Graphene-based 2D materials for rechargeable batteries and hydrogen production and storage: a critical review
Chandra Sekhar
Bongu
 ,
Sehar
Tasleem
,
Mohan Raj
Krishnan
and
Edreese Housni
Alsharaeh
,
Sehar
Tasleem
,
Mohan Raj
Krishnan
and
Edreese Housni
Alsharaeh
 *
*
College of Science and General Studies, AlFaisal University, PO Box 50927, Riyadh, 11533, Saudi Arabia. E-mail: ealsharaeh@alfaisal.edu
First published on 30th July 2024
Abstract
Batteries and hydrogen energy devices are considered the most critical technologies for achieving zero carbon dioxide emissions. However, they still suffer from several limitations, including low efficiency, short cycling life, low storage, and poor safety. With their strong mechanical strength (flexibility), chemical inertness, large surface area, remarkable thermal stability, and excellent electrical and high ion conductivity, graphene can overcome some of the issues associated with batteries and hydrogen energy devices. The properties of various two-dimensional (2D) materials make them potential candidates for a wide range of applications (batteries and hydrogen energy devices), thereby gaining considerable interest. Similarly, graphene has the potential for efficient hydrogen production and storage because of its large surface area and adjustable porosity. Graphene/2D composite materials are promising electrodes for lithium batteries, hydrogen storage, and production applications. This review provides a comprehensive overview of graphene/2D composite materials for lithium batteries and hydrogen storage and production applications.
1. Introduction
The expanding population and growing industrialization have increased the need for energy globally to an unprecedented degree. However, most of our energy comes from fossil fuels, which increase greenhouse gas emissions (GHGs) and contribute to worldwide climate change.1–4 The rising temperature of the earth's has prompted scientists to explore alternative energy sources. Reducing the dependency on fossil fuels and utilizing renewable energy to build new technologies are urgent. As a result, hydrogen (H2) and batteries are considered clean and sustainable energy sources for the future.5–12Batteries can store intermittent energy; thus, they are becoming increasingly popular as renewable sources.13–15 Lithium-ion batteries are the most researched rechargeable batteries owing to their excellent energy density and storage capacity, which are advantageous for various applications and daily energy demands.16–21 Electrode materials are crucial to lithium rechargeable batteries, but the sluggish kinetics and significant volume changes still hinder further applications.16,22–24
H2 is an effective and clean energy source that substitutes fossil fuels.25–27 Hydrogen energy has a gravimetric density roughly seven times higher than that of fossil fuels.28 Hydrogen, a readily available element and a flexible energy carrier, can transform several industries and sectors, including manufacturing, transportation, and power generation. The potential of hydrogen to provide energy without producing hazardous pollutants makes it significant. Since water is the only byproduct of using hydrogen as fuel, hydrogen is a clean and green choice.29 However, even with its enormous promise, there are still some obstacles that must be overcome before hydrogen energy is widely used.30 The main issues are linked to the production and storage of H2 energy.31 Regarding H2 production, novel catalysts and materials, such as nanostructured catalysts, are being investigated to speed up reactions and increase the efficiency for maximizing hydrogen generation.29 Furthermore, the lower volumetric density of hydrogen necessitates huge storage capacities or high pressure. Therefore, advanced catalysts and materials can significantly improve the efficacy and effectiveness of hydrogen storage.32 The common materials used to store hydrogen energy are sodium aluminium hydride (NaAlH4), lithium aluminium hydride (LiAlH4), lithium borohydride (LiBH4), magnesium nickel hydride (Mg2NiH4), sodium borohydride (NaBH4), aluminium hydride (AlH3), zeolites, metal–organic frameworks (MOFs), and covalent organic frameworks (COFs).33–40 However, the drawback of hydrogen adsorption–desorption exists as it lacks reversibility because of its strong bonding force and slow kinetics, necessitating high temperatures for desorption. For the production of hydrogen, titanium dioxide (TiO2), molybdenum disulfide (MoS2), tungsten(VI) oxide (WO3), zinc oxide (ZnO), iron oxide (α-Fe2O3), bismuth vanadate (BiVO4), copper oxide (Cu2O), cadmium sulfide (CdS), and copper/zinc/tungsten (Cu/Zn/W) sulfide are widely employed as electrodes.41–48 However, certain stability and low-efficiency challenges persist, leading to their poor photoelectrochemical (PEC) activity for hydrogen production.49,50 Thus, innovative strategies are needed to address these issues and increase hydrogen generation methods' safety, economy, and efficiency.
Lithium-ion batteries, hydrogen storage, and production electrode material capabilities are found in several carbon-based substrates including porous carbons (PCs), carbon nanotubes (CNTs), MXenes and nanofibres (NFs) with different geometries.51–56 Among all the recently discovered and synthesized nanostructured carbonaceous species, graphene stands out as the most extraordinary material. Their remarkable properties including their enormous surface area, mechanical toughness, and electrical conductivity have revolutionized the study of materials. Consequently, compared to other carbon compounds (PCs, CNTs, MXenes, and NFs), graphene has been used advantageously as prospective substitute electrode materials in many applications for improving particular technical domains, particularly those related to energy generation and storage. Because of their large theoretical SSA (2630 m2 g−1) and ease of functionalization by heteroatoms such as N atoms, graphene offers more electrochemical reaction active sites than other carbon compounds (PCs, CNTs, MXenes, and NFs).
Graphenes were isolated in 2004 and used in energy storage due to their high thermal conductivity, high electrical conductivity, high elasticity and flexibility, and high hardness. Particularly in lithium-ion batteries, they serve as electrodes, conductive materials, and controllers for volume expansion in the electrode material. However, Li ions may ideally be accommodated on both sides of graphene, and their theoretical capacity is at least double that of other carbon-based materials (PCs, CNTs, MXenes, and NFs).57,58 The graphene is a promising substitute for improving H2 generation efficiency. Because of graphene's particular two-dimensional conjugated framework and electrical characteristics, it can be used to increase the photocatalytic efficiency.59 Moreover, the graphene, composed of carbon atoms arranged in a honeycomb lattice, holds the potential for hydrogen storage, giving rise to new hopes for creating an effective solid-state hydrogen storage device.60 In recent years, several papers have been published on graphene composites with 2D materials in batteries and hydrogen storage and production applications. This review aims to assess graphene composites with 2D materials for lithium batteries, hydrogen storage, and production applications.
2. Lithium rechargeable batteries
2.1 Graphene-based 2D materials for lithium-ion batteries (LIBs)
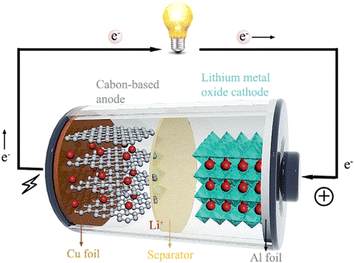 | ||
| Fig. 1 Schematic of the structure and working mechanism of LIBs.66 | ||
“Anode” refers to the negative electrode that releases an electron into the external circuit. An anode collects lithium ions and converts them into active materials. Some characteristics that the anode materials should have include a large energy capacity, excellent charge/ion storage, ion release, long cycle rate, ease of manufacture, safety in use, and cost-effectiveness.67 Since the early 1990s, Sony has been commercially launching LIBs, with graphite was the anode material. Currently, graphite, the most popular anode material in LIBs, has a relatively low theoretical capacity of about 372 mA h g−1 and safety issues related to the insertion of Li+ into the anode structure.68 A graphite anode's limited capacity will prevent it from meeting the demands of the rapidly expanding industries.69 To improve LIB performance and energy density, research is required to fabricate new anode materials with a capacity greater than that of graphite.70
A 2D nanomaterial called graphene is made up of hexagon-shaped carbon atoms with sp2 hybrid orbitals. Graphene has garnered considerable interest across numerous applications since its isolation in 2004.71–77 Graphene, a single layer of graphite, is attracting a lot of attention as a potential anode material due to its enormous specific surface area (2620 m2 g),78 mechanical strength, excellent thermal conductivity (5000 W m−1 K−1),79 electrical conductivity, rapid charge–discharge cycles and a more remarkable ability to hold Li ions and higher capacity (three times that of graphite).80–83 Because of its high energy storage capacity of 669 mA h g−1, good cycling stability, longer interlayer distance of 0.615 nm with mild van der Waals forces, and simple intercalation and deintercalation procedure, graphene is highly suitable for LIB anode applications.84 Numerous scientists have noted that Li+ ion attachments may be facilitated by extra paths provided by graphene sheet defects. The enormous challenge of fabricating LIBs that can provide high power and energy density outputs during charging and discharging may have an answer in this feature. However, porous graphene's high ionic conductivity, surface characteristics, and electric conductivity have attracted considerable attention in battery research.85 Ordered mesoporous graphene nanosheets were synthesized by Fang et al. using a regulated low-concentration mono micelle close-packing assembly technique.86 This anode can hold 833 mA h g−1 at different current densities after multiple cycles. It can give a high reversible capacity of 1040 mA h g−1 at 100 mA g−1. About 255 mA h g−1 of reversible capacity is kept even at a high current density of 5 A g−1. For instance, magnesium oxide and methane were used as starting ingredients in the chemical vapor deposition process to synthesize a hierarchical porous graphene nanomaterial. The Brunauer–Emmett–Teller (BET) surface area and pore size were changed, and this affected the electrochemical properties of LIBs.87 In particular, the heteroatom-doped graphene has demonstrated advances in the electrochemical performance of LIBs. Using the differential electroneutrality of doped nitrogen (N), phosphorus (P), sulfur (S), and boron (B) heteroatoms, a method was devised to increase the relative specific capacity (900 mA h g−1) of LIBs.81,88–92 However, extensive research has revealed that the ionic-steric effect and decreased stability of graphene make it impractical to use it directly as an anode active material in LIBs.93,94 Furthermore, with several advantageous effects, the synthesis of graphene-based composites has been one of the most important avenues for LIB anode active materials. The graphene, particularly with a 2D material, has emerged as a potential anode active material for LIB applications.
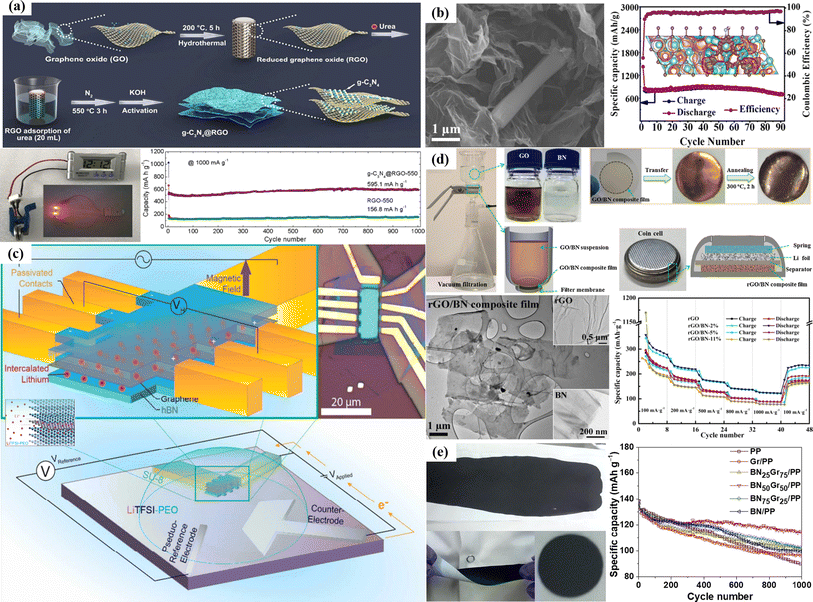 | ||
| Fig. 2 (a) Schematic of the synthesis procedure of g-C3N4@RGO. (b) SEM image and DFT calculations of the C3N4/graphene heterostructure. (c) Hall bar device with the h-BN/graphene/h-BN heterostructure. (d) Vacuum filtration process for preparation, transmission electron microscopy (TEM) image and electrochemical study of the GO/BN composite film. (e) Bilayer BNxGry/PP separator and electrochemical characterization of LIBs.95–99 | ||
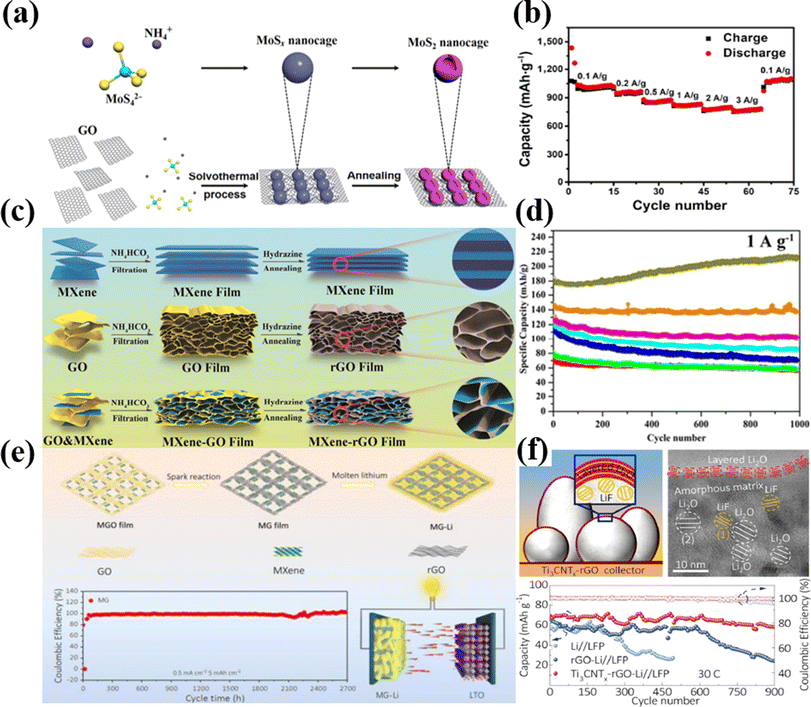 | ||
| Fig. 3 (a) Synthesis process and (b) rate performance of the MoS2/RGO nanocomposite and MoS2 electrode. (c) Fabrication and (d) electrochemical performance of the MXene, rGO and MXene-rGO films at 1 A g−1. (e) Fabrication and coulombic efficiencies of the MG electrode at a current density of 0.5 mA cm−2 with a Li deposition amount of 5 mA h cm−2 for 2700 h. (f) Lithiophilic functional groups result in the formation of a uniform SEI layer (decomposition of LiN(CF3SO2)2); cryo-TEM images of equally dispersed LiF and ordered layered Li2O and superior cycling lifespan over 900 cycles with a capacity retention of 77.7% at 30C of Ti3CNTx/G/Li electrode.101–104 | ||
Cobalt oxide (Co3O4) has been studied as an anode material since it was first used as the anode electrode for LIBs because of its advantages, which include a large theoretical specific capacity of approximately 890 mA h g−1, wide availability, ease of manufacture, and stable chemical properties. Liang Tan et al. used a simple hydrothermal method to make a Co3O4/graphene composite, which was subsequently tested at very low temperatures (Fig. 4a).118 The Co3O4/G anode exhibits a noticeably higher capacity of 605 mA h g−1 at 0.5 A g−1 (Fig. 4b) than that of other anodes at temperatures below zero thanks to the material's creative nanostructure, high conductivity, and extremely good lithiation and delithiation potentials. The inexpensive metal oxide Fe2O3 can displace graphite as the primary anode material for lithium-ion batteries. The redox conversion reaction from Fe2O3 to a combination of Li2O and metallic iron gives a theoretical capacity of up to 1006 mA h g−1. Yu Huang et al. used a simple and effective one-pot hydrothermal process to create a 2D nanostructured Fe2O3/N-rGO composite (Fig. 4c). When tested as LIB anodes, the 2D nanostructured Fe2O3/N-rGO composite demonstrated an exceptional high-rate capacity of 652 mA h g−1 at 15 A g−1 (Fig. 4c).119 A similar Fe2O3/multilayer graphene was synthesized by Junming Xu et al. by a chemical deposition method, which when used as the anode for LIBs exhibited a specific capacity of 1050 mA h g−1 at 0.1C even after 100 cycles.123 Due to their noteworthy characteristics, tin oxide (SnO2) nanoparticles have been thoroughly investigated for various energy-related applications, including LIBs. Naiqing Zhang and coworkers reported hierarchical SnO2 nanoclusters anchored on the graphene sponges (SnO2/GS) via a solvothermal approach with an average diameter of ∼110 nm, as shown in Fig. 4d.120 These hierarchical SnO2/GSs retained a higher reversible capacity of 600 mA h g−1 after over 600 cycles at 4000 mA g−1 (Fig. 4d). Based on a conversion reaction from nickel oxide (NiO) to metallic nickel and Li2O, the estimated theoretical capacity for NiO is 718 mA h g−1. The NiO also suffers from poor rate performance and cycling stability similar to those of other conversion-based anode nanomaterials. NiO composite with graphene is used in the LIB applications to address these obstacles. Ju Fu et al. synthesized hierarchically porous NiO micro-flowers/graphene papers (fNiO/GP) by a facile hydrothermal method followed by annealing in air at 400 °C for 40 min (Fig. 4e).121 Even after 600 cycles at a current density of 1000 mA g−1, fNiO/GP demonstrated high reversible specific capacities of 359 mA h g−1 in lithium-storage applications (Fig. 4e). Similarly, graphene–(Ni–NiO)–C hybrid was hydrothermally treated at 180 °C for 24 h and then carbonized at 700 °C for three hours by Jian-Guo Zhao and colleagues.124 When it was used as the anode material for LIBs, the initial capacity was 711.6 mA h g−1, and after 300 cycles, it increased to 772.1 mA h g−1. Manganese dioxide (MnO2) is plentiful in the Earth's crust, which is a more environmentally friendly metal oxide than other oxides. As an anode for LIBs, MnO2 has a high theoretical capacity of 1232 mA h g−1, enabling a quick charge/discharge rate over a broad potential window. As anode materials for Li-ion batteries, Pedda Masthanaiah Ette et al. revealed that two distinct mesoporous MnO2 were produced from silica templates (MCM-41 and MCM-48) and integrated with graphenes (Fig. 4f).122 Both composites demonstrate improved rate performance, large discharge capacity, and strong cycling stability. When used as LIB electrodes, M8-MnO2-G exhibits a consistent discharge capacity of 1494 mA h g−1 at a rate of 5C (Fig. 4f). The synergistic effects of graphene-integrated mesoporous MnO2 are responsible for the composites' high specific capacities and long cycling performance. LiNi0.8Mn0.1Co0.1O2 as a cathode studied the full cell tests with an M8-MnO2-G composite material anode. The full cell shows a stable capacity up to 200 cycles at a current density of 0.1C.122
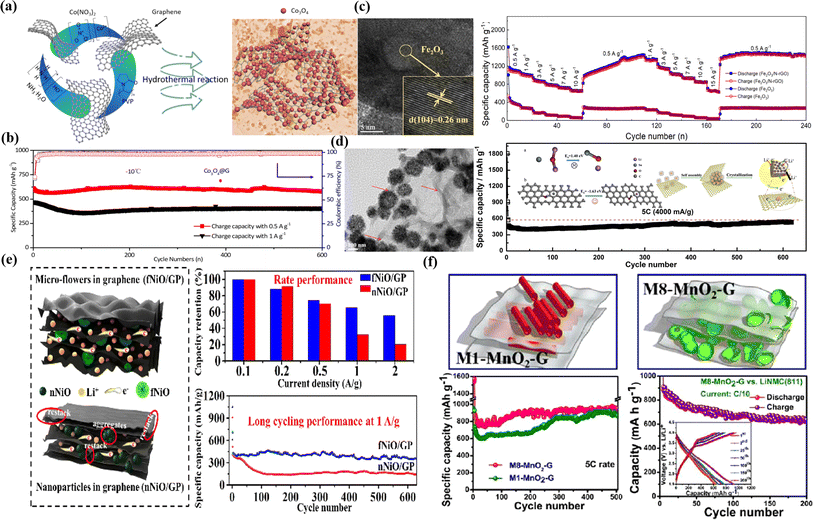 | ||
| Fig. 4 (a) Schematic representation of the preparation and (b) long-cycling performance of Co3O4@G at −10 °C and different current rates. (c) High-resolution transmission electron microscopic (HRTEM) image of the Fe2O3/N-rGO sample and rate capability of Fe2O3/N-rGO and pure Fe2O3 at different current densities. (d) TEM image and long cycling performance of the SnO2@graphene sponges (GS) at a current density of 5C. (e) NiO micro-flowers/graphene paper electrode rate capability at different current densities and long cycling at 1 A g−1. (f) Long cycling performance of the M1-MnO2-G and M8-MnO2-G composite anode material and full cell study of the M8-MnO2-G composite anode with the LiNi0.8Mn0.1Co0.1O2 cathode.118–122 | ||
2.2 Graphene-based 2D materials for lithium–sulfur batteries (LSBs)
Lithium–sulfur batteries are considered the most promising candidates among the battery technologies now used to act as the batteries of the future generation with superior electrochemical performance. The high specific capacity (∼1675 mA h g−1) and high energy density (∼2600 W h kg−1) of lithium–sulfur batteries (LSBs) have gained interest in recent years.125 The present bottlenecks of LSBs include the severe shuttle effect of soluble lithium polysulfides (LiPSs) and slow redox kinetics, which lead to capacity fading and poor rate performance.126 Due to the severe capacity fading (poor cycling stability) and low coulombic efficiency that these devices exhibit, the effective practical implementation of these devices has been put off. Numerous approaches have been proposed to address these problems, some of which involve using 2D-based materials to produce a better component architecture, as will be covered in the following discussion.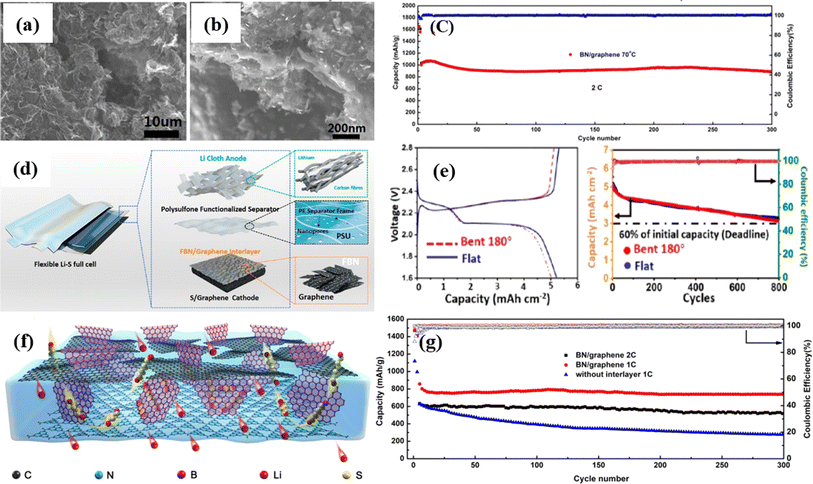 | ||
| Fig. 5 (a and b) SEM images of BN/graphene. (c) Long cycling performance of BN/graphene at 70 °C temperature. (d) Lithium cloth anodes, PSU-Celgard separators, and free-standing graphene/sulfur cathodes shielded by an FBN/G interlayer are used to build flexible Li−S complete cells. (e) Charge/discharge curves and cycling performance of the Li–S pouch cells in the flat state and bent state, respectively, of the FBN/graphene interlayer. (f) Scheme of the multi-functional ion-sieve constructed by three 2D materials (gC3N4, BN and graphene). (g) Coulombic efficiency and discharge capacity of the cell with the BN/graphene interlayer at rates of 1C and 2C.127–129 | ||
Consequently, the resulting flexible Li–S full-cells exhibit excellent electrochemical performance at a high areal capacity of 5.13 mA h cm−2 and a high volumetric and gravimetric energy density of 468 W h L−1 in the folded state, up to 800 cycles (Fig. 5d and e). In contrast to separators, an efficient interlayer must have a high conductivity to maximize the use of active sulfur and boost rate capability. With a polypropylene membrane covered with g-C3N4/h-BN/G, the LSB was built, and after 500 cycles at 1C, it released a discharge capacity of around 600 mA h g−1. The capacity attenuation was less than 0.01% per cycle with a 6 mg cm−2 area S-loading (Fig. 5f and g).
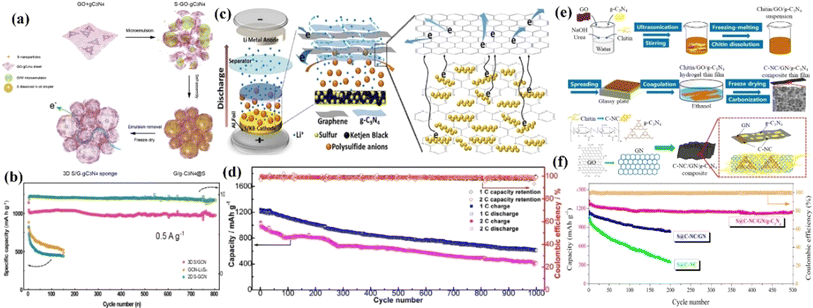 | ||
| Fig. 6 (a) Schematic of the procedure for preparing S/GCN hybrid sponge. (b) Long-term cycling performance of S/GCN, GCN-Li2Sn, and S-GCN cathodes at a low current density of 0.5 A g−1 (0.3C). (c) Schematic of cell configuration with a laminated structure of the g-C3N4/GS cathode interlayer. (d) Cycling performance and capacity retention of the S/KB@C3N4/GS cathode at 1 and 2C for 1000 cycles. (e) Free-standing 3D network cathode (N-doped carbon/graphene/g-C3N4) preparation. (f) Long cycling performance of the S@C-NC, S@C-NC/GN and S@C-NC/GN/g-C3N4 cathodes at 0.5C rate.130–132 | ||
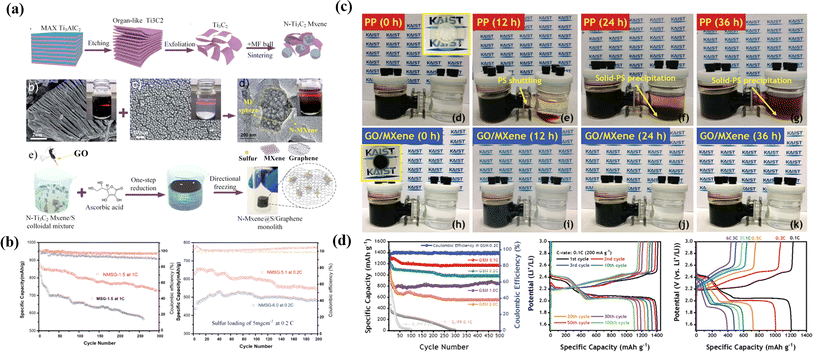 | ||
| Fig. 7 (a) Schematic of the synthesis of N–Ti3C2Tx MXene nanosheets; corresponding SEM and TEM images of Ti3C2Tx flakes, the melamine–formaldehyde (MF) nanosphere and the Ti3C2Tx-wrapped MF nanosphere and schematic of the one-step synthesis process of the freestanding NMXene@S/G composite. (b) Long-term cycling performances of the Li–S cell with NMSG at 1C for 300 cycles. (c) Optical images of the visualized lithium polysulfide diffusion test with a commercially available PP separator and a GO/MXene bilayer composite at 0, 12, 24, and 36 h, consisting of Li2S6 (Left), GM (Middle), and DOL/TEGDME (Right) in a H-type electrolytic cell and electrochemical measurements; long-term cycling test at 0.1, 0.2, 1.0, and 2.0C of GSM and S8/PP cell. (d) Charge–discharge profile at a C rate of 0.1C (200 mA g−1) from the 1st to the 100th cycle; charge–discharge profiles at different C rates (0.1 to 6C).137,138 | ||
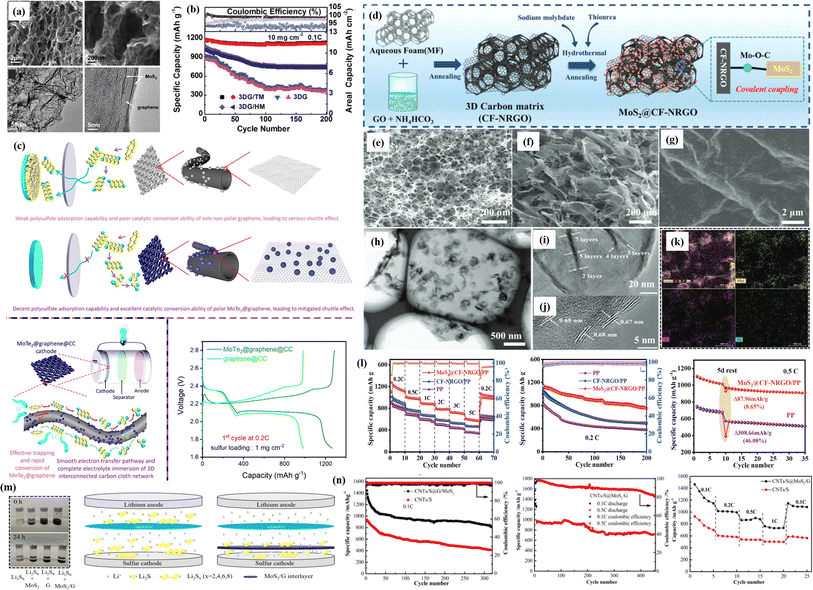 | ||
| Fig. 8 (a) SEM images of 1T MoS2 on the graphene aerogel ((three-dimensional graphene) 3DG/1T MoS2 (TM)). (b) Cycling stability of electrocatalytically active 3DG, 3DG/1H MoS2 (HM), and 3DG/TM as working electrodes vs. Li/Li+ with a catholyte consisting of 1 M Li2S6 at 0.1C rate.139 (c) Polysulfide conversion ability of polar MoTe2@graphene composite as a cathode material for LSBs.140 (d) Schematic of the preparation stages of MoS2@CF-NRGO. SEM images of (e) carbonized MF and (f and g) MoS2@CF-NRGO. (h) TEM and (i and j) HR-TEM images of MoS2@CF-NRGO. (k) EDS mapping images of MoS2@CF-NRGO and the corresponding images of Mo, S, and N. (l) Rate capacity and cycling capabilities at 0.2C of a cell with the MoS2@CF-NRGO/PP separator, CF-NRGO/PP, and PP separators and cycling performance at 0.5C before and after rest of the MoS2@CF-NRGO/PP and PP separators,141 (m) digital photographs of MoS2 and MoS2/G composites in a Li2S6 solution for 0 and 24 h; schematic of the lithium–sulfur battery without and with the MoS2/G interlayer. (n) Cycling performance and coulombic efficiency of the carbon nanotube (CNT)/S@MoS2/G and CNT/S cathodes at 0.1C, cycling performance and coulombic efficiency of the CNT/S@ MoS2/G at 0.5C and rate capability.142 | ||
Additionally, after 200 cycles, the MoS2@CF-NRGO modified separator exhibits a 99.6% average coulombic efficiency, demonstrating an outstanding capacity retention. After ten charge/discharge cycles at 0.5C, Li–S batteries were allowed to rest for five days to examine their self-discharge behavior. Following a five-day rest period, the MoS2@CF-NRGO configuration's discharge capacity drops from 1015.8 to 927.9 mA h g−1, with an average self-discharge value of only 8.6% (Fig. 8l). The molybdenum disulfide/graphene (MoS2/G) interlayer was constructed by Mingang Zhang and his team using a straightforward coating technique, and it was utilized in an LSB separator.142 The color change of the mixed solution can be used to directly compare and evaluate the polysulfide adsorption capacity of composites (Fig. 8m). At 0.1C, the CNT/S@MoS2/G cathode has an initial discharge capacity of 1537.2 mA h g−1. At 0.5C, the CNT/S@MoS2/G cathode has a discharge capacity of 984.9 mA h g−1 (Fig. 8n). With an ultralow capacity decay of 0.061% every cycle, the capacity drops to 713.8 mA h g−1 after 450 cycles. The CNT/S@MoS2/G cathode delivered excellent rate capability. The MoS2/G interlayer, which sits between the cathode and the separator, is thought to have more excellent cycling stability because it confines polysulfide to the cathode and lessens the shuttle effect.
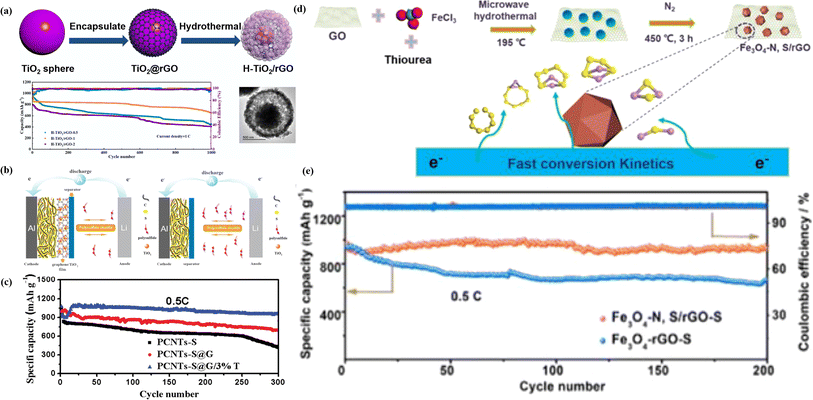 | ||
| Fig. 9 (a) Schematic of the formation and long-term cycling performances at a current density of 1C of H–TiO2/rGO-1.143 (b) Schematic of the electrode configuration for the Li–S battery with a graphene/TiO2 coating film and conventional Li–S battery. (c) Cycling stability of PCNTs–S, PCNTs–S@G, and PCNTs–S@G/3% TiO2 cathodes at 0.5C, and a current density of 0.86 mA cm−2.144 (d) Schematic of the synthesis process of the Fe3O4–N, S/rGO composite. (e) Cycling performance of the Fe3O4–N, S/rGO–S and Fe3O4-rGO–S electrode at 0.5C after 200 cycles.145 | ||
2.3 Graphene-based 2D materials for lithium–O2 and lithium–CO2 batteries
Rechargeable Li–O2 and Li–CO2 batteries, which offer a higher theoretical energy density than that of Li-ion batteries, have recently attracted a lot of attention.146,147 Li–O2 batteries have been developed to fulfil the increased demand for electric energy in today's world. The formation of a lithium peroxide (Li2O2) product, which has a substantially higher energy storage capacity than that of other energy storage systems, is responsible for the high energy density of Li–O2 batteries.148 The high band gap of solid Li2O2, which is probably covering the catalytic sites, makes charge transfer necessary for the breakdown of Li2O2 during the charge process difficult.149 This results in a slow charging process that raises the potentials needed for Li2O2 breakdown, which lowers the battery's energy efficiency and increases the danger of electrolyte degradation.150,151 Furthermore, the size and shape of the Li2O2 product can affect the charge potential. Developing Li–O2 batteries requires improving the materials used in each component of the battery in order to address knotting problems. Two-dimensional materials have recently attracted attention due to their potential to improve the Li–O2 battery performance. This is because of their distinct designs, which have the potential to effectively address a number of issues related to the electrode materials (cathode and anode), solid-state electrolyte, and separator in Li–O2 batteries.152–155 A unique type of MnO2@rGO nanocomposite material was described by Lihua Zhu et al.; it is very effective for Li–O2 batteries and consists of extremely few nano-sized MnO2 grains on the surface of rGO (Fig. 10a). In Li–O2 batteries, MnO2@rGO composites were used as cathode catalysts with a cut-off capacity of 1000 mA h g−1 and a current density of 200 mA g−1 (Fig. 10b).156 Over 60 cycles, batteries based on MnO2@rGO demonstrated a consistent discharge capacity. Strong synergistic effects between the rGO framework and the nano-sized MnO2 particles on its surface are responsible for the exceptional performance. Functionalized graphenes with an optimal bimodal porous structure were produced by Jie Xiao et al. for Li–O2 battery operation (Fig. 10c). A novel air electrode that uses an uncommon hierarchical configuration of functionalized graphene sheets produces the highest capacity of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mA h g−1 at around 2.7 V.85 Yuejiao Li and colleagues described the facet engineering of an ultrathin, two-dimensional Mn3O4 nanosheet on graphene (Mn3O4 NS/G) with the dominating (101) crystal planes. The goal of this catalyst is to generate durable, effective oxygen for high-performance Li–O2 batteries that exhibit ultrahigh capacity and long-term stability, offering a lower charge overpotential (Fig. 10d).157
000 mA h g−1 at around 2.7 V.85 Yuejiao Li and colleagues described the facet engineering of an ultrathin, two-dimensional Mn3O4 nanosheet on graphene (Mn3O4 NS/G) with the dominating (101) crystal planes. The goal of this catalyst is to generate durable, effective oxygen for high-performance Li–O2 batteries that exhibit ultrahigh capacity and long-term stability, offering a lower charge overpotential (Fig. 10d).157
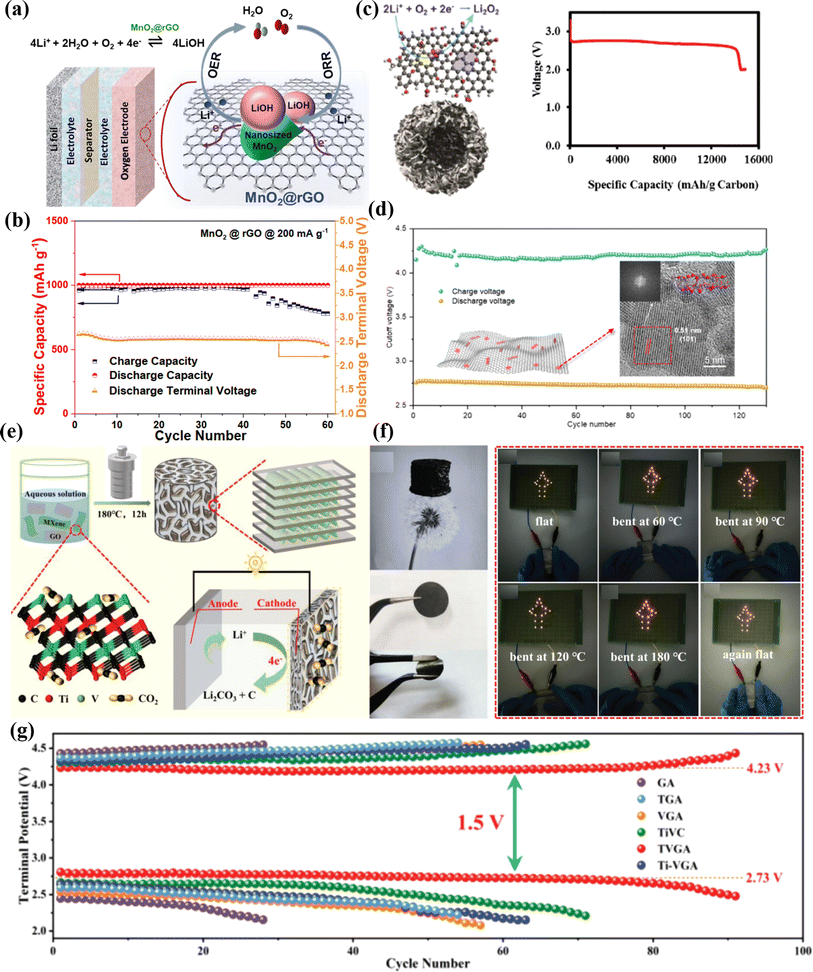 | ||
| Fig. 10 (a) Schematic of the structure catalyzed by MnO2@rGO for the Li–O2 battery. (b) Discharge/charge capacity of MnO2@rGO for the Li–O2 battery at a current density of 200 mA g−1. (c) An optimal bimodal porous structure on a functionalized graphene sheet (FGS) and the discharge curve of a Li–O2 cell using the FGS. (d) Two-dimensional Mn3O4 nanosheets on graphene with charge/discharge voltages. (e) Li–CO2 battery's schematic, optical picture and flexible optical picture of the TiVC/rGO aerogel (TVGA) cathode sheet. (f) LEDs with the flexible Li–CO2 battery. (g) Variation in the terminal polarization of Li–CO2 batteries with different cathodes at a current density of 200 mA h g−1.85,156–158 | ||
Similar to Li–O2 batteries, the Li–CO2 battery is another advanced technology because of its high theoretical energy density of 1876 W h kg−1. Li–CO2 batteries offer a novel method for capturing CO2 and advancing the development of electrochemical energy storage systems of the next generation by using CO2 as the active material at the cathode. Rechargeable Li–CO2 batteries store energy by breaking down the discharge products when exposed to external electricity during the charging process. During discharge, this process produces electricity via a redox reaction between the Li anode and the CO2 cathode, namely Li2CO3.159–161 Zhang Zhang et al. reported the first introduced graphene as a cathode material to improve the performance of Li–CO2 batteries.162 Wei Yu et al. synthesized N, O-diatomic graphene carbon aerogels by a straightforward, one-step thermal approach and used it as a cathode Li–CO2 battery. The resulting Li–CO2 batteries demonstrated an impressive cycling stability of more than 1500 h at 20 μA cm−2 and a significantly improved initial energy efficiency of about 78.46%.160 MXene-based materials have been used as the cathode in Li–CO2 batteries because of their superior interlayer ion transport channels, strong electrical conductivity, and chemical composition's tunability. Wentian Zhao prepared porous TVGA via a hydrothermal reaction and used it as a cathode in Li–CO2 batteries (Fig. 10e). The TVGA cathode has flexible nature, and hence, a flexible Li–CO2 battery was fabricated and used in different bending angles. Light-emitting diodes (LEDs) can be steadily lit by a Li–CO2 battery without any adjustments (Fig. 10f). Fig. 10g compares the cycling performance of Li–CO2 batteries with different cathodes. At a current density of 200 mA g−1, TVGA displays a comparatively low overpotential of 1.5 V.158 The battery has a 91 times stable cycling capacity at 200 mA g−1, with an over potential of 1.5 V for both charging and discharging. These exceptional electrochemical performances are due to the special synergistic interaction between Ti and V in TiVC, which improves the reversible formation and breakdown of the discharge product Li2CO3 by enhancing the interfacial chemical bonding ability, realizing the full exposure of active sites and promoting the catalytic interfacial structural reorganization. In the meantime, the aerogel's rich pore structure helps lower the mass and charge transfer resistance by promoting the diffusion of CO2 and electrolytes in addition to helping to enhance ion transport.
3. Lithium metal anode modification with graphenes
Since lithium metal has the lowest working potential (−3.04 vs. conventional hydrogen electrode) and the largest specific capacity (3860 mA h g−1), it has been considered the best option for lithium-ion battery anodes. The lithium metal anode, a crucial component of the next generation of high-energy density lithium metal batteries (LMBs), has gained significant interest from both industry and academics. However, because of its innate propensity to generate dendrites during cycling, the lower cycling stability and safety concerns have long hindered the adoption of lithium metal anodes. The excessive parasitic interactions between the Li metal dendritic growth and the liquid electrolyte result in the irreversible consumption of both the electrolyte and active lithium. Consequently, the rapid capacity decline and catastrophic cell failure of LMBs are explained by the production of Li dendrites. Several methods have been devised to enhance the lithium metal anode's cycling stability and controlling the Li dendrites.163–169 According to David Mitlin and colleagues, a significant degree of structural and chemical imperfection in graphene leads to unstable SEI formation. This is associated with the rapid decline of coulombic efficiency and the production of filament-like Li dendrites, as well as the consumption of the fluoroethylene carbonate (FEC) additive in the carbonate electrolyte.164 Pinxian Jiang et al. described the graphene hybrid architecture as a lithiophilic host for a high-performance Li metal anode. They utilized it in the LMB to synthesize a nanosheet/graphene heterostructure composite via a polymer-assisted sonochemical process. The Li metal anode that results from the graphene hybrid architectural scaffold shows stable cycling over 200 cycles at 0.5 mA cm−2 (2 mA h) and provides a high energy density of 402 W h kg−1.1704. Hydrogen production and storage
4.1 Sustainable green hydrogen production approaches
Hydrogen (H2) is recognized as an efficient energy carrier as it is an eco-friendly, non-toxic, renewable, and less costly energy source. Currently, H2 is generated from renewable and non-renewable sources, where only 95% of H2 is from non-renewable fossil fuels, and 5% is from renewable sources such as solar and wind.41 The H2 production approaches dependent on non-renewable resources are associated with CO2 emissions, are expensive and energy-intensive, and involve harsh operating conditions.171 Thus, researchers are exploring approaches to generate sustainable, less costly, and zero carbon-emitting H2 energy.172 In terms of renewable energy, the role of abundantly existing solar energy is significant, and two approaches for H2 production can be employed, namely, (1) photocatalytic and (2) photoelectrochemical. Photocatalytic H2 production by water splitting using semiconductor catalysts is a widely researched, less costly, eco-friendly, and effective approach for green H2 generation.173,174 Moreover, PEC water splitting for H2 production is another approach that uses solar-irradiated photoelectrodes and an external bias voltage to suppress charge recombination, leading to excellent overall performance.175| 2H+ + 2e− → H2 | (1) |
| 2H2O + 4h+ → O2 + 4H+ | (2) |
| 2H2O + 2O2 → 2H+ | (3) |
| Photocatalyst | Catalyst loading (mg) | Light source | Sacrificial agent | H2 production (μmol g−1 h−1) | Ref. |
|---|---|---|---|---|---|
| TiO2/rGO/MoS2 | 50 | 3 W 4 LEDs (λ 365 nm) | 10 vol% methyl alcohol | 880 | 176 |
| 2D CN@graphene@CN | 10 | 300 W Xe lamp with 420 nm cut-off filter | 10 vol% TEOA | 5580 | 177 |
| Few-layer graphene (FLG)/g-C3N4 | 0.15 g L−1 | 4 LEDs of 880 W m−2 each (420 nm) | 10% v/v ethylenediaminetetraacetic acid (EDTA) and 3 wt% Pt | 1274 | 178 |
| 0D-2D-2D CdSe QD/B-rGO/O-gC3N4 | 30 | Xe arc lamp (λ > 400 nm) | Triethanolamine (TEOA) | 1435 | 179 |
| Ni2P/rGO/g-C3N4 | 10 | 300 W Xe lamp (λ > 420 nm) | 10 vol% TEOA | 2921 | 180 |
| CdS@h-BN/rGO | 10 | Simulated solar light | 10 vol% triethanolamine (TEA) | 6465.33 | 181 |
| P.RGO/MoS2 | 2 | 400 W Xe lamp | 20% (v/v) TEOA and 1 mL eosin Y | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 230 |
182 |
| 2D/2D MoS2/g-C3N4-Au | 200 | 300 W Xe lamp 420 nm cutoff filter | 20 mL methanol | 1100 | 183 |
| Molybdenum sulfoselenide (MoSSe)@RGO | — | 400 W Xe lamp (λ ≥ 420 nm) | Eosin Y and TEOA | 5016 | 184 |
| GQD/TpPa-1-COF | 20 | 300 W Xe lamp | 0.1 mol per L ascorbic acid | 487 | 185 |
| 2D/2D Cu-tetrahydroxyquinone MOF (CuTHQ)/N-doped graphene (NG) | 20 | 150 W Xe lamp | Methanol (MeOH) | 164 | 186 |
| Co-BDC MOF/GO | — | 150 W halogen lamp | 0.2 M TEOA and EY photosensitizer | 14.5 | 187 |
| 2D/2D PRGO/CdS-diethylenetriamine (DETA) | 50 | 0.35 M Na2S, 0.25 M Na2SO3 and Pt cocatalyst | 300 W Xe lamp with 400 nm cut-off filter | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
188 |
| Cu2O/RGO/BiVO4 | 50 | 30 mL tetracycline | 300 W Xe arc lamp (λ > 420 nm) | 5.95 | 189 |
| rGO-coupled C3N4/C3N5 | 50 | 10 vol% TEOA | 300 W Xe lamp (λ ≥ 400 nm) | 6380 | 190 |
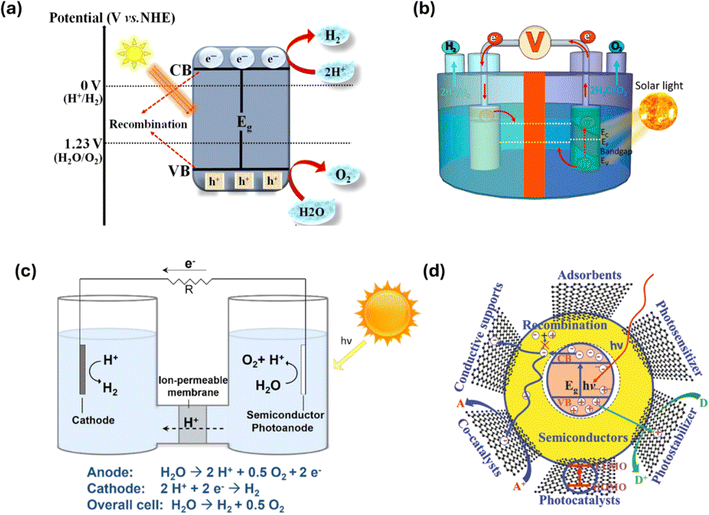 | ||
| Fig. 11 (a) Illustrative overview of the general photocatalytic H2 generation mechanism. (b) Representation of photoanodes and cathode semiconductors for PEC H2 generation. (c) Mechanism of PEC water splitting for H2 generation. (d) Characteristics of graphene for photocatalytic and PEC H2 production.197–199 | ||
The PEC H2 production utilizes a PEC cell comprising two or three electrodes acting as semiconductors for photoelectrodes submerged in an aqueous electrolyte, with the electrodes separated by a membrane.195 Out of the electrodes used, one or two electrodes should have the potential to be activated under irradiation. Normally, the PEC comprises three electrodes for water splitting: a photoanode and a cathode act as active electrodes, while Ag/AgCl acts as a reference electrode. As shown in Fig. 11b, n-type semiconductors have excess electrons and thus are used as photoanodes. Conversely, p-type semiconductors function as photocathodes due to their excess electron–hole pairs. Electrons are excited and move from the VB to the CB when the photoanode is exposed to light with an energy higher than its energy bandgap, followed by the movement of electrons toward the cathode to carry out a reduction reaction, while the holes left behind are involved in the process of oxidation.200,201 With the need for an overpotential for each half-reaction, electrode resistance, loss from charge carriers' recombination, and potential decline at contacts, the energy required for PEC water splitting in practical systems can approach 2 V. To compensate for these losses and supply the energy needed for water splitting, an external bias is frequently used.202Fig. 11c illustrates the steps involved in the PEC water splitting mechanism for H2 generation.
Earlier, various semiconductor materials such as TiO2,207 BiVO4,208 WO3,209 perovskites,210 g-C3N4,211 LDHs,212 ZrO2,213 and CdS214 as photocatalysts have been researched for photocatalytic H2 generation; however, they show poor efficiency towards photocatalytic H2 generation mainly due to lower stability, wide band gap, and charge carrier recombination, whereas the photocatalysts having a narrow band gap suffer from a lower efficiency based on their surface area.215 The utilization of metal-based photocatalysts is also explored extensively; however, metals are toxic and at the same time expensive.216 In this regard, 2D materials as semiconductors for photocatalysis have attracted substantial attention as they offer excellent optical characteristics, good mechanical features, large surface area and less distance for the movement of electron–hole pairs to the solid–water interface.217 Particularly, the heterojunction formation of 2D graphenes having a honeycomb structure with other semiconductor photocatalysts is considered to improve the overall photocatalytic H2 production efficiency.218 This is associated with the existence of an exceptional sp2 hybrid C framework, making it highly conductive for thermal energy, i.e., about 500 W m−1 K−1 and depicts an exceptional charge migration under ambient temperature condition, i.e., about 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1.219 Besides, it shows a zero band gap and an enhanced surface area of about 2600 m2 g−1. Graphene-based photocatalysts depict improved transfer and separation of electron–hole pairs.220 Numerous published works used rGO as one of the standard semiconductor materials for improving the performance. RGO enhances the photocatalytic activity of these composites by facilitating the separation of electron–hole pairs across the catalyst/RGO contact. The photocatalytic H2 production of the nanocomposites is largely dependent on the type of interface and defects present in the produced rGO.221 rGO is unique as it possesses a variety of qualities that promote photocatalytic H2 production efficiency. These properties include increased light utilization, charge carrier separation, intrinsic catalytic activity, and stability under operating conditions. While other photocatalysts could need co-catalysts or extra modifications to operate on par, their appropriateness varies depending on the demands of the given application and optimization techniques.222 The efficient photocatalytic H2 generation by graphene-based photocatalyst materials is due to the following mechanisms (Fig. 11d): (1) reduction of charge carrier recombination, (2) presence of catalytic active sites for reactions, (3) improved adsorption, (4) tunable band gap, and (5) potential to act as photosensitizers and cocatalysts.59 Graphene derivatives including graphene oxide (GO) and reduced graphene oxide (rGO) contain epoxy, hydroxyl, and carboxyl surface groups having O2, acting as catalytic reaction sites and also as supports for the uniform dispersion of metallic ions for nucleation and growth.223 The dark colors of both GO and rGO lead to better absorption of light, expanding the absorption from the UV to the visible region.224
000 cm2 V−1 s−1.219 Besides, it shows a zero band gap and an enhanced surface area of about 2600 m2 g−1. Graphene-based photocatalysts depict improved transfer and separation of electron–hole pairs.220 Numerous published works used rGO as one of the standard semiconductor materials for improving the performance. RGO enhances the photocatalytic activity of these composites by facilitating the separation of electron–hole pairs across the catalyst/RGO contact. The photocatalytic H2 production of the nanocomposites is largely dependent on the type of interface and defects present in the produced rGO.221 rGO is unique as it possesses a variety of qualities that promote photocatalytic H2 production efficiency. These properties include increased light utilization, charge carrier separation, intrinsic catalytic activity, and stability under operating conditions. While other photocatalysts could need co-catalysts or extra modifications to operate on par, their appropriateness varies depending on the demands of the given application and optimization techniques.222 The efficient photocatalytic H2 generation by graphene-based photocatalyst materials is due to the following mechanisms (Fig. 11d): (1) reduction of charge carrier recombination, (2) presence of catalytic active sites for reactions, (3) improved adsorption, (4) tunable band gap, and (5) potential to act as photosensitizers and cocatalysts.59 Graphene derivatives including graphene oxide (GO) and reduced graphene oxide (rGO) contain epoxy, hydroxyl, and carboxyl surface groups having O2, acting as catalytic reaction sites and also as supports for the uniform dispersion of metallic ions for nucleation and growth.223 The dark colors of both GO and rGO lead to better absorption of light, expanding the absorption from the UV to the visible region.224
In the case of PEC H2 production, photoelectrodes must possess the following features: (1) efficient absorption in the visible region, (2) appropriate band gap and positions for band edge to carry out redox reactions, and (3) stable nature in solutions of different pH values.49 PEC H2 production has been extensively studied for photocatalytic semiconductors such as TiO2, MoS2, WO3, ZnO, α-Fe2O3, BiVO4, Cu2O, CdS, and Cu/Zn/W sulfide; however, certain challenges persist, leading to their poor PEC activity. For instance, TiO2,225 WO3,226 and ZnO227 constitute wide band gaps and charge recombination, whereas α-Fe2O3 suffers from the demerits of short lifetime of charges, electron–hole pair recombination and less conductivity,228 BiVO4 also shows charge recombination229 while Cu-based electrodes face the issue of photo corrosion, leading to less efficient PEC performance.230 Lately, C-based materials such as graphene derivatives and rGO were studied to act as supports for developing different photoanodes due to their high surface area, excellent conductivity, charge movement, and stability.231 Along with other semiconductor materials, they are known to show improved overall migration of charges, improve the photocurrent response and inhibit the process of photo corrosion.232
Moreover, regarding heterojunction formation, 2D/2D heterostructures are known to depict excellent interface, leading to an improved surface area and less resistant electron transfer. Compared to the conventional interface, the 2D/2D interface provides a faster transport of charges which reduced their recombination.233 Moreover, 2D/2D heterojunctions help in tuning the band edges for efficient redox reactions.234 The heterojunction also leads to a stable heterostructure, less agglomeration and less photo-corrosion phenomena.235
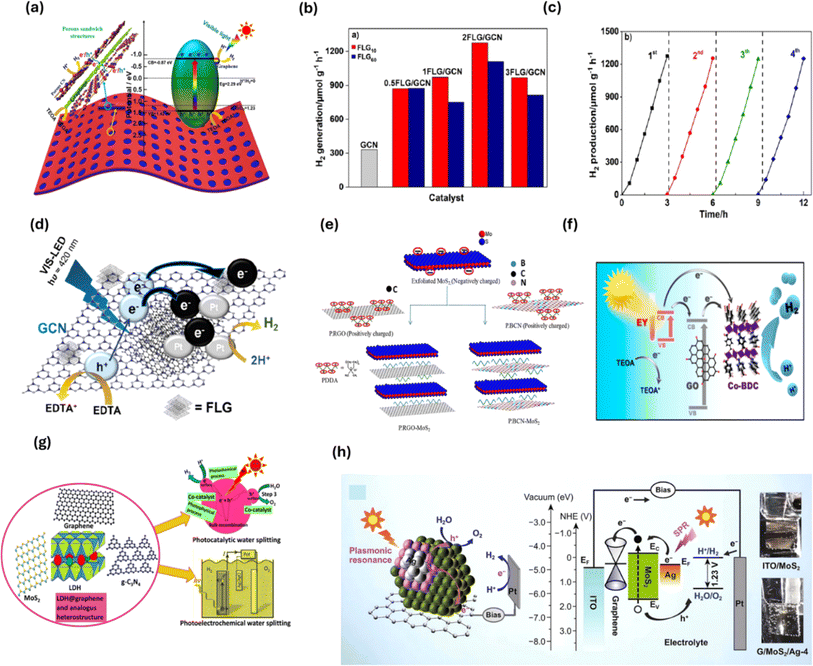 | ||
| Fig. 12 (a) Illustrative overview of the charge transfer mechanism for the photocatalytic H2 production process using 2D CN@graphene@CN loaded with Pt. (b) Rate of photocatalytic H2 production and (c) stability over 4 consecutive cycles using FLG/GCN nanocomposites. (d) Charge transfer mechanism in the FLG/GCN nanocomposite. (e) Overview of the solution-phase electrostatic assembly to synthesize P.rGO and P.BCN. (f) Photocatalytic H2 production mechanism in EY dye-sensitized Co-BDC MOF/GO. (g) Two-dimensional LDH@graphene and g-C3N4 heterojunctions for PEC H2 generation. (h) Overview of the suitable Fermi level alignment and PEC H2 generation in Ag-deposited MoS2/GO.177,178,182,187,237,238 | ||
In conclusion, GO-based photocatalytic nanocomposites showed efficient and economic H2 generation based on the improved surface area, migration of charges, separation of charges and provision of active sites. The incorporation of GO as a cocatalyst was explored to have the potential of replacing noble metals for photocatalytic H2 generation. The 2D/2D GO/g-C3N4 heterojunction incorporated into other semiconductors was extensively studied for H2 generation based on its simple fabrication, non-toxicity, efficient charge separation and transfer and active sites.
In conclusion, the literature to date has shown that adding graphenes at certain interfaces can improve the PEC efficiency of photoelectrodes towards PEC H2 generation. The high electric conductivity of graphenes, which enhances the charge carrier movement across the heterojunction components, is responsible for the significant increases in the efficiency; however, in literature for PEC H2 production using 2D/2D graphene and its derivatives.
4.2 Hydrogen storage using graphene-based nanomaterials
Graphene-based 2D nanomaterials are found to be promising candidates for practical solid-state hydrogen storage applications due to their exceptionally large surface area (2600 m2 g−1), porous structure, and high chemical and thermal stabilities.243 Pristine graphenes generally have low hydrogen storage capacities while functionalizing graphene nanosheets drastically improve their performance.244,245 The functionalization includes doping with hetero atoms such as boron (B) and nitrogen (N), decorating with metal atoms, and defect engineering in the nanosheets (Table 2). Moreover, the graphene nanosheets allow surface curvature tuning, which would help develop a reversible hydrogen storage system with enhanced kinetics.60| Material | Pressure (bar) | Temperature (K) | Hydrogen storage (wt%) | Reference |
|---|---|---|---|---|
| Graphene nanosheets | 100 | 298 | 0.9–1.5 | 244 and 245 |
| 10 | 77 | 1.2 | ||
| Hierarchical graphene | 1 | 77 | 4.0 | 246 |
| Graphene oxide | 50 | 298 | 1.4 | 247 |
| Functionalized graphene with ClSO3H | 2 | 77 | 1.6 | 248 |
| Functionalized graphene with oleum | 2 | 77 | 1.4 | |
| Functionalized graphene with Pt nanoparticles | 57 | 303 | 0.15 | 249 |
| Functionalized graphene with Pd nanoparticles | 40 | 298 | 0.81 | 250 |
| N-doped graphene | 90 | 298 | 1.5 | 251 |
| Li-decorated N-doped penta graphene | — | — | 7.88 | 252 |
| Pd3Co-decorated nitrogen/boron-doped graphene | 30 | 298 | 4.6 | 253 |
| Phosphorous doped graphene | 100 | — | 2.4 | 251 |
| Li-decorated holey graphyne | — | — | 12.8 (simulation result) | 254 |
| Lithium decorated ψ-graphene | — | — | 15.15 (simulation result) | 255 |
| Ti doped ψ-graphene | — | — | 13.14 (simulation result) | 256 |
| Zr decorated ψ-graphene | — | — | 11.3 (simulation result) | 257 |
| Zr-decorated γ-graphyne monolayer | — | — | 7.95 (simulation result) | 258 |
| Nitrogenated holey graphene (C2N) with titanium clusters | — | — | 6.8 | 259 |
| 3D-graphene | 1 | 77 | 1.4 | 260 |
| Transition metal-decorated boron doped twin-graphene | 1 | 77 | 4.95 | 261 |
| Lithium doped fullerene pillared graphene nanocomposites (Li-FPGNs) | — | — | 9.1 | 262 |
| Ni/Pd co-modified graphene | 4 | 298 | 2.65 | 263 |
| Gold decorated graphene nanosheet | — | — | 4.65 | 264 |
| Diatom frustule-graphene | 20 | 298 | 4.83 | 265 |
| Fe–Ti decorated defective graphene | — | — | 5.1 | 266 |
| Ca on graphene | — | — | 5–6 | |
| Mg2.3Y0.1Ni alloy with graphene | — | — | 3.25 | 267 |
The adsorption of hydrogen molecules onto graphene sheets can occur either by physisorption (van der Waals interactions) or by chemisorption (chemical bonds with carbon atoms). The theoretical energy requirement for the physisorption of hydrogen molecules (H2) on single-layer graphenes was evaluated to be in the range of 0.01–0.06 eV (Fig. 13). Even though the energy requirement for hydrogen adsorption onto the graphene sheet is quite low, it still requires low temperatures and high pressures to achieve reasonable hydrogen storage capacities. Chemisorption requires the dissociation of hydrogen molecules, and consequently, the energy requirement is much higher (1.5 eV) (Fig. 13) than that of physisorption. Hydrogen storage capacities of various graphene-based 2D nanomaterials are summarized in Table 2.
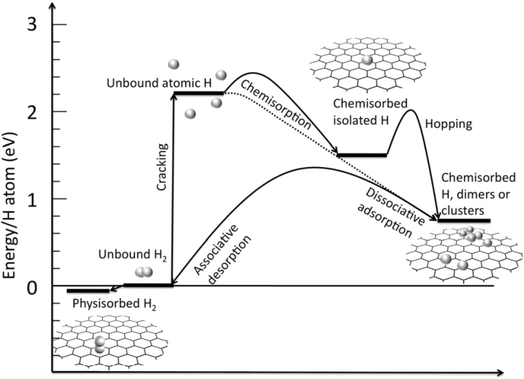 | ||
| Fig. 13 Energy level diagram of hydrogen adsorption–desorption on the graphene monolayer.60 Adapted with permission from RSC. | ||
A significant progress has been made in the field of hydrogen storage using graphene-based 2D materials. Among various graphene-based 2D nanomaterials, alkali, alkaline earth, and transition metal nanoparticle-decorated graphene doped with hetero atoms are found to be potential candidates for this purpose. In addition, the functionalized graphene nanosheets are distinctly advantageous than other carbon allotropes such as fullerene, nanotubes, and porous carbons, as shown in Fig. 14. Nevertheless, the adsorption and desorption properties of hydrogen molecules in 2D nanomaterials are still unclear. Therefore, hydrogen storage at ambient temperatures and pressures using functionalized graphene nanosheets even remains a challenge.
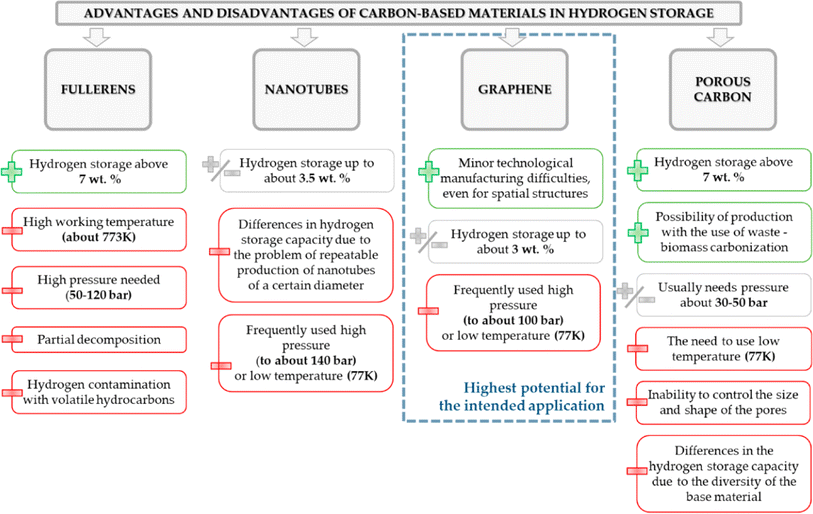 | ||
| Fig. 14 Summary of solid-state hydrogen storage using different carbon-based materials.244 Adapted with permission from MDPI. | ||
5. Summary and outlook
As society's energy needs increase, lithium batteries for energy storage require efficient electrode materials to store energy and the ability to be charged more quickly. Moreover, hydrogen, with the current rate of industrialization leading to greenhouse gas (GHG) emissions, is considered a potential energy carrier that can replace fossil fuels. However, the materials for hydrogen production and storage show challenges of inefficiency and cost-effectiveness. The development of 2D materials may offer a solution to these issues. Graphene is a two-dimensional substance that exhibits remarkable mechanical strength, exceptional conductivity, superb flexibility, high surface area and excellent chemical stability. With these properties, graphenes are desirable for energy storage, hydrogen production and electronic applications (Fig. 15). The synergistic effect of graphenes with 2D composite materials is appropriate for fabricating electrodes of lithium batteries because of their high interlayer adsorption energy and a large specific surface area. As graphenes and 2D composite materials have special features, they can be employed to create stable SEI layers, serve as anode hosts, alter the separator interface (LSB), and change the electrolyte (manage the polysulfides). Additionally, graphenes can enhance the electrical conductivity of two-dimensional materials and lessen volume fluctuations. Apart from this, in terms of H2 production, GO-based photocatalytic nanocomposites show efficient and economic H2 generation based on the improved surface area, efficient migration of charges, separation of charges, and provision of active sites. Moreover, in the case of PEC H2 generation, graphenes show high electric conductivity for efficient charge carrier movement.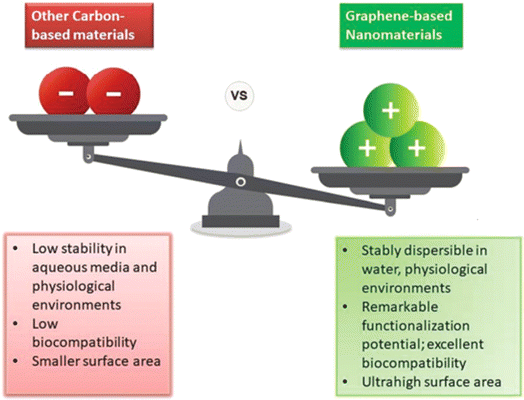 | ||
| Fig. 15 Advantages of the graphene nanomaterial over other carbon-based nanomaterials. Adapted with permission from RSC.268 | ||
Data availability
The data that support the findings of this study are available from the corresponding author.Conflicts of interest
The manuscript was written with contributions from all authors. All authors have approved the final version of the manuscript.References
- Z. Hu, Q. Liu, S.-L. Chou and S.-X. Dou, Two-Dimensional Material-Based Heterostructures for Rechargeable Batteries, Cell Rep. Phys. Sci., 2021, 2(1), 100286 CrossRef CAS.
- J. Wang and W. Azam, Natural Resource Scarcity, Fossil Fuel Energy Consumption, and Total Greenhouse Gas Emissions in Top Emitting Countries, Geosci. Front., 2024, 15(2), 101757 CrossRef CAS.
- I. H. Shah, M. A. Manzoor, W. Jinhui, X. Li, M. K. Hameed, A. Rehaman, P. Li, Y. Zhang, Q. Niu and L. Chang, Comprehensive Review: Effects of Climate Change and Greenhouse Gases Emission Relevance to Environmental Stress on Horticultural Crops and Management, J. Environ. Manage., 2024, 351, 119978 CrossRef CAS.
- M. Shaterabadi, S. Sadeghi and M. A. JirdehiThe Role of Green Hydrogen in Achieving Low and Net-Zero Carbon Emissions: Climate Change and Global Warmin, in Green Hydrogen in Power Systems, Springer, 2024, pp. 141–153 Search PubMed.
- R. Lv, C. Luo, B. Liu, K. Hu, K. Wang, L. Zheng, Y. Guo, J. Du, L. Li and F. Wu, Unveiling Confinement Engineering for Achieving High-Performance Rechargeable Batteries, Adv. Mater., 2024, 2400508 CrossRef CAS.
- S. Wang, L. Wang, S. David, T. Liu, C. Zhan and K. Amine, Correlating Concerted Cations with Oxygen Redox in Rechargeable Batteries, Chem. Soc. Rev., 2024, 53, 3561–3578 RSC.
- Y.-J. Lei, L. Zhao, W.-H. Lai, Z. Huang, B. Sun, P. Jaumaux, K. Sun, Y.-X. Wang and G. Wang, Electrochemical Coupling in Subnanometer Pores/Channels for Rechargeable Batteries, Chem. Soc. Rev., 2024, 53, 3829–3895 RSC.
- A. Risco-Bravo, C. Varela, J. Bartels and E. Zondervan, From Green Hydrogen to Electricity: A Review on Recent Advances, Challenges, and Opportunities on Power-to-Hydrogen-to-Power Systems, Renewable Sustainable Energy Rev., 2024, 189, 113930 CrossRef.
- F. Frieden and J. Leker, Future Costs of Hydrogen: A Quantitative Review, Sustainable Energy Fuels, 2024, 8, 1806–1822 RSC.
- L. Ge, B. Zhang, W. Huang, Y. Li, L. Hou, J. Xiao, Z. Mao and X. Li, A Review of Hydrogen Generation, Storage, and Applications in Power System, J. Energy Storage, 2024, 75, 109307 CrossRef.
- B. S. Zainal, P. J. Ker, H. Mohamed, H. C. Ong, I. Fattah, S. A. Rahman, L. D. Nghiem and T. I. Mahlia, Recent Advancement and Assessment of Green Hydrogen Production Technologies, Renewable Sustainable Energy Rev., 2024, 189, 113941 CrossRef.
- L. Ruihan, H. Feng, X. Ting, L. Yongzhi, Z. Xin and Z. Jiaqi, Progress in the Application of First Principles to Hydrogen Storage Materials, Int. J. Hydrogen Energy, 2024, 56, 1079–1091 CrossRef.
- M. Kurucan, M. Özbaltan, Z. Yetgin and A. Alkaya, Applications of Artificial Neural Network Based Battery Management Systems: A Literature Review, Renewable Sustainable Energy Rev., 2024, 192, 114262 CrossRef.
- A. G. Olabi, Q. Abbas, P. A. Shinde and M. A. Abdelkareem, Rechargeable Batteries: Technological Advancement, Challenges, Current and Emerging Applications, Energy, 2023, 266, 126408 CrossRef CAS.
- F. Mohammadi and M. Saif, A Comprehensive Overview of Electric Vehicle Batteries Market, e-Prime-Advances in Electrical Engineering, Electronics and Energy, 2023, 3, 100127 CrossRef.
- J. Xu, X. Cai, S. Cai, Y. Shao, C. Hu, S. Lu and S. Ding, High-energy Lithium-ion Batteries: Recent Progress and a Promising Future in Applications, Energy Environ. Mater., 2023, 6(5), e12450 CrossRef CAS.
- F. N. U. Khan, M. G. Rasul, A. Sayem and N. K. Mandal, Design and Optimization of Lithium-Ion Battery as an Efficient Energy Storage Device for Electric Vehicles: A Comprehensive Review, J. Energy Storage, 2023, 71, 108033 CrossRef.
- M. Patel, K. Mishra, R. Banerjee, J. Chaudhari, D. Kanchan and D. Kumar, Fundamentals, Recent Developments and Prospects of Lithium and Non-Lithium Electrochemical Rechargeable Battery Systems, J. Energy Chem., 2023, 81, 221–259 CrossRef CAS.
- T. Khan, A. K. Garg, A. Gupta, A. Madan and P. Jain, Comprehensive Review on Latest Advances on Rechargeable Batteries, J. Energy Storage, 2023, 57, 106204 CrossRef.
- S. Nyamathulla and C. Dhanamjayulu, A Review of Battery Energy Storage Systems and Advanced Battery Management System for Different Applications: Challenges and Recommendations, J. Energy Storage, 2024, 86, 111179 CrossRef.
- P. Zhang, Social Issues Caused by Lithium-Ion Batteries, Highl. Sci. Eng. Technol., 2024, 83, 133–138 CrossRef.
- J. Ke, Y. Zhang, Z. Wen, S. Huang, M. Ye, Y. Tang, X. Liu and C. C. Li, Designing Strategies of Advanced Electrode Materials for High-Rate Rechargeable Batteries, J. Mater. Chem. A, 2023, 11(9), 4428–4457 RSC.
- G. Wang, G. Wang, L. Fei, L. Zhao and H. Zhang, Structural Engineering of Anode Materials for Low-Temperature Lithium-Ion Batteries: Mechanisms, Strategies, and Prospects, Nano-Micro Lett., 2024, 16(1), 150 CrossRef.
- Z. Wang, H. Jiang, Y. Zhang, Y. An, C. Wei, L. Tan, S. Xiong, Y. Qian and J. Feng, Application of 2D MXene in Organic Electrode Materials for Rechargeable Batteries: Recent Progress and Perspectives, Adv. Funct. Mater., 2023, 33(12), 2210184 CrossRef.
- J. E. G. Baquero and D. B. Monsalve, From Fossil Fuel Energy to Hydrogen Energy: Transformation of Fossil Fuel Energy Economies into Hydrogen Economies through Social Entrepreneurship, Int. J. Hydrogen Energy, 2024, 54, 574–585 CrossRef.
- T. T. Le, P. Sharma, B. J. Bora, V. D. Tran, T. H. Truong, H. C. Le and P. Q. P. Nguyen, Fueling the Future: A Comprehensive Review of Hydrogen Energy Systems and Their Challenges, Int. J. Hydrogen Energy, 2023, 54, 791–816 CrossRef.
- Q. Hassan, S. Algburi, A. Z. Sameen, H. M. Salman and M. Jaszczur, Green Hydrogen: A Pathway to a Sustainable Energy Future, Int. J. Hydrogen Energy, 2024, 50, 310–333 CrossRef CAS.
- M. S. Ali, M. S. Hossain Khan, R. A. Tuhin, M. A. Kabir, A. K. Azad and O. Farrok, Chapter 9 – Hydrogen Energy Storage and Transportation Challenges: A Review of Recent Advances, in Hydrogen Energy Conversion and Management, ed. Khan, M. M. K., Azad, A. K. and Oo, A. M. T., Elsevier, 2024, pp. 255–287, DOI:10.1016/B978-0-443-15329-7.00001-6.
- F. Alasali, M. I. Abuashour, W. Hammad, D. Almomani, A. M. Obeidat and W. Holderbaum, A Review of Hydrogen Production and Storage Materials for Efficient Integrated Hydrogen Energy Systems, Energy Sci. Eng., 2024, 12, 1934–1968 CrossRef.
- R. Sharma, M. Almáši, S. P. Nehra, V. S. Rao, P. Panchal, D. R. Paul, I. P. Jain and A. Sharma, Photocatalytic Hydrogen Production Using Graphitic Carbon Nitride (GCN): A Precise Review, Renewable Sustainable Energy Rev., 2022, 168, 112776 CrossRef CAS.
- L. Zhang, C. Jia, F. Bai, W. Wang, S. An, K. Zhao, Z. Li, J. Li and H. Sun, A Comprehensive Review of the Promising Clean Energy Carrier: Hydrogen Production, Transportation, Storage, and Utilization (HPTSU) Technologies, Fuel, 2024, 355, 129455 CrossRef CAS.
- R. Singh, A. Altaee and S. Gautam, Nanomaterials in the Advancement of Hydrogen Energy Storage, Heliyon, 2020, 6(7), 04487 CrossRef.
- F. Xu, J. Ren, J. Ma, Y. Wang, K. Zhang, Z. Cao, Q. Sun, S. Wu, G. Li and S. Bai, A Review of Hydrogen Production Kinetics from the Hydrolysis of NaBH4 Solution Catalyzed by Co-Based Catalysts, Int. J. Hydrogen Energy, 2023, 50, 827–844 CrossRef.
- N. S. C. Mazlan, M. F. A. A. H. Yap, M. Ismail, M. S. Yahya, N. A. Ali, N. Sazelee and Y. B. Seok, Reinforce the Dehydrogenation Process of LiAlH4 by Accumulating Porous Activated Carbon, Int. J. Hydrogen Energy, 2023, 48(43), 16381–16391 CrossRef.
- N. Klopčič, I. Grimmer, F. Winkler, M. Sartory and A. Trattner, A Review on Metal Hydride Materials for Hydrogen Storage, J. Energy Storage, 2023, 72, 108456 CrossRef.
- Ç. Yamçıçıer and C. Kürkçü, Investigation of Structural, Electronic, Elastic, Vibrational, Thermodynamic, and Optical Properties of Mg2NiH4 and Mg2RuH4 Compounds Used in Hydrogen Storage, J. Energy Storage, 2024, 84, 110883 CrossRef.
- S. Wang, Z. Zhang, Y. Tan, K. Liang and S. Zhang, Review on the Characteristics of Existing Hydrogen Energy Storage Technologies, Energy Sources, Part A, 2023, 45(1), 985–1006 CrossRef CAS.
- A. Salehabadi, J. Zanganeh and B. Moghtaderi, Mixed Metal Oxides in Catalytic Ammonia Cracking Process for Green Hydrogen Production: A Review, Int. J. Hydrogen Energy, 2024, 63, 828–843 CrossRef CAS.
- A. Yuvaraj, A. Jayarama, D. Sharma, S. S. Nagarkar, S. P. Duttagupta and R. Pinto, Role of Metal-Organic Framework in Hydrogen Gas Storage: A Critical Review, Int. J. Hydrogen Energy, 2024, 59, 1434–1458 CrossRef CAS.
- T. He and Y. Zhao, Covalent Organic Frameworks for Energy Conversion in Photocatalysis, Angew. Chem., Int. Ed., 2023, 62(34), e202303086 CrossRef CAS PubMed.
- M. Rafique, S. Hajra, M. Irshad, M. Usman, M. Imran, M. A. Assiri and W. M. Ashraf, Hydrogen Production Using TiO2-Based Photocatalysts: A Comprehensive Review, ACS Omega, 2023, 8(29), 25640–25648, DOI:10.1021/acsomega.3c00963.
- T. Van Nguyen, M. Tekalgne, T. P. Nguyen, Q. Van Le, S. H. Ahn and S. Y. Kim, Electrocatalysts Based on MoS2 and WS2 for Hydrogen Evolution Reaction: An Overview, Battery Energy, 2023, 2(3), 20220057 CrossRef CAS.
- Z. Ashfaq, T. Iqbal, H. Ali, S. M. Eldin, F. Al-Harbi, M. Arshad and A. M. Galal, Review of Different CdS/TiO2 and WO3/g-C3N4 Composite Based Photocatalyst for Hydrogen Production, Arab. J. Chem., 2023, 105024 CrossRef CAS.
- S. K. Nadikatla, V. B. Chintada, T. R. Gurugubelli and R. Koutavarapu, Review of Recent Developments in the Fabrication of ZnO/CdS Heterostructure Photocatalysts for Degradation of Organic Pollutants and Hydrogen Production, Molecules, 2023, 28(11), 4277 CrossRef PubMed.
- M. Damizia, P. J. Lloreda-Jurado, P. De Filippis, B. De Caprariis, E. Chicardi and R. Sepulveda, Green Hydrogen Production Using Doped Fe2O3 Foams, Int. J. Hydrogen Energy, 2024, 51, 834–845 CrossRef.
- S. Lotfi, M. E. Ouardi, H. A. Ahsaine and A. Assani, Recent Progress on the Synthesis, Morphology and Photocatalytic Dye Degradation of BiVO4 Photocatalysts: A Review, Catal. Rev., 2024, 66(1), 214–258 CrossRef.
- D. K. Becerra-Paniagua, S. Torres-Arellano, C. Martinez-Alonso, E. Luévano-Hipólito and P. Sebastian, Facile and Green Synthesis of Cu/Cu2O Composite for Photocatalytic H2 Generation, Mater. Sci. Semicond. Process., 2023, 162, 107485 CrossRef.
- C. Prasad, N. Madkhali, J. S. Won, J. E. Lee, S. Sangaraju and H. Y. Choi, CdS Based Heterojunction for Water Splitting: A Review, Mater. Sci. Eng. B, 2023, 292, 116413 CrossRef CAS.
- L. Clarizia, M. N. Nadagouda and D. D. Dionysiou, Recent Advances and Challenges of Photoelectrochemical Cells for Hydrogen Production, Curr. Opin. Green Sustainable Chem., 2023, 41, 100825 CrossRef CAS.
- N. Zhao and J. Wang, Solar Full Spectrum Management in Low and Medium Temperature Light-Driven Chemical Hydrogen Synthesis-A Review, Renewable Sustainable Energy Rev., 2024, 196, 114368 CrossRef CAS.
- Z. Chen, Y. Li, L. Wang, Y. Wang, J. Chai, J. Du, Q. Li, Y. Rui, L. Jiang and B. Tang, A Comprehensive Review of Various Carbonaceous Materials for Anode in Lithium-Ion Batteries, Dalton Trans., 2024, 51, 4900–4921 RSC.
- T.-W. Chen, S.-M. Chen, G. Anushya, R. Kannan, P. Veerakumar, A. G. Al-Sehemi, V. Mariyappan, S. Alargarsamy, M. M. Alam and T. C. Mahesh, Electrochemical Energy Storage Applications of Functionalized Carbon-Based Nanomaterials: An Overview, Int. J. Electrochem. Sci., 2024, 100548 CrossRef CAS.
- Y. Wang, Z. Zhang, F. Yuan and B. Wang, Design and Modification of Carbon-Based Materials for High Energy Density Non-Aqueous Aluminum Ion Batteries: A Review, J. Power Sources, 2024, 597, 234110 CrossRef CAS.
- J.-M. Cao, I. V. Zatovsky, Z.-Y. Gu, J.-L. Yang, X.-X. Zhao, J.-Z. Guo, H. Xu and X.-L. Wu, Two-Dimensional MXene with Multidimensional Carbonaceous Matrix: A Platform for General-Purpose Functional Materials, Prog. Mater. Sci., 2023, 135, 101105, DOI:10.1016/j.pmatsci.2023.101105.
- F. Tao, D. Xie, W.-Y. Diao, C. Liu, H.-Z. Sun, W.-L. Li, J.-P. Zhang and X.-L. Wu, Highly Lithiophilic Ti3C2Tx-Mxene Anchored on a Flexible Carbon Foam Scaffolds as the Basis for a Dendrite-Free Lithium Metal Anode, N. Carbon Mater., 2023, 38(4), 765–773, DOI:10.1016/S1872-5805(23)60739-5.
- M. Aizudin, W. Fu, R. P. Pottammel, Z. Dai, H. Wang, X. Rui, J. Zhu, C. C. Li, X.-L. Wu and E. H. Ang, Recent Advancements of Graphene-Based Materials for Zinc-Based Batteries: Beyond Lithium-Ion Batteries, Small, 2024, 20(2), 2305217, DOI:10.1002/smll.202305217.
- H. Liu, X. Liu, W. Li, X. Guo, Y. Wang, G. Wang and D. Zhao, Porous Carbon Composites for Next Generation Rechargeable Lithium Batteries, Adv. Energy Mater., 2017, 7(24), 1700283, DOI:10.1002/aenm.201700283.
- W. Yuan, Y. Zhang, L. Cheng, H. Wu, L. Zheng and D. Zhao, The Applications of Carbon Nanotubes and Graphene in Advanced Rechargeable Lithium Batteries, J. Mater. Chem. A, 2016, 4(23), 8932–8951, 10.1039/C6TA01546H.
- Q. Xiang and J. Yu, Graphene-Based Photocatalysts for Hydrogen Generation, J. Phys. Chem. Lett., 2013, 4(5), 753–759 CrossRef CAS PubMed.
- V. Tozzini and V. Pellegrini, Prospects for Hydrogen Storage in Graphene, Phys. Chem. Chem. Phys., 2013, 15(1), 80–89, 10.1039/C2CP42538F.
- B. M. Khan, W. C. Oh, P. Nuengmatch and K. Ullah, Role of Graphene-Based Nanocomposites as Anode Material for Lithium-Ion Batteries, Mater. Sci. Eng. B, 2023, 287, 116141 CrossRef.
- X. Wang, J. Wang, Z. Chen, K. Yang, Z. Zhang, Z. Shi, T. Mei, J. Qian, J. Li and X. Wang, Yolk-Double Shell Fe3O4@ C@ C Composite as High-Performance Anode Materials for Lithium-Ion Batteries, J. Alloys Compd., 2020, 822, 153656 CrossRef CAS.
- D. Deng, Li-ion Batteries: Basics, Progress, and Challenges, Energy Sci. Eng., 2015, 3(5), 385–418 CrossRef.
- F. Bonaccorso, L. Colombo, G. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Graphene, Related Two-Dimensional Crystals, and Hybrid Systems for Energy Conversion and Storage, Science, 2015, 347(6217), 1246501 CrossRef PubMed.
- E. Morales-Narváez, L. F. Sgobbi, S. A. S. Machado and A. Merkoçi, Graphene-Encapsulated Materials: Synthesis, Applications and Trends, Prog. Mater. Sci., 2017, 86, 1–24 CrossRef.
- L. Zhang, X. Qin, S. Zhao, A. Wang, J. Luo, Z. L. Wang, F. Kang, Z. Lin and B. Li, Advanced Matrixes for Binder-free Nanostructured Electrodes in Lithium-ion Batteries, Adv. Mater., 2020, 32(24), 1908445 CrossRef.
- M. S. Al Ja’farawy, D. N. Hikmah, U. Riyadi, A. Purwanto and H. Widiyandari, A Review: The Development of SiO 2/C Anode Materials for Lithium-Ion Batteries, J. Electron. Mater., 2021, 1–21 Search PubMed.
- X. Li, X. Sun, X. Hu, F. Fan, S. Cai, C. Zheng and G. D. Stucky, Review on Comprehending and Enhancing the Initial Coulombic Efficiency of Anode Materials in Lithium-Ion/Sodium-Ion Batteries, Nano Energy, 2020, 77, 105143 CrossRef.
- H. Lin, N. Lou, D. Yang, R. Jin and Y. Huang, Janus MoSSe/Graphene Heterostructures: Potential Anodes for Lithium-Ion Batteries, J. Alloys Compd., 2021, 854, 157215 CrossRef CAS.
- S. Dhar, T. Dash, B. Palei, T. Rout, S. Biswal, A. Mitra and A. Sahu, Silicon-Graphene Composite Synthesis: Microstructural, Spectroscopic and Electrical Conductivity Characterizations, Mater. Today: Proc., 2020, 33, 5136–5142 CAS.
- X. Zhao, E. Jiaqiang, G. Wu, Y. Deng, D. Han, B. Zhang and Z. Zhang, A Review of Studies Using Graphenes in Energy Conversion, Energy Storage and Heat Transfer Development, Energy Convers. Manage., 2019, 184, 581–599 CrossRef CAS.
- K. Wu, H. Yang, L. Jia, Y. Pan, Y. Hao, K. Zhang, K. Du and G. Hu, Smart Construction of 3D N-Doped Graphene Honeycombs with (NH 4) 2 SO 4 as a Multifunctional Template for Li-Ion Battery Anode:“A Choice That Serves Three Purposes.”, Green Chem., 2019, 21(6), 1472–1483 RSC.
- Z.-S. Wu, G. Zhou, L.-C. Yin, W. Ren, F. Li and H.-M. Cheng, Graphene/Metal Oxide Composite Electrode Materials for Energy Storage, Nano Energy, 2012, 1(1), 107–131 CrossRef CAS.
- N. Li, Z. Chen, W. Ren, F. Li and H.-M. Cheng, Flexible Graphene-Based Lithium Ion Batteries with Ultrafast Charge and Discharge Rates, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(43), 17360–17365 CrossRef CAS PubMed.
- J. Shao, J. Feng, H. Zhou and A. Yuan, Graphene Aerogel Encapsulated Fe-Co Oxide Nanocubes Derived from Prussian Blue Analogue as Integrated Anode with Enhanced Li-Ion Storage Properties, Appl. Surf. Sci., 2019, 471, 745–752 CrossRef CAS.
- N. Antonatos, H. Ghodrati and Z. Sofer, Elements beyond Graphene: Current State and Perspectives of Elemental Monolayer Deposition by Bottom-up Approach, Appl. Mater. Today, 2020, 18, 100502 CrossRef.
- R. Mo, X. Tan, F. Li, R. Tao, J. Xu, D. Kong, Z. Wang, B. Xu, X. Wang and C. Wang, Tin-Graphene Tubes as Anodes for Lithium-Ion Batteries with High Volumetric and Gravimetric Energy Densities, Nat. Commun., 2020, 11(1), 1374 CrossRef CAS PubMed.
- K. Ullah, L. Zhu, Z.-D. Meng, S. Ye, S. Sarkar and W.-C. Oh, Synthesis and Characterization of Novel PtSe 2/Graphene Nanocomposites and Its Visible Light Driven Catalytic Properties, J. Mater. Sci., 2014, 49, 4139–4147 CrossRef CAS.
- S. Z. Hussain, M. Ihrar, S. B. Hussain, W. C. Oh and K. Ullah, A Review on Graphene Based Transition Metal Oxide Composites and Its Application towards Supercapacitor Electrodes, SN Appl. Sci., 2020, 2, 1–23 Search PubMed.
- X. Yu, J. Tang, K. Terabe, T. Sasaki, R. Gao, Y. Ito, K. Nakura, K. Asano and M. Suzuki, Fabrication of Graphene/MoS2 Alternately Stacked Structure for Enhanced Lithium Storage, Mater. Chem. Phys., 2020, 239, 121987 CrossRef.
- Z.-S. Wu, W. Ren, L. Xu, F. Li and H.-M. Cheng, Doped Graphene Sheets as Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries, ACS Nano, 2011, 5(7), 5463–5471 CrossRef.
- M. Mazar Atabaki and R. Kovacevic, Graphene Composites as Anode Materials in Lithium-Ion Batteries, Electron. Mater. Lett., 2013, 9, 133–153 CrossRef.
- Y. Gong, S. Yang, Z. Liu, L. Ma, R. Vajtai and P. M. Ajayan, Graphene-network-backboned Architectures for High-performance Lithium Storage, Adv. Mater., 2013, 25(29), 3979–3984 CrossRef.
- N. Chodankar, A. Nanjundan, D. Losic, D. Dubal and J.-B. Baek, Graphene and Molybdenum Disulphide Hybrids for Energy Applications: An Update, Mater. Today Adv., 2020, 6, 100053 CrossRef.
- J. Xiao, D. Mei, X. Li, W. Xu, D. Wang, G. L. Graff, W. D. Bennett, Z. Nie, L. V. Saraf and I. A. Aksay, Hierarchically Porous Graphene as a Lithium–Air Battery Electrode, Nano Lett., 2011, 11(11), 5071–5078 CrossRef CAS PubMed.
- Y. Fang, Y. Lv, R. Che, H. Wu, X. Zhang, D. Gu, G. Zheng and D. Zhao, Two-Dimensional Mesoporous Carbon Nanosheets and Their Derived Graphene Nanosheets: Synthesis and Efficient Lithium Ion Storage, J. Am. Chem. Soc., 2013, 135(4), 1524–1530, DOI:10.1021/ja310849c.
- X. Zhu, J. Ye, Y. Lu and X. Jia, 3D Graphene Nanostructure Composed of Porous Carbon Sheets and Interconnected Nanocages for High-Performance Lithium-Ion Battery Anodes and Lithium–Sulfur Batteries, ACS Sustain. Chem. Eng., 2019, 7(13), 11241–11249 CrossRef CAS.
- P.-M. Ting, J.-Y. Huang, R. Muruganantham and W.-R. Liu, Nitrogen-Doping Effects on Few-Layer Graphene as an Anode Material for Lithium-Ion Batteries, Mater. Today Commun., 2022, 31, 103498, DOI:10.1016/j.mtcomm.2022.103498.
- Y. S. Yun, V.-D. Le, H. Kim, S.-J. Chang, S. J. Baek, S. Park, B. H. Kim, Y.-H. Kim, K. Kang and H.-J. Jin, Effects of Sulfur Doping on Graphene-Based Nanosheets for Use as Anode Materials in Lithium-Ion Batteries, J. Power Sources, 2014, 262, 79–85 CrossRef CAS.
- D. Cai, C. Wang, C. Shi and N. Tan, Facile Synthesis of N and S Co-Doped Graphene Sheets as Anode Materials for High-Performance Lithium-Ion Batteries, J. Alloys Compd., 2018, 731, 235–242, DOI:10.1016/j.jallcom.2017.10.043.
- C. Zhang, N. Mahmood, H. Yin, F. Liu and Y. Hou, Synthesis of Phosphorus-doped Graphene and Its Multifunctional Applications for Oxygen Reduction Reaction and Lithium Ion Batteries, Adv. Mater., 2013, 25(35), 4932–4937 CrossRef CAS.
- R. Bhushan, P. Kumar and A. K. Thakur, Catalyst-Free Solvothermal Synthesis of Ultrapure Elemental N- and B-Doped Graphene for Energy Storage Application, Solid State Ionics, 2020, 353, 115371, DOI:10.1016/j.ssi.2020.115371.
- X. Lang, X. Wang, Y. Liu, K. Cai, L. Li and Q. Zhang, Cobalt-based Metal Organic Framework (Co-MOFs)/Graphene Oxide Composites as High-performance Anode Active Materials for Lithium-ion Batteries, Int. J. Energy Res., 2021, 45(3), 4811–4820 CrossRef CAS.
- C. Liu, Y. Bai, Y. Zhao, H. Yao and H. Pang, MoS2/Graphene Composites: Fabrication and Electrochemical Energy Storage, Energy Storage Mater., 2020, 33, 470–502 CrossRef.
- S. Wang, Y. Shi, C. Fan, J. Liu, Y. Li, X.-L. Wu, H. Xie, J. Zhang and H. Sun, Layered G-C3N4@ Reduced Graphene Oxide Composites as Anodes with Improved Rate Performance for Lithium-Ion Batteries, ACS Appl. Mater. Interfaces, 2018, 10(36), 30330–30336 CrossRef.
- D. Adekoya, S. Zhang and M. Hankel, 1D/2D C3N4/Graphene Composite as a Preferred Anode Material for Lithium Ion Batteries: Importance of Heterostructure Design via DFT Computation, ACS Appl. Mater. Interfaces, 2020, 12(23), 25875–25883 CrossRef PubMed.
- J. R. Rodriguez, P. J. Kim, K. Kim, Z. Qi, H. Wang and V. G. Pol, Engineered Heat Dissipation and Current Distribution Boron Nitride-Graphene Layer Coated on Polypropylene Separator for High Performance Lithium Metal Battery, J. Colloid Interface Sci., 2021, 583, 362–370, DOI:10.1016/j.jcis.2020.09.009.
- S. F. Zhao, G. A. Elbaz, D. K. Bediako, C. Yu, D. K. Efetov, Y. Guo, J. Ravichandran, K.-A. Min, S. Hong and T. Taniguchi, Controlled Electrochemical Intercalation of Graphene/h-BN van Der Waals Heterostructures, Nano Lett., 2018, 18(1), 460–466 CrossRef.
- H. Li, R. Y. Tay, S. H. Tsang, W. Liu and E. H. T. Teo, Reduced Graphene Oxide/Boron Nitride Composite Film as a Novel Binder-Free Anode for Lithium Ion Batteries with Enhanced Performances, Electrochim. Acta, 2015, 166, 197–205 CrossRef.
- J. Hong, A.-R. Jang, W. B. Park, B. Hou, J.-O. Lee, K.-S. Sohn, S. Cha, Y.-W. Lee and J. I. Sohn, Thermodynamically and Physically Stable Dendrite-Free Li Interface with Layered Boron Nitride Separators, ACS Sustain. Chem. Eng., 2021, 9(11), 4185–4193 CrossRef.
- D. He, Y. Yang, Z. Liu, J. Shao, J. Wu, S. Wang, L. Shen and N. Bao, Solvothermal-Assisted Assembly of MoS 2 Nanocages on Graphene Sheets to Enhance the Electrochemical Performance of Lithium-Ion Battery, Nano Res., 2020, 13, 1029–1034 CrossRef.
- Z. Ma, X. Zhou, W. Deng, D. Lei and Z. Liu, 3D Porous MXene (Ti3C2)/Reduced Graphene Oxide Hybrid Films for Advanced Lithium Storage, ACS Appl. Mater. Interfaces, 2018, 10(4), 3634–3643, DOI:10.1021/acsami.7b17386.
- H. Shi, C. J. Zhang, P. Lu, Y. Dong, P. Wen and Z.-S. Wu, Conducting and Lithiophilic MXene/Graphene Framework for High-Capacity, Dendrite-Free Lithium–Metal Anodes, ACS Nano, 2019, 13(12), 14308–14318, DOI:10.1021/acsnano.9b07710.
- B. Zhang, Z. Ju, Q. Xie, J. Luo, L. Du, C. Zhang and X. Tao, Ti3CNTx MXene/rGO Scaffolds Directing the Formation of a Robust, Layered SEI toward High-Rate and Long-Cycle Lithium Metal Batteries, Energy Storage Mater., 2023, 58, 322–331, DOI:10.1016/j.ensm.2023.03.030.
- X. Zhou, X. Lan, Z. Jiao, H. Zong, P. Zhang and B. Xu, High-Content 1T Phase MoS2 Nanosheets Coupled on Graphene Oxide for Lithium-Ion Batteries, J. Alloys Compd., 2024, 971, 172640, DOI:10.1016/j.jallcom.2023.172640.
- Y.-M. Gao, Y. Liu, K.-J. Feng, J.-Q. Ma, Y.-J. Miao, B.-R. Xu, K.-M. Pan, O. Akiyoshi, G.-X. Wang and K.-K. Zhang, Emerging WS2/WSe2@ Graphene Nanocomposites: Synthesis and Electrochemical Energy Storage Applications, Rare Met., 2024, 43(1), 1–19 CrossRef CAS.
- Y.-L. Wu, J.-B. Hong, W.-X. Zhong, C.-X. Wang, Z.-F. Li and S. Dmytro, Auxiliary Ball Milling to Prepare WS2/Graphene Nanosheets Composite for Lithium-Ion Battery Anode Materials, Tungsten, 2023, 1–10 Search PubMed.
- Y.-T. Du, X. Kan, F. Yang, L.-Y. Gan and U. Schwingenschlögl, MXene/Graphene Heterostructures as High-Performance Electrodes for Li-Ion Batteries, ACS Appl. Mater. Interfaces, 2018, 10(38), 32867–32873 CrossRef CAS PubMed.
- M. S. Mohseni-Salehi, E. Taheri-Nassaj, A. Babaei, A. S. Ghazvini and M. Soleimanzade, V2CTx-carbon Nanotube/Graphene Nanohybrids for Li-Ion Storage Applications, J. Energy Storage, 2024, 85, 111033, DOI:10.1016/j.est.2024.111033.
- J. Aslam and Y. Wang, Metal Oxide Wrapped by Reduced Graphene Oxide Nanocomposites as Anode Materials for Lithium-Ion Batteries, Nanomaterials, 2023, 13(2), 296 CrossRef PubMed.
- K. Ullah, N. Shah, R. Wadood, B. M. Khan and W. C. Oh, Recent Trends in Graphene Based Transition Metal Oxides as Anode Materials for Rechargeable Lithium-Ion Batteries, NanoTrends, 2023, 1, 100004 Search PubMed.
- E. Muchuweni, E. T. Mombeshora, C. M. Muiva and T. S. Sathiaraj, Lithium-Ion Batteries: Recent Progress in Improving the Cycling and Rate Performances of Transition Metal Oxide Anodes by Incorporating Graphene-Based Materials, J. Energy Storage, 2023, 73, 109013, DOI:10.1016/j.est.2023.109013.
- W. Teng, Z. Lu, X. Li, X. wang, J. Liu, J. Dong and D. Nan, High-Performance Flexible SnO2 Anode Boosted by an N-Doped Graphite Coating Layer for Lithium-Ion and Sodium-Ion Batteries, Electrochem. Commun., 2022, 141, 107345, DOI:10.1016/j.elecom.2022.107345.
- T. Liu, Y. Guo, S. Hou, W. Fu, J. Li, L. Meng, C. Mei and L. Zhao, Constructing Hierarchical ZnO@C Composites Using Discarded Sprite and Fanta Drinks for Enhanced Lithium Storage, Appl. Surf. Sci., 2021, 541, 148495, DOI:10.1016/j.apsusc.2020.148495.
- S. Zhu, B. Liang, X. Mou, X. Liang, H. Huang, D. Huang, W. Zhou, S. Xu and J. Guo, In-Situ Synthesis of F-Doped FeOOH Nanorods on Graphene as Anode Materials for High Lithium Storage, J. Alloys Compd., 2022, 905, 164142, DOI:10.1016/j.jallcom.2022.164142.
- X. Lv, Z. Deng, M. Wang and J. Deng, Ti3C2 MXene Derived Carbon-Doped TiO2 Multilayers Anchored with Fe2O3 Nanoparticles as Anode for Enhanced Lithium-Ion Storage, J. Alloys Compd., 2022, 918, 165697, DOI:10.1016/j.jallcom.2022.165697.
- N. Muhammad, G. Yasin, A. Li, Y. Chen, H. M. Saleem, R. Liu, D. Li, Y. Sun, S. Zheng, X. Chen and H. Song, Volumetric Buffering of Manganese Dioxide Nanotubes by Employing ‘as Is’ Graphene Oxide: An Approach towards Stable Metal Oxide Anode Material in Lithium-Ion Batteries, J. Alloys Compd., 2020, 842, 155803, DOI:10.1016/j.jallcom.2020.155803.
- L. Tan, X. Lan, R. Hu, J. Liu, B. Yuan and M. Zhu, Stable Lithium Storage at Subzero Temperatures for High-capacity Co3O4@ Graphene Composite Anodes, ChemNanoMat, 2021, 7(1), 61–70 CrossRef.
- Y. Huang, Y. Li, R. Huang, J. Ji, J. Yao and S. Xiao, One-Pot Hydrothermal Synthesis of N-rGO Supported Fe2O3 Nanoparticles as a Superior Anode Material for Lithium-Ion Batteries, Solid State Ionics, 2021, 368, 115693, DOI:10.1016/j.ssi.2021.115693.
- L. Fan, Y. Zhang, H. Zhou, Z. Guo, Y. Feng and N. Zhang, Kinetically Enhanced Electrochemical Redox Reactions by Chemical Bridging SnO2 and Graphene Sponges toward High-Rate and Long-Cycle Lithium Ion Battery, J. Mater. Sci. Technol., 2021, 88, 250–257, DOI:10.1016/j.jmst.2020.11.082.
- J. Fu, W. Kang, X. Guo, H. Wen, T. Zeng, R. Yuan and C. Zhang, 3D Hierarchically Porous NiO/Graphene Hybrid Paper Anode for Long-Life and High Rate Cycling Flexible Li-Ion Batteries, J. Energy Chem., 2020, 47, 172–179 CrossRef.
- P. M. Ette, D. Bosubabu and K. Ramesha, Graphene Anchored Mesoporous MnO2 Nanostructures as Stable and High-Performance Anode Materials for Li-Ion Batteries, Electrochim. Acta, 2022, 414, 140164, DOI:10.1016/j.electacta.2022.140164.
- J. Xu, D. Xu, J. Wu, J. Wu, J. Zhou, T. Zhou, X. Wang and J. P. Cheng, Ultra-Small Fe2O3 Nanoparticles Anchored on Ultrasonically Exfoliated Multilayer Graphene for LIB Anode Application, Ceram. Int., 2022, 48(21), 32524–32531, DOI:10.1016/j.ceramint.2022.07.198.
- S. Jiang, M. Mao, M. Pang, H. Yang, R. Wang, N. LI, Q. Pan, M. Pang and J. Zhao, Preparation and Performance of a Graphene-(Ni-NiO)-C Hybrid as the Anode of a Lithium-Ion Battery, N. Carbon Mater., 2023, 38(2), 356–365, DOI:10.1016/S1872-5805(22)60647-4.
- C. Bi, M. Zhao, L. Hou, Z. Chen, X. Zhang, B. Li, H. Yuan and J. Huang, Anode Material Options toward 500 W h Kg− 1 Lithium–Sulfur Batteries, Adv. Sci., 2022, 9(2), 2103910 CrossRef CAS.
- Y. Huang, L. Lin, C. Zhang, L. Liu, Y. Li, Z. Qiao, J. Lin, Q. Wei, L. Wang and Q. Xie, Recent Advances and Strategies toward Polysulfides Shuttle Inhibition for High-performance Li–S Batteries, Adv. Sci., 2022, 9(12), 2106004 CrossRef CAS PubMed.
- D. R. Deng, F. Xue, C.-D. Bai, J. Lei, R. Yuan, M. S. Zheng and Q. F. Dong, Enhanced Adsorptions to Polysulfides on Graphene-Supported BN Nanosheets with Excellent Li–S Battery Performance in a Wide Temperature Range, ACS Nano, 2018, 12(11), 11120–11129, DOI:10.1021/acsnano.8b05534.
- B. Yu, Y. Fan, S. Mateti, D. Kim, C. Zhao, S. Lu, X. Liu, Q. Rong, T. Tao and K. K. Tanwar, An Ultra-Long-Life Flexible Lithium–Sulfur Battery with Lithium Cloth Anode and Polysulfone-Functionalized Separator, ACS Nano, 2020, 15(1), 1358–1369 CrossRef PubMed.
- D.-R. Deng, C.-D. Bai, F. Xue, J. Lei, P. Xu, M.-S. Zheng and Q.-F. Dong, Multifunctional Ion-Sieve Constructed by 2D Materials as an Interlayer for Li–S Batteries, ACS Appl. Mater. Interfaces, 2019, 11(12), 11474–11480 CrossRef CAS PubMed.
- J. Zhang, J. Li, W. Wang, X. Zhang, X. Tan, W. Chu and Y. Guo, Microemulsion Assisted Assembly of 3D Porous s/Graphene@ g-C3N4 Hybrid Sponge as Free-standing Cathodes for High Energy Density Li–S Batteries, Adv. Energy Mater., 2018, 8(14), 1702839 CrossRef.
- L. Qu, P. Liu, Y. Yi, T. Wang, P. Yang, X. Tian, M. Li, B. Yang and S. Dai, Enhanced Cycling Performance for Lithium–Sulfur Batteries by a Laminated 2D g-C3N4/Graphene Cathode Interlayer, ChemSusChem, 2019, 12(1), 213–223 CrossRef CAS PubMed.
- X. Wu, S. Li, B. Wang, J. Liu and M. Yu, Free-Standing 3D Network-like Cathode Based on Biomass-Derived N-Doped Carbon/Graphene/g-C3N4 Hybrid Ultrathin Sheets as Sulfur Host for High-Rate Li-S Battery, Renew. Energy, 2020, 158, 509–519 CrossRef CAS.
- J. Nan, X. Guo, J. Xiao, X. Li, W. Chen, W. Wu, H. Liu, Y. Wang, M. Wu and G. Wang, Nanoengineering of 2D MXene-based Materials for Energy Storage Applications, Small, 2021, 17(9), 1902085 CrossRef CAS PubMed.
- M. Shekhirev, C. E. Shuck, A. Sarycheva and Y. Gogotsi, Characterization of MXenes at Every Step, from Their Precursors to Single Flakes and Assembled Films, Prog. Mater. Sci., 2021, 120, 100757 CrossRef CAS.
- Q. Zhong, Y. Li and G. Zhang, Two-Dimensional MXene-Based and MXene-Derived Photocatalysts: Recent Developments and Perspectives, Chem. Eng. J., 2021, 409, 128099 CrossRef CAS.
- F. Zhang, Y. Zhou, Y. Zhang, D. Li and Z. Huang, Facile Synthesis of Sulfur@ Titanium Carbide MXene as High Performance Cathode for Lithium-Sulfur Batteries, Nanophotonics, 2020, 9(7), 2025–2032 CrossRef CAS.
- L. Yuanzheng, Y. Zhicheng, M. Lianghao, L. Buyin and L. Shufa, A Freestanding Nitrogen-Doped MXene/Graphene Cathode for High-Performance Li–S Batteries, Nanoscale Adv., 2022, 4(9), 2189–2195 RSC.
- S. Nam, J. Kim, V. H. Nguyen, M. Mahato, S. Oh, P. Thangasamy, C. W. Ahn and I. Oh, Collectively Exhaustive MXene and Graphene Oxide Multilayer for Suppressing Shuttling Effect in Flexible Lithium Sulfur Battery, Adv. Mater. Technol., 2022, 7(5), 2101025 CrossRef CAS.
- J. He, G. Hartmann, M. Lee, G. S. Hwang, Y. Chen and A. Manthiram, Freestanding 1T MoS 2/Graphene Heterostructures as a Highly Efficient Electrocatalyst for Lithium Polysulfides in Li–S Batteries, Energy Environ. Sci., 2019, 12(1), 344–350 RSC.
- Z. Wei, S. Sarwar, S. Azam, M. R. Ahasan, M. Voyda, X. Zhang and R. Wang, Ultrafast Microwave Synthesis of MoTe2@graphene Composites Accelerating Polysulfide Conversion and Promoting Li2S Nucleation for High-Performance Li-S Batteries, J. Colloid Interface Sci., 2023, 635, 391–405, DOI:10.1016/j.jcis.2022.12.111.
- J. Zhang, G. Xu, Q. Zhang, X. Li, Y. Yang, L. Yang, J. Huang and G. Zhou, Mo-O-C Between MoS2 and Graphene Toward Accelerated Polysulfide Catalytic Conversion for Advanced Lithium-Sulfur Batteries, Adv. Sci., 2022, 9(22), 2201579 CrossRef CAS PubMed.
- X. Fang and M. Zhang, MoS 2/G Interlayer as a Polysulfide Immobilization Apparatus for High-Performance Lithium–Sulfur Batteries, Ionics, 2021, 27, 3875–3885 CrossRef CAS.
- Y. Feng, H. Liu, Y. Liu, F. Zhao, J. Li and X. He, Defective TiO2-Graphene Heterostructures Enabling in-Situ Electrocatalyst Evolution for Lithium-Sulfur Batteries, J. Energy Chem., 2021, 62, 508–515 CrossRef CAS.
- Z. Xiao, Z. Yang, L. Wang, H. Nie, M. Zhong, Q. Lai, X. Xu, L. Zhang and S. Huang, Lithium-Sulfur Batteries: A Lightweight TiO2/Graphene Interlayer, Applied as a Highly Effective Polysulfide Absorbent for Fast, Long-Life Lithium–Sulfur Batteries (Adv. Mater. 18/2015), Adv. Mater., 2015, 27(18), 2890 CrossRef.
- J. Li, Y. Xu, W. Hou and X. Yao, Loading Fe3O4 Nanoparticles on N, S Co-Doped Graphene Suppressing Polysulfides Conversion toward High-Performance Li–S Batteries, J. Mater. Sci., 2023, 58(10), 4552–4564 CrossRef CAS.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J.-M. Tarascon, Li–O2 and Li–S Batteries with High Energy Storage, Nat. Mater., 2012, 11(1), 19–29 CrossRef CAS PubMed.
- D. Geng, N. Ding, T. A. Hor, S. W. Chien, Z. Liu, D. Wuu, X. Sun and Y. Zong, From Lithium-oxygen to Lithium-air Batteries: Challenges and Opportunities, Adv. Energy Mater., 2016, 6(9), 1502164 CrossRef.
- A. Ahmadiparidari, R. E. Warburton, L. Majidi, M. Asadi, A. Chamaani, J. R. Jokisaari, S. Rastegar, Z. Hemmat, B. Sayahpour and R. S. Assary, A Long-cycle-life Lithium–CO2 Battery with Carbon Neutrality, Adv. Mater., 2019, 31(40), 1902518 CrossRef CAS PubMed.
- L. Majidi, P. Yasaei, R. E. Warburton, S. Fuladi, J. Cavin, X. Hu, Z. Hemmat, S. B. Cho, P. Abbasi and M. Vörös, New Class of Electrocatalysts Based on 2D Transition Metal Dichalcogenides in Ionic Liquid, Adv. Mater., 2019, 31(4), 1804453 CrossRef PubMed.
- M. Carboni, A. G. Marrani, R. Spezia and S. Brutti, Degradation of LiTfO/TEGME and LiTfO/DME Electrolytes in Li-O2 Batteries, J. Electrochem. Soc., 2018, 165(2), A118 CrossRef CAS.
- M. D. Bhatt, H. Geaney, M. Nolan and C. O'Dwyer, Key Scientific Challenges in Current Rechargeable Non-Aqueous Li–O 2 Batteries: Experiment and Theory, Phys. Chem. Chem. Phys., 2014, 16(24), 12093–12130 RSC.
- R. Rojaee and R. Shahbazian-Yassar, Two-Dimensional Materials to Address the Lithium Battery Challenges, ACS Nano, 2020, 14(3), 2628–2658 CrossRef CAS PubMed.
- F. Su, X. Hou, J. Qin and Z. Wu, Recent Advances and Challenges of Two-dimensional Materials for High-energy and High-power Lithium-ion Capacitors, Batteries Supercaps, 2020, 3(1), 10–29 CrossRef CAS.
- E. Pomerantseva and Y. Gogotsi, Two-Dimensional Heterostructures for Energy Storage, Nat. Energy, 2017, 2(7), 1–6 Search PubMed.
- H. Tian, Z. W. Seh, K. Yan, Z. Fu, P. Tang, Y. Lu, R. Zhang, D. Legut, Y. Cui and Q. Zhang, Theoretical Investigation of 2D Layered Materials as Protective Films for Lithium and Sodium Metal Anodes, Adv. Energy Mater., 2017, 7(13), 1602528 CrossRef.
- L. Zhu, F. Scheiba, V. Trouillet, M. Georgian, Q. Fu, A. Sarapulpva, F. Sigel, W. Hua and H. Ehrenberg, MnO2 and Reduced Graphene Oxide as Bifunctional Electrocatalysts for Li–O2 Batteries, ACS Appl. Energy Mater., 2019, 2(10), 7121–7131, DOI:10.1021/acsaem.9b01047.
- Y. Li, J. Qin, Y. Ding, J. Ma, P. Das, H. Liu, Z.-S. Wu and X. Bao, Two-Dimensional Mn3O4 Nanosheets with Dominant (101) Crystal Planes on Graphene as Efficient Oxygen Catalysts for Ultrahigh Capacity and Long-Life Li–O2 Batteries, ACS Catal., 2022, 12(20), 12765–12773, DOI:10.1021/acscatal.2c02544.
- W. Zhao, Y. Yang, Q. Deng, Q. Dai, Z. Fang, X. Fu, W. Yan, L. Wu and Y. Zhou, Toward an Understanding of Bimetallic MXene Solid-Solution in Binder-Free Electrocatalyst Cathode for Advanced Li–CO2 Batteries, Adv. Funct. Mater., 2023, 33(5), 2210037 CrossRef CAS.
- F. Liu, J. Zhou, Y. Wang, Y. Xiong, F. Hao, Y. Ma, P. Lu, J. Wang, J. Yin and G. Wang, Rational Engineering of 2D Materials as Advanced Catalyst Cathodes for High-Performance Metal–Carbon Dioxide Batteries, Small Struct., 2023, 4(9), 2300025 CrossRef CAS.
- W. Yu, L. Liu, Y. Yang, N. Li, Y. Chen, X. Yin, J. Niu, J. Wang and S. N. Ding, O-Diatomic Dopants Activate Catalytic Activity of 3D Self-Standing Graphene Carbon Aerogel for Long-Cycle and High-Efficiency Li-CO2 Batteries, Chem. Eng. J., 2023, 465, 142787, DOI:10.1016/j.cej.2023.142787.
- Y. Jiao, J. Qin, H. M. K. Sari, D. Li, X. Li and X. Sun, Recent Progress and Prospects of Li-CO2 Batteries: Mechanisms, Catalysts and Electrolytes, Energy Storage Mater., 2021, 34, 148–170, DOI:10.1016/j.ensm.2020.09.014.
- Z. Zhang, Q. Zhang, Y. Chen, J. Bao, X. Zhou, Z. Xie, J. Wei and Z. Zhou, The First Introduction of Graphene to Rechargeable Li–CO2 Batteries, Angew. Chem., 2015, 127(22), 6650–6653 CrossRef.
- B. Wu, S. Wang, J. Lochala, D. Desrochers, B. Liu, W. Zhang, J. Yang and J. Xiao, The Role of the Solid Electrolyte Interphase Layer in Preventing Li Dendrite Growth in Solid-State Batteries, Energy Environ. Sci., 2018, 11(7), 1803–1810, 10.1039/C8EE00540K.
- W. Liu, Y. Xia, W. Wang, Y. Wang, J. Jin, Y. Chen, E. Paek and D. Mitlin, Pristine or Highly Defective? Understanding the Role of Graphene Structure for Stable Lithium Metal Plating, Adv. Energy Mater., 2019, 9(3), 1802918, DOI:10.1002/aenm.201802918.
- S. Shi, D. Zhou, Y. Jiang, F. Cheng, J. Sun, Q. Guo, Y. Luo, Y. Chen and W. Liu, Lightweight Zn-Philic 3D-Cu Scaffold for Customizable Zinc Ion Batteries, Adv. Funct. Mater., 2024, 34(24), 2312664, DOI:10.1002/adfm.202312664.
- H. Xu, Y. He, Z. Zhang, J. Shi, P. Liu, Z. Tian, K. Luo, X. Zhang, S. Liang and Z. Liu, Slurry-like Hybrid Electrolyte with High Lithium-Ion Transference Number for Dendrite-Free Lithium Metal Anode, J. Energy Chem., 2020, 48, 375–382, DOI:10.1016/j.jechem.2020.02.009.
- M. Li, X. Liu, Q. Li, Z. Jin, W. Wang, A. Wang, Y. Huang and Y. Yang, P4S10 Modified Lithium Anode for Enhanced Performance of Lithium–Sulfur Batteries, J. Energy Chem., 2020, 41, 27–33, DOI:10.1016/j.jechem.2019.03.038.
- Z. Yao, W. Li, T. Chen, X. Xu and Q. Xiao, Recent Progress on Nanomodification Applied in Anodes of Rechargeable Li Metal Batteries, ACS Appl. Energy Mater., 2023, 6(20), 10518–10541, DOI:10.1021/acsaem.3c01657.
- J.-F. Ding, R. Xu, C. Yan, Y. Xiao, L. Xu, H.-J. Peng, H. S. Park, J. Liang and J.-Q. Huang, Review on Nanomaterials for Next-Generation Batteries with Lithium Metal Anodes, Nano Sel., 2020, 1(1), 94–110, DOI:10.1002/nano.202000003.
- P. Jiang, Y. Liao, W. Liu and Y. Chen, Alternating Nanolayers as Lithiophilic Scaffolds for Li-Metal Anode, J. Energy Chem., 2021, 57, 131–139, DOI:10.1016/j.jechem.2020.08.034.
- R. Javaid, Catalytic Hydrogen Production, Storage and Application, Catalysts, 2021, 11(7), 836 CrossRef CAS.
- E. B. Agyekum, C. Nutakor, A. M. Agwa and S. Kamel, A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-up and Its Role in Future Energy Generation, Membranes, 2022, 12(2), 173 CrossRef CAS PubMed.
- Y. Yang, J. Liu, M. Gu, B. Cheng, L. Wang and J. Yu, Bifunctional TiO2/COF S-Scheme Photocatalyst with Enhanced H2O2 Production and Furoic Acid Synthesis Mechanism, Appl. Catal., B, 2023, 333, 122780 CrossRef CAS.
- W. Sun, S. Yang, Y. Liu, C. Shi, W. Shi, X. Lin, F. Guo and Y. Hong, Fabricating Nitrogen-Doped Carbon Dots (NCDs) on Bi3. 64Mo0. 36O6. 55 Nanospheres: A Nanoheterostructure for Enhanced Photocatalytic Performance for Water Purification, J. Phys. Chem. Solids, 2021, 159, 110283 CrossRef CAS.
- W. Zhao, Z. Chen, X. Yang, X. Qian, C. Liu, D. Zhou, T. Sun, M. Zhang, G. Wei and P. D. Dissanayake, Recent Advances in Photocatalytic Hydrogen Evolution with High-Performance Catalysts without Precious Metals, Renewable Sustainable Energy Rev., 2020, 132, 110040 CrossRef CAS.
- J. Liu, P. Wang, J. Fan, H. Yu and J. Yu, In Situ Synthesis of Mo2C Nanoparticles on Graphene Nanosheets for Enhanced Photocatalytic H2-Production Activity of TiO2, ACS Sustainable Chem. Eng., 2021, 9(10), 3828–3837, DOI:10.1021/acssuschemeng.0c08903.
- T. Song, B. Long, S. Yin, A. Ali and G.-J. Deng, Designed Synthesis of a Porous Ultrathin 2D CN@graphene@CN Sandwich Structure for Superior Photocatalytic Hydrogen Evolution under Visible Light, Chem. Eng. J., 2021, 404, 126455, DOI:10.1016/j.cej.2020.126455.
- H. Boumeriame, B. F. Machado, N. M. M. Moura, P. Serp, L. Andrade, T. Lopes, A. Mendes, T. Chafik, E. S. Da Silva and J. L. Faria, Graphitic Carbon Nitride/Few-Layer Graphene Heterostructures for Enhanced Visible-LED Photocatalytic Hydrogen Generation, Int. J. Hydrogen Energy, 2022, 47(61), 25555–25570, DOI:10.1016/j.ijhydene.2022.05.285.
- L. K. Putri, B.-J. Ng, W.-J. Ong, H. W. Lee, W. S. Chang, A. R. Mohamed and S.-P. Chai, Energy Level Tuning of CdSe Colloidal Quantum Dots in Ternary 0D-2D-2D CdSe QD/B-rGO/O-gC3N4 as Photocatalysts for Enhanced Hydrogen Generation, Appl. Catal., B, 2020, 265, 118592, DOI:10.1016/j.apcatb.2020.118592.
- J.-Q. Yan, W. Peng, S.-S. Zhang, D.-P. Lei and J.-H. Huang, Ternary Ni2P/Reduced Graphene Oxide/g-C3N4 Nanotubes for Visible Light-Driven Photocatalytic H2 Production, Int. J. Hydrogen Energy, 2020, 45(32), 16094–16104, DOI:10.1016/j.ijhydene.2020.04.001.
- W. Li, X. Wang, Q. Ma, F. Wang, X. Chu, X. Wang and C. Wang, CdS@h-BN Heterointerface Construction on Reduced Graphene Oxide Nanosheets for Hydrogen Production, Appl. Catal., B, 2021, 284, 119688, DOI:10.1016/j.apcatb.2020.119688.
- K. Pramoda, S. Servottam, M. Kaur and C. N. R. Rao, Layered Nanocomposites of Polymer-Functionalized Reduced Graphene Oxide and Borocarbonitride with MoS2 and MoSe2 and Their Hydrogen Evolution Reaction Activity, ACS Appl. Nano Mater., 2020, 3(2), 1792–1799, DOI:10.1021/acsanm.9b02482.
- F. Pan, M. Khan, T. Lei, M. R. Kamli, J. S. M Sabir, I. Khan and M. Z. Ansari, Visible Light Driven Hydrogen Generation and Pollutant Degradation with Au Loaded 2D/2D Heterojunctional Nanocomposite of MoS2 and g-C3N4, Int. J. Hydrogen Energy, 2024, 51, 1141–1153, DOI:10.1016/j.ijhydene.2023.09.132.
- K. Namsheer, K. Pramoda, K. S. S. Kumar, S. Radhakrishnan and C. S. Rout, Molybdenum Sulfo-Selenide Nanocomposites with Carbon Nanotubes and Reduced Graphene Oxide for Photocatalytic Hydrogen Evolution Reaction, Energy Adv., 2023, 2(10), 1724–1734, 10.1039/D3YA00219E.
- C. Wang, F. Zheng, L. Zhang, J. Yang and P. Dong, Insight into the Role of Graphene Quantum Dots on the Boosted Photocatalytic H2 Production Performance of a Covalent Organic Framework, Appl. Surf. Sci., 2023, 640, 158383, DOI:10.1016/j.apsusc.2023.158383.
- H. Szalad, A. Uscategui, J. Albero and H. García, 2D/2D Cu-Tetrahydroxyquinone MOF/N-Doped Graphene Heterojunction as Photocatalyst for Overall Water Splitting, Int. J. Hydrogen Energy, 2023, 48(33), 12374–12384, DOI:10.1016/j.ijhydene.2022.12.168.
- A. Ejsmont, A. Lewandowska-Andralojc, K. Hauza and J. Goscianska, In Situ Modification of Co-MOF with Graphene Oxide for Enhanced Photocatalytic Hydrogen Production, Int. J. Hydrogen Energy, 2023, 48(24), 8965–8970, DOI:10.1016/j.ijhydene.2022.12.084.
- K. Dai, T. Hu, J. Zhang and L. Lu, Carbon Nanotube Exfoliated Porous Reduced Graphene Oxide/CdS- Diethylenetriamine Heterojunction for Efficient Photocatalytic H2 Production, Appl. Surf. Sci., 2020, 512, 144783, DOI:10.1016/j.apsusc.2019.144783.
- H. Shen, M. Wang, X. Zhang, D. Li, G. Liu and W. Shi, 2D/2D/3D Architecture Z-scheme System for Simultaneous H2 Generation and Antibiotic Degradation, Fuel, 2020, 280, 118618, DOI:10.1016/j.fuel.2020.118618.
- D. Liu, J. Yao, S. Chen, J. Zhang, R. Li and T. Peng, Construction of rGO-Coupled C3N4/C3N5 2D/2D Z-Scheme Heterojunction to Accelerate Charge Separation for Efficient Visible Light H2 Evolution, Appl. Catal., B, 2022, 318, 121822, DOI:10.1016/j.apcatb.2022.121822.
- M. Tahir and N. S. Amin, Advances in Visible Light Responsive Titanium Oxide-Based Photocatalysts for CO2 Conversion to Hydrocarbon Fuels, Energy Convers. Manage., 2013, 76, 194–214 CrossRef CAS.
- T. Grewe, M. Meggouh and H. Tueysuez, Nanocatalysts for Solar Water Splitting and a Perspective on Hydrogen Economy, Chem.–Asian J., 2016, 11(1), 22–42 CrossRef CAS PubMed.
- R. V. Gonçalves, H. Wender, S. Khan and M. A. Melo, Photocatalytic Water Splitting by Suspended Semiconductor Particles, Nanoenergy: Nanotechnology Applied for Energy Production, 2018, pp. 107–140 Search PubMed.
- C. Acar, I. Dincer and G. F. Naterer, Review of Photocatalytic Water-splitting Methods for Sustainable Hydrogen Production, Int. J. Energy Res., 2016, 40(11), 1449–1473 CrossRef CAS.
- S. Tasleem and M. Tahir, Current Trends in Strategies to Improve Photocatalytic Performance of Perovskites Materials for Solar to Hydrogen Production, Renewable Sustainable Energy Rev., 2020, 132, 110073 CrossRef CAS.
- A. Kudo and Y. Miseki, Heterogeneous Photocatalyst Materials for Water Splitting, Chem. Soc. Rev., 2009, 38(1), 253–278 RSC.
- P. Shandilya, R. Sharma, R. K. Arya, A. Kumar, D.-V. N. Vo and G. Sharma, Recent Progress and Challenges in Photocatalytic Water Splitting Using Layered Double Hydroxides (LDH) Based Nanocomposites, Int. J. Hydrogen Energy, 2022, 47(88), 37438–37475 CrossRef CAS.
- W. Nabgan, H. Alqaraghuli, A. Owgi, M. Ikram, D.-V. N. Vo, A. A. Jalil, R. Djellabi, A. H. Nordin and F. Medina, A Review on the Design of Nanostructure-Based Materials for Photoelectrochemical Hydrogen Generation from Wastewater: Bibliometric Analysis, Mechanisms, Prospective, and Challenges, Int. J. Hydrogen Energy, 2023, 52, 622–663 CrossRef.
- X. Li, J. Yu, S. Wageh, A. A. Al-Ghamdi and J. Xie, Graphene in Photocatalysis: A Review, Small, 2016, 12(48), 6640–6696 CrossRef PubMed.
- J. W. Ager, M. R. Shaner, K. A. Walczak, I. D. Sharp and S. Ardo, Experimental Demonstrations of Spontaneous, Solar-Driven Photoelectrochemical Water Splitting, Energy Environ. Sci., 2015, 8(10), 2811–2824 RSC.
- M. Ahmed and I. Dincer, A Review on Photoelectrochemical Hydrogen Production Systems: Challenges and Future Directions, Int. J. Hydrogen Energy, 2019, 44(5), 2474–2507 CrossRef.
- S. K. Saraswat, D. D. Rodene and R. B. Gupta, Recent Advancements in Semiconductor Materials for Photoelectrochemical Water Splitting for Hydrogen Production Using Visible Light, Renewable Sustainable Energy Rev., 2018, 89, 228–248 CrossRef.
- A. Fujishima and K. Honda, Electrochemical Photolysis of Water at a Semiconductor Electrode, Nature, 1972, 238(5358), 37–38 CrossRef PubMed.
- A. Steinfeld, Solar Hydrogen Production via a Two-Step Water-Splitting Thermochemical Cycle Based on Zn/ZnO Redox Reactions, Int. J. Hydrogen Energy, 2002, 27(6), 611–619 CrossRef.
- D. Das and T. N. Veziroglu, Advances in Biological Hydrogen Production Processes, Int. J. Hydrogen Energy, 2008, 33(21), 6046–6057 CrossRef.
- Y. Guan, M. Deng, X. Yu and W. Zhang, Two-Stage Photo-Biological Production of Hydrogen by Marine Green Alga Platymonas Subcordiformis, Biochem. Eng. J., 2004, 19(1), 69–73 CrossRef CAS.
- L. Díaz, V. Rodríguez, M. González-Rodríguez, E. Rodríguez-Castellón, M. Algarra, P. Núñez and E. Moretti, M/TiO 2 (M= Fe, Co, Ni, Cu, Zn) Catalysts for Photocatalytic Hydrogen Production under UV and Visible Light Irradiation, Inorg. Chem. Front., 2021, 8(14), 3491–3500 RSC.
- X. Zhong, Y. Li, H. Wu and R. Xie, Recent Progress in BiVO4-Based Heterojunction Nanomaterials for Photocatalytic Applications, Mater. Sci. Eng. B, 2023, 289, 116278 CrossRef CAS.
- D. Ma, M. Yin, K. Liang, M. Xue, Y. Fan and Z. Li, Simple Synthesis and Efficient Photocatalytic Hydrogen Production of WO3-WS2 and WO3–WS2–MoS2, Mater. Sci. Semicond. Process., 2023, 167, 107788 CrossRef CAS.
- S. Ali and T. Ahmad, Treasure Trove for Efficient Hydrogen Evolution through Water Splitting Using Diverse Perovskite Photocatalysts, Mater. Today Chem., 2023, 29, 101387 CrossRef CAS.
- N. S. Reddy, R. Vijitha, B. R. Naidu, K. K. Rao, H. Chang-Sik and K. Venkateswarlu, Benchmarking Recent Advances in Hydrogen Production Using G-C3N4-Based Photocatalysts, Nano Energy, 2023, 108402 Search PubMed.
- W. Bai, K. Wu, C. Wu, N. Li, Y. Gao and L. Ge, Interfacial Engineering to Construct 2D-2D NiCo-LDH/g-C3N4 Heterojunctions for Enhanced Photocatalytic Hydrogen Production Performance, Int. J. Hydrogen Energy, 2023, 48(44), 16704–16714 CrossRef CAS.
- M. Jiang, M. Huang, J. Cong, Y. Yao, W. Sun and B. Wang, Enhanced Visible-Light Photocatalytic Activity of ZrO2/gCN Composite by Introducing Nitrogen Vacancies with H2 Plasma Treatment, J. Photochem. Photobiol., A, 2024, 448, 115324 CrossRef CAS.
- Z. Qi, J. Chen, Q. Li, N. Wang, S. A. Carabineiro and K. Lv, Increasing the Photocatalytic Hydrogen Generation Activity of CdS Nanorods by Introducing Interfacial and Polarization Electric Fields, Small, 2023, 19(46), 2303318 CrossRef CAS.
- Q. Mao, J. Chen, H. Chen, Z. Chen, J. Chen and Y. Li, Few-Layered 1T-MoS 2-Modified ZnCoS Solid-Solution Hollow Dodecahedra for Enhanced Photocatalytic Hydrogen Evolution, J. Mater. Chem. A, 2019, 7(14), 8472–8484 RSC.
- L. Wang, J. Zhao, H. Liu and J. Huang, Design, Modification and Application of Semiconductor Photocatalysts, J. Taiwan Inst. Chem. Eng., 2018, 93, 590–602 CrossRef CAS.
- S. Chandrappa, D. H. Murthy, N. L. Reddy, S. J. Babu, D. Rangappa, U. Bhargav, V. Preethi, M. M. Kumari and M. V. Shankar, Utilizing 2D Materials to Enhance H2 Generation Efficiency via Photocatalytic Reforming Industrial and Solid Waste, Environ. Res., 2021, 200, 111239 CrossRef CAS.
- X. Li, R. Shen, S. Ma, X. Chen and J. Xie, Graphene-Based Heterojunction Photocatalysts, Appl. Surf. Sci., 2018, 430, 53–107 CrossRef CAS.
- Q. Xiang, J. Yu and M. Jaroniec, Graphene-Based Semiconductor Photocatalysts, Chem. Soc. Rev., 2012, 41(2), 782–796 RSC.
- L. Han, P. Wang and S. Dong, Progress in Graphene-Based Photoactive Nanocomposites as a Promising Class of Photocatalyst, Nanoscale, 2012, 4(19), 5814–5825 RSC.
- A. Mondal, A. Prabhakaran, S. Gupta and V. R. Subramanian, Boosting Photocatalytic Activity Using Reduced Graphene Oxide (RGO)/Semiconductor Nanocomposites: Issues and Future Scope, ACS Omega, 2021, 6(13), 8734–8743, DOI:10.1021/acsomega.0c06045.
- J. Corredor, M. J. Rivero and I. Ortiz, New Insights in the Performance and Reuse of rGO/TiO2 Composites for the Photocatalytic Hydrogen Production, Int. J. Hydrogen Energy, 2021, 46(33), 17500–17506, DOI:10.1016/j.ijhydene.2020.01.181.
- F. Khan, M. S. Khan, S. Kamal, M. Arshad, S. I. Ahmad and S. A. Nami, Recent Advances in Graphene Oxide and Reduced Graphene Oxide Based Nanocomposites for the Photodegradation of Dyes, J. Mater. Chem. C, 2020, 8(45), 15940–15955 RSC.
- J. Yu, L. Zhang and P. Kuang, Graphene Oxide–Metal Oxide and Other Graphene Oxide-Based Composites in Photocatalysis and Electrocatalysis, Elsevier, 2022 Search PubMed.
- A. N. Ghoti, A. B. Patil and S. K. Pardeshi, Li Sensitized CdS/TiO2 Nanocomposite Photoanode for Solar Water Splitting, Hydrogen Generation and Photoelectrochemical (PEC) Performance, Int. J. Hydrogen Energy, 2024, 51, 1586–1597 CrossRef CAS.
- L. Huang, L. He, J. Ni, H. Liu, Z. Xu, C. Gong, Q. Zhang and B. Zhang, WO3/Mo: BiVO4 Heterojunction Structured Photoelectrochemical Sensor for Enhancing Hydrogen Peroxide Monitoring and Mechanism Investigation, Electrochim. Acta, 2023, 439, 141641 CrossRef CAS.
- Z. Masoumi, M. Tayebi, M. Kolaei and B.-K. Lee, Improvement of Surface Light Absorption of ZnO Photoanode Using a Double Heterojunction with α–Fe2O3/g–C3N4 Composite to Enhance Photoelectrochemical Water Splitting, Appl. Surf. Sci., 2023, 608, 154915, DOI:10.1016/j.apsusc.2022.154915.
- A. Z. Khan, T. A. Kandiel, S. Abdel-Azeim, T. N. Jahangir and K. Alhooshani, Phosphate Ions Interfacial Drift Layer to Improve the Performance of CoFe−Prussian Blue Hematite Photoanode toward Water Splitting, Appl. Catal., B, 2022, 304, 121014, DOI:10.1016/j.apcatb.2021.121014.
- L. Fu, Z. Li and X. Shang, Recent Surficial Modification Strategies on BiVO4 Based Photoanodes for Photoelectrochemical Water Splitting Enhancement, Int. J. Hydrogen Energy, 2024, 55, 611–624, DOI:10.1016/j.ijhydene.2023.11.253.
- Q. Wang, Y. Zhang, Y. Liu, K. Wang, W. Qiu, L. Chen, W. Li and J. Li, Photocorrosion Behavior of Cu2O Nanowires during Photoelectrochemical CO2 Reduction, J. Electroanal. Chem., 2022, 912, 116252, DOI:10.1016/j.jelechem.2022.116252.
- R. Chong, Z. Wang, J. Lv, J. Rong, L. Zhang, Y. Jia, L. Wang, Z. Chang and X. Wang, A Hybrid CoOOH-rGO/Fe2O3 Photoanode with Spatial Charge Separation and Charge Transfer for Efficient Photoelectrochemical Water Oxidation, J. Catal., 2021, 399, 170–181, DOI:10.1016/j.jcat.2021.05.006.
- L. Li, B. Li, H. Liu, M. Li and B. Wang, Photoelectrochemical Sensing of Hydrogen Peroxide Using TiO2 Nanotube Arrays Decorated with RGO/CdS, J. Alloys Compd., 2020, 815, 152241, DOI:10.1016/j.jallcom.2019.152241.
- S. Vigneshwaran, P. Karthikeyan, C. M. Park and S. Meenakshi, Boosted Insights of Novel Accordion-like (2D/2D) Hybrid Photocatalyst for the Removal of Cationic Dyes: Mechanistic and Degradation Pathways, J. Environ. Manage., 2020, 273, 111125, DOI:10.1016/j.jenvman.2020.111125.
- D. Vidyasagar, A. Gupta, A. Balapure, S. G. Ghugal, A. G. Shende and S. S. Umare, 2D/2D Wg-C3N4/g-C3N4 Composite as “Adsorb and Shuttle” Model Photocatalyst for Pollution Mitigation, J. Photochem. Photobiol., A, 2019, 370, 117–126, DOI:10.1016/j.jphotochem.2018.10.038.
- H. Che, G. Che, H. Dong, W. Hu, H. Hu, C. Liu and C. Li, Fabrication of Z-Scheme Bi3O4Cl/g-C3N4 2D/2D Heterojunctions with Enhanced Interfacial Charge Separation and Photocatalytic Degradation Various Organic Pollutants Activity, Appl. Surf. Sci., 2018, 455, 705–716, DOI:10.1016/j.apsusc.2018.06.038.
- B. Miao, Y. Zhang, Q. Chen, Y. Zhang, Y. Cao, Z. Bai and L. Chen, Highly Enhanced Photocatalytic Hydrogen Production Performance of Heterostructured Ti3C2/TiO2/rGO Composites, Langmuir, 2022, 38(50), 15579–15591, DOI:10.1021/acs.langmuir.2c02227.
- S. Nayak and K. Parida, Recent Progress in LDH@ Graphene and Analogous Heterostructures for Highly Active and Stable Photocatalytic and Photoelectrochemical Water Splitting, Chem.–Asian J., 2021, 16(16), 2211–2248 CrossRef CAS PubMed.
- D.-B. Seo, T. N. Trung, D.-O. Kim, D. V. Duc, S. Hong, Y. Sohn, J.-R. Jeong and E.-T. Kim, Plasmonic Ag-Decorated Few-Layer MoS 2 Nanosheets Vertically Grown on Graphene for Efficient Photoelectrochemical Water Splitting, Nano-Micro Lett., 2020, 12, 1–14 CrossRef.
- F. Carraro, L. Calvillo, M. Cattelan, M. Favaro, M. Righetto, S. Nappini, I. Píš, V. Celorrio, D. J. Fermín, A. Martucci, S. Agnoli and G. Granozzi, Fast One-Pot Synthesis of MoS2/Crumpled Graphene p–n Nanonjunctions for Enhanced Photoelectrochemical Hydrogen Production, ACS Appl. Mater. Interfaces, 2015, 7(46), 25685–25692, DOI:10.1021/acsami.5b06668.
- Q. Quan, S. Xie, B. Weng, Y. Wang and Y. Xu, Revealing the Double-Edged Sword Role of Graphene on Boosted Charge Transfer versus Active Site Control in TiO2 Nanotube Arrays@ RGO/MoS2 Heterostructure, Small, 2018, 14(21), 1704531 CrossRef.
- S. R. Gujjula, U. Pal, N. Chanda, S. Karingula, S. Chirra, S. Siliveri, S. Goskula and V. Narayanan, Versatile Bifunctional Ag@g-C3N4/r-GO Catalyst for Efficient Photo- and Electrocatalytic H2 Production, Energy Fuels, 2023, 37(13), 9722–9735, DOI:10.1021/acs.energyfuels.3c00518.
- M. Nasr, L. Benhamou, A. Kotbi, N. S. Rajput, A. Campos, A.-I. Lahmar, K. Hoummada, K. Kaja, M. El Marssi and M. Jouiad, Photoelectrochemical Enhancement of Graphene@ WS2 Nanosheets for Water Splitting Reaction, Nanomaterials, 2022, 12(11), 1914 CrossRef PubMed.
- A. K. Geim and K. S. Novoselov, The Rise of Graphene, Nat. Mater., 2007, 6(3), 183–191 CrossRef.
- K. Jastrzębski and P. Kula, Emerging Technology for a Green, Sustainable Energy-Promising Materials for Hydrogen Storage, from Nanotubes to Graphene—a Review, Materials, 2021, 14(10), 2499 CrossRef.
- G. Srinivas, Y. Zhu, R. Piner, N. Skipper, M. Ellerby and R. Ruoff, Synthesis of Graphene-like Nanosheets and Their Hydrogen Adsorption Capacity, Carbon, 2010, 48(3), 630–635, DOI:10.1016/j.carbon.2009.10.003.
- C. X. Guo, Y. Wang and C. M. Li, Hierarchical Graphene-Based Material for over 4.0 Wt% Physisorption Hydrogen Storage Capacity, ACS Sustain. Chem. Eng., 2013, 1(1), 14–18 CrossRef.
- S. H. Aboutalebi, S. Aminorroaya-Yamini, I. Nevirkovets, K. Konstantinov and H. K. Liu, Enhanced Hydrogen Storage in Graphene oxide-MWCNTs Composite at Room Temperature, Adv. Energy Mater., 2012, 2(12), 1439–1446 CrossRef.
- V. Jain and B. Kandasubramanian, Functionalized Graphene Materials for Hydrogen Storage, J. Mater. Sci., 2020, 55(5), 1865–1903 CrossRef.
- C.-C. Huang, N.-W. Pu, C.-A. Wang, J.-C. Huang, Y. Sung and M.-D. Ger, Hydrogen Storage in Graphene Decorated with Pd and Pt Nano-Particles Using an Electroless Deposition Technique, Sep. Purif. Technol., 2011, 82, 210–215 CrossRef CAS.
- V. B. Parambhath, R. Nagar and S. Ramaprabhu, Effect of Nitrogen Doping on Hydrogen Storage Capacity of Palladium Decorated Graphene, Langmuir, 2012, 28(20), 7826–7833 CrossRef CAS PubMed.
- A. Ariharan, B. Viswanathan and V. Nandhakumar, Nitrogen Doped Graphene as Potential Material for Hydrogen Storage, Graphene, 2017, 6(2), 41–60 CrossRef CAS.
- J. Hao, F. Wei, X. Zhang, L. Li, C. Chen, G. Wu, L. Wu, D. Liang, X. Ma, P. Lu and H. Song, An Investigation of Li-Decorated N-Doped Penta-Graphene for Hydrogen Storage, Int. J. Hydrogen Energy, 2021, 46(50), 25533–25542, DOI:10.1016/j.ijhydene.2021.05.089.
- S. S. Samantaray, V. Sangeetha, S. Abinaya and S. Ramaprabhu, Enhanced Hydrogen Storage Performance in Pd3Co Decorated Nitrogen/Boron Doped Graphene Composites, Int. J. Hydrogen Energy, 2018, 43(16), 8018–8025, DOI:10.1016/j.ijhydene.2018.03.078.
- Y. Gao, H. Zhang, H. Pan, Q. Li and J. Zhao, Ultrahigh Hydrogen Storage Capacity of Holey Graphyne, Nanotechnology, 2021, 32(21), 215402, DOI:10.1088/1361-6528/abe48d.
- J. Dewangan, V. Mahamiya, A. Shukla and B. Chakraborty, Lithium Decorated ψ-Graphene as a Potential Hydrogen Storage Material: Density Functional Theory Investigations, Int. J. Hydrogen Energy, 2023, 48(96), 37908–37920, DOI:10.1016/j.ijhydene.2022.10.142.
- B. Chakraborty, P. Ray, N. Garg and S. Banerjee, High Capacity Reversible Hydrogen Storage in Titanium Doped 2D Carbon Allotrope ψ-Graphene: Density Functional Theory Investigations, Int. J. Hydrogen Energy, 2021, 46(5), 4154–4167, DOI:10.1016/j.ijhydene.2020.10.161.
- H. T. Nair, P. K. Jha and B. Chakraborty, High-Capacity Hydrogen Storage in Zirconium Decorated Psi-Graphene: Acumen from Density Functional Theory and Molecular Dynamics Simulations, Int. J. Hydrogen Energy, 2023, 48(96), 37860–37871, DOI:10.1016/j.ijhydene.2022.08.084.
- M. Singh, A. Shukla and B. Chakraborty, High Capacity Hydrogen Storage on Zirconium Decorated γ-Graphyne: A Systematic First-Principles Study, Int. J. Hydrogen Energy, 2023, 48(96), 37834–37846, DOI:10.1016/j.ijhydene.2022.07.062.
- P. Panigrahi, M. Desai, M. K. Talari, H. Bae, H. Lee, R. Ahuja and T. Hussain, Selective Decoration of Nitrogenated Holey Graphene (C2N) with Titanium Clusters for Enhanced Hydrogen Storage Application, Int. J. Hydrogen Energy, 2021, 46(10), 7371–7380, DOI:10.1016/j.ijhydene.2020.11.222.
- Y. Chen, H. Habibullah, G. Xia, C. Jin, Y. Wang, Y. Yan, Y. Chen, X. Gong, Y. Lai and C. Wu, Palladium-Phosphide-Modified Three-Dimensional Phospho-Doped Graphene Materials for Hydrogen Storage, Materials, 2023, 16(12), 4219 CrossRef CAS PubMed.
- S. Dong, E. Lv, J. Wang, C. Li, K. Ma, Z. Gao, W. Yang, Z. Ding, C. Wu and I. D. Gates, Construction of Transition Metal-Decorated Boron Doped Twin-Graphene for Hydrogen Storage: A Theoretical Prediction, Fuel, 2021, 304, 121351, DOI:10.1016/j.fuel.2021.121351.
- C. U. Deniz, H. Mert and C. Baykasoglu, Li-Doped Fullerene Pillared Graphene Nanocomposites for Enhancing Hydrogen Storage: A Computational Study, Comput. Mater. Sci., 2021, 186, 110023, DOI:10.1016/j.commatsci.2020.110023.
- Y. Chen, H. Habibullah, G. Xia, C. Jin, Y. Wang, Y. Yan, Y. Chen, X. Gong, Y. Lai and C. Wu, Hydrogen Storage Properties of Economical Graphene Materials Modified by Non-Precious Metal Nickel and Low-Content Palladium, Inorganics, 2023, 11(6), 251 CrossRef CAS.
- A. L. Triguero and S. Rezaie, Hydrogen Storage Performance of an Au-Decorated Graphene Nanosheet, 2022 Search PubMed.
- S. S. Samantaray, V. Sangeetha, S. Abinaya and S. Ramaprabhu, Diatom Frustule-Graphene Based Nanomaterial for Room Temperature Hydrogen Storage, Int. J. Hydrogen Energy, 2020, 45(1), 764–773, DOI:10.1016/j.ijhydene.2019.10.155.
- R. Nagar, B. P. Vinayan, S. S. Samantaray and S. Ramaprabhu, Recent Advances in Hydrogen Storage Using Catalytically and Chemically Modified Graphene Nanocomposites, J. Mater. Chem. A, 2017, 5(44), 22897–22912, 10.1039/C7TA05068B.
- D. Zhou, C. Zheng, Y. Niu, D. Feng, H. Ren, Y. Zhang and H. Yu, Hydrogen Storage Property Improvement of Ball-Milled Mg2.3Y0.1Ni Alloy with Graphene, Int. J. Hydrogen Energy, 2024, 50, 123–135, DOI:10.1016/j.ijhydene.2023.06.255.
- S. Gulati, Mansi, S. Vijayan, S. Kumar, V. Agarwal, B. Harikumar and R. S. Varma, Magnetic Nanocarriers Adorned on Graphene: Promising Contrast-Enhancing Agents with State-of-the-Art Performance in Magnetic Resonance Imaging (MRI) and Theranostics, Mater. Adv., 2022, 3(7), 2971–2989, 10.1039/D1MA01071A.
| This journal is © The Royal Society of Chemistry 2024 |
