Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synergetic effect of mild hypothermia and antioxidant treatment on ROS–mediated neuron injury under oxygen–glucose deprivation investigated by scanning electrochemical microscopy†
Junjie
Zhang
ab,
Yulin
Liu
ab,
Yuxiang
Zhao
ab,
Siyu
Zhang
ab,
Feng
Xu
 ab and
Fei
Li
ab and
Fei
Li
 *ab
*ab
aThe Key Laboratory of Biomedical Information Engineering of Ministry of Education, School of Life Science and Technology, Xi'an Jiaotong University, Xi'an 710049, P. R. China. E-mail: feili@mail.xjtu.edu.cn
bBioinspired Engineering and Biomechanics Center (BEBC), Xi'an Jiaotong University, Xi'an 710049, P. R. China
First published on 12th November 2024
Abstract
Ischemic stroke and reperfusion injury result in neuronal damage and dysfunction associated with oxidative stress, leading to overproduction of cellular reactive oxygen species (ROS) and reactive nitrogen species (RNS). In situ monitoring of the transient ROS and RNS effluxes during rapid pathologic processes is crucial for understanding the relationship between progression of cell damage and role of oxidative stress, and developing the corresponding neuroprotective strategies. Herein, we built oxygen glucose deprivation (OGD) and mild hypothermic (MH) models to mimic the in vitro conditions of ischemic stroke and MH treatment. We used scanning electrochemical microscopy (SECM) to in situ monitor H2O2 and nitric oxide (NO) effluxes from HT22 cells under the OGD and MH treatment conditions. Through quantitative analysis of the H2O2 and NO efflux results, we found that the cellular oxidative stress was primarily manifested through ROS release under OGD conditions, and the MH treatment partially suppressed the excessive H2O2 and NO production induced by reoxygenation. Moreover, the synergistic therapeutic effect of MH with antioxidant treatment significantly reduced the oxidative stress and enhanced the cell survival. Our work reveals the crucial role of oxidative stress in OGD and reperfusion processes, and the effective improvement of cell viability via combination of MH with antioxidants, proposing promising therapeutic interventions for ischemic stroke and reperfusion injury.
Introduction
Ischemic stroke as one of the leading causes of global death and disability can rapidly induce neuronal apoptosis due to oxygen deprivation and adenosine triphosphate (ATP) depletion caused by blockage of cerebral blood vessels.1,2 Immediate intervention to restore blood flow and salvage neuronal cells is crucial for effective reperfusion.3 However, the process of ischemia and the subsequent reperfusion can lead to extensive free radical production and inflammatory responses that exacerbate cellular damage.4 Mild hypothermia (MH), an intervention to reduce the body's core temperature to a range between 32 °C and 35 °C, has been proven to be beneficial for various brain injuries (e.g., stroke and traumatic brain injury), through attenuating cellular metabolic demands, inhibiting the inflammatory response and activating multiple signaling pathways (e.g., IGF-1R/AKT and IRAK2/NF-κB pathways).5–7 To better understand the underlying mechanisms of ischemia-reperfusion injury and devise effective prevention and treatment strategies, oxygen–glucose deprivation (OGD) and mild hypothermic culture models have been developed and used to stimulate the pathophysiological processes of cerebral ischemic injury and hypothermia therapy in vitro.8–10During OGD and reperfusion, intracellular pathways of generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) are over-activated because of the impaired energy metabolism and subsequent reoxygenation.11 Meanwhile, the reduced synthesis or activity of intercellular endogenous antioxidant defense systems impairs the elimination of accumulation of ROS and RNS, which triggers cellular damage (e.g., lipid peroxidation and organelle dysfunction), ultimately causing neuronal death and brain tissue injury.12–15 Thus, investigation of the dynamics of oxidative stress levels of ROS and RNS of neurons during OGD and reperfusion is crucial for understanding the role of oxidative stress in neuronal injury and the mechanism of ischemic stroke. The conventional methods for measuring cellular ROS and RNS, such as fluorescent staining and spectrophotometry methods, need labelling of cells with specific optical probes and are limited by their long incubation and relatively low quantitative accuracy,16,17 which cannot meet the need of monitoring the rapid progression of the ischemia-reperfusion injury process. Electrochemical methods which are label-free, with high sensitivity and real-time monitoring capabilities, are suitable for noninvasive and quantitative detections of ROS and RNS effluxes. Scanning electrochemical microscopy (SECM) is an electrochemical scanning probe microscope using a μm nm−1-sized electrode as its probe, with the capability to precisely monitor the chemical species released from cells and track the dynamic interfacial processes across the cell membrane through recording faradaic/ion current and potential changes from various charge transfer reactions around cells.18–20 In previous reports, SECM has been applied to in situ characterize the dynamics of ROS and RNS molecules of single living cells in a noninvasive manner,21–23 such as by monitoring the cellular released H2O2 and NO and monitoring the dynamics of intracellular ROS and RNS (including H2O2, ONOO−, NO˙ and NO2−) molecules from several cell lines.24–26 Thus, SECM can be an ideal tool for monitoring the ROS/RNS effluxes of neurons during OGD and MH treatment.
In this work, we investigated the role of oxidative stress and its underlying mechanisms of neuronal injury under ischemic stroke, as well as the synergistic therapeutic effect of physical mild hypothermia combined with chemical drug intervention (Scheme 1). First, to mimic the in vitro conditions of ischemic stroke and MH, we constructed the OGD and MH cell models by selecting HT22 cells, an immortalized mouse hippocampal cell line, as the OGD and reperfusion model representative and culturing them under atmosphere- and temperature-controlled experimental conditions. We determined the durations of OGD and MH processes of HT22 cells via colorimetric and fluorescence characterization studies of cell viability and intracellular ROS and NO levels. Then we employed SECM to in situ monitor the frequencies and amounts of H2O2 and NO effluxes of HT22 cells under OGD and MH treatment alone or in combination with antioxidant (vitamin E in this case) intervention. The results showed that the dramatic release of H2O2 and NO during OGD were eliminated by both the MH treatment alone and the MH combined with vitamin E treatment, and the synergetic effect of MH with antioxidant intervention had the highest efficiency for reducing the oxidative stress level of HT22 cells suffering from OGD and markedly improved the cell viability. Our work contributes to a better understanding of the role of oxidative stress in neuronal injury caused by OGD and the potential therapeutic strategy of MH combined with antioxidants for OGD.
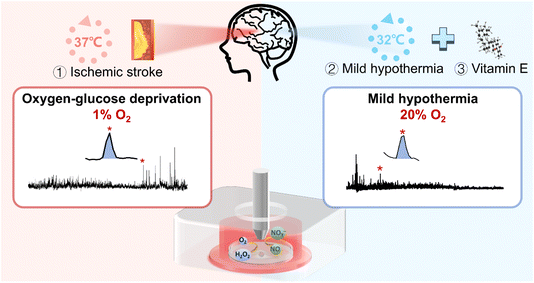 | ||
| Scheme 1 Schematic diagram of application of the SECM platform to in situ monitor H2O2 and NO effluxes of HT22 cells under OGD and MH treatments. | ||
Results and discussion
Oxidative stress of HT22 cells under OGD and MH treatment
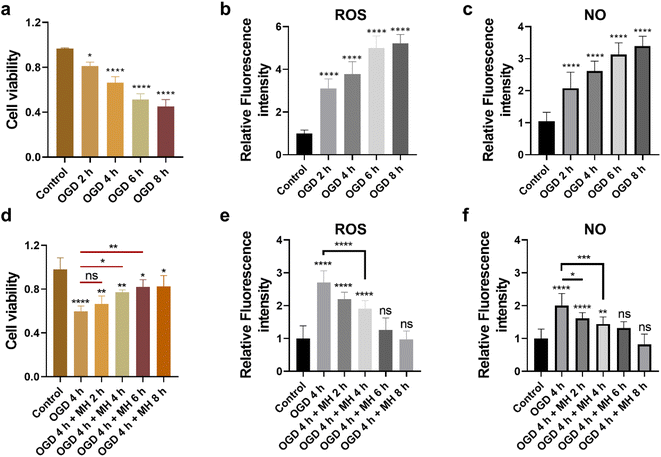
First, to mimic the ischemic stroke and mild hypothermic treatment conditions, we built in vitro cell models using HT22 cells as the ischemic stroke model and then cultured them in glucose-free Earle's balanced salt solution (EBSS) with 1% dissolved oxygen at 37 °C. To investigate the cellular state and oxidative stress level under OGD and MH conditions, we determined the durations of OGD and MH in our experimental system through characterization studies of the three key cellular parameters, i.e., cell viability, and intracellular ROS and NO levels, under OGD and MH processes using colorimetric and fluorescence methods. From Fig. 1a, we observed that the viability of HT22 cells after the OGD process at 0, 2, 4, 6 and 8 h presented a continuous decrease from 81.2% to 66.1%, 51.3% and 45.2%, respectively, compared to that of the control groups of 96.5% under normal physiological conditions (37 °C, high-glucose Dulbecco's modified eagle medium (HG-DMEM), 5% CO2, 20% O2 and 75% N2). This indicates that the HT22 cells were highly sensitive to glucose and oxygen concentrations of the culture medium and the detrimental effects of reduced oxygen availability and energy deprivation on cell viability. From the fluorescence images and statistical results in Fig. S1a and 1b,† we observed that the intracellular ROS levels of HT22 cells after 2, 4, 6, and 8 h of OGD were 3.1, 3.8, 5.0, and 5.2 times higher than those of the control groups. The intracellular NO levels after 2, 4, 6, and 8 h of OGD were 2.1, 2.6, 3.1, and 3.4 times higher than those of the control groups (Fig. 1c and S1b†). These results show that under 2 h of OGD, the cellular oxidative stress levels significantly increased, while the cell viability remained over 80% compared to that of the control group, indicating that the HT22 cells experienced neuronal oxidative injury and could withstand oxidative stress without succumbing to cell death. Additionally, extending OGD to 4 h resulted in a remarkable decrease in cell viability to ∼60%, indicating that the prolonged OGD duration time caused significant cell death likely due to the increased oxidative injury. Thus, the OGD duration of 4 h was selected as the optimal experimental condition of OGD in the subsequent experiments, which allows the examination of both the onset and progression of neuronal oxidative injury.Next, we evaluated the therapeutic intervention of MH during reperfusion via characterization of the cell viability, and intracellular ROS and NO levels of HT22 cells under MH treatment from 0 to 8 h following 4 h of OGD. The cell viability increased to 66%, 77%, and 82%, and remained above 82% after 2, 4, 6 and 8 h of MH treatment, respectively, displaying a significant difference compared to the OGD group (60%) after 4 h of MH treatment (Fig. 1d). This suggests that the MH intervention can effectively ameliorate the neuronal injury induced by OGD. Moreover, the intracellular ROS and NO levels of HT22 cells of the groups after 4 h of MH treatment continuously decreased and presented a significant difference compared to the OGD groups, indicating that the oxidative stress levels of cells decreased after MH treatment for over 4 h (Fig. 1e, f, S1c and d†), while the intracellular ROS and NO levels of HT22 cells after 6 h of MH treatment did not show significant differences compared to the control groups, indicating that MH can effectively suppress the increase in intracellular ROS levels induced by reoxygenation.27 Thus, the MH treatment duration of 4 h was selected as the optimal condition of MH for the following experiments as it can effectively enhance cell viability and reduce oxidative stress in HT22 cells.
SECM platform for monitoring ROS and NO effluxes of HT22 cells under OGD and MH conditions
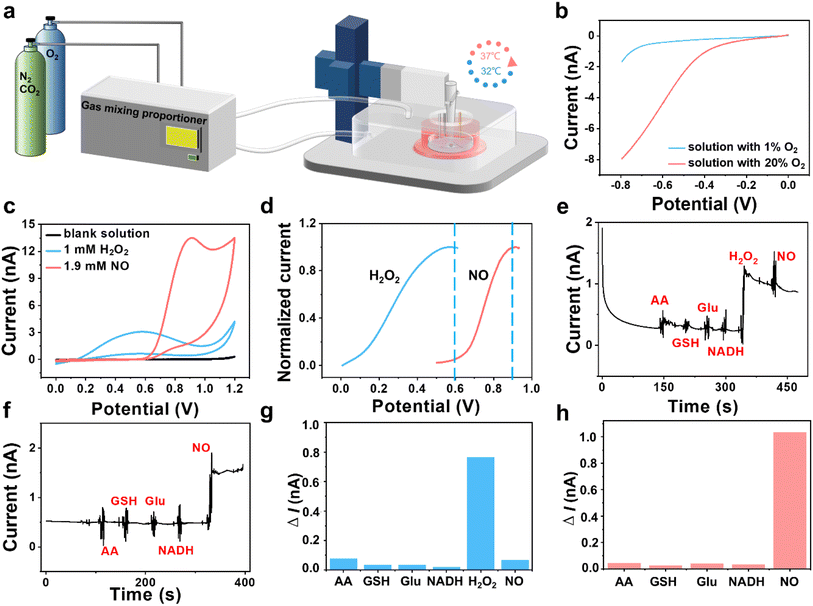
To in situ monitor the H2O2 and NO effluxes of HT22 cells under OGD and MH conditions, we established an atmosphere- and temperature-controlled SECM platform with integrations of a custom atmospheric chamber to regulate the ratios of O2 (1% and 20%) and N2 (94% and 75%) and a culturing chamber to incubate cells at 32 and 37 °C (Fig. 2a). From the linear sweep voltammograms in Fig. 2b, we observed that the oxygen reduction currents were significantly reduced in the solution with 1% dissolved oxygen compared to the solution with 20% dissolved oxygen, confirming the oxygen concentration of the culture medium in our SECM platform was under hypoxic conditions. The temperatures in the system can be warmed up to the preset temperature within 5 min and controlled within a narrow range of ±0.2 °C h−1 (Fig. S2†). Then we used a three-electrode system with a Pt microelectrode as the working electrode to record cyclic voltammograms in the advanced Tyrode's solution after adding H2O2 and NO. Distinct oxidation potential peaks were observed at 0.58 V for H2O2 and 0.9 V for NO with minimal overlap between the two species prior to 0.6 V. And the oxidation current of NO was less than 0.5% of the oxidation current of H2O2 at 0.6 V, indicating that interference between the oxidation currents of H2O2 and NO can be negligible in this case (Fig. 2c and d).Considering the possible interferences from the coexisting redox-active species in cells, such as ascorbic acid (AA), glutathione (GSH), glutamate (Glu) and nicotinamide adenine dinucleotide (NADH),28,29 on our electrochemical monitoring of H2O2 and NO, we used amperometry to evaluate the selectivity of our experimental system. As shown in Fig. 2e, after sequential additions of AA, GSH, Glu, NADH, H2O2 and NO into the advanced Tyrode's solution, an obvious current response at 0.6 V owing to H2O2 oxidation was observed, at which potential NO and other coexisting interferents presented low current responses. As shown in Fig. 2f, NO generated a significant oxidation current at 0.9 V with negligible interference from the other coexisting substances. From the histograms of the current differences at 0.6 V and 0.9 V (Fig. 2g and h), we inferred that the current responses of the interferents were less than 10% of the H2O2 oxidation current at 0.6 V and 5% of the NO oxidation current at 0.9 V, respectively, indicating that the coexisting interferences had a negligible impact on the amperometric detection of H2O2 and NO in our work.
Next, we recorded the current responses of H2O2 and NO released from HT22 cells after adding 2,3-dimethoxy-1,4-naphthalenedione (DMNQ) (a ROS inducer) and L-arginine (L-Arg, a substrate for NO generation by neuronal-type nitric oxide synthase (nNOS)). An obvious current response at 0.6 V was recorded after addition of 30 μM DMNQ, indicating rapid H2O2 production from HT22 cells after adding DMNQ due to the increased extracellular ROS level (Fig. S3a†). Similarly, a rapid increase in the current signal at 0.9 V was observed following addition of 100 μM L-Arg, attributed to the rapid NO production by the intracellular nNOS (Fig. S3b†). These results demonstrate the feasibility of our electrochemical system for monitoring the cellular released H2O2 and NO.
Effects of culture temperature and electrode size on electrochemical measurements of cellular released H2O2 and NO
Based on the Bulter–Volmer equation (eqn (1)) and Strokes–Einstein equation (eqn (2)), the redox currents and the diffusion coefficients of analytes are both affected by the experimental temperature (i.e., OGD (37 °C) and MH (32 °C) in our case).30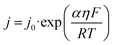 | (1) |
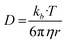 | (2) |
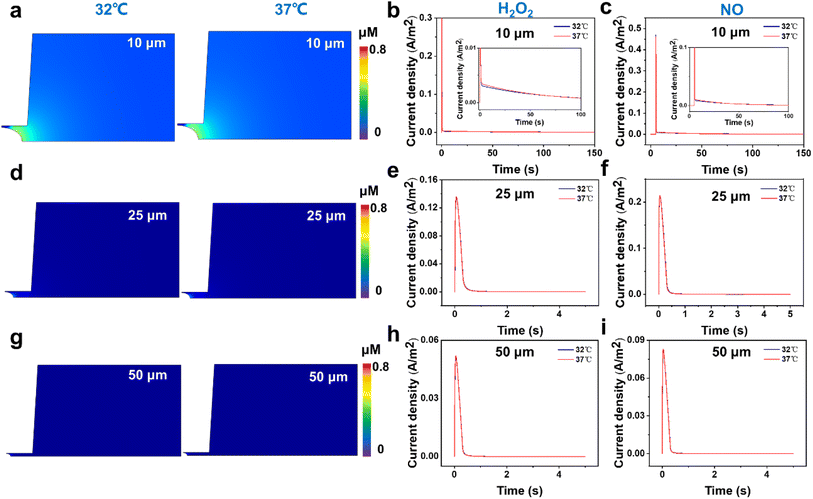
First, from the concentration maps and the amperometric traces of H2O2 and NO effluxes generated by using a 10 μm-in-diameter microdisk electrode in Fig. 3a–c and S4b,† we can see that the H2O2 and NO effluxes mainly diffuse to the bulk solution rather than being localized in the confined space beneath the microelectrode and the cell membrane, suggesting that the 10 μm-in-diameter microdisk electrode is not large enough to capture the total H2O2 and NO effluxes. And the simulated amperometric traces of H2O2 and NO at 37 °C show that the amperometric traces at 37 °C are not well overlapped with the traces at 32 °C. This might be due to the temperature effect on the diffusion coefficients of H2O2 and NO, causing the differences in the collection efficiency, current responses and mass transports of H2O2 and NO effluxes at 32 °C and 37 °C (Fig. 3b and c).
To avoid the temperature effect and achieve better collection efficiency of the cellular released H2O2 and NO, we further analyzed the concentration maps and the amperometric traces of H2O2 and NO effluxes using 25 and 50 μm-in-diameter microdisk microelectrodes as the SECM probes at 32 °C and 37 °C. As shown in Fig. 3d–f and S4c,† the generated H2O2 and NO effluxes are confined within the gaps between the 25 μm-in-diameter microdisk electrodes and the cell surfaces, and the simulated amperometric traces of H2O2 and NO at 37 °C overlap well with the amperometric traces at 32 °C.
These results indicate that the 25 μm-in-diameter electrodes can capture the total released H2O2 and NO effluxes and the temperature effect on the current responses and the mass transports of H2O2 and NO can be neglected. Similar results can be observed from the results of concentration maps and amperometric traces of H2O2 and NO effluxes using a 50 μm-in-diameter microdisk electrode, indicating that a microdisk electrode with a diameter of over 25 μm can collect the cellular released H2O2 and NO in our experimental system (Fig. 3g–i and S4d†). Considering that the smaller electrode size can minimize the background current and improve signal resolution, the 25 μm-in-diameter microdisk electrode was chosen as the SECM probe to monitor the H2O2 and NO effluxes from HT22 cells in our subsequent experiments.
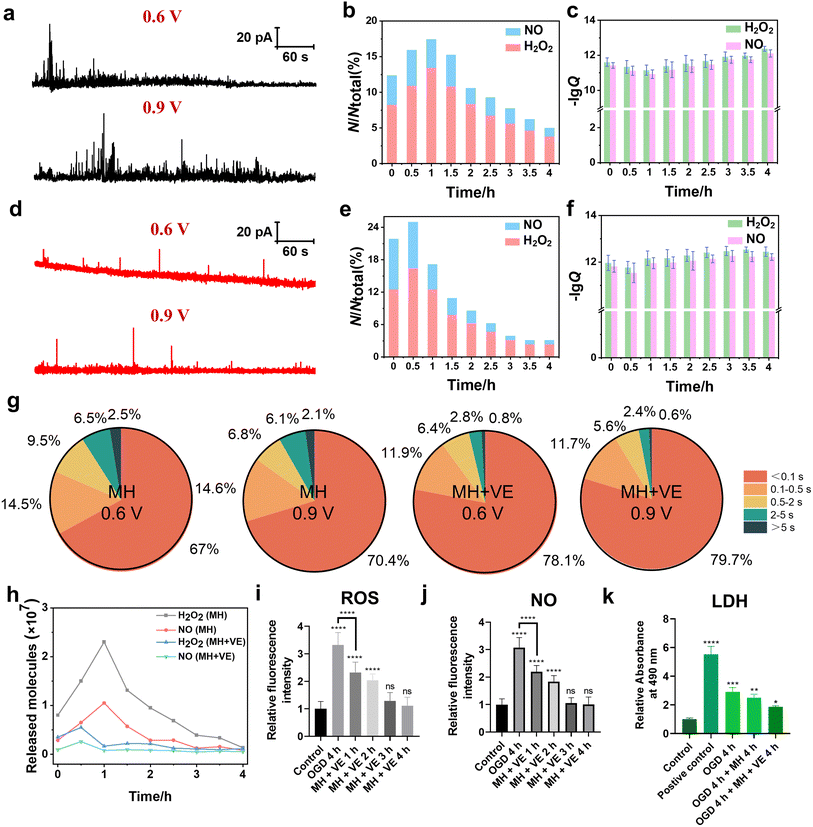
Extracellular ROS and NO effluxes of HT22 cells under OGD in situ monitored by SECM
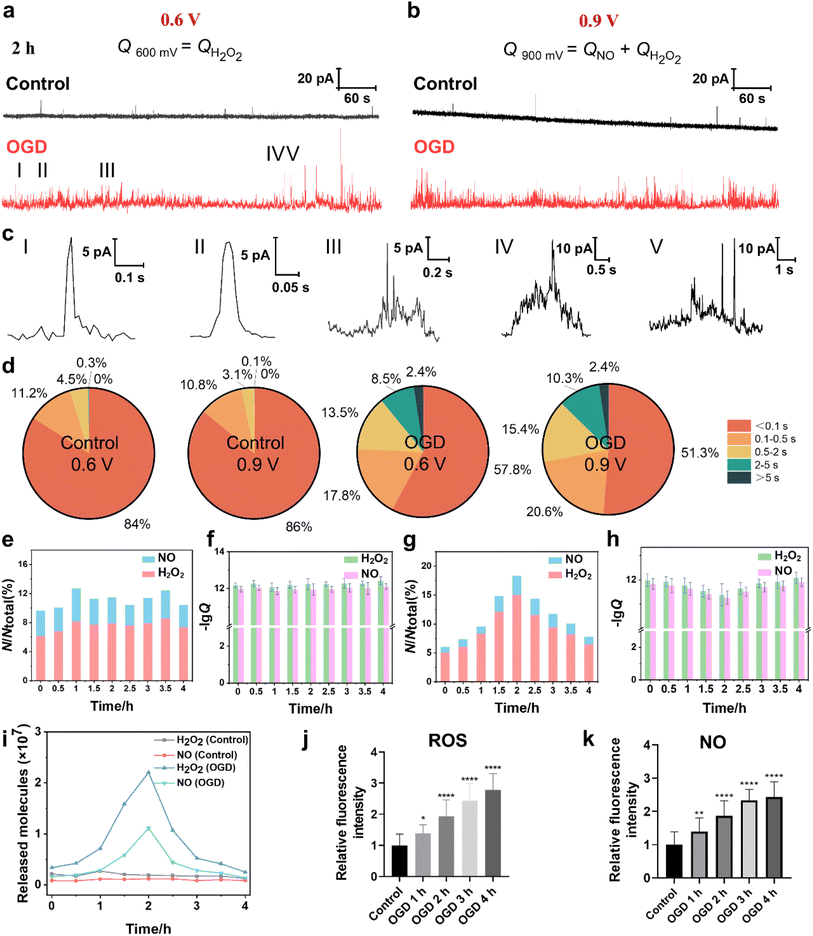
The cellular ROS and NO levels can significantly increase under OGD and reperfusion, triggering cellular dysfunction and even cell death.31 The accumulation of ROS and NO can lead to harmful biochemical reactions inside cells, such as lipid peroxidation of the cell membrane, DNA damage and mitochondrial dysfunction. Thus, we assessed the extent of cellular damage after exposure to OGD and its protective mechanism under MH treatment via quantitatively monitoring the extracellular H2O2 and NO levels using SECM. First, the SECM platform integrated with an adjustable temperature- and atmosphere-controlled chamber described above was used to mimic the normal physiological and OGD conditions. Amperometry was applied to record the oxidative responses of H2O2 and NO effluxes from HT22 cells under OGD every 30 min for 4 h. As shown in Fig. 4a and b, taking the amperometric traces of HT22 cells under OGD for 2 h as a representative example, a series of distinct individual events of H2O2 and NO effluxes from HT22 cells at 0.6 V and 0.9 V, respectively, are observed in the OGD groups compared to the control groups. From the categorized spike types based on the duration and the shape features, the most common spikes, identified with simple events, displayed a single maximum peak within 0.5 s (Fig. 4c(I and II)). Meanwhile, the spikes with complex shape features shown in Fig. 4c(III–V), defined as complex events, have similar characteristics to the neurotransmitter exocytosis and ROS events from neurons.32–34 In addition, 95.2% and 96.8% of the amperometric spikes at 0.6 V and 0.9 V were simple events under the normal physiological conditions, while the proportions of the simple events obviously decreased to 75.6% and 71.9% under OGD (Fig. 4d), indicating the onset of oxidative stress and cellular injury induced by OGD. Additionally, the statistical frequency distributions of H2O2 and NO effluxes maintained a consistent ratio of approximately 6.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.1 over 4 h of incubation under normal physiological conditions, while the ratio shifted dramatically to 8.3
3.1 over 4 h of incubation under normal physiological conditions, while the ratio shifted dramatically to 8.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 under OGD conditions (Fig. 4e), suggesting that the ROS predominated among oxygen-derived free radicals under OGD-induced stress.
1.7 under OGD conditions (Fig. 4e), suggesting that the ROS predominated among oxygen-derived free radicals under OGD-induced stress.
Next, we calculated the charges of the amperometric spikes according to Faraday's law (Q = nzF, zH2O2 = 2 and zNO = 3 in this case), and obtained the Q0.6V and Q0.9V by independent time integrations of i0.6V(t) and i0.9V(t), as well as the number of released molecules (N), which was given by the relative Q.35–38 The averages of NH2O2 and NNO from HT22 cells under normal physiological conditions at 2 h were 0.19 × 107 and 0.12 × 107 molecules, respectively (Fig. 4f). While, during OGD, the HT22 cells exhibited consistent increases of NH2O2 and NNO from 0.34 × 107 and 0.16 × 107 molecules at 0 h, respectively, and reached the maximum values of 2.2 × 107 and 1.1 × 107 molecules at 2 h, respectively. Subsequently, the released NH2O2 and NNO decreased to 0.25 × 107 and 0.14 × 107 molecules at 4 h of OGD (Fig. 4g and i). Moreover, the intracellular ROS levels at 4 h of OGD were 1.38, 1.94, 2.44, and 2.78 times higher than that of the control groups (Fig. 4j and S5a†). And the intracellular NO levels were 1.39, 1.86, 2.33, and 2.43 times higher than that of the control groups (Fig. 4k and S5b†). Both the SECM and fluorescence results show similar trends where the ROS and NO effluxes of HT22 cells rapidly elevated within 2 h of OGD, while the rates of increment subsequently stabilized during 2 to 4 h, revealing that the HT22 cells experienced a significant increase in the oxidative stress level under hypoxia.
A possible reason for the above results might be because the prolonged OGD duration impedes the normal operation of the electron transport chain from the inadequate oxygen and glucose provision.39 This disruption diminishes the potential difference between the internal and external sides of the inner mitochondrial membrane, making it easier for leakage to occur during the electron transport process and thus generating a large amount of ROS and inducing the production of NO.40,41 Simultaneously, OGD causes a stress response of HT22 cells from an increase in the expression of nNOS and thus the synthesis of a large amount of NO.42,43 Furthermore, OGD leads to a decrease in the activities of intracellular antioxidant enzymes (e.g., superoxide dismutase (SOD), catalase (CAT)) and nonenzymatic antioxidants (GSH), which impairs the ability of endogenous antioxidants to scavenge the excessive ROS and promote the accumulation of ROS.44 After OGD for 2 h, the ability to generate ROS and NO within cells gradually diminished due to the insufficient amount of oxygen, which manifested as a reduction in the number of ROS release events.
Extracellular H2O2 and NO effluxes of HT22 cells under MH combined with vitamin E treatment
According to the above experimental results, we found the rapid generation and release of ROS and NO of HT22 cells induced by OGD, which caused an obvious decrease in cell viability. For further developing the neuroprotective treatment methods, reducing the neuronal oxidative stress of HT22 cells can be one of the strategies. MH, one of the clinically used neuroprotective treatment approaches, can effectively inhibit the production of oxygen-derived free radicals and provide neuroprotection after traumatic brain injury and cerebral ischemia through various molecular mechanisms.45 Next, we further explored the therapeutic efficacy of MH through monitoring the H2O2 and NO effluxes of HT22 cells subjected to 4 h of OGD.As shown in Fig. 5a and b, the amperometric traces of H2O2 and NO effluxes of HT22 cells under MH for 1 h exhibited a large amount of release events with a maximum frequency distribution ratio of H2O2 and NO effluxes of 7.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7. This can be possibly attributed to the reoxygenation effect triggered by the massive activation of oxygen resupply to induce the overproduction of various oxygen-derived free radicals within 1 h.46,47 The calculated charges of the amperometric spikes at 0–4 h of MH showed that the average released NH2O2 was 0.80 × 107, 2.3 × 107, 1.0 × 107, 0.39 × 107 and 0.14 × 107 molecules, while NNO was 0.29 × 107, 1.1 × 107, 0.29 × 107, 0.13 × 107 and 0.08 × 107 molecules, respectively (Fig. 5c). These results reveal that the H2O2 and NO effluxes initially increased within the first hour of MH, followed by a continuous decrease, indicating that MH can reduce the oxidative stress due to reoxygenation after OGD.48 Additionally, the fluorescence results of both intracellular ROS and NO levels of HT22 cells under MH showed no obvious difference in the MH groups until 4 h compared to the relative fluorescence intensities of ROS and NO of the control groups (Fig. S6†). And the viability of HT22 cells improved to 75% after 4 h of MH (Fig. S7†), indicating that MH can inhibit the overproduction of ROS and NO of HT22 cells after suffering from OGD. But the efficiency in alleviating cellular oxidative stress and improving cell viability was still limited. Therefore, to substantially reduce the degree of oxidative stress and improve cell survival, it is necessary to combine MH with other neuroprotective strategies to achieve more comprehensive neuroprotective effects.
2.7. This can be possibly attributed to the reoxygenation effect triggered by the massive activation of oxygen resupply to induce the overproduction of various oxygen-derived free radicals within 1 h.46,47 The calculated charges of the amperometric spikes at 0–4 h of MH showed that the average released NH2O2 was 0.80 × 107, 2.3 × 107, 1.0 × 107, 0.39 × 107 and 0.14 × 107 molecules, while NNO was 0.29 × 107, 1.1 × 107, 0.29 × 107, 0.13 × 107 and 0.08 × 107 molecules, respectively (Fig. 5c). These results reveal that the H2O2 and NO effluxes initially increased within the first hour of MH, followed by a continuous decrease, indicating that MH can reduce the oxidative stress due to reoxygenation after OGD.48 Additionally, the fluorescence results of both intracellular ROS and NO levels of HT22 cells under MH showed no obvious difference in the MH groups until 4 h compared to the relative fluorescence intensities of ROS and NO of the control groups (Fig. S6†). And the viability of HT22 cells improved to 75% after 4 h of MH (Fig. S7†), indicating that MH can inhibit the overproduction of ROS and NO of HT22 cells after suffering from OGD. But the efficiency in alleviating cellular oxidative stress and improving cell viability was still limited. Therefore, to substantially reduce the degree of oxidative stress and improve cell survival, it is necessary to combine MH with other neuroprotective strategies to achieve more comprehensive neuroprotective effects.
To further mitigate the oxidative injury of HT22 cells induced by OGD, we added vitamin E (VE), an antioxidant regularly used in clinics, into the cell culture and evaluated the synergistic therapeutic effect of MH treatment with VE. First, to check whether the addition of VE affects our electrochemical detections of H2O2 and NO, we recorded the cyclic voltammogram in the advanced Tyrode's solution after adding VE in the potential range of 0 to 1 V. From Fig. S8,† we can see that there was no oxidation current signal in the potential range of 0.6 to 0.9 V, confirming that VE did not interfere with our electrochemical detection of H2O2 and NO. Then, we added VE into the cell culture medium after MH treatment for 4 h. As shown in Fig. 5d and e, the release events and the frequency distributions of H2O2 and NO effluxes of HT22 cells reached a maximum after 0.5 h of MH combined with VE treatment, indicating the more effective decreases in the released H2O2 and NO effluxes compared to the MH groups (Fig. 5a and b). These results indicate that the synergetic therapeutic effect of MH and VE treatment lead to a more rapid and effective reduction of neuronal oxidative stress levels compared to the MH treatment alone. Additionally, the average release of NH2O2 and NNO from HT22 cells presented a maximum of 0.56 × 107 and 0.26 × 107 molecules, respectively, under 0.5 h of MH with VE treatment (Fig. 5f), which were much lower than the NH2O2 = 1.5 × 107 and NNO = 0.65 × 107 molecules of the MH group at 0.5 h (Fig. 5c). This indicates that the combination of MH with VE treatment can effectively reduce the number of released molecules of H2O2 and NO with a significant decrease in 0.5 h.
Moreover, 90.0% and 91.4% of the amperometric spikes at 0.6 V and 0.9 V were shown to be simple events after addition of VE, suggesting that the oxidative burst in the cells was significantly suppressed with addition of VE (Fig. 5g). And the variation of NH2O2 and NNO during 4 h of MH with VE treatment showed a significant decrement compared to the MH groups (Fig. 5h). The fluorescence results of the ROS and NO effluxes also showed significant decreases compared to the OGD groups. And there was an obvious difference in the group of MH with VE treatment for 1 h compared to the OGD group, demonstrating the rapid and effective scavenging of the released ROS and NO from HT22 cells (Fig. 5i, j and S9†). These results suggest that the oxidative burst of HT22 cells suffering from OGD with reoxygenation is vital for neuronal oxidative injury, and the synergistic effect of MH and VE treatment can significantly ameliorate the oxidative stress levels of HT22 cells from reoxygenation within 1 h.
Next, we further evaluated the therapeutic effect of VE through cell viability and lactate dehydrogenase (LDH) assay experiments. As shown in Fig. S7,† after 4 h of MH with VE treatment, the cell viability was restored to 87.7%, while the extracellular LDH level presented a minimal difference compared to the control group (Fig. 5k), suggesting that the lipid peroxidation injury of HT22 cells was caused by the increased oxidative stress under OGD and thus the degradation of the cell membrane.49,50 The lipophilic VE inhibited the subsequent free radical attack and thus protected the cell membrane.51 These results demonstrate that the MH treatment with antioxidants can significantly improve neuronal survival, and the OGD-induced oxidative stress may be a key factor for the reduction of cellular viability.
Conclusion
In this work, we constructed an atmosphere- and temperature-controlled SECM platform to in situ monitor the released H2O2 and NO of HT22 cells under OGD and MH treatment alongside the antioxidant intervention. The SECM results showed the rapid and dramatic release of H2O2 and NO within 2 h of OGD, while the rates of oxidative stress slowed down during 2–4 h due to the insufficient amount of oxygen, indicating that the neuronal oxidative stress was mainly manifested through ROS release during OGD. The MH treatment partially inhibited the reoxygenation-induced ROS and NO overproduction, which quickly reduced the frequencies and amounts of H2O2 and NO effluxes with the synergistic effect of MH treatment with antioxidant intervention. Our results reveal that the rapid overproduction of ROS and NO of neurons under OGD can impair the redox balance of the cellular antioxidant system, which causes oxidative stress and influences the integrity of the cell membrane through lipid peroxidation, and even results in the loss of cell viability. The synergetic effect of MH and antioxidant intervention can effectively reduce the generation of cellular ROS and NO and mitigate the associated cellular damage. Our work demonstrates that the oxidative stress of HT22 cells under OGD and reperfusion is the primary cause of neuronal injury and highlights the protective effect of the combination of MH treatment with antioxidants, contributing to a better understanding of the pathophysiology of oxidative stress in cerebral ischemia and offering reference for strategies for clinical management of stroke-related conditions. The developed SECM platform equipped with temperature- and atmosphere-controlled functions can also be used as a versatile tool for in situ monitoring the cellular released substances in vitro for study of heat and hypoxia-related diseases.Data availability
Experimental details (including chemicals and materials, fluorescent staining of ROS and NO levels, cytotoxicity assay and LDH leakage assay of HT22 cells during OGD with MH and with/without VE treatment, preparation of SECM probes, amperometric traces of H2O2 and NO released from HT22 cells after the addition of DMNQ and L-Arg, and linear sweep voltammograms of VE in advanced Tyrode's solution), the parameters of the SECM theoretical model and the supporting experimental data are all provided in the ESI.Author contributions
J. J. Z., F. X. and F. L. conceived the ideas and designed the experiments. F. L. directed the cell and SECM experiments. J. J. Z., Y. L. L., Y. X. Z. and S. Y. Z. conducted the experiments and analyzed the data. All authors interpreted data and contributed to the writing of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22127803, 22174106, 12225208), the Fundamental Research Funds for the Central Universities (22127803HZ, SY6J007), and the Young Scholars of Changjiang Scholars Incentive Program of the Ministry of Education of China (Q2022203). We also thank Dr Frank Wang from HEKA Elektronik GmbH for the technical support on the SECM instrument.Notes and references
- M. S. Phipps and C. A. Cronin, Management of acute ischemic stroke, BMJ, 2020, 368, l6983 CrossRef PubMed.
- A. Datta, D. Sarmah, L. Mounica, H. Kaur, R. Kesharwani, G. Verma, P. Veeresh, V. Kotian, K. Kalia, A. Borah, X. Wang, K. R. Dave, D. R. Yavagal and P. Bhattacharya, Cell death pathways in ischemic stroke and targeted pharmacotherapy, Transl. Stroke Res., 2020, 11, 1185–1202 CrossRef PubMed.
- J. Montaner, L. Ramiro, A. Simats, S. Tiedt, K. Makris, G. C. Jickling, S. Debette, J.-C. Sanchez and A. Bustamante, Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke, Nat. Rev. Neurol., 2020, 16, 247–264 CrossRef PubMed.
- S. Orellana-Urzúa, I. Rojas, L. Líbano and R. Rodrigo, Pathophysiology of ischemic stroke: role of oxidative stress, Curr. Pharm. Des., 2020, 26, 4246–4260 CrossRef.
- J. Inamasu and K. Ichikizaki, Mild hypothermia in neurologic emergency: An update, Ann. Emerg. Med., 2002, 40, 220–230 CrossRef PubMed.
- Y. Xiong, A. K. Wakhloo and M. Fisher, Advances in acute ischemic stroke therapy, Circ. Res., 2022, 130, 1230–1251 CrossRef CAS PubMed.
- C. Qin, S. Yang, Y.-H. Chu, H. Zhang, X.-W. Pang, L. Chen, L.-Q. Zhou, M. Chen, D.-S. Tian and W. Wang, Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions, Signal Transduction Targeted Ther., 2022, 7, 215 CrossRef CAS.
- T. Zhou, J. Jiang, M. Zhang, Y. Fu, Z. Yang and L. Jiang, Protective effect of mild hypothermia on oxygen-glucose deprivation injury in rat hippocampal neurons after hypoxia, Mol. Med. Rep., 2013, 7, 1859–1864 CrossRef CAS.
- Z. Gao, Z. Zhang, Q. Bian, Y. Li, D. Ma, Z. Liu and S. Zhang, Mild hypothermia protects rat cortical neurons against oxygen-glucose deprivation/reoxygenation injury via the PI3K/Akt pathway, NeuroReport, 2021, 32, 312–320 CrossRef CAS PubMed.
- T. Zhou, L. Liang, Y. Liang, T. Yu, C. Zeng and L. Jiang, Mild hypothermia protects hippocampal neurons against oxygen-glucose deprivation/reperfusion-induced injury by improving lysosomal function and autophagic flux, Exp. Cell Res., 2017, 358, 147–160 CrossRef CAS PubMed.
- J. J. D. Ho, H. S. J. Man and P. A. Marsden, Nitric oxide signaling in hypoxia, J. Mol. Med., 2012, 90, 217–231 CrossRef CAS PubMed.
- Z.-j. Gao, J. Min, X.-c. Wu, T. Yang, C.-y. Yan, B.-h. Dong and T. Zhang, Repression of neuronal nitric oxide (nNOS) synthesis by MTA1 is involved in oxidative stress-induced neuronal damage, Biochem. Biophys. Res. Commun., 2016, 479, 40–47 CrossRef CAS.
- J. Zhang, Y. Liu, Y. Li, T. Zhu, J. Qiu, F. Xu, H. Zhang and F. Li, In situ and quantitatively imaging of heat-induced oxidative state and oxidative damage of living neurons using scanning electrochemical microscopy, Small Methods, 2022, 6, 2200689 CrossRef CAS PubMed.
- K. Hu, E. Relton, N. Locker, N. T. N. Phan and A. G. Ewing, Electrochemical measurements reveal reactive oxygen species in stress granules, Angew. Chem., Int. Ed., 2021, 60, 15302–15306 CrossRef CAS.
- X.-W. Zhang, Q.-F. Qiu, H. Jiang, F.-L. Zhang, Y.-L. Liu, C. Amatore and W.-H. Huang, Real-time intracellular measurements of ROS and RNS in living cells with single core–shell nanowire electrodes, Angew. Chem., Int. Ed., 2017, 56, 12997–13000 CrossRef CAS PubMed.
- Z. Qiu, X. Li, C. Duan, R. Li and L. Han, Glutaredoxin 1 protects neurons from oxygen-glucose deprivation/reoxygenation (OGD/R)-induced apoptosis and oxidative stress via the modulation of GSK-3β/Nrf2 signaling, J. Bioenerg. Biomembr., 2021, 53, 369–379 CrossRef CAS PubMed.
- S. Sun, F. Hu, J. Wu and S. Zhang, Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons, Redox Biol., 2017, 11, 577–585 CrossRef CAS PubMed.
- D.-D. P. Polcari D and J. Mauzeroll, Scanning electrochemical microscopy: A comprehensive review of experimental parameters from 1989 to 2015, Chem. Rev., 2016, 116, 13234–13278 CrossRef PubMed.
- T.-E. Lin, S. Rapino, H. H. Girault and A. Lesch, Electrochemical imaging of cells and tissues, Chem. Sci., 2018, 9, 4546–4554 RSC.
- T. M. Welle, K. Alanis, M. L. Colombo, J. V. Sweedler and M. Shen, A high spatiotemporal study of somatic exocytosis with scanning electrochemical microscopy and nanoITIES electrodes, Chem. Sci., 2018, 9, 4937–4941 RSC.
- J. Zhang, T. Zhu, J. Lang, W. Fu and F. Li, Recent advances of scanning electrochemical microscopy and scanning ion conductance microscopy for single-cell analysis, Curr. Opin. Electrochem., 2020, 22, 178–185 CrossRef.
- Y. Liu, J. Zhang, Y. Li, Y. Zhao, S. Kuermanbayi, J. Zhuang, H. Zhang, F. Xu and F. Li, Matrix stiffness-dependent microglia activation in response to inflammatory cues: in situ investigation by scanning electrochemical microscopy, Chem. Sci., 2024, 15, 171–184 RSC.
- S. Kuermanbayi, Y. Yang, Y. Zhao, Y. Li, L. Wang, J. Yang, Y. Zhou, F. Xu and F. Li, In situ monitoring of functional activity of extracellular matrix stiffness-dependent multidrug resistance protein 1 using scanning electrochemical microscopy, Chem. Sci., 2022, 13, 10349–10360 RSC.
- J. Zhang, T. Zhu, J. Lang, W. Fu and F. Li, Recent advances of scanning electrochemical microscopy and scanning ion conductance microscopy for single-cell analysis, Curr. Opin. Electrochem., 2020, 22, 178–185 CrossRef.
- X. Zhao, R. Zhu, M. Anikovskiy, Q. Wu and Z. Ding, Profiling H2O2 from single COS-7 cells by means of scanning electrochemical microscopy, Biosens. Bioelectron., 2023, 227, 115123 CrossRef CAS PubMed.
- S. Borgmann, I. Radtke, T. Erichsen, A. Blöchl, R. Heumann and W. Schuhmann, Electrochemical high-content screening of nitric oxide release from endothelial cells, ChemBioChem, 2006, 7, 662–668 CrossRef CAS.
- K. P. Bozem M, V. Mirčeski, E. J. Slowik, I. Bogeski, R. Kappl, C. Heinemann and M. Hoth, Electrochemical Quantification of Extracellular Local H2O2 Kinetics Originating from Single Cells, Antioxid. Redox Signaling, 2018, 29, 501–517 CrossRef.
- M. Hanko, Ľ. Švorc, A. Planková and P. Mikuš, Overview and recent advances in electrochemical sensing of glutathione – a review, Anal. Chim. Acta, 2019, 1062, 1–27 CrossRef CAS PubMed.
- Z. Deng, L. Zhao, H. Zhou, X. Xu and W. Zheng, Recent advances in electrochemical analysis of hydrogen peroxide towards in vivo detection, Process Biochem., 2022, 115, 57–69 CrossRef CAS.
- R. Wei, R. Zhang, Y. Xie, L. Shen and F. Chen, Hydrogen suppresses hypoxia/reoxygenation-induced cell death in hippocampal neurons through reducing oxidative stress, Cell. Physiol. Biochem., 2015, 36, 585–598 CrossRef CAS.
- P. Gründler, A. Kirbs and L. Dunsch, Modern thermoelectrochemistry, ChemPhysChem, 2009, 10, 1722–1746 CrossRef.
- S. Xu, Y. Li, J.-P. Chen, D.-Z. Li, Q. Jiang, T. Wu and X.-Z. Zhou, Oxygen glucose deprivation/re-oxygenation-induced neuronal cell death is associated with Lnc-D63785 m6A methylation and miR-422a accumulation, Cell Death Dis., 2020, 11, 816 CrossRef CAS PubMed.
- S. Majdi, E. C. Berglund, J. Dunevall, A. I. Oleinick, C. Amatore, D. E. Krantz and A. G. Ewing, Electrochemical measurements of optogenetically stimulated quantal amine release from single nerve cell varicosities in drosophila larvae, Angew. Chem., Int. Ed., 2015, 54, 13609–13612 CrossRef CAS PubMed.
- H. Gu, C. Gu, N. Locker and A. G. Ewing, Amperometry and electron microscopy show stress granules induce homotypic fusion of catecholamine vesicles, Angew. Chem., Int. Ed., 2024, 63, e202400422 CrossRef CAS.
- X.-W. Zhang, A. Oleinick, H. Jiang, Q.-L. Liao, Q.-F. Qiu, I. Svir, Y.-L. Liu, C. Amatore and W.-H. Huang, Electrochemical monitoring of ROS/RNS homeostasis within individual phagolysosomes inside single macrophages, Angew. Chem., Int. Ed., 2019, 58, 7753–7756 CrossRef CAS.
- X.-K. Yang, F.-L. Zhang, W.-T. Wu, Y. Tang, J. Yan, Y.-L. Liu, C. Amatore and W.-H. Huang, Quantitative nano-amperometric measurement of intravesicular glutamate content and its sub-quantal release by living neurons, Angew. Chem., Int. Ed., 2021, 60, 15803–15808 CrossRef CAS.
- Y. T. Qi, H. Jiang, W. T. Wu, F. L. Zhang, S. Y. Tian, W. T. Fan, Y. L. Liu, C. Amatore and W. H. Huang, Homeostasis inside single activated phagolysosomes: quantitative and selective measurements of submillisecond dynamics of reactive oxygen and nitrogen species production with a nanoelectrochemical Sensor, J. Am. Chem. Soc., 2022, 144, 9723–9733 CrossRef CAS.
- W. Fan, Y. Zhao, W. Wu, Y. Qin, J. Yan, Y. Liu and W. Huang, Redox homeostasis alteration in Endothelial mechanotransduction monitored by dual stretchable electrochemical sensors, Anal. Chem., 2022, 94, 7425–7432 CrossRef CAS.
- Y. Sun, M.-f. Jin, L. Li, Y. Liu, D. Wang and H. Ni, Genetic inhibition of plppr5 aggravates hypoxic-Ischemie-induced cortical damage and excitotoxic phenotype, Front. Neurosci., 2022, 16, 751489 CrossRef.
- D. Komsiiska, Oxidative stress and stroke: a review of upstream and downstream antioxidant therapeutic options, Comp. Clin. Pathol., 2019, 28, 915–926 CrossRef.
- T. Kahles and R. P. Brandes, Which NADPH oxidase isoform is relevant for ischemic stroke? The case for nox 2, Antioxid. Redox Signal., 2013, 18, 1400–1417 CrossRef CAS PubMed.
- T. Hagen, C. T. Taylor, F. Lam and S. Moncada, Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha, Science, 2003, 302, 1975–1978 CrossRef CAS PubMed.
- J. J. Ho, H. S. Man and P. A. Marsden, Nitric oxide signaling in hypoxia, J. Mol. Med., 2012, 90, 217–231 CrossRef CAS PubMed.
- Z. Wang, Y. Zhao, Y. Hou, G. Tang, R. Zhang, Y. Yang, X. Yan and K. Fan, A thrombin-activated peptide-templated nanozyme for remedying ischemic stroke via thrombolytic and neuroprotective actions, Adv. Mater., 2024, 36, e2210144 CrossRef PubMed.
- T. C. Wu and J. C. Grotta, Hypothermia for acute ischaemic stroke, Lancet Neurol., 2013, 12, 275–284 CrossRef.
- T. Zhou, Y. Liang, L. Jiang, T. Yu, C. Zeng and E. Tao, Mild hypothermia protects against oxygen glucose deprivation/reoxygenation-induced apoptosis via the Wnt/β-catenin signaling pathway in hippocampal neurons, Biochem. Biophys. Res. Commun., 2017, 486, 1005–1013 CrossRef CAS.
- X.-Y. Gao, S.-Z. Zhu, W. Xiang, K.-b. Huang, Y.-F. Hu, Y. Gu and S.-Y. Pan, Prolonged hypothermia exposure diminishes neuroprotection for severe ischemic-hypoxic primary neurons, Cryobiology, 2016, 72, 141–147 CrossRef CAS PubMed.
- D. Wu, M. Li, M. Fisher and X. Ji, Brain cytoprotection of ischemic stroke in the era of effective reperfusion, Sci. Bull., 2022, 67, 2372–2375 CrossRef.
- P. Jovanovic, L. Zoric, I. Stefanovic, B. Dzunic, J. Djordjevic-Jocic, M. Radenkovic and M. Jovanovic, Lactate dehydrogenase and oxidative stress activity in primary open-angle glaucoma aqueous humour, Bosnian J. Basic Med. Sci., 2010, 10, 83–88 CrossRef CAS.
- Y. Yuan, Y. Zhai, J. Chen, X. Xu and H. Wang, Kaempferol ameliorates oxygen-glucose deprivation/reoxygenation-induced neuronal Ferroptosis by activating Nrf2/SLC7A11/GPX4 axis, Biomolecules, 2021, 11, 923 CrossRef CAS.
- E. Niki, Lipid oxidation that is, and is not, inhibited by vitamin E: Consideration about physiological functions of vitamin E, Free Radicals Biol. Med., 2021, 176, 1–15 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc05977h |
| This journal is © The Royal Society of Chemistry 2024 |