Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in irradiation-mediated synthesis and tailoring of inorganic nanomaterials for photo-/electrocatalysis
Shoushuang
Huang
 *ab,
Can
Yue
a,
Kajsa
Uvdal
b and
Zhangjun
Hu
*ab,
Can
Yue
a,
Kajsa
Uvdal
b and
Zhangjun
Hu
 *b
*b
aSchool of Environmental and Chemical Engineering, Shanghai University, Shanghai 200444, China. E-mail: sshuang@shu.edu.cn
bDivision of Molecular Surface Physics & Nanoscience, Department of Physics, Chemistry and Biology, Linköping University, Linköping 58183, Sweden. E-mail: zhangjun.hu@liu.se
First published on 27th November 2024
Abstract
Photo-/electrocatalysis serves as a cornerstone in addressing global energy shortages and environmental pollution, where the development of efficient and stable catalysts is essential yet challenging. Despite extensive efforts, it's still a formidable task to develop catalysts with excellent catalytic behaviours, stability, and low cost. Because of its high precision, favorable controllability and repeatability, radiation technology has emerged as a potent and versatile strategy for the synthesis and modification of nanomaterials. Through meticulous control of irradiation parameters, including energy, fluence and ion species, various inorganic photo-/electrocatalysts can be effectively synthesized with tailored properties. It also enables the efficient adjustment of physicochemical characteristics, such as heteroatom-doping, defect generation, heterostructure construction, micro/nanostructure control, and so on, all of which are beneficial for lowering reaction energy barriers and enhancing energy conversion efficiency. This review comprehensively outlines the principles governing radiation effects on inorganic catalysts, followed by an in-depth discussion of recent advancements in irradiation-enhanced catalysts for various photo-/electrocatalytic applications, such as hydrogen and oxygen evolution reactions, oxygen reduction reactions, and photocatalytic applications. Furthermore, the challenges associated with ionizing and non-ionizing radiation are discussed and potential avenues for future development are outlined. By summarizing and articulating these innovative strategies, we aim to inspire further development of sustainable energy and environmental solutions to drive a greener future.
1 Introduction
In recent years, photocatalysis and electrocatalysis have shown great advantages and broad application prospects in coping with the challenges of renewable clean energy.1 Photocatalysis, driven by solar energy, initiates photocatalytic reactions via the generation of photo-induced electrons and holes, providing a friendly and sustainable way to transform solar energy into chemical energy (e.g. H2 production through water splitting), which is beneficial for tackling environmental challenges.2,3 Electrocatalysis, on the other hand, can realize efficient energy conversion through electrocatalytic reactions on well-designed electrodes, such as hydrogen evolution reaction (HER), oxygen evolution reaction (OER), oxygen reduction reaction (ORR), and so on, opening up broad prospects for the development of efficient energy devices with excellent performances.4–8 In this regard, photocatalysis and electrocatalysis are of great significance for achieving green sustainable development.9 However, the efficiency, selectivity, and stability of photo-/electro-catalytic reactions strongly depend on the utilized nanomaterials. For example, the band gap structure of a photocatalyst directly affects its light absorption and separation efficiency for photo-induced electrons and holes, which subsequently determines its photocatalytic performance.10,11 As a result, the controllable preparation and modification of photo-/electrocatalysts to achieve excellent catalytic activity, selectivity, and stability is essential for advancing catalytic technologies.Currently, there are many ways to fabricate inorganic catalysts with different morphologies, phases and chemical constitutions, mainly including the top down and the bottom-up methods.12–15 However, certain inherent limitations in these methods may restrict their broader practical applications across different contexts. In particular, the “top-down” methods often suffer from inconsistencies in the size of particles, probable contamination from the starting bulk raw materials, and lack of ability to achieve perfectly homogeneous chemical compositions, which brings about their reduced effectiveness in all applications where a high degree of accuracy or uniformity is required. On the other hand, while giving much better control over the size and shape of the catalysts, the bottom-up methods have several drawbacks: complex synthesis processes mostly involving multiple steps, high sensitivity to conditions, variable yields, and difficulties in reproducibility, which are critical constraints when it comes to scaling up production.16 Moreover, both “top-down” and “bottom-up” processes may use toxic and expensive chemicals or harsh conditions, posing environmental and safety concerns. These disadvantages highlight the urgent need for developing new methods to fabricate excellent photo/electro-catalysts.
Recently, the use of radiation technology to control the synthesis and modification of nano-catalysts has received extensive attention.17 Radiation is the emission or transmission of energy via electromagnetic waves or particle beams. The radiation source can be regarded as an energy reservoir, and the energy is released in the form of photons, particles, and other basic carriers. These carriers can transfer energy to matter through direct interactions with atoms and/or molecules, resulting in various unique radiation effects, which are suitable for developing multi-functional nanomaterials for energy storage and conversion (Fig. 1). Recently, several reviews have summarized the use of ion irradiation in the fields of textiles, supercapacitors, microelectronics, and solar cells.10,18–22 However, a comprehensive review encompassing the full spectrum of electromagnetic waves and particle beams is still absent. To this end, this review aims to bridge this gap by thoroughly exploring the characteristics of various ionizing radiation techniques and underscoring the recent progress of ionizing radiation-mediated synthesis and tailoring of inorganic nanomaterials for energy conversion. We begin with a concise overview of the principles behind radiation technology. Next, we discuss the controlled synthesis and modification of inorganic catalysts with various radiation sources, detailing radiation effects on the catalysts, including defect engineering, phase transformation, heterostructure formation, element doping, band gap engineering, morphology control, and so on. Following this, we highlight recent advances in irradiated catalysts for photo-/electrocatalytic applications, emphasizing the significant contributions of irradiation technology in energy and environmental protection. Finally, we articulate our perspective on the prospects and challenges of using ionizing irradiation technology.
 | ||
| Fig. 1 Schematic illustration of the application of ionizing irradiation technology in photo-/electrocatalysis. | ||
2 Fundamentals of radiation technology
2.1 Fundamental concepts
The term “radiation” refers to the transfer of energy through space or any other medium in the form of electromagnetic waves or subatomic particles (Fig. 2a). In the case of electromagnetic radiation, the spectrum extends from the high-energy gamma (γ) rays at one end to long-wave radio waves at the other, all of which propagate at the speed of light and are capable of transporting energy without requiring a physical medium. In contrast, particle beam radiation is made of atomic or subatomic particles, including protons, neutrons, electrons, and ions. These particles carry enough energy that can interact with atoms and molecules directly with different radiation effects, including radiolysis, photolysis, knock-on collisions and photothermal.16,23Fig. 2b and c show six common effects observed in substances exposed to different types of radiation sources. Atomic ionization, a key consequence of radiation, involves the ejection of electrons from atoms or molecules, typically caused by high-energy radiation, such as strong X-rays and alpha, beta and γ-rays. These radiation sources cause electrons in shell and nuclear orbitals to be released, as their photon energies exceed the binding energy of the electrons.24–26 On the other hand, lower-energy radiation, such as soft X-rays, causes only electron transitions to higher energy levels without inducing dislocation due to the absorption of photon energy by the core electrons.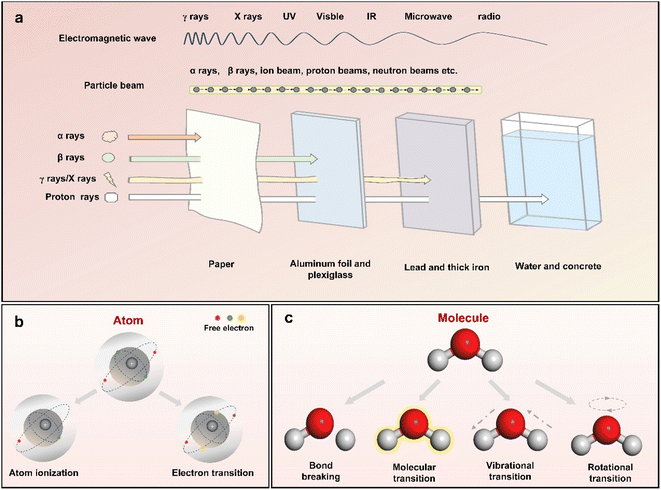 | ||
| Fig. 2 (a) Schematic illustration of the electromagnetic waves and particle beams accompanied by a comparison of their penetrating capabilities. An overview of the six effects of ionizing radiation on (b) atoms and (c) molecules.16 | ||
The response of molecules to irradiation is determined by their structural characteristics. Typically, polar molecules can undergo ionization, leading to the formation of electron–hole pairs. In contrast, electronic excitation is more common in nonpolar molecules and leads to chemical bond dissociation. This phenomenon stems from the intrinsic interactions among electron orbitals that establish chemical bonds. As the energy of radiation extends into the UV, visible light, and infrared ranges, the photon energy becomes more closely aligned with the electronic and vibrational energy levels of molecules. This alignment facilitates molecular and vibrational transitions that promote photochemical reactions and the formation of reactive radicals. At lower energy levels, like those in the microwave range, the photothermal effect induces rotational transitions in polar molecules.
| Category | Subcategory | Energya | Source | Classificationb |
|---|---|---|---|---|
| a These energy ranges are typical and can vary depending on the specific application or source. b There is no clear dividing line between ionizing and non-ionizing radiation in the spectrum of electromagnetic waves. | ||||
| γ-Ray irradiation | −(<0.001 nm) | >1.24 × 106 eV | Electromagnetic wave | Ionizing radiation |
| X-ray irradiation | Hard X-rays (0.001–0.1 nm) | 1.24 × 104–1.24 × 106 eV | Electromagnetic wave | Ionizing radiation |
| Soft X-rays (0.1–10 nm) | 124–1.24 × 104 eV | Electromagnetic wave | Ionizing radiation | |
| Electron beam irradiation | Low energy | 0.1–0.3 MeV | Particle beam | Ionizing radiation |
| Medium energy | 0.3–5 MeV | Particle beam | Ionizing radiation | |
| High energy | 5–10 MeV | Particle beam | Ionizing radiation | |
| Ion beam irradiation | Low energy ion irradiation | Less than 1 keV | Particle beam | Ionizing radiation |
| Medium energy ion-irradiation | A few hundred keV to a few MeV | Particle beam | Ionizing radiation | |
| Swift heavy ion irradiation (SHII) | More than 1 MeV | Particle beam | Ionizing radiation | |
| Neutron beam irradiation | Thermal neutron irradiation | ∼0.025 eV | Particle beam | Ionizing radiation |
| Cold neutron irradiation | 5 × 10−5–0.025 eV | Particle beam | Ionizing radiation | |
| Fast neutron irradiation | 0.1 MeV | Particle beam | Ionizing radiation | |
| Fusion neutron irradiation | 14.1 MeV | Particle beam | Ionizing radiation | |
| Proton beam irradiation | — | A few keV to a few hundred keV | Particle beam | Ionizing radiation |
| UV (ultraviolet) | Extreme UV (10–121 nm) | 10.2–124 eV | Electromagnetic wave | Non-ionizing irradiation |
| Far UV (122–200 nm) | 6.2–10.2 eV | Electromagnetic wave | Non-ionizing irradiation | |
| Middle UV (200–300 nm) | 4.1–6.2 eV | Electromagnetic wave | Non-ionizing irradiation | |
| Near UV (300–400 nm) | 3.1–4.1 eV | Electromagnetic wave | Non-ionizing irradiation | |
| Visible light | Visible light (400–700 nm) | 1.8–3.1 eV | Electromagnetic wave | Non-ionizing irradiation |
| IR (infrared) | Near IR (0.7–1.4 μm) | 0.89–1.8 eV | Electromagnetic wave | Non-ionizing irradiation |
| Middle IR (1.4–3 μm) | 0.41–0.89 eV | Electromagnetic wave | Non-ionizing irradiation | |
| Far IR (3 μm −1 mm) | 1.2 × 10−3–0.41 eV | Electromagnetic wave | Non-ionizing irradiation | |
| Microwave | -(1 mm -1m) | 1.2 × 10−6–1.2 × 10−3 eV | Electromagnetic wave | Non-ionizing irradiation |
2.2 Characteristics of different irradiation technologies
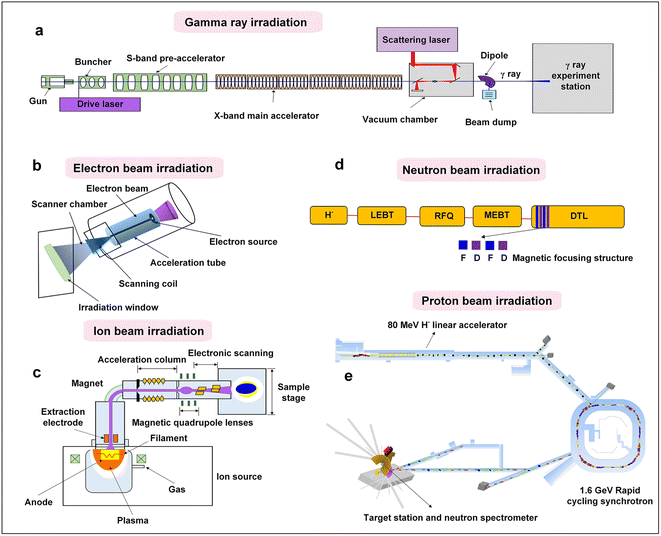
The overall configuration of γ-ray radiation is depicted in Fig. 3a, primarily including the components of the S-band photocathode microwave electron gun, convergence cavity, pre-acceleration section, and X-band main acceleration section. The γ-ray irradiation process begins with the accelerator generating a continuous beam, which is matched and focused. Laser pulses are generated by collision and scattering in the light source, resulting in the generation of a γ-ray pulse in the direction of the electron beam motion. Following the separation of the deflection magnet and electron beam, the generated γ-ray enters the γ-ray transmission section, where it is irradiated onto the substance through the collimation hole. Due to their high energy and strong penetration capabilities, γ-rays can pass entirely through the samples, providing uniform irradiation. The γ-ray irradiation process is straightforward to operate and can be conducted in atmospheric environments. Additionally, the sample station exposed to γ-rays is extensive, covering an area of several square meters.32 These characteristics position γ-ray irradiation as a promising way of realizing the development of highly efficient, cost-effective, mass-produced, and chemically stable catalysts.
Typically, UV wavelengths range from 10 to 400 nm, corresponding to photon energies between 3 and 120 eV.16 UV light can be further categorized into near-ultraviolet, mid-ultraviolet, far-ultraviolet and extreme ultraviolet, in descending order of photon energy. The power, duration, and wavelength of UV irradiation significantly impact the growth of NPs. For example, far-ultraviolet and extreme ultraviolet light can act as ionizing radiation, as its photon energy exceeds atomic ionization thresholds. In contrast, visible light, which spans a wavelength range of 400–700 nm, interacts with catalysts in a different manner. Typically, visible light has photon energies between 1.8 and 3.1 eV, which is perceptible to the human eye.42 Similar to UV irradiation, visible light can ionize solvents through multiphoton effects, generating reactive groups and solvated electrons. Visible light irradiation is cost-effective, highly controllable, environmentally friendly, and safe, and researchers have been experimenting with this technique for decades. Moving further down the spectrum, infrared (IR) light features even lower photon energies and distinct interaction pathways. Infrared light has a lower photon energy than UV light, with wavelengths ranging from 700 nm to 1 mm, and is further classified into near-infrared, mid-infrared, and far-infrared. Infrared frequencies align closely with the vibrational and rotational frequencies of molecular bonds, allowing infrared photons to be absorbed or emitted by molecules during vibrational or rotational transitions. Different from UV and visible light, infrared photons lack sufficient energy to generate free radicals or electrons, thus limiting their use for the synthesis and modification of inorganic nanocrystals. At the longest wavelengths of the non-ionizing spectrum, microwaves offer unique thermal capabilities. Microwaves, with wavelengths between 1 mm and 1 m and photon energies from 1.24 MeV to 1.24 μeV, differ significantly from higher-energy portions of the electromagnetic spectrum. Microwaves are primarily used for the thermal heating of nanomaterials due to their high efficiency and energy conversion rates, though scalability remains a limitation. Non-ionizing irradiation plays an integral role in the synthesis of metals, metal compounds, and carbon materials.43–45 One of the common advantages shared by both non-ionizing and ionizing irradiation is their high versatility across various materials, eliminating the need for conventional heating equipment.16 Consequently, the issues associated with inhomogeneous heat transfer, commonly encountered in traditional wet chemistry, can be effortlessly alleviated.
2.3 Important factors for the radiation process
| j = N/t | (1) |
 | (2) |
3 Synthesis and tailoring of inorganic nanomaterials with ionizing radiation
3.1 Material synthesis
The synthesis of inorganic nanomaterials through radiation represents a cutting-edge way to modify chemical compositions and catalyze atomic-level reactions in solution by using high-energy particles or photons. By carefully controlling irradiation parameters, such as energy, fluence and ion species, the size, morphology and crystallinity can be well controlled, enabling the tailored preparation of catalysts beyond the reach of traditional methods. This process entails irradiating a precursor solution in which solvated electrons and free radicals generated by radiation drive reduction or oxidation reactions, culminating in the nucleation and growth of NPs. Therefore, ionizing radiation synthesis offers distinct advantages, including uniform size distribution, minimized aggregation, and improved purity. In addition, it can operate under ambient conditions and avoid the use of toxic chemicals, making it an efficient and environmentally friendly approach that is essential for the sustainable synthesis of nanocatalysts.In a pioneering study conducted in 1962, Yamazaki and colleagues investigated the effects of γ-rays on the synthesis of Au nanoparticles. They observed the formation of Au sol from an aqueous solution of chloroauric acid under γ-ray irradiation with 60Co as the radiation source.46 Building on this foundational work, subsequent studies have successfully synthesized both mono- and bimetallic nanoparticles using a similar strategy,47–49 which has led to widespread exploration of γ-ray irradiation for the synthesis of metal-based nanomaterials. For instance, Kianfar et al. synthesized Pt NPs/reduced graphene oxide (rGO) hybrids using γ-radiation. The rGO served as an electron sink, facilitating the formation of Pt nanoclusters and increasing the number of active sites.50 They observed that the particle size ranged from 1–8 nm, with an average size of 4.11 nm at an acid ratio (sulfuric to phosphoric acid) of 100. However, at a lower acid ratio of 50, the average size of particles increased to about 71.09 nm. This results indicated that a combination of reducing agents and irradiation creates a highly reducing environment, which favors metal precursor reduction and promotes seed formation and NP growth.16,51 Extending the versatility of γ-ray irradiation synthesis, Yu and colleagues prepared Vulcan xc72-supported Pt NPs by γ-radiation, NaBH4 reduction and polyol reduction methods, respectively.52 They found that the Pt NPs prepared by γ-radiation exhibited higher ORR activity and Pt utilization, even better than that of commercial Pt/C catalysts. Wang et al.53 also reported the preparation of Pt-decorated MoSx catalysts using a two-step γ-ray radiation-induced reduction method in an ethylene glycol medium. The γ-radiation synthesis approach offered a shorter reaction time and higher yield, demonstrating its potential for practical applications.
Most recently, electron beam irradiation has also been utilized for the synthesis of metal–organic frameworks (MOFs). For example, Zhang et al.56 reported the synthesis of HKUST-1@Cu2O heterostructures using γ-radiation under ambient conditions, which exhibited superior catalytic activity in the reduction of p-nitrophenol to p-aminophenol to the pristine HKUST-1. This approach offers advantages in terms of convenience, environmental compatibility, and significant time and energy savings. Furthermore, the γ-radiation-induced post-synthesis technique can be applied to other crystalline porous materials to produce a variety of derivatives, thus expanding its applicability across diverse fields.
Despite the numerous advantages of γ-ray and X-ray irradiation in the synthesis of inorganic nanomaterials, gaining a deeper understanding of the mechanisms underlying crystal formation is essential, as it’s crucial for further optimizing their properties and enabling precise control over the synthesis process to achieve desired performance. A key factor in achieving efficient synthesis through γ-ray and X-ray irradiation lies in the modulation of radiation parameters. Among different parameters, precursor concentration plays a significant role, because it influences the final shape, size, and dispersion of NPs. Theoretically, higher concentrations of precursor tend to produce larger particles with lower dispersion, as the nuclei have more reactive atoms available in solution, promoting growth of nanocrystals. However, the effects of irradiation dose on nanoparticle size remain a subject of ongoing research. Some studies reported a decrease in particle size with increasing dose.57,58 For example, Wiguna et al.59 synthesized Ag NPs using γ-ray irradiation. TEM images showed that the Ag NPs were spherical in shape and the average size became larger with increasing irradiation dose. However, others found that particle size increased with the decrease of dose. This variation highlights the need for precise dose control to optimize particle characteristics. In addition to dose, the dose rate is another critical parameter that influences the growth habits of NPs. The dose rate can affect metal atom production rate, thereby influencing the size and shape of resulting products. According to classical nucleation and growth theory, a higher dose rate leads to a faster production of metal ions, increasing atomic concentration and supersaturation, which reduces critical nucleus size.60 Consequently, smaller sized nuclei form at higher dose rates, resulting in smaller sized nuclei. Recent studies further emphasize the importance of dose rate control in tailoring nanoparticle characteristics. For example, Kepić et al. used low γ-ray doses (1–20 kGy) to prepare Au NPs anchored on RGO sheets, and they observed that higher doses promoted larger particle growth (at 5 and 10 kGy) or a broader particle size distribution (at 20 kGy).61 This result demonstrates that the control of dose rate is important to achieve the desired size and distribution during the process of irradiation-based synthesis.
Recent empirical studies underscore the efficacy of e-beam irradiation for the synthesis of nanomaterials. For example, Lee and colleagues successfully prepared PVP-stabilized Cu NPs in CuSO4 solution dissolved in isopropanol (IPA) by e-beam radiation.62 By controlling the beam energy, beam current, and absorbed dose, the particle sizes of the Cu NPs were well tuned. More specifically, they observed that higher beam energy and beam current produced smaller particles, while an increase in radiation dose led to larger particle sizes. Similarly, Zhou et al. prepared colloidal Ag NPs from an aqueous AgNO3 solution using e-beam irradiation. Their results demonstrated that a higher dose rate was necessary to achieve optimal yields of Ag NPs, which underscores the importance of dose rate control in maximizing nanoparticle production. To further explore the comparative efficacy of electron beam and γ-ray irradiation in radiosynthesis, Nguyen et al. synthesized gold nanoparticles (Au NPs) by exposing an aqueous HAuCl4 solution to both e-beam and γ-ray radiation.63 The results from this work showed that the Au NPs synthesized with e-beam irradiation were significantly smaller than those produced using γ-rays at an equivalent dose rate, highlighting the enhanced precision of e-beam irradiation for achieving finer particle sizes.
Electron beam irradiation has also been demonstrated to be effective in constructing complex core–shell structured nanocatalysts. For instance, Lee et al. used a two-step e-beam irradiation process to fabricate 57FePt@Pt core–shell structures, followed by heat treatment to produce 57FePt@Pt/C catalysts.54 As depicted in Fig. 4a, the Fe core is initially formed by a first e-beam irradiation at 80 kGy, followed by a second e-beam irradiation at 40 kGy to form a Pt shell layer. The TEM image of Fig. 4b confirms the successful synthesis of the core–shell structure. The rapid formation of the 57FePt@Pt/C core–shell structure and its excellent electrocatalytic activity demonstrate the suitability of e-beam irradiation for synthesis.
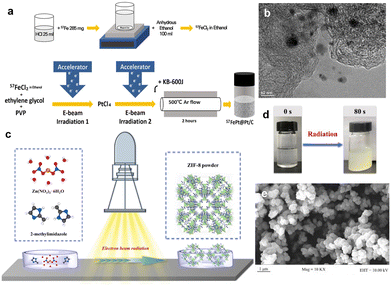 | ||
| Fig. 4 (a) Schematic illustration of the synthesis of 57FePt@Pt/C via electron-beam irradiation. (b) TEM image of 57FePt@Pt/C. Copyright 2023 Wiley-VCH. (c) The synthesis setup of ZIF-8 via electron beam radiation. (d) The photo of ZIF-8 synthesized at an absorbed dose of 50 kGy. (e) SEM image of ZIF-8 at an absorbed dose of 50 kGy.54,55 Copyright 2022, Wiley-VCH. | ||
Beyond inorganic nanomaterials, e-beam irradiation has also shown promise in the synthesis of MOFs. Most recently, Chen et al.55 synthesized ZIF-8 by using high energy e-beam irradiation (Fig. 4c–e). The cumulative absorbed dose was 50 kGy in 80 s. For comparison, ZIF-8 can also be synthesized using conventional solvent heating at 140 °C for 24 h to achieve similar crystallinity and yield. Notably, the energy consumption for e-beam irradiation was two orders of magnitude lower than that required under solvent-heated conditions, suggesting that e-beam irradiation offers ultra-rapid synthesis with significant energy savings. This is because the e-beam radiation effectively activates the whole reaction system, forming highly reactive anionic radicals, leading to a reduction of the energy barrier and significantly increasing the nucleation rate of ZIF-8 nanocrystals. The versatility of e-beam irradiation is further demonstrated in the synthesis of other MOFs, such as MIL-53, MIL-101, and MOF-73, under conditions similar to those used for ZIF-8, proving the effectiveness and broad applicability of e-beam irradiation for the synthesis of MOF materials. In addition, e-beam irradiation has shown potential for synthesizing covalent organic frameworks (COFs). For instance, Wang's group demonstrated that COFs can be rapidly synthesized via e-beam irradiation, with various products obtainable within minutes under ambient conditions.64 This strategy requires only a single radiation source and minimal equipment, presenting a scalable and high-throughput strategy that holds promise for revolutionizing the industrial production of MOFs and COFs.
3.2 Material modifications
The interaction of irradiation with nanoscale systems often leads to the formation of defects, which can impose size constraints in one or more dimensions. These resulting defects can either enhance or restrict their catalytic behaviours, depending on their nature and distribution. Particularly, in some small-volume nanosystems, electronic excitations generated by irradiation, such as those caused by high-energy electrons or particles, tend to disperse more rapidly. This dispersion reduces their localized impacts, thereby diminishing the overall effects of irradiation in such systems. Therefore, in metallic nanosystems, radiation damage is predominantly caused by atomic displacements, leading to the formation of vacancies, interstitials, or other structural defects. In contrast, in insulators, excitation can lead to localized bond breaking. The different responses of these systems to radiation can be exploited to selectively modify the structure of composites, allowing the tailoring of catalysts with excellent photoelectrochemical properties. However, in bulk systems, all energy is eventually absorbed.67 Thus, when energies are high, nanoscale damage is relatively minor as long as displacement cascades do not play a significant role. However, it is not the case that smaller material sizes are better. It is worth noting that the reduction in size leads to a different temperature distribution, resulting in localized temperatures above the melting point, which may have a significant impact on nano-systems, especially in processes such as nanoscale material modification and fabrication. The selection of nanomaterials with appropriate sizes and further exploration of the behaviour of nanosystems under irradiation with energetic particles is crucial for optimizing and controlling the formation of defects, as well as for exploiting the unique properties and functionalities of nanomaterials for various catalytic applications.
For ion beam irradiation, when the incident ions sweep over the surface of the sample, a small number of atoms recoil from the surface. The heavier ions are used to hit the lighter nuclei from the sample and probe the recoil atoms so that the depth distribution of the recoil atoms in the surface region can be obtained. Meanwhile, the deceleration of high-energy ions as they pass through solid matter can be categorized into two mechanisms: electronic stopping and nuclear stopping. A nuclear stopping occurs when the ion collides with the nucleus of the target atom, transferring kinetic energy to the target atom and resulting in translational motion of the target atom. The energy loss is determined by screening the Coulomb interaction. It should be noted that nuclear stopping is only effective for relatively slower and heavier ions of all types. In contrast, electron stopping is influenced by inelastic collisions between moving ions and target electrons, which can be either bound or free. This type of stopping is the result of various physical processes, including ionization of target atoms, electron–phonon coupling, and collective electronic excitation such as plasma. At higher ion energies, electron stopping becomes dominant. The transition from nuclear to electronic stopping depends on the ion mass. For hydrogen ions (protons), electronic stopping is dominant.
Recently, Mravik et al. reported the irradiation of MoS2 nanopowder with C2+ ions (energy 20 keV and 40 keV and ion fluences of 5 ×1014 and 1016 ions per cm2) and H+ ions (energy 30 keV and ion fluences of 1016 and 1017 ions per cm2).68 Stopping and Range of Ions in Matter (SRIM) calculations were performed to understand the effects of different ion irradiation sources on the MoS2 structure. It was found that the lighter H+ ions penetrate deeper compared to the heavier C2+ ions (Fig. 5a). At 20 keV and 40 keV energies, H+ ions produce only 6 vacancies per ion, while C2+ ions produce 189 and 304 vacancies per ion, respectively (Fig. 5b). Typically, incident ions can be halted on the target material by either electronic or nuclear stopping. Thus, the effect of ion irradiation on the material depends on whether the stopping mode predominates. Electronic stopping leads to ionization and electronic excitation, while nuclear stopping causes bond breaking, atomic displacement, vacancy creation and phonon excitation. It can be seen that the main energy loss mechanism for H+ ions is ionization, and therefore H+ ions are predominantly blocked by electrons, while nuclear blocking is negligible. If the incident ion is a C2+ ion, the main energy loss mechanism is also ionization, but there is a significant amount of energy transfer to the target lattice (phonon excitation) and recoils and successive cascades of Mo and S. The main energy loss mechanism is the ionization of the carbon ion. Thus, electronic stagnation dominates, but the contribution of nuclear stagnation is also significant, which is consistent with higher vacancy production. When irradiating a material, the different ion penetration depths in the material may have different effects on the number of vacancies, where the intrinsic reaction mechanisms vary, while the effects of ion energy, fluence and incidence angle on defects such as vacancies remain to be explored.
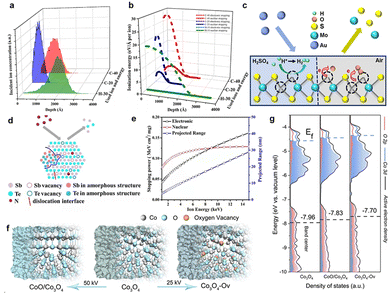 | ||
| Fig. 5 SRIM simulations of ion irradiation effects on MoS2 using H-30 keV, C-20 keV and C-40 keV. (a) The depth of ion density penetration for each ion type; (b) the analysis of electronic and nuclear stopping powers for the irradiated ions; Copyright 2013 Elsevier Ltd. (c) Schematic illustration of electron transfer from MoS2 to O2 adsorbed at defect sites. Copyright 2018, American Chemical Society. (d) Schematic illustration under N+ irradiation. (e) The relationship between nuclear stopping powers, electronic stopping powers and projected range under N+ irradiation. Copyright 2019, Elsevier Ltd. (f) Schematic illustrations of the formation of Co3O4, Co3O4-Ov, and CoO/Co3O4 samples. (g) PDOS plots.28,68–70 Copyright 2020, Wiley-VCH. | ||
Luxa et al.69 investigated the impacts of radiation (S, Se, Te) on the electrocatalytic performance of bulk MoS2 nanosheets. The study was conducted with ionic fluences ranging from 1 × 1014 to 1 × 1016 ions per cm2 at a mean ion energy of 400 keV. Exploring the penetration of ions in materials provides valuable insights into the formation of defects and the distribution of defect concentrations. Thus, SRIM simulations were carried out to investigate the ion beam penetration of MoS2. The simulation projected range (the depth at which the maximum ion injection occurs) decreases as the ion mass is increased. The range of individual projections varies as S (338 nm) > Se (162 nm) > Te (105 nm). He et al.28 fabricated a single layer of MoS2 using 500 keV high-energy Au-ion irradiation. They found that ion irradiation primarily generated S vacancies. As the defect increases, the photoluminescence (PL) peak position shifts from high to low energy levels due to the electron transfer from MoS2 to the adsorbed O2 at the defect sites, resulting in electron depletion and hole enrichment (Fig. 5c).
In addition, the modification of catalysts with ion irradiation is inextricably linked to different electron stopping/nuclear stopping mechanisms. Typically, electronic stopping leads to the ionization of the target by releasing electrons, while nuclear stopping leads to bond breaking, atomic displacement and vacancy formation. It is also important to consider the case of electron stop/nuclear stop crossover. Fig. 5d illustrates the interaction between electron stopping and nuclear stopping, as evidenced by SRIM simulations. The simulations show that the nuclear stop is about 10 times higher for Te ions compared to S ions, and that the nuclear stop is dominant for Te ions, whereas the electronic stop is significant for S and Se ions. In addition, vacancy depth profiles were simulated and displacement energies were calculated to estimate the formation of vacancies (Fig. 5e). The results show that ion irradiation mainly leads to preferential sputtering of S atoms, resulting in S vacancies. In addition, numerical simulations of the vacancy depth profiles show that S vacancies are predominantly produced in the case of Se and Te ion bombardment, whereas this effect is much smaller in the case of bombardment with S ions. Moreover, the formation rate of Mo vacancies is significantly lower due to the higher displacement energy value of Mo. Drawing from the aforementioned findings, it is evident that comprehending interactions between the electronic and nuclear stopping mechanisms and their effects on vacancy formation is critical for customizing ion irradiation processes to achieve specific modifications in material properties.
In Huang's study, N+ ion irradiation was used to modify the crystal and electronic structure of Sb2Te3 nanoplates.71 A series of characterization results revealed that the electronic structure changes induced by irradiation are closely linked to electron stopping and nuclear stopping processes. The relationship between electron stopping, nuclear stopping, incidence range, and energy of N+ ions was further evaluated with SRIM (2013) simulations. The simulation results indicated that the kinetic energy of N+ ions is dissipated through both electronic and nuclear stopping mechanisms, with a primary implantation depth of 29 nm. That's to say the N-doping enhanced the intrinsic activity of the Sb2Te3 catalyst by altering the electronic structure of the surface atoms. Additionally, the electrocatalytic activity and electronic structure of catalysts can be adjusted by introducing various types of defects and increasing the number of dangling bonds around the active site. In particular, the unique ion beam sputtering effect not only strips the surface of the catalyst, but also increases the degree of catalyst roughening, and this irradiated sputtering effect dramatically increases the specific electrochemical area of the catalyst, providing more active sites.
In a recent study, He et al.70 demonstrated the effective regulation of the surface-active electron density of Co3O4 through an argon-ion irradiation method. Through a combination of DFT calculations and characterization of the ultraviolet photoelectron spectrometer spectrum, a notable upshift in the band center of Co3O4 was unveiled (Fig. 5f and g). This shift substantially enhanced the adsorption capacity of the oxygen-containing groups and reduced the barrier for the OER, leading to enhanced performance toward water splitting. These results demonstrated that ion radiation could effectively optimize the surface electron density of catalysts, holding the potential for shaping the future rational design and discovery of superior devices.
Remarkably, ion irradiation has demonstrated remarkable efficacy in augmenting the volume of interfacial area through defect and interface engineering, which significantly enhanced their catalytic performance. Recently, Sun et al.72 tuned and optimized the defects and interfacial engineering on PtPb nanoplates by C+ ion irradiation. By adjusting the C+ ion fluence, the crystalline phase of PtPb nanoplates was transformed from single crystal to polycrystalline, while the surface layer of PtPb nanoplates was distributed with different degrees of dislocation and subcrystalline boundaries, and some of them were even amorphized. Electrochemical measurement results indicated that irradiation-treated PtPd nanoplates with defects and single-crystal/polycrystalline interfaces and appropriate amorphous phases are favourable for the ORR. In a related study, the same group investigated PtPb nanoparticles under nonaqueous conditions through 1 MeV Kr3+ ion irradiation.73 It was found that Kr3+ ion irradiation induces the formation of a crystalline/amorphous phase interface characterized by a unique electronic structure. This interface consisted of a crystalline phase surrounded annularly by an amorphous phase, playing a dominant role in lowering the reaction barrier. DFT calculations further confirmed that the novel interface activated the C–H and O–H bonds, optimized the adsorption of hydroxyl groups and intermediates on the surface and promoted the oxidation reaction, ultimately exhibiting enhanced electrocatalytic activity.
Dong et al. used a controlled flux of F-ion beams to irradiate MoS2, combining doping and defect engineering to investigate whether F-ions can effectively modulate the electrical structure of MoS2 for better HER performance.74Fig. 6a illustrates the schematic structure evolution of MoS2 under irradiation. The pristine MoS2, with a small number of sulfur vacancies, exhibited poor HER catalytic activity. As the ion fluence increased, the concentration of F doping and sulfur vacancies increased proportionally, reaching optimal HER catalytic activity at a fluence of 2 × 1013 ions per cm2. However, when the fluence is further increased to 5 × 1013 ions per cm2, a large number of disordered regions and subgrain boundaries were generated, resulting in a suppression of HER performance. Additionally, the reduced crystallinity of MoS2 nanosheets led to an increased number of grain boundaries, with sulfur vacancy boundaries located within or near these grain boundaries being less catalytically active, thereby diminishing HER performance. Moreover, when the concentration of sulfur vacancies became too high, the intrinsic activity of the sites decreased. Although the number of vacancies correlated positively with ion fluence, excessive lattice symmetry disorder and reduced crystallinity significantly hindered catalytic performance.
Felix et al.75 provided the first comprehensive study on the effects of γ-radiation on the structural, optical, and magnetic properties of monolayer WS2 nanosheets, highlighting significant alterations induced by γ-ray exposure. It was found that the interactions in radiometry, such as photoelectric absorption, Compton scattering, pair production, vacancies and fast electrons, played an essential role in modifying these properties. Collectively, these processes lead to notable changes in the electrical, optical and structural properties of WS2 nanosheets. Experimental results confirmed that the γ-irradiation significantly affected the physical properties of WS2 nanosheets. X-ray photoelectron spectroscopy (XPS) results revealed that irradiation introduces defects, with defect density increasing alongside irradiation dose. Furthermore, Raman spectroscopy tests showed that as the γ-irradiation dose increased, a blue Raman shift of the A1g(Γ) peak was observed, along with an increase in the Raman intensity ratio between the A1g(Γ) and E12g(Γ) modes, and an enhancement in the intensity. Notably, at an irradiation intensity of 400 Gy, a phase transition from antiferromagnetic to ferromagnetic behavior was observed, underscoring the profound impact of γ-radiation on the magnetic properties of the WS2 monolayer.
When a high-energy electron interacts with the nucleus in the target, only a small fraction of the incident electron energy can be transferred to the nucleus due to the conservation of momentum. In this way, high electron energies (greater than or equal to ‘threshold energies’) are required to displace an atom by electron-nucleus scattering.23 For instance, to permanently displace an atom in a graphite structure, about 20 eV of energy must be transferred to a carbon atom with an electron energy of 100 keV. Conversely, electron–electron scattering has the potential to induce ionization or bond breaking even at low electron energies. Although this energy transfer typically doesn't lead to atomic displacement, the localized reaction can still cause damage to the targeted materials. The cross-sections for nuclear and electron scattering decrease as electron energy increases. However, observable electron-nuclear scattering effects only occur at energies above the displacement threshold. Thus, the modification of catalysts with electron beams usually requires high energies, and the concentration of defects can be modulated by adjusting different energies and doses. As a result, a comprehensive understanding of these interactions, along with the threshold energies required for atomic displacements, is essential for controlling and optimizing the performance of catalysts at the nanoscale.
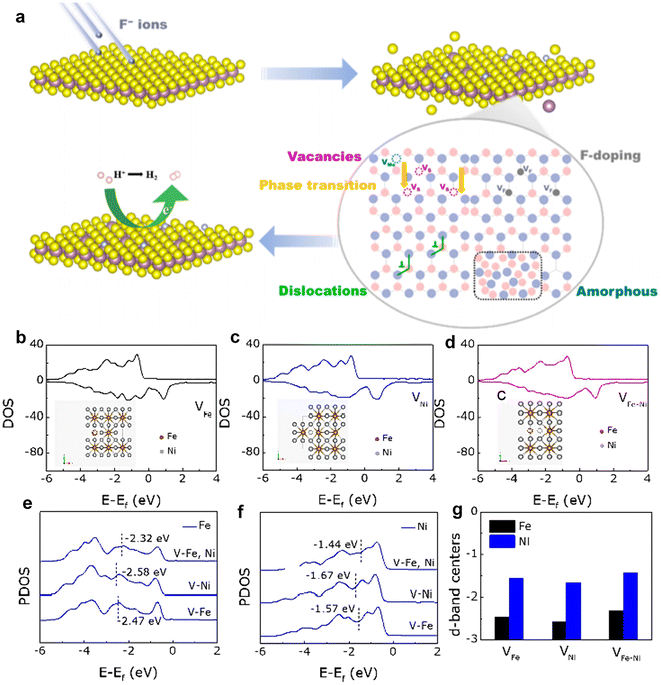
Defects induced by high-energy irradiation play a crucial role in expanding the surface area of catalysts, therefore facilitating the greater absorption of pollutants. For example, Sun et al.76 successfully enhanced the photocatalytic activity of Ag/Bi2WO6/CdWO4 through electron beam irradiation treatment. The intentional irradiation treatment not only induced interface defects but also facilitated the migration of catalytically active substances to the surface of the catalyst, leading to an increase in the content of free hydroxyl groups and significantly boosting the photocatalytic activity. Zhang et al.77 synthesized an FeNi3-16 catalyst with abundant defects by irradiating FeNi3 with H+ ions, which exhibited remarkable electrocatalytic activity towards the OER. The experimental results showed that the introduction of dual metal ion-induced defects activated initially inactive metal sites and significantly improved the OER catalytic performance compared to that of FeNi3. To further explore these effects, different defect models were constructed using DFT calculations, including iron defects (VFe), nickel defects (VNi), and iron and nickel defects (VFe–Ni). The total density of states (TDOS) analysis revealed minimal variation in the electron occupation states. However, partial density of states (PDOS) for Fe and Ni atoms (Fig. 6b–d) showed significant differences. For Fe atoms, the distances between the d-band centers and the Fermi level are −2.47, −2.58, and −2.32 eV, while for Ni atoms, these distances are −1.57, −2.58, and −2.32 eV, respectively. The separation between levels is 1.57, 1.67, and 1.44 eV, respectively (Fig. 6e–g). These results suggest that H+ ion irradiation brings the d-band center closer to the Fermi level, due to the synergistic effects of the dual defects. This closer proximity enhances electron transfer and improves the binding affinity of adsorbed oxygen to metal ions, ultimately boosting OER catalytic activity.
γ-Ray irradiation is produced by the decay of the radioisotope 60Co, and the high-energy photons generated from irradiation have high penetration.78,79 The experimental conditions of γ-ray irradiation are feasible and straightforward, and it can be performed in the ambient environment. Thus, γ-ray irradiation can be performed with a large sample stage area of up to one square meter, making possible the development of catalysts with high efficiency, economy, mass production, and stable chemical properties. γ-Ray irradiation plays an important role in changing the surface structure and electronic properties of catalysts by interacting with matter through processes such as the photoelectric effect, Compton scattering, and the electron pair effect.32,78 In a recent study, Dong et al.32 used 60Co γ-ray irradiation to introduce defects in MoS2 to optimize HER performance. Interestingly, irradiation induced the generation of abundant sulfur (S) vacancies, which not only increased the number of active sites on the surface but also effectively tuned the intrinsic electronic structure of MoS2, thus accelerating reaction kinetics and ultimately enhancing the performance of the HER. It is noteworthy that excessive irradiation led to the passivation of S vacancies by oxygen atoms, which reduced the concentration of S vacancies and exhibited inferior electrocatalytic activity. Thus, the appropriate irradiation dose is crucial for enhancing the HER performance of MoS2 nanosheets. These findings highlight the potential of gamma-ray irradiation as a powerful tool to tailor the properties of catalyst materials and provide insights into the development of advanced highly functional photo-/electrocatalyst catalysts. Similarly, Chavda et al.80 investigated the effect of γ-ray irradiation on the physical properties of the MoS2 monolayer. The DFT calculations indicate that the bandgap of MoS2 decreases as the gamma irradiation dose increases, and the conductivity qualitatively increases due to the creation of additional defect states.
In addition, proton beam irradiation has been increasingly utilized to introduce vacancies into the material. For example, Choi et al.40 found that the ORR performance of the MnO2 catalyst can be effectively boosted by high-energy proton particles (14 MeV). The research found the material surface had produced abundant oxygen vacancies through the radioactive decomposition of water along the proton beam. These irradiation-induced oxygen vacancies create additional levels in the conduction or valence band, narrowing the band gap of MnO2. This narrow band gap enhances the electronic conductivity of oxygen-deficient MnO2, allowing easier electron transfer between MnO2 and oxygen molecules.
Recently, Dong et al.81 investigated the enhancement of HER performance in MoS2 nanosheets through phase transition engineering via 1 MeV e-beam irradiation. Following irradiation, the Raman spectra of MoS2 nanosheets revealed new characteristic peaks, indicating the formation of the 1T-MoS2 phase. Additionally, Fourier transform (FT) analysis of the irradiated samples showed a notable decrease in the Mo–Mo bond length, confirming the phase transition. With increasing electron beam fluence, a higher proportion of 1T phases was observed, activating additional electrocatalytic sites on the inert planes, resulting in better HER performance. Yue et al.82 employed 1 MeV electron beam irradiation to convert 2H–WSe2 to 1T-WSe2, which induced new characteristic peaks for the 1T phase of W at 31.5 and 33.6 eV, corresponding to the W4f5/2 and W4f7/2 states in WSe2. Additionally, new selenium peaks emerged at 54.6 and 55 eV, with binding energies for Se3d5/2 and Se3d3/2 measured at 5 eV. These changes in binding energy indicated alterations in lattice symmetry due to the irradiation. As irradiation fluence increased, the crystalline phase of WSe2 transitioned from 2H to 1T. The 1T-WSe2 structure not only activates previously inert basal planes and increases the total number of active sites but also improves electrical conductivity, thereby enhancing electron transport efficiency and exhibiting enhanced HER performance. Similarly, Jouini et al.83 utilized γ-ray irradiation to induce a phase change from NiO to Ni2O3. Fluorescence quenching behavior indicated the degradation of NiO and its transformation to Ni2O3.
In particular, helium implantation has some advantages in the modification of the shape of the material. As a light element, it can cause little damage to catalysts, and it is easy to form gas bubbles. Recently, Liu and his colleagues85 have developed porous TiO2 nanorod array photoelectrodes by using He+ ion implantation to enhance photoelectrochemical water-splitting. Generally, when He atoms are implanted into TiO2 nanorod arrays, their insolubility in water allows them to be easily trapped by defects, forming bubbles as the fluence is increased or the temperature is raised. The study revealed that the size of the bubbles increased as the irradiation fluence increased. Additionally, annealing the sample in air at 500 °C resulted in larger bubbles and a more uniform distribution. The annealing process allowed the bubbles to absorb other dispersed helium atoms and vacancies. At high enough annealing temperatures, some He atoms escape from the bubbles and form nanoholes. This process generates a large number of nanoholes in the implantation layer of TiO2 nanorod arrays (NRAs). The formation of nanoholes effectively improves the hole trapping rate and charge carrier separation efficiency, and inhibits recombination, which therefore improves the photo-/electrocatalytic performance. Similarly, the combination of He+ ion implantation and post-annealing methods to regulate the morphology of α-Fe2O3 for enhanced photoelectrochemical performance has also been investigated by Wu et al.86 As the irradiation increases, a significant number of nanocavities are formed inside the He+ ion-implanted α-Fe2O3 NRAs. Relevant studies have shown that reasonable control of the parameters of helium ion irradiation fluence and thermal annealing can modulate the catalyst morphology to obtain more efficient catalytic performance.
Dong et al.74 doped F ions into MoS2 by heavy-ion irradiation, achieving successful doping through XPS analysis. The chemical compositions and valence states of MoS2 NSs before and after irradiation are shown in Fig. 7a. Generally, the F 1s peak is located at ∼688 eV in the XPS spectrum. However, F 1s does not show a significant peak in the XPS spectrum of F-2 × 1013. This is because the XPS penetration depth is less than ∼10 nm, which limits the detection of the sample surface. Line-scan profiling in Fig. 7b further confirms successful F doping in MoS2. All of this suggests that ion beam irradiation serves as a novel and effective method for elemental doping.
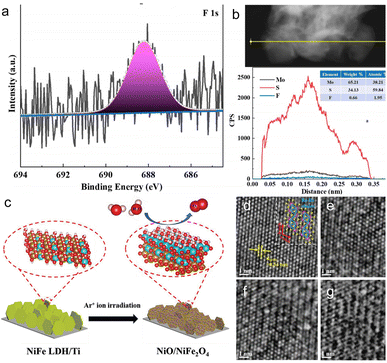 | ||
| Fig. 7 (a) XPS spectra of F 1s from irradiated MoS2 and (b) line-scan profiling analysis of irradiated MoS2. Copyright 2018, American Chemical Society. (c) Schematic illustration of the formation of NiO/NiFe2O4. Copyright 2021, Wiley-VCH. (d) HRTEM images of pristine ReS2 nanosheets with observation direction [001], (e) Ar+ ion beam irradiation of ReS2 nanosheets for 10 s, (f) 30 s, and (g) 60 s.74,84,96,97 Copyright 2018, Wiley-VCH. | ||
γ-Ray irradiation is a promising, convenient and environmentally friendly method for the production of graphene-based nanomaterials. It also contributes to the reduction of metal ions and the modification of the carbon properties and the chemical reactions on the surface. Recently, Rahman et al.98 have synthesized a N-doped RGO-supported Fe-based catalyst with 100 kGy γ-ray irradiation, and the percentage by weight (wt%) of Fe loading is in the range of 10% to 20%. XRD, Raman, FTIR, and EDS characterizations revealed that the γ-ray radiation effectively introduced N and Fe into the sample. The N-doping increases the electron density and forms N-support sites with carbon and iron, which directly improved its ORR performance. Additionally, the strong interactions between Fe-Nx and O2 facilitated rapid electron transfer.
Recently, Ghicov et al.99 investigated the effects of N ion implantation on the structure and doping efficiency of TiO2 nanotubes, using ion fluences of 1 × 1015 and 1 × 1016 ions per cm2 under 60 keV acceleration energy. At a high fluence of 1 × 1016 ions per cm2, the ion implantation disrupts the original morphology of the self-organized TiO2 nanotubes, resulting in complete amorphization of the anatase structure. This amorphous state, however, created traps for photogenerated electron–hole pairs, leading to reduced photocurrents in the UV range. Interestingly, despite these structural alterations, the specific dose of 1 × 1016 ions per cm2 enabled effective N doping, which enhanced the photo-response in the visible range.
Different forms of heteroatom doping play distinct roles in enhancing catalyst properties, necessitating the strategic selection of ions for catalyst irradiation. Recently, Liu et al.100 investigated the effects of noble metal nanoparticles and metal (Fe, V) and non-metal (N) doped TiO2, using energies from 20–50 kV and fluences ranging from 0.1–20 × 1016 ions per cm2. After ion implantation and annealing treatments, the photocatalytic activity of the Au–TiO2 samples was enhanced, which was attributed to the increased absorption of incident light by Au nanoparticles, enhanced local electric field and reactive electron and hole excitation. Additionally, a Schottky junction also formed when Au nanoparticles made direct contact with the TiO2, significantly promoting electron–hole separation and reducing recombination. For Fe-doped TiO2, the mixing of Ti4+ 3d and Fe3+ 3d orbitals introduced new energy levels into the TiO2 band structure, increasing visible light responsiveness. Meanwhile, V doping accelerated the phase transition of TiO2 from anatase to rutile. In the case of N-doped TiO2, experimental and theoretical analyses demonstrated that replacing oxygen with nitrogen created isolated N 2p localized states above the O 2p-dominant valence band. The mixing of N 2p and O 2p states reduced the TiO2 band gap, enhancing visible light absorption. Overall, TiO2 thin films prepared via ion irradiation with Au, Fe, V, and N were highly crystalline, stable, and showed improved photocatalytic activity under visible light. These promising results underscore that different ion sources have varying effects on the structural and physicochemical properties of catalysts, emphasizing the importance of tailored doping strategies.
There are many methods for doping metal ions into wide bandgap materials, such as co-precipitation synthesis and advanced ion implantation.102–105 Unlike the conventional chemical methods that yield unstable metal ion doping, advanced physical doping methods (e.g., ion beam irradiation) can effectively dope metal ions into catalysts in a controlled manner.106 Specifically, ion irradiation not only facilitates metal ion doping but also alters the electronic and surface properties of catalysts, making it particularly effective for doping. In a recent work, hydrothermal growth of ZnO nanorod arrays on fluorine-doped tin oxide (FTO) substrates was reported by Wang et al., followed by doping with varying fluxes (3 × 1015, 5 × 1015, and 2 × 1016 ions per cm2) of Cu ions using ion implantation techniques.107 The doping of Cu ions within the forbidden band gap notably reduces the band gap of ZnO, attributable to the introduction of impurities in the form of Cu2+ and Cu+ states. Electrons in the valence band can be excited to additional copper doping levels and subsequently enter the conduction band of ZnO when exposed to visible light, thereby expanding the light absorption range of the Cu-doped ZnO nanorods. Similarly, Cai et al.108 successfully prepared ZnO NRs doped with V ions by an ion implantation method (energy of 50 keV, flux of 2.5 × 1014–5 × 1015 ions per cm2). The introduction of impurity donor levels through the doping of V4+ ions within the forbidden band has been observed to notably reduce the band gap of ZnO, thereby extending the light absorption of ZnO NRs into the visible region. As a result, the V-ion doped ZnO NRs exhibited superior photocatalytic activity to pristine ZnO. Under visible light irradiation, the V-ion doped ZnO NRs demonstrated the ability to excite additional electrons from the V4+ dopant into the conduction band of ZnO. Furthermore, as the V ion implantation dose increased, so did the charge carrier density, leading to the generation of more electrons and holes for water oxidation reactions.
4 Catalytic applications
4.1 Electrocatalysis
Electrochemical water splitting for hydrogen production is a highly promising strategy for sustainable energy supply, offering a clean alternative to reduce reliance on fossil fuels. However, the inherent slow kinetics of the HER and OER necessitate the use of highly active catalysts.113,114 Noble metals, such as Pt and IrO2, exhibit excellent electrocatalytic activity, but their scarcity and high cost pose significant barriers to large-scale application. Transition metal-based compounds, such as those containing, e.g., Fe, Co, Ni, Mo and W, offer a promising alternative due to their abundance and easy synthesis. However, optimizing these materials to achieve outstanding catalytic activity and high stability for practical applications remains a major challenge. In this context, ionizing and non-ionizing radiation techniques offer unique merits for the development of catalysts toward water splitting (Table 2). By creating atomic-scale defects, activating surface sites, and altering the electronic structure of the catalysts, radiation can significantly enhance their intrinsic electrocatalytic activity.| Catalyst materials | Irradiation sources | Energy | Fluence | Roles | Application | Ref. |
|---|---|---|---|---|---|---|
| Thin-layer MoS2 | γ-Ray irradiation/60Co | — | 100 rad s−1 (0.1, 1, 10, 100 Mrad) | Defect introduction | HER | 32 |
| MoS2 nanosheets | Electron beam irradiation | 1 MeV | 5 × 1014, 5 × 1015, 1 × 1016 e cm−2 | Defect introduction and phase transformation | HER | 81 |
| Bulk MoS2 | Ion beam irradiation/S, Se, and Te | 400 keV | 1 × 1014–1 × 1016 ions per cm2 | Doping and vacancy introduction | HER | 69 |
| Layer MoS2 | Swift heavy ion irradiation/Xe | 91 MeV | 5 × 1012, 2 × 1013 and 5 × 1013 ions per cm2 | Vacancy introduction | HER | 94 |
| MoS2 | Ion beam irradiation/C+ | 3 MeV | 5 × 1012, 2 × 1013 and 5 × 1013 ions per cm2 | Defect engineering | HER | 115 |
| WS2 | Ion beam irradiation/Fe13+ | — | 8 × 1013 and 5 × 1014 ions per cm2 | Fe-doping and vacancy introduction | HER | 95 |
| Bi2Te3 nanosheets | Ion beam irradiation/Fe10+ | 320 keV | 4 × 1014 ions per cm2 | Defect introduction and surface modification | HER | 116 |
| Sb2Te3/Ti | Ion beam irradiation/N+ | 15 keV | 2 × 1014, 2 × 1015, and 2 × 1016 ions per cm2 | Doping and defect introduction | HER | 71 |
| Si | Ion beam irradiation/Ar+ | 90 keV | 5 ×1016–2 × 1017 ions per cm2 | Morphology control | HER | 117 |
| MoSe2 nanosheets | Ion beam irradiation/Ar2+ | — | 5 × 10 14,5 × 10 15 ions per cm2 | Vacancy introduction | HER | 118 |
| WSe2 | Electron beam irradiation | 1 MeV | 1 × 1013, 1 × 1014, 5 × 1014, 1 × 1015, 1 × 1016 e cm−2 | Phase transformation | HER | 82 |
| Ti3C2Tx mxene | γ-ray irradiation/Core fuel bundles | — | 500 Gy h−1 (100, 300 kGy) | Morphology control | HER | 119 |
| 12- Tungstophosphoric acid | Ion beam irradiation/C+ | 10 keV | 5 × 1014–2.5 × 1015 ions per cm2 | Structural modification | HER | 120 |
| Single-layer MoS2 | Ion beam irradiation/Au | 500 keV | 5 × 1011–1 × 1014 ions per cm2 | Defect engineering | HER | 28 |
| CoO/Co3O4 | Ion beam irradiation/Ar+ | 50 keV | 5 × 1015 ions per cm2 | Morphology control/phase transformation | OER | 70 |
| Co3O4 -Ov | Ion beam irradiation/Ar+ | 25 keV | 5 × 1015 ions per cm2 | Oxygen vacancy introduction | OER | 70 |
| NiO/CC | Ion beam irradiation/N+ | 50 keV | 5 × 1015 ions per cm2 | N-doping and vacancy introduction | OER | 121 |
| NiO/NiFe2O4 | Ion beam irradiation/Ar+ | 100 keV | 1, 10, 20, 30, 40 × 1015 ions per cm2 | Heterostructure formation | OER | 96 |
| Pt/N50-GY | γ-Ray irradiation/60Co | — | 140 kGy | Morphology control | ORR | 47 |
| Pt-Ru/HOPG | Ion beam irradiation/N | 100 eV | 4 × 1015–9.6 × 1016 ions per cm2 | Doping and defect | ORR | 166 |
| Fe/N-rGO | γ-Ray irradiation/60Co | — | 100 kGy | Fe/N-doping | ORR | 98 |
| CN/Pt, g-C3N4, Pt | γ-Ray irradiation | — | 20, 60, 100, 140, 180 kGy | Material synthesis | ORR | 167 |
| Pt0.9nm/Pt25Ni75(111) | Ion beam irradiation/N2+, N02 | ≤100 eV | — | Material synthesis | ORR | 168 |
| Pt-CeOx nanowire/C | Proton irradiation | — | 5 kGy | Defect introduction | ORR | 41 |
| MnO2 | Proton irradiation | 14 MeV | — | Vacancy introduction | ORR | 40 |
| Pd/CNT | γ-Ray irradiation/60Co | — | 15, 25, 50, 100 kGy | Material synthesis | ORR | 169 |
| PRGO | γ-Ray irradiation/60Co | 1.33 MeV | 15, 25 kGy | Vacancy introduction | ORR | 129 |
| Pt NPs/GC | Ion beam irradiation/Ar | 380 kev | 7.5 × 1015 ions per cm2 | Defect introduction | ORR | 128 |
| Pt/CN-CB | γ-ray irradiation/60Co | — | 140 kGy | Material synthesis | ORR | 170 |
| Pt/HOPG | Ion beam irradiation/Ar+ | 1 keV | 1.0 × 1014 ions per cm2 | Vacancy introduction | ORR | 171 |
| Pt/HOPG | Ion beam irradiation/Ar | 100 eV | ∼5 × 1016 ions per cm2 | Defect engineering | ORR | 172 |
| Pt/HOPG | Ion beam irradiation/N | 100 eV | ∼5 × 1016 ions per cm2 | N-doping | ORR | 173 |
| Pt–Ru/HOPG | Ion beam irradiation/Ar | 100 eV | 4 × 1015–9.6 × 1016 ions per cm2 | Doping and defect | ORR | 174 |
| PtPb nanoplates | Ion beam irradiation/C+ | 10 eV | 1 × 1016, 2 × 1016, 3 × 1016 ions per cm2 | Defect engineering and interface engineering | ORR | 72,73 |
| GY-PtPd | γ-ray irradiation | — | 150 kGy | Material synthesis | ORR | 175 |
| Fe–N–C | Electron beam irradiation | 10 eV | 80 kGy | Forming Fe–Nx active site | ORR | 176 |
| PtNPs/NrGO | γ-Ray irradiation/60Co | — | 100 kGy | Morphology control | ORR | 177 |
Recently, Xia et al.118 have synthesized defective MoSe2 nanosheets on carbon cloth by a CVD method and it was found that the intentional irradiation with high energy Ar2+ ions (5 × 1014 and 5 × 1015 ions per cm2, respectively) had induced multifold in the basal plane. Four types of vacancy were observed in their work: native Mo and Se vacancy (VMo and VSe), double Se vacancy (VSe2), and the absence of one MoSe2 (VMoSe2). As expected, the irradiated MoSe2 with a dose of 5 × 1015 ions per cm2 displayed a much lower overpotential and Tafel slope than those of pure MoSe2. The improved HER performance can be attributed to the increased catalytic sites and optimized electronic structure due to the formed vacancies. Sun et al.115 utilized C+ ions to irradiate MoS2 with an energy of 3 MeV at various ion fluences (5 × 1012, 2 × 1013, and 5 × 1013 ions per cm2). The ion irradiation activated the inert basal plane of MoS2 NSs by introducing numerous S vacancies, which thereby exhibited much improved HER performance. Additionally, it was found that different ion fluences could modulate the number of S vacancies and amorphous phases on the MoS2 substrate. In particular, the MoS2 nanosheets irradiated at an ion fluence of 2 × 1013 ions per cm2 exhibited the best HER performance with an onset potential of 77 mV and a Tafel slope of 66 mV dec−1. In addition, the ion species, energy, fluence, and other irradiation parameters has distinct effects on vacancy formation within the catalyst. Madauss et al.94 reported a new method to improve the HER performance of MoS2 by using a rapid heavy Xe ion irradiation (irradiation energy 91 MeV). At −0.6 V vs. RHE, the irradiated MoS2 sample exhibited a higher current density (35.3 mA cm−2) than pristine MoS2 (−13.3 mA cm−2). The irradiated samples also had a smaller Tafel slope (104 mV dec−1) compared to pristine MoS2 (106 mV dec−1). He et al.28 fabricated a single layer of MoS2 using 500 keV high-energy Au-ion irradiation. They found that ion irradiation primarily generated S vacancies. As the defect density increases, the photoluminescence (PL) peak first blueshifts and then redshifts due to the electron transfer from MoS2 to the adsorbed O2 at the defect sites.
γ-ray radiation serves as a source of high-energy photons with strong penetrating ability, allowing strong penetration through the sample and ensuring a more uniform irradiation effect. In a recent study, Dong et al.32 employed 60Co γ-ray irradiation to modify MoS2 nanosheets at doses of 0.1, 1, 10, and 100 Mrad to investigate the effect of irradiation fluence on the electrocatalytic performance. A series of characterizations showed that numerous S vacancies were produced on the MoS2 nanosheets after irradiation. Electrochemical measurements showed that at a dose of 10 Mrad, MoS2 nanosheets achieved a low overpotential (η10) value of 269.4 mV to achieve a current density of 10 mA cm−2 with a Tafel slope of 65.96 mV dec−1, which was much smaller than that of bare MoS2 (327.4 mV, 130.88 mV dec−1) and the irradiated samples with 100 krad (121.23 mV dec−1), 1 Mrad (74.97 mV dec−1) and 100 Mrad (74.97 mV dec−1) of MoS2, respectively. The above results indicated that the introduction of S vacancies increased the number of active sites on the MoS2 nanosheets, resulting in better HER performance. Subsequently, the same research group employed 1 MeV electron irradiation to irradiate MoS2 nanosheets, discovering that, besides forming S vacancies, it also induced a transition from the 2-H phase to the 1-T phase.81 This transition can be attributed to the charge redistribution associated with electronic excitation, the formation of S vacancies, and the accumulation of mechanical strain in defective MoS2 nanosheets. The formation of S vacancies and the 1T phase transition during irradiation greatly enhanced the HER activity. In particular, when the irradiation fluence reaches 5 × 1014 e cm−2, a current density of 10 mA cm−2 can be achieved with an initial overpotential of only 141 mV and an overpotential of 235 mV.111 Similarly, Li et al.82 achieved the conversion of 2H-phase tungsten selenide (2H–WSe2) to 1T-phase tungsten selenide (1T-WSe2) under 1 MeV electron irradiation. At an irradiation fluence of 5 × 1014 e cm−2, the minimum onset overpotential of WSe2 was 247 mV (vs. RHE), much lower than that of the as-prepared WSe2 (348 mV.) The Tafel slope of 1T-WSe2 was 67 mV dec−1, which was also lower than that of unirradiated WSe2 (117 mV dec−1). The enhanced electrocatalytic performance of WSe2 under electron beam irradiation is attributed to the presence of 1T-WSe2 components in properly irradiated samples. However, it's important to note that further increases in irradiation fluence lead to agglomeration of WSe2, which severely hindered the exposure of catalytically active sites and resulted in the decrease of HER catalytic activity.92
More recently, in an innovative work by Huang et al.,97 Re vacancy defects were intentionally introduced in ReS2 by a controlled Ar ion beam bombardment. The presence of Re vacancies not only generates enough unsaturated bonds but also affects the intrinsic charge compensation of the S–Re–Re bond, leading to the adsorption of H+ being neither too strong nor too weak. In addition, the vacancy densities increase with the increase of the ion beam intensity. When the irradiation time was extended to 60 s, a significant increase in lattice defects was observed, and most of the zigzag Re–Re chains were disrupted, which impeded the adsorption of H+ at the active site. The HRTEM images, as illustrated in Fig. 7d–g, demonstrate that Ar+ ion beam irradiation is conducive to the formation of Re vacancies with appropriate duration, and it is possible to efficiently control the vacancy density on 2D ReS2 nanosheets.
The hydrophilic/hydrophobic properties have a significant impact on the electrocatalytic performance of catalysts. Enhancing the hydrophilicity of catalysts by various methods and strategies is an encouraging pursuit. Recently, Wang et al.116 investigated the effect of Fe10+ ion irradiation on the HER properties of Bi2Te3 nanosheets deposited on titanium plates. In acidic solution, the HER performance of Bi2Te3 nanosheets was significantly improved after irradiation with Fe10+ ions compared to that of pristine Bi2Te3 nanosheets, with η10 decreasing from 436 mV to 395 mV (Fig. 8a–c). The increase in activity can be attributed to the change of Bi2Te3 surface properties from hydrophobic to hydrophilic by irradiation with iron ions, which promotes the release of H2 bubbles on the catalyst surface and exposes the active sites in time. At the same time, it also prevents large gas bubbles from damaging the electrode, thus improving the stability of the catalyst. This efficient Fe3+ ion irradiation method provides an innovative approach for designing other efficient catalysts.
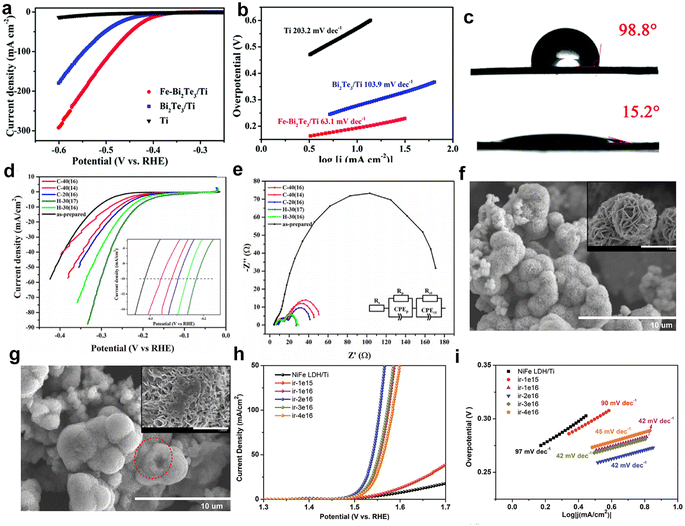 | ||
| Fig. 8 (a) LSV curves of Ti, Bi2Te3/Ti and Fe–Bi2Te3/Ti at a scan rate of 5 m V s−1. (b) Corresponding Tafel plots. (c) Contact angles for Bi2Te3/Ti (top) and Fe–Bi2Te3/Ti (bottom), respectively. Copyright 2020 The Royal Society of Chemistry. The HER properties of MoS2 (d); iR polarization curves; (e) EIS spectrum at an overpotential of 0.2 V vs. RHE; SEM images of as-prepared MoS2 (f) and irradiated sample C-40(16) (g); inset: magnified SEM image of as-prepared and irradiated MoS2. Copyright 2013 Elsevier Ltd. OER performance (h) and Tafel plots (i) of NiO/NiFe2O4 (ir-2e16), NiFe2O4, NiO, and RuO2.68,96,116 Copyright 2021, Wiley-VCH. | ||
To enhance the intrinsic HER catalytic activity for flower-like MoS2, Mravik et al. irradiated MoS2 nanopowder with carbon and hydrogen ions.68 The HER performance of the irradiated samples was tested and the polarization curves of the prepared samples are shown in Fig. 8d. It was observed that all irradiated samples exhibited enhanced HER catalytic activity compared to bare MoS2, achieving an optimal η10 of 213 mV. A similar trend is observed for carbon ion irradiation, with an η10 of 259 mV for sample C-40 (16) and below 280 mV for sample C-40 (14). However, the impact of varying the energy of incident carbon ions was less pronounced.
Apparently, the HER performance of these catalysts was significantly enhanced after irradiation, resulting in a current density at 300 mV that was up to six times higher than that of the non-irradiated sample. Electrode kinetics were evaluated using EIS (Fig. 8e), revealing that the charge transfer resistance (Rct) of irradiated samples was considerably reduced, with hydrogen ion-irradiated samples showing an Rct as low as 12 Ω, up to 14 times lower than that of unirradiated samples. Fig. 8f and g illustrate the morphology of the catalyst before and after irradiation. Initially, the sample displayed spherical flower-like structures composed of nanosheet petals (inset in Fig. 8f). After irradiation, the morphology was altered, with smoother spherical surfaces, disrupted flower-like structures, and partially “opened up” microspheres, as seen in the dashed circles in Fig. 8g, allowing easier access to the interior. Both carbon and hydrogen ion beams produced similar morphological changes. These findings suggest that ion irradiation induces structural defects and morphological changes, which enhance the conductivity and intrinsic activity of the catalysts, which significantly impacted their HER performance.
Wu et al.117 also reported the preparation of nanoporous silica (SiO2) NPs as HER catalysts by irradiation with 90 MeV Ar+ ions and annealing in a vacuum. The effect of fluence on cathode morphology and HER performance was investigated. The η10 of the samples irradiated at fluences of 5 × 1016, 1 × 1017, and 2 × 1017 ions cm−2 were 590, 357, and 483 mV, respectively, which were much smaller than that of the pristine silicon. The improvement in the HER performance was attributed to the formation of a porous structure by the irradiation of Ar+ ions, which facilitated the spillage of the H2 bubbles from the surface of the catalysts, exposing more active sites. The application of irradiation technology in the HER offers new perspectives in the energy fields, but many mechanisms remain to be explored.
Numerous studies have showcased that irradiation can be employed as a distinctive method for engineering efficient and durable OER catalysts for overall water splitting. For example, Zhong et al.96 irradiated a NiFe LDH with Ar+ ions with various fluences. Benefiting from the formed oxygen vacancies and NiO/NiFe2O4 heterostructure, the catalyst with a fluence of 2 × 1016 ions per cm2 presented significantly improved OER performance with an η10 of 279 mV and a low Tafel slope of 42 mV dec−1, superior to that of NiFe LDH/Ti (Fig. 8h–j). Also, the DFT calculations have shown that the NiO/NiFe2O4 heterostructure has optimized the free energy of the oxygen-containing intermediates, thereby showing greater OER performance. Xia et al.121 prepared NiO nanosheets by a hydrothermal method following annealing treatment. Then, N+ ions were irradiated to introduce more oxygen vacancies and dope N into NiO, and the dose of N+ ion beam was 5 × 1015 ions per cm2. The roles of N-doping and O vacancies in the NiO substrate were examined by DFT calculation. The results of the density of states (DOS) showed that the bandgap of NiO decreased from 2.9 eV to 0.56 eV. Meanwhile, the charges are clustered around the O vacancy and N atoms, indicating that it has better electronic conductivity. Thus, the OER catalytic activity of NiO was much improved. In particular, the OER properties of N+-5 × 1015 (5 × 1015 N+ ions per cm2 irradiated samples) were superior to those of NiO/CC (2.32 V, 198.74 mV dec−1), and the potential is 1.98 V at 100 mA cm−2 and Tafel slope is 135.97 mV dec−1. Moreover, the high TOF value of N+-5 × 1015 (92.1 s−1) shows that the electron transfer rate is improved after N+ ion irradiation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 second time chronoamperometric stability test. Chauhan et al.41 fabricated Pt-CeOx/C (Pt/C = 0.02) using 5 kGy proton beam irradiation. Since the free radical density generated by the proton beam irradiation is twice as high as the electron beam irradiation, the surface of the CeOx nanowire is completely transformed into a thin layer of Pt–O–Ce. As expected, the ORR performance is higher than that of the conventional Pt/C (Pt/C = 0.2) and the same as that for Pt-CeOx/C (Pt/C = 0.2). The characterization and surface atomistic simulations indicate that Pt–O–Ce bonds are formed in the defect-rich region of CeOx nanowires, resulting in the maximum ORR activity of the prepared samples. The results suggest that the surface modification of CeOx nanowires by proton beam irradiation can reduce the Pt content and maintain its high activity.
000 second time chronoamperometric stability test. Chauhan et al.41 fabricated Pt-CeOx/C (Pt/C = 0.02) using 5 kGy proton beam irradiation. Since the free radical density generated by the proton beam irradiation is twice as high as the electron beam irradiation, the surface of the CeOx nanowire is completely transformed into a thin layer of Pt–O–Ce. As expected, the ORR performance is higher than that of the conventional Pt/C (Pt/C = 0.2) and the same as that for Pt-CeOx/C (Pt/C = 0.2). The characterization and surface atomistic simulations indicate that Pt–O–Ce bonds are formed in the defect-rich region of CeOx nanowires, resulting in the maximum ORR activity of the prepared samples. The results suggest that the surface modification of CeOx nanowires by proton beam irradiation can reduce the Pt content and maintain its high activity.
As previously mentioned, ionizing irradiation can introduce defects into catalysts through ionization, electron excitation, and cascade collisions. Among these methods, ion irradiation is the most prominent because it allows for the precise control over the energy, type of incident ions, and position of the beam spot.127 This enables adjustment of the irradiation site, doping ion type, and number of defects on the catalysts, which is beneficial for photocatalysis and electrocatalysis. For example, Sun et al.72 reported an effective strategy for optimizing defects and interfaces in intermetallic PtPb nanosheets through 10 MeV C+ ion irradiation. The structure changed from single-crystal to polycrystalline, with the introduction of defects such as dislocations, subgranular boundaries, amorphization, and multiple defect-related interfaces (Fig. 9a–f). The optimized Pt nanoplates exhibited 2.97 times higher ORR specific activity and 3.00 times higher mass activity compared to the pristine Pt nanoplates. Additionally, they also exhibited excellent stability. This work emphasizes the superiority and significance of ion irradiation in inducing and tuning defects and interfaces for better performance. Kakitani et al.128 investigated the interfacial effects of Ar+-induced defects in glassy carbon substrates on the size and electronic structure of Pt NPs. First, a glassy carbon substrate was irradiated with Ar+ ions at 380 keV. Then, Pt nanoparticles were deposited on the substrate by radio frequency magnetron sputtering. The irradiation defects in the carbon substrate promoted the growth of Pt NPs on the substrate, resulting in larger Pt NPs. The DFT calculations revealed that lattice vacancies in the graphite structure lowered the position of the d-band centres, leading to higher ORR activity.
 | ||
| Fig. 9 TEM (a) and HRTEM (b) images of PtPb nanosheets; (c–f) HRTEM image of PtPb/C after C+ irradiation. The inset in (c) is the FFT image of the whole nanosheet. The inset in (e) is the FFT image of the orange region. The red “T” represents dislocations, the blue line represents subcrystalline boundaries, the white line represents crystal orientation, the area surrounded by the yellow curve represents disordered regions, and the orange area represents disordered domains. Copyright 2017 Wiley-VCH. (g) Photocatalytic performance of different ZIF-8 samples. (h) Comparison of the photocatalytic performance of a ZIF-8-1000 kGy and ZnO/ZIF-8 mixture. (i) Photocatalytic degradation of methyl bromide by ZIF-8-1000 kGy.55,72 Copyright 2022, Wiley-VCH. | ||
Devadoss et al.129 demonstrated that RGO films were significantly affected by γ-ray irradiation. The exfoliation and formation of defects on the surface of RGO nanosheets lead to additional edge sites and lots of hydrophilic groups. Meanwhile, oxygen doping into the GO lattice increases the hole size at the Fermi level, leading to the shift of the Fermi level towards positive potential, which is favorable for water oxidation. The enhanced hydrophilicity of 25 kGy C-irradiated RGO also increases hydroxide (OH−) adsorption and facilitates O2 desorption. As a result, at 1.0 V vs. Ag/AgCl, the ORR performance of the 25 kGy-GO catalyst was six times that of untreated RGO. Most recently, Rahman et al.98 synthesized N-doped rGO-supported iron catalysts with different iron loadings using γ-ray irradiation. The results show that γ-ray irradiation introduces both N and Fe into the samples while also generating structural defects. The strong interaction between Fe–Nx and O2 promotes rapid electron transfer. The ORR performance was further influenced by Fe loading, with higher Fe content introduced through irradiation leading to increased ORR activity.
4.2 Photocatalysis
Photocatalysis is a promising approach for producing low-cost hydrogen energy and decomposing organic pollutants.21 However, the limited number of active sites in photocatalysts creates a significant gap between the actual and theoretical efficiencies of photocatalysts. Thus, developing effective modification methods to enhance photocatalytic efficiency is essential to achieving optimal performance in photocatalytic hydrogen production and pollutant decomposition. Irradiation technology enables precise doping and defect control in photocatalysts, allowing for fine-tuning of their electronic structures and optimizing photocatalytic performance (Table 3).| Catalyst materials | Irradiation sources | Energy | Fluence | Roles | Application | Ref. |
|---|---|---|---|---|---|---|
| TiNRs, Ag-TiNRs, Au-TiNRs | Ion beam irradiation/P | 50 keV | 1 × 1014, 1 × 1015, 5 × 1014, 5 × 1015 ions per cm2 | Defect engineering | Degradation of methylene blue | 101 |
| TiO2 | γ-ray irradiation/60Co | 1.2 MeV | 7, 11 kGy | Band gap engineering | Degradation of methylene blue | 130 |
| ZIF-8@ZnO | Electron beam irradiation | 1.5 MeV | 50, 100, 250, 500, 750, 1000 kGy | Material synthesis and heterostructure formation | Degradation of methylene blue | 55 |
| TiO2/CNTs | Proton irradiation | 120 keV | 5 × 1014, 1 × 1016 cm−2 | Defect introduction | Degradation of methylene blue | 131 |
| TiO2 | Electron beam irradiation | 6 MeV | 5, 10, 20 kGy | Structural modification | Degradation of direct blue 1 | 132 |
| MoS2 | γ-ray irradiation/60Co | — | 1, 10, 100, 1000 kGy | Band gap engineering | Degradation of methylene blue | 133 |
| Pb0.98Cu0.01Sr0.01S | γ-ray irradiation/60Co | 25.2 MeV | 90 kGy | Band gap engineering and defect engineering | Degradation of methylene blue | 31 |
| NiO | γ-ray irradiation/60Co | 1.173, 1.332 MeV | 180 Gy to 10 kGy | Phase transformation | Photocatalytic | 83 |
| WO3 | Ion beam irradiation/He2+ | 40 keV | 1 × 1013–1015 ions per cm2 | Morphology control | Degradation of indigo carmine dye and Congo red | 83 |
| WO3 | Ion beam irradiation/He2+ | 40 keV | 1 × 1015 ion per cm2 | Morphology control | Degradation of Rhodamine B | 134 |
| ZnO | Ion beam irradiation/N | 100 keV | 5 × 1014, 1 × 1015, 5 × 1015 ion per cm2 | Band gap engineering and vacancy introduction | Degradation of methyl orange | 135 |
| In2O3:F | γ-ray irradiation/60Co | — | 1, 5, 10, 100 kGy | Band gap engineering and defect engineering | Degradation of methylene blue | 136 |
| Ag/Bi2WO6/CdWO4 | Electron beam irradiation | — | 100 kGy | Defect engineering | Degradation of carmine, rhodamine B and Cr6+ | 76 |
| rGO-TNTAs | γ-ray irradiation/60Co | — | 0, 10, 20, 30, 40 kGy | Reduce GO to partial rGO | Degradation of ethylene (C2H4) | 137 |
| COF | Electron beam irradiation | 1.5 MeV | 5, 100, 300, 500 kGy | Material synthesis | Photoelectrochemical water splitting | 64 |
| WO3 | Ion beam irradiation/Zr+ | 3 keV | 0.33 × 1015, 1 × 1015, 3 × 1015 ions per cm2 | Zr-doping and band gap engineering | Photoelectrochemical water splitting | 138 |
| TiO2 | Ion beam irradiation/Ar+ | 190 keV | 1 × 1012, 5 × 1012, 5 × 1013, 5 × 1014 ions per cm2 | Defect engineering | Photoelectrochemical water splitting | 139 |
| TiO2 nanorods | Ion beam irradiation/N+ | 65 keV | 1 × 1017 ions per cm2 | Nanostructure formation | Photoelectrochemical water splitting | 140 |
| TiO2 nanotubes | Ion beam irradiation/N | 60 keV | 1 × 1015, 1 × 1016 ions per cm2 | N-doping | Photoelectrochemical water splitting | 99 and 141 |
| ZnO nanorod arrays | Ion beam irradiation/Cu | 30 keV | 3 × 105, 5 × 1015, 2 × 1016 ions per cm2 | Doping | Photoelectrochemical water splitting | 107 |
| ZnO nanorod arrays | Ion beam irradiation/V | 50 keV | 2.5 × 1014–5 × 1015 ions per cm2 | Doping | Photoelectrochemical water splitting | 108 |
| TiO2 nanowire arrays | Ion beam irradiation/N | 30 keV | 7 × 1014, 1 × 1016 ions per cm2 | N-doping | Photoelectrochemical water splitting | 142 |
| ZnO nanorod arrays | Ion beam irradiation/N | 30 keV | 1.25 × 1014–5 × 1015 ions per cm2 | N-doping | Photoelectrochemical water splitting | 143 |
| TiO2 nanowire arrays | Ion beam irradiation/C, N | 25, 30 keV | 1 × 1015, 1 × 1016 ions per cm2 | Doping | Photoelectrochemical water splitting | 144 |
| α-Fe2O3 | Ion beam irradiation/Au | 30 keV | 1 × 1016, 2.5 × 1016, 5 × 1016, 1 × 1017 ions per cm2 | Heterostructure formation | Photoelectrochemical water splitting | 145 |
Secondly, the formed ZIF-8@ZnO heterostructure facilitates photogenerated charge separation. These results demonstrate that electron beam irradiation is a promising method for compositing ZIF-8 and forming ZIF-8@ZnO heterostructures, offering significant advantages over conventional solvothermal reactions. Wang et al.111 fabricated CdTe/ZnO heterostructures by N ion irradiation. The photocatalytic activity was improved after N ion irradiation treatment due to the introduction of oxygen vacancy defects. Lv et al.135 fabricated ZnO nanowires on Si substrates via a hydrothermal growth method, followed by an N ion irradiation treatment with an energy of 100 keV (fluences of 5 × 1014, 1 ×1015, and 5 × 1015 ions per cm2). Compared with the as-grown ZnO nanowires, the photocatalytic performance of ZnO heterostructures was improved by the reduction of the band gap and the generation of oxygen vacancies at 5 × 1015 ions per cm2. The reduction of the band gap promotes the transfer of electrons and holes, which improves the charge separation efficiency and visible light absorption. On the other hand, the N ion radiation increases the gap of oxygen vacancies and hinders the recombination of photoexcited electrons and holes. Gallegos et al.132 investigated the degradation of Direct Blue 1 by using a 6 MeV electron beam irradiation of TiO2. The 10 kGy irradiation of TiO2 allows DB1 to degrade more rapidly than the degradation with TiO2 irradiated at 0, 5, and 20 kGy. The absorption rate of the TiO2 irradiated at 10 kGy is lower than that of the other used materials (TiO2 irradiated at 0, 5, and 20 kGy). This is obviously favourable to the degradation of DB1, as excessive adsorption would prevent sufficient light from passing through the photocatalyst, resulting in a reduction in the rate of degradation.
Bamola et al.101 irradiated TiO2 nanorod (TiNR)-based hybrids (Ag-TiNRs and Au-TiNRs) with varying fluences of P ions at 50 keV. They found that Ag-TiNRs irradiated at a fluence of 5 × 1014 ions/cm2 degraded 78% of methyl bromide under visible light irradiation, outperforming samples irradiated at 1 × 1014 ions per cm2 (65%), 1 × 1015 ions per cm2 (74%), and 5 × 1015 ions per cm2 (68%). Notably, the best degradation performance was achieved with Au-TiNRs irradiated at 5 × 1014 ions per cm2, achieving an 84% degradation rate, which was superior to that of samples irradiated at 1 × 1014 ions per cm2 (80%), 1 × 1015 ions per cm2 (76%), and 5 × 1015 ions per cm2 (73%). The study also indicated that these hybrid nanostructures are modified at low fluence, while high fluence leads to structural damage. Low-fluence ion implantation of Ag-TiNRs and Au-TiNRs reduces surface states and enhances charge-harvesting efficiency, with less structural damage compared to high fluence. As a result, charge carriers are more effectively separated in low-fluence hybrid materials, thereby improving photocatalytic activity.
As discussed earlier, e-beam irradiation has proven to be an effective method for surface modification and the induction of microstructural evolution in electrocatalysts. Notably, this technique also has potential applications in the field of photocatalysts.147 For example, Latthe et al.148 altered the micro/nanostructure of photocatalysts by electron beam treatment. The catalytic activity of the irradiated photocatalysts in water splitting was greatly improved. Similarly, Chen et al.149,150 also demonstrated that the oxygen containing groups in the GNSs could be partially removed by medium electron beam irradiation, leading to a significant improvement of the lithium storage performance. In related work, the same group irradiated SnS2 quantum dots (QDs)/amino-functionalized graphene nanosheets (SAGNSs) with electron beams at doses of 70, 140, and 280 kGy.151 They observed that N-doped graphene nanosheets (NGNSs) were successfully prepared by the e-beam irradiation, and the microstructure was further refined. Additionally, it allowed precise control over the crystallinity and lattice defects in SnS2 QDs, influencing the amorphization of NGNSs and the surface oxygen-containing groups. The performance of SAGNSs and SnS2 quantum dots/N-doped graphene nanocomposites (SNGNSs) for degradation of methyl orange (MO) was evaluated under the same experimental conditions. The results showed that the photodegradation rate of SNGNSs reached 95.6% under the irradiation condition of 70 kGy, and that of SAGNSs reached 59.5% after 60 min. The rate constants of SNGNSs were 3.19 times higher than those of SAGNSs at 70 kGy. These findings indicate that e-beam irradiation is a versatile and effective method for the surface modification of graphene-based nanocomposites, enabling the construction of photocatalysts with better performance.
Xie et al.137 utilized γ-ray irradiation to reduce rGO and investigated the photocatalytic properties of rGO-modified TiO2 nanotube arrays (rGO-TNTAs) for ethylene degradation under UV light. It was found that the degradation rate (K) increased first, then decreased, and finally reached the highest value when the dose was 20 kGy. This trend corresponded with a decrease in the ID/IG ratio from 0.9174 to 0.8468. The enhanced photocatalytic activity was attributed to a strong interaction between TiO2 and rGO, facilitated by defects in the rGO structure. After the 20 kGy γ-ray irradiation, optimal changes occurred in the D band to G band intensity ratio of GO, resulting in a 40.9% increase in K for rGO-TNTAs compared to TNTAs following 120 keV proton beam irradiation. Simultaneously, the CNTs experienced damage, a reduction in tube diameter, and a decrease in the Raman peak ID/IG ratio with increased proton fluence. Notably, the nano-TiO2/CNT films demonstrated remarkable MB degradation performance under irradiation. Furthermore, CNTs coated with thinner TiO2 layers exhibited relatively improved photocatalytic activity, likely due to the crystallization of the TiO2 layer and the carrier-trapping effect of the CNTs.
Recently, a research group led by Martinez D conducted a series of experiments to study the photocatalytic properties of WO3 microparticles in organic dyes. They elucidated the size dependence of photocatalytic activity, showing that smaller grain sizes and larger active surface areas corresponded to increased photoactivity and reaction rates.152–155 In related work, Kozovskiy et al.156 studied the effect of low-energy WO3 microparticle irradiation on the structure and photocatalytic activity of organic dyes. Their findings showed that an increase in radiation dose led to small but significant changes in surface area. At a dose of 1013 ions/ per m2, there was a 3.7% increase in Brunauer–Emmett–Teller (BET) surface area. Further dose increases to 1014 and 1015 ions per cm2 resulted in larger increases of 13.8% and 28.1% in BET surface area, respectively. Photocatalytic testing revealed that the initial, non-irradiated WO3 microparticles achieved only 45–50% degradation of indigo carmine dye after 300 min. However, complete degradation was observed after 200 min for microparticles irradiated at 1013 ions per cm2, and after 270 min for those irradiated at 1014 ions per cm2. Notably, partial amorphization at 1015 ions per cm2 led to a reduction in degradation and a decrease in photocatalytic activity. Similarly, in the decomposition of Congo red dye, non-irradiated WO3 microparticles degraded no more than 20% of the initial dye concentration. In contrast, irradiation-modified particles achieved 45–50% degradation. Kozovskiy et al.134 further explored the effects of irradiation on WO3 microparticles in additional experiments. The effect of irradiation of commercial WO3 microparticles with low energy He ions toward Rhodamine B degradation was studied. For the initial microparticles without irradiation, reduction in the maximum absorption intensity takes place and the intensity gradually reaches the minimum after 300 min of testing, whereas in the case of the modified microparticles, the change of the solution is observed after 210 min. The rate of photocatalytic decomposition proceeds much more quickly for the irradiated samples, with a reduction in the band gap value and a shift in the fundamental absorption edge, indicating an obvious change in the electron density of catalysts.
Zhou et al.159 investigated the impact of N implantation on the structure of semiconducting TiO2 at 8 × 1014 ions per cm2 and 1 × 1016 ions per cm2 at 60 keV. At a low irradiation dose, it activated anatase TiO2 nanotubes for noble metal-free photocatalytic H2 generation. Low-dose nitrogen implantation increases the sub-bandgap response of the anatase, which reduces the charge-transfer resistance. However, high doses have an adverse effect on the photocurrent magnitude, leading to excessive charge transfer efficiency. The implantation of various elements in particulate photocatalysts has been shown to significantly enhance photocatalytic performance. Ion beam irradiation is also readily available for various doping modifications of photocatalysts. Additionally, ion implantation introduces oxygen vacancies that lead to an upward shift of the valence band maximum. These findings suggested that the upshift of the conduction and valence bands is beneficial for photoelectrochemical water splitting. Wang et al.107 successfully produced Cu-doped ZnO nanorod arrays under visible light using ion irradiation. The photocurrent density reached 18 μA cm−2 (with respect to the saturated mercuric glycol electrode), which is approximately 11 times higher than that of the undoped ZnO nanorod arrays (0.8 V).
Graphitic carbon nitride (g-C3N4) has been considered as an efficient metal-free catalyst for photocatalytic water splitting, but its performance is constrained by lower charge carrier separation efficiency and less visible light absorption. It has been reported that the visible light absorption and charge carrier separation efficiency of g-C3N4 can be enhanced by introducing carbon vacancies or nitrogen vacancies. There are several methods to produce N or C vacancies in g-C3N4, including hydrogen treatment, calcination in an Ar, N2, or NH3 atmosphere, and thermal polymerization at high temperatures or in strongly alkaline environments.42,160–163 However, the above common chemical methods can only produce one kind of vacancy (VC or VN) and it is very difficult to control its concentration. In addition, these processes are complex and cumbersome and may introduce impurities. To solve this problem, Wang et al.164 have developed an ion beam irradiation method to fabricate defective g-C3N4 nanosheets with the annealing process. They found that the concentration and distribution of incident vacancies can be tuned by incident ion beam energy and fluence. During ion irradiation, vacancies of carbon and nitrogen are simultaneously introduced into g-C3N4 due to the cascade collisions between irradiated ions and target atoms. DFT calculations presented in Fig. 10a–h demonstrate that the introduction of VC with irradiation leads to a narrowing of the bandgap, along with a shifted absorption edge. Local charge density (LDOS) analysis shows that electrons are more inclined around the VN, suggesting effective capture of photogenerated electrons by the VN. This defective state has the potential to improve the performance of photocatalysts and can be used as a high performance photocatalyst for PEC water decomposition.
 | ||
| Fig. 10 Structural models of g-C3N4: (a) pristine, (b) with carbon vacancies, (c) with nitrogen vacancies, and (d) with both carbon and nitrogen vacancies. (e–h) DOS of post-implantation annealed N–TiO2 and prepared N–TiO2. Copyright 2019, Wiley-VCH. (i) XPS spectra of N 1s. (j) Upper panel: XPS Ti 2p spectra for air-annealed pristine TiO2, as-prepared N-TiO2 and annealed N-TiO2. Lower panel: Ti 2p fitted curve for N–TiO2. (k) Relaxation structures of (1) pristine TiO2, (2) interstitial N-doped TiO2, (3) interstitial N-doped TiO2 and (4) substituted N-doped TiO2. (l) Calculated escape energies for various nitrogen chemical states.142,164 Copyright 2015, American Chemical Society. | ||
Wang et al.142 reported the enhancement in the performance of N–TiO2 for visible light-driven PEC water splitting through ion beam irradiation. As shown in Fig. 10i–l, the DFT calculations and XPS characterization revealed that the annealed substituted N dopants became the major introduced species. One can see that the annealed N+ implanted TiO2 exhibited an improved ability to absorb visible light. The optical band gap of the implanted N-TiO2 (2.34 eV) is significantly reduced compared to that of the pristine TiO2 (3.0 eV). On the other hand, the carrier injection efficiency is not significantly improved after N+ implantation, indicating that there is no obvious change in kinetics of the surface water oxidation. The results suggested that N+ ion injection improves the performance of the photoanode mainly by increasing the carrier separation efficiency. After the annealing process, the photocatalytic performance of N+ ion-incorporating TiO2 was improved. The photocurrent density of N-TiO2 after annealing was −1.92 mA cm−2 at 0.5 V vs. Ag/AgCl, which was 4 times higher than that of pure TiO2 nanowires and N+ implanted TiO2 nanowires without the annealing process (Fig. 11a–c). Remarkably, the incident photon-to-current efficiency (IPCE) of N+-implanted TiO2 at 450 nm reaches an impressive 17%, the highest reported among all studies on TiO2-based photoanodes for PEC water splitting without the addition of co-catalysts. However, excessive N+ fluence decreases the PEC performance and separation efficiency of photoexcited carriers at the optimal defect concentration. The samples were subsequently annealed.
 | ||
| Fig. 11 The PEC catalytic properties of TiO2, as-prepared N-TiO2 and annealed N-TiO2. (a) Photoabsorption properties, (b) linear swept voltammetry curves with a 100 mW cm−2 xenon lamp in a 1.0 M NaOH aqueous electrolyte, (c) IPCE spectra collected at 0.5 V vs. Ag/AgCl. The PEC catalytic properties of pristine Fe2O3, Au–Fe2O3-550, and Au–Fe2O3-700. Copyright 2015, American Chemical Society. (d) Mott–Schottky curves of BVO-A and the irradiated BiVO4 films in the dark. (e) EIS curves (inset: the equivalent circuit model) at 1.23 V vs. RHE under AM 1.5 G illumination.145,165 Copyright 2022, ACS Publications. | ||
Ion irradiation can be used to control the implanted elements, and multi-element doping can be achieved and multi-element synergistic modification can be realized. Song et al.144 reported for the first time the utilization of a C/N co-doping strategy to achieve the “Midas Touch” transformation of single-crystal linear rutile TiO2 NW arrays in visible-light PEC water separation. It was demonstrated that good synergistic interactions between C/N dopant atoms ensured a high concentration of substituted n-ti states and Ti3+ morphologies in the C/N co-doped TiO2 NW, which resulted in a significant enhancement of visible-light absorbance, charge separation and transfer efficiency. Therefore, the visible light photoactivity of C/N co-doped TiO2 nanocrystals is nearly 253 times higher than that of pure TiO2 nanocrystals. Moreover, C/N-TiO2 achieved high incident photon/electron conversion efficiency without using any other co-catalysts. The ion implantation doping combined with co-doping strategy has been demonstrated to increase the photoelectrochemical conversion and improve the PEC water separation performance of semiconductor photocatalysts. Wu et al.144 introduced oxygen vacancies into TiO2 by Ar+ ion irradiation, resulting in enhanced photocatalytic performance. They found that low concentrations of oxygen vacancies could form shallow donor energy levels in conduction bands, which can trap photogenerated electrons and release them into the conduction band. The oxygen vacancies generated through irradiation increased the carrier concentration and inhibited photogenerated carrier recombination, thereby improving the PEC water separation properties of TiO2.
Most recently, He et al.145 reported an effective route to improve the PEC water splitting properties of α-Fe2O3 by using Au ion irradiation followed by annealing. After annealing, Au atoms recrystallized, diffused and precipitated on the α-Fe2O3 nanorods to form Au nanoparticles, which promoted stronger contact between the formed Au particles and hematite, enhancing the surface charge injection (C–I) efficiency, which reached 89% at 1.5 V vs. RHE. Furthermore, the Au-implanted Fe2O3 photoelectrode exhibits high stability in a non-photocurrent decay test, with no noticeable photocurrent decay. This work suggests that heterogeneous structures, with embedded metal particles, can be readily formed on film surfaces through ion implantation and subsequent annealing, offering improved PEC performance.
Duan et al.165 fabricated nanoporous BiVO4 with abundant bulk oxygen vacancies through helium ion irradiation followed by vacuum annealing. Under optimal irradiation conditions, the PEC performance of the irradiated samples was significantly improved. As shown in Fig. 11d and e, with an ion flux of 5 × 1013 ions per cm2, the photocurrent density was significantly increased by about 70%, and the photonic current efficiency doubled compared to that of untreated BiVO4. The enhanced PEC performance is attributed to the formation of bulk oxygen vacancies, which improve conductivity, enhance electron–hole pair separation efficiency, and accelerate charge transfer. The results indicate that ion irradiation is a powerful tool to accurately tailor the electronic structure of BiVO4 by inducing oxygen vacancies, thus improving the PEC performance of the material. Moreover, this controllable technique can be further applied to other semiconductor photoelectrodes. The results provide strong evidence that ion beam technology can enhance PEC catalytic performance by manipulating the catalyst structure, implementing co-doping, and generating heterostructures. These findings underscore the importance of accurately controlling irradiation fluence to optimize PEC activity, offering valuable insights for developing advanced photocatalytic materials with tailored performance characteristics.
5 Future perspectives
In summary, this review provides a comprehensive overview of the characteristics of ionizing and non-ionizing radiation, highlighting their advantages over conventional methods for the efficient and rapid synthesis of photo-/electrocatalysts. Additionally, a detailed summary of recent advances in ionizing irradiation for renewable energy materials, particularly in electrocatalytic and photocatalytic applications, is presented. Ionizing radiation enables heteroatom-doping, defect engineering and heterostructure formation. Tables 2 and 3 provide a summary of irradiation applications in the modification and optimization of catalytic materials. Radiation technology has emerged as a promising and efficient tool with applications that can extend to various other fields.(1) Some studies have demonstrated that irradiation technology, different from other chemical methods, can introduce defects within catalysts. Therefore, it becomes crucial to perform targeted simulation calculations of the irradiation effect to guide and predict various doping elements and defect control strategies. SRIM calculations have been employed to simulate the penetration depth of ion beam irradiation in materials. However, accurately determining the penetration depths of electron beams, proton beams, and gamma rays remains a challenge and often requires Monte Carlo simulation methods.
(2) Irradiation has proven to be a highly precise and advanced technique for catalyst modification. For example, it enables the determination of the structure–activity relationship between defect concentration and catalytic properties. However, the underlying mechanisms of the interaction between irradiation processes and structural changes in catalysts require further investigation. The use of in situ characterization technology, such as in situ TEM and Raman measurements, can provide valuable insights into the continuous atomic structural changes in catalyst materials and their intrinsic relationship with the evolution of catalytic properties.
(3) Currently, the modification of catalysts by irradiation primarily relies on a single type of irradiation. This, while effective, may have limitations in fully optimizing catalytic performance for specific applications. By strategically choosing different irradiation types and combining them synergistically, more approaches could be achieved. This will open up the potential to develop novel catalysts with better performance, allowing for applications in new scenarios. For example, such advancements could address critical processes like carbon dioxide reduction and nitrogen fixation, providing pathways for the creation of advanced materials that meet the evolving demands of diverse catalytic applications and align with sustainability goals.
(4) At present, the modification of catalysts through irradiation primarily revolves around precious metal-based materials, due to their unique catalytic properties and broad applications. The continuous improvements in equipment and characterization techniques are paving the way for innovative approaches such as the development of single-atom catalysts. It holds the potential to embrace various catalyst types with tailored structures and enhanced performances, which may revolutionize diverse catalytical applications and contribute to sustainable advancements in materials science.
(5) With the continuous development of various accelerator technologies, the utilization of proton irradiation and neutron irradiation will play an important role in practical industrial production. The high throughput and cost-effectiveness of irradiation technology make it an attractive option for large-scale applications. Its unique ability to modify catalysts at multiple levels, including atomic and molecular levels, presents vast opportunities for improving product quality and reducing costs. This transformative capability contributes significantly to advances in efficiency, sustainability, and innovation across various sectors. The impact of these advanced irradiation techniques on catalysis production needs further exploration and in-depth research. Understanding how these techniques can be harnessed to enhance the catalytic performance of catalysts will undoubtedly uncover new possibilities, driving the evolution of industrial catalysis and paving the way for more sustainable and efficient production methods.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the National Natural Science Foundation of China (12275168), the Science and Technology Commission of Shanghai Municipality (21010500300), Carl Tryggers Foundation for Scientific Research (CTS 24:3314, 23:2433 and 22:2365), STINT Joint China-Sweden Mobility Project (CH2017-7243), and Swedish Government Strategic Research Area in Materials Science on Advanced Functional Materials at Linköping University (SFO-Mat-LiU, No. 2009 0097).Notes and references
- P. Zhou, I. A. Navid, Y. J. Ma, Y. X. Xiao, P. Wang, Z. W. Ye, B. W. Zhou, K. Sun and Z. T. Mi, Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting, Nature, 2023, 613, 66–70 CrossRef CAS PubMed.
- Y. F. Xu, M. Z. Yang, B. X. Chen, X. D. Wang, H. Y. Chen, D. B. Kuang and C. Y. Su, A CsPbBr3 Perovskite Quantum Dot/Graphene Oxide Composite for Photocatalytic CO2 Reduction, J. Am. Chem. Soc., 2017, 139, 5660–5663 CAS.
- M. Y. Ye, Z. H. Zhao, Z. F. Hu, L. Q. Liu, H. M. Ji, Z. R. Shen and T. Y. Ma, 0D/2D Heterojunctions of Vanadate Quantum Dots/Graphitic Carbon Nitride Nanosheets for Enhanced Visible-Light-Driven Photocatalysis, Angew. Chem., Int. Ed., 2017, 56, 8407–8411 CrossRef CAS PubMed.
- F. Guo, T. J. Macdonald, A. J. Sobrido, L. X. Liu, J. R. Feng and G. J. He, Recent Advances in Ultralow-Pt-Loading Electrocatalysts for the Efficient Hydrogen Evolution, Adv. Sci., 2023, 10, 2301098 CrossRef CAS.
- R. Balaji, T. T. Nguyen, M. P. Austeria, D. Kim, J. H. Lee and N. H. Kim, Electronic coupling coordinated vanadium nitride/magnesium oxide hetero-junction for accelerating oxygen reaction and long-life flexible zinc-air batteries, Appl. Catal., B, 2023, 335, 122895 CrossRef CAS.
- J. X. Guo, Y. Zheng, Z. P. Hu, C. Y. Zheng, J. Mao, K. Du, M. Jaroniec, S. Z. Qiao and T. Ling, Direct seawater electrolysis by adjusting the local reaction environment of a catalyst, Nat. Energy, 2023, 8, 264–272 CAS.
- C. Y. Wang, L. C. Yu, F. L. Yang and L. G. Feng, MoS2 nanoflowers coupled with ultrafine Ir nanoparticles for efficient acid overall water splitting reaction, J. Energy Chem., 2023, 87, 144–152 CrossRef CAS.
- T. Hou, T. R. Wei, Y. Y. Wu, L. Zhang, J. Y. Ding, Q. Liu, L. G. Feng and X. J. Liu, FeCu bimetallic clusters for efficient urea production via coupling reduction of carbon dioxide and nitrate, J. Colloid Interface Sci., 2024, 674, 834–840 CrossRef CAS.
- Y. Zhang, Z. H. Li, K. Chen, X. Yang, H. Zhang, X. J. Liu and K. Chu, Promoting Electroreduction of CO2 and NO3- to Urea via Tandem Catalysis of Zn Single Atoms and InO2O3-x, Adv. Energy Mater., 2024, 1614–6832 Search PubMed.
- Y. H. Wang, X. Cheng, K. Zhang, G. Chen, R. Z. Wang and J. J. Zhang, Ion-irradiation of catalyst and electrode materials for water electrolysis/photoelectrolysis cells, rechargeable batteries, and supercapacitors, Adv. Mater., 2022, 3, 7384–7405 RSC.
- S. S. Huang, X. S. Cong, T. Ye, L. B. Liu, K. M. Peng, L. C. Zhang, J. M. Bao, P. Y. Gao, Q. C. Chen and Q. Q. He, Colloidal synthesis of hexagonal CuFe(SxSe1-x)2 nanoplates with exposed highly active (220) facets for boosting overall water splitting, Inorg. Chem. Front., 2023, 10, 2387–2398 RSC.
- Y. Y. Yang, R. G. Wang, L. J. Yang, Y. Jiao and T. Ling, Two dimensional electrocatalyst engineering via heteroatom doping for electrocatalytic nitrogen reduction, Chem. Commun., 2020, 56, 14154–14162 RSC.
- S. B. Lee, J. W. Jung and H. N. Han, Transition to body-centered cubic structure in Au thin films under electron-beam irradiation, Acta Mater., 2023, 247, 118759 CrossRef CAS.
- P. Garrido-Barros, J. Derosa, M. J. Chalkley and J. C. Peters, Tandem electrocatalytic N2 fixation via proton-coupled electron transfer, Nature, 2022, 609, 71 CrossRef CAS.
- Y. B. Kuang, W. Qiao, F. L. Yang and L. G. Feng, Electrochemical hydrogen evolution efficiently boosted by interfacial charge redistribution in Ru/MoSe2 embedded mesoporous hollow carbon spheres, J. Energy Chem., 2023, 85, 447–454 CrossRef CAS.
- K. Guo, A. Baidak and Z. X. Yu, Recent advances in green synthesis and modification of inorganic nanomaterials by ionizing and non-ionizing radiation, J. Mater. Chem. A, 2020, 8, 23029–23058 RSC.
- H. Han, A. Sharma, J. Yoon, Z. Wang, C. Körner, H. Deniz, A. K. Sharma, F. Li, C. Sturm, G. Woltersdorf and S. S. P. Parkin, All-Oxide Metasurfaces Formed by Synchronized Local Ionic Gating, Adv. Mater., 2024, 36, 2401064 CrossRef CAS.
- Z. Y. Yuan, W. Z. Zhou, V. Parvulescu and B. L. Su, Electron beam irradiation effect on nanostructured molecular sieve catalysts, J. Electron Spectrosc. Relat. Phenom., 2003, 129, 189–194 CrossRef CAS.
- Z. Q. Li and F. Chen, Ion beam modification of two-dimensional materials: Characterization, properties, and applications, Appl. Phys. Rev., 2017, 4, 11103 Search PubMed.
- K. Satoh and Y. Oono, Studies on Application of Ion Beam Breeding to Industrial Microorganisms at TIARA, Quantum Beam Sci., 2019, 3, 11 CrossRef CAS.
- X. N. Wang, W. J. Wan, S. H. Shen, H. Y. Wu, H. Z. Zhong, C. Z. Jiang and F. Ren, Application of ion beam technology in (photo)electrocatalytic materials for renewable energy, Appl. Phys. Rev., 2020, 7, 041303 CAS.
- M. Migdal, E. Balcer, L. Bartosik, L. Bak, A. Celinska, J. Cybowska, K. Dobrzelewski, J. Jaroszewicz, K. Jezierski, N. Knake, W. Kubinski, J. Lechniak, M. Lipka, G. Madejowski, A. Malkiewicz, L. Murawski, I. Owsianko, B. Piwowarski, R. Prokopowicz, A. Talarowska, E. Wilinska, T. Witkowski, P. Witkowski, G. Wojtania and M. Wojcik, MARIA Reactor Irradiation Technology Capabilities towards Advanced Applications, Energies, 2021, 14, 8153 CrossRef CAS.
- A. V. Krasheninnikov and F. Banhart, Engineering of nanostructured carbon materials with electron or ion beams, Nat. Mater., 2007, 6, 723–733 CrossRef CAS.
- S. Conrad, P. Kumar, F. Xue, L. M. Ren, S. Henning, C. H. Xiao, K. A. Mkhoyan and M. Tsapatsis, Controlling Dissolution and Transformation of Zeolitic Imidazolate Frameworks by using Electron-Beam-Induced Amorphization, Angew. Chem., Int. Ed., 2018, 57, 13592–13597 CrossRef CAS PubMed.
- S. Ghosh, H. Yun, P. Kumar, S. Conrad, M. Tsapatsis and K. A. Mkhoyan, Two Distinct Stages of Structural Modification of ZIF-L MOF under Electron-Beam Irradiation, Chem. Matter., 2021, 33, 5681–5689 CrossRef CAS.
- Q. L. Chen, C. Dwyer, G. Sheng, C. Z. Zhu, X. N. Li, C. L. Zheng and Y. H. Zhu, Imaging Beam-Sensitive Materials by Electron Microscopy, Adv. Mater., 2020, 32, 1907619 CrossRef CAS.
- B. B. Jin, D. Zhao, F. Liang, L. F. Liu, D. L. Liu, P. Wang and M. Qiu, Electron-Beam Irradiation Induced Regulation of Surface Defects in Lead Halide Perovskite Thin Films, Research, 2021, 9797085 Search PubMed.
- Z. Y. He, R. Zhao, X. F. Chen, H. J. Chen, Y. M. Zhu, H. M. Su, S. X. Huang, J. M. Xue, J. F. Dai, S. Cheng, M. L. Liu, X. W. Wang and Y. Chen, Defect Engineering in Single-Layer MoS2 Using Heavy Ion Irradiation, ACS Appl. Mater. Interfaces, 2018, 10, 42524–42533 CrossRef CAS.
- Y. F. Zhang, J. Shi, C. Chen, N. Li, Z. W. Xu, L. S. Liu, L. H. Zhao, J. Li and M. L. Jing, Structural evolution of defective graphene under heat treatment and gamma irradiation, Phys. E, 2018, 97, 151–154 CrossRef CAS.
- A. Aridi, D. Naoufal, H. El-Rassy and R. Awad, Photocatalytic activity of ZnFeO/NiO nanocomposites carried out under UV irradiation, Ceram. Int., 2022, 48, 30905–30916 CrossRef CAS.
- P. Jeya, S. Keerthana, L. Kungumadevi, R. Yuvakkumar, G. Ravi, A. Kandasami and T. S. Senthil, Gamma irradiation effect on photocatalytic properties of Cu and Sr ions codoped PbS, Environ. Res., 2023, 226, 115651 CrossRef CAS.
- L. Dong, J. Q. Yang, X. Q. Yue, H. M. Geng, W. Q. Li, Y. B. Zhang and X. J. Li, Effects of Co-60 gamma-ray irradiation of thin-layer molybdenum disulfide for the hydrogen evolution reaction, New J. Chem., 2023, 47, 8214–8222 RSC.
- X. H. Fei, W. B. Jia, J. Q. Wang, T. Chen and Y. S. Ling, Study on enzymatic hydrolysis efficiency and physicochemical properties of cellulose and lignocellulose after pretreatment with electron beam irradiation, Int. J. Biol. Macromol., 2020, 145, 733–739 CrossRef CAS PubMed.
- Y. Liu, L. Zhou, F. Ding, S. S. Li, R. Li, Z. G. Li, D. Huang and X. H. Ren, Flame-retardant cotton fabrics modified with phosphoramidate derivative via electron beam irradiation process, J. Ind. Text., 2021, 51, 396–408 CrossRef CAS.
- T. Abou Elmaaty, S. Okubayashi, H. Elsisi and S. Abouelenin, Electron beam irradiation treatment of textiles materials: a review, J. Polym. Res., 2022, 29, 117 CrossRef CAS.
- S. Yeo, J. Han, S. Bae and D. S. Lee, Coherence in defect evolution data for the ion beam irradiated graphene, Sci. Rep., 2018, 8, 13973 CrossRef PubMed.
- R. C. Ramola, S. Negi, R. C. Singh and F. Singh, Gas sensing response of ion beam irradiated Ga-doped ZnO thin films, Sci. Rep., 2022, 12, 22351 CrossRef CAS.
- Z. X. Qu, C. J. Yu, Y. T. Wei, X. P. Su and A. B. Du, Thermal conductivity of boron carbide under fast neutron irradiation, J. Adv. Ceram., 2022, 11, 482–494 CrossRef CAS.
- D. Nesheva, Electron and Neutron Beam Irradiation Effects in Homogeneous and Nanostructured Oxides, ACS Omega, 2023, 8, 12603–12612 CrossRef CAS.
- Y. Choi, D. Lim, E. Oh, C. Lim and S. H. Baeck, Effect of proton irradiation on electrocatalytic properties of MnO2 for oxygen reduction reaction, J. Mater. Chem. A, 2019, 7, 11659–11664 RSC.
- S. Chauhan, T. Mori, T. Kobayashi, S. Yamamoto, S. Ito, G. Auchterlonie, R. Wepf, S. Ueda and F. Ye, Surface layer of Pt-O-Ce bonds on CeOx-nanowire with high ORR activity converted by proton beam irradiation, J. Am. Chem. Soc., 2021, 104, 1945–1952 CAS.
- Y. F. Li, M. Yang, Y. Xing, X. C. Liu, Y. Yang, X. Wang and S. Y. Song, Preparation of Carbon-Rich g-C3N4 Nanosheets with Enhanced Visible Light Utilization for Efficient Photocatalytic Hydrogen Production, Small, 2017, 13, 1701552 CrossRef.
- L. Chetibi, D. Hamana, M. M. Silvan and S. Achour, Electrochemical synthesis of graphite nanorings under UV irradiation, Mater. Lett., 2024, 372, 137024 CrossRef CAS.
- R. Sharma, S. Gyergyek and S. M. Andersen, Microwave-Assisted Scalable Synthesis of Pt/C: Impact of the Microwave Irradiation and Carrier Solution Polarity on Nanoparticle Formation and Aging of the Support Carbon, ACS Appl. Energy Mater., 2022, 5, 705–716 CrossRef CAS.
- Y. M. Sun, X. L. Hu, W. Luo, H. H. Xu, C. C. Hu and Y. H. Huang, Synthesis of Amorphous FeOOH/Reduced Graphene Oxide Composite by Infrared Irradiation and Its Superior Lithium Storage Performance, ACS Appl. Mater. Interfaces, 2013, 5, 10145–10150 CrossRef CAS.
- H. Fujita, M. Izawa and H. Yamazaki, γ-Ray-induced Formation of Gold Sol from Chloroauric Acid Solution, Nat. Commun., 1962, 196, 666–667 CrossRef CAS.
- W. Wang, F. T. Yao, M. Zeng, M. F. Pei, C. Y. Min, Z. W. Xu, R. Q. Shao, S. K. Liu, H. T. Shi and Y. H. Xia, Sp-nitrogen and gamma-ray modulating multiply gamma-graphyne for anchoring Pt nanoparticles to boost oxygen reduction activity and stability, Appl. Mater., 2022, 29, 101626 Search PubMed.
- Z. Y. Zhang, T. M. Nenoff, J. Y. Huang, D. T. Berry and P. P. Provencio, Room Temperature Synthesis of Thermally Immiscible Ag-Ni Nanoalloys, J. Phys. Chem. C, 2009, 113, 1155–1159 CrossRef CAS.
- F. Ksar, L. Ramos, B. Keita, L. Nadjo, P. Beaunier and H. Remita, Bimetallic Palladium-Gold Nanostructures: Application in Ethanol Oxidation, Chem. Matter., 2009, 21, 3677–3683 CrossRef CAS.
- S. Kianfar, A. N. Golikand and B. Zarenezhad, Experimental and artificial intelligence for modeling the cyclic voltammogram behavior of Pt/reduced graphene oxide nanocatalyst synthesized using gamma irradiation at different experimental conditions of graphene oxide, J. Solid State Electrochem., 2022, 26, 2195–2207 CrossRef CAS.
- D. C. Clifford, C. E. Castano and J. V. Rojas, Supported transition metal nanomaterials: Nanocomposites synthesized by ionizing radiation, Radiat. Phys. Chem., 2017, 132, 52–64 CrossRef CAS.
- H. Y. Park, D. S. Yang, D. Bhattacharjya, M. Y. Song and J. S. Yu, A highly efficient carbon-supported Pt electrocatalyst prepared by γ-irradiation for cathodic oxygen reduction, Int. J. Hydrogen Energy, 2014, 39, 1688–1697 CrossRef CAS.
- Y. C. Wang, S. X. Li, X. Y. Que, Z. Y. Zhang, L. Xu, Y. Wang, J. Peng, J. Q. Li, S. L. Hu, Y. Y. Ao and M. L. Zhai, Low loading of Pt in radiation-synthesized Pt-MoS/KB nanocomposites for enhancing the electrocatalytic hydrogen evolution reaction, J. Mater. Chem. A, 2024, 12, 18476–18486 RSC.
- C. Lee, Y. R. Uhm, G. M. Sun, H. Choi-Yim, J. H. Park and T. Ha, Synthesis of Electrocatalyst FePt@Pt/C Using Electron Beam Irradiation, Phys. Status Solidi A, 2024, 221, 2300941 CrossRef CAS.
- J. C. Chen, M. X. Zhang, S. T. Zhang, K. C. Cao, X. Z. Mao, M. J. Zhang, L. W. He, X. Dong, J. Shu, H. C. Dong, F. W. Zhai, R. F. Shen, M. J. Yuan, X. F. Zhao, G. Z. Wu, Z. F. Chai and S. Wang, Metal-Organic Framework@Metal Oxide Heterostructures Induced by Electron-Beam Radiation, Angew. Chem., Int. Ed., 2022, 61, 202212532 CrossRef.
- M. X. Zhang, J. C. Chen, X. F. Zhao, X. Z. Mao, C. Y. Li, J. Diwu, G. Z. Wu, Z. F. Chai and S. O. Wang, A MOF@Metal Oxide Heterostructure Induced by Post-Synthetic Gamma-Ray Irradiation for Catalytic Reduction, Angew. Chem., Int. Ed., 2024, 63, e202405213 CrossRef CAS PubMed.
- Q. Zhang, S. Y. Ye, X. M. Chen, X. L. Song, L. Q. Li and X. Huang, Photocatalytic degradation of ethylene using titanium dioxide nanotube arrays with Ag and reduced graphene oxide irradiated by γ-ray radiolysis, Appl. Catal., B, 2017, 203, 673–683 CrossRef CAS.
- K. Naghavi, E. Saion, K. Rezaee and W. M. M. Yunus, Influence of dose on particle size of colloidal silver nanoparticles synthesized by gamma radiation, Radiat. Phys. Chem., 2010, 79, 1203–1208 CrossRef CAS.
- P. A. Wiguna, D. Djuhana, C. Imawan and A. Umar, Physicochemical properties of colloidal Ag/PVA nanoparticles synthesized by gamma irradiation, J. Phys.: Conf. Ser., 2020, 1428, 012022 CrossRef CAS.
- Z. I. Ali, O. A. Ghazy, G. Meligi, H. H. Saleh and M. Bekhit, Radiation-Induced Synthesis of Copper/Poly(vinyl alcohol) Nanocomposites and Their Catalytic Activity, Adv. Polym. Technol., 2018, 37, 365–375 CrossRef CAS.
- D. P. Kepic, A. M. Stefanovic, M. D. Budimir, V. B. Pavlovic, A. Bonasera, M. Scopelliti and B. M. Todorovic-Markovic, Gamma rays induced synthesis of graphene oxide/gold nanoparticle composites: structural and photothermal study, Radiat. Phys. Chem., 2023, 202, 110545 CrossRef CAS.
- J. H. Park, H. W. Kim, H. S. Kang, Y. H. Koo and B. C. Lee, Size control of copper nanoparticle by electron beam irradiation, Mater. Res. Innovations., 2014, 18, 678–684 CrossRef.
- T. D. Nguyen, J. D. Kim, M. G. So and K. S. Kim, Experimental measurements of gold nanoparticle nucleation and growth by citrate reduction of HAuCl4, Adv Powder Technol., 2010, 21, 111–118 CrossRef.
- M. X. Zhang, J. C. Chen, S. T. Zhang, X. Q. Zhou, L. W. He, M. V. Sheridan, M. J. Yuan, M. J. Zhang, L. Chen, X. Dai, F. Y. Ma, J. D. Wang, J. T. Hu, G. Z. Wu, X. Q. Kong, R. H. Zhou, T. E. Albrecht-Schmitt, Z. F. Chai and S. Wang, Electron Beam Irradiation as a General Approach for the Rapid Synthesis of Covalent Organic Frameworks under Ambient Conditions, J. Am. Chem. Soc., 2020, 142, 9169–9174 CrossRef CAS.
- T. M. Tang, Z. L. Wang and J. Q. Guan, A review of defect engineering in two-dimensional materials for electrocatalytic hydrogen evolution reaction, Chin. J. Catal., 2022, 43, 636–678 CrossRef CAS.
- J. Li, G. S. Li, J. H. Wang, C. L. Xue, X. S. Li, S. Wang, B. Q. Han, M. Yang and L. P. Li, A novel core-double shell heterostructure derived from a metal-organic framework for efficient HER, OER and ORR electrocatalysis, Inorg. Chem. Front., 2020, 7, 191–197 RSC.
- F. Banhart, Irradiation effects in carbon nanostructures, Rep. Prog. Phys., 1999, 62, 1181–1221 CrossRef CAS.
- J. R. Mravik, I. Milanovic, S. M. Govedarovic, A. Mrakovic, E. Korneeva, I. S. Simatovic and S. Kurko, Improvement of MoS2 electrocatalytic activity for hydrogen evolution reaction by ion irradiation, Int. J. Hydrogen Energy, 2023, 48, 38676–38685 CrossRef.
- J. Luxa, V. Mazanek, A. Mackova, P. Malinsky, S. Akhmadaliev and Z. Sofer, Tuning of electrocatalytic properties of MoS2 by chalcogenide ion implantation, Appl. Mater., 2019, 14, 216–223 Search PubMed.
- D. He, X. Y. Song, W. Q. Li, C. Y. Tang, J. C. Liu, Z. J. Ke, C. Z. Jiang and X. H. Xiao, Active Electron Density Modulation of Co3O4-Based Catalysts Enhances their Oxygen Evolution Performance, Angew. Chem., Int. Ed., 2020, 59, 6929–6935 CrossRef CAS.
- L. Q. Huang, Q. T. Wang, H. P. Liu, Y. X. Wu, Y. X. Yang, G. F. Ma, Z. Q. Lei and S. F. Ren, N plus irradiation regulates surface defects and doping towards efficient hydrogen evolution reaction on Sb2Te3, Appl. Surf. Sci., 2023, 609, 155347 CrossRef CAS.
- Y. J. Sun, Y. X. Liang, M. C. Luo, F. Lv, Y. N. Qin, L. Wang, C. Xu, E. G. Fu and S. J. Guo, Defects and Interfaces on PtPb Nanoplates Boost Fuel Cell Electrocatalysis, Small, 2018, 14, 1702259 CrossRef.
- Y. X. Liang, Y. J. Sun, X. Y. Wang, E. G. Fu, J. Zhang, J. L. Du, X. D. Wen and S. J. Guo, High electrocatalytic performance inspired by crystalline/amorphous interface in PtPb nanoplate, Nanoscale, 2018, 10, 11357–11364 RSC.
- L. Dong, J. Q. Yang, X. Q. Yue, S. L. Dong, W. Q. Li and X. J. Li, Defect Engineering of MoS2 Nanosheets by Heavy-Ion Irradiation for Hydrogen Evolution, ACS Appl. Nano Mater., 2023, 6, 18858–18868 CrossRef CAS.
- J. F. Felix, A. F. da Silva, S. W. da Silva, F. Qu, B. Qiu, J. F. Ren, W. M. de Azevedo, M. Henini and C. C. Huang, A comprehensive study on the effects of gamma radiation on the physical properties of a two-dimensional WS2 monolayer semiconductor, Nanoscale Horiz., 2020, 5, 259–267 RSC.
- R. R. Sun, J. Wang, T. L. Yang, R. He, K. H. Xue, L. Wang, X. L. Yu, J. T. Wang, T. Yang and W. L. Wang, Electron beam irradiation treatment of Ag/Bi2WO6/CdWO4 heterogeneous material with enhanced photocatalytic activity, New J. Chem., 2019, 43, 13764–13774 RSC.
- R. F. Zhang, C. Y. Qi, X. P. Gao, Y. Y. Li and B. Wang, FeNi3 nanosheets with multiple defects induced by H plus -ion irradiation show enhanced electrocatalytic action during the oxygen evolution reaction, FlatChem, 2024, 45, 100649 CrossRef CAS.
- X. L. Wu, X. J. Zheng, G. B. A. Zhang, X. N. Chen and J. W. Ding, Tightly-bound trion and bandgap engineering via gamma-ray irradiation in the monolayer transition metal dichalcogenide WSe2, Nanotechnology, 2021, 32, 1361–6528 Search PubMed.
- G. Y. Zhao, H. Deng, N. Tyree, M. Guy, A. Lisfi, Q. Peng, A. A. Yan, C. Wang and Y. Lan, Recent Progress on Irradiation-Induced Defect Engineering of Two-Dimensional 2H-MoS2 Few Layers, Appl. Sci., 2019, 9, 648 CrossRef.
- C. P. Chavda, A. Srivastava, E. Vaughan, J. W. Wang, M. R. Gartia and G. Veronis, Effect of gamma irradiation on the physical properties of MoS2 monolayer, Phys. Chem. Chem. Phys., 2023, 25, 22359–22369 RSC.
- L. Dong, J. Q. Yang, X. Q. Yue, W. Q. Li, Y. H. Jing, Y. B. Zhang and X. J. Li, The effects of the fluence of electron irradiation on the structure and hydrogen evolution reaction performance of molybdenum disulfide, J. Mater. Chem. C, 2022, 10, 7839–7848 RSC.
- X. Q. Yue, J. Q. Yang, W. Q. Li, Y. H. Jing, L. Dong, Y. B. Zhang and X. J. Li, Electron Irradiation Induces the Conversion from 2H-WSe2 to 1T-WSe2 and Promotes the Performance of Electrocatalytic Hydrogen Evolution, ACS Sustain. Chem. Eng., 2022, 10, 2420–2428 CrossRef CAS.
- K. Jouini, A. Raouafi, W. Dridi, M. Daoudi, A. B. Mustapha, R. Chtourou and F. Hosni, Investigation of gamma-ray irradiation induced phase change from NiO to Ni2O3 for enhancing photocatalytic performance, Optik, 2019, 195, 163109 CrossRef CAS.
- A. Kozlovskiy, M. Zdorovets, I. Kenzhina, A. Berguzinov, D. Tishkevich, T. Zubar and A. Trukhanov, The study of the applicability of ionizing radiation to increase the photocatalytic activity of TiO2 thin films, J. Nanostruct. Chem., 2020, 10, 331–346 CrossRef CAS.
- Y. C. Liu, S. H. Shen, F. Ren, J. N. Chen, Y. M. Fu, X. D. Zheng, G. X. Cai, Z. Xing, H. Y. Wu and C. Z. Jiang, Fabrication of porous TiO2 nanorod array photoelectrodes with enhanced photoelectrochemical water splitting by helium ion implantation, Nanoscale, 2016, 8, 10642–10648 RSC.
- H. Y. Wu, L. Wu, S. H. Shen, Y. C. Liu, G. X. Cai, X. N. Wang, Y. H. Qiu, H. Z. Zhong, Z. Xing, J. Tang, Z. Q. Dai, C. Z. Jiang and F. Ren, Enhanced photoelectrochemical performance of an alpha-Fe2O3 nanorods photoanode with embedded nanocavities formed by helium ions implantation, Int. J. Hydrogen Energy, 2020, 45, 9408–9415 CrossRef CAS.
- V. Stará, P. Procházka, D. Marecek, T. Sikolaa and J. Cechal, Ambipolar remote graphene doping by low-energy electron beam irradiation, Nanoscale, 2018, 10, 17520–17524 RSC.
- Y. Ling, Z. H. Yang, Q. Zhang, Y. F. Zhang, W. W. Cai and H. Cheng, A self-template synthesis of defect-rich WS2 as a highly efficient electrocatalyst for the hydrogen evolution reaction, Chem. Commun., 2018, 54, 2631–2634 RSC.
- D. Escalera-Lopez, R. Griffin, M. Isaacs, K. Wilson, R. E. Palmer and N. V. Rees, Electrochemical sulfidation of WS2 nanoarrays: Strong dependence of hydrogen evolution activity on transition metal sulfide surface composition, Electrochem. Commun., 2017, 81, 106–111 CrossRef CAS.
- J. J. Duan, S. Chen, B. A. Chambers, G. G. Andersson and S. Z. Qiao, 3D WS2 Nanolayers@Heteroatom-Doped Graphene Films as Hydrogen Evolution Catalyst Electrodes, Adv. Mater., 2015, 27, 4234–4241 CrossRef CAS PubMed.
- D. Q. Gao, B. R. Xia, C. R. Zhu, Y. H. Du, P. X. Xi, D. S. Xue, J. Ding and J. Wang, Activation of the MoSe2 basal plane and Se-edge by B doping for enhanced hydrogen evolution, J. Mater. Chem. A, 2018, 6, 510–515 RSC.
- Y. Yin, Y. M. Zhang, T. L. Gao, T. Yao, X. H. Zhang, J. C. Han, X. J. Wang, Z. H. Zhang, P. Xu, P. Zhang, X. Z. Cao, B. Song and S. Jin, Synergistic Phase and Disorder Engineering in 1T-MoSe2 Nanosheets for Enhanced Hydrogen-Evolution Reaction, Adv. Mater., 2017, 29, 1700311 CrossRef.
- X. Shang, K. L. Yan, Z. Z. Liu, S. S. Lu, B. Dong, J. Q. Chi, X. Li, Y. R. Liu, Y. M. Chai and C. G. Liu, Oxidized carbon fiber supported vertical WS2 nanosheets arrays as efficient 3 D nanostructure electrocatalyts for hydrogen evolution reaction, Appl. Surf. Sci., 2017, 402, 120–128 CrossRef CAS.
- L. Madauss, I. Zegkinoglou, H. V. Muinos, Y. W. Choi, S. Kunze, M. Q. Zhao, C. H. Naylor, P. Ernst, E. Pollmann, O. Ochedowski, H. Lebius, A. Benyagoub, B. Ban-d'Etat, A. T. C. Johnson, F. Djurabekova, B. Roldan Cuenya and M. Schleberger, Highly active single-layer MoS2 catalysts synthesized by swift heavy ion irradiation, Nanoscale, 2018, 10, 22908–22916 RSC.
- D. L. Cao, T. M. Zhang, J. Zeng, L. Cai, X. F. Pu, J. M. Qian, D. Q. Gao and J. Liu, Fe13+-ion irradiated WS2 with multi-vacancies and Fe dopants for hydrogen evolution reaction, FlatChem, 2021, 27, 2452–2627 CrossRef.
- H. Z. Zhong, G. P. Gao, X. N. Wang, H. Y. Wu, S. H. Shen, W. B. Zuo, G. X. Cai, G. Wei, Y. Shi, D. J. Fu, C. Z. Jiang, L. W. Wang and F. Ren, Ion Irradiation Inducing Oxygen Vacancy-Rich NiO/NiFe2O4 Heterostructure for Enhanced Electrocatalytic Water Splitting, Small, 2021, 17, 2103501 CrossRef CAS.
- W. T. Huang, Q. W. Zhou, S. Q. Su, J. Li, X. B. Lu, X. S. Gao, X. Wang, M. L. Jin, G. F. Zhou, Z. Zhang and J. M. Liu, Ion Beam Defect Engineering on ReS2/Si Photocathode with Significantly Enhanced Hydrogen Evolution Reaction, Adv. Mater. Interfaces, 2019, 6, 1801663 CrossRef.
- K. R. Rahman, K. Y. Kok, W. Y. Wong, H. Yang and K. L. Lim, Effect of Iron Loading on the Catalytic Activity of Fe/N-Doped Reduced Graphene Oxide Catalysts via Irradiation, Appl. Sci., 2021, 11, 431 CrossRef.
- A. Ghicov, J. M. Macak, H. Tsuchiya, J. Kunze, V. Haeublein, L. Frey and P. Schmuki, Ion implantation and annealing for an efficient N-doping of TiO2 nanotubes, Nano Lett., 2006, 6, 1080–1082 CrossRef CAS.
- D. Liu, F. Ren, G. X. Cai, Y. C. Liu, M. Q. Hong, J. J. Ying, Y. Liu, J. Zhou, W. Wu, X. H. Xiao and C. Z. Jiang, Fabrication of TiO2-based composite films by sequential ion implantation and subsequent annealing, Mater. Res. Express, 2014, 1, 25703 CrossRef CAS.
- P. Bamola, S. Rawat, M. Tanwar, K. Asokan, C. Dwivedi, R. Kumar and H. Sharma, Effect of low energy ion irradiation on TiO2-based hybrid nanostructures for enhanced photocatalytic activity, Eur. Phys. J.: Spec. Top., 2022, 231, 2941–2949 CAS.
- M. Takeuchi, S. Sakai, M. Matsuoka and M. Anpo, Preparation of the visible light responsive TiO2 thin film photocatalysts by the RF magnetron sputtering deposition method, Res. Chem. Intermed., 2009, 35, 973–983 CrossRef CAS.
- H. Narayan, H. Alemu, L. Macheli, M. Thakurdesai and T. K. G. Rao, Synthesis and characterization of Y3+-doped TiO2 nanocomposites for photocatalytic applications, Nanotechnology, 2009, 20, 255601 CrossRef.
- M. M. Ba-Abbad, A. A. H. Kadhum, A. Mohamad, M. S. Takriff and K. Sopian, Visible light photocatalytic activity of Fe3+-doped ZnO nanoparticle prepared via sol-gel technique, Chemosphere, 2013, 91, 1604–1611 CrossRef CAS.
- A. P. Bhirud, S. D. Sathaye, R. P. Waichal, L. K. Nikam and B. B. Kale, An eco-friendly, highly stable and efficient nanostructured p-type N-doped ZnO photocatalyst for environmentally benign solar hydrogen production, Green Chem., 2012, 14, 2790–2798 RSC.
- M. Ni, M. K. H. Leung, D. Y. C. Leung and K. Sumathy, A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production, Renewable Sustainable Energy Rev., 2007, 11, 401–425 CrossRef CAS.
- M. Wang, F. Ren, G. X. Cai, Y. C. Liu, S. H. Shen and L. J. Guo, Activating ZnO nanorod photoanodes in visible light by Cu ion implantation, Nano Res., 2014, 7, 353–364 CrossRef CAS.
- L. Cai, F. Ren, M. Wang, G. X. Cai, Y. B. Chen, Y. C. Liu, S. H. Shen and L. J. Guo, V ions implanted ZnO nanorod arrays for photoelectrochemical water splitting under visible light, Int. J. Hydrogen Energy, 2015, 40, 1394–1401 CrossRef CAS.
- D. H. Deng, K. S. Novoselov, Q. Fu, N. F. Zheng, Z. Q. Tian and X. H. Bao, Catalysis with two-dimensional materials and their heterostructures, Nat. Nanotechnol., 2016, 11, 218–230 CrossRef CAS PubMed.
- J. Sitek, K. Czerniak-Losiewicz, A. P. Gertych, M. Giza, P. Dabrowski, M. Rogala, K. Wilczynski, A. Kaleta, S. Kret, B. R. Conran, X. C. Wang, C. McAleese, M. Macha, A. Radenovic, M. Zdrojek, I. Pasternak and W. Strupinski, Selective Growth of van der Waals Heterostructures Enabled by Electron-Beam Irradiation, ACS Appl. Mater. Interfaces, 2023, 15, 33838–33847 CrossRef CAS.
- Y. Z. Wang, W. Li, Y. M. Feng, S. S. Lv, M. Y. Li and Z. C. Li, Nitrogen ion irradiation effect on enhancing photocatalytic performance of CdTe/ZnO heterostructures, Front. Mater. Sci., 2018, 12, 392–404 CrossRef.
- S. Dhara, Formation, dynamics, and characterization of nanostructures by ion beam irradiation, Crit. Rev. Solid State Mater. Sci., 2007, 32, 1–50 CrossRef CAS.
- P. Z. Chen, K. Xu, S. Tao, T. P. Zhou, Y. Tong, H. Ding, L. D. Zhang, W. S. Chu, C. Z. Wu and Y. Xie, Phase-Transformation Engineering in Cobalt Diselenide Realizing Enhanced Catalytic Activity for Hydrogen Evolution in an Alkaline Medium, Adv. Mater., 2016, 28, 7527–7532 CrossRef CAS.
- B. A. Zhang, H. Y. Qin, L. C. Diao, N. Q. Zhao, C. S. Shi, E. Z. Liu and C. N. He, Strongly coupled hollow-oxide/phosphide hybrid coated with nitrogen-doped carbon as highly efficient electrocatalysts in alkaline for hydrogen evolution reaction, J. Catal., 2019, 377, 582–588 CrossRef CAS.
- C. Sun, P. P. Wang, H. Wang, C. Xu, J. T. Zhu, Y. X. Liang, Y. Su, Y. N. Jiang, W. Q. Wu, E. G. Fu and G. F. Zou, Defect engineering of molybdenum disulfide through ion irradiation to boost hydrogen evolution reaction performance, Nano Res., 2019, 12, 1613–1618 CrossRef CAS.
- Q. T. Wang, K. Cui, J. Li, Y. X. Wu, Y. X. Yang, X. Z. Zhou, G. F. Ma, Z. W. Yang, Z. Q. Lei and S. F. Ren, Iron ion irradiated Bi2Te3 nanosheets with defects and regulated hydrophilicity to enhance the hydrogen evolution reaction, Nanoscale, 2020, 12, 16208–16214 RSC.
- L. Wu, F. Ren, G. X. Cai, Z. Xing, H. Y. Wu, X. D. Zheng, X. N. Wang and C. Z. Jiang, Fabrication of nanoporous Si electrocathode by high-energy argon ion irradiation for improved electrocatalytic hydrogen production, Int. J. Hydrogen Energy, 2018, 43, 64–71 CrossRef CAS.
- B. R. Xia, T. T. Wang, X. D. Jiang, T. M. Zhang, J. Li, W. Xiao, P. X. Xi, D. Q. Gao, D. S. Xue and J. Ding, Ar2+ Beam Irradiation-Induced Multivancancies in MoSe2 Nanosheet for Enhanced Electrochemical Hydrogen Evolution, ACS Energy Lett., 2018, 3, 2167–2172 CrossRef CAS.
- M. T. Mehran, M. M. Baig, F. Shahzad, S. R. Naqvi and S. Iqbal, Gamma irradiated structural modification of Ti3C2Tx for high performance supercapacitors and the hydrogen evolution reaction, New J. Chem., 2023, 47, 7205–7210 RSC.
- Z. Mravik, D. Bajuk-Bogdanovic, A. Mrakovic, L. Vukosavljevic, I. Trajic, J. Kovac, D. Perusko, N. Gavrilov and Z. Jovanovic, Structural and electrochemical properties of carbon ion beam irradiated 12-tungstophosphoric acid, Radiat. Phys. Chem., 2021, 183, 109422 CrossRef CAS.
- B. R. Xia, T. T. Wang, X. D. Jiang, J. Li, T. M. Zhang, P. Xi, D. Q. Gao and D. S. Xue, N+-ion irradiation engineering towards the efficient oxygen evolution reaction on NiO nanosheet arrays, J. Mater. Chem. A, 2019, 7, 4729–4733 RSC.
- T. T. Li, T. Y. Jing, D. W. Rao, S. Mourdikoudis, Y. P. Zuo and M. Y. Wang, Two-dimensional materials for electrocatalysis and energy storage applications, Inorg. Chem. Front., 2022, 9, 6008–6046 RSC.
- D. Voiry, H. Yamaguchi, J. W. Li, R. Silva, D. C. B. Alves, T. Fujita, M. W. Chen, T. Asefa, V. B. Shenoy, G. Eda and M. Chhowalla, Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution, Nat. Mater., 2013, 12, 850–855 CrossRef CAS.
- G. Q. Li, D. Zhang, Q. Qiao, Y. F. Yu, D. Peterson, A. Zafar, R. Kumar, S. Curtarolo, F. Hunte, S. Shannon, Y. M. Zhu, W. T. Yang and L. Y. Cao, All The Catalytic Active Sites of MoS2 for Hydrogen Evolution, J. Am. Chem. Soc., 2016, 138, 16632–16638 CrossRef CAS PubMed.
- J. H. Kim, D. H. Youn, K. Kawashima, J. Lin, H. Lim and C. B. Mullins, An active nanoporous Ni(Fe) OER electrocatalyst via selective dissolution of Cd in alkaline media, Appl. Catal., B, 2018, 225, 1–7 CrossRef CAS.
- S. D. Bhoyate, J. Kim, F. M. de Souza, J. R. Y. Lin, E. H. Lee, A. Kumar and R. K. Gupta, Science and engineering for non-noble-metal-based electrocatalysts to boost their ORR performance: A critical review, Coord. Chem. Rev., 2023, 474, 214854 CrossRef CAS.
- Y. H. Wang, X. Cheng, K. Zhang, G. Chen, R. Z. Wang and J. J. Zhang, Ion-irradiation of catalyst and electrode materials for water electrolysis/photoelectrolysis cells, rechargeable batteries, and supercapacitors, Mater. Adv., 2022, 3, 7384–7405 RSC.
- K. Kakitani, T. Kimata, T. Yamaki, S. Yamamoto, T. Taguchi, T. Kobayashi, W. Mao and T. Terai, The interface between platinum nanoparticle catalysts and an Ar+-irradiated carbon support, Surf. Coat. Technol., 2018, 355, 259–263 CrossRef CAS.
- A. Devadoss, S. Ramasundaram, K. Asokan, B. Kim and S. Pitchaimuthu, Enhanced water oxidation catalytic performance of graphene oxide by gamma ray irradiation post-treatment, Mater. Lett., 2019, 241, 31–34 CrossRef CAS.
- A. Bourezgui, I. Kacem, M. Daoudi and A. F. Al-Hossainy, Influence of Gamma-Irradiation on Structural, Optical and Photocatalytic Performance of TiO2 Nanoparticles Under Controlled Atmospheres, J. Electron. Mater., 2020, 49, 1904–1921 CrossRef CAS.
- Y. Chen, H. Y. Zhao, Y. Y. Wu, X. Q. Huang, L. Wang and B. Guo, Effects of proton irradiation on structures and photo-catalytic property of nano-TiO2/CNTs films, Radiat. Phys. Chem., 2018, 153, 79–85 CrossRef CAS.
- E. Gallegos, F. M. Bisesti, K. Vaca-Escobar, C. Santacruz, L. Fernandez, A. Debut and P. J. Espinoza-Montero, Degradation of Direct Blue 1 through Heterogeneous Photocatalysis with TiO2 Irradiated with E-Beam, Processes, 2020, 8, 1181 CrossRef CAS.
- Y. P. He, X. Xiang, L. J. Sun, C. X. Tian, G. X. Yang and X. T. Zu, Effect of gamma-irradiation on adsorption and photocatalytic ability of MoS2 nanomaterials, Int. J. Mod. Phys. B, 2019, 33, 1250275 Search PubMed.
- A. L. Kozlovskiy, A. Alina and M. V. Zdorovets, Study of the effect of ion irradiation on increasing the photocatalytic activity of WO3 microparticles, J. Mater. Sci.: Mater. Electron., 2021, 32, 3863–3877 CrossRef CAS.
- S. H. Lv, M. Y. Li, M. L. Qiu, Y. Z. Wang, F. H. Dong, C. H. Chen, J. P. Cheng and Z. C. Li, Effect of nitrogen ion irradiation treatment to the enhancement of ZnO photocatalytic performance, Surf. Interface Anal., 2020, 52, 348–354 CrossRef CAS.
- C. Nefzi, N. Beji, M. Souli, A. Mejri, S. Alleg and N. Kamoun-Turki, Effect of gamma-irradiation on optical, structural and electrical properties of In2O3: F thin films for photocatalysis application, Opt Laser. Technol., 2019, 112, 85–92 CrossRef CAS.
- X. T. Xie, L. Q. Li, S. Y. Ye, Q. Zhang, X. M. Chen and X. Huang, Photocatalytic degradation of ethylene by TiO2 nanotubes/reduced graphene oxide prepared by gamma irradiation, Radiat. Phys. Chem., 2020, 169, 108371 CrossRef.
- H. Y. Wu, F. Ren, Z. Xing, X. D. Zheng, L. Wu and C. Z. Jiang, Cathodic shift of onset potential for water oxidation of WO3 photoanode by Zr+ ions implantation, J. Appl. Phys., 2017, 121, 085305 CrossRef.
- H. Y. Wu, Z. W. Wang, S. X. Jin, X. Z. Cao, F. Ren, L. Wu, Z. Xing, X. N. Wang, G. X. Cai and C. Z. Jiang, Enhanced photoelectrochemical performance of TiO2 through controlled Ar+ ion irradiation: A combined experimental and theoretical study, Int. J. Hydrogen Energy, 2018, 43, 6936–6944 CrossRef CAS.
- X. D. Zheng, S. H. Shen, F. Ren, G. X. Cai, Z. Xing, Y. C. Liu, D. Liu, G. Z. Zhang, X. H. Xiao, W. Wu and C. Z. Jiang, Irradiation-induced TiO2 nanorods for photoelectrochemical hydrogen production, Int. J. Hydrogen Energy, 2015, 40, 5034–5041 CrossRef CAS.
- A. Ghicov, J. M. Macak, H. Tsuchiya, J. Kunze, V. Haeublein, S. Kleber and P. Schmuki, TiO2 nanotube layers: Dose effects during nitrogen doping by ion implantation, Chem. Phys. Lett., 2006, 419, 426–429 CrossRef CAS.
- G. M. Wang, X. H. Xiao, W. Q. Li, Z. Y. Lin, Z. P. Zhao, C. Chen, C. Wang, Y. J. Li, X. Q. Huang, L. Miao, C. Z. Jiang, Y. Huang and X. F. Duan, Significantly Enhanced Visible Light Photoelectrochemical Activity in TiO2 Nanowire Arrays by Nitrogen Implantation, Nano Lett., 2015, 15, 4692–4698 CrossRef CAS.
- M. Wang, F. Ren, J. G. Zhou, G. X. Cai, L. Cai, Y. F. Hu, D. N. Wang, Y. C. Liu, L. J. J. Guo and S. H. Shen, N Doping to ZnO Nanorods for Photoelectrochemical Water Splitting under Visible Light: Engineered Impurity Distribution and Terraced Band Structure, Sci. Rep., 2015, 5, 12925 CrossRef CAS.
- X. Y. Song, W. Q. Li, D. He, H. Y. Wu, Z. J. Ke, C. Z. Jiang, G. M. Wang and X. H. Xiao, The "Midas Touch" Transformation of TiO2 Nanowire Arrays during Visible Light Photoelectrochemical Performance by Carbon/Nitrogen Coimplantation, Adv. Energy Mater., 2018, 8, 1800165 CrossRef.
- D. He, X. Y. Song, Z. J. Ke, X. H. Xiao and C. Z. Jiang, Construct Fe2+ species and Au particles for significantly enhanced photoelectrochemical performance of alpha-Fe2O3 by ion implantation, Sci. China Mater., 2018, 61, 878–886 CrossRef CAS.
- C. L. Zhang, R. H. Ma, Q. We, M. R. Yang, R. Cao and X. N. Zong, Photocatalytic degradation of organic pollutants in wastewater by heteropolyacids: a review, J. Coord. Chem., 2021, 74, 1751–1764 CrossRef CAS.
- R. T. Huang, L. J. Wang, Q. Zhang, Z. W. Chen, Z. Li, D. Y. Pan, B. Zhao, M. H. Wu, C. M. L. Wu and C. H. Shek, Irradiated Graphene Loaded with SnO2 Quantum Dots for Energy Storage, ACS Nano, 2015, 9, 11351–11361 CrossRef CAS.
- S. S. Latthe, S. An, S. Jin and S. S. Yoon, High energy electron beam irradiated TiO2 photoanodes for improved water splitting, J. Mater. Chem. A, 2013, 1, 13567–13575 RSC.
- L. M. Wang, T. L. Zhang, P. Y. Li, W. X. Huang, J. L. Tang, P. Y. Wang, J. Liu, Q. X. Yuan, R. Bai, B. Li, K. Zhang, Y. L. Zhao and C. Y. Chen, Use of Synchrotron Radiation-Analytical Techniques To Reveal Chemical Origin of Silver-Nanoparticle Cytotoxicity, ACS Nano, 2015, 9, 6532–6547 CrossRef CAS PubMed.
- S. S. Huang, W. F. Wang, D. Y. Chen, Z. J. Hu, Y. Jiang, Z. W. Chen, Z. Li, D. Y. Pan and B. Zhao, Enhancing lithium-ion batteries performance via electron-beam irradiation strategies: A case study of graphene aerogels loaded with SnO2 quantum dots, Electrochim. Acta, 2018, 281, 769–776 CrossRef CAS.
- D. Y. Chen, S. S. Huang, R. T. Huang, Q. Zhang, T. T. Le, E. B. Cheng, R. Yue, Z. J. Hu and Z. W. Chen, Electron Beam-Induced Microstructural Evolution of SnS2 Quantum Dots Assembled on N-Doped Graphene Nanosheets with Enhanced Photocatalytic Activity, Adv. Mater. Interfaces, 2019, 6, 1801759 CrossRef.
- D. S. Martinez, A. Martinez-de la Cruz and E. L. Cuellar, Photocatalytic properties of WO3 nanoparticles obtained by precipitation in presence of urea as complexing agent, Appl. Catal., A, 2011, 398, 179–186 CrossRef.
- A. B. D. Nandiyanto, F. Triawan, R. Firly, A. G. Abdullah, Y. Aono, K. Inaba and K. Kishimoto, Identification of Micro-Mechanical Characteristics of Monoclinic Tungsten Trioxide Microparticles by Nanoindentation Technique, Mater. Phys. Mech., 2019, 42, 323–329 CAS.
- D. Sanchez-Martinez, A. Martinez-de la Cruz and E. Lopez-Cuellar, Synthesis of WO3 nanoparticles by citric acid-assisted precipitation and evaluation of their photocatalytic properties, Mater. Res. Bull., 2013, 48, 691–697 CrossRef CAS.
- A. Martinez-de la Cruz, D. S. Martinez and E. L. Cuellar, Synthesis and characterization of WO3 nanoparticles prepared by the precipitation method: Evaluation of photocatalytic activity under vis-irradiation, Solid State Sci., 2010, 12, 88–94 CrossRef CAS.
- A. L. Kozlovskiy and M. V. Zdorovets, Study of the photocatalytic activity of irradiated WO3 microparticles, Appl. Phys. A, 2020, 126, 638 CrossRef CAS.
- P. B. Johnson, A. Markwitz and P. W. Gilberd, Shallow nanoporous surface layers produced by helium ion implantation, Adv. Mater., 2001, 13, 997–1000 CrossRef CAS.
- M. Bruel, Application of hydrogen ion beams to Silicon On Insulator material technology, Nucl. Instrum. Methods Phys. Res., Sect. B, 1996, 108, 313–319 CrossRef CAS.
- X. M. Zhou, V. Haublein, N. Liu, N. T. Nguyen, E. M. Zolnhofer, H. Tsuchiya, M. S. Killian, K. Meyer, L. Frey and P. Schmuki, TiO2 Nanotubes: Nitrogen-Ion Implantation at Low Dose Provides Noble-Metal-Free Photocatalytic H2 Evolution Activity, Angew. Chem., Int. Ed., 2016, 55, 3763–3767 CrossRef CAS PubMed.
- S. S. Lee, H. Zhu, E. Q. Contreras, A. Prakash, H. L. Puppala and V. L. Colvin, High Temperature Decomposition of Cerium Precursors To Form Ceria Nanocrystal Libraries for Biological Applications, Chem. Matter., 2012, 24, 424–432 CrossRef CAS.
- X. B. Li, G. Hartley, A. J. Ward, P. A. Young, A. F. Masters and T. Maschmeyer, Hydrogenated Defects in Graphitic Carbon Nitride Nanosheets for Improved Photocatalytic Hydrogen Evolution, J. Phys. Chem. C, 2015, 119, 14938–14946 CrossRef CAS.
- P. Niu, G. Liu and H. M. Cheng, Nitrogen Vacancy-Promoted Photocatalytic Activity of Graphitic Carbon Nitride, J. Phys. Chem. C, 2012, 116, 11013–11018 CrossRef CAS.
- H. J. Yu, R. Shi, Y. X. Zhao, T. Bian, Y. F. Zhao, C. Zhou, G. I. N. Waterhouse, L. Z. Wu, C. H. Tung and T. R. Zhang, Alkali-Assisted Synthesis of Nitrogen Deficient Graphitic Carbon Nitride with Tunable Band Structures for Efficient Visible-Light-Driven Hydrogen Evolution, Adv. Mater., 2017, 29, 1605148 CrossRef.
- X. N. Wang, L. Wu, Z. W. Wang, H. Y. Wu, X. M. Zhou, H. Y. Ma, H. Z. Zhong, Z. Xing, G. X. Cai, C. Z. Jiang and F. Ren, C/N Vacancy Co-Enhanced Visible-Light-Driven Hydrogen Evolution of g-C3N4 Nanosheets Through Controlled He+ Ion Irradiation, Sol. RRL, 2019, 3, 1800298 CrossRef.
- H. Duan, H. Y. Wu, H. Z. Zhong, X. N. Wang, W. J. Wan, D. R. Li, G. X. Cai, C. Z. Jiang and F. Ren, Improving PEC Performance of BiVO4 by Introducing Bulk Oxygen Vacancies by He Ion Irradiation, J. Phys. Chem. C, 2022, 126, 7688–7695 CrossRef CAS.
- S. Pylypenko, A. Queen, T. S. Olson, A. Dameron, K. O'Neill, K. C. Neyerlin, B. Pivovar, H. N. Dinh, D. S. Ginley, T. Gennett and R. O'Hayre, Tuning Carbon-Based Fuel Cell Catalyst Support Structures via Nitrogen Functionalization. I. Investigation of Structural and Compositional Modification of Highly Oriented Pyrolytic Graphite Model Catalyst Supports as a Function of Nitrogen Implantation Dose, J. Phys. Chem. C, 2011, 115, 13667–13675 CrossRef CAS.
- X. Shi, W. Wang, X. R. Miao, F. Tian, Z. W. Xu, N. Li and M. L. Jing, Constructing Conductive Channels between Platinum Nanoparticles and Graphitic Carbon Nitride by Gamma Irradiation for an Enhanced Oxygen Reduction Reaction, ACS Appl. Mater. Interfaces, 2020, 12, 46095–46106 CrossRef CAS PubMed.
- M. Asano, R. Kawamura, N. Todoroki and T. Wadayama, ORR Properties for Model Pt-Shell Layers Prepared on Nitrogen-Beam Irradiated Pt25Ni75(111) Substrate, ECS Trans., 2016, 75, 809–814 CrossRef CAS.
- T. F. Choo, N. M. Zali, N. U. Saidin and K. Y. Kok, Gamma Radiolysis-Synthesized Carbon Nanotube-Supported Palladium as Electrocatalyst for Oxygen Reduction Reaction, Electrocatalysis, 2023, 14, 418–428 CrossRef CAS.
- L. S. Liu, X. Shi, W. Wang, M. F. Pei, C. X. Hong, Y. L. Xue, Z. W. Xu, F. Tian and X. F. Guo, Carbon nitride/positive carbon black anchoring Pt NPs assembled by gamma-rays as ORR catalyst with excellent stability, Nanotechnology, 2021, 32, 345601 CrossRef CAS.
- H. Okazaki, K. Kakitani, T. Kimata, A. Idesaki, H. Koshikawa, D. Matsumura, S. Yamamoto and T. Yamaki, Changes in electronic structure of carbon supports for Pt catalysts induced by vacancy formation due to Ar+ irradiation, J. Chem. Phys., 2020, 152, 124708 CrossRef CAS PubMed.
- Y. K. Zhou, T. Holme, J. Berry, T. R. Ohno, D. Ginley and R. O'Hayre, Dopant-Induced Electronic Structure Modification of HOPG Surfaces: Implications for High Activity Fuel Cell Catalysts, J. Phys. Chem. C, 2010, 114, 506–515 CrossRef CAS.
- Y. K. Zhou, R. Pasquarelli, T. Holme, J. Berry, D. Ginley and R. O'Hayre, Improving PEM fuel cell catalyst activity and durability using nitrogen-doped carbon supports: observations from model Pt/HOPG systems, J. Mater. Chem., 2009, 19, 7830–7838 RSC.
- S. Pylypenko, A. Queen, T. S. Olson, A. Dameron, K. O'Neill, K. C. Neyerlin, B. Pivovar, H. N. Dinh, D. S. Ginley, T. Gennett and R. O'Hayre, Tuning Carbon-Based Fuel Cell Catalyst Support Structures via Nitrogen Functionalization. II. Investigation of Durability of Pt-Ru Nanoparticles Supported on Highly Oriented Pyrolytic Graphite Model Catalyst Supports As a Function of Nitrogen Implantation Dose, J. Phys. Chem. C, 2011, 115, 13676–13684 CrossRef CAS.
- W. Wang, S. S. Liu, C. Y. Min, M. Zeng, H. T. Shi, R. Q. Shao, S. K. Liu, Z. W. Xu, Y. H. Xia and N. Li, A Cathode Material of Fuel Cells: F-Doped gamma-Graphyne/PtPd Nanocomposite from Plasma Activation and Gamma Irradiation, ACS Appl. Energy Mater., 2022, 5, 2036–2044 CrossRef CAS.
- Y. R. Uhm, G. M. Sun, J. Lee, C. S. Kim, J. H. Park and T. Ha, Fe-Nx active sites in Fe-N-C electrocatalysts synthesized using electron beam irradiation, J. Korean Phys. Soc., 2023, 82, 286–292 CrossRef CAS.
- W. Wang, X. M. Zhao, H. T. Shi, L. S. Liu, H. Deng, Z. W. Xu, F. Tian and X. R. Miao, Shape inducer-free polygonal angle platinum nanoparticles in graphene oxide as oxygen reduction catalyst derived from gamma irradiation, J. Colloid Interface Sci., 2020, 575, 1–15 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |