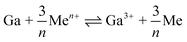Open Access Article
Open Access ArticleGallium-based liquid metals as reaction media for nanomaterials synthesis
Ming
Wang
 and
Yiliang
Lin
and
Yiliang
Lin
 *
*
Department of Chemical and Biomolecular Engineering, National University of Singapore, Engineering Drive 4, 117585, Singapore. E-mail: y.lin@nus.edu.sg
First published on 6th March 2024
Abstract
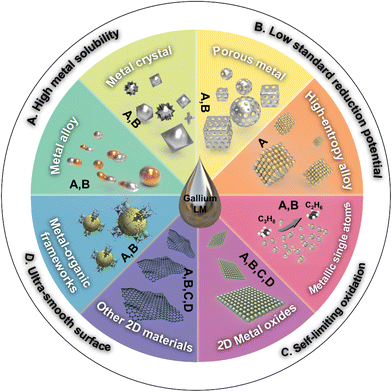
Gallium-based liquid metals (LMs) and their alloys have gained prominence in the realm of flexible and stretchable electronics. Recent advances have expanded the interest to explore the electron-rich core and interface of LMs to synthesize various nanomaterials, where Ga-based LMs serve as versatile reaction media. In this paper, we delve into the latest developments within this burgeoning field. Our discussion begins by elucidating the unique attributes of LMs that render them suitable as reaction media, including their high metal solubility, low standard reduction potential, self-limiting oxidation and ultra-smooth and “layer” surface. We then provide a comprehensive categorized summary of utilizing these features to fabricate a variety of nanomaterials, including pure metallic materials (metal alloys, metal crystals, porous metals, high-entropy alloys and metallic single atoms), metal–inorganic compounds (2D metal oxides, 2D metallic inorganic compounds and 2D graphitic materials), as well as metal–organic composites (metal–organic frameworks). This paper concludes by discussing the current challenges in this field and exploring potential future directions. The versatility and unique properties of Ga-based LMs are poised to play a pivotal role in the future of nanomaterial science, paving the way for more efficient, sustainable, and innovative technological solutions.
1 Introduction
Liquid metals (LMs) with low melting points are increasingly garnering interest in several critical fields, owing to their unique combination of room temperature fluidity, metallic conductivity and superior thermal conductivity. Among them, mercury (Hg), cesium (Cs) and rubidium (Rb) are being progressively eliminated from use due to their high chemical instability, radioactivity or toxicity.1,2 Gallium (Ga) is known for its non-toxicity and has a melting point of 29.7 °C. Ga uniquely exhibits both metallic and covalent bonding, allowing it to remain a liquid over a broad temperature range (up to approximately 2000 °C), while maintaining a low vapor pressure.2,3 Additionally, it exhibits remarkable stability in air and water.3 Apart from Ga, Ga-based LMs can be derived from binary or ternary mixtures of other post-transition metals (indium (In), tin (Sn), aluminum (Al), zinc (Zn), Hg, etc.).4,5 This approach allows for adjusting the melting point range from 13.2 to 27.0 °C, offering versatile applications in various fields.These LMs exhibit properties characteristic of both metallic and covalent bonds, marked by an abundance of free electrons and disordered ions.6,7 Consequently, various metal elements can dissolve, precipitate, diffuse mutually and form intermetallic compounds within the LM matrix, making them ideal reaction media for materials synthesis.8–11 Significantly, Ga features a low standard reduction potential (E0[Ga3+/Ga0] = −0.529 V vs. the standard hydrogen electrode (SHE)), endowing it with a highly active surface for catalytic or reduction interface reactions.12,13 More interestingly, LMs spontaneously form an ultra-thin oxide layer when exposed to air.14,15 This oxide skin, with weak adhesion to the nonpolar surface of the flowing metallic matrix, is easily transferable to any desired substrate.16,17 However, upon the removal of the oxide skin, LMs can provide atomically smooth interfaces, making them suitable as soft and ultra-smooth templates for low temperature deposition and 2D material growth.18,19
In this review, we focus on the latest developments in the synthesis of exciting nanomaterials utilizing Ga-based LMs as reaction media. We begin with a concise overview of the distinctive characteristics pertinent to Ga-based LM reactors, including their high metal solubility, low standard reduction potential, self-limiting oxidation as well as ultra-smooth and “layer” surface. Consequently, we highlight recent application examples and fabrication strategies in the synthesis of diverse materials, such as metal alloys, metal crystals, porous metals, high-entropy alloys, metallic single atoms, 2D metal oxides, 2D metallic inorganic compounds, 2D graphitic materials and metal–organic frameworks, as illustrated in Fig. 1. Lastly, we discuss the existing challenges and explore potential opportunities in the realm of LM reactors, aiming to shed light on their future prospects and applications.
2 Attributes of Ga-based liquid metals
2.1 High metal solubility in LMs
Distinct from conventional liquid solvents, Ga-based LMs stand out as dense and highly interactive liquid solvents.9 Their broad melting range, reductive fluid environment and electron-rich composition, and the abundance of vacancies in their bulk allow them to solvate or disperse a wide range of metallic elements (Fig. 2a). This assortment includes various post-transition metals, transition metals, lanthanides, alkali metals and rare-earth elements, and even some semimetals like silicon,20 all maintained in a neutral state to form eutectic alloys or composites.2,4,21,22 Notably, these alloys or composites can serve as unique stand-alone droplet microreactors, promoting liquid–solid interfacial interactions within the droplet. This facilitates the formation of ordered crystalline phases of intermetallic compounds even below the melting temperature of the individual elements involved.6,23,24 Prominent examples include eutectic alloys of Ga and In (EGaIn) and galinstan (eutectic alloys of Ga, In and Sn) with melting points of 15.4 °C and 13.2 °C, respectively (Table 1).18 The high metal solubility of Ga-based LMs renders them valuable in various scientific applications, particularly in the synthesis of new materials and compounds.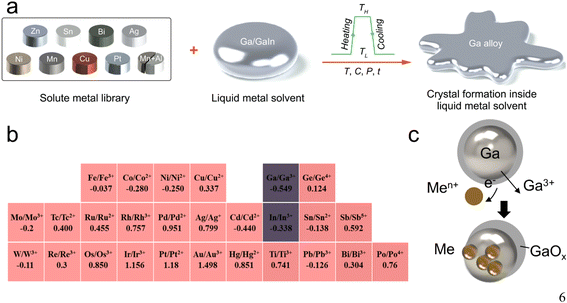 | ||
| Fig. 2 Ga-based LMs have high metal solubility and a low standard reduction potential. (a) The procedures for forming metal alloys or growing metal crystals in liquid Ga solvents. Reprinted with permission.9 Copyright (2022), American Association for the Advancement of Science. (b) Standard reduction potentials for selected elements in the periodic table; elements marked in red could be reduced by Ga. (c) Sketch of the GRR scheme based on liquid Ga. Metal salt ions (Men+) pass through the pores in the Ga oxide shell and are reduced to their metal form by Ga. | ||
| Alloyed elements | Element A at (%) | Element B at (%) | Element C at (%) | Element D at (%) | Melting point (°C) |
|---|---|---|---|---|---|
| Ga | 100 | 0 | 0 | 0 | 29.8 |
| Ga/In (EGaIn) | 85.8 | 14.2 | 0 | 0 | 15.4 |
| Ga/Sn | 91.7 | 8.3 | 0 | 0 | 21.0 |
| Ga/Al | 97.6 | 2.4 | 0 | 0 | 25.9 |
| Ga/Zn | 96.1 | 3.9 | 0 | 0 | 24.7 |
| Ga/Hg | 98.0 | 2.0 | 0 | 0 | 27.0 |
| Ga/In/Sn (galinstan) | 78.3 | 14.9 | 6.8 | 0 | 13.2 |
| Ga/In/Zn | 67.0 | 29.0 | 4.0 | 0 | 13.0 |
| Ga/In/Sn/Zn | 51.0 | 25.0 | 13.0 | 1.0 | 3.0 |
2.2 Low standard reduction potential
In the electrochemical series, Ga is in such a position (E0Ga/Ga3+ = −0.549) that it can theoretically reduce metal ions with a higher standard electrode potential than itself to their metallic form (Fig. 2b).24–27 This characteristic facilitates surface modification of LMs through a galvanic replacement reaction (GRR), as illustrated in Fig. 2c. The general principle of this reaction is summarized as the following equation,where the standard electrode potential value of the element ‘Me’ (metals) should be greater than that of Ga, as highlighted by the elements marked in red in the periodic table (Fig. 2b).28
In addition to the reduced-state metallic materials, non-metallic structures such as reduced state graphene oxide (rGO), can also be obtained on LM surfaces via the electrochemical reactions.29 These surface modifications not only improve stability under harsh conditions, but also modulate fundamental properties such as catalytic activity.
2.3 Oxide skin on the LM surface
Upon exposure to oxygen (>1 part per million), Ga rapidly forms a self-limiting oxide skin,30 and the self-limiting oxidation process can be explained by the Cabrera–Mott metal oxidation model (Fig. 3a).31 The thickness of the final oxide layer, typically just a few nanometers, has been confirmed through various methods, such as X-ray reflectivity analysis,32 transmission electron microscopy (TEM) (Fig. 3b)33 and angle-resolved X-ray photoelectron spectroscopy (XPS).34 This atomically thin interfacial oxide is regarded as a naturally occurring 2D material.35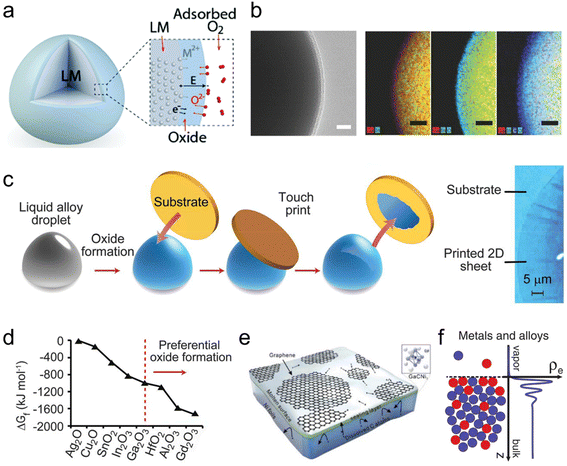 | ||
| Fig. 3 Unique surface properties of Ga-based LMs. (a) Schematic diagram of the Cabrera–Mott metal oxidation model. Reprinted with permission.16 Copyright (2021), Royal Society of Chemistry. (b) TEM image and elemental mapping of the EGaIn nanoparticles with an oxide skin. Scale bars are 5 nm for the TEM image and 10 nm for the elemental mapping. Reprinted with permission.33 Copyright (2015), Wiley-VCH. (c) Schematic of oxide layer exfoliation and translocation on LMs. (d) ΔGf of formation for different metal oxides. The oxides leading to the maximum reduction in ΔGf (to the right of the red dashed line) are predicted to dominate the interface. Reprinted with permission.35 Copyright (2017), American Association for the Advancement of Science. (e) Chemical vapor deposition (CVD) growth of 2D GaCNi3 on the liquid Ga ultra-smooth surface. Reprinted with permission.38 Copyright (2015), American Chemical Society. (f) Schematics of the atomic surface structure and electron density profile ρe(z) of LMs. Surface layered structures are observed for both metals and alloys. Reprinted with permission.39 Copyright (2006), American Association for the Advancement of Science. | ||
The oxide skin significantly alters the physical and chemical properties of Ga-based LMs, affecting their surface chemistry, wetting behavior and rheological properties.36 Furthermore, the oxide skin also enhances the adhesion of the metal substrate, allowing it to firmly adhere (or ‘wet’) more efficiently with many substrates, particularly like glass, quartz, SiO2/Si wafers or polyimide films.37 The non-polar and mobile nature of the parent metal also make the oxide skin on the surface less attractive, allowing for easy transfer of the oxide layer to desired substrates or its layering into a colloidal suspension (Fig. 3c). Moreover, this facile method to acquire 2D metal oxides is not limited to single-phase metal surfaces. Integrating desired metal elements into LMs can create alloys, and on the surface of LM alloys, metal elements compete for presence in the 2D oxide layer. The oxide that leads to the largest reduction in Gibbs free energy (ΔGf) will dominate the surface, allowing controllable modification of the oxide layer by the addition of different selected metals (Fig. 3d).35
2.4 Ultra-smooth and “layer” surface
Interestingly, upon the removal of oxide skin (i.e., with acid), LMs feature an atomically smooth and highly reactive surface, characterized by abundance of many quasi-free electrons and positive ions.2 The ultra-smooth surface template characteristic of LMs makes them exceptionally suitable for the deposition of 2D layered materials. Such a surface offers significant advantages, including prevention of inhomogeneous nucleation, minimization of defects and reduction of grain boundary occurrence (Fig. 3e).52,53 Moreover, near the surface of LMs, there is a relaxation in interatomic interactions, differing from those in the metal core. The variation leads to a change in density from the body of the metal to the liquid–gas interface, resulting in the liquid core of the metal exhibiting a state of almost free electrons, while the surface becomes layered and ordered (Fig. 3f).2,40 This surface “layering”, typically only two to three atomic diameters thick, is observed in many oxide-free LMs and their alloy droplets.39 This surface structure plays a crucial role in the distinctive properties and applications of LMs, particularly in the field of low-dimensional materials synthesis.3 Liquid metal reactors for nanomaterials synthesis
3.1 Metallic nanomaterials
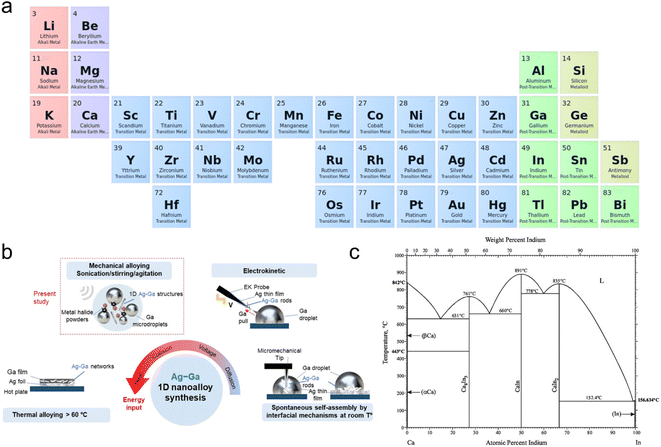 | ||
| Fig. 4 Liquid Ga can serve as a reaction medium. (a) Elements that may get dissolved in Ga.4,53 (b) Schematic representation of the synthesis methods of the LM alloy, taking Ag–Ga alloys as an example. Reprinted with permission.6 Copyright (2022), American Chemical Society. (c) Analyzed Ca–In phase diagram alongside the corresponding experimental data. Reprinted with permission.54 Copyright (2016), ASM International. | ||
Additionally, Ga possesses a low standard reduction potential, allowing it to undergo galvanic replacement with a wide range of metals. This unique attribute makes Ga-based LMs a versatile template for GRR. Leveraging this, bimetallic nanoparticles (NPs), metallic crystals or metal shells in their reduced state can be synthesized through the GRR. The reaction is controlled by the disparity in the standard reduction potentials between the two metal/metal ion couples involved,55 such as Ga with Cu2+, Ag+, Co3+, Ni2+, Cd2+, Sn2+, Pt2+, Au3+ and others (Fig. 5a).27,55–57 Notably, recent studies highlight the efficiency and versatility of sonochemical synthesis in producing Ga-based nano-alloys.49 Sonochemical-assisted synthesis, when applied in metal-ionic solutions, facilitates the growth of crystalline nanostructures on the surface of LM NPs,13 such as 1D Ag–Ga nano-alloys (Fig. 4b and 5b),6 binary and ternary alloy nanoparticles (Fig. 5c),56 and core–shell nanoparticles (LM core and WOx, MoOx, VOx, MnO2 shell) (Fig. 5d).58 These nanostructures or nanoparticles can be optimized by fine-tuning the chemical concentration, the sequence of addition and introducing oxidizing skin inhibitors. When modulating the sonochemical-assisted GRR process of a Ga–chloroauric acid (HAuCl4) system and adding NaH2PO4 inhibitors, unprecedented Au@Ga oxide and hollow Ga oxide nanostructures were obtained.59 Bimetallic NPs, synthesized through the GRR strategy with Ga, such as Ga–Pd, Ga–Pt and Ga–Ni, find diverse applications in catalysis and photocatalysis.56,60,61 Furthermore, this approach can further create metal-shelled or hollow-shelled nanoparticles, which hold promise for various applications, including nanoreactors,62 catalysis,63 drug delivery64 and diagnostic imaging.65
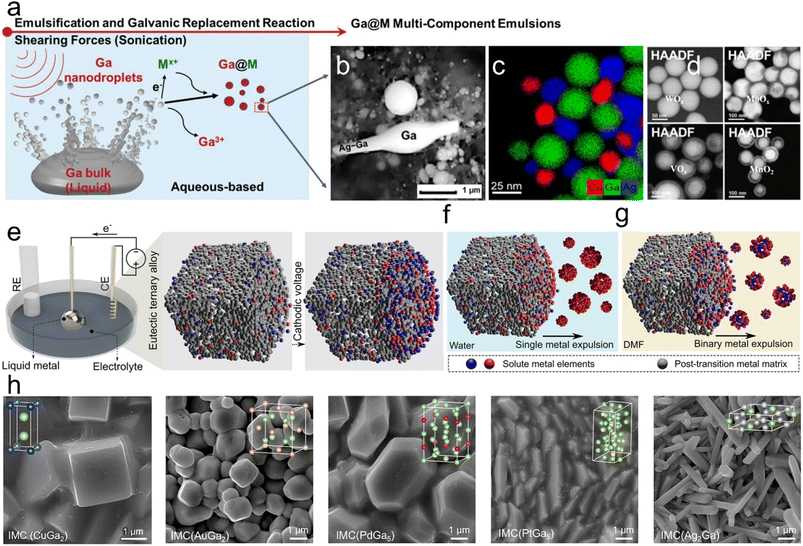 | ||
| Fig. 5 Galvanic replacement reaction (GRR) and metal expulsion based on the liquid Ga system. (a) Schematic of the ultrasound induced GRR between metal ions and Ga. Metal salt ions (Mx+) are reduced by Ga to form Ga@M bimetallic composites. Reprinted with permission.58 Copyright (2021), Wiley-VCH. (b) High-magnification SEM (HM-SEM) images of typical 1D Ag nanoneedles with Ga nanoparticles. Reprinted with permission.6 Copyright (2022), American Chemical Society. (c) Energy dispersive X-ray spectroscopy (EDXS) elemental maps of Ag–Cu–Ga ternary alloy nanoparticles. Reprinted with permission.56 Copyright (2020), American Chemical Society. (d) High-angle annular dark-field (HAADF) image of the Ga core and WOx, MoOx, VOx and MnO2 shells. Reprinted with permission.58 Copyright (2021), Wiley-VCH. (e) Schematic diagram of an electrochemical device for applying an electrical potential to the Ga alloy surface. Expulsion of the (f) monophasic metals in aqueous electrolytes and (g) binary metals in non-aqueous electrolytes. Reprinted with permission.8 Copyright (2022), American Chemical Society. (h) SEM images of CuGa2, AuGa2, PdGa5, PtGa5 and Ag2Ga intermetallic crystals. Reprinted with permission.11 Copyright (2023), American Chemical Society. | ||
Intriguingly, when a solute metal is fully dissolved in a Ga solvent at high temperatures and subsequently becomes supersaturated upon cooling, intermetallic crystals composed of Ga and the solute metal can crystallize within the LMs.9 Physical perturbations at the interfaces of LMs, such as applying electric potential or controlling the melting temperature, can induce phase separation of solute metals into their zero-valent form, a phenomenon known as ‘metal expulsion’. This results in the complete separation of metal entities from the LMs. For example, alloys, such as Ga–In–Sn and In–bismuth (Bi)–Sn, can be utilized as electrochemical cathodes to initiate the separation of the metal phases at the LM–electrolyte interface by applying a cathodic potential, with the type of metal emission depending on the electrolyte (Fig. 5e–g).8 Moreover, the interfacial crystal formation within metallic liquid–solid coexisting systems leads to unique growth dynamics, crystal structures and arrangements that are distinct from traditional solvent precipitation (Fig. 5h). This LM-based intermetallic crystal growth approach holds promise for designing bimetallic or multi-metal structures useful for catalysis,66 sensing67 and energy storage.11,68
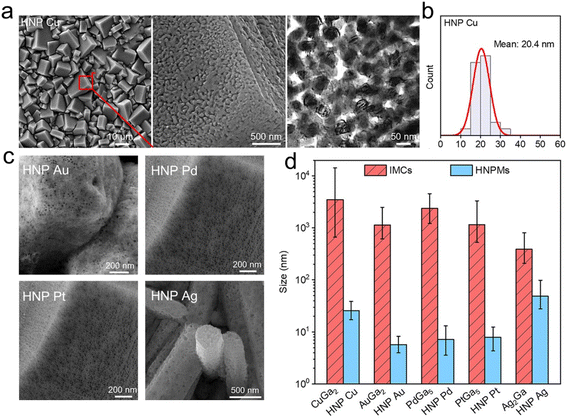 | ||
| Fig. 6 Porous characterization of hierarchical nanoporous metals. (a) SEM and TEM images of hierarchical nanoporous (HNP) Cu show the micro/submicro cuboids decorated with nanoscale ligaments and pores. (b) Pore size distribution of the Cu indicates nanoscale ligaments and pores of about 20.4 nm. (c) SEM images of the nanoporous Au, Pd, Pt and Ag reveal the versatility of this strategy. (d) Characteristic sizes of the hierarchical porosities of intermetallic crystals and HNP metals. Reprinted with permission.11 Copyright (2023), American Chemical Society. | ||
The easy alloying properties of Ga-based LMs, coupled with the negative mixing enthalpy with other metal elements, make them an ideal matrix for preparing complex multicomponent HEAs.80 Specifically, the negative mixing enthalpy can reduce the ΔGf, enabling effective creation of homogeneous alloys through liquid Ga-assisted synthesis methods, overcoming the challenges of elemental segregation and immiscibility in alloy systems. For instance, it is feasible to create an equiatomic HEA of gallium, indium, tin and zinc (GaInSnZn) by employing a technique that involves induction-melting high-purity elements of In, Sn and Zn in equal ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) within a Ga solvent (Fig. 7a). The resulting HEA is a highly conductive fluid, promising for flexible electronic applications (Fig. 7b).81 Additionally, a novel and versatile strategy has recently been developed for synthesizing HEA NPs from various metal elements (Cu, Pd, Ni, Mn, Al, In, Rh, Pt, Co and Mg) with Ga-based LMs as reaction media and reservoirs under mild conditions (923 K) (Fig. 7c and d).82 Ga's relatively negative mixing enthalpy with other solute elements results in HEAs with a highest percentage of solid solution for compositions with the same mixing enthalpy (Fig. 7e).82 This method represents a significant advancement in the field of materials science, particularly in the synthesis of HEAs, enabling the creation of customizable metal nanoparticles under mild conditions. The nanostructures of HEAs, with their high specific surface area, strong synergistic effects, customizable compositional variations and significant lattice distortions, expand their potential applications.82,83 This facile strategy makes them an ideal platform for a variety of surface reactions,84,85 making them applicable in fields such as multiphase catalysis,83 energy storage86 and bio/plasma imaging.86,87
1) within a Ga solvent (Fig. 7a). The resulting HEA is a highly conductive fluid, promising for flexible electronic applications (Fig. 7b).81 Additionally, a novel and versatile strategy has recently been developed for synthesizing HEA NPs from various metal elements (Cu, Pd, Ni, Mn, Al, In, Rh, Pt, Co and Mg) with Ga-based LMs as reaction media and reservoirs under mild conditions (923 K) (Fig. 7c and d).82 Ga's relatively negative mixing enthalpy with other solute elements results in HEAs with a highest percentage of solid solution for compositions with the same mixing enthalpy (Fig. 7e).82 This method represents a significant advancement in the field of materials science, particularly in the synthesis of HEAs, enabling the creation of customizable metal nanoparticles under mild conditions. The nanostructures of HEAs, with their high specific surface area, strong synergistic effects, customizable compositional variations and significant lattice distortions, expand their potential applications.82,83 This facile strategy makes them an ideal platform for a variety of surface reactions,84,85 making them applicable in fields such as multiphase catalysis,83 energy storage86 and bio/plasma imaging.86,87
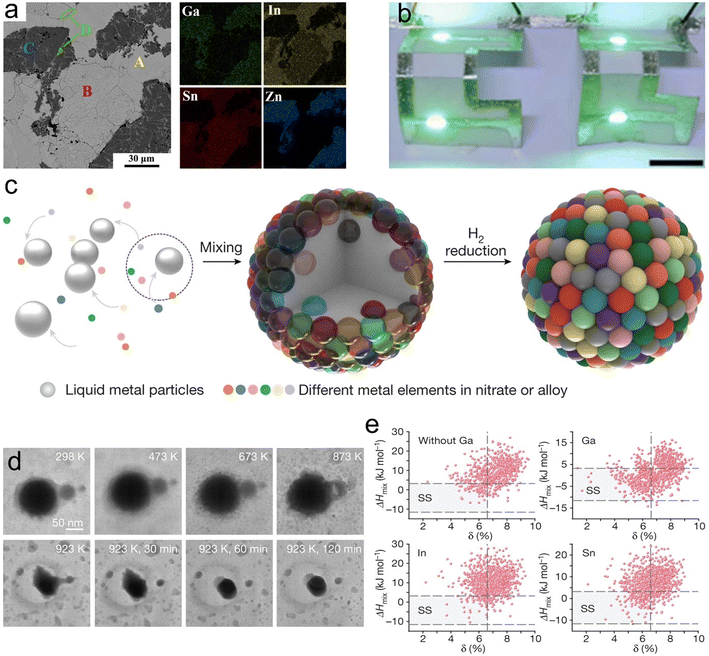 | ||
| Fig. 7 Ga-based high entropy alloys (HEAs). (a) Back scattering electron (BSE) image of GaInSnZn HEAs; elemental mapping shows that the components in the alloy are phase separated. (b) GaInSnZn-based direct-writing 3D electronic circuits; scale bar is 2 cm. Reprinted with permission.81 Copyright (2022), Elsevier B.V. (c) Schematic diagram of the LM reservoir-assisted synthesis process for HEA NPs. Various metal salt precursors are mixed, thermally decomposed, hydrogen reduced and alloyed in liquid metal reservoirs. (d) The TEM image of the process of conversion from precursors into HEA NPs shows that the HEANPs were formed on a large scale after 30 min at 923 K. (e) The distribution of the mixing enthalpy (ΔHmix) and the atom radii difference factor (δ) of the HEAs without Ga and containing Ga, In and Sn. With the introduction of Ga, more data points were available to satisfy the ΔHmix and δ in the solid solution phase region. Reprinted with permission.82 Copyright (2023), Springer Nature. | ||
Ga single atoms, with their inherent catalytic properties, can be prepared through impregnation and reduction under a high temperature atmosphere.91–93 For instance, Ga SAs were successfully synthesized by reducing Ga metal salt precursors under a high temperature atmosphere. The confinement effect of oxygen vacancies (VO) plays a crucial role in this process, favoring the synthesis of well-isolated Ga SAs. This results in maximizing the availability of Ga–VO reaction sites, thereby significantly enhancing catalytic activity and selectivity.92 Another example is the creation of Ga single atom-coordinated hollow ZSM-5 catalysts via wet chemistry combined with high-temperature treatment. These encapsulated Ga SA catalysts exhibit high catalytic conversion efficiency, excellent reusability, and resistance to sintering, attributed to the presence of Ga single atoms.93 Besides the high-temperature and wet-chemical synthesis strategies, laser irradiation is a more facile and effective method to synthesize Ga SAs at room temperature.91 Additionally, the fluid nature of the LMs enables the mobility of Ga single atoms, and a series of Ga single atoms with different coordination environments (P, S and N atom doping) have been synthesized (Fig. 8a and b). The fluxional feature of Ga single atoms enhances the active sites, improving catalytic stability and boosting activity in CO2 reduction for CO formation.94
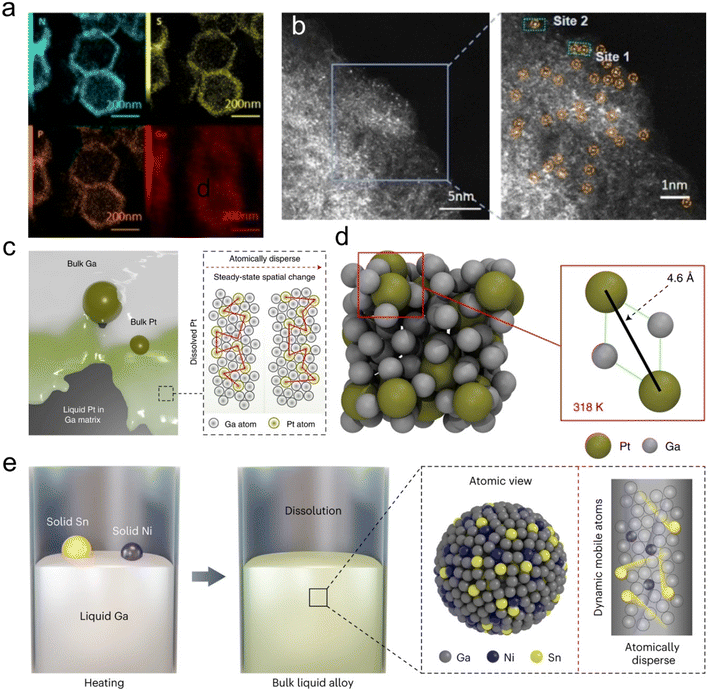 | ||
| Fig. 8 Ga single atoms (SAs) or metal SAs produced with Ga assistance. (a) EDS elemental mapping of the Ga-N3S-PC catalyst confirms the distribution of P, S, N, and Ga elements. (b) The atomic phase image of the Ga-N3S-PC catalyst shows the dispersed Ga single atoms. Reprinted with permission.94 Copyright (2022), Wiley-VCH. (c) Schematic representation of the Ga–Pt system and its atomic distribution. Pt is atomically dispersed and maintains dynamics in Ga solvents. (d) Representative snapshots of the atomic configurations of Pt in Ga in the bulk. Two Pt atoms dispersed in Ga are 4.6 Å apart, indicating that Pt remains in the liquid state. Reprinted with permission.95 Copyright (2022), Springer Nature. (e) Schematic representation of the preparation and atomic mobility of the liquid GaSn0.029Ni0.023 catalyst. Sn and Ni remain atomically dispersed and active in the Ga matrix. Reprinted with permission.96 Copyright (2023), Springer Nature. | ||
Besides Ga SAs, the metal-fluid nature of Ga-based metal alloys results in solute elements to be atomically isolated, resembling solute single atoms of other metallic elements (Fig. 8c). For instance, in a Ga–platinum (Pt) catalyst, liquid Ga serves as a support matrix for Pt, and these Pt atoms are uniformly dispersed within the liquid Ga matrix, effectively preventing atomic aggregation and resulting in the formation of “liquid” Pt single atoms (Fig. 8d). This unique catalytic system accelerates various catalytic reactions and significantly improves kinetics at low temperatures.95 Similarly, in the GaSn0.029Ni0.023 liquid alloy catalyst, the Ga solvent plays a crucial role in achieving well-dispersed tin (Sn) and nickel (Ni) atomic configurations, resulting in a highly selective catalytic synthesis of propylene (Fig. 8e).96 In addition, liquid Ga also assists in generating Pd single atoms through physical vapor deposition of Pd–Ga alloys in an ultrahigh vacuum enviroment.97 An another notable aspect of liquid Ga is its abundant vacancies, which allow it to incorporate various metallic elements as a second phase. Within this system, the active second-phase element is dynamically and atomically isolated within the liquid Ga-rich phase of these binary eutectic alloys, contrasting with solid metals. Such alteration in their electronic structure leads to significantly enhanced catalytic properties, offering possibility in future studies to use lower energies (under mild conditions) for breaking metal bonds to obtain various SAs. Furthermore, the liquid matrix provides exceptional fluidity, deformability and self-healing capabilities, beneficial for product separation, maintaining catalytic system integrity even in the face of external damage and reducing interface polar impedance. For example, dynamic dispersion of Sn atoms in the liquid Ga matrix can maintain long-term integrity of the catalytic conversion of lithium polysulfides (LiPSs), thereby improving their overall catalytic performance.98
3.2 Metal compound nanomaterials (inorganics)
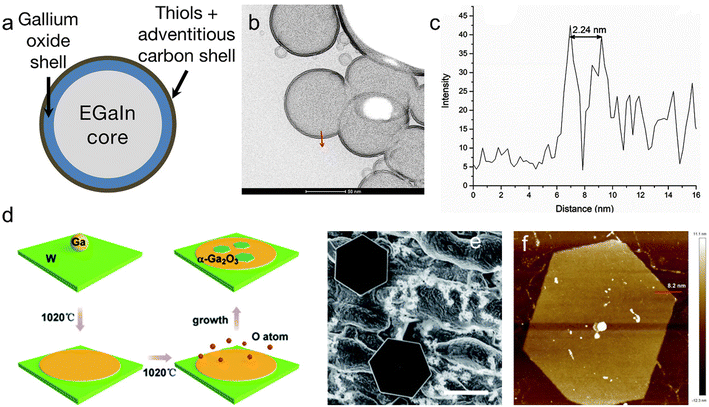 | ||
| Fig. 9 Ga oxide layer, 2D Ga2O3. (a) Schematic showing thiolated molecule functionalized EGaIn nanoparticles. (b) Representative HAADF image of 1-day old thiophenol-functionalized EGaIn nanoparticles after processing with a sobel filter to reveal Ga oxide shell thickness. (c) Intensity peaks extracted from scanning transmission electron microscopy (STEM) images of the nanoparticles. The average Ga oxide shell thickness values were hTP,1day = 1.23 ± 0.20 nm and hTP,28days = 2.12 ± 0.26 nm. Reprinted with permission.101 Copyright (2018), American Chemical Society. (d) Schematic diagram of the growth process of hexagonal α-Ga2O3 crystals. As the temperature increases, Ga spreads out on the substrate to form a flat surface, and O atoms diffuse into the surface of liquid Ga to form hexagonal α-Ga2O3. (e) The SEM image shows that the Ga2O3 crystals are regular hexagonal in shape and extremely homogeneous. (f) Atomic force microscope (AFM) image of the hexagonal Ga2O3 crystals. Reprinted with permission.102 Copyright (2021), Royal Society of Chemistry. | ||
Furthermore, the alloying of Ga-based LMs allows the formation of co-alloyed 2D metal oxides at the LM–air interface.35 In these LM alloys, the surface oxides are determined by the oxide with the lower Gibbs free energy, typically leading to a binary oxide on the surface.104 Moreover, the oxides that lead to the largest reduction in ΔGf are the main products, and LM eutectic alloys, such as EGaIn, mainly consist of Ga oxides in their surface oxide composition (Fig. 10a).105 However, selecting specific alloying elements can control the oxide layers, allowing the spontaneous formation of high-melting-point oxides like HfO2, Gd2O3 and Al2O3, instead of Ga2O3 (Fig. 10b), and XPS shows exfoliated 2D materials consisting entirely of oxides of solute elements (Fig. 10c). This method offers a controllable method to synthesize high-quality 2D metallic oxides at or near room temperature.35 Notably, this principle could be extended to other LMs (i.e., Sn–Bi alloys and Bi–Sb alloys) to obtain Bi2O3-doped SnO nanosheets and ultra-thin α-Sb2O3 layers, respectively.106,107 For further examples and detailed insights into metal oxide synthesis using non-Ga-based LMs, readers are encouraged to consult recent reviews on this topic.17,21
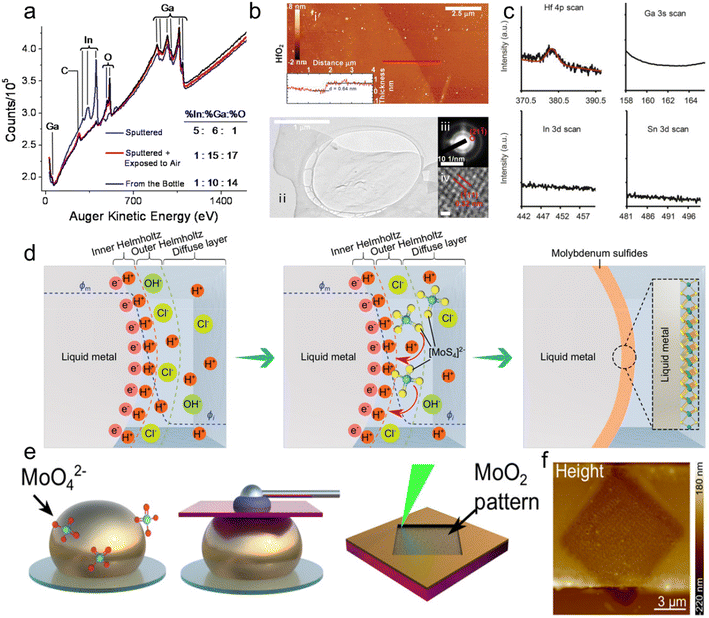 | ||
| Fig. 10 2D metal oxides formed on the LM alloy surface and metal oxides produced by the reaction of electrolyte solutions with LM surfaces. (a) Auger spectra of EGaIn samples from the bottle (black), removed from the outer skin (blue) and exposed to air after removing outer layer. It shows that the oxide layer of EGaIn is mainly composed of Ga2O3. Reprinted with permission.105 Copyright (2008), Wiley-VCH. (b) Characterization of metal oxides from the GaInSn alloy containing 1% Hf. i, AFM images, with thickness profile (inset) determined at the red line as indicated. ii, TEM image. iii, selected-area electron diffraction (SAED). iv, high-resolution TEM images (scale bar, 0.5 nm). The as-grown HfO2 sheets are ultrathin and polycrystalline. (c) XPS spectra of the exfoliated metal oxides from the GaInSn alloy containing 1% Hf shows that the oxides are composed exclusively of HfO2. Reprinted with permission.35 Copyright (2017), American Association for the Advancement of Science. (d) Schematic diagram showing dynamic electric double layer (EDL) formation on LM surfaces, where [MoS4]2− reacts with H+ in the Helmholtz layer to form 2D MoS2. Reprinted with permission.35 Copyright (2020), Wiley-VCH. (e) Schematic diagram of the preparation and patterning process of the 2D MnO2 material. MnO2 was prepared by introducing molybdate precursors around EGaIn droplets. A laser writing technique was used to prepare patterned MnO2. (f) AFM image of the laser-annealed pattern shows 45 nm reduction in thickness. Reprinted with permission.112 Copyright (2021), American Chemical Society. | ||
Interestingly, in the presence of different temperatures, Galinstan undergoes a transition from self-limiting Cabrera–Mott oxidation to a more complex, non-self-limiting reaction, forming heterogeneous oxide regions or shells containing Ga, In and Sn.16,108,109 The formation of LM oxides is influenced by several factors: (1) Temperature. Temperature plays a crucial role, as higher temperatures boost oxidation through increased thermal energy and favorable thermodynamics. (2) Environment. The environmental conditions, particularly the presence and pressure of oxygen, significantly impact the rate of oxidation. (3) Interfacial properties. Interfacial properties including surface contaminants, tension, and wetting determine oxide layer morphology and thickness. (4) LM composition. The composition of the LM, along with dissolution kinetics, and mechanical aspects influence oxide skin formation. By controlling these factors, it is possible to tailor LM oxides for specific applications and uses.109 Furthermore, oxide layers can be acquired by introducing gases into LM melts in various environments (such as aqueous, organic, or inorganic electrolyte solutions) to go beyond self-limiting oxidation. For instance, GaOOH microcrystals form when liquid Ga is sonicated in the presence of water,110 while γ-AlOOH is synthesized through the interaction of the Galinstan–Al alloy surface with water.111
Liquid Ga serves as an excellent template for 2D material growth due to its atomic-level ultra-smooth surface and strong reducibility.2 In acidic solutions, the Ga surface readily attracts H+ to exhibit a dynamic electric double layer (EDL) (Fig. 10d).18,112 The self-generated potential, along with excess electrons on the Ga surface, provides an ideal environment for reducing metal anions at the interface.112 One notable example is the reduction of the molybdate precursors (MoO42−) templated onto the liquid–liquid interface, leading to the formation of hydrated molybdenum oxide (H2MoO3) (Fig. 10e). These are subsequently transformed into 2D MoO2via laser writing techniques (Fig. 10f).112 Similarly, atomic-thickness hydrated MnO2 sheets and the inorganic network of nano-MnO2 are synthesized by an electric coupling substitution reaction between permanganate ions and EGaIn.12,113 Additionally, 2D CuOx nanomaterials are produced by galvanic replacement between Cu(OH)3− and liquid Ga.14 The electron accumulation on the Ga surface creates a reactive surface potential, driving the reduction and self-deposition of precursor metal cations. For example, significantly crystallized rhombohedral layered Bi2Te3 platelets were formed when metal-based cations (specifically Bi3+–HTeO2+ in acidic environments) are introduced to the smooth and oxide-free EGaIn surfaces.114 These 2D metal oxides derived from Ga-based LMs have been explored for a variety of potential applications, including electronic devices,115 spintronics,116 photocatalysts,117 energy storage systems,118 sensors119 and more,120,121 highlighting their versatility and potential in various technological fields.
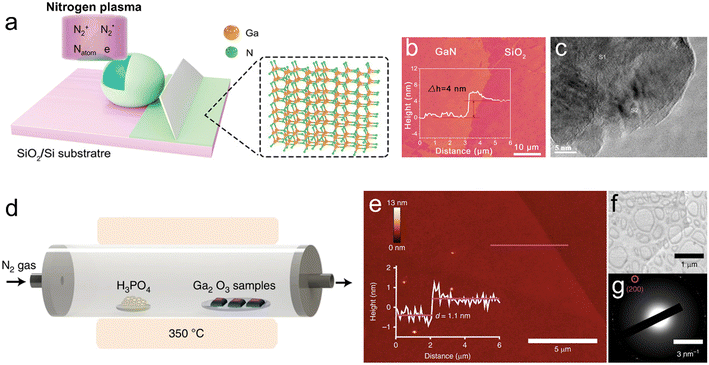 | ||
| Fig. 11 2D GaN semiconductors were directly derived from LM Ga and GaPO4 nanosheets were transformed from Ga2O3. (a) Schematic illustration of the formation of a GaN layer on Ga via a nitrogen-plasma reaction mechanism. The GaN layer adheres to the substrate by vdW forces. (b) AFM image shows the exfoliated GaN layer at 4 nm height. The inset shows the step height profile of the GaN layer. (c) High-resolution TEM images confirm the crystallization of the GaN nanosheets. Reprinted with permission.127 Copyright (2022), Wiley-VCH. (d) Schematic diagram of the vapor phase reaction system for the synthesis of GaPO4 nanosheets. (e) AFM topography of a GaPO4 nanosheet and the height profile along the magenta line. (f) TEM micrograph of the GaPO4 film shows a translucent sheet-like morphology. (g) The SAED pattern of the TEM micrograph of the GaPO4 nanosheet confirms its crystalline structure. Reprinted with permission.103 Copyright (2018), Springer Nature. | ||
Additionally, other chemical modifications or CVD vapor conversion of Ga2O3 can produce other 2D materials. For instance, centimeter-scale Ga2O3 is produced through substrate printing methods, followed by a chemical vapor-phase reaction with phosphoric acid to form 2D GaPO4 nanosheets (Fig. 11d–g).103 Similarly, chlorination and low-temperature sulfurization steps can transform Ga2O3 and In2O3 into 2D GaS and In2S3, respectively.115 Extrusion printing and ammonolysis of Ga2O3 and In2O3 as precursors, with InN requiring an extra bromination step, creates 2D GaN and InN nanosheets.128 The resultant 2D GaN films are utilized to construct patterned GaN-based photodetector arrays through vdW transfer, UV lithography patterning and Ti/Au thermal evaporation.129 Electrochemical substitution represents another notable technique for synthesizing 2D materials using LM reactors, which leverages the dynamic EDL of LMs. For instance, an aqueous solution of the (NH4)2MoS4 precursor reduced on the EGaIn surface produces expansive planar MoS2 with an oxide-free EGaIn interface acting as both a reduction agent and a template.18
Moreover, LMs in their molten state exhibit an amorphous and isotropic atomic structure, which helps in mitigating the negative effects typically associated with grain boundaries. The increased mobility of atoms and electrons in the liquid phase promotes the spontaneous assembly of 2D materials on the LM surface. When the LM is subsequently solidified, this process triggers the self-limited growth of 2D materials, resulting in the attainment of a strictly uniform monolayer. The unique shear transformation of LMs facilitates the ultraclean sliding transfer process of the deposited planar materials on the LM interface.19 For instance, 2D layered chalcogenide crystals, such as GaSe and GaxIn1−xSe, have been achieved using Ga or EGaIn through low-temperature vapor-phase growth.130 Alternatively, centimeter-size GaSe crystals were prepared through atmospheric pressure CVD in liquid Ga-assisted synthesis at a higher temperature (Fig. 12a) After removal from the synthesis boat, large-scale GaSe nanosheets with a smooth surface, a single-crystalline structure and high quality were obtained (Fig. 12b–d).131
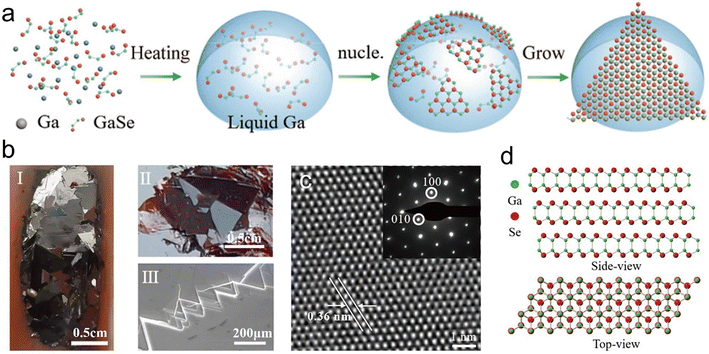 | ||
| Fig. 12 The growth of GaSe crystals on the liquid Ga surface. (a) Schematic representation of the GaSe 2D crystal formation process. GaSe powder melts in liquid Ga, and then GaSe crystals precipitate and grow on the liquid Ga surface. (b) Optical images of (I) GaSe in liquid Ga and (II) stripped down, and (III) SEM image of crystals, showing the triangle morphology and smooth surface. (c) High resolution TEM (HRTEM) image of the GaSe crystal. The inset selected area electron diffraction (SAED) pattern shows a single-crystalline structure. (d) Schemes of the atomic structure of GaSe viewed from top and side. Reprinted with permission.131 Copyright (2021), Tsinghua University Press and Springer-Verlag GmbH, Germany, part of Springer Nature. | ||
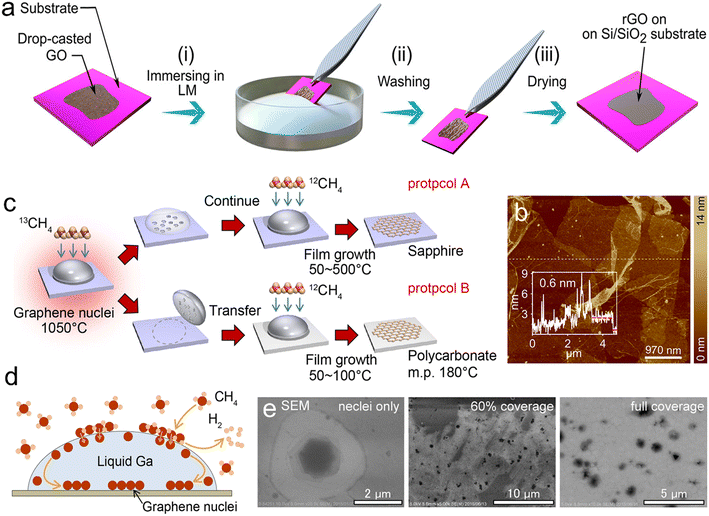 | ||
| Fig. 13 Liquid Ga for the synthesis of graphene 2D materials. (a) Schematic representation of a GO layer immobilized on a Si/SiO2 substrate reduced by liquid Ga. (b) AFM images and the thickness profile of the rGO sample showing a monolayer rGO sheet with a thickness of 0.6 nm. Reprinted with permission.19 Copyright (2021), American Chemical Society. (c) Schematic diagrams of two methods (A, continuous and B, stepwise) for graphene edge growth using liquid Ga. The process involves the formation of graphene nuclei using 13C CH4 at 1050 °C for 300 s and graphene edge growth with 12CH4 at a lower temperature. (d) Mechanism of graphene nuclei generation and film growth. (e) SEM images of graphene grown at 100 °C with different coverage; 0% (13C nuclei only), 60% (partly covered) and 100% (full coverage). After the high-temperature growth of GO nuclei with 13C-CH4 and further low-temperature growth with 12C-CH4, the product still contains only 13C. Reprinted with permission.132 Copyright (2017), Springer Nature. | ||
Liquid metals not only serve as a common interface for the retention and generation of planar materials, but they also exhibit a natural catalytic effect, particularly effective in breaking C–O, C–N and C–H bonds in organic molecules and reconstructing them as graphitic C–C bonds at room temperature. Ga-based LMs, doped with metals such as In and Sn, are especially conducive to dissociating organic precursors to form free radicals for adsorption. Upon leveraging this capability, a wide range of carbon-containing sheets can be fabricated, ranging from graphene-like planar surfaces to highly porous 2D graphitic material sheets, in both monolayers and multilayers.133 A practical application of this is the continuous production of high-quality GO sheets using liquid Ga as a catalyst and diluted 13C/12C methane as the CVD source gas (Fig. 13c–e). These Ga catalysts facilitate catalytic conversion on various substrates, such as sapphire or plastic polycarbonate, at a temperature near room temperature.132
3.3 Metal–organic frameworks
Metal–organic frameworks (MOFs) represent a category of porous polymeric materials, wherein metal ions are interconnected by organic bridging ligands.134 MOFs have attracted wide interest in the fields of energy storage,135 small molecule detection,136 optical sensors,137 catalysis,138 and electromagnetic wave absorption due to their excellent properties such as high internal surface area, tunable pore size, ordered crystal structure, and excellent chemical stability.136,139 Ga-based LMs have demonstrated impressive capabilities as fluid electrodes in the electrochemical synthesis of MOFs. These Ga-based LMs act as activators, accelerating electrosynthesis and enabling reactions at lower onset potentials. Moreover, when submerged in an electrolyte solution, solute metals in the Ga-based LMs dynamically migrate and accumulate at the interface, enhancing electrode interfacial exposure and boosting the reaction efficiency (Fig. 14a).140 Additionally, the electrification of LMs induces interfacial Marangoni flow, facilitating the continuous progression of chemical reactions through releasing adhered reaction products from the electrode surface to enhance the overall efficiency and effectiveness of the electrochemical synthesis (Fig. 14b).141 Notable examples of this application include the production of layered Zn-MOFs using a liquid Ga–Zn alloy as the working electrode26 as well as synthesizing zeolitic imidazolate frameworks (ZIFs)-8 through anodic oxidation of a Ga–Zn liquid alloy (Fig. 14c and d).141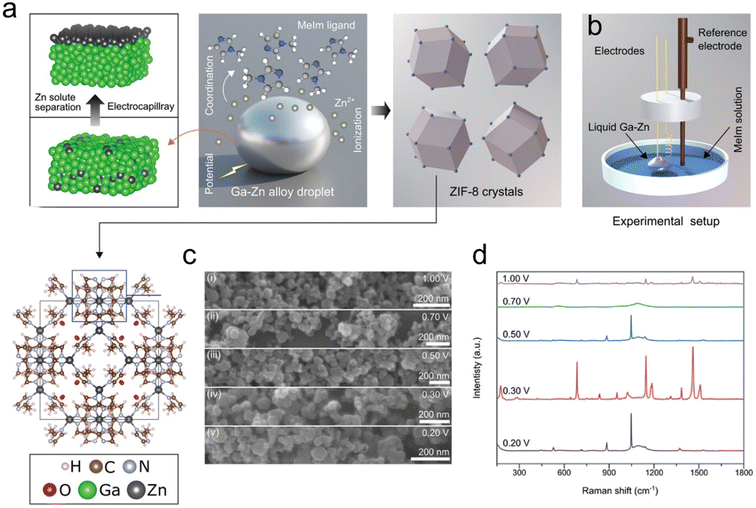 | ||
| Fig. 14 GaZn alloys for the synthesis of ZIF-8. (a) Schematic diagram of the electrosynthesis of ZIF-8. The solute metal Zn in GaZn alloys migrates to the surface and is ionized into Zn2+, which coordinates with deprotonated organic linkers to form the polyhedral ZIF-8. (b) The setup of the three-electrode cell, in which a Ga–Zn alloy was used as an anode for the electrosynthesis of ZIF-8. (c) SEM images of products synthesized at different voltages showing a rhombic polyhedral geometry. (i) 0.20 V; (ii) 0.30 V; (iii) 0.50 V; (iv) 0.70 V; (v) 1.00 V versus SCE, respectively. (d) Raman spectra of products synthesized at different voltages. The curve corresponding to 0.3 V shows all the characteristic peaks of ZIF-8, which indicates the successful synthesis of ZIF-8. Reprinted with permission.141 Copyright (2023), Wiley-VCH. | ||
4 Challenges and opportunities
Although there have been notable achievements in utilizing Ga-based LMs as solvents or reaction media for preparing various functional materials, this research area remains largely exploratory with vast untapped potential. This review identifies key challenges and promising directions for future research on LM reactors, aiming to stimulate further investigation in this area. The primary considerations can be outlined as follows:(1) Ga-based liquid metal solvents offer the ability to dissolve various metal elements, creating alloys that blend the advantages of each component. Future research interest has surged in exploring the doping of Ga-based metal alloys with different components and ratios to produce a range of functional alloys, such as corrosion resistance, magnetism, high strength, flexibility, shape memory, elastocaloric properties, etc.
(2) The sonochemical-assisted GRR strategy, relying on Ga, can be used to fabricate metal nanocrystals, multi-alloyed NPs and core–shell structure NPs. However, the nanostructures generated through electrocoupling substitution and sonication processes exhibit stochastic characteristics, leading to uncertainties in dimensions, elemental distributions, and morphologies.59 This lack of control limits the application of uniform and specific nanostructured NPs. Therefore, further research should focus on precisely regulating the NP structure.
(3) Nanoporous metals’ properties can be significantly affected by the challenge of precisely controlling the residual content of sub-inert metals.142 Furthermore, the microstructure of stabilized nanoporous metals is essential for their applications in catalysts, sensing, and other areas. These two aspects are also significant considerations in Ga solvent-based dealloying strategies.
(4) HEA NPs can be synthesized by a variety of methods, such as carbothermal shock synthesis, mechanical alloying, microwave heating, wet chemistry, liquid laser ablation, chemical dealloying, etc.143 However, these methods always require the application of extreme heating temperatures to provide high mixing entropy.82 The easy alloying property of liquid Ga makes it possible to synthesize HEA NPs at low temperatures. Future investigations could focus on the utilization of liquid Ga in diverse synthesis techniques for HEA NPs, aiming to uncover more efficient, gentle and cost-effective methods for HEA NP synthesis.
(5) The fluid-like, ultra-smooth surfaces of Ga-based LMs are extensively used in synthesizing 2D materials. Yet, an underexplored aspect is their short-range atomic “layer” surface in the near-surface region. Current research lacks evidence of the specific impact of this near-surface atomic layering on materials synthesis in Ga-based LM systems. Future studies could focus more on how liquid Ga's layered surfaces influence the interfacial growth of crystals and 2D materials.
(6) Single atom (SA) catalysts are prized for their maximum atom utilization and high catalytic selectivity. Common SA preparation processes, such as atomic deposition and wet chemistry, often demand harsh conditions (such as high temperatures, high pressures, strong acids or tedious procedures), while the solute elements in Ga-based metal alloy catalysts are mobile and atomically separated. Those solute elements resemble single atoms, and the solvent Ga effectively prevents bonding between solute elements, suggesting the possibility of producing metal SAs under milder conditions, which could facilitate large-scale production of SA catalysts. There is considerable potential in using Ga-based solvents for producing SA catalysts for a wide range of applications. Also, Ga SAs have been less explored so far, and Ga's fluxional nature at the single atomic level and the corresponding catalytic mechanism due to inherent mobility require deeper investigation.
(7) In synthesizing 2D metal oxides, the strategies utilizing LMs’ self-limiting oxidation combined with subsequent transfer stripping techniques offer compelling advantages. However, two key areas need further optimization. Firstly, achieving complete, single-attempt delamination of all 2D metal oxides presents a challenge. Imperfect contact between the substrate and the LM surface, along with inconsistent application of contact force, can lead to the rupture of the oxide layer or restrict the size of the 2D metal oxide. Secondly, issues with substrate bonding or uneven force application can result in residual LM being left on the substrate. This is a significant hurdle in the fabrication of high-purity and high-precision electronic devices.
(8) Currently, the exploration of MOF production using LM reactors is in its early stages, primarily focusing on Zn-based ZIFs. Considering LMs’ capability to alloy with various metal elements and the potential universal applicability of the electrochemical synthesis method discussed in this review, future research could pivot towards electrochemically synthesizing a diverse range of metal-based MOFs using Ga–X alloys as anodes (where ‘X’ represents other metal elements). Such an efficient production method might have the potential to significantly expand the use of MOF materials, especially if the range of metal elements in LM-based MOF systems can be broadened.
5 Conclusion
Ga-based room temperature LMs possess a wonderful combination of metallic and liquid solvent features, including high metal solubility, low standard reduction potential, self-limiting oxidation, and ultra-smooth surfaces. This unique combination positions Ga-based LMs as exceptional reaction media for the exploration and fabrication of a diverse range of nanomaterials. In this review, we summarize the recent developments for the fabrication of pure metallic materials (metal alloys, metal crystals, porous metals, high-entropy alloys and metallic single atoms), metal–inorganic compounds (2D metal oxides, 2D metallic inorganic compounds and 2D graphitic materials), and metal–organic composites (metal–organic frameworks) from liquid Ga reactors.Looking forward, the potential applications and innovations in nanomaterial synthesis using Ga-based LMs are vast and largely untapped. As researchers continue to unravel the complexities and capabilities of these fascinating materials, we anticipate a surge in groundbreaking discoveries and applications across various fields, including electronics, catalysis, energy storage, and environmental technology. The versatility and unique properties of Ga-based LMs are poised to play a pivotal role in the future of materials science, paving the way for more efficient, sustainable, and innovative technological solutions.
Author contributions
Ming Wang: writing – original draft and review and editing. Yiliang Lin: supervision, writing – original draft, and review and editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors are grateful for the financial support from the NUS Start-up Grant for this work.References
- Q. Wang, Y. Yu and J. Liu, Adv. Eng. Mater., 2018, 20, 1700781 CrossRef.
- F.-M. Allioux, M. B. Ghasemian, W. Xie, A. P. O'Mullane, T. Daeneke, M. D. Dickey and K. Kalantar-Zadeh, Nanoscale Horiz., 2022, 7, 141–167 RSC.
- M. J. Regan, E. H. Kawamoto, S. Lee, P. S. Pershan, N. Maskil, M. Deutsch, O. M. Magnussen, B. M. Ocko and L. E. Berman, Phys. Rev. Lett., 1995, 75, 2498–2501 CrossRef CAS PubMed.
- T. Daeneke, K. Khoshmanesh, N. Mahmood, I. A. de Castro, D. Esrafilzadeh, S. J. Barrow, M. D. Dickey and K. Kalantar-zadeh, Chem. Soc. Rev., 2018, 47, 4073–4111 RSC.
- S.-Y. Tang, C. Tabor, K. Kalantar-Zadeh and M. D. Dickey, Annu. Rev. Mater. Res., 2021, 51, 381–408 CrossRef CAS.
- C. A. Echeverria, J. Tang, Z. Cao, D. Esrafilzadeh and K. Kalantar-Zadeh, ACS Appl. Nano Mater., 2022, 5, 6820–6831 CrossRef CAS.
- S. Chen, H.-Z. Wang, R.-Q. Zhao, W. Rao and J. Liu, Matter, 2020, 2, 1446–1480 CrossRef.
- M. Baharfar, J. Zheng, R. Abbasi, S. Lim, V. Kundi, P. V. Kumar, Md. A. Rahim, C. Zhang, K. Kalantar-Zadeh and M. Mayyas, Chem. Mater., 2022, 34, 10761–10771 CrossRef CAS.
- S. A. Idrus-Saidi, J. Tang, S. Lambie, J. Han, M. Mayyas, M. B. Ghasemian, F.-M. Allioux, S. Cai, P. Koshy and P. Mostaghimi, Science, 2022, 378, 1118–1124 CrossRef CAS PubMed.
- H. Liu, K. Wang, K. E. Aasmundtveit and N. Hoivik, J. Electron. Mater., 2012, 41, 2453–2462 CrossRef CAS.
- Y. Chi, P. V. Kumar, J. Zheng, C. Kong, R. Yu, L. Johnston, M. B. Ghasemian, Md. A. Rahim, T. Kumeria, D. Chu, X. Lu, G. Mao, K. Kalantar-Zadeh and J. Tang, ACS Nano, 2023, 17, 17070–17081 CrossRef CAS PubMed.
- M. B. Ghasemian, M. Mayyas, S. A. Idrus-Saidi, M. A. Jamal, J. Yang, S. S. Mofarah, E. Adabifiroozjaei, J. Tang, N. Syed, A. P. O'Mullane, T. Daeneke and K. Kalantar-Zadeh, Adv. Funct. Mater., 2019, 29, 1901649 CrossRef.
- M. K. Akbari, Z. Hai, Z. Wei, R. K. Ramachandran, C. Detavernier, M. Patel, J. Kim, F. Verpoort, H. Lu and S. Zhuiykov, J. Mater. Chem. C, 2019, 7, 5584–5595 RSC.
- H. Li, R. Abbasi, Y. Wang, F. M. Allioux, P. Koshy, S. A. Idrus-Saidi, M. A. Rahim, J. Yang, M. Mousavi, J. Tang, M. B. Ghasemian, R. Jalili, K. Kalantar-Zadeh and M. Mayyas, J. Mater. Chem. C, 2020, 8, 1656–1665 RSC.
- L. Duan, T. Zhou, Y. Zhang, J. Zhao, H. Zheng, B. Zi, J. Zhang, Q. Li, J. Liu and Q. Liu, Adv. Mater., 2023, 35, 2210515 CrossRef CAS PubMed.
- A. Goff, P. Aukarasereenont, C. K. Nguyen, R. Grant, N. Syed, A. Zavabeti, A. Elbourne and T. Daeneke, Dalton Trans., 2021, 50, 7513–7526 RSC.
- P. Aukarasereenont, A. Goff, C. K. Nguyen, C. F. McConville, A. Elbourne, A. Zavabeti and T. Daeneke, Chem. Soc. Rev., 2022, 51, 1253–1276 RSC.
- Y. Wang, M. Mayyas, J. Yang, J. Tang, M. B. Ghasemian, J. Han, A. Elbourne, T. Daeneke, R. B. Kaner and K. Kalantar-Zadeh, Adv. Funct. Mater., 2021, 31, 2005866 CrossRef CAS.
- M. Baharfar, M. Mayyas, M. Rahbar, F.-M. Allioux, J. Tang, Y. Wang, Z. Cao, F. Centurion, R. Jalili, G. Liu and K. Kalantar-Zadeh, ACS Nano, 2021, 15, 19661–19671 CrossRef CAS PubMed.
- M. K. Sunkara, S. Sharma, R. Miranda, G. Lian and E. C. Dickey, Appl. Phys. Lett., 2001, 79, 1546–1548 CrossRef CAS.
- S. Zhao, J. Zhang and L. Fu, Adv. Mater., 2021, 33, 2005544 CrossRef CAS PubMed.
- Y. Ding, M. Zeng and L. Fu, Matter, 2019, 1, 1099–1103 CrossRef.
- K. Y. Kwon, S. Cheeseman, A. Frias-De-Diego, H. Hong, J. Yang, W. Jung, H. Yin, B. J. Murdoch, F. Scholle, N. Crook, E. Crisci, M. D. Dickey, V. K. Truong and T. Kim, Adv. Mater., 2021, 33, 2104298 CrossRef CAS PubMed.
- J.-H. Fu, T.-Y. Liu, Y. Cui and J. Liu, Adv. Mater. Interfaces, 2021, 8, 2001936 CrossRef CAS.
- E. A. Sharova, A. S. Falchevskaya, S. S. Leonchuk, A. V. Redkov, V. Nikolaev and V. V. Vinogradov, Chem. Commun., 2023, 59, 10928–10931 RSC.
- J. Zheng, M. Mousavi, M. Baharfar, A. Sharma, T. Kumeria, J. Han, P. Kumar, K. Kalantar-Zadeh and M. Mayyas, J. Mater. Chem. C, 2022, 10, 14963–14970 RSC.
- A. S. Falchevskaya, A. Y. Prilepskii, S. A. Tsvetikova, E. I. Koshel and V. V. Vinogradov, Chem. Mater., 2021, 33, 1571–1580 CrossRef CAS.
- A. Jannat, Q. Yao, A. Zavabeti, N. Syed, B. Y. Zhang, T. Ahmed, S. Kuriakose, M. Mohiuddin, N. Pillai, F. Haque, G. Ren, D. M. Zhu, N. Cheng, Y. Du, S. A. Tawfik, M. J. S. Spencer, B. J. Murdoch, L. Wang, C. F. McConville, S. Walia, T. Daeneke, L. Zhu and J. Z. Ou, Mater. Horiz., 2020, 7, 827–834 RSC.
- Y. Wang, S. Wang, H. Chang and W. Rao, Adv. Mater. Interfaces, 2020, 7, 2000626 CrossRef CAS.
- M. J. Regan, H. Tostmann, P. S. Pershan, O. M. Magnussen, E. DiMasi, B. M. Ocko and M. Deutsch, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 55, 10786–10790 CrossRef CAS.
- N. Cabrera and N. F. Mott, Rep. Prog. Phys., 1949, 12, 163 CrossRef CAS.
- M. J. Regan, P. S. Pershan, O. M. Magnussen, B. M. Ocko, M. Deutsch and L. E. Berman, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 55, 15874–15884 CrossRef CAS.
- Y. Lin, C. Cooper, M. Wang, J. J. Adams, J. Genzer and M. D. Dickey, Small, 2015, 11, 6397–6403 CrossRef CAS PubMed.
- R. N. S. Sodhi, P. Brodersen, L. Cademartiri, M. M. Thuo and C. A. Nijhuis, Surf. Interface Anal., 2017, 49, 1309–1315 CrossRef CAS.
- A. Zavabeti, J. Z. Ou, B. J. Carey, N. Syed, R. Orrell-Trigg, E. L. Mayes, C. Xu, O. Kavehei, A. P. O'Mullane and R. B. Kaner, Science, 2017, 358, 332–335 CrossRef CAS PubMed.
- W. Babatain, M. S. Kim and M. M. Hussain, Adv. Funct. Mater., 2023, 2308116 CrossRef.
- R. S. Datta, N. Syed, A. Zavabeti, A. Jannat, M. Mohiuddin, M. Rokunuzzaman, B. Y. Zhang, M. A. Rahman, P. Atkin, K. A. Messalea, M. B. Ghasemian, E. D. Gaspera, S. Bhattacharyya, M. S. Fuhrer, S. P. Russo, C. F. McConville, D. Esrafilzadeh, K. Kalantar-Zadeh and T. Daeneke, Nat. Electron., 2020, 3, 51–58 CrossRef CAS.
- L. Chen, Z. Kong, S. Yue, J. Liu, J. Deng, Y. Xiao, R. G. Mendes, M. H. Rümmeli, L. Peng and L. Fu, Chem. Mater., 2015, 27, 8230–8236 CrossRef CAS.
- O. G. Shpyrko, R. Streitel, V. S. K. Balagurusamy, A. Y. Grigoriev, M. Deutsch, B. M. Ocko, M. Meron, B. Lin and P. S. Pershan, Science, 2006, 313, 77–80 CrossRef CAS PubMed.
- K. Kalantar-Zadeh, J. Tang, T. Daeneke, A. P. O'Mullane, L. A. Stewart, J. Liu, C. Majidi, R. S. Ruoff, P. S. Weiss and M. D. Dickey, ACS Nano, 2019, 13, 7388–7395 CrossRef CAS PubMed.
- M. G. Kanatzidis, R. Pöttgen and W. Jeitschko, Angew. Chem., Int. Ed., 2005, 44, 6996–7023 CrossRef CAS PubMed.
- A. Turchanin and W. Freyland, Chem. Phys. Lett., 2004, 387, 106–109 CrossRef CAS.
- I. S. Golovin, Materials, 2023, 16, 2365 CrossRef CAS PubMed.
- A. P. Rao, M. T. Cook, H. L. Hall and M. B. Shattan, Atoms, 2019, 7, 84 CrossRef CAS.
- J. Li, Z. Chen, J. Jing and J. Hou, J. Mater. Sci. Technol., 2020, 41, 33–42 CrossRef CAS.
- T. Itami, R. Xu and W. van der Lugt, J. Alloys Compd., 1993, 201, 37–41 CrossRef CAS.
- Z. Yao, X. Tian, L. Jiang, H. Hao, G. Zhang, S. Wu, Z. Zhao and N. Gerile, J. Alloys Compd., 2015, 637, 431–435 CrossRef CAS.
- M. M. Yazdanpanah, S. A. Harfenist, A. Safir and R. W. Cohn, J. Appl. Phys., 2005, 98, 073510 CrossRef.
- J. Tang, J. Tang, M. Mayyas, M. B. Ghasemian, J. Sun, M. A. Rahim, J. Yang, J. Han, D. J. Lawes, R. Jalili, T. Daeneke, M. G. Saborio, Z. Cao, C. A. Echeverria, F.-M. Allioux, A. Zavabeti, J. Hamilton, V. Mitchell, A. P. O'Mullane, R. B. Kaner, D. Esrafilzadeh, M. D. Dickey and K. Kalantar-Zadeh, Adv. Mater., 2022, 34, 2105789 CrossRef CAS PubMed.
- N. R. Wood, A. I. Wolsiefer, R. W. Cohn and S. J. Williams, Electrophoresis, 2013, 34, 1922–1930 CrossRef CAS PubMed.
- M. A. H. Khondoker and D. Sameoto, Smart Mater. Struct., 2016, 25, 093001 CrossRef.
- D. Wang, J. Ye, Y. Bai, F. Yang, J. Zhang, W. Rao and J. Liu, Adv. Mater., 2023, 2303533 CrossRef CAS PubMed.
- L. Wang and J. Liu, Front. Energy, 2013, 7, 317–332 CrossRef.
- H. Okamoto, J. Phase Equilib. Diffus., 2016, 37, 350–362 CrossRef CAS.
- O. Oloye, C. Tang, A. Du, G. Will and A. P. O'Mullane, Nanoscale, 2019, 11, 9705–9715 RSC.
- L. Castilla-Amorós, D. Stoian, J. R. Pankhurst, S. B. Varandili and R. Buonsanti, J. Am. Chem. Soc., 2020, 142, 19283–19290 CrossRef PubMed.
- F. Hoshyargar, J. Crawford and A. P. O'Mullane, J. Am. Chem. Soc., 2017, 139, 1464–1471 CrossRef CAS PubMed.
- L. Ren, N. Cheng, X. Man, D. Qi, Y. Liu, G. Xu, D. Cui, N. Liu, J. Zhong, G. Peleckis, X. Xu, S. X. Dou and Y. Du, Adv. Mater., 2021, 33, 2008024 CrossRef CAS PubMed.
- H. Lu, S.-Y. Tang, J. Zhu, X. Huang, H. Forgham, X. Li, A. Shen, G. Yun, J. Hu, S. Zhang, T. P. Davis, W. Li and R. Qiao, Adv. Funct. Mater., 2024, 34, 2311300 CrossRef CAS.
- N. Taccardi, M. Grabau, J. Debuschewitz, M. Distaso, M. Brandl, R. Hock, F. Maier, C. Papp, J. Erhard, C. Neiss, W. Peukert, A. Görling, H.-P. Steinrück and P. Wasserscheid, Nat. Chem., 2017, 9, 862–867 CrossRef CAS PubMed.
- D. Esrafilzadeh, A. Zavabeti, R. Jalili, P. Atkin, J. Choi, B. J. Carey, R. Brkljača, A. P. O'Mullane, M. D. Dickey, D. L. Officer, D. R. MacFarlane, T. Daeneke and K. Kalantar-Zadeh, Nat. Commun., 2019, 10, 865 CrossRef PubMed.
- W. Zhu, Z. Chen, Y. Pan, R. Dai, Y. Wu, Z. Zhuang, D. Wang, Q. Peng, C. Chen and Y. Li, Adv. Mater., 2019, 31, 1800426 CrossRef PubMed.
- B. Li and H. C. Zeng, Adv. Mater., 2019, 31, 1801104 CrossRef.
- L. Wang, J. Wan, J. Wang and D. Wang, Small Struct., 2021, 2, 2000041 CrossRef CAS.
- E. Yasun, S. Gandhi, S. Choudhury, R. Mohammadinejad, F. Benyettou, N. Gozubenli and H. Arami, J. Drug Delivery Sci. Technol., 2020, 60, 102094 CrossRef CAS PubMed.
- Z. Weng, W. Liu, L.-C. Yin, R. Fang, M. Li, E. I. Altman, Q. Fan, F. Li, H.-M. Cheng and H. Wang, Nano Lett., 2015, 15, 7704–7710 CrossRef CAS PubMed.
- R. Lin, E. Hu, M. Liu, Y. Wang, H. Cheng, J. Wu, J.-C. Zheng, Q. Wu, S. Bak, X. Tong, R. Zhang, W. Yang, K. A. Persson, X. Yu, X.-Q. Yang and H. L. Xin, Nat. Commun., 2019, 10, 1650 CrossRef PubMed.
- F. Lin, D. Nordlund, Y. Li, M. K. Quan, L. Cheng, T.-C. Weng, Y. Liu, H. L. Xin and M. M. Doeff, Nat. Energy, 2016, 1, 1–8 Search PubMed.
- J.-C. Shao and H.-J. Jin, J. Mater. Sci., 2020, 55, 8337–8345 CrossRef CAS.
- Y. Zhang, Q. Bai, W. Yang and Z. Zhang, Sci. China: Technol. Sci., 2021, 64, 2229–2236 CrossRef CAS.
- X. Guo, C. Zhang, Q. Tian and D. Yu, Mater. Today Commun., 2021, 26, 102007 CrossRef CAS.
- A. Wittstock, V. Zielasek, J. Biener, C. M. Friend and M. Bäumer, Science, 2010, 327, 319–322 CrossRef CAS PubMed.
- J. Biener, A. Wittstock, L. A. Zepeda-Ruiz, M. M. Biener, V. Zielasek, D. Kramer, R. N. Viswanath, J. Weissmüller, M. Bäumer and A. V. Hamza, Nat. Mater., 2009, 8, 47–51 CrossRef CAS PubMed.
- H.-J. Qiu, X. Li, H.-T. Xu, H.-J. Zhang and Y. Wang, J. Mater. Chem. C, 2014, 2, 9788–9799 RSC.
- J. T. Zhang and C. M. Li, Chem. Soc. Rev., 2012, 41, 7016–7031 RSC.
- W. Yuan, Y. Tang, X. Yang and Z. Wan, Appl. Energy, 2012, 94, 309–329 CrossRef CAS.
- Y. Zhang, X. Yang and P. K. Liaw, JOM, 2012, 64, 830–838 CrossRef CAS.
- S. H. Shim, H. Pouraliakbar and S. I. Hong, Mater. Sci. Eng., 2021, 825, 141875 CrossRef CAS.
- W.-Y. Ching, S. San, J. Brechtl, R. Sakidja, M. Zhang and P. K. Liaw, npj Comput. Mater., 2020, 6, 1–10 CrossRef.
- A. Takeuchi and A. Inoue, Mater. Trans., 2005, 46, 2817–2829 CrossRef CAS.
- J. Bai, X. Jin, H. Yang and J. Qiao, J. Alloys Compd., 2022, 919, 165736 CrossRef CAS.
- G. Cao, J. Liang, Z. Guo, K. Yang, G. Wang, H. Wang, X. Wan, Z. Li, Y. Bai, Y. Zhang, J. Liu, Y. Feng, Z. Zheng, C. Lu, G. He, Z. Xiong, Z. Liu, S. Chen, Y. Guo, M. Zeng, J. Lin and L. Fu, Nature, 2023, 619, 73–77 CrossRef CAS PubMed.
- Y. Yao, Z. Huang, P. Xie, S. D. Lacey, R. J. Jacob, H. Xie, F. Chen, A. Nie, T. Pu, M. Rehwoldt, D. Yu, M. R. Zachariah, C. Wang, R. Shahbazian-Yassar, J. Li and L. Hu, Science, 2018, 359, 1489–1494 CrossRef CAS PubMed.
- P. Xie, Y. Yao, Z. Huang, Z. Liu, J. Zhang, T. Li, G. Wang, R. Shahbazian-Yassar, L. Hu and C. Wang, Nat. Commun., 2019, 10, 4011 CrossRef PubMed.
- S. Gao, S. Hao, Z. Huang, Y. Yuan, S. Han, L. Lei, X. Zhang, R. Shahbazian-Yassar and J. Lu, Nat. Commun., 2020, 11, 2016 CrossRef CAS PubMed.
- N. A. Frey, S. Peng, K. Cheng and S. Sun, Chem. Soc. Rev., 2009, 38, 2532–2542 RSC.
- M. B. Cortie and A. M. McDonagh, Chem. Rev., 2011, 111, 3713–3735 CrossRef CAS PubMed.
- Q. Sun, N. Wang, T. Zhang, R. Bai, A. Mayoral, P. Zhang, Q. Zhang, O. Terasaki and J. Yu, Angew. Chem., Int. Ed., 2019, 58, 18570–18576 CrossRef CAS PubMed.
- L. Peng, L. Shang, T. Zhang and G. I. N. Waterhouse, Adv. Energy Mater., 2020, 10, 2003018 CrossRef CAS.
- X.-F. Yang, A. Wang, B. Qiao, J. Li, J. Liu and T. Zhang, Acc. Chem. Res., 2013, 46, 1740–1748 CrossRef CAS PubMed.
- Y. Lin, X. Gao, J. Yue, Y. Fang, J. Shi, L. Meng, C. Clayton, X.-X. Zhang, F. Shi and J. Deng, Nat. Chem., 2023, 15, 119–128 CrossRef CAS PubMed.
- M. Zhang, W. Xu, C.-L. Ma, J. Yu, Y.-T. Liu and B. Ding, ACS Nano, 2022, 16, 4186–4196 CrossRef CAS PubMed.
- L. Wu, J. Xin, Y. Wang, K. Zhang, J. Zhang, J. Sun, R. Zou and J. Liang, J. Energy Chem., 2023, 84, 363–373 CrossRef CAS.
- Z. Zhang, J. Zhu, S. Chen, W. Sun and D. Wang, Angew. Chem., Int. Ed., 2023, 62, e202215136 CrossRef CAS PubMed.
- M. A. Rahim, J. Tang, A. J. Christofferson, P. V. Kumar, N. Meftahi, F. Centurion, Z. Cao, J. Tang, M. Baharfar and M. Mayyas, Nat. Chem., 2022, 14, 935–941 CrossRef CAS PubMed.
- J. Tang, A. J. Christofferson, J. Sun, Q. Zhai, P. V. Kumar, J. A. Yuwono, M. Tajik, N. Meftahi, J. Tang, L. Dai, G. Mao, S. P. Russo, R. B. Kaner, M. A. Rahim and K. Kalantar-Zadeh, Nat. Nanotechnol., 2023, 1–5 Search PubMed.
- M. Kettner, S. Maisel, C. Stumm, M. Schwarz, C. Schuschke, A. Görling and J. Libuda, J. Catal., 2019, 369, 33–46 CrossRef CAS.
- Y. Qi, N. Li, K. Zhang, Y. Yang, Z. Ren, J. You, Q. Hou, C. Shen, T. Jin and Z. Peng, Adv. Mater., 2022, 34, 2204810 CrossRef CAS PubMed.
- T. Liu, P. Sen and C.-J. Kim, J. Microelectromech. Syst., 2012, 21, 443–450 CAS.
- M. D. Dickey, R. C. Chiechi, R. J. Larsen, E. A. Weiss, D. A. Weitz and G. M. Whitesides, Adv. Funct. Mater., 2008, 18, 1097–1104 CrossRef CAS.
- Z. J. Farrell and C. Tabor, Langmuir, 2018, 34, 234–240 CrossRef CAS PubMed.
- M. Li, L. Li, Y. Fan, L. Huang, D. Geng and W. Yang, Nanoscale Adv., 2021, 3, 4411–4415 RSC.
- N. Syed, A. Zavabeti, J. Z. Ou, M. Mohiuddin, N. Pillai, B. J. Carey, B. Y. Zhang, R. S. Datta, A. Jannat, F. Haque, K. A. Messalea, C. Xu, S. P. Russo, C. F. McConville, T. Daeneke and K. Kalantar-Zadeh, Nat. Commun., 2018, 9, 3618 CrossRef PubMed.
- T. Daeneke, P. Atkin, R. Orrell-Trigg, A. Zavabeti, T. Ahmed, S. Walia, M. Liu, Y. Tachibana, M. Javaid, A. D. Greentree, S. P. Russo, R. B. Kaner and K. Kalantar-Zadeh, ACS Nano, 2017, 11, 10974–10983 CrossRef CAS PubMed.
- R. C. Chiechi, E. A. Weiss, M. D. Dickey and G. M. Whitesides, Angew. Chem., Int. Ed., 2008, 47, 142–144 CrossRef CAS PubMed.
- M. B. Ghasemian, A. Zavabeti, M. Mousavi, B. J. Murdoch, A. J. Christofferson, N. Meftahi, J. Tang, J. Han, R. Jalili, F.-M. Allioux, M. Mayyas, Z. Chen, A. Elbourne, C. F. McConville, S. P. Russo, S. Ringer and K. Kalantar-Zadeh, Adv. Mater., 2021, 33, 2104793 CrossRef CAS PubMed.
- K. A. Messalea, N. Syed, A. Zavabeti, M. Mohiuddin, A. Jannat, P. Aukarasereenont, C. K. Nguyen, M. X. Low, S. Walia, B. Haas, C. T. Koch, N. Mahmood, K. Khoshmanesh, K. Kalantar-Zadeh and T. Daeneke, ACS Nano, 2021, 15, 16067–16075 CrossRef CAS PubMed.
- A. Martin, W. Kiarie, B. Chang and M. Thuo, Angew. Chem., Int. Ed., 2020, 59, 352–357 CrossRef CAS PubMed.
- A. Martin, C. Du, B. Chang and M. Thuo, Chem. Mater., 2020, 32, 9045–9055 CrossRef CAS.
- M. A. Creighton, M. C. Yuen, M. A. Susner, Z. Farrell, B. Maruyama and C. E. Tabor, Langmuir, 2020, 36, 12933–12941 CrossRef CAS PubMed.
- A. Zavabeti, B. Y. Zhang, I. A. de Castro, J. Z. Ou, B. J. Carey, M. Mohiuddin, RobiS. Datta, C. Xu, A. P. Mouritz, C. F. McConville, A. P. O'Mullane, T. Daeneke and K. Kalantar-Zadeh, Adv. Funct. Mater., 2018, 28, 1804057 CrossRef.
- Y. Wang, M. Mayyas, J. Yang, M. B. Ghasemian, J. Tang, M. Mousavi, J. Han, M. Ahmed, M. Baharfar, G. Mao, Y. Yao, D. Esrafilzadeh, D. Cortie and K. Kalantar-Zadeh, ACS Appl. Mater. Interfaces, 2021, 13, 53181–53193 CrossRef CAS PubMed.
- S. Cai, M. B. Ghasemian, M. A. Rahim, M. Baharfar, J. Yang, J. Tang, K. Kalantar-Zadeh and F.-M. Allioux, Nanoscale, 2023, 15, 4291–4300 RSC.
- M. Mousavi, M. B. Ghasemian, J. Han, Y. Wang, R. Abbasi, J. Yang, J. Tang, S. A. Idrus-Saidi, X. Guan, M. J. Christoe, S. Merhebi, C. Zhang, J. Tang, R. Jalili, T. Daeneke, T. Wu, K. Kalantar-Zadeh and M. Mayyas, Appl. Mater. Today, 2021, 22, 100954 CrossRef.
- B. J. Carey, J. Z. Ou, R. M. Clark, K. J. Berean, A. Zavabeti, A. S. R. Chesman, S. P. Russo, D. W. M. Lau, Z.-Q. Xu, Q. Bao, O. Kavehei, B. C. Gibson, M. D. Dickey, R. B. Kaner, T. Daeneke and K. Kalantar-Zadeh, Nat. Commun., 2017, 8, 14482 CrossRef CAS PubMed.
- X. Wang, R. Quhe, Y. Zhi, Z. Liu, Y. Huang, X. Dai, Y. Tang, Z. Wu and W. Tang, Superlattices Microstruct., 2019, 125, 330–337 CrossRef CAS.
- M. Akatsuka, Y. Kawaguchi, R. Itoh, A. Ozawa, M. Yamamoto, T. Tanabe and T. Yoshida, Appl. Catal., B, 2020, 262, 118247 CrossRef CAS.
- X. Tang, X. Huang, Y. Huang, Y. Gou, J. Pastore, Y. Yang, Y. Xiong, J. Qian, J. D. Brock, J. Lu, L. Xiao, H. D. Abruña and L. Zhuang, ACS Appl. Mater. Interfaces, 2018, 10, 5519–5526 CrossRef CAS PubMed.
- G. Litrico, P. Proulx, J.-B. Gouriet and P. Rambaud, Adv. Powder Technol., 2015, 26, 1–7 CrossRef CAS.
- M. Wang, Z. Lai, X. Jin, T. Sun, H. Liu and H. Qi, Adv. Funct. Mater., 2021, 31, 2101957 CrossRef CAS.
- M. Wang, X. Feng, X. Wang, S. Hu, C. Zhang and H. Qi, J. Mater. Chem. A, 2021, 9, 24539–24547 RSC.
- G. Shen, D. Chen, P.-C. Chen and C. Zhou, ACS Nano, 2009, 3, 1115–1120 CrossRef CAS PubMed.
- P. Krempl, G. Schleinzer and W. Wallnöfer, Sens. Actuators, A, 1997, 61, 361–363 CrossRef CAS.
- S. N. Mohammad, A. A. Salvador and H. Morkoc, Proc. IEEE, 1995, 83, 1306–1355 CrossRef CAS.
- H. Khan, N. Mahmood, A. Zavabeti, A. Elbourne, M. A. Rahman, B. Y. Zhang, V. Krishnamurthi, P. Atkin, M. B. Ghasemian, J. Yang, G. Zheng, A. R. Ravindran, S. Walia, L. Wang, S. P. Russo, T. Daeneke, Y. Li and K. Kalantar-Zadeh, Nat. Commun., 2020, 11, 3449 CrossRef CAS PubMed.
- V. Krishnamurthi, H. Khan, T. Ahmed, A. Zavabeti, S. A. Tawfik, S. K. Jain, M. J. S. Spencer, S. Balendhran, K. B. Crozier, Z. Li, L. Fu, M. Mohiuddin, M. X. Low, B. Shabbir, A. Boes, A. Mitchell, C. F. McConville, Y. Li, K. Kalantar-Zadeh, N. Mahmood and S. Walia, Adv. Mater., 2020, 32, 2004247 CrossRef CAS PubMed.
- Q. Li, B.-D. Du, J.-Y. Gao, B.-Y. Xing, D.-K. Wang, J.-F. Ye and J. Liu, Adv. Mater. Technol., 2022, 7, 2200733 CrossRef CAS.
- N. Syed, A. Zavabeti, K. A. Messalea, E. Della Gaspera, A. Elbourne, A. Jannat, M. Mohiuddin, B. Y. Zhang, G. Zheng, L. Wang, S. P. Russo, D. Esrafilzadeh, C. F. McConville, K. Kalantar-Zadeh and T. Daeneke, J. Am. Chem. Soc., 2019, 141, 104–108 CrossRef CAS PubMed.
- Y. Du, S. Yin, Y. Li, J. Chen, D. Shi, E. Guo, H. Zhang, Z. Wang, Q. Qin and C. Zou, Small Methods, 2023, 2300175 Search PubMed.
- Y. Zhou, B. Deng, Y. Zhou, X. Ren, J. Yin, C. Jin, Z. Liu and H. Peng, Nano Lett., 2016, 16, 2103–2107 CrossRef CAS PubMed.
- Z. Chen, Q. Chen, Z. Chai, B. Wei, J. Wang, Y. Liu, Y. Shi, Z. Wang and J. Li, Nano Res., 2022, 15, 4677–4681 CrossRef CAS.
- J. Fujita, T. Hiyama, A. Hirukawa, T. Kondo, J. Nakamura, S. Ito, R. Araki, Y. Ito, M. Takeguchi and W. W. Pai, Sci. Rep., 2017, 7, 12371 CrossRef PubMed.
- M. Mayyas, H. Li, P. Kumar, M. B. Ghasemian, J. Yang, Y. Wang, D. J. Lawes, J. Han, M. G. Saborio, J. Tang, R. Jalili, S. H. Lee, W. K. Seong, S. P. Russo, D. Esrafilzadeh, T. Daeneke, R. B. Kaner, R. S. Ruoff and K. Kalantar-Zadeh, Adv. Mater. Interfaces, 2020, 32, 2001997 CrossRef CAS PubMed.
- S. L. James, Chem. Soc. Rev., 2003, 32, 276–288 RSC.
- X. Hu, T. Huang, G. Zhang, S. Lin, R. Chen, L.-H. Chung and J. He, Coord. Chem. Rev., 2023, 475, 214879 CrossRef CAS.
- B. Wang, J.-H. Liu, J. Yu, J. Lv, C. Dong and J.-R. Li, J. Hazard. Mater., 2020, 382, 121018 CrossRef CAS PubMed.
- A. S. Efimova, P. V. Alekseevskiy, M. V. Timofeeva, Y. A. Kenzhebayeva, A. O. Kuleshova, I. G. Koryakina, D. I. Pavlov, T. S. Sukhikh, A. S. Potapov, S. A. Shipilovskikh, N. Li and V. A. Milichko, Small Methods, 2023, 7, 2300752 CrossRef CAS PubMed.
- S. Wang, Z. Ai, X. Niu, W. Yang, R. Kang, Z. Lin, A. Waseem, L. Jiao and H.-L. Jiang, Adv. Mater., 2023, 35, 2302512 CrossRef CAS PubMed.
- R. Shu, J. Wu and X. Yang, Composites, Part A, 2023, 173, 107677 CrossRef CAS.
- A. V. Parmuzina and O. V. Kravchenko, Int. J. Hydrogen Energy, 2008, 33, 3073–3076 CrossRef CAS.
- J. Zheng, A. Sharma, T. Kumeria, Y. Chi, M. B. Ghasemian, G. Mao, J. Tang, P. Kumar, Md. A. Rahim and K. Kalantar-Zadeh, Adv. Funct. Mater., 2023, 2300969 CrossRef.
- H. Kwon, H.-N. Barad, A. R. Silva Olaya, M. Alarcón-Correa, K. Hahn, G. Richter, G. Wittstock and P. Fischer, ACS Appl. Mater. Interfaces, 2023, 15, 5620–5627 CrossRef CAS PubMed.
- D. Bridges, D. Fieser, J. J. Santiago and A. Hu, Metals, 2023, 13, 1193 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |