Open Access Article
Open Access ArticleBiological activity of vanadium pincer complexes†
Luis Humberto
Delgado-Rangel
 a,
Viviana
Reyes-Márquez
a,
Viviana
Reyes-Márquez
 b,
María Esther
Moreno-Narváez
b,
María Esther
Moreno-Narváez
 a,
Alberto
Aragón-Muriel
a,
Alberto
Aragón-Muriel
 c,
Jesús R.
Parra-Unda
c,
Jesús R.
Parra-Unda
 d,
J. Antonio
Cruz-Navarro
d,
J. Antonio
Cruz-Navarro
 a,
Mayra A.
Martínez-Torres
a,
Mayra A.
Martínez-Torres
 a,
Hugo
Valdés
a,
Hugo
Valdés
 e and
David
Morales-Morales
e and
David
Morales-Morales
 *a
*a
aInstituto de Química, Universidad Nacional Autónoma de México, Ciudad Universitaria, Circuito Exterior s/n, Ciudad de México C.P. 04510, Mexico. E-mail: damor@unam.mx
bDepartamento de Ciencias Químico-Biológicas, Universidad de Sonora, Luis Encinas y Rosales S/N, Hermosillo 83000, Mexico
cGrupo de Investigaciones Bioquímicas (GIB), Universidad del Magdalena, Santa Marta 470004, Colombia
dUnidad de Investigaciones en Salud Pública “Dra. Kaethe Willms”, Facultad de Ciencias Químico Biológicas, Universidad Autónoma de Sinaloa, Culiacán 80010, Mexico
eDepartamento de Química Orgánica y Química Inorgánica, Instituto de Investigación Química “Andrés M. del Río” (IQAR), Facultad de Farmacia, Universidad de Alcalá, Alcalá de Henares, 28805 Madrid, Spain
First published on 15th November 2024
Abstract
This review focuses on the biological activity of vanadium pincer complexes, exploring their applications as activators or inhibitors of enzymatic function, antioxidants, and agents with potential therapeutic effects. Specifically, their capacities as antidiabetic, antibacterial, antiviral, antiparasitic, and anticancer agents are examined in detail. The use of pincer ligands for biological applications has grown enormously in the last ten years, as these ligands can confer to the complexes they form properties such as enhanced stability, improved bioavailability, and greater specificity for pharmacological targets, potentially leading to pharmacological synergy. Additionally, the use of vanadium in the development of pharmacologically active compounds has gained attention due to its role in certain biological processes and its ability to interact with proteins by mimicking phosphorus atoms, making vanadium-containing molecules of significant interest for further study.
Introduction
Vanadium is a transition metal widely distributed in the Earth's crust and located in the first transition series of the periodic table, specifically in group 5.1 It exhibits a broad range of oxidation states, from −3 to +5, with V(III), V(IV), and V(V) species being the most biologically relevant.2 Vanadium is considered a trace element with essential roles in both biology and medicine, typically in the form of coordination complexes, where its oxidation state is +5 or +4.2The biological importance of vanadium is underscored by the evolution of several enzyme systems that contain this metal in their active sites.3 Examples include vanadium-dependent haloperoxidases4 and vanadium-containing nitrogenases.5–9 Additionally, vanadium plays an important role in insulin regulation in higher organisms, though the exact mechanisms remain unclear.10–12
Over the past three decades, the development of metallodrugs based on coordination compounds has expanded rapidly, incorporating a variety of transition metals and ligands with specific structures.13–22 Given vanadium's biological relevance,23,24 it is not surprising that many vanadium-based complexes have been proposed for the treatment of various diseases.25–33 Vanadium complexes have shown promising activities as anticancer agents18,26,27,34–39 due to their ability to inhibit biological processes, crucial for cancer cell growth. Furthermore, they have been tested for the treatment of diabetes,40,41 antibacterial,35,42,43 antifungal,43,44 and parasitic diseases (vide infra).45–49
The biological activity of these complexes can be modulated by selecting the appropriate ligand.49,50 In this review, we focus on pincer ligands,51 which provide a robust platform that stabilizes different oxidation states of the metal center and can be easily functionalized and tuned.52 Pincer ligands are tridentate compounds that coordinate to a metal fragment in a meridional fashion. When a pincer ligand coordinates with a metal center, forms a pincer complex, which is typically stable under thermal and moisture conditions due to the chelation effect. Although these properties have traditionally been valued in the design of catalysts53–81 and materials,82–84 recent studies have explored their use in tailoring complexes with anticancer,85–93 antiparasitic,94 and antibacterial activities.45–96
Thus, in this review, we examine vanadium pincer complexes with biological applications, specifically focusing on their biodistribution, enzyme inhibition and activation capabilities, antioxidant properties, and their potential as antidiabetic, antibacterial, antiviral, antiparasitic, and anticancer agents. For comparative purposes, we have also included complexes with tetra- and penta-dentate ligands.
Biodistribution of vanadium
Vanadium was identified as a trace element96 essential for certain organisms such as ascidians,97 polychaete worms,98,99 and Amanita mushrooms.100,101 However, information regarding its essentiality for mammals and humans remained limited. Vanadium occurred in nature in oxidation states ranging from +2 to +5 and played a role in some biochemical processes in mammals. Importantly, vanadium is not considered carcinogenic, although it has been detected in cancer cells interacting with enzymes.34 While vanadium compounds proved toxic in high quantities,102 they have been investigated for therapeutic uses, particularly in treating diseases such as cancer and diabetes (vide infra).Studies in mice provided insights into the absorption, distribution, and excretion of vanadium. Analyses showed that vanadium was primarily absorbed in the bone, followed by the liver, kidneys, and spleen. The extent of vanadium absorption depended on particle size and dosage. Smaller particle sizes and higher doses led to increased vanadium accumulation in the bone and liver, particularly in diabetic mice compared to non-diabetic mice.96 Additionally, a study with vanadium dioxide derivatives demonstrated differential absorption, with the unabsorbed portion excreted through faeces within 28 days.96 After treatment cessation, the concentration of accumulated vanadium in organs decreased over 14 days until reaching basal levels in some tissues.
Simple inorganic vanadium salts like sodium metavanadate (Na2VO3) and vanadyl sulphate (VOSO4) demonstrated medical potential but exhibited low absorption, higher toxicity, and were primarily excreted through faeces and urine. Given these limitations, researchers developed new vanadium compounds to improve their biological activity.33
An example of biodistribution and pharmacokinetics in vanadium-based anticancer agents was performed using vanadocene dichloride (VDC).103 Studies in mice revealed that VDC levels decreased in the blood and small intestine, while vanadium accumulated in the kidneys and liver. It was also well-established that some vanadium species interacted with various biomolecules, including citrate, lactate, oxalate, and amino acids. Vanadium also bounds to proteins such as transferrin, albumin, and immunoglobulins.104
Vanadium pincer complexes were identified in biological systems.51 A vanadium-pyrroloquinoline quinone complex was found in methanol dehydrogenase and a bacterial dehydrogenase enzyme. Tridentate dioxidovanadium complexes were evaluated for their antidiabetic activity as inhibitors of insulin-related enzymes. These complexes, with promising inhibition of α-amylase and α-glucosidase, suggested potential as insulin enzyme inhibitors.105
The biodistribution of vanadium compounds is influenced by factors such as stability, coordination geometry, electric charge, hydrolipophilicity balance, substituents, and redox properties. Vanadium pincer complexes, in particular, were explored for their potential to improve solubility, absorption, and biodistribution in humans. These compounds offered versatile therapeutic potential, with better absorption and targeted intra-/intercellular interactions, which enhanced therapeutic efficacy.33
Overall, research on the biodistribution of vanadium has provided fundamental knowledge on the pharmacokinetic and pharmacodynamic parameters of vanadium compounds. However, further studies are needed on this topic to deepen our knowledge of vanadium pincer complexes specifically, and thus the following sections explore their potential in the treatment of diseases such as cancer and diabetes, as well as their interactions at the enzymatic level (vide infra).
Vanadium-mediated enzyme activation and inhibition
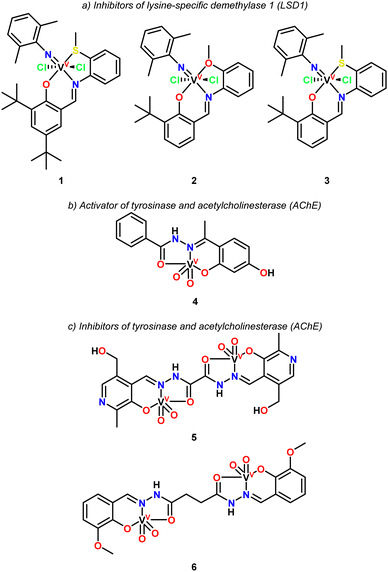
The exploration of vanadium-mediated enzyme activation and inhibition systems is of vital importance to understand the mechanisms by which the desired pharmacological action occurs.In 2019, Lu and coworkers reported the synthesis of three vanadium pincer complexes (Fig. 1, complexes 1–3) and their evaluation as inhibitors of lysine-specific demethylase 1 (LSD1),106 an enzyme implicated in the progression of various diseases, including several types of cancer.
Structural differences in the pincer ligands had a significant impact on the inhibition rates. Hence, complexes 1 and 3 containing an ONS pincer ligand exhibited greater inhibition compared to complex 2 including in its structure an ONO pincer ligand. Complex 1 demonstrated the highest inhibition rate, reaching 70%, followed by complex 3 at 44%, and complex 2 with the lowest inhibition rate of 28%. The presence of a sulfur atom in the pincer ligand (ONS) resulted in a significantly enhanced activity compared to the oxygen derivative (ONO), as observed in the comparison between complexes 2 and 3. Additionally, the introduction of an extra tert-butyl group in the pincer ligand further boosted the activity, with complex 1 being more active than complex 3.
In contrast, the free pincer ligands showed no significant inhibitory activity against LSD1 under the same conditions, highlighting the crucial role of the vanadium center in the complexes. All compounds were tested under similar conditions using a concentration of 30 μM.
The IC50 value of complex 1 was determined to be 19.0 μM, which is lower than that of the reference compound tranylcypromine (TCP) (26.1 μM for TCP vs. 19.0 μM for complex 1). Furthermore, complex 1 was evaluated for its inhibition of monoamine oxidases (MAO), as LSD1 shares a similar amino acid sequence with MAO. Only weak inhibition of MAO-A/B was observed at concentrations of 30 μM and 60 μM, demonstrating that complex 1 is selective for LSD1.
While some vanadium compounds can act as enzyme inhibitors, the presence of vanadium in organisms is essential for the functionality and activation of numerous proteins and enzymes. In particular, several vanadium complexes, primarily based on vanadate (VO4)3−, have the ability to activate enzymes due to their remarkable chemical similarity to phosphate groups, allowing vanadate to act as a phosphate competitor. Furthermore, vanadate compounds can form stable complexes with target enzymes such as phosphatases, kinases, nucleases, ATPases, glucose-6-phosphate dehydrogenase, and tyrosine kinases, all of which play critical roles in cell regulation processes.107
In the specific case of vanadium complexes with pincer-type ligands, with the capacity to act as enzymatic activators, Back and coworkers reported the activation properties of a series of pentacoordinated dioxidovanadium(V) complexes (4–6) on tyrosinase and acetylcholinesterase (AChE).108 Among these, only complex 4 demonstrated ability to activate tyrosinase and AChE by 11.5% and 47%, respectively, while complexes 5 and 6 exhibited inhibitory effects. An in silico evaluation of complex 4 suggested that the presence of a hydroxyl group mediates its interaction with the enzymes through hydrophobic and van der Waals forces. Reports on enzyme activation by vanadium complexes are scarce, as most studies focus on their inhibitory effects on specific enzymes.
Antioxidant activity of vanadium pincer complexes
An important part to consider for a better therapeutic treatment is the reduction of oxidative stress, which occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the body's ability to neutralize these harmful molecules or repair the resulting damage. ROS, including free radicals like superoxide and hydroxyl radicals, are highly reactive and can cause significant harm to cellular components such as lipids, proteins, and DNA. This oxidative damage is closely associated with the development and progression of several chronic diseases, including cancer, cardiovascular conditions, neurodegenerative disorders, and diabetes.To counteract oxidative stress, the body relies on a complex antioxidant defence system consisting of enzymatic antioxidants (such as superoxide dismutase and catalase) and non-enzymatic antioxidants (such as vitamins C and E). However, when oxidative stress overwhelms these natural defences, external antioxidants may be required to restore balance and prevent further cellular damage.
Vanadium compounds have recently gained attention for their potential antioxidant properties.109 This interest stems from their ability to scavenge free radicals, reduce ROS production, and enhance the activity of endogenous antioxidant enzymes. Studies suggest that vanadium may help mitigate oxidative damage in various biological systems, presenting potential therapeutic applications in conditions where oxidative stress plays a critical role.110Fig. 2 shows some representative examples of vanadium pincer complexes with antioxidant activity.
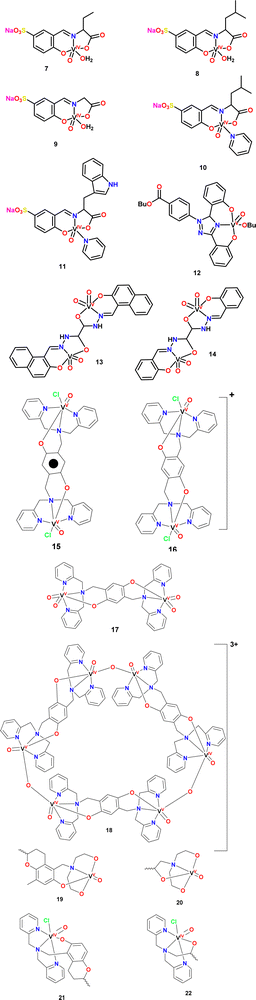
Adam and coworkers reported the synthesis of a series of oxovanadium(IV) complexes with salicylideneamino ligands (complexes 7–11).111 The antioxidant activity of these vanadium complexes was evaluated, focusing on their effectiveness in inhibiting superoxide radicals, a major component of ROS. Excessive ROS production can lead to cellular damage when it exceeds the body's antioxidant defense mechanisms, such as superoxide dismutase (SOD). The results indicated that the vanadium complexes effectively inhibit superoxide anion radical formation. Notably, complexes 7, 8 and 9 exhibited significant SOD-like activity, with inhibition percentages of 87%, 85%, and 91%, respectively, highlighting their strong antioxidant potential.
In contrast, complexes 10 and 11 showed relatively lower inhibition values, at 65% and 59%, respectively, indicating a lesser but still notable antioxidant capacity. Further analysis using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical assay revealed that complex 9 demonstrated substantial antioxidant activity, with a 55% inhibition rate. This value, exceeding the 50% threshold, confirms the superior antioxidant potential of complex 9 compared to the other vanadium pincer complexes analyzed for Adam and coworkers.
The antioxidant efficacy of complex 12 and its deferasirox (DFX) ligand was evaluated through the reduction of DPPH absorbance, with the IC50 parameter used for quantification.112 The analysis showed that complex 12 exhibits significantly higher antioxidant activity compared to the standard antioxidant, butylated hydroxytoluene (BHT). Also, complex 12 demonstrated a remarkable IC50 value of 0.1 mM, substantially lower than that of BHT (IC50 = 3.6 mM). A lower IC50 value signifies greater radical scavenging capacity and enhanced antioxidant activity, highlighting the strong efficacy of complex 12.113
This finding is consistent with previous studies on metal complexes, suggesting that the high activity is likely due to the metal complex and the inherent properties of the metal ion itself. In contrast, the free DFX ligand showed a high IC50 value, indicating minimal antioxidant activity.
Shi and coworkers presented the antioxidant activity of the vanadium pincer complexes 13 and 14,114 which were evaluated using various assays to assess its ability to mimic SOD activity, scavenge ABTS radicals, and neutralize hydroxyl radicals. The complexes demonstrated notable SOD-like activity, with specific values ranging from 0.24 to 0.89 U mg−1. Remarkably when these complexes were conjugated with bovine serum albumin (BSA) to form hybrid proteins, their SOD activity increased significantly. This enhancement suggests that the protein environment provided by BSA stabilizes the active form of the complexes, enabling more efficient catalysis of superoxide radicals. Moreover, the hybrid proteins exhibit greater antioxidant activity compared to the standalone complexes, likely due to a combination of factors such as stabilization, improved solubility, and potentially altered electronic properties within the protein microenvironment.
From the reviewed works regarding the antioxidant activity of pincer-type vanadium complexes, this property appears to operate through two distinct pathways, (1) enhancing the activity of enzymes that catalyse free radical reduction (particularly SOD), and (2) directly acting as free radical reducing agents, such as DPPH.
Despite this, it is important to remember that vanadium can act as an oxidizing agent and produce reactive species H2O2, such is the case described by Stylianou et al.115 for pincer-type complexes derived from iminopyridine hydroquinonate (complexes 15–18). The authors suggest that O2 is activated by vanadium metal, in such a way that the coordination of O2 with metal ions produces an increase in one-electron the reduction potential of O2 making it kinetically more reactive. Which is resumed in the interaction of O2 with V(IV) and then the oxidation of hydroquinone to semiquinone giving place to the production of H2O2 in the process. Keeping this in mind, we can deduce that vanadium complexes have a protective effect against oxidative stress, and also an oxidizing effect that can be direct or indirect. As exemplified by the complexes studied by Ioanna Hadjiadamouy et al.,116 who prepared vitamin E derivatives (α-tocopherol) that coordinate as pincer-type ligands to vanadium IV and V species. The authors performed the assessment of the radical scavenging capacity (RSC), to eliminate the DPPH˙ radical. The study showed that β-tocDEA and β-tocDPA exhibit antioxidant activity almost five times lower than α-tocopherol. Contrary to expectations, where the inhibition of the DPPH radical was the goal, the results indicated that complexes 19–22 seemingly acted as initiators in the formation of radicals rather than inhibiting the DPPH radical in solution. These results, beyond being counterproductive, are of great interest since examination of the cytotoxicity of these complexes, demonstrated great cytotoxic activity in both normal and cancer cells in μM (MRC5, LMS and U2OS cells) and nM concentrations (HeLa and HEK293). The same authors commented that detailed analysis of the mechanism by which the cytotoxic activity proceeds is necessary, but they also suggest that the complexes cause oxidative stress in cancer cell lines.116
Having reviewed the enzyme inhibitory and activating activity of vanadium pincer-type complexes, in addition to the possible behaviours they may have with respect to their capacity as oxidizing or antioxidant agents. The use of this type of compounds as antidiabetic, antibacterial, antiviral, antiparasitic and anticancer agents is reviewed in more detail next.
Antidiabetic activity of vanadium pincer complexes
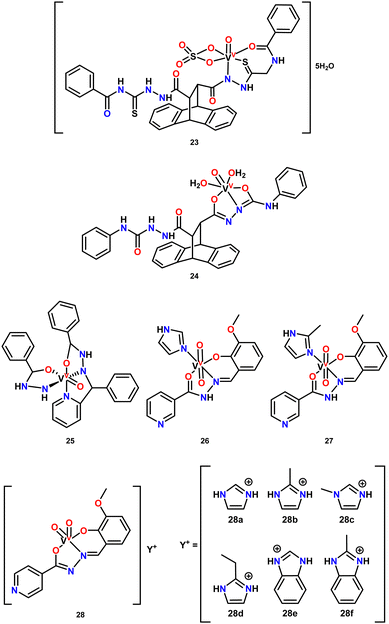
Vanadium compounds have also garnered attention for their insulin-mimetic117 or insulin-enhancing properties by stimulating glucose uptake in glucose-metabolizing cells, making them ideal candidates for the treatment of diabetes mellitus—a prevalent metabolic disorder characterized by elevated blood glucose levels.40,41 Several vanadium compounds have shown promising potential for the development of orally administered treatments, which could replace insulin injections, offering a less painful and more cost-effective option.118 The vanadium complexes studied exhibit various oxidation states and ligand types. In this review, we focus specifically on vanadium pincer complexes, which, although still underexplored as antidiabetic agents, have shown promising properties that could expand their therapeutic use in managing this metabolic disorder.El-Gammal and coworkers reported the synthesis and potential medicinal application of two new VO(IV) complexes featuring pincer ligands (Fig. 3, complexes 23 and 24), derived from thiosemicarbazide ligands, for the oral treatment of diabetes.119 The complexes were tested in diabetic rats, which were administered a water suspension at a dose of 5 mg kg−1 body weight per day, orally, for 30 days. The results showed that complex 23 exhibited a stronger hypolipidemic effect, which the authors attributed to the normalization of glucose metabolism, inhibition of lipolysis by vanadyl ions through tyrosine phosphorylation, inhibition of HMG-CoA synthase, and the regulation of lipogenic enzyme gene expression.
Patel and coworkers on the other hand synthesized and studied the antidiabetic potential of a new mixed-ligand oxovanadium(IV) complex with a tridentate Schiff base (Fig. 3, complex 25).120 The activity of complex 25 was assessed using an α-glucosidase inhibition assay, with acarbose as the standard. The results demonstrated moderate inhibition activity, with percentage inhibition at 200 μM and IC50 values of 14.75 μM for the complex compared to 18.59 μM for acarbose. Based on these findings, the authors suggested that this complex could serve as a potential α-glucosidase inhibitor.
In another study by Patel and coworkers, two new dioxidovanadium(V) complexes (26 and 27) bearing a tridentate ONO donor nicotinic acid ligand and imidazoles were investigated for their in vitro antidiabetic activity.106 The activity was evaluated through α-amylase and α-glucosidase inhibition assays. Both complexes showed inhibitory effects against α-glucosidase, with IC50 values of 153.037 and 32.542 μg mL−1 for 26 and 27, respectively, with 27 demonstrating a potency comparable to the standard, acarbose, suggesting its potential as a potent α-glucosidase inhibitor.
Contrarily, in the α-amylase inhibition assay, complex 26 exhibited the lowest IC50 value (23.669 μg mL−1), while complex 27 had the highest IC50 value (182.901 μg mL−1), indicating a varied response. Lastly, for β-glucosidase inhibition, 27 displayed the highest IC50 value (2021.770 μg mL−1), whereas 26 exhibited a moderate IC50 value (282.050 μg mL−1).
These results led the researchers to conclude that both complexes, 23 and 24, hold promise as potential antidiabetic agents due to their significant inhibition activities, which were concentration-dependent.
More recently, the same research group investigated the in vitro antidiabetic activity of new anionic dioxidovanadium(v) complexes with a hydrazone as pincer ligand (28a–f) using α-glucosidase, β-glucosidase, and α-amylase inhibition assays.121 The α-glucosidase inhibition assay revealed that complex 28f was the most active in the series, with an IC50 value of 53.88 μg mL−1. However, in the β-glucosidase inhibition assay, this complex displayed the lowest inhibition (IC50 = 843.41 μg mL−1), while complex 28c was the most potent β-glucosidase inhibitor, with an IC50 value of 227.45 μg mL−1.
Additionally, in the α-amylase inhibition study, complex 28d exhibited the lowest IC50 value (82.99 μg mL−1), while the other complexes showed moderate activity compared to the control acarbose, with IC50 values ranging from 100 to 270 μg mL−1. The inhibition activity of these complexes was concentration-dependent, consistent with findings from their previous work.
Interestingly, the researchers observed that complexes containing electron repelling moieties exhibited better inhibition activity, leading them to conclude that these vanadium complexes hold promise as potential antidiabetic agents.
Antibacterial and antiviral activity of vanadium pincer complexes
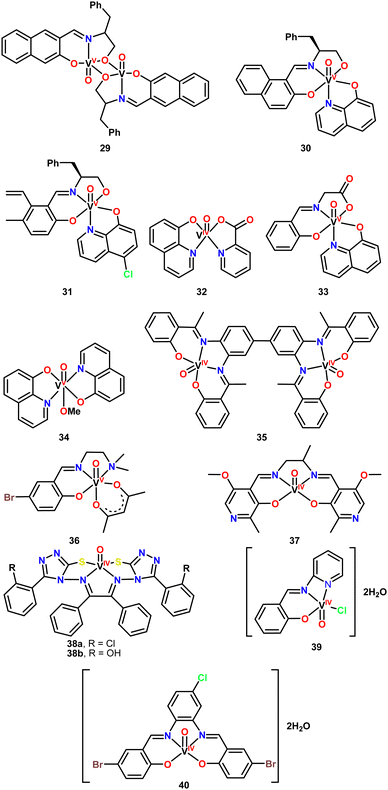
In 2014, Pessoa and coworkers leveraged the well-established biological activity of hydroxyquinolines to prepare six vanadium complexes featuring this naturally occurring ligand (Fig. 4, complexes 29–34).122,123 Complex 29 is a dinuclear complex, bridged by two oxygen atoms from the ONO pincer ligands and does not contain a hydroxyquinoline ligand. The other five complexes are mononuclear, including two non-pincer complexes, 32 and 34. Notably, the pincer ONO ligands are Schiff bases, which are easily synthesized and can be readily tuned for specific applications.The biological evaluation of complexes 29–34 aimed to assess their antibacterial and anti-tumor activities. The antituberculosis effect of the complexes was specifically studied to determine their antibacterial properties. For this purpose, the Resazurin assay was employed to determine the minimal inhibitory concentration (MIC). A Mycobacterium tuberculosis strain was prepared and incubated for 7–10 days, with the vanadium complexes tested at concentrations ranging from 0.25 to 250 μg mL−1 in DMSO as solvent. The bacterial cultures and the complexes were mixed in microplates and incubated for 7 days at 37 °C. Resazurin was then added, and the plates were incubated for an additional 24 h. The results demonstrated good antituberculosis activity, with the vanadium complexes showing bacterial growth inhibition like that of commercial antibiotics. Complexes 30, 32, 33, and 34 exhibited MIC values of 1.5 μg mL−1, while the commercial antibiotics gentamicin and ciprofloxacin had MIC values of 1 μg mL−1 and 2–4 μg mL−1, respectively, under similar conditions.
Interestingly, the presence of a chlorine atom in the 8-hydroxyquinoline ligand negatively impacted the activity of complex 31, which showed no activity against M. tuberculosis. Similar results were observed for the dimeric complex 29. Furthermore, other complexes lacking 8-hydroxyquinoline also demonstrated no activity, indicating that the presence of the hydroxyquinoline ligand is fundamental to the antibacterial efficacy of these complexes.
The essential role of vanadium in the biological activity was confirmed by testing copper analogues of the complexes under similar conditions, which showed no activity. This suggests that vanadium, the pincer ligand, and 8-hydroxyquinoline work synergistically to produce active species.
Despite the promising activity of the vanadium complexes against some strains, their potency remains lower than that of drugs currently used for tuberculosis treatment, such as isoniazid, which has a MIC of 0.03 μg mL−1. Further structural modifications of the complexes could enhance their activity and elucidating the mechanism of action may provide insights for designing more effective vanadium-based complexes.
Rajavel and coworkers reported the synthesis of a new tetradentate Schiff base ligand and its dinuclear metal complexes.124 They described the vanadium derivative (Fig. 4, complex 35), along with complexes of other transition metals such as copper, zinc, and nickel. The Schiff base ligand and its complexes were obtained in good yields and thoroughly characterized using various spectroscopic techniques.
The biological activity of the complexes was tested as bacterial growth inhibitors against Gram-negative Klebsiella pneumoniae and Gram-positive Staphylococcus pyogenes strains using the disk agar diffusion method. The complexes and the free ligand were evaluated at concentrations of 50, 100, and 150 μg mL−1, with tetracycline and chloramphenicol serving as reference drugs for the Gram-positive and Gram-negative strains, respectively. The complexes exhibited higher antibacterial activity compared to the free ligand, with a clear concentration-dependent effect, as indicated by the measured inhibition zones.
Interestingly, the vanadium complex demonstrated better performance against the Gram-negative strain compared to the Gram-positive strain, which is known for its thicker lipopolysaccharide cell wall. This behaviour aligns with chelation theory, which suggests that the inclusion of the metal increases the lipophilicity of the complex, facilitating its penetration through the bacterial cell wall. However, despite the increased activity of the complexes, their efficacy was still lower than that of the reference drugs.
Khalaji and Grivani synthesized a Schiff base pincer ligand and its vanadium(IV) complex (Fig. 4, complex 36).125 The pincer ligand coordinates to oxovanadium through the imine nitrogen, phenolic oxygen, and amino nitrogen in the equatorial plane, with two oxygen atoms from the acetylacetone ligand completing the metal center's coordination sphere.
The antimicrobial activity of complex 36 and its free pincer ligand was evaluated using the disk agar diffusion method. The activity was tested against Gram-negative strains (Escherichia coli, Klebsiella pneumoniae, Salmonella enteritidis) and Gram-positive strains (Staphylococcus aureus and Bacillus cereus). For this experiment, a solution with a concentration of 100 mg mL−1 of each compound was prepared and applied to bacterial cultures, which were then incubated for 24 h at 37 °C.
Contrary to expectations, the free ligand demonstrated better antibacterial activity than complex 36, with inhibition zones of 45.3 mm compared to 20.3 mm, respectively. Additionally, no significant differences in activity were observed between Gram-positive and Gram-negative strains.
In 2021, Azizi and coworkers published a study on a vanadium complex featuring a tetradentate Schiff base with N, N, O, O donor atoms.126 The structure of complex 37 exhibits a square pyramidal geometry, where the vanadium is coordinated by two nitrogen and two oxygen atoms from the ligand, with an oxo group (V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) occupying the apical position (Fig. 4).
O) occupying the apical position (Fig. 4).
The biological activity of complex 37 was evaluated through antibacterial screening using the determination of MIC via the dilution method and minimal bactericidal concentration (MBC) in agar medium. Serial dilutions of the complex in the micromolar range were prepared to assess its ability to inhibit bacterial growth in Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli and Pseudomonas aeruginosa) strains. The complex, controls, and reference drug (tetracycline) were incubated with bacterial cultures for 24 h at 37 °C, after which the MIC and MBC values were determined.
Complex 37 did not exhibit antibacterial activity against E. coli. However, slight bacterial growth inhibition was observed for P. aeruginosa and S. aureus, though the complex was less active than tetracycline. The likely reason for the limited activity is that the lipophilic character of complex 37 may not have been sufficient to facilitate strong interactions with the bacterial cell walls.
In 2021, Sharma and coworkers reported the synthesis of two triazole ligands and their corresponding oxovanadium complexes (Fig. 4, complexes 38a–b).127 The Schiff base ligands contain two triazole units, with either a hydroxyl or methyl substituent on the adjacent aromatic ring. These ligands coordinate the oxovanadium moiety in a tetradentate fashion via nitrogen and sulfur atoms, resulting in a square pyramidal geometry. Triazole moieties are commonly found in biologically active compounds,128–134 which prompted the researchers to evaluate the antibacterial activity of both the free ligands and their vanadium complexes.
The antibacterial activity was assessed using the agar diffusion method, targeting Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus. The compounds were tested at concentrations ranging from 4 mg mL−1 to 0.05 mg mL−1 in DMSO, with neomycin serving as the reference drug.
The results showed that the antibacterial activity of the free ligands and their vanadium complexes was comparable, with inhibition zones for both Gram-negative and Gram-positive strains being similar. This is notable, as Gram-positive bacteria typically show greater susceptibility to antibiotics due to their thinner cell wall. However, the observed activities were lower than those of neomycin.
Recently, Abdel-Rahman and coworkers synthesized an ONN pincer ligand and its corresponding vanadium complex (Fig. 4, complex 39).135 The biological activity of these compounds was investigated through antibacterial, cytotoxic, and antioxidant assays, as well as molecular docking studies. The antibacterial activity was assessed using the disc diffusion and microdilution methods. Solutions of the free ligand and the vanadium complex were prepared in DMSO at a final concentration of 20 μM and tested against four bacterial strains: two Gram-negative (Escherichia coli, Klebsiella pneumoniae) and two Gram-positive (Staphylococcus aureus, Streptococcus mutans). Gentamicin and ampicillin were used as reference drugs for Gram-negative and Gram-positive bacteria, respectively. After 24 h of incubation, the inhibition zones were measured.
The free ligand exhibited lower antibacterial activity compared to complex 39. Interestingly, complex 39 showed similar activity to its cobalt and chromium analogs, although it was less effective than the nickel derivative. It is important to note that the concentration used in this assay was lower than those reported in other studies, making direct comparisons with emerging compounds challenging. The authors suggest that the observed results are consistent with previous studies, where the enhanced activity of metal complexes is attributed to the chelation theory. They also propose that the azomethine group plays a crucial role in interacting with the bacterial cell wall through hydrogen bonding.
Abdel-Rahman and coworkers synthesized a tetracoordinated Schiff base ligand and its corresponding oxovanadium complex (Fig. 4, complex 40).136 To investigate the biological activity of the ligand and complex 40, the authors conducted antibacterial, antifungal, antioxidant, cytotoxic, and DNA binding assays. The antibacterial tests were performed using the agar disc diffusion method and MIC determination by dilution. The compounds were tested at concentrations of 15 and 30 μM against Gram-negative Serratia marcescens and Escherichia coli, as well as Gram-positive Micrococcus luteus, with ofloxacin serving as the positive control. The results were reported as an activity index (%), which compared the inhibition zones of the compounds to those of the reference drug.
Consistent with previous studies, complex 40 exhibited greater antibacterial activity than the free ligand, with the highest efficacy observed against Micrococcus luteus. The inhibition zone for this strain was similar to that of ofloxacin and larger than those observed for other metal complexes, such as zinc.
Antiparasitic activity of vanadium pincer compounds
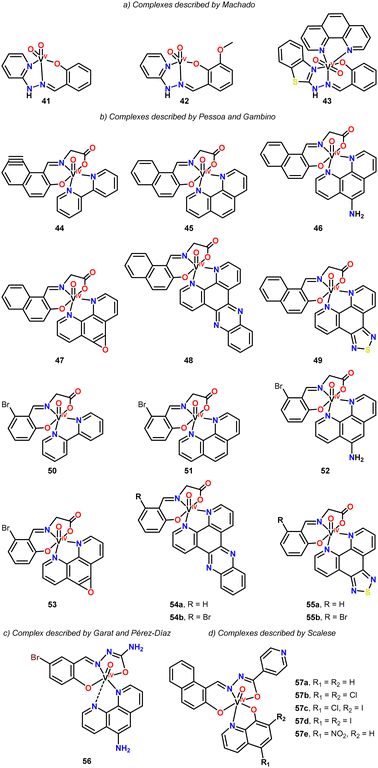
The antiparasitic activity of vanadium complexes has been extensively studied in recent years, targeting various species of parasites, including Entamoeba histolytica, Leishmania species, and Trypanosoma.45,108,137 Among the earliest studies of vanadium complexes with antiparasitic properties in the 21st century, their action against Entamoeba histolytica, Leishmania amazonensis, Leishmania donovani, and Trypanosoma cruzi has been reported.138–141The use of coordination or organometallic vanadium compounds for the treatment of parasitic diseases has emerged as a promising alternative, showing encouraging results. However, a review of recent literature indicates that, over the past decade, vanadium compounds featuring pincer-type ligands have been reported only sparingly.142–144Fig. 5 presents some representative examples. Despite their limited number, the compounds that have been studied exhibit intriguing properties, maintaining them as a focal point of ongoing research.
In 2014, Machado and coworkers reported the synthesis of new vanadium-based agents targeting Trypanosoma cruzi, using NNO pincer ligands (Fig. 5, complexes 41–43).50 These complexes were evaluated against the epimastigote form of the Trypanosoma cruzi strain Dm28c. Complexes 41 and 42 exhibited lower activity compared to their respective free pincer ligands. However, the incorporation of a benzothiazole fragment into the pincer ligand along with 1,10-phenanthroline produced a more potent compound. Complex 43, in particular, demonstrated a 10-fold reduction in IC50 compared to the free ligands, achieving values similar to those of the reference drug, Nifurtimox. This enhanced activity is likely due to the interaction between DNA and the 1,10-phenanthroline ligand, as it is well known that the planarity of this ligand facilitates such interactions.
In 2017, Pessoa and Gambino investigated the anti-Trypanosoma cruzi activity of 14 vanadium complexes featuring ONO pincer ligands and N^N chelates (Fig. 5, complexes 44–55).38,145 The complexes were evaluated against the epimastigote form of the T. cruzi strain CL Brener, and their selectivity toward the parasite was assessed using VERO cells as a mammalian model. The complexes generally exhibited IC50 values in the low micromolar range (1.4 to 4.7 μM), which were comparable to or even lower than the activity of the reference drug, Nifurtimox (2.76 ± 0.19 μM). Additionally, the complexes demonstrated moderate to good selectivity toward the parasite, with selectivity indices (SI) ranging from 7 to 58.
The activity of the vanadium complexes was found to be influenced by the N^N chelate ligands. Complexes containing the bipyridine ligand were the least active, whereas those with the dipyridophenazine ligand exhibited the highest activity. The authors further concluded that these compounds induce apoptosis and cause alterations in the mitochondrial membrane potential in T. cruzi, suggesting this as the mechanism of cell death.
In 2020, Garat and Pérez-Díaz synthesized a pincer vanadium complex featuring 5-bromosalicylaldehyde semicarbazone as the pincer ligand, along with 1,10-phenanthrolin-5-amine as the chelating N^N ligand (Fig. 5, complex 56).146 The complex exhibited an IC50 value of 3.76 ± 0.08 μM in a proliferation assay against T. cruzi, a value comparable to that of the reference drug nifurtimox (2.8 ± 0.2 μM). Regarding the mechanism of parasite growth inhibition, the study determined that neither apoptosis nor necrosis were involved; instead, the complex induced a growth arrest phenomenon in the parasite.
In 2021, Scalese and coworkers synthesized five vanadium ONO-pincer complexes featuring 8-hydroxyquinoline derivatives (Fig. 5, complexes 57a–e).147 These complexes exhibited activity against both the epimastigote and trypomastigote forms of T. cruzi (CL Brener strain). The IC50 values for epimastigotes ranged from 3.45 to 7.70 μM, while for trypomastigotes, the IC50 values were comparable to those of Nifurtimox (0.29–3.02 μM). Notably, trypomastigotes were more sensitive to the vanadium complexes, showing IC50 values that were seven to 70 times lower than those of the reference drug. Additionally, the selectivity of the complexes towards the parasite increased significantly when halogen substituents were included in the hydroxyquinoline moiety.
Anticancer activity of vanadium pincer complexes
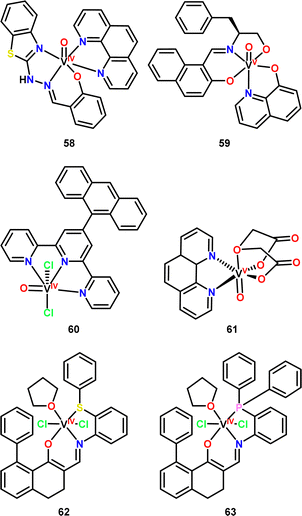
Over the past decade, research into the anticancer activity of vanadium compounds has intensified in response to the urgent need for new therapeutic agents to combat cancer. In this context, vanadium complexes with pincer-type ligands have gained attention. Many of these pincer-type vanadium compounds have been tested against various cancer cell lines, including human ovarian carcinoma cells (MCF-7),50,106,148–152 breast adenocarcinoma cells (A278038,50,122,148,153 and A2780cisR122), prostate cancer cells (PC3),38,50 cervical cancer cells (HeLa,154–157 SiHa,154 and C33A154), hepatocellular carcinoma cells (HEP G2),107,158–161 lung carcinoma cells (A549),151,158 colorectal adenocarcinoma cells (HT-29,157 SW480,153,158 and SW620158), epidermal carcinoma cells (KB-3-1),153 epithelial human breast cancer cells (MDAMB231),38 human osteosarcoma cells (MG-63 and HOS),162 human gastric cancer cells (MGC803),163 human esophageal cancer cells (EC109), colon carcinoma cells (HCT-116),150 and ovary cells (CHO-K1).159Vanadium pincer-type compounds commonly feature heteroatoms such as N, O, P, and S in various triad arrangements to coordinate with the metal centre. The most prevalent configurations include NNN, NNO, ONO, OOO, ONP, and ONS, as illustrated in Fig. 6,50,122,162,163 highlighting the diverse possibilities available for the synthesis and exploration of these compounds in anticancer applications.
Some authors have highlighted the increase in anticancer activity of compounds when moving from the use of the ligands alone to the use of vanadium complexes,150,151,155 even surpassing the cytotoxic capacity of cisplatin.151 It is likely that several ligands exhibit little or no cytotoxic activity in such an way that many authors omit the comparison with their corresponding complexes, only highlighting the cytotoxic activity of the vanadium compounds. Thus, the selectivity or stability of the complex is directly dependant on the ligand used. And it is common to observe the use of co-ligands such as 1,10-phenantholine (As observed in complexes 58 and 6150,162) to increase the stability of the complexes, as shown in a study by Szklarzcwics et al.160 where they state that this type of co-ligands increases the lifetime of the complex by slowing down the oxidation process.
Conclusions
The use of vanadium complexes for pharmacological applications has seen a significant rise in recent years, particularly in addressing diseases such as cancer, diabetes, parasitic infections, and bacterial infections. Despite this progress, the number of studies focusing specifically on vanadium compounds with tridentate pincer-type ligands remains limited. Fortunately, there is a growing trend in exploring these compounds due to the unique properties they offer, particularly in terms of stability, selectivity, and tunability. Vanadium pincer complexes are distinguished by their strong chemical and biological properties, making them promising candidates for a variety of therapeutic applications. Pincer ligands, which commonly contain donor atoms such as nitrogen, oxygen, sulfur, or phosphorus, form highly stable chelating structures. This stability is crucial for ensuring the vanadium complexes remain intact in harsh biological environments, allowing them to exert their pharmacological effects. Additionally, the ease with which pincer ligands can be functionalized provides flexibility in tailoring these complexes for specific biological targets.One notable aspect of vanadium pincer complexes is their multifunctionality. Several of these complexes have demonstrated efficacy in multiple therapeutic areas, such as showing both antiviral and antibacterial activity. This multifunctionality makes these complexes particularly attractive for further research, as they offer the potential for developing multi-targeted therapies. The ability to address multiple pathways or diseases with a single compound is increasingly important in precision medicine and could lead to more effective treatment strategies.
Vanadium's ability to exist in multiple oxidation states (III, IV, V) further enhances the potential of these pincer complexes in pharmacology. This redox flexibility allows vanadium to participate in a wide range of biological processes, including enzymatic regulation and modulation of oxidative stress. This is particularly relevant for diseases like cancer and diabetes, where oxidative stress and signaling dysregulation are key drivers of disease progression. The ability of vanadium pincer complexes to interact with these processes makes them valuable candidates for therapeutic intervention.
Despite the promising properties of vanadium pincer complexes, challenges remain in improving their specificity for biological targets. The use of molecular modeling, structure–activity relationship (SAR) studies, and high-throughput screening will likely play a critical role in addressing this issue. These tools will enable researchers to better understand how vanadium complexes interact with their biological targets, facilitating the design of more selective and potent compounds. Additionally, minimizing the toxicity of vanadium compounds is essential for their clinical use. Careful selection of ligands that enhance bioavailability and reduce the overall toxicity of the complexes will be important in moving these compounds forward in the drug development pipeline.
Hence, vanadium pincer complexes represent a valuable class of compounds for therapeutic development, offering stability, versatility, and multifunctionality. Bidentate N^N ligands further enhance their potential by providing a flexible framework for optimizing biological activity. While more research is needed to fully explore their potential, particularly in improving selectivity and reducing toxicity, vanadium pincer complexes hold great promise for the future of pharmacology, particularly in the treatment of complex diseases such as cancer, diabetes, and infections.
It is important to note that there are currently no drugs including vanadium compounds as active ingredients. It is possible that in the future drugs may be developed that include vanadium pincer-type complexes as active ingredients, emphasizing the relevance of pincer-type ligands to provide greater stability to the complexes they form without inhibiting their biological activity. To do this, it would be necessary to further continue the study of this type of molecules, both in terms of their biological activity and what involves the absorption and elimination of these compounds from the body, in order to shed further light into pharmacokinetic and pharmacodynamic studies.
Author contributions
Writing – original draft preparation, L. H. D.-R., V. R.-M., M. E. M.-N., A. A.-M., J. R. P.-V., J. A. C.-N., M. A. M.-T., H. V. and D. M.-M.; execution and drawing, L. H. D.-R., V. R.-M., M. E. M.-N., A. A.-M., J. R. P.-V., J. A. C.-N., M. A. M.-T., H. V. and D. M.-M.;. writing – review and editing, L. H. D.-R., H. V. and D. M.-M.; visualization and supervision, L. H. D.-R., H. V. and D. M.-M.; funding acquisition, D. M.-M. and H. V. All authors have read and agreed to the published version of the manuscript.Data availability
The data supporting this article has been included as part of the ESI.†Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The financial support of this research by PAPIIT-DGAPA-UNAM (PAPIIT IN223323) and CONACYT A1-S-033933 is gratefully acknowledged.Notes and references
- M. Biswas, K. Kanta Choudhury, A. Banerjee and R. K. Pathak, Coord. Chem. Rev., 2024, 517, 216026 CrossRef CAS.
- J. Costa Pessoa, E. Garribba, M. F. A. Santos and T. Santos-Silva, Coord. Chem. Rev., 2015, 301–302, 49–86 CrossRef CAS.
- J. Costa Pessoa, J. Inorg. Biochem., 2015, 147, 4–24 CrossRef CAS.
- J. M. Winter and B. S. Moore, J. Biol. Chem., 2009, 284, 18577–18581 CrossRef CAS.
- D. Rehder, J. Inorg. Biochem., 2000, 80, 133–136 CrossRef CAS PubMed.
- P. E. M. Siegbahn and W.-J. Wei, Phys. Chem. Chem. Phys., 2024, 26, 1684–1695 RSC.
- D. Sippel and O. Einsle, Nat. Chem. Biol., 2017, 13, 956–960 CrossRef CAS PubMed.
- C. S. Harwood, Annu. Rev. Microbiol., 2020, 74, 247–266 CrossRef CAS.
- M. Rohde, K. Grunau and O. Einsle, Angew. Chem., Int. Ed., 2020, 59, 23626–23630 CrossRef CAS PubMed.
- S. Treviño and A. Diaz, J. Inorg. Biochem., 2020, 208, 111094 CrossRef PubMed.
- M. C. Cam, R. W. Brownsey and J. H. McNeill, Can. J. Physiol. Pharmacol., 2000, 78, 829–847 CrossRef CAS PubMed.
- S. M. Brichard and J.-C. Henquin, Trends Pharm. Sci., 1995, 16, 265–270 CrossRef CAS PubMed.
- E. Rufino-Felipe, R. Colorado-Peralta, V. Reyes-Márquez, H. Valdés and D. Morales-Morales, Anti-Cancer Agents Med. Chem., 2021, 21, 938–948 CrossRef CAS PubMed.
- W. Liu and R. Gust, Coord. Chem. Rev., 2016, 329, 191–213 CrossRef CAS.
- K. D. Mjos and C. Orvig, Chem. Rev., 2014, 114, 4540–4563 CrossRef CAS.
- W. Liu and R. Gust, Chem. Soc. Rev., 2013, 42, 755–773 RSC.
- A. Casini, C. Gabbiani, E. Michelucci, G. Pieraccini, G. Moneti, P. J. Dyson and L. Messori, J. Biol. Inorg. Chem., 2009, 14, 761–770 CrossRef CAS PubMed.
- L. Ignacio Esteban, C.-V. Juan Fernando, V. Ana Laura Di and E. Susana Beatriz, Curr. Med. Chem., 2017, 24, 112–148 CrossRef PubMed.
- R. T. Mertens, S. Gukathasan, A. S. Arojojoye, C. Olelewe and S. G. Awuah, Chem. Rev., 2023, 123, 6612–6667 CrossRef CAS.
- Y. C. Ong, S. Roy, P. C. Andrews and G. Gasser, Chem. Rev., 2019, 119, 730–796 CrossRef CAS.
- B. Englinger, C. Pirker, P. Heffeter, A. Terenzi, C. R. Kowol, B. K. Keppler and W. Berger, Chem. Rev., 2019, 119, 1519–1624 CrossRef CAS.
- X. Wang, X. Wang, S. Jin, N. Muhammad and Z. Guo, Chem. Rev., 2019, 119, 1138–1192 CrossRef CAS PubMed.
- D. Rehder, Inorg. Chim. Acta, 2017, 455, 378–389 CrossRef CAS.
- D. Rehder, Metallomics, 2015, 7, 730–742 CrossRef CAS.
- K. Hashmi, Satya, S. Gupta, A. Siddique, T. Khan and S. Joshi, J. Trace Elem. Med. Biol., 2023, 79, 127245 CrossRef CAS.
- A. L. De Sousa-Coelho, G. Fraqueza and M. Aureliano, Pharmaceuticals, 2024, 17, 12 Search PubMed.
- E. Kioseoglou, S. Petanidis, C. Gabriel and A. Salifoglou, Coord. Chem. Rev., 2015, 301–302, 87–105 CrossRef CAS.
- D. Rehder, Inorg. Chim. Acta, 2020, 504, 119445 CrossRef CAS.
- D. Rehder, ChemTexts, 2018, 4, 20 CrossRef.
- E. Del Carpio, L. Hernández, C. Ciangherotti, V. Villalobos Coa, L. Jiménez, V. Lubes and G. Lubes, Coord. Chem. Rev., 2018, 372, 117–140 CrossRef CAS PubMed.
- D. Rehder, Future Med. Chem., 2016, 8, 325–338 CrossRef CAS.
- J. C. Pessoa, S. Etcheverry and D. Gambino, Coord. Chem. Rev., 2015, 301–302, 24–48 CrossRef CAS PubMed.
- A. P. Singh, S. Roy and I. C. Maurya, Transition Met. Chem., 2024, 49, 101–119 CrossRef CAS.
- S. Kumar, S. Kumari, R. Karan, A. Kumar, R. K. Rawal and P. Kumar Gupta, Inorg. Chem. Commun., 2024, 161, 112014 CrossRef CAS.
- J. Wang, B. Huang, L. Wang, G. Jiang, J. Cheng, Y. Xiong, J. Wang and X. Liao, J. Chem. Res., 2021, 45, 1016–1021 CrossRef CAS.
- K. Saraswathi and S. Angappan, Mini-Rev. Med. Chem., 2021, 21, 1909–1924 CrossRef.
- Saswati, P. Adão, S. Majumder, S. P. Dash, S. Roy, M. L. Kuznetsov, J. Costa Pessoa, C. S. B. Gomes, M. R. Hardikar, E. R. T. Tiekink and R. Dinda, Dalton Trans., 2018, 47, 11358–11374 RSC.
- G. Scalese, M. F. Mosquillo, S. Rostán, J. Castiglioni, I. Alho, L. Pérez, I. Correia, F. Marques, J. Costa Pessoa and D. Gambino, J. Inorg. Biochem., 2017, 175, 154–166 CrossRef CAS.
- N. Ladislav and B. K. Samuel, J. Cancer Res. Updates, 2014, 3, 97–102 Search PubMed.
- K. H. Thompson and C. Orvig, J. Inorg. Biochem., 2006, 100, 1925–1935 CrossRef CAS PubMed.
- K. H. Thompson, J. Lichter, C. LeBel, M. C. Scaife, J. H. McNeill and C. Orvig, J. Inorg. Biochem., 2009, 103, 554–558 CrossRef CAS PubMed.
- B. Priya, A. Kumar and N. Sharma, J. Chem. Res., 2020, 44, 460–470 CrossRef CAS.
- B. Priya, A. Kumar and N. Sharma, Aust. J. Chem., 2020, 73, 61–72 CrossRef CAS.
- S. Sharma, D. Das, B. Sadhu and N. Sharma, J. Coord. Chem., 2022, 75, 689–706 Search PubMed.
- C.-T. Brenda, R.-F. Norma, R.-L. Marcela, L.-V. Nelly and F. Teresa, J. Trace Elem. Med. Biol., 2023, 78, 127201 CrossRef CAS.
- B. M. Dorsey, C. C. McLauchlan and M. A. Jones, Front. Chem., 2018, 6, 109 CrossRef.
- A. T. Christensen, C. C. McLauchlan, A. Dolbecq, P. Mialane and M. A. Jones, Oxid. Med. Cell. Longevity, 2016, 2016, 9025627 CrossRef.
- P. d A. Machado, V. Z. Mota, A. C. d L. Cavalli, G. S. G. de Carvalho, A. D. Da Silva, J. Gameiro, A. Cuin and E. S. Coimbra, Acta Trop., 2015, 148, 120–127 CrossRef CAS.
- J. Benítez, I. Correia, L. Becco, M. Fernández, B. Garat, H. Gallardo, G. Conte, M. L. Kuznetsov, A. Neves, V. Moreno, J. Costa Pessoa and D. Gambino, Z. Anorg. Allg. Chem., 2013, 639, 1417–1425 CrossRef.
- I. Machado, M. Fernández, L. Becco, B. Garat, R. F. Brissos, N. Zabarska, P. Gamez, F. Marques, I. Correia, J. Costa Pessoa and D. Gambino, Inorg. Chim. Acta, 2014, 420, 39–46 CrossRef CAS.
- J. L. Nevarez, A. Turmo, J. Hu and R. P. Hausinger, ChemCatChem, 2020, 12, 4242–4254 CrossRef CAS.
- D. M. Roddick, Top. Organomet. Chem., 2013, 40, 49–88 CrossRef CAS.
- H. Valdés and D. Morales-Morales, in Catalysis for a Sustainable Environment, ed. A. J. L. Pombeiro, M. Sutradhar and E. C. B. A. Alegria, 2024, ch. 18, pp. 389–408 Search PubMed.
- H. Valdés, R. Osorio-Yañez, E. Rufino-Felipe and D. Morales-Morales, in Comprehensive Organometallic Chemistry IV, ed. G. Parkin, K. Meyer and D. O’hare, Elsevier, Oxford, 4th edn, 2022, vol. 7, pp. 816–867 Search PubMed.
- H. Valdes, J. M. German-Acacio, G. van Koten and D. Morales-Morales, Dalton Trans., 2022, 51, 1724–1744 RSC.
- H. Valdés, E. Rufino-Felipe and D. Morales-Morales, J. Organomet. Chem., 2019, 898, 120864 CrossRef.
- H. Valdés, M. A. García-Eleno, D. Canseco-Gonzalez and D. Morales-Morales, ChemCatChem, 2018, 10, 3136–3172 CrossRef.
- J. H. Choi, H. Valdés, D. Pingen and M. H. G. Prechtl, Pincer Compounds: Chemistry and Applications, Elsevier, 2018, pp. 273–294 Search PubMed.
- Pincer Compounds Chemistry and Applications, ed. D. Morales-Morales, Elsevier, 2018 Search PubMed.
- M. Asay and D. Morales-Morales, Top. Organomet. Chem., 2016, 54, 239–268 CrossRef CAS.
- D. Morales-Morales and C. Jensen, The Chemistry of Pincer Compounds, 2007 Search PubMed.
- D. Morales-Morales, Rev. Soc. Quim. Mex., 2004, 48, 338–346 CAS.
- D. Benito-Garagorri and K. Kirchner, Acc. Chem. Res., 2008, 41, 201–213 CrossRef CAS.
- Y. Wang, Z. Huang, G. Liu and Z. Huang, Acc. Chem. Res., 2022, 55, 2148–2161 CrossRef CAS.
- T. Zell and D. Milstein, Acc. Chem. Res., 2015, 48, 1979–1994 CrossRef CAS.
- L. Alig, M. Fritz and S. Schneider, Chem. Rev., 2019, 119, 2681–2751 CrossRef CAS.
- J. Choi, A. H. R. MacArthur, M. Brookhart and A. S. Goldman, Chem. Rev., 2011, 111, 1761–1779 CrossRef CAS.
- A. Kumar, T. M. Bhatti and A. S. Goldman, Chem. Rev., 2017, 117, 12357–12384 CrossRef CAS PubMed.
- N. Selander and K. J. Szabó, Chem. Rev., 2011, 111, 2048–2076 CrossRef CAS PubMed.
- M. E. Van Der Boom and D. Milstein, Chem. Rev., 2003, 103, 1759–1792 CrossRef CAS PubMed.
- F. Bertini, M. Glatz, N. Gorgas, B. Stoger, M. Peruzzini, L. F. Veiros, K. Kirchner and L. Gonsalvi, Chem. Sci., 2017, 8, 5024–5029 RSC.
- P. Kang, T. J. Meyer and M. Brookhart, Chem. Sci., 2013, 4, 3497–3502 RSC.
- E. Peris and R. H. Crabtree, Chem. Soc. Rev., 2018, 47, 1959–1968 RSC.
- A. Kasera, J. P. Biswas, A. Ali Alshehri, S. Ahmed Al-Thabaiti, M. Mokhtar and D. Maiti, Coord. Chem. Rev., 2023, 475, 214915 CrossRef CAS.
- H. Li, B. Zheng and K. W. Huang, Coord. Chem. Rev., 2015, 293–294, 116–138 CrossRef CAS.
- M. Q. Slagt, D. A. P. v Zwieten, A. J. C. M. Moerkerk, R. J. M. K. Gebbink and G. V. Koten, Coord. Chem. Rev., 2004, 248, 2275–2282 CrossRef CAS.
- H. A. Younus, N. Ahmad, W. Su and F. Verpoort, Coord. Chem. Rev., 2014, 276, 112–152 CrossRef CAS.
- J. M. Serrano-Becerra and D. Morales-Morales, Curr. Org. Synth., 2009, 6, 169–192 CrossRef CAS.
- D. Morales-Morales, Mini-Rev. Org. Chem., 2008, 5, 141–152 CrossRef CAS.
- K. J. Szabó, Top. Organomet. Chem., 2013, 40, 203–242 CrossRef PubMed.
- H.-T. Zhang and M.-T. Zhang, Top. Organomet. Chem., 2021, 68, 379–449 CrossRef CAS.
- H. Valdés, J. M. Germán-Acacio and D. Morales-Morales, in Organic Materials as Smart Nanocarriers for Drug Delivery, ed. A. M. Grumezescu, Elsevier - William Andrew, 2018, pp. 245–291 Search PubMed.
- H. Valdés, L. González-Sebastián and D. Morales-Morales, J. Organomet. Chem., 2017, 845, 229–257 CrossRef.
- M. Papanikolaou, S. Hadjithoma, O. Keramidas, C. Drouza, A. Amoiridis, A. Themistokleous, S. C. Hayes, H. N. Miras, P. Lianos, A. C. Tsipis, T. A. Kabanos and A. D. Keramidas, Inorg. Chem., 2024, 63, 3229–3249 CrossRef CAS PubMed.
- S. Wu, Z. Wu, Q. Ge, X. Zheng and Z. Yang, Org. Biomol. Chem., 2021, 19, 5254–5273 RSC.
- A. S. Estrada-Montaño, A. D. Ryabov, A. Gries, C. Gaiddon and R. Le Lagadec, Eur. J. Inorg. Chem., 2017, 1673–1678 CrossRef.
- A. Oulmidi, S. Radi, A. Idir, A. Zyad, I. Kabach, M. Nhiri, K. Robeyns, A. Rotaru and Y. Garcia, RSC Adv., 2021, 11, 34742–34753 RSC.
- A. Sánchez-Mora, E. Briñez, A. Pico, L. González-Sebastián, J. Antonio Cruz-Navarrro, A. Arenaza-Corona, N. Puentes-Díaz, J. Alí-Torres, V. Reyes-Márquez and D. Morales-Morales, Chem. Biodiversity, 2024, 21, e202400995 CrossRef.
- S. G. Churusova, D. V. Aleksanyan, E. Y. Rybalkina, O. Y. Susova, V. V. Brunova, R. R. Aysin, Y. V. Nelyubina, A. S. Peregudov, E. I. Gutsul, Z. S. Klemenkova and V. A. Kozlov, Inorg. Chem., 2017, 56, 9834–9850 CrossRef CAS PubMed.
- Y. Ye, S. Wu, Z. Wu, Q. Lei, X. Yang, H. Cai, T. Jiang, Y. Chen, M. Dai and Z. Yang, J. Organomet. Chem., 2024, 1020, 123346 CrossRef CAS.
- L. Tabrizi and H. Chiniforoshan, Dalton Trans., 2017, 46, 14164–14173 RSC.
- D. V. Aleksanyan, A. V. Konovalov, S. G. Churusova, E. Y. Rybalkina, A. S. Peregudov, S. A. Aksenova, E. I. Gutsul, Z. S. Klemenkova and V. A. Kozlov, Pharmaceutics, 2023, 15, 1088 CrossRef CAS.
- J.-J. Qu, L.-L. Shi, Y.-B. Wang, J. Yan, T. Shao, X.-Q. Hao, J.-X. Wang, H.-Y. Zhang, J.-F. Gong and B. Song, Molecules, 2022, 27, 3106 CrossRef CAS PubMed.
- F. Salsi, G. Bulhões Portapilla, K. Schutjajew, M. Roca Jungfer, A. Goulart, A. Hagenbach, S. de Albuquerque and U. Abram, Eur. J. Inorg. Chem., 2019, 4455–4462 CrossRef CAS.
- A. Aragón-Muriel, B. A. Aguilar-Castillo, E. Rufino-Felipe, H. Valdés, L. González-Sebastián, R. N. Osorio-Yáñez, Y. Liscano, V. Gómez-Benítez, D. Polo-Cerón and D. Morales-Morales, Polyhedron, 2022, 227, 116115 CrossRef.
- A. Aragón-Muriel, V. Reyes-Márquez, F. Cañavera-Buelvas, J. R. Parra-Unda, F. Cuenú-Cabezas, D. Polo-Cerón, R. Colorado-Peralta, G. V. Suárez-Moreno, B. A. Aguilar-Castillo and D. Morales-Morales, Inorganics, 2022, 10, 134 CrossRef.
- S.-Y. Tan, X.-Z. Chen, A. Cao and H. Wang, Biol. Trace Elem. Res., 2023, 201, 2917–2926 CrossRef CAS PubMed.
- T. Ueki, N. Yamaguchi, Romaidi, Y. Isago and H. Tanahashi, Coord. Chem. Rev., 2015, 301–302, 300–308 CrossRef CAS.
- D. Fattorini, A. Notti, M. Nigro and F. Regoli, Environ. Sci. Pollut. Res., 2010, 17, 220–228 CrossRef CAS PubMed.
- Y. Nobuo, Y. Masafumi, K. Kei and U. Tatsuya, Zool. Sci., 2016, 33, 266–271 Search PubMed.
- S. Braeuer, M. Walenta, L. Steiner and W. Goessler, J. Anal. At. Spectrom., 2021, 36, 954–967 RSC.
- J. J. R. Fraústo da Silva, Chem. Speciation Bioavailability, 1989, 1, 139–150 CrossRef.
- M. Kaya, K. Çavuşoğlu, E. Yalçin and A. Acar, Sci. Rep., 2023, 13, 8493 CrossRef CAS PubMed.
- J. H. Toney, M. S. Murthy and T. J. Marks, Chem. – Biol. Interact., 1985, 56, 45–54 CrossRef CAS PubMed.
- J. C. Pessoa, M. F. A. Santos, I. Correia, D. Sanna, G. Sciortino and E. Garribba, Coord. Chem. Rev., 2021, 449, 214192 CrossRef CAS.
- N. Patel, A. K. Prajapati, R. N. Jadeja, R. N. Patel, S. K. Patel, I. P. Tripathi, N. Dwivedi, V. K. Gupta and R. J. Butcher, Polyhedron, 2020, 180, 114434 CrossRef CAS.
- L.-P. Lu, J.-H. Liu, S.-H. Cen, Y.-L. Jiang and G.-Q. Hu, Bioorg. Med. Chem. Lett., 2019, 29, 681–683 CrossRef CAS PubMed.
- A. A. Sharfalddin, I. M. Al-Younis, H. A. Mohammed, M. Dhahri, F. Mouffouk, H. Abu Ali, M. J. Anwar, K. A. Qureshi, M. A. Hussien, M. Alghrably, M. Jaremko, N. Alasmael, J. I. Lachowicz and A.-H. Emwas, Inorganics, 2022, 10, 244 CrossRef CAS.
- O. A. Chaves, M. C. C. de Oliveira, C. M. C. de Salles, F. M. Martins, B. A. Iglesias and D. F. Back, J. Inorg. Biochem., 2019, 200, 110800 CrossRef CAS PubMed.
- W. Feng, X. Han, H. Hu, M. Chang, L. Ding, H. Xiang, Y. Chen and Y. Li, Nat. Commun., 2021, 12, 2203 CrossRef CAS PubMed.
- M. Aureliano, A. L. De Sousa-Coelho, C. C. Dolan, D. A. Roess and D. C. Crans, Int. J. Mol. Sci., 2023, 24, 5382 CrossRef CAS PubMed.
- M. S. S. Adam and H. Elsawy, J. Photochem. Photobiol., B, 2018, 184, 34–43 CrossRef CAS.
- T. Zandvakili, S. J. Fatemi, S. Y. Ebrahimipour, H. Ebrahimnejad, M. Dusek and V. Eigner, ChemistrySelect, 2021, 6, 10405–10411 CrossRef CAS.
- J. Shi, Y. Wei, Y. Zhang, J. Tang, H. Bian, Q. Yu and F. Huang, Polyhedron, 2019, 162, 81–90 CrossRef CAS.
- M. Stylianou, C. Drouza, J. Giapintzakis, G. I. Athanasopoulos and A. D. Keramidas, Inorg. Chem., 2015, 54, 7218–7229 CrossRef CAS.
- I. Hadjiadamou, M. Vlasiou, S. Spanou, Y. Simos, G. Papanastasiou, E. Kontargiris, I. Dhima, V. Ragos, S. Karkabounas, C. Drouza and A. D. Keramidas, J. Inorg. Biochem., 2020, 208, 111074 CrossRef CAS PubMed.
- K. H. Thompson, J. H. McNeill and C. Orvig, Chem. Rev., 1999, 99, 2561–2572 CrossRef CAS.
- K. H. Thompson and C. Orvig, J. Chem. Soc., Dalton Trans., 2000, 2885–2892 RSC.
- O. A. El-Gammal, M. Gaber and S. A. Mandour, Appl. Organomet. Chem., 2020, 34, e5699 CrossRef CAS.
- R. N. Patel and Y. P. Singh, J. Mol. Struct., 2018, 1153, 162–169 CrossRef CAS.
- N. Patel, A. K. Prajapati, R. N. Jadeja, I. P. Tripathi and N. Dwivedi, J. Indian Chem. Soc., 2021, 98, 100047 CrossRef CAS.
- I. Correia, P. Adão, S. Roy, M. Wahba, C. Matos, M. R. Maurya, F. Marques, F. R. Pavan, C. Q. F. Leite, F. Avecilla and J. Costa Pessoa, J. Inorg. Biochem., 2014, 141, 83–93 CrossRef CAS PubMed.
- P. Adão, M. L. Kuznetsov, S. Barroso, A. M. Martins, F. Avecilla and J. C. Pessoa, Inorg. Chem., 2012, 51, 11430–11449 CrossRef PubMed.
- P. Jayaseelan, E. Akila, M. Usha Rani and R. Rajavel, J. Saudi Chem. Soc., 2016, 20, 625–634 CrossRef CAS.
- B. S. Far, G. Grivani, A. D. Khalaji, M. Khorshidi and A. Gholizadeh, J. Mol. Struct., 2019, 1197, 361–368 CrossRef CAS.
- S. Askarian, S. A. Beyramabadi, F. Badmasti, F. S. Heravi, A. M. A. Tabrizi, H. Azizi, M. A. Mohaghegh, A. Morsali, A. Bazian, M. R. Bozorgmehr and O. Azizi, J. Mol. Struct., 2021, 1246, 131189 CrossRef CAS.
- B. P. Sharma, S. K. Pandey, B. P. Marasini, S. Shrestha and M. L. Sharma, J. Nepal Chem. Soc., 2021, 42, 56–63 CrossRef.
- O. Gupta, T. Pradhan and G. Chawla, J. Mol. Struct., 2023, 1274, 134487 CrossRef CAS.
- U. Salma, S. Ahmad, M. Zafer Alam and S. A. Khan, J. Mol. Struct., 2024, 1301, 137240 CrossRef CAS.
- A. K. Agrahari, P. Bose, M. K. Jaiswal, S. Rajkhowa, A. S. Singh, S. Hotha, N. Mishra and V. K. Tiwari, Chem. Rev., 2021, 121, 7638–7956 CrossRef CAS PubMed.
- K. Kacprzak, I. Skiera, M. Piasecka and Z. Paryzek, Chem. Rev., 2016, 116, 5689–5743 CrossRef CAS PubMed.
- V. K. Tiwari, B. B. Mishra, K. B. Mishra, N. Mishra, A. S. Singh and X. Chen, Chem. Rev., 2016, 116, 3086–3240 CrossRef CAS PubMed.
- N. Z. Fantoni, A. H. El-Sagheer and T. Brown, Chem. Rev., 2021, 121, 7122–7154 CrossRef CAS PubMed.
- A. Moulin, M. Bibian, A.-L. Blayo, S. El Habnouni, J. Martinez and J.-A. Fehrentz, Chem. Rev., 2010, 110, 1809–1827 CrossRef CAS PubMed.
- L. H. Abdel-Rahman, M. S. S. Adam, N. Al-Zaqri, M. R. Shehata, H. El-Sayed Ahmed and S. K. Mohamed, Arab. J. Chem., 2022, 15, 103737 CrossRef CAS.
- L. H. Abdel-Rahman, M. T. Basha, B. S. Al-Farhan, M. R. Shehata and E. M. Abdalla, Appl. Organomet. Chem., 2022, 36, e6484 CrossRef CAS.
- D. Gambino, Coord. Chem. Rev., 2011, 255, 2193–2203 CrossRef CAS.
- N. Bharti, Shailendra, M. T. Gonzalez Garza, D. E. Cruz-Vega, J. Castro-Garza, K. Saleem, F. Naqvi, M. R. Maurya and A. Azam, Bioorg. Med. Chem. Lett., 2002, 12, 869–871 CrossRef CAS.
- G. R. Noleto, A. L. R. Mercê, M. Iacomini, P. A. J. Gorin, V. T. Soccol and M. B. M. Oliveira, Mol. Cell. Biochem., 2002, 233, 73–83 CrossRef CAS.
- A. K. Haldar, S. Banerjee, K. Naskar, D. Kalita, N. S. Islam and S. Roy, Exp. Parasitol., 2009, 122, 145–154 CrossRef CAS.
- C. Urquiola, M. Vieites, G. Aguirre, A. Marín, B. Solano, G. Arrambide, P. Noblía, M. L. Lavaggi, M. H. Torre, M. González, A. Monge, D. Gambino and H. Cerecetto, Biorg. Med. Chem., 2006, 14, 5503–5509 CrossRef CAS.
- M. Fernández, L. Becco, I. Correia, J. Benítez, O. E. Piro, G. A. Echeverria, A. Medeiros, M. Comini, M. L. Lavaggi, M. González, H. Cerecetto, V. Moreno, J. C. Pessoa, B. Garat and D. Gambino, J. Inorg. Biochem., 2013, 127, 150–160 CrossRef PubMed.
- G. Scalese, J. Benítez, S. Rostán, I. Correia, L. Bradford, M. Vieites, L. Minini, A. Merlino, E. L. Coitiño, E. Birriel, J. Varela, H. Cerecetto, M. González, J. C. Pessoa and D. Gambino, J. Inorg. Biochem., 2015, 147, 116–125 CrossRef CAS.
- M. Fernández, J. Varela, I. Correia, E. Birriel, J. Castiglioni, V. Moreno, J. Costa Pessoa, H. Cerecetto, M. González and D. Gambino, Dalton Trans., 2013, 42, 11900–11911 RSC.
- G. Scalese, I. Correia, J. Benítez, S. Rostán, F. Marques, F. Mendes, A. P. Matos, J. Costa Pessoa and D. Gambino, J. Inorg. Biochem., 2017, 166, 162–172 CrossRef CAS PubMed.
- M. F. Mosquillo, P. Smircich, A. Lima, S. A. Gehrke, G. Scalese, I. Machado, D. Gambino, B. Garat and L. Pérez-Díaz, Bioinorg. Chem. Appl., 2020, 2020, 1634270 Search PubMed.
- G. Scalese, I. Machado, G. Salinas, L. Pérez-Díaz and D. Gambino, Molecules, 2021, 26, 5375 CrossRef CAS.
- I. Correia, S. Roy, C. P. Matos, S. Borovic, N. Butenko, I. Cavaco, F. Marques, J. Lorenzo, A. Rodríguez, V. Moreno and J. C. Pessoa, J. Inorg. Biochem., 2015, 147, 134–146 CrossRef CAS.
- S. Y. Ebrahimipour, I. Sheikhshoaie, A. C. Kautz, M. Ameri, H. Pasban-Aliabadi, H. Amiri Rudbari, G. Bruno and C. Janiak, Polyhedron, 2015, 93, 99–105 CrossRef CAS.
- M. S. S. Adam, O. M. El-Hady and F. Ullah, RSC Adv., 2019, 9, 34311–34329 RSC.
- F. Chen, Z. Gao, C. You, H. Wu, Y. Li, X. He, Y. Zhang, Y. Zhang and B. Sun, Dalton Trans., 2019, 48, 15160–15169 RSC.
- P. Mokhtari and G. Mohammadnezhad, Polyhedron, 2022, 215, 115655 CrossRef CAS.
- C. R. Kowol, N. V. Nagy, T. Jakusch, A. Roller, P. Heffeter, B. K. Keppler and É. A. Enyedy, J. Inorg. Biochem., 2015, 152, 62–73 CrossRef CAS.
- R. S. Nair, M. Kuriakose, V. Somasundaram, V. Shenoi, M. R. P. Kurup and P. Srinivas, Life Sci., 2014, 116, 90–97 CrossRef CAS PubMed.
- S. P. Dash, A. K. Panda, S. Pasayat, R. Dinda, A. Biswas, E. R. T. Tiekink, S. Mukhopadhyay, S. K. Bhutia, W. Kaminsky and E. Sinn, RSC Adv., 2015, 5, 51852–51867 RSC.
- A. Kumar, I. Pant, A. Dixit, S. Banerjee, B. Banik, R. Saha, P. Kondaiah and A. R. Chakravarty, J. Inorg. Biochem., 2017, 174, 45–54 CrossRef CAS PubMed.
- S. Lima, A. Banerjee, G. Sahu, S. A. Patra, K. Sahu, T. Sasamori, G. Sciortino, E. Garribba and R. Dinda, J. Inorg. Biochem., 2022, 233, 111853 CrossRef CAS PubMed.
- A. Adach, M. Daszkiewicz, M. Tyszka-Czochara and B. Barszcz, RSC Adv., 2015, 5, 85470–85479 RSC.
- M. Tyszka-Czochara, A. Adach, T. Grabowski, P. Konieczny, P. Pasko, J. Ortyl, T. Świergosz and M. Majka, Int. J. Mol. Sci., 2021, 22, 1802 CrossRef CAS PubMed.
- J. Szklarzewicz, A. Jurowska, M. Hodorowicz, G. Kazek, M. Głuch-Lutwin, J. Sapa and M. Papież, J. Mol. Struct., 2021, 1224, 129205 CrossRef CAS.
- G. Kazek, M. Głuch-Lutwin, B. Mordyl, E. Menaszek, M. Kubacka, A. Jurowska, D. Cież, B. Trzewik, J. Szklarzewicz and M. A. Papież, Pharmaceuticals, 2024, 17, 229 CrossRef CAS PubMed.
- A. Tesmar, D. Wyrzykowski, R. Kruszyński, K. Niska, I. Inkielewicz-Stępniak, J. Drzeżdżon, D. Jacewicz and L. Chmurzyński, Biometals, 2017, 30, 261–275 CrossRef CAS PubMed.
- L.-P. Lu, F.-Z. Suo, Y.-L. Feng, L.-L. Song, Y. Li, Y.-J. Li and K.-T. Wang, Eur. J. Med. Chem., 2019, 176, 1–10 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj04551c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |