Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Removal of Pb-based compounds mediated by graphene oxide-like materials obtained from Sargassum: unravelling key features of their interaction using density functional theory and spectroscopic methods†
Sandra Julieta
Gutierrez-Ojeda
a,
Rocío
Martínez-Flores
*b,
Raúl
Pareja-Rodríguez
c,
Geonel
Rodriguez-Gattorno
c,
Rodrigo
Ponce-Pérez
*d,
María G.
Moreno-Armenta
d and
Jonathan
Guerrero-Sánchez
 d
d
aInstituto de Física, Universidad Nacional Autónoma de México, 04510 Ciudad de México, Mexico
bTecnológico Nacional de México, TecNM, Instituto Tecnológico Superior de Monclova, Carretera 57 km. 4.5, 25733 Monclova, Coahuila, Mexico. E-mail: rocio.mf@monclova.tecnm.mx
cDepartamento de Física Aplicada, CINVESTAV-IPN, Carr. Antigua a Progreso km. 6, CORDEMEX, 97310 Mérida, Yucatán, Mexico
dCentro de Nanociencias y Nanotecnología, Universidad Nacional Autónoma de México, Km. 107, Apdo. 14 Carretera Tijuana, Ensenada, Baja California, Mexico. E-mail: rponce@ens.cnyn.unam.mx
First published on 23rd August 2024
Abstract
Graphene oxide obtained from biomass possesses a rich variety of properties and applications. Sargassum, a macroalgae abundant in the Caribbean Sea, has been proposed as a viable and cost-effective biomass. Graphene oxide derived from Sargassum has an unprecedented selectivity for Pb capture in the hydrocerussite phase. This study presents exhaustive theoretical and experimental efforts to understand the interaction between Pb-based molecules (Pb, Pb(CO3)3, and Pb(OH)2) with representative models of the functional groups present in graphene oxide (COOH, OOH, OH, and O). Our results demonstrate that CO3 enhances the Pb adsorption on the graphene oxide surface. Hydrogen bonds and van der Waals interactions between CO3 of the Pb(CO3)3 with the GO surface are the driving forces to improve the Pb capturing process and further hydrocerussite formation on GOs. Although Pb and Pb(OH)2 can also be trapped by graphene oxide, it is less probable from an energetic point of view. Here, we demonstrate that low-cost graphene oxide obtained from Sargassum can be useful in environmental remediation.
Environmental significanceGraphene oxide (GO) obtained from Sargassum has demonstrated its viability in removing heavy metals such as Pb from water because of their intrinsic functional groups on their surface. Using Sargassum as a precursor of GOs, we can reduce their impact on Mexican coasts, and also, GOs help us to remove Pb2+ from water by the formation of hydrocerussite minerals. |
Introduction
Water pollution is one of the most critical global challenges. Heavy metals are a major concern among water contaminants due to their toxicity and carcinogenic effects.1 Also, they are not biodegradable and tend to bioaccumulate, increasing their concentration in living organisms. The most common water contaminants are those obtained from petroleum refineries and electronic waste: antimony (Sb), arsenic (As), cadmium (Cd), and chromium (Cr). Heavy metal contaminants such as mercury (Hg) and lead (Pb) mainly stem from pesticides, fertilizers, insecticides, and industrial waste.2Lead ion (Pb2+) is one of the most toxic ions since it can severely harm human bodies.3,4 As a result, the efficient separation/removal of Pb2+ from aqueous solutions is critical and has attracted much attention.5 Consequently, multiple approaches have been developed to remove Pb from water. For example, chemical precipitation,6 ion exchange, and electrochemical removal.7 However, the facilities, operation, and maintenance are significant disadvantages. Despite this, adsorption has been considered one of the most economical and standard methods in water purification. In this sense, oxygen-containing functional groups perform remarkably, such as carbon nanotubes, clay minerals, metal oxides, and bio-adsorbents.8–13
Graphene oxide (GO), consisting of a hexagonal network of covalently bonded carbon (C) atoms, has attracted multidisciplinary research due to its two-dimensional structure and unique physicochemical properties.14 GOs with oxygen-containing functional groups are very suitable for pollutant trapping due to their excellent remotion ability.15,16 GOs can be obtained by conventional thermal decomposition of biomass.17,18 Surprisingly, GOs from Sargassum sp. have shown impressive Pb2+ absorption capacities by forming a very stable mineral phase known as hydrocerussite.19 This is unexpected since GOs from Sargassum tend to have a high calcium concentration, which supposedly interferes with lead absorption.20 Understanding the carbonaceous residue interaction with Pb2+ is important because they are the primary materials for removing this toxic metal. In this way, Pareja-Rodríguez et al.19 suggest that the interface displays high interaction with Pb2+ in aqueous solutions due to functional groups (OH, C–O, CO3). They suggested that the functional groups in GOs obtained from Sargassum play an important role in Pb2+ removal because of their interaction with CO3 groups, inducing a hydrophilic character and chemical reactivity superior to graphene. However, an atomic scale analysis to understand how Pb-based compounds from hydrocerussite minerals are removed has yet to be reported.
With this precedent, we investigate the Pb-based compounds' remotion mechanisms by GOs using quantum mechanics calculations in combination with experimental characterization techniques: Raman, infrared (FTIR), and X-ray photoelectron spectroscopy (XPS). We account for Pb0, Pb(CO3)3, and Pb(OH)2 molecules (in which Pb is a cation Pb2+) adsorbed on the GO functionalized surface. The results denote that the unique features of some functional groups in GOs obtained from Sargassum allow Pb2+ capture, generating H bonds and weak van der Waals interactions that enhance the surface interaction and hydrocerussite growth on the functionalized monolayer.
Methods
Computational methods
The adsorption of Pb0, Pb(CO3)3, and Pb(OH)2 onto GOs was studied by DFT calculations in the absence of temperature. The calculations were done using the Vienna Ab initio Simulation Package (VASP)21–23 code. The electronic states were expanded using the projector-augmented wave (PAW) basis24,25 with a cutoff energy of 480 eV. The exchange–correlation energies were treated using the generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) parametrization.26 The DFT-D3 correction of Grimme is adopted to describe the van der Waals (vdW) interaction.27 The GOs were simulated using the supercell method. Since our calculations are periodic, the supercell includes a vacuum space perpendicular to graphene larger than 15 Å to avoid interaction between periodic slabs. Also, the supercell is formed by 5 graphene unit cells of length along the x and y axis to form a 5 × 5 × 1 periodicity. In the geometry optimization, the convergence criterion for all force components must be less than 0.026 eV Å−1 and total energy differences are less than 1 × 10−4 eV. The Brillouin zone is sampled with a gamma-centered k-points grid of 7 × 7 × 1, employing the Monkhorst–Pack scheme.28 Non-covalent interactions were calculated using the CRITIC2 software.29 For more details about parameter optimization, see the ESI.†Experimental characterization techniques
The Raman spectra were obtained using an Alpha 300 WITEC equipment (ALPHA 300RA, Lise-Meitner, Germany), utilizing a Ne (blue) laser with a λ = 488 nm. To ensure the safety of the samples, the laser power was maintained below 10 mW throughout the measurements to prevent potential sample damage caused by laser irradiation. X-ray photoelectron spectroscopy analysis was conducted using a K-Alpha Thermo Scientific (XPS, Waltham, USA) spectrometer with an Al Kα X-ray monochromatic source (1486.6 eV) at 12 kV and 40 W. The analyzed area was 400 μm2, using an incident relative angle of 30°. Before analysis, the surface of the samples was treated by argon (Ar) erosion for 30 s (3 kV and 30 W). The deconvolution of the XPS spectra was performed with different mathematical functions (Lorentzian, Gaussian, and their combinations). X-ray diffraction patterns were collected using a Bruker D-8 Advance diffractometer (Bruker, Billerica, USA) operating at 40 kV and 30 mA in the angular range 5–60° at a step interval of 0.02° and a counting time of 0.5 s with Cu-Kα1 radiation.Results and discussion
First, the graphene monolayer was optimized from scratch. Our calculated cell parameter is a = 2.47 Å, which agrees with previous reports.30 Since pristine graphene is not reactive, H, O, or OH functionalized graphene promotes active sites, conferring physicochemical properties suitable for Pb-based compound removal. Characterization techniques allowed us to differentiate the geometries of carbon atoms and detect the presence of functional groups (OH, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, COOH, and CO32−). All these functional groups may provide the chemical characteristics to GOs for acting as a very efficient adsorbent material.
O, COOH, and CO32−). All these functional groups may provide the chemical characteristics to GOs for acting as a very efficient adsorbent material.
Previously, Paez-Ornelas et al.31 reported four stable GO configurations containing COOH, OH, and O functional groups. We reproduce their models from scratch using a graphene layer. The atomic representation of the structures can be seen in Fig. 1, where the models are labeled as C1, C2, C3, and C4. These models align with the experimentally observed functional groups, so we use them to study the Pb-based compound capture.
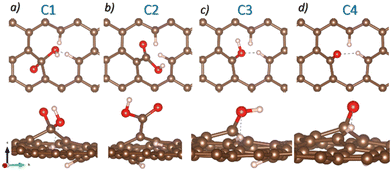 | ||
| Fig. 1 Top and side views of the four GO atomic models. a) C1, b) C2, c) C3, and d) C4. Brown, red, and white spheres represent C, O, and H atoms. | ||
The adsorption energy (Eads) of the functional groups or Pb-based molecules follows the equation:
| Eads = Eslab − Eref − Efunct group/molecule | (1) |
Although Eads allows us to see that all functional groups favorably adsorb on the graphene sheet, and we cannot compare the stability of these models since they have a different number of atoms. In this way, the defect formation energy (DFE) formalism – that does not depend on the number of atoms but on the chemical potentials – is appropriate to describe our system's stability.32–34 The DFE is defined as
 | (2) |
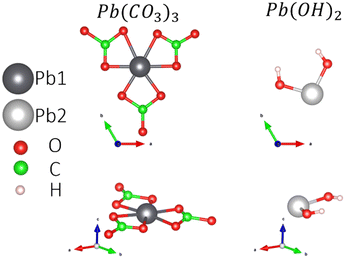
Experimentally, a flake shape is observed when the Pb2+-containing moieties are adsorbed on GOs.19 The flake shape on the surface is similar in morphology to hydrocerussite ([Pb3(CO3)2(OH)2]), as reported by Siidra et al.35 This mineral has unit cell parameters of a = 5.248(3) and c = 23.66(1) Å. Additionally, it has the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m with Wyckoff positions 6c and 18h for Pb1 and Pb2, respectively.
m with Wyckoff positions 6c and 18h for Pb1 and Pb2, respectively.
To understand the Pb-based compound adsorption process on the GOs, we accounted for the hydrocerussite constituents: the mineral cerussite, Pb(CO3), and Pb(OH)2 sheets, which contain OH groups and Pb2+. So, the isolated Pb0 and the molecules Pb(CO3)3 (see the ESI† for more details) and Pb(OH)2 are adsorbed on the four possible functional groups in the GOs. Fig. 2 shows the atomic configurations of the molecules considered in this work. The treated constituents from the hydrocerussite cif file were simulated in a cubic box of 20 Å.
Table 1 summarizes the DFE values for the Pb0, Pb(CO3)3, and Pb(OH)2 molecules adsorbed on the four GO models. For each molecule adsorbed in a different functional group, we consider different orientations for the interaction to find the most stable configuration; in the manuscript, we focus on the most stable model for each case.
| GO configuration | DFE (eV Å−2) | ||
|---|---|---|---|
| Pb0 | Pb(CO3)3 | Pb(OH)2 | |
| C1 | −0.10 | −0.58 | −0.25 |
| C2 | −0.16 | −0.66 | −0.30 |
| C3 | −0.06 | −0.59 | −0.22 |
| C4 | −0.07 | −0.55 | −0.20 |
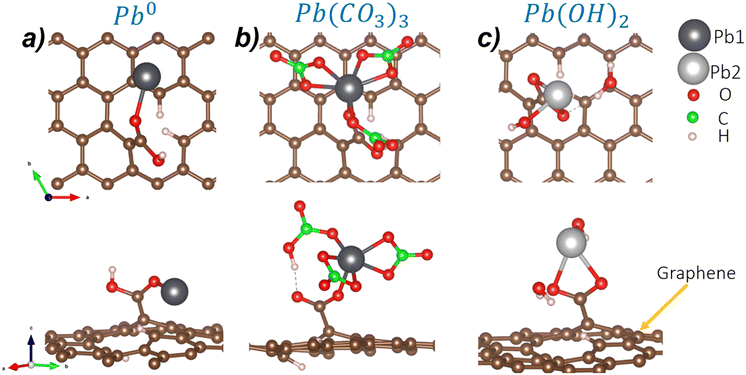
First, we focus on the isolated Pb0. Regarding the COOH functionalized GOs (C2 model), Pb0 can interact with the O from the CO or the OH group. The interaction with the CO group is the most favorable configuration with a DFE value of −0.16 eV A−2. The atomistic representation of this configuration is depicted in Fig. 3a. Here, Pb0 bonds to O from the carbonyl group with a bond distance of 2.25 Å. Also, the surface–Pb separation distance is 2.82 Å. These results show that Pb–O bonds are stable on GOs. The C1, C3, and C4 configurations display higher defective formation energies (−0.10 eV Å−2, −0.06 eV Å−2, −0.07 eV Å−2, respectively), and are schematized in Fig. S3 of the ESI.†
In the case of the Pb(CO3)3 molecule (Pb2+), the most stable interaction is with the C2 GO configuration with a DFE of −0.66 eV Å−2. Here, the adsorption takes place in the carbonyl group. Also, we notice a ligand exchange between the OH group from GOs and one of the carbonate groups of the Pb(CO3)3 molecule. After the interaction, Pb is still sixfold coordinated, retaining the stability of the Pb2+ moiety, as shown in Fig. 3b. The Pb1–O bond distance is 2.25 Å, while the distance between the surface and the Pb1 atom is 4.57 Å. Although the C1 functional group is similar to C2, the Pb(CO3)3 adsorption on the C1 site is less stable by 0.08 eV Å−2, probably because ligand exchange does not happen spontaneously compared to the C1 site. Also, as the COOH functional group in C1 is formed in C of the graphene structure, steric effects may induce the large formation energy.
On the other hand, C3 and C4 sites are less stable by 0.07 eV Å−2 and 0.11 eV Å−2, respectively. In C3, ligand exchange appears; however, there is no evident chemical interaction between Pb and O, which is the main reason behind the lower DFE. Finally, C4 is the site with the larger DFE because weak vdW interactions hold the O–Pb(CO3)3 interaction. Although the C1, C3, and C4 functional groups are less stable, experimentally, it has been shown that they may participate under N and air atmospheres.19 Considering the previous results, COOH groups are the most prominent for Pb2+ adsorption on GOs.5 Fig. S4† shows the less stable models for Pb(CO3)3 adsorption.
Finally, we analyze the adsorption of the Pb(OH)2 molecule (Pb2+) on the different GO models. The DFE displays that the C2 configuration, again, is the most stable due to the interaction between an H atom from the COOH group and the OH group from the Pb(OH)2 molecule, generating a physisorbed H2O molecule and chemical O–Pb–O bonds with distances of 2.43 Å and 2.38 Å with the COO functional group (see Fig. 3c). Also, the Pb2–surface distance is 5.03 Å. When the molecule is adsorbed on C1, the DFE is higher. In this case, an H2O molecule is generated due to the ligand exchange between the OH group of the C1 functional group and one OH of the Pb(OH)2 molecule. The H2O molecule interacts through weak vdW interactions with one O atom of the COO group, and the Pb attaches to the remaining O atom. The main difference between C1 and C2 is the Pb bonding; in C1, it forms one bond, while in C2, it is two-fold coordinated. Pb(OH)2 adsorption on C3 and C4 sites shows less stable DFEs by 0.08 eV Å−2 and 0.10 eV Å−2, respectively. See Fig. S5† for structural details of the unstable adsorption sites. Table 2 summarizes the bond distances obtained in the most stable cases for each molecule.
| System | Bond length (Å) | ||||
|---|---|---|---|---|---|
| Pb1–O | Pb2–O | Pb2–OH | Pb1–surface | Pb2–surface | |
| C2–Pb0 | 2.25 | — | — | 2.82 | — |
| C2–Pb(CO3)3 | 2.23 | — | — | 4.57 | — |
| C2–Pb(OH)2 | — | 2.43 | 2.13 | — | 5.03 |
Upon analyzing all the possible adsorption sites for the Pb-based molecules on the different functional groups, we noticed that the C2 configuration is the most viable configuration to trap Pb through oxygen interaction and ligand exchange. As evidenced in the experiment, all the functional groups trap Pb, however, COOH is the most efficient and desirable for its removal.
To identify the different kinds of interactions present in the adsorption of Pb0, Pb(CO3)3, and Pb(OH)2 on COOH functionalized GO surface, we investigate the non-covalent interactions (NCIs).36,37 The NCI is a useful tool that helps us distinguish between the different interactions in our systems. We investigated the NCIs by calculating the reduced density gradient, s[ρ(r)]:
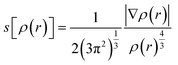 | (3) |
Fig. 4 exhibits the NCI index for the adsorption of Pb and Pb compounds on the GOs. Fig. 4a displays their corresponding isosurface (s = 0.5 a.u), where blue isosurfaces show attractive interactions, red color indicates repulsive interactions, and green isosurfaces are vdW interactions. The results show attractive interaction between Pb and O from COOH; similar behavior is noticed between Pb and the GO surface, and vdW forces are noticed around the attractive interaction. Fig. 4b shows the s[ρ(r)] vs. sign(λ2)ρ(r) plot, where the purple surface denotes all the interactions in the system and the green surface represents the Pb–surface interaction. We focus on the low s and low ρ regions to distinguish the non-covalent interactions. The sharp peak observed at −0.07 corresponds with the bond Pb–O formed after the interaction, while the peak at −0.05 is attributed to the interaction that ocuurs between Pb and graphene. Also, the green surface at low s and close to zero ρ indicates vdW interactions between Pb and the surface.
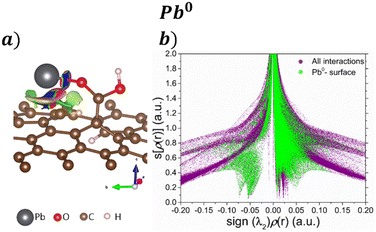 | ||
| Fig. 4 a) NCI isosurfaces with s = 0.05 a.u. and their corresponding b) s[ρ(r)] vs. sign(λ2)ρ(r) graph for the adsorption Pb0 on the GO surface. | ||
Their corresponding isosurfaces (s = 0.5 a.u.) and s[ρ(r)] vs. sign(λ2)ρ(r) graph for the adsorption of Pb(CO3)3 molecule on the COOH functionalized GOs are displayed in Fig. 5a and b respectively. In the graph, the purple color surface denotes all the interactions in the system. After the ligand exchange reaction, the blue sharp peak at −0.05 is for the H bond formed between a CO3 and the COOH functional group. Fig. 5a shows that the H bond has an attractive character. The sharp orange peak at 0.075 corresponds to the Pb–O interaction, and the result suggests that the bond formed is ionic. The vdW interactions between the CO3 groups and the surface are seen in Fig. 5a, denoted by the green isosurface. This is corroborated in Fig. 5b, where the green surface is observed at low s and low ρ.
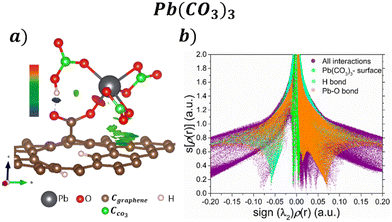 | ||
| Fig. 5 a) NCI isosurfaces with s = 0.05 a.u. and b) s[ρ(r)] vs. sign(λ2)ρ(r) graph for the adsorption Pb(CO3)3 on the GO surface. | ||
Regarding the Pb(OH)2 adsorption, their corresponding isosurface (s = 0.5 a.u.) and s[ρ(r)] vs. sign(λ2)ρ(r) plot are depicted in Fig. 6a and b, respectively. Pb interacts with the COOH functional group by forming two Pb–O bonds, corresponding with the sharp peaks at −0.05 and 0.06, after a ligand exchange reaction producing a water molecule. The water molecule still interacts with the Pb molecule and the GO surface by weak interactions. Also, according to Fig. 6a, a kind of H bond interaction between the O from COO and the H from H2O has a repulsive character. Such interaction corresponds to the sharp peak at 0.03 in Fig. 6b.
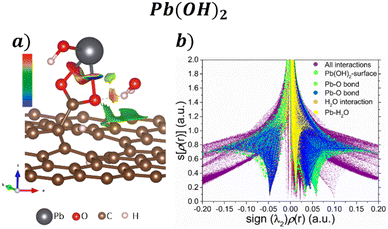 | ||
| Fig. 6 a) NCI isosurface with s = 0.05 a.u. and b) s[ρ(r)] vs. sign(λ2)ρ(r) graph for the adsorption Pb(OH)2 on the GO surface. | ||
Experimental characterization
The graphene oxide derived from Sargassum sp. was obtained by thermal decomposition at 420 °C without atmosphere control, as previously reported by Pareja-Rodríguez et al.19 As a result, they observed the presence of OH, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH and CO32− functional groups onto GOs. As proof of concept, we study the GOs after the interaction with the Pb-based compounds using spectroscopic techniques such as Raman and XPS. The results are shown in Fig. 7. The Raman spectra (see Fig. 7a) show the typical pattern of the graphene oxide-like materials.38 However, compared with the GOs without interaction with Pb2+ reported by Pareja-Rodríguez et al.,19 we notice the new bands at 562 and 1088 cm−1, indicating the presence of vibrational modes associated with –(COO)Pb bonds.5,39 These vibrations suggest the formation of bonds between the GOs functional groups and Pb2+ ions via the coordination or complexation with the carboxylate (COO−) groups.
O, C–OH and CO32− functional groups onto GOs. As proof of concept, we study the GOs after the interaction with the Pb-based compounds using spectroscopic techniques such as Raman and XPS. The results are shown in Fig. 7. The Raman spectra (see Fig. 7a) show the typical pattern of the graphene oxide-like materials.38 However, compared with the GOs without interaction with Pb2+ reported by Pareja-Rodríguez et al.,19 we notice the new bands at 562 and 1088 cm−1, indicating the presence of vibrational modes associated with –(COO)Pb bonds.5,39 These vibrations suggest the formation of bonds between the GOs functional groups and Pb2+ ions via the coordination or complexation with the carboxylate (COO−) groups.
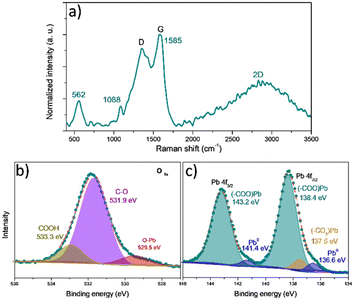 | ||
| Fig. 7 Experimental characterization of GO–Pb2+: a) Raman, b) O 1s, and c) Pb 4f high-resolution XPS spectra. | ||
Additionally, the G peak position shift from 1538 to 1585 cm−1 can be attributed to physical stress and electron transfers between functional groups of the GOs (mainly COOH) and Pb2+ ions.5 The G peak shift towards higher values indicates that GOs experience an increase in electron density. This shift suggests that the interaction with Pb2+ leads to an alteration in the electronic environment of the GOs, potentially due to electron transfer from the functional groups to the GO structure.5,40 The observed changes in the Raman spectrum of GOs provide clear evidence of chemical modifications occurring on their surface; these changes highlight the surface reactivity of GOs and the formation of new chemical species due to interaction with Pb2+ ions.
Pareja-Rodríguez et al.19 provided the FTIR spectra of GOs before and after interacting with Pb2+ ions. The bands in 1421, 874, and 711 cm−1 are mainly associated with carbonates (CaCO3).19,41 The slight change in the position and intensity of the carbonate-related bands after the interaction with Pb2+ justifies the theoretical calculations since weak interactions dominate. The carbonates are not the main active sites on the GO surface for interacting with Pb2+ ions. The band at 1105 cm−1, associated with C–O stretching vibrations of COOH groups,5,19 decreases appreciably in intensity and shifts to lower wavenumber values, indicating the formation of chemical bonds with an element of highly reduced molecular mass such as Pb. Such a shift towards lower wavenumbers may indicate a change in the chemical environment, suggesting the involvement of the COOH groups in the interaction with Pb2+ ions. According to Raman spectroscopy, the interaction between the COOH groups and Pb2+ ions can form –(COO)Pb bonds. These results support the theoretical calculations demonstrating that COOH groups are active sites where the Pb2+ ions adsorb on the GO surface.
To evaluate the chemical bonding state of the GO surface after Pb interaction, XPS analysis was performed. The high-resolution O 1s spectra (Fig. 7b) show three individual components located at 533.3, 531.3, and 529.7 eV that corresponds with the signal of carboxylic groups (COOH),5,42 the presence of O bonded to tetrahedral carbon (O–Csp3),5,42 associated with ethers and alcohols (C–O–C and C–OH), and the O bonded to Pb (O–Pb),43 respectively. Besides, Fig. S6† displays the high-resolution O 1s spectra before the adsorption of Pb-based molecules. Notice a shift to higher bond energies – after the adsorption of Pb – of the peaks associated with the COOH groups and the C–O–C and C–OH groups, which supposes a change in the electron population in the GOs because of the presence of a massive atom such as Pb.
Table 3 presents the main functional groups' relative proportions. In GOs, the main oxygenated groups (in order of their relative proportion, calculated by the area under the curve of each component in the O 1s spectrum) promote multifunctional character and defects in their structure. The surface oxygen content (sOc) is related to the rigidity of graphene materials. Graphene oxide with a high sOc is very flexible, whereas reduced graphene oxide with a lower sOc has higher rigidity.44 After interaction with lead, the formation of O–Pb bonds is observed,5 and an important decrease (from 9.35 to 5.14%) in the relative proportion of COOH groups coincides with the theoretical prediction that these are the groups where the interaction with Pb-based compounds is more favored. This interaction between Pb-based compounds and the COOH groups could lead to a redistribution of electron density within the functional groups, decreasing electron density on the oxygen atoms forming the COOH groups. The presence of Pb2+ cations can induce polarization of electron density due to their positive charge. This electronic interaction can cause a redistribution of electron density within the COOH group, leading to a decrease in electron density at the oxygen atoms and an increase in the binding energy.5,42
| Before | After | ||||
|---|---|---|---|---|---|
| GOs | A (u2) | RP (%) | GO–Pb | A (u2) | RP (%) |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121.88 121.88 |
2.35 | O–Pb | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 946.60 946.60 |
3.64 |
| C–O | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 606.80 606.80 |
21.53 | C–O | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 541.00 541.00 |
21.32 |
| COOH | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 218.65 218.65 |
9.35 | COOH | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526.16 526.16 |
5.14 |
| O1s (total) | 430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 057.64 057.64 |
438![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 645.93 645.93 |
|||
The high-resolution Pb 4f spectrum (Fig. 7c) displays two peaks, Pb 4f7/2 and Pb 4f5/2 at 138.4 and 143.2 eV, respectively. The Pb 4f5/2 signal is decomposed in two peaks at 143.2 eV, attributed to the COO−–Pb+ species, indicating the presence of Pb2+ coordinated with carboxylate groups (COO−).5,42 The small peaks at 141.4 and 136.6 eV are associated with Pb0 species due to photon-induced surface damage.43 The peak at this binding energy suggests that a minor amount of metallic lead is present (as observed in the EDS mapping in Fig. S7†) on the surface of the GO–Pb. The Pb 4f7/2 peak at 138.4 eV, corresponds to COO−–Pb+ species,5,42 and another peak with a minor relative proportion at 137.5 eV corresponds to Pb–CO3 bonds,43 and this is consistent with the deconvolution of the O 1s peak. The higher relative proportion of the species (COO−–Pb+) explains why most Pb2+ ions and the GO surface interactions are via carboxylic (COOH) groups.
Proposed mechanisms of Pb2+ adsorption
Once we have enough experimental and theoretical information about the adsorption of Pb2+ onto GOs, we propose the next adsorption mechanism.Carboxylic groups (–COOH−) and carbonate (CO32−) on the surface of GOs contain oxygen atoms that can form chemical bonds with Pb2+. These active sites facilitate the chemisorption of Pb2+ onto the GO surface through attractive forces and the formation of coordination bonds (COO−–Pb+):
| −COOH− + Pb2+ → −COOPb+ + H+ | (4) |
A chemisorption mechanism carries out the removal process of the Pb2+ ions due to electrostatic attractions between the Pb2+ ions and the GO surface functional groups in a basic medium (pH = 8.7, determined using a pH meter); this promotes the precipitation of Pb(OH)2, as can be seen in the logarithmic distribution diagram presented in Fig. S8† generated according to the experimental conditions reported by Pareja-Rodríguez et al.19 The effect of the increase of the ionic strength, the product of the solubility of the salts contained in the GOs, and the presence of Cl− ions increases the dissociation of this precipitate because of the functional groups on the surface; then hydrocerussite is formed. The solubility of this type of lead hydroxo-carbonate has been reported by Fiorito et al.;41 its formation presupposes a considerable free energy change in the system, justifying the spontaneous transition of hydroxide to hydroxo-carbonate. The formation of this compound can be described as:
| 3PbOH+ + 2CaCO3 → Pb3(CO3)2(OH)2 + 2Ca2+ + OH− | (5) |
Conclusions
To investigate the Pb2+ remotion and hydrocerussite formation using graphene oxide obtained from Sargassum sp. we focused on understanding the experimentally identified hydrocerussite mineral component (Pb0, Pb(CO3)3, Pb(OH)2) interaction with graphene oxide models (functionalized with COOH, OH, and O). The formation energy indicates that the COOH functional group is the most stable. Pb adsorption is through the oxygen atom in the COOH group. To understand the chemical interaction between the adsorbed molecules and the GO surface, non-covalent interactions (NCIs) were calculated. The NCI depicts that the CO3 has van der Waals-like weak attractive interactions that enhance the Pb adsorption on the GO surface. However, repulsive and attractive interactions are present in Pb(OH)2; this consolidates the idea that the presence of CO3 enhances the Pb2+ removal process. Therefore, using Sargassum-based GOs is efficient for Pb2+ removal through hydrocerussite formation.Author contributions
The manuscript was written through the contributions of all the authors. All the authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank DGAPA-UNAM projects IG101124, IA100624, and IN101523 for their financial support. Calculations were performed in the DGCTIC-UNAM Supercomputing Center, project no. LANCAD-UNAM-DGTIC-150, LANCAD-UNAM-DGTIC-368 and LANCAD-UNAM-DGTIC-422. We thank E. Murillo, E. Aparicio-Ceja, and Aldo Rodriguez-Guerrero for their technical support and useful discussions. All authors thank J. I. Paez-Ornelas for the table of contents image and useful comments and discussions.Notes and references
- C. Zamora-Ledezma, D. Negrete-Bolagay, F. Figueroa, E. Zamora-Ledezma, M. Ni, F. Alexis and V. H. Guerrero, Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods, Environ. Technol. Innovation, 2021, 22, 101504 CrossRef CAS.
- S. T. Song, N. Saman, K. Johari and H. Mat, Removal of Hg(II) from Aqueous Solution by Adsorption Using Raw and Chemically Modified Rice Straw As Novel Adsorbents, Ind. Eng. Chem. Res., 2013, 52, 13092 CrossRef CAS.
- M. L. Sall, A. Karim, D. Diaw, D. Gningue-Sall, S. E. Aaron and J. J. Aaron, Toxic heavy metals: impact on the environment and human health, and treatment with conducting organic polymers, a review, Environ. Sci. Pollut. Res., 2020, 27, 29927 CrossRef CAS PubMed.
- R. A. Goyer, Toxic and essential metal interactions, Annu. Rev. Nutr., 1997, 17, 37 CrossRef CAS PubMed.
- M. T. Nguyen, J. Zhang, V. Prabhakaran, S. Tan, E. T. Baxter, V. Shutthanandan, G. E. Johnson, R. Rousseau and V. A. Glezakou, Graphene Oxide as a Pb(II) Separation Medium: Has Part of the Story Been Overlooked?, JACS Au, 2021, 1, 766 CrossRef CAS PubMed.
- O. Surucu, Trace determination of heavy metals and electrochemical removal of lead from drinking water, Chem. Pap., 2021, 75, 4227 CrossRef CAS.
- Q. Chen, Y. Yao, X. Li, J. Lu, J. Zhou and Z. Huang, Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates, Journal of Water Process Engineering, 2018, 26, 289 CrossRef.
- C. L. Chen, X. K. Wang and M. Nagatsu, Europium Adsorption on Multiwall Carbon Nanotube/Iron Oxide Magnetic Composite in the Presence of Polyacrylic Acid, Environ. Sci. Technol., 2009, 43, 2362 CrossRef CAS PubMed.
- X. Ren, C. Chen, M. Nagatsu and X. Wang, Carbon nanotubes as adsorbents in environmental pollution management: A review, Chem. Eng. J., 2011, 170, 395 CrossRef CAS.
- A. Sarı, M. Tuzen and M. Soylak, Adsorption of Pb(II) and Cr(III) from aqueous solution on Celtek clay, J. Hazard. Mater., 2017, 144, 41 CrossRef PubMed.
- C. Chen, X. Yang, J. Wei, X. Tan and X. Wang, Eu(III) uptake on rectorite in the presence of humic acid: A macroscopic and spectroscopic study, J. Colloid Interface Sci., 2013, 393, 249 CrossRef CAS PubMed.
- R. Zhang, C. Chen, J. Li and X. Wang, Investigation of interaction between U(VI) and carbonaceous nanofibers by batch experiments and modeling study, J. Colloid Interface Sci., 2015, 460, 237 CrossRef CAS PubMed.
- J. Li, X. Wang, G. Zhao, C. Chen, Z. Chai, A. Alsaedi, T. Hayat and X. Wang, Metal–organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions, Chem. Soc. Rev., 2018, 48, 2322 RSC.
- X. M. Huang, L. Z. Liu, S. Zhou and J. J. Zhao, Physical properties and device applications of graphene oxide, Front. Phys., 2020, 15, 33301 CrossRef.
- Y. Sun, S. Yang, G. Zhao, Q. Wang and X. Wang, Adsorption of Polycyclic Aromatic Hydrocarbons on Graphene Oxides and Reduced Graphene Oxides, Chem. – Asian J., 2013, 8, 2755 CrossRef CAS PubMed.
- T. Wen, X. Wu, X. Tan, X. Wang and A. Xu, One-Pot Synthesis of Water-Swellable Mg–Al Layered Double Hydroxides and Graphene Oxide Nanocomposites for Efficient Removal of As(V) from Aqueous Solutions, ACS Appl. Mater. Interfaces, 2013, 5, 3304 CrossRef CAS PubMed.
- G. Duran-Jimenez, T. Monti, J. J. Titman, V. Hernandez-Montoya, S. W. Kingman and E. R. Binner, New insights into microwave pyrolysis of biomass: Preparation of carbon-based products from pecan nutshells and their application in wastewater treatment, J. Anal. Appl. Pyrolysis, 2017, 124, 113 CrossRef.
- V. Kirubakaran, V. Sivaramakrishnan, R. Nalini, T. Sekar, M. Premalatha and P. Subramanian, A review on gasification of biomass, Renewable Sustainable Energy Rev., 2019, 13, 179 CrossRef.
- R. Pareja-Rodríguez, Y. Freile-Pelegrín, D. Robledo, M. Ruiz-Gómez, R. Martínez-Flores and G. Rodríguez-Gattorno, Self-generated active sites in graphene oxide-like materials by controlling the oxidative decomposition reactions of Sargassum, J. Environ. Chem. Eng., 2021, 9, 106551 CrossRef.
- Q. Shi, G. E. Sterbinsky, V. Prigiobbe and X. Meng, Mechanistic Study of Lead Adsorption on Activated Carbon, Langmuir, 2018, 34, 13565 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci., 1996, 6, 15 CrossRef CAS.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS PubMed.
- P. E. Blochl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef PubMed.
- G. Kresse and D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- H. Monkhorst and J. Pack, Special points for Brillouin-zone integrations, Phys. Rev. B: Solid State, 1976, 13, 5188 CrossRef.
- A. Otero-de-la-Roza, E. R. Johnson and V. Luaña, Critic2: A program for real-space analysis of quantum chemical interactions in solids, Comput. Phys. Commun., 2014, 185, 1007 CrossRef CAS.
- J. M. Galicia-Hernández, E. Chigo Anota, M. T. Romero de la Cruz, M. González Melchor and G. Hernández Cocoletzi, First principles studies of the graphene-phenol interactions, J. Mol. Model., 2012, 18, 3857 CrossRef PubMed.
- J. I. Paez-Ornelas, H. N. Fernandez-Escamilla, H. A. Borbón-Nuñez, H. Tiznado, T. Noboru and J. Guerrero-Sánchez, A first-principles study of the atomic layer deposition of ZnO on carboxyl functionalized carbon nanotubes: the role of water molecules, Phys. Chem. Chem. Phys., 2021, 23, 3467 RSC.
- G. X. Qian, R. M. Martin and D. J. Chadi, First-principles study of the atomic reconstructions and energies of Ga- and As-stabilized GaAs(100) surfaces, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 38, 7649 CrossRef CAS PubMed.
- J. Ruiz-González, G. H. Cocoletzi and L. Morales de la Garza, Modeling the Electronic structure and stability of three aluminum nitride phases, Rev. Mex. Fis., 2021, 67, 343 Search PubMed.
- S. J. Gutiérrez-Ojeda, R. Ponce-Pérez, J. Guerrero-Sánchez, R. García-Diaz, F. Sánchez-Ochoa, Ma. G. Moreno-Armenta and G. H. Cocoletzi, Modeling the half-metallicity of the CrN/GaN (111) heterostructure, Appl. Surf. Sci., 2021, 566, 150637 CrossRef.
- O. Siidra, D. Nekrasova, W. Depmeier, N. Chukanov, A. Zaitsev and R. Turner, Hydro-cerussite-related minerals and materials: structural principles, chemical variations and infrared spectroscopy, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2018, 74, 182 CrossRef CAS PubMed.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, Revealing Noncovalent Interactions, J. Am. Chem. Soc., 2010, 132, 6498 CrossRef CAS PubMed.
- J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J. P. Piquemal, D. N. Beratan and W. Yang, NCIPLOT: A Program for Plotting Noncovalent Interaction Regions, J. Chem. Theory Comput., 2011, 7, 625 CrossRef PubMed.
- Y. Wang, D. C. Alsmeyer and R. L. Mccreery, Raman Spectroscopy of Carbon Materials: Structural Basis of Observed Spectra, Chem. Mater., 1990, 2, 557 CrossRef CAS.
- L. Burgio, R. J. H. Clark and S. Firth, Raman spectroscopy as a means for the identification of plattnerite (PbO2), of lead pigments and of their degradation products, Analyst, 2001, 126, 222 RSC.
- W. Peng, H. Li, Y. Liu and S. Song, Comparison of Pb(II) adsorption onto graphene oxide prepared from natural graphites: Diagramming the Pb(II) adsorption sites, Appl. Surf. Sci., 2016, 364, 620 CrossRef CAS.
- E. Fiorito, G. E. Porcedda, L. Brundu, C. Passiu, D. Atzei, G. Ennas, B. Elsener, M. Fantauzzi and A. Rossi, Calcium carbonate as sorbent for lead removal from wastewaters, Chemosphere, 2022, 296, 133897 CrossRef CAS PubMed.
- Z. Guo, J. Zhang, Y. Kang and H. Liu, Rapid and efficient removal of Pb(II) from aqueous solutions using biomass-derived activated carbon with humic acid in-situ modification, Ecotoxicol. Environ. Saf., 2017, 145, 442 CrossRef CAS PubMed.
- H. Wang, A. Zhou, F. Peng, H. Yu and J. Yang, Mechanism study on adsorption of acidified multiwalled carbon nanotubes to Pb(II), J. Colloid Interface Sci., 2017, 316, 277 CrossRef PubMed.
- Z. Guo, P. Zhang, C. Xie, E. Voyiatzis, K. Faserl, A. J. Chetwynd, F. A. Monikh, G. Melagraki, Z. Zhang, W. J. G. M. Peijnenburg, A. Afantitis and C. Chen, Iseult Lynch, Defining the Surface Oxygen Threshold That Switches the Interaction Mode of Graphene Oxide with Bacteria, ACS Nano, 2023, 17, 6350 CrossRef CAS PubMed.
- A. Alfarra, E. Frackowiak and F. Béguin, The HSAB concept as a means to interpret the adsorption of metal ions onto activated carbons, Appl. Surf. Sci., 2004, 228, 84 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Less stable DFT models, O 1s XPS, GO-Pb EDS, and XRD. See DOI: https://doi.org/10.1039/d4en00301b |
| This journal is © The Royal Society of Chemistry 2024 |