Trends in protein derived materials for wound care applications
Muhammad
Zubair
 a,
Saadat
Hussain
b,
Mujeeb-
ur-Rehman
b,
Ajaz
Hussain
c,
Muhammad Ehtisham
Akram
c,
Sohail
Shahzad
d,
Zahid
Rauf
e,
Maria
Mujahid
d and
Aman
Ullah
a,
Saadat
Hussain
b,
Mujeeb-
ur-Rehman
b,
Ajaz
Hussain
c,
Muhammad Ehtisham
Akram
c,
Sohail
Shahzad
d,
Zahid
Rauf
e,
Maria
Mujahid
d and
Aman
Ullah
 *a
*a
aLipids Utilization Lab, Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada T6G 2P5. E-mail: ullah2@ualberta.ca
bLEJ Nanotechnology Center, HEJ Research Institute of Chemistry, ICCBS, University of Karachi, Karachi-75270, Pakistan
cInstitute of Chemical Sciences, Bahauddin Zakariya University, Multan 60800, Punjab, Pakistan
dDepartment of Chemistry, University of Sahiwal, Sahiwal 57000, Pakistan
ePakistan Forest Institute (PFI), Peshawar 25130, Khyber Pakhtunkhwa, Pakistan
First published on 1st November 2024
Abstract
Natural resource based polymers, especially those derived from proteins, have attracted significant attention for their potential utilization in advanced wound care applications. Protein based wound care materials provide superior biocompatibility, biodegradability, and other functionalities compared to conventional dressings. The effectiveness of various fabrication techniques, such as electrospinning, phase separation, self-assembly, and ball milling, is examined in the context of developing protein-based materials for wound healing. These methods produce a wide range of forms, including hydrogels, scaffolds, sponges, films, and bioinspired nanomaterials, each designed for specific types of wounds and different stages of healing. This review presents a comprehensive analysis of recent research that investigates the transformation of proteins into materials for wound healing applications. Our focus is on essential proteins, such as keratin, collagen, gelatin, silk, zein, and albumin, and we emphasize their distinct traits and roles in wound care management. Protein-based wound care materials show promising potential in biomedical engineering, offering improved healing capabilities and reduced risks of infection. It is crucial to explore the potential use of these materials in clinical settings while also addressing the challenges that may arise from their commercialization in the future.
1. Introduction
Effective wound care is of great importance in healthcare due to the growing prevalence of acute and chronic injuries.1 Managing chronic wounds can be especially challenging, as they are often caused by conditions such as diabetes, disorders affecting the cardiovascular system, and reduced mobility.2 The National Institute of Health (NIH) projects that the number of people with chronic wounds will increase from 6 million to 77 million by 2060 if the current management or treatment methods do not improve.3,4 According to the World Health Organization (WHO), burn injuries result in approximately 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fatalities and over 11 million cutaneous wounds per year.5 Due to the diverse nature of wounds, they may be classified based on their dimensions, types, locations, depths, and thickness.6 Open wounds and closed wounds are two distinct categories; open wounds are characterized by damage to the mucous membrane, which allows foreign substances to infiltrate the tissue, such as abrasion, laceration, or avulsion.7 In contrast, closed wounds do not involve any damage to the skin surface, they instead cause tissue damage and bleeding beneath the skin, which results in blisters, seromas, or contusions.8
000 fatalities and over 11 million cutaneous wounds per year.5 Due to the diverse nature of wounds, they may be classified based on their dimensions, types, locations, depths, and thickness.6 Open wounds and closed wounds are two distinct categories; open wounds are characterized by damage to the mucous membrane, which allows foreign substances to infiltrate the tissue, such as abrasion, laceration, or avulsion.7 In contrast, closed wounds do not involve any damage to the skin surface, they instead cause tissue damage and bleeding beneath the skin, which results in blisters, seromas, or contusions.8
In recent times, the focus of biomedical engineering has been on developing biodegradable wound dressings composed of natural and synthetic polymers. These dressings are designed to provide efficient and rapid treatment for wound injuries while simultaneously minimizing the potential for bacterial infections.9 This method, which utilizes biopolymers, is considered to have significant promise for enhancing skin restoration and recovery. It is reported to overcome the constraints associated with autografting, debridement, and allografting techniques.10 The rise of persistent high-risk wounds, particularly in the elderly, immunocompromised individuals, and diabetics, has enhanced the relevance of wound management. Four successive physiological phases, namely coagulation, inflammation, cell proliferation, and tissue regeneration, make up the intricate process of wound healing. Bacteria can readily infect the wound during these processes, increasing inflammation and inhibiting wound healing.11 Currently, most commercial dressings available are made from cotton-based sterile gauze, which often has large pores, making it challenging to prevent the entry and growth of germs. Infection is a crucial issue that can slow down the healing process of wounds.12 To promote better wound healing, advanced wound dressings can be designed to protect the wound from bacteria and maintain moisture.13 These dressings offer comfort, allow oxygen to penetrate, and shield the wound bed from mechanical shock. The materials used in wound dressings must also satisfy specific criteria, including nontoxicity, biocompatibility, and biodegradability.14
Natural polymers that are biodegradable, biocompatible, and have chemical properties similar to the extracellular matrix (ECM) are promising materials that can be used as efficient wound dressing applications. Living organisms produce these polymers which consist of proteins and carbohydrates that combine chemically. Natural polymers, unlike synthetic ones, are predominantly hydrophilic and biocompatible, as illustrated in Table 1. These characteristics make them particularly suitable for biomedical applications.15 Recently, a study reported by Wei and coworkers synthesized gelatin-based anti-inflammatory hydrogels with carboxymethyl chitosan, aloe vera, and glutaraldehyde, showing elasticity, self-repair, shear properties, and anti-S. aureus and E. coli activities, and 12-hour lomefloxacin release, suitable for wound dressings.16 Reisi-Vanani et al. developed wound dressings from thymus oil, PVA, gelatin, and licorice extract, producing smooth nanofibers with enhanced diameter, strong resistance to S. aureus and K. pneumoniae, and efficient fibroblast migration in vitro.17 Gong et al. (2023) developed a biphasic aerogel-hydrogel (AHB-gel) dressing using β-lactoglobulin fibrils (BLGFs) and polyvinyl alcohol (PVA), enhancing liquid interaction, softness, and in vitro biocompatibility. In vivo, studies confirmed the effectiveness of AHB-gel dressings with BLGFs for hemostasis and wound healing.18 Wang et al. (2023) developed a genipin-cross-linked carboxymethyl chitosan-gelatin hydrogel delivering dimethyloxallyl glycine (DMOG) for improved wound healing. The study indicated enhanced cell migration and proliferation in vitro, and the co-loaded drug hydrogels significantly accelerated in vivo wound recovery.19 Jia et al. (2024) created a sodium alginate (SA) hydrogel with recombinant human collagen III (rhCol III) for site-specific, sustained release of extracellular vehicles (EVs). The rhCol III/SA-EVs hydrogel's antioxidant and anti-inflammatory properties aid in healing diabetic wounds, characterized by hyper-inflammation and high oxidative stress. This multifunctional hydrogel offers sustained EV release for multimodal wound healing therapy in diabetic mice.20 Sellappan & Manoharan (2024) developed an antibacterial wound dressing using C. roseus leaf extract and M. recutita chamomile flower, incorporating green synthesized ZnO into keratin and alginate based dressings. The biopolymeric mats exhibited better mechanical, thermal, and hydrophilic properties, and allowed oxygen and water vapor permeability, promoting cell–material interactions. The mat also showed potent antibacterial activity against E. coli and B. subtilis.21 Similarly, phloretin and γ-cyclodextrin complex-incorporated polycaprolactone and silk protein nanofiber wound dressings displayed antibacterial activity against S. aureus, antioxidant capacity, blood compatibility, improved cell viability,22 and suitable physical and chemical properties for promoting diabetic wound healing and reducing bacterial infections.
| Sr. no. | Feature | Protein-based wound care materials | Synthetic polymer-based materials | Ref. |
|---|---|---|---|---|
| 1 | Biocompatibility | Highly biocompatible, composed of natural materials like keratin, silk, and collagen, which lowers the possibility of allergic responses | Exhibit reduced biocompatibility, requiring alterations to minimize immune responses or inflammatory reactions | 25 |
| 2 | Healing promotion | Collagen and silk proteins, for example, actively stimulate tissue regeneration to improve wound healing | Provide defensive barriers and inactive support, they lack the ability to promote active healing processes | 26 |
| 3 | Biodegradability | Biodegradable and readily absorbed by the body | Non-biodegradable or degrading slowly | 27 |
| 4 | Moisture Regulation | Maintain optimal moisture levels | Certain materials can hold water, they often need extra processing to replicate the conditions found in natural wounds | 28 |
| 5 | Mechanical Properties | Exhibiting exceptional elasticity and durability | Potentially possesses increased stiffness or necessitates substantial alterations to align with the mechanical characteristics of biological tissues | 29 |
| 6 | Antimicrobial properties | Some proteins possess inherent antimicrobial properties (e.g., keratin), reducing infection risk | Do not possess innate antimicrobial properties; they require special coatings or substances to achieve such properties | 30 31 |
| 7 | Cost and availability | Cost may be higher due to the use of natural ingredients, and the supply could be constrained by the availability of biological resources | Abundantly available, cost-effective and easy to scale up | 30 |
| 8 | Environmental impact | Eco-friendly, with minimal harmful environmental | Commonly synthesized from petroleum-based resources, leading to environmental issues | 31 |
| 9 | Customizability | Ability of modification is constrained by its biological nature, though enhancements can be achieved through techniques such as crosslinking or genetic manipulation | Offers extensive customization options; can be tailored and developed with characteristics | 32 |
| 10 | Risk of disease transmission | If not properly obtained and handled, there exists a slight, albeit possible, chance of transmitting diseases | While there is no danger of transmitting biological diseases, it may trigger reactions to foreign substances in the body | 29 |
| 11 | Adhesion to tissue | Superior adherence to tissues occurs through natural interactions with components of cells | Enhancing adhesion to biological tissues might necessitate the use of adhesives or modifications to the surface | 26 33 |
Despite abundant research on natural polymers and their potential for wound treatment, there is a lack of commercial natural polymer-based wound dressings in the market. The disconnect between academic research and commercial products creates a gap in the market where not all the capabilities of natural polymers are utilized to treat different types of wounds. Given the various forms of wounds, it is crucial to have access to a broad range of wound dressings with diverse compositions and structures. Natural polymer-based wound dressings often exhibit the ideal qualities for treating wounds, but the primary differences between them are cost and patient comfort.23
Proteins are a necessary component of almost all tissues and have been widely used in tissue repair. Proteins have good biocompatibility, low immunogenicity, and regulated disintegration.24 The advantages of protein-based biomaterials compensate for the limitations of traditional non-biogenic materials. The present review article aims to provide a comprehensive analysis of studies conducted within the past five years, which focus on protein transformation into wound care materials using various chemical methods, their different 7forms and various proteins that are used so far in the wound care applications aimed at accelerating the wound healing process. Additionally, this review delves into the attributes of keratin, collagen, gelatin, silk, zein and albumin derived material for wound care applications in great detail. Lastly, the review concludes by highlighting the limitations and challenges associated with using protein wound care materials.
2. Modification methods to design protein based wound healing materials
Various approaches can be employed to design wound healing materials using proteins (summarized in Table 2), which promote tissue regeneration, minimize the risk of infection, and facilitate a conducive environment for healing.| Methods | Description | Advantages | Disadvantages | Examples |
|---|---|---|---|---|
| Electrospinning | Produces nano-scale fibers from protein solutions using electric field | High surface area, biocompatibility and fine nano-fibers | Limited control over fiber diameter and protein complexity | Zein, soy, pea proteins, casein and lactoferrin based materials |
| Phase separation | Separates homogeneous solution into phases forming scaffolds or wound dressings | Simple, scalable, porous structures and favorable for cell growth | Limited control over pore size | Protein-based polymers and Coacervates |
| Self-assembly | Spontaneous arrangement of molecules into ordered structures | Biodegradable, non-toxic, controllable size and biocompatibility | Complex design requirements | Gelatin-recombinant type III hydrogels, aneroin-based hydrogels and Fibronectin-based hydrogels |
| Ball milling | Grinds materials into nanoparticles using stainless steel balls | High surface area, customizable size and suitable for drug delivery | Energy-intensive, potential contamination | Metal-based Nps and protein-based Nps (PBNps) |
2.1. Electrospinning
Electrospinning is used to synthesize extremely thin fibers, ranging from micro to nano scales, by applying an electric field to a protein solution or molten form.34,35 There are three main ways of electrospinning, which are blend spinning, co-axial spinning and emulsion spinning.36 In blend spinning, the drug or active therapeutic agent is mixed into the protein liquid, which is then subjected to a high voltage.37 This method is used to produce fine nano fibers derived from proteins that can be used as a wound healing material.38 The use of natural proteins instead of harsh chemicals is a key aspect of the process. However, the complexity of proteins presents challenges due to variations in size, charge, and bonding types.39 So, it could be overcome by the choice of suitable material. Furthermore, cross-linking and blending with synthetic polymers like polyvinyl alcohol (PVA), polycaprolactone (PCL) and polyethylene oxide (PEO) improved their compatibility with the process.40,41 Plant and animal proteins can be used in electrospinning processes to create various drug delivery systems. For example, zein, soy and pea proteins are commonly utilized.42 A study reported zein in combination with polymers to create antimicrobial wound dressings through methods like UV cross-linking or co-axial electrospinning.17 Soy protein isolates exhibit beneficial and potentially harmful properties, when combined with suitable polymers through electrospinning, i.e., as antioxidants and allergens.43 Pea proteins are also isolated via electrospinning and contain globulins and albumin as major proteins.44 However, pea protein nanofibers face challenges such as non-uniform structure and size.42 Cinnamaldehyde, when combined with pea protein through electrospinning, has proven to be an effective antibacterial agent for wound healing.45 A significant portion of animal-based proteins originates from milk. For instance, casein, whey, lactoferrin, and lysozyme possess innate antimicrobial characteristics.46 This is due to their ability to bind to iron and break down bacteria. Consequently, these proteins are valuable components in wound healing materials.40Lactoferrin is a protein with a wide range of beneficial properties, including its ability to fight bacteria, reduce inflammation, and protect against oxidative damage.47 Researchers have found that it can be combined with materials like polycaprolactone (PCL), polyacetic acid (PLA), and gelatin using electrospinning.48 Moreover, lactoferrin can be applied to the affected area of the skin to prevent the growth of harmful bacteria.49,50
2.2. Phase separation
Phase separation is a process in which a homogeneous solution is separated into multiple phases, typically a rich polymer phase and a phase with a higher solvent concentration.51 This technique can be utilized in wound healing materials to develop porous structures.52 A polymer solution containing protein-based polymers and a solvent is placed in a mold or spread over a surface.53 The polymer-rich phase solidifies upon evaporation of the solvent, leaving behind a porous structure that can be utilized as a scaffold or wound dressing.53,54Certain aspects of proteins are considered in the process of phase separation; for example how they rearrange themselves during evaporation as its rate determines the pore size of the scaffold.55 During this process, depending on the length and amount of the proteins, two different structures can be formed i.e., clumps and small jelly-like globs known as coacervates. The coacervates possess special properties that change with the size of the protein. They act as good adsorbers and can support the wound healing process.56
2.3. Self-assembly
Self-assembly refers to the process by which molecules or materials spontaneously arrange themselves into ordered structures without the need for external influences.57 Self-assembly involves the use of molecules, such as peptides, that have been designed to interact with one another in a specific way. It results in structures that resemble natural extracellular matrix components. These structures can create a favourable environment for cell growth and tissue regeneration. By manipulating the materials dimensions and pore structure through self-assembly processes, we can create wound dressings with specific desired characteristics.58 Self-assembled hydrogels are designed for wound dressing because they are biodegradable, non-toxic and safe for the skin. Hydrogels like gelatin-recombinant type III have an amazing ability to respond to the body's requirements by forming microscopic structures that enhance wound healing. Work has been reported where rat skin cell regeneration is quicker as compared to the control group.59 Hydrogels are particularly beneficial for managing the various stages of the healing process.43 For instance, chronic wounds like diabetic ulcers can complicate the healing process. So, hydrogels facilitate the healing process due to a moist environment.60 Self-assembling peptides automatically arrange themselves into a structure that conforms to the wound without any external assistance. This concept is analogous to the self-assembly of settling material, such as aneroin-based hydrogels,61 organophosphorous hydrolyse combined with lucine, fibronectin (large glycoprotein found in blood plasma) for sensitive body parts like eyes,62 Bovine Serum Albumin based self-assembly hydrogels63etc.Self-assembled hydrogels have diverse applications in wound healing such as hemostasis, infection prevention, and inflammation control.64 These materials are designed to deliver drugs, cytokines, or cells to the wound site, they degrade into bio-active peptides or natural amino acids for tissue repair.65 Advanced self-assembled materials enable spatio-temporal analysis throughout various healing stages, offering long-term, multi-modal treatments and regulating the wound microenvironment.66
2.4. Ball milling
The process of acquiring extremely fine particles known as nanoparticles (Nps) involves obtaining them via pore uniformity.67 Nps possess a high surface area and can absorb moisture. They provide a suitable environment that is beneficial for wound healing.68 Nps can be derived from either metal-based or protein-based sources (PBNps).69 These particles can be combined with drugs to serve as a matrix that delivers the drug to the wound site and addresses wound-related issues during the process of regeneration and healing.70 This process generates a fine powder with specific electrical and chemical properties. This powdered form is further grinded for several hours to attain the more porous Nps.71 The heat generated from the continuous movement of the balls helps to control the size of the Nps produced. These particles can be customized based on the weight and number of balls used.72 They are then shaped as needed for a specific wound and mixed with drugs or antibiotics to serve as a wound dressing to prevent exposure to dirt and microorganisms.732.5. Physical cross linking
Physical crosslinking technique involves connecting proteins such as those found in soybeans through non-covalent interactions. Wound care materials like hydrogels are formed by various physical interactions, including hydrogen bonding, electrostatic and ionic interactions, hydrophobic interactions, and chain entanglement. Altering physical factors can induce changes in proteins, as seen in soy protein, where a folded conformation develops from an initially random coiled structure. Further aggregation of soy protein results in the creation of a hydrogel, which is a 3D networked structure. For topical application or wound care, hydrogels are produced using methods such as freeze-thawing, self-assembly, and heat treatment, without the addition of chemicals.74Another method is freeze–thawing, which involves subjecting the protein to repeated cycles of freezing and thawing without chemical linkers. This process crystallizes the protein to a higher degree, forming a 3D hydrogel with enhanced elasticity and interconnections. Increasing the number of cycles leads to more crystalline regions in the hydrogels, resulting in a stiffer structure. Varshney and colleagues conducted a study where they employed the freeze–thaw method to design gels from soya protein isolates (SPI) and polyvinyl alcohol (PVA). They found that increasing the freezing time and number of cycles improved the mechanical properties of the gel. The interaction between the –COOH group of SPI and the –OH group of PVA resulted in a gel with improved biocompatibility, proliferation, and cell attachment.75
The layer-by-layer assembly offers another approach to creating protein-based nanoparticles for wound care. This process involves depositing oppositely charged polyelectrolytes onto a charged layer, which can be either a protein or a nanoparticle with the opposite charge. This technique allows for the design of protein-based nanomaterials with specific surface charges and controlled sizes, enabling features such as the regulated release of therapeutic agents or drugs.76,77
Zhou and coworkers developed a self-assembled bio-gel using SPI, polydopamine-reduced graphene oxide (PGO), and gallic acid (GA) in a dopamine-glycerol/water solvent through self-assembly.78 Similarly, researchers employed self-assembly to produce beta-conglycinin/bacterial cellulose (BC) and glycinin/BC membranes. These combinations stabilized soybean release, enhancing wound healing by reducing inflammation and promoting angiogenesis and collagen deposition. The glycinin /BC membrane demonstrated superior wound healing compared to beta-conglycinin /BC.79 Heating proteins unfolds molecules, revealing active sites and promoting aggregation through covalent and noncovalent bonds, forming a gel network at high concentrations. Chien et al. developed an injectable, biocompatible, and biodegradable SPI hydrogel dressing with sustained drug release, as confirmed by in vivo and in vitro analyses.80
2.6. Chemical cross linking
This technique involves creating covalent bonds between soy protein chains, resulting in a more robust interconnected structure with enhanced spatial networks. Hydrogels modified through chemical means demonstrate superior physical stability and mechanical strength. Cross-linking agents can form bonds with soy protein molecules by interacting with various reactive groups, including hydroxyl (–OH), amino (–NH2), and carboxyl (–COOH). This process generates a network-like structure, reinforcing the protein material. Due to their ability to produce a wide range of desired properties, cross-linking agents are the most commonly used method for modifying soy protein hydrogels.81 Epichlorohydrin (ECH) is a frequently used chemical linking agent. The most prevalent chemical modification methods involve the use of chemical cross-linking agents and photopolymerization.82Photopolymerization employs visible or ultraviolet light to initiate the polymerization reaction in the creation of photopolymerized hydrogels. The molecules become excited upon absorbing energy from UV or visible light, generating free radicals. These radicals then trigger the formation of unsaturated bonds in monomeric units, which subsequently undergo polymerization to form hydrogels. A study reported the hybrid hydrogels of SPI and silk fibroin (SF) by subjecting them to 50 W (LED) visible light for 2 minutes. The SPI hydrogel produced through photo cross-linking exhibited superior water resistance and mechanical strength compared to hydrogels obtained through conventional methods.83
This method improves protein solubility and distribution by altering the pH of the solution to a value considerably different from the protein's isoelectric point, causing the protein to unfold. The solution is then cast into a mold and dehydrated to form a film. The drying process can be expedited using ambient temperature, infrared radiation, or microwave energy.84. In a study by Zhao et al., a composite film was developed by blending hydroxypropyl chitosan (HPCS) and soy protein isolate (SPI). Utilizing the solution casting technique, they discovered that a 50% SPI content yielded a film with ideal cytocompatibility and hemocompatibility. This film demonstrated enhanced wound healing, accelerated skin regeneration, and notably improved granulation tissue formation and collagen deposition.85
2.7. Genetically engineered protein nanomaterials
The field of genetically engineered protein-based nanomaterials has seen growing interest in creating protein-based nanomaterials with particular attributes such as size, controlled release, and stability.86 This method involves modifying proteins genetically or chemically to change their amino acid sequence. Furthermore, specific functional groups are incorporated at regular intervals to control the size and shape of the resulting nanomaterial, which is formed through the interaction of these introduced functional groups.3. Forms of proteins derived wound healing materials
3.1. Hydrogels
Protein-based hydrogels are a promising option for wound healing due to their flexibility, smooth surfaces, and high water content.43 These hydrogels can adapt to different temperatures, pH levels, and the presence of specific ions or enzymes, making them suitable for treating wounds at various stages of healing.60 Functional hydrogels can be categorized as those that can adjust their surface in response to the changing requirements of a wound. This ability allows the hydrogel to adapt to the specific needs of the wound at any given moment, whether it requires acceleration or deceleration.87 Hydrogels have been successfully synthesized that can respond to the wound's requirements in a timely and effective manner.62,63,61,66 Different types of hydrogels can be categorized based on their capacity to sustain their structural and functional properties under diverse circumstances.88 One such type of hydrogel is the peptide-based hydrogel, which can rapidly stop bleeding by forming a barrier at the site of the wound. This process occurs more rapidly than other hemostatic materials, such as gauze or chitosan.89 Hydrogels infused with antimicrobial peptides (AMPs) demonstrate a broad spectrum of antimicrobial activity and makes them ideal to prevent wound infections.90 Moreover, they can be combined with fungicides to boost their antimicrobial properties, making them effective as antiseptics during the healing process.91 Peptide-based hydrogels which contain anti-inflammatory drugs are used for the controlled and targeted drug release to reduce and minimize inflammation during wound healing.66 Protein-based hydrogels can serve as a scaffold that encourages cell growth, migration, and tissue repair. Their ability to adhere to cells can be enhanced via the incorporation of bioactive epitopes.92 The use of keratin protein hydrogels and silk protein fibers in regenerative medicine is aimed at resolving the widespread issue of cartilage defects in conditions such as osteoarthritis (OA), as well as healing irregular wound surfaces.93 These hydrogels and fibers are particularly beneficial due to their cell adhesion and mechanical properties, making them well-suited for drug injection and cartilage regeneration.24,90 Researchers have successfully developed a hydrogel system using polypeptide proteins derived from bovine serum albumin (BSA) sourced from cows and silver ions (Ag+).94,95 It results in a sterile environment with strong antibacterial properties and improved blood vessel formation.96An innovative injectable polypeptide-protein hydrogel was created for treating infected wounds by forming a porous structure through the coordination of thiolated BSA protein and thiolated polypeptide with Ag+ ions.95 The hydrogel demonstrated efficient gelation and self-healing properties, along with biological functions such as vascularization and antibacterial activity, due to the S–Ag coordination in the presence of Ag+ ions.97In vitro studies confirmed its role in the healing process, while in vivo experiments on infected mouse wounds revealed its antibacterial effect, wound healing, hair follicle formation, and angiogenesis. These findings highlight the hydrogel's potential in treating infected wounds.98
3.2. Scaffold
Healing can be impeded in the inflammatory stage by excess fluid impacting nearby cells, infections, or other health issues.99 Appropriate wound dressings are crucial to promote healing and preventing infections. Although widely used, common dressings like cotton, bandages, and gauzes have limitations.100 These typical materials show rapid drying, which is unfavourable healing environment, and get adhered to the wound and skin, causing discomfort during removal.101 To tackle these problems, biomaterial-based scaffolds offer structural support and an ideal environment for cell regeneration, acting similarly to natural tissues.43 These three-dimensional (3D) scaffolds have been utilized in a variety of sectors, such as bone, muscle, and skin regeneration.102,103 Effective wound healing requires a scaffold that exhibits high porosity, substantial surface area-to-volume ratio, interconnectivity, flexibility, biocompatibility, and biodegradability.55 Moreover, its degradation rate is synchronized with tissue regeneration that promotes cell attachment, growth, and specialization.104,105Scaffold construction employs diverse techniques which modify properties significantly. Despite the availability of various methods, developing scalable and cost-effective approaches for producing customized scaffolds in large quantities remains a continuous struggle.106,107 An ideal wound healing scaffold should possess appropriate physical and mechanical properties to prevent infections, promote cell adhesion and proliferation while absorbing fluids and maintaining a moist environment.108 Conventional materials, such as cotton pads, have limited fluid retention capacity.100 Therefore, the optimal scaffold should exhibit high absorbency, porosity, and the ability to retain fluids and swell effectively.109,110
3.3. Sponges
Various types of sponges, having mineral skeletons and scleroproteins, play a crucial role in wound care applications.111 The use of special patterns on wound dressings is essential for accelerating healing by appropriately directing cells.112 Although substantial knowledge exists regarding two-dimensional(2D) dressings, research is scarce on three-dimensional dressings that facilitate healing from the exterior to the interior.113–115 Sponge shape influences blood vessel formation, with Radial-196 showing the highest increase in CD31(cluster of differentiation 31) marker as compared to Radial-40, Radial-15, and random sponges.116 Human umbilical vein endothelial cells (HUVECs) produce vascular endothelial growth factor (VEGF), which promotes angiogenesis.117 Western blotting revealed the highest levels of VEGF, phosphorylated akt (p-AKT), endothelial nitric oxide synthase (eNOS), and VE-Cadherin in HUVECs on Radial-196. In vivo studies confirmed superior blood vessel development in the Radial-196 group, indicating that specific sponge shapes enhance angiogenesis.118 Researchers utilized ice templating to form layered, ridged sponge structures for promoting fluid flow and organized cell alignment, thus expediting cell movement.119 Extended sponge shapes boosted protein production for angiogenesis. The Radial-196 sponge was most effective in swiftly healing deep skin wounds in rats, resulting in increased blood vessel formation.120 The application of natural components like proteins can be harnessed to fabricate radial sponges. These sponges possess a design that promotes healing, initiating from the periphery and progressing toward the center. The development of these sponges at decreased temperatures, with thicker layers and ridges, is advantageous for accelerating wound healing.116,1213.4. Films
Films are developed from various materials, including natural proteins derived from silk, soy, or egg.122,123 They have thin layers or sheets and have been applied in a variety of uses, such as wound healing and tissue regeneration. Similarly, they serve as carriers for drug delivery.46 The dressing materials resemble films and are designed to enhance the attributes of wound dressings, such as tensile strength and flexibility.124 Some recently reported films include the following, bovine serum albumin (BSA) films are created through a process that entails protein fibrillation and reverse dialysis.125 These films are biodegradable, non-toxic, and have a variable thickness. They exhibit high transparency and exceptional durability across various solutions.38 Films composed of renewable resources such as soybean polysaccharide and catechol-functionalized soy protein isolate are utilized in a combined form to improve mechanical properties.61 Studies demonstrated a 44% improvement in tensile strength for soy protein isolate (SPI) films, and polydopamine-modified microcrystalline celluloses (MCC) enhanced the duration of usage by 82.3% in comparison to SPI films.126 Egg white is transformed into insoluble hydrogels with the help of 5,6-dihydroxyindole (DHI), which leads to the formation of a black, water-soluble artificial biomelanin (ABM) film.127Silk fibroin and chitin are combined to synthesize films with remarkable dimensional structure, which have applications in electronic and smart contact lenses.128 Plasticizers such as glycerol are added to replace water to increase the flexibility of silk fibroin films. It stabilizes the helical structures in the films.73 These films can be easily molded and adapted to fit any wound location on the upper skin of the body. Films that incorporate bitter vetch protein sourced from Vicia Ervilia display remarkable tensile strength when combined with a small amount of glycerol and the positively charged compound spermidine.129 Spermidine functions as a plasticizer, enhancing the flexibility and extensibility of the films. Additionally, these films exhibit improved barrier properties against gases and water vapor.73
3.5. Protein bioinspired nanomaterials
Bioinspired protein-based nanomaterials (PBNs) are the smallest particles used to control physical and chemical properties. Their high surface area aids in the adsorption of vapors and air molecules, making them an effective alternative for wound healing environments.124,130 Nanoparticles are frequently paired with antibiotics and medications to support wound healing throughout its stages. This material combination has diverse uses, such as tissue engineering, drug delivery, disease diagnosis, and fluid or exudate management.131 Serum albumin-based nanomaterials, such as nanoparticles coated with serum albumin or peptides, improve stem cell adhesion and proliferation, significantly enhancing bone cell growth and function in vivo.125 This accelerates the usually slow bone regeneration process, aiding faster bone repair in athletes with fractures.73Silk nanomaterials are ideal for repairing damaged corneal tissue due to their transparency, flexibility, and slow degradation.132 These nanoparticles can be functionalized with specific molecules to enhance cell attachment, growth, and corneal regeneration. Consequently, they are advantageous in optical surgeries and can expedite healing for individuals recovering from eye injuries or surgeries.124 These nanomaterials are similar to the extracellular matrix (ECM) in our bodies and help in repairing damaged blood vessels. They mainly deal with an inner layer of skin injuries which are sometimes not detectable by the naked eye. An interesting fact is that we can use them for a long time unless the wound heals and it does not affect the skin cells during the process.133 It safeguards the wound from external bacterial or viral infections. A 3D environment constructed with fibrin nanomaterials can encourage the growth of blood vessels by releasing various growth factors in pre-established settings. This facilitates the transformation of stem cells into muscle cells and accelerates the regeneration process.62 Another example of a material used in dental gums regeneration is silk fibroin nanoparticles (Nps). By combining these Nps with a sensor molecule called basic fibroblast growth factor (bFGF), the dental pulp stem cells can grow more effectively. This process can result in the formation of tissue that is similar to natural tissue and the development of blood vessels within the new tissue.134 A comparison of different forms of proteins derived materials for wound care applications is summarized in the Table 3.
| Material | Properties | Advantages | Limitations |
|---|---|---|---|
| Hydrogels | Biodegradable, porous and moist support | Enhances wound healing and tissue regeneration | Limited mechanical strength |
| Scaffolds | 3D structure, biocompatible and porous | Enhances tissue regeneration | Commercial scalability challenges |
| Sponges | Porous, radial shape and promotes angiogenesis | Accelerates wound healing and blood vessel growth | Variable efficacy |
| Films | Thin, flexible and biodegradable | Wound protection and drug delivery | Limited durability |
| Protein-based nanoparticles (NPs) | High surface area and biocompatible | Enhances stem cell adhesion and bone regeneration | Potential toxicity |
4. Protein based wound care materials
Polymers that are derived from natural resources, and extensively utilized in medical and pharmaceutical sectors are favoured due to their hydrophilic properties, biocompatibility, non-toxicity, biodegradability, affordability, and cost-effectiveness.135,136 Additionally, some natural polymers possess inherent antibacterial activity, making them suitable for wound dressing applications. Among the antibacterial natural polymers that have been studied in recent years are aloe vera, chitosan, curcumin, honey, keratin, pectin, and propolis. Carbohydrates and proteins are the two main classes of natural polymers that can be obtained from either animal or plant sources. For instance, chitosan, silk, and honey are derived from animal sources, while pectin, aloe vera, and cellulose are extracted from plants. Conversely, collagen, gelatin, and keratin are widely used animal-derived proteins.6The sequence of amino acids that form peptide bonds and bind together to form the basic structure of proteins interact to form a three-dimensional structure. Hydrophobic, electrostatic, and hydrogen bonds, as well as covalent bonds like disulfide bonds, are all present in protein interactions. Because proteins include a variety of functional groups that may change chemically, enzymatically, and physically, they can be designed for different uses. Protein-based biopolymers 3D structure and functional characteristics are closely linked. Foaming/emulsibility and fat binding ability are primarily influenced by hydrophilic–hydrophobic and hydrophobic interactions, respectively, whereas hydro-solubility, swelling capacity, and gelation capability are pertinent to hydrophilic interactions within the protein network.137
Since biopolymers have been used for years to heal wounds and illnesses, the use of protein based biomaterials in the biomedical field is not new. The advancement of methods to assess and classify protein materials as well as better extraction procedures from their sources have influenced trends in protein based biopolymers for medicinal applications. Biomedical applications often utilize non-immunogenic proteins to create nano/microfibers due to their exceptional fiber-forming properties, high nutritional value, relatively biocompatible polymers, and other advantages.138
The exceptional qualities and distinctive tunability of protein-based biomaterials remain intriguing as the biomedical industry turns its attention to smart materials. They may be made to react to a variety of stimuli, such as temperature changes, oxidative agents, pH changes, and even the presence of specific biomolecules. Additionally, protein based biomaterials provide a multitude of potential for the development of precisely engineered biomaterials since they can be made to self-assemble into a wide variety of forms, from porous architectures to nano-sized materials.139
4.1. Keratin derived materials
Keratin, often found in natural materials like feathers, hair, hooves, horns, and wool, is a widespread choice for its high sulfur content and other functional groups in creating these materials.140 Researchers are particularly interested in incorporating keratin-based formulations (KFs) due to their compatibility with living tissues and biocompatibility. KFs have proven useful in various fields, such as drug delivery, wound healing, and the development of artificial tissues.141,142 Additionally, studies are being carried out to explore the combination of keratin with other natural or synthetic materials to create new products. However, it is important to note that the specific peptide composition and molecular weight of keratin extracted from different sources can significantly affect its properties and performance.141,143 Unlike other natural polymers like starch, gelatin, and chitosan, the complex three-dimensional structure of keratin requires the use of powerful chemicals for extraction due to its unique characteristics.144 As a wound dressing material, keratin shows great potential due to its numerous beneficial properties. These include its capacity to biodegrade, exhibit biocompatibility, allow water vapor transmission, and form gel-like structures. Keratin-based dressings, such as films, hydrogels, sponges, and patches, help promote the healing process by aligning with the body's natural healing mechanisms.144–146 The use of keratin and eco-friendly solvents makes it a practical and cost-effective choice.Antibacterial, antifungal, and cytocompatible polysulfobetaine/keratin-based hydrogels incorporating chlorhexidine were reported through free radical polymerization of sulfobetaine and oxidative self-crosslinking of reduced keratin. These hydrogels exhibited release behaviors of acidity, glutathione, and trypsin, leading to the rapid release of chlorhexidine within the wound matrix. The hydrogels were transformed into powders (xerogels) and applied to wounds, where they reformed in situ by absorbing wound fluid. In vivo testing on infected wounds showed that the xerogel powder dressing reduced inflammation and promoted collagen deposition, resulting in accelerated wound closure. The study demonstrated that the prepared xerogel powder has great potential for promoting wound healing.147 A nanofibrous hydrogel composed of keratin and poly(L-lactate-caprolactone) (PLCL) copolymer, incorporating fibroblast growth factor (FGF-2), was developed using the low-pressure filtration-assisted method in one study. The hydrogel was designed to mimic the dermis, with keratin representing the dermis and PLCL replicating it. The hydrogel was highly porous, biodegradable, and biocompatible, and in vivo experiments demonstrated enhanced re-epithelialization, collagen deposition, hair follicle regeneration, and new blood vessel formation. Moreover, the incorporation of FGF-2 resulted in a superior wound-healing effect as exhibited in. Fig. 1. The study provided an in-depth explanation of the role and mechanism of the bilayer hydrogel dressing in wound healing and resolved the issue of poor interface adhesion of electrospinning nanofibers.148
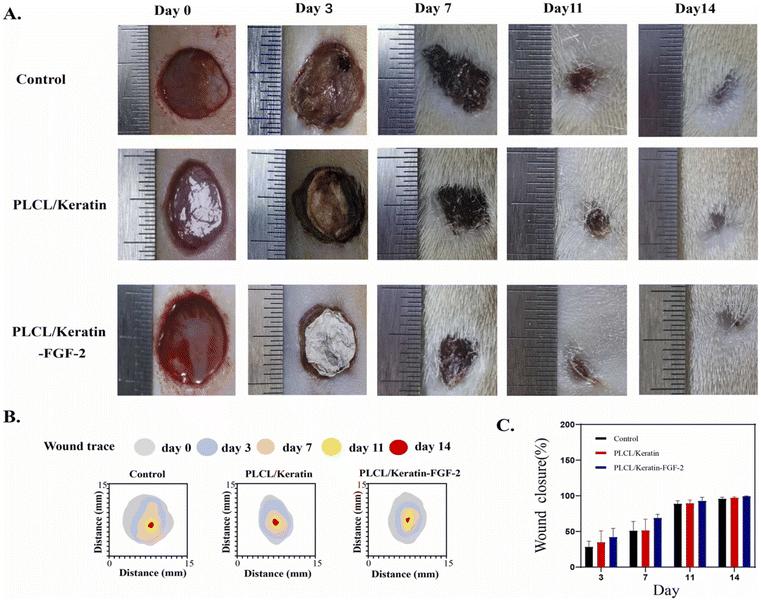 | ||
| Fig. 1 In vivo studies of wound healing process. (A) Representative photographs of wound tissues in different groups on days 0, 3, 7, 11, and 14. (B) Traces of wound-bed closure in three groups during 14-day observation. (C) Wound healing rate of samples in different groups. Reproduced from ref. 148 with permission from Elsevier, copyright 2023. | ||
According to Demir et al., both xanthan/gelatin and keratin/xanthan/gelatin hydrogel dressings with exceptional fluid absorption capacity have been developed for the local delivery of vitamin C. The xanthan/gelatin hydrogels were prepared through crosslinking with varying concentrations of glycerol. The addition of keratin with the xanthan/gelatin/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) hydrogel, improved its mechanical properties, collagen synthesis, and viability of L929 fibroblasts, as well as the release of protein. Both xanthan/gelatin and keratin/xanthan/gelatin hydrogels containing vitamin C exhibited excellent water absorption capabilities, making them suitable for exudate wounds. All the synthesized hydrogels tested inhibited bacterial growth, indicating that both xanthan/gelatin and keratin/xanthan/gelatin hydrogels incorporating vitamin C can be utilized as wound dressing materials.149
2) hydrogel, improved its mechanical properties, collagen synthesis, and viability of L929 fibroblasts, as well as the release of protein. Both xanthan/gelatin and keratin/xanthan/gelatin hydrogels containing vitamin C exhibited excellent water absorption capabilities, making them suitable for exudate wounds. All the synthesized hydrogels tested inhibited bacterial growth, indicating that both xanthan/gelatin and keratin/xanthan/gelatin hydrogels incorporating vitamin C can be utilized as wound dressing materials.149
Kaviyashri and colleagues examined the potential of keratin, activated carbon, and aqueous garlic extract as components of wound dressings. The study revealed that activated carbon exhibited effective adsorption of keratin due to its numerous pores, making it a suitable carrier. The presence of 5% to 10% garlic extract was found to inhibit bacterial growth effectively. Based on these findings, the study concluded that these components could be optimized and combined to design an ideal dressing material for wound repair.150 On the other hand, carboxymethyl cellulose (CMC) was used by Sadeghi and coworkers to develop novel antibacterial sponge-type dressings using keratin, derived from human hair, for the topical delivery system for clindamycin. In addition, the researchers incorporated halloysite nanotubes to facilitate the sustained release of the antibiotic. The addition of larger amounts of keratin to CMC hydrogels resulted in improved water stability, slower clindamycin release, enhanced antibacterial activity, and increased cell attachment and proliferation, although it did not significantly affect water vapor transmission rate.151 In another study, Yao et al. reported a bilayer wound dressing that consisted of a gelatin/keratin nanofibrous mat as the inner layer and a commercial polyurethane dressing as the outer layer. Scanning electron microscope (SEM) analysis revealed a uniform morphology without beads and with an average fiber diameter of 160.4 nm. In vitro analysis using L929 fibroblast cells showed that the gelatin/keratin nanofibrous mat promoted cell attachment and proliferation. Furthermore, the gelatin/keratin/polyurethane bilayer wound dressing demonstrated a better healing response by producing more blood vessels and reducing the wound area at 4 and 14 days compared to the bilayer membrane without keratin, gauze, and the commercial wound dressing (Comfeel),152 as depicted in Fig. 2.
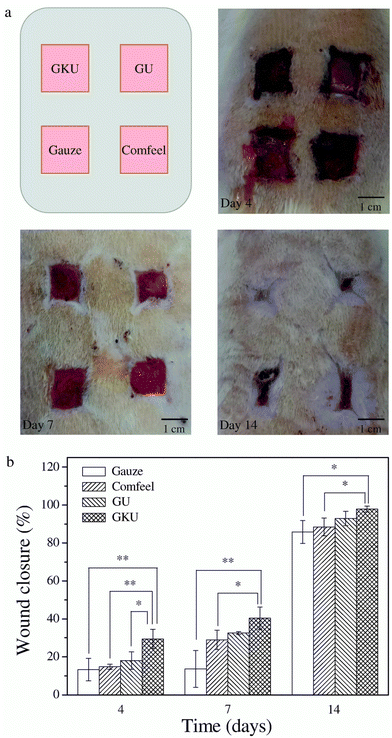 | ||
| Fig. 2 In vivo study of healed wounds. (a) Macroscopic appearance of the wounds after treatment with the gelatin/keratin/commercial polyurethane (GKU) membrane, gelatin/keratin (GU) membrane, gauze and Comfeel® on the fourth, seventh and fourteenth postoperative days. (b) Wound closure with the healing time (4, 7 and 14 days). Reproduced from ref. 152 with permission from Elsevier, copyright 2017. | ||
Keratin from chicken feathers was also utilized to synthesize three types of nonwoven dressings, including pure keratin, keratin-sodium alginate, and keratin-chitosan compositions. These dressings were analyzed using FTIR and SEM, and their physical properties, such as thickness, air permeability, and areal density, indicated their potential use as wound dressings. The keratin-sodium alginate and keratin-chitosan dressings exhibited antibacterial activity against both Gram-negative and Gram-positive bacterial strains, with inhibition zones greater than 2.0 cm. Further investigation of these dressings’ potential was assessed through cytotoxicity, cell viability, and in vivo studies on Albino Wistar rats. The results revealed that the prepared materials were non-toxic and provided good support for cell viability. Wound dressings composed of keratin-chitosan and keratin-sodium alginate exhibited complete wound healing in rat models within 15, 17, 21, and 23 days, respectively. In contrast, dressings made solely of keratin proved to be less efficient in promoting wound closure.153
4.2. Gelatin derived materials
Gelatin is commonly utilized in wound dressings because it has a molecular resemblance to the extracellular matrix of human tissues and organs. Its properties, such as biocompatibility, biodegradability, cell-interactivity, non-immunogenicity, and processability, make it an excellent biopolymer for wound care applications (Table 4).154,155 Additionally, gelatin's low antigenicity makes it suitable for various biomedical applications. However, gelatin's hydrophilic nature requires crosslinking to improve its mechanical strength and stability, ensuring insolubility in biological environments.155 The studies reported the use of enzymatic techniques with transglutaminase or chemical techniques with carbodiimides, fructose, diepoxy, genipin, diisocyanates, glutaraldehyde, formaldehyde or dextran dialdehyde.156,157| Material shape | Composition | Active ingredients | Method of fabrication | Animal Tested | Wound size | Healing time | Notable findings | Ref. |
|---|---|---|---|---|---|---|---|---|
| N.A: not available. | ||||||||
| Fibrous scaffolds | PCL, gelatin, ε-polylysine | — | Fused deposition modeling and electrospinning | S. aureus, P. aeruginosa, E. coli | N. A | N. A | Antibacterial, skin cell toxicity | 162 |
| Hydrogels | Xanthan, gelatin, keratin | Vitamin C | Crosslinking with glycerol | N.A | N. A | N. A | Biocompatible, sustained release up to 100 hours, good mechanical properties | 149 |
| Bilayer sponge/nanofibers | Gelatin, carrageenan | Fibrin | Cross-linking of gelatin with sodium tripolyphosphate, solution casting, electrospinning | Male Wister rats | 10 mm | 14 days | Tensile strength increased by addition of carrageenan/fibrin layer, adhesion and proliferation of L929 cells, angiogenic potential | 163 |
| Hydrogels | Gelatin, dimethyl aminoethyl methacrylate | — | Chemical and physical cross-linking | Wister rats | — | 21 days | Mechanical strength improved by cross-linking, biodegradable, non-toxic | 164 |
| Hydrogels | Gelatin, oxidized chondroitin sulfate | Curcumin loaded chitosan nanoparticles | Freeze drying | — | — | — | Non-toxic, In vitro Curcumin stable release | 165 |
| Electrospun membranes | Poly(L-lactide-co-glycolide)/gelatin | Zinc oxide nanoparticles | Electrospinning | Rat | 10 mm | 14 days | Cytocompatible, hemostatic, antibacterial | 166 |
| Nanocomposite hydrogels | Modified gelatin/iron | Camellia sinensis extract | Metal organic framework (MOF) | B. serous, S. aureus, S. mutans, P. aeruginosa, E. coli, K. pneumoniae | — | — | Antibacterial, sustained release, adding MOF increased water absorption | 167 |
| Hydrogels | Modified hyaluronate, gelatin | Doxycycline | Crosslinking by boronate ester and encapsulation of doxycycline | Rats | 20 mm burn wound | 14 days | Antibacterial, bioadhesive, injectable, biocompatible | 168 |
| Film | Carboxymethyl chitosan-gelatin- | mesoporous silica nanoparticles containing Myrtus communis L. extract | — | Mice | 7 mm | 6 to 12 days | Antioxidant, increased tensile strength by adding nanoparticles, reduced cytotoxicity, reduced drug release | 169 |
| Film | Gelatin/Persian gum/bacterial nanocellulose | Frankincense essential oil and Teucrium polium extract | Solution casting | S. aureus, P. aeruginosa, E. coli, A. baumannii | — | — | Better anti-inflammatory and antibacterial activity, biocompatible | 170 |
To tackle the challenge of rapid degradation and poor adhesion in gelatin-based traditional dressings in fluid environments, Lin and colleagues developed more adhesive dressings using gelatin, silica, and 3-glycidoxypropyltrimethoxysilane as a coupling agent. By employing the sol–gel method to create hydrogels, the stability of the structure was enhanced through the formation of covalent bonds between gelatin and silica, as a result of the coupling reaction. Moreover, dopamine was added to further increase adhesiveness. The study showed that these hybrid dressings were 2.5 times more adhesive to soft tissues than pure gelatin in humid conditions. In vitro and in vivo tests also revealed their superior healing capabilities as exhibited in Fig. 3.158
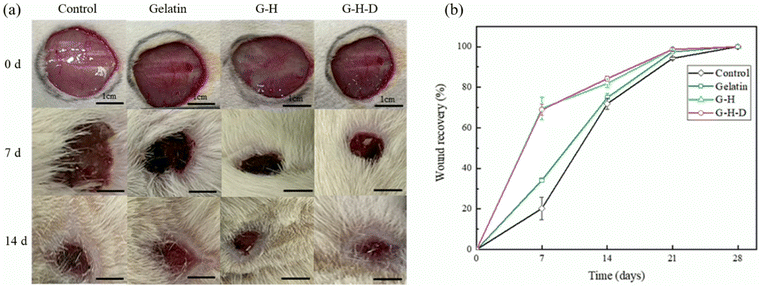 | ||
| Fig. 3 (a) In vivo skin wound healing assessment. Untreated (control), gelatin, gelatin-silica hybrid (G-H) and gelatin-silica-dopamine (G-H-D) samples were applied to a 2 cm diameter round wound created using a surgical scalpel for wound recovery observation over different time periods (b) the wound recovery trend was calculated based on the changes in wound area observed in (a) Reproduced from ref. 158 with permission from Elsevier, copyright 2024. | ||
Developing wound dressings that can effectively control rapid bleeding during accidents and surgeries is a significant challenge. Li and his team developed effective sponges for wounds based on methacrylated gelatin-dopamine, quaternized chitosan, and glycerol to control rapid bleeding. The results demonstrated that these composite sponges were biocompatible, could absorb water, displayed good self-adhesion properties, and exhibited better antibacterial activity compared to commercial gelatin and chitosan dressings. Animal studies conducted on rat tail and liver bleeding models showed that the hemostasis time and blood loss in these prepared dressings were better than those of commercial gelatin and chitosan dressings. Therefore, these dressings/sponges have significant potential to be employed as hemostatic agents for surgeries and emergency accident treatments.159 Khan et al., have developed a multilayer nanofibrous wound dressing using electrospinning, chemical functionalization, and electrospray techniques with a layer-by-layer method. The dressing comprises a coating of diethylenetriamine-functionalized polyacrylonitrile TiO2 nanoparticles and a bioderived gelatin layer. It includes an outer layer that acts as a barrier against pathogens, an interlayer that kills microbes, and a contact layer for improved biocompatibility and cell viability. The nanofibrous membranes demonstrated antibacterial properties, and showed better cell morphology, proliferation, and viability in comparison to 3T3 (3-day transfer) fibroblasts (Fig. 4), making them suitable for wound healing applications.160
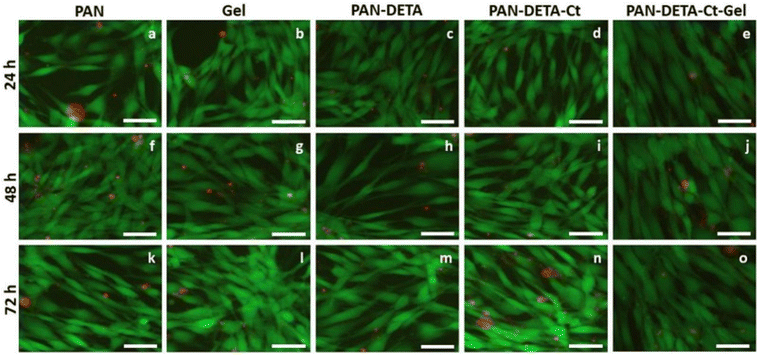 | ||
| Fig. 4 Fibroblast (3T3) cell morphology on the various NFs membrane scaffolds at 24, 48 and 72 h. Reproduced from ref. 160 with permission from Elsevier, copyright 2023. | ||
In response to the growing need for plant derived antibiotic alternatives, Vanani et al. have developed antibacterial core–shell nanofibers as wound healing scaffolds to evaluate their synergistic effect. The scaffolds were composed of PVA/gelatin/thymus essential oil as the core and PVA/gelatin/licorice extract as the shell. The nanofibers were found to be free of beads, smooth, and had an average diameter of 119 nm. The addition of essential oil and licorice extract increased the diameter of the nanofibers. The nanofibrous scaffolds exhibited antibacterial properties, were non-hemolytic, and promoted the viability and proliferation of L929 fibroblasts, (Fig. 5) showing them as potential candidates for use as wound dressings.17
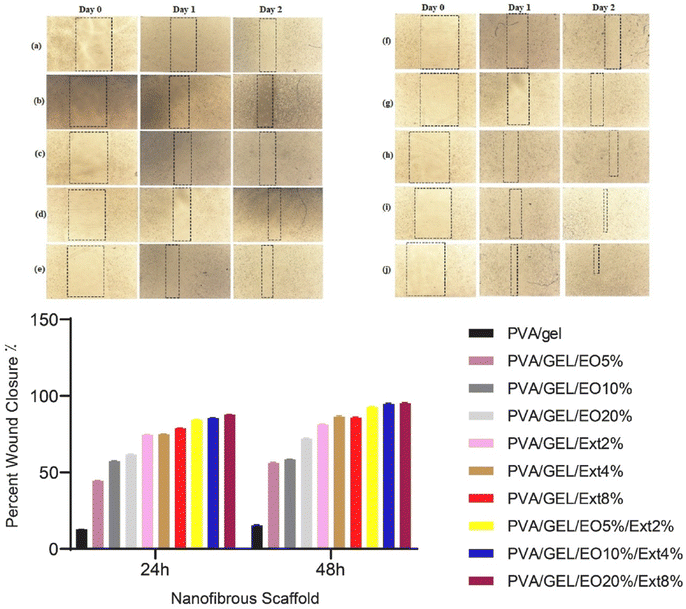 | ||
| Fig. 5 Wound healing micrographs and fibroblast migration into the scratch area after one- and two-days cell culture on the nanofibrous scaffolds. Reproduced from ref. 17 with permission from Elsevier, copyright 2017. | ||
Chronic wound healing is often hindered by infections, creating a need for wound dressings that can release antibacterial and antioxidant substances. These advanced dressings are highly sought after due to their potential to combat infection-related complications in the healing process. Lv and his team have developed nanofibrous membranes composed of polycaprolactone (PCL) and gelatin, incorporating varying ratios of curcumin and borneol using the electrospinning technique. The resulting membranes demonstrated excellent water absorption capabilities, improved mechanical properties, and enhanced dissolution of curcumin. Additionally, the membranes were shown to exhibit antibacterial and antioxidant properties, while also demonstrating biocompatibility. These features, combined with the use of biodegradable polymers, an eco-friendly production method, and promising results in living organisms, indicate the potential for large-scale production.161
4.3. Collagen derived materials
Collagen's diverse applications are well-recognized, encompassing its utilization as a material for sutures, in viscous formulations, as lenses for bandages, for grafts to replace vitreous humor, and as a protective agent during surgical procedures.171 Collagen is a crucial protein in humans that is abundant and plays a vital role in maintaining the structural integrity of the skin. It functions as a scaffold within the extracellular matrix (ECM) and serves as an essential signalling molecule.172,173 Numerous approaches have been tested to evaluate the use of collagen in wound healing (Table 5). They have been employed as scaffolds or matrices in soft tissue repair, hemostasis, tissue engineering, and more recently nano-medication.174–180| Material shape | Composition | Active ingredients | Method of fabrication | Animal tested | Wound size | Healing time | Notable findings | Ref. |
|---|---|---|---|---|---|---|---|---|
| N.A: not available. | ||||||||
| Hydrogel | Collagen, xanthum gum | Ketorolac, methylene blue, dexamethasone and quinic acid | Interaction of functional groups | Fibroblast cells | N. A | N. A | Controlled release of ketorolac and methylene blue | 200 |
| Hydrogels | Collagen-polyurethane-alginate | Ketorolac | Electrostatic interaction | E. coli, In vitro wound healing on fibroblast cells, anti-cancer activity on colon and breast cancerous cells | N. A | N. A | Proliferation of fibroblast and monocytes, anti-cancer activity against breast and colon cancer, anti-bacterial activity against E. coli | 201 |
| Vesicles | Peptides [CLP (G8): (GPO)8GG or (GPO)8GC and ELP (F6): (VPGFG)6G′] | Vancomycin | Vancomysin encapsulation in prepared vesicles and in liposome | Methicillin-resistant Staphylococcus aureus | — | — | Sustained release and higher % encapsulation compared to liposomal version Novel antibiotic delivery system | 202 |
| Infuse | Recombinant human bone morphogenetic protein-2 (rhBMP-2) on an absorbable collagen sponge (ACS) | rhBMP-2 | rhBMP-2 absorbed on ACS | Non-human primates | — | — | Osteoinductive autograft replacement | 203 |
| Hydrogels | Collagen | Gallic acid and naproxen Metal nanoparticles | Through polymerization and cross linking | — | — | — | Potential drug delivery agents, anti-microbial hydrogel films Higher encapsulation and release of drugs | 204 |
| Scaffold | Collagen-chondroitin sulfate scaffold | Stromal derived factor-1 alpha (SDF-1α] | Dehydrothermal treatment | Human umbilical vein endothelial cells | — | — | Enhanced pro angionic response for wound healing | 205 |
| Scaffold | Collagen/heparin bi-affinity multilayer delivery system (CHBMDS) | CBD-bFGF (a collagen-binding domain (CBD)) | Specific or electrostatic interaction | SD rat | — | 5 weeks | Sustained and localized release of CBD-bFGF Enhanced angiogenesis | 206 |
| Nanofiber | Collagen and chitosan | Curcumin | Electrospinning | Escherichia coli Pseudomonas aeruginosa, and Staphylococcus aureus | — | — | Sustained release of curcumin up to 72 hours, tend to be potential patches for wound healing | 207 |
| Hydrogel | Collagen and chitosan | Cross linked by dialdehyde starch | Mixing different ratios | — | — | — | Characterized as potential biocompatible wound dressing | 208 |
| Hydrogel | Human like collagen and chitosan | Crosslinked via dialdehyde starch | Mixing in different ratios | In vitro and In vivo Model (post injection) | — | 1, 9, 12 and 28 weeks | Reduces inflammation, can be potential skin patch scaffolds, wrinkle treatments, and tissue cavity fillers | 209 |
Collagen is mixed with natural and synthetic polymers, including alginate, chitosan, hyaluronic acid, elastin, polyethylene oxide and silk fibroin, etc., to develop collagen based wound dressings.181–183 These modified fabrics have also additives such as antibiotics,184 insulin,185 or gold nanoparticles186,187 and have primarily been studied in small animal wound healing models or in vitro investigations. Although initial findings are promising, a comprehensive evaluation of the effectiveness of antibacterial wound dressings for clinical diabetic foot ulcers remains inconclusive. To establish the true efficacy of this treatment, more extensive and rigorous clinical studies are necessary.
In scaffolds and matrices, collagen is applied as a surface coating to maintain a moist environment and enhance cell adhesion.188 Water retention is essential for preserving moisture in the wound bed. In vitro studies on collagen have demonstrated that the arginine-glycine-aspartic acid (RGD) sequences interact with cell integrins, promoting the attachment and movement of fibroblasts and keratinocytes. In recent years, there has been growing interest in collagen nanostructures. A relatively new material has emerged, consisting of collagen reduced to nanoparticulate dimensions. This nanoscale form provides an enhanced surface area to volume ratio. A key advantage of these nano-collagens is their ability to be delivered to specific targets using a material that is biocompatible with the microenvironment of wounds.189,190 One constraint is the lack of understanding and investigation into these nanoparticles, necessitating more in-depth study. A recent pilot study using Porcine derived hydrolyzed collagen (PDHC) to treat chronic ulcers of various causes revealed that the product was safe to use and sped up the healing process.191 The large-scale production of recombinant human collagens from non-animal sources may face challenges due to the potential requirement for post-translational proline hydroxylation. Following the identification of collagen-like proteins (Scl1 and Scl2) in Streptococcus pyogenes, researchers developed constructs in a recombinant E. coli system to explore large-scale production methods. Utilizing bacterial collagens provides a synthetic approach to creating non-animal collagen without specific bioactivity, allowing for customized interactions. An experiment using human mesenchymal stem cells demonstrated this system's potential for chondrogenesis.192 Research findings indicate that recombinant human collagen shows promise as a future wound healing agent.
Shields of collagen were synthesized as corneal bandages to aid in the healing of wounds following radial keratomy, corneal transplantation, keratorefractive surgery, and epithelial debridement procedures. When applied to the eye, the thin collagen films take on the shape of the cornea, allow for adequate oxygen transmission to support corneal metabolism, and function as temporary bandages. When the shields dissolve, a layer of collagen solution is left behind that appears to lubricate the eye's surface, reduce rubbing of the lids against the cornea, and promote epithelial healing.193–196 Li Zhiye and colleagues recently reported a study on phycocyanin-loaded hydrogels made from collagen, chitosan, and genipin. The study evaluated the biocompatibility and cell migration potential of these hydrogels in vitro. A gel containing collagen/chitosan (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75) demonstrated the best performance in promoting cell migration. Subsequent in vivo studies confirmed these results, establishing nanocomposite hydrogel as the most effective formulation for promoting diabetic wound healing through increased cell migration and reduced MMP-9 expression.197 Malathi and coworkers studied about the use of collagen-based zinc oxide nanoparticles (ZnO NPs) for wound healing. Their study proved that the developed nanocomposite enhanced the rate of wound healing to a high level. These innovative bio-nanocomposites possess several advantageous properties including strong antibacterial properties, non-toxicity, short, adjustable, economical, and large-scale fabrication process.198 Recently, researchers developed a novel wound dressing made from a blend of fish collagen, oxidized sodium alginate derived from seaweed, borax (a boron compound), and polyvinyl alcohol (a plastic-like material). This dressing is designed to effectively heal deep wounds. The fish-skin collagen-based dressing is self-healing, injectable, adheres to various surfaces, and degrades naturally in the body. In experiments involving mice, the dressing demonstrated superior performance compared to traditional gauze, rapidly stopping bleeding and accelerating wound healing by stimulating skin cell growth, collagen production, and new blood vessel formation.199
75) demonstrated the best performance in promoting cell migration. Subsequent in vivo studies confirmed these results, establishing nanocomposite hydrogel as the most effective formulation for promoting diabetic wound healing through increased cell migration and reduced MMP-9 expression.197 Malathi and coworkers studied about the use of collagen-based zinc oxide nanoparticles (ZnO NPs) for wound healing. Their study proved that the developed nanocomposite enhanced the rate of wound healing to a high level. These innovative bio-nanocomposites possess several advantageous properties including strong antibacterial properties, non-toxicity, short, adjustable, economical, and large-scale fabrication process.198 Recently, researchers developed a novel wound dressing made from a blend of fish collagen, oxidized sodium alginate derived from seaweed, borax (a boron compound), and polyvinyl alcohol (a plastic-like material). This dressing is designed to effectively heal deep wounds. The fish-skin collagen-based dressing is self-healing, injectable, adheres to various surfaces, and degrades naturally in the body. In experiments involving mice, the dressing demonstrated superior performance compared to traditional gauze, rapidly stopping bleeding and accelerating wound healing by stimulating skin cell growth, collagen production, and new blood vessel formation.199
4.4. Silk fibroin based materials
Silk fibroin and its derivatives are being assessed for their potential application in wound dressings due to their biocompatibility, exceptional mechanical strength, biodegradability and high water absorption capacity. Additionally, silk fibroin has been shown to promote cell migration and proliferation, which is essential for accelerating wound healing.114,128,210–213 The Food and Drug Administration (FDA) has approved the use of silk as a surgical mesh due to its utility as a stitching material and the fact that it has minimal negative effects. Silk fibroin, the primary constituent of silk produced by silkworms, functions in a coordinated manner to promote wound healing. It exerts control over cellular processes on both a macroscopic and molecular scale. By activating a pathway known as NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells) signaling, silk fibroin accelerates the healing process. This signalling mechanism is crucial for various cellular activities, such as cell adhesion, proliferation, and anti-inflammatory responses.214Tatlisulu et al. (2024) explored the use of honeybee silk (HS) as an alternative to silkworm silk in tissue engineering (TE) applications. Their research revealed the potential of HS, a previously unexplored material, for TE purposes, paving the way for new research directions. The study findings, based on the materials employed and the favorable results obtained, indicate that the developed CH-HS scaffold displays antibacterial qualities and exhibits cellular characteristics. These discoveries hold promise for additional biomedical applications, particularly in the realm of wound healing.215 In another study, a natural hydrogel composed of silk fibroin (SF) and soybean protein isolate (SPI) was prepared using the main components. To enhance the bioactivity of the hydrogel, quercetin was incorporated to create an SF/SPI-Q hydrogel. The resulting hydrogel was effective in accelerating wound healing in an infected burn wound model as shown in Fig. 6. The SF/SPI-Q hydrogels promoted the re-epithelialization process, collagen synthesis, and neovascularization in burn wounds. Additionally, they exhibited anti-inflammatory effects that further helped reduce inflammation. Therefore, the SF/SPI-Q hydrogels have the potential to be one of the most effective treatments for wound healing.82
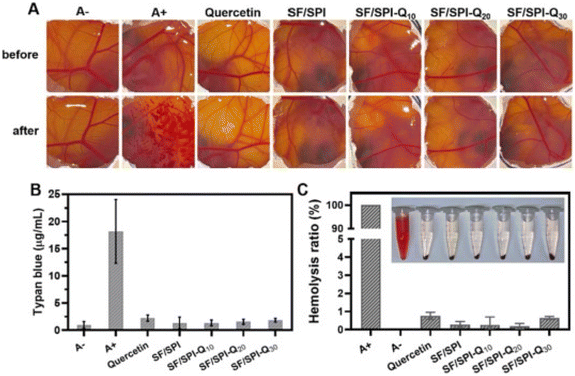 | ||
| Fig. 6 (A) Effect of different treatments on chorioallantoic membrane (CAM)-trypan blue stained assay CAM. A−: negative control (0.9% NaCl solution); A+: positive control (0.1 M NaOH). (B) The amount of trypan blue adsorbed on CAM after different treatments. (C) Hemolysis ratio of quercetin, silk fibroin (SF)/soybean protein isolate (SPI), SF/SPI-quercetin (Q) 10%, SF/SPI-Q 20% and SF/SPI-Q 30% hydrogels; A−: negative control (0.9% NaCl solution); A+: positive control (H2O). Reproduced from ref. 82 with permission from Elsevier, copyright 2024. | ||
A study was conducted by Shuiqing and their colleagues to construct a scaffold using silk fibroin nanofibers films (SNF) to promote wound healing and prevent scar formation. The scaffold was engineered to resemble the extracellular matrix of the skin, and the resulting silk film exhibited greater mechanical strength and hydrophobicity, protecting the wound and dermis from external stimuli. Additionally, the scaffold displayed improved water uptake, which facilitated moisture retention at the wound site. This feature, along with the scaffold's resemblance to the components of normal epidermis and dermis, inhibited scar formation. In vitro and in vivo studies confirmed the scaffold's effectiveness in promoting tissue regeneration and preventing scar formation216 as exhibited in Fig. 7.
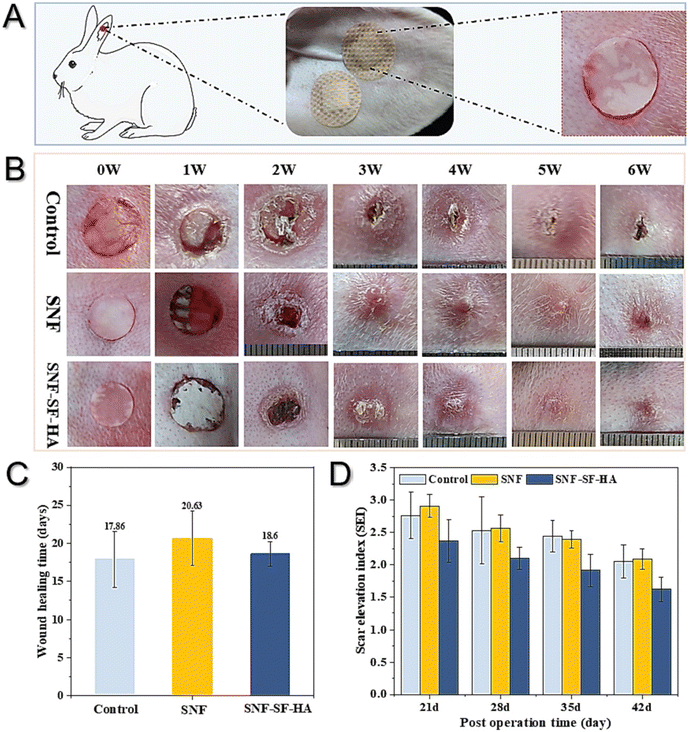 | ||
| Fig. 7 Observations of wound healing and scarring in the rabbit ear, after treatment with different artificial grafts. (A) Scheme of scaffold application in a rabbit ear wound model, (B) wound changes, including control (untreated), SNF films, and SNF-silk fibroin (SF)-hyaluronic acid (HA) scaffold, (C) wound closure time, (D) scar elevation index. Reproduced from ref. 216 with permission from Elsevier, copyright 2024. | ||
Silk sericin based hydrogels with plant extracts were synthesized by Zahoor and coworkers. They studied their efficacy in promoting wound healing in mice with alloxan-induced diabetes. Researchers produced 6 mm excision wounds and then applied topical hydrogel treatments. Results indicated that all hydrogel-treated groups showed significantly higher wound contraction from day 3 to day 11 compared to the negative control, with the 4% sericin + 4% banyan + 4% onion hydrogel performing best as shown in Fig. 8. Serum levels of anti-inflammatory cytokine Interleukin-10 and tissue inhibitor metalloproteinase (TIMP) were significantly higher in the hydrogel groups, while pro-inflammatory cytokines tumour necrosis factor-α and Interleukin-6, and matrix metalloproteinases MMP (matrix metalloproteinases)-2 and MMP-9, were significantly lower.217 Silk fibroin hydrogels show potential as burn wound dressings due to their regenerative properties, but difficult gelation conditions limit their clinical application. Sushma Indrakumar and his colleagues employed a white light-responsive photopolymerization technique for gelation through tyrosine photooxidation. A silk fibroin gel-incorporated dressing (SFD) was developed to fit irregular burn surfaces. The mild gelation conditions enabled drug incorporation for localized delivery. The dressing demonstrated favourable swelling capacity and moisture retention. In vitro, cytocompatibility was assessed using HaCaT cells, and in vivo performance was evaluated on a rodent model with a second-degree burn, showing scarless healing in SFD-treated groups via gross and histological analyses. The SFD developed in this study shows promise as an advanced burn wound care solution.218
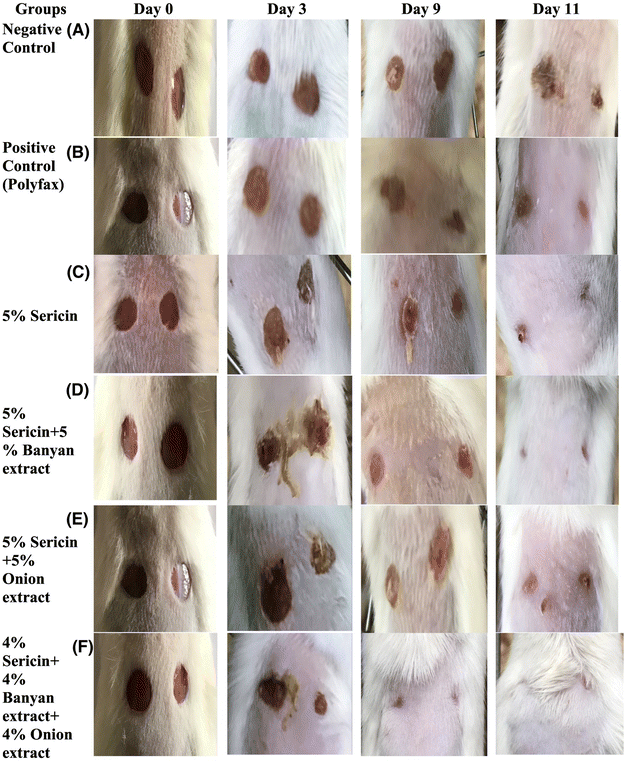 | ||
| Fig. 8 Wound healing area at different post-wounding days in mice of (A) negative control; (B) positive control; (C) sericin based treatment group; (D) sericin + banyan based treatment group; (E) sericin + onion based treatment group and (F) sericin + banyan + onion based treatment group. Reproduced from ref. 217 with permission from Elsevier, copyright 2023. | ||
A study revealed that sericin, a bio-waste product derived from the degumming of silk cocoons, effectively exfoliates the MoS2 layers, resulting in improved dispersity and stability of MoS2 nanosheets (MoS2-NSs). MoS2-NS/Sericin maintains its photothermal properties when illuminated by an 808 nm light source, exhibits strong antibacterial activity, and accelerates wound healing by promoting fibroblast migration. In vitro experiments have shown that MoS2-NS/Sericin scavenges reactive oxygen species (ROS) during the inflammatory stage of wound healing and transforms M1-type macrophages into M2-type, which supports recovery. Full-thickness skin wound tests conducted on rats demonstrated that MoS2/Sericin, under 808 nm irradiation, optimally promotes wound healing. Consequently, MoS2-NS/Sericin holds significant potential for treating bacteria-infected wounds.219
Regenerated silk, derived from protein extracted from Bombyx mori silkworm cocoons, exhibits numerous desirable properties. These include high transparency, compatibility with biological systems, ability to degrade naturally, and suitability for modification with optically active nanoparticles and biochemical markers. Its porous structure facilitates the transfer of nutrients and oxygen while also providing protection against bacterial infections in wounds, making it an ideal material for wound dressing applications. Various chemical and physical cues can enhance the bioactivity of silk fibroin (SF) wound matrices, simultaneously optimizing these cues is challenging due to their complex interactions during the fabrication process.220–222 A recent study explored a biodegradable silk-curcumin composite for extended drug release in wound treatment. The study found that curcumin integrated into the silk surface exhibited sustained release for 10 days, significantly outperforming traditional wound care drug delivery systems. The silk's superhydrophobic nature prevented wound wetting, while its pH sensitivity allowed for visual monitoring of wound healing progress.223 Another study utilized β-Sheet β-rich silk nanofiber (BSNFs) and amorphous silk nanofibers to create bioactive matrices with various cues optimized through aqueous media self-assembly.224,225 Additionally, BSNFs were employed to develop an anisotropic gel under electric field influence. Nerve growth factors (NGF) were immobilized within this hydrogel to fabricate bioactive systems, providing multiple physical and biological cues for addressing spinal cord injuries.226 Similarly, researchers designed a silk fibroin-based matrix with adjusted hierarchical microstructures and introduced various physical cues. An anisotropic porous scaffold was created using silk fibroin nanofibers under an electric field. This engineered nanomaterial demonstrated improved cell migration, leading to more effective wound healing.227 The incorporation of Desferrioxamine (DFO) into a silk nanofiber hydrogel, demonstrated prolonged drug release over a 40-day period. Both in vitro and in vivo analyses revealed improved endothelial cell migration and gene expression, along with a decrease in inflammatory macrophages.228 In a similar study, Sang and colleagues developed scaffolds with a microstructure resembling the extracellular matrix, using amorphous silk fibroin. These scaffolds showed potential for tissue repair and regenerative medicine applications.228 Gang and coworkers engineered scaffolds using β-sheet rich silk nanofiber infused with deferoxamine. They combined deferoxamine-loaded BSNF with amorphous silk nanofibers (ASNFs) under an electric field to create a uniform mixture. This novel scaffold exhibited enhanced wound healing efficacy in both in vitro and in vivo.229
Bari et al. investigated silk sericin (SS) microparticles for non-surgical intervertebral disk degeneration treatment, enhancing SS with growth factors, platelet lysate (PL), and platelet-poor plasma (PPP). Spray-drying produced smooth microparticles, and the PL and PPP combination promoted nucleus pulposus cell growth. SS microparticles, with or without PPP, exhibited antioxidant properties by reducing reactive oxygen species (ROS). Co-incubating SS microparticles with PL protected nucleus pulposus cells from hydrogen peroxide-induced oxidative stress. This indicates that SS microparticles with PL + PPP are promising for non-invasive drug delivery in regenerative medicine and wound treatment.230 Karaly et al. developed an aerosolized nano-powder using Avicenna marina extract and neomycin-loaded SF nanoparticles for wound healing. The extract exhibited antioxidant, antibacterial, and cell proliferation properties, effectively combating various bacterial strains. In vitro and in vivo studies demonstrated superior wound healing, achieving complete closure within 24 hours in fibroblast scratch assays and enhancing fibroblast proliferation while reducing inflammation in rodent models, suggesting its potential for wound healing applications.231
4.5. Zein based materials
Zein, a cost-effective amphiphilic prolamine,232 is an excellent candidate for wound healing applications due to its inherent characteristics such as non-toxicity, non-inflammatory stimulation, biodegradability, flexibility, and biocompatibility.233,234 Moreover, zein based wound care materials have unique features, including resistance to water, heat, abrasion, and humidity, make them an attractive option for a range of biomedical applications232,235,236 for wound care applications (Table 6).| Material | Composition | Active ingredient | Method | Animal tested | Wound size | Healing time | Notable findings | Ref. |
|---|---|---|---|---|---|---|---|---|
| N.A: not available. | ||||||||
| Hydrogel | Pectin | Doxorubicin | Liquid–liquid dispersion method | N/A | N. A | N. A | Controlled release of Doxorubicin | 248 |
| Zein solution | Propylene glycol alginate | Curcumin | Hydrogen bonding | — | N. A | N. A | Sustained release of curcumin | 249 |
| Zein solution | Polyacrylate, glycerin | β-Carotene | Hydrogen bonding, hydrophobic interactions | Female Sprague-Dawley rats | — | 21 days | Promotion of wound healing and collagen synthesis | 250 |
| Zein nanoparticles | Glycerin | Quercetin | Electrostatic interactions | N/A | — | N/A | Delivery and controlled release Higher % encapsulation efficiency | 251 |
| Emulsion gel | Sodium alginate | Curcumin and resveratrol | Electrospinning | — | — | — | Encapsulation of nutrients | 252 |
| Membrane nanoparticles | Oleic acid, pectin | 253— | Solvent evaporation | — | — | — | The viscous behavior of pectin was changed | 253 |
| Nanoparticles | Chitosan-polyvinyl alcohol, Fe3+ | Gentamicin | Liquid–liquid dispersion method, imine bond | E. coli (K12) and S. aureus (ATCC 96) | — | — | Synergistic antibacterial effect, against drug-resistant bacteria. | 254 |
| Nanofilm | Zein prolamine, tea carbon spot, CaO2 | Ir-Zein protien | Electrospinning | Male Sprague-Dawley (SD) rats | 15 mm | 10 days | A versatile antibacterial wound dressing, promoted diabetic wound healing | 243 |
| Zein nanofiber | Zein/CeNP1%, 3%, and 5% NFs | Cerium oxide nanoparticles | Electrospinning | E. coli, Pseudomonas aeruginosa, and Staphylococcus aureus | — | — | Significant mechanical, antioxidant, and cytotoxicity properties | 255 |
| Nanofiber | Zein protein and tungsten oxide | Tungsten oxide | Electrospinning | Melanoma cell lines | — | — | Promising and safe candidate for anticancer applications | 256 |
Using phase separation methodology, Lu et al. developed ivermectin (IVM)-loaded zein microspheres with an average diameter of 1 μm. The researchers proposed these zein microspheres as potential nanocarriers, noting that their dimensions were suitable for macrophage uptake. The study concluded that the sustained-release properties of zein microspheres could be beneficial in creating scaffolds that promote cell growth and tissue engineering. Zein-based composites have diverse applications, including drug delivery systems, tissue engineering, bone reconstruction, and wound repair.237 In a related investigation on biomimetic mineralization, Yao et al. conducted a study where hydroxyapatite (HA) nanocrystals were formed on zein fibers produced through electrospinning. The researchers reported that the HA crystals were irregularly dispersed on the nanofibers, and these biomineralized structures had a beneficial effect on osteoblast proliferation. Additionally, Zhang et al. explored the formation of calcium phosphate using zein as a template and examined the process of biomineralization at the interface between air and concentrated simulated body fluid (SBF).238 Studies have shown that applying a continuous calcium phosphate layer to pure zein film enhances its mechanical properties, including hardness and modulus. Additionally, research indicates that zein films with biomineralization are excellent for supporting fibroblast cell attachment, multiplication (Fig. 9), and development, owing to their water-attracting nature and mechanical characteristics. These films also have potential applications as a biometric scaffold in bone tissue regeneration.
 | ||
| Fig. 9 Fluorescence micrographs of the fibroblast cells that were cultured for 7 days on (a) the pure zein film and zein/minerals films biomimetic mineralized for (b) 0.5 h, (c) 1 h, and (d) 2 h. Reproduced from ref. 238 with permission from the American Chemical Society, copyright 2013. | ||
Studies on zein composites have explored combining zein with other substances to enhance matrix properties. Zhang et al. used zein as an additive to impart antibacterial qualities, creating a zein protein composite by mixing it with silver nanoparticles. They found no significant difference in the effects on Escherichia coli and Staphylococcus aureus between silver nanoparticle composites and silver alone. However, silver samples with acidified zein showed superior bacterial inhibition. The study suggested that zein improves hemocompatibility and broadens wound care applications. To avoid potential toxicity, the silver content in the composite must be optimized.239
Agnes Gagliardi and colleagues developed a biocompatible, eco-friendly gel composed of zein loaded with rutin. They assessed its efficacy in vitro and in vivo for treating burns and sores compared to DuoDERM®. The gel showed improved migration and rapid gap closure within 24 hours in vitro, and a 90% reduction in wound area within 10 days in vivo in Wistar rats, outperforming both the free form and standard gel. Additionally, the gel significantly reduced inflammatory markers like TNF-α, IL-1β, IL-6, and IL-10.240 Similarly, Momgain et al. developed zein nanoparticles incorporating Moringa oleifera leaf extracts via nanoprecipitation for wound treatment. They optimized the formulation, prepared a gel, and characterized it for pH, spreadability, extrudability, and homogeneity. Testing in an animal model demonstrated significant wound healing activity. The study suggested Moringa oleifera leaves as a superior intervention for wound healing over other oral or topical extracts, indicating zein-loaded Moringa oleifera extract's potential as a diabetic wound healing agent.241
A study conducted by Gabriela et al. utilized electrospinning to prepare zein nanofibers reinforced with graphene oxide, enhancing their mechanical properties. The team incorporated various concentrations of curcumin into these GO-strengthened nanofibers to boost bioavailability, adhesion, and oxygenation. In vitro testing of this curcumin-loaded GO-Zein nanofiber composite showed improved safety, with evidence of cell growth throughout the fiber structure. The engineered nanofibers displayed a biphasic release pattern in vitro, suggesting cell proliferation. However, the effectiveness of these nanofibers in living organisms remains to be investigated.242 Lenian Zhou and colleagues developed zein proteins with hydrophilic and hydrophobic properties, loaded with CaO2/carbon dots (CD), for improved healing of diabetic wounds. The modified zein film with irradiated (Ir-Zein) calcium oxide nanoparticles and carbon dots used as a wound dressing demonstrated remarkable effectiveness in accelerating the healing of diabetic wounds. The study demonstrated the effectiveness of the CaO2/CD@Ir-Zein film in enhancing the progression of chronic wounds from the inflammatory stage to skin repair243 as shown in Fig. 10.
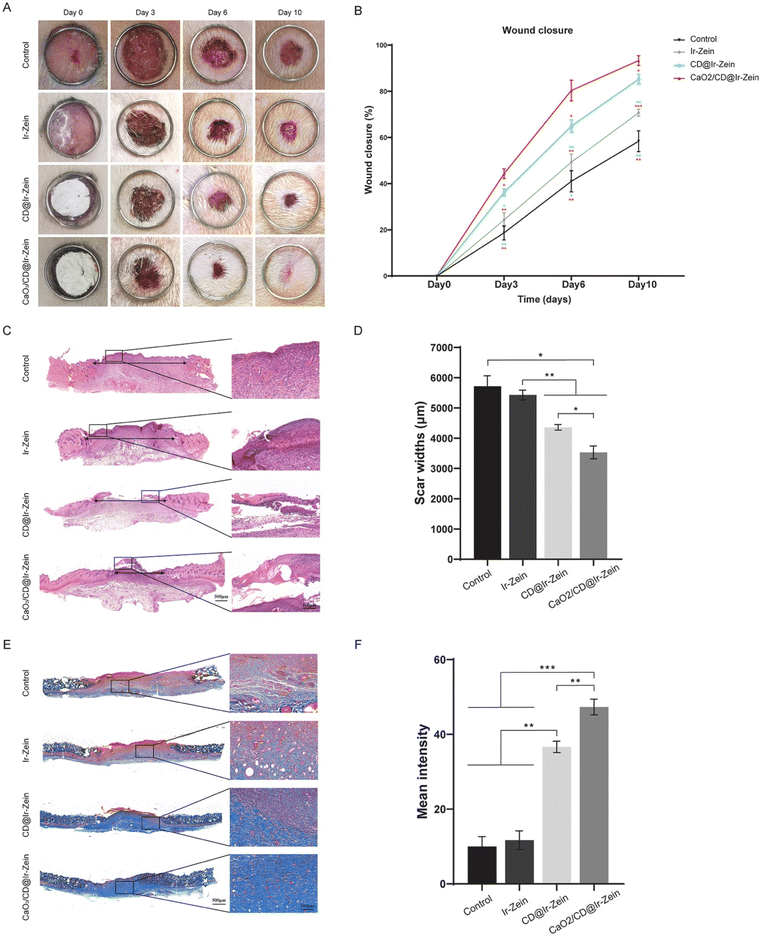 | ||
| Fig. 10 In vivo assessment of CaO2/CD@Ir-Zein for diabetic wound healing in rat. (A) The representative images of full-thickness skin wounds after treatment with control, Ir-Zein, CD@Ir-Zein, and CaO2/CD@Ir-Zein. (B) Change of wound closure percentages with time. (C) H&E staining of the wound on day 10. (D) Quantitative analysis of scar widths in re-epithelialization. (E) Masson's trichrome staining of tissue regeneration within the wound on day 10. (F) Quantitative analysis of mean intensity. Reproduced from ref. 243 with permission from Elsevier, copyright 2024. | ||
Nasrin Salehi and colleagues developed curcumin-loaded zein nanofibers as a wound dressing with poly(sodium 4-styrene sulfonate) serving as a polyanion and poly(diallyldimethylammonium chloride) (PDADMAC) acting as a polycation, using electrospun nanofibers. The designed nanofiber facilitated the controlled release of curcumin, which exhibited antioxidant activity, and the PDADMAC demonstrated antibacterial properties. The nanofiber also exhibited better cell migration and adhesion, and cell attachment.244
Moiz Uddin Khan and his fellow researchers developed an innovative invasive thermo-responsive hydrogel that combines glycerophosphate and biologically active zein for tissue engineering applications. Chitosan (CS) and hydroxyapatite (HA) were used to fabricate the gel. The gel exhibited a solution phase between 4 to 10 °C and transitioned to a gel at body temperature within 4 to 6 minutes. The gel's mechanical strength (52.2 MPa at 40% strain) was significantly enhanced by the addition of zein, and it demonstrated good injectability and ease of shaping into complex structures for treatment.245
Zein scaffolds were transformed into fibrous structures resembling the extracellular matrix using electrospinning. The zein scaffold was crosslinked with trimethylpropane triglycidyl ether and then evaluated for human mesenchymal stem cell (MSC) adhesion, growth, and infiltration into the scaffold in vitro. The study revealed that the plant-derived zein scaffold was a potential carrier for MSCs for tissue engineering applications, offering an alternative to animal-derived gelatin protein-based carriers.246 Biopolymer-based films, such as chitosan and zein, may be more effective than conventional bandages in promoting wound healing. These films can store and release beneficial molecules like ellagic acid to combat infection and promote healing over time. The films demonstrated bactericidal and fungicidal properties against samples of bacteria and fungi while retaining their elasticity and comfort. Additionally, one of the formulations showed an accelerated wound healing rate in animal models, reducing inflammation, and could be a promising candidate for future wound dressings.247
4.6. Albumin based materials
Albumin is a protein found in large quantities in the bloodstream, making it a promising option for various therapeutic uses, such as wound healing. This is due to its inherent properties, including non-immunogenicity, biocompatibility, and antibacterial characteristics, as well as its ability to promote angiogenesis.257–259 The studies have shown that albumin-based nano-materials have either gone through clinical trials or have already obtained FDA approval.260 The use of albumin, which is both hydrophilic and hydrophobic, makes it a safer and non-toxic option for encapsulating drugs as a biocompatible nanocarrier.261–263 The albumin nanoclusters were reported by the incorporation of diethylenetriamine diazeniumdiolate (DT/NO) through electrostatic interaction and cross-linking. These nanoclusters were capable of releasing nitric oxide and exhibited a prominent anti-bacterial effect against methicillin-resistant Staphylococcus aureus (MRSA)-infected wounds. Additionally, the nanoclusters showed a remarkable in vivo anti-bacterial effect, which enhanced wound healing in an MRSA-challenged wound mouse model. The amount of NO released was sufficient to ensure the effectiveness of the nanoclusters against MRSA-infected wounds264 as exhibited in Fig. 11.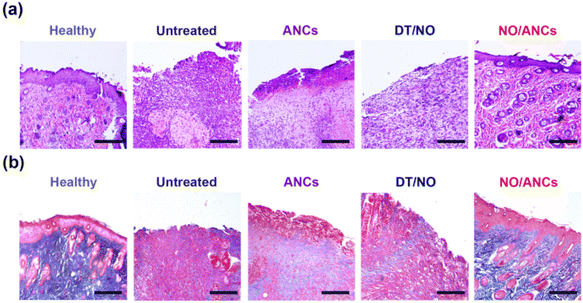 | ||
| Fig. 11 Histological assessments. (a) Representative microscopic images of the tissue sections treated with or without NO/ANCs, ANCs, and DT/NO after H&E staining. (b) Representative microscopic images of the tissue sections after Masson's trichrome staining. The scale bar represents 200 μm. Reproduced from ref. 264 with permission from Springer Nature, copyright 2024. | ||
Fatemeh Saadat prepared nanofibers containing albumin and caffeine to enhance wound healing. The application of these nanofibers to wounds resulted in improved blood flow to the affected area, thus speeding up the recovery process. The study found that caffeine played a crucial role in stimulating the growth of new blood vessels in the skin, which led to more effective wound closure.265 In another study, Mohammed A. Naseer and fellows designed albumin based composite material with greater biodegradability and biocompatibility for enhanced wound healing applications. Novel sutures were designed using extrusion method from human albumin serum. Promising results were obtained from physicochemical and mechanical tests of the designed suture. The designed suture is proposed to be used as a 3D filament to design custom scaffolds.266 Furthermore, Elias Madadian and colleagues have developed a hybrid foam for use as a wound dressing. Sodium alginate and albumin were used as the main components, with calcium chloride mist serving as a cross-linking agent. The researchers employed rhodamine B as their model drug in the study. The designed scaffold underwent porosity, degradation, mechanical, and drug release tests. The results showed exceptional mechanical properties and sustained drug release. Additionally, they found that altering the concentrations of sodium alginate, albumin, and the crosslinking agent could regulate the drug release.267 A study conducted by Neives Vanaclocha et al. discovered that individuals with higher serum prealbumin levels experienced improved re-epithelialization and wound healing compared to those with lower prealbumin levels. Patients with higher prealbumin levels demonstrated a shorter time to full wound recovery and were found to be an independent predictor of successful graft outcomes.268 Adnan and his colleagues developed a hydrogel comprised of bovine serum albumin-riboflavin retinoic acid. This bovine serum albumin (BSA)-riboflavin retinoic acid (BHG) hydrogel increased the expression of the transglutaminase-2 enzyme, leading to improved epithelial cell regeneration and Wnt-B-caretin signaling, which promotes stromal cell growth. The study showed promise for healing wounded corneas and could be further explored for wound healing and progenitor cell remodeling in an in vivo model.269 Recently Jia and fellows270 developed human serum albumin-Zn-vascular endothelial growth factor (HMS-Zn@VEGF) microspheres, integrating antibacterial and angiogenic human serum albumin to address infected wounds. In vitro experiments showed total bacterial elimination with one application, while rat studies demonstrated fast wound healing via decreased inflammation, improved collagen production, and enhanced angiogenesis (eee Fig. 12). VEGF was successfully loaded onto the microspheres through HSA adsorption on zinc ions and His-tagged VEGF crosslinking. The microspheres displayed potent, broad-spectrum antibacterial activity and promoted neovascularization, confirming their biocompatibility, antimicrobial efficacy, and angiogenic properties, indicating their potential as an infected wound treatment.
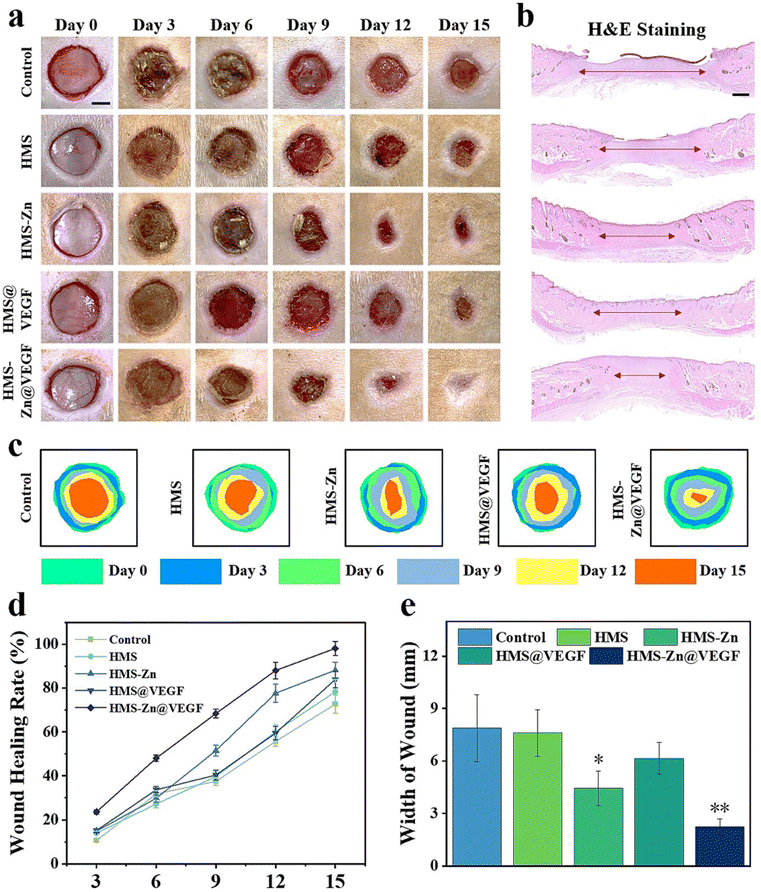 | ||
| Fig. 12 HMS-Zn@VEGF promoted healing of infected wounds in rats (a) representative pictures of different treatment groups on days 0, 3, 6, 9, 12, and 15. The scale bar is 50 mm. (b) H&E staining of the wound. The scale bar is 1000 μm. (c) Mockup of wound healing. (d) Healing curves of infected wounds in each group of rats. (e) Quantitative statistics on wound width. Reproduced from ref. 270 with permission from Elsevier, copyright 2024. | ||
Moreover, researchers have synthesized a wound dressing that accelerates the healing of diabetic wounds by using a composite hydrogel containing bovine serum albumin and aloe vera. This hydrogel is unique due to its porous structure, self-fluorescence, and biocompatibility, which were achieved without adding any toxic chemicals or dyes. In preclinical studies, this hydrogel showed promising results in promoting collagen synthesis and angiogenesis, leading to faster healing. Additionally, this hydrogel can be 3D printed for customized wound care treatments.271
5. Challenges and future perspectives
Protein-derived wound care materials face several challenges. The lack of preclinical research and limitations in biomimetic wound dressings pose significant problems. Regulating the breakdown rate of biomimetic substances is also challenging. While these dressings aim to create a moist healing environment, their ability to retain moisture can fluctuate greatly, potentially causing the wound bed to become excessively dry or waterlogged.77,272 Additionally, the elastic and strength properties of these dressings may not always meet the specific needs of the wound site. The intricate production processes raise concerns about consistency between batches, which is crucial for ensuring reproducible clinical outcomes. Inconsistencies in manufacturing can undermine the reliability and effectiveness of these dressings in wound healing.273,274A further crucial issue is the long-term safety and biocompatibility of protein derived materials used in wound healing and dressings. Despite their promising potential for wound care applications, it is vital to confirm their compatibility with biological systems and establish a comprehensive safety profile for successful clinical use.275,276 Finally, there is an urgent need for more comprehensive clinical trials to fully determine the efficacy and safety of wound dressings. Although preclinical studies and early-stage clinical trials often show promising results, the transition to routine clinical application can reveal unexpected complications, such as allergic responses, increased infection risks, or long-term biocompatibility issues.277
The field of wound healing has seen remarkable progress due to innovations in protein based materials. Various techniques for creating protein derived wound care products yield specific forms and notable advantages for different wound types. These materials are excellent in facilitating the biochemical processes necessary for wound repair. Research indicates that variations in cellular shape, movement, specialization, and survival significantly impact the healing process.278,279 It is anticipated that next-generation wound care technologies incorporating advanced proteins will precisely track crucial healing indicators, such as oxygen concentration and heat, and make appropriate modifications. These innovative devices are expected to relay collected data to medical professionals for further evaluation.
Despite progress, challenges persist in the field of protein-based wound care technologies. To achieve efficient and cell-specific transformation, these technologies require refinement without causing any adverse effects. Optimal wound healing treatments should enable precise, autonomous delivery of multiple molecules at the wound site to speed up the healing process. Advancements necessitate identifying specific wounds, protein mechanism of action and developing materials with adjustable properties. Continued research in this field is crucial for the development of translational wound care materials and their applications. Prospect studies should emphasize developing methods and mechanistic insights to produce different forms of wound care materials from abundant protein resources. Only a small number of protein based wound dressings have made it to the commercialization stage, despite substantial research on the use of proteins in wound healing applications. The majority of research on proteins in wound healing has primarily focused on their ability to promote cell migration and their antimicrobial properties. Nevertheless, there is a lack of research on multifunctional smart systems derived from proteins, especially regarding smart dressings that demonstrate specific stimulus-responsive behaviors. In the future, the focus should be on developing intelligent systems substantiated by understanding healing and infection mechanisms, particularly in the context of chronic wounds. These systems can potentially showcase the effectiveness of proteins in promoting the healing process.
6. Summary
Protein derived wound care materials are frequently utilized and have the potential to replace traditional materials due to their hydrophilic properties, biodegradability, abundance in nature, and ease of access. Considering the numerous forms of skin injury, such as burns, abrasions, tears, and diseases, the appropriate type of wound and the protein derived materials employed for treatment should be determined based on the specific type of damage. There are many proteins available to transform into wound care materials. However, keratin, collagen, gelatin, zein, albumin and silk are the most studied proteins for wound care management. The development of advanced wound dressings necessitates the utilization of sophisticated techniques, such as electrospinning, phase separation, self-assembly, and ball milling, which are intended to promote tissue regeneration and minimize the risk of infection. Studies have shown that incorporating proteins with other polymeric materials improves their characteristics, making them appropriate for application in advanced wound care products. These attributes include antibacterial and antifungal hydrogels, nanofibrous hydrogels, and bilayer wound dressings, all of which exhibit improved healing, cell viability, and antimicrobial activity. In the future, it is anticipated that proteins will become more widely available in medical markets, with doctors recommending their use for a range of wound care applications. However, we will require collaborative action from a diverse group of professionals, including researchers, medical practitioners, pharmacists, biochemists, and government officials to achieve this goal.Author contributions
Muhammad Zubair: conceptualization, writing – original draft, – review & editing. Saadat Hussain: writing. Mujeeb-ur-Rehman: writing and reviewing, Ajaz Hussain: writing and reviewing. Muhammad Ehtisham Akram: writing. Sohail Shahzad: writing and reviewing, Zahid Rauf: writing and editing, Maria Mujahid: writing, Aman Ullah: supervision, review & editing.Data availability
This review article is based on previous studies that have been already reported. Therefore, there are no new data to reveal. All referenced studies have been properly cited, showing respect for the intellectual property of their original authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the use of Paperpal for paraphrasing assistance in the preparation of this review article, which helped improve the clarity and coherence of the text.References
- A. Sharma, D. Sharma and F. Zhao, Adv. Healthcare Mater., 2023, 12, 2300556 CrossRef PubMed.
- L. Su, Y. Jia, L. Fu, K. Guo and S. Xie, Heliyon, 2023, e22520 CrossRef.
- G. C. Gurtner, S. Werner, Y. Barrandon and M. T. Longaker, Nature, 2008, 453, 314–321 Search PubMed.
- C. K. Sen, Adv. Wound Care, 2021, 10, 281–292 Search PubMed.
- A. Markiewicz-Gospodarek, M. Kozioł, M. Tobiasz, J. Baj, E. Radzikowska-Büchner and A. Przekora, Int. J. Environ. Res. Public Health, 2022, 19, 1338 CrossRef PubMed.
- W. Huang, Y. Wang, Z. Huang, X. Wang, L. Chen, Y. Zhang and L. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 41076–41088 Search PubMed.
- S.-K. Han, in Innovations and Advances in Wound Healing, Springer, 2023, pp. 1–42 Search PubMed.
- T. Velnar, T. Bailey and V. Smrkolj, J. Int. Med. Res., 2009, 37, 1528–1542 CrossRef.
- E. M. Tottoli, R. Dorati, I. Genta, E. Chiesa, S. Pisani and B. Conti, Pharmaceutics, 2020, 12, 735 CrossRef PubMed.
- M. Mirhaj, S. Labbaf, M. Tavakoli and A. M. Seifalian, Int. Wound J., 2022, 19, 1934–1954 CrossRef PubMed.
- B. Balakrishnan, M. Mohanty, P. Umashankar and A. Jayakrishnan, Biomaterials, 2005, 26, 6335–6342 CrossRef PubMed.
- E. Rezvani Ghomi, S. Khalili, S. Nouri Khorasani, R. Esmaeely Neisiany and S. Ramakrishna, J. Appl. Polym. Sci., 2019, 136, 47738 CrossRef.
- M. Farahani and A. Shafiee, Adv. Healthcare Mater., 2021, 10, 2100477 CrossRef PubMed.
- B. Gupta, R. Agarwal and M. Alam, Indian J. Fibre Text. Res., 2010, 174–187 Search PubMed.
- J.-S. Jeon, H.-T. Kim, M.-G. Kim, M.-S. Oh, S.-R. Hong, M.-H. Yoon, H.-C. Shin, J.-H. Shim, N. A. Afifi and A. Hacımüftüoğlu, Chromatographia, 2016, 79, 851–860 CrossRef.
- C. Wei, S. Xing, Y. Li, M. Koosha, S. Wang, H. Chen, Y. Zhai, L. Wang, X. Yang and R. Fakhrullin, Int. J. Biol. Macromol., 2024, 261, 129720 CrossRef PubMed.
- V. Reisi-Vanani, S. Hosseini, E. Soleiman-Dehkordi, S. N. Boroujeni, M. Farzan, V. V. Ebani, M. Gholipourmalekabadi, K. Lozano and Z. Lorigooini, J. Drug Delivery Sci. Technol., 2023, 81, 104282 CrossRef.
- M. Gong, H. Shi, Z. Hu, F. Wang, M. Dong, R. Lei, Z. Zeng, Y. Wang and J. Chen, Chem. Eng. J., 2023, 473, 145394 CrossRef.
- X. Wang, J. Wu, M. Wang, C. Lu, W. Li, Q. Lu, Y. Li, B. Lian and B. Zhang, J. Biomed. Mater. Res., 2023, 111, 404–414 CrossRef.
- Y. Jia, Y. Han, Y. Zhang, L. Li, B. Zhang and X. Yan, Regener. Ther., 2024, 27, 329–341 CrossRef.
- L. K. Sellappan and S. Manoharan, Int. J. Biol. Macromol., 2024, 259, 129162 CrossRef PubMed.
- T. Zhao, N. Wang, Y. Wang, J. Yang, Y. Tang, Y. Wang, H. Wei, J. Yang, T. Yu and X. Sun, Int. J. Biol. Macromol., 2024, 135724 CrossRef.
- Y. Long, M. Bai, X. Liu, W. Lu, C. Zhong, S. Tian, S. Xu, Y. Ma, Y. Tian and H. Zhang, Carbohydr. Polym., 2022, 297, 119974 CrossRef PubMed.
- W. Wei, Y. Ma, X. Yao, W. Zhou, X. Wang, C. Li, J. Lin, Q. He, S. Leptihn and H. Ouyang, Bioact. Mater., 2021, 6, 998–1011 Search PubMed.
- R. A. M. Osmani, E. Singh, K. Jadhav, S. Jadhav and R. Banerjee, in Applications of Advanced Green Materials, Elsevier, 2021, pp. 573–630 Search PubMed.
- A. Basit, H. Yu, L. Wang, M. A. Uddin, Y. Wang, K. M. Awan, B. E. Keshta and M. O. Malik, Eur. Polym. J., 2024, 113260 CrossRef.
- M. R. Alam, M. A. Shahid, S. Alimuzzaman and A. N. Khan, Biomed. Eng. Adv., 2022, 4, 100064 CrossRef.
- V. Gounden and M. Singh, Gels, 2024, 10, 43 CrossRef PubMed.
- A. Sadeghianmaryan, N. Ahmadian, S. Wheatley, H. A. Sardroud, S. A. S. Nasrollah, E. Naseri and A. Ahmadi, Int. J. Biol. Macromol., 2024, 131207 CrossRef.
- G. Satchanska, S. Davidova and P. D. Petrov, Polymers, 2024, 16, 1159 CrossRef PubMed.
- H. Zhang, X. Lin, X. Cao, Y. Wang, J. Wang and Y. Zhao, Bioact. Mater., 2024, 33, 355–376 Search PubMed.
- P. C. Pires, F. Damiri, E. N. Zare, A. Hasan, R. E. Neisiany, F. Veiga, P. Makvandi and A. C. Paiva-Santos, Int. J. Biol. Macromol., 2024, 130296 CrossRef.
- S. Cai, C. Wu, W. Yang, W. Liang, H. Yu and L. Liu, Nanotechnol. Rev., 2020, 9, 971–989 CrossRef.
- M. N. Uddin, M. Jobaer, S. I. Mahedi and A. Ali, J. Text. Inst., 2023, 114, 1592–1617 CrossRef.
- D. Ji, Y. Lin, X. Guo, B. Ramasubramanian, R. Wang, N. Radacsi, R. Jose, X. Qin and S. Ramakrishna, Nat. Rev. Methods Primers, 2024, 4, 1 CrossRef.
- M. Ahmadi Bonakdar and D. Rodrigue, Macromol, 2024, 4, 58–103 Search PubMed.
- A. Moreira, D. Lawson, L. Onyekuru, K. Dziemidowicz, U. Angkawinitwong, P. F. Costa, N. Radacsi and G. R. Williams, J. Controlled Release, 2021, 329, 1172–1197 CrossRef PubMed.
- M. Hayat, S. A. R. Bukhari and M. Irfan, Biotechnol. J., 2023, 2300279 CrossRef.
- A. Miserez, J. Yu and P. Mohammadi, Chem. Rev., 2023, 123, 2049–2111 CrossRef.
- A. Akhmetova and A. Heinz, Pharmaceutics, 2020, 13, 4 CrossRef PubMed.
- Z. Terzopoulou, A. Zamboulis, I. Koumentakou, G. Michailidou, M. J. Noordam and D. N. Bikiaris, Biomacromolecules, 2022, 23, 1841–1863 CrossRef.
- E. Zdraveva, V. Gaurina Srček, K. Kraljić, D. Škevin, I. Slivac and M. Obranović, Polymers, 2023, 15, 2684 CrossRef PubMed.
- M. Pandian, G. Reshma, C. Arthi, M. Másson and J. Rangasamy, Eur. Polym. J., 2023, 198, 112390 CrossRef.
- P. Farshi, S. N. Mirmohammadali, B. Rajpurohit, J. S. Smith and Y. Li, J. Agric. Food Res., 2023, 100927 Search PubMed.
- M. Raeisi, M. A. Mohammadi, V. Bagheri, S. Ramezani, M. Ghorbani, M. Tabibiazar, O. Coban, R. Khoshbakht, S. Marashi and S. Noori, Biointerface Res. Appl. Chem., 2023, 13, 486 Search PubMed.
- A. Fatima, S. Adeel, M. A. Qayyum and H. A. Tanveer, in Biopolymers in the Textile Industry: Opportunities and Limitations, Springer, 2024, pp. 273–313 Search PubMed.
- M. A. M. Fathil and H. Katas, Pharmaceutics, 2023, 15, 991 CrossRef PubMed.
- Z. Zhang, Y. Zhang, Y. Guo, C. Qian, K. Chen, S. Fang, A. Qiu, L. Zhong, J. Zhang and R. He, J. Biomater. Appl., 2024, 39, 48–57 CrossRef PubMed.
- X. Cao, Y. Ren, Q. Lu, K. Wang, Y. Wu, Y. Wang, Y. Zhang, X.-s. Cui, Z. Yang and Z. Chen, Front. Nutr., 2023, 9, 1018336 CrossRef PubMed.
- C. Coccolini, E. Berselli, C. Blanco-Llamero, F. Fathi, M. B. P. Oliveira, K. Krambeck and E. B. Souto, Int. J. Pept. Res. Ther., 2023, 29, 71 CrossRef.
- Z. Jiang, Z. Zheng, S. Yu, Y. Gao, J. Ma, L. Huang and L. Yang, Pharmaceutics, 2023, 15, 1829 CrossRef.
- C. Yang, Z. Zhang, L. Gan, L. Zhang, L. Yang and P. Wu, Int. J. Mol. Sci., 2023, 24, 7319 CrossRef PubMed.
- F. Chen, X. Li, Y. Yu, Q. Li, H. Lin, L. Xu and H. C. Shum, Nat. Commun., 2023, 14, 2793 CrossRef PubMed.
- Y. Yang, Y. Ru, T. Zhao and M. Liu, Chem, 2023, 3113–3137 Search PubMed.
- A. R. Calore, V. Srinivas, L. Groenendijk, A. Serafim, I. C. Stancu, A. Wilbers, N. Leoné, A. A. Sanchez, D. Auhl and C. Mota, Acta Biomater., 2023, 156, 158–176 CrossRef.
- X. Peng, Y. Li, T. Li, Y. Li, Y. Deng, X. Xie, Y. Wang, G. Li and L. Bian, Adv. Sci., 2022, 9, 2203890 CrossRef PubMed.
- L. Hu, S. Zhou, X. Zhang, C. Shi, Y. Zhang and X. Chen, Polymers, 2024, 16, 2097 CrossRef PubMed.
- Y. Nie, X. Han, Z. Ao, S. Ning, X. Li and D. Han, Mater. Chem. Front., 2021, 5, 7022–7031 RSC.
- J. Huang, X. Lei, Z. Huang, Z. Rong, H. Li, Y. Xie, L. Duan, J. Xiong, D. Wang and S. Zhu, Int. J. Bioprint., 2022, 8, 517 CrossRef PubMed.
- Y. Xu, Q. Hu, Z. Wei, Y. Ou, Y. Cao, H. Zhou, M. Wang, K. Yu and B. Liang, Biomater. Res., 2023, 27, 36 CrossRef.
- F. Wang, C. Yang and X. Hu, in Lightweight Materials from Biopolymers and Biofibers, ACS Publications, 2014, pp. 177–208 Search PubMed.
- Y. Hu, H. Shi, X. Ma, T. Xia, Y. Wu, L. Chen, Z. Ren, L. Lei, J. Jiang and J. Wang, Acta Biomater., 2023, 159, 128–139 CrossRef PubMed.
- P. T. Smith, B. Narupai, J. H. Tsui, S. C. Millik, R. T. Shafranek, D.-H. Kim and A. Nelson, Biomacromolecules, 2019, 21, 484–492 CrossRef PubMed.
- A. Sola, A. Trinchi and A. J. Hill, Smart Mater. Manuf., 2023, 1, 100013 Search PubMed.
- N. Falcone, M. Ermis, D. G. Tamay, M. Mecwan, M. Monirizad, T. G. Mathes, V. Jucaud, A. Choroomi, N. R. de Barros and Y. Zhu, Adv. Healthcare Mater., 2023, 12, 2301096 CrossRef CAS PubMed.
- T. Guan, J. Li, C. Chen and Y. Liu, Adv. Sci., 2022, 9, 2104165 CrossRef CAS PubMed.
- K. Bischoff, C. Esen and R. Hellmann, Nanomaterials, 2023, 13, 2693 CrossRef CAS.
- L. Doveri, G. Dacarro, Y. A. D. Fernandez, M. Razzetti, A. Taglietti, G. Chirico, M. Collini, I. Sorzabal-Bellido, M. Esparza and C. Ortiz-de-Solorzano, Colloids Surf., B, 2023, 227, 113373 CrossRef.
- A. A. E.-S. A. E.-K. Nafeh, I. M. A. E.-A. Mohamed and M. F. Foda, Nanomaterials, 2024, 14, 1254 CrossRef.
- A. A. Aljabali, M. Rezigue, R. H. Alsharedeh, M. A. Obeid, V. Mishra, A. Serrano-Aroca, M. El-Tanani and M. M. Tambuwala, Ther. Delivery, 2022, 13, 321–338 CrossRef.
- B. A. Hemdan, G. K. Hassan, A. B. Abou Hammad and A. M. El Nahrawy, Microbial Nanotechnology: Green Synthesis and Applications, 2021, pp. 155–190 Search PubMed.
- S. Kumar, B. Kumar, R. Sehgal, M. Wani, D. Kumar, M. D. Sharma, V. Singh, R. Sehgal and V. Kumar, in Nanoparticles reinforced metal nanocomposites: mechanical performance and durability, Springer, 2023, pp. 209–235 Search PubMed.
- D. Zhang and Y. Wang, Int. J. Mol. Sci., 2019, 20, 3054 CrossRef.
- C. Cai, W. Li, X. Zhang, B. Cheng, S. Chen and Y. Zhang, Adv. Wound Care, 2024 DOI:10.1089/wound.2024.0024.
- N. Varshney, A. K. Sahi, S. Poddar, N. K. Vishwakarma, G. Kavimandan, A. Prakash and S. K. Mahto, ACS Appl. Mater. Interfaces, 2022, 14, 14033–14048 CrossRef.
- W. Li, X. Lei, H. Feng, B. Li, J. Kong and M. Xing, Pharmaceutics, 2022, 14, 297 CrossRef.
- M. U. A. Khan, M. A. Aslam, R. A. Rahman, M. F. B. Abdullah, A. Mehmood and G. M. Stojanović, J. Biomater. Sci., Polym. Ed., 2024, 1–44 Search PubMed.
- D. Zhou, H. Liu, L. Han, D. Liu, X. Liu, Q. Yan, D. He, Z. Li, X. Lu and C. Jiang, Chem. Eng. J., 2023, 469, 143914 CrossRef.
- W. He, J. Xu, Y. Zheng, J. Chen, Y. Yin, D. A. Mosselhy, F. Zou, M. Ma and X. Liu, Int. J. Biol. Macromol., 2022, 211, 754–766 CrossRef PubMed.
- K. B. Chien, E. J. Chung and R. N. Shah, J. Biomater. Appl., 2014, 28, 1085–1096 CrossRef.
- J. Amirian, Y. Zeng, M. I. Shekh, G. Sharma, F. J. Stadler, J. Song, B. Du and Y. Zhu, Carbohydr. Polym., 2021, 251, 117005 CrossRef PubMed.
- J. Li, Y. Li, C. Guo and X. Wu, Chem. Eng. J., 2024, 481, 148458 CrossRef.
- P. Dorishetty, R. Balu, A. Sreekumar, L. de Campo, J. P. Mata, N. R. Choudhury and N. K. Dutta, ACS Sustainable Chem. Eng., 2019, 7, 9257–9271 CrossRef.
- S. Kaya and A. Kaya, J. Food Eng., 2000, 43, 91–96 CrossRef.
- Y. Zhao, Z. Wang, Q. Zhang, F. Chen, Z. Yue, T. Zhang, H. Deng, C. Huselstein, D. P. Anderson and P. R. Chang, Int. J. Biol. Macromol., 2018, 118, 1293–1302 CrossRef PubMed.
- D. Diaz, A. Care and A. Sunna, Genes, 2018, 9, 370 CrossRef.
- W. Zhang, X. Li, W. Chen, X. Huang, T. Hua, J. Hu, J. Zhu, S. Ye and X. Li, RSC Adv., 2024, 14, 18317–18329 RSC.
- M. H. Norahan, S. C. Pedroza-González, M. G. Sánchez-Salazar, M. M. Álvarez and G. T. de Santiago, Bioact. Mater., 2023, 24, 197–235 Search PubMed.
- H. Shao, X. Wu, Y. Xiao, Y. Yang, J. Ma, Y. Zhou, W. Chen, S. Qin, J. Yang and R. Wang, Int. J. Biol. Macromol., 2024, 129752 CrossRef.
- Z. Arabpour, F. Abedi, M. Salehi, S. M. Baharnoori, M. Soleimani and A. R. Djalilian, Int. J. Mol. Sci., 2024, 25, 1982 CrossRef PubMed.
- N. Rezaei, H. G. Hamidabadi, S. Khosravimelal, M. Zahiri, Z. A. Ahovan, M. N. Bojnordi, B. S. Eftekhari, A. Hashemi, F. Ganji and S. Darabi, Int. J. Biol. Macromol., 2020, 164, 855–862 CrossRef.
- C. D. Spicer, Polym. Chem., 2020, 11, 184–219 RSC.
- Y. Lyu, Y. Liu, H. He and H. Wang, Gels, 2023, 9, 431 CrossRef.
- X. Liu, Z. Guo, J. Wang, W. Shen, Z. Jia, S. Jia, L. Li, J. Wang, L. Wang and J. Li, Adv. Healthcare Mater., 2024, 2303824 CrossRef.
- Z. Yu, Z. Wang, Y. Chen, Y. Wang, L. Tang, Y. Xi, K. Lai, Q. Zhang, S. Li and D. Xu, Biomaterials, 2024, 122772 Search PubMed.
- F. Kong, N. Mehwish and B. H. Lee, Acta Biomater., 2023, 157, 67–90 CrossRef.
- S. Wei, Z. Wang, X. Liang, T. Xiong, Z. Kang, S. Lei, B. Wu and B. Cheng, Am. J. Transl. Res., 2023, 15, 4467 Search PubMed.
- C. Chen, L. Chen, C. Mao, L. Jin, S. Wu, Y. Zheng, Z. Cui, Z. Li, Y. Zhang and S. Zhu, Small, 2023, 2306553 Search PubMed.
- V. Kumar, A. Kumar, N. S. Chauhan, G. Yadav, M. Goswami and G. Packirisamy, ACS Appl. Bio Mater., 2022, 5, 2726–2740 CrossRef.
- R. Yadav, R. Kumar, M. Kathpalia, B. Ahmed, K. Dua, M. Gulati, S. Singh, P. J. Singh, S. Kumar and R. M. Shah, J. Mater. Chem. B, 2024, 12, 7977–8006 RSC.
- C. Shi, C. Wang, H. Liu, Q. Li, R. Li, Y. Zhang, Y. Liu, Y. Shao and J. Wang, Front. Bioeng. Biotechnol., 2020, 8, 182 CrossRef PubMed.
- N. Bhardwaj, D. Chouhan and B. B. Mandal, in Functional 3D tissue engineering scaffolds, Elsevier, 2018, pp. 345–365 Search PubMed.
- P. Chocholata, V. Kulda and V. Babuska, Materials, 2019, 12, 568 CrossRef.
- I. Negut, G. Dorcioman and V. Grumezescu, Polymers, 2020, 12, 2010 CrossRef.
- A. Rahmani Del Bakhshayesh, N. Annabi, R. Khalilov, A. Akbarzadeh, M. Samiei, E. Alizadeh, M. Alizadeh-Ghodsi, S. Davaran and A. Montaseri, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 691–705 CrossRef.
- Y. P. Afsharian and M. Rahimnejad, Polym. Test., 2021, 93, 106952 CrossRef.
- R. B. Attasgah, B. Velasco-Rodríguez, A. Pardo, J. Fernández-Vega, L. Arellano-Galindo, L. C. Rosales-Rivera, G. Prieto, S. Barbosa, J. F. A. Soltero and M. Mahmoudi, Iscience, 2022, 25, 104019 CrossRef.
- M. Rasouli, M. Soleimani, S. Hosseinzadeh and J. Ranjbari, J. Polym. Environ., 2023, 31, 4621–4640 CrossRef CAS.
- T. Su, M. Zhang, Q. Zeng, W. Pan, Y. Huang, Y. Qian, W. Dong, X. Qi and J. Shen, Bioact. Mater., 2021, 6, 579–588 Search PubMed.
- G.-M. Lanno, C. Ramos, L. Preem, M. Putrins, I. Laidmae, T. Tenson and K. Kogermann, ACS Omega, 2020, 5, 30011–30022 CrossRef.
- T. Nawaz, L. Gu, J. Gibbons, Z. Hu and R. Zhou, Biomimetics, 2024, 9, 373 CrossRef PubMed.
- Kirti and S. S. Khora, Encyclopedia of Marine Biotechnology, 2020, vol. 2, pp. 1175–1193 Search PubMed.
- Y. Feng, X. Li, Q. Zhang, S. Yan, Y. Guo, M. Li and R. You, Carbohydr. Polym., 2019, 216, 17–24 CrossRef PubMed.
- M. Pollini and F. Paladini, Materials, 2020, 13, 3361 CrossRef PubMed.
- S. Singaravelu, G. Ramanathan, M. Raja, N. Nagiah, P. Padmapriya, K. Kaveri and U. T. Sivagnanam, Int. J. Biol. Macromol., 2016, 86, 810–819 CrossRef PubMed.
- J. Li, L. Xiao, S. Gao, H. Huang, Q. Lei, Y. Chen, Z. Chen, L. Xue, F. Yan and L. Cai, Adv. Healthcare Mater., 2023, 12, 2202737 CrossRef.
- S. Chandel, R. Kumaragurubaran, H. Giri and M. Dixit, in Vascular Hyperpermeability: Methods and Protocols, Springer, 2023, pp. 147–162 Search PubMed.
- E. Tassara, C. Oliveri, L. Vezzulli, C. Cerrano, L. Xiao, M. Giovine and M. Pozzolini, Mar. Drugs, 2023, 21, 428 CrossRef PubMed.
- H. Zhou, L. Chen, C. Huang, Z. Jiang, H. Zhang, X. Liu, F. Zhu, Q. Wen, P. Shi and K. Liu, J. Nanobiotechnol., 2024, 22, 530 CrossRef PubMed.
- D. W. Green, J.-M. Lee and H.-S. Jung, Tissue Eng., Part B, 2015, 21, 438–450 CrossRef.
- J. E. Brown, J. E. Moreau, A. M. Berman, H. J. McSherry, J. M. Coburn, D. F. Schmidt and D. L. Kaplan, Adv. Healthcare Mater., 2017, 6, 1600762 CrossRef.
- G. Egan, An investigation into silk fibroin for diverse wound healing applications, 2023, DOI:10.48730/54mr-wj35.
- A. Krishna, V. Arya, D. Geethanjali, J. Joji and N. John, in Advances in Bionanocomposites, Elsevier, 2024, pp. 211–224 Search PubMed.
- K. DeFrates, T. Markiewicz, P. Gallo, A. Rack, A. Weyhmiller, B. Jarmusik and X. Hu, Int. J. Mol. Sci., 2018, 19, 1717 CrossRef.
- Z. A. Raza, S. Khalil, M. I. Majeed and T. Sarwar, Polym. Bull., 2023, 80, 2019–2043 CrossRef CAS.
- L. R. Amado, K. de Souza Silva and M. A. Mauro, Food Biophys., 2024, 19, 256–268 CrossRef.
- F. Guarderas, Y. Leavell, T. Sengupta, M. Zhukova and T. L. Megraw, Adv. in Skin & Wound Care, 2016, 29, 131–134 Search PubMed.
- P. P. Patil, M. R. Reagan and R. A. Bohara, Int. J. Biol. Macromol., 2020, 164, 4613–4627 CrossRef CAS.
- S. Saray Tarkasheh, M. Alizadeh, S. Amiri and I. Karimi Sani, J. Polym. Environ., 2024, 1–22 Search PubMed.
- S. Chen, X. Tong, Y. Huo, S. Liu, Y. Yin, M. L. Tan, K. Cai and W. Ji, Adv. Mater., 2024, 2406192 CrossRef PubMed.
- A. Ghareeb and M. Shalaby, bioRxiv, 2023, DOI:10.1101/2023.02.21.529363.
- B. Pourjabbar, E. Biazar, S. Heidari Keshel and A. Baradaran-Rafii, Int. Wound J., 2023, 20, 484–498 CrossRef PubMed.
- T. Selvaras, S. A. Alshamrani, R. Gopal, S. K. Jaganathan, S. Sivalingam, S. Kadiman and S. Saidin, J. Biomed. Mater. Res., Part B, 2023, 111, 1171–1181 CrossRef.
- M. Fathi-Achachelouei, D. Keskin, E. Bat, N. E. Vrana and A. Tezcaner, J. Biomed. Mater. Res., Part B, 2020, 108, 2041–2062 CrossRef PubMed.
- I. Benalaya, G. Alves, J. Lopes and L. R. Silva, Int. J. Mol. Sci., 2024, 25, 1322 CrossRef.
- D. Verma, M. Okhawilai, S. Nangan, V. K. Thakur, S. Gopi, K. Kuppusamy, M. Sharma and H. Uyama, Nano-Struct. Nano-Objects, 2024, 37, 101086 CrossRef.
- M. Rostami, M. Yousefi, A. Khezerlou, M. A. Mohammadi and S. M. Jafari, Food Hydrocolloids, 2019, 97, 105170 CrossRef.
- S. R. Falsafi, H. Rostamabadi, K. Nishinari, R. Amani and S. M. Jafari, Food Chem., 2022, 374, 131826 CrossRef PubMed.
- H. Rostamabadi, E. Assadpour, H. S. Tabarestani, S. R. Falsafi and S. M. Jafari, Trends Food Sci. Technol., 2020, 100, 190–209 CrossRef.
- S. Feroz, N. Muhammad, J. Ratnayake and G. Dias, Bioact. Mater., 2020, 5, 496–509 Search PubMed.
- K. M. Salleh and N. F. Abd Rashid, in Polymer Composites Derived from Animal Sources, Elsevier, 2024, pp. 219–242 Search PubMed.
- S. Soleymani Eil Bakhtiari and S. Karbasi, J. Biomater. Sci., Polym. Ed., 2024, 35, 916–965 CrossRef PubMed.
- S. Banasaz and V. Ferraro, Polymers, 2024, 16, 1999 CrossRef.
- S. Sharma, H. Rostamabadi, S. Gupta, A. K. Nadda, M. S. Kharazmi and S. M. Jafari, Eur. Polym. J., 2022, 111614 CrossRef.
- L. Wang, Y. Shang, J. Zhang, J. Yuan and J. Shen, Adv. Colloid Interface Sci., 2023, 103012 CrossRef.
- M. El-Azazy, in Protein-Based Biopolymers, Elsevier, 2023, pp. 59–91 Search PubMed.
- Y. Shang, P. Wang, X. Wan, L. Wang, X. Liu, J. Yuan, B. Chi and J. Shen, Int. J. Biol. Macromol., 2023, 242, 124754 CrossRef PubMed.
- M. Zhang, S. Xu, C. Du, R. Wang, C. Han, Y. Che, W. Feng, C. Wang, S. Gao and W. Zhao, Colloids Surf., B, 2023, 222, 113119 CrossRef.
- G. C. Demir, Ö. Erdemli, D. Keskin and A. Tezcaner, Int. J. Pharm., 2022, 614, 121436 CrossRef.
- Y. Kaviyashri, T. Arunachalam and B. Paramasivan, Mater. Today: Proc., 2021, 47, 321–325 Search PubMed.
- S. Sadeghi, J. Nourmohammadi, A. Ghaee and N. Soleimani, Int. J. Biol. Macromol., 2020, 147, 1239–1247 CrossRef PubMed.
- C.-H. Yao, C.-Y. Lee, C.-H. Huang, Y.-S. Chen and K.-Y. Chen, Mater. Sci. Eng., C, 2017, 79, 533–540 CrossRef.
- O. Shanmugasundaram, K. S. Z. Ahmed, K. Sujatha, P. Ponnmurugan, A. Srivastava, R. Ramesh, R. Sukumar and K. Elanithi, Mater. Sci. Eng., C, 2018, 92, 26–33 CrossRef PubMed.
- S. P. Ndlovu, K. Ngece, S. Alven and B. A. Aderibigbe, Polymers, 2021, 13, 2959 CrossRef.
- P. Pankongadisak, U. R. Ruktanonchai, P. Supaphol and O. Suwantong, Polym. Adv. Technol., 2017, 28, 849–858 CrossRef.
- J. Dias, S. Baptista-Silva, C. De Oliveira, A. Sousa, A. L. Oliveira, P. Bártolo and P. Granja, Eur. Polym. J., 2017, 95, 161–173 CrossRef.
- M.-k. Yeh, Y.-m. Liang, K.-m. Cheng, N.-T. Dai, C.-c. Liu and J.-j. Young, Polymer, 2011, 52, 996–1003 CrossRef.
- Y.-C. Lin, H.-Y. Wang, Y.-C. Tang, W.-R. Lin, C.-L. Tseng, C.-C. Hu and R.-J. Chung, Int. J. Biol. Macromol., 2024, 258, 128845 CrossRef PubMed.
- W. Li, Z. Su, Y. Hu, L. Meng, F. Zhu, B. Xie, J. Wan and Q. Wu, Int. J. Biol. Macromol., 2023, 241, 124102 CrossRef.
- R. Khan, S. Haider, M. U. A. Khan, A. Haider, S. I. Abd Razak, A. Hasan, R. Khan and M. U. Wahit, Int. J. Biol. Macromol., 2023, 253, 127169 CrossRef PubMed.
- Y. Lv, Z. Yu, C. Li, J. Zhou, X. Lv, J. Chen, M. Wei, J. Liu, X. Yu and C. Wang, Int. J. Biol. Macromol., 2022, 219, 1227–1236 CrossRef CAS PubMed.
- E. R. Ghomi, V. Chellappan, R. E. Neisiany, N. Dubey, K. Amuthavalli, N. K. Verma, R. Lakshminarayanan and S. Ramakrishna, Compos. Sci. Technol., 2023, 110402 Search PubMed.
- M. Alizadeh, S. Salehi, M. Tavakoli, M. Mirhaj, J. Varshosaz, N. Kazemi, S. Salehi, M. Mehrjoo and S. A. M. Abadi, Int. J. Biol. Macromol., 2023, 233, 123491 CrossRef PubMed.
- B. Pourbadiei, M. A. A. Monghari, H. M. Khorasani and A. Pourjavadi, J. Photochem. Photobiol., B, 2023, 112750 CrossRef.
- M. Soltani, M. H. Nazarpak, A. Zamani and A. Solouk, Mater. Today Commun., 2023, 35, 106167 CrossRef.
- Z. Yuan, L. Zhang, H. Zheng, M. Shafiq, J. Song, B. Sun, E.-N. Mohamed, E.-H. Hany, Y. Morsi and C. Huang, J. Drug Delivery Sci. Technol., 2023, 89, 105072 CrossRef.
- S. Hezari, A. Olad and A. Dilmaghani, Int. J. Biol. Macromol., 2022, 218, 488–505 CrossRef PubMed.
- Y. Hu, B. Yu, Y. Jia, M. Lei, Z. Li, H. Liu, H. Huang, F. Xu, J. Li and Z. Wei, Acta Biomater., 2023, 164, 151–158 CrossRef PubMed.
- Y. Haririan, A. Asefnejad, H. Hamishehkar and M. R. Farahpour, Int. J. Biol. Macromol., 2023, 253, 127081 CrossRef PubMed.
- S. Abdollahi and Z. Raoufi, J. Drug Delivery Sci. Technol., 2022, 72, 103423 CrossRef.
- D. DeVore, D. Wise, D. Trantolo, D. Altobelli, M. Yaszemski and J. Gresser, Encyclopedic Handbook of Biomaterials and Bioengineering, Marcel Dekker, New York, 1995, pp. 1233–1260 Search PubMed.
- G. Tronci, in Advanced Textiles for Wound Care, Elsevier, 2019, pp. 363–389 Search PubMed.
- S. S. Mathew-Steiner, S. Roy and C. K. Sen, Bioengineering, 2021, 8, 63 CrossRef PubMed.
- H. Elgharably, S. Roy, S. Khanna, M. Abas, P. DasGhatak, A. Das, K. Mohammed and C. K. Sen, Wound Repair Regen., 2013, 21, 473–481 CrossRef PubMed.
- M. Marchand, C. Monnot, L. Muller and S. Germain, Semin. Cell Dev. Biol., 2019, 89, 147–156 CrossRef CAS.
- M. S. El Masry, S. Chaffee, P. D. Ghatak, S. S. Mathew-Steiner, A. Das, N. Higuita-Castro, S. Roy, R. A. Anani and C. K. Sen, FASEB J., 2019, 33, 2144 CrossRef CAS.
- X. Liu, C. Zheng, X. Luo, X. Wang and H. Jiang, Mater. Sci. Eng., C, 2019, 99, 1509–1522 CrossRef CAS PubMed.
- H. Elgharably, K. Ganesh, J. Dickerson, S. Khanna, M. Abas, P. D. Ghatak, S. Dixit, V. Bergdall, S. Roy and C. K. Sen, Wound Repair Regen., 2014, 22, 720–729 CrossRef PubMed.
- P. J. Kallis and A. J. Friedman, J. Drugs Dermatol., 2018, 17, 403–408 CAS.
- E. O. Osidak, V. I. Kozhukhov, M. S. Osidak and S. P. Domogatsky, Int. J. Bioprint., 2020, 6, 270 CrossRef CAS PubMed.
- A. Hernández-Rangel and E. S. Martin-Martinez, J. Biomed. Mater. Res., Part A, 2021, 109, 1751–1764 CrossRef PubMed.
- S. K. Ramadass, L. S. Nazir, R. Thangam, R. K. Perumal, I. Manjubala, B. Madhan and S. Seetharaman, Colloids Surf., B, 2019, 175, 636–643 CrossRef.
- R.-E. Geanaliu-Nicolae and E. Andronescu, Materials, 2020, 13, 5641 CrossRef PubMed.
- L. Shi, F. Lin, M. Zhou, Y. Li, W. Li, G. Shan, Y. Xu, J. Xu and J. Yang, J. Biomater. Appl., 2021, 36, 219–236 CrossRef PubMed.
- A. Ehterami, M. Salehi, S. Farzamfar, A. Vaez, H. Samadian, H. Sahrapeyma, M. Mirzaii, S. Ghorbani and A. Goodarzi, Int. J. Biol. Macromol., 2018, 117, 601–609 CrossRef PubMed.
- M.-Y. Bai, F.-Y. Ku, J.-F. Shyu, T. Hayashi and C.-C. Wu, Polymers, 2021, 13, 516 CrossRef PubMed.
- P. Boomi, R. Ganesan, G. Prabu Poorani, S. Jegatheeswaran, C. Balakumar, H. Gurumallesh Prabu, K. Anand, N. Marimuthu Prabhu, J. Jeyakanthan and M. Saravanan, Int. J. Nanomed., 2020, 7553–7568 CrossRef PubMed.
- V. D. Constantin, A. Carâp, S. Bobic, V. Budu, M. A. Kaya, S. Marin, M. M. Marin and B. Socea, Tissue engineering-collagen sponge dressing for chronic wounds, International Conference on Advanced Materials and Systems (ICAMS). The National Research & Development Institute for Textiles and Leather-INCDTP, 2018, 63–68 Search PubMed.
- S. Lo and M. B. Fauzi, Pharmaceutics, 2021, 13, 316 CrossRef PubMed.
- S. Mondal, G. Hoang, P. Manivasagan, M. S. Moorthy, T. T. V. Phan, H. H. Kim, T. P. Nguyen and J. Oh, Ceram. Int., 2019, 45, 2977–2988 CrossRef.
- I. Wiser, E. Tamir, H. Kaufman, E. Keren, S. Avshalom, D. Klein, L. Heller and E. Shapira, Wounds, 2019, 31, 103–107 Search PubMed.
- P. A. Parmar, J. P. St-Pierre, L. W. Chow, J. L. Puetzer, V. Stoichevska, Y. Y. Peng, J. A. Werkmeister, J. A. Ramshaw and M. M. Stevens, Adv. Healthcare Mater., 2016, 5, 1656–1666 CrossRef PubMed.
- D. E. Poland and H. E. Kaufman, J. Cataract Refractive Surg., 1988, 14, 489–491 CrossRef.
- J. Robin, C. Keys, L. Kaminski and M. Viana, Invest. Ophthalmol. Visual Sci., 1990, 31, 1294–1300 Search PubMed.
- G. J. Shaker, S. Ueda, J. A. LoCascio and J. V. Aquavella, Invest. Ophthalmol. Visual Sci., 1989, 30, 1565–1568 Search PubMed.
- R. H. Marmer, J. Cataract Refractive Surg., 1988, 14, 496–499 CrossRef PubMed.
- Z. Li, C. Qian, X. Zheng, X. Qi, J. Bi, H. Wang and J. Cao, Int. J. Biol. Macromol., 2024, 266, 131220 CrossRef.
- S. Malathi, P. Balashanmugam, T. Devasena and S. N. Kalkura, J. Drug Delivery Sci. Technol., 2021, 63, 102498 CrossRef.
- P.-F. Cai, B.-D. Zheng, Y.-L. Xu, B.-X. Li, Z.-Y. Liu, Y.-Y. Huang, J. Ye and M.-T. Xiao, Int. J. Biol. Macromol., 2024, 266, 131179 CrossRef.
- J. E. Gutierrez-Reyes, M. Caldera-Villalobos, J. A. Claudio-Rizo, D. A. Cabrera-Munguía, J. J. Becerra-Rodriguez, F. Soriano-Corral and A. Herrera-Guerrero, Biomed. Mater., 2023, 18, 035011 CrossRef.
- R. Lara-Rico, C. M. López-Badillo, J. A. Claudio-Rizo, D. A. Cabrera-Munguía, J. J. Becerra-Rodríguez, R. Espinosa-Neira and B. R. Cruz-Ortiz, Biopolymers, 2023, e23538 CrossRef.
- J. Hwang, H. Huang, M. O. Sullivan and K. L. Kiick, Mol. Pharm., 2023, 20, 1696–1708 CrossRef.
- W. F. McKay, S. M. Peckham and J. M. Badura, Int. Orthop., 2007, 31, 729–734 CrossRef PubMed.
- M. Sahiner, D. Alpaslan and B. O. Bitlisli, Polym. Bull., 2014, 71, 3017–3033 CrossRef.
- A. L. Laiva, R. M. Raftery, M. B. Keogh and F. J. O'Brien, Int. J. Pharm., 2018, 544, 372–379 CrossRef.
- W. Hao, J. Han, Y. Chu, L. Huang, Y. Zhuang, J. Sun, X. Li, Y. Zhao, Y. Chen and J. Dai, Macromol. Biosci., 2018, 18, 1800086 CrossRef PubMed.
- M. Castellano, A. Dodero, S. Scarfi, S. Mirata, M. Pozzolini, E. Tassara, A. Sionkowska, K. Adamiak, M. Alloisio and S. Vicini, Polymers, 2023, 15, 2931 CrossRef.
- F. Valipour, E. Z. Rahimabadi and H. Rostamzad, Int. J. Biol. Macromol., 2023, 253, 126704 CrossRef.
- X. Ma, J. Deng, Y. Du, X. Li, D. Fan, C. Zhu, J. Hui, P. Ma and W. Xue, J. Mater. Chem. B, 2014, 2, 2749–2763 RSC.
- F. P. Seib, G. T. Jones, J. Rnjak-Kovacina, Y. Lin and D. L. Kaplan, Adv. Healthcare Mater., 2013, 2, 1606–1611 CrossRef.
- V. Werner and L. Meinel, Eur. J. Pharm. Biopharm., 2015, 97, 392–399 CrossRef.
- H. Tao, D. L. Kaplan and F. G. Omenetto, Adv. Mater., 2012, 24, 2824–2837 CrossRef PubMed.
- J. Zhang, E. Pritchard, X. Hu, T. Valentin, B. Panilaitis, F. G. Omenetto and D. L. Kaplan, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11981–11986 CrossRef PubMed.
- D. W. Infanger, W. Abdel-Naby, J. J. Kalal, N. B. Paulson, Y. Bai and B. D. Lawrence, Invest. Ophthalmol. Vis. Sci., 2019, 60, 2820–2820 Search PubMed.
- S. Tatlisulu, E. Ozgor, D. Kavaz and M. B. Djamgoz, J. Med. Biol. Eng., 2024, 44, 266–279 CrossRef.
- S. Zhou, Q. Wang, W. Yang, L. Wang, J. Wang, R. You, Z. Luo, Q. Zhang and S. Yan, Int. J. Biol.Macromol, 2024, 255, 128350 CrossRef CAS.
- S. Zahoor, H. M. Tahir, S. Ali, A. Ali, A. Muzamil, Z. Murtaza and N. Zahoor, Int. J. Biol. Macromol., 2023, 242, 125184 CrossRef PubMed.
- S. Indrakumar, A. Joshi, T. K. Dash, V. Mishra, B. Tandon and K. Chatterjee, Int. J. Biol. Macromol., 2023, 233, 123569 CrossRef.
- L. Qiu, L. Duan, H. Lin, M. Wang, H. Liang, G. Peng, X. Yang, Y. Si and S. Yi, Adv. Fiber Mater., 2024, 1–18 Search PubMed.
- M. H. Khosropanah, M. A. Vaghasloo, M. Shakibaei, A. L. Mueller, A. M. Kajbafzadeh, L. Amani, I. Haririan, A. Azimzadeh, Z. Hassannejad and M. M. Zolbin, J. Tissue Eng. Regener. Med., 2022, 16, 91–109 CrossRef.
- Q. Wang, S. Zhou, L. Wang, R. You, S. Yan, Q. Zhang and M. Li, Composites, Part B, 2021, 224, 109165 CrossRef.
- A. Kumar Sahi, S. Gundu, P. Kumari, T. Klepka and A. Sionkowska, Biomimetics, 2023, 8, 55 CrossRef PubMed.
- N. J. Prakash, D. Shanmugarajan, B. Kandasubramanian, P. Khot and K. Kodam, Mater. Today Chem., 2023, 27, 101289 CrossRef.
- D. Lin, M. Li, L. Wang, J. Cheng, Y. Yang, H. Wang, J. Ye and Y. Liu, Adv. Funct. Mater., 2024, 34, 2405255 CrossRef.
- Q. Lu, F. Zhang, W. Cheng, X. Gao, Z. Ding, X. Zhang, Q. Lu and D. L. Kaplan, Adv. Healthcare Mater., 2021, 10, 2100427 CrossRef PubMed.
- X. Gao, W. Cheng, X. Zhang, Z. Zhou, Z. Ding, X. Zhou, Q. Lu and D. L. Kaplan, ACS Appl. Mater. Interfaces, 2022, 14, 3701–3715 CrossRef PubMed.
- G. Lu, Z. Ding, Y. Wei, X. Lu, Q. Lu and D. L. Kaplan, ACS Appl. Mater. Interfaces, 2018, 10, 44314–44323 CrossRef PubMed.
- Z. Ding, Y. Zhang, P. Guo, T. Duan, W. Cheng, Y. Guo, X. Zheng, G. Lu, Q. Lu and D. L. Kaplan, ACS Biomater. Sci. Eng., 2021, 7, 1147–1158 CrossRef PubMed.
- G. Xu, L. Xiao, P. Guo, Y. Wang, S. Ke, G. Lyu, X. Ding, Q. Lu and D. L. Kaplan, ACS Biomater. Sci. Eng., 2023, 9, 5813–5823 CrossRef PubMed.
- B. G. Bernardes, A. Veiga, J. Barros, C. A. García-González and A. L. Oliveira, Int. J. Mol. Sci., 2024, 25, 3133 CrossRef PubMed.
- A. H. Karaly, W. A. Sarhan and I. M. El-Sherbiny, Int. J. Biol. Macromol., 2021, 182, 413–424 CrossRef PubMed.
- L. Han, J. Zhu, K. L. Jones, J. Yang, R. Zhai, J. Cao and B. Hu, Int. J. Biol. Macromol., 2024, 132796 CrossRef.
- H. Hodaei, Z. Esmaeili, Y. Erfani, S. S. Esnaashari, M. Geravand and M. Adabi, Sci. Rep., 2024, 14, 23796 CrossRef PubMed.
- N. Ibrahem Khaled and D. Santhiya, Adv. Compos. Mater., 2024, 33, 324–346 CrossRef.
- S. Tortorella, M. Maturi, V. V. Buratti, G. Vozzolo, E. Locatelli, L. Sambri and M. C. Franchini, RSC Adv., 2021, 11, 39004–39026 RSC.
- E. Oleandro, M. Stanzione, G. G. Buonocore and M. Lavorgna, Nanomaterials, 2024, 14, 414 CrossRef PubMed.
- H. M. Lai and G. W. Padua, Cereal Chem., 1997, 74, 771–775 CrossRef.
- C.-Y. Zhang, W. Zhang, H.-B. Yao, H.-Z. Zhu, L.-B. Mao and S.-H. Yu, Cryst. Growth Des., 2013, 13, 3505–3513 CrossRef CAS.
- B. Zhang, Y. Luo and Q. Wang, Biomacromolecules, 2010, 11, 2366–2375 CrossRef CAS.
- A. Gagliardi, E. Giuliano, S. Voci, N. Costa, S. Bulotta, M. C. Salvatici, N. Ambrosio, D. Paolino, F. Siddique and M. Majid, Int. J. Biol. Macromol., 2024, 269, 132071 CrossRef CAS PubMed.
- A. Mamgain, R. Kenwat and R. Paliwal, Int. J. Biol. Macromol, 2024, 263, 130314 CrossRef CAS PubMed.
- G. L. Breitenbach, B. S. Caldas, M. C. G. Pellá, E. C. Muniz and D. C. Dragunski, Colloids and Surfaces A: Physicochemical and E. Aspects, 2024, 134872 CrossRef CAS.
- L. Zhou, S. Guo, Z. Dong, P. Liu, W. Shi, L. Shen and J. Yin, Mater. Today Adv., 2024, 21, 100458 CrossRef CAS.
- N. Salehi, A. Ghaee, H. Moris, S. Derhambakhsh, M. M. Sharifloo and F. Safshekan, Biomed. Mater., 2024, 19, 025044 CrossRef.
- M. U. din Khan, A. Afzaal, M. A. Gilani, S. Perveen, F. Sharif, A. Asif, A. Faisal, M. S. Nazir, O. Huck and S. Tabassum, Smart Mater. Struct, 2024, 33, 085007 CrossRef.
- A. Limaye, V. Perumal, C. M. Karner and T.L. Livingston Arinzeh, Adv. Nanobiomed. Res., 2024, 4, 2300104 CrossRef PubMed.
- W. de Souza Tavares, G. A. V. Barreto, E. P. Pinto, P. G. de Barros Silva and F. F. O. de Sousa, J. Drug Delivery Sci. Technol., 2023, 88, 104942 CrossRef.
- P. Kaushik, E. Priyadarshini, K. Rawat, P. Rajamani and H. Bohidar, Int. J. Biol. Macromol., 2020, 152, 1027–1037 CrossRef PubMed.
- F. Liu, R. Li, L. Mao and Y. Gao, Food Chem., 2018, 255, 390–398 CrossRef PubMed.
- X.-W. Chen, S.-Y. Fu, J.-J. Hou, J. Guo, J.-M. Wang and X.-Q. Yang, Food Chem., 2016, 211, 836–844 CrossRef PubMed.
- S. Chen, Y. Han, Y. Wang, X. Yang, C. Sun, L. Mao and Y. Gao, Food Chem., 2019, 276, 322–332 CrossRef PubMed.
- J. Yan, X. Liang, C. Ma, D. J. McClements, X. Liu and F. Liu, Food Hydrocolloids, 2021, 113, 106473 CrossRef.
- S. Soltani and A. Madadlou, Food Hydrocolloids, 2015, 43, 664–669 CrossRef.
- M. Bavya, K. V. Rohan, G. Gaurav and R. Srivasatava, Mater. Sci. Eng., C, 2019, 95, 226–235 CrossRef.
- N. Fereydouni and M. E. Astaneh, J. Mol. Struct., 2023, 136006 CrossRef.
- G. El Fawal, A. M. Omar and M. M. Abu-Serie, Sci. Rep., 2023, 13, 22216 CrossRef PubMed.
- M. Karimi, S. Bahrami, S. B. Ravari, P. S. Zangabad, H. Mirshekari, M. Bozorgomid, S. Shahreza, M. Sori and M. Hamblin, Expert Opin. Drug Delivery, 2016, 13, 1609–1623 CrossRef PubMed.
- T. P. H. Hutapea, K. A. Madurani, M. Y. Syahputra, M. N. Hudha, A. N. Asriana and F. Kurniawan, J. Sci.: Adv. Mater. Devices, 2023, 8, 100549 Search PubMed.
- F. Kong, N. Mehwish and B. H. Lee, Acta Biomater., 2023, 157, 67–90 CrossRef PubMed.
- E. S. Lee and Y. S. Youn, J. Pharm. Invest., 2016, 46, 305–315 CrossRef.
- A. Spada, J. Emami, J. A. Tuszynski and A. Lavasanifar, Mol. Pharmaceutics, 2021, 18, 1862–1894 CrossRef PubMed.
- K. Naik, P. Singh, M. Yadav, S. K. Srivastava, S. Tripathi, R. Ranjan, P. Dhar, A. K. Verma, S. Chaudhary and A. S. Parmar, J. Mater. Chem. B, 2023, 11, 8142–8158 RSC.
- S. Homaeigohar, T.-Y. Tsai, E. S. Zarie, M. Elbahri, T.-H. Young and A. R. Boccaccini, Mater. Sci. Eng., C, 2020, 116, 111248 CrossRef PubMed.
- D. Kwak, J. Lee, J. Kim, H. Kim, J.-Y. Lee, D.-D. Kim and J. W. Yoo, J. Pharm. Invest., 2024, 54, 51–60 CrossRef.
- F. Saadat and A. F. AzarbayjaniAlbumin-loaded nanofiber for topical wound healing, 2024, https://doi.org/10.21203/rs.3.rs-2523511/v2.
- M. A. Naser, A. M. Sayed, W. Abdelmoez, M. T. El-Wakad and M. S. Abdo, Sci. Rep., 2024, 14, 7912 CrossRef CAS PubMed.
- E. Madadian, E. Naseri, R. Legault and A. Ahmadi, 3D Print. Addit. Manuf., 2024, 11, e1175–e1185 CrossRef.
- N. Vanaclocha, L. M. Gómez, M. D. P. Del Caz, V. V. Vanaclocha and F. J. M. Alonso, Burns, 2024, 50, 903–912 CrossRef.
- A. AliKhan, A. Masmali, S. Alanazi, T. Almubrad and S. Akhtar, Therapeutic efficacy of bovine serum albumin-riboflavin-retinoic acid formulated hydrogel on corneal wound healing and progenitor cell remodeling: An ex vivo study 2024, DOI:10.21203/rs.3.rs-4407359/v1.
- Z. Jia, D. Lin, C. Tang, X. Sun, L. Cao and L. Liu, Mater. Des., 2024, 239, 112810 CrossRef CAS.
- K. Naik, P. Singh, M. Yadav, S. K. Srivastava, S. Tripathi, R. Ranjan, P. Dhar, A. K. Verma, S. Chaudhary and A. S. Parmar, J. Mater. Chem. B, 2023, 11, 8142–8158 RSC.
- W. Zhao, X. Yang and L. Li, ACS Appl. Mater. Interfaces, 2024, 16, 40356–40370 CrossRef PubMed.
- P. Sanjarnia, M. L. Picchio, A. N. P. Solis, K. Schuhladen, P. M. Fliss, N. Politakos, L. Metterhausen, M. Calderón and E. R. Osorio-Blanco, Adv. Drug Delivery Rev., 2024, 115217 CrossRef PubMed.
- F. Xu, C. Dawson, M. Lamb, E. Mueller, E. Stefanek, M. Akbari and T. Hoare, Front. Bioeng. Biotechnol., 2022, 10, 849831 CrossRef PubMed.
- L. Yan, Y. Wang, J. Feng, Y. Ni, T. Zhang, Y. Cao, M. Zhou and C. Zhao, Front. Endocrinol., 2024, 15, 1430543 CrossRef PubMed.
- J. Nandhini, E. Karthikeyan and S. Rajeshkumar, Biomed. Technol., 2024, 6, 26–45 CrossRef.
- N. I. M. Fadilah, M. Maarof, A. Motta, Y. Tabata and M. B. Fauzi, Biomedicines, 2022, 10, 2226 CrossRef PubMed.
- Y. Wang, K. Vizely, C. Y. Li, K. Shen, A. Shakeri, R. Khosravi, J. R. Smith, E. A. I. Alteza, Y. Zhao and M. Radisic, Regener. Biomater., 2024, 11, rbae032 CrossRef PubMed.
- N. J. Murugan, S. Cariba, S. Abeygunawardena, N. Rouleau and S. L. Payne, Cell. Mol. Life Sci., 2024, 81, 9 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |
