seems to not be the case or cannot be clearly derived.For PFBA, PFHxA, PFOA, PFDA, PFBS and PFHxS loads in the commercial sewer or the overall influent are not statistically higher than in the municipal sewer. This indicates that these compounds are mainly influenced by consumer product use and diffuse inputs characteristic for an urban area, such as stormwater runoff or groundwater infiltration, which are considered equally intensive within the city network. In order to examine the possibility that these diffuse inputs could dilute, and therefore affect the actual concentration of PFAS reaching the sewers, we compared the wastewater discharge with calculated stormwater runoff, which was based on the official meteorological data. No correlation was found, meaning that we saw no noticeable trend of PFAS dilution due to stormwater input. Indeed, rainwater could dilute the PFAS discharged load, but it is also possible for it to be a PFAS contributor, either in the form of stormwater runoff, or through contaminated rainwater itself. As for the groundwater infiltration into sewers, no actual discharge data was available. The mass balance estimations conducted by Krlovic et al.23 for the same study area already suggested that these diffuse pathways can play a relevant role for the emission of PFAS into the sewer system, but new dedicated surveys are needed to quantify them. For remaining substances (PFNA, PFDS, 8:2 FTS and ADONA) the results, and therefore their interpretation is not as clear due to high shares of censored values.
Further strengthening the notion that 6:2 FTS and PFOS are strongly influenced by commercial activities is the variability of concentrations throughout the year-long sampling campaign (ESI 1†). Regardless of the time of year, these two compounds were consistently present in sewer C at a significantly higher level than in sewer M and with less variability than what was observed with other examined compounds. It is assumed that this is because commercial activities rarely cease to completely operate for a prolonged period of time, whereas other PFAS inputs are unpredictable and can occur in short, intense periods. An example of this can be an increased influx of people due to tourism season, resulting in a higher use of PFAS-containing consumer products. This could explain the much higher variability in the rest of the examined compounds.
To contextualize the results within a broader context or PFAS emissions in the urban water environment, the influent concentrations of the most frequently detected PFAS compounds in this study are compared to other published results of PFAS in raw wastewater from European cities (Table 3). PFAS compounds in our study are generally within the range of what was found in other publications, except in the case of 6:2 FTS, which was up to four times as high in a study by Gobelius et al.18 The authors noted that the area was influenced by a highly contaminated firefighting training site, which was not the case for our city and could thus explain the discrepancy, given that 6:2 FTS is the main PFAS found in newer AFFF formulations.38,39 However, more research on 6:2 FTS in European WWTPs are needed, as they are still largely missing from European-centered literature.8,40
| Publication | PFBA | PFHxA | PFOA | PFBS | PFHxS | PFOS | 6:2 FTS | Site details |
|---|---|---|---|---|---|---|---|---|
| a Few values available and no LOD/LOQ values available, hence average estimated with censored values set to 0. | ||||||||
| This study | 13.1 | 17.8 | 5.3 | 8.3 | 5.4 | 11.6 | 23.7 | Large central European city, single municipal WWTP, no heavy industry |
| 18 | 35 | 6.5 | 4.6 | 4.1 | 19 | 9 | 109 | Mainly domestic wastewater, Uppsala, Sweden |
| 75 | 15 | 8.9 | 4.9 | 34 | 9.1 | 56 | Domestic, industrial and commercial wastewater mixture, Uppsala, Sweden | |
| 21 | 5.4 | 2.05 | <LOD | 4.95 | 3.2 | 10.0 | NA | WWTP Leibnitz, Austria |
| 0.8 | 1.42 | <LOD | 1.7 | 1.1 | 3.8 | NA | WWTP Gösendorf, Austria | |
| 36 | 4.8 | 1.8 | 3.4 | n.d. | 6.9 | 11.1 | NA | Mean value of 10 WWTPs, predominantly municipal, with some industrial discharge |
| 6 | <LOD | 5.0 | 4.7 | 1.5 | 4.3 | 7.0 | NA | Municipal WWTP, Bromma, Sweden |
| <LOD | 6.7 | 2.9 | 0.2 | 0.4 | 1.8 | NA | Municipal WWTP, Bollebygd, Sweden | |
| <LOD | 3.8 | 3.2 | 1.1 | 1.5 | 3.3 | NA | Municipal WWTP, Umeå, Sweden | |
| 37 | NA | 1.7 | 16.5 | NA | 6.0 | 13.4 | NA | 80% municipal, 20% industrial wastewater, Athens, Greece |
| NA | <LOQ | 4.2 | NA | 6.8 | 3.5 | NA | 100% municipal wastewater, Athens, Greece | |
In the years since the 2009 amendment to the Stockholm Convention on Persistent Organic Pollutants41 PFOS was phased out of most of the consumer products. In more recent literature, concentrations of PFOS in apparel were mostly near or below the limit of quantification,42–44 and even if a higher-concentration example of apparel was taken into account in an emissions model, it would still not contribute significantly to the overall environmental emissions,12,45 let alone into the wastewater. The experimentally observed trend of an increase of PFAS concentration via the aging of apparel still does not explain the high PFOS loads in the wastewater. Apparel artificial aging experiment studies saw minimal to no increase in concentration of PFOS, in contrast to other substances from this group, which in some cases exhibited an increase up to 100-fold.46,47 With respect to cosmetic products, the vast majority that could be washed down the drain exhibited PFOS concentrations below LOQ.11,48 Yet in spite of these findings, the frequency of occurrence and the magnitude of PFOS in this assessment is one of the highest exhibited of all the examined PFAS, indicating that other, non-consumer related emissions are contributing to the load of PFOS to a greater extent.
In order to explain the unidentified loads, the framework of Krlovic et al.23 was utilized. This framework was designed to estimate the loadings of select PFAS in a municipal WWTP influent, based on available literature and consumer datasets. The sources included consumer products and diffuse inputs but did not consider commercial activities of an industrial nature, due to the lack of robust quantitative information. The authors have argued that the lack of inclusion of commercial inputs might have been responsible for the poor results of the model for certain PFAS, such as PFOS. In Fig. 4, the modeled values are compared with the per-person loads of sewer M, sewer C, and influent in order to identify the sources of each examined PFAS and any discrepancies in expected versus observed loads. If there were no significant discrepancies between the modeled results and the sewers observed in this work, it would indicate that those PFAS species would be ubiquitously present, thus most likely stemming from diffuse and consumer inputs. Conversely, a discrepancy between the modeled results with the sewer C loads would indicate that there is an additional input corresponding to the commercial nature of the catchment.
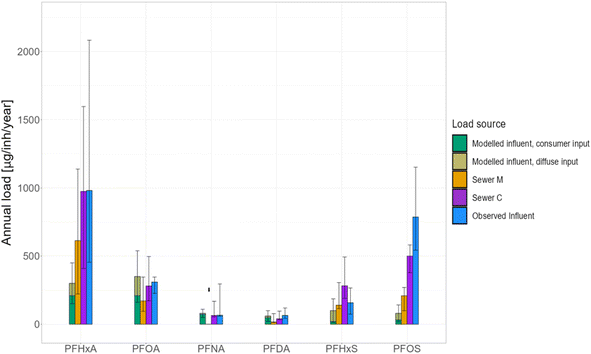 | ||
| Fig. 4 Modeled results from Krlovic et al.23 2024, compared to the loads calculated in this study. The upper section of the model bars represents the portion of the loads attributed to diffuse inputs, while the rest is attributed to consumer product use and activities. The water balance segment of the model has been adjusted to correspond to the meteorological situation of the sampling year. For PFNA in sewer M, only loads calculated from uncensored values are presented. | ||
The comparison of results was rather variable across PFAS. In the case of PFOS, the model could not account for more than 10% of the observed annual load in the total influent, and could account for a larger fraction when compared with sewer M. Sewer C load was more than double that of sewer M, and the influent PFOS load was the highest of all. These findings strengthen the argument that PFOS emission into municipal wastewater can be attributed to sources other than consumer product usage and diffuse inputs, such as commercial activities. The even higher per-person loads of PFOS in the influent have driven the search for further suspected emitters within the city boundaries. Additional business entities in other catchments within the studied city were identified, that fit the profile of potential PFOS emitters. These include the manufacturing of coatings, paints, and varnishes,49 as well as printing ink manufacturing.50 Moreover, Kibbey and colleagues51 investigated different emission hotspots to distinguish the sources based on the composition of the contaminated area. Among the included hotspots were the commercial activities related to metal plating, coatings and tanneries. In all of the cases where this was considered, PFOS was found in much higher magnitudes than any of the other examined PFAS. Although not directly related to the activities within the city that we monitored, these findings indicate the omnipresence of PFOS in areas of commerce. PFHxA loads, despite not being statistically different among the sewer catchments in this project, were not explained to a larger extent by the model. This is most likely due to the consumer data used in the model being from a time before the phaseout of longer chained PFAS, which are consequently more in line with the modeled values. Although the commercial sewer values were higher than those in the municipal, the uncertainty across all three catchments was too high to claim with certainty that commercial activities result in higher PFHxA emissions.
The aforementioned modeling procedure has not been conducted for 6:2 FTS due to lack of a complete dataset covering all relevant flows, but there are arguments that can explain the discrepancy of its loading among different areas of this study. Although this compound has been found in a vast array of products, such as cosmetics52 and textiles,44 it has been shown to have numerous applications in the industrial/commercial sector, more diverse compared to other examined PFAS. The commercial application possibilities include handling lithium-ion batteries, circuit board production, textile manufacturing and protection, paper and packaging, chrome plating, and automotive parts.5,24 A noteworthy finding is that, since the official phaseout of PFOS, 6:2 FTS has been used instead due to its structural similarity to PFOS, replacing it in industries where it was usually used.53,54 However, the heterogeneity of 6:2 FTS detection in products and processes, as well as the lack of information regarding commercial emitters means that 6:2 FTS can be released from a variety of different sources. A stronger focus, therefore, needs to be applied in the examination of this compound from a commercial and industrial perspective.
8:2 FTS and PFDS also exhibited significant differences when comparing per-person loads, but there were caveats that made it impossible to quantitatively interpret the findings for these substances. For instance, 8:2 FTS was found below LOQ in the majority of samples taken in the commercial sewer, and the influent loads are significantly higher than those from sewer M. Given that 8:2 FTS is found both in consumer products52 and commercial applications,55 the argument for such a result is that the inputs were not captured by a rather small number of data points collected during the sampling campaign. A similar situation was noted for PFDS, as it was the only instance of a compound where the municipal per-person loads were significantly higher than those found in the commercial sewer, with the influent results not considered due to the number of censored values. If the sources of PFDS were coming exclusively from municipal and diffuse processes, we would expect no statistical difference across the sampled sites. Indeed, according to the CHEMSEC database, PFDS was found solely in consumer products, specifically textile, leather, cleaning, and painting products, but no commercial processes.24 Therefore, we argue that this result is more likely an example of a statistical anomaly, further propagated by the ROS imputation. ADONA is another example where more focus needs to be directed. As of now, no commercial processes or consumer products are directly associated with ADONA concentrations, aside from its use as a fluoropolymer processing aid,5 which is not part of the activities within the monitored city. In our study, ADONA loads were mostly found in sewer M and influent, but not in sewer C, raising the question as to whether the result is another example of a monitoring anomaly or rather an example of unpredictable diffuse inputs (i.e., stormwater runoff or groundwater introduction). Overall, the concentrations close to LOQ meant that these substances could not be completely validated in this case study.
5 Conclusions
The presented study has assessed the differentiation in source allocation of PFAS with respect to contrasting sewage regions, based on their prevailing purpose. To the best of our knowledge, this study is the first of its kind to conduct a comprehensive comparison of PFAS within sewage, with respect to activities associated with each catchment. 6:2 FTS and PFOS loads were significantly higher in the commercial and total influent areas when compared with the municipal area. The rest of the examined PFAS did not exhibit such a difference among any of the observed sewers. This is a clear indication that there is a strong influence of commercial activities on the overall occurrence and emission intensity of 6:2 FTS and PFOS. 8:2 FTS and PFDS also exhibited significant differences, but the lack of uncensored values above the appropriate threshold of 75% needed for ROS handling meant that a statistical difference could not be identified with certainty. The remainder of the observed PFAS, however, are most likely not influenced by commercial activities but are rather dictated by consumer behavior and diffuse inputs, which are characteristic of a municipal setting. The divergence in the PCA results of ammonium ion concentrations from 6:2 FTS and PFOS further supports the argument that these two substances are emitted mostly from commercial activities, given that ammonium is a reliable indicator of the magnitude of human presence in an area. Moreover, 6:2 FTS is structurally similar to PFOS, allowing emitters to change formulations and retain desired characteristics. This means that loads of these two substances can be expected to come from similar emitters within an urban wastewater setting, based on their commercial purpose. This finding, therefore, marks an important contribution to the distinction of PFAS sources in an urban wastewater setting. Future work related to PFAS in urban wastewater should focus on a few different aspects. Wastewater discharges from suspected commercial emitters should be monitored to establish the occurrence and magnitude of PFAS from specific commercial sources, which are, in general, missing from peer-reviewed literature. Further, the relevance of specific sources could be investigated via a survey with sampling points located in urban sub-catchments with unique characteristics related to commercial activities.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study has been conducted within the project FATERISK-Aqua, which is funded by the Vienna Science and Technology Fund (WWTF) [10.47379/ESR20013]. The authors acknowledge TU Wien Bibliothek for financial support through its Open Access Funding Programme.References
- R. C. Buck, J. Franklin, U. Berger, J. M. Conder, I. T. Cousins and P. de Voogt,
et al., Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins, Integr. Environ. Assess. Manage., 2011, 7(4), 513–541 CrossRef CAS PubMed
.
- C. McCarthy, W. Kappleman and W. DiGuiseppi, Ecological Considerations of Per- and Polyfluoroalkyl Substances (PFAS), Curr. Pollut. Rep., 2017, 3(4), 289–301 CrossRef CAS
.
- OECD, Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs), 2018, available from, https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7%26doclanguage=en.
- J. B. Brown, J. M. Conder, J. A. Arblaster and C. P. Higgins, Assessing Human Health Risks from Per- and Polyfluoroalkyl Substance (PFAS)-Impacted Vegetable Consumption: A Tiered Modeling Approach, Environ. Sci. Technol., 2020, 54(23), 15202–15214 CrossRef CAS PubMed
.
- J. Glüge, M. Scheringer, I. T. Cousins, J. C. DeWitt, G. Goldenman and D. Herzke,
et al., An overview of the uses of per- and polyfluoroalkyl substances (PFAS), Environ. Sci.: Processes Impacts, 2020, 22(12), 2345–2373 RSC
.
- M. Filipovic and U. Berger, Are perfluoroalkyl acids in waste water treatment plant effluents the result of primary emissions from the technosphere or of environmental recirculation?, Chemosphere, 2015, 129, 74–80 CrossRef CAS PubMed
.
- S. Kurwadkar, J. Dane, S. R. Kanel, M. N. Nadagouda, R. W. Cawdrey and B. Ambade,
et al., Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution, Sci. Total Environ., 2022, 809, 151003 CrossRef CAS PubMed
.
- S. P. Lenka, M. Kah and L. P. Padhye, A review of the occurrence, transformation, and removal of poly- and perfluoroalkyl substances (PFAS) in wastewater treatment plants, Water Res., 2021, 199, 117187 CrossRef CAS PubMed
.
- S. Kittlaus, M. Clara, J. van Gils, O. Gabriel, M. B. Broer and G. Hochedlinger,
et al., Coupling a pathway-oriented approach with tailor-made monitoring as key to well-performing regionalized modelling of PFAS emissions and river concentrations, Sci. Total Environ., 2022, 849, 157764 CrossRef CAS PubMed
.
- T. P. Knepper, T. Frömel, C. Gremmel, I. van Driezum, H. Weil and R. Vestergren, et al., Understanding the exposure pathways of per- and polyfluoroalkyl substances (PFASs) via use of PFASs - Containing products – risk estimation for man and environment [Internet]. 06844 Dessau-Roßlau, Germany: Federal Environment Agency Germany, 2014, [cited 2021 Jul 11] p. 139. Report No.: (UBA-FB) 001935/E. available from: https://www.umweltbundesamt.de/publikationen/understanding-the-exposure-pathways-of-per.
- K. W. Pütz, S. Namazkar, M. Plassmann and J. P. Benskin, Are cosmetics a significant source of PFAS in Europe? product inventories, chemical characterization and emission estimates, Environ. Sci.: Processes Impacts, 2022, 24(10), 1697–1707 RSC
.
- R. Vestergren, D. Herzke, T. Wang and I. T. Cousins, Are imported consumer products an important diffuse source of PFASs to the Norwegian environment?, Environ. Pollut., 2015, 198, 223–230 CrossRef CAS
.
- G. Codling, H. Yuan, P. D. Jones, J. P. Giesy and M. Hecker, Metals and PFAS in stormwater and surface runoff in a semi-arid Canadian city subject to large variations in temperature among seasons, Environ. Sci. Pollut. Res., 2020, 27(15), 18232–18241 CrossRef CAS PubMed
.
- E. F. Houtz and D. L. Sedlak, Oxidative Conversion as a Means of Detecting Precursors to Perfluoroalkyl Acids in Urban Runoff, Environ. Sci. Technol., 2012, 46(17), 9342–9349 CrossRef CAS PubMed
.
- J. Reinikainen, N. Perkola, L. Äystö and J. Sorvari, The occurrence, distribution, and risks of PFAS at AFFF-impacted sites in Finland, Sci. Total Environ., 2022, 829, 154237 CrossRef CAS PubMed
.
- I. T. Cousins, J. H. Johansson, M. E. Salter, B. Sha and M. Scheringer, Outside the Safe Operating Space of a New Planetary Boundary for Per- and Polyfluoroalkyl Substances (PFAS), Environ. Sci. Technol., 2022, 56(16), 11172–11179 CrossRef CAS PubMed
.
- M. Clara, S. Scharf, S. Weiss, O. Gans and C. Scheffknecht, Emissions of perfluorinated alkylated substances (PFAS) from point sources—identification of relevant branches, Water Sci. Technol., 2008, 58(1), 59–66 CrossRef CAS PubMed
.
- L. Gobelius, L. Glimstedt, J. Olsson, K. Wiberg and L. Ahrens, Mass flow of per- and polyfluoroalkyl substances (PFAS) in a Swedish municipal wastewater network and wastewater treatment plant, Chemosphere, 2023, 336, 139182 CrossRef CAS PubMed
.
- C. Gallen, G. Eaglesham, D. Drage, T. H. Nguyen and J. F. Mueller, A mass estimate of perfluoroalkyl substance (PFAS) release from Australian wastewater treatment plants, Chemosphere, 2018, 208, 975–983 CrossRef CAS PubMed
.
- H. Hamid and L. Li, Role of wastewater treatment plant in environmental cycling of poly- and perfluoroalkyl substances, Ecocycles, 2016, 2(2), 43–53 CrossRef
.
- V. Müller, A. Kindness and J. Feldmann, Fluorine mass balance analysis of PFAS in communal waters at a wastewater plant from Austria, Water Res., 2023, 244, 120501 Search PubMed
.
-
Environmental Health Matters Initiative, Division on Earth and Life Studies, National Academies of Sciences, Engineering, and Medicine. Understanding, Controlling, and Preventing Exposure to PFAS, Proceedings of a Workshop in Brief, ed. A. Johnson, M. Shelton-Davenport and E. Mantus, National Academies Press, Washington, D.C, 2020, [cited 2024 Aug 16]. available from: https://www.nap.edu/catalog/25856 Search PubMed
.
- N. Krlovic, E. Saracevic, J. Derx, C. Gundacker, J. Krampe and M. Zessner,
et al., A source-based framework to estimate the annual load of PFAS in municipal wastewater, Sci. Total Environ., 2024, 920, 170997 CrossRef CAS PubMed
.
-
CHEMSEC, Chemsec PFAS Guide, 2023, Chemsec PFAS Guide, available from: https://pfas.chemsec.org/ Search PubMed
.
- F. Been, L. Rossi, C. Ort, S. Rudaz, O. Delémont and P. Esseiva, Population Normalization with Ammonium in Wastewater-Based Epidemiology: Application to Illicit Drug Monitoring, Environ. Sci. Technol., 2014, 48(14), 8162–8169 CrossRef CAS PubMed
.
- K. M. Udert, T. A. Larsen and W. Gujer, Fate of major compounds in source-separated urine, Water Sci. Technol., 2006, 54(11–12), 413–420 CrossRef CAS PubMed
.
-
USEPA, Draft Method 1633 Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS, USEPA, 2021, available from: https://www.epa.gov/system/files/documents/2021-09/method_1633_draft_aug-2021.pdf Search PubMed
.
-
M. Pagano and K. Favreau, Principles of Biostatistics, Chapman and Hall/CRC, 2nd edn, 2018, [cited 2023 Jan 17]. available from: https://www.taylorfrancis.com/books/9780429489624 Search PubMed
.
- A. J. Turkson, F. Ayiah-Mensah and V. Nimoh, Handling Censoring and Censored Data in Survival Analysis: A Standalone Systematic Literature Review, Int. J. Math. Math. Sci., 2021, 1–16 CrossRef
.
-
D. R. Helsel, Statistics for Censored Environmental Data Using Minitab and R, Wiley series in statistics in practice, Wiley, Hoboken, N.J, 2nd edn, 2012, p. 324 Search PubMed
.
-
L. Lee, NADA: Nondetects and Data Analysis for Environmental Data, The Comprehensive R Archive Network, 2020, available from: https://cran.r-project.org/web/packages/NADA/index.html Search PubMed
.
-
R Core Team, R: A Language and Environment for Statistical, R Foundation for Statistical Computing, Vienna, Austria, 2022, available from: https://www.R-project.org/ Search PubMed
.
- W. H. Kruskal and W. A. Wallis, Use of Ranks in One-Criterion Variance Analysis, J. Am. Stat. Assoc., 1952, 47(260), 583–621 CrossRef
.
- H. B. Mann and D. R. Whitney, On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other, Ann. Math. Stat., 1947, 18(1), 50–60 CrossRef
.
-
C. E. Bonferroni, Teoria statistica delle classi e calcolo delle probabilità, Pubblicazioni R Ist Super Sci Econ E Commer Firenze, 1936 Search PubMed
.
- M. Lorenzo, J. Campo, M. Morales Suárez-Varela and Y. Picó, Occurrence, distribution and behavior of emerging persistent organic pollutants (POPs) in a Mediterranean wetland protected area, Sci. Total Environ., 2019, 646, 1009–1020 CrossRef CAS PubMed
.
- O. S. Arvaniti, E. I. Ventouri, A. S. Stasinakis and N. S. Thomaidis, Occurrence of different classes of perfluorinated compounds in Greek wastewater treatment plants and determination of their solid–water distribution coefficients, J. Hazard. Mater., 2012, 239–240, 24–31 CrossRef CAS PubMed
.
- X. Dauchy, V. Boiteux, C. Bach, C. Rosin and J. F. Munoz, Per- and polyfluoroalkyl substances in firefighting foam concentrates and water samples collected near sites impacted by the use of these foams, Chemosphere, 2017, 183, 53–61 CrossRef CAS PubMed
.
-
K. E. M. I. Chemical, Analysis of Selected Fire-Fighting Foams on the Swedish Market 2014 PM 6/15, 2015, Article number: 511 163. kemikalieinspektionen.se, available from, https://www.kemi.se/download/18.6df1d3df171c243fb23960dd/1591097411709/pm-6-15.pdf Search PubMed
.
- O. S. Arvaniti and A. S. Stasinakis, Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment, Sci. Total Environ., 2015, 524–525, 81–92 CrossRef CAS PubMed
.
-
United Nations, Stockholm Convention on Persistent Organic Pollutants (POPs), as Ammended in 2009, United Nations, 2009 Search PubMed
.
- C. Gremmel, T. Frömel and T. P. Knepper, Systematic determination of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in outdoor jackets, Chemosphere, 2016, 160, 173–180 CrossRef CAS PubMed
.
-
L. Hanssen and D. Herzke, Investigation of Outdoor Textiles with Respect to Determine the Conent of Ionic Perfluorinated Substances (PFAS), Kjeller: NILU, 2015, Report No.: M306 Search PubMed
.
- A. K. Tokranov, N. Nishizawa, C. A. Amadei, J. E. Zenobio, H. M. Pickard and J. G. Allen,
et al., How Do We Measure Poly- and Perfluoroalkyl Substances (PFASs) at the Surface of Consumer Products?, Environ. Sci. Technol. Lett., 2019, 6(1), 38–43 CrossRef CAS PubMed
.
- M. Kotthoff, J. Müller, H. Jürling, M. Schlummer and D. Fiedler, Perfluoroalkyl and polyfluoroalkyl substances in consumer products, Environ. Sci. Pollut. Res., 2015, 22(19), 14546–14559 CrossRef CAS
.
- I. van der Veen, S. Schellenberger, A. C. Hanning, A. Stare, J. de Boer and J. M. Weiss,
et al., Fate of Per- and Polyfluoroalkyl Substances from Durable Water-Repellent Clothing during Use, Environ. Sci. Technol., 2022, 56(9), 5886–5897 CrossRef CAS PubMed
.
- I. van der Veen, A. C. Hanning, A. Stare, P. E. G. Leonards, J. de Boer and J. M. Weiss, The effect of weathering on per- and polyfluoroalkyl substances (PFASs) from durable water repellent (DWR) clothing, Chemosphere, 2020, 249, 126100 CrossRef CAS PubMed
.
-
Ministry of Environment and Food Denmark, Risk Assessment of Fluorinated Substances in Cosmetic Products, Danish Environmental Protection Agency, 2018 Search PubMed
.
- X. Liu, Z. Guo, K. A. Krebs, R. H. Pope and N. F. Roache, Concentrations and trends of perfluorinated chemicals in potential indoor sources from 2007 through 2011 in the US, Chemosphere, 2014, 98, 51–57 CrossRef CAS PubMed
.
-
D. Herzke, S. Posner and E. Olsson, Survey, Screening and Analyses of PFCs in Consumer Products. RISE, 2009, available from: http://www.swereaivf.se Search PubMed
.
- T. C. G. Kibbey, R. Jabrzemski and D. M. O'Carroll, Source allocation of per- and polyfluoroalkyl substances (PFAS) with supervised machine learning: Classification performance and the role of feature selection in an expanded dataset, Chemosphere, 2021, 275, 130124 CrossRef CAS PubMed
.
- L. Schultes, R. Vestergren, K. Volkova, E. Westberg, T. Jacobson and J. P. Benskin, Per- and polyfluoroalkyl substances and fluorine mass balance in cosmetic products from the Swedish market: implications for environmental emissions and human exposure, Environ. Sci.: Processes Impacts, 2018, 20(12), 1680–1690 RSC
.
- W. J. Backe, T. C. Day and J. A. Field, Zwitterionic, Cationic, and Anionic Fluorinated Chemicals in Aqueous Film Forming Foam Formulations and Groundwater from U.S. Military Bases by Nonaqueous Large-Volume Injection HPLC-MS/MS, Environ. Sci. Technol., 2013, 47(10), 5226–5234 CrossRef CAS PubMed
.
- X. Yang, J. Huang, K. Zhang, G. Yu, S. Deng and B. Wang, Stability of 6:2 fluorotelomer sulfonate in advanced oxidation processes: degradation kinetics and pathway, Environ. Sci. Pollut. Res., 2014, 21(6), 4634–4642 CrossRef CAS PubMed
.
- PubChem. PubChem, 2022, [cited 2022 Feb 7]. CAS2019 (JP2009145658), available from, https://pubchem.ncbi.nlm.nih.gov/compound/3016044#section=Use-and-Manufacturing.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00415a |
| This journal is © The Royal Society of Chemistry 2024 |