Open Access Article
Open Access ArticleDye adsorption-assisted colloidal dispersion of single-walled carbon nanotubes in polar solvents†
Akiho
Horibe
a,
Tomoko
Murayama
b,
Tsuyoshi
Kawai
 b and
Yoshiyuki
Nonoguchi
b and
Yoshiyuki
Nonoguchi
 *a
*a
aFaculty of Materials Science and Engineering, Kyoto Institute of Technology, Kyoto 606-8585, Japan. E-mail: nonoguchi@kit.ac.jp
bDivision of Materials Science, Nara Institute of Science and Technology, Ikoma 630-0192, Japan
First published on 18th September 2023
Abstract
Micellar surfactants with amphiphilic chemical structures are mostly used to disperse single-walled carbon nanotubes (SWCNTs) in water. However, there is little systematic knowledge regarding the use of low-molecular-weight nonmicellar adsorbates as efficient surfactants for colloidal SWCNT dispersions, which limits the number of available solvents. In this article, we present an empirical rule for adsorbate-based dispersants required for SWCNT dispersion in polar organic solvents and water. First, we demonstrate that SWCNTs can be dispersed in aqueous media with the aid of the non-aqueous stilbene backbone compound amsonic acid. The impact of nonmicellar physical adsorption on dispersion was systematically examined using low-molecular-weight compounds, including Fluorescent Brighteners 28 and 220. This discovery has increased the availability of solvents such as water, polar organic solvents such as alcohols, and surfactants such as widely used organic dyes. The results of our study indicate that effective nonmicellar dispersants should include acid–base-carrying structures. The present findings have elucidated the colloidal chemistry of nanocarbon materials with significant potential for application as low-molecular-weight adsorbate-enhanced multifunctional inks.
Introduction
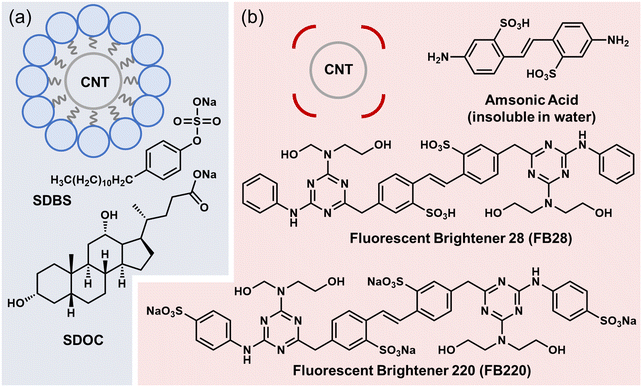
The colloidal dispersion of single-walled carbon nanotubes (SWCNTs) in a solvent is an important step for fabricating their various forms (e.g. composites and semitransparent thin films),1–3 as well as separating them in terms of electronic types, such as semiconducting and metallic forms.4–6 SWCNTs are widely recognised as promising mechanical, electronic, and thermal substances, but are also known to be insoluble in most solvents without surfactants, making it difficult to fabricate SWCNT-impregnated materials in solution. Colloidal dispersion has been achieved through the chemical and physical functionalization of SWCNTs. Chemical grafting enables the dispersion of SWCNTs in arbitrary solvents, ranging from water to hydrophobic media.7,8 In most cases, this approach degrades the intrinsic properties of SWCNTs, including their electronic transport and photochemical response. Precise chemical functionalization is a contemporary issue for elucidating strong localised (trapped) photoluminescence and its use in emerging quantum bit spinning as a quantum information technology.9–13 To extract the intrinsic properties of SWCNTs for most uses, damage (defects) derived from chemical processing should be minimised.Physical molecular adsorption was investigated for the colloidal dispersion of SWCNTs, which allowed the separation of semiconducting and metallic SWCNTs as well as the fabrication of thin-film electronics. In the early stages, micellar surfactants such as sodium dodecyl sulfate, sodium dodecylbenzenesulfonate (SDBS), and sodium deoxycholate (SDOC) were reported to efficiently isolate and stabilise SWCNTs in water (Fig. 1a),14–18 leading to the development of aqueous inks with long shelf times. In addition to aqueous micellar dispersion, dispersion based on molecular adsorption is achieved using 1) DNA and protein wrapping,19–23 and 2) approaches using planar organic compounds24–28 as surfactants. Micelle formation is, in principle, limited to water. Therefore, an understanding of molecular adsorption-assisted dispersion is required for processing SWCNTs in various organic solvents. Conjugated polymers with long alkyl side chains are known to disperse SWCNTs in low-polarity solvents such as toluene.29–33 The strong affinity derived from π–π interactions between the tubular SWCNT surface and the planar backbones of conjugated polymers is believed to overcome the self-aggregation force of SWCNTs. Cellulosic polymers and some types of vinyl polymers have also been reported to aid in the colloidal dispersion of SWCNTs in arbitrary organic solvents.34–36 A recent study based on artificial intelligence-assisted feature extraction suggested that weak interactions such as hydrogen bonding could be a key factor in the design of efficient surfactants.36
 | ||
| Fig. 1 Surfactant structures. (a) Typical micelle-type surfactants. (b) Non-micellar surfactants. Abbreviations: CNT, carbon nanotube; SDBS, sodium dodecylbenzenesulfonate; SDOC, sodium deoxycholate. | ||
Compared to polymer surfactants, there are few studies on surfactants derived from low-molecular-weight molecules for efficient SWCNT dispersion,24–28,37,38 except for those on well-known micellar solutions. Owing to the limitations of such reports, the general design of efficient surfactants remains unexplored. Herein, we report empirical guidelines for dispersants required for SWCNT dispersion in water and polar organic solvents. First, we demonstrate that a non-aqueous stilbene backbone compound, amsonic acid (Fig. 1b), can aid in the aqueous dispersion of SWCNTs without yielding significant precipitates after high-speed centrifugation. This suggests that the physical adsorption of small compounds without micelle formation can alter the dispersibility of SWCNTs. Based on this chemical backbone, Fluorescent Brightener 220 (FB220) and Fluorescent Brightener 28 (FB28) were then used to investigate the mechanistic effect of nonmicelle physical adsorption on dispersion. The findings from this study could expand the scope of available surfactants to include various organic dyes and solvents ranging from water to polar organic solvents, such as alcohols. Based on preliminary controlled experiments, we propose that strategies for nonmicellar and efficient dispersion include acid–base carrying structures.
Results and discussion
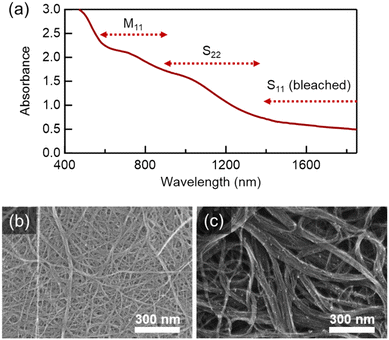
We demonstrated that SWCNTs can form colloidal dispersions in the presence of several dispersants, including amsonic acid, FB28, and FB220 (Fig. 2). In contrast to conventional amphiphilic surfactants such as SDOC, several adsorbates help SWCNTs disperse not only in water, but also in ethanol. As micelle formation aided by hydrophobic interactions requires an aqueous medium, the alcohol dispersion observed here could be driven by distinct mechanisms.In this study, we found that stilbene derivatives, including amsonic acid, were beneficial for the colloidal dispersion of SWCNTs in water (Fig. 2a). The mean diameter of SWCNTs (OCSiAl Tuball) is assumed to be 1.6 nm, and the semiconducting SWCNTs therein possess the first and second optical transition energies of approximately 0.6 eV and 1.1 eV, corresponding approximately to the wavelengths of 2060 nm and 1145 nm, respectively.39 In the absorption spectra for amsonic acid-assisted SWCNT dispersion (Fig. 2a), the first optical transition at approximately 2060 nm was breached, presumably due to the chemical doping of SWCNTs by the adsorbates.40 Observed peaks in the near-infrared region were consistent with the second optical transition of semiconducting SWCNTs (approximately 1145 nm) and the first transition of metallic SWCNTs (approximately 760 nm) of 1.6 nm in diameter. Therefore, adsorbates such as amsonic acid can serve as surfactants, leading to the colloidal stability of SWCNTs in water. High-resolution scanning tunnelling electron microscopy (STEM) revealed that the SWCNTs were well-exfoliated after amsonic-acid-assisted dispersion (Fig. 2b), whereas the highly bundled SWCNTs were observed after dispersion without amsonic acid (Fig. 2c). Notably, amsonic acid is insoluble in water, whereas a stable SWCNT dispersion can form in its presence. Additionally, other adsorbates in this study (e.g. FB28 and FB220) were not soluble, but were dispersed as nanoparticles in water (Fig. S1†). Correspondingly, optical microscopy detected little amounts of SWCNTs in the dispersion with FB220, due to detection resolution (Fig. S2†). This result strongly suggests that the roles of stilbene-based adsorbates, such as amsonic acid, in SWCNT dispersion are distinct from those in micelle-assisted dispersion.
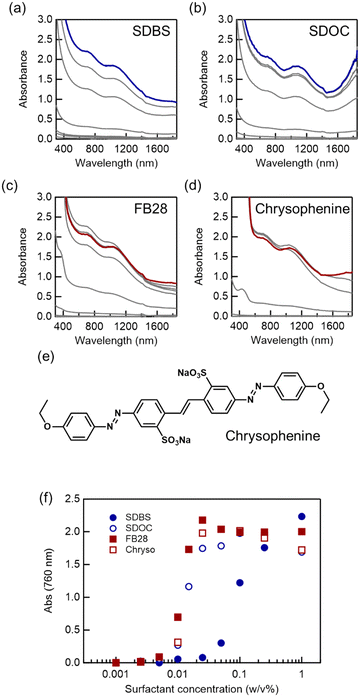
We then examined the dispersibility of the SWCNTs with various surfactants, while keeping the amount of feed constant at 0.25 mg mL−1. To confirm the reliability of our experiments, we compared the new adsorbates based on organic dyes (FB28 and chrysophenine) with conventional micellar surfactants, such as SDBS and SDOC (Fig. 3). Refractive index measurements demonstrated that unlike micellar compounds such as SDBS and SDOC, FB28 exhibited no phase transition between 0.001 and 0.5 mg mL−1 (Fig. S3†). The absorption results indicated that all chemicals aided in the dispersion of the SWCNTs in aqueous solution. Significantly, we confirmed very low thresholds in the concentration (approximately 0.01 mg mL−1) for the dispersion with adsorbate-type surfactants, compared to micelle-type surfactants. The apparent thresholds for FB28 and chrysophenine were almost ten times lower than that of SDBS and slightly lower than that of SDOC, which is the best surfactant for large-diameter SWCNTs. A thin SWCNT dispersion was also obtained with FB28, even below the apparent dispersion threshold of 0.01 mg mL−1, unlike the conventional micellar surfactants. This difference also suggests that the adsorbate-type dispersion is associated with a different dispersion mechanism from that of the micelle-assisted method.
When investigating adsorbate-assisted dispersion, we noticed that dispersant compounds possess common features: first, they are planar, based on large aromatic backbones, or with the aid of classical hydrogen bonding inside the compounds. Second, their structures contain both acidic and basic functional groups, although the reason for this is not clear at present. Most dispersants were planar due to a large aromatic backbone or intramolecular hydrogen bonding. We tentatively assume that the aromatic backbone interacts with the SWCNT outer surface by π–π stacking, while acidic and basic functional groups interact very well with water molecules and hydroxyl groups of alcohol by hydration reaction, H-bonding, etc. Based on these characteristics, we found that a wide range of organic dyes can be used as surfactants for the colloidal dispersion of SWCNTs in water, even in polar organic solvents (Fig. 4). Suitable chemical structures of organic dyes range from azobenzene and stilbene derivatives to phthalocyanines with acid and base functional groups (e.g. sulfonate for acids and amino, azo, and triazine groups for bases). Some formed stable colloidal SWCNT dispersions in ethylene glycol, methanol, ethanol, and isopropyl alcohol. Such alcohol dispersions are important for many composite applications. The mixtures of SWCNTs and organic dyes mostly lost their dispersibility in N-methylpyrrolidone, except for the mixture containing Reactive Blue 12, suggesting that the strong adsorption of organic dyes onto the SWCNTs could completely alter the dispersibility of their derived composites.
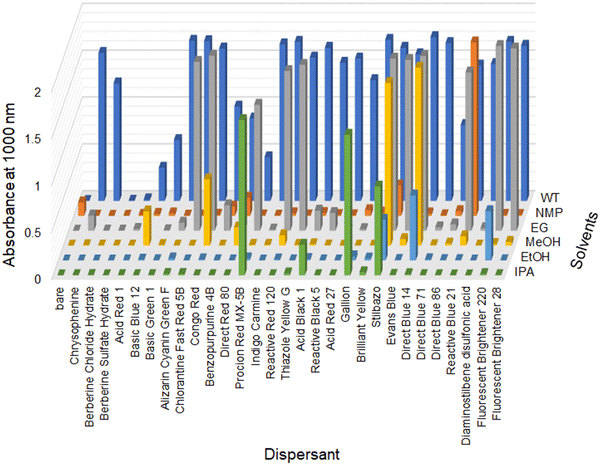 | ||
| Fig. 4 S22 absorbance (1000 nm) of SWCNT dispersions with the aid of various organic dyes in water (WT), N-methyl-pyrrolidone (NMP), and several alcohol solvents (ethylene glycol (EG), methanol (MeOH), ethanol (EtOH), and isopropyl alcohol (IPA)). The input amount of SWCNTs and the surfactant concentration were 0.25 mg mL−1 and 1.0 w/v%, respectively. The optical length was set to 2 mm. There are few SWCNT precipitates after centrifugation when the absorbance is above 1.8, indicating quantitative dispersion under the present condition (0.25 mg mL−1). Chemical structures used here are listed in the ESI† Fig. S4. | ||
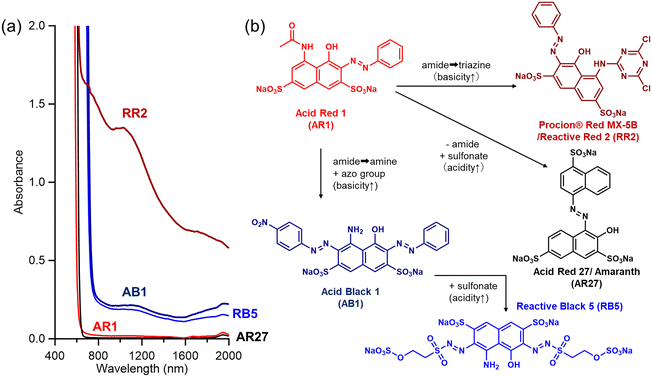
We further examined the effects of the acid and base strengths on the dispersibility of the adsorbate-based surfactants. Under the present dispersion conditions (i.e. 0.25 mg mL−1 SWCNTs, 0.25 w/v% surfactants, 11 W sonication power, 10 min, ice bath) and centrifugation conditions (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190g, 10 min), we found that a very dilute aqueous dispersion can be obtained with the aid of Acid Red 1 (red line and compound in Fig. 5). The dispersibility was found to decrease for the dispersion with the acid compound Acid Red 27 (black line and compound in Fig. 5), in which the sulfonate group was introduced to Acid Red 1. In contrast, using more basic compounds, such as Acid Black 1 and Reactive Black 5 (navy and blue lines and compounds in Fig. 5), led to a better dispersion. Furthermore, the planar compound Reactive Red 2 (called Procion Red MX-5B) with an Acid Red 1 backbone and a very strong base triazine group formed the best dispersion with few precipitates after centrifugation (brown line and compound in Fig. 5). As such, the results of the preliminary control experiments support the importance of the adsorbate acid–base balance when preparing colloidal SWCNT dispersions.
190g, 10 min), we found that a very dilute aqueous dispersion can be obtained with the aid of Acid Red 1 (red line and compound in Fig. 5). The dispersibility was found to decrease for the dispersion with the acid compound Acid Red 27 (black line and compound in Fig. 5), in which the sulfonate group was introduced to Acid Red 1. In contrast, using more basic compounds, such as Acid Black 1 and Reactive Black 5 (navy and blue lines and compounds in Fig. 5), led to a better dispersion. Furthermore, the planar compound Reactive Red 2 (called Procion Red MX-5B) with an Acid Red 1 backbone and a very strong base triazine group formed the best dispersion with few precipitates after centrifugation (brown line and compound in Fig. 5). As such, the results of the preliminary control experiments support the importance of the adsorbate acid–base balance when preparing colloidal SWCNT dispersions.
Conclusions
We prepared colloidally stable dispersions of SWCNTs in water and polar organic solvents such as alcohol with the aid of organic adsorbates. Strong adsorbates based on organic dyes altered the dispersibility of SWCNTs in a manner different from well-studied micelle-assisted dispersions. Efficient surfactants based on organic dye adsorbates are likely to possess both acidic and basic functional groups, as well as mostly planar backbone structures. Both features are associated with hydrogen bonding, although it is unclear whether hydrogen bonding is necessary. The present findings concerning structural requirements are expected to increase the number of known suitable surfactants in water and organic solvents. Future studies may focus on lowering the polarity of dispersion solvents to the levels of ester, ether, and other low-polarity chemicals. We believe that our findings will lay the groundwork for the development of functional inks and composites carrying well-dispersed nanocarbon materials at high weight fractions.Experimental
All chemicals were used as received. The suppliers are listed in the ESI.† The SWCNT inks (OCSiAl Tuball, Batch No. 01RW02.N1.208) were prepared using a probe sonication system (QSonica Q125, 11 W (tuned)). Typically, 5 mg of SWCNTs was charged in an aqueous solution of 0.25 w/v% organic dyes and then sonicated for 10 min in an ice bath. Some organic dyes are insoluble and are subjected to sonication with SWCNTs. After centrifugation (Kubota 5500) for ten minutes at 23 °C and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190g, the upper 75% of the supernatant was collected by gentle pipetting to avoid disturbances.
190g, the upper 75% of the supernatant was collected by gentle pipetting to avoid disturbances.
The absorption spectra were recorded using a UV-vis–NIR spectrophotometer with Si, InGaAs, and PbS photodetectors (Shimadzu UV3600Plus). Cuvettes (2 mm) were used for the liquid-form absorbance measurements. The morphology was observed using STEM in secondary electron detection mode (200 kV, Hitachi HD2700). The presence of micelles was determined using a refractometer (Anton Parr Abbemat Performance 300). The particle diameter distribution was estimated using dynamic light scattering (Otsuka Electronics ELSZ-1000ZS).
Author contributions
YN conceived and supervised the study. AH and TM conducted the experiments. AH, TK, and YN drafted the manuscript and all authors contributed to the final manuscript. YN secured research funding.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr. Yoko Matsuzawa and Dr. Hirokuni Jintoku (National Institute of Advanced Industrial Science and Technology) for technical consultation concerning the dispersion with Fluorescent Brightener 28. This study was supported financially by the MEXT Leading Initiative for Excellent Young Researchers. This study is based on the results obtained from the JPNP20004 project subsidised by the New Energy and Industrial Technology Development Organization.Notes and references
- Y. Kang and T. A. Taton, J. Am. Chem. Soc., 2003, 125, 5650–5651 CrossRef CAS PubMed.
- Z. Wu, Z. Chen, X. Du, J. M. Logan, J. Sippel, M. Nikolou, K. Kamaras, J. R. Reynolds, D. B. Tanner, A. F. Hebard and A. G. Rinzler, Science, 2004, 305, 1273–1276 CrossRef CAS.
- A. Sobolkina, V. Mechtcherine, V. Khavrus, D. Maier, M. Mende, M. Ritschel and A. Leonhardt, Cem. Concr. Compos., 2012, 34, 1104–1113 CrossRef CAS.
- M. S. Arnold, A. A. Green, J. F. Hulvat, S. I. Stupp and M. C. Hersam, Nat. Nanotechnol., 2006, 1, 60–65 CrossRef CAS.
- H. Liu, D. Nishide, T. Tanaka and H. Kataura, Nat. Commun., 2011, 2, 309 CrossRef.
- C. Y. Khripin, J. A. Fagan and M. Zheng, J. Am. Chem. Soc., 2013, 135, 6822–6825 CrossRef CAS.
- J. Zhu, J. Kim, H. Peng, J. L. Margrave, V. N. Khabashesku and E. V. Barrera, Nano Lett., 2003, 3, 1107–1113 CrossRef CAS.
- C. A. Dyke and J. M. Tour, Chemistry, 2004, 10, 812–817 CrossRef CAS.
- S. Ghosh, S. M. Bachilo, R. A. Simonette, K. M. Beckingham and R. B. Weisman, Science, 2010, 330, 1656–1659 CrossRef CAS PubMed.
- M. S. Hofmann, J. T. Glückert, J. Noé, C. Bourjau, R. Dehmel and A. Högele, Nat. Nanotechnol., 2013, 8, 502–505 CrossRef CAS.
- Y. Miyauchi, M. Iwamura, S. Mouri, T. Kawazoe, M. Ohtsu and K. Matsuda, Nat. Photonics, 2013, 7, 715–719 CrossRef CAS.
- Y. Piao, B. Meany, L. R. Powell, N. Valley, H. Kwon, G. C. Schatz and Y. Wang, Nat. Chem., 2013, 5, 840–845 CrossRef CAS PubMed.
- X. Ma, L. Adamska, H. Yamaguchi, S. E. Yalcin, S. Tretiak, S. K. Doorn and H. Htoon, ACS Nano, 2014, 8, 10782–10789 CrossRef CAS PubMed.
- M. J. O'Connell, S. M. Bachilo, C. B. Huffman, V. C. Moore, M. S. Strano, E. H. Haroz, K. L. Rialon, P. J. Boul, W. H. Noon, C. Kittrell, J. Ma, R. H. Hauge, R. B. Weisman and R. E. Smalley, Science, 2002, 297, 593–596 CrossRef.
- C. Richard, F. Balavoine, P. Schultz, T. W. Ebbesen and C. Mioskowski, Science, 2003, 300, 775–778 CrossRef CAS.
- M. F. Islam, E. Rojas, D. M. Bergey, A. T. Johnson and A. G. Yodh, Nano Lett., 2003, 3, 269–273 CrossRef CAS.
- W. Wenseleers, I. I. Vlasov, E. Goovaerts, E. D. Obraztsova, A. S. Lobach and A. Bouwen, Adv. Funct. Mater., 2004, 14, 1105–1112 CrossRef CAS.
- K. Yurekli, C. A. Mitchell and R. Krishnamoorti, J. Am. Chem. Soc., 2004, 126, 9902–9903 CrossRef CAS.
- M. Zheng, A. Jagota, E. D. Semke, B. A. Diner, R. S. McLean, S. R. Lustig, R. E. Richardson and N. G. Tassi, Nat. Mater., 2003, 2, 338–342 CrossRef CAS PubMed.
- X. Tu, S. Manohar, A. Jagota and M. Zheng, Nature, 2009, 460, 250–253 CrossRef CAS PubMed.
- M. Kwak, J. Gao, D. K. Prusty, A. J. Musser, V. A. Markov, N. Tombros, M. C. A. Stuart, W. R. Browne, E. J. Boekema, G. ten Brinke, H. T. Jonkman, B. J. van Wees, M. A. Loi and A. Herrmann, Angew. Chem., Int. Ed., 2011, 50, 3206–3210 CrossRef CAS PubMed.
- T. Shiraki, A. Tsuzuki, F. Toshimitsu and N. Nakashima, Chem. – Eur. J., 2016, 22, 4774–4779 CrossRef CAS.
- X. Liang, H. Li, J. Dou, Q. Wang, W. He, C. Wang, D. Li, J.-M. Lin and Y. Zhang, Adv. Mater., 2020, 32, e2000165 CrossRef.
- H. Murakami, T. Nomura and N. Nakashima, Chem. Phys. Lett., 2003, 378, 481–485 CrossRef CAS.
- K. S. Chichak, A. Star, M. V. P. Altoé and J. F. Stoddart, Small, 2005, 1, 452–461 CrossRef CAS PubMed.
- A. Ikeda, Y. Tanaka, K. Nobusawa and J.-I. Kikuchi, Langmuir, 2007, 23, 10913–10915 CrossRef CAS.
- K. Nobusawa, A. Ikeda, J.-I. Kikuchi, S.-I. Kawano, N. Fujita and S. Shinkai, Angew. Chem., Int. Ed., 2008, 47, 4577–4580 CrossRef CAS PubMed.
- B. Koh, G. Kim, H. K. Yoon, J. B. Park, R. Kopelman and W. Cheng, Langmuir, 2012, 28, 11676–11686 CrossRef CAS.
- J. Chen, H. Liu, W. A. Weimer, M. D. Halls, D. H. Waldeck and G. C. Walker, J. Am. Chem. Soc., 2002, 124, 9034–9035 CrossRef CAS.
- A. Nish, J.-Y. Hwang, J. Doig and R. J. Nicholas, Nat. Nanotechnol., 2007, 2, 640–646 CrossRef CAS.
- F. Chen, B. Wang, Y. Chen and L.-J. Li, Nano Lett., 2007, 7, 3013–3017 CrossRef CAS PubMed.
- F. Cheng, P. Imin, C. Maunders, G. Botton and A. Adronov, Macromolecules, 2008, 41, 2304–2308 CrossRef CAS.
- N. Stürzl, F. Hennrich, S. Lebedkin and M. M. Kappes, J. Phys. Chem. C, 2009, 113, 14628–14632 CrossRef.
- Y. Kim, M. Chikamatsu, R. Azumi, T. Saito and N. Minami, Appl. Phys. Express, 2013, 6, 025101 CrossRef.
- G. Mazzotta, M. Dollmann, S. N. Habisreutinger, M. G. Christoforo, Z. Wang, H. J. Snaith, M. K. Riede and R. J. Nicholas, ACS Appl. Mater. Interfaces, 2019, 11, 1185–1191 CrossRef CAS.
- Y. Nonoguchi, T. Miyao, C. Goto, T. Kawai and K. Funatsu, Adv. Mater. Interfaces, 2022, 9, 2101723 CrossRef CAS.
- Y. Kato, M. Fukuzawa, F. Toshimitsu and N. Nakashima, Chem. Lett., 2015, 44, 566–567 CrossRef CAS.
- H. Jintoku, T. Sato, T. Nakazumi, Y. Matsuzawa, H. Kihara and M. Yoshida, ACS Appl. Mater. Interfaces, 2017, 9, 30805–30811 CrossRef CAS.
- T. Saito, S. Ohmori, B. Shukla, M. Yumura and S. Iijima, Appl. Phys. Express, 2009, 2, 095006 CrossRef.
- Y. Nonoguchi, A. Tani, T. Murayama, H. Uchida and T. Kawai, Chem. – Asian J., 2018, 13, 3942–3946 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Particle size analysis with dynamic light scattering. Refractive index for the confirmation of micelle formation. See DOI: https://doi.org/10.1039/d3lf00119a |
| This journal is © The Royal Society of Chemistry 2024 |