Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Overcoming the energy–water nexus in dry regions – water-positive production of green hydrogen carriers and base chemicals: the DryHy project – technical aspects†
Victor
Selmert‡
 *a,
Leandros
Paschalidis‡
*a,
Leandros
Paschalidis‡
 *a,
Nicolas
Kruse§
*a,
Nicolas
Kruse§
 a,
Steffen
Dirkes
a,
Steffen
Dirkes
 a,
Ansgar
Kretzschmar
a,
Ansgar
Kretzschmar
 a,
Gbenga
Jerome
ab,
Carl
Jung
a,
Gbenga
Jerome
ab,
Carl
Jung
 ab,
Lu
Xu
ab,
Nils
Beltermann
c,
Hermann
Tempel
ab,
Lu
Xu
ab,
Nils
Beltermann
c,
Hermann
Tempel
 a,
Roland
Peters
a,
Roland
Peters
 a,
Remzi Can
Samsun
a,
Remzi Can
Samsun
 a and
Rüdiger-A.
Eichel
a and
Rüdiger-A.
Eichel
 abd
abd
aForschungszentrum Jülich GmbH, Institute of Energy Technologies, Fundamental Electrochemistry, IET-1, Wilhelm-Johnen-Straße, 52428 Jülich, Germany. E-mail: v.selmert@fz-juelich.de; l.paschalidis@fz-juelich.de
bInstitute of Physical Chemistry, RWTH Aachen University, 52074 Aachen, Germany
cForschungszentrum Jülich GmbH, Institute of Energy Technologies, Electrochemical Process Engineering, IET-4, Wilhelm-Johnen-Straße, 52428 Jülich, Germany
dFaculty of Mechanical Engineering, RWTH Aachen University, 52062 Aachen, Germany
First published on 25th February 2025
Abstract
The application of Direct Air Capture (DAC) for extracting CO2 from the atmosphere has a great potential to reduce net CO2 emissions and help achieve climate goals. Besides storing the separated CO2, it can be used as a carbon feedstock for producing CO2-neutral e-fuels, marking a critical research focus area. Despite advancements in various DAC technologies and processes, their large-scale implementation remains limited, among other reasons, because of the large amounts of energy required to power such processes. This article explores the utilization of DAC for water-conscious production of methanol in sunny regions, using cost-efficient photovoltaic power. The selected approach is presented, which involves a process on demonstrator scale with amine-based DAC for CO2 and water separation from air, high-temperature electrolysis using solid oxide electrolysis cells (SOEC) for syngas production, and subsequent methanol synthesis. We also discuss alternative methods, potential locations, and implementation strategies, highlighting the advantages but also the challenges of producing green methanol in sunny regions outside Germany.
1 Introduction
The anthropogenic climate change is one of the grand challenges of this century. Due to the vast emissions of greenhouse gases such as CO2, the average surface temperature has risen by 1.09 °C compared to the pre-industrial (1850–1900) age and is expected to exceed 2 °C by 2100 if emissions continue unabated.1 In order to mitigate climate change a carbon neutral economy has to be achieved by preventing CO2 emissions and employing negative emission technologies (NET) for processes that cannot be made CO2 neutral.2,3 There are two key approaches for this transformation: a fuel shift from fossil energy carriers to renewable production of electricity and the development of circular CO2-technologies, often termed as power-to-X.4For Germany, studies show that it will be challenging to satisfy the energy demand with domestic renewable power production.5–7 Consequently, energy imports will play an important role in achieving climate neutrality, as these studies assume that domestic renewable power generation will be complemented by green energy imports, especially in periods of low energy output from wind and solar. Additionally, renewable power can be harvested more efficiently in other regions. For example, Fig. 1 shows the average photovoltaic potential around the globe. As anticipated, regions around the subtropics exhibit significantly higher solar potential compared to northern countries. This includes most of Africa, especially the north and the south, Australia, Mexico, the USA, Chile as well as Arabia. This geographic advantage is crucial for the efficient and cost-effective supply of renewable power.
 | ||
| Fig. 1 The average photovoltaic power potential around the globe. Figure adapted from “Solar resource map” © 2021 Solargis licensed under CC BY-SA 4.0 (https://solargis.com). | ||
However, most of these regions contain a vast amount of dry land with low infrastructure and low energy demand, e.g., wide parts of Africa, Arabia or Australia. Therefore, there is a dire need for energy carrier technologies to transfer the energy from its point of generation to the demand side. Energy transfer via high-voltage transmission lines is often not an option, as the suitable infrastructure at the generation side might be missing and transport over extremely large distances (e.g., continents) is not viable.8 Therefore, alternative energy carriers are necessary such as hydrogen and its derivatives.2,9,10 Here the energy is stored in chemical bonds and transportation of the chemical compounds can be facilitated by pipelines, tank vessels and fuel trucks. Additionally, producing hydrogen or its derivatives from renewable power has the added benefit that these compounds can be used as commodity chemicals as well and thereby defossilize the chemical industry.
Among the potential candidates, methanol appears to be very promising: firstly, methanol is a liquid with reasonable volumetric energy density making transportation, e.g., in a tank vessel, more feasible compared to hydrogen and secondly, allows for easy handling and storage.11–13 Moreover, as of today, the market size of methanol is already large with more than 100 Mt y−1 (ref. 13 and 14) owing to its use in fuel blending or use in the chemical industry, e.g., in the methanol-to-olefin process for the synthesis of polymers. Lastly, the synthesis of methanol from syngas is well established, which eliminates the need to develop entirely new synthesis routes.15
Nonetheless, there are multiple challenges for the synthesis of cost-efficient, green methanol. First and foremost, cost-efficient renewable power generation is needed, as many studies on the production of e-fuels show that the production costs are mainly dependent on the costs of the primary energy source.17–19 Additionally, to produce methanol, a CO2-negative carbon feedstock is required, e.g., biomass or CO2 from direct air capture (DAC), and a hydrogen source, typically water. However, as illustrated in Fig. 2, the aridity index is particular low in subtropical regions,16 which coincide with areas identified as having a high photovoltaic power potential in Fig. 1. This indicates an inverse correlation between high solar irradiation and the availability of water, with regions experiencing very high solar irradiation typically being arid.20 Thus, in order to avoid competing with drinking water, it is necessary to generate green methanol water-neutral or even water-positive.
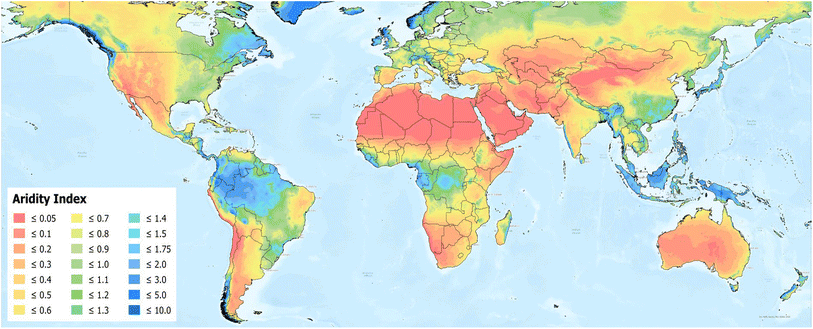 | ||
| Fig. 2 The aridity index around the globe. The aridity index is defined as the mean annual precipitation in relation to the mean annual reference evapotranspiration.16 Figure adapted from Zomer et al.16 licensed under CC BY-SA 4.0. | ||
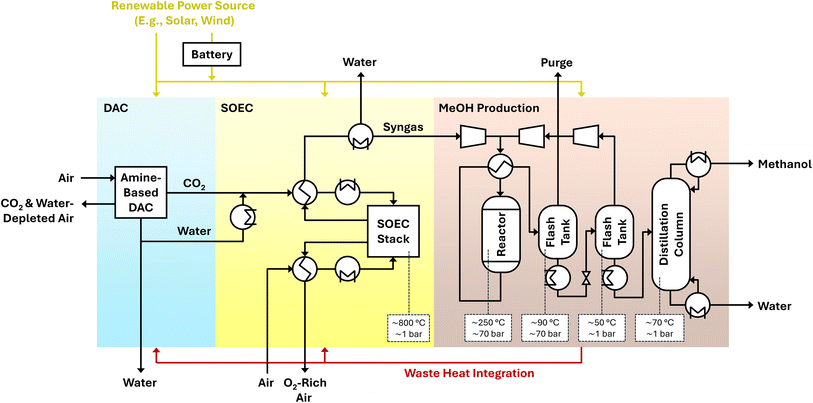
The water-positive generation of green methanol is the main focus of the DryHy project. DryHy employs amine-based DAC technologies, which belong to the most advanced and most energy efficient DAC processes that are currently available.21 In addition, amine-based DAC does not only separate CO2, but also separates water, which is typically an undesired byproduct in DAC processes. However, DryHy employs an innovative approach whereby the water produced as a byproduct of the DAC is utilized for electrolysis, in contrast to the depletion of local water sources, which is a consequence of common synthesis approaches for e-fuels. The DAC device for CO2 and H2O separation from air is combined with a downstream solid oxide electrolyzer cell (SOEC) for syngas production and a methanol reactor.
This approach will be implemented on a demonstrator scale with the objective of bridging the gap between small-scale research and industrial application, thereby facilitating the transition of the technology to a more mature market position. With this, the DryHy project aims to provide technologies for achieving the climate goals, while increasing the energy security through diversification and lowering costs by methanol production in regions with cheap renewable power generation. Additionally, the project aims at establishing new partnerships and opening up new market options. In the following, the approach, synergies and challenges of the DryHy process are discussed from a technical perspective.
2 The DryHy approach
The conceptual design of the DryHy process is presented in a process flow diagram in Fig. 3. The designed process comprises three sub-processes. First, the DAC process extracts both water and CO2 from ambient air and separates them. Any surplus water captured is purified for use as drinking water. The captured water and carbon dioxide are then directed to a co-electrolysis process, where syngas is produced via high-temperature electrolysis using solid oxide electrolysis cells (SOEC). In the final sub-process, the produced syngas is converted into methanol.Solar energy powers the entire process. Depending on economic considerations, the plant will either operate only during daylight hours, with a hot stand-by phase during the night, or continuously over the 24 hour cycle using a suitable photovoltaic energy storage such as batteries. Additionally, there is potential for heat integration due to the exothermic reactions occurring in the methanol production sub-process.
3 Direct air capture
The term DAC encompasses a range of technologies that facilitate the capture of CO2 from the atmosphere and subsequent enrichment. To achieve this at the low concentration of 400 ppm CO2 in air, processes are required that selectively bind CO2. Typically, the strong interaction of carbon dioxide, a Lewis acid, with a corresponding Lewis base is exploited. For example, amines in amine-based DAC or an aqueous hydroxide solution in calcium looping can be suitable bases to bind CO2. To release CO2 and regenerate the base, heat is usually used.Numerous technologies for direct air capture have been developed.22–24 Of these, however, amine-based chemisorption of carbon dioxide and calcium looping are the most advanced and technologically mature.21,23,25,26 Since the DryHy process focuses on the demonstration level, only these two technologies, which are available beyond the laboratory scale, are discussed in detail in this article.
As depicted in Fig. 4a, calcium looping consist of two cycles, a sodium-based cycle and a calcium-based cycle. In the sodium cycle, CO2 is captured by contacting air with an aqueous solution of sodium hydroxide, which dissolves the CO2 as sodium carbonate. Subsequently, the carbonate solution is mixed with calcium hydroxide, precipitating the carbonate as calcium carbonate and regenerating the sodium hydroxide. In the calcium cycle, the precipitate is dried and calcined at temperatures exceeding 700 °C to form gaseous CO2 and solid calcium oxide.25 Finally, the calcium oxide is regenerated to calcium hydroxide by the addition of water.
While calcium looping can capture CO2 very selectively with high purity and reasonable energy consumption (6–10 GJ t−1),21 there are some drawbacks. Firstly, a calciner unit requires high temperatures, which are usually provided by burning fuels inside the reactor. In order to achieve carbon neutrality and pure CO2 as product, a carbon neutral fuel is needed and pure oxygen not to dilute the CO2 with nitrogen from air.25 Secondly, contacting large amounts of air with an aqueous sodium hydroxide solution results in a significant amount of water evaporation and thus, a high water consumption. For example, Carbon Engineering specified the water consumption of their technology to 4.7 tons per ton of CO2.27 In particular, when considering the context of water-conscious CO2 capture in arid regions, this technology is not an appropriate solution.
Amine-based DAC utilizes chemisorption of CO2 on porous, amine-functionalized adsorbents.28 CO2, a Lewis acid, interacts strongly with the amine groups, which act as Lewis base, forming a carbamate in dry conditions.25 In the presence of water, the water itself can adsorb on the amine groups as well due to their polar nature. However, unlike most adsorbents, where a competitive adsorption between water and CO2 is observed, amine-based adsorbents feature a cooperative effect.29,30 In the presence of water, the CO2 forms a bicarbonate with water at the amine group leading to an enhanced CO2 capacity.25
For the separation process usually a temperature-vacuum-swing adsorption (TVSA) approach is used (Fig. 4b):21 at ambient conditions, air is streaming through a packed bed of the solid amine adsorbent. Once the adsorbent is saturated, CO2 is released shifting the equilibrium by increasing the temperature (usually 80–130 °C)21 and applying vacuum. After the bed is cooled down again, the cycle can start again.
In comparison to calcium looping, the TVSA process uses much lower temperatures for regeneration, making the integration of waste heat possible. However, the TVSA process is a discontinuous process. Thus, to achieve near-continuous production, typically multiple packed bed shifted in cycle time are used. In addition, alternatives to TVSA processes are being developed that move the adsorbent itself through zones of adsorption and regeneration leading to a continuous production of CO2.31
Usually the separation of water from air besides CO2 is regarded as disadvantageous, as the desorption of additional water increases the energy demand during regeneration. However, at the current state it is not possible to capture only CO2 without capturing water in amine-based DAC. In addition, the energy consumption of amine-based DAC is usually estimated to be about 4.8–8.3 GJ t−1,21,32,33 which is comparable to the estimated energy consumption of calcium looping. Accordingly, there is no discernible advantage in terms of energy efficiency when employing calcium looping instead of the water-positive amine-based DAC. In the context of the DryHy project, the water, which is obtained as byproduct from the amine-based DAC, is essential in order to provide water for the production of methanol. The amount of water depends on adsorbent and process design as well as weather conditions. Fasihi et al. reported a mass ratio of 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for water to CO2 of the Climeworks DAC technology.32 However, data in this regard is scarce and further research especially in regard to temperature and relative humidity dependence is needed, as the amount of water separated will likely differ in northern Europe from a placement in an arid region in northern Africa as well as vary over time as the weather conditions change.
1 for water to CO2 of the Climeworks DAC technology.32 However, data in this regard is scarce and further research especially in regard to temperature and relative humidity dependence is needed, as the amount of water separated will likely differ in northern Europe from a placement in an arid region in northern Africa as well as vary over time as the weather conditions change.
Usually a CO2 purity of about 90% up to more than 99.9% can be achieved with this technology.32 Besides water, other contaminants include mostly N2 and O2 from air. In addition, ammonia might be present in traces as well from the decomposition of the adsorbent, which, due to its high solubility in water, will be mostly present in the aqueous phase. Reports of trace impurities such as NOx, SOx or H2S in the product gas are scarce. SOx has been reported to adsorb irreversibly on the amines leading to blocking of the adsorption site and degradation of the adsorbent.25,34 Nonetheless, it will be important to keep track of the traces of sulfur components in the product gas, as these can cause severe degradation of the SOEC.
4 Solid oxide electrolyser cells
In DryHy the conversion of water and carbon dioxide to syngas is achieved using Solid Oxide Electrolyzer Cells (SOEC).35–38 Solid oxide electrolysis is a high-temperature technology that uses a solid oxide ceramic material as the electrolyte and can be used to split water, carbon dioxide, or a mixture of both into hydrogen, carbon monoxide, and oxygen. SOEC is a promising technology for renewable power generation integration, enabling efficient hydrogen production, carbon recycling, and the synthesis of syngas for sustainable fuel and chemical production.39The SOEC technology enables the direct conversion of water and CO2 into syngas (a mixture of hydrogen and carbon monoxide) at high temperatures (usually 600–850 °C) and atmospheric pressure.39,40 The main electrochemical reactions that take place inside the SOEC when it operates in co-electrolysis mode are given below:
 | (1) |
 | (2) |
Further, the reverse water-gas shift reaction takes place and is often considered to reach equilibrium rapidly at operation conditions.
| CO2 + H2 ⇌ CO + H2O | (3) |
Depending on the operation temperature, methanation might occur to some extent inside the SOEC. Both methanation reactions presented are exothermic and favored by lower temperatures and higher pressures.
| CO + 3H2 ⇌ CH4 + H2O | (4) |
| CO2 + 4H2 ⇌ CH4 + 2H2O | (5) |
In DryHy, the SOEC sub-process comprises an evaporator for the production of steam, two heat exchangers and two heating plates, the SOEC stack, as well as a condenser for water condensation and separation from the stack outlet stream (Fig. 3).
Over the last half-century, electrolysis technologies have rapidly developed. Some of the most important ones are: alkaline electrolysis, proton exchange membrane (PEM) electrolysis and solid oxide electrolysis (SOE).35,38,41,42 While all three technologies are mainly developed for hydrogen production, solid oxide electrolysis can be effectively used for syngas production.
The SOEC distinguishes itself from other electrolysis technologies for two main reasons. Firstly, the high temperature facilitates faster kinetics and higher ionic conductivity, resulting in a decrease of the ohmic resistance of the solid oxide electrolyte and other cell components. The superior kinetics of SOEC technology are evident when comparing the area-specific resistance (ASR) of various electrolysis methods, with SOEC exhibiting lower resistances compared to alkaline and proton exchange membrane (PEM) electrolysis systems.39 Lower resistance leads to reduced voltage losses and less electrical power consumption. Finally, due to the higher operation temperatures, it requires less electrical work and can make use of more heat instead, this way a higher percentage of the total energy demand can be provided through heat. This is best exemplified by thermodynamics as shown below.
| ΔG = ΔH − TΔS | (6) |
In the above equation, ΔG is the change in Gibbs free energy and equals the electrical work that must be added to the system, while TΔS (the product of temperature times the entropy change, which is positive for electrolysis mode) equals the heat that needs to be added to the system. For the same ΔH (the enthalpy change does not change significantly as temperature increases43,44), the electrical work that must be added to the system decreases with increasing temperature. At the same time, the heat that must be added to the system increases.40 Overall, the SOEC achieves the conversion of CO2 and water in a single system with high energy efficiency and the flexibility to vary between heat and electrical supply to a certain degree. The high energy efficiency of solid oxide electrolysis cell systems has already been demonstrated experimentally in small-scale systems for hydrogen production45,46 and generally accepted to be higher than this of other electrolysis technologies.47 Within the DryHy project, energy efficiencies for syngas production will also be assessed using a demonstration system.
It is worth noting that producing a syngas mixture is not the only option for producing methanol and other green hydrogen carriers. Synthesis routes from a mixture of H2 and CO2 are also possible and circumvent the need for CO2 reduction to CO via SOEC.48,49 Techno-economic analyses done by Zhang et al. show the syngas pathway to be a feasible approach for methanol production,50,51 and, showed it to produce methanol at a lower price than the alternative that starts from a mixture of H2 and CO2.49 Several techno-economic analyses have been conducted in the past years on the power-to-methanol topic,52–54 a comprehensive techno-economic analysis, incorporating the specific features of the DryHy process, will also be conducted during the project to benchmark its performance against alternative approaches.55
The SOEC technology also has certain challenges. The degradation of solid oxide electrolysis cells poses a significant challenge to their long-term stability and performance. The rate of degradation depends on a number of factors including the selected material, fabrication methods, and operation conditions. The operation mode and conditions that impact the long-term stability of a SOEC comprise the operation temperature, current density, as well as the composition and purity of the feed gas.43,56,57
In the relevant literature a wide range of degradation rates have been reported for solid oxide cells. This is to be expected, since degradation rates depend on operating mode and conditions, feed composition and stack/system design. Some studies report degradation rates of 3.96%/1000 h,57 while others report significantly lower degradation rates of 0.6%/1000 h (ref. 58) or even 0.33%/1000 h (ref. 39) (percentages correspond to cell voltage change).58 Therefore, it is important to point out that even though SOEC offers faster kinetics and higher energy efficiency, the (often) faster degradation of the cells47 means that they might eventually be outperformed by other electrolysis methods. To the best of our knowledge, there's no techno-economic study comparing electrolysis technologies taking into account cell degradation.
One potential cause of degradation is the redox cycling of nickel (Ni) in the Ni/yttria-stabilized zirconia (YSZ) electrode. This occurs when the reducing atmosphere, typically maintained by recirculation of hydrogen or syngas, is interrupted. In such cases, Ni oxidizes to NiO, leading to a volumetric expansion. This volumetric expansion generates mechanical stress within the electrode, causing cracks, delamination, and severe structural damage. Redox cycling results in irreversible degradation by compromising electron conduction pathways and reducing the electrochemical surface area, further weakening the electrode–electrolyte interface.59,60 It is therefore essential to implement the necessary mechanisms and safety measures to maintain a reductive atmosphere, even in the event of an unforeseen shutdown.
Further, nickel migration and agglomeration within the Ni/YSZ cermet contribute to long-term degradation. Ni particles coarsen and migrate under high temperatures and current densities, leading to a reduction in active sites for electrochemical reactions. This depletion of Ni from localized areas impairs the overall efficiency of the cell, as fewer catalytic sites remain for the electrochemical processes to occur.59,60
Another reason for degeneration is the formation and deposition of solid carbon in downstream system components and within the SOEC fuel electrode itself. The deposition of solid carbon, including the formation of carbon nanotubes, within the fuel electrode of the SOEC can block active sites, disrupt electron pathways, and cause additional structural stress. Carbon deposition in downstream components can also lead to blockages of, e.g., heat exchangers, negatively affecting system performance and durability through increased pressure losses, reduced heat transfer surface as well as metal dusting. Preventing carbon formation is essential, particularly in high-carbon-content environments or when operating with syngas, but it is manageable by careful selection of operation conditions.61–63
Finally, SOEC degradation is accelerated by impurities such as silicon, chromium, and sulfur, which may originate from system components or feed gases. These impurities can poison the active catalyst sites, and accelerate material breakdown, impacting both the electrode and electrolyte.60,64 Mitigating degeneration is crucial for developing more durable SOECs. Current research focuses on optimizing material composition, improving electrode stability, reducing the impact of redox cycling and carbon deposition, and optimizing operating parameters.59,60
5 Methanol production
The production of methanol from syngas is a well-established and thoroughly studied process.15,65–68 The most commonly used process variant comprises a high-pressure tubular flow reactor, two flash tanks, and a distillation column for downstream processing. The reactor is packed with a catalyst composed of CuO/ZnO/Al2O3 and operates under conditions of 240–270 °C and 50–100 bar.69 Within the reactor, the following reactions occur:| CO + 2H2 ⇌ CH3OH | (7) |
| CO2 + 3H2 ⇌ CH3OH + H2O | (8) |
| CO + H2O ⇌ CO2 + H2 | (9) |
The methanol process presents some potential for heat integration. The reactions inside the methanol reactor are overall exothermic, producing heat that can be used upstream either for the DAC or the steam generation. Note that the second reaction (cf.eqn (8)) produces water as a by-product. The flash tanks separate the gaseous components such as carbon monoxide and hydrogen to be recycled, while the distillation column purifies methanol by separating it from water.
The composition of the syngas plays an important role in the efficiency and topology of the methanol production process. For example, when CO2 is present in the syngas, water will be produced which will need to be separated via an energy-intensive distillation process, leading to lower overall process efficiencies. In addition, the formed water in CO2-rich methanol synthesis can inhibit the reaction kinetics due to water adsorption known as reversible effect.70–72 Long-term stability may be crucially affected by the formed water in CO2-based methanol synthesis leading to sintering of the catalyst which causes a frequent replacement of catalyst regarded as drawback in terms of economic performance.65,70,71,73 Furthermore, higher CO2 content will lead to lower methanol single pass conversion. Thus, limiting the amount of carbon dioxide in the produced syngas might be beneficial.
Alternatively, the carbon dioxide can be separated at the start of the methanol process with absorption and recycled upstream to avoid distillation downstream. But the presence of high CO and low CO2 contents in syngas leads to elevated formation of by-products like higher alcohols.74,75 All these alternatives must be studied and optimized to ensure that an optimal process design is found for the DryHy concept.
6 Process implementation
The combination of these three technologies has great potential, but for their implementation in the DryHy process, several challenges must be investigated and overcome. Primarily, the impact of impurities from DAC on the SOEC needs rigorous experimental examination, with particular focus on the influence of sulfuric components (SOx), as these can cause degradation.56 However, unless placed in the vicinity of an emitting source (e.g., industry or volcano) the concentration of sulfuric components is expected to be negligible small. For example, the SO2 concentration is typically far below 1 ppm.76 Additionally, data indicates that sulfuric components such as SO2 are irreversibly adsorbed in the DAC.34 Nonetheless, this should be closely monitored as data in this regard is still scarce.As mentioned above, other typical components include nitrogen and oxygen from air as well as small amounts of ammonia from possible degradation of the adsorbent. The ammonia does not present an issue, especially in small traces, as it thermally decomposes to nitrogen and hydrogen in the temperature range used in the SOEC.77
Any oxygen in form of O2 present in the CO2 can react with the produced hydrogen to water and thus, lower the efficiency of the SOEC. However, as oxygen is typically only present in low concentrations (well below 2%), the efficiency loss is limited. Additionally, this side reactions produces heat and, therefore, lowers the heating demand of the SOEC. Consequently, it has to be seen, if and to what extend oxygen actually impacts the efficiency.
As an inert gas, nitrogen does not impact the SOEC significantly, but will be relevant in the methanol reactor. For efficient syngas usage, any syngas that did not react in the methanol reactor in the first pass is recycled to be used again. Consequently, any nitrogen contamination will accumulate in the loop and, therefore, increase the amount of gas that needs to be purged. To avoid emitting poisonous gas, the offgas will be burnt, oxidizing H2 and CO to H2O and CO2, respectively. Since the CO2 stems from the DAC, the overall process is still CO2-negative, but the purge will slightly lower the efficiency. Other significant emissions are not expected from the DryHy process.
Since the DAC is not only removing CO2 but also water from the air, the impact of DAC on the water content in the local atmosphere has to be monitored. Especially, a potential impact on the rain building mechanisms has to be studied. As this will strongly depend on the size of the DAC plant, a simulation approach is a suitable way to investigate this correlation for various plant sizes.
Furthermore, the formation of carbon post-SOEC presents a considerable challenge. Fig. 5 delineates the conditions under which carbon formation can occur spontaneously at the outlet of an isothermal SOEC for various carbon-to-hydrogen ratios. The Boudouard equilibrium, methane reforming and methane decomposition that lead to the formation of solid carbon are shown below.
| 2CO ⇌ CO2 + C(s) | (10) |
| CH4 + 2CO ⇌ 2H2O + 3C(s) | (11) |
| CH4 ⇌ 2H2 + C(s) | (12) |
 | ||
| Fig. 5 Carbon formation limit as a function of temperature and utilization of CO2 and water as well as the H/C ratio. Carbon formation is thermodynamically favored when operating in conditions above the corresponding curve. The methodology for the calculations is given in the ESI.† | ||
Carbon formation can be avoided either by staying in the “thermodynamic-safe region” (below the respective carbon formation boundary) or by operating in a region, where carbon formation is spontaneous, but suppressed by a rapid cool-down to overcome the kinetics of the carbon-forming reactions. These two scenarios are depicted for a feed with a hydrogen-to-carbon atom ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (the required ratio for methanol production). In the first scenario (shown with a gray arrow in Fig. 5), high utilization rates are achieved (roughly 85%) and rapid cooling is applied to overcome the kinetics of carbon formation. Alternatively, the second scenario (shown with a black arrow on Fig. 5) maintains low utilization rates (roughly 50%) to remain within the “thermodynamic-safe region” and definitively avoids carbon formation.
1 (the required ratio for methanol production). In the first scenario (shown with a gray arrow in Fig. 5), high utilization rates are achieved (roughly 85%) and rapid cooling is applied to overcome the kinetics of carbon formation. Alternatively, the second scenario (shown with a black arrow on Fig. 5) maintains low utilization rates (roughly 50%) to remain within the “thermodynamic-safe region” and definitively avoids carbon formation.
The operation mode of the SOEC has implications on the process layout as well. For operation in the thermodynamic safe region, a significant amount of CO2 and water is left in the product gas, which has to be separated before feeding it to the methanol reactor. Otherwise, a significant amount of water would be produced together with methanol, which would require energy-intense separation. Additionally, a higher hydrogen-to-carbon ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 would be necessary for the reaction of CO2 to methanol, while also shifting the equilibrium more towards the educts.65 Consequently, a low conversion to methanol per pass would be the result if CO2 is present in significant amounts. To avoid this, any CO2 leaving the SOEC could be separated, e.g., by amine washing, while most of the water can be condensed. Subsequently, both, CO2 and water, can be recycled to the SOEC. However, the separation of water and CO2 will add to the energy requirements of the process.
1 would be necessary for the reaction of CO2 to methanol, while also shifting the equilibrium more towards the educts.65 Consequently, a low conversion to methanol per pass would be the result if CO2 is present in significant amounts. To avoid this, any CO2 leaving the SOEC could be separated, e.g., by amine washing, while most of the water can be condensed. Subsequently, both, CO2 and water, can be recycled to the SOEC. However, the separation of water and CO2 will add to the energy requirements of the process.
The alternative, applying a high utilization and preventing carbon formation by rapid cool-down, can be carried out (e.g., by spraying the hot syngas with water or mixing it with steam and later on separating the water with condensation) without the necessity for additional carbon dioxide separation processes, as the CO2 content in the syngas is low. But in turn, a device with rapid cooling ability is required, and fast cooldown of the produced syngas likely means that little to no heat can be recovered by the hot syngas stream. For the process layout, it will be essential to thoroughly analyze, which of these two approaches is overall more energy efficient and offers more benefits.
Further, the dynamic power supply necessitates study. It is imperative to develop effective ramp-up and ramp-down strategies, as well as to establish a hot stand-by operation mode to manage the entire power cycle efficiently.
For future developments, it is also important to consider the scalability of the DryHy process and its technologies. The scale-up of DAC systems is one of the major research focuses of this technology with companies such as Climeworks or Carbon Engineering pushing the limits of the current DAC plant size. For amine-based solid sorbent DAC, a modular approach is possible and often deployed.21 In a modular design, the DAC system is partitioned into modules of the same size, which enable mass production of the same unit and bringing down production costs.21 Nonetheless, at the current state, DAC is an expensive technology and capital and operational costs need to come down in the future to make DAC-based process approaches economically more viable.
Similarly, issues with scaling up the SOEC technology must also be considered. A recent review by Jolaoso et al.78 found four main obstacles in scaling up the SOEC technology. Namely, making the SOEC stacks larger and more robust, overcoming the degradation issues, the integration of renewable power sources, and assessing how competitive they are with their competitors, namely alkaline and PEM electrolysis. The DryHy project presents a great opportunity for addressing these challenges.
Since methanol synthesis, especially from syngas being rich in CO, is an industrially proven technology already operated in large units, scaling the process is not seen as a problem.79 Moreover, even adaptions of the reactor to the DryHy context are not expected to cause hindering issues because of the use of fixed-bed technology which is known as unproblematic in up-scaling.73
Finally, the economic and social dimensions of implementing the DryHy strategy must also be addressed to ensure its viability. Techno-economic analysis should optimize the capital and operational costs and provide deep insights into the cost structures as well as the sensitivity of the process towards efficiency losses. Material intensities and criticalities should be carefully analyzed as well, including the emissions from materials produced (cradle-to-grave life cycle analysis). Additionally, it is relevant to evaluate the effectiveness of the DryHy process chain in comparison to alternative power-to-X routes, especially in the context of application in arid regions. A comprehensive examination of the economic implications can be found in another source.55
Similarly, establishing cooperations, and building public acceptance in regions suitable for the DryHy process will be crucial for transferring the process into application as well. Therefore, the technology transfer has to be investigated by fostering the exchange with local stakeholders, establishing collaborations and adapting the DryHy concept to locally accepted business models. For this purpose, it is important to make sure that the technical expertise is available at the desired location. Overall, comprehensive studies and strategic planning are essential to overcome these multifaceted challenges and achieve effective implementation.
7 Conclusions
Combining DAC with SOEC and renewable power sources offers a promising solution for producing climate-neutral methanol and other green hydrogen carriers. Amine-based DAC can provide CO2 as well as water, which can be directly and efficiently converted into syngas utilizing the SOEC. Subsequently, the generation of methanol (or fuels) from syngas is a straight-forward process.Harvesting solar energy and producing methanol in sun-rich regions, such as Africa, is especially beneficial for the DryHy process. While the high photovoltaic power potential reduces costs, the separation of water from air by the DAC conserves water resources, which are usually scarce in dry regions. In the future, this approach can foster economic growth and stability, benefiting both local and global markets. Moreover, the transport and utilization of green methanol can leverage existing infrastructure, making it economically viable and sustainable.
Challenges remain in implementing these technologies. The effect of impurities in the CO2 and water mixture from the DAC on the SOEC and methanol reactor needs to be studied closely. Nitrogen and oxygen can potentially lower the efficiency of the process, whereas trace impurities such as sulfuric components can lead to degradation of the SOEC. Additionally, not much data is available on the effect of temperature and humidity on the water-to-CO2 ratio supplied by the DAC and its impact on the DryHy process chain. Finally, the system needs to be designed to adapt to fluctuating energy supplies. Thus, future research should focus on optimizing dynamic behavior and developing storage technologies for energy and intermediate products as well.
In summary, DAC combined with renewable power generation and SOEC offers a sustainable method for green methanol production, supporting climate goals and economic benefits. Continued research and pilot projects are essential to overcome challenges and fully realize this technology's potential.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funding provided by the Federal Ministry of Education and Research (BMBF) within “Project DryHy: Water-positive generation of hydrogen and e-fuels in arid regions (Phase 1).” (FKZ: 03SF0716). The authors would like to express their gratitude to SBC Lehmann for his valuable assistance in designing the graphics for Fig. 4.Notes and references
- IPCC, Climate Change 2021 – the Physical Science Basis, Cambridge University Press, 2021 Search PubMed.
- IEA, Net Zero by 2050 – A Roadmap for the Global Energy Sector, 2021 Search PubMed.
- Climate Change 2022 – Impacts, Adaptation and Vulnerability, ed. Intergovernmental Panel on Climate Change, Cambridge University Press, 2023 Search PubMed.
- S. R. Foit, I. C. Vinke, L. G. J. de Haart and R.-A. Eichel, Angew. Chem., Int. Ed., 2017, 56, 5402–5411 Search PubMed.
- Prognos, Öko-Institut and Wuppertal-Institut, Klimaneutrales Deutschland 2045: Wie Deutschland seine Klimaziele schon vor 2050 erreichen kann: Langfassung, https://www.agora-energiewende.de/publikationen/klimaneutrales-deutschland-2045-1 Search PubMed.
- Bundesministerium für Wirtschaft und Energie, Nationales Reformprogramm 2020 – Die Nationale Wasserstoffstrategie, https://www.bmwk.de/Redaktion/DE/Publikationen/Energie/die-nationale-wasserstoffstrategie.html Search PubMed.
- Agora Industry and TU Hamburg, Wasserstoff-Importoptionen für Deutschland: Analyse mit einer Vertiefung zu Synthetischem Erdgas (SNG) bei nahezu geschlossenem Kohlenstoffkreislauf, https://www.agora-energiewende.de/publikationen/wasserstoff-importoptionen-fuer-deutschland Search PubMed.
- D. DeSantis, B. D. James, C. Houchins, G. Saur and M. Lyubovsky, iScience, 2021, 24, 103495 Search PubMed.
- D. Teichmann, W. Arlt, P. Wasserscheid and R. Freymann, Energy Environ. Sci., 2011, 4, 2767 RSC.
- T. Rüde, S. Dürr, P. Preuster, M. Wolf and P. Wasserscheid, Sustainable Energy Fuels, 2022, 6, 1541–1553 Search PubMed.
- F. Schorn, J. L. Breuer, R. C. Samsun, T. Schnorbus, B. Heuser, R. Peters and D. Stolten, Adv. Appl. Energy, 2021, 3, 100050 CrossRef CAS.
- A. Ullah, N. A. Hashim, M. F. Rabuni and M. U. Mohd Junaidi, Energies, 2023, 16, 1482 CrossRef CAS.
- S. Kang, F. Boshell, A. Goeppert, S. G. Prakash, I. Landälv and D. Saygin, Innovation Outlook: Renewable Methanol, International Renewable Energy Agency, Abu Dhabi, 2021 Search PubMed.
- Methanol Market Services Asia, Methanol Price and Supply/Demand, 2024, https://www.methanol.org/methanol-price-supply-demand/ Search PubMed.
- G. Liu, H. Hagelin-Weaver and B. Welt, Waste, 2023, 1, 228–248 CrossRef.
- R. J. Zomer, J. Xu and A. Trabucco, Sci. Data, 2022, 9, 409 CrossRef PubMed.
- D. Heß, M. Klumpp and R. Dittmeyer, Nutzung von CO2 aus Luft als Rohstoff für synthetische Kraftstoffe und Chemikalien Search PubMed.
- P. Heinzmann, S. Glöser-Chahoud, N. Dahmen, U. Langenmayr, D. Pfleger and F. Schultmann, Techno-ökonomische Bewertung der Produktion regenerativer synthetischer Kraftstoffe Search PubMed.
- D. H. König, M. Freiberg, R.-U. Dietrich and A. Wörner, Fuel, 2015, 159, 289–297 CrossRef.
- A. S. Richey, B. F. Thomas, M.-H. Lo, J. T. Reager, J. S. Famiglietti, K. Voss, S. Swenson and M. Rodell, Water Resour. Res., 2015, 51, 5217–5238 CrossRef PubMed.
- N. McQueen, K. V. Gomes, C. McCormick, K. Blumanthal, M. Pisciotta and J. Wilcox, Prog. Energy, 2021, 3, 032001 CrossRef CAS.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. Mac Dowell, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- E. S. Sanz-Pérez, C. R. Murdock, S. A. Didas and C. W. Jones, Chem. Rev., 2016, 116, 11840–11876 CrossRef PubMed.
- X. Zhu, W. Xie, J. Wu, Y. Miao, C. Xiang, C. Chen, B. Ge, Z. Gan, F. Yang, M. Zhang, D. O'Hare, J. Li, T. Ge and R. Wang, Chem. Soc. Rev., 2022, 51, 6574–6651 RSC.
- A. Goeppert, M. Czaun, G. K. Surya Prakash and G. A. Olah, Energy Environ. Sci., 2012, 5, 7833 RSC.
- A. Yagmur Goren, D. Erdemir and I. Dincer, Environ. Res., 2024, 240, 117503 Search PubMed.
- D. W. Keith, G. Holmes, D. St. Angelo and K. Heidel, Joule, 2018, 2, 1573–1594 CrossRef CAS.
- M. Yang, C. Ma, M. Xu, S. Wang and L. Xu, Curr. Pollut. Rep., 2019, 5, 272–293 CrossRef CAS.
- R. Veneman, N. Frigka, W. Zhao, Z. Li, S. Kersten and W. Brilman, Int. J. Greenhouse Gas Control, 2015, 41, 268–275 CrossRef CAS.
- H. Zhang, A. Goeppert, G. A. Olah and G. S. Prakash, J. CO2 Util., 2017, 19, 91–99 Search PubMed.
- P. Eisenberger and Global Thermostat Operations LLC, System and Method for Carbon Dioxide Capture and Sequestration, US Pat., US8500855B2, 2013 Search PubMed.
- M. Fasihi, O. Efimova and C. Breyer, J. Cleaner Prod., 2019, 224, 957–980 CrossRef CAS.
- A. Sinha and M. J. Realff, AIChE J., 2019, 65, e16607 CrossRef.
- R. A. Khatri, S. S. C. Chuang, Y. Soong and M. Gray, Energy Fuels, 2006, 20, 1514–1520 CrossRef CAS.
- R. Peters, R. Deja, L. Blum, V. N. Nguyen, Q. Fang and D. Stolten, Int. J. Hydrogen Energy, 2015, 40, 7103–7113 Search PubMed.
- L. Blum, Q. Fang, L. G. J. de Haart, J. Malzbender, N. Margaritis, N. H. Menzler and R. Peters, ECS Trans., 2017, 78, 1791 CrossRef CAS.
- L. G. J. de Haart, S. B. Beale, R. Deja, L. Dittrich, T. Duyster, Q. Fang, S. Foit, S. Gross-Barsnick, N. Margaritis, U. de Haart, I. Hoven, N. Kruse, C. Lenser, Q. Ma, N. H. Menzler, D. Naumenko, M. Nohl, R. Peters, D. Sebold, F. Thaler, W. Tiedemann, I. Unachukwu, B. A. Varghese, V. Vibhu, I. C. Vinke, S. E. Wolf, S. Zhang, J. Zurek and L. Blum, ECS Trans., 2021, 103, 299 CrossRef CAS.
- S. E. Wolf, F. E. Winterhalder, V. Vibhu, L. G. J. de Haart, O. Guillon, R.-A. Eichel and N. H. Menzler, J. Mater. Chem. A, 2023, 11, 17977–18028 RSC.
- A. Hauch, R. Küngas, P. Blennow, A. B. Hansen, J. B. Hansen, B. V. Mathiesen and M. B. Mogensen, Science, 2020, 370, eaba6118 CrossRef CAS PubMed.
- Y. Shi, N. Cai, T. Cao and J. Zhang, High-Temperature Electrochemical Energy Conversion and Storage Fundamentals and Applications, CRC Press, 1st edn, 2019 Search PubMed.
- P. Fernández-Arias, A. Antón-Sancho, G. Lampropoulos and D. Vergara, Appl. Sci., 2024, 14, 2524 CrossRef.
- S. Mucci, A. Mitsos and D. Bongartz, Comput. Chem. Eng., 2023, 175, 108260 CrossRef CAS.
- Y. Wang, W. Li, L. Ma, W. Li and X. Liu, J. Mater. Sci. Technol., 2020, 55, 35–55 CrossRef CAS.
- S. H. Jensen, P. H. Larsen and M. Mogensen, Int. J. Hydrogen Energy, 2007, 32, 3253–3257 CrossRef CAS.
- R. Peters, W. Tiedemann, I. Hoven, R. Deja, N. Kruse, Q. Fang, D. Schäfer, F. Kunz, L. Blum, R. Peters and R.-A. Eichel, J. Electrochem. Soc., 2023, 170, 044509 CrossRef CAS.
- R. Peters, N. Kruse, W. Tiedemann, I. Hoven, R. Deja, D. Schäfer, F. Kunz and R.-A. Eichel, ECS Trans., 2023, 111, 1657 Search PubMed.
- R. A. Abdelsalam, M. Mohamed, H. E. Z. Farag and E. F. El-Saadany, Energy Convers. Manage., 2024, 319, 118907 Search PubMed.
- P. Li, S. Gong, C. Li and Z. Liu, Clean Energy, 2022, 6, 202–210 CrossRef.
- P. Battaglia, G. Buffo, D. Ferrero, M. Santarelli and A. Lanzini, J. CO2 Util., 2021, 44, 101407 CrossRef CAS.
- H. Zhang and U. Desideri, Energy, 2020, 199, 117498 CrossRef CAS.
- H. Zhang, L. Wang, J. Van herle, F. Maréchal and U. Desideri, Energies, 2019, 12, 3742 CrossRef CAS.
- S. Schemme, J. L. Breuer, M. Köller, S. Meschede, F. Walman, R. C. Samsun, R. Peters and D. Stolten, Int. J. Hydrogen Energy, 2020, 45, 5395–5414 CrossRef CAS.
- F. Schorn, J. L. Breuer, R. C. Samsun, T. Schnorbus, B. Heuser, R. Peters and D. Stolten, Adv. Appl. Energy, 2021, 3, 100050 CrossRef CAS.
- M. Decker, F. Schorn, R. C. Samsun, R. Peters and D. Stolten, Appl. Energy, 2019, 250, 1099–1109 CrossRef.
- H. Wenzel, G. Müller, F. Harzendorf, T. Schöb, F. Kullmann, J. M. Weinand and D. Stolten, Nexus, 2025, 2, 100054 CrossRef.
- A. Hauch and P. Blennow, Solid State Ionics, 2023, 391, 116127 CrossRef CAS.
- A. D. N. Kamkeng and M. Wang, Chem. Eng. J., 2022, 429, 132158 CrossRef CAS.
- Q. Fang, U. de Haart, D. Schäfer, F. Thaler, V. Rangel-Hernandez, R. Peters and L. Blum, J. Electrochem. Soc., 2020, 167, 144508 CrossRef CAS.
- H. Choi, J. Shin, C. Yeon, S.-Y. Park, S.-T. Bae, J. W. Kim, J.-H. Lee, J.-W. Park, C.-W. Lee, K. J. Yoon and H. J. Chang, Energy Environ. Sci., 2024, 17, 5410–5420 RSC.
- M. S. Khan and R. Knibbe, Fuel Electrode Materials for Solid Oxide Electrolysis Cells (SOECs), Springer International Publishing, 1st edn, 2023, pp. 91–115 Search PubMed.
- Y. Tao, S. D. Ebbesen and M. B. Mogensen, J. Electrochem. Soc., 2014, 161, F337 CrossRef CAS.
- V. Subotić, P. Harter, M. Kusnezoff, T. W. Napporn, H. Schroettner and C. Hochenauer, Sustainable Energy Fuels, 2021, 5, 2065–2076 RSC.
- B. Königshofer, M. Höber, G. Nusev, P. Boškoski, D. Juricic, N. Margaritis, C. Hochenauer and V. Subotic, J. Power Sources, 2023, 556, 232404 CrossRef.
- D. Schäfer, L. Queda, V. Nischwitz, Q. Fang and L. Blum, Processes, 2022, 10(3), 598 CrossRef.
- V. Dieterich, A. Buttler, A. Hanel, H. Spliethoff and S. Fendt, Energy Environ. Sci., 2020, 13, 3207–3252 RSC.
- I. Sharma, V. Shah and M. Shah, Environ. Technol. Innovation, 2022, 28, 102589 CrossRef CAS.
- M. T. Luu, D. Milani, A. Bahadori and A. Abbas, J. CO2 Util., 2015, 12, 62–76 CrossRef CAS.
- F. Nestler, M. Krüger, J. Full, M. J. Hadrich, R. J. White and A. Schaadt, Chem. Ing. Tech., 2018, 90, 1409–1418 CrossRef CAS.
- M. Pérez-Fortes, J. C. Schöneberger, A. Boulamanti and E. Tzimas, Appl. Energy, 2016, 161, 718–732 CrossRef.
- A. Prašnikar, A. Pavlišič, F. Ruiz-Zepeda, J. Kovač and B. Likozar, Ind. Eng. Chem. Res., 2019, 58, 13021–13029 CrossRef.
- M. B. Fichtl, D. Schlereth, N. Jacobsen, I. Kasatkin, J. Schumann, M. Behrens, R. Schlögl and O. Hinrichsen, Appl. Catal., A, 2015, 502, 262–270 CrossRef CAS.
- J. T. Sun, I. S. Metcalfe and M. Sahibzada, Ind. Eng. Chem. Res., 1999, 38, 3868–3872 CrossRef CAS.
- G. Donati and R. Paludetto, Catal. Today, 1997, 34, 483–533 CrossRef CAS.
- F. Pontzen, W. Liebner, V. Gronemann, M. Rothaemel and B. Ahlers, Catal. Today, 2011, 171, 242–250 CrossRef CAS.
- F. Nestler, J. Voß, A. Fastabend, T. Niemeier, H. Ruland and M. J. Hadrich, Chem. Ing. Tech., 2024, 96, 1166–1176 CrossRef CAS.
- J. A. Adame, A. Notario, F. Villanueva and J. Albaladejo, Sci. Total Environ., 2012, 429, 281–291 CrossRef CAS PubMed.
- S. R. Arsad, P. J. Ker, M. A. Hannan, S. G. Tang, N. R S, C. F. Chau and T. Mahlia, Int. J. Hydrogen Energy, 2024, 50, 447–472 CrossRef CAS.
- L. A. Jolaoso, I. T. Bello, O. A. Ojelade, A. Yousuf, C. Duan and P. Kazempoor, Int. J. Hydrogen Energy, 2023, 48, 33017–33041 CrossRef CAS.
- S. Schemme, J. L. Breuer, R. C. Samsun, R. Peters and D. Stolten, J. CO2 Util., 2018, 27, 223–237 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4se01783h |
| ‡ These authors contributed equally to this work. |
| § Current address: German Aerospace Center (DLR), Institute of Future Fuels, Linder Hoehe, 51147 Cologne, Germany. |
| This journal is © The Royal Society of Chemistry 2025 |