Sustainable valorisation of food waste into engineered biochars for CO2 capture towards a circular economy
Wenhui
Jia†
a,
Shuangjun
Li†
b,
Junyao
Wang†
c,
Jonathan T. E.
Lee
 d,
Carol Sze Ki
Lin
d,
Carol Sze Ki
Lin
 e,
Ondřej
Mašek
f,
Huiyan
Zhang
e,
Ondřej
Mašek
f,
Huiyan
Zhang
 a and
Xiangzhou
Yuan
a and
Xiangzhou
Yuan
 *a
*a
aMinistry of Education of Key Laboratory of Energy Thermal Conversion and Control, School of Energy and Environment, Southeast University, Nanjing 210096, China. E-mail: yuanxz@seu.edu.cn
bDepartment of Chemical & Biological Engineering, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul 02841, Korea
cSchool of Materials and Energy, Guangdong University of Technology, Guangzhou 510006, China
dEnvironmental Research Institute, National University of Singapore, Singapore 138602, Singapore
eSchool of Energy and Environment, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong, China
fUK Biochar Research Centre, School of GeoSciences, University of Edinburgh, Crew Building, Alexander Crum Brown Road, Edinburgh EH9 3FF, UK
First published on 2nd January 2024
Abstract
The large amount of food waste generated worldwidely has significant adverse environmental impacts to our entire ecosystem, highlighting the urgent need for a historic resolution to achieve sustainable managment of food waste as well as its circular economy. In this regard, preparation of engineered biochars from food waste has garnered significant attention for CO2 capture, as this upcycling potential could play a significant role in advancing the concept of a negative carbon circular economy. Hence, this review holistically explores the potential of food waste-derived engineered biochars as CO2 adsorbents, not only from sample-level to process-level CO2 adsorption, but also from a life-cycle perspective. Sample-level CO2 adsorption is examined in terms of synthetic methods and procedures, focusing on application and optimisation of carbonisation, activation, and surface modification processes. The application of machine learning for guiding syntheses of high-performance CO2 adsorbents derived from food waste is also dicussed. Process-level CO2 adsorption is examined in terms of two primary cycling configurations, namely pressure swing adsorption and temperature swing adsorption, whose efficiency is critical for commercialisation. In addition, a comprehensive life-cycle assessment is performed to provide a novel and timely overview of the environmental impacts of CO2 adsorption using food waste-derived engineered biochars. This review demonstrates the viability and potential of integrating food waste-derived engineered biochars with carbon capture technologies to afford an environmentally friendly innovation for sustainable food waste management and climate change mitigation, which is benefical to achieving UN Sustainable Development Goals including Goals 11–13.
Introduction
Approximately one-third of total global food resources, equivalent to 1.3 billion tons annually, is wasted in food production and consumption, resulting in a broad range of adverse environmental impacts.1,2 Greenhouse gas (GHG) emissions are one of the most serious environmental impacts,3 with 8–10% of global GHG emissions attributable to unsustainable management of food waste.4 For example, the U.S. Environmental Protection Agency (EPA) highlighted that due to mismanagement of food waste, 170 million metric tons of carbon dioxide (CO2) equivalent GHGs are emitted annually, an amount that is equal to the annual CO2 emissions of 42 coal-fired power plants.5 Moreover, food waste generation has been increasing globally and it is now included in three of the United Nations’ 17 Sustainable Development Goals (UN SDGs), namely goal 11: sustainable cities and communities, goal 12: sustainable consumption and production patterns, and goal 13: climate action.6 Food waste, as a typical type of biomass but commonly mismanaged, leads to serious health and environmental issues. It is closely associated with human activities and is the most easily generated, collected, and accumulated in urban environments. It is worth noting that food waste is seasonal and regional properties, which is one key difference from general biomass, i.e., wood biomass and rice husk. Therefore, to attain global environmental sustainability, there is a pressing need to transition from the prevailing linear paradigm of food consumption and production to a circular paradigm, where unavoidable residues from the farming and food industry are valorised.Sustainable valorisation of food waste at a global scale is essential for reducing environmental and economic burdens of food waste and thereby achieving a circular economy.7,8 Biochemical approaches (i.e., anaerobic digestion, fermentation, and composting) and thermochemical approaches (i.e., hydrothermal carbonisation (HTC), pyrolysis/co-pyrolysis, and gasification) are widely used to upcycle food waste into value-added bioenergy and solid carbonaceous materials,2 and additional pretreatments for size reduction are commonly needed to improve conversion efficiency.7 However, there are three major challenges associated with biochemical approaches, namely that (1) they are inherently slow (i.e., can take up to several months) and typically require large reactors, and their conversion efficiency is low owing to the diversity and complexity of food waste; (2) they rely on microorganisms, which are susceptible to variations in operating conditions, such that precise control is required to optimise product yields, energy exergy, and economic feasibility, and (3) they require additional rebuilding treatments to transform main products with low-molecular weight into high-molecular weight variants, resulting in energy-inefficient conversion.2
The aforementioned challenges have driven investigations into thermochemical approaches, which offer fast reaction rates, mild operating conditions, controllable product compositions, and high feasibility for commercial applications.9 Gasification is a thermochemical approach that efficiently converts solid carbonaceous materials into syngas i.e., carbon monoxide (CO) and hydrogen (H2) in a gasifier under high-temperature and low-oxygen conditions.10,11 Owing to the high volatile matter content (<80%) of food waste, it is regarded as a promising alternative for energy production, as it solidifies energy security via a waste-to-energy strategy.9,12 Pyrolysis of food waste occurs at relatively low temperatures (300–600 °C) in an oxygen-free atmosphere and results in upcycling of food waste into highly heterogeneous solid, liquid, and gaseous products.13 Pyrolysis is classified as either slow pyrolysis (which mainly generates biochar) or fast (flash) pyrolysis (which mainly generates bio-oil) and has wide applications in research and industry. HTC is widely considered to be a practical and suitable approach for valorising high-moisture content food waste to produce value-added carbonaceous materials that are termed ‘hydrochar’.10 However, it remains difficult to upcycle food waste into biofuels in a profitable manner with zero carbon emissions. This has led to numerous research on valorisation of food waste into high-performance carbonaceous materials for environmental protections and various energy conversion and storage applications.10,14
Current research on organic waste-derived carbonaceous materials for energy and environmental applications have been reviewed.10,26,27 These reviews have highlighted that conversion of organic waste into carbonaceous materials for CO2 capture is sustainable and practical, especially in the context of carbon neutrality.28,29 Moreover, food waste-derived high-performance engineered biochars have been researched as materials for CO2 capture (Table 1), which is a feasible approach for simultaneously mitigating climate change and achieving sustainable waste management.30,31 Therefore, there is an urgent need for a comprehensive review of current research on food waste-derived CO2 adsorbents to cover recent advances, existing challenges, and future perspectives. As summarized in Fig. 1, this review (1) examines the synthesis routes from food waste to high-performance CO2 adsorbents (including machine learning-aided optimization of these routes), (2) evaluates capture performance of CO2 adsorbents from both sample- and process-level perspectives, and (3) assesses their environmental impacts from a life-cycle perspective. Thus, this review reveals key aspects of design and optimization of food waste-derived CO2 adsorbents and provides guidelines for large-scale CO2 capture deployment. It is hoped that this will assist reseracher from academic and industrial communities and/or even policymakers from governmental agencies, who are striving to achieve goals such as carbon neutrality, sustainable waste management, circular carbon economy, and even UN SDGs.
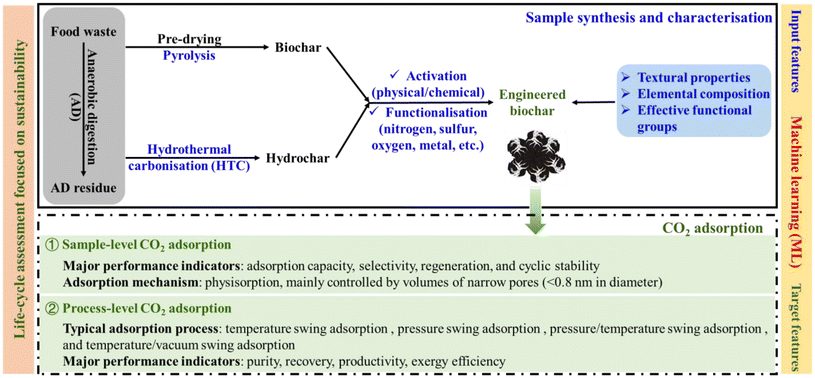 | ||
| Fig. 1 Sustainable valorisation of food waste into engineered biochars for carbon dioxide (CO2) capture, as part of a circular economy. | ||
| Feedstock | Synthesis approach | Textural properties | N content (%) | CO2 adsorption capacity | CO2/N2 selectivity | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonisation | Activation | Surface functionalisation | Specific surface area (m2 g) | Total pore volume (cm3 g−1) | 25 °C & 1 bar | 0 °C & 0.1 bar | ISAT method | Henry's law | |||
| Notes. KOH = potassium hydroxide; K2CO3 = potassium carbonate; K2C2O4 = potassium oxalate; ISAT = initial surface absorption test; CO2 = carbon dioxide; N2 = nitrogen. | |||||||||||
| Crab shells | Pyrolysis at 400 °C for 1.5 h | KOH activation at 600 °C for 1.5 h (2) | — | 1004 | 0.46 | 6.2 | 4.37 | — | 19 (15/85) | — | 15 |
| Fish scales | Pyrolysis at 330 °C for 3 h | KOH activation at 600 °C for 1 h (1) | — | 1479 | 0.88 | — | 2.80 | — | — | 16 | |
| Rotten strawberries | Hydrothermal carbonization at 180 °C for 12 h | KOH activation at 600 °C (2) | — | 935 | 0.42 | 3.68 | 3.63 | 5.27 | 16 (10/90) | — | 17 |
| Shrimp shells | Pyrolysis at 400 °C for 1.5 h | KOH activation at 700 °C for 1 h (1) | — | 1406 | 0.72 | 2.16 | 4.20 | 6.69 | 23 (15/85) | — | 18 |
| Coconut shells | Pyrolysis at 500 °C for 2 h | K2CO3 activation at 600 °C for 1 h (3) | Urea modification at 350 °C for 2 h in air | 1082 | 0.39 | 2.74 | 3.71 | 5.12 | 13 (10/90) | 19 | |
| Pineapple waste | Hydrothermal carbonisation at 210 °C for 10 h with alkali metal oxalate | K2C2O4 activation at 700 °C for 2 h | — | 1076 | 0.08 | 0.33 | 4.25 | 5.32 | 27 (10/90) | 32 | 20 |
| Garlic peels | Hydrothermal carbonisation at 220 °C for 24 h followed by pyrolysis at 400 °C for 2 h | KOH activation at 600 °C for 1 h (2) | — | 947 | 0.51 | — | 4.22 | 6.25 | — | 21 | |
| Sugarcane bagasse | Pyrolysis at 600 °C for 0.5 h | KOH activation at 600 °C for 1 h (2) | Urea solution | 1113 | 0.57 | 1.98 | 4.80 | — | — | 22 | 22 |
| Coffee grounds | — | K2CO3 activation at 700 °C for 5 h (1) | — | 1476 | 0.61 | 1.67 | 4.54 | 7.02 | 16 (20/80) | — | 23 |
| K2CO3 activation at 800 °C for 1 h (1) | 1692 | 0.71 | 1.51 | 4.46 | 7.18 | 14 (20/80) | |||||
| Food waste and wood waste | 10 kW fixed-bed downdraft gasifier | KOH activation at 850 °C for 2 h (1) | — | 841 | 0.85 | — | 3.23 | — | 24 | ||
| Mixed food waste | pyrolysis at 400 °C for 1 h | KOH activation at 600 °C for 1 h (2) | — | 807 | 0.41 | 1.6 | 2.54 | — | 25 | ||
Advances in food waste-derived CO2 adsorbents
Conventional synthetic procedures
Carbonisation and activation have been widely used to synthesise high-performance CO2 adsorbents from food waste.30–32 Slow pyrolysis has been considered one of the most promising and practical routes for carbonization of biomass and its waste to produce biochars.10,33 However, direct pyrolysis is not practically applicable to food waste because its high moisture content requires an energy-intensive pre-drying process for upcycling food waste into biochars (as shown in Fig. 1). Therefore, HTC, which is a thermochemical conversion performed in the presence of water at 180–265 °C and 2–6 MPa for 5–240 min,10 has attracted much attention to produce food waste-derived hydrochar,34,35 due to that HTC does not require pre-drying treatment of food waste and requires the addition of little or no water. It suggests that HTC reduces both energy and water consumptions, simultaneously.Biochar and hydrochar have poor textural properties, and thus have been subjected to physical or chemical activation to enlarge their surface areas and generate microporous structures, which facilitate high-performance CO2 adsorption.30,31 Physical activation is typically performed using CO2, steam, and air as activation agents, and increases the porosity and functional groups of biochars or/hydrochars in an eco-friendly and cost-effective manner. CO2 is the most widely used in physical activation, due to its relatively green characteristics and low reactivity at high activation temperatures (i.e., >700 °C).36 Moreover, the off-gas produced by physical activation with CO2 can be reused as an activation agent after simple combustion,37 minimizing additional CO2 emissions and achieving a closed carbon loop. Compared with physical activation of food waste, chemical activation is typically conducted at lower temperatures and with shorter residence time, and produces engineered biochar with more well-developed pore structures and high yields of final products. Potassium hydroxide (KOH), phosphoric acid (H3PO4), and zinc chloride (ZnCl2) are the chemical agents that are most commonly used for chemical activation.31 For example, Ma et al.38 treated hazelnut shells with KOH at 650 °C for 1 h to form an engineered biochar with a surface area of 2134 m2 g−1 and total pore volume of 0.96 cm3 g−1. However, the aforementioned chemical agents are toxic, hazardous, and corrosive, and thus damage equipment and can pollute the environment if not subjected to secondary treatments. This indicates that additional investigations on environmental impacts of chemical activation is warranted. In addition, from a life-cycle perspective, the use of chemical agents significantly contributes to environmental impacts, and the trade-off between performance enhancement and synthesis methods needs to be well considered for practical applications.
Another effective process to increase adsorption performance of CO2 adsorbents, especially in terms of their adsorption capacity for CO2 and selectivity for CO2 over other gases, is functionalisation, which generates active sites for efficient adsorption of CO2 molecules.39–41 Nitrogen (N), sulfur (S), and metal oxides (e.g., MgO, CuO), are the main sources of heteroatoms that are added to CO2 adsorbents to increase the number and basicity of active sites on their surfaces. Compared with single-doping treatment, dual-doping and triple-doping treatment provides more active sites on CO2 adsorbents, resulting in them exhibiting excellent CO2 adsorption.42 Furthermore, several typical biomass waste-derived engineered biochars have a high content of functional groups as they are naturally rich in heteroatoms. This means that the prepared biochar could have naturally added heteroatoms, thereby exhibiting the characteristic of enhanced adsorption performance as well. For example, shrimp shell-derived engineered biochar contains 2.86 wt% N,43 and willow catkin-derived engineered biochar contains 2.56 wt% S and 4.62 wt% N.44 Jelleyfish-based engineered biochar was proved rich in heteroatoms (Na, P, N, Ca and Mg), the functional groups existed were confirmed advantageous for CO2 capture.45 And nitrogen-rich seaweed-based engineered biochar has demonstrated excellent CO2 adsorption capacity, and its mechanism has been studied using density functional theory. This suggests that surface functionalisation is a useful and practical additional treatment for increasing the CO2 capture performance of a food-waste-derived engineered biochar that contains insufficient functional groups on its surface.
Conventional synthetic approaches
Optimisation of synthesis conditions (in terms of operating temperature, residence time, and heating rate) for carbonisation and activation (or surface functionalisation) of food waste is critical for producing engineered biochar with excellent CO2 capture performance. Current synthesis approaches are summarized as (1) intuition-based approaches, (2) one-factor-at-a-time approaches, and (3) design of experiment via response surface methodology (RSM).46,47 The first two approaches are frequently integrated to develop engineered biochar for CO2 capture, but (3) is widely considered as a more efficient way to synthesise engineered biochar samples for CO2 capture. However, (1), (2), and (3) are all time- and labour-intensive, and it is difficult to determine the underlying relationships between synthetic conditions and CO2 capture performance of the resulting engineered biochar materials. Thus, it remains highly challenging to directly optimize the production of engineered biochars such that they exhibit high-performance CO2 capture.Machine learning-aided synthesis approaches
Machine learning (ML) is one of the most widespread data-driven approaches in many fields. For example, ML approaches have been applied in many studies on material discovery, process optimization, and environmental protection48–50 as they provide valuable insights that aid the understanding, interpretation, and inverse design of complicated systems. Moreover, as shown in Fig. 2, recent studies have verified that ML is an efficient and practical tool for developing engineered biochars for CO2 capture.50–52 Data collection, formatting, and pre-processing are the essential steps in the application of ML for predicting CO2 capture performance of engineered biochars. Collected and formatted dataset are invariably lacking some data, meaning that data imputation and/or discarding is a critical step that must be conducted prior to ML investigations.50,52 For example, Yuan et al. collected 632 datapoints from 76 peer-reviewed publications and ultimately used 527 of them without missing data values for predicting the CO2 adsorption capacities of biomass waste-derived porous carbon materials.50 In addition, tree-based ML algorithms (i.e., random, forest, decision trees, gradient boosting decision trees, and light gradient boosting tree) are widely used to develop prediction models for engineered biochar-based CO2 capture. First, pre-processed data are randomly divided into a training dataset (70%–90%) and a test dataset (30%–10%), which are then used for training and testing ML models. The current major findings are that (1) different models, such as random forest and gradient boosting decision trees, exhibit high accuracy and predictive performance, and (2) textural properties play a more critical role than chemical composition in engineered biochar-based CO2 adsorption. It is the basic and plain application of ML algorithms to develop predictive ML models for CO2 capture performance, the more interesting and critical thing is to apply ML modeling approaches to design specific CO2 adsorbents and adsorption parameters with the aim of practical CO2 capture applications.53 However, it remains challenging to directly design engineered biochars with high CO2 capture performance. This is because recent research has focused on forward prediction of CO2 adsorption performance but not backward design of high-performance engineered biochar-based CO2 adsorbents.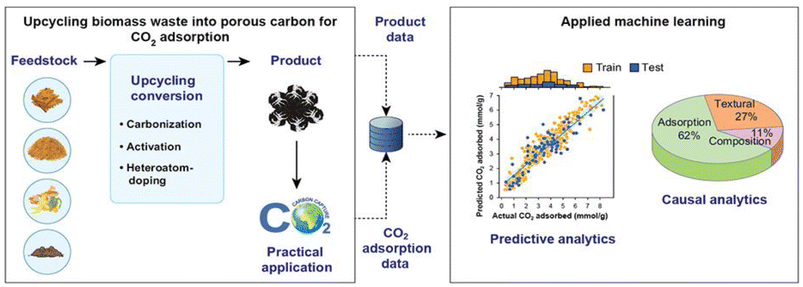 | ||
| Fig. 2 Application of machine learning (ML) for upcycling food waste into engineered biochars for carbon dioxide (CO2) capture. | ||
Cyclic performance evaluation for post-combustion CO2 capture
Sample-level characterisations of the aforementioned textural properties and gas adsorption performances of food waste-derived engineered biochars suggest that they are effective CO2 adsorbents.31 However, process-level characterization via experimental and/or simulation approaches are needed to determine the practicality of food waste-derived CO2 adsorbents. For example, cyclic performances of food waste-derived engineered biochars-based post-combustion CO2 capture must be quantitatively assessed. Shen54 and Yuan et al.30,31 have reported that the CO2 adsorption performance of an engineered biochar is primarily affected by its characteristics (i.e., textural properties and surface functionality) and the adsorption conditions (i.e., temperature, pressure, humidity, and CO2 concentration). In addition, Bernardo et al.55 highlighted the cyclic performances and dynamic behaviours of newly developed CO2 adsorbents as emerging research trends.Research on cyclic performance has been extensively explored in recent studies, various factor-driven processes were discussed separately. Álvarez-Gutiérrez et al.56 valorised cherry stones into engineered biochars to separate CO2 and methane (CH4) gas mixtures via a pressure swing adsorption (PSA) process, thereby affording fuel-grade methane. They used three cyclic performance indicators, CO2 working capacity, CH4 productivity, and CH4 purity as the target parameters, and found that an adsorption pressure of 5 bar was effective for PSA using cherry stone-derived adsorbents. Surra et al.57 developed a maize cob waste-derived engineered biochar that exhibited great potentials for CO2 separation and biogas upgrading. In addition, Liang et al.58 noted that their prepared popcorn-derived porous carbon reached a CO2 adsorption capacity of 4.60 mmol g−1 at 1066 mbar and 25 °C. Du et al.59 prepared engineered biochar with high CO2 adsorption properties using cauliflower and demonstrated 3.1 mmol g−1 CO2 adsorption capacity at 1 bar and 25 °C. The breakthrough curves and adsorption isotherms of cherry stone-derived engineered biochar filled gaps between the sample- and process-level investigations, providing critical knowledge to design, summarize, and validate the PSA process.56,57 The addition of a vacuum step into the PSA process creates the vacuum-pressure swing adsorption (VPSA) process, which uses food waste-derived engineered biochars as CO2 adsorbents. Majchrzak-Kucęba et al.60 upcycled coconut shells into an engineered biochar for a bench-scale VPSA process, verifing as a practical and feasible way for adsorbing CO2 emitted from cement plants. They also reported that CO2 purity increased but CO2 recovery decreased as the flow rate of feeding gas and working cycle time increased. In addition, Majchrzak-Kucęba et al.61 developed the dual-reflux vacuum-pressure adsorption (DR-VPSA) process, aiming to improve both CO2 recovery and purity. In a pilot-scale application, they divided a single adsorber into a two-stage reactor that completely separated CO2 from inlet gas using various engineered biochar-based CO2 adsorbents. In addition, they determined that sample-level characterisations, such as isotherm and working capacity, selectivity, renderability, and cyclic stability, are essential for accelerating process-level investigations.
Bahamon et al.62 applied the grand canonical Monte Carlo (GCMC) simulation method to obtain pure and multi-component gas adsorption data, and then used working capacity, purity, and energy consumption as major indicators to evaluate their performance of various CO2 adsorption processes (i.e., PSA, temperature swing adsorption (TSA), and vacuum swing adsorption (VSA)) using date seed-derived engineered biochars. Bahamon et al.62 concluded that two of the major indicators, i.e. working capacity and purity, were not significantly affected by pre-treatment of pre-adsorbed water. Food waste-derived engineered biochars have been widely investigated in laboratory-scale CO2 adsorption studies. These engineered biochars have exhibited favourable textural properties and demonstrated excellent equilibrium and dynamic adsorption performance, as discussed in section 2. However, it remains challenging to directly use food waste-derived engineered biochar in commercial-scale CO2 capture applications. Therefore, to facilitate practical applications, process design and optimization for CO2 adsorption is urgently needed to determine optimal cycle configuration and operation conditions while using food waste-derived engineered biochars as CO2 adsorbents. Fig. 3 depicts the most commonly used cycle configuration. To the best of our knowledge, few investigations of biochar have been performed to examine process-level CO2 adsorption,62 and these provided useful guidelines and valuable insights on food waste-derived engineered biochars for CO2 adsorption. In addition, this review examines several process configurations to show the CO2 capture applications of food waste-derived engineered biochars. To bridge the gaps between sample synthesis and practical CO2 capture, there is a need for focused collection of essential data on the sample-level CO2 adsorption performance of food waste-derived engineered biochars to facilitate the evaluation and optimization of the process-level CO2 adsorption performance of such engineered biochars.63
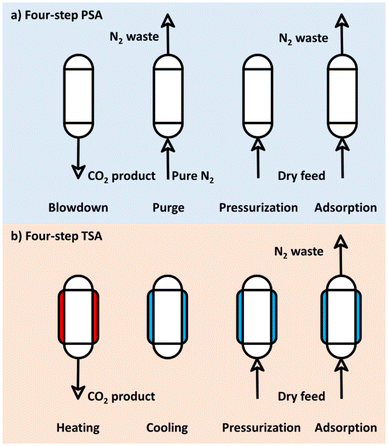 | ||
| Fig. 3 Typical pressure and temperature difference driven carbon dioxide (CO2) adsorption cycle configurations (PSA = pressure swing adsorption; TSA = temperature swing adsorption; N2 = nitrogen). | ||
Pressure-driven CO2 adsorption
The PSA process configuration was pioneered by Skarstrom in 1960. It exploits a pressure difference to achieve CO2 separation; that is, a high-pressure condition is used to adsorb CO2 gas, and then a low-pressure condition is used to desorb CO2 gas.64 Dual-step PSA is the basic PSA process, whereas four-step PSA is the most commonly used PSA process, with the four-step being adsorption, blow-down, purging, and pressurization. A high working capacity of the CO2 adsorbent is obtained via the pressurization step, and extra energy is consumed by compression and vacuum generation.65 Commercial deployment of the four-step PSA process employs physical adsorbents (especially engineered biochars) in packed-bed reactors, owing to their ease of handling, high stability and safety, low energy consumptions, low capital investment cost, and high deployment feasibility.66Pressure-driven CO2 adsorption using engineered biochars as adsorbents is summarized in Table 2. For example, Drage et al.67 investigated the necessity of adsorbent pretreatment for achieving high CO2 uptake. Specifically, they examined the CO2 working capacity of engineered biochars at atmospheric and elevated pressures (up to 4.1 MPa), using a single PSA process as the cycle configuration. Their major findings highlighted that cyclic adsorption capacity was affected by adsorption capacity and isotherm shape. Siqueira et al.68 devised a mathematical model to describe the PSA process and then validated this model with a pilot-scale PSA experimental data using commercial activated carbon in a packed-bed reactor. Only tests of equilibrium CO2 uptake measurements and adsorption kinetics were required for determining process-level investigations, and the major indicators for evaluating cyclic performance, namely product purity, recovery, and productivity, were obtained after performing a process-level numerical simulation. Decreases in pressure in the packed-bed reactor in the PSA process were considered; these occur as the pressure-driven process is negatively affected by the unreasonable flow resistance. In addition, the application of adsorbent monoliths was found to increase mass transfer rates and reduce pressure decreases in the packed-bed reactor.69 A three-dimensional (3D)-printed activated carbon monolith was used for CO2 adsorption,70 thereby demonstrating a novel synthetic route for enhancing cyclic gas separation performance.
| Mixture treated | Process configuration | Research method | Pressure range (MPa) | Sample-level characterisation | Process-level evaluation | Results and discussion | Ref. |
|---|---|---|---|---|---|---|---|
| Notes. PSA = pressure swing adsorption; VSA = vacuum swing adsorption; VPSA = vacuum pressure swing adsorption; CO = carbon monoxide; CO2 = carbon dioxide; H2 = hydrogen; N2 = nitrogen; CH4 = methane; O2 = oxygen; 3D = three-dimensional. | |||||||
| CO2/N2 | PSA | Simulation | 0.1–4.1 | CO2 and N2 isotherms measurement | CO2 adsorption capacity | Cyclic adsorption capacity depends on adsorption capacity and isotherm shape. | 67 |
| CO2/N2 | Four-step PSA | Experiment and simulation | 0.6, 1.2, 2.0 | Single and binary isotherms measurement | CO2 purity, recovery, and productivity | Commercial activated carbon showed high CO2 adsorption capacity. The mathematical model devised to describe the PSA process validated by experimental data. | 68 |
| CO2/CH4 | Four-step PSA | Experiment | 0.3, 0.5, 1.0 | CO2 and CH4 isotherms, heat capacity measurement | CO2 purity, recovery, and productivity | 3D-printed adsorbent monoliths displayed PSA performance competitive with that of conventionally manufactured structured adsorbents. | 70 |
| CO2/CH4 | Two-step PSA | Simulation | 0.1–0.5 | CO2 and CH4 isotherms, and binary gas breakthrough curves | CO2 purity, recovery | Devised short-cut model that simply, but accurately clarifies the physical phenomenon in the PSA process, and screened performance of CO2 adsorption by engineered biochars | 71 |
| CO2/N2/O2 | Five-step VSA | Experiment | — | Binary and ternary isotherms, and breakthrough curves | CO2 purity, recovery | Vacuum level found to play an important role in the cyclic performance. | 73 |
| CO2/N2 | Four-step PSA | Simulation | Not mentioned | CO2 and N2 isotherms | CO2 purity, recovery, productivity, energy consumption | It including process optimisation tools early in the adsorbent development workflow can be beneficial adsorption metrics proposed should be used with caution. | 75 |
| CO2/N2 | Six-step VSA | Simulation | Low to 0.005 | CO2 and N2 isotherms, uptake rates | Measurement of CO2 purity, recovery, productivity, and energy consumption | Detailed process modelling, simulation and optimisation strategies are the most reliable way to qualitatively and quantitatively evaluate potential adsorbents for CO2 capture. | 76 |
| CO2/N2 | VPSA | Experiment | Low to 0.030 | CO2 and N2 isotherms measurement | CO2 working capacity | Porous structure and unique surface properties of biochar make it an effective CO2 adsorbent, including in the VPSA process. | 77 |
| H2/CO2/CH4/CO/N2 | 10-step VPSA | Simulation | 0.05–0.50 | Adsorption isotherms and kinetic measurement | H2 purity and recovery | Multi-objective optimization methods can be used to design the VPSA process using engineered biochars as adsorbents. | 78 |
| CO2/N2 | Five-step VPSA | Simulation | 0.03–0.40 | CO2 and N2 adsorption isotherms measurement | CO2 working capacity, CO2 recovery, actual work consumption, second-law efficiency | Effect of adsorbent isotherm type on energy efficiency of the VPSA process clarified. | 79 |
However, the above-mentioned mathematical models are too complex to be used for describing the physical processes occurring in packed-bed reactors, as it requires time-consuming and labour-intensive simulations, especially when comparing cyclic performances of PSA processes using various carbonaceous materials as CO2 adsorbents. Fortunately, Álvarez-Gutiérrez et al.71 presented a straightforward model of engineered biochar-based PSA processes. They formed two groups to classify four-step PSA processes: one group is comprising the pressurisation and adsorption steps, and the other group is comprising the depressurisation and purging steps. Next, with reference to a series of basic assumptions, they determined the mass, momentum, and energy conservation in the packed-bed reactor. In addition, only adsorption isotherms of pure gas component were required to characterize the sample-level process in this simplified model. However, some other process-level indicators, including bed density and porosity, are required for studying PSA processes. A pressure-driven cycle configuration that employs a step involving adsorbent regeneration under vacuum conditions is referred to as a VSA process. A VSA process is simple because CO2 desorption is readily conducted under vacuum, whereas CO2 adsorption occurs at atmospheric pressure.72 A VSA process can also be used to treat flue gas emitted from power plants, even though its pressure is slightly above atmospheric pressure and adsorbent regeneration occurs under low-pressure conditions. The level of vacuum required in the regeneration step is affected by the rectangular shape of CO2 adsorption isotherms, and engineered biochars exhibited better CO2 recoverability under a moderate vacuum level.73 Zhang and Webley72 and Haghpanah et al.74 both determined that a VSA process results in a better CO2 separation process than a PSA process. They devised a simplified model for rapid process simulation to evaluate the cyclic performance of solid CO2 adsorbents.75 They obtained basic measurements of pure-component isotherms of CO2 adsorbents, which was necessary for the process simulation. Sample-level measurements revealed the adsorbent metrics, namely CO2 adsorption capacity, gas selectivity, and gas working capacity (all of which can be obtained from the adsorption isotherms). The performance indicators, namely purity, recovery, energy consumption, and productivity, were considered as optimisation objectives for cyclic process-level CO2 adsorption. The adsorbent metrics were used to screen promising and practical adsorbents for use in designing process-level CO2 capture using food waste-derived engineered biochars. Moreover, Nikolaidis et al.76 established mathematical models to describe the physical phenomena occurring in the PSA/VSA process, and concluded that product purity, recovery, productivity, and energy consumption were the optimisation objectives for process-level CO2 capture. Furthermore, they designed a dual-bed VSA cycle consisting of six steps, including a counter-current pressurisation with the light product, adsorption, pressure equalization, blowdown, evacuation, and counter-current re-pressurisation, for investigating CO2 separation performance. They found that this novel cyclic process is potentially feasible for engineered biochar-based CO2 adsorption.
As mentioned above, the PSA process adsorbs CO2 at super-ambient pressure and desorbs CO2 at near-ambient pressure, whereas the VSA process adsorbs CO2 at nearly atmospheric pressure and desorbs CO2 under a vacuum conditions. A combination of the PSA and VSA processes, denoted as the VPSA process, has attracted much attention; its adsorption step is performed at above atmospheric pressure, and its desorption step is performed under vacuum. Izabela and Marcelina77 reported that engineered biochar-based adsorbents, even with poor CO2 uptake in sample-level CO2 adsorption, achieved high CO2 working capacities in a VPSA process. They also tested biomass-based carbonaceous materials in this VPSA process, highlighting their potential as practical CO2 adsorbents for commercial CO2 capture. Crucially, only CO2 uptake obtained from sample-level CO2 adsorption is necessary to enable preliminary design of a VPSA process. Moreover, more complex VPSA configurations have been devised to solve specific problems. For example, Xiao et al.78 developed a 10-step VPSA cycle for H2 purification, which contains several additional steps of pressure equalisation compared with the basic VPSA. They employed commercial engineered biochars as CO2 adsorbents for evaluating H2 separation performance, and also they compared the performance of various engineered biochars in different process configurations (i.e., PSA and VPSA). In addition, they used sample-level experimental data to determine multi-component gas adsorption and CO2 adsorption kinetics to establish heat and mass transfer models, and then identified engineered biochars’ solid thermal conductivities and heat transfer coefficients. Zhou et al.80 developed a new VPSA cyclic process for nitrogen (N2)/CH4/CO2 mixture separation using engineered biochars as CO2 adsorbents in a packed-bed reactor. They showed that engineered biochars are viable as gas adsorbents in a VPSA process, demonstrating good potential for further development.
As vacuum regeneration is a time-saving process, VPSA processes are especially suitable for industrial-scale CO2 capture applications. A high CO2 working capacity can be obtained in the CO2 adsorption (i.e., physisorption) step at higher than atmospheric pressures, and the vacuum-level desorption step is suitable for the regeneration of engineered biochars. However, the compressor and vacuum pump used for CO2 adsorption and desorption are energy-intensive equipments, suggesting that energy consumption must be reduced or energy efficiency improved in VPSA processes. Zhao et al.79 designed a research framework to evaluate the energy efficiency of a VPSA process for CO2 capture. They obtained the isotherm parameters and physical properties of CO2 adsorbents (including commercial engineered biochars) and applied these in a process-level CO2 adsorption simulation. They employed energy efficiency indicators, namely minimum separation work, consumption of actual work, and second-law efficiency, as cyclic performance indicators together with other classic indicators, e.g., product purity, recovery, and productivity. They concluded that the second-law efficiency of the VPSA cycle was lower than 10%, indicating that it had large energy-saving potential and isotherm shape profoundly affected energy efficiency indicators. For example, they found that the proportionality factor of CO2 working capacity – a parameter they devised that represents the steepness of an isotherm – needed to be low to obtain high second-law efficiency. This may indicate that this is an important parameter for consideration when designing high-performance food waste derived-engineered biochars for CO2 capture.
Temperature-driven CO2 adsorption
A short regeneration time is essential for pressure-driven processes and facilitates industrial-scale CO2 adsorption applications. However, high-grade energy (i.e., electrical energy) is required to drive both a compressor and a vacuum pump, hence it remains challenging to achieve energy-saving targets for these pressure-driven processes via re-design and optimisation. Therefore, temperature-driven CO2 adsorption has attracted much attention, given the temperature-dependance of the working capacities of engineered biochar-based CO2 adsorbents. Compared with PSA processes, TSA process consume much less energy81,82 and have lower CO2 emissions when using renewable energy (i.e., low-grade thermal energy). Typical investigations of temperature-driven CO2 capture process are summarized in Table 3, and the data needed from sample-level CO2 adsorption processes for evaluating process-level CO2 capture performance are also indicated.| Mixture treated | Process configuration | Research approach | Temperature range (°C) | Sample-level characterisation | Process-level evaluation | Results and discussion | Ref. |
|---|---|---|---|---|---|---|---|
| CO2/N2 | Four-step TSA | Simulation | 100–120 | CO2 and N2 isotherms, adsorbent heat capacity measurement | CO2 purity, recovery, productivity, energy consumption, second-law efficiency, capture cost | Comprehensive analysis conducted using cyclic performance indicators to investigate performance in separating CO2 from a post-combustion stream; adsorbent selection found to require multi-index evaluation. | 83 |
| CO2/N2 | Four-step TSA (with heat recovery) | Simulation | 55, 65, 75, 85 | CO2 and N2 isotherms, adsorbent heat capacity measurement | CO2 working capacity, recovery, exergy efficiency | Cyclic performance evaluation results demonstrate that heat recovery has a positive influence on the TSA process | 84 |
| CO2/N2 | Four-step TSA (with internal heat and mass recovery) | Simulation | 55, 65, 75, 85 | CO2 and N2 isotherms, adsorbent heat capacity measurement | CO2 working capacity, recovery, exergy efficiency | The main parameters, e.g. regeneration heat and exergy efficiency, are determined based on the characteristics of activated carbon, and the internal heat and mass recovery steps are effective. | 85 |
| CO2/N2 | Four-step TSA (with sound assisted) | Experiment | 40, 70, 100, 130, 150 | None | CO2 working capacity, purity, recovery | Heating very efficient for desorbing carbon dioxide, and sound-assisted strategy is effective. | 86 |
| CO2/N2 | Four-step TSA (sound assisted) | Experiment | 40, 70, 100, 130, 150 | Adsorption dynamic measurement | CO2 productivity, working capacity, capture energy cost | Preliminary energy and feasibility estimations suggest that the sound-assisted TSA configuration may be industrially competitive. | 87 |
| CO2/N2/H2O | Four-step VTSA | Experiment | 70 | Adsorption dynamic measurement | Working capacity | Devised model is a good approximation of the physical phenomenon in fixed bed filling with engineered biochars. | 88 |
| CO2/N2 | Five-step VTSA | Experiment and simulation | 70 | CO2 and N2 isotherms, breakthrough curves, adsorbent heat capacity, thermal conductivity measurement | CO2 purity, recovery | Prediction of the characteristic parameters of cyclic configurations analysed was accurate, different process configurations can be compared fairly. | 89 |
| CO2/N2 | Two-step TSA | Experiment | 100–120 | Static gas adsorption, dynamic adsorption tests | Working capacity, energy consumption | Adsorption/desorption system integrated with radiative cooling and solar heating devised, thus avoiding use of energy for heating and cooling processes; represents proof-of-concept work that may pave an alternative way to for developing energy-saving TSA techniques. | 90 |
Zhao et al.83 investigated the basic four-step TSA process consisting of pressurization, adsorption, heating, and cooling steps. They established a shortcut model to describe the physical process occurring in a packed-bed reactor and simulated in detail the steps of the TSA process. Several typical CO2 adsorbents (i.e., commercial activated carbon adsorbents) were compared, with their CO2 and N2 isotherms and physical properties (i.e., specific heat capacity, density, and isosteric heat of adsorption) regarded as the input features (the same scenario evidently applies to biochar as well). The CO2 selectivity, recovery, purity, productivity, and other energy-efficiency indicators (i.e., specific thermal energy consumption, minimum separation work, and the second-law efficiency) were calculated as the target features. Following this research, Jiang et al.84 further developed a four-step TSA process equipped with a heat recovery step, and calculated its energy-efficiency indicators (i.e., minimum separation work, and exergy efficiency), which they compared in detailed with those of the basic four-step TSA process. They concluded that the heat recovery step was an effective and practical route for heat re-utilisation, as it increased the theoretical exergy efficiency by approximately 20%–30% in the four-step TSA CO2 capture process. In addition, Jiang et al.85 studied the four-step TSA CO2 adsorption process incorporated an internal heat recovery, internal mass recovery, and internal heat/mass recovery step, respectively, and showed that these processes exhibited greater energy efficiency than the basic four-step TSA CO2 adsorption process.
Raganati et al.86 determined that the main limitation of the conventional TSA process is the dilution of CO2 by the purging gas, and also used engineered biochars as CO2 adsorbents in a laboratory-scale experimental study. The CO2 purity decreased with the introduction of N2 purging, and thus, the regeneration process that separated the heating and purging steps was studied. The main focus was the trade-off between an increase in CO2 recovery and a decrease in CO2 purity with the introduction of N2 purging. They employed an indirect heating method integrated with additional force enhancement, i.e., a sound-assisted TSA process. Raganati et al.87 also experimentally investigated a laboratory-scale TSA process in a sound-assisted fluidised-bed reactor, and tested the CO2 working capacity, dynamic breakthrough, and regeneration performance of CO2 adsorbents. Moreover, they examined effects of adsorption/desorption temperatures and CO2 partial pressure on cyclic performance. The abovementioned comprehensive research framework is suitable for the design and optimisation of cyclic configurations using food waste-derived engineered biochars as CO2 adsorbents.
The heating and cooling steps used in the CO2 adsorption–desorption processes of the conventional TSA process render it unsuitable for used in a rapid cyclic fashion. Therefore, the vacuum step is combined with a heating regeneration method to give the vacuum-temperature swing adsorption (VTSA) process. This process reduces the temperature and pressure differences required for process-level CO2 adsorption, thereby shortening residence time for cyclic CO2 adsorption–desorption processes, and increasing CO2 productivity and recovery. Plaza et al.88 used a mathematical model to simulate a VTSA process and then used laboratory-scale experimental data to validate the model. Specifically, they studied a four-step VTSA process, consisting of adsorption, heating and evacuation, cooling, and pressurisation and purging, in a laboratory-scale fixed bed adsorption unit. The physical aspects (mass, energy, and momentum conservation) in the packed-bed reactor were examined by establishing non-isothermal non-adiabatic dynamic model. In addition, adsorption isotherms of pure component were collected to quantify the gas adsorption amounts, and breakthrough experiments were conducted to evaluate overall mass transfer resistance. Durán et al.89 explored a five-step VTSA process based on a conventional VSA process and developed three columns to decrease its energy requirements. They assessed detailed process configurations in a laboratory-scale fixed-bed reactor, using commercial activated carbon as a CO2 adsorbent. Li et al.63 proved that a VTSA process was superior to both TSA and pressure-temperature swing adsorption processes. The methodology devised for evaluating the cyclic CO2 adsorption performance of engineered biochars could guide food waste-derived engineered biochar-based CO2 capture well.
As mentioned, a possible energy-saving route involves the use of low-grade thermal energy in temperature-driven CO2 adsorption, i.e., integrating renewable energy (i.e., solar thermal energy or geothermal energy) with a TSA process. Dang et al.90 investigated the integration of a TSA process with passive radiative cooling and solar heating routes in a packed-bed reactor using commercial activated carbon as CO2 adsorbent. Their results demonstrated that they were viable as energy-saving TSA processes. They measured CO2 adsorption isotherms and breakthrough from sample-level CO2 adsorption data, and examined a packed bed's radiative cooling properties and solar heating effects. Their findings suggested that the integration of renewable energy with food waste-derived engineered biochar-based CO2 capture is a promising and practical strategy to achieve both carbon neutrality and sustainable waste management.
Other CO2 adsorption processes
In addition to temperature- and pressure-driven CO2 adsorption processes, other CO2 adsorption processes have been developed to evaluate CO2 working capacities using engineered biochars as CO2 adsorbents. For example, electric swing adsorption (ESA) has been considered as a promising route to accelerate the heating step by using a low-voltage electric current via establishment of the Joule effect. Lillia et al.91 developed a temperature/electric swing adsorption (T/ESA) process for CO2 adsorption that comprised eight steps, including adsorption, recuperative pre-heating, steam heating, electric heating, purge-to-capture, purge-to recycle, thermal recovery, and cooling. As both steam heating and electric heating were energy-consuming steps, the sensible heat was recycled in the thermal recovery step and then used for the recuperative pre-heating step. The T/ESA process was developed based on CO2 gas adsorption isotherms, adsorbent density and porosity, heat capacity, mass transfer coefficient, and electric resistance, which were obtained from sample-level CO2 adsorption experiments. This T/ESA process was thus found to be applicable to the conventional natural gas combined cycle with an exhaust gas recycle. Zhao et al.92 experimentally studied a combined electrical and vacuum swing CO2 adsorption (VESA) process to evaluate the cyclic performance of a novel hybrid monolith. Their findings revealed that the CO2 purity obtained from the VESA process was up to 33% higher than the CO2 purity (17%–23%) obtained from a simple VSA process, validating the VESA process as more promising than a VSA process for CO2 adsorption.The microwave swing adsorption (MSA) process was devised as an alternative to the TSA process; the MSA involves a fast, contactless, and direct heating route, which is benefical to increasing CO2 working capacity and accelerating CO2 desorption.93 It was found that rapid adsorbent regeneration can be achieved using the MSA process, and also that energy consumption can be reduced by integrating renewable energy with electrified CO2 capture. Yassin et al.94 performed detailed comparisons between an MSA process and a conventional TSA process using a rotatory fixed-bed reactor filled with CO2 adsorbents. They evaluated CO2 adsorption performance in terms of CO2 uptake capacity, regeneration efficiency, and rate of regeneration. And they also considered power consumption per adsorbent mass as a CO2-adsorption performance indicator. They concluded that MSA was an energy-saving process, because the power consumed by microwave regeneration was 18.69% less than conventional TSA process.
In addition, other environmental factors have been investigated. For example, Querejeta et al.95 studied the effect of humidity on CO2 adsorption capacity using K2CO3-doped engineered biochar samples in a packed-bed reactor. The results revealed that carbonisation occurred in the packed-bed reactor and improved the CO2 adsorption capacity. In another typical example, Cuesta and Song96 devised a novel pH-based swing CO2 adsorption process by using activated carbon black adsorbents and immobilised carbonic anhydrase biocatalysts for ambient CO2 capture. This proof-of-concept study revealed a new way to drive CO2 enriched from a dilute source to a sink for storage or usage.
Life-cycle assessment of food-waste derived CO2 adsorbents
Use of food waste-derived engineered biochars for CO2 capture contributes to sustainable management of food waste and carbon neutrality,97 thereby mitigating two critical environmental problems. Life-cycle assessment (LCA) is widely considered to be a powerful and practical tool for fully investigating environmental impacts, thereby affording guidelines for researchers and policymakers.Life cycle assessment of CO2 adsorbents
The synthesis of CO2 adsorbents, particularly green CO2 adsorbents from waste materials, is considered an emerging technology. Thus, LCA of this emerging technology has received attention in recent years. Table 4 provides a detailed summary of LCAs of various types of CO2 adsorbents. Researchers have studied biomass, particularly food waste, which is transformed into biochars for use as CO2 adsorbents. These studies have evaluated the environmental sustainability of this approach using LCA. The food waste that has been used has included waste oyster shells,98 coconut shells,99,100 wheat flour and glucose,101 and mixed food waste.25 Wang et al.98 conducted a preliminary and simplified LCA to compare the CO2 emissions of two calcium oxide (CaO)-based adsorbents derived from waste oyster shells and limestone. Their results indicated that net negative emissions were more easily achieved using oyster shells than limestone. Zhu et al.101 conducted a preliminary LCA of five kinds of CO2 adsorbents synthesised from glucose or wheat flour to provide optimisation information, from an environmental perspective, at the early development stage. Yuan et al.25 assessed the environmental impacts of an engineered biochar derived from general mixed food waste and its application for industrial CO2 capture. They found that their technical route had high potential to achieve a closed carbon loop.| Type of CO2 adsorbent | Framework/origin | Functional unit | System boundary | Inventory data | Environmental impact category | GWP performance (kg CO2 eq.) | Impact assessment | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1. Climate change, 2. Ozone depletion, 3. Respiratory inorganics, 4. Acidification terrestrial and freshwater, 5. Eutrophication freshwater, 6. Eutrophication marine, 7. Eutrophication terrestrial, 8. Ionizing radiation, 9. Photochemical ozone formation, 10. Cancer human health effects, 11. Non-cancer human health effects, 12. Ecotoxicity freshwater, 13. Land use, 14. Water scarcity, 15. Resource use, energy carriers, 16. Resource use, mineral and metals, 17. Smog, 18. Fossil fuel depletion, 19. Marine aquatic ecotoxicity, 20. Human toxicity, 21. Terrestrial ecotoxicity, 22. Photochemical oxidation, 23. Fine particulate matter formation, 24. Abiotic depletion potential, and 25. Primary energy demand. | ||||||||
| CaO-based sorbent | Oyster shell | 1 kg sorbent | Raw material acquisition, production, CO2 adsorption, recycle and disposal | Laboratory-scale experiment | 1 | −4.4–0 | Midpoint and endpoint | 98 |
| CaO-based sorbent | Limestone | 1 kg sorbent | −3.7–0.7 | |||||
| Physiosorbed carbon nanotube (CNT) supported polyethylenimine (PEI) sorbent | CNT | 1 kg sorbent (1 kg CO2 adsorbed) | Production, CO2 adsorption | Laboratory-scale experiment | 1–5, 10, 11, 17, 18 | 209 | Midpoint | 102 |
| Covalently bound CNT supported PEI sorbent | CNT | 326 | ||||||
| Amine on alumina sorbent | Alumina | 1 kg CO2 adsorbed | Production and disposal | Scientific paper and database | 1–16 | 12.76 E10–3 | Midpoint | 99 |
| Amine on silica sorbent | Silica | 23.53 E10–3 | ||||||
| Amine on cellulose sorbent | Cellulose | 46 E10–3 | ||||||
| Potassium carbonate on silica sorbent | Silica | 9.38 E10–3 | ||||||
| Potassium carbonate on activated carbon sorbent | Coconut shell | 11.54 E10–3 | ||||||
| Anionic exchange Resin sorbent | Resin | 36.30 E10–3 | ||||||
| Activated carbon | Persian ironwood biomass | 1 kg CO2 adsorption | Production | Laboratory-scale experiment and simulation for scale up | 1, 2, 4, 5, 9, 12, 18–22 | 9.501 | Midpoint | 103 |
| Copper oxide-activated carbon | Persian ironwood biomass | 26.367 | ||||||
| Zeolite (13X-APG) | FAU zeolite | 1 kg CO2 adsorbed | Production, CO2 adsorption and disposal | Laboratory-scale experiment | 1, 2, 4, 5, 12, 16, 18–22 | 4.2 | Midpoint | 100 |
| Carbon molecular sieve (CMS) (CMS-330) | Coconut shell | 7.73 | ||||||
| Biochar | Glucose and sodium | 1 kg CO2 uptake | Production, CO2 uptake | Laboratory-scale experiment | 1, 2, 4–6, 8–14, 16, 18, 19, 21, 23 | 666.26 | Midpoint | 101 |
| Biochar treated with urea | Glucose and sodium | 679.89 | ||||||
| Biochar treated with cobalt acetate | Glucose and sodium | 586.41 | ||||||
| Biochar | Wheat flour, Glucose and sodium | 138.93–309.43 | ||||||
| Biochar treated with dicyandiamide | Wheat flour, Glucose and sodium | 108.36–459.11/ | ||||||
| Biochar | Mixed food waste obtained from the Korea Institute of Civil Engineering and Building Technology | 1 kg adsorbent | Production | Laboratory-scale experiment | 1, 2, 4, 5, 8, 9, 14, 23, 24, 25 | 12.87 | Midpoint | 25 |
| Biochar | Raw material acquisition, Production, CO2 adsorption, disposal | −5.4 | Midpoint | |||||
Environmental impacts of food waste-derived CO2 adsorbents
LCA results from different studies cannot be compared due to the studies having used different system boundaries and functional units (FUs). As shown in Fig. 4, FUs that have been commonly used are 1 kg of adsorbent and 1 kg of CO2 adsorbed/captured, with cradle-to-gate and cradle-to-grave, respectively, as system boundaries. Specifically, the cradle-to-gate system boundary assesses the impacts of biochar until it leaves the factory gate while the cradle-to-grave system boundary also includes the use and end life of biochar.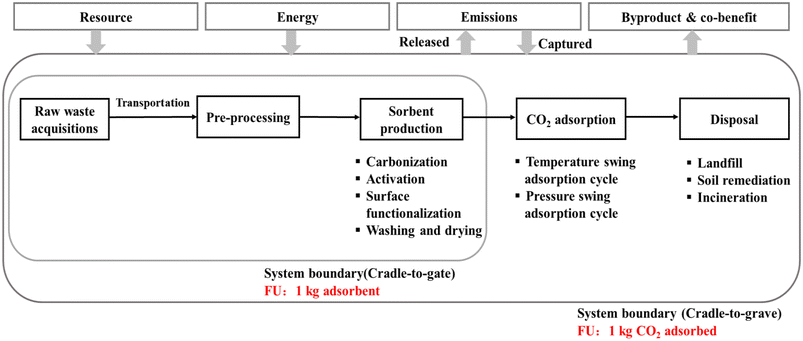 | ||
| Fig. 4 A general system boundary for food waste derived carbon dioxide (CO2) adsorbent (FU = functional unit). | ||
We highlight that in both studies, a thorough consideration of all potential carbon emissions during the processes is required, including the additional emissions as by-product. The life cycle equivalent CO2 emissions of the aforementioned CaO-based adsorbent derived from oyster shells achieved negative CO2 emissions when coupled with 10 cycles of CO2 carbonation/calcinations.98 Moreover, it was assumed that no CO2 emissions occurred during the CO2 adsorption cycle due to waste heat being utilised for CO2 adsorbent regeneration. Deutz and Bardow99 showed that the carbon footprint (11.54 10−3 kgCO2-equivalents per kg CO2 captured) of a coconut-derived adsorbent performed well compared with five other types of CO2 adsorbents. Yuan et al.25 estimated that the life-cycle CO2 equivalent emissions of food waste-derived engineered biochar was 12.87 kg within a cradle-to-gate system boundary, which is on the same order of magnitude as engineered biochars prepared from other types of biomass. However, this process may lead to non-negligible impacts on primary energy consumption and water depletion. In addition, in the cradle-to-grave assessment, the CO2 equivalent emissions of engineered biochar in carbon capture applications play a larger proportion. This implies that a one-sided pursuit of reducing CO2 equivalent carbon emissions caused by energy consumption during sample preparation may have negative effects, and optimizing the process to improve sample performance may yield great benefits.
Challenges and future prospects
Currently, LCA is widely regarded as an important and powerful tool for assessing the overall performance of emerging CO2 adsorbents derived from food waste. However, there have been insufficient LCA studies in this field and they lack a consensus framework. Firstly, the technical route of upcycling food waste into engineered biochars for CO2 adsorption involves multiple functions, including waste management, production of CO2 adsorbents, and CO2 mitigation. Thus, inconsistent FUs have been used to describe this process, which makes it difficult to compare the use of different carbon precursors and adsorbent preparation pathways. Secondly, as shown in Table 4, the system boundaries considered vary significantly, and as shown in Fig. 4, few studies have provided a complete cradle-to-gate or cradle-to-grave assessment. Penultimately, the CO2 adsorption process has been oversimplified in most studies, and few studies have considered the actual life span of CO2 adsorbents. In actual industrial scenarios, the condition of the CO2 sources, the types of CO2 adsorption cycles, the energy consumed by adsorbent regenerations, and the total cyclic number of CO2 adsorbents vary significantly and have a considerable influence on LCA results. Finally, laboratory-scale experiment data have been used as the core inventory data in almost all studies, because this technology remains in its early stages. Thus, these data may not necessarily parallel industrial scale data, resulting in rather high potential uncertainty. Moreover, few studies have conducted uncertainty and sensitivity assessments of key factors.Conclusions and perspectives
This review extensively explored whether food waste-derived engineered biochars have been found to be effective as CO2 adsorbents, by examining studies on sample preparation with different capture processes (including both sample- and process-level) and on environmental impacts with comprehensive LCAs. This review revealed the viability of food waste-derived engineered biochars as promising alternative materials for CO2 capture. Moreover, this review addressed challenges in sample preparation process, harnessing ML for optimisation, tailoring cyclic process, and embracing holistic LCA, thereby significantly contributing to the understanding and advancement of sustainable food waste upcycling and carbon capture technologies within the context of a circular economy. It's worth mentioning that wasting food itself is not considered a sustainable practice, by utilizing the wastes generated during the grain production process, such as banana peels, potato peels, these can still be regarded as effective sources of engineered biochars.Owing to the high moisture content of food waste, anaerobic digestion followed by HTC is highly preferred for upcycling food waste into value-added CH4 (or H2) and carbonaceous materials. In particularly, the hydrochar obtained in this approach can be further converted into effective catalysts via anaerobic digestion, which enhancing CH4 or H2 yields.104 Only a simple batch reactor is required for valorising food waste into valuable gaseous and solid products, making it practical and feasible for commercialisation. Activation and surface functionalisation have been widely used to develop engineered biochars with significant enhancement of their potential applications, especially for CO2 adsorption. From a life-cycle perspective, HTC, avoiding energy consumption for pre-drying treatment of food waste, is classified as a green technical route to upcycle food waste into solid carbon materials. The environmental impacts potentially introduced by the chemical agents during activation and surface functionalisation also need to be assessed in the context of the trade-off with performance enhancement.
Within the context of carbon neutrality, this review comprehensively addressed food waste-derived engineered biochars for CO2 capture at both the sample-level and process-level processes. Conventional synthetic routes for high-performance CO2 adsorbents are both time- and labour-intensive, therefore, ML is considered as an emerging technology for accelerating synthesis of biomass-based CO2 adsorbents and effectively providing valuable guidelines for inverse design of engineered biochars with high-performance CO2 capture. These results promote commercial applications of engineered biochar-based CO2 adsorption approaches. As ML is a data-driven approach, data collection and pre-processing treatment need to be improved, which are critical for providing accurate performance prediction and valuable guidelines for CO2 adsorbent synthesis. Moreover, various factor-driven CO2 adsorption processes, including TSA and PSA processes were reviewed and compared in detail. Research on a wide range of cyclic processes has underscored the necessity of tailoring cycle configurations to match the characteristics of food waste-derived engineered biochars. The judicious selection of cyclic processes, with a particular focus on adsorption isotherm profiles, plays a pivotal role in enhancing cyclic performance.
When targeting the UN SDGs and striving towards achieving a circular economy, the environmental impacts of emerging technical routes need to be comprehensively assessed from a life-cycle perspective. This review evaluated studies on CO2 adsorption using food waste-derived engineered biochars with the consideration of various system boundaries (i.e., cradle-to-gate and cradle-to-grave). These studies suggested that food waste-derived engineered biochars for CO2 capture has great potential to achieve a closed carbon loop and is thus a promising alternative to conventional CO2 adsorption methods. However, these studies highlighted that there is currently no standardized research approach to provide a unified assessment for the selection of different boundaries, FUs, and environmental impact parameters. The LCA-based conclusions have limited applicabilities and cannot be compared across different studies horizontally. Considering the uncertainties of laboratory-scale investigations, more comprehensive assessments need to be performed to ensure that food waste-derived engineered biochars makes substantial contributions to a circular economy and environmental sustainability.
Author contributions
J. Wen, J. Wang, and S. Li contributed equally to this work. J. Wen – conceptualization, investigation, visualization, writing – original draft, review & editing; J. Wang and S. Li – investigation, data curation, formal analysis, visualization, writing – original draft, review & editing; J.T.E. Lee – writing – review & editing; C.S.K. Lin, O. Mašek, and H. Zhang – supervision, writing – review & editing; X. Yuan – supervision, visualization, validation, funding acquisition, writing – original draft, review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Start-up Research Fund of Southeast University (RF1028623274) and Youth Program of National Natural Science Foundation of China (Grant No. 72104257). J. T. E. Lee was supported by the National Research Foundation, Prime Minister's Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.References
- A. Srivastava and A. Mishra, Environ. Sustainability, 2022, 5, 401–421 CrossRef CAS
.
- E. O. Ebikade, S. Sadula, Y. Gupta and D. G. Vlachos, Green Chem., 2021, 23, 2806–2833 RSC
.
- K. Schanes, K. Dobernig and B. Gözet, J. Cleaner Prod., 2018, 182, 978–991 CrossRef
.
-
UNEP, Food Waste Index Report 2021, United Nations Environment Programme, Nairobi, 2021 Search PubMed
.
-
USEPA, From Farm to Kitchen: The Environmental Impacts of U.S. Food Waste, U.S. Environmental Protection Agency, 2021 Search PubMed
.
-
UnitedNations, Transforming Our World: The 2030 Agenda for Sustainable Development, Department of Economic and Social Affairs, United Nations, 2015 Search PubMed
.
- P. Roy, A. K. Mohanty, P. Dick and M. Misra, ACS Environ. Au, 2023, 3, 58–75 CrossRef CAS PubMed
.
- J. Aschemann-Witzel, D. Asioli, M. Banovic, M. A. Perito, A. O. Peschel and V. Stancu, Trends Food Sci. Technol., 2023, 132, 132–137 CrossRef CAS
.
- P. Murugesan, V. Raja, S. Dutta, J. A. Moses and C. Anandharamakrishnan, Sci. Total Environ., 2022, 851, 157955 CrossRef CAS PubMed
.
- X. Yuan, P. D. Dissanayake, B. Gao, W. J. Liu, K. B. Lee and Y. S. Ok, J. Environ. Manage., 2021, 296, 113128 CrossRef CAS PubMed
.
- S. Yadav and D. Singh, Carbon Res., 2023, 2, 34 CrossRef
.
- S. Moogi, S.-H. Jang, G. H. Rhee, C. H. Ko, Y. J. Choi, S. H. Lee, P. L. Show, K.-Y. A. Lin and Y.-K. Park, Chemosphere, 2022, 287, 132224 CrossRef CAS PubMed
.
- G. Su, H. C. Ong, I. M. R. Fattah, Y. S. Ok, J. H. Jang and C. T. Wang, Sci. Total Environ., 2022, 809, 151170 CrossRef CAS PubMed
.
- L. Dai, O. Karakas, Y. Cheng, K. Cobb, P. Chen and R. Ruan, Chem. Eng. J., 2023, 453, 139725 CrossRef CAS
.
- T. Chen, S. Deng, B. Wang, J. Huang, Y. Wang and G. Yu, RSC Adv., 2015, 5, 48323–48330 RSC
.
- B. Huang, H. Shao, N. Liu, Z. J. Xu and Y. Huang, RSC Adv., 2015, 5, 88171–88175 RSC
.
- L. Yue, L. Rao, L. Wang, L. Wang, J. Wu, X. Hu, H. DaCosta, J. Yang and M. Fan, Ind. Eng. Chem. Res., 2017, 56, 14115–14122 CrossRef CAS
.
- F. Yang, J. Wang, L. Liu, P. Zhang, W. Yu, Q. Deng, Z. Zeng and S. Deng, ACS Sustainable Chem. Eng., 2018, 6, 15550–15559 CrossRef CAS
.
- L. Yue, Q. Xia, L. Wang, L. Wang, H. DaCosta, J. Yang and X. Hu, J. Colloid Interface Sci., 2018, 511, 259–267 CrossRef CAS PubMed
.
- M. Y. Zhu, W. Q. Cai, F. Verpoort and J. B. Zhou, Chem. Eng. Res. Des., 2019, 146, 130–140 CrossRef CAS
.
- G.-g. Huang, Y.-f. Liu, X.-x. Wu and J.-j. Cai, New Carbon Mater., 2019, 34, 247–257 CrossRef CAS
.
- J. Han, L. Zhang, B. Zhao, L. Qin, Y. Wang and F. Xing, Ind. Crops Prod., 2019, 128, 290–297 CrossRef CAS
.
- M.-J. Kim, S. W. Choi, H. Kim, S. Mun and K. B. Lee, Chem. Eng. J., 2020, 397, 125404 CrossRef CAS
.
- A. D. Igalavithana, S. W. Choi, P. D. Dissanayake, J. Shang, C. H. Wang, X. Yang, S. Kim, D. C. W. Tsang, K. B. Lee and Y. S. Ok, J. Hazard. Mater., 2020, 391, 121147 CrossRef CAS PubMed
.
- X. Yuan, J. Wang, S. Deng, P. D. Dissanayake, S. Wang, S. You, A. C. K. Yip, S. Li, Y. Jeong, D. C. W. Tsang and Y. S. Ok, ACS Sustainable Chem. Eng., 2022, 10, 13026–13036 CrossRef CAS
.
- S. Joseph, G. Saianand, M. R. Benzigar, K. Ramadass, G. Singh, A.-I. Gopalan, J. H. Yang, T. Mori, A. H. Al–Muhtaseb, J. Yi and A. Vinu, Adv. Sustainable Syst., 2020, 5, 2000169 CrossRef
.
- R. Chakraborty, V. K, M. Pradhan and A. K. Nayak, J. Mater. Chem. A, 2022, 10, 6965–7005 RSC
.
- L. Wang, J. Deng, X. Yang, R. Hou and D. Hou, Carbon Res., 2023, 2, 2 CrossRef
.
- W. Zhang, X. Qiu, C. Wang, L. Zhong, F. Fu, J. Zhu, Z. Zhang, Y. Qin, D. Yang and C. C. Xu, Carbon Res., 2022, 1, 14 CrossRef
.
- S. Li, X. Yuan, S. Deng, L. Zhao and K. B. Lee, Renewable Sustainable Energy Rev., 2021, 152, 111708 CrossRef CAS
.
- X. Yuan, J. Wang, S. Deng, M. Suvarna, X. Wang, W. Zhang, S. T. Hamilton, A. Alahmed, A. Jamal, A.-H. A. Park, X. Bi and Y. S. Ok, Renewable Sustainable Energy Rev., 2022, 162, 112413 CrossRef CAS
.
- W. Liu, H. Jiang and H. Q. Yu, Chem. Rev., 2015, 115, 12251–12285 CrossRef CAS PubMed
.
- X. Yuan, Y. Shen, P. A. Withana, O. Mašek, C. S. K. Lin, S. You, F. M. G. Tack and Y. S. Ok, Chem. Eng. J., 2023, 469, 143783 CrossRef CAS
.
- M. Pecchi, M. Baratieri, J. L. Goldfarb and A. R. Maag, Bioresour. Technol., 2022, 348, 126799 CrossRef CAS PubMed
.
- H. S. Le, W.-H. Chen, S. F. Ahmed, Z. Said, N. Rafa, A. T. Le, Ü Ağbulut, I. Veza, X. P. Nguyen, X. Q. Duong, Z. Huang and A. T. Hoang, Bioresour. Technol., 2022, 363, 127958 CrossRef PubMed
.
- H. S. Kambo and A. Dutta, Renewable Sustainable Energy Rev., 2015, 45, 359–378 CrossRef CAS
.
- X. Yuan, N. M. Kumar, B. Brigljević, S. Li, S. Deng, M. Byun, B. Lee, C. S. K. Lin, D. C. W. Tsang, K. B. Lee, S. S. Chopra, H. Lim and Y. S. Ok, Green Chem., 2022, 24, 1494–1504 RSC
.
- C. Ma, T. Lu, J. Shao, J. Huang, X. Hu and L. Wang, Sep. Purif. Technol., 2022, 281, 119899 CrossRef CAS
.
- X. Yuan, S. Li, S. Jeon, S. Deng, L. Zhao and K. B. Lee, J. Hazard. Mater., 2020, 399, 123010 CrossRef CAS PubMed
.
- L. Rao, S. Liu, L. Wang, C. Ma, J. Wu, L. An and X. Hu, Chem. Eng. J., 2019, 359, 428–435 CrossRef CAS
.
- C. Song, B. Zhang, L. Hao, J. Min, N. Liu, R. Niu, J. Gong and T. Tang, Green Energy Environ., 2022, 7, 411–422 CrossRef CAS
.
- G. Nazir, A. Rehman and S.-J. Park, J. CO2 Util., 2021, 51, 101641 CrossRef CAS
.
- A. K. Mondal, K. Kretschmer, Y. Zhao, H. Liu, H. Fan and G. Wang, Microporous Mesoporous Mater., 2017, 246, 72–80 CrossRef CAS
.
- Y. Li, G. Wang, T. Wei, Z. Fan and P. Yan, Nano Energy, 2016, 19, 165–175 CrossRef CAS
.
- S. Ha, S. G. Jeong, S. Myeong, C. lim and Y.-S. Lee, J. CO2 Util., 2023, 76, 102589 CrossRef CAS
.
- M. V. Gil, M. Martínez, S. García, F. Rubiera, J. J. Pis and C. Pevida, Fuel Process. Technol., 2013, 106, 55–61 CrossRef CAS
.
- S. Li, M. K. Cho, K. B. Lee, S. Deng, L. Zhao, X. Yuan and J. Wang, Sci. Total Environ., 2022, 834, 155262 CrossRef CAS PubMed
.
- J. Li, K. Lim, H. Yang, Z. Ren, S. Raghavan, P.-Y. Chen, T. Buonassisi and X. Wang, Matter, 2020, 3, 393–432 CrossRef
.
- H. Yin, M. Xu, Z. Luo, X. Bi, J. Li, S. Zhang and X. Wang, Green Energy Environ., 2022, 9(1), 54–70 CrossRef
.
- X. Yuan, M. Suvarna, S. Low, P. D. Dissanayake, K. B. Lee, J. Li, X. Wang and Y. S. Ok, Environ. Sci. Technol., 2021, 55, 11925–11936 CrossRef CAS PubMed
.
- X. Ma, W. Xu, R. Su, L. Shao, Z. Zeng, L. Li and H. Wang, Sep. Purif. Technol., 2023, 306, 122521 CrossRef CAS
.
- X. Zhu, D. C. W. Tsang, L. Wang, Z. Su, D. Hou, L. Li and J. Shang, J. Cleaner Prod., 2020, 273, 122915 CrossRef CAS
.
- X. Yuan, J. Li, J. Y. Lim, A. Zolfaghari, D. S. Alessi, Y. Wang, X. Wang and Y. S. Ok, ACS EST Water, 2023 DOI:10.1021/acsestwater.3c00215
.
- Y. Shen, Fuel Process. Technol., 2022, 236, 107437 CrossRef CAS
.
- M. Bernardo, N. Lapa, I. Fonseca and I. A. A. C. Esteves, Front. Energy Res., 2021, 9, 625188 CrossRef
.
- N. Álvarez-Gutiérrez, S. García, M. V. Gil, F. Rubiera and C. Pevida, Energy Fuels, 2016, 30, 5005–5015 CrossRef
.
- E. Surra, R. P. P. L. Ribeiro, T. Santos, M. Bernardo, J. P. B. Mota, N. Lapa and I. A. A. C. Esteves, J. Environ. Chem. Eng., 2022, 10(1), 107065 CrossRef CAS
.
- T. Liang, C. Chen, X. Li and J. Zhang, Langmuir, 2016, 32, 8042–8049 CrossRef CAS PubMed
.
- J. Du, Y. Yu, H. Lv, C. Chen, J. Zhang and A. Chen, J. Nanopart. Res., 2018, 20, 1–12 CrossRef CAS
.
- I. Majchrzak-Kucęba, D. Wawrzyńczak and A. Ściubidło, J. CO2 Util., 2022, 61, 102027 CrossRef
.
- I. Majchrzak-Kucęba, D. Wawrzyńczak, A. Ściubidło, J. Zdeb, W. Smółka and A. Zajchowski, J. CO2 Util., 2019, 29, 1–11 CrossRef
.
- D. Bahamon, A. E. Ogungbenro, M. Khaleel, M. R. M. Abu-Zahra and L. F. Vega, Ind. Eng. Chem. Res., 2020, 59, 7161–7173 CrossRef CAS
.
- S. Li, M.-K. Cho, X. Yuan, S. Deng, H. Li, L. Zhao, R. Zhao, Y. Wang, J. Wang and K. B. Lee, Fuel, 2023, 331, 125599 CrossRef CAS
.
- A. W. Dowling, S. R. R. Vetukuri and L. T. Biegler, AIChE J., 2012, 58, 3777–3791 CrossRef CAS
.
- S. García, C. F. Martín, J. J. Pis, F. Rubiera and C. Pevida, Energy Procedia, 2013, 37, 127–133 CrossRef
.
- J. A. Delgado, M. A. Uguina, J. L. Sotelo, B. Ruíz and J. M. Gómez, Adsorption, 2006, 12, 5–18 CrossRef CAS
.
- T. C. Drage, J. M. Blackman, C. Pevida and C. E. Snape, Energy Fuels, 2009, 23, 2790–2796 CrossRef CAS
.
- R. M. Siqueira, G. R. Freitas, H. R. Peixoto, J. F. d. Nascimento, A. P. S. Musse, A. E. B. Torres, D. C. S. Azevedo and M. Bastos-Neto, Energy Procedia, 2017, 114, 2182–2192 CrossRef CAS
.
- F. Rezaei and P. Webley, Sep. Purif. Technol., 2010, 70, 243–256 CrossRef CAS
.
- S. Lawson, Q. Al-Naddaf, K. Newport, A. Rownaghi and F. Rezaei, Ind. Eng. Chem. Res., 2021, 60, 16445–16456 CrossRef CAS
.
- N. Álvarez-Gutiérrez, M. V. Gil, F. Rubiera and C. Pevida, J. CO2 Util., 2018, 28, 207–215 CrossRef
.
- J. Zhang and P. A. Webley, Environ. Sci. Technol., 2008, 42, 563–569 CrossRef CAS PubMed
.
- S. Divekar, S. Dasgupta, A. Arya, P. Gupta, S. Singh and A. Nanoti, Sep. Purif. Technol., 2020, 234, 115594 CrossRef CAS
.
- R. Haghpanah, A. Majumder, R. Nilam, A. Rajendran, S. Farooq, I. A. Karimi and M. Amanullah, Ind. Eng. Chem. Res., 2013, 52, 4249–4265 CrossRef CAS
.
- A. K. Rajagopalan, A. M. Avila and A. Rajendran, Int. J. Greenhouse Gas Control, 2016, 46, 76–85 CrossRef CAS
.
- G. N. Nikolaidis, E. S. Kikkinides and M. C. Georgiadis, Ind. Eng. Chem. Res., 2016, 55, 635–646 CrossRef CAS
.
- I. Majchrzak-Kucęba and M. Sołtysik, J. Therm. Anal. Calorim., 2020, 142, 267–273 CrossRef
.
- J. Xiao, A. Mei, W. Tao, S. Ma, P. Bénard and R. Chahine, Energies, 2021, 14(9), 2450 CrossRef CAS
.
- R. Zhao, S. Deng, S. Wang, L. Zhao, Y. Zhang, B. Liu, H. Li and Z. Yu, Appl. Therm. Eng., 2018, 128, 818–829 CrossRef CAS
.
- Y. Zhou, D. Qu, Z. Qian, Y. Yang, P. Li and A. E. Rodrigues, Fluid Phase Equilib., 2022, 561, 113541 CrossRef CAS
.
- J. Merel, M. Clausse and F. Meunier, Ind. Eng. Chem. Res., 2008, 47, 209–215 CrossRef CAS
.
- A. Ntiamoah, J. Ling, P. Xiao, P. A. Webley and Y. Zhai, Ind. Eng. Chem. Res., 2016, 55, 703–713 CrossRef CAS
.
- R. Zhao, L. Liu, L. Zhao, S. Deng, S. Li and Y. Zhang, Renewable Sustainable Energy Rev., 2019, 114, 109285 CrossRef CAS
.
- L. Jiang, A. P. Roskilly and R. Z. Wang, Energy Convers. Manage., 2018, 165, 396–404 CrossRef CAS
.
- L. Jiang, R. Q. Wang, A. Gonzalez-Diaz, A. Smallbone, R. O. Lamidi and A. P. Roskilly, Appl. Therm. Eng., 2020, 169, 396–404 CrossRef
.
- F. Raganati, P. Ammendola and R. Chirone, Sep. Purif. Technol., 2016, 167, 24–31 CrossRef CAS
.
- F. Raganati, R. Chirone and P. Ammendola, Ind. Eng. Chem. Res., 2020, 59, 3593–3605 CrossRef CAS
.
- M. G. Plaza, I. Durán, F. Rubiera and C. Pevida, Energy Procedia, 2017, 114, 2353–2361 CrossRef CAS
.
- I. Durán, F. Rubiera and C. Pevida, Chem. Eng. J., 2020, 382, 122841 CrossRef
.
- Y.-X. Dang, P. Tan, B. Hu, C. Gu, X.-Q. Liu and L.-B. Sun, Green Energy Environ., 2022 DOI:10.1016/j.gee.2022.08.004
.
- S. Lillia, D. Bonalumi, C. Grande and G. Manzolini, Int. J. Greenhouse Gas Control, 2018, 74, 155–173 CrossRef CAS
.
- Q. Zhao, F. Wu, Y. Men, X. Fang, J. Zhao, P. Xiao, P. A. Webley and C. A. Grande, Chem. Eng. J., 2019, 358, 707–717 CrossRef CAS
.
- Y. Gomez-Rueda, B. Verougstraete, C. Ranga, E. Perez-Botella, F. Reniers and J. F. M. Denayer, Chem. Eng. J., 2022, 446, 137345 CrossRef CAS
.
- M. M. Yassin, J. A. Anderson, G. A. Dimitrakis and C. F. Martín, Sep. Purif. Technol., 2021, 276, 119326 CrossRef CAS
.
- N. Querejeta, F. Rubiera and C. Pevida, J. Energy Chem., 2019, 34, 208–219 CrossRef
.
- A. R. Cuesta and C. Song, Appl. Energy, 2020, 280, 116003 CrossRef CAS
.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. M. Dowell, Energy Environ. Sci., 2018, 11, 1062–1176 RSC
.
- T. Wang, D. C. Xiao, C. H. Huang, Y. K. Hsieh, C. S. Tan and C. F. Wang, J. Hazard. Mater., 2014, 270, 92–101 CrossRef CAS PubMed
.
- S. Deutz and A. Bardow, Nat. Energy, 2021, 6, 203–213 CrossRef CAS
.
- R. Gonzalez-Olmos, A. Gutierrez-Ortega, J. Sempere and R. Nomen, J. CO2 Util., 2022, 55, 101791 CrossRef CAS
.
- Y. Zhu, X. Ge, Y. Li, W. Hou, J. Ma and H. Li, Pol. J. Environ. Stud., 2022, 31, 2973–2986 CrossRef CAS
.
- F. Wu, Z. Zhou, S. Temizel-Sekeryan, R. Ghamkhar and A. L. Hicks, J. Cleaner Prod., 2020, 270, 122465 CrossRef CAS
.
- M. Nowrouzi, H. Abyar, H. Younesi and E. Khaki, J. CO2 Util., 2021, 47, 101491 CrossRef CAS
.
- X. Yuan, Y. Cao, J. Li, A. K. Patel, C. D. Dong, X. Jin, C. Gu, A. C. K. Yip, D. C. W. Tsang and Y. S. Ok, Biotechnol. Adv., 2023, 67, 108181 CrossRef PubMed
.
Footnote |
| † These authors contributed equally as first authors. |
| This journal is © The Royal Society of Chemistry 2024 |
