Open Access Article
Open Access ArticleA critical review of a hidden epidemic: examining the occupational and environmental risk factors of chronic kidney disease of unknown etiology (CKDu)
Madeleine Bradley a,
Danielle Land
a,
Danielle Land ac,
Darrin A. Thompson
ac,
Darrin A. Thompson bd and
David M. Cwiertny
bd and
David M. Cwiertny *abe
*abe
aUniversity of Iowa, Department of Civil & Environmental Engineering, Iowa City, IA, USA. E-mail: david-cwiertny@uiowa.edu
bUniversity of Iowa, Center for Health Effects of Environmental Contamination, Iowa City, IA, USA
cMichigan State University-Hurley Children's Hospital Pediatric Public Health Initiative, Charles Stewart Mott Department of Public Health, Michigan State University, Flint, MI, USA
dUniversity of Iowa, Department of Occupational and Environmental Health, Iowa City, IA, USA
eUniversity of Iowa, Department of Chemistry, Iowa City, IA, USA
First published on 4th November 2024
Abstract
The global burden of chronic kidney disease (CKD) in terms of mortality and disability-adjusted life years has increased, and this trend is expected to worsen over the next few decades. The primary cause of CKD is known to be due to hypertension and diabetes, however, over the last three decades, a form of CKD has been described in people without any known risk factors. These cases can be described as chronic kidney disease of an unknown etiology (CKDu). Cases of CKDu are rising primarily among rural agricultural communities in affected regions and occur mostly among young male farmers. There is no agreement on whether CKDu in these emerging clusters represents a single disease or a group of different diseases. As such, hypothesized causes of CKDu development include chronic occupational heat stress and dehydration, as well as exposure to environmental contaminants and agrochemicals, such as heavy metals and pesticides. The purpose of this critical review is to bring together the current literature on proposed CKDu etiologies, specifically those related to work in the agricultural sector. This review examines what is known about these occupational and environmental factors and their potential impact on the widespread epidemics of CKDu.
Environmental significanceThis review examines the hidden epidemic of chronic kidney disease of unknown etiology (CKDu), a severe form of chronic kidney disease unrelated to traditional risk factors emerging in clusters predominantly in low- to middle-income countries. There is no agreement on whether CKDu within these clusters represents a single disease or a group of different diseases. Hypothesized causes of CKDu include chronic occupational heat stress and dehydration, as well as exposure to environmental contaminants and agrochemicals. This review examines what is known about these environmental factors and their potential impact on the widespread epidemics of CKDu. |
1. Introduction
Chronic kidney disease (CKD) is a potentially life-threatening condition that requires prompt and sustained medical attention.1 It has become a major public health issue in recent decades, as awareness and recognition of the condition's high burden of disease continues to grow.2 In 1990, CKD was ranked as the 17th leading cause of death globally. In 2017 this rose to 12th, indicating that both prevalence and burden of disease are growing.2 Estimates from 2017 found CKD to be responsible for up to 1.2 million deaths worldwide, and this number is projected to reach between 2.2 and 4.0 million deaths by 2040.2,3 While chronic kidney disease is commonly related to known risk factors like diabetes, hypertension, and glomerulonephritis,4 an epidemic of new CKD cases of unknown cause (CKDu) has been growing across the globe.5–9 In these cases, a patient has none of the known risk factors thought to cause CKD, and the exact cause remains unidentified.6,9,10The total extent of CKDu worldwide is still unknown due to a lack of quality data from many low-income countries.11,12 The first descriptions of CKDu came from Sri Lanka in the 1990s and Costa Rica in the 2000s, when disease hot spots started to appear, but models suggest CKDu in Costa Rica could be traced back to the 1970s.13,14 CKDu hotspots, or areas of high disease prevalence, include Central America and South Asia, chiefly Sri Lanka, India, El Salvador, Costa Rica, and Nicaragua.15–17 Cases there are being recognized particularly in rural areas and regions of agricultural activity.5,13 CKDu has also been referred to as chronic kidney disease of a nontraditional etiology (CKDnt), mesoamerican nephropathy (MEN), and chronic interstitial nephritis of agricultural communities (CINAC).13,18 This variable terminology coupled with varying case definitions may have hindered broader understanding of the condition and its global burden until recent years due to limited literature comparability and data sharing.11,13
With little global acknowledgement of CKDu and varying terminology, tracking and treatment of the condition in certain regions of the world has been a challenge, and much is still unknown about the disease.2,19 Many hypotheses have been put forth suggesting potential causes of these unknown cases of CKD, but a definitive etiology is yet to be identified.13,20
The purpose of this critical review is to bring together the current literature on proposed CKDu etiologies, specifically those related to occupational and environmental risk factors. The review followed a structured approach and was conducted by searching the databases PubMed, Scopus and Google Scholar using key words, including “CKDu”, “etiology” “environmental factors”, and “agriculture”. Over 150 publications were reviewed, with 115 cited here. Content reviewed was limited to that published in the last 30 years.
2. Chronic kidney disease
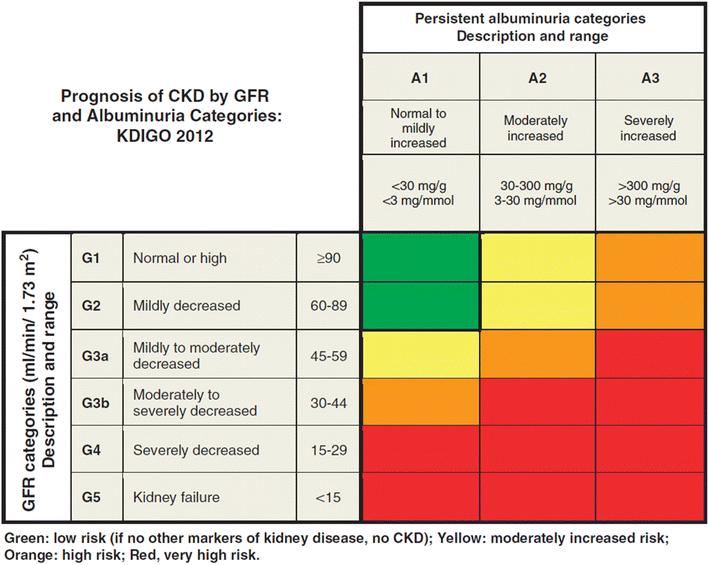
It is estimated that around 10% of the global population is currently affected by CKD, with many unaware of their condition.21 In early stages of the disease patients often present with no symptoms,22 but as CKD progresses, scarring and nephron loss can occur, causing the kidneys to gradually lose their ability to function.23 Further progression can lead to end-stage renal disease (ESRD), which is associated with kidney failure leading to heavy disease burden and premature death.4Recognition of CKD has grown over recent years, as increased awareness of the disease in the research community led to the development of a more specific classification for diagnosis.24 In 2013, a joint international effort involving the U.S. Centers for Disease Control and Prevention (CDC), the Pan American Health Organization, and the Latin American Society of Nephrology and Hypertension led to the development of a set of clinical guidelines for defining and diagnosing CKD. These guidelines have been laid out by an international body that provides recommendations on the evaluation and management of kidney diseases called Kidney Disease: Improving Global Outcomes, or KDIGO.25,26 According to the internationally accepted definition, chronic kidney disease is present when “there are abnormalities in the function or structure of the kidney that are present for greater than three months, with implications for health”.25
2.1. Diagnosing CKD
The kidneys serve a variety of purposes in the body. These include key endocrine and metabolic roles but, most importantly, the primary function of the kidneys is to maintain normal internal homeostasis – such as normal electrolyte, fluid, and acid–base balance, and the excretion of water-soluble waste products from the body.25 Nephrons, the functional units of the kidneys, remove excessive levels of solutes like electrolytes, which prevents blood from becoming toxic.27 Additionally, the kidneys produce important hormones like renin and erythropoietin, which keep the human body functioning properly.24 When the kidneys are not working efficiently, individuals can develop symptoms like weight loss, poor appetite, infections, and impairments in physical and cognitive functioning.22,27Diagnosing CKD can be a challenge, however, as there is not always a clear indicator of disease. Unlike many other diseases, CKD is often asymptomatic until it is very advanced.2,4 The best estimates of kidney function can be determined by testing an individual's glomerular filtration rate and albuminuria levels.4,25 The glomerular filtration rate measures the volume of blood that is filtered by the glomeruli, tiny filters in the kidneys, each minute, whereas a test of the albuminuria level measures the amount of the protein albumin that is present in the urine.28,29 When the glomerular filtration rate is low and there is evidence of increased albuminuria, the individual is suffering from some level of kidney disease.4,25
After being diagnosed, CKD is classified by cause and then further staged based on rates of eGFR and albuminuria (Fig. 1). These levels are important in determining the risks an individual may have after developing CKD and can help to determine the severity of the disease.4,30 To progress between each category a patient must show prolonged elevated albuminuria levels and continual decreases in the estimated glomerular filtration rate (eGFR).4,17 Patients can progress rapidly through the stages to kidney failure within months, or it can take years of follow up before a patient will become severely ill with the disease.25,26
2.2. Known CKD risk factors
The most common risk factors for CKD include diabetes and hypertension.24 Of kidney failure cases in the U.S. between 2015 and 2017, 75% had primary diagnoses of diabetes or hypertension.31,32 However, factors like age, race, and socioeconomic status are also thought to play a role in determining the risk of developing CKD.2 Large scale screening programs implemented in the U.S., Australia, and Norway in the 2000s revealed that over 10% of the population had markers of disease.2 It is important to note that despite high prevalence of the disease in the general population, it is not recommended to screen individuals for CKD without a history of known risk factors or symptoms.24 However, most cases of chronic kidney disease begin asymptomatically,22 and without a thorough understanding of all of the risk factors that could cause CKD, many CKD patients remain unaware of their condition.22.3. CKD treatment and associated costs
In both developed and developing countries, it is estimated that fewer than 10% of patients with CKD know that they have the disease.33 This results in medical resources being primarily geared towards providing treatments for ESRD and not the earlier stages of CKD. While there is currently no cure for chronic kidney disease, available treatments can prevent the disease from progressing to a more advanced stage of CKD.2,34 The best approach to managing CKD is early detection so that measures can be put in place to limit diseases progression and reduce cardiovascular disease, before the disease has time to greatly impair kidney functions or cause kidney failure.35 Things such as dietary changes, exercise, medications, and treatment of known risk factors (e.g. diabetes and/or hypertension) are the primary approaches for CKD treatment by medical professionals.25,36 These interventions can improve outcomes for both renal and cardiovascular functions, slowing or preventing the progression to ESRD.2The treatment costs of CKD started rising after the 1960s when renal replacement techniques (RRT) became available.2,36 If CKD progresses and kidney failure occurs, patients will need RRT in the form of regular dialysis or kidney transplants, both of which are costly and not easily obtained in most low- and middle-income countries around the world.12,36 This is also the case in high income countries, where the high cost of long-term dialysis is a barrier to many people receiving treatment.37 Estimates show that around 2.5 million patients worldwide currently undergo dialysis or await kidney transplants, and this need is expected to double to 5.4 million by 2030.2 While large, this number only represents around 10% of those who currently require treatment to live, leaving 90% of those with kidney failure without access to necessary lifesaving treatment.24
CKD and its projected growth represent a major public health and economic challenge worldwide. Though many people are not treated, the availability of renal replacement therapies is increasing, and as more people live longer with the disease, the total disease burden of CKD grows.2,36 Compared to worldwide rates, the U.S. and Mexico were found to have some of the highest CKD burdens, which can be largely attributed to similarly high rates of diabetes and hypertension.38,39 In the U.S., treatment for CKD is thought to exceed 46 billion U.S. dollars each year.24,36 Additionally, those with CKD may also develop comorbidities such as cardiovascular disease, anemia, bone disorders, and diseases of the nervous system, which can add to healthcare costs.24
3. Chronic kidney disease of an unknown etiology
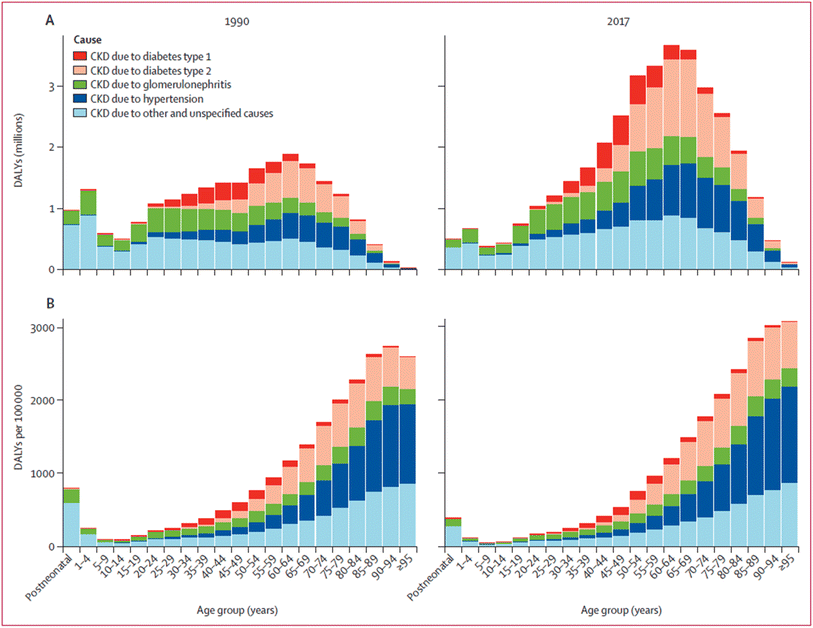
Over the last few decades, an increase in cases of CKD and CKD mortality has been observed, particularly in rural areas of Central America and South Asia.40,41 As previously discussed, many cases of CKD are related to risk factors such as diabetes, hypertension, and aging. However, some cases have an unknown origin, and these instances can be described as cases of chronic kidney disease of unknown etiology, or CKDu. Regional clustering of this disease suggests CKDu is an environmentally induced problem.42Bikbov et al.,2 shows the distribution of disability-adjusted life years (or DALYs) of CKD in 1990 and 2017 based on the underlying cause (Fig. 2). DALYs represent the sum of the years of potential life that is lost due to premature mortality or the years of life lost due to a disability, and it is a valuable metric because it exemplifies the overall burden of a disease, not just the case load.2 While the largest contribution of CKD DALYs in 2017 was due to traditional risk factors, such as diabetes, a substantial number of DALYs across all age groups were deemed to be caused by ‘other and unspecified causes’. These cases were estimated to have the highest age standardized rate of DALYs across all causes of CKD in 2017 at 138.8 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in the population.2
000 in the population.2
CKDu is a global disease affecting low-, middle-, and high-income countries, highlighting the urgency in understanding its risk factors and causes.43 The global burden of CKD and CKDu is increasing faster in low- and middle-income countries, and even within economically developing nations, those affected by CKDu are usually from the most underserved populations, including in the U.S. among immigrant agricultural workers38,44 and Aboriginal groups in central Australia and New Zealand.11,45 CKDu is a diagnosis of exclusion and characteristics of the disease may vary in different geographic locations.43,46 To date, there is no conclusive evidence linking CKDu to any single etiological cause despite consistent association across regions with male sex, rural residence, and farm labor.11
4. Proposed etiologies
While many etiologies have been proposed for CKDu, none have been definitively recognized in any region of the world.47 Regional epidemics tend to have common demographics, disease presentation, clinical features, and renal histopathology (i.e., kidney tissue effects).48 However, study designs and outcome measurements differ between studies significantly so that it is difficult to identify underlying etiology or if the clusters of diseases across different geographical locations are a single or a group of different diseases.15 Studies have noted clinical differences between CKD and CKDu, though there is no agreement on a clinical or histopathological feature that can be diagnostic in CKDu.1 In CKD caused by hypertension and diabetes, the glomeruli are predominantly affected, thus proteinuria (presence of protein in urine) is an early sign of disease.46 Unlike common CKD, CKDu is generally recognized as a disease process affecting the tubulo-interstitial compartment of the kidney, and proteinuria is considered inconsistent with a diagnosis of CKDu, making this commonly used marker ineffective for diagnosis or screening.46,49 The Disadvantaged Population estimated Glomerular Filtration Rate (eGFR) Epidemiology Study (DEGREE) definition of CKDu even excludes cases with heavy proteinuria.50 In fact, one proposed definition of CKDu is kidney damage with an eGFR of <60 mL min−1/1.73 m2 in the absence of diabetes, hypertension, heavy proteinuria, and structural renal disease.51–53 Nevertheless, proteinuria continues to be used as an early indicator of disease by the Department of Health in Sri Lanka and all other CKDu-affected countries.48Hotspots of CKDu have mostly been reported in agricultural communities in Central America and South Asia, and suspected in locations such as Africa, the Middle East, and North America. Clusters of CKDu have been described in predominantly rural, agricultural communities and appear to affect lower socioeconomic classes that work in farm labor in tropical climates.54 CKDu is characterized as slowly progressing and asymptomatic until advanced stages of the sickness, which is described as an incurable and slowly deteriorating disorder.55 This can lead to high mortality rates and disease burden, especially in CKDu hotspots. Major hotspots of CKDu disease prevalence include Central American countries such as Nicaragua, El Salvador, Costa Rica, and Mexico, as well as South Asia including India and Sri Lanka.13,38 Because there is evidence from some histopathological studies that CDKu patterns are similar (if not the same) across regional epidemics, identification of risk factors would be impactful not only regionally but also globally in understanding chronic kidney disease.
Based on current evidence, there is a clear association between the distribution of CKDu cases and rural and agricultural communities.56 Even though some of those who are affected are not agricultural workers, a majority live in communities close to some form of agriculture. This suggests some condition or combination of conditions related to rural agricultural settings is a possible factor contributing to widespread CKDu. Proposed causation typically begins with occupational, social, or environmental exposures, which are then tied directly to secondary factors like heat or exposure to pesticides, heavy metals, or contaminated food.57 Based on this premise and by simplifying the web of causation from Gunatilake et al.,57 three main subgroups of etiologies have been identified that are the most salient in the discussion of the origins of CKDu. These include heat stress, drinking water contamination, and agrochemical exposures. Below, the relevant literature is synthesized surrounding these three etiologies. Other proposed factors not discussed in this review include genetics, ethnicity, alcoholism, altitude, the use of nonsteroidal drugs, and more.25 While some literature has found associations between these factors and chronic kidney disease of unknown origin, evidence is generally limited.1 Ultimately, more research is needed to determine whether any are likely drivers of disease.
4.1. Heat stress nephropathy
A significant body of research published on the topic has suggested that heat stress neuropathy due to repeated dehydration and strenuous manual labor is the main risk factor for the development of CKDu.58–60 The association between occupational heat exposure with kidney injury has been mainly reported in tropical regions. A combination of heat stress, water shortage, and overexertion can initiate several physiologic processes that lead to kidney injury, and repeated kidney injury may subsequently lead to CKD.59 Heat stress occurs when the body cannot dissipate excess heat, leading to a rise in core temperature and increased heart rate.61 This can happen due to a combination of factors such as high air temperature, humidity, physical exertion, and clothing.61 When the body absorbs more heat than it can release, it can result in various heat-related illnesses like heat stroke, exhaustion, cramps, and rashes.61Experts suggest that CKDu may be a form of heat-stress nephropathy and “may represent one of the first epidemics due to global warming”.59 As accelerating climate change brings increased heat extremes, there is an urgent need to identify its impact on climate-related health crises.59,62,63 The area of climate and health should be a focus in future research of CKDu to identify, and subsequently prevent and treat, its cause. Future research should also focus on investigation of environmental contaminants and co-occurrence impacts on CKDu.
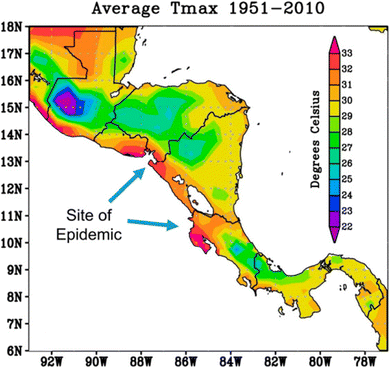
Central America has been the most studied region surrounding heat stress nephropathy. Researchers have shown that in Costa Rica, CKDu was probably present as far back as the 1970's in the Guanacaste Province on the Pacific Coast.16,64 By 2010, the prevalence of CKDu in this area had grown ten-fold in men and four-fold in women.16,64 While the study could not definitively attribute the increase in cases to rising temperatures, it noted heat stress and dehydration as possible stressors.16 Another study suggested a possible link between increase in cases in Costa Rica with climate change.59 Over that time (1970s to 2010), maximum temperatures in Central America rose by 0.2 degrees Celsius, with the number of extremely hot days increasing by 30–75%.59
For this region, Fig. 3 illustrates that the higher average maximum temperatures fall along the Pacific Coast, the main region of the epidemic in Central America. However, suggesting that a warming climate correlates with a rise in CKDu cases does not necessarily prove that a link exists, and fails to account for other factors that could be at play. CKDu is thought to be most prevalent among sugar cane workers in Central America, occurring more commonly in men,65 and thus it could be related to occupational heat stress. Nevertheless, women and men who are not sugarcane workers are still suffering from CKDu in Central America,56 indicating that there may be co-existent risk factors (e.g., agrochemical exposure or contaminated drinking water). More research targeted to these epidemic sites is recommended to determine possible causation more definitively, and additional contributing risk factors, between heat stress and kidney disease.
The relationship between CKDu and heat has also been found in other studies with specific groups of agricultural workers. In a group of sugarcane workers with normal preharvest kidney function, researchers found that the development of acute kidney injury (AKI) during the harvest season was a common occurrence, developing in 34 of 326 workers.65 Of the workers who had developed work-related AKI and participated in at least one follow up visit at 6 and 12 months, 11 experienced a >30% decrease in eGFR, an indication of CKD, while 18 stayed the same or improved.65 Researchers attributed the frequency with which CKDu was found in the sugarcane workers to their work in such extreme heat conditions, but of the 326 study participants, all were males who worked in sugar cane fields, and other occupational or socioeconomic factors were not ruled out.65 A more recent study published in 2023 focused on a cohort of 569 outdoor workers in El Salvador and Nicaragua and examined how occupation related to heat stress and heat strain.66 Of the five industries represented among the cohort (sugarcane, corn, plantain, brickmaking, and construction workers), sugarcane workers and Nicaraguan agrochemical applicators performed more strenuous work and experienced greater levels of heat strain and subsequent impairment of kidney function (defined by eGFR < 90 mL min−1/1.73 m2).66 Another review analyzed longitudinal data from more than 800 sugarcane cutters and found inflammation biomarkers and fever were associated with kidney injury.67
Although few studies have examined the impact of heat stress and strain on kidney function in populations in the U.S., available evidence suggests the presence of CKDu hotspots in North America.38,68 In a sample of 471 agriculture workers across 29 farms in California, heavy occupational workload and piece-rate work (i.e., compensation per task completed) were associated with AKI.69 Additionally, evidence of AKI across a single work shift was found in 14.9% of participants in this group and increased workload was associated with a nearly two-fold increase in the odds of developing AKI at work.69 A study of 192 agricultural workers in Florida found that for each 5 degree (Fahrenheit) increase in the heat index, the odds of AKI increased by 47%.68
Recently, researchers have warned against accepting the heat stress neuropathy hypothesis as the leading cause of CKDu because it could be detrimental in the effort to search for other possible etiological factors, such as exposure to environmental toxins.46,56,70,71 One research team found that high temperatures did not have any significant impact on CKDu development in El Salvador,72 and another concluded that the trends in CKDu in Central America cannot be fully explained by the heat stress hypothesis.73 Herath et al.,56 based on a review of heat-related literature, found inadequate evidence to support a causal relationship between heat stress and CKDu. They argue that CKDu is not observed in other tropical regions of the world where workers are exposed to heat stress, and that the existence of cases of CKDu in populations that have not been exposed to heat stress would lead to the assumption that other factors are more likely to be at play.46,56,70,71,74 A systematic review by Chapman et al., found no consistent evidence that supports an association between CKD and heat-stress or dehydration, though they did find statistically significant associations for the use of pesticides.75 We note, as also suggested by Mendley et al.,13 cases in these other regions of the world may simply remain unreported due to a lack of quality data.
An intervention program study conducted in El Salvador and Nicaragua showed that the implementation of so called water-rest-shade programs was able to reduce the incidence of kidney damage in workers that were high risk.76 The program, an intervention adapted from the U.S. Occupational Health and Safety Administration (OSHA) was conducted two months into a five month harvest period. Each worker was provided with a camelback for water and stations for water refills, as well as nearby shade tents and mandatory 10 to 15 minute rest periods every 1 to 1.5 hours. Results showed that those who participated in the intervention program had less of a decrease in their eGFR levels than the control group. This was observed both across a workday and across the entire harvest, though sample size was limited.76 While this association does not prove causality for developing the disease, the finding that rest and shade (i.e., less heat stress) leads to a reduction in kidney damage is important and should be explored further. It also represents a promising, relatively easy to implement best practice for protecting the kidney health of workers in occupations prone to heat stress.
4.2. Drinking water source and quality
The characteristic, unique geographical distribution of CKDu cases suggests the possible involvement of exposure to contaminated drinking water.77 Consistent exposures to contaminated drinking water have been found to increase risks of developing cancer and disorders of the kidney, liver, and reproductive organs.78 A lack of quality drinking water has also been linked to cases of CKDu.14,79–83 Thus, it is an important factor to consider in furthering our understanding of possible etiologies.A number of studies have postulated a role for groundwater quality in disease incidence and progression, suggesting it is a significant contributor to the etiology of CKDu.9,84,85 In Sri Lanka, CKDu is more prominent among communities that consume groundwater as their main source of drinking water, with more than 87% of the population living in CKDu-endemic regions relying on wells.80 Additionally, previous research has shown that people who drink well water are more vulnerable to the disease compared to those who consume water from public water supplies, natural springs, rainwater, and surface waters.9 In these same CKDu-endemic regions, very low (1.5%) or zero prevalence of CKDu was reported from those who consumed spring water.9,86 Data has also shown that CKDu patients tend to cluster toward the lower elevations of local tanks and canal systems, further implying a hydro-geochemical relationship.6,86,87 Recently, one study in Central India found CKDu cases had significantly higher surface water usage as their drinking water source (OR 3.178, 95% CI 1.029–9.818).88
The most studied environmentally induced causative factor for CKDu is high fluoride (F−) concentration in drinking water.85 Fluorine is a naturally abundant chemical element, and due to water–rock interactions within aquifers fluoride is commonly present in groundwater. Kidneys are one of the most important organs that remove fluoride from the body, with about 60% of the daily fluoride that is absorbed by healthy adults ultimately excreted in urine.89 Therefore, the kidney is one of the most exposed soft tissues to fluoride in the body.89 A 2006 report by the U.S. National Research Council stressed the dangers of fluoride on renal tissue, stating that “human kidneys concentrate fluoride as much as 50-fold from plasma to urine. Portions of the renal system may therefore be at higher risk of fluoride toxicity than most soft tissues”.90 Some of the major adverse effects on kidneys attributed to excessive fluoride consumption include inhibition in tubular reabsorption, changes in urinary ion excretion, and disruption of collage biosynthesis in the body.91,92
Due to the health risks associated with long-term exposure to high fluoride concentrations, the World Health Organization (WHO) recommends a maximum acceptable limit of 1.5 mg L−1 of fluoride in drinking water with an optimal range between 0.5 and 1.0 mg L−1.90 The United States began fluoridating drinking water in public waters systems in 1950 to prevent tooth decay.92 The recommended U.S. drinking water fluoride concentration is 0.7 mg L−1, and the current enforceable drinking water standard (i.e., Maximum Contaminant Level or MCL) for fluoride is 4.0 mg L−1.93 However, some evidence suggests that excessive consumption of fluoride may have clinical consequences such as adverse effects on the kidneys, including at concentrations below the U.S. enforceable limit.91 Studies have noted that increased fluoride consumption among children is a risk factor for renal damage,94,95 even at concentrations as low as 2.0 mg L−1 in drinking water, due to their ability to retain absorbed fluoride.91,96,97
Fluoride as a causative factor for CKDu in Sri Lanka is of particular interest due to the remarkably confined geographical distribution of cases in the country.47,48 Most CKDu-affected areas in Sri Lanka are within the dry zone, an area that receives little rainfall and experiences prolonged dry periods. Additionally, groundwater fluoride concentrations in the dry zone are relatively high (0.5 mg L−1) compared to the wet zone (<0.5 mg L−1), where incidents of CKDu are minimal.6,48 One study analyzed 1304 water samples from wells in all districts in Sri Lanka from 2010 to 2014, finding evidence of multiple drinking water contaminants.81 Results showed that the Sri Lankan well water was highly contaminated with fluoride, with 20% of wells containing a concentration > 1.0 mg L−1 (above the Sri Lankan standard of 1.0 mg L−1). Of these wells, 9.9% had concentrations above the WHO's standard of 1.5 mg L−1, with the highest district measuring 7.0 mg L−1. Additionally, a majority of water samples showed high water hardness, with 42.2% of samples having hardness levels (in mg L−1 as CaCO3) greater than or equal to 180 mg L−1. Wells were also found to have elevated levels of aluminum, nitrate, arsenic and manganese, but the researchers did not associate these to possible CKDu risk factors. Based on spatial association, fluoride contamination and water hardness were proposed as suspected risk factors for CKDu.81
In another study, Balasooriya et al.80 compared hydrogeochemical data from wells of consumers affected and unaffected by CKDu in Sri Lanka. A total of 63 groundwater well samples were taken, with one third (19) taken from wells used by CKDu patients. Significantly higher values of pH, total hardness, electrical conductivity, alkalinity, and calcium (Ca2+), magnesium (Mg2+), and F− were observed for wells used by CKDu patients when compared to wells used by study participants without CKDu. Mg2+ was found to be the most prominent factor for water hardness in the wells of CKDu affected consumers, and the combination of fluoride and hard water was suggested as a potential risk factor for CKDu in the region. The median F− concentration for non-CKDu regions was 0.49 mg L−1 compared to 0.63 mg L−1 for CKDu regions, with CKDu wells ranging from concentrations of 0.23 mg L−1 to 5.00 mg L−1. Similarly, another study by Shi et al.98 compared 52 groundwater samples from CKDu-impacted wells to 18 groundwater samples from non-CKDu impacted wells, based on official reports on CKDu well distribution in Sri Lanka. This study found significantly higher Si4+ and F− concentrations in CKDu groundwater sample.98
Gobalarajah et al.20 collected 38 water samples in Thunukkai Division in Mullaitivu District, Sri Lanka and investigated the association between physicochemical characteristics of the drinking water and serum creatinine levels (a measure of kidney function) of CKDu patients. Of the 38 samples, 35 were taken from areas CKDu patients lived and 3 were collected as controls where there were no records of CKDu patients. The authors found that six explanatory variables accounted for 51% of variation in serum creatinine concentration of the CKDu patients: F−, phosphate, TDS, total hardness, and arsenic (R2 = 0.5109). Serum creatinine levels of the CKDu patients were significantly and negatively correlated with phosphate (R = −0.628, p < 0.001), as likelihood of CKDu occurrence decreased by 0.211 times for every unit increase in phosphate content. This negative influence of phosphate ions in water on serum creatinine of CKDu patients had been noted in an earlier study.99 The study also found that TDS (R = 0.271) and As (R = 0.304) concentrations were significantly positively correlated with creatinine levels (p < 0.05).20 Although the authors found F− was not significantly correlated to serum creatinine levels, they noted multiple studies in the past had found significant relationships between F− content and CKDu occurrence.80
While fluoride has been linked to incidence of CKDu, fluoride as a single causative factor would not explain the absence of CKDu in areas located in endemic regions with similar geographic and socio-economic characteristics. Additionally, CKDu has been diagnosed in individuals who used to drink low fluoride water. Other groundwater constituents, such as hardness9 and more recently, silica,100,101 have been linked to the occurrence of CKDu. For example, one study found groundwater in CKDu endemic regions had higher average Si compared to non-CKDu regions in Sri Lanka (p < 0.05).98
Correlation between high water hardness and CKDu occurrence has been extensively reported in the literature.20,42 Imbulana et al.102 found higher numbers of CKDu cases in areas using finished (i.e., after treatment) drinking water with high levels of total dissolved solids, major ions (calcium, magnesium, sodium, potassium, chloride, and sulphate) and high hardness. Many earlier studies have reported a relationship between water hardness and CKDu occurrence, with one study demonstrating that 96% of patients who developed CKDu had been exposed to hard or very hard water over a period of 5 years.82 Hard water is attributable to the presence of polyvalent cations including calcium, magnesium, strontium, and iron, which will be present together with carbonate, bicarbonate, sulfate, and chloride anions.103 These hardness-causing minerals enter groundwater during naturally-occurring hydrogeochemical processes, along with other elements commonly found in the geogenic environment.20,104 Research has suggested that hard water in combination with other elements or contaminants may increase the risk of CKDu incidence, as the presence of hardness may modify chemical forms of fluoride and trace elements in the water, acting as a secondary factor for disease etiology.9,83,105,106 A recent study published in 2024 suggested Mg hardness combined with excess F− could produce nephrotoxic complexes that may trigger kidney damage in CKDu endemic areas of Sri Lanka.107 Another found exposure to Sri Lanka's groundwater caused kidney damage in zebrafish, and the authors concluded these findings suggested high water hardness and fluorine could be the inducible environmental factors for CKDu.106
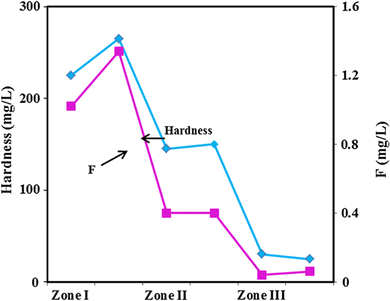
Fig. 4 from Wasana et al.9 shows such a correlation between high hardness, high fluoride, and CKDu prevalence. For this study, researchers divided a larger, high-risk region for CKDu in Sri Lanka into three sub zones. Zone 1 describes areas of high CKDu prevalence with a majority of residents being affected by the disease, and the main water resources are either dug or tube well water. Zone 2 refers to areas of low CKDu prevalence with the majority unaffected by disease, and the main water resource in this zone is mostly dug well water. Zone 3 encompasses areas that are within the larger high-risk region, but there is no prevalence of disease and residents here use spring water. After plotting fluoride and hardness of drinking water against prevalence of CKDu, Fig. 4 reveals a strong correlation between the high-prevalence areas of zone 1 and high levels of hardness and fluoride. Zone 2, which has less CKDu prevalence, also exhibits lower levels of hardness and fluoride, and in zone 3 where the disease is not present, these levels decrease even further.
A follow up study with mice corroborated the possible combined effects of fluoride and hard water on CKDu.78 The authors suggested that synergic effects of cadmium, fluoride, and water hardness could lead to kidney damage in mice, even at WHO maximum recommended levels. Toxic synergistic effects of cadmium, fluoride, and hardness were more pronounced when present in water at relatively higher concentrations (e.g., double the WHO maximum levels). Notably, when the mice were treated with drinking water excluding one or two of the elements, evidence of renal injury was absent.
While there is no consensus on any of these factors as a definitive cause of CKDu, sufficient evidence has established a direct or indirect relationship between disease occurrence and groundwater use. Additionally, some studies have suggested that providing water that meets drinking water standards and guidelines helps reduce the occurrence of CKDu. For example, low incidence of disease has been noted among people who consume more pristine natural spring water and treated water from large municipal water systems while living in hotspot regions of CKDu.102,108 One study in Sri Lanka found the highest risk for CKDu was in participants who consume well water (OR 2.52, 95% CI 1.12–5.70).82 Reducing excessive contents of fluoride, hardness, and other dissolved ions in groundwater has been effective in reducing the incidence of CKDu and protects residents from other health impacts related to consumption of drinking water with high fluoride and hardness.102
4.3. Nephrotoxic heavy metals in drinking water
Trace heavy metals are common environmental pollutants that have been associated with impaired kidney function in epidemiological studies. Many such metals are nonessential for human health, toxic at low doses, and persistent in the environment. Potentially nephrotoxic heavy metals include cadmium (Cd), mercury (Hg), lead (Pb), chromium (Cr), uranium (U), gold (Au), platinum (Pt), cobalt (Co), nickel (Ni), vanadium (V), and thallium (Tl); and metalloids such as arsenic (As) and antimony (Sb).6 Heavy metals can bioaccumulate in the kidney, which increases the risk of kidney damage from both high-level acute and low-level chronic exposures.109,110 Some studies have identified heavy metals and metalloids (notably, arsenic, cadmium, and lead) as causative factors for CKDu, although some others have found conflicting results.110In Western Australia, CKD has become endemic for Aboriginal people living in the area and researchers believe a high proportion of these cases are CKDu.45 In an examination of drinking water quality in the affected communities, exceedingly high levels of both nitrate and uranium in the groundwater were observed.45 Each of these components are thought to be nephrotoxic, but they can also combine to form a highly nephrotoxic compound called uranyl nitrate. Uranyl nitrate [UO2(NO3)2] is a complex of the common uranium ion uranyl (UO22+) and nitrate (NO3−), and it is highly soluble in water and is thus absorbed by the gastrointestinal tract more readily than low soluble uranium compounds.45 Uranium's main target is the kidneys, and kidney damage has been seen in humans and animals after inhaling or ingesting uranium compounds.111 However, ingesting water-soluble uranium compounds (e.g., uranyl nitrate) is known to result in kidney effects at lower doses than following exposure to insoluble uranium compounds.111
Other contaminants thought to be linked to CKDu include cadmium, lead, and arsenic, which have each been found in drinking water, foods, and soils in areas of high CKDu prevalence.112 Although the United States has not yet reported hot spots of CKDu incidence, low levels of lead in community water systems have been associated with lower hemoglobin levels and higher erythropoietin stimulating agent (ESA) use among patients with end stage kidney disease (ESKD), suggesting that even low levels of lead can have impacts on kidney health in vulnerable patients.113 Arsenic has received considerable attention over the years as a potential driver for CKDu due to widespread groundwater contamination. One study in the US investigated the relationship between urine arsenic levels and the presence of albuminuria, a marker of kidney damage, in American Indian adults from rural areas of Arizona, Oklahoma, and North and South Dakota.114 In a cross-sectional study, the researchers found urine arsenic concentrations were associated with increased albuminuria in a rural US population with a high burden of diabetes and obesity.114
Conversely, many research groups have reported large variations in concentrations of heavy metals in drinking water sources used by CKDu patients, and nearly all studies detected amounts of contaminants below the maximum acceptable levels by the WHO.6,80,110 Additionally, concentrations of heavy metals and metalloids have generally not shown significant variation between endemic and non-endemic areas. For example, when analyzing spatial relationships between high disease prevalence and drinking water concentrations of arsenic, lead, cadmium, or uranium, Rango et al.115 found no significant relationship linking any contaminant to CKDu. Thus, although most evidence suggests that heavy metals and metalloids do not cause CKDu on their own, given their ability to bioaccumulate in tissues, more work is needed in order to understand or eliminate heavy metals as a cause of CKDu.
4.4. Agrochemical exposures
Given the prevalence of CKDu in agricultural communities and among rural agricultural workers, multiple studies have aimed to determine the effect that pesticides and other agrochemicals can have on CKDu development. Experimental studies have suggested a relationship between pesticide exposure and impaired renal function, but few high-quality epidemiological studies exist that directly examine this relationship. One such study is the Agricultural Health Study (AHS), a longitudinal cohort study of agricultural workers and their families from Iowa and North Carolina.116 Based on data from the Agricultural Health Study, a smaller cohort study of licensed pesticide applicators was evaluated. ESRD risk was associated with the highest tertile of intensity-weighted use of the herbicides atrazine, metolachlor, alachlor, paraquat, and pendimethalin when compared to no use.116 Researchers observed a significant exposure-response trend with increasing use levels for all of these herbicides. Additionally, ESRD risk was associated with the highest tertile of metalaxyl use, a fungicide (HR = 1.92; 95% CI: 1.01, 3.66). The risk of developing ESRD was greater for study participants who had to undergo multiple doctor visits (HR = 2.13; 95% CI: 1.17, 3.89) or hospitalizations due to pesticide use (HR = 3.05; 95% CI: 1.67, 5.58).116 These findings support an association between ESRD and chronic exposure to certain pesticides.The authors conducted another study investigating the relationship between ESRD among wives of licensed pesticide applicators in the same AHS cohort.117 The rate of ESRD was significantly elevated among women who reported the highest versus the lowest cumulative pesticide use (HR: 4.22; 95% CI: 1.26, 14.20). Among women who never applied pesticides, ESRD was associated with whether their husband ever used paraquat (HR = 1.99; 95% CI: 1.14, 3.47) and butylate (HR = 1.71; 95% CI: 1.00, 2.95). These findings suggest ESRD may be associated with direct and/or indirect exposure to pesticides.
Additional studies have corroborated the connection between pesticide use and diminished kidney function. A study of a community in Mexico found that kidney function tests of pesticide-exposed farmworkers were worse than tests of workers in an unexposed control group.118 Another found lower eGFR among pesticide applicators who had ever used the herbicides pendimethalin, atrazine, and dicamba compared with applicators who never used each pesticide. Prior use of pendimethalin (OR = 1.6, 95% CI: 1.1, 2.2) and atrazine (OR = 1.8, 95% CI: 1.0, 3.0) was associated with elevated odds of CKD.119 In a 2019 study, patients with Mesoamerican nephropathy (a form of CKDu) with exposure to any agrochemical (OR = 4.86; 95% CI: 1.82, 12.96) or paraquat (OR = 12.25; 95% CI: 1.51, 99.36) was associated with kidney failure compared to non-exposed patients.120
Glyphosate has been identified as a possible agrochemical that could lead to kidney damage.57 Some have suggested this could be because of its unique metal chelating properties, which allow it to form a complex with nephrotoxic metals.79 Glyphosate is one of the most widely used herbicides in the modern world, and has often been reported as widely used in hot spot areas of the CKDu epidemic. Indeed, one study in Sri Lanka found that participants had a higher risk of CKDu if they had a history of working with spray glyphosate (OR 5.12, 95% CI 2.33–11.26).82
Co-exposure to the pesticide paraquat along with glyphosate was also cited as a possible risk factor for developing chronic kidney disease.57,116 Indirect exposure to the pesticides paraquat and butylate were found to be associated with increased ESRD rates, with those exposed developing ESRD at more than twice the rates of those who had no exposure. The synergistic effects of both glyphosate and paraquat have also been described as playing a role in renal damage.57,105,121
Another widely studied chemical class proposed as a causative factor in CKDu development is organochlorine pesticides (OCPs). Ghosh et al.21 compared levels of pesticides in the blood in healthy controls, CKDu, and CKD of known etiology participant groups. They detected nine OCPs in both groups of CKD patients and in healthy controls. In CKDu patients, increased levels of four pesticides, α-HCH, aldrin, β-endosulfan, and p,p′-DDE were observed when compared to levels in healthy controls. Two pesticides exhibited significant association with CKDu when using patient with CKD of known origin as the reference group: β-endosulfan (OR = 2.16) and p,p′-DDE (OR = 3.20).21 This indicates that there is a potential association between certain OCP exposures and the development of CKDu. Notably, these study participants were not directly involved in agricultural activity or manufacturing, but the authors suggest they were likely exposed through environmental contamination.
As with each of the previously discussed proposed etiologies, the literature is not conclusive nor extensive enough to determine if agrochemicals are a definitive cause of CKDu and conflicting opinions exist. A review of 25 epidemiological studies from Meso-America found no significant associations between CKDu and pesticide exposure.122 Another determined that while agrochemicals may contribute to the genesis of CKDu, they are unlikely to be the sole or main cause.123 Valcke et al.124 found that existing studies did not provide enough evidence for an association between pesticides and regional CKDu epidemics. However, due to poor pesticide exposure assessment, the authors stated that the role of nephrotoxic agrochemicals cannot be discarded, and future research should assess lifetime exposures to relevant pesticides while also looking at potential interactions with other major risk factors.124
4.5. Multifactorial causes
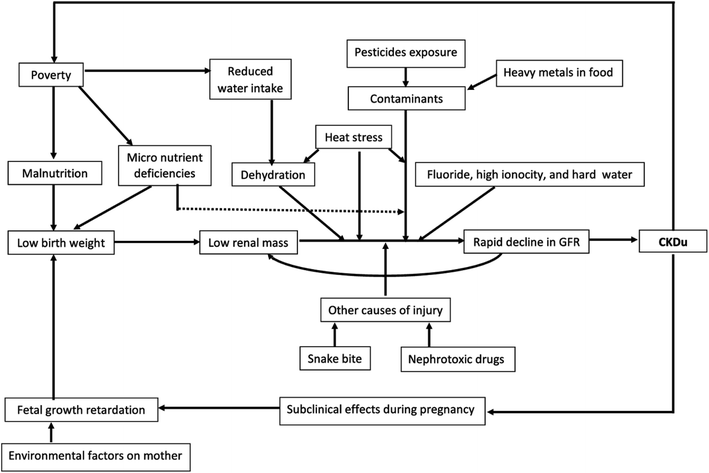
A growing number of studies have suggested that multiple factors combine to cause CKDu, and refer to CKDu as chronic kidney disease of multi-factorial origin.125–127 Some studies suggest a multifactorial approach because no single cause has been determined and the strong association with agricultural occupations. For instance, a system dynamics model (Fig. 5) would incorporate exposures from pesticides and heavy metals, drinking hard water with high levels of fluoride, poverty, low birth weight, micronutrient deficiencies and heat stress. Instead of using linear models, Jayasinghe and Zhu8 also incorporate feedback loops into their system dynamics model, which they propose is a more effective way of analyzing disease. The authors hypothesized that by using this approach, researchers can gain a better understanding of how various factors impact the disease and predict disease hot spots and system vulnerabilities. For instance, factors may overlap, such as exposure to heat stress, contaminated drinking water, and dehydration. In this way of thinking, it may be easier to quantify the interactions between different factors and unravel the potential etiologies.5. Discussion: knowledge gaps and research needs
Based on our review of the literature, a summary of proposed etiologies, supporting evidence for each etiology, and associated areas of future research are presented in Table 1 and discussed below. Since a standardized definition for CKD was established, use of this definition has been largely accepted across the world. One of the major, remaining challenges of studying CKDu is there is no universally accepted definition, and differences remain in how CKDu is diagnosed, leading to large regional disparities in research approaches.128,129 While traditional CKD treatment involves control of diabetic state, blood pressure, and other risk factors, the efficacy of these therapies slowing CKDu progression is unknown and, in some cases, could potentially exacerbate it.128 In order to effectively treat and prevent CKDu, a consensus definition is crucial to identify the underlying cause or causes. In this review, we investigate three broadly identified potential etiologies of CKDu.| Proposed etiology | Supporting evidence | Areas of need for future study |
|---|---|---|
| Heat stress | • Warmer temperatures have been correlated with increases in CKDu cases in Central America, and regions that were hottest had the highest prevalence of disease | • Determine if there are other hot regions of the world experiencing similar CKDu prevalence that have been previously overlooked due to limitations in medical care and diagnosis |
| • Sugarcane workers, who are objectively exposed to the highest levels of heat stress, were identified as the most at risk for CKDu | • Improve understanding as to why some hot regions of the world do not experience high levels of CKDu | |
| • Cross sectional studies have identified changes in kidney function during harvests, with the most change coming from those with the highest workloads | • Most supporting evidence for the heat stress hypothesis comes from Central America, but relatively less research has been done elsewhere (e.g., Sri Lanka or India). A broad scale harvest and non-harvest study comparing kidney function should be completed to attempt to isolate heat as a variable | |
| • Heat stress interventions in Central America have shown promise in reducing kidney injury | • Why has CKDu prevalence emerged recently? Is this emergence due to increased awareness, warming induced by climate change, or other factors | |
| Contaminated drinking water | • There are direct adverse effects on the kidneys from excess fluoride, and the kidney is exposed to higher concentrations of fluoride than most other soft tissues | • For often suggested contaminants linked to CKDu (e.g., fluoride, hardness causing elements), studies are necessary to establish concentration thresholds and exposure durations associated with CKDu onset. Additional studies should also aim to establish a physiological mechanism by which these contaminants lead to kidney disease |
| • Fluoride and high-water hardness have been shown in multiple studies to correlate to high levels of CKDu | • Larger longitudinal studies that measure levels of contamination should be conducted to determine how contaminants might affect CKDu prevalence over time | |
| • Women and children who are not exposed to certain contaminants occupationally are still becoming ill, making drinking water a possible pathway | • More studies are needed to understand the synergistic effects that contaminant mixtures may exhibit. Testing more combinations of contaminants, as has been done to some extent with simultaneous measurement of fluoride, cadmium, and high hardness, could provide insightful results | |
| • Strong associations exist between drinking water from abandoned wells and developing CKDu | • Broader spectrum analytical techniques (e.g., non-targeted methods for both organic and inorganic contaminants) should be applied to water samples to look for previously overlooked drinking water contaminants in CKDu affected populations | |
| • CKDu was found significantly less or was not present in areas that used water from natural springs | ||
| • Australian Aboriginal communities and those in proximity had much higher rates of renal disease, with drinking water as a common environmental factor | ||
| Agrochemical exposure | • Many pesticides and herbicides have been linked to kidney damage; for example, glyphosate and paraquat are very widely used and have been linked to kidney damage through epidemiological and animal studies | • Biomonitoring studies should continue to focus on the degree of occupational exposure to pesticides for agricultural workers with CKDu. Such work will help to determine if there is a causal mechanism at play based on duration or quantity of agrochemical exposure |
| • CKDu is occurring distinctly in agricultural regions, which could correlate with high interaction with pesticides | • Occupations outside of farming have also been affected by CKDu, thus it is important to investigate other routes for agrochemical exposure beyond occupational (e.g., through drinking water contamination or airborne drift to nearby communities) | |
| • Large cohort studies have found a correlation between increased use of certain pesticides and end stage renal disease | • Agrochemicals or pesticides encompass a very large group of chemical contaminants, including both active ingredients and other chemicals in more complex formulations. More definitive research is needed linking certain commonly used active ingredients and agrochemical formulations to kidney damage, with further epidemiological studies conducted with at risk populations | |
| • The emerging CKDu epidemics of the 1990s coincide with a global increase in use of agrochemicals | • There is need for improved understanding as to why certain agricultural regions of the world are not experiencing high levels of CKDu despite comparable patterns in agrochemical use | |
| • The higher risk of developing CKDu in men versus women has been related to occupational pesticide exposure | ||
| • The low, but present, risk for women and children in proximity to pesticides could be through indirect contamination or pesticide drift | ||
| Multifactorial approach | • Chronic diseases are commonly multifactorial (i.e., diabetes, cancer) | • Large epidemiological studies testing the weight of multiple factors, including social determinants, on disease prevalence could help elucidate how factors may work in combination to cause kidney disease and those populations most at risk |
| • Multiple of the suggested etiologies may be working in combination (e.g., heat stress and agrochemical exposure) to drive kidney disease in certain populations | • To support such work, there is need to make more readily available and better coordinate the use of existing datasets on global CKDu incidence and associated data relevant to exposure to proposed risk factors (e.g., drinking water quality, pesticide use) | |
| • Protocols for studying CKDu should be standardized so that future work consistently builds off existing literature toward a more definitive understanding of CKDu root causes |
5.1. Heat stress
The only variable currently known to be constant across all regions with CKDu is the presence of heat, and therefore the ability to undergo heat stress. Collectively, research has demonstrated that a combination of extended heat exposure coupled with episodes of dehydration has the potential to cause damage or at least stress to the kidneys. If CKDu cases are a type of heat stress nephropathy, it would explain the similar epidemics occurring among agricultural workers laboring in high-heat conditions. However, if this is the case, it could be assumed that the same types of epidemics that are emerging in South Asia and Central America should be happening in equally warm areas of Africa, the Middle East, and Australia. There is some evidence of small disease clusters in these areas, like in the Aboriginal communities of Australia, but large-scale CKDu hotspots have not yet been observed. Of course, the most at-risk subjects in these areas are usually from impoverished communities where medical care may be scarce, and diagnosis would be hard to confirm.Most data to date related to heat stress and CKDu seems to be concentrated in specific locations (e.g., Central America), and more research is needed in other areas of the globe establishing heat stress as a potential risk factor. Additionally, few large-scale epidemiological studies have been conducted that isolate heat stress as a potential risk factor, and more are needed. One study that conducted a longitudinal assessment of kidney function in migrant farm workers in 2021 stated most studies related to CKDu and AKI focus on workload, heat stress, and hydration and that there is a need for studies that can evaluate occupational risk factors in combination with heat stress and dehydration.58
5.2. Contaminated drinking water
Some research has suggested that by providing drinking water that is compliant with water quality standards and/or access to advanced drinking water treatment (e.g., reverse osmosis), CKDu occurrence is reduced. Additionally, lower prevalence of CKDu has been shown among those who consume water from areas with natural springs. Accordingly, this has led many to conclude that CKDu is linked to drinking water quality and the presence of contaminants of either natural (e.g., hardness causing elements) or anthropogenic (e.g., agrochemical) origin.There is need to develop a priority list of drinking water contaminants that should be the focus of future studies looking at potential associations with CKDu. From the existing literature, fluoride, hardness-causing elements, as well as select metals (e.g., cadmium, arsenic) and agrochemicals (e.g., nitrate and glyphosate) are high-priority contaminants that should be included in future studies focusing on links between drinking water quality and CKDu. These should be the focus of both fundamental toxicological studies establishing the mechanism, dose–response behavior, and relevant exposure timescales for onset of kidney disease, as well as larger longitudinal studies focused on population health over time. There is also the need to better account for complex mixtures of contaminants in drinking water. Though acceptable drinking water quality has been defined through guidelines and standards from the WHO and other regulatory bodies, water quality standards focus on individual contaminants, and do not account for possible synergistic effects of trace metals and other constituents, like hardness-causing elements, present in mixtures. Additional research is needed to better characterize the composition of mixtures using broader spectrum, non-targeted analytical approaches and the adverse impact on kidney health of such mixtures.
5.3. Agrochemical exposure
CKDu is often observed in agricultural workers and in areas of intense agricultural activity. Moreover, several pesticides widely used around the globe are known to negatively impact kidney function. For example, glyphosate is regulated in US drinking water for its potential to cause kidney damage. Accordingly, many have suggested exposure to agrochemicals like pesticides can explain CKDu occurrence in certain, but not all, communities (e.g., CKDu in populations that are unrelated to farming, such as mining and construction occupations).Most studies to date that have suggested links between pesticides and CKDu cases contained unspecific and unquantified exposure measurements, thereby limiting the ability to make a significant conclusion about whether agrochemicals play a role in CKDu development. Future research should prioritize biomonitoring in affected populations, particularly agricultural workers, to strengthen possible associations between CKDu and pesticide exposure. Moreover, pesticide active ingredients are applied in formulations, which are mixtures of both the active and inert ingredients. Additional research should be careful to distinguish between potential exposure to active ingredients and formulation mixtures when assessing potential links to CKDu incidence. To address CKDu incidence beyond agricultural workers, more studies are needed establishing non-occupational exposure routes to pesticide active ingredients such as contaminated drinking water or inhalation of airborne drift. Studies should prioritize those agrochemicals known or suspected to exhibit nephrotoxicity but should not overlook the potential for previously unrecognized toxicity for other agrochemicals, highlighting the need for ongoing toxicity studies to explore adverse health outcomes resulting from long-term exposure to widely used agrochemicals and agrochemical mixtures.
5.4. Other research needs
It is unclear if the cause for CKDu is the same across all affected regions or if they are different from region to region. No studies have found a definitive association with any single biological, agrochemical, or hydrogeochemical etiological factor. Few studies have considered the interplay of multiple risk factors or the potential role of social determinants of health. A significant challenge is that there is often little to no overlap in CKDu study designs, limiting attempts to better understand commonalities and differences of disease between regions. Finding causative risk factors across regions is challenging when using study data at a single point in time, further complicating efforts to associate any given risk factor with clinical and pathological findings. There are also limitations in data availability from region to region, which further limits comparison of global CKDu occurrence.6. Conclusion
Chronic kidney disease is treatable and deserves more attention globally. Unlike the global decline seen in other non-communicable disease over the past few decades, CKD has not seen the same extent of improvement. An active public health response would include effective management of risk factors at both the individual and primary care levels. However, for a large portion of those with chronic kidney disease, their primary risk factor is not known. This complicates intervention, which is predicated on a thorough understanding of all risk factors and how exactly they cause disease. Despite this uncertainty, public health measures could be implemented using interventions known or suspected to reduce CKDu incidence such as providing clean drinking water or implementing a shade-rest program for workers vulnerable to heat stress. While the current CKDu epidemics remain unexplained, it is still likely that an etiology will be confirmed with more research and global awareness of the disease. For example, two other forms of nephropathy, Itai-Itai disease in Japan and Balkan endemic nephropathy (BEN), had etiologies that were uncovered in 1968 and 1993, respectively, following multiple decades of research and funding.85There is ample evidence that CKDu is caused by common but not necessarily singular factors across the globe. This is supported by the fact that regional epidemics are occurring simultaneously on opposite quadrants of the world, with many having similar occupational, geographical, and socioeconomic characteristics. Based on reasonably consistent findings in terms of pathologies, it is likely that some sort of toxin or contaminant is a factor in each of these epidemics. However, it appears that no single hypothesis that has been put forth thus far can explain in totality the findings and distribution of the disease since its initial discovery. Moreover, there is little agreement between researchers as to the combination of exposures and environmental toxins that might lead to the manifestation of CKDu. The research that exists is insufficient, failing to strongly support any single etiology, and ultimately more research is needed to further establish root causes of CKDu.
Data availability
No new data were created or analyzed during this study. Data sharing is not applicable to this article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Research by the Center for Health Effects of Environmental Contamination at the University of Iowa is made possible through funding from the State of Iowa and the Iowa Department of Natural Resources. The authors would also like to acknowledge the anonymous reviewers whose comments and recommendations improved the clarity and impact of this work.References
- S. Gunawardena, M. Dayaratne, H. Wijesinghe and E. Wijewickrama, A systematic review of renal pathology in chronic kidney disease of uncertain etiology, Kidney Int. Rep., 2021, 6(6), 1711–1728, DOI:10.1016/j.ekir.2021.03.898.
- B. Bikbov, C. A. Purcell, A. S. Levey, M. Smith, A. Abdoli, M. Abebe, O. M. Adebayo, M. Afarideh, S. K. Agarwal and M. Agudelo-Botero, Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017, Lancet, 2020, 395(10225), 709–733, DOI:10.1016/S0140-6736(20)30045-3.
- V. A. Luyckx, Z. Al-Aly, A. K. Bello, E. Bellorin-Font, R. G. Carlini, J. Fabian, G. Garcia-Garcia, A. Iyengar, M. Sekkarie and W. Van Biesen, Sustainable development goals relevant to kidney health: an update on progress, Nat. Rev. Nephrol., 2021, 17(1), 15–32, DOI:10.1038/s41581-020-00363-6.
- A. C. Webster, E. V. Nagler, R. L. Morton and P. Masson, Chronic kidney disease, Lancet, 2017, 389(10075), 1238–1252, DOI:10.1016/S0140-6736(16)32064-5.
- P. Stalin, A. J. Purty and G. Abraham, Distribution and determinants of chronic kidney disease of unknown etiology: a brief overview, Indian J. Nephrol., 2020, 30(4), 241–244, DOI:10.4103/ijn.IJN_313_18.
- S. Imbulana and K. Oguma, Groundwater as a potential cause of Chronic Kidney Disease of unknown etiology (CKDu) in Sri Lanka: a review, J. Water Health, 2021, 19(3), 393–410, DOI:10.2166/WH.2021.079.
- N. Pearce, B. Caplin, N. Gunawardena, P. Kaur, C. O'callaghan-Gordo and T. Ruwanpathirana, CKD of unknown cause: a global epidemic?, Kidney Int. Rep., 2019, 4(3), 367–369, DOI:10.1016/j.ekir.2018.11.019.
- S. Jayasinghe and Y.-G. Zhu, Chronic kidney disease of unknown etiology (CKDu): using a system dynamics model to conceptualize the multiple environmental causative pathways of the epidemic, Sci. Total Environ., 2020, 705, 135766, DOI:10.1016/j.scitotenv.2019.135766.
- H. Wasana, D. Aluthpatabendi, W. Kularatne, P. Wijekoon, R. Weerasooriya and J. Bandara, Drinking water quality and chronic kidney disease of unknown etiology (CKDu): synergic effects of fluoride, cadmium and hardness of water, Environ. Geochem. Health, 2016, 38(1), 157–168, DOI:10.1007/s10653-015-9699-7.
- M. Kulathunga, M. A. Wijayawardena, R. Naidu and A. Wijeratne, Chronic kidney disease of unknown aetiology in Sri Lanka and the exposure to environmental chemicals: a review of literature, Environ. Geochem. Health, 2019, 41, 2329–2338, DOI:10.1007/s10653-019-00264-z.
- J. Pett, F. Mohamed, J. Knight, C. Linhart, N. J. Osborne and R. Taylor, Two decades of chronic kidney disease of unknown aetiology (CKDu) research: existing evidence and persistent gaps from epidemiological studies in Sri Lanka, Nephrology, 2022, 27(3), 238–247, DOI:10.1111/nep.13989.
- J. W. Stanifer, A. Muiru, T. H. Jafar and U. D. Patel, Chronic kidney disease in low-and middle-income countries, Nephrol. Dial. Transplant., 2016, 31(6), 868–874, DOI:10.1093/ndt/gfv466.
- S. R. Mendley, A. Levin, R. Correa-Rotter, B. R. Joubert, E. A. Whelan, B. Curwin, E. H. Koritzinsky, D. M. Gaughan, P. L. Kimmel and S. Anand, Chronic kidney diseases in agricultural communities: report from a workshop, Kidney Int., 2019, 96(5), 1071–1076, DOI:10.1016/j.kint.2019.06.024.
- P. Vlahos, S. L. Schensul, S. Anand, E. Shipley, S. Diyabalanage, C. Hu, T. Ha, A. Staniec, L. Haider and J. J. Schensul, Water sources and kidney function: investigating chronic kidney disease of unknown etiology in a prospective study, npj Clean Water, 2021, 4(1), 50, DOI:10.1038/s41545-021-00141-2.
- O. John, B. Gummudi, A. Jha, N. Gopalakrishnan, O. P. Kalra, P. Kaur, V. Kher, V. Kumar, R. S. Machiraju and N. Osborne, Chronic kidney disease of unknown etiology in India: what do we know and where we need to go, Kidney Int. Rep., 2021, 6(11), 2743–2751, DOI:10.1016/j.ekir.2021.07.031.
- C. Wesseling, B. V. W. De Joode, J. Crowe, R. Rittner, N. A. Sanati, C. Hogstedt and K. Jakobsson, Mesoamerican nephropathy: geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica, Occup. Environ. Med., 2015, 72(10), 714–721, DOI:10.1136/oemed-2014-102799.
- V. M. Campese, The unresolved epidemic of chronic kidney disease of uncertain origin (CKDu) around the world: a review and new insights, Clin. Nephrol., 2021, 95(2), 65, DOI:10.5414/cn110186.
- M. Madero, Is an Environmental Nephrotoxin the Primary Cause of CKDu (Mesoamerican Nephropathy)? Commentary, Kidney360, 2020, 1(7), 602–603, DOI:10.34067/KID.0003412020.
- N. P. Singh, A. K. Gupta, G. Kaur and T. Khanna, Chronic Kidney Disease of Unknown Origin—What Do We Know, J. Assoc. Physicians India, 2020, 68, 76–79 Search PubMed , https://europepmc.org/abstract/MED/32009367.
- K. Gobalarajah, P. Subramaniam, U. A. Jayawardena, G. Rasiah, S. Rajendra and J. Prabagar, Impact of water quality on chronic kidney disease of unknown etiology (CKDu) in Thunukkai Division in Mullaitivu District, Sri Lanka, BMC Nephrol., 2020, 21, 1–11, DOI:10.1186/s12882-020-02157-1.
- R. Ghosh, M. Siddarth, N. Singh, V. Tyagi, P. K. Kare, B. D. Banerjee, O. P. Kalra and A. K. Tripathi, Organochlorine pesticide level in patients with chronic kidney disease of unknown etiology and its association with renal function, Environ. Health Prev. Med., 2017, 22, 1–8, DOI:10.1186/s12199-017-0660-5.
- K. Broberg, Water, rest, shade: can simple actions stop an epidemic of chronic kidney disease of unknown etiology among farm workers in Central America?, Scand. J. Work, Environ. Health, 2018, 44(1), 1–2, DOI:10.5271/sjweh.3699.
- N. Jayatilake, S. Mendis, P. Maheepala and F. R. Mehta, Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country, BMC Nephrol., 2013, 14(1), 1–13, DOI:10.1186/1471-2369-14-180.
- Y. Zhou and J. Yang, Chronic kidney disease: overview, Chronic Kidney Disease: Diagnosis and Treatment, 2020, pp. 3–12 Search PubMed.
- A. Levin, P. E. Stevens, R. W. Bilous, J. Coresh, A. L. De Francisco, P. E. De Jong, K. E. Griffith, B. R. Hemmelgarn, K. Iseki and E. J. Lamb, Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease, Kidney Int. Suppl., 2013, 3(1), 1–150 CrossRef.
- S. A. Keogh, J. H. Leibler, C. M. Sennett Decker, J. J. Amador Velázquez, E. R. Jarquin, D. Lopez-Pilarte, R. Garcia-Trabanino, I. S. Delgado, Z. E. Petropoulos and D. J. Friedman, High prevalence of chronic kidney disease of unknown etiology among workers in the Mesoamerican Nephropathy Occupational Study, BMC Nephrol., 2022, 23(1), 238, DOI:10.1186/s12882-022-02861-0.
- A. Y. Rosinger, H. Bethancourt, Z. S. Swanson, R. Nzunza, J. Saunders, S. Dhanasekar, W. L. Kenney, K. Hu, M. J. Douglass and E. Ndiema, Drinking water salinity is associated with hypertension and hyperdilute urine among Daasanach pastoralists in Northern Kenya, Sci. Total Environ., 2021, 770, 144667, DOI:10.1016/j.scitotenv.2020.144667.
- A. M. Cusumano, C. Tzanno-Martins and G. J. Rosa-Diez, The glomerular filtration rate: from the diagnosis of kidney function to a public health tool, Front. Med., 2021, 8, 769335, DOI:10.3389/fmed.2021.769335.
- P. E. De Jong, S. J. Bakker and R. T. Gansevoort, What to Measure—Albuminuria or Total Proteinuria?, Am. J. Kidney Dis., 2011, 57(1), 1–2, DOI:10.1053/j.ajkd.2010.11.005.
- D. Abdissa, Purposeful review to identify risk factors, epidemiology, clinical features, treatment and prevention of chronic kidney disease of unknown etiology, Int. J. Nephrol. Renovascular Dis., 2020, 367–377, DOI:10.2147/IJNRD.S283161.
- R. Saran, Y. Li, B. Robinson, K. C. Abbott, L. Y. Agodoa, J. Ayanian, J. Bragg-Gresham, R. Balkrishnan, J. L. Chen, E. Cope, P. W. Eggers, D. Gillen, D. Gipson, S. M. Hailpern, Y. N. Hall, K. He, W. Herman, M. Heung, R. A. Hirth, D. Hutton, S. J. Jacobsen, K. Kalantar-Zadeh, C. P. Kovesdy, Y. Lu, M. Z. Molnar, H. Morgenstern, B. Nallamothu, D. V. Nguyen, A. M. O’hare, B. Plattner, R. Pisoni, F. K. Port, P. Rao, C. M. Rhee, A. Sakhuja, D. E. Schaubel, D. T. Selewski, V. Shahinian, J. J. Sim, P. Song, E. Streja, M. K. Tamura, F. Tentori, S. White, K. Woodside and R. A. Hirth, US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States, Am. J. Kidney Dis., 2016, 67(suppl. 1), S1, DOI:10.1053/j.ajkd.2015.12.014.
- K. L. Johansen, G. M. Chertow, R. N. Foley, D. T. Gilbertson, C. A. Herzog, A. Ishani, A. K. Israni, E. Ku, M. K. Tamura and S. Li, US renal data system 2020 annual data report: epidemiology of kidney disease in the United States, Am. J. Kidney Dis., 2021, 77(4), A7–A8 CrossRef PubMed.
- D. Alfego, J. Ennis, B. Gillespie, M. J. Lewis, E. Montgomery, S. Ferrè, J. A. Vassalotti and S. Letovsky, Chronic kidney disease testing among at-risk adults in the US remains low: real-world evidence from a national laboratory database, Diabetes Care, 2021, 44(9), 2025–2032 CrossRef CAS PubMed.
- N. D. Eneanya, L. E. Boulware, J. Tsai, M. A. Bruce, C. L. Ford, C. Harris, L. S. Morales, M. J. Ryan, P. P. Reese and R. J. Thorpe Jr, Health inequities and the inappropriate use of race in nephrology, Nat. Rev. Nephrol., 2022, 18(2), 84–94, DOI:10.1038/s41581-021-00501-8.
- A. Francis, M. N. Harhay, A. C. Ong, S. L. Tummalapalli, A. Ortiz, A. B. Fogo, D. Fliser, P. Roy-Chaudhury, M. Fontana and M. Nangaku, Chronic kidney disease and the global public health agenda: an international consensus, Nat. Rev. Nephrol., 2024, 1–13 Search PubMed.
- T. Liyanage, T. Ninomiya, V. Jha, B. Neal, H. M. Patrice, I. Okpechi, M.-H. Zhao, J. Lv, A. X. Garg and J. Knight, Worldwide access to treatment for end-stage kidney disease: a systematic review, Lancet, 2015, 385(9981), 1975–1982, DOI:10.1016/S0140-6736(14)61601-9.
- J. Himmelfarb, R. Vanholder, R. Mehrotra and M. Tonelli, The current and future landscape of dialysis, Nat. Rev. Nephrol., 2020, 16(10), 573–585 CrossRef PubMed.
- D. J. Aguilar and M. Madero, Other potential CKD hotspots in the world: the cases of Mexico and the United States, Semin. Nephrol., 2019, 39(3), 300–307, DOI:10.1016/j.semnephrol.2019.02.008.
- D. J. Smith, L. M. Pius, L. C. Plantinga, L. M. Thompson, V. Mac and V. S. Hertzberg, Heat stress and kidney function in farmworkers in the US: a scoping review, J. Agromed., 2022, 27(2), 183–192, DOI:10.1080/1059924X.2021.1893883.
- X. Feng, N. Hou, Z. Chen, J. Liu, X. Li, X. Sun and Y. Liu, Secular trends of epidemiologic patterns of chronic kidney disease over three decades: an updated analysis of the Global Burden of Disease Study 2019, BMJ Open, 2023, 13(3), e064540 CrossRef PubMed.
- C. P. Kovesdy, Epidemiology of chronic kidney disease: an update 2022, Kidney Int. Suppl., 2022, 12(1), 7–11 CrossRef.
- W. Udeshani, N. H. Koralegedara, S. Gunatilake, S.-L. Li, X. Zhu and R. Chandrajith, Geochemistry of groundwater in the semi-arid crystalline terrain of Sri Lanka and its health implications among agricultural communities, Water, 2022, 14(20), 3241, DOI:10.3390/w14203241.
- S. Nayak, T. Rehman, K. Patel, P. Dash, A. Alice, S. Kanungo, S. K. Palo and S. Pati, Factors associated with chronic kidney disease of unknown etiology (CKDu): a systematic review, Healthcare, 2023, 11(4), 551, DOI:10.3390/healthcare11040551.
- D. J. Smith, V. Mac, L. M. Thompson, L. Plantinga, L. Kasper and V. S. Hertzberg, Using occupational histories to assess heat exposure in undocumented workers receiving emergent renal dialysis in Georgia, Workplace Health & Saf., 2022, 70(5), 251–258, DOI:10.1177/21650799211060695.
- J. Rajapakse, S. Rainer-Smith, G. J. Millar, P. Grace, A. Hutton, W. Hoy, C. Jeffries-Stokes and B. Hudson, Unsafe drinking water quality in remote Western Australian Aboriginal communities, Geogr. Res., 2019, 57(2), 178–188, DOI:10.1111/1745-5871.12308.
- A. Trivedi and S. Kumar, Chronic kidney disease of unknown origin: think beyond common etiologies, Cureus, 2023, 15(5), e38939, DOI:10.7759/cureus.38939.
- J. H. Redmon, K. E. Levine, J. Lebov, J. Harrington and A. Kondash, A comparative review: Chronic Kidney Disease of unknown etiology (CKDu) research conducted in Latin America versus Asia, Environ. Res., 2021, 192, 110270, DOI:10.1016/j.envres.2020.110270.
- S. J. Wimalawansa and C. B. Dissanayake, Factors affecting the environmentally induced, chronic kidney disease of unknown aetiology in dry zonal regions in tropical countries—novel findings, Environments, 2019, 7(1), 2, DOI:10.3390/environments7010002.
- T. S. C. Gunasekara, P. M. C. De Silva, C. Herath, S. Siribaddana, N. Siribaddana, C. Jayasumana, S. Jayasinghe, M. Cardenas-Gonzalez and N. Jayasundara, The utility of novel renal biomarkers in assessment of chronic kidney disease of unknown etiology (CKDu): a review, Int. J. Environ. Res. Publ. Health, 2020, 17(24), 9522 CrossRef CAS PubMed.
- B. Caplin, K. Jakobsson, J. Glaser, D. Nitsch, V. Jha, A. Singh, R. Correa-Rotter and N. Pearce, International collaboration for the epidemiology of eGFR in low and middle income populations-rationale and core protocol for the disadvantaged populations eGFR epidemiology study (DEGREE), BMC Nephrol., 2017, 18, 1–8 CrossRef PubMed.
- S. A. Hamilton, W. P. Nakanga, J. E. Prynn, A. C. Crampin, D. Fecht, P. Vineis, B. Caplin, N. Pearce and M. J. Nyirenda, Prevalence and risk factors for chronic kidney disease of unknown cause in Malawi: a cross-sectional analysis in a rural and urban population, BMC Nephrol., 2020, 21, 1–12 CrossRef PubMed.
- L. A. Inker, B. C. Astor, C. H. Fox, T. Isakova, J. P. Lash, C. A. Peralta, M. K. Tamura and H. I. Feldman, KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD, Am. J. Kidney Dis., 2014, 63(5), 713–735 CrossRef PubMed.
- A. S. Levey, K.-U. Eckardt, Y. Tsukamoto, A. Levin, J. Coresh, J. Rossert, D. D. Zeeuw, T. H. Hostetter, N. Lameire and G. Eknoyan, Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO), Kidney Int., 2005, 67(6), 2089–2100 CrossRef PubMed.
- S. Nayak-Rao, Profile of chronic kidney disease from a nephrology underserviced region in North Eastern India: a preliminary report from a single center in Assam, Int. Urol. Nephrol., 2024, 56(3), 1103–1108, DOI:10.1007/s11255-023-03736-5.
- W. V. D. Priyadarshani, A. F. D. De Namor and S. R. P. Silva, Rising of a global silent killer: critical analysis of chronic kidney disease of uncertain aetiology (CKDu) worldwide and mitigation steps, Environ. Geochem. Health, 2023, 45(6), 2647–2662, DOI:10.1007/s10653-022-01373-y.
- C. Herath, C. Jayasumana, P. M. C. De Silva, P. C. De Silva, S. Siribaddana and M. E. De Broe, Kidney diseases in agricultural communities: a case against heat-stress nephropathy, Kidney Int. Rep., 2018, 3(2), 271–280, DOI:10.1016/j.ekir.2017.10.006.
- S. Gunatilake, S. Seneff and L. Orlando, Glyphosate's synergistic toxicity in combination with other factors as a cause of chronic kidney disease of unknown origin, Int. J. Environ. Res. Publ. Health, 2019, 16(15), 2734, DOI:10.3390/ijerph16152734.
- N. López-Gálvez, R. Wagoner, R. A. Canales, K. Ernst, J. L. Burgess, J. De Zapien, C. Rosales and P. Beamer, Longitudinal assessment of kidney function in migrant farm workers, Environ. Res., 2021, 202, 111686, DOI:10.1016/j.envres.2021.111686.
- J. Glaser, J. Lemery, B. Rajagopalan, H. F. Diaz, R. García-Trabanino, G. Taduri, M. Madero, M. Amarasinghe, G. Abraham and S. Anutrakulchai, Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy, Clin. J. Am. Soc. Nephrol., 2016, 11(8), 1472–1483, DOI:10.2215/CJN.13841215.
- P. N. Kulasooriya, K. B. Jayasekara, T. Nisansala, S. Kannangara, R. Karunarathna, C. Karunarathne, M. Wikramarathne and S. M. Albert, Utility of self-reported heat stress symptoms and NGAL biomarker to screen for chronic kidney disease of unknown origin (CKDu) in Sri Lanka, Int. J. Environ. Res. Publ. Health, 2021, 18(19), 10498, DOI:10.3390/ijerph181910498.
- Health, N. I. F. O. S. A., Heat Stress and Workers, 2024, https://www.cdc.gov/niosh/heat-stress/about/index.html Search PubMed.
- C. Sorensen and R. Garcia-Trabanino, A new era of climate medicine—addressing heat-triggered renal disease, N. Engl. J. Med., 2019, 381(8), 693–696, DOI:10.1056/NEJMp1907859.
- M. Al-Bouwarthan, A. A. Almulla and M. Yaseen, The impact of heat on kidney health: A PRISMA-compliant bibliometric analysis, Medicine, 2022, 101(36), e30328, DOI:10.1097/MD.0000000000030328.
- C. Wesseling, Is an environmental nephrotoxin the primary cause of CKDu (Mesoamerican nephropathy)? CON, Kidney360, 2020, 1(7), 596–601, DOI:10.34067/KID.0002922020.
- J. Kupferman, O. Ramírez-Rubio, J. J. Amador, D. López-Pilarte, E. H. Wilker, R. L. Laws, C. Sennett, N. V. Robles, J. L. Lau and A. J. Salinas, Acute kidney injury in sugarcane workers at risk for Mesoamerican nephropathy, Am. J. Kidney Dis., 2018, 72(4), 475–482, DOI:10.1053/j.ajkd.2018.04.014.
- Z. E. Petropoulos, S. A. Keogh, E. Jarquín, D. López-Pilarte, J. J. Amador Velázquez, R. García-Trabanino, M. R. Amador Sánchez, R. Guevara, A. Gruener and D. R. Allen, Heat stress and heat strain among outdoor workers in El Salvador and Nicaragua, J. Expo. Sci. Environ. Epidemiol., 2023, 33(4), 622–630, DOI:10.1038/s41370-023-00537-x.
- E. Hansson, J. Glaser, K. Jakobsson, I. Weiss, C. Wesseling, R. A. Lucas, J. L. K. Wei, U. Ekström, J. Wijkström and T. Bodin, Pathophysiological mechanisms by which heat stress potentially induces kidney inflammation and chronic kidney disease in sugarcane workers, Nutrients, 2020, 12(6), 1639, DOI:10.3390/nu12061639.
- J. Mix, L. Elon, V. V. T. Mac, J. Flocks, E. Economos, A. J. Tovar-Aguilar, V. S. Hertzberg and L. A. Mccauley, Hydration status, kidney function, and kidney injury in Florida agricultural workers, J. Occup. Environ. Med., 2018, 60(5), e253–e260, DOI:10.1097/JOM.0000000000001261.
- S. Moyce, T. Armitage, D. Mitchell and M. Schenker, Acute kidney injury and workload in a sample of California agricultural workers, Am. J. Ind. Med., 2020, 63(3), 258–268, DOI:10.1002/ajim.23076.
- N. Pearce and B. Caplin, Let's Take the Heat Out of the CKDu Debate: More Evidence Is Needed, BMJ Publishing Group Ltd, 2019, pp. 357–359 Search PubMed.
- C. L. Chapman, H. W. Hess, R. A. Lucas, J. Glaser, R. Saran, J. Bragg-Gresham, D. H. Wegman, E. Hansson, C. T. Minson and Z. J. Schlader, Occupational heat exposure and the risk of chronic kidney disease of nontraditional origin in the United States, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 2021, 321(2), R141–R151 CrossRef CAS PubMed.
- D. R. Vandervort, D. L. López, C. M. Orantes and D. S. Rodríguez, Spatial distribution of unspecified chronic kidney disease in El Salvador by crop area cultivated and ambient temperature, MEDICC Rev., 2014, 16, 31–38, DOI:10.37757/mr2014.v16.n2.6.
- P. Ordunez, F. J. Nieto, R. Martinez, P. Soliz, G. P. Giraldo, S. A. Mott and W. E. Hoy, Chronic kidney disease mortality trends in selected Central America countries, 1997–2013: clues to an epidemic of chronic interstitial nephritis of agricultural communities, J. Epidemiol. Community Health, 2018, 72(4), 280–286, DOI:10.1136/jech-2017-210023.
- P. M. C. De Silva, E. Ekanayake, T. Gunasekara, W. G. Thakshila, P. Sandamini, P. Abeysiriwardhana, K. Nishara, A. Harishchandra, P. C. De Silva and N. Siribaddana, Occupational heat exposure alone does not explain chronic kidney disease of uncertain aetiology (CKDu) in Sri Lanka, J. Clim. Change Health, 2022, 8, 100143 CrossRef.
- E. Chapman, M. M. Haby, E. Illanes, J. Sanchez-Viamonte, V. Elias and L. Reveiz, Risk factors for chronic kidney disease of non-traditional causes: a systematic review, Pan American Journal of Public Health, 2019, 43, e35 Search PubMed.
- D. H. Wegman, J. Apelqvist, M. Bottai, U. Ekström, R. García-Trabanino, J. Glaser, C. Hogstedt, K. Jakobsson, E. Jarquín and R. A. Lucas, Intervention to diminish dehydration and kidney damage among sugarcane workers, Scand. J. Work, Environ. Health, 2018, 16–24, DOI:10.5271/sjweh.3659.
- R. Chandrajith, N. Nanayakkara, C. Zwiener, C. Daniel, K. Amann and J. A. Barth, Geochemical Characteristics of Groundwater Consumed by Patients with Chronic Kidney Disease with Unknown Aetiology in the Crystalline Dry Zone Terrain of Sri Lanka, Expo. Health, 2024, 16(1), 183–195, DOI:10.1007/s12403-023-00547-y.
- H. Wasana, G. D. Perera, P. D. S. Gunawardena, P. S. Fernando and J. Bandara, WHO water quality standards vs synergic effect (s) of fluoride, heavy metals and hardness in drinking water on kidney tissues, Sci. Rep., 2017, 7(1), 1–6, DOI:10.1038/srep42516.
- C. Jayasumana, S. Gunatilake and P. Senanayake, Glyphosate, hard water and nephrotoxic metals: are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka?, Int. J. Environ. Res. Publ. Health, 2014, 11(2), 2125–2147, DOI:10.3390/ijerph110202125.
- S. Balasooriya, H. Munasinghe, A. Herath, S. Diyabalanage, O. Ileperuma, H. Manthrithilake, C. Daniel, K. Amann, C. Zwiener and J. A. Barth, Possible links between groundwater geochemistry and chronic kidney disease of unknown etiology (CKDu): an investigation from the Ginnoruwa region in Sri Lanka, Expo. Health, 2020, 12, 823–834, DOI:10.1007/s12403-019-00340-w.
- H. a. S. Herath, K. Kubota, T. Kawakami, S. Nagasawa, A. Motoyama, S. Weragoda, G. T. Chaminda and S. Yatigammana, Potential risk of drinking water to human health in Sri Lanka, Environ. Forensics, 2017, 18(3), 241–250, DOI:10.1080/15275922.2017.1340364.
- C. Jayasumana, P. Paranagama, S. Agampodi, C. Wijewardane, S. Gunatilake and S. Siribaddana, Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka, Environ. Health, 2015, 14(1), 1–10, DOI:10.1186/1476-069X-14-6.
- R. Chandrajith, C. Dissanayake, T. Ariyarathna, H. Herath and J. Padmasiri, Dose-dependent Na and Ca in fluoride-rich drinking water—another major cause of chronic renal failure in tropical arid regions, Sci. Total Environ., 2011, 409(4), 671–675, DOI:10.1016/j.scitotenv.2010.10.046.
- S. Weragoda and T. Kawakami, Evaluation of groundwater quality in 14 districts in Sri Lanka: a collaboration research between Sri Lanka and Japan, Trends in Asian Water Environmental Science and Technology, 2016, pp. 151–155 Search PubMed.
- O. Hettithanthri, S. Sandanayake, D. Magana-Arachchi, R. Wanigatunge, A. U. Rajapaksha, X. Zeng, Q. Shi, H. Guo and M. Vithanage, Risk factors for endemic chronic kidney disease of unknown etiology in Sri Lanka: retrospect of water security in the dry zone, Sci. Total Environ., 2021, 795, 148839, DOI:10.1016/j.scitotenv.2021.148839.
- J. Jayasekara, D. Dissanayake, S. Adhikari and P. Bandara, Geographical distribution of chronic kidney disease of unknown origin in North Central Region of Sri Lanka, Ceylon Med. J., 2013, 58(1), 6–10, DOI:10.4038/cmj.v58i1.5356.
- N. Ranasinghe, E. Kruger, R. Chandrajith and M. Tennant, Groundwater fluoride in Sri Lanka: opportunities to mitigate the risk at maximum contaminant level, Ceylon Med. J., 2018, 63(4), 174–179, DOI:10.4038/cmj.v63i4.8768.
- M. Atlani, A. Kumar, R. Ahirwar, M. Meenu, S. K. Goel, R. Kumari, A. Anirudhan, S. Vallamshetla and G. S. T. Reddy, Heavy metal association with chronic kidney disease of unknown cause in central India-results from a case-control study, BMC Nephrol., 2024, 25(1), 120 CrossRef CAS.
- L. Wu, C. Fan, Z. Zhang, X. Zhang, Q. Lou, N. Guo, W. Huang, M. Zhang, F. Yin and Z. Guan, Association between fluoride exposure and kidney function in adults: a cross-sectional study based on endemic fluorosis area in China, Ecotoxicol. Environ. Saf., 2021, 225, 112735, DOI:10.1016/j.ecoenv.2021.112735.
- National Research Council, in Fluoride in Drinking Water: A Scientific Review of EPA's Standards, The National Academies Press, Washington, DC, 2006, DOI:10.17226/11571.
- R. Dharmaratne, Exploring the role of excess fluoride in chronic kidney disease: a review, Hum. Exp. Toxicol., 2019, 38(3), 269–279, DOI:10.1177/0960327118814161.
- S. Peckham and N. Awofeso, Water fluoridation: a critical review of the physiological effects of ingested fluoride as a public health intervention, Sci. World J., 2014, 2014, 293019, DOI:10.1155/2014/293019.
- United States Environmental Protection Agency, National Primary Drinking Water Regulations, 2024, https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations Search PubMed.
- J.-L. Liu, T. Xia, Y.-Y. Yu, X.-Z. Sun, Q. Zhu, W. He, M. Zhang and A. Wang, The dose-effect relationship of water fluoride levels and renal damage in children, Weisheng Yanjiu, 2005, 34(3), 287–288 CAS , https://europepmc.org/abstract/MED/16111031.
- X. Xiong, J. Liu, W. He, T. Xia, P. He, X. Chen, K. Yang and A. Wang, Dose–effect relationship between drinking water fluoride levels and damage to liver and kidney functions in children, Environ. Res., 2007, 103(1), 112–116, DOI:10.1016/j.envres.2006.05.008.
- J. Ekstrand, S. J. Fomon, E. E. Ziegler and S. E. Nelson, Fluoride pharmacokinetics in infancy, Pediatr. Res., 1994, 35(2), 157–163, DOI:10.1203/00006450-199402000-00006.
- R. W. Dharmaratne, Fluoride in drinking water and diet: the causative factor of chronic kidney diseases in the North Central Province of Sri Lanka, Environ. Health Prev. Med., 2015, 20, 237–242 CrossRef CAS.
- Q. Shi, Z. Gao, H. Guo, X. Zeng, S. Sandanayake and M. Vithanage, Hydrogeochemical factors controlling the occurrence of chronic kidney disease of unknown etiology (CKDu), Environ. Geochem. Health, 2023, 45(5), 2611–2627 CrossRef CAS PubMed.
- H. Eddington, R. Hoefield, S. Sinha, C. Chrysochou, B. Lane, R. N. Foley, J. Hegarty, J. New, D. J. O'donoghue and R. J. Middleton, Serum phosphate and mortality in patients with chronic kidney disease, Clin. J. Am. Soc. Nephrol., 2010, 5(12), 2251–2257, DOI:10.2215/CJN.00810110.
- S. Mascarenhas, S. Mutnuri and A. Ganguly, Deleterious role of trace elements–silica and lead in the development of chronic kidney disease, Chemosphere, 2017, 177, 239–249 CrossRef CAS PubMed.
- S. Mascarenhas, S. Mutnuri and A. Ganguly, Silica-a trace geogenic element with emerging nephrotoxic potential, Sci. Total Environ., 2018, 645, 297–317, DOI:10.1016/j.scitotenv.2018.07.075.
- S. Imbulana, K. Oguma and S. Takizawa, Evaluation of groundwater quality and reverse osmosis water treatment plants in the endemic areas of Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka, Sci. Total Environ., 2020, 745, 140716, DOI:10.1016/j.scitotenv.2020.140716.
- W. Botheju, J. Liyanage and S. Kannangara, The groundwater geochemistry and the human health risk assessment of drinking water in an area with a high prevalence of chronic kidney disease of unknown etiology (CKDu), Sri Lanka, J. Chem., 2021, 2021, 1–18, DOI:10.1155/2021/1755140.
- R. Chandrajith and S. Diyabalanage, Geo-environmental assessment of geochemistry of groundwater and associated human health risks in the dry zone terrain of Sri Lanka, J. Natl. Sci. Found. Sri Lanka, 2022, 50, 213–229, DOI:10.4038/jnsfsr.v50i0.11239.
- J. C. Ulrich, K. Hoffman, T. Gunasekara, P. Sandamini, B. P. Jackson, P. M. C. De Silva, N. Jayasundara and P. L. Ferguson, Glyphosate and fluoride in high-hardness drinking water are positively associated with chronic kidney disease of unknown etiology (CKDu) in Sri Lanka, Environ. Sci. Technol. Lett., 2023, 10(10), 916–923, DOI:10.1021/acs.estlett.3c00504.
- Y.-F. Yang, W.-G. Li, P.-P. Wen, P.-P. Jia, Y.-Z. Li, T.-Y. Li and D.-S. Pei, Exposure to Sri Lanka's local groundwater in a CKDu prevalent area causes kidney damage in zebrafish, Aquat. Toxicol., 2022, 251, 106276 CrossRef CAS.
- K. Priyadarshanee, Z. Pang, E. Edirisinghe, H. Pitawala, J. Gunasekara, W. Wijesooriya, Y. Hao, Y. Bao and J. Tian, Synergic Origin and Evolution of TDS, Mg and Fluoride in Groundwater as Relative to Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka, Water, 2024, 16(11), 1606 CrossRef CAS.
- K. B. Jayasekara, D. M. Dissanayake, R. Sivakanesan, A. Ranasinghe, R. H. Karunarathna and G. W. G. P. Kumara, Epidemiology of chronic kidney disease, with special emphasis on chronic kidney disease of uncertain etiology, in the north central region of Sri Lanka, J. Epidemiol., 2015, 25(4), 275–280, DOI:10.2188/jea.JE20140074.
- M. R. Kulathunga, M. A. Wijayawardena, R. Naidu, S. J. Wimalawansa and M. M. Rahman, Health risk assessment from heavy metals derived from drinking water and rice, and correlation with CKDu, Front. Water, 2022, 3, 786487, DOI:10.3389/frwa.2021.786487.
- S. Xu, S.-L. Li, F. Yue, C. Udeshani and R. Chandrajith, Natural and anthropogenic controls of groundwater quality in Sri Lanka: implications for chronic kidney disease of unknown etiology (CKDU), Water, 2021, 13(19), 2724, DOI:10.3390/w13192724.
- S. Keith, O. Faroon, N. Roney, et al., Toxicological Profile for Uranium, in Agency for Toxic Substances and Disease Registry (US), Atlanta (GA), 2013, https://www.ncbi.nlm.nih.gov/books/NBK158802/ Search PubMed.
- T. A. Jayalal, Chronic kidney disease of uncertain aetiology: adding vital piece of information to the national project team report of Sri Lanka, BMC Nephrol., 2015, 16(1), 1–3, DOI:10.1186/s12882-015-0211-5.
- J. Danziger, K. J. Mukamal and E. Weinhandl, Associations of community water lead concentrations with hemoglobin concentrations and erythropoietin-stimulating agent use among patients with advanced CKD, J. Am. Soc. Nephrol., 2021, 32(10), 2425–2434, DOI:10.1681/ASN.2020091281.
- L. Y. Zheng, J. G. Umans, M. Tellez-Plaza, F. Yeh, K. A. Francesconi, W. Goessler, E. K. Silbergeld, E. Guallar, B. V. Howard and V. M. Weaver, Urine arsenic and prevalent albuminuria: evidence from a population-based study, Am. J. Kidney Dis., 2013, 61(3), 385–394, DOI:10.1053/j.ajkd.2012.09.011.
- T. Rango, M. Jeuland, H. Manthrithilake and P. Mccornick, Nephrotoxic contaminants in drinking water and urine, and chronic kidney disease in rural Sri Lanka, Sci. Total Environ., 2015, 518, 574–585, DOI:10.1016/j.scitotenv.2015.02.097.
- J. F. Lebov, L. S. Engel, D. Richardson, S. L. Hogan, J. A. Hoppin and D. P. Sandler, Pesticide use and risk of end-stage renal disease among licensed pesticide applicators in the Agricultural Health Study, Occup. Environ. Med., 2016, 73(1), 3–12, DOI:10.1136/oemed-2014-102615.
- J. F. Lebov, L. S. Engel, D. Richardson, S. L. Hogan, D. P. Sandler and J. A. Hoppin, Pesticide exposure and end-stage renal disease risk among wives of pesticide applicators in the Agricultural Health Study, Environ. Res., 2015, 143, 198–210, DOI:10.1016/j.envres.2015.10.002.
- R. Payán-Rentería, G. Garibay-Chavez, R. Rangel-Ascencio, V. Preciado-Martínez, L. Muñoz-Islas, C. Beltrán-Miranda, S. Mena-Munguía, L. Jave-Suárez, A. Feria-Velasco and R. De Celis, Effect of chronic pesticide exposure in farm workers of a Mexico community, Arch. Environ. Occup. Health, 2012, 67(1), 22–30, DOI:10.1080/19338244.2011.564230.
- J. J. Shearer, D. P. Sandler, G. Andreotti, K. Murata, S. Shrestha, C. G. Parks, D. Liu, M. C. Alavanja, O. Landgren and L. E. B. Freeman, Pesticide use and kidney function among farmers in the Biomarkers of Exposure and Effect in Agriculture study, Environ. Res., 2021, 199, 111276, DOI:10.1016/j.envres.2021.111276.
- M. W. Holliday Jr, Q. Li, E. G. Bustamante, J. Niu, L. Huang, I. M. Espina, J. R. Dominguez, L. Truong, K. O. Murray and L. Fan, Potential mechanisms involved in chronic kidney disease of unclear etiology, Clin. J. Am. Soc. Nephrol., 2022, 17(9), 1293–1304, DOI:10.2215/CJN.16831221.
- R. Babich, J. C. Ulrich, E. D. V. Ekanayake, A. Massarsky, P. M. C. De Silva, P. M. Manage, B. P. Jackson, P. L. Ferguson, R. T. Di Giulio and I. A. Drummond, Kidney developmental effects of metal-herbicide mixtures: implications for chronic kidney disease of unknown etiology, Environ. Int., 2020, 144, 106019, DOI:10.1016/j.envint.2020.106019.
- M. Gonzalez-Quiroz, N. Pearce, B. Caplin and D. Nitsch, What do epidemiological studies tell us about chronic kidney disease of undetermined cause in Meso-America? A systematic review and meta-analysis, Clin. Kidney J., 2018, 11(4), 496–506, DOI:10.1093/ckj/sfx136.
- S. J. Wimalawansa, The role of ions, heavy metals, fluoride, and agrochemicals: critical evaluation of potential aetiological factors of chronic kidney disease of multifactorial origin (CKDmfo/CKDu) and recommendations for its eradication, Environ. Geochem. Health, 2016, 38(3), 639–678, DOI:10.1007/s10653-015-9768-y.
- M. Valcke, M.-E. Levasseur, A. S. Da Silva and C. Wesseling, Pesticide exposures and chronic kidney disease of unknown etiology: an epidemiologic review, Environ. Health, 2017, 16(1), 1–20, DOI:10.1186/s12940-017-0254-0.
- S. J. Wimalawansa, Escalating chronic kidney diseases of multi-factorial origin in Sri Lanka: causes, solutions, and recommendations, Environ. Health Prev. Med., 2014, 19(6), 375–394 CrossRef.
- S. J. Wimalawansa, Escalating chronic kidney diseases of multi-factorial origin (CKD-mfo) in Sri Lanka: causes, solutions, and recommendations—update and responses, Environ. Health Prev. Med., 2015, 20(2), 152–157 CrossRef.
- S. J. Wimalawansa, Public health interventions for chronic diseases: cost–benefit modelizations for eradicating chronic kidney disease of multifactorial origin (CKDmfo/CKDu) from tropical countries, Heliyon, 2019, 5(10), e02309 CrossRef.
- I. R. Rao, A. Bangera, S. P. Nagaraju, S. V. Shenoy, R. A. Prabhu, D. Rangaswamy and M. V. Bhojaraja, Chronic kidney disease of unknown aetiology: a comprehensive review of a global public health problem, Trop. Med. Int. Health, 2023, 28(8), 588–600 CrossRef.
- S. E. Claudel, M. Chan, M. K. Scammell and S. S. Waikar, Challenges and Opportunities: Studying CKDu in the United States, Kidney360, 2024, 10, 34067 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |